
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 18 February 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.829878
This article is part of the Research TopicPathogenesis, Immune Escape, Prognosis and Novel Management of Lymphoid Proliferative DisordersView all 18 articles
 Ziyuan Shen1†
Ziyuan Shen1† Yingliang Jin2†
Yingliang Jin2† Qian Sun3†
Qian Sun3† Shuo Zhang3
Shuo Zhang3 Xi Chen1
Xi Chen1 Lingling Hu3
Lingling Hu3 Chenlu He1
Chenlu He1 Ying Wang4
Ying Wang4 Qinhua Liu5
Qinhua Liu5 Hao Zhang6
Hao Zhang6 Xin Liu6
Xin Liu6 Ling Wang7
Ling Wang7 Jun Jiao7
Jun Jiao7 Yuqing Miao8
Yuqing Miao8 Weiying Gu9
Weiying Gu9 Fei Wang9
Fei Wang9 Chunling Wang10
Chunling Wang10 Yuye Shi10
Yuye Shi10 Jingjing Ye11
Jingjing Ye11 Taigang Zhu12
Taigang Zhu12 Cai Sun3
Cai Sun3 Xuguang Song3
Xuguang Song3 Linyan Xu3
Linyan Xu3 Dongmei Yan3
Dongmei Yan3 Haiying Sun3
Haiying Sun3 Jiang Cao3
Jiang Cao3 Depeng Li3
Depeng Li3 Zhenyu Li3
Zhenyu Li3 Zhao Wang13
Zhao Wang13 Shuiping Huang2*
Shuiping Huang2* Kailin Xu3*and
Kailin Xu3*and  Wei Sang3* on behalf of HHLWG 14
Wei Sang3* on behalf of HHLWG 14Hemophagocytic lymphohistiocytosis (HLH) is an immune disorder with rapid progression and poor survival. Individual treatment strategy is restricted, due to the absence of precise stratification criteria. In this multicenter retrospective study, we aimed to develop a feasible prognostic model for adult HLH in China. A total of 270 newly diagnosed patients of adult HLH were retrieved from the Huaihai Lymphoma Working Group (HHLWG), of whom 184 from 5 medical centers served as derivation cohort, and 86 cases from 3 other centers served as validation cohort. X-Tile program and Maxstat analysis were used to identify optimal cutoff points of continuous variables; univariate and multivariate Cox analyses were used for variable selection, and the Kaplan–Meier curve was used to analyze the value of variables on prognosis. The C-index, Brier Score, and calibration curve were used for model validation. Multivariate analysis showed that age, creatinine, albumin, platelet, lymphocyte ratio, and alanine aminotransferase were independent prognostic factors. By rounding up the hazard ratios from 6 significant variables, a maximum of 9 points was assigned. The final scoring model of HHLWG-HPI was identified with four risk groups: low risk (≤3 pts), low-intermediate risk (4 pts), high-intermediate risk (5-6 pts), and high risk (≥7 pts), with 5-year overall survival rates of 68.5%, 35.2%, 21.3%, and 10.8%, respectively. The C-indexes were 0.796 and 0.758 in the derivation and validation cohorts by using a bootstrap resampling program. In conclusion, the HHLWG-HPI model provides a feasible and accurate stratification system for individualized treatment strategy in adult HLH.
Hemophagocytic lymphohistiocytosis (HLH) is a severe hyperinflammatory syndrome characterized by excessive activation of T cells and macrophages and is classified into primary/hereditary (pHLH) and secondary/acquired (sHLH). Primary HLH is a fatal disease and usually develops in infancy or early childhood with a median survival of 2 months if without hematopoietic stem cell transplantation (1). Secondary HLH can be initiated by a large variety of inducements that activate the immune system, such as infections, autoimmune diseases, and tumors (2). Epstein–Barr virus is a common pathogenic factor of HLH, accounting for about 70% of infection-associated HLH (3, 4), and the most common cause of tumor-associated HLH is non-Hodgkin’s lymphoma (5, 6).
The widely used diagnostic criteria for HLH are HLH-2004 and HScore (7, 8). Currently, the recommended initial therapeutic regimens are HLH-94 and HLH-2004 (8, 9). Wang et al. proposed the DEP regimen as a salvage therapy, which showed an encouraging overall response rate (76.2%) in adult refractory and relapsed HLH (10). Due to the complexity of etiology and the heterogeneity of clinical manifestations, there is a lack of precise prognostic stratification and unified individualized treatment criteria in adult HLH.
In recent years, numerous studies have explored the prognostic factors of HLH in pediatric patients. Q. et al. proposed that the lymphocyte subset was essential for prognosis (11), and Pan et al. revealed that a higher disseminated intravascular coagulation (DIC) score and lower albumin, hemoglobin, and platelet levels were negative prognostic factors in malignancy-associated HLH (12). Schram et al. confirmed that platelets and alanine aminotransferase were independent factors for overall survival (OS) in adult HLH (13). Zhou et al. confirmed that high ferritin levels (>1,050 μg/l) were associated with poor survival (14). However, other studies revealed that ferritin was not an independent prognostic indicator for adult HLH. So, the value of ferritin on the prognosis of HLH was still controversial (15, 16). Furthermore, the heterogeneity and genetic abnormalities of HLH increase the difficulty of individualized treatment for adult HLH (5, 17, 18). Therefore, there is an urgent need to establish a prognostic stratification system for adult HLH.
Based on multicenter data from the Huaihai Lymphoma Working Group (HHLWG) in China, we carried out this retrospective study to explore the prognosis of adult HLH and attempted to establish a novel prognostic model to guide precise stratification for individualized treatment.
Data from five centers of HHLWG in this study served as the derivation cohort. The five centers are (1) Affiliated Hospital of Xuzhou Medical University (n = 75) (2), the Affiliated Hospital of Jining Medical University (n = 38) (3), Yancheng First People’s Hospital (n = 37) (4), Huai’an First People’s Hospital (n = 22), and (5) Taian Central Hospital (n = 12).
Data from three centers of HHLWG in this study served as the external validation cohort. The three centers are (1) The First Affiliated Hospital of Anhui Medical University (n = 40) (2), The First People’s Hospital of Changzhou (n = 30), and (3) Qilu Hospital of Shandong University (n = 16). Study approval was obtained from the independent Ethics Committees of each participating center in HHLWG and met the Helsinki Declaration. Patients over 18 years old with newly diagnosed HLH retrieved from the above centers between January 1, 2013, and August 19, 2020, were included. Median follow-up was 30.6 months [95% CI (22.2–38.9)] in the derivation cohort and 54.8 months [95% CI (25.3–84.3)] in the validation cohort. Figure 1 shows the flowchart of the inclusion and exclusion processes in this study.
At admission, the following variables were collected: age, gender, etiologies, ferritin, triglycerides (TG), fibrinogen (FIB), lactate dehydrogenase (LDH), creatinine (Cr), alanine aminotransferase (ALT), hemoglobin (Hb), platelet (PLT), lymphocyte ratio (LYR), albumin (ALB), fever, EBV infection, presence or absence of splenomegaly, and therapeutic regimens.
The diagnosis of HLH was established according to HLH-2004 diagnostic guidelines (8). Five of eight criteria are required to make a diagnosis of HLH (1): fever (2); splenomegaly (3); cytopenias (affecting ≥2 of 3 lineages in the peripheral blood: hemoglobin <90 g/l (in infants: hemoglobin < 100 g/l), platelets < 100 × 109/l, neutrophils < 1.0 × 109/ (4); hypofibrinogenemia and/or hypertriglyceridemia: fasting triglycerides ≥ 3.0 mmol/l; fibrinogen ≤1.5 g/l) (5); hemophagocytosis in bone marrow, spleen, or lymph nodes (6); low or absent NK-cell activity (according to local laboratory reference) (7); ferritin ≥ 500 μg/l; and (8) soluble CD25 (soluble IL-2 receptor) ≥ 2,400 U/ml. All pathological biopsies were double blinded reviewed by at least two pathologists.
Follow-up was conducted by consulting inpatient medical records and making phone calls. We followed up all the patients until February 19, 2021, or the death of patients. Overall survival (OS) was calculated as the interval between the time of diagnosis and death from any cause or the last follow-up. The survival status of all patients was confirmed with death records or a telephone call to next of kin (if patient died during the follow-up) or to the patients themselves.
Based on the data from HHLWG, we attempted to develop a prognostic index model for adult HLH, the HHLWG-HPI. Data were presented as numbers (percentages) for categorical variables and median (interquartile range, IQR) for all continuous variables. Outliers were verified by the hospital medical record system. All cases were required to have complete clinical information in order to avoid unnecessary bias. The Shapiro–Wilk test was used to test the normality of numerical variables. Differences in clinical factors between etiology groups were analyzed by using the Kruskal–Wallis test and analysis of variance (ANOVA) test. Continuous variables were transformed into categorical variables by X-Tile program (Yale University, New Haven, CT, USA) (19) and Maxstat analysis (titled as Maximally Selected Rank Statistics). The X-Tile program can help divide patients into subgroups by determining the optimal cutoff points of a continuous or ordinal categorical variable based on the maximum χ2 statistic value on the log-rank test (20). The Cox proportional hazard model was used to analyze the univariate association between prognostic factors and OS. All variables with p<0.1 in univariate analysis were kept in the multivariate analysis by using backward selection for the best predictor set, and Akaike information criteria (AIC) was used to evaluate the model. All statistical tests were two-sided, and the statistical significance was set at p < 0.05.
The prognostic index (HHLWG-HPI) was derived from the prognostic model that was identified using the Cox proportional hazards model. Index scores were assigned proportionally to the estimates of the relative contribution of the independent factors in the HHLWG-HPI model. The proximity of Kaplan–Meier was used to stratify patients into risk strata based on each score value.
The model was internally validated using a bootstrap resampling procedure (1,000 iterations) with a relatively corrected Harrell’s C-statistics (C-index). A C-index score around 0.70 indicates a good model (21). Brier Score is another score function that measures the accuracy of probabilistic prediction. In survival analysis, the Brier Score measures the mean of the difference between the observed and the estimated survival beyond a certain time. The score value ranges from 0 to 1, and a higher score indicates higher inaccuracy (22, 23). The calibration curve for probability of survival was used to show optimal agreement between prediction and actual observation. External validation of the HHLWG-HPI was performed using data from 3 medical centers of HHLWG. The C-index and calibration curve were based on the basis of the regression analysis. The statistical analysis was performed by SPSS statistics for Windows, Version 19.0 (Armonk, NY: IBM Corp.), and R software (version 4.0.3; http://www.Rproject.org).
There were 434 HLH patients retrieved from HHLWG, of whom 354 were older than 18 years. Eighty-four patients were ineligible for inclusion, so 270 patients were eventually included in the whole cohort. The derivation cohort consisted of 184 cases, and the external validation cohort consisted of 86 patients. The characteristics of patients in both cohorts were compared, as shown in Table 1. The median age was 56 (IQR, 46–66) years, and 53.6% patients were male in the derivation cohort. Noticeably, patients in the derivation cohort were younger compared with the validation cohort (median age 56 vs. 61 years). Of the 184 patients, infections (n = 88) and tumor (n = 64) were the most frequent underlying etiologies, accounting for 83%. The results of the Kruskal–Wallis test showed that the lymphocyte ratio was significantly different among etiology groups, with the highest value in the infection-associated group.
In the whole cohort (n = 270), infection (n = 119) and tumor (n = 97) were the most frequent underlying etiologies, accounting for 80%. There was no significant difference among different etiology groups (p = 0.092, Figure 2A). The survival curves of different etiologies groups were examined by KM analysis, and the results showed that the OS of patients in the infection-associated group was significantly higher than that in the tumor-associated group (χ2 = 6.400, p = 0.011), and there was no significant difference between the OS of patients in the infection-associated group and that in the autoimmunity-associated group (χ2 = 0.895, p = 0.344). The results showed that the 5-year survival rates were significantly different in infection-associated, autoimmunity-associated, other etiologies, and tumor-associated groups (p < 0.01).
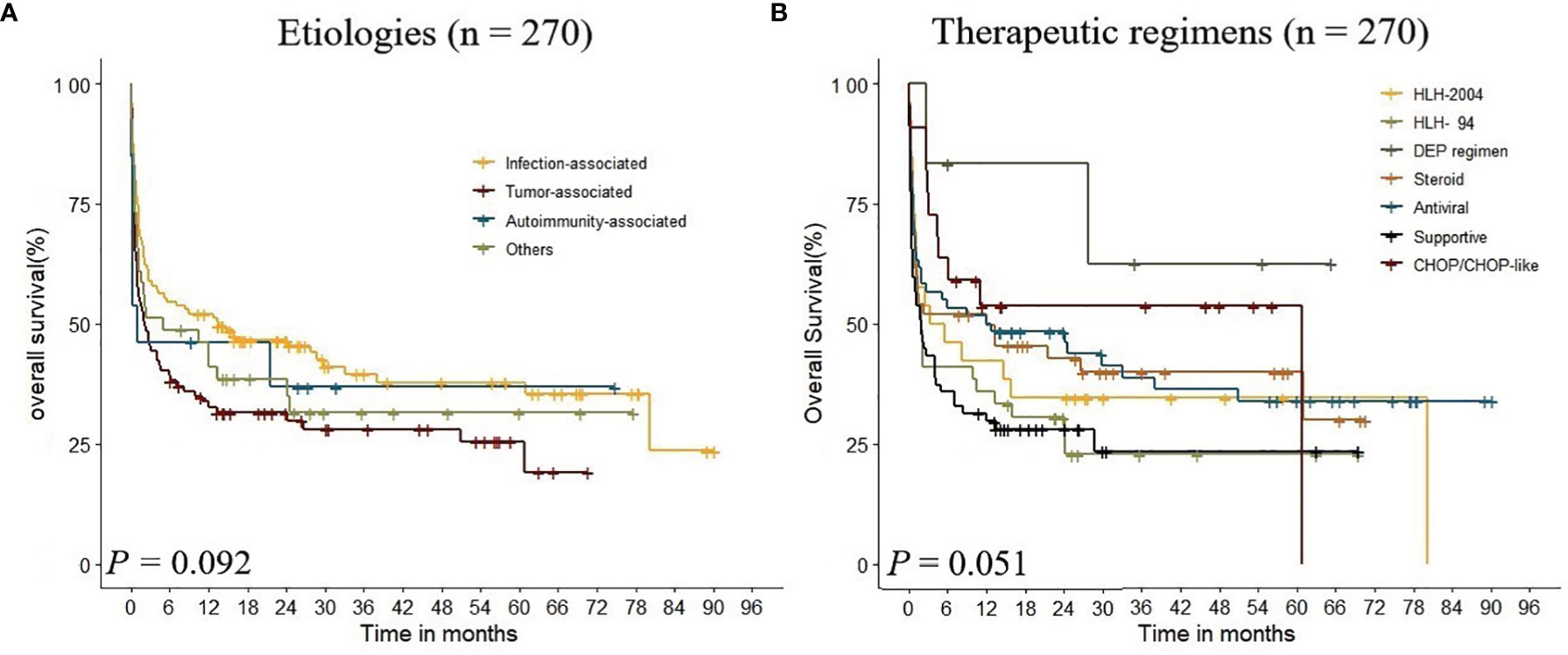
Figure 2 Kaplan–Meier analysis estimate of survival rate in adult HLH according to the underlying etiologies and therapeutic regimens.
EBV-DNA data were available in 163 evaluable patients, of whom 82 exceeded 1,000 copies/ml. KM analysis showed that high EBV-DNA level was associated with poor outcome, with only 39% of 5-year OS. Maxstat analysis was performed in 105 cases with explicit value, and the optimal cutoff value of EBV-DNA was 1,520 copies/ml. The influence of different etiologies on the prognosis of adult HLH was further explored. According to KM analysis results, we found that there were no significant differences between EBV infection and other groups in prognosis (p > 0.05). In addition, we explored the prognostic value of ferritin in adult HLH. Of the 270 patients, the ferritin levels of 230 were above 500 ng/ml, and there was no survival difference between them and those with ferritin levels below 500 (p = 0.99). Of the 173 patients with explicit values that could be evaluated, an optimal cutoff value could not be obtained.
In this study, patients received regimens of HLH-94 (n = 39), HLH-2004 (n = 26), DEP regimen (n = 6), steroid (n = 50), antiviral (n = 60), CHOP/CHOP-like (n = 22), and supportive treatment (n = 67). The 1-year OS of each treatment group was 42.3%, 35.9%, 83.3%, 52.0%, 51.7%, 31.3%, and 53.7%, respectively. KM analysis indicated that there was no significant difference in therapeutic regimens on the prognosis of adult HLH (p = 0.051, Figure 2B). The results showed that the 5-year survival rates were significantly different among DEP regimen, HLH-94 regimen, HLH-2004 regimen, and steroid regimen groups (p < 0.01).
In patients with evaluable immune globulin (IgA, IgG, and IgM) and lymphocyte subset data, the correlations between immune status and survival were analyzed. The linear association of all immune factors was measured by Pearson’s correlation. The results suggested that CD8 had a significant positive linear association with IgG (r = 0.334, p = 0.015) and CD3 was negatively correlated with IgM (r = -0.383, p = 0.004).
KM analysis indicated that there was no significant difference in immune globulin and lymphocyte subsets according to reference ranges (Figure 3). By Maxstat analysis, the optimal cutoff points for CD4+, CD8+ proportion, and CD4+/CD8+ ratio were 20.13, 19, and 3.24, respectively. Based on those cutoff points above, the survival of patients could be stratified.
In this study, continuous variables included in this study were age, lactate dehydrogenase (LDH), hemoglobin (Hb), platelet (PLT), fibrinogen (FIB), lymphocyte ratio (LYR), albumin (ALB), triglycerides (TG), creatinine (Cr), and alanine aminotransferase (ALT). The optimal cutoff values of TG, FIB, and ALT obtained by maximally selected rank statistics (Figure 4A) were 1.41, 1.2, and 40, respectively. Based on these cutoff values, we divided patients into higher group and lower group. X-Tile software was used to determine the optimal cutoff points for age, ALB, Hb, PLT, Cr, and LYR (Figure 4B). The best cutoff values for age were 50 and 65, for ALB 29.8 and 35.2, for Hb 68 and 104, for PLT 25 and 72, for Cr 53 and 66, and for LYR 21.1 and 45.8.
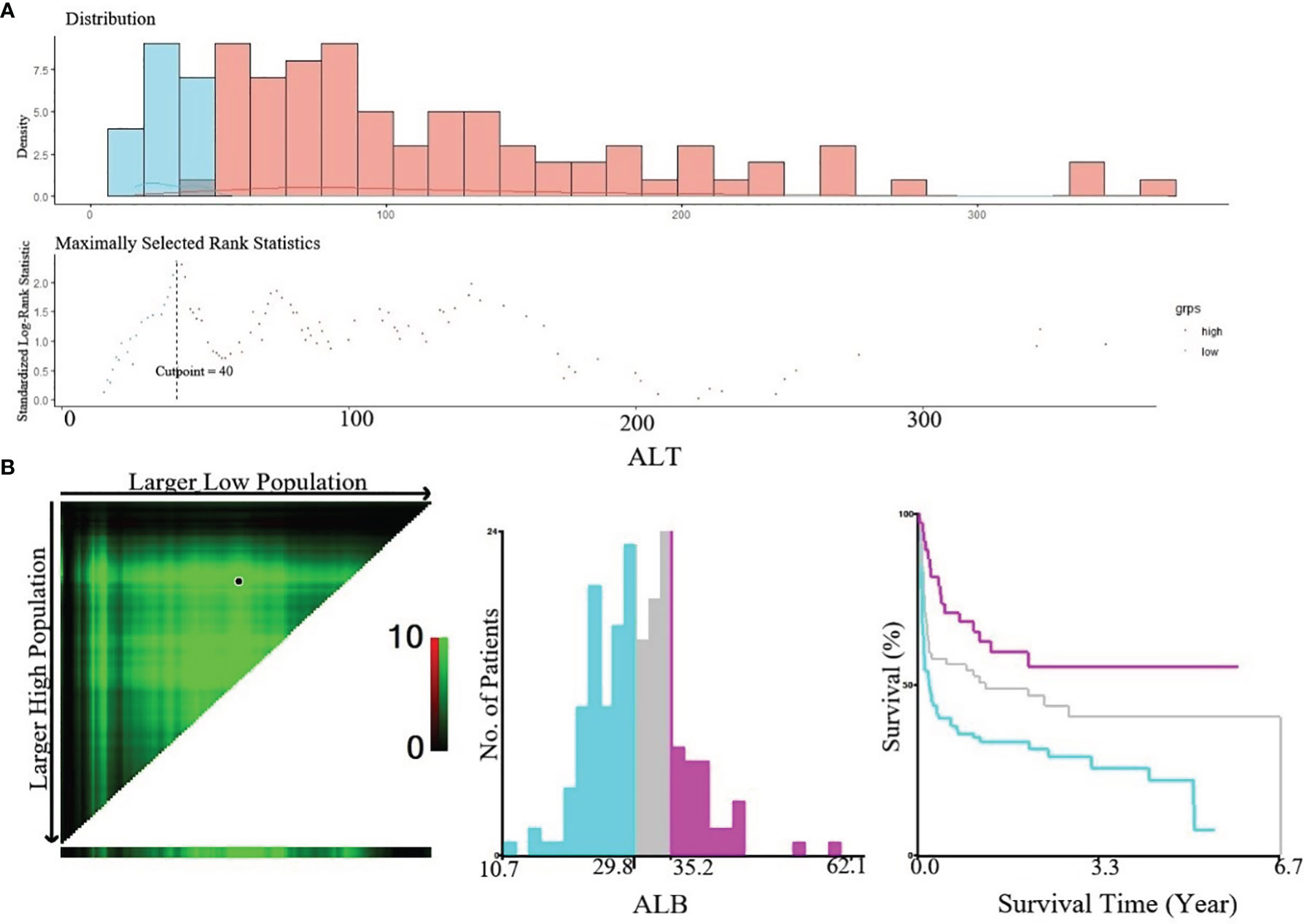
Figure 4 (A) Cut-off point of ALT defined by using maximally selected log-rank statistics. The estimated optimal cut-off point of ALT was 40 U/L; (B) X-Tile analysis of OS according to Alb. The black circles highlighted the optimal cut-off values which were presented in histograms.
The univariate analysis showed that age, Cr, ALB, PLT, LYR, Hb, FIB, ALT, etiologies, and gender significantly affected survival in the derivation cohort, whereas hepatosplenomegaly did not. Age, Cr, and ALB appeared to be stronger predictors (p < 0.001). The correlations between the clinical characteristics at diagnosis and OS are shown in Table 2.
In the multivariate Cox regression model, age was stratified into three groups (18–49, 50–65, and > 65 years); albumin in three groups (<29.8, 29.8–35.2, and >35.2 g/l); creatinine in three groups (<53, 53–66, and >66 μmol/l); lymphocyte ratio in two groups (≤45.8 and >45.8%); alanine aminotransferase in two groups (≤40 and >40), and platelet in two groups (≤72 and >72 × 109/l). After rounding up the hazard ratios of the significant variables, the current HHLWG-HPI used 6 factors with a maximum of 9 scoring points (Table 3). Additionally, we assessed the accuracy using C-index and we also calculated the error of the model fitting on survival data using Brier Score in the internal validation. On average, the derivation cohort generated a high C-index (0.796) and a low brier score (0.184). The calibration curve for the probability of 5-year OS showed a good correlation between the actual observed outcome and the prediction by HHLWG-HPI (Figure 5A). Using this index, four risk groups were formed: low risk (LR, ≤3 pts), low-intermediate risk (LIR, 4 pts), high-intermediate risk (HIR, 5–6 pts), and high risk (HR, ≥7 pts) (Figure 6A). There was clearly a difference in OS between each of these risk groups (global comparison p < 0.001; LR vs. LIR p = 0.012; LIR vs. HIR p = 0.024; HIR vs. HR p = 0.014). This model showed precise stratification of outcomes with 5-year OS of 68.5%, 35.2%, 21.3%, and 10.8%, respectively.
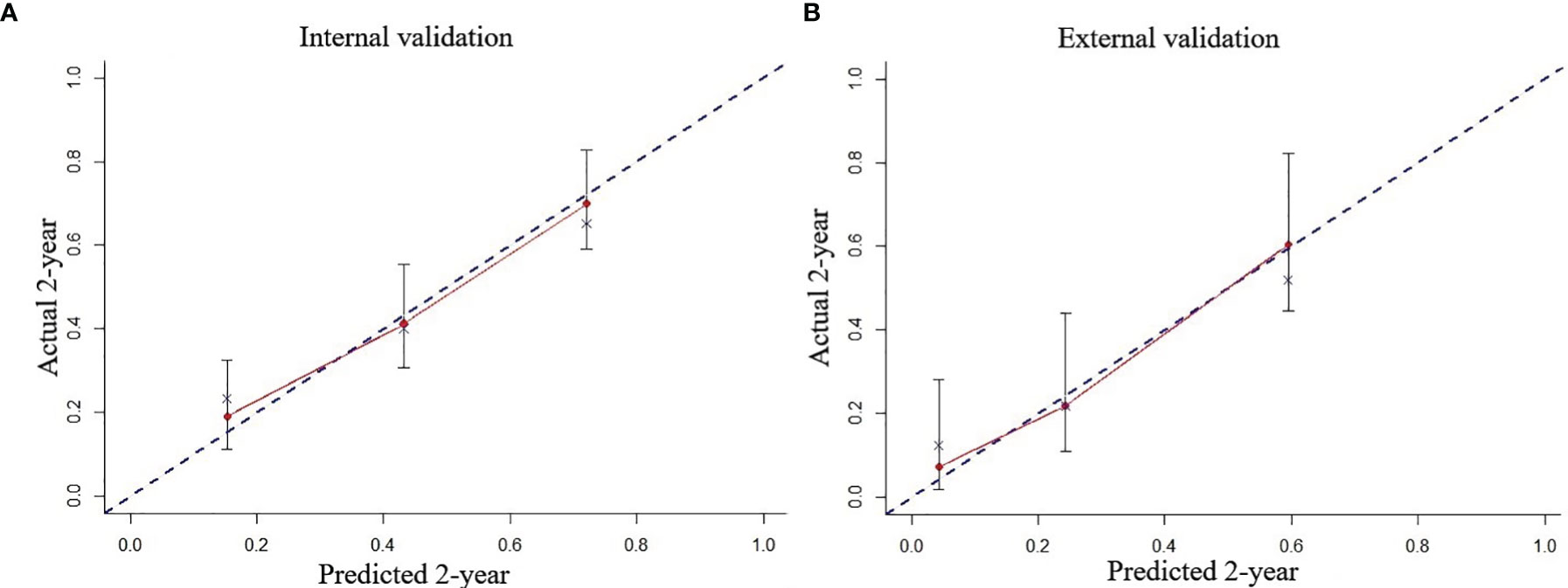
Figure 5 The predicted probability of 2-year OS by HHLWG-HPI was plotted on the x‐axis, and the actual 2-year OS was plotted on the y‐axis in internal (A) and external (B) validation.
We further validated the HHLWG-HPI externally by the calibration curve and by computing the C-index and Brier Score in an independent validation cohort of 86 patients. The C-index was 0.758, and the Brier Score was 0.176 (Figure 5B). Noticeably, 25.58% of patients were classified as high risk. KM analysis also showed that there were significant differences in OS between the four groups, confirming the reproducibility of the HHLWG-HPI (Figure 6B).
In this retrospective study, multiple regimens were adopted for adult HLH, and patients in the DEP regimen group had the best 5-year OS (62.5%). However, it was confusing that patients in the HLH-94 regimen group had poor survival (5-year OS 22.9%), even worse than that in steroid regimen group (5-y OS 30%), which was probably a consequence of improper individual treatment strategies. Thus, we used the HHLWG-HPI model to verify the whole cohort. Chi-square analysis results suggested that the proportion of HIR/HR patients in the HLH-94 regimen was significantly higher than that in the CHOP/CHOP-like regimen (χ2 = 6.608, p = 0.010). Similarly, the proportion of HIR/HR patients in the supportive treatment group was higher than that in the HLH-2004 regimen (χ2 = 5.955, p = 0.015) and CHOP/CHOP-like regimen (χ2 = 17.161, p < 0.001). Due to the lack of accurate risk stratification, the individual therapeutic regimen could not be reasonably selected, resulting in the confusing survival data in different groups.
Hemophagocytic lymphohistiocytosis is a rare, life-threatening disorder with excessive immune activation. The therapeutic regimens available for the treatment of adult HLH vary widely in intensity (24, 25). Due to the lack of an accurate prognostic stratification system, there are no individualized therapeutic criteria for adult HLH (26, 27). Therefore, based on feasible clinical variables, we first established the HHLWG-HPI model, providing a novel stratification system for adult HLH.
Several studies have shown that albumin, age, and alanine aminotransferase can be independent prognostic factors for HLH (13, 28). Based on the results of this cohort study, KM analysis showed that etiologies were not independent factors for the prognosis of adult HLH. However, further analysis between subgroups found that the survival rate of patients in the infection-associated group was significantly higher than that in the tumor-associated group. Of 270 cases, EBV-DNA data were available in 163 evaluable patients, and KM analysis showed that a high EBV-DNA level was associated with poor outcome. We also attempted to explore the effect of ferritin on the prognosis of adult HLH, but approximately 36% data were missing, and there was no correlation between ferritin level and the prognosis of adult HLH. Although therapeutic regimens were not independent factors for the prognosis, the 5-year survival rate of the patients in the DEP regimen was higher than that in the HLH-94 regimen, HLH-04 regimen, and steroid regimen in this study. In addition, we proved that the levels of immune globulin were not associated with survival. However, patients with a low ratio of CD4/CD8 were with better prognosis.
Multivariate analysis showed that age, albumin, creatinine, alanine transaminase, lymphocyte ratio, and platelet were independent prognostic factors for adult HLH, and these variables were grouped by respective optimal cutoff points. X-Tile program results suggested that platelet >72 × 109/l could predict a better outcome and older age at onset, and ALT ≥ 40 U/l and Cr>66 μmol/l were positively associated with poor survival, which were consistent with previous reports (26, 29). After the model iterations of multivariate analysis, we developed the HHLWG-HPI model with a maximum of score of 9 and 4 risk groups. The 5-year OS of the high-risk group in the derivation cohort was higher than that in the validation cohort (10.8% vs. 4.5%), and we found that the median age of patients in the validation population was higher, which may offer an explanation of a lower OS in the validation cohort. In addition, the derivation cohort generated a high C-index (0.703) and a low Brier Score (0.189), and the C-index and Brier Score were 0.721 and 0.169, respectively. It is worth noting that both cohorts are unselected and from real-world settings.
The HLH-94 regimen and the HLH-2004 regimen are still the current first-line treatments for HLH. Wang et al. showed that the DEP regimen was an optimal salvage therapy for adult HLH with an overall response of 76.2% (10), which is worth noting. In this real-world retrospective study, the DEP regimen showed the best clinical response, but HLH-94, HLH-2004 regimen, and supportive treatment groups showed poor clinical response. The differences in clinical response among treatment groups may be due to different risks of patients, so the HHLWG-IPI model was used to validate the whole cohort, and the results suggested that the proportion of HIR/HR patients in the HLH-94 regimen was significantly higher than that in the CHOP/CHOP-like regimen (Table 4). There were also a high proportion of HIR/HR patients in steroid regimen and antiviral regimen groups, but the 5-year OS of patients was even higher than that in the HLH-94 regimen group, which was worth further discussion in the following studies. In this study, all patients in the DEP regimen were classified into low-risk and low-intermediate-risk groups, indicating that the DEP regimen could also be used for the treatment of patients at low-risk and low-intermediate-risk groups. However, due to the limited sample size, further studies are needed.
In conclusion, based on multicenter data of HHLWG, we developed the HHLWG-HPI model, which will potentially provide criteria for accurate stratification and individual treatment strategy in adult HLH. However, in this retrospective study, we did not collect the data of genetic measurements, sCD25, and pro-inflammatory markers, which may reduce model sensitivity and specificity. Further prospective multicenter studies are urgently needed to validate the model.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Study approval was obtained from the independent Ethics Committees of each participating center in HHLWG. The patients/participants provided their written informed consent to participate in this study.
WS, SH, and KX: designed this study. ZS and CH: analysis and interpretation. ZS, YJ, QS, SZ, XC, LH, YW, QL, HZ, XL, LW, JJ, YM, WG, FW, CW, YS, JY, TZ, CS, XS, LX, DY, HS, JC, DL, ZL, and ZW: acquisition of data. JC, DL, ZL, and ZW provided the advices of this study. All authors contributed to the article and approved the submitted version.
This study was funded by the Natural Science Foundation of Jiangsu Province, Grant/Award Number: BK20171181; Jiangsu Key Research and Development Project of Social Development, Grant/Award Number: BE2019638; and Young Medical Talents of Jiangsu Science and Education Health Project, Grant/Award Number: QNRC2016791.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Thanks are given to the Huaihai Lymphoma Working Group (HHLWG) for its participation in this study.
1. Pachlopnik Schmid J, Cote M, Menager MM, Burgess A, Nehme N, Menasche G, et al. Inherited Defects in Lymphocyte Cytotoxic Activity. Immunol Rev (2010) 235:10–23. doi: 10.1111/j.0105-2896.2010.00890.x
2. Jing-Shi W, Yi-Ni W, Lin W, Zhao W. Splenectomy as a Treatment for Adults With Relapsed Hemophagocytic Lymphohistiocytosis of Unknown Cause. Ann Hematol (2015) 94:753–60. doi: 10.1007/s00277-014-2276-9
3. Maakaroun NR, Moanna A, Jacob JT, Albrecht H. Viral Infections Associated With Haemophagocytic Syndrome. Rev Med Virol (2010) 20:93–105. doi: 10.1002/rmv.638
4. Lai W, Wang Y, Wang J, Wu L, Jin Z, Wang Z. Epstein-Barr Virus-Associated Hemophagocytic Lymphohistiocytosis in Adults and Adolescents-A Life-Threatening Disease: Analysis of 133 Cases From a Single Center. Hematology (2018) 23:810–6. doi: 10.1080/10245332.2018.1491093
5. Riviere S, Galicier L, Coppo P, Marzac C, Aumont C, Lambotte O, et al. Reactive Hemophagocytic Syndrome in Adults: A Retrospective Analysis of 162 Patients. Am J Med (2014) 127:1118–25. doi: 10.1016/j.amjmed.2014.04.034
6. Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP. Prognostic Factors and Outcomes of Adults With Hemophagocytic Lymphohistiocytosis. Mayo Clin Proc (2014) 89:484–92. doi: 10.1016/j.mayocp.2013.12.012
7. Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, et al. Development and Validation of the HScore, A Score for the Diagnosis of Reactive Hemophagocytic Syndrome. Arthritis Rheumatol (2014) 66:2613–20. doi: 10.1002/art.38690
8. Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and Therapeutic Guidelines for Hemophagocytic Lymphohistiocytosis. Pediatr Blood Cancer (2007) 48:124–31. doi: 10.1002/pbc.21039
9. Henter J. HLH-94: A Treatment Protocol for Hemophagocytic Lymphohistiocytosis. Med Pediatr Oncol (1997) 28:342–7. doi: 10.1002/(SICI)1096-911X(199705)28:5<342::AID-MPO3>3.0.CO;2-H
10. Wang Y, Huang W, Hu L, Cen X, Li L, Wang J, et al. Multicenter Study of Combination DEP Regimen as a Salvage Therapy for Adult Refractory Hemophagocytic Lymphohistiocytosis. Blood (2015) 126:2186–92. doi: 10.1182/blood-2015-05-644914
11. An Q, Fang DH, Xuan CM, Xu SM, Jin MW, Ji Q. Lymphocyte Subsets in Children With Hemophagocytic Lymphohistiocytosis (HLH) and Its Clinical Significance. Eur Rev Med Pharmacol Sci (2018) 22:2000–4. doi: 10.26355/eurrev_201804_14728
12. Pan H, Huo Y, Sun L. Comparison Between Clinical Features and Prognosis of Malignancy- and Non-Malignancy-Associated Pediatric Hemophagocytic Lymphohistiocytosis. BMC Pediatr (2019) 19:468. doi: 10.1186/s12887-019-1702-5
13. Zhou J, Zhou J, Wu ZQ, Goyal H, Xu HG. A Novel Prognostic Model for Adult Patients With Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis (2020) 15:215. doi: 10.1186/s13023-020-01496-4
14. Zhou J, Zhou J, Shen DT, Goyal H, Wu ZQ, Xu HG. Development and Validation of the Prognostic Value of Ferritin in Adult Patients With Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis (2020) 15:71. doi: 10.1186/s13023-020-1336-6
15. Lin TF, Ferlic-Stark LL, Allen CE, Kozinetz CA, McClain KL. Rate of Decline of Ferritin in Patients With Hemophagocytic Lymphohistiocytosis as a Prognostic Variable for Mortality. Pediatr Blood Cancer (2011) 56:154–5. doi: 10.1002/pbc.22774
16. Schram AM, Campigotto F, Mullally A, Fogerty A, Massarotti E, Neuberg D, et al. Marked Hyperferritinemia Does Not Predict for HLH in the Adult Population. Blood (2015) 125:1548–52. doi: 10.1182/blood-2014-10-602607
17. Hayden A, Park S, Giustini D, Lee AY, Chen LY. Hemophagocytic Syndromes (HPSs) Including Hemophagocytic Lymphohistiocytosis (HLH) in Adults: A Systematic Scoping Review. Blood Rev (2016) 30:411–20. doi: 10.1016/j.blre.2016.05.001
18. Miao Y, Zhu HY, Qiao C, Xia Y, Kong Y, Zou YX, et al. Pathogenic Gene Mutations or Variants Identified by Targeted Gene Sequencing in Adults With Hemophagocytic Lymphohistiocytosis. Front Immunol (2019) 10:395. doi: 10.3389/fimmu.2019.00395
19. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: A New Bio-Informatics Tool for Biomarker Assessment and Outcome-Based Cut-Point Optimization. Clin Cancer Res (2004) 10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713
20. Kano K, Yamada T, Yamamoto K, Komori K, Watanabe H, Hara K, et al. Association Between Lymph Node Ratio and Survival in Patients With Pathological Stage II/III Gastric Cancer. Ann Surg Oncol (2020) 27:4235–47. doi: 10.1245/s10434-020-08616-1
21. Harrell FE Jr, Lee KL, Mark DB. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat Med (1996) 15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
22. Chaudhary K, Poirion OB, Lu L, Garmire LX. Deep Learning-Based Multi-Omics Integration Robustly Predicts Survival in Liver Cancer. Clin Cancer Res (2018) 24:1248–59. doi: 10.1158/1078-0432.CCR-17-0853
23. Zhang X, Li Y, Akinyemiju T, Ojesina AI, Buckhaults P, Liu N, et al. Pathway-Structured Predictive Model for Cancer Survival Prediction: A Two-Stage Approach. Genetics (2017) 205:89–100. doi: 10.1534/genetics.116.189191
24. Ambruso DR, Hays T, Zwartjes WJ, Tubergen DG, Favara BE. Successful Treatment of Lymphohistiocytic Reticulosis With Phagocytosis With Epipodophyllotoxin VP 16-213. Cancer (1980) 45:2516–20. doi: 10.1002/1097-0142(19800515)45:10<2516::aid-cncr2820451008>3.0.co;2-v
25. Henter JI, Elinder G, Finkel Y, Soder O. Successful Induction With Chemotherapy Including Teniposide in Familial Erythrophagocytic Lymphohistiocytosis. Lancet (1986) 2:1402. doi: 10.1016/s0140-6736(86)92047-7
26. Arca M, Fardet L, Galicier L, Riviere S, Marzac C, Aumont C, et al. Prognostic Factors of Early Death in a Cohort of 162 Adult Haemophagocytic Syndrome: Impact of Triggering Disease and Early Treatment With Etoposide. Br J Haematol (2015) 168:63–8. doi: 10.1111/bjh.13102
27. Zhou M, Li L, Zhang Q, Ma S, Sun J, Zhu L, et al. Clinical Features and Outcomes in Secondary Adult Hemophagocytic Lymphohistiocytosis. QJM (2018) 111:23–31. doi: 10.1093/qjmed/hcx183
28. Huang J, Yin G, Duan L, Tian T, Xu J, Wang J, et al. Prognostic Value of Blood-Based Inflammatory Biomarkers in Secondary Hemophagocytic Lymphohistiocytosis. J Clin Immunol (2020) 40:718–28. doi: 10.1007/s10875-020-00801-x
Keywords: hemophagocytic lymphohistiocytosis, adult, multicenter, prognostic model, stratification
Citation: Shen Z, Jin Y, Sun Q, Zhang S, Chen X, Hu L, He C, Wang Y, Liu Q, Zhang H, Liu X, Wang L, Jiao J, Miao Y, Gu W, Wang F, Wang C, Shi Y, Ye J, Zhu T, Sun C, Song X, Xu L, Yan D, Sun H, Cao J, Li D, Li Z, Wang Z, Huang S, Xu K and Sang W (2022) A Novel Prognostic Index Model for Adult Hemophagocytic Lymphohistiocytosis: A Multicenter Retrospective Analysis in China. Front. Immunol. 13:829878. doi: 10.3389/fimmu.2022.829878
Received: 06 December 2021; Accepted: 28 January 2022;
Published: 18 February 2022.
Edited by:
Katy Rezvani, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Xiao-Dong Mo, Peking University People’s Hospital, ChinaCopyright © 2022 Shen, Jin, Sun, Zhang, Chen, Hu, He, Wang, Liu, Zhang, Liu, Wang, Jiao, Miao, Gu, Wang, Wang, Shi, Ye, Zhu, Sun, Song, Xu, Yan, Sun, Cao, Li, Li, Wang, Huang, Xu and Sang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Sang, eHlmeWxibDUxNUB4emhtdS5lZHUuY24=; Kailin Xu, bGlobWRAMTYzLmNvbQ==; Shuiping Huang, aHNwQHh6aG11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.