- 1Department of Dermatology, Michigan Medicine, University of Michigan, Ann Arbor, MI, United States
- 2Department of Biostatistics, School of Public Health, University of Michigan, Ann Arbor, MI, United States
- 3Department of Dermatology, Northwestern Medicine, Northwestern University, Chicago, IL, United States
- 4Department of Urology, Northwestern Medicine, Northwestern University, Chicago, IL, United States
- 5Department of Computational Medicine and Bioinformatics, Michigan Medicine, University of Michigan, Ann Arbor, MI, United States
Immune-mediated skin conditions (IMSCs) are a diverse group of autoimmune diseases associated with significant disease burden. Atopic dermatitis and psoriasis are among the most common IMSCs in the United States and have disproportionate impact on racial and ethnic minorities. African American patients are more likely to develop atopic dermatitis compared to their European American counterparts; and despite lower prevalence of psoriasis among this group, African American patients can suffer from more extensive disease involvement, significant post-inflammatory changes, and a decreased quality of life. While recent studies have been focused on understanding the heterogeneity underlying disease mechanisms and genetic factors at play, little emphasis has been put on the effect of psychosocial or psychological stress on immune pathways, and how these factors contribute to differences in clinical severity, prevalence, and treatment response across ethnic groups. In this review, we explore the heterogeneity of atopic dermatitis and psoriasis between African American and European American patients by summarizing epidemiological studies, addressing potential molecular and environmental factors, with a focus on the intersection between stress and inflammatory pathways.
Introduction
Over 84 million Americans are impacted by at least one skin disease (1), posing significant health and economic burden. Atopic dermatitis (AD) and psoriasis are among the most common and widely studied immune-mediated skin conditions (IMSCs). With recent advances in genomic technology, studies over the past decade (2–8) have focused on elucidating the underlying mechanisms of disease pathogenesis for AD and psoriasis. More specifically, these studies have explored the genetic and molecular factors associated with the disease pathophysiology. However, the primary racial makeup of these studies has been predominantly European American (EA). While AD and psoriasis affect populations of different origins, their burden is exacerbated in some ethnic minority groups (9–11). Yet, there is a paucity of molecular studies describing the driving factors behind ethnic heterogeneity in the severity, presentation, and predominance of IMSCs.
Environmental factors, such as stress, are known to play roles in shaping the onset and clinical severity of IMSCs (12–14). While chronic stressors may impact any individual, unique psychosocial factors such as racism, discrimination, and acculturative stress (anxiety or tension related to efforts to adapt to the values of dominant culture within a society) are unique among ethnic minorities (15). Studies from the US Department of Health and Human Services Office of Minority Health show that African American (AA) adults living below the poverty line are twice as likely to experience psychological distress (16) and the downstream effects of these stressors can result in decreased likelihood of receiving medical care (17, 18). It is therefore important to understand the influence of stressors, including psychosocial stress, on the pathogenesis of IMSCs, especially as this may play role in the ethnic differences seen across common IMSCs.
In this review, we explore the heterogeneity in AD and psoriasis across AA and EA patients by summarizing epidemiological studies, as well as the potential molecular and environmental factors involved in disease pathogenesis. We also place particular focus on the intersections between known stress pathways and IMSC inflammatory pathways in the literature.
Atopic Dermatitis
Epidemiology
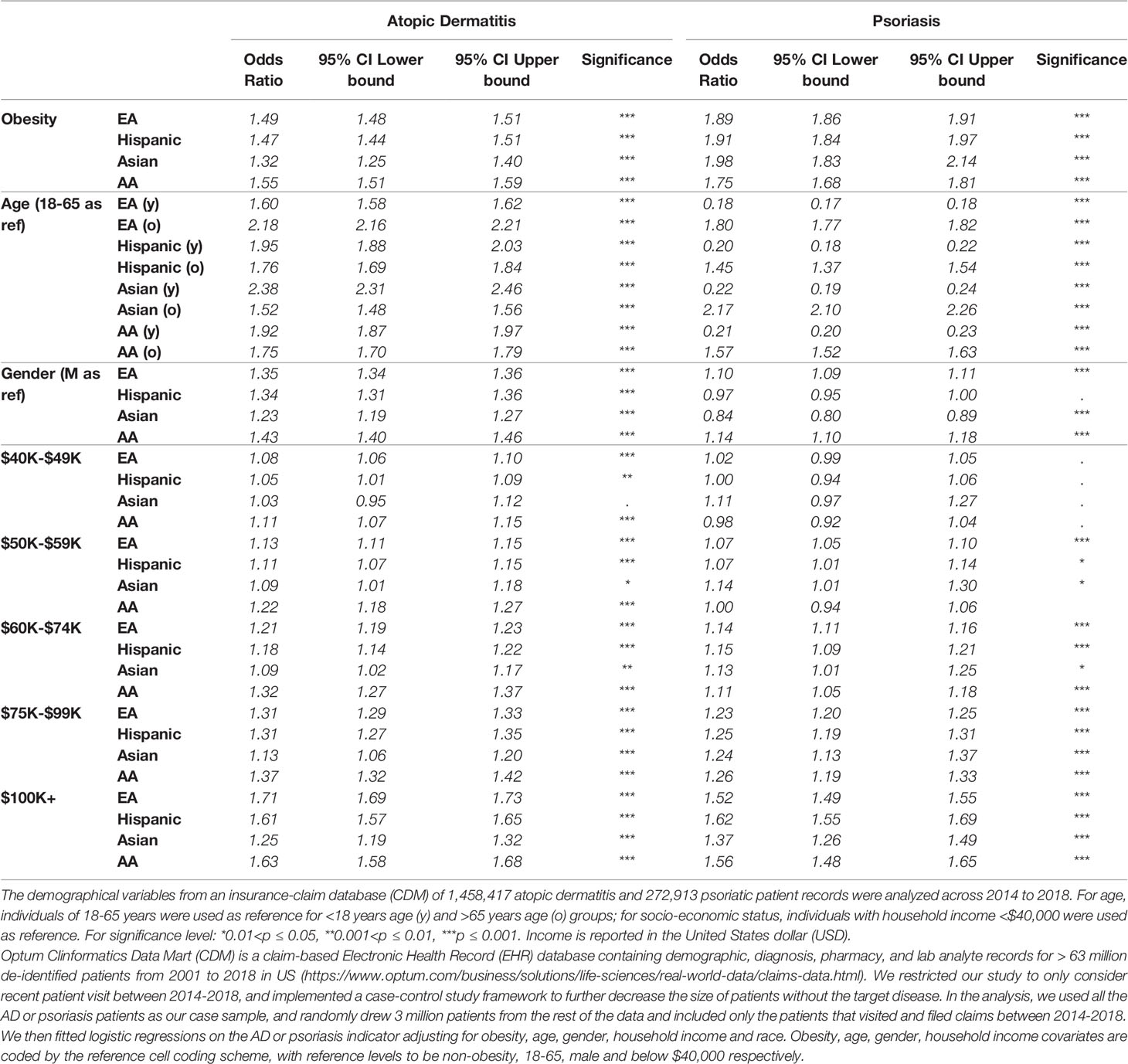
Atopic dermatitis (AD) is an inflammatory skin condition affecting more than 18 million adults in the United States (19, 20). This condition classically presents with pruritic, erythematous plaques involving the flexor surfaces, particularly in the antecubital and popliteal fossae. In Fitzpatrick skin types IV through VI however, eczematous patches often appear brown, purple, or ashen grey in color (11). Clinically, AD often presents with greater involvement of the flexural surfaces in adults, however patients of African descent are more likely to present with more prominent involvement of the extensor surfaces (21). Previous epidemiological studies using self-reported ethnic information highlight a slightly higher prevalence of AD in AA patients when compared with EAs (19.3% versus 16.1%) (22). This predominance is also seen at young age, with AA children found to be 1.7 times more likely to develop AD compared to their EA counterparts, even after adjusting for health insurance and socioeconomic status (11). In addition to reported racial differences in AD prevalence, AA children as a group has been reported to have more severe disease than EA children (23); the study also suggested structural racism or the increased proportion of AA children living in lower income, segregated communities with exposure to greater environmental stressors, are associated with disease severity. To further validate and understand the epidemiological factors involved, we studied the demographical variables from an insurance-claim database, Optum Clinformatics Data Mart (CDM), consisting of 1,458,417 AD patient records across 2014 to 2018 (Table 1). As expected, the association of AD-related clinical visits was significantly stronger at younger age (<18 years) for all ethnic groups compared with our reference age group (18-65 years) (OR:1.60, 1.95, 2.38, 1.92 for EA, Hispanic, Asian and AA populations, respectively). The older patient population group (>65 years) also had significantly stronger association with AD clinical visits (OR=2.18, 1.76, 1.52, 1.75 for the same four ethnic groups, respectively). The data importantly highlights that gender factors had the largest effect sizes in AA (e.g. OR=1.43 for female) compared to the other ethnic groups (OR between 1.23-1.35 for female). Patients with higher income are also associated with higher prevalance of clinical visit for AD.
Genetics
While the cause of AD is complex, studies over the last decade have provided insights into genetic and environmental factors associated with disease pathogenesis and severity. Filaggrin (FLG), a protein encoding gene from the epidermal differentiation complex (EDC) and expressed in the keratinized layer of the epidermis, plays an important role in skin barrier function, for example by promoting keratinocyte differentiation and rapid cell death (24). FLG expression is immune-modulated by both aryl hydrocarbon receptor signaling and cytokines, resulting in dysregulation within the lesional skin (25). The locus harboring FLG has been identified as one of the strongest genetic signals associated with AD (26). Carriers of the FLG loss-of-function (LOF) variants have increased odds (3-fold) of having AD. While LOF FLG mutations are risk factors for the development of AD in patients of European and Asian descent, this association has not been reported among individuals of African descent. In fact, loss of function FLG mutations are thought to be less common in AA patients with AD when compared to EAs (27), and a recent study suggests that the common FLG mutation found in AA patients are distinct from those in EA and Asians (28). Nevertheless, decreased expression and mutations of filaggrin-2 (FLG2), also from EDC, have been associated with persistent symptoms of AD in AA, while such variations are absent or infrequently found in AD patients of European ancestry (29). The first genome-wide significant association at rs3811419 for AD in AA patients was found to be an expression quantitative trait loci (eQTL) for THEM4 (a gene associated with allergy), and FLG-AS1 (a non-coding RNA that overlaps the filaggrin gene) in blood. The association between the risk allele of this eQTL locus and increased expression of FLG-AS1 can potentially offer an alternative mechanism explaining skin barrier deficiency in AA, although further research is required (30).
While AA patients are more likely to present with more severe AD than EAs patients (22), a recent study challenged the perception that racial heterogeneity in the severity of AD is genetically driven (31). This study found that observed differences of AD in AA patients, including disease severity, were not associated with a continuous measure of African genetic ancestry. This finding supports epidemiologic, rather than genetic, associations with disease severity and suggests the potential roles of other factors such as environmental components (e.g., stress, pollution, social determinants of health) in disease heterogeneity and their influence on disease pathogenesis.
Stress and Immunologic Parameters
Though most studies describing the molecular signature of AD have been conducted in patients of European ancestry, a recent study by Wongvibulsin et al. confirmed previously reported Th2/Th22 skewing in AA patients with AD, along with upregulated Th1 cytokines in lesional skin of AD, contribute to the increased disease severity in AA patients (32). The authors also reported elevated serum C-reactive protein (CRP), ferritin, and blood eosinophils in AA patients when compared to EA patients with AD. In addition, Schmeer et al. measured serum CRP levels across ethnic groups in children aged 2 to 10 years and found significantly higher serum measurements in AA and Hispanic children when compared to EA children (33). In adults, increased activity of two essential pro-inflammatory transcription control pathways, NFĸB and AP-1, was found in AA subjects who also independently reported experiencing greater perceived racial discrimination as assessed through a 17-item Perceived Ethnic Discrimination Questionnaire—Community Version (34) when compared to EA subjects. These findings suggest that psychosocial stressors may play a role in the increased activation of pro-inflammatory pathways.
Existing studies have proposed several mechanisms in the intersection of psychological stress and AD. The first involves an association between the hypothalamus-pituitary-adrenal (HPA) axis and AD (35). AD has been linked to HPA axis alterations, which contribute to several downstream pathological changes in the skin. Adrenocorticotropic hormone (ACTH), which promotes glucocorticoid secretion, can create a negative feedback loop in the HPA axis in response to stress, and early life adversity can modulate the regulation of HPA axis through epigenetic modification of the glucocorticoid receptors in the skin (36). An important modulator in the HPA axis and AD pathogenesis is IL-18, a member of the IL-1 cytokine family implicated in various immune-mediated skin disease including AD, psoriasis, alopecia areata, dermatomyositis, and cutaneous lupus erythematous (37–41). ACTH can also activates caspase-1 and keratin 1, leading to keratinocyte production of IL-18 (42). In the absence of IL-12, IL-18 has been shown to modulate the Th2 pathway, inducing expression of IL-4, IL-13, and IgE by basophils (43) The downstream effect of these expressed factors has been linked to AD pathogenesis. IL-13 is a prominent Th2 cytokine (6, 44); and serum IgE levels are elevated among AA patients with AD when compared to all other racial/ethnic groups (22). Lastly, IL-18 is thought to directly activate mast cells, leading to release of the enzyme chymase which cleaves pro-IL-18 and potentially accelerates the inflammatory response in AD lesions (45). Existing evidence shows higher clinical AD severity index score (SCORing Atopic Dermatitis or SCORAD) correlate with increased serum IL-18 concentration in AD patients (46). However, as far as the authors are aware, no studies have compared HPA modulation and IL-18 expression across different ethnic groups in AD patients.
Another possible mechanism for the intersection of stress with AD pathogenesis involves chronic psychological stressors that induce serum epinephrine, norepinephrine, and cortisol levels, triggering a shift to a Th2 cytokine profile (47). Though this has not been substantiated in AD through measurement of serum levels, salivary cortisol level has been correlated with SCORAD index scores among patients with elevated stress levels (48). In addition, genetic variants of interferon regulatory factor 2 (IRF2), a protein with crucial roles in immune response, including the regulation of IFNγ and basophil expansion, as well as the transcription of gasdermin D, are associated with AD risk in both AA and EAs (49). Gasdermin D is a critical mediator of inflammatory pathologies, with its non-canonical inflammasome signaling pathway leading to proteolytic activation of IL-1B and IL-18. The pro-inflammatory cytokine, IL-1B, along with TNF-alpha induce expression of 11 beta-hydroxysteriod dehydrogenase, are critical enzymes involved in cortisol synthesis and hypothesized to modulate pro-inflammatory cytokine expression in keratinocytes (50). Our recent work using skin and 3D human skin equivalents (HSE) also demonstrates that skin from AA patients exhibits stronger inflammatory response when compared with that from EA patients. The differentially expressed genes (DEG) in AA skin include those that encode immunoglobulins and their receptors such as FCER1G; proinflammatory genes such as TNF, IL-32; and different EDC and keratin genes. By investigating the effect of TNF signaling on HSE, we further demonstrated enhanced TNF pro-inflammatory effects in AA HSE (51).
Psoriasis
Epidemiology
Psoriasis is a chronic IMSC with variable prevalence across populations. It has a lower prevalence among AA patients when compared with EAs (0.22% to 1.9% in AA vs 1.28% to 3.6% in EA) in the United States (52). Nevertheless, AA patients have been found to have more extensive disease involvement and higher rate of comorbidities including diabetes, hypertension, and hyperlipidemia, after controlling for age and body mass index, when directly compared to EAs (53, 54). While erythematous plaques with thick overlying scale is characteristic of plaque psoriasis, AA patients often present with less conspicuous erythema and a higher degree of dyspigmentation (55), which can often take months to years to resolve. These pigmentary changes can often be of equal or greater concern to patients than the psoriasis itself and contributes to the report of increased disease severity, greater psychological impact, treatment dissatisfaction, and decreased quality of life among AA patients (9, 54). There is currently little consensus regarding gender differences in psoriasis (56), particularly for underrepresented ethnic groups.
We used the CDM insurance-claim database to review the associated demographic factors with 272,913 psoriatic patients with diagnosis between 2014 and 2018 (Table 1). Specifically, the impact of gender on psoriasis was found to be significantly different across ethnic groups, with EA and AA women having higher psoriasis diagnosis rates compared to men (OR=1.10, 1.14); in contrast, the gender effect was not significant in Hispanic patients (p=0.098), and within the Asian population, females had significantly lower rate (OR=0.84). Previous studies showed ambiguous results when estimating the prevalence of psoriasis in each gender, but overall the differences are very minimal (57). We also observed that psoriasis risk was significantly higher for people with obesity across different ethnic groups, and the association between clinical visits for psoriasis was significantly lower among patients with lower income.
Genetics
Like AD, psoriasis has a complex genetic architecture with >80 different disease susceptibility loci identified, the majority of which only contribute to a modest effect of disease association. The HLA-Cw6 is the most prominent disease-associated signal, with >4 OR being revealed (58), and multiple different work have also highlighted other independent signals in the MHC region (59, 60). Despite >10 years since the first GWAS for psoriasis was conducted, large-scale genetic studies for psoriasis have been exclusively been based on EA, Chinese, and Japanese populations (4, 7, 61–64). Up to now, only very limited small GWAS study on psoriasis is based on African ancestry (65). While this can be attributed by the lower incidence rate of psoriasis and the more complex design for GWAS in individuals of African ancestry, the lack of diversity in genetic research for psoriasis needs to be addressed in order to understand the disease heterogeneity and to facilitate the fine-mapping of ethnic-shared/unique causal variations.
Stress and Immunologic Parameters
Increased corticotropin-releasing hormone (CRH) from stress leads to increased serum cortisol levels and decreased brain derived neurotrophic factor (BDNF) (66). CRH, encoded by the CRHR-1 gene, is a peptide hormone that is essential in the physiologic response to stress. Elevation in serum CRH levels is associated with exposure to stress in psoriatic and AD patients, providing a link between stress and the HPA in both conditions (67). In psoriatic patients, increased serum CRH and decreased skin CRHR-1 expression have also been linked to the induction of vascular endothelial growth factor (VEGF) release from mast cells (67). VEGF is a known growth factor involved in the pathogenesis of psoriatic lesions and it is therefore hypothesized that these pathways are linked through increased levels of CRH playing a role in the activation of mast cells that release VEGF (68). CRH has also been implicated in stimulating the production of IL-6 and IL-11, and the downregulation of IL-1B, IL-2, and IL-18 in keratinocytes (69, 70); and a previous work has found elevated serum cortisol level in psoriatic patients when compared to healthy controls under increased psychosocial stress (71). These authors proposed that psoriatic patients have a robust neuroendocrine response in the presence of acute stressors, increasing vulnerability to psoriatic activity. On the other hand, localized glucocorticoid deficiency in psoriatic skin is associated with epidermal differentiation and inflammatory response, and restoring glucocorticoid biosynthesis can normalize these processes (72). Topical glucocorticoid has been shown to be responded by AA skin in a stronger degree, with the response of AA skin associating with inflammation and metabolic disruptions while the genes responding to glucocorticoid in EA are associated with cell barrier modifications (73). Animal studies have demonstrated the reduction of brain-derived neurotrophic factor (BDNF), a protein with skin related functions in humans including the induction of apoptosis in basal keratinocytes, in response to acute stress (66, 74, 75). These findings suggest that psychosocial stress as a potential factor linking decreased BDNF levels in psoriatic patients. Nevertheless, there is still very limited study that describes the differential impact of stress on chemokine or cytokine expression among AA and EA patients with psoriasis, which requires attention and future investigations.
Conclusion
AD and psoriasis are two of the most common IMSCs that can have significant impact on the quality of life in patients, especially in racial/ethnic minorities. Individuals of African ancestry are more likely to develop AD in childhood, experience more severe disease, and can have atypical presentation in adulthood with greater involvement of the extensor surfaces. Though the prevalence of psoriasis is lower among AA patients, they can suffer from more extensive disease involvement, experience significant post-inflammatory changes, and report decreased quality of life. Previous studies have attempted to account for the differences in AD or psoriasis disease severity and prevalence through variable demographic and genetic components; however, as highlighted in a recent review, the differential severity of AD between AA and EA patients cannot solely be explained by association with genetic ancestry (31). Research on the ethnic heterogeneity of IMSCs should place more focus on the role of psychosocial stressors on inflammatory cytokine and chemokine expression and overall disease pathogenesis of these conditions. We review existing studies examining the role of psychological or psychosocial stress at the molecular level in AD and psoriasis, highlighting the role of the HPA axis and IL-18 in AD, CRH and BDNF in psoriasis, and cortisol levels in both.
Author Contributions
LT and TJ planned and designed the work. QL, SS, and MP conducted the EHR analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Psoriasis Foundation (LT, MP, and JG), and awards from the National Institutes of Health (K01AR072129 to LT; 1P30AR075043 to LT, MP, and JG). MP, LT, TJ were also supported by the Dermatology Foundation.
Conflict of Interest
JG has served as a consultant to AbbVie, Eli Lilly, Almirall, Celgene, BMS, Janssen, Prometheus, TimberPharma, Galderma, Novatis, MiRagen, AnaptysBio and has received research support from AbbVie, SunPharma, Eli Lilly, Kyowa Kirin, Almirall, Celgene, BMS, Janssen, Prometheus, and TimberPharma. LT has received support from Janssen and Galderma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. AAD. Burden of Skin Disease (2016). Available at: https://www.aad.org/member/clinical-quality/clinical-care/bsd.
2. Baurecht H, Hotze M, Brand S, Buning C, Cormican P, Corvin A, et al. Genome-Wide Comparative Analysis of Atopic Dermatitis and Psoriasis Gives Insight Into Opposing Genetic Mechanisms. Am J Hum Genet (2015) 96:104–20. doi: 10.1016/j.ajhg.2014.12.004
3. Li B, Tsoi LC, Swindell WR, Gudjonsson JE, Tejasvi T, Johnston A, et al. Transcriptome Analysis of Psoriasis in a Large Case-Control Sample: RNA-Seq Provides Insights Into Disease Mechanisms. J Invest Dermatol (2014) 134:1828–38. doi: 10.1038/jid.2014.28
4. Patrick MT, Stuart PE, Raja K, Gudjonsson JE, Tejasvi T, Yang J, et al. Genetic Signature to Provide Robust Risk Assessment of Psoriatic Arthritis Development in Psoriasis Patients. Nat Commun (2018) 9:4178. doi: 10.1038/s41467-018-06672-6
5. Patrick MT, Stuart PE, Zhang H, Zhao Q, Yin X, He K, et al. Causal Relationship and Shared Genetic Loci Between Psoriasis and Type 2 Diabetes Through Trans-Disease Meta-Analysis. J Invest Dermatol (2021) 141:1493–502. doi: 10.1016/j.jid.2020.11.025
6. Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, et al. Atopic Dermatitis Is an IL-13 Dominant Disease With Greater Molecular Heterogeneity Compared to Psoriasis. J Invest Dermatol (2019) 139:1480–9. doi: 10.1016/j.jid.2018.12.018
7. Tsoi LC, Stuart PE, Tian C, Gudjonsson JE, Das S, Zawistowski M, et al. Large Scale Meta-Analysis Characterizes Genetic Architecture for Common Psoriasis Associated Variants. Nat Commun (2017) 8:15382. doi: 10.1038/ncomms15382
8. Uppala R, Tsoi LC, Harms PW, Wang B, Billi AC, Maverakis E, et al. “Autoinflammatory Psoriasis”-Genetics and Biology of Pustular Psoriasis. Cell Mol Immunol (2021) 18:307–17. doi: 10.1038/s41423-020-0519-3
9. Alexis AF, Blackcloud P. Psoriasis in Skin of Color: Epidemiology, Genetics, Clinical Presentation, and Treatment Nuances. J Clin Aesthet Dermatol (2014) 7:16–24. Retrieved from: https://jcadonline.com
10. Kaufman BP, Alexis AF. Psoriasis in Skin of Color: Insights Into the Epidemiology, Clinical Presentation, Genetics, Quality-Of-Life Impact, and Treatment of Psoriasis in Non-White Racial/Ethnic Groups. Am J Clin Dermatol (2018) 19:405–23. doi: 10.1007/s40257-017-0332-7
11. Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic Dermatitis in Diverse Racial and Ethnic Groups-Variations in Epidemiology, Genetics, Clinical Presentation and Treatment. Exp Dermatol (2018) 27:340–57. doi: 10.1111/exd.13514
12. Kantor R, Silverberg JI. Environmental Risk Factors and Their Role in the Management of Atopic Dermatitis. Expert Rev Clin Immunol (2017) 13:15–26. doi: 10.1080/1744666X.2016.1212660
13. Prescott SL, Larcombe DL, Logan AC, West C, Burks W, Caraballo L, et al. The Skin Microbiome: Impact of Modern Environments on Skin Ecology, Barrier Integrity, and Systemic Immune Programming. World Allergy Organ J (2017) 10:29. doi: 10.1186/s40413-017-0160-5
14. Vojdani A. A Potential Link Between Environmental Triggers and Autoimmunity. Autoimmune Dis (2014) 2014:437231. doi: 10.1155/2014/437231
15. Nevid JS, Rathus SA. Psychology and the Challenges of Life: Adjustment in the New Millennium. 10th. Hoboken, New Jersey: Wiley (2007).
16. CDC. Health. Atlanta, Georgia:United States (2017). Available at: https://www.cdc.gov/nchs/data/hus/hus17.pdf.
17. Lee C, Ayers SL, Kronenfeld JJ. The Association Between Perceived Provider Discrimination, Healthcare Utilization and Health Status in Racial and Ethnic Minorities. Ethn Dis (2009) 19:330–7. Retrieved from: https://www.ethndis.org/edonline/index.php/ethndis
18. Peek ME, Wagner J, Tang H, Baker DC, Chin MH. Self-Reported Racial Discrimination in Health Care and Diabetes Outcomes. Med Care (2011) 49:618–25. doi: 10.1097/MLR.0b013e318215d925
19. Hanifin JM, Reed ML, Eczema P, Impact Working G. A Population-Based Survey of Eczema Prevalence in the United States. Dermatitis (2007) 18:82–91. doi: 10.2310/6620.2007.06034
20. Silverberg JI. Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatol Clinics (2017) 35:283–9. doi: 10.1016/j.det.2017.02.002
21. Vachiramon V, Tey HL, Thompson AE, Yosipovitch G, Lotti R, Dallaglio K, et al. Atopic Dermatitis in African American Children: Addressing Unmet Needs of a Common Disease. Pediatr Dermatol (2012) 29:395–402. doi: 10.1111/j.1525-1470.2012.01740.x
22. Brunner PM, Guttman-Yassky E. Racial Differences in Atopic Dermatitis. Ann Allergy Asthma Immunol (2019) 122:449–55. doi: 10.1016/j.anai.2018.11.015
23. Tackett KJ, Jenkins F, Morrell DS, McShane DB, Burkhart CN. Structural Racism and Its Influence on the Severity of Atopic Dermatitis in African American Children. Pediatr Dermatol (2020) 37:142–6. doi: 10.1111/pde.14058
24. Gutowska-Owsiak D, de la Serna JB, Fritzsche M, Naeem A, Podobas EI, Leeming M, et al. Orchestrated Control of Filaggrin-Actin Scaffolds Underpins Cornification. Cell Death Dis (2018) 9:412. doi: 10.1038/s41419-018-0407-2
25. Furue M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17a, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int J Mol Sci (2020) 21:5382. doi: 10.3390/ijms21155382
26. O’Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in Atopic Dermatitis. J Allergy Clin Immunol (2008) 122:689–93. doi: 10.1016/j.jaci.2008.08.002
27. Margolis DJ, Apter AJ, Gupta J, Hoffstad O, Papadopoulos M, Campbell LE, et al. The Persistence of Atopic Dermatitis and Filaggrin (FLG) Mutations in a US Longitudinal Cohort. J Allergy Clin Immunol (2012) 130:912–7. doi: 10.1016/j.jaci.2012.07.008
28. Zhu Y, Mitra N, Feng Y, Tishkoff S, Hoffstad O, Margolis D. FLG Variation Differs Between European Americans and African Americans. J Invest Dermatol (2021) 141:1855–7. doi: 10.1016/j.jid.2020.12.022
29. Margolis DJ, Gupta J, Apter AJ, Ganguly T, Hoffstad O, Papadopoulos M, et al. Filaggrin-2 Variation Is Associated With More Persistent Atopic Dermatitis in African American Subjects. J Allergy Clin Immunol (2014) 133:784–9. doi: 10.1016/j.jaci.2013.09.015
30. Almoguera B, Vazquez L, Mentch F, March ME, Connolly JJ, Peissig PL, et al. Novel Locus for Atopic Dermatitis in African Americans and Replication in European Americans. J Allergy Clin Immunol (2019) 143:1229–31. doi: 10.1016/j.jaci.2018.10.038
31. Abuabara K, You Y, Margolis DJ, Hoffmann TJ, Risch N, Jorgenson E. Genetic Ancestry Does Not Explain Increased Atopic Dermatitis Susceptibility or Worse Disease Control Among African American Subjects in 2 Large US Cohorts. J Allergy Clin Immunol (2020) 145:192–8.e11. doi: 10.1016/j.jaci.2019.06.044
32. Wongvibulsin S, Sutaria N, Kannan S, Alphonse MP, Belzberg M, Williams KA, et al. Transcriptomic Analysis of Atopic Dermatitis in African Americans Is Characterized by Th2/Th17-Centered Cutaneous Immune Activation. Sci Rep (2021) 11:11175. doi: 10.1038/s41598-021-90105-w
33. Schmeer KK, Tarrence J. Racial-Ethnic Disparities in Inflammation: Evidence of Weathering in Childhood? J Health Soc Behav (2018) 59:411–28. doi: 10.1177/0022146518784592
34. Brondolo E, Kelly KP, Coakley V, Gordon T, Thompson S, Levy E, et al. The Perceived Ethnic Discrimination Questionnaire: Development and Preliminary Validation of a Community Version. J Appl Soc Psychol (2006) 35:335–65. doi: 10.1111/j.1559-1816.2005.tb02124.x
35. Lin TK, Zhong L, Santiago JL. Association Between Stress and the HPA Axis in the Atopic Dermatitis. Int J Mol Sci (2017) 18:2131. doi: 10.3390/ijms18102131
36. Liu PZ, Nusslock R. How Stress Gets Under the Skin: Early Life Adversity and Glucocorticoid Receptor Epigenetic Regulation. Curr Genomics (2018) 19:653–64. doi: 10.2174/1389202919666171228164350
37. Lebre MC, Antons JC, Kalinski P, Schuitemaker JH, van Capel TM, Kapsenberg ML, et al. Double-Stranded RNA-Exposed Human Keratinocytes Promote Th1 Responses by Inducing a Type-1 Polarized Phenotype in Dendritic Cells: Role of Keratinocyte-Derived Tumor Necrosis Factor Alpha, Type I Interferons, and Interleukin-18. J Invest Dermatol (2003) 120:990–7. doi: 10.1046/j.1523-1747.2003.12245.x
38. Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 Regulates Both Th1 and Th2 Responses. Annu Rev Immunol (2001) 19:423–74. doi: 10.1146/annurev.immunol.19.1.423
39. Tsoi LC, Gharaee-Kermani M, Berthier CC, Nault T, Hile GA, Estadt SN, et al. IL18-Containing 5-Gene Signature Distinguishes Histologically Identical Dermatomyositis and Lupus Erythematosus Skin Lesions. JCI Insight (2020) 5:e139558. doi: 10.1172/jci.insight.139558
40. Wang D, Drenker M, Eiz-Vesper B, Werfel T, Wittmann M. Evidence for a Pathogenetic Role of Interleukin-18 in Cutaneous Lupus Erythematosus. Arthritis Rheum (2008) 58:3205–15. doi: 10.1002/art.23868
41. Wittmann M, Macdonald A, Renne J. IL-18 and Skin Inflammation. Autoimmun Rev (2009) 9:45–8. doi: 10.1016/j.autrev.2009.03.003
42. Roth W, Kumar V, Beer HD, Richter M, Wohlenberg C, Reuter U, et al. Keratin 1 Maintains Skin Integrity and Participates in an Inflammatory Network in Skin Through Interleukin-18. J Cell Sci (2012) 125:5269–79. doi: 10.1242/jcs.116574
43. Yoshimoto T, Tsutsui H, Tominaga K, Hoshino K, Okamura H, Akira S, et al. IL-18, Although Antiallergic When Administered With IL-12, Stimulates IL-4 and Histamine Release by Basophils. Proc Natl Acad Sci USA (1999) 96:13962–6. doi: 10.1073/pnas.96.24.13962
44. Tsoi LC, Rodriguez E, Stolzl D, Wehkamp U, Sun J, Gerdes S, et al. Progression of Acute-to-Chronic Atopic Dermatitis Is Associated With Quantitative Rather Than Qualitative Changes in Cytokine Responses. J Allergy Clin Immunol (2019) 145:1406–15. doi: 10.1016/j.jaci.2019.11.047
45. Yoshimoto T, Mizutani H, Tsutsui H, Noben-Trauth N, Yamanaka K, Tanaka M, et al. IL-18 Induction of IgE: Dependence on CD4+ T Cells, IL-4 and STAT6. Nat Immunol (2000) 1:132–7. doi: 10.1038/77811
46. Zedan K, Rasheed Z, Farouk Y, Alzolibani AA, Bin Saif G, Ismail HA, et al. Immunoglobulin E, Interleukin-18 and Interleukin-12 in Patients With Atopic Dermatitis: Correlation With Disease Activity. J Clin Diagn Res (2015) 9:WC01–5. doi: 10.7860/JCDR/2015/12261.5742
47. Suarez AL, Feramisco JD, Koo J, Steinhoff M. Psychoneuroimmunology of Psychological Stress and Atopic Dermatitis: Pathophysiologic and Therapeutic Updates. Acta Derm Venereol (2012) 92:7–15. doi: 10.2340/00015555-1188
48. Mizawa M, Yamaguchi M, Ueda C, Makino T, Shimizu T. Stress Evaluation in Adult Patients With Atopic Dermatitis Using Salivary Cortisol. BioMed Res Int (2013) 2013:138027. doi: 10.1155/2013/138027
49. Gao PS, Leung DY, Rafaels NM, Boguniewicz M, Hand T, Gao L, et al. Genetic Variants in Interferon Regulatory Factor 2 (IRF2) Are Associated With Atopic Dermatitis and Eczema Herpeticum. J Invest Dermatol (2012) 132:650–7. doi: 10.1038/jid.2011.374
50. Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, et al. Gasdermin D Plays a Vital Role in the Generation of Neutrophil Extracellular Traps. Sci Immunol (2018) 3:eaar6689. doi: 10.1126/sciimmunol.aar6689
51. Klopot A, Baida G, Kel A, Tsoi LC, Perez White BE, Budunova I. Transcriptome Analysis Reveals Intrinsic Proinflammatory Signaling in Healthy African American Skin. J Invest Dermatol (2021) S0022-202X(21)02400-3. doi: 10.1016/j.jid.2021.09.031
52. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis Prevalence Among Adults in the United States. J Am Acad Dermatol (2014) 70:512–6. doi: 10.1016/j.jaad.2013.11.013
53. Gelfand JM, Stern RS, Nijsten T, Feldman SR, Thomas J, Kist J, et al. The Prevalence of Psoriasis in African Americans: Results From a Population-Based Study. J Am Acad Dermatol (2005) 52:23–6. doi: 10.1016/j.jaad.2004.07.045
54. Kerr GS, Qaiyumi S, Richards J, Vahabzadeh-Monshie H, Kindred C, Whelton S, et al. Psoriasis and Psoriatic Arthritis in African-American Patients–the Need to Measure Disease Burden. Clin Rheumatol (2015) 34:1753–9. doi: 10.1007/s10067-014-2763-3
55. McMichael AJ, Vachiramon V, Guzman-Sanchez DA, Camacho F. Psoriasis in African-Americans: A Caregivers’ Survey. J Drugs Dermatol (2012) 11:478–82. Retrieved from: https://jddonline.com
56. Iskandar IYK, Parisi R, Griffiths CEM, Ashcroft DM. Systematic Review Examining Changes Over Time and Variation in the Incidence and Prevalence of Psoriasis by Age and Gender. Br J Dermatol (2021) 184:243–58. doi: 10.1111/bjd.19169
57. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project team, et al. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J Invest Dermatol (2013) 133:377–85. doi: 10.1038/jid.2012.339
58. Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 New Psoriasis Susceptibility Loci Highlights the Role of Innate Immunity. Nat Genet (2012) 44:1341–8. doi: 10.1038/ng.2467
59. Fan X, Yang S, Huang W, Wang ZM, Sun LD, Liang YH, et al. Fine Mapping of the Psoriasis Susceptibility Locus PSORS1 Supports HLA-C as the Susceptibility Gene in the Han Chinese Population. PloS Genet (2008) 4:e1000038. doi: 10.1371/journal.pgen.1000038
60. Knight J, Spain SL, Capon F, Hayday A, Nestle FO, Clop A, et al. Conditional Analysis Identifies Three Novel Major Histocompatibility Complex Loci Associated With Psoriasis. Hum Mol Genet (2012) 21:5185–92. doi: 10.1093/hmg/dds344
61. Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A Large-Scale Genetic Association Study Confirms IL12B and Leads to the Identification of IL23R as Psoriasis-Risk Genes. Am J Hum Genet (2007) 80:273–90. doi: 10.1086/511051
62. Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-Wide Scan Reveals Association of Psoriasis With IL-23 and NF-kappaB Pathways. Nat Genet (2009) 41:199–204. doi: 10.1038/ng.311
63. Ogawa K, Okada Y. The Current Landscape of Psoriasis Genetics in 2020. J Dermatol Sci (2020) 99:2–8. doi: 10.1016/j.jdermsci.2020.05.008
64. Yin X, Low HQ, Wang L, Li Y, Ellinghaus E, Han J, et al. Genome-Wide Meta-Analysis Identifies Multiple Novel Associations and Ethnic Heterogeneity of Psoriasis Susceptibility. Nat Commun (2015) 6:6916. doi: 10.1038/ncomms7916
65. Bejaoui Y, Witte M, Abdelhady M, Eldarouti M, Abdallah NMA, Elghzaly AA, et al. Genome-Wide Association Study of Psoriasis in an Egyptian Population. Exp Dermatol (2019) 28:623–7. doi: 10.1111/exd.13926
66. Bath KG, Schilit A, Lee FS. Stress Effects on BDNF Expression: Effects of Age, Sex, and Form of Stress. Neuroscience (2013) 239:149–56. doi: 10.1016/j.neuroscience.2013.01.074
67. Vasiadi M, Therianou A, Sideri K, Smyrnioti M, Sismanopoulos N, Delivanis DA, et al. Increased Serum CRH Levels With Decreased Skin CRHR-1 Gene Expression in Psoriasis and Atopic Dermatitis. J Allergy Clin Immunol (2012) 129:1410–3. doi: 10.1016/j.jaci.2012.01.041
68. Alexopoulos A, Chrousos GP. Stress-Related Skin Disorders. Rev Endocr Metab Disord (2016) 17:295–304. doi: 10.1007/s11154-016-9367-y
69. Park HJ, Kim HJ, Lee JH, Lee JY, Cho BK, Kang JS, et al. Corticotropin-Releasing Hormone (CRH) Downregulates Interleukin-18 Expression in Human HaCaT Keratinocytes by Activation of P38 Mitogen-Activated Protein Kinase (MAPK) Pathway. J Invest Dermatol (2005) 124:751–5. doi: 10.1111/j.0022-202X.2005.23656.x
70. Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-Releasing Hormone Inhibits Nuclear factor-kappaB Pathway in Human HaCaT Keratinocytes. J Invest Dermatol (2003) 121:1496–9. doi: 10.1111/j.1523-1747.2003.12612.x
71. de Brouwer SJ, van Middendorp H, Stormink C, Kraaimaat FW, Sweep FC, de Jong EM, et al. The Psychophysiological Stress Response in Psoriasis and Rheumatoid Arthritis. Br J Dermatol (2014) 170:824–31. doi: 10.1111/bjd.12697
72. Sarkar MK, Kaplan N, Tsoi LC, Xing X, Liang Y, Swindell WR, et al. Endogenous Glucocorticoid Deficiency in Psoriasis Promotes Inflammation and Abnormal Differentiation. J Invest Dermatol (2017) 137:1474–83. doi: 10.1016/j.jid.2017.02.972
73. Lili LN, Klopot A, Readhead B, Baida G, Dudley JT, Budunova I. Transcriptomic Network Interactions in Human Skin Treated With Topical Glucocorticoid Clobetasol Propionate. J Invest Dermatol (2019) 139:2281–91. doi: 10.1016/j.jid.2019.04.021
74. Brunoni AR, Lotufo PA, Sabbag C, Goulart AC, Santos IS, Bensenor IM. Decreased Brain-Derived Neurotrophic Factor Plasma Levels in Psoriasis Patients. Braz J Med Biol Res (2015) 48:711–4. doi: 10.1590/1414-431x20154574
Keywords: African America, psoriasis, atopic dermatitis, stress, minority
Citation: Jamerson TA, Li Q, Sreeskandarajan S, Budunova IV, He Z, Kang J, Gudjonsson JE, Patrick MT and Tsoi LC (2022) Roles Played by Stress-Induced Pathways in Driving Ethnic Heterogeneity for Inflammatory Skin Diseases. Front. Immunol. 13:845655. doi: 10.3389/fimmu.2022.845655
Received: 30 December 2021; Accepted: 23 March 2022;
Published: 28 April 2022.
Edited by:
Lisa G. Rider, National Institute of Environmental Health Sciences (NIH), United StatesReviewed by:
Lennart Matthias Roesner, Hannover Medical School, GermanyCopyright © 2022 Jamerson, Li, Sreeskandarajan, Budunova, He, Kang, Gudjonsson, Patrick and Tsoi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lam C. Tsoi, YWxleHRzb2lAbWVkLnVtaWNoLmVkdQ==
 Taylor A. Jamerson
Taylor A. Jamerson Qinmengge Li2
Qinmengge Li2 Irina V. Budunova
Irina V. Budunova Jian Kang
Jian Kang Johann E. Gudjonsson
Johann E. Gudjonsson Matthew T. Patrick
Matthew T. Patrick Lam C. Tsoi
Lam C. Tsoi