- 1Department of Oncology, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
- 2Department of Pathology, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
- 3Department of Medical, Shanghai OrigiMed Co., Ltd, Shanghai, China
Soft-tissue sarcomas (STS), with over 100 different histologic subtypes, are rare tumors that account for 1% of all adult malignancies. Immune checkpoint inhibitors (ICIs) display certain benefits in some subtypes, especially in undifferentiated pleomorphic sarcoma (UPS), alveolar soft part sarcoma (ASPS), and leiomyosarcoma (LMS). However, efficacy is difficult to predict. High tumor mutational burden (TMB-H) and programmed death-ligand 1 (PD-L1) expression are the strongest features associated with the efficacy of immunotherapy, although they are rarely found in STS patients. Until now, whether or not PD-L1 expression and TMB are related to the efficacy of immunotherapy has not been determined. In this study, we report data obtained from two STS patients, one ASPS and one UPS with a high TMB, that benefited from anlotinib combined with toripalimab following resistance to anlotinib monotherapy. A 26 year-old female patient was diagnosed with ASPS. PD-L1 was negative. Next generation sequencing (NSG) revealed ASPSCR1-TFE3 fusion and TMB-H. Following eight months of anlotinib monotherapy, the patient’s disease progressed but continued to benefit from subsequent use of anlotinib combined with toripalimab for 19 months. Another 63 year-old male patient was diagnosed with UPS. PD-L1 was positive and NGS revealed TMB-H. Following 19 months of anlotinib monotherapy, the patient’s disease progressed but continued to benefit from subsequent use of anlotinib combined with toripalimab. DFS is 23 months to follow-up time. The results presented are the first to report the relationship between TMB and the efficacy of immunotherapy in STS. Based on our results, we hypothesis that anlotinib combined with toripalimab is effective for the treatment of some advanced ASPS or UPS. TMB may be a potential predictive biomarker for ICI treatment and deserves additional study.
Introduction
Soft-tissue sarcomas (STS) are a rare and heterogeneous group of tumors that represent less than one percent of all adult malignancies (1). STS contains more than 100 different histologic and molecular subtypes, each of which exhibits different clinical manifestations. Treatments include chemotherapy, targeted therapy, and immunotherapy. Since some subtypes of STS are chemoresistant, chemosensitivity is dependent on specific histologic type. Novel target drugs have been approved, including pazopanib, regorafenib, and anlotinib (approved in China) (2–4). Immune checkpoint inhibitors (ICIs) have indicated initial efficacy in some subtypes, especially in myxofibrosarcoma, undifferentiated pleomorphic sarcoma (UPS), alveolar soft part sarcoma (ASPS), cutaneous angiosarcoma, undifferentiated sarcomas, and leiomyosarcoma (5–8). ICIs plus anti-VEGR therapy have indicated preliminary activity, particularly in patients with ASPS (9). However, these therapies are still not perfect and predictive biomarkers are unclear. Tumor mutational burden (TMB) and programmed death-ligand 1 (PD-L1) expression are the strongest features associated with the efficacy of immunotherapy treatment, but are rarely found in STS patients. As such, identifying patients that will benefit from ICI treatment is crucial.
In this study, we report data obtained from one ASPS and one UPS patient with high TMB (TMB-H) following anlotinib resistance that received anlotinib combined with toripalimab therapy, and achieved a partial response (PR) and stable disease (SD), respectively. Our data suggest that TMB-H may be a potential predictive biomarker for immunotherapy.
Case 1
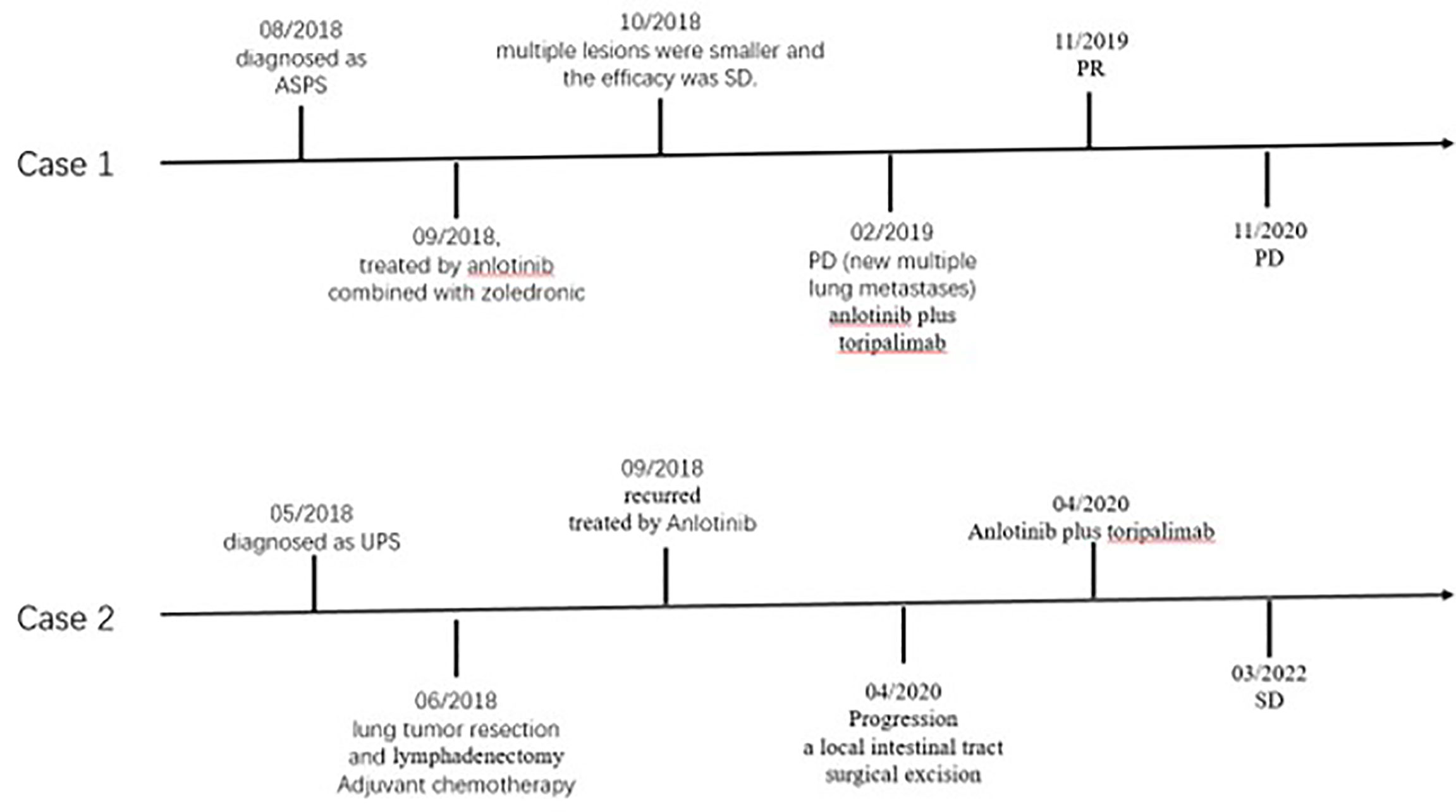
In July 2018, a 26 year-old female patient underwent bone emission computed tomography (ECT), positron emission tomography (PET), and magnetic resonance imaging (MRI) examinations for bone pain in our hospital. These examinations revealed a right thigh mass and abnormal activation of multiple focal bone metabolism, indicating a malignant bone tumor with metastases. In August 2018, a biopsy of tumor mass and a sacroiliac joint biopsy were performed. The pathological diagnosis report indicated that the mass on the outer right thigh was consistent with alveolar soft part sarcoma (ASPS) (Figure 1). Additionally, next generation sequencing (NGS) of biopsy tissue yielded 13.1 muts/Mb of TMB, determined as TMB-Hbased on the previous data (10) andpositive ASPSCR1-TFE3 fusion. Immunohistochemistry (IHC) assay using 28-8 antibody showed tumor cells were PD-L1 negative. The neutrophil-to-lymphocyte ratio (NLR) was 2.17. In September 2018, anlotinib (12 mg, qd, d1-14, q3w) combined with zoledronic acid was used to treat the tumor. A PET/CT re-examination in October 2018 showed that multiple lesions were smaller than previously recorded. According to RECIST 1.1, the efficacy was stable disease (SD). However, in February 2019, following eight months of treatment, disease progression (PD) was observed (new multiple lung metastases occurred, Figure 2A), and soreness of the shoulder and upper arm was reported to be aggravated. Based on previous treatment and NGS results, a treatment of anlotinib combined with toripalimab (240 mg, ivd, d1, q3w, a PD-1 blocker drug) began in May 2019. Soreness was relieved after one week. In November 2019, CT re-examination indicated that the efficacy evaluation was a partial response (PR) (Figure 2B). Anlotinib combined with toripalimab was continued as treatment, and re-examination in March 2020 suggested that the disease was stable (Figure 2C). Bone metastasis was SD, and ostalgia worsened in July 2020. However, pain was reduced after replacing bisphosphonates with denosumab. A CT scan revealed that the disease was PD in December 2020. Progression free survival (PFS) was 19 months (Figure 3).
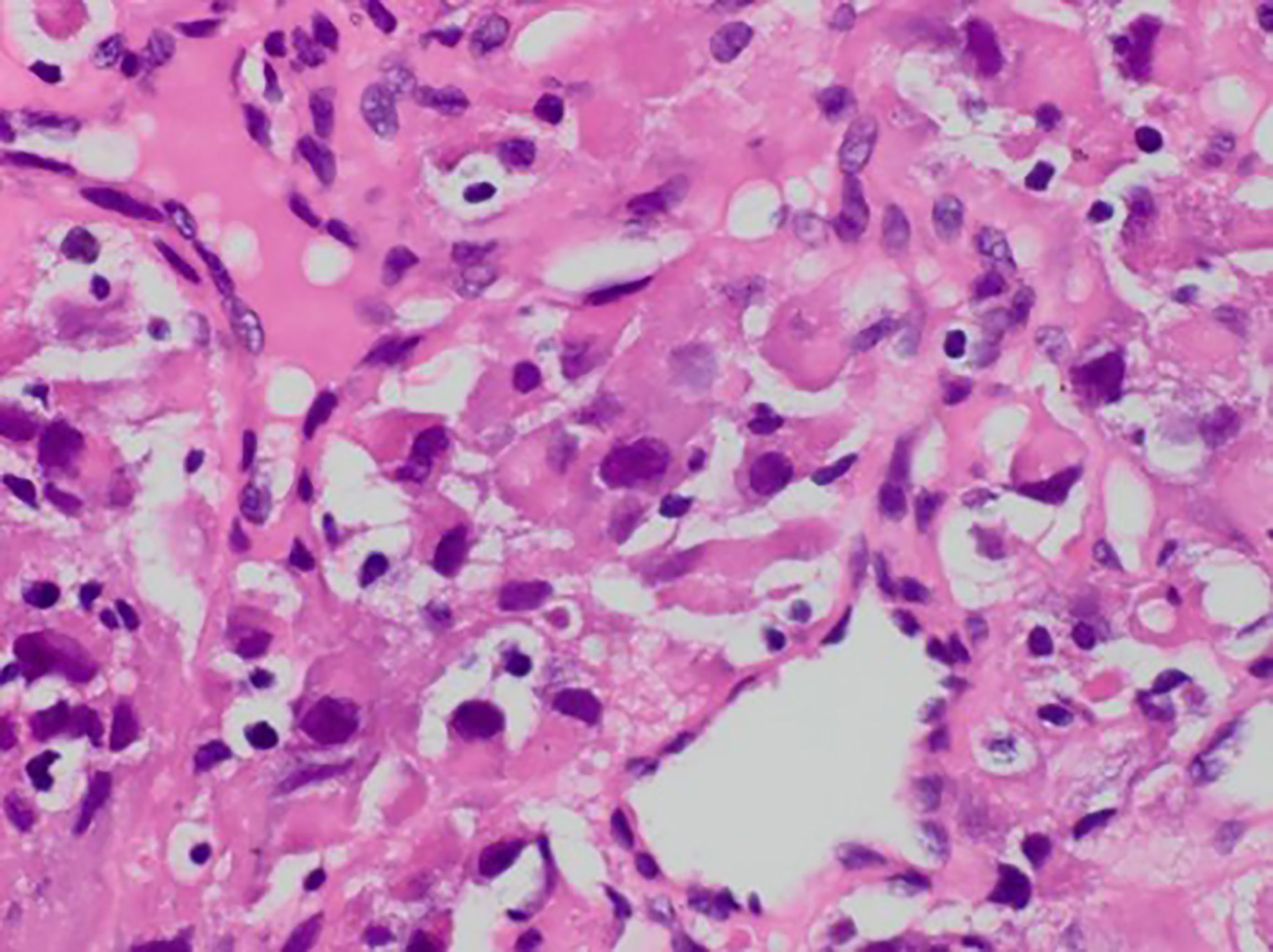
Figure 1 Postoperative pathology revealed obvious heterogeneity in tumor cells with hyperchromatic, irregular nuclei and a foamy cytoplasm (HE, 400x).
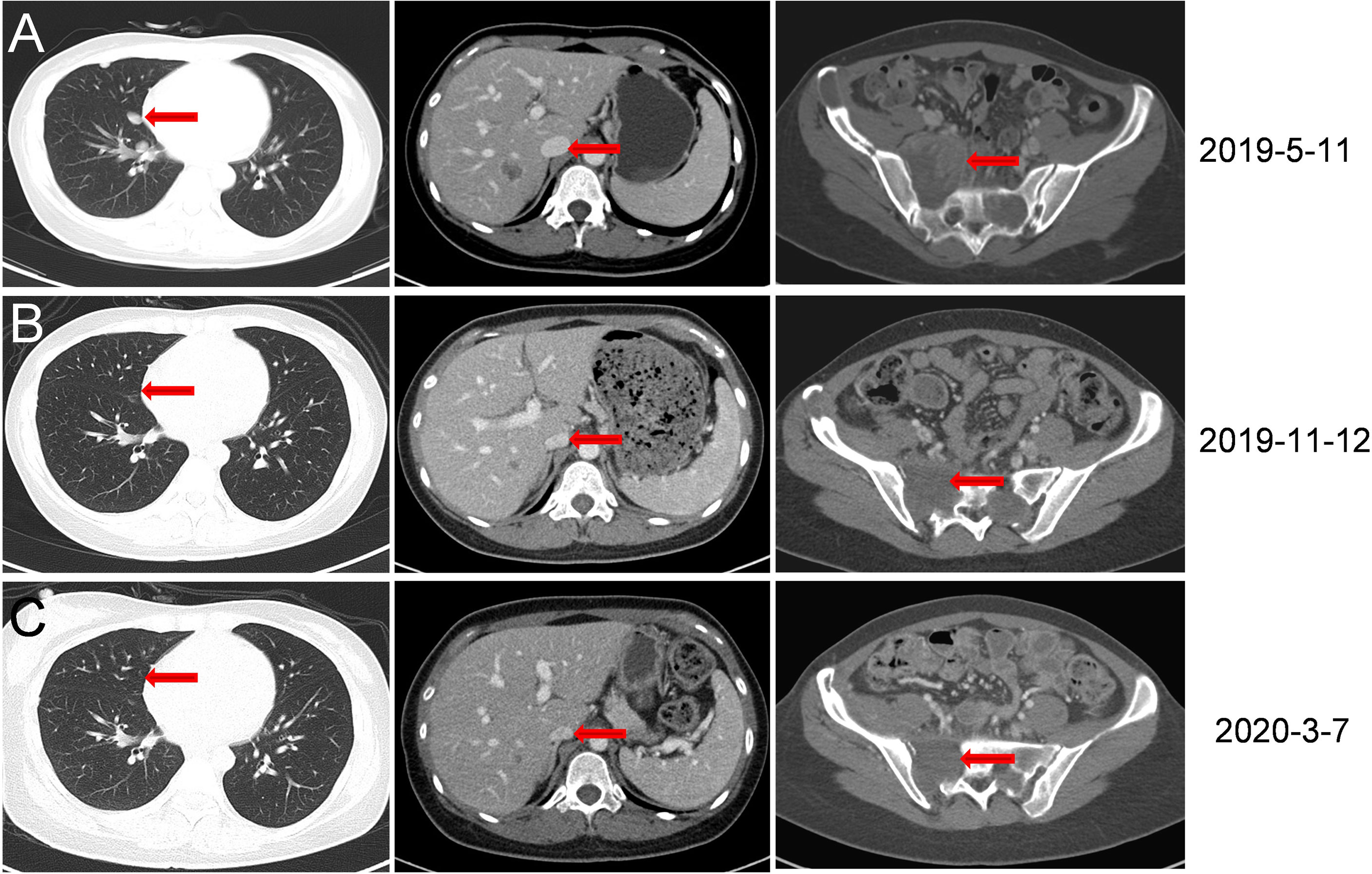
Figure 2 CT scan images during treatment. (A) In May 2019, eight months following anlotinib treatment, new multiple lung metastases were observed. (B) In November 2019, six months following anlotinib combined with toripalimab treatment, lesions in the lung disappeared. Liver and sacral vertebrae metastases shrank. (Size was reduced from 5.5 cm × 5.4 cm to 3.5 cm × 3.8 cm). (C) In March 2020, CT re-examination indicated stable disease.
Case 2
In May 2018, a 63 year-old male patient came to our hospital due to cough and blood-streaked sputum. A CT scan revealed left lung nodules and lymphadenectasis in the mediastinum and left hilar and suspected metastasis of left adrenal nodule (Figure 4A). The clinical stage was T2bN2Mx. The patient underwent a left upper lung tumor resection combined with a lymphadenectomy in June 2018. Intraoperative rapid cryopathology was performed and the pathological diagnosis was lung adenocarcinoma. However, postoperative pathology confirmed the pathological diagnosis was UPS (Figure 5). Immunohistochemistry showed vimentin was positive, and TTF1, CK5/6, and CK7 were negative. Surgical tissues were sent for NGS testing. The results of NGS indicated TMB-H (10.0 muts/Mb) (10). PD-L1 expression in tumor (using the 28-8 IHC assay) was 90%. The patient received pemetrexed plus platinum as adjuvant chemotherapy. The tumor recurred three months later (Figure 4B). Anlotinib was then administered orally at once, and the efficacy was SD. However, following 19 months of the treatment, the patient presented with abdominal pain and a small bowel mass was found, indicating tumor progression (Figure 4C). In April 2020, the other lesions are stable, therefore a local intestinal tract surgical excision was performed and the pathology showed UPS (Figure 5). Anlotinib combined with toripalimab treatment (240 mg, ivd, d1, q3w) was then administered. In July 2020, a CT re-examination indicated that tumors had shrunk (Figure 4D), and the efficacy evaluation was SD. Treatment with combination therapy is currently ongoing. In March 2022, a CT re-examination indicated the efficacy was SD (Figure 4E) and PFS is 23 months (until the time of manuscript submission) (Figure 3).
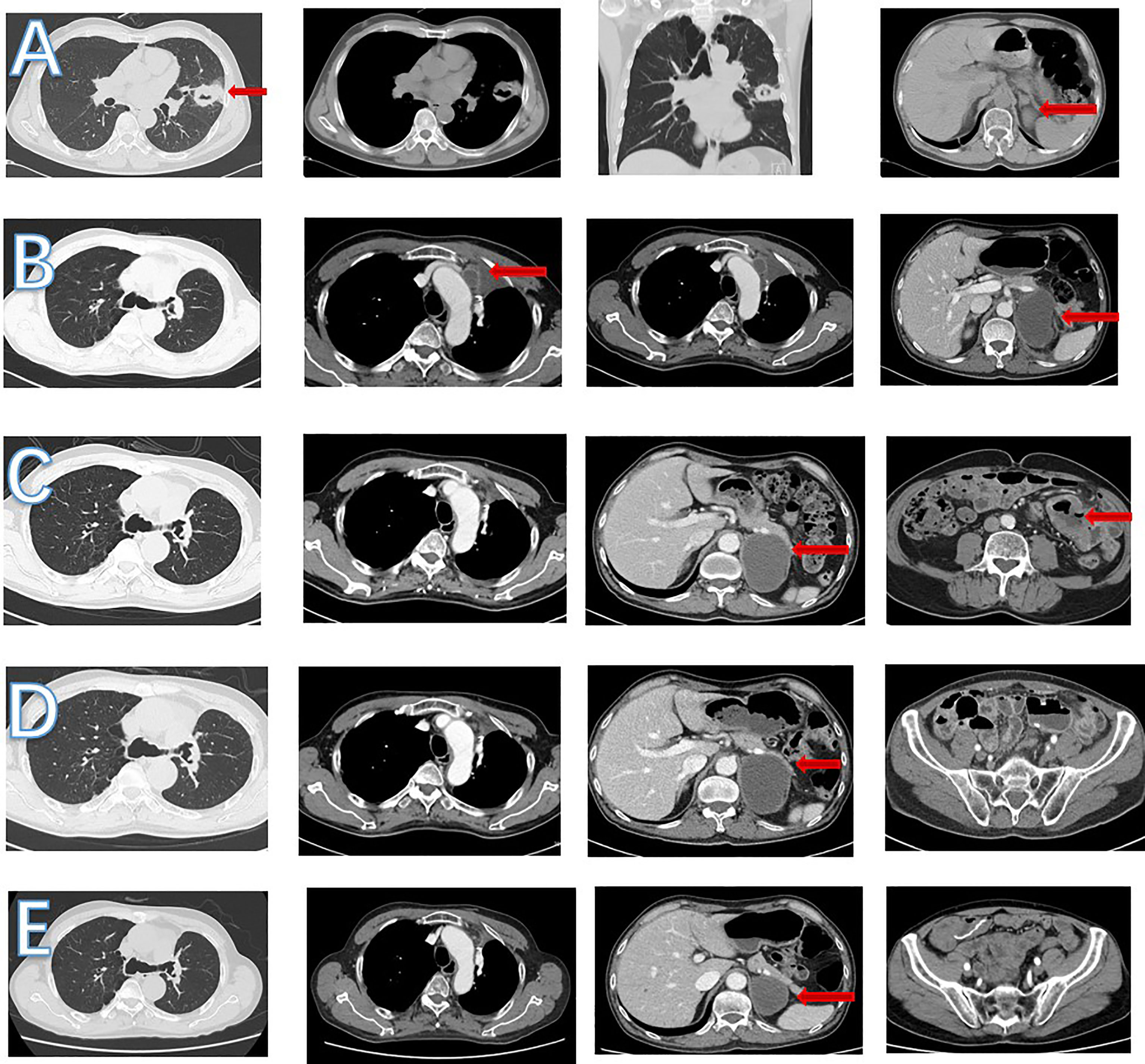
Figure 4 CT scan images during treatment. (A) In June 2018, left lung nodules and suspected metastasis of left adrenal nodule. (B) In September 2018, enlarged mediastinal lymph nodule and increased left adrenal nodule. (C) In April 2020, a new small bowel mass was found, left adrenal nodule was stable and mediastinal lymph nodule shrank. (D) In July 2020, left adrenal and mediastinal lymph nodule was stable. No small intestinal tumor was found. (E) In March 2022, left adrenal nodule shrank and mediastinal lymph nodule was stable.
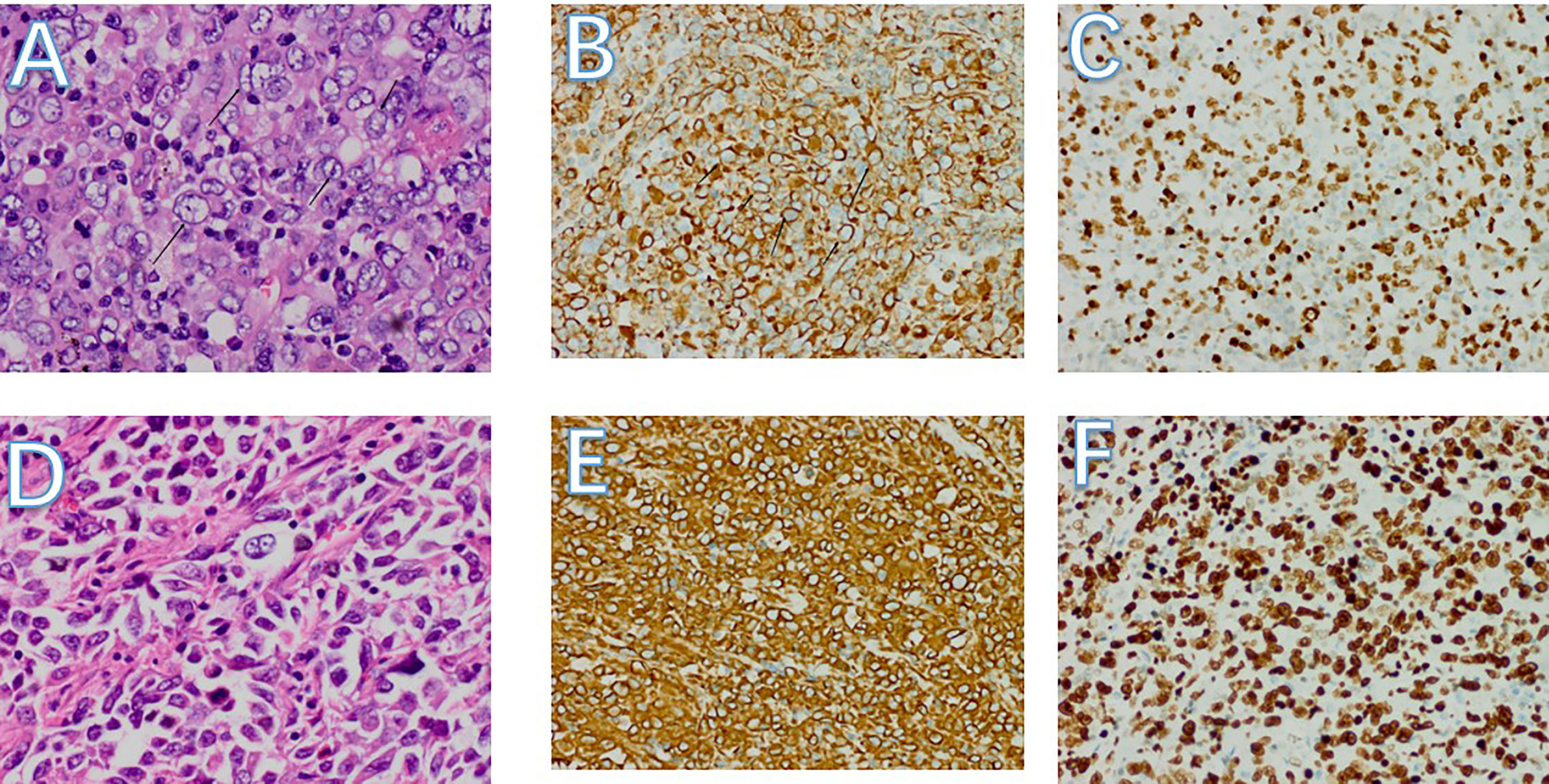
Figure 5 (A-C) Pathology of left lung mass. (A) Pulmonary tumors with cytologic atypia,different nuclear sizes. Most of the nuclei are vacuolated, some nucleoli can be seen, and mitotic figures are obvious (HE, 400x). (B) Diffuse cytoplasmic positivity in tumor cells (Vimentin 200x). C:tumor cells are nuclear-positive, showing a hyperproliferative state. (ki67 200x) (D-F) Pathology of small bowel mass. (D) Small bowel tumors with different nuclear sizes. Most of the nuclei are vacuolated, some nucleoli can be seen, and mitotic figures are obvious (HE, 400x). (E) Diffuse cytoplasmic positivity in tumor cells (Vimentin 200x). (F) Tumor cells are nuclear-positive, showing a hyperproliferative state. (ki67 200x).
Discussion
ICIs, consisting of PD-L1, programmed cell death protein-1 (PD-1), and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) inhibitors, have revolutionized the treatment of human solid tissue tumors (11). Some studies indicate that ICIs are also effective in STS (5–7), although the results are heterogeneous for different subtypes. Monotherapy pembrolizumab in a Phase 2 clinical trial indicated that seven (18%) of 40 patients had an objective response for four (40%) of ten patients with UPS, two (20%) of ten patients with liposarcoma, and one (10%) of ten patients with synovial sarcoma. In another clinical trial of 38 patients treated with nivolumab and ipilimumab, the confirmed objective response rate (ORR) was 16% and demonstrated promising efficacy for certain sarcoma subtypes (UPS, LMS, myxofibrosarcoma, and angiosarcoma) (6). Higher levels of efficacy were determined for gene expression related to antigen presentation and T-cell infiltration in UPS and LMS, as compared to synovial sarcoma and liposarcoma (12). These outcomes partly explain the positive results for UPS and LMS. However, the exact reason why ICIs is effective remained elusive.
Targeted therapy for sarcoma is largely achieved with small-molecule, multi-target, anti-vascular drugs, including pazopanib, regorafenib and anlotinib, approved for the treatment of different types of sarcomas (2–4). Single-agent tyrosine kinase inhibitor (TKI) drugs are effective for the treatment of some sarcomas. Combined treatments maybe improve efficacy. In recent years, TKIs combined with ICIs have attracted extensive attention. In a single-arm clinical trial, the median PFS of the ASPS subgroup treated with axitinib plus pembrolizumab was 12.4 months, and the evaluated 3-month progression-free survival for ASPS was 72.7% (9), and the ORR was 60.0%, indicating that TKIs combined with ICIs may have preliminary activity in patients with advanced sarcomas, particularly in patients with ASPS (9).
ASPS is an extremely rare subtype of STS, with only one diagnosis per 10 million people per year, accounting for approximately 0.5% of all sarcomas (13). Although ASPS is often reported as an indolent disease as compared to other subtypes of sarcoma, many patients have distant metastases or multiple local recurrences at diagnosis; and the prognosis is poor (14). Conventional chemotherapy has little therapeutic influence on ASPS (15). Molecular analyses of ASPS has revealed a specific ASPSCR1-TFE3 gene fusion that causes the upregulation of angiogenesis and proliferation transcripts (16), providing evidence for the application of TKIs directed at vascular endothelial growth factor (VEGF). Anlotinib, pazopanib, and sunitinib have been recommended as a first-line therapy for ASPS (4, 17, 18). Additionally, ICIs have shown good efficacy for ASPS (19). For example, the PD-L1 inhibitor atezolizumab has been shown to achieve a 42% objective response (20). Toripalimab is a recombinant humanized anti-PD-1 monoclonal antibody, independently developed in China. In a Phase I clinical trial (JS001) of toripalimab for advanced or refractory ASPS, one and two of 12 total patients achieved CR and PR, respectively. The median PFS (mPFS) is expected to be 12.4 months (21). For this trial, the ORR, disease control rate (DCR), and median overall survival (OS) were 25.0% (3/12), 91.7% (11/12), and 34.7 months, respectively, in ASPS patients with toripalimab treatment (22). The combination of TKIs and ICIs have been investigated in advanced sarcomas (9). One possibility in explaining the therapeutic effect of the immune checkpoint blockade in ASPS is ASPSCR1-TFE3 fusion. Such fusion could theoretically serve as a highly selective tumor-specific antigen (23).
Immunotherapy has shown preliminary influence in the treatment of sarcoma, however, clear predictive biomarkers remain uncertain. PD-L1 expression andTMB-H can predict the efficacy of immunotherapy in most tumors (24, 25), although no affirmation has been drawn for STS. Some studies suggest that specific subtypes of UPS and LMS sarcoma may predict the response to immunotherapy (7). However, PD-L1 status, TMB, and microsatellite instability status have not been investigated.
A past study investigated the expression of PD-L1 in sarcoma and found a positive rate of only 12%; one positive outcome resulted from a radiation associated pleomorphic sarcoma and the other from a spindle cell sarcoma (26). Another study indicated intra-tumoral infiltration of PD1-positive lymphocytes and PD-L1 expression (no tumor expression) in 65% and 58% of STS, respectively (27); patients with a PD1 (+)/PD-L1 (+) pattern had the shortest survival period. In the SARC028 clinical trial, PD-L1 was at the 1% threshold in only three (4%) of 70 patients and all three patients had UPS. Of the three positive patients, only two had a response: one had a complete response and the other had a partial response (5). In a trial of axitinib plus pembrolizumab, tumor cell PD-L1 expression was positive in 15 (52%) of 29 patients, although neither PD-L1 positivity nor an increased tumor-infiltrating lymphocyte score correlated with progression-free survival of longer than six months, or achieved a partial response. A baseline neutrophil-to-lymphocyte ratio of more than five is associated with a progressive response (9). Therefore, the expression of PD-L1 cannot predict the efficacy of immunotherapy in some STS patients. However, due to the lack of data and the heterogeneity of sarcoma, such a conclusion is contradictory. Our second patient with high PD-L1 expression combined with TMB-H suggests that PD-L1 expression may be a positive biomarker for predicting efficacy in UPS patients.
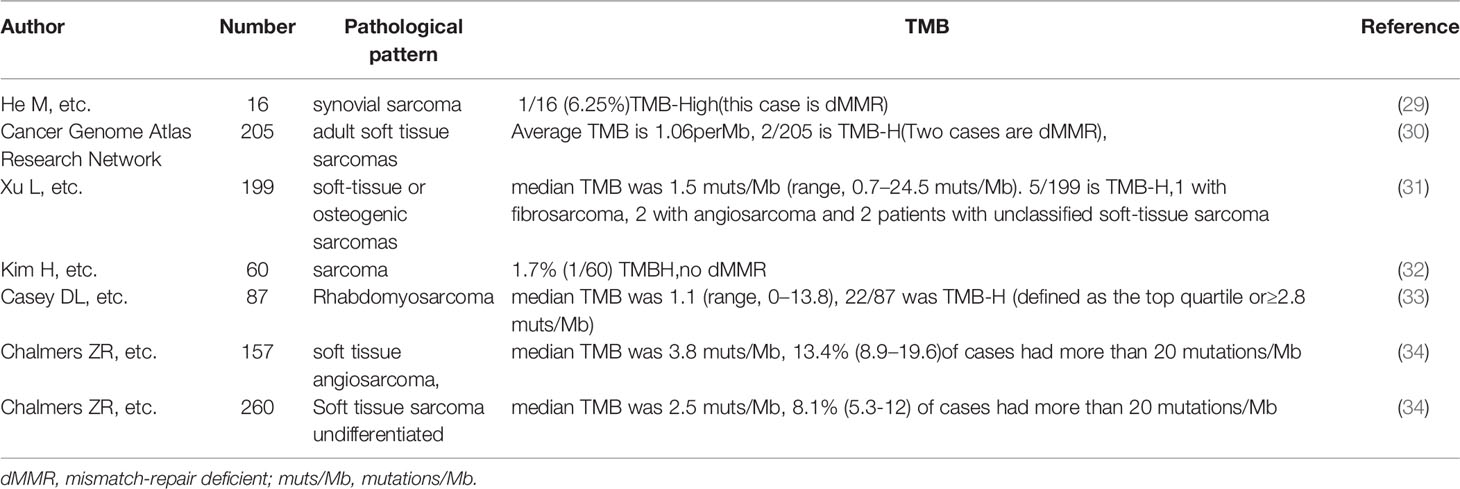
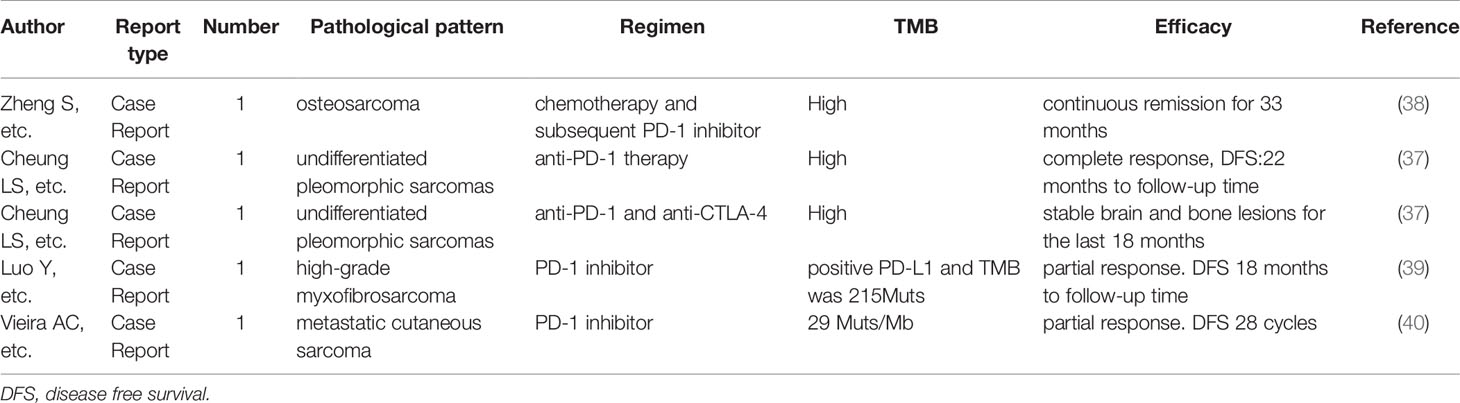
TMB is often detected in various tumors and is related to the efficacy of ICI treatment (28). Yet, the TMB of STS has been reported to be low (Table 1) (34, 35). A study indicated that the TMB of 68 STS patients was relatively low (median: 2.05 per Mb) (36). Previous studies have also indicated that ASPS displays minimal differences in mutations (9). However, both of our patients with TMB-H benefit from anlotinib combined with toripalimab treatment, suggesting that TMB may be a potential biomarker for therapy. A previously published case report found that a UPS patient with high TMB was effectively treated with a single anti-PD-1 agent (37). Some cases show the same result (Table 2). As such, our results indicate that combination therapy may be effective in anti-vascular, therapy-resistant patients with TMB-H.
An Open-Label Phase IB trial found that the combination of toripalimab plus axitinib is tolerable and displays promising antitumor activity in patients with treatment-naive metastatic mucosal melanoma (41). The finding suggests that toripalimab combined with anti-vascular targeted drugs may have a synergistic effect. Another case report also indicated that the combination of ICIs with anti-VEGF has satisfactory curative effect (42, 43). Similarly, for our cases, following resistance to anlotinib monotherapy and by considering TMB-H, our STS and the ASPS patient were both treated with anlotinib combined with toripalimab and reached SD and PR, respectively.
To our knowledge, this is the first report for the combination of anti-VEGF and PD-L1 inhibition for TMB-H STS. Our results are encouraging. However, the following limitations exist for our results : 1. We could not confirm whether or not the effect was due to a single immunotherapy or to the synergy of combined treatment. 2. Our case reports only provide preliminary conclusions, although they, at least, indicated that TMB-H may be a potential biomarker. 3. Specific reasons for effectiveness of the combined treatment remains elusive.
Large randomized controlled trials should be conducted in order to examine the potential benefits of the PD-1/PD-L1 blockade, either alone or in combination with targeted therapy. To determine patients most likely to benefit from ICIs; and to evaluate TMB, the tumor microenvironment, gene expression, and genetic alterations, as well as to explore underlying cellular and molecular mechanisms, future studies are warranted.
Conclusion
Anlotinib combined with toripalimab was shown to be an effective therapy in advanced STS or ASPS patients with metastases, specifically for TMB-H STS. Our study indicates that anti-VEGF TKIs combined with ICIs may be an effective choice for the treatment of STS or ASPS. The two cases examined suggest that TMB-H may be a potential predictor. This hypothesis will need to be tested in a larger clinical trial that encompasses robust correlative studies. The ongoing clinical trial NCT02609984, NCT02815995, and NCT02636725 will confirm the efficacy of ICIs and explore predictive marker.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics committee of Guangdong Provincial Hospital of Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conception/Design: YL and HZ; Provision of study material or patients: YL and YHL; Collection and/or assembly of data: YQ, XC, and XQ; Data analysis and interpretation: YY, XD, and YC; Manuscript writing: YL and MX; Final approval of manuscript: YL and HZ. All authors have read and approved the submitted version of the manuscript.
Conflict of Interest
Authors MX and YC are employed by Shanghai OrigiMed Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We owe thanks to the patients and their family. We thank OrigiMed for NGS technical support and scientific comments.
References
1. Clark MA, Fisher C, Judson I, Thomas JM. Soft-Tissue Sarcomas in Adults. N Engl J Med (2005) 353(7):701–11. doi: 10.1056/NEJMra041866
2. van der Graaf WTA, Blay J-Y, Chawla SP, Kim D-W, Bui-Nguyen B, Casali PG, et al. Pazopanib for Metastatic Soft-Tissue Sarcoma (PALETTE): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet (2012) 379(9829):1879–86. doi: 10.1016/S0140-6736(12)60651-5
3. Mir O, Brodowicz T, Italiano A, Wallet J, Blay J-Y, Bertucci F, et al. Safety and Efficacy of Regorafenib in Patients With Advanced Soft Tissue Sarcoma (REGOSARC): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Oncol (2016) 17(12):1732–42. doi: 10.1016/S1470-2045(16)30507-1
4. Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients With Refractory Metastatic Soft-Tissue Sarcoma. Clin Cancer Res (2018) 24(21):5233–8. doi: 10.1158/1078-0432.CCR-17-3766
5. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in Advanced Soft-Tissue Sarcoma and Bone Sarcoma (SARC028): A Multicentre, Two-Cohort, Single-Arm, Open-Label, Phase 2 Trial. Lancet Oncol (2017) 18(11):1493–501. doi: 10.1016/S1470-2045(17)30624-1
6. D'Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab With or Without Ipilimumab Treatment for Metastatic Sarcoma (Alliance A091401): Two Open-Label, Non-Comparative, Randomised, Phase 2 Trials. Lancet Oncol (2018) 19(3):416–26. doi: 10.1016/S1470-2045(18)30006-8
7. Monga V, Skubitz KM, Maliske S, Mott SL, Dietz H, Hirbe AC, et al. A Retrospective Analysis of the Efficacy of Immunotherapy in Metastatic Soft-Tissue Sarcomas. Cancers (2020) 12(7):1873. doi: 10.3390/cancers12071873
8. Burgess MA, Bolejack V, Tine BAV, Schuetze S, Hu J, D’Angelo SP, et al. Multicenter Phase II Study of Pembrolizumab (P) in Advanced Soft Tissue Sarcoma (STS) and Bone Sarcomas (BS): Final Results of SARC028 and Biomarker Analyses. J Clin Oncol (2017) 35(Supplement; Abstract 11008). doi: 10.1200/JCO.2017.35.15_suppl.11008
9. Wilky BA, Trucco MM, Subhawong TK, Florou V, Park W, Kwon D, et al. Axitinib Plus Pembrolizumab in Patients With Advanced Sarcomas Including Alveolar Soft-Part Sarcoma: A Single-Centre, Single-Arm, Phase 2 Trial. Lancet Oncol (2019) 20(6):837–48. doi: 10.1016/S1470-2045(19)30153-6
10. Cao J, Chen L, Li H, Chen H, Yao J, Mu S, et al. An Accurate and Comprehensive Clinical Sequencing Assay for Cancer Targeted and Immunotherapies. Oncologist (2019) 24(12):e1294–302. doi: 10.1634/theoncologist.2019-0236
11. Schreiber RD, Old LJ, Smyth MJ. Cancer Immunoediting: Integrating Immunity's Roles in Cancer Suppression and Promotion. Science (2011) 331(6024):1565–70. doi: 10.1126/science.1203486
12. Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, et al. T-Cell Infiltration and Clonality Correlate With Programmed Cell Death Protein 1 and Programmed Death-Ligand 1 Expression in Patients With Soft Tissue Sarcomas. Cancer (2017) 123(17):3291–304. doi: 10.1002/cncr.30726
13. Penel N, Coindre JM, Giraud A, Terrier P, Ranchere-Vince D, Collin F, et al. Presentation and Outcome of Frequent and Rare Sarcoma Histologic Subtypes: A Study of 10,262 Patients With Localized Visceral/Soft Tissue Sarcoma Managed in Reference Centers. Cancer (2018) 124(6):1179–87. doi: 10.1002/cncr.31176
14. Portera CA, Ho V, Patel SR, Hunt KK, Feig BW, Respondek PM, et al. Alveolar Soft Part Sarcoma: Clinical Course and Patterns of Metastasis in 70 Patients Treated at a Single Institution. Cancer (2001) 91(3):585–91. doi: 10.1002/1097-0142(20010201)91:3<585::AID-CNCR1038>3.0.CO;2-0
15. Reichardt P, Lindner T, Pink D, Thuss-Patience PC, Kretzschmar A, D?Rken B. Chemotherapy in Alveolar Soft Part Sarcomas. What Do We Know? Eur J Cancer (2003) 39(11):1511–6. doi: 10.1016/S0959-8049(03)00264-8
16. Stockwin LH, Vistica DT, Kenney S, Schrump DS, Butcher DO, Raffeld M, et al. Gene Expression Profiling of Alveolar Soft-Part Sarcoma (ASPS). BMC Cancer (2009) 9:22. doi: 10.1186/1471-2407-9-22
17. Stacchiotti S, Mir O, Le Cesne A, Vincenzi B, Fedenko A, Maki RG, et al. Activity of Pazopanib and Trabectedin in Advanced Alveolar Soft Part Sarcoma. Oncologist (2018) 23(1):62–70. doi: 10.1634/theoncologist.2017-0161
18. Stacchiotti S, Negri T, Zaffaroni N, Palassini E, Morosi C, Brich S, et al. Sunitinib in Advanced Alveolar Soft Part Sarcoma: Evidence of a Direct Antitumor Effect. Ann Oncol Off J Eur Soc Med Oncol (2011) 22(7):1682–90. doi: 10.1093/annonc/mdq644
19. Groisberg R, Hong DS, Behrang A, Hess K, Janku F, Piha-Paul S, et al. Characteristics and Outcomes of Patients With Advanced Sarcoma Enrolled in Early Phase Immunotherapy Trials. J Immunother Cancer (2017) 5(1):100. doi: 10.1186/s40425-017-0301-y
20. Coyne GO SE, Moore N, Robert M, Naoko T, Lamin J, et al. Phase II Study of Atezolizumab in Patients With Alveolar Soft Part Sarcoma. In: Connective Tissue Oncology Society Annual Meeting, 2018, vol. Abstr 3042818. Rome, Italy (2018).
21. Yang S, Yang J, Han Y, Jiang S, Jiang S. Effect of JS001, a Monoclonal Antibody Targeting Programed Death-1(PD-1), on Responses and Disease Control in Patients With Advanced or Refractory Alveolar Soft Part Sarcoma:Results From a Phase 1 Trial. J Clin Oncol (2018) 36(15_suppl):11572. doi: 10.1200/JCO.2018.36.15_suppl.11572
22. Yang J, Dong L, Yang S, Han X, Han Y, Jiang S, et al. Safety and Clinical Efficacy of Toripalimab, a PD-1 mAb, in Patients With Advanced or Recurrent Malignancies in a Phase I Study. Eur J Cancer (2020) 130:182–92. doi: 10.1016/j.ejca.2020.01.028
23. Conley AP, Trinh VA, Zobniw CM, Posey K, Martinez JD, Arrieta OG, et al. Positive Tumor Response to Combined Checkpoint Inhibitors in a Patient With Refractory Alveolar Soft Part Sarcoma: A Case Report. J Glob Oncol (2018) 4:1–6. doi: 10.1200/JGO.2017.009993
24. Duffy MJ, Crown J. Biomarkers for Predicting Response to Immunotherapy With Immune Checkpoint Inhibitors in Cancer Patients. Clin Chem (2019) 65(10):1228–38. doi: 10.1373/clinchem.2019.303644
25. Cao D, Xu H, Xu X, Guo T, Ge W. High Tumor Mutation Burden Predicts Better Efficacy of Immunotherapy: A Pooled Analysis of 103078 Cancer Patients. Oncoimmunology (2019) 8(9):e1629258. doi: 10.1080/2162402X.2019.1629258
26. D'Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin L-X, Carvajal RD, et al. Prevalence of Tumor-Infiltrating Lymphocytes and PD-L1 Expression in the Soft Tissue Sarcoma Microenvironment. Hum Pathol (2015) 46(3):357–65. doi: 10.1016/j.humpath.2014.11.001
27. Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, et al. Tumor Infiltrating PD1-Positive Lymphocytes and the Expression of PD-L1 Predict Poor Prognosis of Soft Tissue Sarcomas. PloS One (2013) 8(12):e82870. doi: 10.1371/journal.pone.0082870
28. Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. New Engl J Med (2017) 377(25):2500–1. doi: 10.1056/NEJMc1713444
29. He M, Abro B, Kaushal M, Chen L, Chen T, Gondim M, et al. Tumor Mutation Burden and Checkpoint Immunotherapy Markers in Primary and Metastatic Synovial Sarcoma. Hum Pathol (2020) 100:15–23. doi: 10.1016/j.humpath.2020.04.007
30. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell (2017) 171(4):950–65.e28. doi: 10.1016/j.cell.2017.10.014
31. Xu L, Xie X, Shi X, Zhang P, Liu A, Wang J, et al. Potential Application of Genomic Profiling for the Diagnosis and Treatment of Patients With Sarcoma. Oncol Lett (2021) 21(5):353. doi: 10.3892/ol.2021.12614
32. Kim H, Hong JY, Lee J, Park SH, Park JO, Park YS, et al. Clinical Sequencing to Assess Tumor Mutational Burden as a Useful Biomarker to Immunotherapy in Various Solid Tumors. Ther Adv Med Oncol (2021) 13:1758835921992992. doi: 10.1177/1758835921992992
33. Casey DL, Wexler LH, Pitter KL, Samstein RM, Slotkin EK, Wolden SL. Genomic Determinants of Clinical Outcomes in Rhabdomyosarcoma. Clin Cancer Res an Off J Am Assoc Cancer Res (2020) 26(5):1135–40. doi: 10.1158/1078-0432.CCR-19-2631
34. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational Burden. Genome Med (2017) 9(1):34. doi: 10.1186/s13073-017-0424-2
35. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational Heterogeneity in Cancer and the Search for New Cancer-Associated Genes. Nature (2013) 499(7457):214–8. doi: 10.1038/nature12213
36. Xu L-B, Zhao Z-G, Xu S-F, Zhang X-X, Liu T, Jing C-Y, et al. The Landscape of Gene Mutations and Clinical Significance of Tumor Mutation Burden in Patients With Soft Tissue Sarcoma Who Underwent Surgical Resection and Received Conventional Adjuvant Therapy. Int J Biol Markers (2020) 35(3):14–22. doi: 10.1177/1724600820925095
37. Cheung LS, Chen L, Oke TF, Schaffer TB, Boudadi K, Ngo JT, et al. Anti-PD-1 Elicits Regression of Undifferentiated Pleomorphic Sarcomas With UV-Mutation Signatures. J Immunother Cancer (2021) 9(6):e002345. doi: 10.1136/jitc-2021-002345
38. Zheng S, Wang F, Huang J, Zhou Y, Yang Q, Qian G, et al. Case Report: Sequential Chemotherapy and Immunotherapy Produce Sustained Response in Osteosarcoma With High Tumor Mutational Burden. Front Endocrinol (Lausanne) (2021) 12:625226. doi: 10.3389/fendo.2021.625226
39. Luo Y, Min L, Zhou Y, Tang F, Lu M, Xie H, et al. Remarkable Response to Anti-PD1 Immunotherapy in Refractory Metastatic High-Grade Myxofibrosarcoma Patient: A Case Report. Medicine (2021) 100(12):e25262. doi: 10.1097/MD.0000000000025262
40. Vieira AC, Megid TBC, Melo R, Muniz D, Salgues ACR, Barbosa FG, et al. Response to Anti-PD1 Immunotherapy in Patients With Metastatic Cutaneous Sarcoma: Case Reports and Literature Review. Oxf Med Case Rep (2020) 2020(1):omz138. doi: 10.1093/omcr/omz138
41. Sheng X, Yan X, Chi Z, Si L, Cui C, Tang B, et al. Axitinib in Combination With Toripalimab, a Humanized Immunoglobulin G Monoclonal Antibody Against Programmed Cell Death-1, in Patients With Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial. J Clin Oncol Off J Am Soc Clin Oncol (2019) 37(32):2987–99. doi: 10.1200/JCO.19.00210
42. Xu Z, Zhang Y, Yu Y-H. Successful Treatment of Advanced Alveolar Soft Part Sarcoma With Camrelizumab Combined With Apatinib: A Case Report. Ann Palliat Med (2021) 10(1):785–92. doi: 10.21037/apm-20-2275
Keywords: soft-tissue sarcomas, tumor mutational burden (TMB), immune checkpoint inhibitors, anlotinib, toripalimab
Citation: Li Y, Liu Y, Qu Y, Chen X, Qu X, Ye Y, Du X, Cheng Y, Xu M and Zhang H (2022) Case Report: Two Cases of Soft-Tissue Sarcomas: High TMB as a Potential Predictive Biomarker for Anlotinib Combined With Toripalimab Therapy. Front. Immunol. 13:832593. doi: 10.3389/fimmu.2022.832593
Received: 10 December 2021; Accepted: 11 April 2022;
Published: 06 May 2022.
Edited by:
Liangfang Shen, Central South University, ChinaReviewed by:
Yoshiyuki Suehara, Juntendo University, JapanXinjun Wang, Second Affiliated Hospital of Zhengzhou University, China
Copyright © 2022 Li, Liu, Qu, Chen, Qu, Ye, Du, Cheng, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Li, eW9uZ2ppZTg4bmV0QDE2My5jb20=; Haibo Zhang, aGFpYm96aEBhbGl5dW4uY29t
 Yong Li
Yong Li Yihong Liu1
Yihong Liu1 Yanchun Qu
Yanchun Qu Mian Xu
Mian Xu