
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 28 February 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.822996
This article is part of the Research Topic Diagnosis and Treatment of Autoimmune Liver Diseases: Current Issues and Future Perspectives View all 7 articles
 Angela Ceribelli1,2
Angela Ceribelli1,2 Natasa Isailovic1
Natasa Isailovic1 Carolina Gorlino1
Carolina Gorlino1 Roberto Assandri3
Roberto Assandri3 Matteo Vecellio1
Matteo Vecellio1 Maria De Santis1,2
Maria De Santis1,2 Minoru Satoh4
Minoru Satoh4 Carlo Selmi1,2*
Carlo Selmi1,2*Introduction: Antimitochondrial antibodies (AMAs) are the hallmark of primary biliary cholangitis (PBC) but can be identified also in patients with connective tissue disease, namely, systemic sclerosis (SSc). Protein immunoprecipitation (IP) and IP-Western blot (WB) can be used to confirm AMA positivity directed at the pyruvate dehydrogenase complex (PDC) subunits E1α, E1β, E2/E3, and E3BP in patients showing a cytoplasmic reticular pattern at indirect immunofluorescence when performed in a screening setting before the onset of overt cholestasis in rheumatic patients.
Patients and Methods: We studied sera from 285 patients affected by connective tissue disease [SSc, n = 144; dermato/polymyositis (DM/PM), n = 56; and undifferentiated connective tissue disease (UCTD), n = 85] by indirect immunofluorescence (IIF), protein-IP, and IP-WB to identify specific PDC subunits recognized by AMA.
Results: Twenty percent (57/285) of sera from patients with connective tissue disease had a cytoplasmic reticular pattern at IIF, and in 77% (44/57, including 20 SSc, 12 PM/DM, and 12 UCTD) of these, we detected different titers of autoantibodies against the PDC subunits, specifically against PDC-E2. Among these sera, 4 (9%) tested positive for anti-E1α, 15 (34%) for anti-E1β, and 16 (36%) for anti-E3BP. Four of the 20 AMA-positive SSc cases (20%) had been already diagnosed with PBC, and all were positive for autoantibodies against the subunits PDC-E2, E3, and E3BP.
Conclusions: Using IIF and IP, we confirm that autoantibodies against the PDC components are detected in rheumatic patients with PBC or without liver dysfunction. In view of the strong predictive value of AMA for PBC, a strict follow-up of these latter patients is warranted for an early diagnosis of the disease.
Serum antimitochondrial antibodies (AMA) are directed against several components of the mitochondrial membrane and represent the hallmark of primary biliary cholangitis (PBC) (1). Serum AMA can be detected using routine laboratory techniques, namely, immunoblotting, ELISA, and indirect immunofluorescence (IIF) on rodent kidney/stomach/liver tissue sections (2), usually requested when a clinical suspect of PBC is present. However, AMA positivity has been reported by the International Consensus on Antinuclear Antibody (ANA) Patterns (ICAP; www.autoab.org) as a coarse granular filamentous staining extending throughout the cytoplasm by IIF on cellular substrates such as HEp-2 cells (AC-21), usually performed as a screening test for rheumatic diseases. Thus, AMA presence may be identified by IIF before the clinical suspect of PBC, and this is fundamental for the early diagnosis of PBC and for the correct therapeutic approach before the onset of PBC severe complications such as cirrhosis and liver failure (2, 3). In particular, early AMA identification has been proposed to significantly reduce the need for medical therapies, the severity of PBC clinical features, the need for liver transplantation, and the risk of life-threatening complications (4). Routine commercial tests used for AMA identification, including ELISA and immunoblot, and AMA analysis on triple tissue slides are largely used worldwide for PBC diagnosis but usually when a clinical suspect of PBC is already present (5) while being not routinely used as screening methods for autoantibody (autoAb) analysis as for IIF on HEp-2 slides and immunoprecipitation (IP).
Most PBC-specific AMAs bind to a trypsin-sensitive antigen of the inner mitochondrial membrane previously named M2 (6), mainly targeting the E2 component that forms the core structure of the pyruvate dehydrogenase complex (PDC) in the majority of patients with PBC, but the E1 and E3 subunits are also recognized (7), along with E3BP (initially known as “protein X”) (8). We have previously described the protein-IP pattern of AMA in 15% of our cohort of patients with systemic sclerosis (SSc) (9), showing that AMAs have a strong positive cytoplasmic reticular staining using IIF on HEp-2 cells and not standard IIF on tissue slides, as described also in a recent article by Calise et al. (10).
We report herein the prevalence of serum AMAs in a cohort of patients with different connective tissue diseases, moving from the cytoplasmic reticular pattern detected by IIF on HEp-2 cells (AC-21) and then detecting by IP-Western blot (WB) the different patterns of recognition of AMA of the PDC subunits.
We collected serum samples and clinical information from 285 patients consecutively followed from 2013 to 2019 in our Outpatient clinic for SSc (n = 144) (11), dermato/polymyositis (DM/PM) (n = 56) (12), and undifferentiated connective tissue disease (UCTD; n = 85) (13) according to the most recent classification criteria. None of these patients had been included in our previous study (9). Internationally accepted criteria were used to confirm the diagnosis of PBC (14, 15). Patients who had internal organ involvement consistent with SSc but did not fulfill the American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) criteria were defined as SSc sine scleroderma (16) and very early diagnosis of SSc (VEDOSS) as defined by Minier et al. (17). Pulmonary and cardiac diseases related to the systemic autoimmune disease were defined in our cohort based on established parameters (18, 19). Negative controls are healthy subjects without a history suggestive of systemic autoimmune rheumatic disease and seronegative at our laboratory analysis. As for controls used in the described experiments, in detail, we used (i) cytoplasmic-reticular pattern by IIF (AC-21) and the speckled cytoplasmic pattern by IIF (AC-20) both provided by ICAP as positive controls; (ii) 10 negative controls for each IIF, IP, and IP-WB analysis, as described in the Materials and Methods section; (iii) the positive and negative controls for AMA tissue analysis were included in the used kit (Astra Formedic, Milan, Italy).
Liver laboratory tests, i.e., aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyltransferase (gammaGT), alkaline phosphatase (ALP), and bilirubin (total and direct), were obtained within 3 months from the blood sampling for autoAbs and, when available, liver histology was also recorded.
The present study was approved by the institutional review board of the Humanitas Research Hospital, and a signed informed consent was obtained from all subjects in accordance with the Declaration of Helsinki and its subsequent modifications.
Sera were obtained from whole blood through centrifugation at 2,000g for 15 min and then stored at −20°C until use. Antinuclear and cytoplasmic antibodies were tested by IIF on HEp-2 ANA slides (INOVA Diagnostics, San Diego, CA, USA) using serial dilution of human sera (1:80; 1:160; 1:320; 1:640; 1:1,280) of patients and healthy patients’ sera as control for autofluorescence followed by AlexaFluor488 AffiniPure F(ab′)2 fragment goat anti-human IgG, Fcγ fragment specific (Jackson ImmunoResearch Europe Ltd., Suffolk, UK) as previously described (20). Images were acquired using the Olympus BX53 Upright fluorescence microscope.
After HEp-2 IIF analysis, we performed the IIF analysis on tissue slides for all the patients who had variable expression of autoAbs directed against the PDC, following the manufacturer’s instructions (Astra Formedic, Milan, Italy). The kit included also positive and negative controls, and protocol procedures are the same as described above for IIF on HEp-2 slides. IIF patterns were reported according to the ICAP nomenclature (https://www.anapatterns.org; Supplementary Table 1), and we used their reference sera (including AMA) as positive control for autoAb analysis (10).
Sera showing a cytoplasmic-reticular pattern by IIF (AC-21) and the control sample that showed speckled cytoplasmic pattern by IIF (AC-20) were tested by IP-WB for AMA component analysis as previously described (9). In detail, 50 μl of candidate sera were cross-linked to the protein-A Sepharose beads using dimethyl pimelimidate (DMP) (Merck KGaA, Burlington, USA) and then immunoprecipitated with cell extract from 5 × 106 K562 cells/sample. Proteins were then fractionated by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose filter, probed with 1:500 of mouse monoclonal anti-human PDH E1α antibody (Novus Biologicals, Littleton, CO, USA) for a 41-kDa protein identification, followed by horseradish peroxidase (HRP) goat anti-mouse IgG (1:10,000 dilution; Thermo Fisher, Waltham, MA, USA). The same procedure was used to identify the other bands of the complex; in detail, we used mouse anti-human PDH E1β (1:500 dilution; Novus Biological, Littleton, CO, USA) for the 34-kDa protein; mouse anti-human PDH protein X/E3BP (1:1,000 dilution; Novus Biological, Littleton, CO, USA) for the 54-kDa proteins; mouse anti-human PDH E2/E3 proteins of 58 kDa and 74 kDa (1:10,000 dilution; Abcam, Cambridge, UK) followed by goat anti-mouse IgG (Thermo Fisher, Waltham, MA, USA). Signal development was performed by Immobilon Western Chemiluminescent HRP substrate (Millipore, Darmstadt, Germany) and acquired using ChemiDoc (Bio-Rad, CA, USA).
Serum autoAbs were screened by protein IP using 35S-methionine-labeled K562 cell extract followed by SDS-PAGE and autoradiography and by RNA-IP using unlabeled K562 cell extract followed by urea-PAGE and silver staining, as described previously (20, 21). In the presence of possible autoAb positivity in the controls’ cohort, we used commercial ELISA to validate results from protein or RNA IP to confirm positivity for anti-HMGCoA and -RNA Polymerase III antibodies.
Continuous variables included in the results were evaluated using Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). However, statistical comparisons were not included due to the small number of cases in each subcategory.
In our cohort, 20% (57/285) of sera had a cytoplasmic reticular pattern at IIF as described both on tissue slides and on HEp-2 cells (Figure 1) that we define possibly related to AMA positivity as specified by the ICAP website (AC-21 cytoplasmic pattern, www.anapatterns.org). When analyzing potential differences in IIF patterns between the different IP-WB antibody profiles against the AMA antigens, described in our previous report (9), 44/57 (77%) of these IIF samples showed a variable recognition of the PDC antigens (Figure 2), in particular, all sera reacted against the PDC-E2 subunit while a lower variable percentage was directed against the other subunits: 16/44 (36%) against E3BP, 15/44 (34%) against E1β, 8/44 (18%) against E3, and only 4/44 (9%) against the E1α subunit. Moving to the possible clinical significance of these results, in particular, for the known association of AMA and PBC in rheumatic patients affected by SSc, we confirmed the elevated number of SSc patients with these autoAbs, 20/44 (45%) SSc (2 diffuse, 14 limited, and 4 sine scleroderma) patients, but we also identified PDC-positive patients with additional diagnosis, in particular, 12/44 (27%) PM/DM (4 DM, 8 PM) and 12/44 (27%) UCTD patients, whose main features are shown in Table 1.
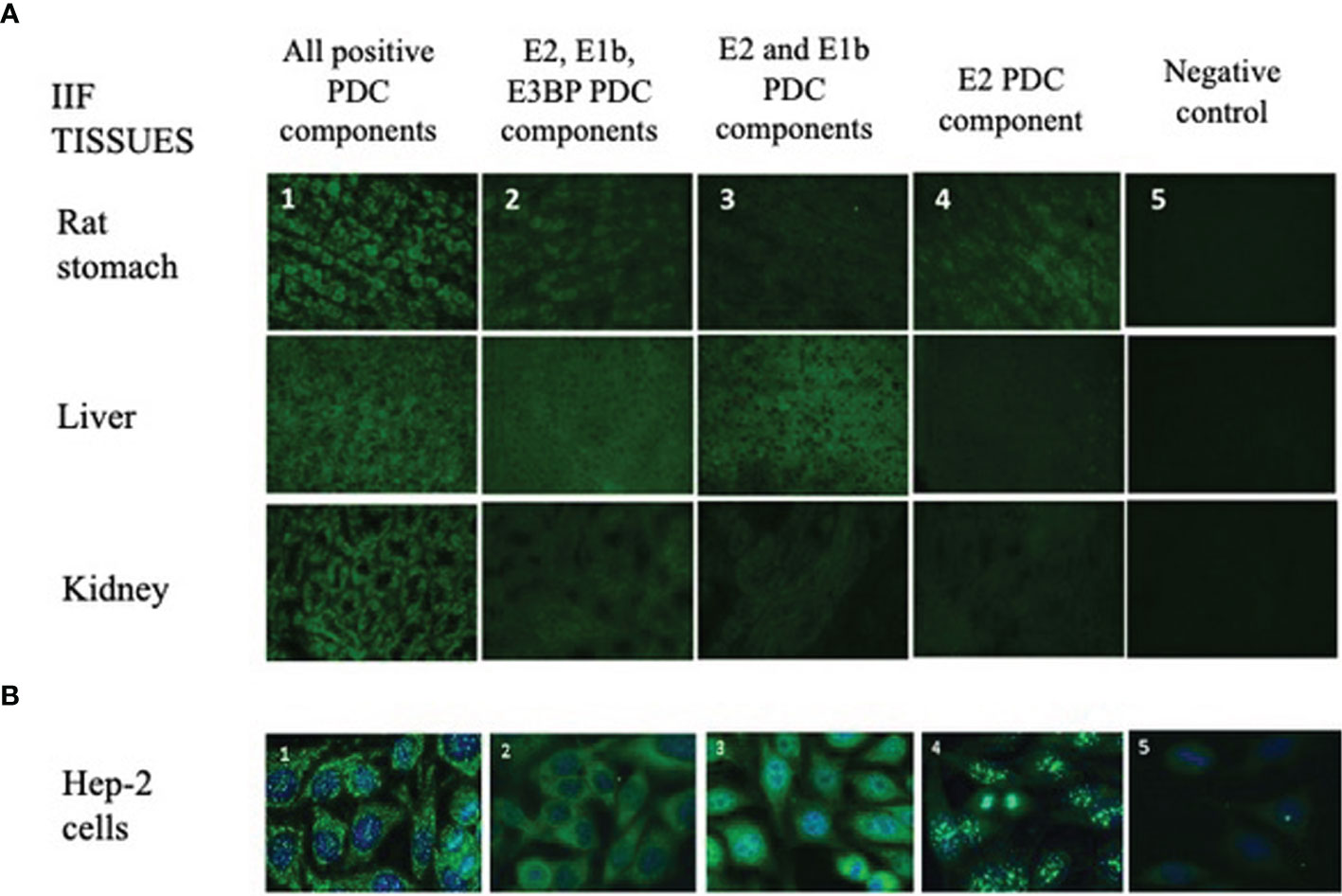
Figure 1 Different IIF patterns of AMA-positive patients on tissue sections and HEp-2 cell slides. (A) The IIF analysis on tissues (first row: rat stomach; second row: liver; third row: kidney) is used in routine laboratories as a screening technique of AMA-positive samples, and it shows different IIF intensities concordant with the different expressions of the PDC autoantigens (1, 2, 3, 4, 5; ×40 objective). 1: IIF pattern of a patient positive for all PDC autoantibodies (E1a, E1b, E2, E3, E3bp). 2: IIF pattern of a patient with autoantibodies to only E2, E1b, and E3BP components. 3: IIF pattern of a patient with autoantibodies to only E2 and E1b components. 4: IIF of a patient positive only for the E2 component. 5: Negative control. (B) Different IIF patterns of AMA-positive sera on HEp-2 cell slides. This method shows different cytoplasmic staining patterns with variable recognition of the PDC antigens (1, 2, 3, 4, 5) using the ×40 objective. These 5 panels correspond to the same 5 panels and patients’ samples shown in panel (A). AMA, antimitochondrial antibodies; IIF, indirect immunofluorescence; PDC, pyruvate dehydrogenase complex.
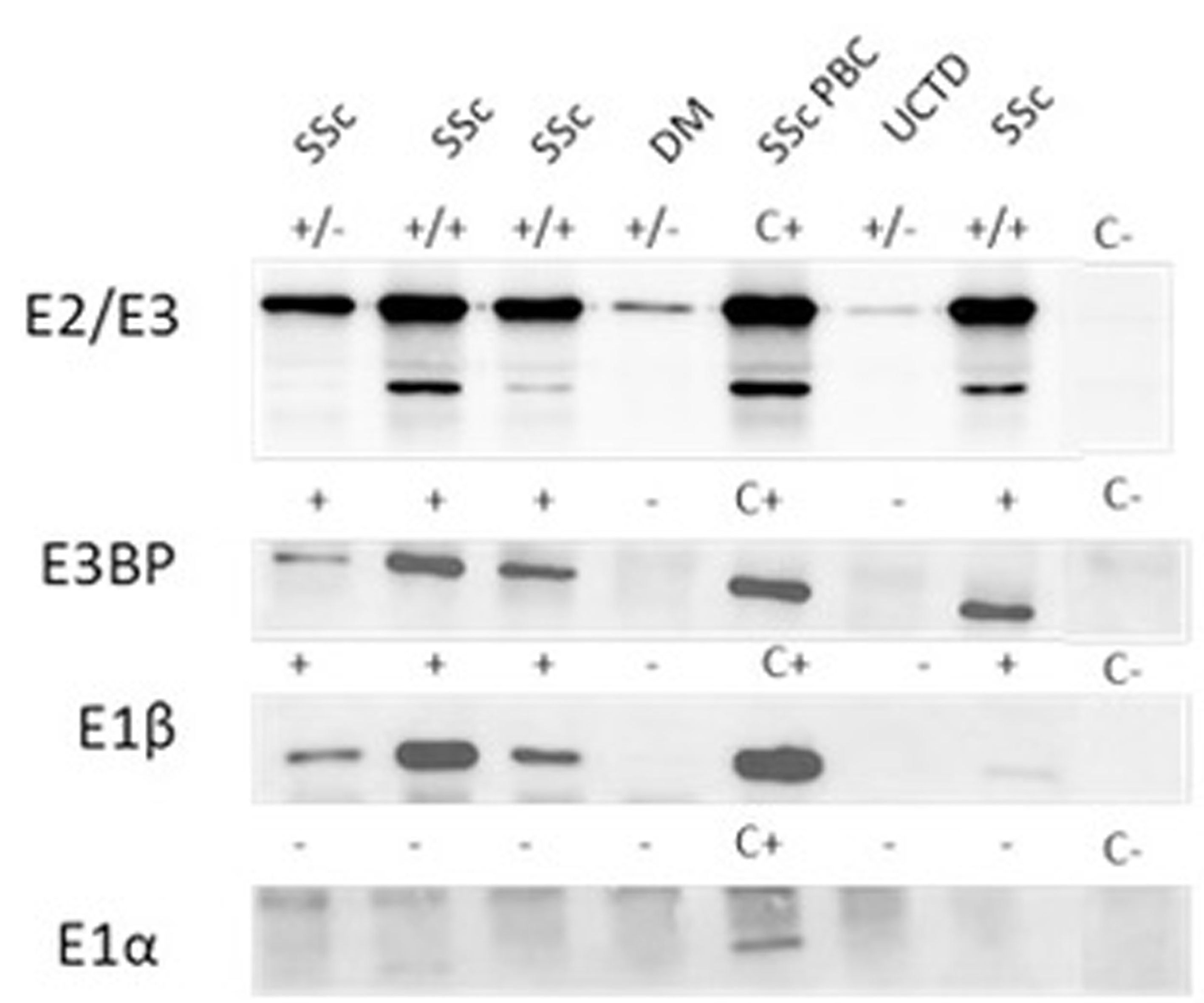
Figure 2 Autoantibody reactivity to PDC antigens by IP-WB. Different reactivities directed against the PDC subunits (E2/E3, E3BP, E1β, E1α) in AMA-positive representative sera from patients with SSc with and without PBC, one patient with DM and one with UCTD. C+, positive control; C-, negative control; +/- refers to the positivity of only one of the two components of the E2/E3 complex; +/+ both components of the E2/E3 complex are positive; - negative IP-WB band. AMA, antimitochondrial antibodies; DM, dermatomyositis; IP-WB, immunoprecipitation-Western Blot; PBC, primary biliary cholangitis; PDC, pyruvate dehydrogenase complex; SSc, scleroderma, systemic sclerosis; UCTD, undifferentiated connective tissue disease.
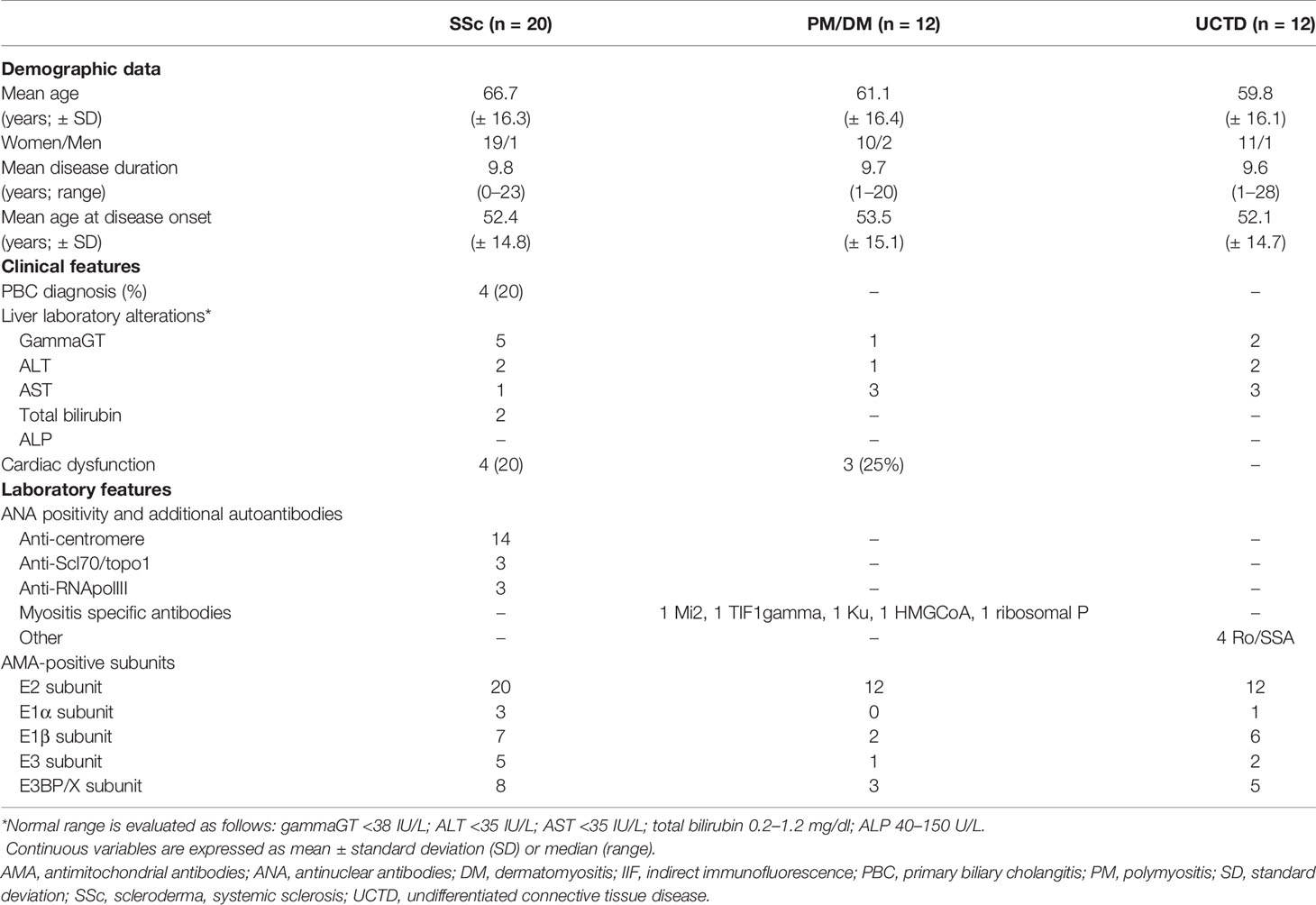
Table 1 Main feature data of the 44 rheumatic patients with cytoplasmic reticular pattern by HEp-2 IIF analysis, then tested for AMA components.
In the SSc cohort with cytoplasmic reticular AMA pattern at IIF (AC-21), 20% (4/20) had a previous diagnosis of PBC undergoing UDCA treatment, while no UCTD or PM/DM case had a confirmed diagnosis of PBC. When performing IIF on tissue slides, we confirmed that all the 4 patients with AMA had the typical IIF pattern on tissue slides. In the group of 44 patients with positive autoAbs against the 5 PDC subunits, 8 (18%) had elevated GGT, 5 (11%) had elevated ALT, and 7 (16%) had elevated AST, while 2 (5%) had increased total bilirubin, and only one patient had increased ALP (Table 1) but not enough criteria to fulfill a diagnosis of PBC except for 4 SSc cases who had a defined PBC diagnosis and were in hepatologic follow-up and treatment. In the PM/DM cohort, AMA positivity defined by cytoplasmic reticular IIF (AC-21) and variable expression of PBC components by IP-WB (Figures 2, 3) were associated with cardiac dysfunction in 1 case each of aortic valve dysfunction, pericardial effusion, and arrhythmia, as previously reported (22).
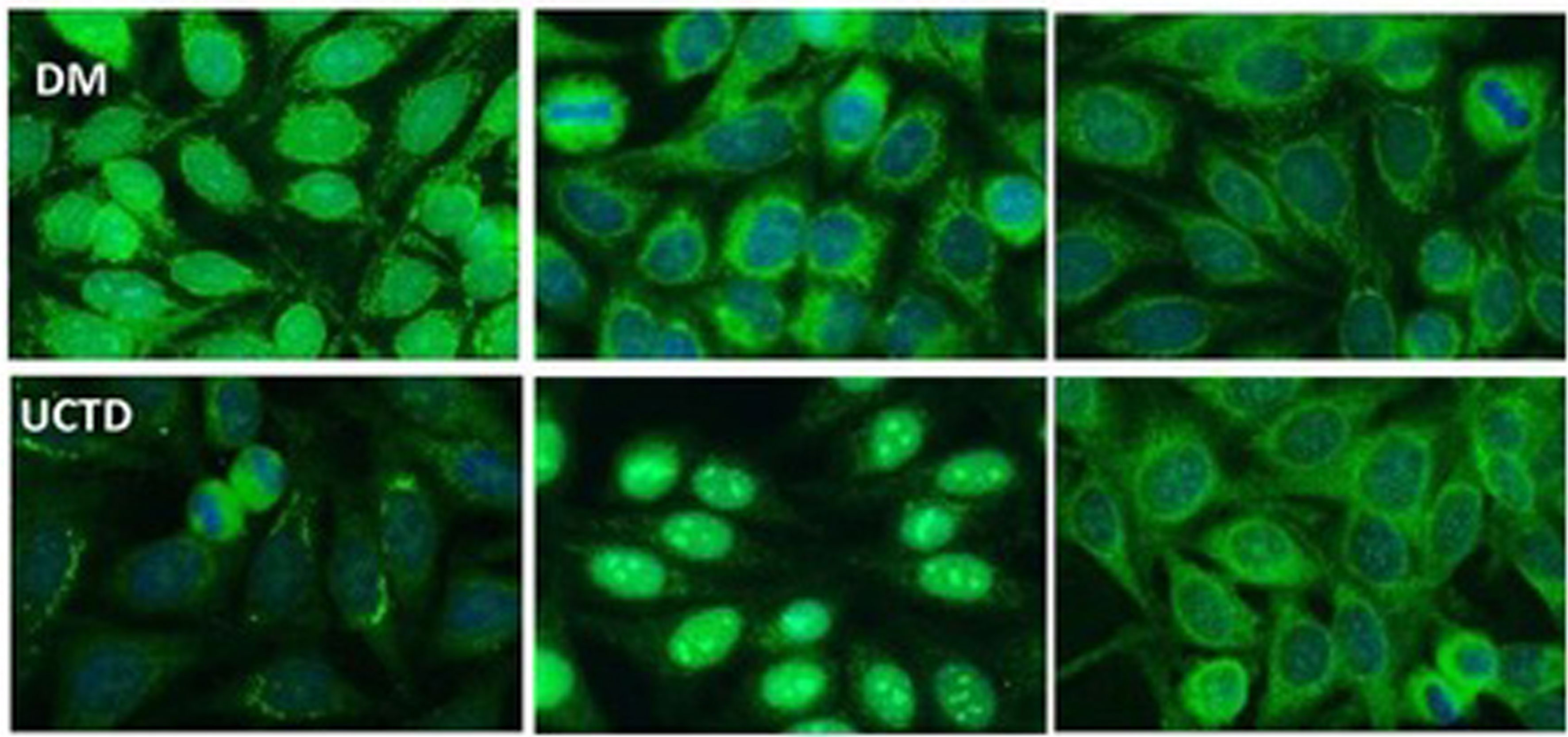
Figure 3 Representative cytoplasmic reticular patterns observed by IIF HEp-2 in AMA-positive patients. In the DM row, we show IIF on HEp2 cells of a DM patient with cytoplasmic staining pattern limited around the nuclei, not spread in the whole cytoplasm as in the case of cytoplasmic reticular AMA-like typical pattern. In the UCTD row, the IIF HEp-2 pattern shows a differential distribution of reticular areas. AMA, antimitochondrial antibodies; IIF, indirect immunofluorescence; DM, dermatomyositis; UCTD, undifferentiated connective tissue disease.
Further IIF ANA patterns described in AMA-positive subjects are listed in the Supplementary Table 1, and they include anti-centromere (ACA) in 14/44 (32%; AC-3), fine nuclear speckled pattern in 8/44 (18%; AC-4), and other patterns (i.e., nuclear large/coarse speckles, nuclear dots, punctate nuclear envelope, clumpy nucleolar, nucleolar homogeneous, polar/Golgi like, and mitotic NuMa-like) in <10% of cases (see Supplementary Table 1 for ICAP nomenclature). Only two AMA-positive sera (2/44, 5%) were negative for ANA at titers ≥1:160.
Additional autoAbs reported in the 20 SSc patients with variable expression of PDC antigens are anti-Scl70/topoisomerase I antibodies (3/20, 15%), RNApolIII (3/20, 15%), and 1 anti-Th/To positive (1/20, 5%). In the PM/DM and UCTD patients, we detected rare specificities such as anti-Mi2, anti-TIF1γ, anti-ribosomal P, anti-Ku, and anti-HMGCoA in less than 5% of cases each, as confirmed in our research laboratory by combining techniques such as protein IP, RNA IP, IP-WB, and specific ELISA identified according to previous protocols (9, 20, 21).
The first aim of our analysis was to identify AMA-positive samples before the onset of PBC in patients affected by SSc and other rheumatic diseases (1). For this purpose, we started from a screening technique represented by HEp-2 cell IIF to select samples with a cytoplasmic reticular pattern suggestive for AMA as described by ICAP (AC-21 pattern; www.autoab.org). We did not use IIF on tissue slides as screening technique but only to characterize the different expressions of AMA antigens directed against the PDC. Moreover, AMA confirmation was obtained by protein IP as previously described (9). Our results show that a cytoplasmic reticular pattern was present in 20% of rheumatic patients affected in particular by SSc, UCTD, and PM/DM. The identification of AMA by protein IP needs to be confirmed by IP-WB using specific antibodies directed against the different PDC components, as these proteins can have a molecular weight similar to other autoantigens and they need to be further analyzed by IP-WB for confirmation. In fact, PDC proteins have molecular weights around 55 kDa (E3BP and E2), similar to autoantigens such as Ro60, Ro52, or Jo-1 that are common in rheumatic diseases; thus, it is necessary to confirm AMA positivity by IP-WB.
In 2018, the ICAP Committee (www.autoab.org) described a reference serum for cytoplasmic reticular IIF pattern (AC-21 in the ICAP nomenclature) with an antigen association with PDC-E2/M2, BCOADC-E2, OGDC-E2, E1α subunit of PDC, and E3BP/protein X, and we used their standard as positive control for our analysis (10). They described a clinical significance of this pattern as commonly found in PBC, but also in SSc and PBC-SSc overlap syndrome (23), as confirmed in our cohort of rheumatic patients.
In real-life IIF analysis, AMA positivity can be an isolated cytoplasmic pattern with different titers, or it can be associated with additional IIF patterns such as the ANA patterns described in our cohort. Thus, it is necessary to use additional methods to confirm the AMA positivity suspected by IIF through the use of additional tests such as protein IP and IP-WB for each antigenic component recognized by AMA on the PDC. Surprisingly, all our tested patients were positive for autoAbs against E2, the main protein of the PDC on the inner mitochondria membrane (24) that is also the protein used for routine tests worldwide and extended disease-specific immunoassays for the liver profile (6). AMAs also target E1 (composed of the units α and β) and E3 subunits to a lesser extent (25–27) and, similarly, the E3BP component. From a clinical point of view, the majority of PBC patients react with the PDH-E2 component of the PDC targeted by AMA (6), and in our cohort, the second most common autoAb after anti-E2 was anti-E3BP (36%), while E1β follows as third component (34%), and the other components are detected at much lower frequency (8).
As for IIF on tissue sections, this test is routinely used for diagnosis of AMA in clinical laboratories—thanks to its technical simplicity and cost-effectiveness. However, IIF lacks both specificity and sensitivity, and AMA cannot be detected by IIF in up to 10% of PBC patients diagnosed with standard diagnostic criteria (28). These results were confirmed in our analysis, as we observed very low IIF intensity in tissues, especially when not all antigens of the PDC were recognized by the patient’s serum (Figure 1). Surprisingly, IIF on HEp-2 slides was more sensitive if taking in consideration the different forms of cytoplasmic reticular patterns to screen for the presence of anti-E2 antibodies, as shown in Figures 2, 3.
AMAs are the hallmark of PBC, and they can be identified in rheumatic patients mainly with diagnosis of SSc (9). In our study, we confirm that half of our tested patients were affected by SSc, and among them, only a small number of cases had liver abnormalities. This aspect is important if we consider that AMA can be identified much earlier than clinical and biochemical manifestations, and in particular, the reactivity with PDC-E2 and/or E3BP seems to be strongly predictive of the presence of PBC (29, 30). On the other hand, no PM/DM patient and UCTD patient have autoimmune liver disease based on routine laboratory testing, and recently, AMA positivity has been reported in association with severe cardiac involvement (such as arrhythmia, myocarditis, and cardiomyopathy) more than PBC in AMA-positive myositis patients (22), even though we observed this association in a limited number of cases in this cohort and in our previous analysis of a larger cohort of PM/DM patients (31).
In conclusion, as shown in Figure 4, we strongly encourage clinical laboratory technicians to report a cytoplasmic reticular staining (AC-21) when analyzing ANA samples of rheumatic patients by HEp-2 cell IIF and to specify any cytoplasmic positivity as described by the ICAP Committee, as this may be the first sign of the presence of AMA. Furthermore, recognition of different components of PDC in AMA-positive PBC patients may define possible clinical association with liver and cardiac disease, and AMA may be detected in rheumatic disease patients without PBC and liver function alterations even years before the clinical manifestations of the liver disease. Thus, AMA positivity needs to be reported to the clinicians even when a cytoplasmic reticular IIF pattern is present (AC-21) to maintain a strict follow-up of these patients for the early diagnosis of PBC or any other liver dysfunction. Then, if IP and IP-WB are available, they may be used for two reasons. First, these methods are used to screen for known and rare autoantigen specificities, from the common ones to the rare autoantigens that may characterize rare diseases such as SSc and PM/DM included in our study, without the need for additional testing. Second, in this screening phase, IP and IP-WB allow the identification of AMAs with the variable patterns described in the present article, thus allowing early diagnosis of PBC (before the clinical suspect based on liver function alterations) on the basis of the different AMA IP patterns. It is clear that when IP and IP-WB cannot be performed, this method must be replaced by conventional methods, but we suggest to ask for these additional tests as soon as possible when the AC-21 pattern is reported in the ANA IIF HEp-2 cell report.
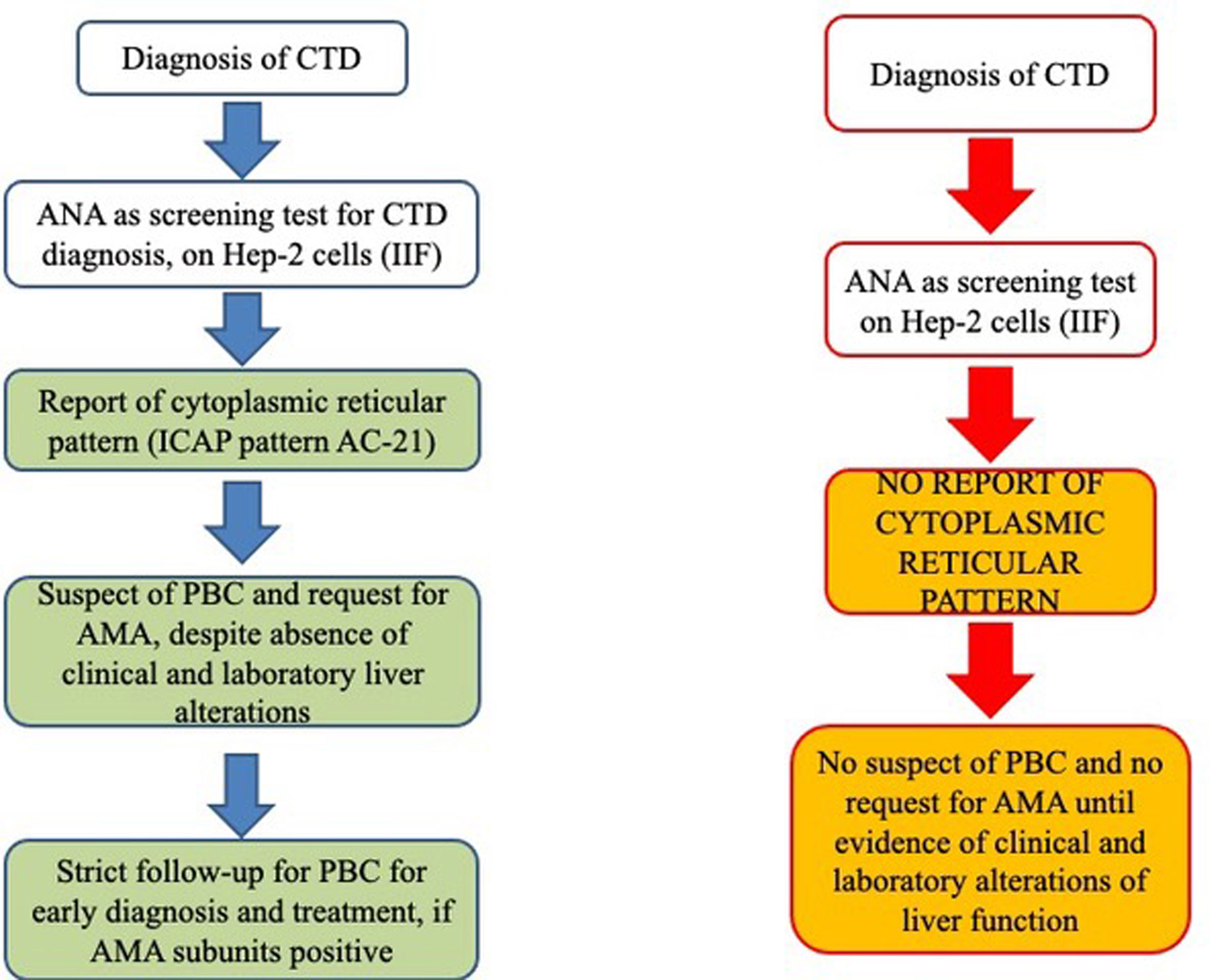
Figure 4 Schematic representation of our proposed working model (left flow chart) from the report of cytoplasmic patterns at immunofluorescence to additional tests for AMA identification, before the onset of clinical features suggestive of altered liver function (right flow chart). AMA, antimitochondrial antibodies; ANA, antinuclear antibodies; CTD, connective tissue disease; ICAP, International Consensus on Antinuclear Antibody Patterns; IIF, indirect immunofluorescence; PBC, primary biliary cholangitis.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
The studies involving human participants were reviewed and approved by Humanitas Research Center Ethics Committee, Rozzano, Milan, Italy. The patients/participants provided their written informed consent to participate in this study.
Authors equally contributed to the article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.822996/full#supplementary-material
Supplementary Table 1 | ANA patterns recognized beside the cytoplasmic reticular pattern in 44 rheumatic patients with different diagnosis as described. All the numbers are shown in the total lane are shown as % and absolute number in brackets. ACA, anti-centromere antibodies; ANA, anti-nuclear antibodies; DM, dermatomyositis; PM, polymyositis, SSc, scleroderma, systemic sclerosis; UCTD, undifferentiated connective tissue disease.
1. Muratori L, Granito A, Muratori P, Pappas G, Bianchi FB. Antimitochondrial Antibodies and Other Antibodies in Primary Biliary Cirrhosis: Diagnostic and Prognostic Value. Clin Liver Dis (2008) 12:261–76. doi: 10.1016/j.cld.2008.02.009
2. Vierling JM. Primary Biliary Cirrhosis and Autoimmune Cholangiopathy. Clin Liver Dis (2004) 8:177–94. doi: 10.1016/S1089-3261(03)00132-6
3. Muratori P, Granito A, Ferri S, Pappas G, Volta U, Menichella R, et al. Multiple Nuclear Dots and Rim-Like/Membranous IgG Isotypes in Primary Biliary Cirrhosis. Autoimmunity (2009) 42:224–7. doi: 10.1080/08916930802709133
4. Wesierska-Gadek J, Penner E, Battezzati PM, Selmi C, Zuin M, Hitchman E, et al. Correlation of Initial Autoantibody Profile and Clinical Outcome in Primary Biliary Cirrhosis. Hepatology (2006) 43:1135–44. doi: 10.1002/hep.21172
5. Benson GD, Kikuchi K, Miyakawa H, Tanaka A, Watnik MR, Gershwin ME. Serial Analysis of Antimitochondrial Antibody in Patients With Primary Biliary Cirrhosis. Clin Dev Immunol (2004) 11:129–33. doi: 10.1080/10446670410001722113
6. Han E, Jo SJ, Lee H, Choi AR, Lim J, Jung ES, et al. Clinical Relevance of Combined Anti-Mitochondrial M2 Detection Assays for Primary Biliary Cirrhosis. Clin Chim Acta (2017) 464:113–7. doi: 10.1016/j.cca.2016.11.021
7. Cancado EL, Harriz M. The Importance of Autoantibody Detection in Primary Biliary Cirrhosis. Front Immunol (2015) 6:309. doi: 10.3389/fimmu.2015.00309
8. Palmer JM, Jones DE, Quinn J, McHugh A, Yeaman SJ. Characterization of the Autoantibody Responses to Recombinant E3 Binding Protein (Protein X) of Pyruvate Dehydrogenase in Primary Biliary Cirrhosis. Hepatology (1999) 30:21–6. doi: 10.1002/hep.510300106
9. Ceribelli A, Isailovic N, De Santis M, Generali E, Satoh M, Selmi C. Detection of Anti-Mitochondrial Antibodies by Immunoprecipitation in Patients With Systemic Sclerosis. J Immunol Methods (2018) 452:1–5. doi: 10.1016/j.jim.2017.10.001
10. Calise SJ, Zheng B, Hasegawa T, Satoh M, Isailovic N, Ceribelli A, et al. Reference Standards for the Detection of Anti-Mitochondrial and Anti-Rods/Rings Autoantibodies. Clin Chem Lab Med (2018) 56:1789–98. doi: 10.1515/cclm-2017-1152
11. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Ann Rheum Dis (2013) 72:1747–55. doi: 10.1136/annrheumdis-2013-eular.238
12. Targoff IN, Miller FW, Medsger TA Jr., Oddis CV. Classification Criteria for the Idiopathic Inflammatory Myopathies. Curr Opin Rheumatol (1997) 9:527–35. doi: 10.1097/00002281-199711000-00008
13. Mosca M, Tani C, Vagnani S, Carli L, Bombardieri S. The Diagnosis and Classification of Undifferentiated Connective Tissue Diseases. J Autoimmun (2014) 48-49:50–2. doi: 10.1016/j.jaut.2014.01.019
14. Bowlus CL, Gershwin ME. The Diagnosis of Primary Biliary Cirrhosis. Autoimmun Rev (2014) 13:441–4. doi: 10.1016/j.autrev.2014.01.041
15. Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary Biliary Cirrhosis. Hepatology (2009) 50:291–308. doi: 10.1002/hep.22906
16. Poormoghim H, Lucas M, Fertig N, Medsger TA Jr. Systemic Sclerosis Sine Scleroderma: Demographic, Clinical, and Serologic Features and Survival in Forty-Eight Patients. Arthritis Rheum (2000) 43:444–51. doi: 10.1002/1529-0131(200002)43:2<444::AID-ANR27>3.0.CO;2-G
17. Minier T, Guiducci S, Bellando-Randone S, Bruni C, Lepri G, Czirjak L, et al. Preliminary Analysis of the Very Early Diagnosis of Systemic Sclerosis (VEDOSS) EUSTAR Multicentre Study: Evidence for Puffy Fingers as a Pivotal Sign for Suspicion of Systemic Sclerosis. Ann Rheum Dis (2014) 73:2087–93. doi: 10.1136/annrheumdis-2013-203716
18. De Santis M, Bosello SL, Peluso G, Pinnelli M, Alivernini S, Zizzo G, et al. Bronchoalveolar Lavage Fluid and Progression of Scleroderma Interstitial Lung Disease. Clin Respir J (2012) 6:9–17. doi: 10.1111/j.1752-699X.2010.00228.x
19. De Santis M, Bosello S, La Torre G, Capuano A, Tolusso B, Pagliari G, et al. Functional, Radiological and Biological Markers of Alveolitis and Infections of the Lower Respiratory Tract in Patients With Systemic Sclerosis. Respir Res (2005) 6:96. doi: 10.1186/1465-9921-6-96
20. Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Franceschini F, Quinzanini M, et al. Anti-MJ/NXP-2 Autoantibody Specificity in a Cohort of Adult Italian Patients With Polymyositis/Dermatomyositis. Arthritis Res Ther (2012) 14:R97. doi: 10.1186/ar3822
21. Ceribelli A, Cavazzana I, Franceschini F, Airo P, Tincani A, Cattaneo R, et al. Anti-Th/To Are Common Antinucleolar Autoantibodies in Italian Patients With Scleroderma. J Rheumatol (2010) 37:2071–5. doi: 10.3899/jrheum.100316
22. Albayda J, Khan A, Casciola-Rosen L, Corse AM, Paik JJ, Christopher-Stine L. Inflammatory Myopathy Associated With Anti-Mitochondrial Antibodies: A Distinct Phenotype With Cardiac Involvement. Semin Arthritis Rheum (2018) 47:552–6. doi: 10.1016/j.semarthrit.2017.06.004
23. Shuai Z, Wang J, Badamagunta M, Choi J, Yang G, Zhang W, et al. The Fingerprint of Antimitochondrial Antibodies and the Etiology of Primary Biliary Cholangitis. Hepatology (2017) 65:1670–82. doi: 10.1002/hep.29059
24. Jiang J, Baiesc FL, Hiromasa Y, Yu X, Hui WH, Dai X, et al. Atomic Structure of the E2 Inner Core of Human Pyruvate Dehydrogenase Complex. Biochemistry (2018) 57:2325–34. doi: 10.1021/acs.biochem.8b00357
25. Leung PS, Coppel RL, Ansari A, Munoz S, Gershwin ME. Antimitochondrial Antibodies in Primary Biliary Cirrhosis. Semin Liver Dis (1997) 17:61–9. doi: 10.1055/s-2007-1007183
26. Villalta D, Sorrentino MC, Girolami E, Tampoia M, Alessio MG, Brusca I, et al. Autoantibody Profiling of Patients With Primary Biliary Cirrhosis Using a Multiplexed Line-Blot Assay. Clin Chim Acta (2015) 438:135–8. doi: 10.1016/j.cca.2014.08.024
27. Bassendine MF, Jones DE, Yeaman SJ. Biochemistry and Autoimmune Response to the 2-Oxoacid Dehydrogenase Complexes in Primary Biliary Cirrhosis. Semin Liver Dis (1997) 17:49–60. doi: 10.1055/s-2007-1007182
28. Tanaka A, Miyakawa H, Luketic VA, Kaplan M, Storch WB, Gershwin ME. The Diagnostic Value of Anti-Mitochondrial Antibodies, Especially in Primary Biliary Cirrhosis. Cell Mol Biol (Noisy-le-grand) (2002) 48:295–9.
29. Roll J, Boyer JL, Barry D, Klatskin G. The Prognostic Importance of Clinical and Histologic Features in Asymptomatic and Symptomatic Primary Biliary Cirrhosis. N Engl J Med (1983) 308:1–7. doi: 10.1056/NEJM198301063080101
30. Mitchison HC, Bassendine MF, Hendrick A, Bennett MK, Bird G, Watson AJ, et al. Positive Antimitochondrial Antibody But Normal Alkaline Phosphatase: Is This Primary Biliary Cirrhosis? Hepatology (1986) 6:1279–84. doi: 10.1002/hep.1840060609
Keywords: antimitochondrial antibodies (AMAs), primary biliary cholangitis (PBC), protein immunoprecipitation, IP-Western blot, pyruvate dehydrogenase complex (PDC)
Citation: Ceribelli A, Isailovic N, Gorlino C, Assandri R, Vecellio M, De Santis M, Satoh M and Selmi C (2022) Antigen Reactivity and Clinical Significance of Autoantibodies Directed Against the Pyruvate Dehydrogenase Antigen Complex in Patients With Connective Tissue Disease. Front. Immunol. 13:822996. doi: 10.3389/fimmu.2022.822996
Received: 26 November 2021; Accepted: 04 February 2022;
Published: 28 February 2022.
Edited by:
Alessandro Granito, University of Bologna, ItalyReviewed by:
Xavier Bossuyt, KU Leuven, BelgiumCopyright © 2022 Ceribelli, Isailovic, Gorlino, Assandri, Vecellio, De Santis, Satoh and Selmi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo Selmi, carlo.selmi@hunimed.eu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.