
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 26 January 2022
Sec. Inflammation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.816149
 Hailin Liu1,2,3†
Hailin Liu1,2,3† Jialing Hu1,2,3†
Jialing Hu1,2,3† Qingcui Zheng1,2,3
Qingcui Zheng1,2,3 Xiaojin Feng1,2,3
Xiaojin Feng1,2,3 Fenfang Zhan1,2,3
Fenfang Zhan1,2,3 Xifeng Wang2,4
Xifeng Wang2,4 Guohai Xu1,2*
Guohai Xu1,2* Fuzhou Hua1,2*
Fuzhou Hua1,2*Mechanical damage is one of the predisposing factors of inflammation, and it runs through the entire inflammatory pathological process. Repeated or persistent damaging mechanical irritation leads to chronic inflammatory diseases. The mechanism of how mechanical forces induce inflammation is not fully understood. Piezo1 is a newly discovered mechanically sensitive ion channel. The Piezo1 channel opens in response to mechanical stimuli, transducing mechanical signals into an inflammatory cascade in the cell leading to tissue inflammation. A large amount of evidence shows that Piezo1 plays a vital role in the occurrence and progression of chronic inflammatory diseases. This mini-review briefly presents new evidence that Piezo1 responds to different mechanical stresses to trigger inflammation in various tissues. The discovery of Piezo1 provides new insights for the treatment of chronic inflammatory diseases related to mechanical stress. Inhibiting the transduction of damaging mechanical signals into inflammatory signals can inhibit inflammation and improve the outcome of inflammation at an early stage. The pharmacology of Piezo1 has shown bright prospects. The development of tissue-specific Piezo1 drugs for clinical use may be a new target for treating chronic inflammation.
The human body is permanently exposed to mechanical forces, either passively applied or generated inside cells (1, 2). Cells can sense whether the mechanical stress of the microenvironment changes and can adapt to altered mechanical demands. Most physiological processes are related to mechanical force, which is also one of the initiating factors of tissue damage and inflammation (3). Mechanical force-related inflammation is caused by mechanical force damage to tissues. In general, mechanical forces can be divided based on the tissue bed or the type of force, such as hemodynamic, stretch, and stiffness forces. Inflammation-induced by different mechanical forces has different pathological processes. Generally speaking, when destructive mechanical force is applied to cells, in addition to direct mechanical force damage, mechanical force induces cells to secrete proinflammatory factors and cause indirect damage. When inflammatory factors stimulates factors, local blood vessels experience transient constriction followed by vasodilation through a nerve reflex, increased vascular permeability, and leakage of intravascular fluid through the vessel wall to the outside of the vessel. Inflammatory mediators can induce immune cells to adhere and migrate from the vascular endothelium to the injured site, resulting in inflammatory cell infiltration. Inflammatory cells remove necrotic tissue cells on the one hand and damage normal tissue on the other. Under the stimulation of inflammatory factors and tissue disintegration products, the corresponding growth factors are released, the number of proliferating cells increases locally in inflammation, and the inflammation has turned into a chronic process (4).
Normal inflammation is time-limited as an adaptive defense response of the body, occurring when the injury factor is present and disappearing when the injury is removed. If an acute mechanical injury is encountered, the production of inflammation in the organism is localized, transient, and belongs to acute inflammation (5). Harmful mechanical force signals persist and damage tissues leading to chronic inflammatory diseases. Such as mechanical pressure overload leading to osteoarthritis and lumbar degeneration (6, 7), disturbed haemodynamic shear forces damage vascular endothelial cells and ultimately lead to atherosclerosis (8), fat inflammation and insulin resistance induced by fat cell enlargement eventually progress to obesity and diabetes (9), overload of myocardium induces myocardial inflammation, which leads to long-term myocardial fibrosis and cardiac hypertrophy, etc. (10). The most critical features of chronic tissue inflammation are infiltration of inflammatory cells (such as lymphocytes, plasma cells, and monocytes) within the lesion, tissue destruction, and often more pronounced proliferation of fibrous connective tissue, blood vessels, and epithelial, glandular, and parenchymal cells to replace and repair the damaged tissue. Active inflammation, tissue destruction, and repair responses coincide in chronic inflammation. The ultimate harm of chronic inflammation is mainly the damage to essential organs such as the brain, heart, kidney, etc. It is easy to cause disability, affects labour ability and quality of life, and costly medical expenses, which increases society and families’ economic burden (11).
Various mechanical factors can induce chronic inflammation, but cells can perceive noxious stimuli through mechanical transduction (12). All cells exhibit mechanical sensitivity and convert mechanical signals into electrical or chemical signals (13). Piezo1 channel is a newly discovered mechanically sensitive ion channel (MSC), an effective mechanical sensor for cells (14). The latest data shows that the Piezo1 channel is a trimeric structure. In plan view, it looks like a propeller blade or three-pointed tooth, with an ion penetration hole in the middle and a cap on the top [CED (C-terminal extracellular domain)] (15). In a lateral view, the Piezo1 channels distributed on the cell membrane and the lipid membranes surrounding the channels interact to form a dome-like structure and use the trimeric structure described above to mediate mechanical force transmission by a lever principle mechanism. This unique feature is considered essential for force sensing (16). Piezo1 is embedded in the lipid bilayer, which is sensitive to local and global pressure in the bilayer. The Piezo1 channel is mainly located in the plasma membrane. It has been reported to be located in the endoplasmic reticulum and the cytoplasmic compartment, and nuclear envelope near the nucleus (17–21). Piezo1 enables cells to sense various “outside-in” and “inside-out” mechanical forces, including radial pressure, membrane stretching, compression, shear stress, matrix stiffness, ultrasound, matrix nano topology, and osmotic pressure (22). When mechanical stimulation hits the cell membrane, the stress will be distributed to all components, including the double layer, cytoskeleton (CSK) and extracellular matrix (ECM), which converge on the Piezo1 channel and induce the Piezo1 channel from a closed state turn to an open state. The Piezo1 channel allows Ca2+, K+ and Na+ plasma to flow when it is open (Figure 1). Piezo1 regulates various functions such as protein synthesis, secretion, migration, proliferation and apoptosis under mechanical pressure (23).
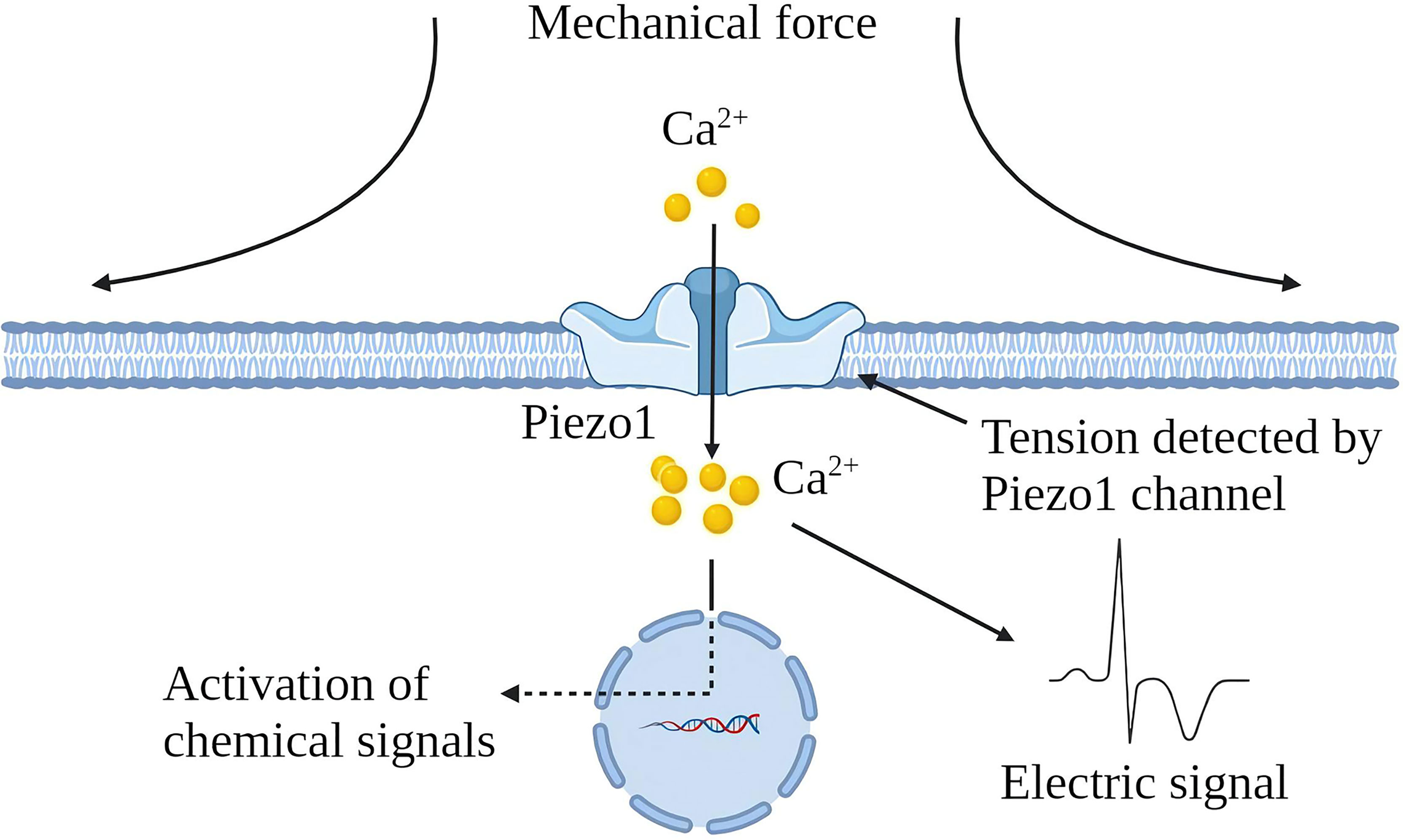
Figure 1 Schematic diagram of Piezo1 channel activation by mechanical force. The Piezo1 channel is a trimeric structure located in the plasma membrane. The mechanical force exerted on the cell membrane causes the opening of the Piezo1 channel, leading to the influx of extracellular Ca2+ and transducing mechanical signals into electrical and chemical signals in the cell.
After mechanical stress damages the tissue and induces tissue inflammation, the progress of inflammation is often accompanied by changes in the mechanical properties of the tissue (24). For example, if edema and necrosis occur after substantial organ injury, the local tissue will relax, and the pressure will decrease (25). If the exudate in the alveoli replaces the air, the density increases, and the pressure increases (26). If the proliferation is based on scar tissue rather than healthy epithelial tissue, there is an increase in local density and increased tissue rigidity (27). Immune cells “squeeze out” from blood vessels and migrate to inflammation sites also show a significant degree of mechanical plasticity (28). As a component of cellular perception of mechanical forces, Piezo1 senses mechanical stress to initiate inflammation and senses changes in local mechanical stress in inflamed tissues and participates in the development of inflammation. As a mechanosensor, Piezo1 senses mechanical forces and initiates inflammation. It also senses changes in local mechanical stress in inflamed tissues and participates in the development of inflammation (29). In acute inflammation, the destructive mechanical forces are released, the inflammatory necrosis clears, and a new balance of Piezo1 function on the cells is reached. The uncontrolled function of Piezo1 in tissues is an essential factor in the development of acute inflammation to chronic. In osteoarthritis, Piezo1 induces cartilage apoptosis and inflammation, inflammatory exudation leads to increased intra-articular interstitial fluid and increased intra-articular pressure, and the increased pressure initiates apoptotic and inflammatory programs (30, 31). In myocardial fibrosis, increasing atrial fibroblast load or heart stretching can activate Piezo1 to increase Ca2+ Inflow and promote inflammation and the proliferation and fibrosis of fibroblasts (32). Myocardial fibrosis reduces cardiac compliance and increases the cardiac load, further exacerbating fibrosis. The increase of ECM in the process of lung injury repair will increase the stiffness of the tissue, and the increase of the tissue stiffness will activate the Piezo1 on the fibroblasts, promoting the proliferation of fibroblasts, and the production of ECM components, enhancing and accelerating lung fibrosis (33). In the process of chronic inflammation, Piezo1 perceives changes in tissue “homeostasis” and dysfunction of tissue Piezo1 often accelerates the development of chronic inflammation (Figure 2).
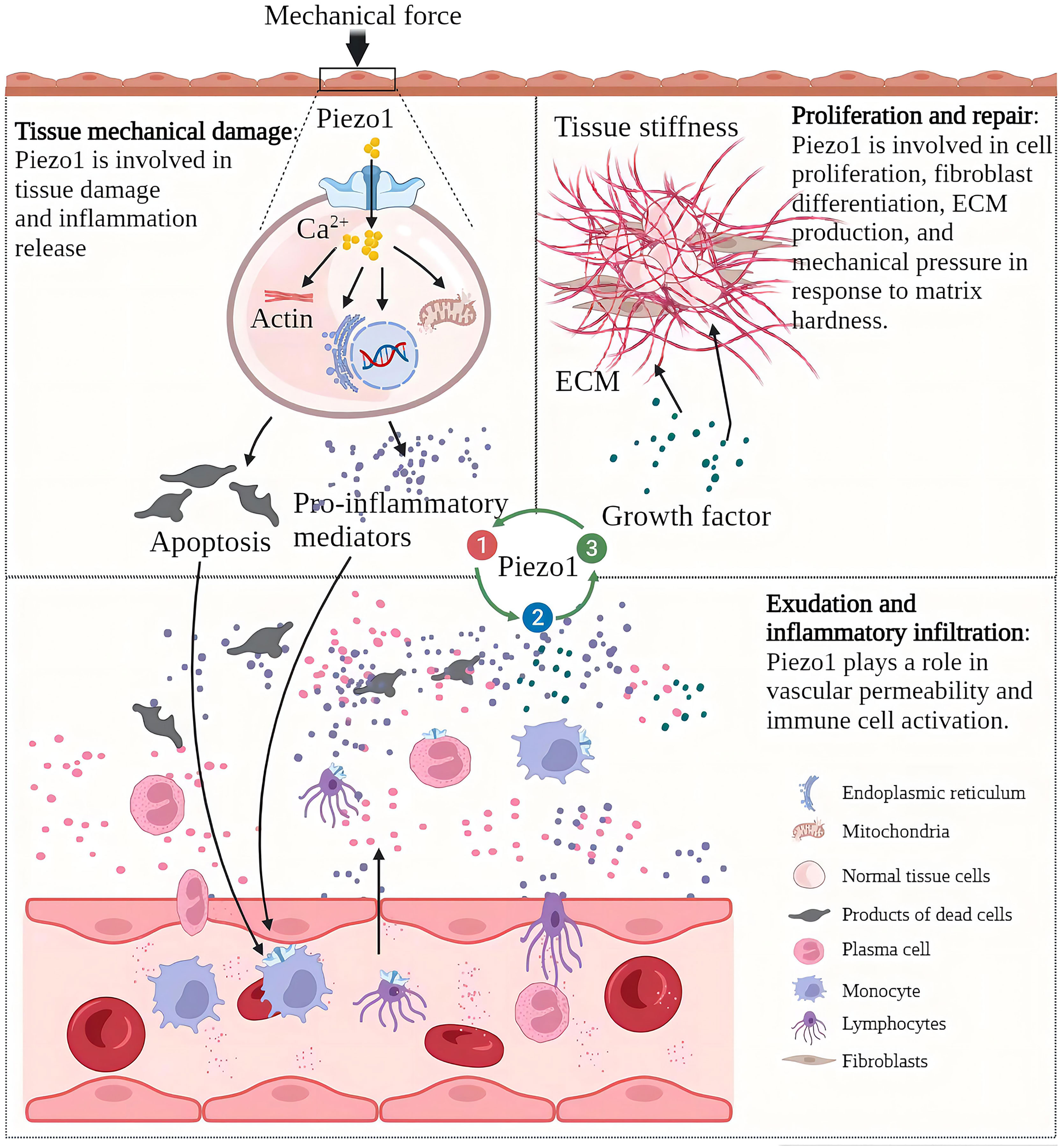
Figure 2 Piezo1 is involved in various pathological chronic inflammation processes and acts as a sensor and effector of mechanical stress within tissues in inflammation. Chronic inflammation is characterized by tissue injury, inflammatory infiltration, and proliferative repair. The Piezo1 channel transduces mechanical forces into intracellular inflammatory signals to induce injury and inflammation, and the development of inflammation alters the mechanical properties of tissues. Piezo1 senses changes in mechanical stress in the local environment and regulates the progression of chronic inflammation.
Piezo1 is widely expressed in various mechanically sensitive cells, and Piezo1 expression has been upregulated in various chronic inflammatory tissues (34) (Figure 3). Here, we collate the evidence that Piezo1 is involved in a variety of chronic inflammatory diseases, highlight how Piezo1 initiates inflammatory responses in mechanotransduction. Piezo1 can play a vital role in developing chronic inflammatory diseases by transducing mechanical stimuli into intracellular pro-inflammatory signals in response to various transient or sustained injurious mechanical signals. Based on the role of Piezo1 in inflammatory signal transduction, a large amount of evidence shows that inhibiting Piezo1 can block inflammation in the early onset of chronic inflammatory diseases.
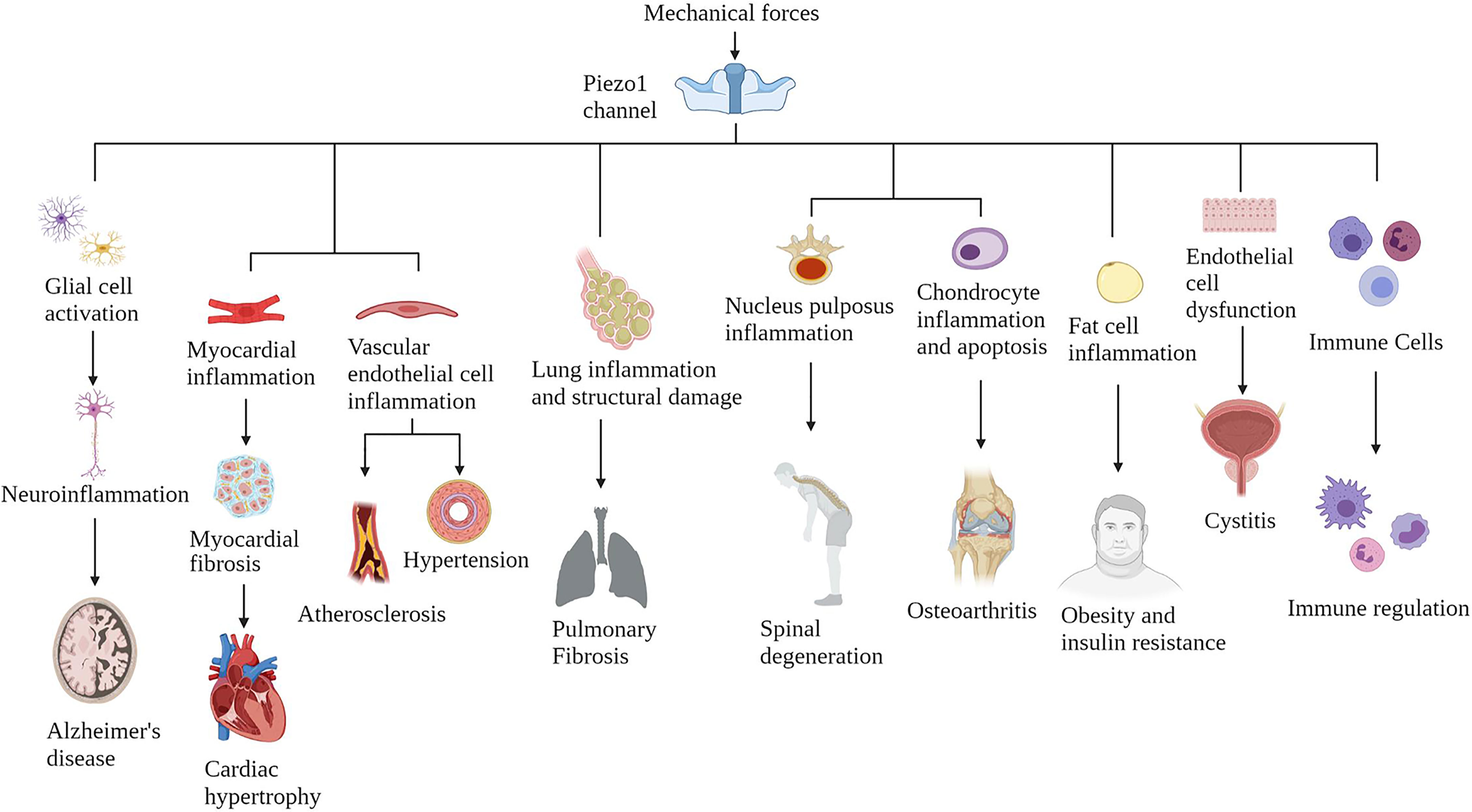
Figure 3 The Piezo1 channel responds to various mechanical force activation and induces chronic inflammatory diseases in multiple systems. The Piezo1 channel transduces mechanical stimulation into an intracellular signal cascade, leading to tissue damage and inflammation.
Both central neurons and glial cells have a high degree of mechanical sensitivity and can respond to changes in the stiffness of the surrounding environment and react quickly (35, 36). Normal brain tissue cells are in a quiet environment rich in polysaccharides, and changes in the stiffness of the brain matrix will affect the development and neurophysiology of brain cells (37). β-amyloid(Aβ) plaque deposits and neuroinflammation are signs of AD, and increased brain stiffness is detected with Aβ plaque deposits (38). β-amyloid plaques are very brittle and rigid in structure (stiffness is about 3×109Pa), and the surface morphology of the brain tissue deposited by the plaques will become rougher. Astrocytes are the most abundant type of glial cells in the brain, and they play an established role in maintaining neuronal function (39). Peripheral infection and ageing can upregulate the expression of Piezo1 in reactive cortical astrocytes (36). Increased expression of Piezo1 in reactive astrocytes exposed to Aβ is not detected in non-AD brains (36). In the brain of AD, astrocytes are more reactive and release pro-inflammatory cytokines more. Piezo1 may potentially regulate the signal transduction of reactive astrocytes, thereby affecting the phenotype of astrocytes. Rough Aβ plaques also induce dynamic changes in astrocyte-neuron interactions, affecting neurotransmission, signal gradients, and the interconnection between synapses (40).
Microglia are the brain’s resident immune cells, and it is currently believed that they originated from monocytes in the bone marrow. In AD brains, microglia accumulate around Aβ plaques and invade their nuclei for phagocytic clearance of Aβ. Aβ stimulates microglia to trigger the release of various pro-inflammatory cytokines, leading to neuroinflammation, neuronal dysfunction, and death. Piezo1 plays multiple roles in the inflammatory activation of microglia. On the one hand, microglia express TLR4, which is an innate immune receptor (41). Aβ and infection-related bacterial lipopolysaccharide (LPS) activates TLR4 while upregulating Piezo1. Piezo1 may coordinate TLR signals and induce Ca2+ influx, and the latter may regulate cellular immune activity through activation of the CaMKII-Mst1/2-Rac axis (42). Therefore, Piezo1 may be involved in the innate immune regulation of glial cells (42). On the other hand, stiff amyloid plaques may upregulate Piezo1 in microglia, thereby affecting the immune activity of microglia. In vitro, microglia cultured on stiffness gradient hydrogels migrate to harder areas and are more reactive in harder areas (43). Piezo1 also regulates microglial function in acute hyperglycemic stress, indicating that Piezo1 plays an essential role in hyperglycemic brain injury (44) .In the AD brain, amyloid deposition and neuroinflammation may form a vicious circle. Aβ plaque deposits harden the brain matrix and pro-inflammatory activation of glial cells. A large number of pro-inflammatory factors aggravate neuronal damage, and nerve damage will further release amyloid (Figure 4). Although elevated expression of Piezo1 was not detected in AD brain neurons, María Velasco-Estevez et al. (45) showed that neuronal Piezo1 overactivation induces neural demyelination and nerve damage. Piezo1 inhibits axonal regeneration and affects repair after nerve injury (46). This may partly explain the pathogenesis of AD. The accumulation of Aβ is a long-term chronic process. When the balance between the production and elimination of Aβ is broken, the cognitive ability of patients with a large amount of Aβ accumulation will rapidly decline (47, 48).
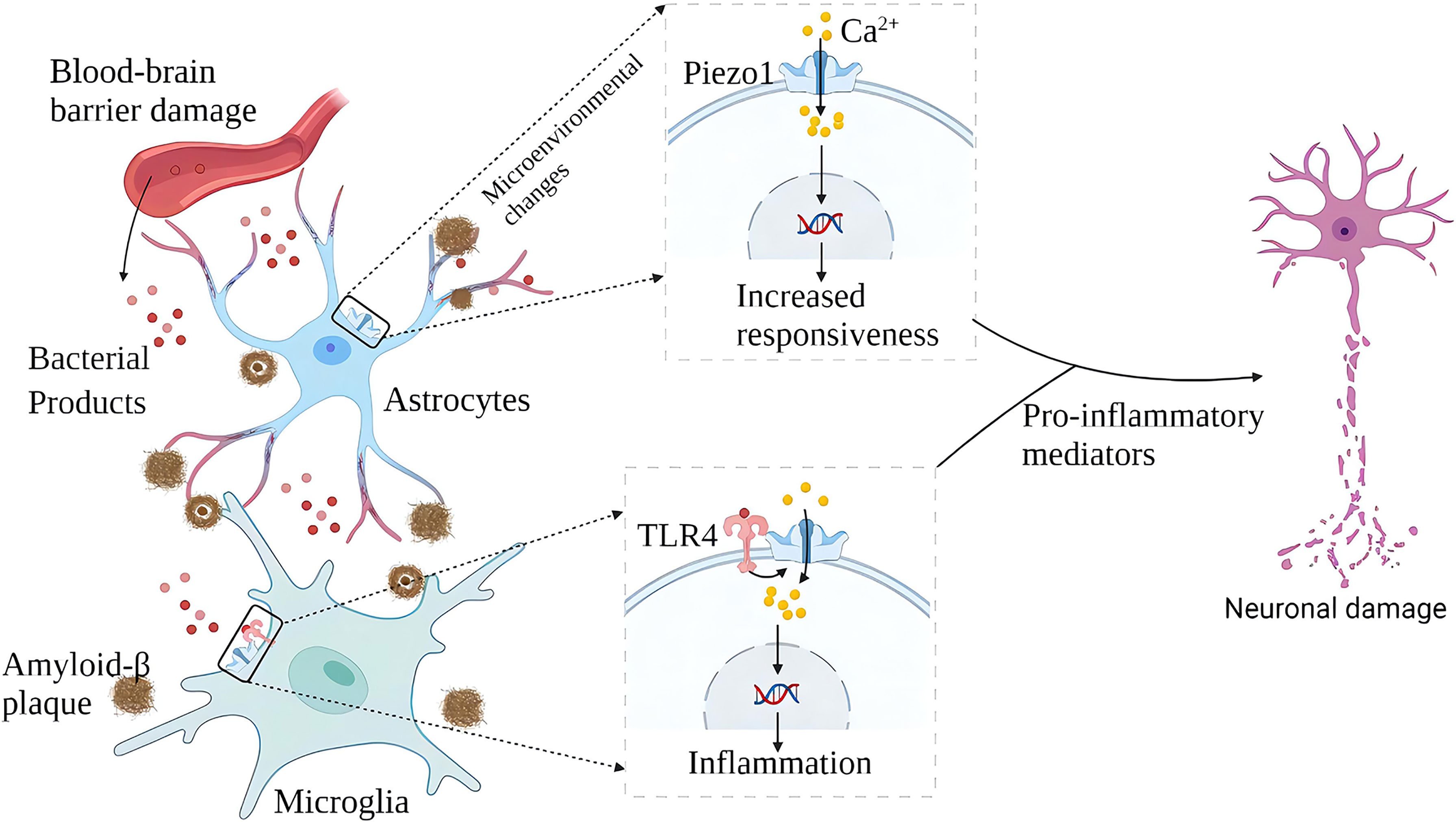
Figure 4 Schematic diagram of the role of Piezo1 in AD. In AD, plaque deposited brain tissue has increased stiffness, increased expression of Piezo1 in astrocytes and microglia exposed to amyloid plaques, and increased inflammatory reactivity. Upregulation of Piezo1 in cerebral vascular endothelial cells induced by various mechanical factors damages the vascular endothelium, leading to increased blood-brain barrier permeability and more bacterial products entering the brain. Bacterial products such as LPS activate TLR4 to initiate microglial innate immune responses, and Piezo1 coordinates TLR4 signaling and induces Ca2+ inward flow, regulating cellular immune activity. Chronic inflammation and neuronal damage lead to cognitive impairment.
In the cardiovascular system, myocardial contraction pumps blood into the arteries, and blood flow creates friction and pressure on the blood vessels (49). MSCs are widely expressed in the cardiovascular system, sensing changes in mechanical forces and transducing mechanical signals into chemical and electrical signals (50). Piezo1 is one of the cardiovascular sense and plays a crucial role in cardiovascular development, blood pressure regulation, hypertensive vascular remodeling, and other physiology and pathology (51–54). Cardiac fibroblasts play an essential role in the normal physiological function of the heart and its response to damage or stress (55). Piezo1 helps atrial fibroblasts to adapt to changes in the hardness of the surrounding matrix stiffness and modulates fibroblast mechanical properties (56). Mechanical stretching in vitro induces activation of atrial fibroblasts, which stimulates the production of ECM and stimulates the production of chemokines, such as monocyte chemoattractant protein (MCP)-1 and MCP-3, and triggers typical inflammatory pathways (57). Increased cardiomyocyte load or cardiac stretch can activate Piezo1 to increase Ca2+ influx, leading to increased expression of the inflammatory/mast cell factor IL-6 mRNA. IL-6 is a pleiotropic pro-inflammatory cytokine that promotes the proliferation and fibrosis of cardiac fibroblasts (32). Myocardial fibrosis (MF) reduces cardiac compliance and pumping function (58) (Figure 5).
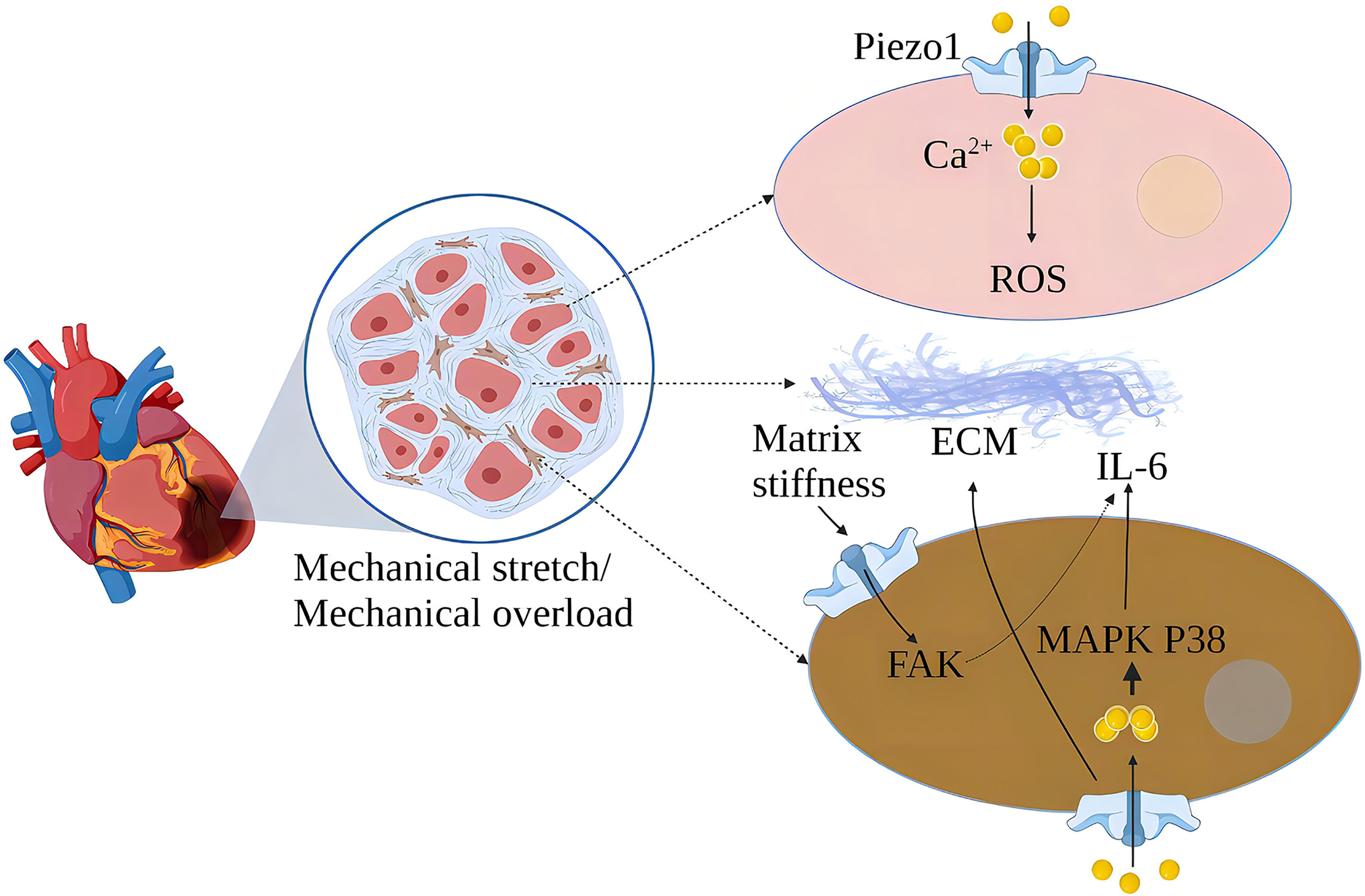
Figure 5 Schematic diagram of the mechanism of action of Piezo1 in myocardial fibrosis. Piezo1 helps cardiomyocytes transduce mechanical stretch into intracellular Ca2+ signaling and ROS signaling, and mechanical overload leads to increased Piezo1 expression in cardiomyocytes, ultimately leading to cardiomyopathy. In vitro, mechanical stretch-induced fibroblast Piezo1 opening and promoted IL-6 mRNA expression through the MAPKP38 signaling pathway. Mechanical stretch induces fibroblasts to increase ECM secretion, and increased matrix stiffness may further upregulate Piezo1 expression and promote fibrosis progression.
Atherosclerosis (AS) is a multifactorial inflammatory disease, vascular endothelial cells (ECs) injury is the initiating factor of AS. After an endothelial injury, cholesterol and lipids in the blood are deposited under the endothelium and attract monocytes to accumulate, monocytes differentiate into macrophages, phagocytic lipids are transformed into foam cells, and foam cells secrete pro-inflammatory factors (59). Vascular ECs continue to be subjected to the shearing force of the blood flow. In AS, Piezo1 is associated with ECs damage and macrophage activation (60, 61). The damage and anti-damage effects of Piezo1 depend on the type of blood flow signal. Blood flow includes both laminar and turbulent flow. In general, laminar flow leads to NO formation, barrier protection and protect against inflammation, while disturbed blood flow leads to vasoconstriction, permeable barriers, and inflammatory signaling - atherosclerosis develops. ECs in continuous laminar flow is subjected to shear forces only in the direction of the cell axis. In contrast, cells in turbulent blood flow are subjected to forces in a random direction (62). Turbulence activation of Piezo1 channels on vascular endothelial cells induces inflammatory signaling via integrin-associated PECAM-1/VE-calmodulin/VEGFR2 and PI-3-kinase-dependent activation, leading to FAK-dependent NF-κB activation, as well as reduced eNOS activation by promoting cAMP degradation via activation of phosphodiesterase 4D. NF-κB activation leads to the expression of leukocyte adhesion molecules including VCAM-1 and ICAM-1 and chemokines including CCL2, which promotes the progression of AS (63–65). NF-κB also promotes NLRP3 assembly, caspase-1 triggering, and IL-1β production (66).
Atherosclerotic plaque leads to arterial stenosis and high blood flow shear stress, which can activate a variety of monocyte activation through Piezo1, promote the adhesion of muscle cells to stimulated endothelial cells, phagocytic activity and low-oxidized density lipoprotein uptake and cytokine expression (67) (Figure 6). Stenting to correct disturbed blood flow to laminar flow effectively protects ECs (68). It has been confirmed that laminar flow induces the release of ATP through the Piezo1 channel on the ECs. It then activates the downstream signal mediated by P2Y2/Gq/G11, which further leads to the phosphorylation of AKT and the release of eNOS to participate in blood pressure regulation and anti-atherosclerosis (69). Expanding blood flow channels to reduce high shear stress can also reduce the pro-inflammatory effect of monocytes (67).
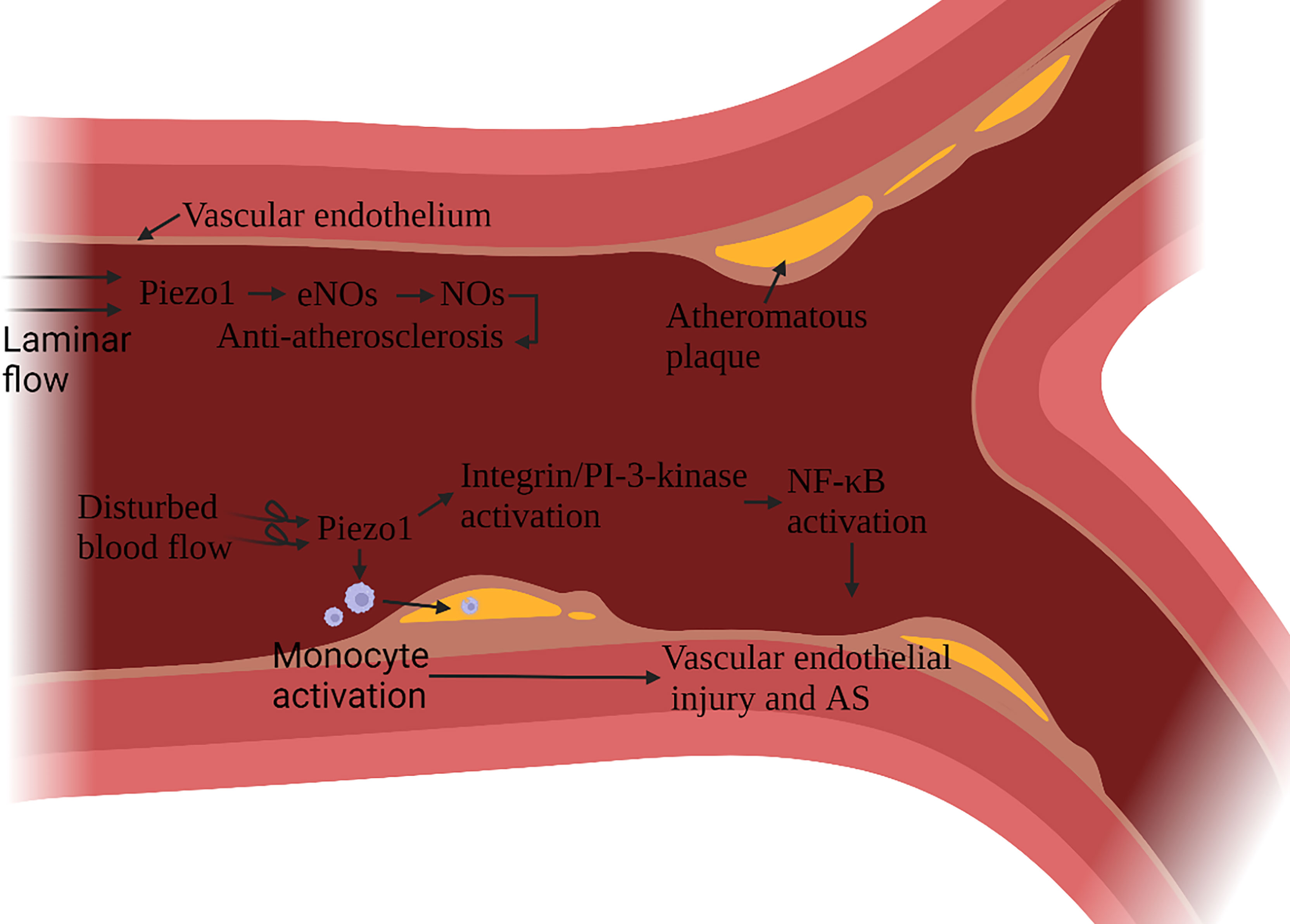
Figure 6 Schematic diagram of the role of Piezo1 in atherosclerosis. Piezo1 on vascular endothelial cells senses the shearing force of blood flow, and laminar flow produces NO through Piezo1, which has the effect of protecting vascular endothelium. Disturbed blood flow shear force activates Piezo1, activates inflammatory pathways through integrin activation and PI3K-related signaling pathways to cause vascular endothelial inflammation, reduces eNOS activation, and induces monocytes to accumulate to the injured site, accelerating the formation of atherosclerotic plaques.
Piezo1 is necessary to regulate NO formation, vascular tone, and blood pressure (69). Damage to vascular ECs resulted in vascular sclerosis and increased peripheral resistance, which is one of the essential mechanisms for the occurrence and development of hypertension. IL-6 produced by endothelial cell injury stimulates the synthesis of several acute-phase reactive proteins, including CRP, serum amyloid A, fibrinogen, TNFα and IL-1β. IL-6 can cause ECs dysfunction, increases peripheral vascular resistance and raising blood pressure (70). IL-6 also stimulates the adhesion and aggregation of neutrophils in capillaries, aggravating inflammation and endothelial damage (71). Piezo1 plays an essential role in the remodeling of congenital arteries (72, 73). In addition, Piezo1 is up-regulated in rats with Ang II-induced heart failure, indicating that Piezo1 is also involved in arterial remodeling after injury (74). Excessive activation of Piezo1 in cardiomyocytes leads to excessive ROS and causes cytotoxicity (55). In addition to oxidative stress, ROS can also inactivate the vasodilation function of NO, reducing the amount of NO that can play a role, leading to the consequences of hypertension (75). Piezo1 may also regulate blood pressure by participating in the rat’s motor booster reflex (76).
Pulmonary fibrosis is the end-stage changes of a large group of lung diseases characterized by the proliferation of fibroblasts and the accumulation of a large amount of extracellular matrix accompanied by inflammatory damage and destruction of tissue structure. Normal alveolar tissue is damaged and undergoes abnormal repair, leading to abnormal structure (scar formation) (77). Lung tissue is a highly mechanized organ. Piezo1 responds to lung mechanical stress and is involved in the development of pulmonary fibrosis through multiple mechanisms.
Alveolar surfactants play a role in reducing alveolar surface tension and maintaining lung compliance (78). The membrane tension generated by alveolar swelling activates Piezo1 in alveolar type I epithelial cells (AT I), and the Piezo1-mediated rise in Ca2+ activates pannexin hemichannels and leads to ATP release. The released ATP stimulates the nearby AT II to release alveolar surfactants (79). High alveolar surface tension leads to alveolar surfactant dysfunction. Alveolar surfactant dysfunction is associated with repeated or continuous damage to the alveolar epithelium and pulmonary fibrosis (80). Animal models of alveolar hypertonicity show different signaling pathways that induce pulmonary fibrosis through a mechanical transduction. These include the Rho/rho-related protein kinase (ROCK), corticotrophin-related transcription factor-A (MRTF-A) and yes-associated protein 1 (YAP)/(transcriptional co-activator with PDZ binding motif) TAZ signaling pathways (81). YAP and TAZ accumulate in cell nuclei exposed to high mechanical stress or undergoing deformation and cytoskeletal tension. YAP/TAZ exerts its pro-fibrosis effect through interaction with nuclear transcription factors and activation of different ECM genes (82). Mechanical ventilation is the primary method for treating acute respiratory distress syndrome (ARDS). Relative to the tiny tidal volume, the excessive expansion of lung volume in mechanical ventilation will cause ventilator-related lung injury (VILI). Piezo1 played a crucial role in VILI after ARDS. Mechanical stretching activates Piezo1 and increases the activity of calcium-dependent calpain to destroy the lung endothelial barrier. Mechanical stretching also induces the activation of Piezo1 channels in AT II. The increase of intracellular Ca2+ induces cell apoptosis and abnormal secretion of alveolar surfactant (83), thereby aggravating lung damage and inflammation in patients with ARDS (84). Cyclic stretching activates Piezo1 during mechanical ventilation and affects VILI development by promoting RhoA/ROCK1 signaling. Ionizing radiation upregulates the Piezo1 channel in ATII and regulates the radiation-induced epithelial-mesenchymal transition by forming positive feedback with TGF-β1. The repair of lung tissue after injury increased the stiffness of the extracellular matrix (ECM), leading to the nuclear localization of YAP in fibroblasts, which subsequently showed increased production of ECM components. This may increase the stiffness of the ECM again and start a vicious circle, which may eventually lead to fibrosis.
Periodic hydrostatic pressure in the lungs can activate monocyte Piezo1 and cause Ca2+ influx, phosphorylation of transcription factor cJun, and expression of endothelin 1 (ET1), which in turn activates hypoxia-inducible factor 1α (HIF1α) via the endothelin receptor type B (EDNRB), thus facilitating transcription of an effective, prolonged pro-inflammatory mediator program (85). This effect of Piezo1 also increases the secretion of chemokine CXCL2 in monocytes, allowing neutrophils to migrate from the blood to the lungs along the CXCL2 gradient and inducing neutrophils to clear bacteria (86). Knockout of the Piezo1 gene in mice has a protective effect on pulmonary fibrosis induced by bleomycin (85) (Figure 7).
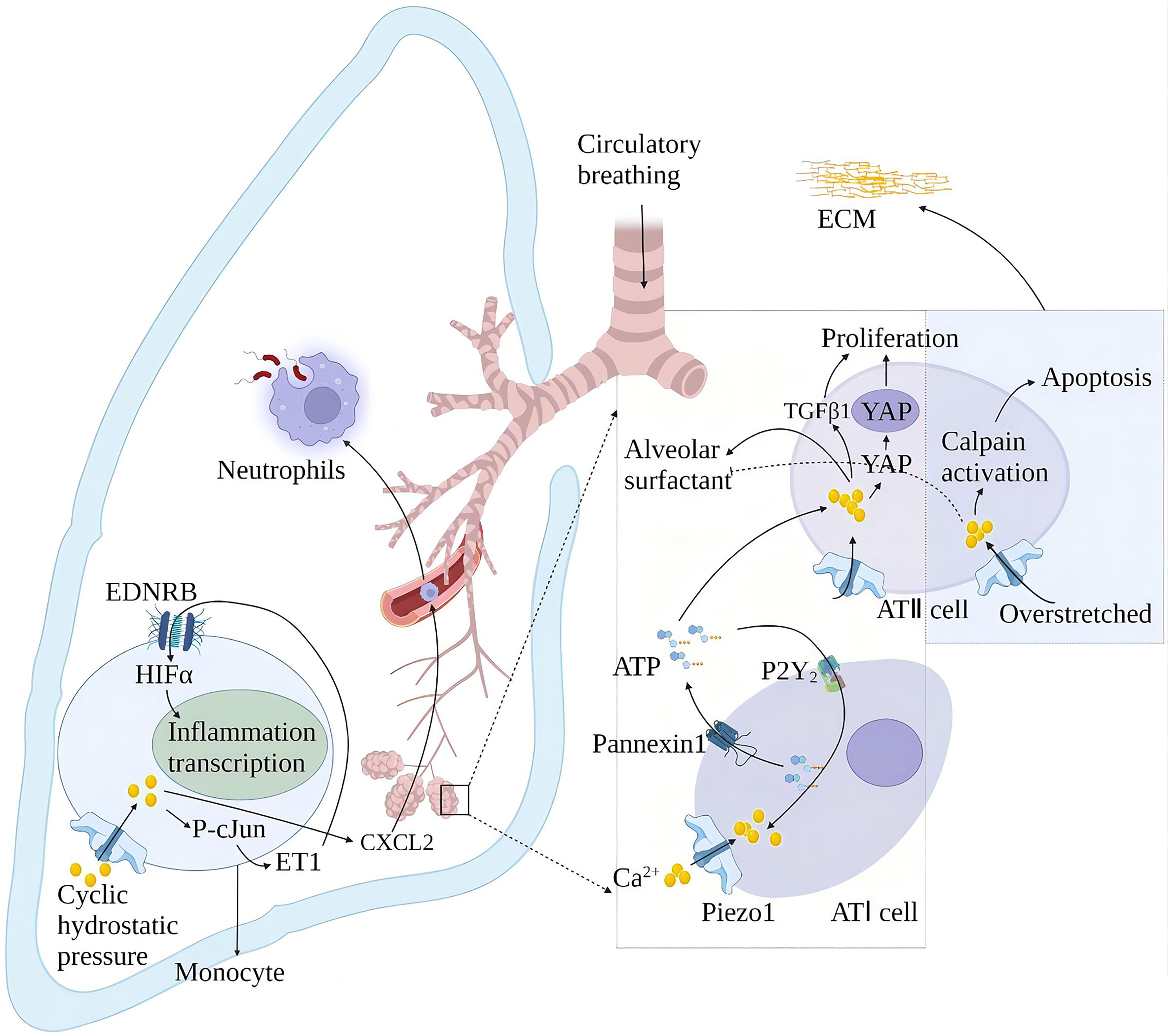
Figure 7 Schematic diagram of the role of Piezo1 in innate lung immunity and pulmonary fibrosis. Periodic hydrostatic pressure in the lungs can activate monocyte Piezo1 to cause Ca2+ influx, phosphorylation of transcription factor cJun, and expression of endothelin 1 (ET1), which in turn activates HIF1α via the endothelin receptor type B (EDNRB), Thereby promoting the transcription of pro-inflammatory mediator programs. Piezo1 also increased the secretion of the chemokine CXCL2 in monocytes in the lung, inducing neutrophils to migrate from the blood vessels to the lungs to clear bacteria. Piezo1 senses alveolar tone and is involved in the secretion of alveolar surfactant and proliferation of ATII. Alveolar hyperextension leads to reduced secretion of alveolar surfactant and apoptosis of ATII, exacerbating lung injury and inflammation in ARDS patients. Piezo1 upregulation in alveoli induces an epithelial-mesenchymal transition with increased extracellular matrix (ECM) stiffness and promotes pulmonary fibrosis.
Piezo1 is a major skeletal mechanosensor that regulates skeletal homeostasis. Piezo1 plays an essential role in skeletal growth and development by affecting osteoblast-osteoclast crosstalk in response to mechanical forces (87–92). Mechanical forces at the physiological level are the basis for normal bone and joint function, and excessive mechanical loading of the bones can lead to inflammation and degenerative degeneration. In osteoarthritis (OA), upregulation of chondrocyte Piezo1 expression under high strain mechanical stress consistently enhances Ca2+ signaling leading to apoptosis (30, 31). Furthermore, Other studies suggest that MAPK/ERK5 and MAPK/ERK1/2 are downstream signal molecules mediated by Piezo1 channels to mediate late excessive apoptosis of chondrocytes under high-strain stimulation (93). After chondrocyte apoptosis, it will stimulate the environment in the joint and produce a large amount of oxygen free radicals and inflammatory mediators (such as IL-1β, TNFα, PE, etc.) to damage the new cartilage tissue and blood vessels. Chondrocyte loss and chronic inflammatory infiltration increase interarticular fluid, exerting pressure on intra- and extra-articular structures, further activating mechanoreceptors and injury receptors and creating a vicious cycle (94) (Figure 8).
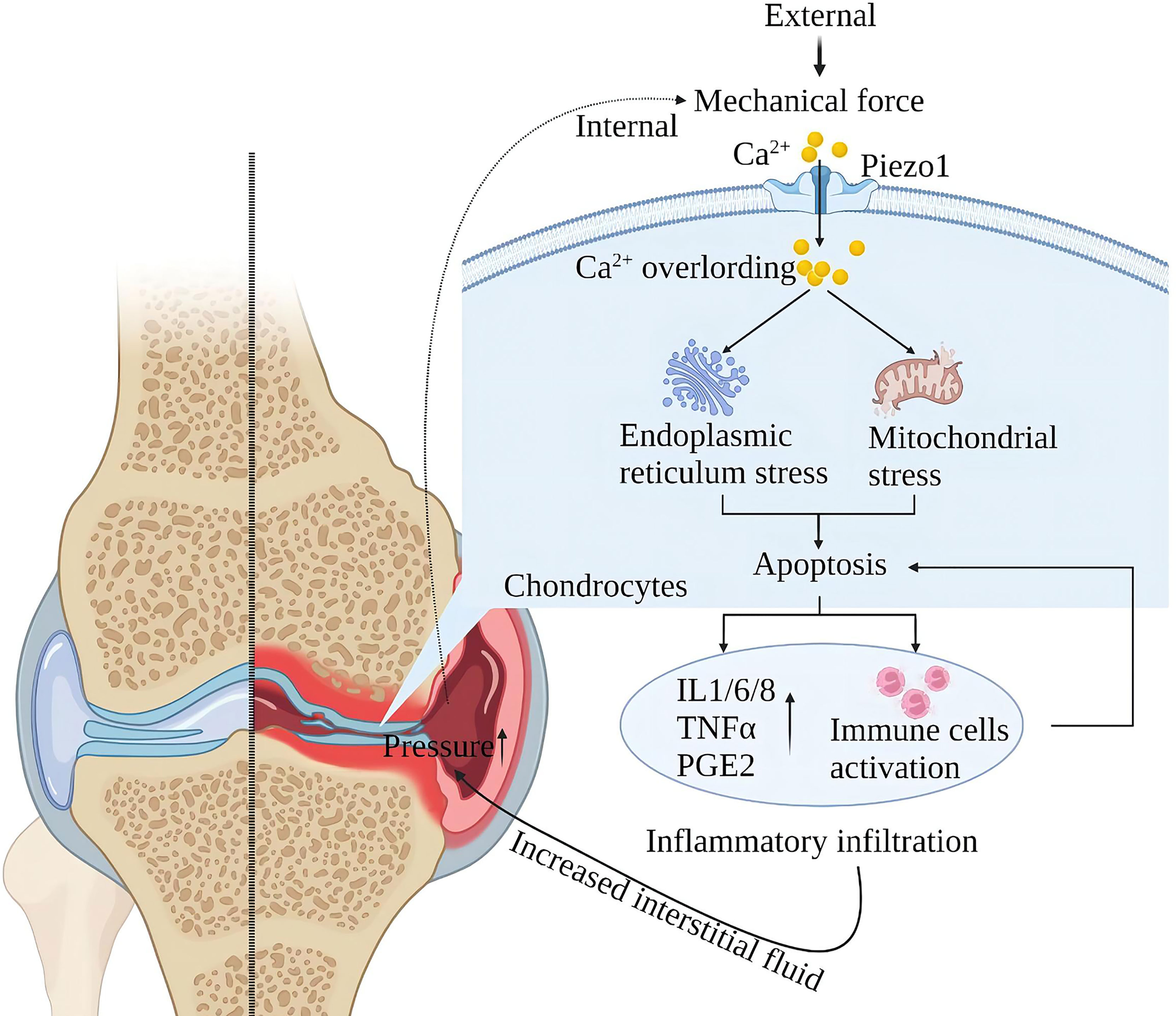
Figure 8 Schematic diagram of the role of Piezo1 in OA. Mechanical force activates Piezo1 on articular cartilage cells, induces Ca2+ overload, endoplasmic reticulum, and mitochondrial stress, leading to inflammation and apoptosis of chondrocytes. Cartilage cell damage leads to exudation of interstitial fluid and inflammatory infiltration. Inflammatory infiltration damages the nascent cartilage tissue, and exudation leads to increased intra-articular pressure, further activating Piezo1, creating a vicious injury cycle.
A single impact applied to the lumbar disc without causing structural damage to the lumbar spine also triggered significant upregulation of Piezo1, NLRP3 inflammatory vesicles, catabolic (MMP-9, MMP-13) and pro-inflammatory gene (IL-1β) expression and induced disc degeneration (IDD). Piezo1 inhibition reduces mechanical shock-induced activation of NLRP3 inflammasome and IL-1β expression in nucleus pulposus cells (95). In addition, G protein-coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocytes in osteoarthritis via suppression of Piezo1 (96). Mechanical stretching of the lumbar spine upregulates Piezo1, NLRP3, ASC and IL-1β in nucleus pulposus cells. In terms of mechanism, Piezo1 activation increases the cytoplasmic Ca2+ load, and Ca2+ activates the NF-κB pathway and increases the expression of NLRP3, proIL-1β and proIL-18. Ca2+ further stimulates inflammasome assembly, enhances the activation of caspase-1, and promotes the secretion of IL-1β (66). In the intervertebral disc, the rigid ECM around the nucleus pulposus tissue activates the Piezo1 channel of the nucleus pulposus cells to increase intracellular reactive oxygen species (ROS) and the expression of GRP78 and CHOP, which contributes to oxidative stress and endoplasmic reticulum stress. The continuous mechanical stress damage of the intervertebral disc leads to the death of cartilage cells, the reduction of extracellular matrix, the increase of various inflammatory factors, and finally, progress to lumbar degeneration (97) (Figure 9).
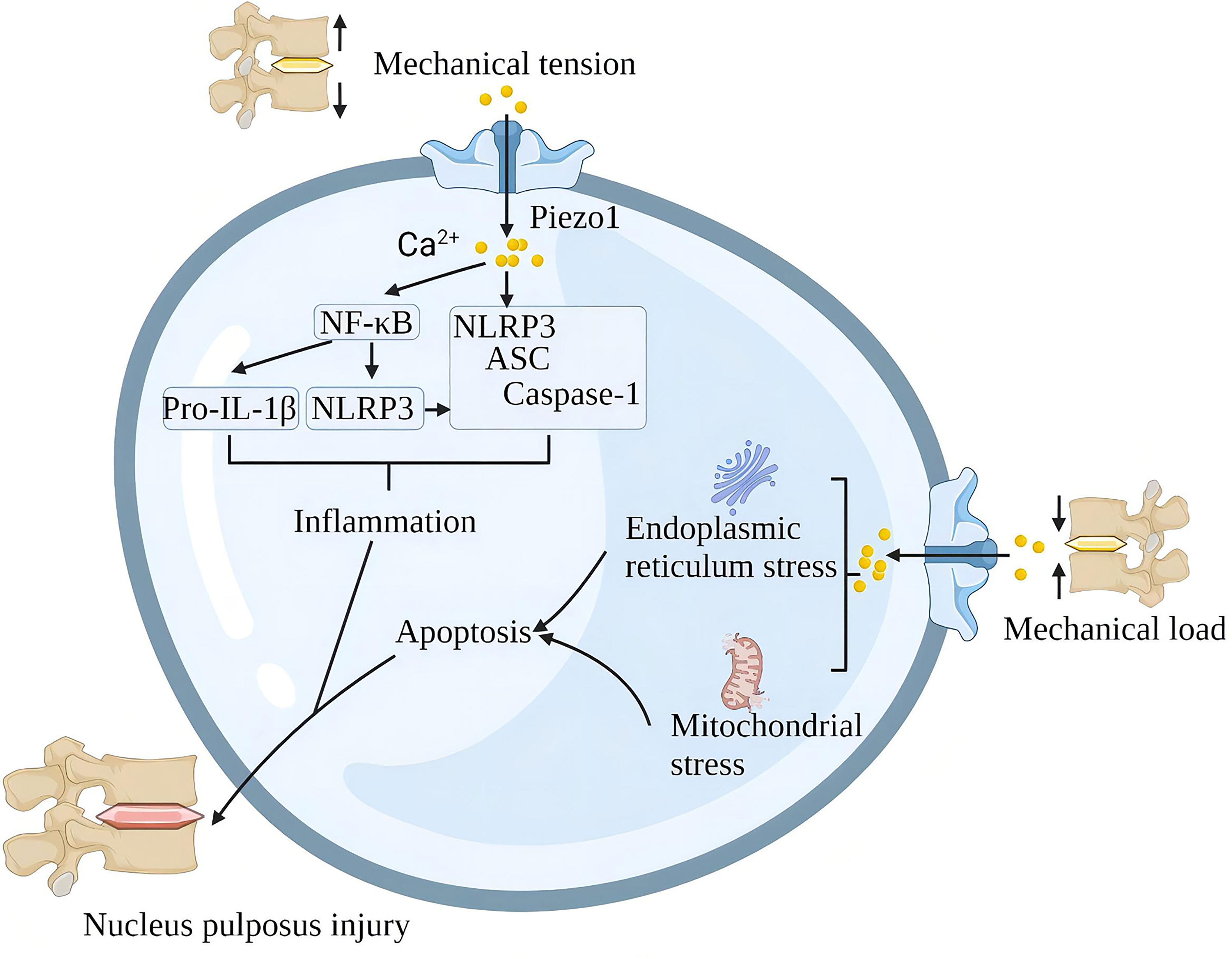
Figure 9 Schematic diagram of the role of Piezo1 in spinal degeneration. Piezo1 on the nucleus pulposus cells senses mechanical stretching and induces Ca2+ influx, which initiates the inflammatory pathways of NF-κB and NLRP3. Mechanical shock induces mitochondria and endoplasmic reticulum stress through Piezo1, leading to apoptosis of nucleus pulposus cells. Chronic inflammation and loss of nucleus pulposus cells lead to spinal degeneration.
Obesity is due to excessive calorie intake, which leads to an increase in the size of fat cells (hypertrophy) and the number of cells (hyperplasia) (98). Hypertrophy promotes adipose tissue inflammation and insulin resistance. Proliferation leads to smaller fat cells, less adipose tissue inflammation and better insulin sensitivity (99). Piezo1 on the fat cell membrane can sense membrane tension from the inside out. The function of Piezo1 is essential for systemic lipid metabolism and insulin sensitivity (100). Piezo1 opening in developing mature adipocytes leads to the release of FGF1, which induces the Differentiation of adipocyte precursors through the activation of FGF-receptor-1, suggesting that Piezo1 opening is a signal that promotes the Differentiation of adipocyte precursors (101). Piezo1 -/- mice showed defects in the differentiation of adipocyte precursors into mature adipocytes when fed a high-fat diet (HFD), increased number of hypertrophic adipocytes, increased white adipose tissue (WAT) inflammation, and obesity, and reduced insulin sensitivity (9). The expression of Piezo1 was significantly increased in the white adipose tissue of obese mice, the mechanism of Piezo1 in obesity and insulin resistance needs further study (9) (Figure 10).
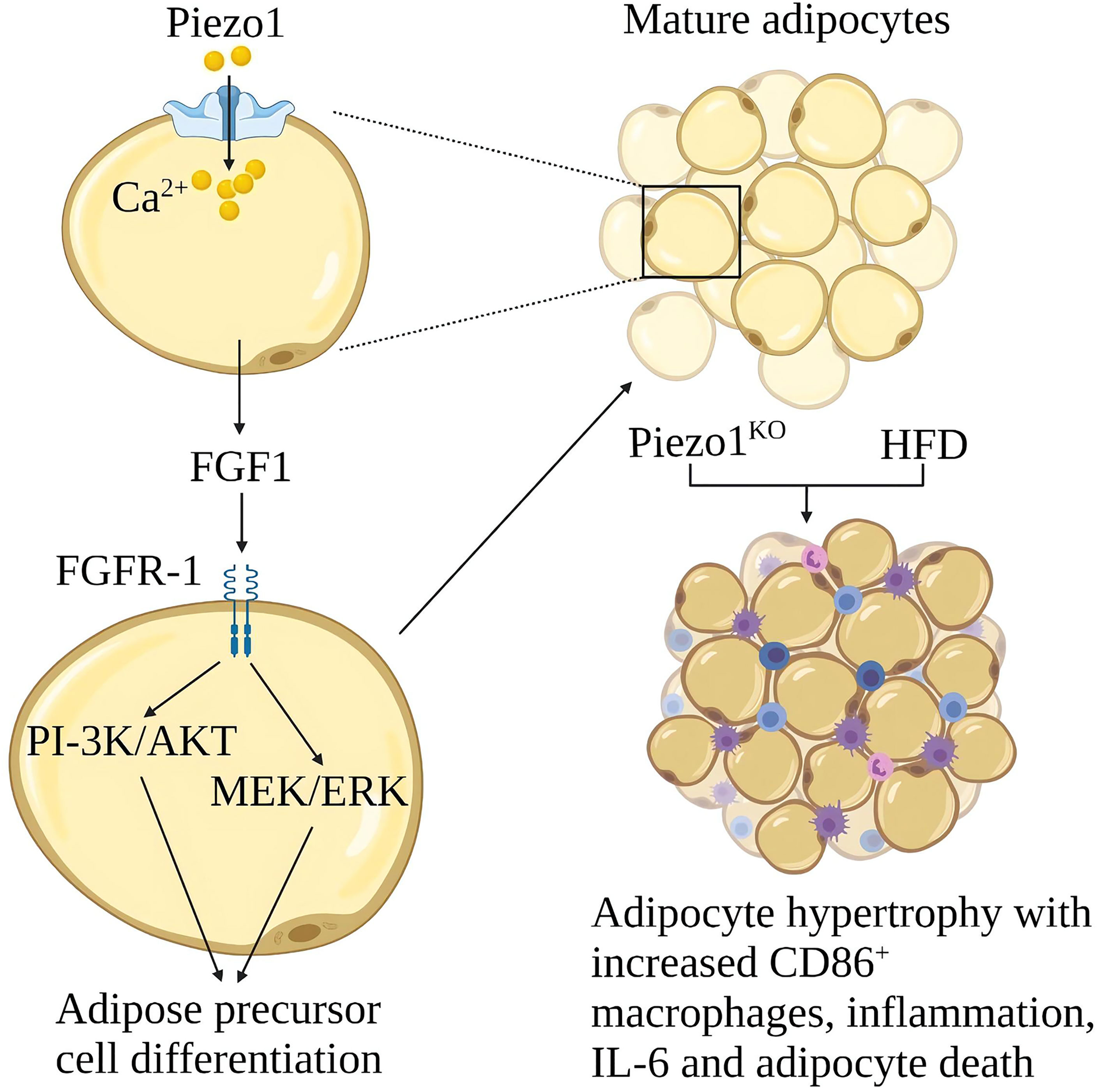
Figure 10 Schematic diagram of the role of Piezo1 in obesity and insulin resistance. Opening Piezo1 on mature adipocytes promotes FGF1 secretion, which induces adipose precursor cell differentiation. Mice lacking Piezo1 in adipocytes developed adipose progenitor cell differentiation disorder and increased hypertrophy after a high-fat diet, with increased CD86+ macrophages, inflammation, IL-6, and adipocyte death.
Chronic cystitis mainly manifests as chronic inflammation infiltration of the bladder and severe urinary frequency, urgency, lower abdominal pain, painful urination, hematuria and other symptoms (102). Piezo1 is widely expressed in the urinary system (103, 104). Qian Liu et al. (105)found that the expression of Piezo1 in bladder Cajal interstitial-like cells (ICC-LC) in the urothelium and mesenchyme of the cystitis rat model induced by cyclophosphamide (CYP) for 48 hours was significantly increased. CYP treatment for eight days resulted in a more significant up-regulation of Piezo1 in chronic bladder inflammation. ICC-LCs plays a vital role in regulating bladder activity, The abnormal number and function of bladder ICC-LCs are related to bladder inflammation (106–108). In terms of mechanism, Piezo1 and NCX reversely cooperate to trigger an increase in the continuous influx of Ca2+ into the bladder ICC-LCs. In bladder ICC-LCs, Na+/Ca2+ exchanger (NCX)-mediated ion transport is critical to cytoplasmic Ca2+ homeostasis in bladder ICC-LCs. NCX usually maintains Ca2+ homeostasis by removing Ca2+ from the cell forward and bringing Ca2+ into the cell in a reverse mode (109). In rats with chronic cystitis, the NCX reverse mode in the bladder ICC-LCs were relatively activated and cooperated with Piezo1. During chronic cystitis, mechanically activated Piezo1 promotes the flow of Ca2+ and Na+ into ICC-LCs. On the other hand, Piezo1 and NCX1 work in reverse to pump large amounts of Ca2+ to cause cellular Ca2+ overload and induce hyperfunction of the bladder (Figure 11). SiRNA targeting Piezo1 significantly reduced the reverse mode NCX1 on the ICC-LCs membrane and the increased Ca2+ and Na+ in the cytoplasm. GsMTx4(an inhibitor of Piezo1) can significantly improve the urodynamic abnormalities related to cystitis, significantly reduce the maximum bladder pressure (MBP), significantly extend the systolic interval (ICI), and relieve the symptoms of cystitis.
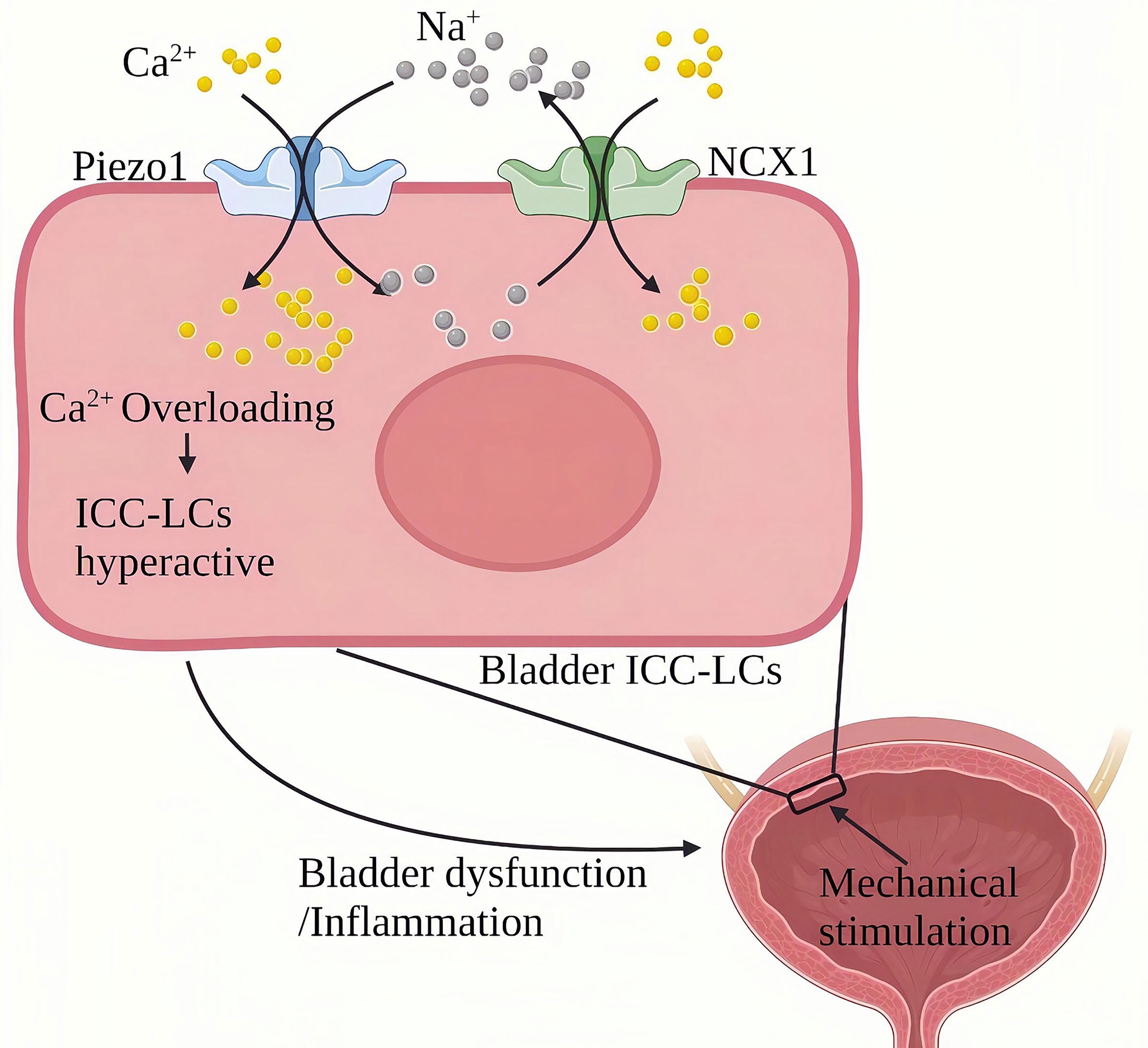
Figure 11 Schematic diagram of the role of Piezo1 in chronic bladder inflammation. The ICC-LC in the bladder senses mechanical stimulation and works in reverse with NCX to trigger the continuous flow of Ca2+ into the ICC-LC, leading to Ca2+ overload and ICC-LC dysfunction and inducing bladder function abnormalities and inflammation.
The cytoplasmic Ca2+ under resting conditions is ∼10-7M, 104 times lower than Ca2+ in the extracellular milieu (∼10-3M), various cell stimulations can promote the increase of cytoplasmic Ca2+ (110). Cytoplasmic Ca2+ mainly comes from extracellular and intracellular Ca2+ storage (endoplasmic reticulum (ER) and sarcoplasmic reticulum in muscle cells) (111, 112). The intracellular Ca2+ and Ca2+ signals must be strictly controlled. Ca2+ is the second messenger in the cell, and Ca2+, including inflammation, controls every cell aspect.
Based on the localization of Piezo1 in the cell, Piezo1 activation would not only trigger extracellular Ca2+ inward flow, but Piezo1 also promotes Ca2+ release from the Ca2+ pool (113). María Velasco-Estevez found that in the presence of extracellular Ca2+, Yoda1(an activator of Piezo1) initially enhances intracellular Ca2+ levels, most likely through the membrane-bound Piezo1 channel through Ca2+ influx, which may cause calcium-induced calcium release process (CICR) from intracellular stores (81). Fam38A mediates integrin activation by recruiting the small GTPase R-Ras to the ER, which activates the calcium-activated protease calpain by increasing Ca2+ release from cytoplasmic stores (18). The consumption of Ca2+ in the internal storage can activate the Ca2+ release-activated channel (CRAC) in the outer membrane, thereby enhancing the influx of extracellular Ca2+ and promoting the replenishment of the internal Ca2+ storage (114). Upregulation of Piezo1 in pancreatic acinar cells can induce PLA2 to activate TRPV4 channels and cause a continuous increase in Ca2+ (115). Hamza Atcha’s study (61)showed that the positive feedback regulation between Piezo1 and cytoskeletal actin promotes the activation of macrophage inflammation. Piezo1 enhanced the formation of F-actin, and actin polymerization promoted Piezo1-mediated Ca2+ activity, indicating a potential positive feedback regulation between ion channel activity and cytoskeleton. The transient or continuous increase of Ca2+ breaks the Ca2+ homeostasis, and the enhancement of the Ca2+ signal induces inflammation and other cellular events through a signal cascade.
The function of Piezo1 and its downstream mechanism have obvious differences in various tissue inflammations. Piezo1-mediated Ca2+ signaling participates in the occurrence and development of inflammation through a variety of signaling pathways (Table 1), including the classic inflammation pathway Jak/Stat: IL-6 receptor family (56, 120), TLR (9, 42) and NF-κB (66, 117, 121); Inflammasome NLRP3 (66, 95); Ca2+-sensitive MAPK family (ERK, JNK, P38) (116, 120, 122, 123), AKT/mTOR (60, 69)、β1 integrin (18); As well as focal adhesion kinase (FAK) related to inflammation-related mechanical transformation (60, 124), proline-rich tyrosine kinase2 (Pyk2) (125); Calcineurin (RACK1), calcium/calmodulin-dependent protein kinase II (CaMKII-Mst1/2-Rac) and calpain1 (18). Although the downstream mechanisms involved in Piezo1 are different in different organizations and occasions, they all eventually lead to inflammatory events.
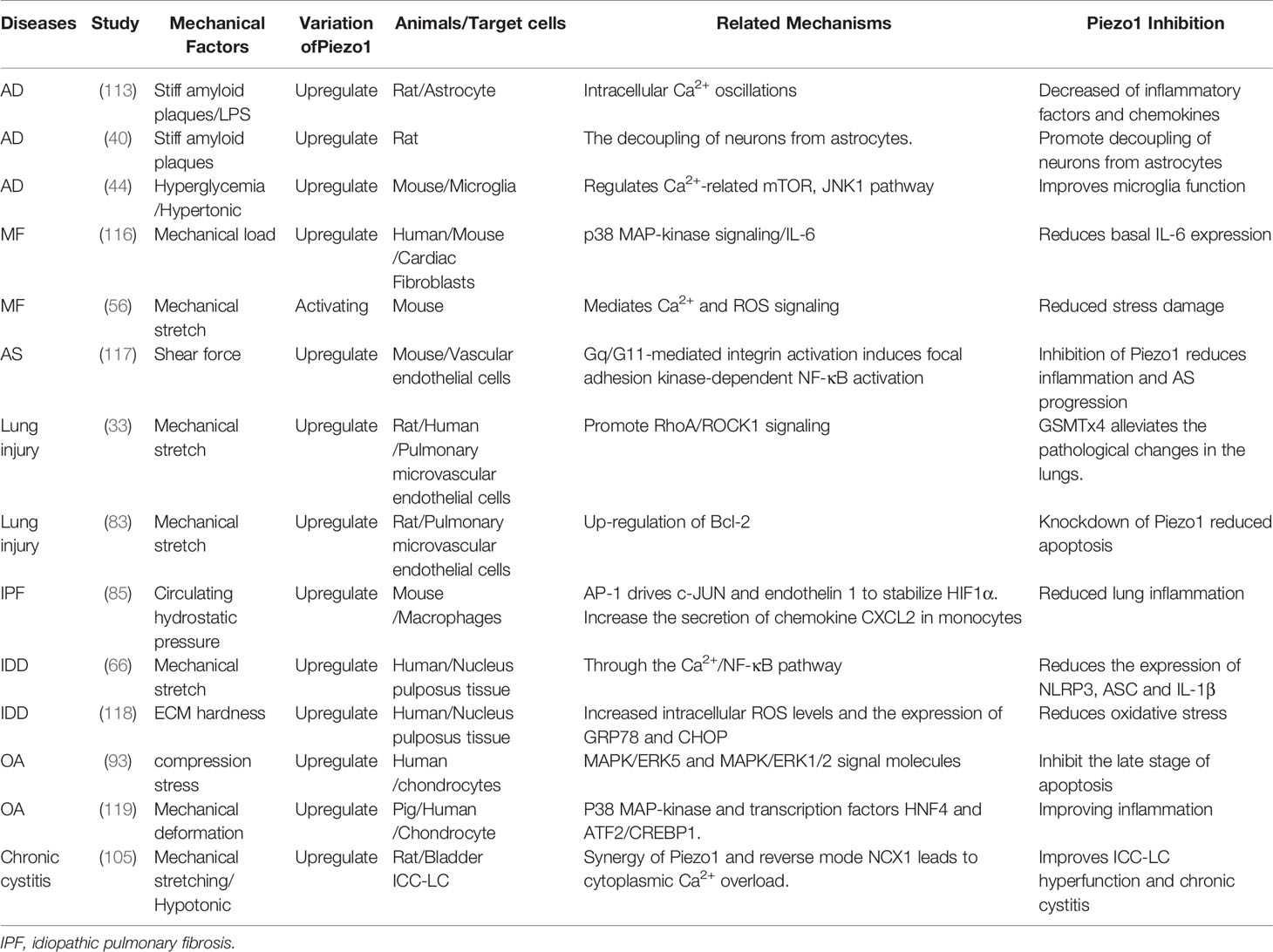
Table 1 Current mechanisms are linking Piezo1 to the development and progression of chronic inflammatory diseases.
Chronic inflammation is often accompanied by physical and psychological symptoms, including pain and mood changes. Pain is an adaptive response of the body to injury, and these symptoms have a serious impact on the quality of life during chronic inflammation (126). Nociception provides a means of neurofeedback that allows the central nervous system (CNS) to detect and avoid toxic and potentially destructive stimuli (for example, heat, cold, mechanical, or chemical stimuli) inactive and passive environments (127).The accumulation of K+ and H+ in local lesions during inflammation, especially inflammatory mediators such as prostaglandin, serotonin, bradykinin, and other stimulating nociceptors, is the main cause of pain (127). Exudation in the inflammatory lesion causes tissue swelling, increased tension, and compression of nerve endings can cause pain. In addition, the inflamed organ enlarges, which increases the tension of the capsule rich in sensory nerve endings, and the nerve endings are stretched and cause pain. Cytokines can also affect CNS activity through various body fluids and neural communication pathways (such as the vagus nerve afferent nerve). Recent studies have suggested that many C-type nerve fibers feel strong stimulation under normal conditions but do not cause pain. However, under the action of inflammatory mediators, these nociceptive fibers are more sensitive to mechanical stimuli, even if they are mildly stimulated. It can also cause pain.
The research of Piezo1 channel in pain is still in the early stage. Piezo2 has been confirmed to be related to various pain (128). Piezo1 and Piezo2 channels are both expressed in sensory neurons. Piezo2 is highly expressed in dorsal root ganglion neurons of various sizes; the most prominent is a giant diameter neuron involved in mediating touch and proprioception. In contrast, Piezo1 is selectively expressed in smaller DRG neurons that mediate nociception, indicating its potential role in pain (129). Knockout of Piezo2 in the same mouse sensory nerve will damage the sense of touch, but will make the mice sensitive to mechanical pain, indicating a negative interaction between Piezo1 and Piezo2.
Recently, Piezo1 was confirmed to be expressed in the trigeminal ganglion and is involved in the mechanical transduction of migraine. In ex vivo hemiskull preparation, Yoda1 activates the continuous nociceptive discharge of the meningeal branch of the trigeminal nerve to a large extent (130). Interestingly, Mingmin Zhang’s research results show that Piezo1extensive expression in sensory neurons will reduce rather than cause mechanical pain responses (131). Another study (132) showed that Piezo1 was involved in mediating the reduction of pain threshold caused by sleep deprivation, while microinjection of GSMTx4 or PD151746(a calpain inhibitor) partially reversed the pain threshold. Therefore, the research of Piezo1 in pain still needs further exploration.
Inflammation-induced mechanical injury is mainly sterile, and antibiotic treatment is ineffective because there is no specific pathogen, such as bacterial infection (127). The treatment of aseptic inflammation should be tailored to the different etiologies. Based on understanding the pathogenesis of chronic tissue inflammation caused by mechanical force imbalance, reducing destructive mechanical force is important to prevent and treat chronic tissue inflammation. Many targeted preventive measures have been put forward clinically, and significant effects have been achieved. For example, joint braking and non-steroidal anti-inflammatory drugs are early treatments for osteoarthritis (133, 134). Patients with lumbar spine degeneration should rest in bed to relieve spinal pressure (135, 136). Stent implantation can correct local disordered hemodynamics and effectively delay the progression of atherosclerosis (62, 137). Piezo1 channel provides solid theoretical support for these clinical prevention measures. This also reminds us that for chronic inflammation caused by mechanical damage, a good lifestyle is better than all treatments after inflammation (138, 139). However, most chronic tissue inflammation has an insidious onset, and damaging mechanical signals are usually long-standing before clinical symptoms. Therefore, Piezo1 pharmacology is of particular importance.
Currently, only few drugs are used in pharmacological studies of the Piezo1 channel (Table 2). They include lanthanides Gd3+ and La3+, aminoglycoside antibiotics (such as streptomycin and gentamicin), Ruthenium red (RR) (16), and GsMTx-4 peptide isolated from tarantula spider toxins (143). They block most MSCs, including Piezo1, and inhibit MA currents induced by Piezo1 (157). Mechanistically, GsMTx4 can bind to the cell membrane. In the resting state, GsMTx4 is stabilized by six lysine residues on the surface. When the membrane tension increases, the lateral pressure in the phospholipid bilayer decreases, and the penetration distance of GsMTx4 on the cell membrane increases, which in turn causes the outer layer of phospholipid molecules to relax locally, thereby reducing the efficiency of force transduction from the lipid bilayer to the channel (143). Another class of compounds, such as amphiphilic chlorpromazine and LPC, has been shown to activate prokaryotic and eukaryotic MSCs (158, 159). Yoda1 is a new class of synthetic small molecule compounds that can specifically activate the Piezo1 channel. Yoda1 at a micromolar concentration induces a strong Ca2+ response in cells transfected with human or mouse Piezo1 (140). Jedi1/2 is a new type of hydrophilic Piezo1 chemical activator, which acts through the peripheral blades and uses a peripheral lever-like device composed of blades and light beams to gate the central ion conduction hole (142). Recently, an analogue of Yoda1, named Dooku1 (147), and tubeimoside I (TBMS1) identified in traditional Chinese medicine (148), reversibly antagonizes the activation of Piezo1 induced by Yoda1 through competition for specific channel binding sites.
The activity of the Piezo1 channel can also be adjusted physically. With the advantages of high resolution and good penetration, ultrasound technology has been widely used in clinical practice. In 2018, the Maduke research team discovered that the Piezo1 could be activated by 43MHz ultrasound outside the body to become open (160). A shock wave (SW) is a mechanical energy wave generated by the medium’s highly rapid compression and accumulation through vibration or high-speed motion (161, 162). The mechanical stress generated by the extracorporeal shock wave stimulates the cells to make the cell membrane structure elastically deform, activate the mechanosensitive ion channel protein on the cell membrane, and produce a series of biological effects (163, 164). Electricity plays a vital role in wound healing by activating Piezo1 channels and Ca2+ influx (154). Knockdown of Piezo1 partially reversed radiation-induced in vitro epithelial-mesenchymal transition and played a therapeutic role in bleomycin-induced pulmonary fibrosis in rats (155). It also suggests the potential role of Piezo1 in tumor radiotherapy. Magnetic nanocomposite materials can activate Piezo1 to induce osteoblast differentiation and promote bone repair (156). Mechanical forces are the main mechanism for activation of Piezo1 channels. The use of physical methods to control the expression of Piezo1 has excellent clinical application prospects (Table 2).
As a newly discovered mechanically sensitive ion channel, Piezo1 plays a vital role in inflammation triggered by mechanical stress imbalance and changes in the local environment of cells. Piezo1 transduces mechanical damage signals into intracellular inflammatory cascades to drive inflammation. Changes in local pressure during the progression of inflammation may further affect the outcome of inflammation through Piezo1 (165, 166). Mechanical stress and inflammation are both dynamic processes. The link between Piezo1 and inflammation is a growing field that helps us understand how individual cells respond to the environment’s continuous and diverse mechanical stimuli.
The discovery of Piezo1 provides new ideas for the treating chronic inflammatory diseases. Inhibiting the transduction of mechanical damage signals at an early stage can reduce the incidence and improve the outcome of chronic inflammation. Although the function of Piezo1 in inflammation has been established, its pharmacological effects are still limited to low-affinity drugs (activators and blockers) with poor solubility and stability. They cannot be used in vivo (167). Therefore, more work is needed to determine more modifiers.
When Piezo1 is used as a treatment strategy, understanding how tissue-specific factors regulate the sensitivity of Piezo1 channels will improve the specificity of any potential treatment. For example, in AS, inhibition of Piezo1 is not the best way to fight AS because it brings about vascular tension and hypertension (168). In addition, some diseases are not caused by changes in the Piezo1 channel’s state but by changes in the Piezo1 channel itself (169, 170). Therefore, Piezo1 drugs with tissue specificity may be the dawn of future disease treatment, and the development of drugs targeting the downstream pathway of Piezo1 is also an alternative approach. These strategies may help reduce disease and treatment costs under mechanical stress, especially chronic inflammatory diseases.
Similar to Piezo1, Piezo2 is a newly discovered mechanosensitive ion channel. Piezo2 has a similar structure to Piezo1 as well as mechanosensitivity (171). Piezo2 is expressed on and exerts mechanotransduction in various mechanosensitive tissues and is associated with various inflammatory diseases (172–174). Human Piezo2 defects and Piezo2 gain-of-function mutations are associated with respiratory insufficiency at birth, chronic obstructive pulmonary disease, and adult sleep apnea (175–177). Piezo2-deficient mice cannot respond to the punctate and dynamic allodynia induced by capsaicin-induced inflammation and nerve damage, indicating that Piezo2 mediates sensitized mechanical pain caused by inflammation and nerve damage (178). Piezo2 may also be a therapeutic strategy for chronic inflammation induced by mechanical stress. Piezo1 and Piezo2 also have signal crosstalk. Piezo1 and Piezo2 are highly expressed in articular chondrocytes and jointly promote Ca2+ signal transduction in chondrocytes caused by mechanical force (31). Inhibition of Piezo1 and Piezo2 reduces the death of cartilage cells after mechanical injury in vivo, but the mechanism of how Piezo1 and Piezo2 channels work together at the molecular level remains unclear.
In addition to the piezoelectric family, MSC also includes degenerations/acid-sensitive channels, TREK/TRAAK, transient receptor potential channels, mechanically sensitive channels of the TMEM16 superfamily, OSCA/TMEM63, these ion channels can sense various forms of force, and Conversion of mechanical stimuli into electrical or chemical signals (179). Some ion channel-mediated signals are also active participants in inflammation (180–182). In the acinar cells of patients with acute pancreatitis, the Piezo1 of the acinar cells is upregulated and induced PLA2 to activate the TRPV4 channel to cause the continuous increase of Ca2+ and damage the mitochondrial function (115). Piezo1 responds to long-term shear stress and regulates the activation of TRPV4 channels in vascular endothelial cells, resulting in continuous Ca2+ inflow (183). The connection between Piezo1 and other MSCs deserves further study.
In short, as a sensory component of mechanical signals, the relationship between Piezo1 channels and inflammation is precise and complex. The discovery of Piezo1 provides a new direction for the treatment of diseases. It inhibits the occurrence and development of inflammatory events in the early stage after mechanical damage is an effective treatment for the prevention and treatment of chronic inflammatory diseases. Piezo1 pharmacology has shown promising prospects. The link between the Piezo1 channel and chronic inflammation and Piezo1 drugs for clinical application will focus on future research.
HL and JH performed the literature search and manuscript writing. ZQ edited and revised the manuscript. XF, FZ, and XW edited the manuscript. GX and FH guided and revised this manuscript. All authors have read and agreed to the published version of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (82060219, 81860259); Natural Science Foundation of Jiangxi Province (20202BAB206033 and 20202BABL206016); Youth Team Project of the Second Affiliated Hospital of Nanchang University (2019YNTD12003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The figures were created with BioRender.com under paid subscription.
1. Fischer LS, Rangarajan S, Sadhanasatish T, Grashoff C. Molecular Force Measurement With Tension Sensors. Annu Rev Biophys (2021) 50:595–616. doi: 10.1146/annurev-biophys-101920-064756
2. Tschumperlin DJ. Why Stress Matters: An Introduction. Methods Mol Biol (2021) 2299:159–69. doi: 10.1007/978-1-0716-1382-5_12
3. Kendroud S, Fitzgerald LA, Murray and A. Hanna. Physiology I, StatPearls Publishing LLC. Physiology, Nociceptive Pathways. Treasure Island (FL: StatPearls Publishing LLC (2021).
4. Stone WL, Basit H, Burns B. Pathology, Inflammation. Treasure Island (FL: StatPearls Publishing LLC (2021).
5. Hannoodee S, Nasuruddin DN. Acute Inflammatory Response. Treasure Island (FL: StatPearls Publishing LLC (2021).
6. Tavakoli J, Diwan AD, Tipper JL. Advanced Strategies for the Regeneration of Lumbar Disc Annulus Fibrosus. Int J Mol Sci (2020) 21(14). doi: 10.3390/ijms21144889
7. Jiang W, Liu H, Wan R, Wu Y, Shi Z, Huang W. Mechanisms Linking Mitochondrial Mechanotransduction and Chondrocyte Biology in the Pathogenesis of Osteoarthritis. Ageing Res Rev (2021) 67:101315. doi: 10.1016/j.arr.2021.101315
8. Shinge SAU, Zhang D, Achu Muluh T, Nie Y, Yu F. Mechanosensitive Piezo1 Channel Evoked-Mechanical Signals in Atherosclerosis. J Inflamm Res (2021) 14:3621–36. doi: 10.2147/JIR.S319789
9. Zhao C, Sun Q, Tang L, Cao Y, Nourse JL, Pathak MM, et al. Mechanosensitive Ion Channel Piezo1 Regulates Diet-Induced Adipose Inflammation and Systemic Insulin Resistance. Front Endocrinol (Lausanne) (2019) 10:373. doi: 10.3389/fendo.2019.00373
10. Paulus WJ, Zile MR. From Systemic Inflammation to Myocardial Fibrosis: The Heart Failure With Preserved Ejection Fraction Paradigm Revisited. Circ Res (2021) 128(10):1451–67. doi: 10.1161/CIRCRESAHA.121.318159
11. Pahwa R, Goyal A, Bansal P, Jialal I. Chronic Inflammation. Treasure Island (FL: StatPearls Publishing LLC (2021).
12. Joshi H, Morley SC. Cells Under Stress: The Mechanical Environment Shapes Inflammasome Responses to Danger Signals. J Leukoc Biol (2019) 106(1):119–25. doi: 10.1002/JLB.3MIR1118-417R
13. Fang XZ, Zhou T, Xu JQ, Wang YX, Sun MM, He YJ, et al. Structure, Kinetic Properties and Biological Function of Mechanosensitive Piezo Channels. Cell Biosci (2021) 11(1):13. doi: 10.1186/s13578-020-00522-z
14. Satoh K, Hata M, Takahara S, Tsuzaki H, Yokota H, Akatsu H, et al. A Novel Membrane Protein, Encoded by the Gene Covering KIAA0233, Is Transcriptionally Induced in Senile Plaque-Associated Astrocytes. Brain Res (2006) 1108(1):19–27. doi: 10.1016/j.brainres.2006.06.050
15. Kamajaya A, Kaiser JT, Lee J, Reid M, Rees DC. The Structure of a Conserved Piezo Channel Domain Reveals a Topologically Distinct β Sandwich Fold. Structure (2014) 22(10):1520–7. doi: 10.1016/j.str.2014.08.009
16. Guo YR, MacKinnon R. Structure-Based Membrane Dome Mechanism for Piezo Mechanosensitivity. Elife (2017) 6. doi: 10.7554/eLife.33660
17. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are Essential Components of Distinct Mechanically Activated Cation Channels. Science (2010) 330(6000):55–60. doi: 10.1126/science.1193270
18. McHugh BJ, Buttery R, Lad Y, Banks S, Haslett C, Sethi T. Integrin Activation by Fam38A Uses a Novel Mechanism of R-Ras Targeting to the Endoplasmic Reticulum. J Cell Sci (2010) 123(Pt 1):51–61. doi: 10.1242/jcs.056424
19. Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, et al. Piezo Proteins are Pore-Forming Subunits of Mechanically Activated Channels. Nature (2012) 483(7388):176–81. doi: 10.1038/nature10812
20. Miyamoto T, et al. Functional Role for Piezo1 in Stretch-Evoked Ca²⁺ Influx and ATP Release in Urothelial Cell Cultures. J Biol Chem (2014) 289(23):16565–75. doi: 10.1074/jbc.M113.528638
21. Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, Davey CF, et al. Mechanical Stretch Triggers Rapid Epithelial Cell Division Through Piezo1. Nature (2017) 543(7643):118–21. doi: 10.1038/nature21407
22. Bagriantsev SN, Gracheva EO, Gallagher PG. Piezo Proteins: Regulators of Mechanosensation and Other Cellular Processes. J Biol Chem (2014) 289(46):31673–81. doi: 10.1074/jbc.R114.612697
23. Ridone P, Vassalli M, Martinac B. Piezo1 Mechanosensitive Channels: What are They and Why are They Important. Biophys Rev (2019) 11(5):795–805. doi: 10.1007/s12551-019-00584-5
24. Li M, Yin H, Yan Z, Li H, Wu J, Wang Y, et al. The Immune Microenvironment in Cartilage Injury and Repair. Acta Biomater (2021) S1742-7061(21)00812-6. doi: 10.1016/j.actbio.2021.12.006
25. Adigun R, Basit H, Murray J. Cell Liquefactive Necrosis. Treasure Island (FL: StatPearls Publishing LLC (2021).
26. Zeng C, Lagier D, Lee JW, Vidal Melo MF. Perioperative Pulmonary Atelectasis: Part I. Biology and Mechanisms. Anesthesiology (2021) 136(1):181–205. doi: 10.1097/ALN.0000000000003943
27. Guillamat-Prats R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells (2021) 10(7):1729. doi: 10.3390/cells10071729
28. Porritt RA, Zemmour D, Abe M, Lee Y, Meena Narayanan M, Teixeira de Carvalho T, et al. NLRP3 Inflammasome Mediates Immune-Stromal Interactions in Vasculitis. Circ Res (2021) 129:e183–e200. doi: 10.1161/CIRCRESAHA.121.319153
29. Orsini EM, Perelas A, Southern BD, Grove LM, Olman MA, Scheraga RG. Stretching the Function of Innate Immune Cells. Front Immunol (2021) 12:767319. doi: 10.3389/fimmu.2021.767319
30. Amin AK, Huntley JS, Bush PG, Simpson AH, Hall AC. Chondrocyte Death in Mechanically Injured Articular Cartilage–The Influence of Extracellular Calcium. J Orthop Res (2009) 27(6):778–84. doi: 10.1002/jor.20809
31. Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, et al. Synergy Between Piezo1 and Piezo2 Channels Confers High-Strain Mechanosensitivity to Articular Cartilage. Proc Natl Acad Sci USA (2014) 111(47):E5114–22. doi: 10.1073/pnas.1414298111
32. Banerjee I, Fuseler JW, Intwala AR, Baudino TA. IL-6 Loss Causes Ventricular Dysfunction, Fibrosis, Reduced Capillary Density, and Dramatically Alters the Cell Populations of the Developing and Adult Heart. Am J Physiol Heart Circ Physiol (2009) 296(5):H1694–704. doi: 10.1152/ajpheart.00908.2008
33. Zhang Y, Jiang L, Huang T, Lu D, Song Y, Wang L, et al. Mechanosensitive Cation Channel Piezo1 Contributes to Ventilator-Induced Lung Injury by Activating RhoA/ROCK1 in Rats. Respir Res (2021) 22(1):250. doi: 10.1186/s12931-021-01844-3
34. Richardson J, Kotevski A, Poole K. From Stretch to Deflection: The Importance of Context in the Activation of Mammalian, Mechanically Activated Ion Channels. FEBS J (2021). doi: 10.1111/febs.16041
35. Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, et al. Mechanosensing is Critical for Axon Growth in the Developing Brain. Nat Neurosci (2016) 19(12):1592–8. doi: 10.1038/nn.4394
36. Segel M, Neumann B, Hill MFE, Weber IP, Viscomi C, Zhao C, et al. Niche Stiffness Underlies the Ageing of Central Nervous System Progenitor Cells. Nature (2019) 573(7772):130–4. doi: 10.1038/s41586-019-1484-9
37. Ryu Y, Iwashita M, Lee W, Uchimura K, Kosodo Y. A Shift in Tissue Stiffness During Hippocampal Maturation Correlates to the Pattern of Neurogenesis and Composition of the Extracellular Matrix. Front Aging Neurosci (2021) 13:709620. doi: 10.3389/fnagi.2021.709620
38. Velasco-Estevez M, Mampay M, Boutin H, Chaney A, Warn P, Sharp A, et al. Infection Augments Expression of Mechanosensing Piezo1 Channels in Amyloid Plaque-Reactive Astrocytes. Front Aging Neurosci (2018) 10:332. doi: 10.3389/fnagi.2018.00332
39. Semyanov A, Verkhratsky A. Astrocytic Processes: From Tripartite Synapses to the Active Milieu. Trends Neurosci (2021) 44(10):781–92. doi: 10.1016/j.tins.2021.07.006
40. Blumenthal NR, Hermanson O, Heimrich B, Shastri VP. Stochastic Nanoroughness Modulates Neuron-Astrocyte Interactions and Function via Mechanosensing Cation Channels. Proc Natl Acad Sci USA (2014) 111(45):16124–9. doi: 10.1073/pnas.1412740111
41. Rodríguez-Gómez JA, Kavanagh E, Engskog-Vlachos P, Engskog MKR, Herrera AJ, Espinosa-Oliva AM, et al. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells (2020) 9(7):1717. doi: 10.3390/cells9071717
42. Geng J, Shi Y, Zhang J, Yang B, Wang P, Yuan W, et al. TLR4 Signalling via Piezo1 Engages and Enhances the Macrophage Mediated Host Response During Bacterial Infection. Nat Commun (2021) 12(1):3519. doi: 10.1038/s41467-021-23683-y
43. Moshayedi P, Ng G, Kwok JC, Yeo GS, Bryant CE, Fawcett JW, et al. The Relationship Between Glial Cell Mechanosensitivity and Foreign Body Reactions in the Central Nervous System. Biomaterials (2014) 35(13):3919–25. doi: 10.1016/j.biomaterials.2014.01.038
44. Liu H, Bian W, Yang D, Yang M, Luo H, et al. Inhibiting the Piezo1 Channel Protects Microglia From Acute Hyperglycaemia Damage Through the JNK1 and mTOR Signalling Pathways. Life Sci (2021) 264:118667. doi: 10.1016/j.lfs.2020.118667
45. Velasco-Estevez M, Gadalla KKE, Liñan-Barba N, Cobb S, Dev KK, Sheridan GK. Inhibition of Piezo1 Attenuates Demyelination in the Central Nervous System. Glia (2020) 68(2):356–75. doi: 10.1002/glia.23722
46. Song Y, Li D, Farrelly O, Miles L, Li F, Kim SE, et al. The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron (2019) 102(2):373–389.e6. doi: 10.1016/j.neuron.2019.01.050
47. Rahman MM, Lendel C. Extracellular Protein Components of Amyloid Plaques and Their Roles in Alzheimer’s Disease Pathology. Mol Neurodegener (2021) 16(1):59. doi: 10.1186/s13024-021-00465-0
48. Ullah R, Park TJ, Huang X, Kim MO. Abnormal Amyloid Beta Metabolism in Systemic Abnormalities and Alzheimer’s Pathology: Insights and Therapeutic Approaches From Periphery. Ageing Res Rev (2021) 71:101451. doi: 10.1016/j.arr.2021.101451
49. Tanaka K, Joshi D, Timalsina S, Schwartz MA. Early Events in Endothelial Flow Sensing. Cytoskeleton (Hoboken) (2021) 78(6):217–31. doi: 10.1002/cm.21652
50. Wang Y, Shi J, Tong X. Cross-Talk Between Mechanosensitive Ion Channels and Calcium Regulatory Proteins in Cardiovascular Health and Disease. Int J Mol Sci (2021) 22(16). doi: 10.3390/ijms22168782
51. Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ, et al. Piezo1 Channels Sense Whole Body Physical Activity to Reset Cardiovascular Homeostasis and Enhance Performance. Nat Commun (2017) 8(1):350. doi: 10.1038/s41467-017-00429-3
52. Beech DJ, Kalli AC. Force Sensing by Piezo Channels in Cardiovascular Health and Disease. Arterioscler Thromb Vasc Biol (2019) 39(11):2228–39. doi: 10.1161/ATVBAHA.119.313348
53. Douguet D, Patel A, Xu A, Vanhoutte PM, Honoré E. Piezo Ion Channels in Cardiovascular Mechanobiology. Trends Pharmacol Sci (2019) 40(12):956–70. doi: 10.1016/j.tips.2019.10.002
54. Miron TR, Flood ED, Tykocki NR, Thompson JM, Watts SW. Identification of Piezo1 Channels in Perivascular Adipose Tissue (PVAT) and Their Potential Role in Vascular Function. Pharmacol Res (2021) 175:105995. doi: 10.1016/j.phrs.2021.105995
55. Jiang F, Yin K, Wu K, Zhang M, Wang S, Cheng H, et al. The Mechanosensitive Piezo1 Channel Mediates Heart Mechano-Chemo Transduction. Nat Commun (2021) 12(1):869. doi: 10.1038/s41467-021-21178-4
56. Emig R, Knodt W, Krussig MJ, Zgierski-Johnston CM, Gorka O, Groß O, et al. Piezo1 Channels Contribute to the Regulation of Human Atrial Fibroblast Mechanical Properties and Matrix Stiffness Sensing. Cells (2021) 10(3):663. doi: 10.3390/cells10030663
57. Lindner D, Zietsch C, Tank J, Sossalla S, Fluschnik N, Hinrichs S, et al. Cardiac Fibroblasts Support Cardiac Inflammation in Heart Failure. Basic Res Cardiol (2014) 109(5):428. doi: 10.1007/s00395-014-0428-7
58. Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 Mediates Myocardial Fibrosis, Concentric Hypertrophy, and Diastolic Dysfunction in Rats. Hypertension (2010) 56(2):225–31. doi: 10.1161/HYPERTENSIONAHA.109.148635
59. Lanzer P, Hannan FM, Lanzer JD, Janzen J, Raggi P, Furniss D, et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J Am Coll Cardiol (2021) 78(11):1145–65. doi: 10.1016/j.jacc.2021.06.049
60. Zhang L, Li Y, Ma X, Liu J, Wang X, Zhang L, et al. Ginsenoside Rg1-Notoginsenoside R1-Protocatechuic Aldehyde Reduces Atherosclerosis and Attenuates Low-Shear Stress-Induced Vascular Endothelial Cell Dysfunction. Front Pharmacol (2020) 11:588259. doi: 10.3389/fphar.2020.588259
61. Atcha H, Jairaman A, Holt JR, Meli VS, Nagalla RR, Veerasubramanian PK, et al. Mechanically Activated Ion Channel Piezo1 Modulates Macrophage Polarization and Stiffness Sensing. Nat Commun (2021) 12(1):3256. doi: 10.1038/s41467-021-23482-5
62. Chiu JJ, Chien S. Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives. Physiol Rev (2011) 91(1):327–87. doi: 10.1152/physrev.00047.2009
63. Mohan S, Mohan N, Sprague EA. Differential Activation of NF-Kappa B in Human Aortic Endothelial Cells Conditioned to Specific Flow Environments. Am J Physiol (1997) 273(2 Pt 1):C572–8. doi: 10.1152/ajpcell.1997.273.2.C572
64. Nagel T, Resnick N, Dewey CF Jr, Gimbrone MA Jr., et al. Vascular Endothelial Cells Respond to Spatial Gradients in Fluid Shear Stress by Enhanced Activation of Transcription Factors. Arterioscler Thromb Vasc Biol (1999) 19(8):1825–34. doi: 10.1161/01.ATV.19.8.1825
65. Feaver RE, Gelfand BD, Wang C, Schwartz MA, Blackman BR, et al. Atheroprone Hemodynamics Regulate Fibronectin Deposition to Create Positive Feedback That Sustains Endothelial Inflammation. Circ Res (2010) 106(11):1703–11. doi: 10.1161/CIRCRESAHA.109.216283
66. Sun Y, Leng P, Song M, Li D, Guo P, Xu X, et al. Piezo1 Activates the NLRP3 Inflammasome in Nucleus Pulposus Cell-Mediated by Ca(2+)/NF-κb Pathway. Int Immunopharmacol (2020) 85:106681. doi: 10.1016/j.intimp.2020.106681
67. Baratchi S, Zaldivia MTK, Wallert M, Loseff-Silver J, Al-Aryahi S, Zamani J, et al. Transcatheter Aortic Valve Implantation Represents an Anti-Inflammatory Therapy Via Reduction of Shear Stress-Induced, Piezo-1-Mediated Monocyte Activation. Circulation (2020) 142(11):1092–105. doi: 10.1161/CIRCULATIONAHA.120.045536
68. Schirmer CM, Malek AM. Patient Based Computational Fluid Dynamic Characterization of Carotid Bifurcation Stenosis Before and After Endovascular Revascularization. J Neurointerv Surg (2012) 4(6):448–54. doi: 10.1136/neurintsurg-2011-010070
69. Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial Cation Channel PIEZO1 Controls Blood Pressure by Mediating Flow-Induced ATP Release. J Clin Invest (2016) 126(12):4527–36. doi: 10.1172/JCI87343
70. Kang S, Kishimoto T. Interplay Between Interleukin-6 Signaling and the Vascular Endothelium in Cytokine Storms. Exp Mol Med (2021) 53(7):1116–23. doi: 10.1038/s12276-021-00649-0
71. Nordlohne J, von Vietinghoff S. Interleukin 17A in Atherosclerosis - Regulation and Pathophysiologic Effector Function. Cytokine (2019) 122:154089. doi: 10.1016/j.cyto.2017.06.016
72. Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, et al. Piezo1, a Mechanically Activated Ion Channel, is Required for Vascular Development in Mice. Proc Natl Acad Sci USA (2014) 111(28):10347–52. doi: 10.1073/pnas.1409233111
73. Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep (2015) 13(6):1161–71. doi: 10.1016/j.celrep.2015.09.072
74. Liang J, Huang B, Yuan G, Chen Y, Liang F, Zeng H, et al. Stretch-Activated Channel Piezo1 is Up-Regulated in Failure Heart and Cardiomyocyte Stimulated by AngII. Am J Transl Res (2017) 9(6):2945–55.
75. Agita A, Alsagaff MT. Inflammation, Immunity, and Hypertension. Acta Med Indones (2017) 49(2):158–65.
76. Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The Mechano-Gated Channel Inhibitor GsMTx4 Reduces the Exercise Pressor Reflex in Decerebrate Rats. J Physiol (2016) 594(3):641–55. doi: 10.1113/JP271714
77. Smith ML. The Histologic Diagnosis of Usual Interstitial Pneumonia of Idiopathic Pulmonary Fibrosis. Where We Are and Where We Need to Go. Mod Pathol (2021) 35(Suppl 1):8–14. doi: 10.1038/s41379-021-00889-5
78. Pioselli B, Salomone F, Mazzola G, Amidani D, Sgarbi E, Amadei F, et al. Pulmonary Surfactant: A Unique Biomaterial With Life-Saving Therapeutic Applications. Curr Med Chem (2021) 29(3):526–90. doi: 10.2174/0929867328666210825110421
79. Diem K, Fauler M, Fois G, Hellmann A, Winokurow N, Schumacher S, et al. Mechanical Stretch Activates Piezo1 in Caveolae of Alveolar Type I Cells to Trigger ATP Release and Paracrine Stimulation of Surfactant Secretion From Alveolar Type II Cells. FASEB J (2020) 34(9):12785–804. doi: 10.1096/fj.202000613RRR
80. Lopez-Rodriguez E, Boden C, Echaide M, Perez-Gil J, Kolb M, Gauldie J, et al. Surfactant Dysfunction During Overexpression of TGF-β1 Precedes Profibrotic Lung Remodeling In Vivo. Am J Physiol Lung Cell Mol Physiol (2016) 310(11):L1260–71. doi: 10.1152/ajplung.00065.2016
81. Deng Z, Fear MW, Suk Choi Y, Wood FM, Allahham A, Mutsaers SE, et al. The Extracellular Matrix and Mechanotransduction in Pulmonary Fibrosis. Int J Biochem Cell Biol (2020) 126:105802. doi: 10.1016/j.biocel.2020.105802
82. Sun M, Sun Y, Feng Z, Kang X, Yang W, Wang Y, et al. New Insights Into the Hippo/YAP Pathway in Idiopathic Pulmonary Fibrosis. Pharmacol Res (2021) 169:105635. doi: 10.1016/j.phrs.2021.105635
83. Liang GP, Xu J, Cao LL, Zeng YH, Chen BX, Yang J, et al. Piezo1 Induced Apoptosis of Type II Pneumocytes During ARDS. Respir Res (2019) 20(1):118. doi: 10.1186/s12931-019-1083-1
84. Jiang L, Zhang Y, Lu D, Huang T, Yan K, Yang W, et al. Mechanosensitive Piezo1 Channel Activation Promotes Ventilator-Induced Lung Injury via Disruption of Endothelial Junctions in ARDS Rats. Biochem Biophys Res Commun (2021) 556:79–86. doi: 10.1016/j.bbrc.2021.03.163
85. Solis AG, Bielecki P, Steach HR, Sharma L, Harman CCD, Yun S, et al. Mechanosensation of Cyclical Force by PIEZO1 is Essential for Innate Immunity. Nature (2019) 573(7772):69–74. doi: 10.1038/s41586-019-1485-8
86. Sato Y, Tansho-Nagakawa S, Ubagai T, Ono Y. Analysis of Immune Responses in Acinetobacter Baumannii-Infected Klotho Knockout Mice: A Mouse Model of Acinetobacter Baumannii Infection in Aged Hosts. Front Immunol (2020) 11:601614. doi: 10.3389/fimmu.2020.601614
87. Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M, et al. Stimulation of Piezo1 by Mechanical Signals Promotes Bone Anabolism. Elife (2019) 8:e49631. doi: 10.7554/eLife.49631
88. Sun W, Chi S, Li Y, Ling S, Tan Y, Xu Y, et al. The Mechanosensitive Piezo1 Channel Is Required for Bone Formation. Elife (2019) 8:e47454. doi: 10.7554/eLife.47454
89. Yoneda M, Suzuki H, Hatano N, Nakano S, Muraki Y, Miyazawa K, et al. PIEZO1 and TRPV4, Which Are Distinct Mechano-Sensors in the Osteoblastic MC3T3-E1 Cells, Modify Cell-Proliferation. Int J Mol Sci (2019) 20(19):4960. doi: 10.3390/ijms20194960
90. Wang L, You X, Lotinun S, Zhang L, Wu N, Zou W. Mechanical Sensing Protein PIEZO1 Regulates Bone Homeostasis via Osteoblast-Osteoclast Crosstalk. Nat Commun (2020) 11(1):282. doi: 10.1038/s41467-019-14146-6
91. Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong Q, et al. Piezo1/2 Mediate Mechanotransduction Essential for Bone Formation Through Concerted Activation of NFAT-YAP1-ß-Catenin. Elife (2020) 9:e52779. doi: 10.7554/eLife.52779
92. Hendrickx G, Fischer V, Liedert A, von Kroge S, Haffner-Luntzer M, Brylka L, et al. Piezo1 Inactivation in Chondrocytes Impairs Trabecular Bone Formation. J Bone Miner Res (2021) 36(2):369–84. doi: 10.1002/jbmr.4198
93. Li XF, Zhang Z, Li XD, Wang TB, Zhang HN. Mechanism of the Piezo1 Protein-Induced Apoptosis of the Chondrocytes Through the MAPK/ERK1/2 Signal Pathway. Zhonghua Yi Xue Za Zhi (2016) 96(31):2472–7.
94. Hwang HS, Kim HA. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int J Mol Sci (2015) 16(11):26035–54. doi: 10.3390/ijms161125943
95. Sun Z, Zheng X, Li S, Zeng B, Yang J, Ling Z, et al. Single Impact Injury of Vertebral Endplates Without Structural Disruption, Initiates Disc Degeneration Through Piezo1 Mediated Inflammation and Metabolism Dysfunction. Spine (Phila Pa 1976) (2021). doi: 10.1097/BRS.0000000000004203
96. Sun Y, Leng P, Guo P, Gao H, Liu Y, Li C, et al. G Protein Coupled Estrogen Receptor Attenuates Mechanical Stress-Mediated Apoptosis of Chondrocyte in Osteoarthritis via Suppression of Piezo1. Mol Med (2021) 27(1):96. doi: 10.1186/s10020-021-00360-w
97. Rauck RL, Gargiulo CA, Ruoff GE, Schnitzer TJ, Trapp RG. Chronic Low Back Pain: New Perspectives and Treatment Guidelines for Primary Care: Part II. Manag Care Interface (1998) 11(3):71–5.
98. Ghaben AL, Scherer PE. Adipogenesis and Metabolic Health. Nat Rev Mol Cell Biol (2019) 20(4):242–58. doi: 10.1038/s41580-018-0093-z
99. Rosen ED, Spiegelman BM. What We Talk About When We Talk About Fat. Cell (2014) 156(1-2):20–44. doi: 10.1016/j.cell.2013.12.012
100. Greenhill C. Plasticity of Fat Cells. Nat Rev Endocrinol (2018) 14(9):504. doi: 10.1038/s41574-018-0053-x
101. Wang S, Cao S, Arhatte M, Li D, Shi Y, Kurz S, et al. Adipocyte Piezo1 Mediates Obesogenic Adipogenesis Through the FGF1/FGFR1 Signaling Pathway in Mice. Nat Commun (2020) 11(1):2303. doi: 10.1038/s41467-020-16026-w
102. Lim Y, O’Rourke S. Interstitial Cystitis. Treasure Island (FL: StatPearls Publishing LLC (2021).
103. Geng J, Geng J, Zhao Q, Zhang T, Xiao B. In Touch With the Mechanosensitive Piezo Channels: Structure, Ion Permeation, and Mechanotransduction. Curr Top Membr (2017) 79:159–95. doi: 10.1016/bs.ctm.2016.11.006
104. Dalghi MG, Clayton DR, Ruiz WG, Al-Bataineh MM, Satlin LM, Kleyman TR, et al. Expression and Distribution of PIEZO1 in the Mouse Urinary Tract. Am J Physiol Renal Physiol (2019) 317(2):F303–f321. doi: 10.1152/ajprenal.00214.2019
105. Liu Q, Sun B, Zhao J, Wang Q, An F, Hu X, et al. Increased Piezo1 Channel Activity in Interstitial Cajal-Like Cells Induces Bladder Hyperactivity by Functionally Interacting With NCX1 in Rats With Cyclophosphamide-Induced Cystitis. Exp Mol Med (2018) 50(5):1–16. doi: 10.1038/s12276-018-0088-z
106. Juszczak K, Maciukiewicz P, Drewa T, Thor PJ. Cajal-Like Interstitial Cells as a Novel Target in Detrusor Overactivity Treatment: True or Myth? Cent Eur J Urol (2014) 66(4):413–7. doi: 10.5173/ceju.2013.04.art5
107. Liu Q, Long Z, Dong X, Zhang T, Zhao J, Sun B, et al. Cyclophosphamide-Induced HCN1 Channel Upregulation in Interstitial Cajal-Like Cells Leads to Bladder Hyperactivity in Mice. Exp Mol Med (2017) 49(4):e319. doi: 10.1038/emm.2017.31
108. Sun B, Dong X, Zhao J, Yang Z, Zhang Y, Li L. Differentiation of Human Urine-Derived Stem Cells Into Interstitial Cells of Cajal-Like Cells by Exogenous Gene Modification: A Preliminary Study. Biochem Biophys Res Commun (2020) 523(1):10–7. doi: 10.1016/j.bbrc.2019.09.121
109. Ander BP, Hurtado C, Raposo CS, Maddaford TG, Deniset JF, Hryshko LV, et al. Differential Sensitivities of the NCX1.1 and NCX1.3 Isoforms of the Na+-Ca2+ Exchanger to Alpha-Linolenic Acid. Cardiovasc Res (2007) 73(2):395–403. doi: 10.1016/j.cardiores.2006.09.013
110. Kavalali ET. Neuronal Ca(2+) Signalling at Rest and During Spontaneous Neurotransmission. J Physiol (2020) 598(9):1649–54. doi: 10.1113/JP276541
111. Berridge MJ. The Versatility and Complexity of Calcium Signalling. Novartis Found Symp (2001) 239:52–64; discussion 64-7, 150-9. doi: 10.1002/0470846674.ch6
112. Strehler EE, Treiman M. Calcium Pumps of Plasma Membrane and Cell Interior. Curr Mol Med (2004) 4(3):323–35. doi: 10.2174/1566524043360735
113. Velasco-Estevez M, Rolle SO, Mampay M, Dev KK, Sheridan GK. Piezo1 Regulates Calcium Oscillations and Cytokine Release From Astrocytes. Glia (2020) 68(1):145–60. doi: 10.1002/glia.23709
114. Prakriya M. The Molecular Physiology of CRAC Channels. Immunol Rev (2009) 231(1):88–98. doi: 10.1111/j.1600-065X.2009.00820.x
115. Swain SM, Romac JM, Shahid RA, Pandol SJ, Liedtke W, Vigna SR, et al. TRPV4 Channel Opening Mediates Pressure-Induced Pancreatitis Initiated by Piezo1 Activation. J Clin Invest (2020) 130(5):2527–41. doi: 10.1172/JCI134111
116. Blythe NM, Muraki K, Ludlow MJ, Stylianidis V, Gilbert HTJ, Evans EL, et al. Mechanically Activated Piezo1 Channels of Cardiac Fibroblasts Stimulate P38 Mitogen-Activated Protein Kinase Activity and Interleukin-6 Secretion. J Biol Chem (2019) 294(46):17395–408. doi: 10.1074/jbc.RA119.009167
117. Albarrán-Juárez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, et al. Piezo1 and G(q)/G(11) Promote Endothelial Inflammation Depending on Flow Pattern and Integrin Activation. J Exp Med (2018) 215(10):2655–72. doi: 10.1084/jem.20180483
118. Wang B, Ke W, Wang K, Li G, Ma L, Lu S, et al. Mechanosensitive Ion Channel Piezo1 Activated by Matrix Stiffness Regulates Oxidative Stress-Induced Senescence and Apoptosis in Human Intervertebral Disc Degeneration. Oxid Med Cell Longev (2021) p:8884922. doi: 10.1155/2021/8884922
119. Lee W, Nims RJ, Savadipour A, Zhang Q, Leddy HA, Liu F, et al. Inflammatory Signaling Sensitizes Piezo1 Mechanotransduction in Articular Chondrocytes as a Pathogenic Feed-Forward Mechanism in Osteoarthritis. Proc Natl Acad Sci USA (2021) 118(13):e2001611118. doi: 10.1073/pnas.2001611118
120. Lohberger B, Kaltenegger H, Weigl L, Mann A, Kullich W, Stuendl N, et al. Mechanical Exposure and Diacerein Treatment Modulates Integrin-FAK-MAPKs Mechanotransduction in Human Osteoarthritis Chondrocytes. Cell Signal (2019) 56:23–30. doi: 10.1016/j.cellsig.2018.12.010
121. Jin Y, Li J, Wang Y, Ye R, Feng X, Jing Z, et al. Functional Role of Mechanosensitive Ion Channel Piezo1 in Human Periodontal Ligament Cells. Angle Orthod (2015) 85(1):87–94. doi: 10.2319/123113-955.1
122. Shen Y, Pan Y, Guo S, Sun L, Zhang C, Wang L. The Roles of Mechanosensitive Ion Channels and Associated Downstream MAPK Signaling Pathways in PDLC Mechanotransduction. Mol Med Rep (2020) 21(5):2113–22. doi: 10.3892/mmr.2020.11006
123. Liu S, Xu X, Fang Z, Ning Y, Deng B, Pan X, et al. Piezo1 Impairs Hepatocellular Tumor Growth via Deregulation of the MAPK-Mediated YAP Signaling Pathway. Cell Calcium (2021) 95:102367. doi: 10.1016/j.ceca.2021.102367
124. Chen X, Wanggou S, Bodalia A, Zhu M, Dong W, Fan JJ, et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron (2018) 100(4):799–815.e7. doi: 10.1016/j.neuron.2018.09.046
125. Mousawi F, Peng H, Li J, Ponnambalam S, Roger S, Zhao H, et al. Chemical Activation of the Piezo1 Channel Drives Mesenchymal Stem Cell Migration via Inducing ATP Release and Activation of P2 Receptor Purinergic Signaling. Stem Cells (2020) 38(3):410–21. doi: 10.1002/stem.3114
126. Chávez-Castillo M, Ortega Á, Cudris-Torres L, Duran P, Rojas M, Manzano A, et al. Specialized Pro-Resolving Lipid Mediators: The Future of Chronic Pain Therapy? Int J Mol Sci (2021) 22(19):10370. doi: 10.3390/ijms221910370
127. Armstrong SA, Herr MJ. Physiology, Nociception. Treasure Island (FL: StatPearls Publishing LLC (2021).
128. Wang L, Zhou H, Zhang M, Liu W, Deng T, Zhao Q, et al. Structure and Mechanogating of the Mammalian Tactile Channel PIEZO2. Nature (2019) 573(7773):225–9. doi: 10.1038/s41586-019-1505-8
129. Wang J, La JH, Hamill OP. PIEZO1 Is Selectively Expressed in Small Diameter Mouse DRG Neurons Distinct From Neurons Strongly Expressing Trpv1. Front Mol Neurosci (2019) 12:178. doi: 10.3389/fnmol.2019.00178
130. Mikhailov N, Leskinen J, Fagerlund I, Poguzhelskaya E, Giniatullina R, Gafurov O, et al. Mechanosensitive Meningeal Nociception via Piezo Channels: Implications for Pulsatile Pain in Migraine? Neuropharmacology (2019) 149:113–23. doi: 10.1016/j.neuropharm.2019.02.015
131. Zhang M, Wang Y, Geng J, Zhou S, Xiao B. Mechanically Activated Piezo Channels Mediate Touch and Suppress Acute Mechanical Pain Response in Mice. Cell Rep (2019) 26(6):1419–31.e4. doi: 10.1016/j.celrep.2019.01.056
132. Ma T, Wang YY, Lu Y, Feng L, Yang YT, Li GH, et al. Inhibition of Piezo1/Ca(2+)/calpain Signaling in the Rat Basal Forebrain Reverses Sleep Deprivation-Induced Fear Memory Impairments. Behav Brain Res (2022) 417:113594. doi: 10.1016/j.bbr.2021.113594
133. Maqbool M, Fekadu G, Jiang X, Bekele F, Tolossa T, Turi E, et al. An Up to Date on Clinical Prospects and Management of Osteoarthritis. Ann Med Surg (Lond) (2021) 72:103077. doi: 10.1016/j.amsu.2021.103077
134. Tan BY, Thach T, Munro YL, Skou ST, Thumboo J, Car J, et al. Complex Lifestyle and Psychological Intervention in Knee Osteoarthritis: Scoping Review of Randomized Controlled Trials. Int J Environ Res Public Health (2021) 18(23):12757. doi: 10.3390/ijerph182312757
135. Benzakour T, Igoumenou V, Mavrogenis AF, Benzakour A. Current Concepts for Lumbar Disc Herniation. Int Orthop (2019) 43(4):841–51. doi: 10.1007/s00264-018-4247-6
136. Frost BA, Camarero-Espinosa S, Foster EJ. Materials for the Spine: Anatomy, Problems, and Solutions. Mater (Basel) (2019) 12(2):253. doi: 10.3390/ma12020253
137. Nakazawa G, Yazdani SK, Finn AV, Vorpahl M, Kolodgie FD, Virmani R. Pathological Findings at Bifurcation Lesions: The Impact of Flow Distribution on Atherosclerosis and Arterial Healing After Stent Implantation. J Am Coll Cardiol (2010) 55(16):1679–87. doi: 10.1016/j.jacc.2010.01.021
138. Modarresi Chahardehi A, Masoumi SA, Bigdeloo M, Arsad H, Lim V. The Effect of Exercise on Patients With Rheumatoid Arthritis on the Modulation of Inflammation. Clin Exp Rheumatol (2021).
139. Wawrzyniak-Gramacka E, Hertmanowska N, Tylutka A, Morawin B, Wacka E, Gutowicz M, et al. The Association of Anti-Inflammatory Diet Ingredients and Lifestyle Exercise With Inflammaging. Nutrients (2021) 13(11):3696. doi: 10.3390/nu13113696
140. Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, et al. Chemical Activation of the Mechanotransduction Channel Piezo1. Elife (2015) 4:e07369. doi: 10.7554/eLife.07369
141. Zhao W, Wei Z, Xin G, Li Y, Yuan J, Ming Y, et al. Piezo1 Initiates Platelet Hyperreactivity and Accelerates Thrombosis in Hypertension. J Thromb Haemost (2021) 19(12):3113–25. doi: 10.1111/jth.15504
142. Wang Y, Chi S, Guo H, Li G, Wang L, Zhao Q, et al. A Lever-Like Transduction Pathway for Long-Distance Chemical- and Mechano-Gating of the Mechanosensitive Piezo1 Channel. Nat Commun (2018) 9(1):1300. doi: 10.1038/s41467-018-03570-9
143. Bae C, Sachs F, Gottlieb PA. The Mechanosensitive Ion Channel Piezo1 Is Inhibited by the Peptide Gsmtx4. Biochemistry (2011) 50(29):6295–300. doi: 10.1021/bi200770q
144. Lawrence KM, Jones RC, Jackson TR, Baylie RL, Abbott B, Bruhn-Olszewska B, et al. Chondroprotection by Urocortin Involves Blockade of the Mechanosensitive Ion Channel Piezo1. Sci Rep (2017) 7(1):5147. doi: 10.1038/s41598-017-04367-4
145. Maneshi MM, Ziegler L, Sachs F, Hua SZ, Gottlieb PA. Enantiomeric Aβ Peptides Inhibit the Fluid Shear Stress Response of PIEZO1. Sci Rep (2018) 8(1):14267. doi: 10.1038/s41598-018-32572-2
146. Shi J, Hyman AJ, De Vecchis D, Chong J, Lichtenstein L, Futers TS, et al. Sphingomyelinase Disables Inactivation in Endogenous PIEZO1 Channels. Cell Rep (2020) 33(1):108225. doi: 10.1016/j.celrep.2020.108225
147. Evans EL, Cuthbertson K, Endesh N, Rode B, Blythe NM, Hyman AJ, et al. Yoda1 Analogue (Dooku1) Which Antagonizes Yoda1-Evoked Activation of Piezo1 and Aortic Relaxation. Br J Pharmacol (2018) 175(10):1744–59. doi: 10.1111/bph.14188
148. Liu S, Pan X, Cheng W, Deng B, He Y, Zhang L, et al. Tubeimoside I Antagonizes Yoda1-Evoked Piezo1 Channel Activation. Front Pharmacol (2020) 11:768. doi: 10.3389/fphar.2020.00768
149. Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 Channels Inhibits Mechanosensitive Piezo Channel Activity by Depleting Membrane Phosphoinositides. Sci Signal (2015) 8(363):ra15. doi: 10.1126/scisignal.2005667
150. Romero LO, Massey AE, Mata-Daboin AD, Sierra-Valdez FJ, Chauhan SC, Cordero-Morales JF, et al. Dietary Fatty Acids Fine-Tune Piezo1 Mechanical Response. Nat Commun (2019) 10(1):1200. doi: 10.1038/s41467-019-09055-7
151. Short B. Cholesterol Helps PIEZO1 Use the Force. J Gen Physiol (2020) 152(8):e202012693. doi: 10.1085/jgp.202012693
152. Qiu Z, Guo J, Kala S, Zhu J, Xian Q, Qiu W, et al. The Mechanosensitive Ion Channel Piezo1 Significantly Mediates In Vitro Ultrasonic Stimulation of Neurons. iScience (2019) 21:448–57. doi: 10.1016/j.isci.2019.10.037
153. Shen X, Song Z, Xu E, Zhou J, Yan F. Sensitization of Nerve Cells to Ultrasound Stimulation Through Piezo1-Targeted Microbubbles. Ultrason Sonochem (2021) 73:105494. doi: 10.1016/j.ultsonch.2021.105494
154. Kim TH, Jeon WY, Ji Y, Park EJ, Yoon DS, Lee NH, et al. Electricity Auto-Generating Skin Patch Promotes Wound Healing Process by Activation of Mechanosensitive Ion Channels. Biomaterials (2021) 275:120948. doi: 10.1016/j.biomaterials.2021.120948
155. Huang JQ, Zhang H, Guo XW, Lu Y, Wang SN, Cheng B, et al. Mechanically Activated Calcium Channel PIEZO1 Modulates Radiation-Induced Epithelial-Mesenchymal Transition by Forming a Positive Feedback With TGF-β1. Front Mol Biosci (2021) 8:725275. doi: 10.3389/fmolb.2021.725275
156. Hao L, Li L, Wang P, Wang Z, Shi X, Guo M, et al. Synergistic Osteogenesis Promoted by Magnetically Actuated Nano-Mechanical Stimuli. Nanoscale (2019) 11(48):23423–37. doi: 10.1039/C9NR07170A
157. Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, et al. Identification of a Peptide Toxin From Grammostola Spatulata Spider Venom That Blocks Cation-Selective Stretch-Activated Channels. J Gen Physiol (2000) 115(5):583–98. doi: 10.1085/jgp.115.5.583
158. Hamill OP, McBride DW Jr. The Pharmacology of Mechanogated Membrane Ion Channels. Pharmacol Rev (1996) 48(2):231–52.
160. Prieto ML, Firouzi K, Khuri-Yakub BT, Maduke M. Activation of Piezo1 But Not Na(V)1.2 Channels by Ultrasound at 43 MHz. Ultrasound Med Biol (2018) 44(6):1217–32.
161. Ha CH, Kim S, Chung J, An SH, Kwon K. Extracorporeal Shock Wave Stimulates Expression of the Angiogenic Genes via Mechanosensory Complex in Endothelial Cells: Mimetic Effect of Fluid Shear Stress in Endothelial Cells. Int J Cardiol (2013) 168(4):4168–77. doi: 10.1016/j.ijcard.2013.07.112
162. Li D, Pellegrino A, Hallack A, Petrinic N, Jérusalem A, Cleveland RO. Response of Single Cells to Shock Waves and Numerically Optimized Waveforms for Cancer Therapy. Biophys J (2018) 114(6):1433–9. doi: 10.1016/j.bpj.2017.09.042
163. d’Agostino MC, Craig K, Tibalt E, Respizzi S. Shock Wave as Biological Therapeutic Tool: From Mechanical Stimulation to Recovery and Healing, Through Mechanotransduction. Int J Surg (2015) 24(Pt B):147–53. doi: 10.1016/j.ijsu.2015.11.030
164. Modena DAO, da Silva CN, Grecco C, Guidi RM, Moreira RG, Coelho AA, et al. Extracorporeal Shockwave: Mechanisms of Action and Physiological Aspects for Cellulite, Body Shaping, and Localized Fat-Systematic Review. J Cosmet Laser Ther (2017) 19(6):314–9. doi: 10.1080/14764172.2017.1334928
165. Nilius B, Honoré E. Sensing Pressure With Ion Channels. Trends Neurosci (2012) 35(8):477–86. doi: 10.1016/j.tins.2012.04.002
166. Douguet D, Honoré E. Mammalian Mechanoelectrical Transduction: Structure and Function of Force-Gated Ion Channels. Cell (2019) 179(2):340–54. doi: 10.1016/j.cell.2019.08.049
167. Xiao B. Levering Mechanically Activated Piezo Channels for Potential Pharmacological Intervention. Annu Rev Pharmacol Toxicol (2020) 60:195–218. doi: 10.1146/annurev-pharmtox-010919-023703
168. Iring A, Jin YJ, Albarrán-Juárez J, Siragusa M, Wang S, Dancs PT, et al. Shear Stress-Induced Endothelial Adrenomedullin Signaling Regulates Vascular Tone and Blood Pressure. J Clin Invest (2019) 129(7):2775–91. doi: 10.1172/JCI123825
169. Alper SL. Genetic Diseases of PIEZO1 and PIEZO2 Dysfunction. Curr Top Membr (2017) 79:97–134. doi: 10.1016/bs.ctm.2017.01.001
170. Martin-Almedina S, Mansour S, Ostergaard P. Human Phenotypes Caused by PIEZO1 Mutations; One Gene, Two Overlapping Phenotypes? J Physiol (2018) 596(6):985–92. doi: 10.1113/JP275718
171. Syeda R. Physiology and Pathophysiology of Mechanically Activated PIEZO Channels. Annu Rev Neurosci (2021) 44:383–402. doi: 10.1146/annurev-neuro-093020-120939
172. Romero LO, Caires R, Nickolls AR, Chesler AT, Cordero-Morales JF, Vásquez V. A Dietary Fatty Acid Counteracts Neuronal Mechanical Sensitization. Nat Commun (2020) 11(1):2997. doi: 10.1038/s41467-020-16816-2
173. Heyburn L, Abutarboush R, Goodrich S, Urioste R, Batuure A, Wheel J, et al. Repeated Low-Level Blast Acutely Alters Brain Cytokines, Neurovascular Proteins, Mechanotransduction, and Neurodegenerative Markers in a Rat Model. Front Cell Neurosci (2021) 15:636707. doi: 10.3389/fncel.2021.636707
174. Liu M, Li Y, Zhong J, Xia L, Dou N. The Effect of IL-6/Piezo2 on the Trigeminal Neuropathic Pain. Aging (Albany NY) (2021) 13(10):13615–25. doi: 10.18632/aging.202887
175. Delle Vedove A, Storbeck M, Heller R, Hölker I, Hebbar M, Shukla A. Biallelic Loss of Proprioception-Related PIEZO2 Causes Muscular Atrophy With Perinatal Respiratory Distress, Arthrogryposis, and Scoliosis. Am J Hum Genet (2016) 99(6):1406–8.
176. Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, et al. Piezo2 Senses Airway Stretch and Mediates Lung Inflation-Induced Apnoea. Nature (2017) 541(7636):176–81. doi: 10.1038/nature20793
177. Wu J, Zhong L, Chen T, Qiu L, Li YF. A Rare Case With Compound Heterozygous Mutations of Piezo-Type Mechanosensitive Ion Channel Component 2 (PIEZO2) Induced Tracheobronchomalacia. Chin Med J (Engl) (2021) 134(10):1254–6. doi: 10.1097/CM9.0000000000001500
178. Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kühnemund J, et al. The Mechanosensitive Ion Channel Piezo2 Mediates Sensitivity to Mechanical Pain in Mice. Sci Transl Med (2018) 10(462):eaat9897. doi: 10.1126/scitranslmed.aat9897
179. Jin P, Jan LY, Jan YN. Mechanosensitive Ion Channels: Structural Features Relevant to Mechanotransduction Mechanisms. Annu Rev Neurosci (2020) 43:207–29. doi: 10.1146/annurev-neuro-070918-050509
180. Schumacher MA. Transient Receptor Potential Channels in Pain and Inflammation: Therapeutic Opportunities. Pain Pract (2010) 10(3):185–200. doi: 10.1111/j.1533-2500.2010.00358.x
181. La JH, Gebhart GF. Colitis Decreases Mechanosensitive K2P Channel Expression and Function in Mouse Colon Sensory Neurons. Am J Physiol Gastrointest Liver Physiol (2011) 301(1):G165–74. doi: 10.1152/ajpgi.00417.2010
182. Silverman HA, Chen A, Kravatz NL, Chavan SS, Chang EH. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front Immunol (2020) 11:590261. doi: 10.3389/fimmu.2020.590261
Keywords: Piezo1, chronic inflammation, mechanical force, Ca 2+, pharmacology, MSc
Citation: Liu H, Hu J, Zheng Q, Feng X, Zhan F, Wang X, Xu G and Hua F (2022) Piezo1 Channels as Force Sensors in Mechanical Force-Related Chronic Inflammation. Front. Immunol. 13:816149. doi: 10.3389/fimmu.2022.816149
Received: 16 November 2021; Accepted: 03 January 2022;
Published: 26 January 2022.
Edited by:
Teun J. De Vries, VU Amsterdam, NetherlandsReviewed by:
Jian Shi, University of Leeds, United KingdomCopyright © 2022 Liu, Hu, Zheng, Feng, Zhan, Wang, Xu and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuzhou Hua, aHVhZnV6aG91QDEyNi5jb20=; Guohai Xu, eHVndW9oYWkxQHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.