- 1Department of Gynaecology, the Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Digestive Internal Medicine, the Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 3Department of Neurosurgery, the Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 4Department of Neurology Medicine, the Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 5Department of Hematology, the Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 6Institute of Biotherapy for Hematological Malignancies, Shandong University, Jinan, China
- 7Shandong University-Karolinska Institute Collaboration Laboratory for Stem Cell Research, Shandong University, Jinan, China
Our previous study showed that interferon gamma (IFN-γ) might enhance the immunosuppressive properties of mesenchymal stem cells (MSCs) by upregulating the expression of indoleamine 2,3-dioxygenease. Therefore, we treated experimental autoimmune encephalomyelitis (EAE) mice, an animal model of multiple sclerosis (MS), with IFN-γ-primed human umbilical cord MSCs (IFN-γ-hUCMSCs). This study aimed to investigate the potential therapeutic effects of IFN-γ-hUCMSCs transplantation and to identify the biological pathways involved in EAE mice. Firstly, the body weights and clinical scores of EAE mice were recorded before and after treatment. Then, the inflammatory cytokine levels in splenic cell supernatants were quantified by enzyme-linked immunosorbent assay. Finally, the mRNA expression levels of signal transducer and activator of transduction 3 (STAT3), retinoic acid-related orphan receptor gamma t (ROR-γt), and forkhead box P3 (Foxp3) were detected by quantitative reverse transcription polymerase chain reaction. We observed that IFN-γ-hUCMSCs transplantation significantly alleviated body weight loss and decreased the clinical scores of mice. Additionally, IFN-γ-hUCMSCs transplantation could regulate the production of inflammatory cytokines, interleukin (IL)-10 and IL-17, thereby showing more potent treatment efficacy than human umbilical cord MSCs (hUCMSCs) transplantation (p < 0.05). Compared with the EAE group, the expressions of STAT3 and ROR-γt in the transplantation groups were significantly decreased, but the expression of Foxp3 was significantly upregulated in the IFN-γ-hUCMSCs transplantation group compared to that in the hUCMSCs transplantation group. We assumed that IFN-γ-hUCMSCs may affect the balance of T helper 17 (Th17) cells/regulatory T cells (Tregs) through the Foxp3/ROR-γt/STAT3 signaling pathway to reduce the inflammatory response, thereby improving the clinical symptoms of EAE mice. Our study demonstrated that transplantation of IFN-γ-hUCMSCs could reduce inflammation in EAE mice via the Foxp3/ROR-γt/STAT3 signaling pathway, highlighting the therapeutic effects of IFN-γ-hUCMSCs in patients with MS.
Introduction
Multiple sclerosis (MS) is a common cause of neurological disability among young people, resulting in increased economic and social costs (1, 2). MS has been investigated over a century, and although much has been discovered about the immunobiology, genetics, and epidemiology of this disease, the treatment outcomes remain unsatisfactory.
MS is characterized by inflammation-mediated demyelination of the white matter tracts with partial preservation of axons (3). Experimental autoimmune encephalomyelitis (EAE) is an animal model of MS that is particularly useful for testing new therapeutic approaches against MS (4, 5). Studies indicated that T helper (Th) cells, mainly Th1 and Th17 cells, which are characterized by the production of interleukin (IL)-17, are involved in the pathogenicity of MS and EAE, whereas regulatory T cells (Tregs) can maintain the autoimmune response (6, 7). One of the root causes of EAE is the decrease in the expression of forkhead box P3 (Foxp3)-expressing anti-autoimmune Tregs and an associated increase in autoimmune Th1 and Th17 cells (8). Moreover, Th17 cells, rather than Th1 cells, are important in autoimmune inflammatory conditions (9). Therefore, the balance of Th17/Tregs may play an important role in the modulation of EAE. IL-10 is an anti-inflammatory cytokine that plays a crucial role in preventing inflammatory and autoimmune pathologies and is involved in the regulation of the Janus kinase/signal transducers and activators of transduction (JAK/STAT) signaling pathway. Genetic deletion of STAT3 in T cells has been shown to abrogate Th17 differentiation, suggesting that STAT3 is a potential therapeutic target for Th17-mediated diseases (10). Tregs can exert their functions by releasing inhibitory cytokines (IL-10 and IL-35) (11). Therefore, the production of inflammatory cytokines (IL-10 and IL-17) and the activation of the JAK/STAT signaling pathway were observed in our study.
With the development of novel treatment methods for autoimmune diseases, transplantation of mesenchymal stem cells (MSCs) has become popular as an effective strategy to counteract the progression of autoimmune disorders. Evidence suggests that MSCs can exert anti-inflammatory and immunomodulatory effects in various tissues, lower the clinical scores, and reduce central nervous system (CNS) leukocyte infiltration in EAE mice (12, 13). Our previous study showed that the immunosuppressive properties of MSCs may be enhanced by interferon gamma (IFN-γ) due to the upregulation of the tryptophan-catabolizing enzyme indoleamine 2,3-dioxygenease (IDO) (14). Therefore, we tried to treat EAE with IFN-γ-primed human umbilical cord MSCs (IFN-γ-hUCMSCs) and investigated their potential therapeutic effects on EAE mice.
Materials and Methods
Culture of hUCMSCs and IFN-γ-hUCMSCs
Human umbilical cord specimens were obtained from healthy Chinese young women who received cesarean section at the Second Hospital of Shandong University under sterile conditions. Informed consent was obtained from all participants before the collection of human umbilical cord specimens. This study was approved by the Ethics Committee of the Second Hospital of Shandong University, China.
The hUCMSCs were isolated and cultured, and the phenotype of the hUCMSCs was identified as described in our previous study (14). The umbilical cord was cut into small sections, following two washes with phosphate-buffered saline (PBS). The pieces were digested with collagenase for 1 h and trypsin-EDTA (Gibco, Waltham, MA, USA) for 30 min. The specimens were then filtered, the cells cultured in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (HyClone, Logan, UT, USA), and were fixed with 10% defined fetal bovine serum (Gibco), epidermal growth factor, basic fibroblast growth factor (both from PeproTech, Cranbury, NJ, USA), L-glutamine (Gibco), and penicillin–streptomycin solution (HyClone) with 5% CO2 at 37°C for 72 h. After 3–5 days, non-adherent cells were removed and the medium was replenished. When the density of the cells reached 80%, they were digested with trypsin–EDTA at room temperature and passaged into new culture dishes. The expressions of the cell surface markers, including CD105, CD90, CD73, CD45, CD34, and HLA-DR (human leukocyte antigen—DR isotype), were evaluated using a LSRFortessa™ flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Then, partial human umbilical cord MSCs (hUCMSCs) were pretreated with IFN-γ (20 ng/ml, 48 h). Cell suspensions were prepared at a density of 1 × 107 cells/ml.
The whole process was performed in a Good Manufacturing Practice (GMP) laboratory.
EAE Grouping and Cell Transplantation
Female C57BL/6J mice (6–8 weeks old; Biotechnology Co., Ltd., Beijing, China) were housed under a 12:12-h light/dark cycle in temperature- and humidity-controlled rooms. Mice were immunized with the myelin oligodendrocyte glycoprotein peptide 35-55 [MOG35-55; GL Biochem (Shanghai) Ltd., Shanghai, China] according to a previously published protocol (15). Priming mice to induce EAE involved complete Freund adjuvant (Sigma, St. Louis, MO, USA) containing 4 mg/ml Mycobacterium tuberculosis (strain H37Ra; Difco, Franklin Lakes, NJ, USA) and 200 μg MOG35-55. On days 0 and 2 post-immunization, 200 ng of pertussis toxin (Sigma) was administered intraperitoneally. Then, the mice were divided into three groups (n = 8 per group): the EAE group, the hUCMSCs transplantation (EAE+hUCMSCs) group, and the IFN-γ-hUCMSCs transplantation (EAE+IFN-γ-hUCMSCs) group. Fourteen days after immunization, each mouse in the transplantation groups was injected with 100 μl of the cell suspension (1 × 107 cells/ml) via the tail vein. The mice in the EAE group were injected with 100 μl of PBS instead. All animal experiments were performed per the Ethical Committee for Animal Experiments of Shandong University, China.
Body Weight and Clinical Score Assessment
After immunization, the mice were weighed and evaluated for signs of neurological disability. Clinical disability was scored as follows: 0, no paralysis; 1, loss of tail tone; 2, hindlimb weakness; 3, hindlimb paralysis; 4, hindlimb and forelimb paralysis; and 5, dying or dead (16). The scorers were blinded to the treatment groups.
Splenocyte Culture and Detection of Inflammatory Cytokines
Two weeks after cell transplantation, the mice were anesthetized and sacrificed, and the spleens were removed. Splenocytes were cultured as described previously (17).
The spleen tissue was passed through a 100-μm nylon mesh screen to prepare single-cell suspensions, followed by removal of red blood cells with lysis buffer and incubation for 5 min. The suspensions were centrifuged (4°C at 1,200 rpm) and the supernatants collected. Splenocytes were washed twice with RPMI 1640 and adjusted to a density of 1 × 106 cells/ml. The cells were incubated at 37°C for 2 h and then stimulated with MOG35-55 (15 μg/ml) for 48 h at 37°C in a 5% CO2 incubator. After 3 days, the supernatants were harvested and stored at −80°C for cytokine detection using enzyme-linked immunosorbent assay (ELISA).
The contents of IL-10 and IL-17 in the supernatants were assessed using commercially available ELISA kits (Proteintech, Shanghai, China) according to the manufacturer’s instructions.
Real-Time Fluorescence Quantitative PCR
Total RNA from the lumbar myeloid tissue of mice was extracted using the TRIzol reagent (Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions 2 weeks after cell transplantation. First-strand complementary DNA (cDNA) was synthesized using HiScript II Q RT SuperMix (Vazyme, Nanjing, China). Primers were supplied by Sangon Biotech (Shanghai, China). The primer sequences for STAT3, ROR-γt, and Foxp3 are listed in Table 1. Quantitative real-time PCR (qRT-PCR) was performed with AceQ qPCR SYBR Green Master Mix (Vazyme) using the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). PCR was performed in triplicate, and all results were normalized to the expression of GAPDH using the 2−ΔΔCt method.
Statistical Analysis
Data were recorded separately and expressed as the mean ± standard deviation. Data were analyzed using one-way ANOVA and Tukey’s multiple comparisons test. Statistical significance was set at p < 0.05. All data were analyzed using SPSS software (version 23.0).
Results
Effects of hUCMSCs and IFN-γ-hUCMSCs Transplantations on the Body Weights and Clinical Scores of EAE Mice
The body weights and clinical scores of mice were recorded and assessed after immunization. After approximately 2 weeks, EAE mice began to lose weight, and the clinical scores started to increase. We observed that transplantation of hUCMSCs and IFN-γ-hUCMSCs significantly alleviated body weight loss and decreased the clinical scores of mice, especially in the IFN-γ-hUCMSCs transplantation group (Figure 1).

Figure 1 Effects of transplantation of human umbilical cord mesenchymal stem cells (hUCMSCs) and IFN-γ-primed hUCMSCs (IFN-γ-hUCMSCs) on the body weights and clinical scores of experimental autoimmune encephalomyelitis (EAE) mice. Transplantation of hUCMSCs and IFN-γ-hUCMSCs significantly alleviated the body weight loss and clinical scores of mice, especially in the IFN-γ-hUCMSCs transplantation group. *p < 0.05 (n = 8 per group).
Inflammatory Cytokines of Splenocyte Culture Supernatants
To investigate the changes in the inflammatory cytokines after cell transplantations, the contents of IL-10 and IL-17 in splenocyte culture supernatants were tested using ELISA. We discovered that transplantation of hUCMSCs and IFN-γ-hUCMSCs could increase the concentration of IL-10, especially in the IFN-γ-hUCMSCs group. Conversely, IL-17, the pro-inflammatory cytokine, was observed to be remarkably lower in both cell transplantation groups, especially in the IFN-γ-hUCMSCs group (Figure 2). The results showed that transplantation of IFN-γ-hUCMSCs could regulate the inflammation response more effectively than hUCMSCs in EAE mice.
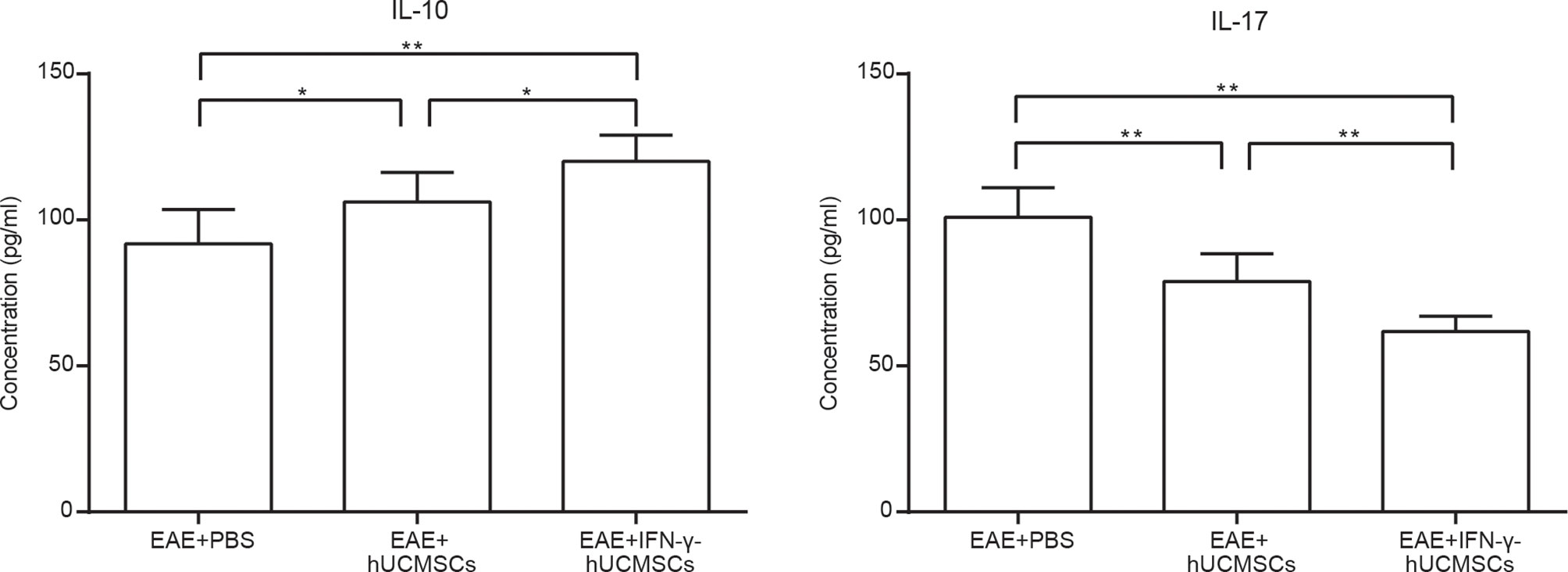
Figure 2 Effects of transplantation of human umbilical cord mesenchymal stem cells (hUCMSCs) and IFN-γ-primed hUCMSCs (IFN-γ-hUCMSCs) on the levels of IL-10 and IL-17 in experimental autoimmune encephalomyelitis (EAE) mice. Cell transplantation could increase the concentration of IL-10 and reduce the concentration of IL-17, especially in the IFN-γ-hUCMSCs transplantation group. *p < 0.05, **p < 0.01 (n = 8 per group).
mRNA Expression Levels of Foxp3, ROR-γt, and STAT3
The messenger RNA (mRNA) expression levels of Foxp3, ROR-γt, and STAT3 were determined using qRT-PCR in all transplantation groups (Figure 3). Compared with those in the EAE group, the expressions of STAT3 and ROR-γt in the cell transplantation groups were significantly decreased, but the expression of Foxp3 was upregulated. However, the increase in Foxp3 expression was more significant in the IFN-γ-hUCMSCs transplantation group than that in the hUCMSCs transplantation group.
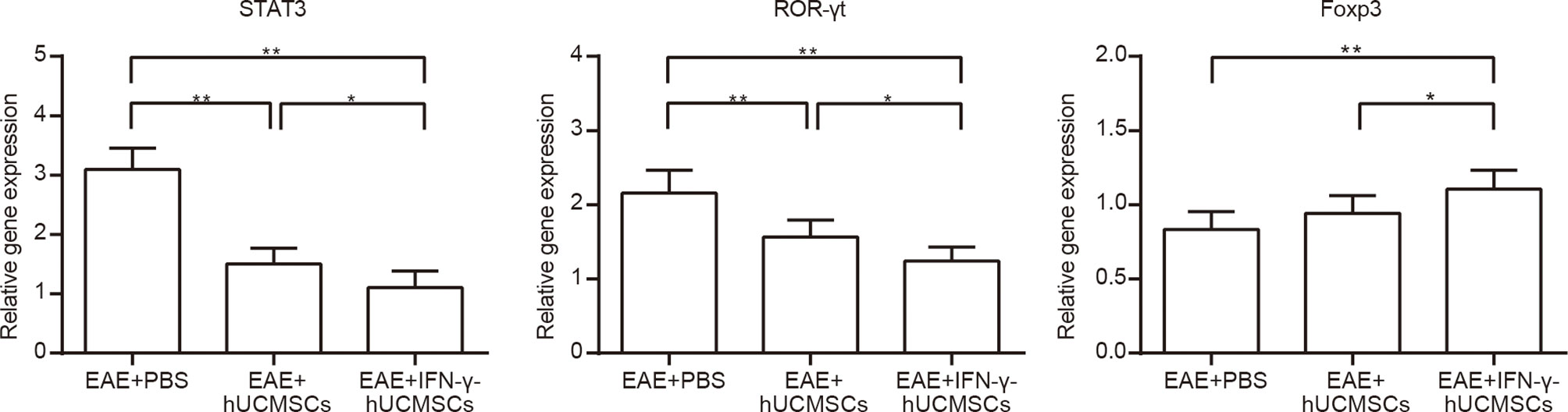
Figure 3 Messenger RNA (mRNA) expressions of Foxp3, ROR-γt, and STAT3 in the cell transplantation groups. The expressions of STAT3 and ROR-γt were significantly downregulated and that of Foxp3 was upregulated in the cell transplantation groups, especially in the IFN-γ-primed human umbilical cord mesenchymal stem cells (IFN-γ-hUCMSCs) transplantation group. *p < 0.05, **p < 0.01, (n = 8 per group).
Discussion
MS is a demyelinating disorder induced by activation of the autoimmune system. The treatments for MS mainly include the administration of glucocorticoids in the acute phase, disease modification therapy (DMT) to reduce the inflammatory activity and improve long-term prognosis, and symptomatic treatment of complications; however, the treatment outcomes are not satisfactory. Therefore, it is important to discover effective therapeutic strategies for patients with MS. MSCs have shown strong immunomodulatory functions in many diseases, especially autoimmune diseases (18). Therefore, transplantation of MSCs is a promising therapeutic strategy for patients with MS.
Studies have shown that MSCs may suppress the activity of B cells and reduce autoantibody production (18). In addition, they can promote the expansion of Tregs by secreting IL-10, IDO, and transforming growth factor beta (TGF-β) (19, 20). Moreover, MSCs can suppress the differentiation and proliferation of Th cells and increase the suppressive proportion of B cells via IDO (21, 22), and IFN-γ is the main inducer of IDO (23). IFN-γ-primed MSCs (IFN-γ-primed MSCs) showed stronger ability to regulate the inflammation and immune suppression than MSCs in vitro (24). Therefore, we investigated the effects of IFN-γ-hUCMSCs on EAE mice established for MS studies.
In our study, we observed that IFN-γ-hUCMSCs transplantation significantly alleviated body weight loss and decreased the clinical scores of mice. In addition, IFN-γ-hUCMSCs transplantation could regulate the production of inflammatory cytokines (IL-10 and IL-17), thereby showing significantly higher potent treatment efficacy than hUCMSCs transplantation. IL-10, along with its receptors, plays an important role in the pathogenesis of various diseases, including infectious, inflammatory, and autoimmune diseases (25, 26). Studies have shown that bone marrow-derived MSCs can inhibit Th17 cell differentiation via IL-10 secretion (27). IL-17 is a signature cytokine of Th17 cells. The orphan nuclear receptor ROR-γt is the master regulator that drives the differentiation of Th17 cells (28). Recent evidence has shown that ROR-γt can potently upregulate IL-17 reporter activity without the involvement of any other factors (29). Furthermore, IL-10 may suppress the expression of ROR-γt. Foxp3+ Tregs are a special lineage of cells central to the maintenance of immunological tolerance (11, 30). They function as transcriptional repressors for various transcription factors. Foxp3 directly interacts with ROR-γt through the exon 2 region of Foxp3 (29). STAT3 signaling is essential for the induction of ROR-γt and the subsequent Th17 cell differentiation, and the SRY-related high-mobility group (HMG) box5 (Sox5) and c-Maf may cooperatively induce Th17 cell differentiation via the induction of ROR-γt as downstream targets of STAT3 (31, 32). Therefore, we speculated whether the changes in the levels of inflammatory cytokines (IL-10 and IL-17) were related to the mRNA expressions of ROR-γt, Foxp3, and STAT3.
As mentioned above, the imbalance of Th17/Tregs is involved in the pathogenesis of EAE. STAT3 and ROR-γt can promote the differentiation of Th17 cells. Foxp3 is necessary for Tregs to exert immunosuppressive function and is negatively regulated by STAT3. Genetic deletion of STAT3 in T cells has been shown to abrogate Th17 differentiation, suggesting that the inhibition of STAT3 can tilt the balance of Th17/Tregs toward Tregs. Combined with the results of our study, we observed that, compared to those in the EAE group, the expressions of STAT3 and ROR-γt in the transplantation groups were significantly decreased, but the expression of Foxp3 was upregulated, especially in the IFN-γ-hUCMSCs transplantation group. We assumed that IFN-γ-hUCMSCs may affect the Th17/Tregs balance through the Foxp3/ROR-γt/STAT3 signaling pathway to reduce the inflammatory response, thereby improving the clinical symptoms in EAE mice. This study has some possible limitations. The inhibition experiment should be studied in the future.
In summary, we found that IFN-γ-hUCMSCs could significantly alleviate the body weight loss and clinical scores of EAE mice and regulate the production of inflammatory cytokines via the Foxp3/ROR-γt/STAT3 signaling pathway.
Conclusion
Our study demonstrated that transplantation of IFN-γ-hUCMSCs could reduce inflammation in EAE mice via the Foxp3/ROR-γt/STAT3 signaling pathway, which highlights the therapeutic effects of IFN-γ-hUCMSCs in patients with MS. These results suggest that transplantation of IFN-γ-hUCMSCs may be a potential therapy for MS.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Hospital of Shandong University. The patients/participants provided written informed consent to participate in this study. The animal study was reviewed and approved by Ethics Committee of the Second Hospital of Shandong University.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation of China (81870848), the Fundamental Research Funds of Chinese Academy of Medical Sciences(2019-RC-HL-026), Shandong University Multidisciplinary Research and Innovation Team of Young Scholars(2020QNQT019).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.835345/full#supplementary-material
References
1. van der Feen FE, de Haan GA, van der Lijn I, Heersema DJ, Meilof JF, Heutink J. Independent Outdoor Mobility of Persons With Multiple Sclerosis - A Systematic Review. Mult Scler Relat Disord (2019) 37(undefined):101463. doi: 10.1016/j.msard.2019.101463
2. Nicholas JA, Electricwala B, Lee LK, Johnson KM. Burden of Relapsing-Remitting Multiple Sclerosis on Workers in the US: A Cross-Sectional Analysis of Survey Data. BMC Neurol (2019) 19(1):258. doi: 10.1186/s12883-019-1495-z
3. Magliozzi R, Howell OW, Durrenberger P, Aricò E, James R, Cruciani C, et al. Meningeal Inflammation Changes the Balance of TNF Signalling in Cortical Grey Matter in Multiple Sclerosis. J Neuroinflamm (2019) 16(1):259. doi: 10.1186/s12974-019-1650-x
4. Lunin SM, Khrenov MO, Glushkova OV, Parfenyuk SB, Novoselova TV, Novoselova EG. Protective Effect of PBCA Nanoparticles Loaded With Thymulin Against the Relapsing-Remitting Form of Experimental Autoimmune Encephalomyelitis in Mice. Int J Mol Sci (2019) 20(21):5374. doi: 10.3390/ijms20215374
5. Seifert HA, Gerstner G, Kent G, Vandenbark AA, Offner H. Estrogen-Induced Compensatory Mechanisms Protect IL-10-Deficient Mice From Developing EAE. J Neuroinflamm (2019) 16(1):195. doi: 10.1186/s12974-019-1588-z
6. Engler JB, Heckmann NF, Jäger J, Gold SM, Friese MA. Pregnancy Enables Expansion of Disease-Specific Regulatory T Cells in an Animal Model of Multiple Sclerosis. J Immunol (Baltimore Md 1950) (2019) 203(7):1743–52. doi: 10.4049/jimmunol.1900611
7. Li YH, Xu F, Thome R, Guo MF, Sun ML, Song GB, et al. Mdivi-1, a Mitochondrial Fission Inhibitor, Modulates T Helper Cells and Suppresses the Development of Experimental Autoimmune Encephalomyelitis. J Neuroinflamm (2019) 16(1):149. doi: 10.1186/s12974-019-1542-0
8. Pahan S, Pahan K. Mode of Action of Aspirin in Experimental Autoimmune Encephalomyelitis. DNA Cell Biol (2019) 38(7):593–6. doi: 10.1089/dna.2019.4814
9. Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 Drives a Pathogenic T Cell Population That Induces Autoimmune Inflammation. J Exp Med (2005) 201(2):233–40. doi: 10.1084/jem.20041257
10. Mbanefo E, Yan M, Kang M, Alhakeem S, Jittayasothorn Y, Yu C, et al. STAT3-Specific Single Domain Nanobody Inhibits Expansion of Pathogenic Th17 Responses and Suppresses Uveitis in Mice. Front Immunol (2021) 12:724609. doi: 10.3389/fimmu.2021.724609
11. Pandiyan P, Zhu J. Origin and Functions of Pro-Inflammatory Cytokine Producing Foxp3+ Regulatory T Cells. Cytokine (2015) 76(1):13–24. doi: 10.1016/j.cyto.2015.07.005
12. Rafieemehr H, Kheyrandish M, Soleimani M. Neuroprotective Effects of Transplanted Mesenchymal Stromal Cells-Derived Human Umbilical Cord Blood Neural Progenitor Cells in EAE. Iranian J Allergy Asthma Immunol (2015) 14(6):596–604.
13. Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer's Disease. Neurochem Res (2018) 43(11):2165–77. doi: 10.1007/s11064-018-2641-5
14. Zhou X, Liu X, Liu L, Han C, Xie Z, Liu X, et al. Transplantation of IFN-γ Primed hUCMSCs Significantly Improved Outcomes of Experimental Autoimmune Encephalomyelitis in a Mouse Model. Neurochem Res (2020) 45(7):1510–7. doi: 10.1007/s11064-020-03009-y
15. O'Neill EJ, Day MJ, Wraith DC. IL-10 Is Essential for Disease Protection Following Intranasal Peptide Administration in the C57BL/6 Model of EAE. J Neuroimmunol (2006) 178:1–8. doi: 10.1016/j.jneuroim.2006.05.030
16. Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, et al. Injection of Adult Neurospheres Induces Recovery in a Chronic Model of Multiple Sclerosis. Nature (2003) 422(6933):688–94. doi: 10.1038/nature01552
17. Liu R, Zhang Z, Lu Z, Borlongan C, Pan J, Chen J, et al. Human Umbilical Cord Stem Cells Ameliorate Experimental Autoimmune Encephalomyelitis by Regulating Immunoinflammation and Remyelination. Stem Cells Dev (2013) 22(7):1053–62. doi: 10.1089/scd.2012.0463
18. Yang C, Wu M, You M, Chen Y, Luo M, Chen Q. The Therapeutic Applications of Mesenchymal Stromal Cells From Human Perinatal Tissues in Autoimmune Diseases. Stem Cell Res Ther (2021) 12(1):103. doi: 10.1186/s13287-021-02158-3
19. Nauta AJ, Fibbe WE. Immunomodulatory Properties of Mesenchymal Stromal Cells. Blood (2007) 110(10):3499–506. doi: 10.1182/blood-2007-02-069716
20. Al-Massri KF, Ahmed LA, El-Abhar HS. Mesenchymal Stem Cells in Chemotherapy-Induced Peripheral Neuropathy: A New Challenging Approach Which Requires Further Investigations. J Tissue Eng Regen Med (2019) 14(1). doi: 10.1002/term.2972
21. François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC Suppression Correlates With Cytokine Induction of Indoleamine 2,3-Dioxygenase and Bystander M2 Macrophage Differentiation. Mol Ther J Am Soc Gene Ther (2012) 20(1):187–95. doi: 10.1038/mt.2011.189
22. Li H, Deng Y, Liang J, Huang F, Qiu W, Zhang M, et al. Mesenchymal Stromal Cells Attenuate Multiple Sclerosis IDO-Dependent Increasing the Suppressive Proportion of CD5+ IL-10+ B Cells. Am J Trans Res (2019) 11(9):5673–88.
23. Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-Dioxygenase Is a Signaling Protein in Long-Term Tolerance by Dendritic Cells. Nat Immunol (2011) 12(9):870–8. doi: 10.1038/ni.2077
24. de Witte SFH, Merino AM, Franquesa M, Strini T, van Zoggel JAA, Korevaar SS, et al. Cytokine Treatment Optimises the Immunotherapeutic Effects of Umbilical Cord-Derived MSC for Treatment of Inflammatory Liver Disease. Stem Cell Res Ther (2017) 8(1):140. doi: 10.1186/s13287-017-0590-6
25. Shao M, Wang D, Zhou Y, Du K, Liu W. Interleukin-10 Delivered by Mesenchymal Stem Cells Attenuates Experimental Autoimmune Myocarditis. Int Immunopharmacol (2020) 81:106212. doi: 10.1016/j.intimp.2020.106212
26. Wu X, Tian J, Wang S. Insight Into Non-Pathogenic Th17 Cells in Autoimmune Diseases. Int Immunopharmacol (2018) 9:1112. doi: 10.3389/fimmu.2018.01112
27. Qu X, Liu X, Cheng K, Yang R, Zhao RC. Mesenchymal Stem Cells Inhibit Th17 Cell Differentiation by IL-10 Secretion. Exp Hematol (2012) 40(9):761–70. doi: 10.1016/j.exphem.2012.05.006
28. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The Orphan Nuclear Receptor RORgammat Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell (2006) 126(6):1121–33. doi: 10.1016/j.cell.2006.07.035
29. Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, et al. Foxp3 Inhibits RORgammat-Mediated IL-17a mRNA Transcription Through Direct Interaction With RORgammat. J Biol Chem (2008) 283(25):17003–8. doi: 10.1074/jbc.M801286200
30. Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the Regulatory T Cell Lineage In Vivo. Science (New York NY) (2010) 329(5999):1667–71. doi: 10.1126/science.1191996
31. Tanaka S, Suto A, Iwamoto T, Kashiwakuma D, Kagami S, Suzuki K, et al. Sox5 and C-Maf Cooperatively Induce Th17 Cell Differentiation via RORγt Induction as Downstream Targets of Stat3. J Exp Med (2014) 211(9):1857–74. doi: 10.1084/jem.20130791
Keywords: IFN-γ-primed human umbilical cord mesenchymal stem cell (IFN-γ-hUCMSCs), multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE), indoleamine 2,3-dioxygenease (IDO), Foxp3/ROR-γt/STAT3 signaling pathway
Citation: Ling X, Wang T, Han C, Wang P, Liu X, Zheng C, Bi J and Zhou X (2022) IFN-γ-Primed hUCMSCs Significantly Reduced Inflammation via the Foxp3/ROR-γt/STAT3 Signaling Pathway in an Animal Model of Multiple Sclerosis. Front. Immunol. 13:835345. doi: 10.3389/fimmu.2022.835345
Received: 14 December 2021; Accepted: 31 January 2022;
Published: 01 March 2022.
Edited by:
Jianwei Pan, First Affiliated Hospital, School of Medicine, Zhejiang University, ChinaReviewed by:
Wenjie You, Shandong Provincial Hospital, ChinaHuaqiu Zhang, Huazhong University of Science and Technology, China
Copyright © 2022 Ling, Wang, Han, Wang, Liu, Zheng, Bi and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Zhou, enh5c2R1QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiao Ling
Xiao Ling Teng Wang
Teng Wang Chao Han
Chao Han Pin Wang
Pin Wang Xiaoli Liu
Xiaoli Liu Chengyun Zheng
Chengyun Zheng Jianzhong Bi
Jianzhong Bi Xiaoyan Zhou
Xiaoyan Zhou