- 1Clinical Immunology, Angioedema and Allergy Unit, The Zabludowicz Center for Autoimmune Diseases, Sheba Medical Center, Ramat Gan, Israel
- 2Department of Internal Medicine “C”, Shamir Medical Center, Zerifin, Israel
- 3Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel
- 4Hematology Institute and Blood Bank, Meir Medical Center, Kfar Saba, Israel
- 5The National Hemophilia Center and Thrombosis Unit, Amalia Biron Research Institute of Thrombosis and Hemostasis, Sheba Medical Center, Ramat Gan, Israel
Background: Antiphospholipid syndrome (APS) is an acquired hypercoagulable condition associated with antiphospholipid antibody (aPL) presence. Data on re-thrombosis following APS-diagnosis are limited.
Methods: This is a retrospective analysis of new thrombotic events among primary APS (pAPS) patients followed for up to 15 years in three medical centers in Israel.
Results: Among 312 primary-APS patients, 143 (46%) had new thrombotic event classified to three patterns: (1) Arterial—associated with heart valve disease (OR 7.24, 95% C.I. 2.26–24.6), hypertension (OR 3, 95% C.I. 1.44–6.25), elevated anti-B2-GPI IgM (OR 1.04, 95% C.I. 0.996–1.08), arterial thrombosis at presentation (OR 1.74 95% C.I. 0.992–3.26), and older age (41 vs. 34 years, p < 0.001). (2) Venous—linked with venous thrombosis at presentation (OR 12.9, 95% C.I. 5.27–31.6, p < 0.001), heart valve disease (OR 9.81 95% C.I. 1.82–52.9, p = 0.018), aGAPSS (OR 1.15 95% C.I. 1.02–1.29), and younger age (31 vs. 36.5 years, p = 0.001); and (3) Combined pattern—associated with heart valve disease (OR 40.5 95% C.I. 7.7–212) and pulmonary embolism (OR 7.47 95% C.I. 1.96–28.5). A 4th variant “the Breakthrough pattern” defined by re-thrombosis despite prophylactic therapy was observed in 100/143 (70%) patients and linked with heart valve disease (OR 8. 95% C.I. 2.43–26.3), venous thrombosis at presentation (OR 2.61 95% C.I. 1.47–4.66), leg ulcers (OR 12.2, 95% C.I. 1.4–107), hypertension (OR 1.99, 95% C.I. 0.92–4.34), and higher aGAPSS (OR 1.08, 95% C.I. 0.99–1.18).
Conclusion: In this real-life observation, re-thrombosis was common among pAPS patients including in those recommended to receive prophylactic therapy. Different patterns of recurrence were identified and linked with presenting symptoms, specific serological markers, APS manifestations, and comorbidities. Studies that will address interventions to prevent recurrences of APS-related events are needed.
Introduction
The antiphospholipid syndrome (APS) is an autoimmune acquired coagulopathy characterized by thrombosis and obstetric morbidity in the presence of antiphospholipid antibodies (aPLs). Since first described in the 1980s, classification criteria of APS are based on the concomitant presence of typical clinical features and aPLs. These criteria, though designed for classification, are often used for diagnosis (1). Additional features currently defined as the “non-criteria” manifestations, such as thrombocytopenia, autoimmune hemolytic anemia (AIHA), heart valve disease, and non-thrombotic neurological manifestations, have been related to APS (2). Lastly, an aggressive subset of this disease termed catastrophic APS (cAPS) is documented in 1% of patients and withhold poor outcomes (2, 3).
Factors predictive of APS course and prognosis are not well defined, and upon APS diagnosis treatment protocols typically follow general guidelines comprising antiplatelet and/or anticoagulants (4). Recurrent APS-related events are common and difficult to predict (5–9). Thereby, the Global Antiphospholipid Score (GAPSS) was developed for the assessment of thrombosis risk in APS. This score include 6 factors, namely, seropositivity for anti-cardiolipin, anti-B2GPI, lupus anticoagulant, and anti-phosphatidylserine–prothrombin complex antibodies, as well as dyslipidemia and hypertension (10). The GAPSS score is rarely used because of the scarcity of laboratories performing the anti-phosphatidylserine–prothrombin complex assay and is commonly replace by a modified GAPSS (aGAPSS) (11). The latter has been validated in several cohorts; nonetheless, it has some limitations such as the simplicity of aPL assessment with no isotype or titer measurement as well as the lack of non-criteria manifestations. Other risk factors have related to recurrence of APS manifestation such as the presence of LAC or triple aPL positivity (i.e., the concomitant presence of anti-cardiolipin (aCL) and anti-β2‐glycoprotein I (aβ2GPI) and lupus anticoagulants (LAC) (8, 12). Of note, the occurrence of arterial thrombosis has long been considered a risk factor of aggressive disease, with some advocating a more intensive treatment, although this is not a matter of consensus (4, 13–15).
Currently, the risk of APS recurrence is difficult to assess in general and particularly at presentation of disease. In this multicenter study, we evaluated the associations between features presented early/at diagnosis of primary APS, and recurrence of thrombotic events during the long-term follow-up.
Patients and Methods
Patients
This is a retrospective study of primary APS patients diagnosed according to the international (Sydney) classification criteria for the antiphospholipid syndrome (1). Data were retrieved from medical records of sequential patients treated in three large centers in Israel (Sheba-Tel Hashomer, Meir, and Shamir Medical Centers) during January 2004 to December 2019. This study was performed in accordance with the declaration of Helsinki and in agreement and approved for this study by the Sheba Medical Center Review Board.
Patients who at presentation of APS or at any point of the disease fulfilled the criteria of systemic lupus erythematous disease, based on the relevant diagnostic criteria (16, 17), or another systemic autoimmune disease were excluded. All patients were treated in specialized centers, and decisions upon follow-up and therapy were at their specialist discretion.
Temporal associations were established between the first clinical event which led to APS diagnosis (i.e., presenting symptom) and recurrent thrombosis. For this purpose, three clinical patterns of recurrence during follow-up were defined: [1] “arterial pattern” (e.g., stroke, limb ischemia, myocardial infarction); [2] “venous pattern” (i.e., deep vein thrombosis, pulmonary embolism presented); [3] “combined pattern” (APS-related events of mixed origin during follow-up, i.e., arterial and obstetric; venous and obstetric; arterial and venous; and arterial, venous, and obstetric). Additionally, a fourth variant, the “breakthrough pattern”, was defined by recurrence of thrombotic events despite recommended anti-thrombotic therapy, regardless of type of thrombosis. Patients with each recurrence pattern were compared with primary APS patients who have had no thrombosis during follow-up.
Demographic characteristics (age, sex, age at diagnosis, length of follow-up, treatments); presenting APS classification clinical criteria (i.e., thrombotic or obstetric events and aPL serology); concomitant conditions (hypertension, smoking, diabetes mellitus, and dyslipidemia); non-criteria APS-related manifestations manifesting at any time during the disease course (heart valve disease (Libman–Sacks endocarditis), livedo reticularis, leg ulcers, migraine, epilepsy, autoimmune hemolytic anemia, thrombocytopenia, leukopenia); APS-related outcomes (death, catastrophic APS, aGAPSS, bleeding events); and therapies prescribed at any point of the disease were also collected.
Serology and Scores
The presence of anti-cardiolipin (aCL) and anti-β2‐glycoprotein I (aβ2GPI) of the IgG and IgM isotypes was measured by enzyme‐linked immunosorbent assay (ELISA) or by a multiplex system. The kits that were used were all commercial (ELISA—aB2GPI by AESKU Diagnostics (Wendelsheim, Germany) and aCL by Varelisa (Pharmacia Diagnostics, Stockholm, Sweden); Bioplex both aB2GPI and aCL by Bio-Rad, Hercules, CA, USA). B2GPI and ACL were considered positive if antibody levels were above 20 MPL units (IgG phospholipid units or IgM phospholipid units), or if >99th percentile or according to the manufacturer’s instructions were present in a minimum of two tests performed at least 12 weeks apart were obtained. Very high titers were considered as fourfold or higher of the upper normal limit as specified for each kit. Lupus anticoagulant (LA) activity was detected by coagulation assays in routine use at each center and was consistent with the International Society of Thrombosis and Hemostasis guidelines (18). The LA assays were modified in 2016; up until 2016, LA activity was measured by LA-responsive activated partial thromboplastin time (aPTT) aPL (by Stago, confirmed using the Actem FS Kit by Siemens, Erlangen, Germany), and from 2016, LA activity was measured by a combination of silicon clotting time and the use of the Russell Viper Venom Kit (by IL, Bedford, MA, USA). In case of anticoagulation treatment or spontaneous INR >1.5, patients’ plasma was mixed with normal plasma in order to reduce false positivity. Positivity was defined as single, double, or triple positive according to the number of different positive tests obtained.
In this study, we used the validated aGAPSS (11, 19) which allots 3 points for dyslipidemia, 1 for arterial hypertension, 5 for anti-cardiolipin antibodies IgG/IgM, 4 for anti-β2 glycoprotein IgG/IgM, and 4 for lupus anticoagulant. Catastrophic APS (cAPS) was defined according to the International Task Force on CAPS criteria (2).
Statistical Analysis
The data were analyzed using BMDP software (BMDP Statistical Software, University of California Press, Berkeley, LA, USA). Pearson’s chi-square test or Fisher’s exact test (two-tailed) was used for the analysis of between-group differences in discrete variables, and analysis of variance (ANOVA) was used for comparing continuous variables. Using those variables found to be significant (<0.10) on univariate analysis, we applied a stepwise logistic regression in order to determine those variables most significantly associated with each outcome. Choices of variables to include in our stepwise analysis were drawn from the number of patients who were to prognosticate; thus, for every 5 patients, 1 variable was allowed. Patients with missing relevant data were excluded from the analysis. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated, and a p-value of ≤0.05 was considered significant.
Results
We included 312 primary APS patients in this study. The combined duration of follow-up was 3,182 patient-years (mean 10 ± 7 years; Table 1). APS-related events during follow-up (excluding the initial “classification” event) were documented in 180 (57.6%) patients, of whom 143/180 (79%) experienced an additional thrombotic event while in 37/180 (21%) female patients’ obstetric complications were the recurrent event. Thus, 169/312 (54.2%) patients that had no thrombotic event during follow-up were compared to different patterns of thrombotic recurrences. There were 100 among 143 (70%) patients who suffered from recurrent thrombosis despite preventive therapy. Notably in our entire cohort, 95.5% (298/312) received guideline-based therapy (Table 1). During the entire study period, 31 bleeding events were reported in 27/312 (8.6%) patients, of which 16/312 (5.1%) were defined as major bleeding resulting in a bleeding rate of 0.97 events/100 patient years.
Four distinct patterns of new thrombotic events during follow-up were identified in this study, namely, “arterial”, “venous”, “combined”, and “breakthrough” (Figure 1). Notably, longer follow-up was linked with more recurrence regardless of the pattern.
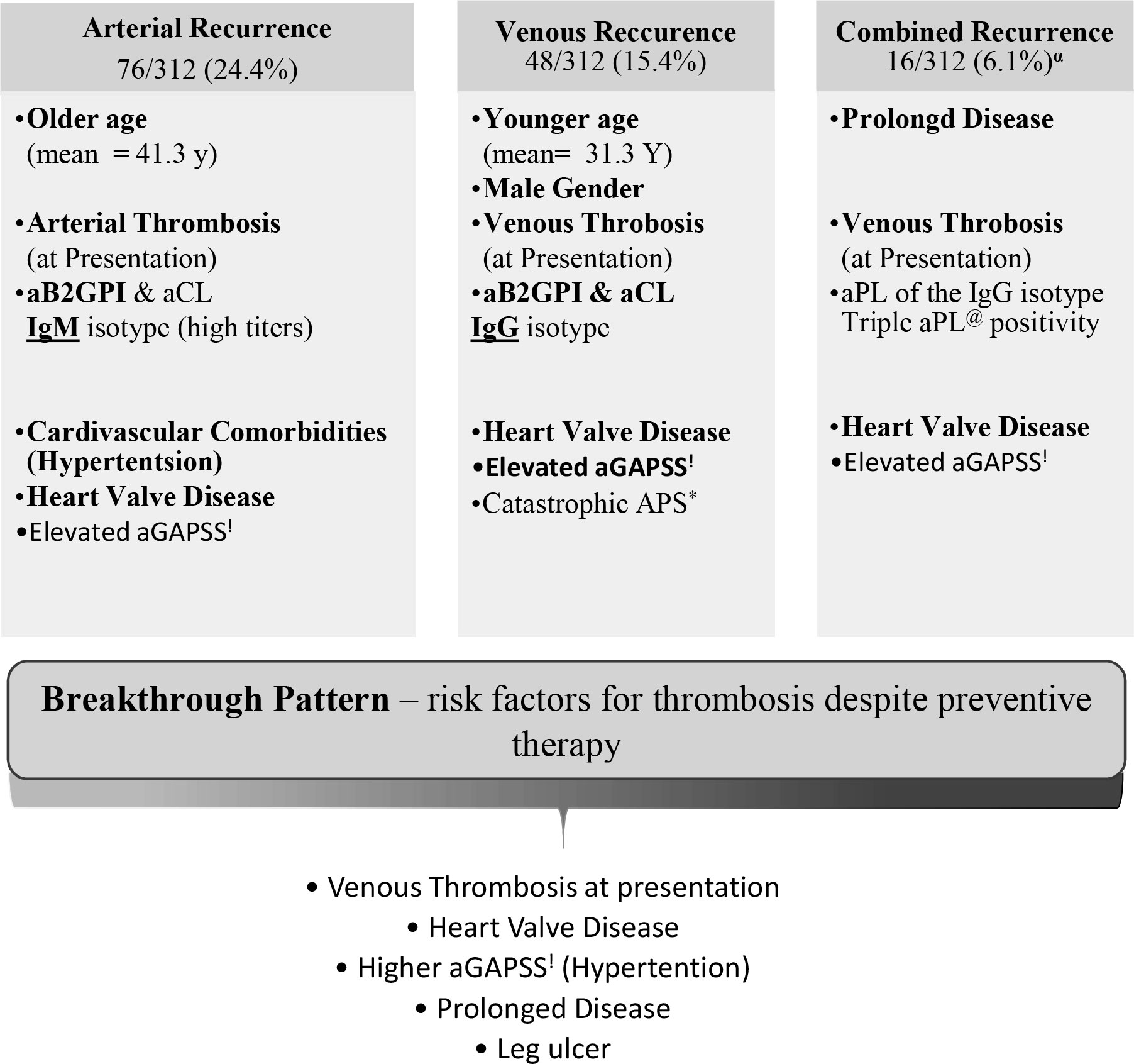
Figure 1 Patterns of thrombotic recurrence in the primary Antiphospholipid Syndrome (n=143). All patients with at least one thrombotic event after established antiphospholipid syndrome. αSubset of patients with multiple types of thrombosis (i.e., arterial and venous; arterial and obstetric; venous and obstetric; arterial, venous and obstetric). *Antiphospholipid. @Antiphospholipid antibody.!Adjusted Global Antiphospholipid Syndrome.
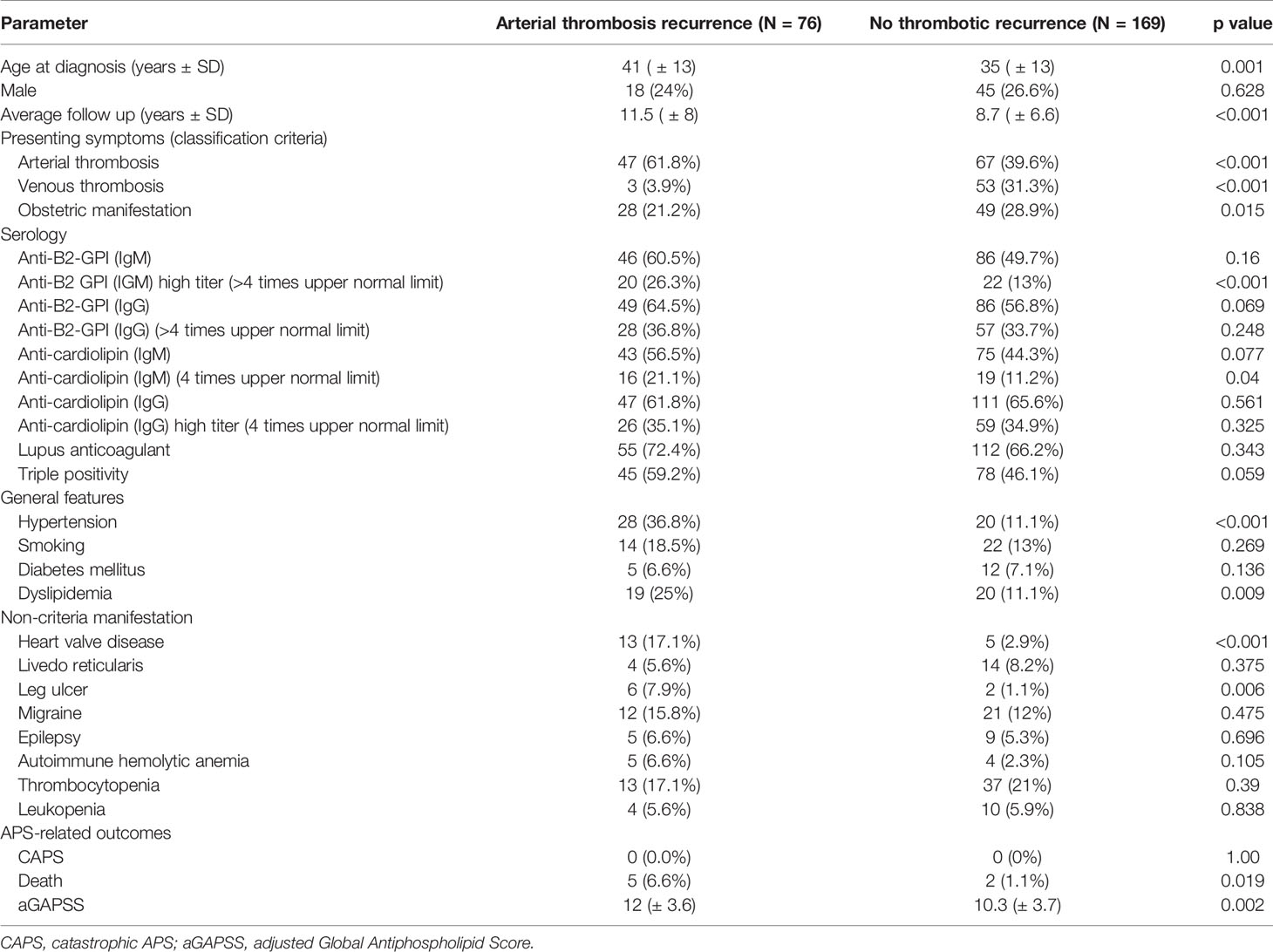
The “arterial” pattern was documented in 76/312 (24.4%) primary APS (pAPS) patients (Table 2). These events were associated with older age (41.3 ± 13.2 vs. 34.2 ± 13.2; p < 0.001) and occurred mainly among patients with arterial thrombosis at APS diagnosis. Arterial thrombosis during follow-up was also more common in patients with very high titer (>4-fold upper normal limit) aPLs of the IgM isotype (both anti-B2-GPI and anti-cardiolipin) hypertension, dyslipidemia, heart valve disease, higher aGAPSS, and higher mortality in comparison to patients with no recurrence during follow-up (Table 2). Interestingly, when comparing aPLs among patients with the arterial pattern of recurrence to our entire cohort of primary APS patients (including those with other patterns of re-thrombosis), the link with aPLs of the IgM isotype remained persistent regardless of titers (B2GPI IgM 64.5% vs. 43.6%, p = 0.002, aCL IgM 56.5% vs. 39% p = 0.001). In the stepwise regression analysis, the most important factors related to the “arterial” pattern of recurrence were heart valve disease (OR 7.24, 95% C.I. 2.26–24.6), hypertension (OR 3, 95% C.I. 1.44–6.25), elevated anti-B2-GPI IgM (OR 1.04, 95% C.I. 0.996–1.08), and arterial thrombosis at presentation (OR 1.74 95% C.I. 0.992–3.26), with area under the curve of 0.726.
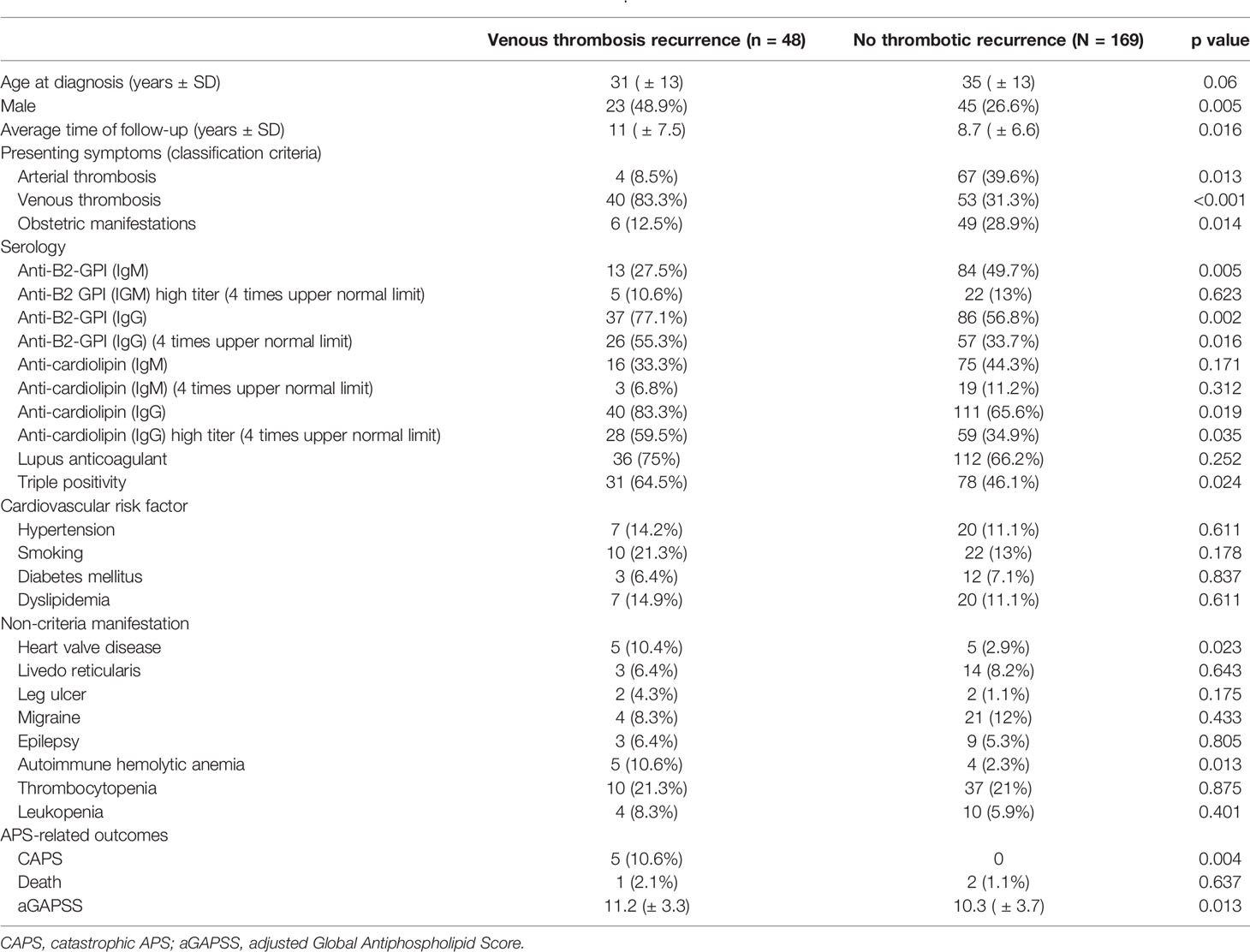
The “venous” pattern was observed in 48/312 (15.4%) (Table 3). This was associated with younger age (31.3 ± 12.7 vs. 36.7 ± 13.5 p = 0.011), male gender, and venous thrombosis at APS presentation. This pattern was also linked with anti-cardiolipin and anti-B2GPI, but of the IgG isotype, triple aPL positivity, heart valve disease, and aGAPSS. On stepwise regression analysis, the three parameters mostly related to this pattern were venous thrombosis at presentation (OR 12.9, 95% C.I. 5.27–31.6, p < 0.001), heart valve disease (OR 9.81 95% C.I. 1.82–52.9, p = 0.018), and aGAPSS (OR 1.15 95% C.I. 1.02–1.29), with area under the curve of 0.825.
The “combined” pattern in which more than one type of APS-related event occurred during follow-up was documented in 19/312 (6.1%) patients. Combined events including arterial thrombosis and obstetric morbidity in 2 patients, arterial and venous thrombosis in 9 patients, venous thrombosis and obstetric morbidity in 4 patients, and lastly 4 patients have had all three—arterial thrombosis, venous thrombosis, and obstetric morbidity. This pattern was associated with longer disease course and follow-up by 6.3 years, venous thrombosis as the presenting symptom, and aPL triple positivity as well as IgG isotypes (Table 4). For this relatively more severe phenotype, we also evaluated interactions with non-criteria manifestations at any time during the course of the disease; thus notably, this pattern was associated with heart valve disease, livedo reticularis, and leg ulcers. The aGAPSS score was significantly elevated in this group (11.9 vs. 10.3 in the control group, p = 0.013). Lastly, patients with this pattern presented more often with cAPS. Using a stepwise regression analysis, we identified 2 statistically significant factors which had the most impact on this phenotype: heart valve disease (OR 40.5 95% C.I. 7.7–212) and pulmonary embolism (OR 7.47 95% C.I. 1.96–28.5), with area under the curve of 0.805.

Table 4 Serological Clinical parameters of OAPS patients with thrombosis (OAPSt) and without thrombosis during follow up (OAPSnt).
The “breakthrough pattern” addressed the occurrences of APS-related events despite preventive therapy in our entire cohort regardless of presenting symptoms or type of new thrombotic event. This variant was documented in 100/143 (70%) pAPS patients, of whom 81 (81%) were treated with anticoagulants ± antiplatelet agents (Table 5). In comparison to the patients who have had no thrombosis during follow-up, the patients with the “breakthrough pattern” were more likely to be treated with the combination of warfarin and antiplatelet agent (27% vs. 14.6%, p = 0.012) and less likely to be treated with antiplatelet therapy only (8% vs. 17.5%, p = 0.026). The “breakthrough” pattern was also associated with venous thrombosis at presentation, prolonged duration of disease (>4 years on average), limb ischemia/ulcers, and heart valve disease. Using a stepwise regression analysis, we identified five factors which had the strongest association with this pattern, namely, heart valve disease (OR 8 95% C.I. 2.43–26.3), venous thrombosis at presentation (OR 2.61 95% C.I. 1.47–4.66), leg ulcers (OR 12.2, 95% C.I. 1.4–107), hypertension (OR 1.99, 95% C.I. 0.92–4.34), and higher aGAPSS (OR 1.08, 95% C.I. 0.99–1.18), with area under the curve of 0.716.
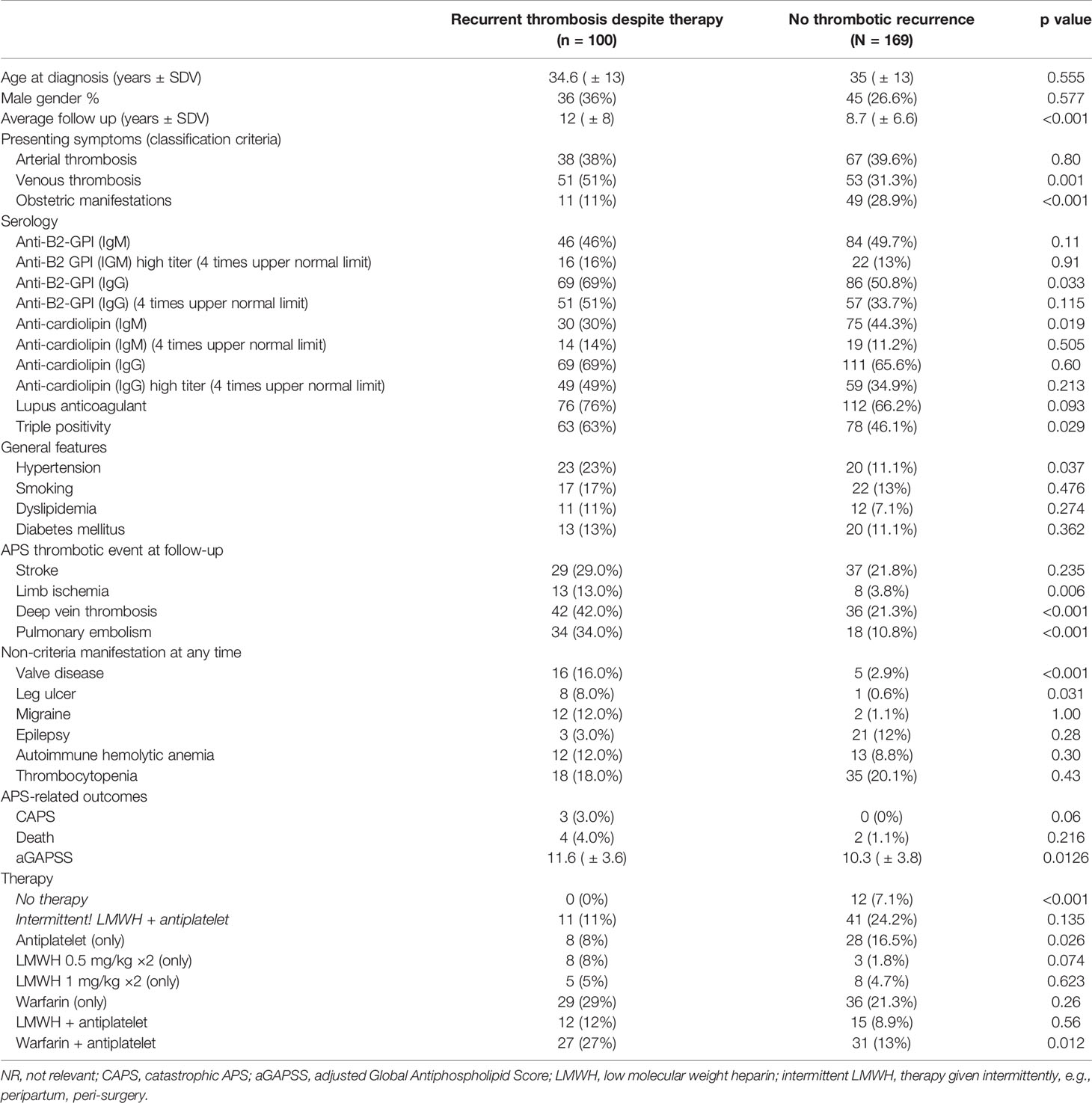
Table 5 Clinical and serological parameters associated with the “breakthrough” pattern—e.g., APS-related event despite preventive therapy (anti-aggregation or anticoagulation).
The “breakthrough pattern” addressed the occurrences of APS-related events despite preventive therapy in our entire cohort regardless of presenting symptoms or type of new thrombotic event. This variant was documented in 100/143 (70%) pAPS patients, of whom 81 (81%) were treated with anticoagulants ± antiplatelet agents (Table 5). In comparison to the patients who have had no thrombosis during follow-up, the patients with the “breakthrough pattern” were more likely to be treated with the combination of warfarin and antiplatelet agent (27% vs. 14.6%, p = 0.012) and less likely to be treated with antiplatelet therapy only (8% vs. 17.5%, p = 0.026). The “breakthrough” pattern was also associated with venous thrombosis at presentation, prolonged duration of disease (>4 years on average), limb ischemia/ulcers, and heart valve disease. Using a stepwise regression analysis, we identified five factors which had the strongest association with this pattern, namely, heart valve disease (OR 8 95% C.I. 2.43–26.3), venous thrombosis at presentation (OR 2.61 95% C.I. 1.47–4.66), leg ulcers (OR 12.2, 95% C.I. 1.4–107), hypertension (OR 1.99, 95% C.I. 0.92–4.34), and higher aGAPSS (OR 1.08, 95% C.I. 0.99–1.18), with area under the curve of 0.716.
Discussion
In this study, we aimed to determine risk factors linked with recurrent thrombosis among APS patients in Israel. Focusing on primary APS (pAPS) enabled us to minimize confounding factors related to SLE or other systemic autoimmune disease activity and/or therapy. We therefore studied the course of 312 pAPS patients for an average duration of more than 10 years. During this period, 143 (46%) experienced a new thrombotic event which occurred in 100 (70%) of them despite receiving guideline-appropriate antithrombotic treatment at the time of recurrence.
Few studies have focused on thrombosis recurrence in APS, and most have included mixed populations of patients with primary and/or secondary APS. Among these studies, a thrombotic recurrence rate of 25%–30% during 4 to 10 years of follow-up has been reported (6–9, 19–21). In a recent study, with an average follow-up of 18 years, a higher rate of 44% new thrombotic events was observed (5) and stands in agreement with our prolonged study. A novel aspect of our report is the definition of patterns of recurrence, of which two patterns of recurrence termed “combined” and “breakthrough” were strongly linked with longer duration of disease. This is consistent with the Piedmont cohort in which thrombosis recurrence despite preventive therapy (“breakthrough”) was reported to increase over time (22). Finally, long-term follow-up would increase the probability of a patient to develop cardiovascular comorbidity which is known to aggravate outcome in APS patients (7, 23, 24). Other plausible explanations for the relatively higher rate of thrombosis in our study may be attributed to the selection of patients only with pAPS, the high rate of triple aPL positivity (57%) documented in our cohort (8), and the as-yet unidentified genetic or environmental factors specific to Israeli patients.
Currently, there are gaps in our knowledge of APS patterns of recurrence. In this study, we attempted to identify features associated with recurrent thrombosis that may allow a more tailored approach to follow-up and treatment of pAPS patients. We identified 4 patterns of thrombotic recurrence, namely, “arterial”, “venous”, “combined”, and “breakthrough”, each associated with different risk factors that can be easily evaluated in any APS clinic explicitly APS-presenting symptom, patients’ age, aPL isotypes, and comorbidities (e.g., non-criteria manifestation, hypertension, and others).
In our cohort, we found that the significance clinical predictive factor for arterial, venous, or combined recurrence was the nature of the initial thrombosis: arterial recurrence correlating with arterial events at diagnosis and venous and combined recurrence correlating with venous thrombosis at presentation. Similar correlations between APS-defining events and type of recurrence have been previously reported in some cohorts (6, 21, 25, 26) but not in all studies (8, 22).
A prominent feature among APS patients with all types of recurrence was the presence of heart valve disease. While this manifestation is not considered an APS criterion, our cohort stresses the clinical importance of this non-criteria feature. Currently, little is known about the role of heart valve disease as a prognostic factor of APS. One report has stated that in obstetric APS patients heart valve disease was associated with an elevated risk of thrombosis (27). Whether heart valve disease in APS is a consequence of thrombotic complication or a manifestation of immune activation is yet to be determined; nonetheless, it seems to be a strong predictor of further event and thus it seems prudent to include regular assessment of the heart valves in primary APS patients.
Herein, another interesting link with pattern of APS recurrence was the presence of specific aPLs as well as the gravity of triple aPL positivity. The anti-phospholipid antibodies are not only nowadays regarded as classification criteria of APS but also clearly linked with the pathogenesis and severity of disease (8, 28, 29). In particular, Pengo et al. showed that the triple positivity of aPL is predictive of worst outcomes (8). Others demonstrated that IgG aCL is predictive of new thrombotic events (7) whereas LAC was related to adverse obstetric outcomes (30, 31). Recently, we reported a correlation between criteria and non-criteria aPL profiles and APS phenotype at presentation (29). In the current study, we found a small but significant association between high-titer aB2GPI of the IgM isotype and recurrence arterial thrombosis, while venous recurrence was associated with aB2GPI and aCL of the IgG isotypes as well as high titers. This is in agreement with other reports, especially of the association between aPL-IgM isotypes with older age, arterial events (8, 29), and stroke (32). Interestingly, elevated IgG subtypes (both at normal and very high titers) are associated.
The aGAPSS is nowadays the most validated score for APS recurrence and in our study was ubiquitous among all phenotypes. Previous studies have shown that the aGAPSS score is useful to predict the thrombosis (11) and rate of thrombosis among obstetric APS patients (27). The APS ACTION study had demonstrated this score to be significant in predicting thrombotic recurrence (13), although relatively lower scores for aGAPSS were reported in this study population for those with and without recurrence. This stands in agreement with our data, and it is our belief that the relatively higher aGAPSS scores in our cohort provide another explanation to the relatively high rate of thrombosis observed in this study.
Interpreting aGAPSS in regard to therapeutic decisions might be somewhat of a challenge, as this score is based on two pillars: one is the cardiovascular risk factors and the other is the serological positivity of aPL antibodies. Tailoring the therapy according to this score as well as patient phenotypes might be a feasible option in APS clinics. Nonetheless, it may be postulated that addressing comorbidities in the therapeutic arsenal for APS is of the essence, while other immunomodulatory interventions might be considered as treatments with hydroxychloroquine (33, 34) or anti-CD20 monoclonal antibodies (35) for patients at a higher risk of recurrence.
Last but not least, tailoring of APS therapy according to the initial thrombotic event had been proposed in the past especially in regard to arterial thrombosis (36). More recently, in close proximity to the end of our study in 2019, the new European League against Rheumatism (EULAR) recommendations for management of APS were published, in which intensifying therapy for patients with recurrent events and potentially for those presenting with arterial thrombosis and/or a high-risk aPL profile was also suggested (4). This stands in support with our data, as the vast majority of patients with the “breakthrough” pattern in this study were treated with anticoagulant ± antiplatelet therapies at the usual doses. Intensifying anti-thrombotic therapy is frequently limited by the fear of increased bleeding. In this regard, the overall rate of any bleeding in our cohort was 0.65% annually and the major bleeding rate was 0.97 events per 100 patient years. According to a recent published analysis, this rate of bleeding can be considered as low in comparison to the general population of patients treated with anticoagulants (37).
Our study has several limitations that derived from its retrospective nature. These include incomplete and possible inaccurate reporting especially given the prolonged duration of follow-up and the inability to verify patient adherence to antithrombotic treatments. In light of the retrospective design of the study, the definition of thrombosis despite preventive therapy was defined by the treating physician. INR levels were too often uncertain at the time of thrombosis; thus, we have chosen to rely on treating physician appraisal. Notably, the breakthrough pattern was not compared to patients with recurrence but not of the breakthrough pattern, as the latter was a too-small group and thus was compared to patients with no recurrence at all. However, we believe that these limitations are compensated for by the multicenter nature of this study which is relatively large and the well-defined cohort of primary APS patients. In addition, the similarities regarding mortality rates, thrombosis recurrence, and incidence of cAPS between our population and other published cohorts support the validity of our data.
In summary, and in agreement with previous studies (9, 13, 22, 38), we found that during a relatively long follow-up almost half (46%) of pAPS patients suffered a new thrombotic event. Given the challenge to foresee the risk of APS recurrence, we identified 4 phenotypes of recurrence: arterial, venous, combined, and breakthrough. Each of these patterns was linked with specific risk factors that can be assessed in every APS clinic and may enable physicians to better allocate patients at a higher risk and potentially tailor interventions accordingly.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Sheba Medical Center Helsinki Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SN and NA-L contributed to the conception and design of the study. SN, SH, and NA-L organized the database. SN performed the statistical analysis. SN and NA-L wrote the first draft of the manuscript. ME wrote sections of the manuscript. All authors contributed to the manuscript revision and read and approved the submitted version.
Funding
Publication fees were funded by the grant from the Bayern Scholarship for research in the field of thrombosis and coagulation disorders granted to SN by Israeli Association of Internal Medicine.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International Consensus Statement on an Update of the Classification Criteria for Definite Antiphospholipid Syndrome (APS). J Thromb Haemost JTH (2006) 4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Cervera R, Tektonidou M, Espinosa G, Cabral A, González E, Erkan D, et al. Task Force on Catastrophic Antiphospholipid Syndrome (APS) and Non-Criteria APS Manifestations (I): Catastrophic APS, APS Nephropathy and Heart Valve Lesions. Lupus (2011) 20(2):165–73. doi: 10.1177/0961203310395051
3. Rodríguez-Pintó I, Moitinho M, Santacreu I, Shoenfeld Y, Erkan D, Espinosa G, et al. Catastrophic Antiphospholipid Syndrome (CAPS): Descriptive Analysis of 500 Patients From the International CAPS Registry. Autoimmun Rev (2016) 15(12):1120–4. doi: 10.1016/j.autrev.2016.09.010
4. Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR Recommendations for the Management of Antiphospholipid Syndrome in Adults. Ann Rheum Dis (2020) 78:1296–304.
5. Taraborelli M, Reggia R, Dall’Ara F, Fredi M, Andreoli L, Gerosa M, et al. Longterm Outcome of Patients With Primary Antiphospholipid Syndrome: A Retrospective Multicenter Study. J Rheumatol (2017) 44(8):1165–72. doi: 10.3899/jrheum.161364
6. Erkan D, Yazici Y, Sobel R, Lockshin MD. Primary Antiphospholipid Syndrome: Functional Outcome After 10 Years. J Rheumatol (2000) 27(12):2817–21.
7. Turiel M, Sarzi-Puttini P, Peretti R, Rossi E, Atzeni F, Parsons W, et al. Thrombotic Risk Factors In Primary Antiphospholipid Syndrome: A 5-Year Prospective Study. Stroke (2005) 36(7):1490–4. doi: 10.1161/01.STR.0000170645.40562.09
8. Pengo V, Ruffatti A, Legnani C, Gresele P, Barcellona D, Erba N, et al. Clinical Course of High-Risk Patients Diagnosed With Antiphospholipid Syndrome. J Thromb Haemost (2010) 8(2):237–42. doi: 10.1111/j.1538-7836.2009.03674.x
9. Jackson WG, Oromendia C, Unlu O, Erkan D, DeSancho MT. Recurrent Thrombosis in Patients With Antiphospholipid Antibodies and Arterial Thrombosis on Antithrombotic Therapy. Blood Adv (2017) 1(25):2320–4. doi: 10.1182/bloodadvances.2017008185
10. Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: The Global Anti-Phospholipid Syndrome Score. Rheumatology (2013) 52(8):1397–403. doi: 10.1093/rheumatology/kes388
11. Radin M, Schreiber K, Costanzo P, Cecchi I, Roccatello D, Baldovino S, et al. The Adjusted Global AntiphosPholipid Syndrome Score (aGAPSS) for Risk Stratification in Young APS Patients With Acute Myocardial Infarction. Int J Cardiol (2017) 240:72–7. doi: 10.1016/j.ijcard.2017.02.155
12. Galli M, Luciani D, Bertolini G, Barbui T. Lupus Anticoagulants are Stronger Risk Factors for Thrombosis Than Anticardiolipin Antibodies in the Antiphospholipid Syndrome: A Systematic Review of the Literature. Blood (2003) 101(5):1827–32. doi: 10.1182/blood-2002-02-0441
13. Radin M, Sciascia S, Erkan D, Pengo V, Tektonidou MG, Ugarte A, et al. The Adjusted Global Antiphospholipid Syndrome Score (aGAPSS) and the Risk of Recurrent Thrombosis: Results From the APS ACTION Cohort. Semin Arthritis Rheumatol (2019) 49(3):464–8. doi: 10.1016/j.semarthrit.2019.04.009
14. Les I, Ruiz-Irastorza G, Khamashta MA. Intensity and Duration of Anticoagulation Therapy in Antiphospholipid Syndrome. Semin Thromb Hemost (2012) 38(4):339–47. doi: 10.1055/s-0032-1304720
15. Dufrost V, Risse J, Reshetnyak T, Satybaldyeva M, Du Y, Yan X-X, et al. Increased Risk of Thrombosis in Antiphospholipid Syndrome Patients Treated With Direct Oral Anticoagulants. Results from an international patient-level data meta-analysis. Autoimmun Rev (2018) 17(10):1011–21. doi: 10.1016/j.autrev.2018.04.009
16. Hochberg M. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum (1997) 40(9):1725–. doi: 10.1002/art.1780400928
17. Petri M, Orbai A-M, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and Validation of the Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol (2012) 64(8):2677–86. doi: 10.1002/art.34473
18. Brandt JT, Barna LK, Triplett DA. Laboratory Identification of Lupus Anticoagulants: Results of the Second International Workshop for Identification of Lupus Anticoagulants. On behalf of the Subcommittee on Lupus Anticoagulants/Antiphospholipid Antibodies of the ISTH. Thromb Haemost (1995) 74(6):1597–603. doi: 10.1055/s-0038-1649988
19. Fernandez Mosteirin N, Saez Comet L, Salvador Osuna C, Calvo Villas JM, Velilla Marco J. Independent Validation of the Adjusted GAPSS: Role of Thrombotic Risk Assessment in the Real-Life Setting. Lupus (2017) 26(12):1328–32. doi: 10.1177/0961203317703493
20. Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramón E, et al. Morbidity and Mortality in the Antiphospholipid Syndrome During a 10-Year Period: A Multicentre Prospective Study of 1000 Patients. Ann Rheum Dis (2015) 74(6):1011–8. doi: 10.1136/annrheumdis-2013-204838
21. Grika EP, Ziakas PD, Zintzaras E, Moutsopoulos HM, Vlachoyiannopoulos PG. Morbidity, Mortality, and Organ Damage in Patients With Antiphospholipid Syndrome. J Rheumatol (2012) 39(3):516–23. doi: 10.3899/jrheum.110800
22. Bazzan M, Vaccarino A, Stella S, Sciascia S, Montaruli B, Bertero MT, et al. Patients With Antiphosholipid Syndrome and Thrombotic Recurrences: A Real World Observation (the Piedmont Cohort Study). Lupus (2016) 25(5):479–85. doi: 10.1177/0961203315617538
23. Ruiz-Irastorza G, Cuadrado M, Ruiz-Arruza I, Brey R, Crowther M, Derksen R, et al. Evidence-Based Recommendations for the Prevention and Long-Term Management of Thrombosis in Antiphospholipid Antibody-Positive Patients: Report of a Task Force at the 13th International Congress on Antiphospholipid Antibodies. Lupus (2011) 20(2):206–18. doi: 10.1177/0961203310395803
24. Chighizola CB, Andreoli L, Gerosa M, Tincani A, Ruffatti A, Meroni PL. The Treatment of Anti-Phospholipid Syndrome: A Comprehensive Clinical Approach. J Autoimmun (2018) 90:1–27. doi: 10.1016/j.jaut.2018.02.003
25. Finazzi G, Brancaccio V, Moia M, Ciaverella N, Mazzucconi MG, Schinco PC, et al. Natural History and Risk Factors for Thrombosis in 360 Patients With Antiphospholipid Antibodies: A Four-Year Prospective Study From the Italian Registry. Am J Med (1996) 100(5):530–6. doi: 10.1016/S0002-9343(96)00060-5
26. Rosove MH, Brewer PM. Antiphospholipid Thrombosis: Clinical Course After the First Thrombotic Event in 70 Patients. Ann Intern Med (1992) 117(4):303–8. doi: 10.7326/0003-4819-117-4-303
27. de Jesús GR, Sciascia S, Andrade D, Barbhaiya M, Tektonidou M, Banzato A, et al. Factors Associated With First Thrombosis in Patients Presenting With Obstetric Antiphospholipid Syndrome (APS) in the APS Alliance for Clinical Trials and International Networking Clinical Database and Repository: A Retrospective Study. BJOG Int J Obstet Gynaecol (2019) 126(5):656–61.
28. Alessandri C, Agmon-Levin N, Conti F, Perricone C, Ortona E, Pendolino M, et al. Anti-Mutated Citrullinated Vimentin Antibodies in Antiphospholipid Syndrome: Diagnostic Value and Relationship With Clinical Features. Immunol Res (2017) 65(2):524–31. doi: 10.1007/s12026-017-8899-x
29. Volkov I, Seguro L, Leon EP, Kovács L, Roggenbuck D, Schierack P, et al. Profiles of Criteria and non-Criteria Anti-Phospholipid Autoantibodies are Associated With Clinical Phenotypes of the Antiphospholipid Syndrome. Auto- Immun Highlights (2020) 11(1):8. doi: 10.1186/s13317-020-00131-3
30. Vinatier D, Dufour P, Cosson M, Houpeau JL. Antiphospholipid Syndrome and Recurrent Miscarriages. Eur J Obstet Gynecol Reprod Biol (2001) 96(1):37–50. doi: 10.1016/S0301-2115(00)00404-8
31. Al Jameil N, Tyagi P, Al Shenefy A. Incidence of Anticardiolipin Antibodies and Lupus Anticoagulant Factor Among Women Experiencing Unexplained Recurrent Abortion and Intrauterine Fetal Death. Int J Clin Exp Pathol (2015) 8(3):3204–9.
32. Urbanski G, Yelnik CM, Maillard H, Launay D, Dubucquoi S, Hachulla E, et al. Antiphospholipid Syndrome With Isolated Isotype M Anticardiolipin and/or Anti-B2GPI Antibody Is Associated With Stroke. Stroke (2018) 49(11):2770–2. doi: 10.1161/STROKEAHA.118.023021
33. Sayar Z, Moll R, Isenberg D, Cohen H. Thrombotic Antiphospholipid Syndrome: A Practical Guide to Diagnosis and Management. Thromb Res (2021) 198:213–21. doi: 10.1016/j.thromres.2020.10.010
34. Kravvariti E, Koutsogianni A, Samoli E, Sfikakis PP, Tektonidou MG. The Effect of Hydroxychloroquine on Thrombosis Prevention and Antiphospholipid Antibody Levels in Primary Antiphospholipid Syndrome: A Pilot Open Label Randomized Prospective Study. Autoimmun Rev (2020) 19(4):102491. doi: 10.1016/j.autrev.2020.102491
35. Agmon-Levin N, Berman M, Harel L, Lidar M, Drori T, Hajyahia S, et al. Rituximab for Refractory Manifestations of the Antiphospholipid Syndrome: A Multicentre Israeli Experience. Clin Exp Rheumatol (2020) 39(5):1049–55. doi: 10.1136/lupus-2020-eurolupus.12
36. Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GRV. The Management of Thrombosis in the Antiphospholipid-Antibody Syndrome. N Engl J Med (1995) 332(15):993–7. doi: 10.1056/NEJM199504133321504
37. Khan F, Tritschler T, Kimpton M, Wells PS, Kearon C, Weitz JI, et al. Long-Term Risk for Major Bleeding During Extended Oral Anticoagulant Therapy for First Unprovoked Venous Thromboembolism. Ann Intern Med (2021) 174(10):1420–9. doi: 10.7326/M21-1094
Keywords: antiphospholipid syndrome, thrombosis - immunology, recurrence, cardiovascular disease, AGAPSS, therapy
Citation: Niznik S, Rapoport MJ, Avnery O, Lubetsky A, Haj Yahia S, Ellis MH and Agmon-Levin N (2022) Patterns of Recurrent Thrombosis in Primary Antiphospholipid Syndrome—Multicenter, Real-Life Long-Term Follow-Up. Front. Immunol. 13:843718. doi: 10.3389/fimmu.2022.843718
Received: 26 December 2021; Accepted: 15 March 2022;
Published: 19 April 2022.
Edited by:
Marie-Agnes Dragon-Durey, Université Paris Descartes, FranceReviewed by:
Rohan Willis, University of Texas Medical Branch at Galveston, United StatesAleksandra Antovic, Karolinska Institutet, Sweden
Copyright © 2022 Niznik, Rapoport, Avnery, Lubetsky, Haj Yahia, Ellis and Agmon-Levin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nancy Agmon-Levin, TmFuY3kuQWdtb24tTGV2aW5Ac2hlYmEuaGVhbHRoLmdvdi5pbA==
 Stanley Niznik
Stanley Niznik Micha J. Rapoport2,3
Micha J. Rapoport2,3 Soad Haj Yahia
Soad Haj Yahia