- 1Guanghua Clinical Medical College, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Department of Rheumatology, Shanghai Guanghua Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Department of Neurology, Rizhao Hospital of Traditional Chinese Medicine, Rizhao, China
- 4Integrated Traditional Chinese and Western Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
Precise expression and regulation of genes in the immune system is important for organisms to produce strong immunity towards pathogens and limit autoimmunity. In recent years, an increasing number of studies has shown that long noncoding RNAs (lncRNAs) are closely related to immune function and can participate in regulating immune responses by regulating immune cell differentiation, development, and function. As immune cells, the polarization response of macrophages (Mφs) plays an important role in immune function and inflammation. LncRNAs can regulate the phenotypic polarization of Mφs to M1 or M2 through various mechanisms; promote pro-inflammatory or anti-inflammatory effects; and participate in the pathogenesis of cancers, inflammatory diseases, infections, metabolic diseases, and autoimmune diseases. In addition, it is important to explore the regulatory mechanisms of lncRNAs on the dynamic transition between different Mφs phenotypes. Thus, the regulatory role of lncRNAs in the polarization of Mφs and their mechanism are discussed in this review.
Introduction
Macrophages (Mφs) are vital antigen-presenting cells with high heterogeneity and plasticity in the human immune system (1). Mφs can induce multiple polarization processes and exert different functions depending on the conditions (2). The local cytokine environment can lead to polarization of Mφs. Therefore, according to their expression of cell surface markers, the production of specific factors, and biological activities, Mφs are classified into the M1-like and M2-like types (3). M1 and M2 polarization is a remarkable characteristic of Mφs involved in numerous biological processes. M1 Mφs are known as classical Mφs and are activated by bacterial lipopolysaccharide or interferon (IFN)-γ. To produce and secrete higher levels of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-12, and cyclooxygenase-21. M1 Mφs have robust antimicrobial functions and inhibit tumor growth (4). In M2 Mφs, signal transducer and activator of transcription 6 (STAT6) is activated by IL-4 receptor α (IL-4Rα) and M2 Mφs are polarized by Th2 cytokine IL-4/IL-13. M2 Mφs have anti-inflammatory and strong phagocytic abilities to eliminate apoptotic cells and are useful for chronic infection treatment and wound healing (5). Moreover, according to the different stimuli received, M2 Mφs can be further divided into four different types: M2a, M2b, M2c, and M2d (6) (See Figure 1).
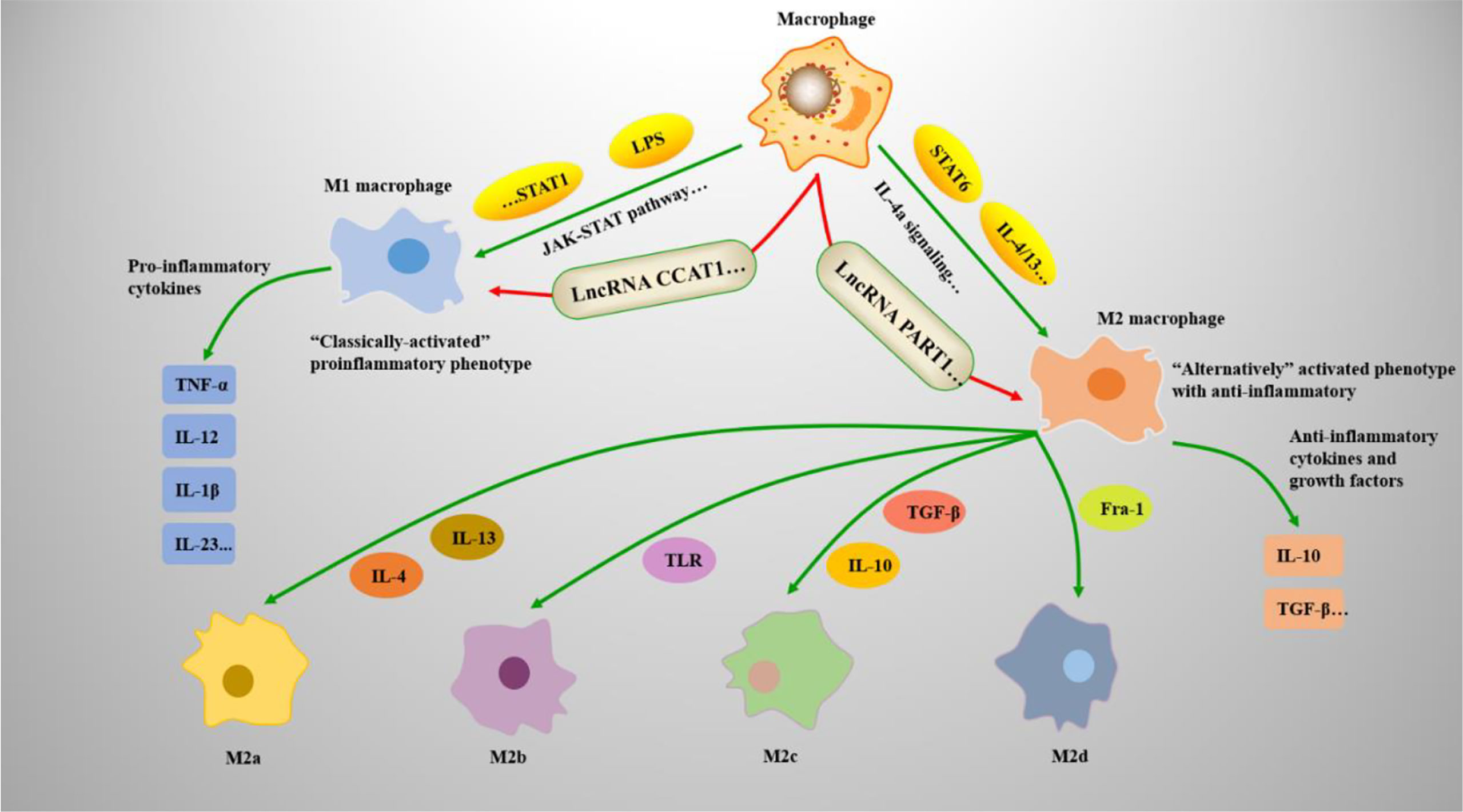
Figure 1 Mφs polarization mechanisms and types. Mφs adopt a proinflammatory M1 phenotype or anti-inflammatory M2 phenotype. (1) Under the action of the JAK-STAT pathway, STAT1 dimerizes and reacts with interferon (IFN)-γ to induce M1-associated genes, such as TNF-α and IL-12. Lipopolysaccharide (LPS) also induces M1-associated genes by forming the IFN-stimulated gene factor 3 (ISGF3) complex. (2) IL-4, IL-13, and STAT6 are associated with M2 Mφ polarization. STAT6 mediates IL-4a signaling and regulates M2-associated genes, such as IL-10 and TGF-β; (3) the M2 phenotype can differentiate into M2a, M2b, M2c, and M2d under the induction of related genes.
Precise regulation of Mφs polarization is a key step in controlling immune system function, clearing pathogens, and defending against external evil. The evidence accumulated in the past decade shows that lncRNAs are widely expressed and plays a key role in gene regulation. LncRNAs are a group of non-coding RNAs >200 nucleotides in length and composed of molecules transcribed by different types of RNA polymerase II (7). LncRNAs could regulate chromatin function, change the stability and translation of cytoplasmic mRNAs, and interfere with the signaling pathways. These functions influence gene expression in different biological and pathological environments, such as immunoreactive diseases (8). In addition, lncRNAs are responsible for the integrity of nuclear structure and can regulate the expression of nearby genes or genes in other parts of the cell by interacting with proteins, RNAs and DNAs (7). LncRNAs are a potential biomarker (9) and act as crucial regulators of Mφs polarization in response to intracellular or extracellular stimulation. various lncRNAs exhibit different functions in Mφs infiltration and polarization and regulate the secretion of inflammatory cytokines released by Mφs (10). Recent studies indicated that lncRNAs could participate in M1 or M2 polarization and exert multifunctional roles in disease development (11) (See Tables 1, 2).
Here, we review the current understanding of lncRNA functions in Mφs polarization. Using different disease models, we reviewed that different lncRNAs regulate the polarization of Mφs via different signaling pathways and act on different targets; we also explain the mechanism of these effects.
LncRNAs Regulate Mφs Polarization in Various Cancers
Tumor-associated macrophages (TAMs), which are specialized phagocytic cells, play an important role in the pathology and pathogenesis of cancers and are responsible for modulating the tumor microenvironment (TME) (95) (See Table 1). The microenvironment signals exposed to Mφs are the key factors that determine the phenotype of Mφs; these microenvironment signals selectively regulate their functions, including M1 and M2 polarization (96). At present, TAMs are mainly divided into two subtypes (See Figure 2): M1 TAMs of classical activation pathway and M2 TAMs of alternative activation pathway. M1 TAMs have significant anti-tumor effect. In the TME, it can identify tumor cells and kill tumor cells through the corresponding signaling pathway. According to the different pathways of action, it can be divided into direct killing mechanism and antibody-dependent cytotoxicity (ADCC) mechanism (97). M2 TAMs can significantly promote tumor growth. First of all, M2 TAMs secrete cytokines such as IL-6 and CXCL-8 into the TME and directly promotes the growth of tumor cells (98). Secondly, M2 TAMs can also block the inducible nitric oxide synthase (iNOS) pathway, reduce the synthesis of NO, accelerate the production of polyamines, and then promote the proliferation of tumor cells (99). M2 TAMs also promote angiogenesis of tumor cells by secreting vascular endothelial growth factor (VEGF) and IL-17, and can release matrix metalloproteinase (MMP)-2, MMP-9 and other substances to degrade extracellular matrix, promote vascular endothelial migration and induce angiogenesis (100, 101). M1 and M2 TAMs existed in all stages of the tumor, mainly M1 in the early stage and M2 in the middle and late stages of the tumor. With the progression of tumor, M1 is gradually polarized to M2, and the increase number of M2 TAMs also indicates a poor prognosis. Clinical studies have confirmed that M1 TAMs polarize to M2 TAMs induced by proliferator-activated receptor (PPAR)-γ. The increase in the proportion of M2 TAMs are closely related to tumor cell proliferation and tumor tissue vascular density (MVD) (102). A new study has shown that the inhibition of nuclear factor-kappa B (NF-κB) activity and the increase of STAT3 activity in Mφs represent a tumor-mediated mechanism that triggers M1/M2 deviation (103). Moreover, lncRNAs are increasingly appreciated as important regulators of gene expression (104), and multiple studies have showed (105, 106) that lncRNAs have a significant role in the regulation of TAM polarization in TME and functions principally as diagnostic and therapeutic biomarkers. Because lncRNAs are highly stable, specific and easy to detect, and can be expressed abnormally under different pathophysiological conditions (107); it can be used as a regulatory molecule of gene expression, acting as oncogenes or tumor suppressors, thus playing a role and directly involved in the occurrence of cancer, apoptosis, cell migration and invasion (108, 109). As a potential biomarker for diagnosis and prediction, lncRNAs have a wide range of functions in the occurrence and development of various human diseases, especially in the occurrence and development of many cancers.
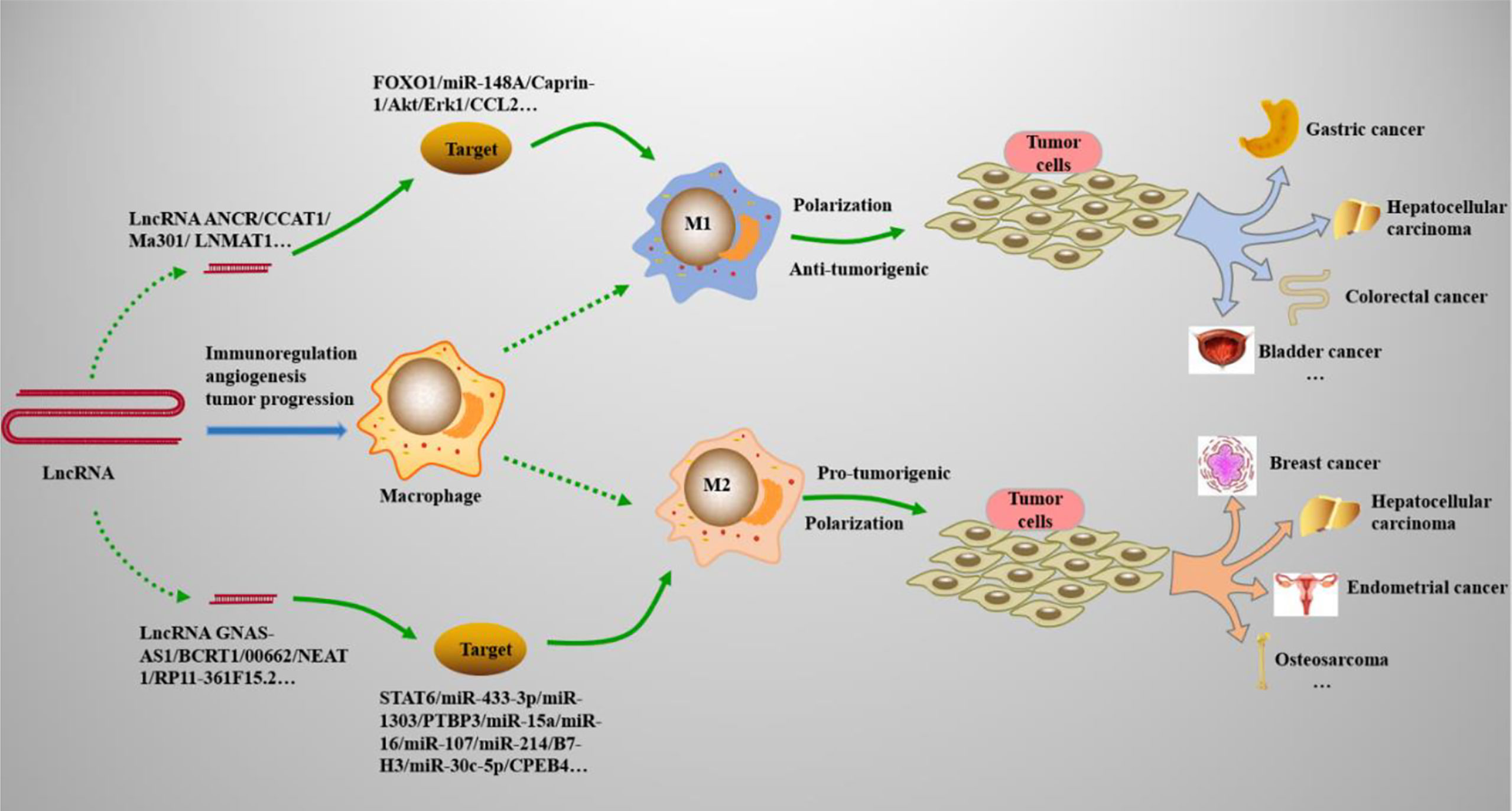
Figure 2 LncRNAs regulate Mφs polarization in cancers. Many types of lncRNAs play important roles in cancers by acting on the M1 and M2 polarization processes of Mφs. (1) M1 polarization can resist tumor cells and play an immunomodulatory role in tumor microenvironments, such as gastric cancer, bladder cancer, and colorectal cancer. (2) M2 polarization promotes the growth, migration, and proliferation of tumor cells, and is involved in the progression of cancers, such as breast cancer, hepatocellular carcinoma, and osteosarcoma.
Respiratory System Cancers
Lung cancer is the most common respiratory cancer, with non-small cell lung cancer (NSCLC) accountings for approximately 85% of all lung cancers as the most common cancer worldwide. Increasing evidence has shown that Mφs and lncRNAs are involved in the development of inflammation and malignant transformation of tumor cells. LncRNAs are also involved in the pathogenesis of lung cancer. Li et al. (40) used lncRNAGNAS-AS1, to explore the role of GNAS-AS1 in the development of NSCLC. These experimental studies showed that lncRNAGNAS-AS1 was highly expressed in M2 Mφs and NSCLC cell lines, which was significantly higher than that in M1 Mφs, and promoted the polarization of M2 Mφs and progression of NSCLC by directly inhibiting miR4319 and increasing the level of N-terminalEF-handcalciumbindingprotein3 (NECAB3). In addition, lncRNAGNAS-AS1 was also highly expressed in lung TAMs and clinical tumor tissues. Therefore, lncRNAGNAS-AS1 promoted the polarization of M2 Mφs and induced the progression of NSCLC through the miR-4319/NECAB3M2 axis in the tumor microenvironment, showing potential as a target for treating NSCLC. Another study on lung cancer confirmed that lncRNA XIST can also play a role by regulating Mφs. The expression of lncRNA XIST in M2 Mφs was significantly higher than that in M1 Mφs. Down-regulation of lncRNA XIST could significantly inhibit IL-4-induced M2 polarization, resulting in downregulation of M2 specific markers such as IL-10 and CD163. Due to the direct interaction between T-cell-specific transcription factor (TCF)-4 and lncRNA XIST, this inhibition was stopped by the overexpression of TCF-4. However, lncRNA XIST knockout can restore IL-4-induced M2 polarization, further indicating the importance of TCF-4 in M2 Mφs and TAMs-induced proliferation, migration and invasion of lung cancer cells. The results showed that lncRNA XIST positively regulated the M2 polarization and interacted with TCF-4, thus regulating the development of lung cancer (41).
Urinary System Cancers
Prostate cancer (PC) is one of the most common tumors in the urinary system, and methods for its diagnosis and treatment have rapidly progressed in recent years; however, therapeutic effects remain unsatisfactory (110). Recently, the therapeutic value of lncRNAs in regulating Mφs in the TME has been widely explored, and a Chinese research team has found (44) that lncRNA LINC00467 may be beneficial for treating PC. Additionally, the lncRNA LINC00467 mainly exists in the cytoplasm, and its expression is upregulated in PC tissues and cells to enhance polarization of the M2 phenotype via the miR-494-3p/STAT3 axis. While M2 polarization can activate the STAT3 pathway, enhance the tumor enhancement of M2, and thus promote the growth and migration of PC cells (44).
Compared with PC, bladder cancer is among the most serious malignant tumors worldwide. Although the incidence of muscle-invasive bladder cancer (MIBC) is low, the overall mortality rate is high. Chen et al. (15) found that a new lncRNA named as LNMAT1 through sequencing of five MIBC organizations. Its expression in MIBC tissues was significantly upregulated and positively correlated with the intensity of Mφs infiltration around the tumors. Further experiments showed that overexpression of LNMAT1 in vivo activated Mφs polarization by mediating heterogeneous nuclear Ribonucleoprotein L (hnRNPL) to promote the transcription of its target gene, CCL2. Furthermore, this lncRNA can induce the expression of CCL2 in bladder cancer cells, upregulate CCL2 to stimulate TAMs to secrete VEGF-C, participate in lymphogenesis, promote lymphatic metastasis and lymphangiogenesis, and accelerate the invasiveness of bladder cancer cells. These findings elaborate that LNMAT1 regulates the polarization of TAMs and mediates lymphatic metastasis, revealing a carcinogenic role for LNMAT1 in bladder cancer progression. Differential genes of lncRNA LINC01140 and fibroblast growth factor 9 (FGF9) were found in another study of an online microarray expression profile (GSE77952) of MIBC (39). By the analysis of polymerase chain reaction (PCR) and enzyme linked immunosorbent assay (ELISA), revealed a positive correlation between the expression of LINC01140 and FGF9. FGF9 and LINC01140 were highly expressed in M2 Mφs; after knocking down FGF9 and LINC01140, the viability and invasive ability of MIBC cells were significantly inhibited, leading to a decrease in the M2 markers IL-10 and Arg1 and weakened M2 polarization. In addition, by using the competing endogenous RNA mechanism, it was found that inhibition of miR-140-5p significantly reversed the expression and effects of LINC01140 and FGF9, thus affecting M2 polarization. Finally, LINC01140, miR-140-5p and FGF9 form a lncRNA-miRNA-mRNA mechanism axis, which influences M2 polarization by regulating the TME, thereby affecting the progression of MIBC.
Digestive System Cancers
Oral squamous cell carcinoma (OSCC) is a digestive system cancer that is also a common type of head and neck cancer. The occurrence and progress of OSCC are closely related to Mφs. M2 Mφs accelerate the progression of OSCC through a complex cross-talk mechanism and are regulated by lncRNAs. The NF-κB signaling plays an important role in tumor development. Ai et al. (42) found that deletion of lncRNA DCST1AS1 hindered the progression of OSCC by inhibiting the NF-κB signaling pathway. Its overexpression can cause M2 polarization to can promote the growth and differentiation of OSCC cells and accelerate the development of cancer by regulating the tumor immune environment. Another lncRNA, LBX1-AS1, is secreted by RBPJ-overexpression (RBPJ-OE) Mφs and belongs to the exocrine RNA. Its mechanism is mediated via the miR-182-5p/FOXO3 pathway. As a competing endogenous RNA, LBX1-AS1 stimulates Mφs by acting on miR-182-5p and fork head box protein O3 (FOXO3), causing Mφs polarization and activating Mφs to regulate the development of tumor cells (49).
Gastric cancer (GC) is a common disease affecting the digestive system. In recent years, research on GC has mainly focused on the molecular mechanism and attempts to identify new therapeutic targets. LncRNAs and Mφs polarization were shown to be closely related to the development of GC. M1 polarization can kill tumor cells and inhibit tumor lymphangiogenesis and angiogenesis (111). Overexpression of lncRNA ANCR in Mφs significantly reduced the expression of the M1 polarization marker IL-1β and IL-6, thus inhibiting M1 polarization. Co-culture of Mφs with GC cells showing high expression of lncRNA ANCR resulted in significantly enhanced invasiveness and migration of GC cells. FOXO1 is the target gene of the lncRNA ANCR. On one hand, FOXO1 can promote Mφs to produce inflammatory factors and induce M1 polarization. In contrast, lncRNA ANCR accelerates the ubiquitination of FOXO1 and inhibits its expression. After downregulation of FOXO1, M1 polarization was also inhibited. Based on these results, overexpression of lncRNA ANCR can downregulate FOXO1, thus inhibiting M1 polarization and promoting the development of GC cells (12). Other lncRNA can affect GC progression by influencing M2 polarization. M2 Mφs can induce GC cells growth, and lncRNA HCG18 can positively regulate M2 polarization. Specifically, lncRNA HCG18 can bind to miR-875-3p and target krüppel-like factor4 (KLF4) regulation, as well as upregulate KLF4 by reducing the expression of miR-875-3p in Mφs, thereby promoting M2 polarization. M2 Mφs have anti-inflammatory effects, secrete inflammatory cytokines to kill tumor cells, slow GC development, and play a role in immune surveillance (45).
Hepatocellular carcinoma (HCC) is a common tumor of the digestive system with a high incidence in Asia. M2 Mφs can promote tumor angiogenesis and induce cancer cell metastasis, but the mechanism of Mφs polarization is unclear. Four studies have explained the role of this mechanism in HCC development through the regulation of different lncRNAs on M2 polarization. It has been found (26) that M2 Mφs can promote human umbilical vein endothelial cell angiogenesis when M2-THP-1 cells are co-cultured with these cells. In mouse model of H22 tumor cells, lncRNA CENDE downregulated the expression of CD163, STAT-6, and VEGF by promoting M2 polarization, thus indirectly regulating tumor angiogenesis. In a study of SMCC-7721 cells, the supernatant of TAMs significantly promoted cell invasiveness. The expression of lncRNA GAS5 is downregulated in M2 Mφs and TAMs, which has been shown to be related to Mφs polarization in the progression of HCC. This lncRNA plays a regulatory role by regulating the expression of the target protein phosphatase and tensin homolog (PTEN) to inhibit M2 polarization of TAMs in SMCC-7721 cells (22). M2 polarization can also interact with the miR-372-3p/TLR4 axis, thus affecting the delivery of lncRNA PART1 into liver cancer tissues and cells via tumor-derived extracellular vesicles, inducing the upregulation of PART1 and Toll-like receptor 4 (TLR4) and downregulation of miR-372-3p, and promoting the proliferation and invasion of liver cancer cells. Additionally, miR-372-3p can bind to PART1 and negatively regulate TLR4, act on extracellular vesicles, and induce M2 polarization in HCC tissues, thus promoting the occurrence of HCC (23). The microRNA-15a-5p/CXCL17 axis is another regulatory pathway of M2 polarization in HCC. A previous study showed (24) that lncRNA DLX6-AS1, miR-15a-5p, and CXCL17 were highly expressed in HCC cells, and exosomes were isolated from HCC cells and co-cultured with M2 Mφs. The results showed that extracellular ubiquitin induced and intracellular HCC-exosomes transported DLX6-AS1 to Mφs, targeted binding to miR-15a-5p, and regulated CXCL17 expression to stimulate M2 polarization. In addition, Wnt ligands in tumor cells can stimulate M2 polarization, which can jointly accelerate the development and metastasis of HCC cells in vivo and in vitro. Furthermore, numerous studies have shown that different lncRNAs (lncRNA COX-2, lncRNA TUC339, and lncRNA Ma301) induce M1 and M2 polarization in the tumor microenvironment through different action targets (E-cadherin, caprin-1) and pathways (Akt/Erk1pathway), decrease the levels of iNOS and TNF-α in M1 Mφs, and increase the levels of Arg-1 and Fizz-1 in M2 Mφs. LncRNAs also promotes the production of various inflammatory factors, chemokines and exocrine, thus acting on the immune response of tumors and inhibiting pathological processes such as immune escape and invasion of HCC. These studies provides a new theoretical basis for treating HCC (46–48).
With the aging of the population and poor lifestyle, the global morbidity and mortality of colorectal cancer (CRC) are increasing each year. In epigenetic studies, lncRNAs were confirmed to be closely related to the pathogenesis of CRC. For example, lncRNA RP4 functions in CRC by binding to miR7-5p (112). Previous studies also showed that Mφs are involved in the pathogenesis of numerous cancers. Is there a regulatory relationship between lncRNAs and Mφs that interfere with the progression of CRC? Related studies found that lncRNAs HLA-F-AS1, NBR2, PTTG3P, and RPPH1 induce the polarization response of tumor-associated Mφs by acting on the corresponding targets or pathways, thus participating in the pathological development of CRC. Mechanistically, lncRNA MIR155HG (31) and lncRNA HLA-F-AS1 (27) can competitively bind with miR-650 and miR-375, reverse-regulate the expression of miR-650 and miR-375, and indirectly target annexin A2 (ANXA2) and profilin 1 (PFN1). Inhibiting of miR-650 and miR-375 promoted the expression of ANXA2 and PFN1 in CRC-derived extracellular vesicles. This mechanism further stimulated increased M2 Mφs marker expression, induced M2 Mφs phenotypic polarization, and accelerated CRC cells invasion. In contrast, lncRNA NBR2 can activate M1 polarization, inhibit M2 polarization, and regulate the polarization of TAMs to promote the release of inflammatory factors such as TNF-α, IL1-β, and IL-10, thus acting on the TME. However, after knockout of the lncRNA NBR2 gene, these effects were reversed (28). A similar process occurs with lncRNA PTTG3P (29). Under the action of hypoxia-inducible factor-1-α (HIF-1α), the hypoxia environment induces the overexpression of PTTG3P to promotes M2 polarization and stimulates tumor development. In addition, exosomes can mediate Mφs polarization. Liang et al. (30) found that the expression of lncRNA RPPH1 is upregulated in CRC, which is related to poor prognosis. In terms of the mechanism, RPPH1 binds to β-III tubulin and prevents its ubiquitination, which induces the transformation of epithelial-mesenchymal transition and accelerates the metastasis of cancer cells. Further studies showed that RPPH1 is closely related to exosomes, which can transfer into Mφs by exosomes to mediate M2 polarization and promote tumor growth and migration.
Reproductive System Cancers
Breast cancer occurs following uncontrolled proliferation of breast epithelial cells via the action of various carcinogenic factors and is considered as a reproductive system cancer. Modern studies showed that the lncRNA-miRNA-mRNA axis plays an important role in cancer progression. The lncRNA BCRT1/miR-1303/PTBP3 and lncRNA Xist/miR-101-3p/KLF6/C/EBPα can regulate the progression of breast cancer. In the lncRNA BCRT1/miR-1303/PTBP3 axis (18), polypyrimidine tract binding protein 3 (PTBP3) promotes breast cancer, and the expression of lncRNA BCRT1 in breast cancer tissues is significantly increased, which is related to the poor prognosis of patients. In the mechanism of action, lncRNA BCRT1 can bind to miR-1303 and act on PTBP3 to inhibit its expression. After overexpression, lncRNA BCRT1 significantly promotes M2 polarization mediated by exosomes. After M2 polarization, inflammatory factors such as TNF-α and IL-6 are produced, which promote cancer in tumor microenvironment; this effect is more obvious in hypoxic environments. Phenotypic transformation of TAMs mediates tumor immune escape. Zhao et al. (50) found that the expression of lncRNA Xist and C/EBPα was upregulated in M1 Mφs, whereas the expression of miR-101-3p was downregulated. LncRNA Xist knockout inhibited the expression of C/EBPα and KLF6 and induced the transformation from M1 to M2, thus promoting the proliferation and migration of breast cancer cells. Therefore, lncRNA Xist competes with miR-101-3p to regulate the expression of C/EBPα and KLF6 to mediate Mφs polarization, promote the expression of lncRNA Xist in M1 Mφs and inhibit the expression of miR-101-3p in M2 Mφs, thus inhibiting the progression of breast cancer. TAMs reprogram their specific functional phenotype in the tumor environment to promote cancer progression and metastasis. Zhou et al. (16) showed that lncRNA p21 was significantly upregulated in Mφs. Knockout of P21 led to mouse double minute 2 (MDM2)-induced proteasome-dependent degradation of p53 and activated the NF-κB/STAT3 pathway, which promoted Mφs polarization to pro-inflammatory M1 and accelerated the progression of breast cancer. These results show that lncRNA p21 plays a key role in regulating the function of TAMs and can be a new therapeutic target for breast cancer.
In addition, after Zong (19) successfully induced M2 polarization by IL-4/IL-13 experimentally, the RNA level of lncRNA SNHG1 determined using by qRT-PCR was increased, indicating that this lncRNA is involved in M2 polarization. Further experiments showed that lncRNA SNHG1 knockout inhibited phosphorylation of STAT6 and indirectly weakened M2 polarization. Subsequent transplantation of Mφs mixed with other cells accelerated the growth of breast cancer cells. The lncRNA SNHG1 regulates M2 polarization to affects cell growth and invasion. LncRNA 00337 (20) and lncRNA 00514 (21) have similar mechanisms and can both affect the development of breast cancer by regulating M2 TAMs polarization.
Other Systemic Cancers
In addition to the above common cancers, Mφs polarization is involved in the pathological mechanisms of osteosarcoma and brain tumors. Osteosarcoma is common in children and adolescents. The lncRNA LOC100129620 can promote the proliferation and migration of osteosarcoma cells in vivo (33) by binding to miR-335-3p and regulating cyclin-dependent kinases 6 (CDK6), thus promoting angiogenesis and Mφs polarization. In contrast, LOC100129620 gene knockout decreased the infiltration and M2 TAMs polarization, and inhibited the development of osteosarcoma. In glioblastoma multiforme, Zhang found (38) that coagulation factor X (FX) was highly expressed and positively correlated with the concentration of TAMs. FX is secreted in the tumor microenvironment, has a strong chemotactic effect on Mφs, promotes the phosphorylation and activation of ERK1/2 and AKT in Mφs, and stimulates Mφs to M2 polarization. In addition, FX was identified as the target gene of miR-338-3p. The lncRNA CASC2c interacts with miR-338-3p and binds with FX to jointly inhibit the expression of FX and finally inhibit M2 polarization.
In summary, lncRNAs and Mφs play important role in multi-system cancers such as the respiratory, digestive, and reproductive systems. Through the analysis of the results of the above studies, it can be found that the mechanism of lncRNAs regulating Mφs polarization is mainly through the lncRNA-miRNA-mRNA axis. A large number of animal experiments and clinical trials have confirmed that M2 TAMs play an important role in tumor growth, invasion and metastasis, while M1 TAMs can inhibit tumor formation. It is also found that M2 polarization occurs more frequently and the mechanism of action is more complex than that of M1 in the occurrence and development of cancers. The presence of a large number of M2 Mφs in tumor tissue indicates a poor prognosis, which has a positive reference value for the clinical treatment of M2 Mφs. In the TME, TAMs produce a polarization response, secrete inflammatory factors and chemokines, regulate immune responses, and participate in the occurrence and development of cancers, which is important in cancer pathogenesis. Therefore, blocking Mφs from reaching tumor tissue, clearing Mφs and inhibiting the activation or repolarization of TAMs in TME has become a hot issue in the field of tumor immune research. Based on the understanding of the function of TAMs and the relationship between M1 and M2 Mφs, reversing the polarization of M1 to M2 Mφs is likely to be a key target for future tumor therapy. However, tumor treatment is a systematic project, and the effect of any single treatment is very limited. Regulating Mφs polarization through lncRNAs acting on different targets and pathways can provide new hope for the treatment of many types of cancers. Through the study of these lncRNAs in vivo and in vitro, TAMs can be polarized into pro-inflammatory or anti-inflammatory state, so as to develop new strategies to change the phenotype of Mφs. These cell therapies can be further combined with currently available chemotherapy and radiotherapy options to improve therapeutic effectiveness (113).
LncRNAs Regulate Mφs Polarization in Inflammatory Diseases
In addition to resisting pathogens, Mφs play a key role in eliminating inflammation and repairing damaged tissues. The polarization response of Mφs is essential for immunity and dynamic balance. In the early stage of tissue injury, Mφs are activated to produce a pro-inflammatory effect, thereby removing cell fragments and pathogens (114). During this phase of transformation, Mφs change their phenotype and play a key role in inflammation regression and tissue repair (115).
Osteoarthritis (OA) is a joint degenerative disease that often occurs in the elderly, and affects joint activity and function. Currently, there is no efficient and standardized treatment. A large number of studies has shown that many inflammatory cells and inflammatory factors, such as IL-6, IL10, TNF-α, are involved in the pathogenesis of OA (116). For example, M1 Mφs secrete pro-inflammatory cytokines. Under the influence of the inflammatory microenvironment, M1 Mφs are activated and secrete high levels of inflammatory mediators, infiltrating synovial tissues and chondrocytes, leading to apoptosis and accelerating the development of OA, which is considered as a key factors regulating the pathogenesis of OA (117). The results of a previous study suggested (58) that osteopontin (OPN) affect M1 polarization. Overexpression of OPN can promote M1 Mφs polarization, produce pro-inflammatory cytokines and degrading enzymes, and act on chondrocytes to exert a cytotoxic effect. Further experiments showed that lncRNA XIST acts on OPN indirectly. It binds to miR-376c-5p competitively through the competing endogenous RNA mechanism, thus weakening the inhibition of miR-376c-5p on OPN, and then affects M1 polarization. In addition, Xist gene knockout affects M1 Mφs and chondrocyte apoptosis. The effect of the lncRNA XIST/miR-376c-5p/OPN axis on M1 Mφs and the inflammatory microenvironment may be a new mechanism of apoptosis in OA chondrocytes. A similar mechanism was found in the lncRNA IGHCγ1 (90). This lncRNA is mainly located in the cytoplasm of Mφs and competitively binds to miR-6891-3p via the NF-κB signaling pathway, and regulates TLR4 to inhibit Mφs proliferation and inflammation, ultimately affecting OA development.
M1 and M2 polarization play important roles in lung inflammation and injury (118). Targeting Mφs polarization and affecting the inflammatory microenvironment may be useful for treating pneumonia. LncRNA GAS5 plays a key role in cell proliferation, invasion, apoptosis, and Mφs polarization (119). In a study of childhood pneumonia, Chi et al. (53), showed that overexpression of lncRNA GAS5 inhibits JAK2/STAT3 signal transduction, which promotes M1 polarization. The specific mechanism involves the miRNA-mRNA action axis: GAS5 combined with miR-455-5p can promote the expression of SOCS3, and then inhibit the JAK2/STAT3 pathway, thus promoting phenotypic polarization of Mφs from M2 to M1. These findings have potential for the development of therapeutic strategies against pneumonia. The mechanisms by which lncRNAs regulate M1 or M2 polarization in the pneumonia microenvironment are similar; for example, lncRNA p21 and lncRNA NKILA promote M2 polarization through NF-κB signaling pathway (54, 64). Moreover, through the PI3K/AKT pathway, lncRNA Gm16410 reduces the expression of TNF-α in Mφs, inhibits inflammation, and stimulates polarization of Mφs induced by PM2.5, which participates in the activation of Mφs and process of inflammation (78). In addition, these effects have been described in numerous studies.
Mφs have also been widely reported to be involved in the pathogenesis of cardiovascular inflammatory diseases. Viral myocarditis is a type of heart inflammatory disease caused by viruses, that can lead to heart failure in severe cases. There is increasing evidence that Mφs polarization plays an important role in coxsackievirus B3-induced viral myocarditis. Zhang et al. (67) found that lncRNA AK085865 can increase the susceptibility to viral myocarditis by regulating the Mφs polarization. It specifically binds to interleukin enhancer-binding factor (ILF2) and promotes M2 Mφ polarization by negatively regulating the ILF2-ILF3 complex-mediated miRNA-192 pathway. Overexpression of miRNA-192 can also effectively stimulate the transformation of myocardial infiltrating Mφs to the M2 phenotype. This reveals the key mechanism by which lncRNA AK085865 regulates Mφs polarization and act on myocarditis. LncRNA MEG3 participates in the regulation of M1/M2 Mφs via another pathway (68); it regulates tumor necrosis factor receptor-associated factor 6 (TRAF6) expression by binding to miR-233. Inhibitory MEG3 can downregulate the NF-κB pathway; inhibited the expression of iNOS, TNF-α, and CD86; reduced the expression of M1 Mφs; promoted the expression of Arg-1 and Fizz-1; increased the M2 polarization, and finally reduced cardiomyocyte injury.
In traumatic diseases, lncRNA GBP9 regulates Mφs polarization and reduces the inflammatory response after spinal cord injury through corresponding pathways. Studies have shown that after spinal cord injury, Mφs first differentiate into M1 Mφs under the stimulation of lipopolysaccharide and TNF-α, and TLRs on the cell surface are activated, which induces the recruitment of downstream protein myeloid differentiation factor 88, activates NF-κB, JAK-STAT, and other pathways; and promotes M1 to release TNF-α, CCL8, and other molecules (57, 120).
Moreover, M2 Mφs surface receptors that bind to IL-4 and IL-13 can promote the phosphorylation of STAT6 and stimulate M2 Mφs. M2 Mφs can highly express IL-10, transforming growth factor (TGF)-β and neurotrophic factors, which can inhibit the nerve cell apoptosis and pro-inflammatory effects of M1, as well as promote nerve tissue repair (121). Zhou et al. found (57) that lncRNA GBP9 competitively binds to miR-34a to eliminate the inhibition of suppressor of cytokine signaling-3 (SOCS3), thus regulating STAT1/STAT6 signaling and Mφs polarization. After M1 polarization, M1 showed stronger phagocytosis and antigen presentation ability. In M1 Mφs, lncRNA GBP9 overexpression significantly decreased the expression of p-STAT1, SOCS3, IL-6, and IL-12, whereas in M2 Mφs, the same overexpression increased the concentration of SOCS3 and decreased the production of p-STAT6, IL-10, and TGF-β1, indicating that lncRNA GBP9 overexpression promoted M1 polarization. In contrast, after silencing lncRNA GBP9, the M2 polarization response was enhanced, nerve repair factor was secreted, and spinal cord injury was improved.
LncRNAs Regulate Mφs Polarization in Infections
M1 Mφs have a strong cytotoxic effect on infected cells and are resistant to infection. Human immunodeficiency virus (HIV) infection poses a challenge to human health. Studies have shown that Mφs polarization is associated with HIV infection and that these cells are immune to virus-induced cell death. M1 Mφs play an important role in early antiviral immune responses and late restorative responses in the process of HIV infection (122). These Mφs show good prospects for treating HIV infection by blocking the aggregation of Mφs at the inflammatory site or changing the Mφs polarization. Boliar et al.’ data (92) showed that the expression of lncRNA SAF was significantly upregulated in HIV-1-infected Mφs. Downregulation of SAF can increase the activity of caspase-3/7 in virus-infected Mφs. This caspase-induced apoptosis occurs only in HIV-1-infected Mφs, but not in other cells, resulting in a significant reduction of HIV-1 virus in Mφs. Therefore, targeting of lncRNA SAF is a potential method for inducing the death of Mφs infected by HIV-1.
LncRNAs Regulate Mφs Polarization in Metabolic Diseases
Recent studies revealed that the mechanism by which lncRNAs regulate Mφs are important in the development of metabolic diseases, such as diabetes. Diabetes mellitus is related to insulin resistance. It can promote inflammatory responses and induce neurovascular complications through the proliferation, polarization, and dysfunction of Mφs (123). Das et al. (86) studied the role of lncRNA Dnm3os in diabetes. The results showed that in Mφs, a decrease in nucleolin and overexpression of Dnm3os changed the modification of intracellular histone, enhanced the promoter H3K9ac, recruited histone acetyltransferase, and targeted upregulated the genes related to inflammation and immune response such as NKx3-2 AP1, STAT, and IRF1, which promoted phagocytosis, whereas knockout of Dnm3os weakened these responses. In addition, nucleolin gene knockout increased the expression of pro-inflammatory factor IL-6 in Mφs, which is further enhanced by lncRNA Dnm3os-regulated Mφs. Diabetic peripheral neuropathy (DPN) is the main complication of type 2 diabetes mellitus. It has been reported that the expression of M1 Mφs in patients with DPN is significantly increased and mediates the secretion of inflammatory factors such as IL-1 and IL-6, which promotes the occurrence and development of DPN (4). Further studies (52) showed that in DPN, lncRNA HCG18 competitively binds to miR-146a and regulates TNF receptor associated factor 6 (TRAF6) to promote M1 polarization and inflammatory factor secretion. MiR-146a can negatively regulate the expression of TRAF6, whereas inhibition of TRAF6 can reverse the promoting effect of HCG18 on M1 Mφs. HCG18 stimulates Mφ activation via the TRAF6/NF-κB pathway. Therefore, lncRNA HCG18 can promote the proliferation of M1 Mφs in DPN and accelerate the development of disease by regulating the miR-146a/TRAF6 axis.
LncRNAs Regulate Mφs Polarization in Autoimmune Diseases
Rheumatoid arthritis (RA) is a common autoimmune disease with a complex pathogenesis, and its incidence is increasing. In RA, TNF-α produced by M1 Mφs can trigger synovial cells to produce cytokines, leading to the development of arthritis. Infiltrating Mφs in the joint synovium plays an important role in the pathogenesis of RA, and the degree of infiltration is related to the activity of the disease and degree of joint erosion (124). A study showed (61) that silencing of lncRNA H19 altered the expression of lipopolysaccharide in Mφs from patients with RA, induced M1 polarization, and reduced the expression of factors such as IL-6 and CXCL10. However, overexpression of H19 showed the opposite effects by promoting M1 polarization and aggravating arthritis in mice. In practice, lncRNA H19 is highly expressed in patients with RA. Increased expression of KDM6A, promotes M1 polarization and aggravates arthritis. Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) characterized by extensive inflammation, demyelination and axonal loss (125, 126). Lesions often occur in the brain and spinal cord. Due to unknown etiology and complex pathophysiological mechanisms, the etiology and pathogenesis of MS have not been fully elucidated (127, 128). Microglia can be divided into M1 and M2 functional subtypes; they play a central role in inflammatory process and neuronal destruction in MS (129). It has been found that M2 polarized microglia can promote neuronal survival, neurite growth and oligodendrocyte progenitor cell (OPC) differentiation (130, 131). In epigenetics, lncRNA Gas5 is an epigenetic regulator of microglial polarization. It is highly expressed in microglia of patients with MS and can inhibit M2 polarization of microglia. The specific mechanism is that Gas5 suppresses the transcription of TRF4, a key factor regulating M2 polarization, through recruiting the polycomb repressive complex 2 (PRC2), thus inhibiting M2 polarization. Therefore, this study suggests that lncRNA Gas5 may be a promising target for the treatment of MS (76).
Conclusion
Mφs constantly convert between two extreme polarization states, M1 and M2 Mφs. Mφs are involved in the pathogenesis and progression of many diseases. Identifying the key regulators of Mφs polarization is important for developing treatments for diseases. An increasing number of lncRNAs has been identified in the process of Mφ polarization. There is a close relationship between lncRNAs and Mφs, which play an important regulatory role in Mφs. Their multifunctional capabilities and heterogeneous working mechanisms make them potential targets for treating diseases. In conclusion, lncRNAs exhibit tremendous effects on Mφs differentiation and polarization. The interaction between lncRNAs and Mφs is involved in the development of multiple systemic diseases and provides useful targets for the interference of macrophage involved disorders.
However, compared with the study of small molecule RNA, lncRNAs are still in the initial stage, and their function and regulation mechanism still need to be further clarified. In order to fully realize the therapeutic value of lncRNAs and the development of corresponding therapeutic drugs, there are still some difficulties to be overcome. First of all, we need to improve the method of transporting lncRNAs to specific areas. Because of the long length of lncRNA, it is difficult to deliver full-length lncRNAs, so we need an accurate and efficient means of transport to ensure the stability of lncRNAs. Secondly, we need to establish a complete, detailed and specific lncRNAs database to record the specific regulatory cell types and target genes of each lncRNAs. Finally, we need to solve the difficulty of finding homologous lncRNA. Especially in animal experiments, due to the epigenetic differences between animals and humans, the human lncRNAs may not exist in animals. This requires techniques such as gene knockout to build animal models, which makes the experiment more difficult. In recent years, with the deepening of research, the experimental techniques related to lncRNAs have been continuously developed, an increasing number of lncRNAs have been found, and play an important role in the occurrence and development of various diseases, and can be used as genes related to diagnosis and treatment. In particular, there is an increasing number of studies on lncRNAs regulating Mφs polarization and participating in the development of various diseases, which provides a new research content for future research. On the basis of fully understanding the mechanism of action, appropriate genetic engineering drugs are developed to achieve the purpose of prevention, diagnosis and treatment.
Author Contributions
PJ wrote the manuscript. XL conceived the study. All authors have reviewed and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
References
1. Dong R, Zhang B, Tan B, Lin N. Long non-Coding RNAs as the Regulators and Targets of Macrophage M2 Polarization. Life Sci (2021) 266:118895. doi: 10.1016/j.lfs.2020.118895
2. Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of Macrophage Polarization in Autoimmunity. Immunology (2018) 154:186–95. doi: 10.1111/imm.12910
3. Murray PJ. Macrophage Polarization. Annu Rev Physiol (2017) 79:541–66. doi: 10.1146/annurev-physiol-022516-034339
4. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili S-A, Mardani F, et al. Macrophage Plasticity, Polarization, and Function in Health and Disease. J Cell Physiol (2018) 233:6425–40. doi: 10.1002/jcp.26429
5. Atri C, Guerfali F, Laouini D. Role of Human Macrophage Polarization in Inflammation During Infectious Diseases. Int J Mol Sci (2018) 19:1801. doi: 10.3390/ijms19061801
6. Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage Subsets in Atherosclerosis. Nat Rev Cardiol (2015) 12:10–7. doi: 10.1038/nrcardio.2014.173
7. Chen L-L. Linking Long Noncoding RNA Localization and Function. Trends Biochem Sci (2016) 41:761–72. doi: 10.1016/j.tibs.2016.07.003
8. Statello L, Guo C-J, Chen L-L, Huarte M. Gene Regulation by Long Non-Coding RNAs and its Biological Functions. Nat Rev Mol Cell Biol (2021) 22:96–118. doi: 10.1038/s41580-020-00315-9
9. Zhou X, Xie D, Huang J, Lu A, Wang R, Jin Y, et al. Therapeutic Effects of (5R)-5-Hydroxytriptolide on Fibroblast-Like Synoviocytes in Rheumatoid Arthritis via lncRNA WAKMAR2/miR-4478/E2F1/p53 Axis. Front Immunol (2021) 12:605616. doi: 10.3389/fimmu.2021.605616
10. Sica A, Mantovani A. Macrophage Plasticity and Polarization: In Vivo Veritas. J Clin Invest (2012) 122:787–95. doi: 10.1172/JCI59643
11. Mohapatra S, Pioppini C, Ozpolat B, Calin GA. Non-Coding RNAs Regulation of Macrophage Polarization in Cancer. Mol Cancer (2021) 20:24. doi: 10.1186/s12943-021-01313-x
12. Xie C, Guo Y, Lou S. LncRNA ANCR Promotes Invasion and Migration of Gastric Cancer by Regulating FoxO1 Expression to Inhibit Macrophage M1 Polarization. Dig Dis Sci (2020) 65:2863–72. doi: 10.1007/s10620-019-06019-1
13. Liu J, Ding D, Jiang Z, Du T, Liu J, Kong Z. Long non-Coding RNA CCAT1/miR-148a/Pkcζ Prevents Cell Migration of Prostate Cancer by Altering Macrophage Polarization. Prostate (2019) 79:105–12. doi: 10.1002/pros.23716
14. Ahmad I, Valverde A, Naqvi RA, Naqvi AR. Long Non-Coding RNAs RN7SK and GAS5 Regulate Macrophage Polarization and Innate Immune Responses. Front Immunol (2020) 11:604981. doi: 10.3389/fimmu.2020.604981
15. Chen C, He W, Huang J, Wang B, Li H, Cai Q, et al. LNMAT1 Promotes Lymphatic Metastasis of Bladder Cancer via CCL2 Dependent Macrophage Recruitment. Nat Commun (2018) 9:3826. doi: 10.1038/s41467-018-06152-x
16. Zhou L, Tian Y, Guo F, Yu B, Li J, Xu H, et al. LincRNA-P21 Knockdown Reversed Tumor-Associated Macrophages Function by Promoting MDM2 to Antagonize* P53 Activation and Alleviate Breast Cancer Development. Cancer Immunol Immunother (2020) 69:835–46. doi: 10.1007/s00262-020-02511-0
17. Liu S-Q, Zhou Z-Y, Dong X, Guo L, Zhang K-J. LncRNA GNAS-AS1 Facilitates ER+ Breast Cancer Cells Progression by Promoting M2 Macrophage Polarization via Regulating miR-433-3p/GATA3 Axis. Biosci Rep (2020) 40:BSR20200626. doi: 10.1042/BSR20200626
18. Liang Y, Song X, Li Y, Chen B, Zhao W, Wang L, et al. LncRNA BCRT1 Promotes Breast Cancer Progression by Targeting miR-1303/PTBP3 Axis. Mol Cancer (2020) 19:85. doi: 10.1186/s12943-020-01206-5
19. Zong S, Dai W, Guo X, Wang K. LncRNA-SNHG1 Promotes Macrophage M2-Like Polarization and Contributes to Breast Cancer Growth and Metastasis. Aging (2021) 13:23169–81. doi: 10.18632/aging.203609
20. Xing Z, Zhang M, Liu J, Liu G, Feng K, Wang X. LINC00337 Induces Tumor Development and Chemoresistance to Paclitaxel of Breast Cancer by Recruiting M2 Tumor-Associated Macrophages. Mol Immunol (2021) 138:1–9. doi: 10.1016/j.molimm.2021.07.009
21. Tao S, Chen Q, Lin C, Dong H. Linc00514 Promotes Breast Cancer Metastasis and M2 Polarization of Tumor-Associated Macrophages via Jagged1-Mediated Notch Signaling Pathway. J Exp Clin Cancer Res (2020) 39:191. doi: 10.1186/s13046-020-01676-x
22. Wang X, Li F, Zhao W, Gao Z, Shen B, Xu H, et al. Long non-Coding RNA GAS5 Overexpression Inhibits M2-Like Polarization of Tumour-Associated Macrophages in SMCC-7721 Cells by Promoting PTEN Expression. Int J Exp Pathol (2020) 101:215–22. doi: 10.1111/iep.12374
23. Zhou J, Che J, Xu L, Yang W, Zhou W, Zhou C. Tumor-Derived Extracellular Vesicles Containing Long Noncoding RNA PART1 Exert Oncogenic Effect in Hepatocellular Carcinoma by Polarizing Macrophages Into M2. Dig Liver Dis (2021) S1590-8658(21)00374–1. doi: 10.1016/j.dld.2021.07.005
24. Wang L, Lin J, Ma X, Xu D, Shi C, Wang W, et al. Exosomal DLX6-AS1 From Hepatocellular Carcinoma Cells Induces M2 Macrophage Polarization to Promote Migration and Invasion in Hepatocellular Carcinoma Through microRNA-15a-5p/CXCL17 Axis. J Exp Clin Cancer Res (2021) 40:177. doi: 10.1186/s13046-021-01973-z
25. Chen J, Huang Z-B, Liao C-J, Hu X-W, Li S-L, Qi M, et al. LncRNA TP73-AS1/miR-539/MMP-8 Axis Modulates M2 Macrophage Polarization in Hepatocellular Carcinoma via TGF-β1 Signaling. Cell Signal (2020) 75:109738. doi: 10.1016/j.cellsig.2020.109738
26. Han C, Yang Y, Sheng Y, Wang J, Li W, Zhou X, et al. The Mechanism of lncRNA-CRNDE in Regulating Tumour-Associated Macrophage M2 Polarization and Promoting Tumour Angiogenesis. J Cell Mol Med (2021) 25:4235–47. doi: 10.1111/jcmm.16477
27. Zhang J, Li S, Zhang X, Li C, Zhang J, Zhou W. LncRNA HLA-F-AS1 Promotes Colorectal Cancer Metastasis by Inducing PFN1 in Colorectal Cancer-Derived Extracellular Vesicles and Mediating Macrophage Polarization. Cancer Gene Ther (2021) 28:1269–84. doi: 10.1038/s41417-020-00276-3
28. Lai F, Zhang H, Xu B, Xie Y, Yu H. Long non-Coding RNA NBR2 Suppresses the Progress of Colorectal Cancer In Vitro and In Vivo by Regulating the Polarization of TAM. Bioengineered (2021) 12:5462–75. doi: 10.1080/21655979.2021.1958558
29. Wang Y, Yu G, Liu Y, Xie L, Ge J, Zhao G, et al. Hypoxia-Induced PTTG3P Contributes to Colorectal Cancer Glycolysis and M2 Phenotype of Macrophage. Biosci Rep (2021) 41:BSR20210764. doi: 10.1042/BSR20210764
30. Liang Z, Liu H, Wang F, Xiong L, Zhou C, Hu T, et al. LncRNA RPPH1 Promotes Colorectal Cancer Metastasis by Interacting With TUBB3 and by Promoting Exosomes-Mediated Macrophage M2 Polarization. Cell Death Dis (2019) 10:829. doi: 10.1038/s41419-019-2077-0
31. Zhou L, Li J, Liao M, Zhang Q, Yang M. LncRNA MIR155HG Induces M2 Macrophage Polarization and Drug Resistance of Colorectal Cancer Cells by Regulating ANXA2. Cancer Immunol Immunother (2021). doi: 10.1007/s00262-021-03055-7
32. Yang D, Liu K, Fan L, Liang W, Xu T, Jiang W, et al. LncRNA RP11-361F15.2 Promotes Osteosarcoma Tumorigenesis by Inhibiting M2-Like Polarization of Tumor-Associated Macrophages of CPEB4. Cancer Lett (2020) 473:33–49. doi: 10.1016/j.canlet.2019.12.041
33. Chen Y, Tang G, Qian H, Chen J, Cheng B, Zhou C, et al. LncRNA LOC100129620 Promotes Osteosarcoma Progression Through Regulating CDK6 Expression, Tumor Angiogenesis, and Macrophage Polarization. Aging (2021) 13:14258–76. doi: 10.18632/aging.203042
34. Gao Y, Fang P, Li W-J, Zhang J, Wang G-P, Jiang D-F, et al. LncRNA NEAT1 Sponges miR-214 to Regulate M2 Macrophage Polarization by Regulation of B7-H3 in Multiple Myeloma. Mol Immunol (2020) 117:20–8. doi: 10.1016/j.molimm.2019.10.026
35. Liu F, Zhang T, Zou S, Jiang B, Hua D. B7-H3 Promotes Cell Migration and Invasion Through the Jak2/Stat3/MMP9 Signaling Pathway in Colorectal Cancer. Mol Med Rep (2015) 12:5455–60. doi: 10.3892/mmr.2015.4050
36. Zhou Y, Zhao W, Mao L, Wang Y, Xia L, Cao M, et al. Long non-Coding RNA NIFK-AS1 Inhibits M2 Polarization of Macrophages in Endometrial Cancer Through Targeting miR-146a. Int J Biochem Cell Biol (2018) 104:25–33. doi: 10.1016/j.biocel.2018.08.017
37. Cao J, Dong R, Jiang L, Gong Y, Yuan M, You J, et al. LncRNA-MM2P Identified as a Modulator of Macrophage M2 Polarization. Cancer Immunol Res (2019) 7:292–305. doi: 10.1158/2326-6066.CIR-18-0145
38. Zhang Y, Feng J, Fu H, Liu C, Yu Z, Sun Y, et al. Coagulation Factor X Regulated by CASC2c Recruited Macrophages and Induced M2 Polarization in Glioblastoma Multiforme. Front Immunol (2018) 9:1557. doi: 10.3389/fimmu.2018.01557
39. Wu S, Xu R, Zhu X, He H, Zhang J, Zeng Q, et al. The Long Noncoding RNA LINC01140/miR-140-5p/FGF9 Axis Modulates Bladder Cancer Cell Aggressiveness and Macrophage M2 Polarization. Aging (2020) 12:25845–64. doi: 10.18632/aging.202147
40. Li Z, Feng C, Guo J, Hu X, Xie D. GNAS-AS1/miR-4319/NECAB3 Axis Promotes Migration and Invasion of non-Small Cell Lung Cancer Cells by Altering Macrophage Polarization. Funct Integr Genomics (2020) 20:17–28. doi: 10.1007/s10142-019-00696-x
41. Sun Y, Xu J. TCF-4 Regulated lncRNA-XIST Promotes M2 Polarization Of Macrophages And Is Associated With Lung Cancer. OncoTargets Ther (2019) 12:8055–62. doi: 10.2147/OTT.S210952
42. Ai Y, Liu S, Luo H, Wu S, Wei H, Tang Z, et al. lncRNA DCST1-AS1 Facilitates Oral Squamous Cell Carcinoma by Promoting M2 Macrophage Polarization Through Activating NF-κb Signaling. J Immunol Res (2021) 2021:1–9. doi: 10.1155/2021/5524231
43. Liu G, Du X, Xiao L, Zeng Q, Liu Q. Activation of FGD5-AS1 Promotes Progression of Cervical Cancer Through Regulating BST2 to Inhibit Macrophage M1 Polarization. J Immunol Res (2021) 2021:1–12. doi: 10.1155/2021/5857214
44. Jiang H, Deng W, Zhu K, Zeng Z, Hu B, Zhou Z, et al. LINC00467 Promotes Prostate Cancer Progression via M2 Macrophage Polarization and the miR-494-3p/STAT3 Axis. Front Oncol (2021) 11:661431. doi: 10.3389/fonc.2021.661431
45. Xin L, Wu Y, Liu C, Zeng F, Wang J-L, Wu D-Z, et al. Exosome-Mediated Transfer of lncRNA HCG18 Promotes M2 Macrophage Polarization in Gastric Cancer. Mol Immunol (2021) 140:196–205. doi: 10.1016/j.molimm.2021.10.011
46. Li X, Lei Y, Wu M, Li N. Regulation of Macrophage Activation and Polarization by HCC-Derived Exosomal lncRNA Tuc339. Int J Mol Sci (2018) 19:2958. doi: 10.3390/ijms19102958
47. Ye Y, Xu Y, Lai Y, He W, Li Y, Wang R, et al. Long non-Coding RNA Cox-2 Prevents Immune Evasion and Metastasis of Hepatocellular Carcinoma by Altering M1/M2 Macrophage Polarization. J Cell Biochem (2018) 119:2951–63. doi: 10.1002/jcb.26509
48. Luo H-L, Luo T, Liu J-J, Wu F-X, Bai T, Ou C, et al. Macrophage Polarization-Associated lnc-Ma301 Interacts With Caprin-1 to Inhibit Hepatocellular Carcinoma Metastasis Through the Akt/Erk1 Pathway. Cancer Cell Int (2021) 21:422. doi: 10.1186/s12935-021-02133-1
49. Ai Y, Wei H, Wu S, Tang Z, Li X, Zou C. Exosomal LncRNA LBX1-AS1 Derived From RBPJ Overexpressed-Macrophages Inhibits Oral Squamous Cell Carcinoma Progress via miR-182-5p/FOXO3. Front Oncol (2021) 11:605884. doi: 10.3389/fonc.2021.605884
50. Zhao Y, Yu Z, Ma R, Zhang Y, Zhao L, Yan Y, et al. lncRNA-Xist/miR-101-3p/KLF6/C/Ebpα Axis Promotes TAM Polarization to Regulate Cancer Cell Proliferation and Migration. Mol Ther Nucleic Acids (2021) 23:536–51. doi: 10.1016/j.omtn.2020.12.005
51. Hu J, Zhang L, Liechty C, Zgheib C, Hodges MM, Liechty KW, et al. Long Noncoding RNA GAS5 Regulates Macrophage Polarization and Diabetic Wound Healing. J Invest Dermatol (2020) 140:1629–38. doi: 10.1016/j.jid.2019.12.030
52. Ren W, Xi G, Li X, Zhao L, Yang K, Fan X, et al. Long non-Coding RNA HCG18 Promotes M1 Macrophage Polarization Through Regulating the miR-146a/TRAF6 Axis, Facilitating the Progression of Diabetic Peripheral Neuropathy. Mol Cell Biochem (2021) 476:471–82. doi: 10.1007/s11010-020-03923-3
53. Chi X, Ding B, Zhang L, Zhang J, Wang J, Zhang W. lncRNA GAS5 Promotes M1 Macrophage Polarization via miR-455-5p/SOCS3 Pathway in Childhood Pneumonia. J Cell Physiol (2019) 234:13242–51. doi: 10.1002/jcp.27996
54. Zhang X-Y, Chen Z-C, Zhang L-X, Li X-S, Guo Y-L, Tian C-J, et al. LincRNA-P21 Promotes Classical Macrophage Activation in Acute Respiratory Distress Syndrome by Activating NF-κb. Exp Lung Res (2020) 46:174–84. doi: 10.1080/01902148.2020.1758246
55. He W, Che H, Jin C, Li Y, Li F, Zhou R. LncRNA AFAP1-AS1 Promotes M1 Polarization of Macrophages and Osteogenic Differentiation of Valve Interstitial Cells. J Physiol Biochem (2021) 77:461–8. doi: 10.1007/s13105-021-00821-0
56. Sun F, Guo Z, Zhang C, Che H, Gong W, Shen Z, et al. LncRNA NRON Alleviates Atrial Fibrosis Through Suppression of M1 Macrophages Activated by Atrial Myocytes. Biosci Rep (2019) 39:BSR20192215. doi: 10.1042/BSR20192215
57. Zhou J, Li Z, Wu T, Zhao Q, Zhao Q, Cao Y. LncGBP9/miR-34a Axis Drives Macrophages Toward a Phenotype Conducive for Spinal Cord Injury Repair via STAT1/STAT6 and SOCS3. J Neuroinflamm (2020) 17:134. doi: 10.1186/s12974-020-01805-5
58. Li L, Lv G, Wang B, Kuang L. XIST/miR-376c-5p/OPN Axis Modulates the Influence of Proinflammatory M1 Macrophages on Osteoarthritis Chondrocyte Apoptosis. J Cell Physiol (2020) 235:281–93. doi: 10.1002/jcp.28968
59. Jiang M, Dai J, Yin M, Jiang C, Ren M, Tian L. LncRNA MEG8 Sponging miR-181a-5p Contributes to M1 Macrophage Polarization by Regulating SHP2 Expression in Henoch-Schonlein Purpura Rats. Ann Med (2021) 53:1576–88. doi: 10.1080/07853890.2021.1969033
60. Li X, Liu R, Wang Y, Zhu W, Zhao D, Wang X, et al. Cholangiocyte-Derived Exosomal lncRNA H19 Promotes Macrophage Activation and Hepatic Inflammation Under Cholestatic Conditions. Cells (2020) 9:190. doi: 10.3390/cells9010190
61. Zhu X, Zhu Y, Ding C, Zhang W, Guan H, Li C, et al. LncRNA H19 Regulates Macrophage Polarization and Promotes Freund’s Complete Adjuvant-Induced Arthritis by Upregulating KDM6A. Int Immunopharmacol (2021) 93:107402. doi: 10.1016/j.intimp.2021.107402
62. Luo Y-Y, Yang Z-Q, Lin X-F, Zhao F-L, Tu H-T, Wang L-J, et al. Knockdown of lncRNA PVT1 Attenuated Macrophage M1 Polarization and Relieved Sepsis Induced Myocardial Injury via miR-29a/HMGB1 Axis. Cytokine (2021) 143:155509. doi: 10.1016/j.cyto.2021.155509
63. Han X, Huang S, Xue P, Fu J, Liu L, Zhang C, et al. LncRNA PTPRE-AS1 Modulates M2 Macrophage Activation and Inflammatory Diseases by Epigenetic Promotion of PTPRE. Sci Adv (2019) 5:eaax9230. doi: 10.1126/sciadv.aax9230
64. Li Q, Lu L, Li X, Lu S. Long non-Coding RNA NKILA Alleviates Airway Inflammation in Asthmatic Mice by Promoting M2 Macrophage Polarization and Inhibiting the NF-κb Pathway. Biochem Biophys Res Commun (2021) 571:46–52. doi: 10.1016/j.bbrc.2021.07.023
65. Xia L, Wang X, Liu L, Fu J, Xiao W, Liang Q, et al. Lnc-BAZ2B Promotes M2 Macrophage Activation and Inflammation in Children With Asthma Through Stabilizing BAZ2B pre-mRNA. J Allergy Clin Immunol (2021) 147:921–932.e9. doi: 10.1016/j.jaci.2020.06.034
66. Pei W, Zhang Y, Li X, Luo M, Chen T, Zhang M, et al. LncRNA AK085865 Depletion Ameliorates Asthmatic Airway Inflammation by Modulating Macrophage Polarization. Int Immunopharmacol (2020) 83:106450. doi: 10.1016/j.intimp.2020.106450
67. Zhang Y, Li X, Wang C, Zhang M, Yang H, Lv K. lncRNA AK085865 Promotes Macrophage M2 Polarization in CVB3-Induced VM by Regulating ILF2-ILF3 Complex-Mediated miRNA-192 Biogenesis. Mol Ther Nucleic Acids (2020) 21:441–51. doi: 10.1016/j.omtn.2020.06.017
68. Xue Y, Zhang S, Zheng C, Li Y, Zhang L, Su Q, et al. Long non-Coding RNA MEG3 Inhibits M2 Macrophage Polarization by Activating TRAF6 via microRNA-223 Down-Regulation in Viral Myocarditis. J Cell Mol Med (2020) 24:12341–54. doi: 10.1111/jcmm.15720
69. Wang B, Li X, Hu W, Zhou Y, Din Y. Silencing of lncRNA SNHG20 Delays the Progression of Nonalcoholic Fatty Liver Disease to Hepatocellular Carcinoma via Regulating Liver Kupffer Cells Polarization. IUBMB Life (2019) 71:1952–61. doi: 10.1002/iub.2137
70. Wang W, Guo Z-H. Downregulation of lncRNA NEAT1 Ameliorates LPS-Induced Inflammatory Responses by Promoting Macrophage M2 Polarization via miR-125a-5p/TRAF6/TAK1 Axis. Inflammation (2020) 43:1548–60. doi: 10.1007/s10753-020-01231-y
71. Chen J, Zhou R, Liang Y, Fu X, Wang D, Wang C. Blockade of lncRNA-ASLNCS5088–enriched Exosome Generation in M2 Macrophages by GW4869 Dampens the Effect of M2 Macrophages on Orchestrating Fibroblast Activation. FASEB J (2019) 33:12200–12. doi: 10.1096/fj.201901610
72. Liu D, Wei Y, Liu Y, Wu T, Hu J, Lu H. The Long Non-Coding RNA NEAT1/miR-224-5p/IL-33 Axis Modulates Macrophage M2a Polarization and A1 Astrocyte Activation. Mol Neurobiol (2021) 58:4506–19. doi: 10.1007/s12035-021-02405-x
73. Ma W, Zhang W, Cui B, Gao J, Liu Q, Yao M, et al. Functional Delivery of lncRNA TUG1 by Endothelial Progenitor Cells Derived Extracellular Vesicles Confers Anti-Inflammatory Macrophage Polarization in Sepsis via Impairing miR-9-5p-Targeted SIRT1 Inhibition. Cell Death Dis (2021) 12:1056. doi: 10.1038/s41419-021-04117-5
74. Zhang P, Lu B, Zhang Q, Xu F, Zhang R, Wang C, et al. LncRNA NEAT1 Sponges MiRNA-148a-3p to Suppress Choroidal Neovascularization and M2 Macrophage Polarization. Mol Immunol (2020) 127:212–22. doi: 10.1016/j.molimm.2020.08.008
75. Han Y-B, Tian M, Wang X-X, Fan D-H, Li W-Z, Wu F, et al. Berberine Ameliorates Obesity-Induced Chronic Inflammation Through Suppression of ER Stress and Promotion of Macrophage M2 Polarization at Least Partly via Downregulating lncRNA Gomafu. Int Immunopharmacol (2020) 86:106741. doi: 10.1016/j.intimp.2020.106741
76. Sun D, Yu Z, Fang X, Liu M, Pu Y, Shao Q, et al. Lnc RNA GAS 5 Inhibits Microglial M2 Polarization and Exacerbates Demyelination. EMBO Rep (2017) 18:1801–16. doi: 10.15252/embr.201643668
77. Li N, Liu Y, Cai J. LncRNA MIR155HG Regulates M1/M2 Macrophage Polarization in Chronic Obstructive Pulmonary Disease. BioMed Pharmacother (2019) 117:109015. doi: 10.1016/j.biopha.2019.109015
78. Xu J, Xu H, Ma K, Wang Y, Niu B, Zhang L, et al. lncRNA Gm16410 Mediates PM2.5-Induced Macrophage Activation via PI3K/AKT Pathway. Front Cell Dev Biol (2021) 9:618045. doi: 10.3389/fcell.2021.618045
79. Gao X, Ge J, Li W, Zhou W, Xu L. LncRNA KCNQ1OT1 Ameliorates Particle-Induced Osteolysis Through Inducing Macrophage Polarization by Inhibiting miR-21a-5p. Biol Chem (2018) 399:375–86. doi: 10.1515/hsz-2017-0215
80. Li Y, Sun T, Shen S, Wang L, Yan J. LncRNA DYNLRB2-2 Inhibits THP-1 Macrophage Foam Cell Formation by Enhancing Autophagy. Biol Chem (2019) 400:1047–57. doi: 10.1515/hsz-2018-0461
81. Simion V, Zhou H, Haemmig S, Pierce JB, Mendes S, Tesmenitsky Y, et al. A Macrophage-Specific lncRNA Regulates Apoptosis and Atherosclerosis by Tethering HuR in the Nucleus. Nat Commun (2020) 11:6135. doi: 10.1038/s41467-020-19664-2
82. Sun C, Fu Y, Gu X, Xi X, Peng X, Wang C, et al. Macrophage-Enriched lncRNA RAPIA: A Novel Therapeutic Target for Atherosclerosis. Arterioscler Thromb Vasc Biol (2020) 40:1464–78. doi: 10.1161/ATVBAHA.119.313749
83. Wang L, Xia J, Ke Z, Zhang B. Blockade of NEAT1 Represses Inflammation Response and Lipid Uptake via Modulating miR-342-3p in Human Macrophages THP-1 Cells. J Cell Physiol (2019) 234:5319–26. doi: 10.1002/jcp.27340
84. Hu X, Ma R, Fu W, Zhang C, Du X. LncRNA UCA1 Sponges miR-206 to Exacerbate Oxidative Stress and Apoptosis Induced by Ox-LDL in Human Macrophages. J Cell Physiol (2019) 234:14154–60. doi: 10.1002/jcp.28109
85. Wang Z, Kun Y, Lei Z, Dawei W, Lin P, Jibo W. LncRNA MIAT Downregulates IL-1β, TNF-α to Suppress Macrophage Inflammation But is Suppressed by ATP-Induced NLRP3 Inflammasome Activation. Cell Cycle (2021) 20:194–203. doi: 10.1080/15384101.2020.1867788
86. Das S, Reddy MA, Senapati P, Stapleton K, Lanting L, Wang M, et al. Diabetes Mellitus–Induced Long Noncoding RNA Dnm3os Regulates Macrophage Functions and Inflammation via Nuclear Mechanisms. Arterioscler Thromb Vasc Biol (2018) 38:1806–20. doi: 10.1161/ATVBAHA.117.310663
87. Zgheib C, Hodges MM, Hu J, Liechty KW, Xu J. Long non-Coding RNA Lethe Regulates Hyperglycemia-Induced Reactive Oxygen Species Production in Macrophages. PloS One (2017) 12:e0177453. doi: 10.1371/journal.pone.0177453
88. Liu X, Li C, Zhu J, Li W, Zhu Q. Dysregulation of FTX/miR-545 Signaling Pathway Downregulates Tim-3 and is Responsible for the Abnormal Activation of Macrophage in Cirrhosis. J Cell Biochem (2019) 120:2336–46. doi: 10.1002/jcb.27562
89. Wang Y, Luo Y, Yao Y, Ji Y, Feng L, Du F, et al. Silencing the lncRNA Maclpil in Pro-Inflammatory Macrophages Attenuates Acute Experimental Ischemic Stroke via LCP1 in Mice. J Cereb Blood Flow Metab (2020) 40:747–59. doi: 10.1177/0271678X19836118
90. Zhang P, Sun J, Liang C, Gu B, Xu Y, Lu H, et al. lncRNA IGHC γ 1 Acts as a ceRNA to Regulate Macrophage Inflammation via the miR-6891-3p/TLR4 Axis in Osteoarthritis. Mediators Inflamm (2020) 2020:1–11. doi: 10.1155/2020/9743037
91. Stapleton K, Das S, Reddy MA, Leung A, Amaram V, Lanting L, et al. Novel Long Noncoding RNA, Macrophage Inflammation-Suppressing Transcript (MIST ), Regulates Macrophage Activation During Obesity. Arterioscler Thromb Vasc Biol (2020) 40:914–28. doi: 10.1161/ATVBAHA.119.313359
92. Boliar S, Gludish DW, Jambo KC, Kamng’ona R, Mvaya L, Mwandumba HC, et al. Inhibition of the lncRNA SAF Drives Activation of Apoptotic Effector Caspases in HIV-1–Infected Human Macrophages. Proc Natl Acad Sci (2019) 116:7431–8. doi: 10.1073/pnas.1818662116
93. Vollmers AC, Covarrubias S, Kuang D, Shulkin A, Iwuagwu J, Katzman S, et al. A Conserved Long Noncoding RNA, GAPLINC, Modulates the Immune Response During Endotoxic Shock. Proc Natl Acad Sci (2021) 118:e2016648118. doi: 10.1073/pnas.2016648118
94. Shen M, Pan X, Gao Y, Ye H, Zhang J, Chen Y, et al. LncRNA CRNDE Exacerbates IgA Nephropathy Progression by Promoting NLRP3 Inflammasome Activation in Macrophages. Immunol Invest (2021) 1–13. doi: 10.1080/08820139.2021.1989461
95. Meurette O, Mehlen P. Notch Signaling in the Tumor Microenvironment. Cancer Cell (2018) 34:536–48. doi: 10.1016/j.ccell.2018.07.009
96. Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage Polarization in Tumour Progression. Semin Cancer Biol (2008) 18:349–55. doi: 10.1016/j.semcancer.2008.03.004
97. Hao N-B, Lü M-H, Fan Y-H, Cao Y-L, Zhang Z-R, Yang S-M. Macrophages in Tumor Microenvironments and the Progression of Tumors. Clin Dev Immunol (2012) 2012:1–11. doi: 10.1155/2012/948098
98. Duluc D, Delneste Y, Tan F, Moles M-P, Grimaud L, Lenoir J, et al. Tumor-Associated Leukemia Inhibitory Factor and IL-6 Skew Monocyte Differentiation Into Tumor-Associated Macrophage-Like Cells. Blood (2007) 110:4319–30. doi: 10.1182/blood-2007-02-072587
99. Español A, Dasso M, Cella M, Goren N, Sales ME. Muscarinic Regulation of SCA-9 Cell Proliferation via Nitric Oxide Synthases, Arginases and Cyclooxygenases. Role of the Nuclear Translocation Factor-κb. Eur J Pharmacol (2012) 683:43–53. doi: 10.1016/j.ejphar.2012.03.013
100. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat Rev Clin Oncol (2017) 14:399–416. doi: 10.1038/nrclinonc.2016.217
101. Wang R, Zhang J, Chen S, Lu M, Luo X, Yao S, et al. Tumor-Associated Macrophages Provide a Suitable Microenvironment for non-Small Lung Cancer Invasion and Progression. Lung Cancer (2011) 74:188–96. doi: 10.1016/j.lungcan.2011.04.009
102. Herwig MC, Bergstrom C, Wells JR, Höller T, Grossniklaus HE. M2/M1 Ratio of Tumor Associated Macrophages and PPAR-Gamma Expression in Uveal Melanomas With Class 1 and Class 2 Molecular Profiles. Exp Eye Res (2013) 107:52–8. doi: 10.1016/j.exer.2012.11.012
103. Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, et al. P50 Nuclear Factor-κb Overexpression in Tumor-Associated Macrophages Inhibits M1 Inflammatory Responses and Antitumor Resistance. Cancer Res (2006) 66:11432–40. doi: 10.1158/0008-5472.CAN-06-1867
104. Hou L, Heilbronner U, Degenhardt F, Adli M, Akiyama K, Akula N, et al. Genetic Variants Associated With Response to Lithium Treatment in Bipolar Disorder: A Genome-Wide Association Study. Lancet (2016) 387:1085–93. doi: 10.1016/S0140-6736(16)00143-4
105. Kim T, Croce CM. Long Noncoding RNAs: Undeciphered Cellular Codes Encrypting Keys of Colorectal Cancer Pathogenesis. Cancer Lett (2018) 417:89–95. doi: 10.1016/j.canlet.2017.12.033
106. Wang L, Cho KB, Li Y, Tao G, Xie Z, Guo B. Long Noncoding RNA (lncRNA)-Mediated Competing Endogenous RNA Networks Provide Novel Potential Biomarkers and Therapeutic Targets for Colorectal Cancer. Int J Mol Sci (2019) 20:E5758. doi: 10.3390/ijms20225758
107. Boon RA, Jaé N, Holdt L, Dimmeler S. Long Noncoding RNAs. J Am Coll Cardiol (2016) 67:1214–26. doi: 10.1016/j.jacc.2015.12.051
108. Fattahi S, Kosari-Monfared M, Golpour M, Emami Z, Ghasemiyan M, Nouri M, et al. LncRNAs as Potential Diagnostic and Prognostic Biomarkers in Gastric Cancer: A Novel Approach to Personalized Medicine. J Cell Physiol (2020) 235:3189–206. doi: 10.1002/jcp.29260
109. Previdi MC, Carotenuto P, Zito D, Pandolfo R, Braconi C. Noncoding RNAs as Novel Biomarkers in Pancreatic Cancer: What do We Know? Future Oncol (2017) 13:443–53. doi: 10.2217/fon-2016-0253
110. Litwin MS, Tan H-J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA (2017) 317:2532. doi: 10.1001/jama.2017.7248
111. Tedesco S, Bolego C, Toniolo A, Nassi A, Fadini GP, Locati M, et al. Phenotypic Activation and Pharmacological Outcomes of Spontaneously Differentiated Human Monocyte-Derived Macrophages. Immunobiology (2015) 220:545–54. doi: 10.1016/j.imbio.2014.12.008
112. Liu M-L, Zhang Q, Yuan X, Jin L, Wang L-L, Fang T-T, et al. Long Noncoding RNA RP4 Functions as a Competing Endogenous RNA Through miR-7-5p Sponge Activity in Colorectal Cancer. World J Gastroenterol (2018) 24:1004–12. doi: 10.3748/wjg.v24.i9.1004
113. Pirlog R, Cismaru A, Nutu A, Berindan-Neagoe I. Field Cancerization in NSCLC: A New Perspective on MicroRNAs in Macrophage Polarization. Int J Mol Sci (2021) 22:746. doi: 10.3390/ijms22020746
114. Oishi Y, Manabe I. Macrophages in Inflammation, Repair and Regeneration. Int Immunol (2018) 30(11):511–28. doi: 10.1093/intimm/dxy054
115. Oishi Y, Manabe I. Macrophages in Age-Related Chronic Inflammatory Diseases. NPJ Aging Mech Dis (2016) 2:16018. doi: 10.1038/npjamd.2016.18
116. Griffin TM, Scanzello CR. Innate Inflammation and Synovial Macrophages in Osteoarthritis Pathophysiology. Clin Exp Rheumatol (2019) 37 Suppl 120:57–63.
117. Gaffney L, Warren P, Wrona EA, Fisher MB, Freytes DO. Macrophages’ Role in Tissue Disease and Regeneration. In: Kloc M, editor. Macrophages. Results and Problems in Cell Differentiation. Cham: Springer International Publishing (2017). p. 245–71. doi: 10.1007/978-3-319-54090-0_10
118. Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu Z, et al. MiR-127 Modulates Macrophage Polarization and Promotes Lung Inflammation and Injury by Activating the JNK Pathway. J Immunol (2015) 194:1239–51. doi: 10.4049/jimmunol.1402088
119. Simion V, Haemmig S, Feinberg MW. LncRNAs in Vascular Biology and Disease. Vascul Pharmacol (2019) 114:145–56. doi: 10.1016/j.vph.2018.01.003
120. Kacimi R, Giffard RG, Yenari MA. Endotoxin-Activated Microglia Injure Brain Derived Endothelial Cells via NF-κb, JAK-STAT and JNK Stress Kinase Pathways. J Inflamm (2011) 8:7. doi: 10.1186/1476-9255-8-7
121. Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, et al. Akt1 and Akt2 Protein Kinases Differentially Contribute to Macrophage Polarization. Proc Natl Acad Sci (2012) 109:9517–22. doi: 10.1073/pnas.1119038109
122. Burdo TH, Walker J, Williams KC. Macrophage Polarization in AIDS: Dynamic Interface Between Anti-Viral and Anti-Inflammatory Macrophages During Acute and Chronic Infection. J Clin Cell Immunol (2015) 6:333.
123. Li S, Reddy MA, Cai Q, Meng L, Yuan H, Lanting L, et al. Enhanced Proatherogenic Responses in Macrophages and Vascular Smooth Muscle Cells Derived From Diabetic Db/Db Mice. Diabetes (2006) 55:2611–9. doi: 10.2337/db06-0164
124. Udalova IA, Mantovani A, Feldmann M. Macrophage Heterogeneity in the Context of Rheumatoid Arthritis. Nat Rev Rheumatol (2016) 12:472–85. doi: 10.1038/nrrheum.2016.91
125. Masoumi F, Ghorbani S, Talebi F, Branton WG, Rajaei S, Power C, et al. Malat1 Long Noncoding RNA Regulates Inflammation and Leukocyte Differentiation in Experimental Autoimmune Encephalomyelitis. J Neuroimmunol (2019) 328:50–9. doi: 10.1016/j.jneuroim.2018.11.013
126. Ontaneda D, Thompson AJ, Fox RJ, Cohen JA. Progressive Multiple Sclerosis: Prospects for Disease Therapy, Repair, and Restoration of Function. Lancet (2017) 389:1357–66. doi: 10.1016/S0140-6736(16)31320-4
127. Steinman L. Immunology of Relapse and Remission in Multiple Sclerosis. Annu Rev Immunol (2014) 32:257–81. doi: 10.1146/annurev-immunol-032713-120227
128. Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple Sclerosis. Lancet (2018) 391:1622–36. doi: 10.1016/S0140-6736(18)30481-1
129. Gordon S. Alternative Activation of Macrophages. Nat Rev Immunol (2003) 3:23–35. doi: 10.1038/nri978
130. Miron VE, Boyd A, Zhao J-W, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 Microglia and Macrophages Drive Oligodendrocyte Differentiation During CNS Remyelination. Nat Neurosci (2013) 16:1211–8. doi: 10.1038/nn.3469
Keywords: long noncoding RNAs, regulation, macrophages, polarization, diseases
Citation: Jiang P and Li X (2022) Regulatory Mechanism of lncRNAs in M1/M2 Macrophages Polarization in the Diseases of Different Etiology. Front. Immunol. 13:835932. doi: 10.3389/fimmu.2022.835932
Received: 15 December 2021; Accepted: 10 January 2022;
Published: 25 January 2022.
Edited by:
Guan-Jun Yang, Ningbo University, ChinaReviewed by:
Kun Yang, East Tennessee State University, United StatesJun Li, Capital Medical University, China
Copyright © 2022 Jiang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaopeng Li, MTE1MDYzNTY5NUBxcS5jb20=
 Ping Jiang
Ping Jiang Xiaopeng Li3,4*
Xiaopeng Li3,4*