
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 16 February 2022
Sec. Vaccines and Molecular Therapeutics
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.814429
Objectives: To evaluate the safety of each anti-TNF therapy for patients with rheumatoid arthritis (RA) and then make the best choice in clinical practice.
Methods: We searched PUBMED, EMBASE, and the Cochrane Library. The deadline for retrieval is August 2021. The ORs, Confidence Intervals (CIs), and p values were calculated by STATA.16.0 software for assessment.
Result: 72 RCTs involving 28332 subjects were included. AEs were more common with adalimumab combined disease-modifying anti-rheumatic drugs (DMARDs) compared with placebo (OR = 1.60, 95% CI: 1.06, 2.42), DMARDs (1.28, 95% CI: 1.08, 1.52), etanercept combined DMARDs (1.32, 95% CI: 1.03, 1.67); certolizumab combined DMARDs compared with placebo (1.63, 95% CI: 1.07, 2.46), DMARDs (1.30, 95% CI: 1.10, 1.54), etanercept combined DMARDs (1.34, 95% CI: 1.05, 1.70). In SAEs, comparisons between treatments showed adalimumab (0.20, 95% CI: 0.07, 0.59), etanercept combined DMARDs (0.39, 95% CI: 0.15, 0.96), golimumab (0.19, 95% CI: 0.05, 0.77), infliximab (0.15, 95% CI: 0.03,0.71) decreased the risk of SAEs compared with golimumab combined DMARDs. In infections, comparisons between treatments showed adalimumab combined DMARDs (0.59, 95% CI: 0.37, 0.95), etanercept (0.49, 95% CI: 0.28, 0.88), etanercept combined DMARDs (0.56, 95% CI: 0.35, 0.91), golimumab combined DMARDs (0.51, 95% CI: 0.31, 0.83) decreased the risk of infections compared with infliximab combined DMARDs. No evidence indicated that the use of TNF-α inhibitors influenced the risk of serious infections, malignant tumors.
Conclusion: In conclusion, we regard etanercept monotherapy as the optimal choice for RA patients in clinical practice when the efficacy is similar. Conversely, certolizumab + DMARDs therapy is not recommended.
Systematic Review Registration: identifier PROSPERO CRD42021276176.
Rheumatoid arthritis (RA) is one of the most prevalent chronic inflammatory diseases, which can cause cartilage and bone damage as well as a disability that carries a substantial burden for both the individual and society (1). Currently, antitumors necrosis factor (anti-TNF) therapy has been established as an efficacious therapeutic strategy in RA (2). TNF-α is a pro-inflammatory cytokine known to have a key role in the pathogenesis of chronic immune-mediated diseases (3). Five TNF-α inhibitors have received regulatory approval for clinical use in rheumatology: adalimumab, golimumab, infliximab, certolizumab, and etanercept (4). They are commonly used in the treatment of rheumatoid arthritis.
Besides therapeutic effects, some studies reported that TNF-α inhibitors may also cause some adverse effects in patients with RA (5–8). Although there have been some pair-wise meta-analyses and network meta-analyses that evaluate the safety of different TNF-α inhibitors therapies for patients with RA. Nevertheless, most of the trials only focused on total AEs and SAEs or just one kind of detailed AEs, and some of the initial meta-analyses were contradicted by subsequent studies. For instance, Bongartz et al. reported that RA patients who were treated by anti-TNF therapies had an increased risk of serious infections and malignancies (9), while another trial evaluating malignancy risk in RA patients concluded that there was no significant evidence of an increased risk of malignancy using TNF-α inhibitors (10).
To evaluate the safety of TNF-α inhibitors in patients with RA, we choose six safety outcomes to systematically assess 10 anti-TNF therapies from 72 RCTs with a sample size of 28332 patients. Our network meta-analysis seeks to infer the risk of adverse effects of two therapies in patients with rheumatoid arthritis by direct and indirect comparisons. Simultaneously, it extracts and analyzes data from all randomized control trials (RCTs) to select the best therapy. The objective of the current study is to better characterize the safety of each anti-TNF therapy for patients with RA and then make the best choice in clinical practice.
We searched PUBMED, EMBASE, and the Cochrane Library with the terms of drugs (adalimumab, certolizumab, etanercept, infliximab, and golimumab) and diseases (rheumatoid arthritis). After matching each “drug” and “disease”, restricting search results with the condition “randomized controlled trial”, we finally form the retrieval expressions that adapt to different databases. The deadline for retrieval is August 2021. Two investigators performed the literature screening according to the inclusion and exclusion criteria independently. The repeated studies were excluded firstly. Afterward, excluded unrelated studies by reading the titles and abstracts. The literature that met the inclusion and exclusion criteria was further screened by reading the full text. Disagreements were resolved by consensus Equations.
RCTs associated with adalimumab, certolizumab, etanercept, infliximab, and golimumab in the treatment of rheumatic diseases are included. Subjects should be greater than or equal to 18 years old and should be diagnosed with rheumatoid arthritis according to American College of Rheumatology criteria or other authoritative criteria. Disease progression, race, nationality, and complications are not limited. For the types of interventions, the experimental groups use TNF-α inhibitors, with or without disease-modifying antirheumatic drugs (DMARDs). The control groups use placebo (with or without DMARDs) or DMARDs alone.
RCTs that accord with any of the following criteria will be excluded: (1) studies with no accessible records of AE, SAE, malignant tumors, infections, severe infections, or malignant tumors (requiring intravenous antibiotic treatment or hospitalization or threatening patient’s life); (2) repetitive studies with shorter follow-up time; (3) studies with improper control (other therapy in experimental group or control group); (4) studies with Jadad score lower than or equal to 3 points; (5) studies with full texts not available.
Data extraction was performed independently by He Bei and Li Yun, and the EndNote software was used to filter duplications and irrelevant literature by reading titles and abstracts. The remaining articles were then browsed in full text to determine whether they met the inclusion criteria. After removing ineligible publications, the two reviewers independently extracted data from each study, and disagreements were resolved by reaching a consensus. From each eligible study, we extracted and summarized the following details: the first author, year of publication, country, the total number of participants, type of TNF-α inhibitors, age range, follow-up time, duration of trials.
Two investigators independently assessed each study’s risk of bias as low, unclear, and high. Disagreements were resolved by consensus. The items included: Random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; other bias.
Two reviewers independently used the modified Jadad scale to assess the quality of RCTs (randomized control trials). NOS includes three aspects (selection, comparability, and exposure for case-control studies or outcomes for cohort studies), as well as scores of 4, 2, and 3, respectively. The modified Jadad scale comprises four parts: generation of the allocation sequence, concealment of allocation, blinding, and incomplete outcome data, and scores of 2, 2, 2, and 1 for four parts, respectively. Studies with scores of 1-3 were considered to be of low quality; 4-7 high quality.
Network meta-analysis was performed to compare each of the 10 anti-TNF therapies. Based on the multivariate framework, the network meta-analysis was conducted using frequency theory, and two program packages, network, and mvmeta, developed by STATA 16 software based on multiple regression theory, were used for statistical analysis. Firstly, an evidence network diagram was drawn to show the comparison between interventions, and the consistency test was conducted according to the existence of closed rings. Second, for counting data, OR was used for calculation, the network meta of adverse drug reactions was analyzed, 95% confidence interval was used for all effect sizes, and 95%CI of OR did not cross effect line 1, indicating that P<0.05 was statistically significant. SUCRA analysis was used to seek therapies that had the highest probability of adverse events, with the higher the SUCRA value, the higher the risk. Stata 16.0 draws a comparative-correction funnel plot to determine whether there is a small sample effect in the analysis and recognition network, to evaluate the publication bias of the final screening. All tests were two-sided with a significance level of 0.05.
By searching databases, we retrieved 3200 original records. After excluding duplicates and irrelevant articles, 211 full-text articles were assessed for eligibility. By reading full-text, 72 articles met the inclusive criteria and exclusive criteria (11–82). The following diagram of the study selection process for this meta-analysis is shown in Figure 1. The 72 articles included 28332 patients, followed up for about 16-104 weeks. 72 articles involved RCT experiments, including 21 adalimumab trials, 13 certolizumab trials, 21 etanercept trials, 9 golimumab trials, and 8 infliximab trials. Table 1 summarizes the relevant characteristics.
58 articles (12, 15, 16, 19, 21–26, 28–38, 40–42, 44–47, 49–56, 58–69, 71–75, 77, 79–82) reported the occurrence of AEs and 23778 RA patients was included. The network of eligible comparisons is shown in Figure 2. Network meta-analysis showed that adalimumab combined DMARDs compared with placebo therapy statistically significantly increased the risk of AEs by 60% (1.60, 95% CI: 1.06, 2.42); compared with DMARDs, the risk of AEs increased by 28% (1.28, 95% CI: 1.08, 1.52) (Table 2 and Figure 3). Certolizumab also found that compared with placebo therapy, the risk of AE increased by 127% (2.27, 95% CI: 1.22, 4.24). In addition, certolizumab combined DMARDs compared with placebo therapy statistically significantly increased the risk of AEs by 63% (1.63, 95% CI: 1.07, 2.46); compared with DMARDs, the risk of AEs increased by 30% (1.30, 95% CI: 1.10, 1.54). Comparisons between treatments showed certolizumab combined DMARDs increased the risk of AEs compared with etanercept combined DMARDs (1.34, 95% CI: 1.05, 1.70); adalimumab combined DMARDs increased the risk of AEs compared with etanercept combined DMARDs (1.32, 95% CI: 1.03, 1.67) (Table 2). There was no statistically significant difference between other comparisons.
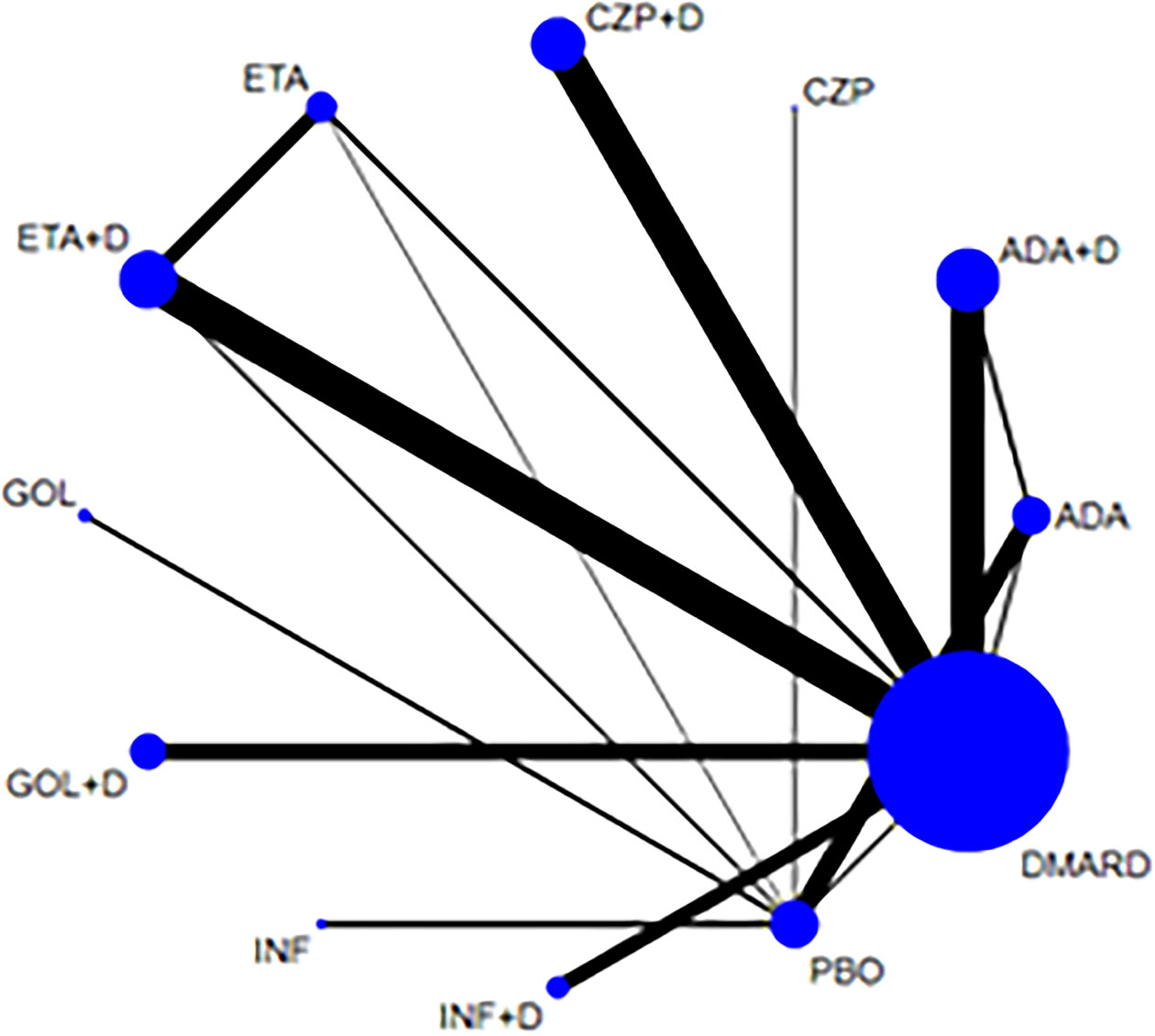
Figure 2 Network of treatment comparisons for adverse events. The size of the circles corresponds to the total number of people. Direct comparable treatments are connected with a line. ADA, adalimumab; + D, plus DMARD; CZP, certolizumab; ETA, etanercept; GOL, golimumab; INF, infliximab; PBO, placebo; DMARD, disease-modifying anti-rheumatic drugs.
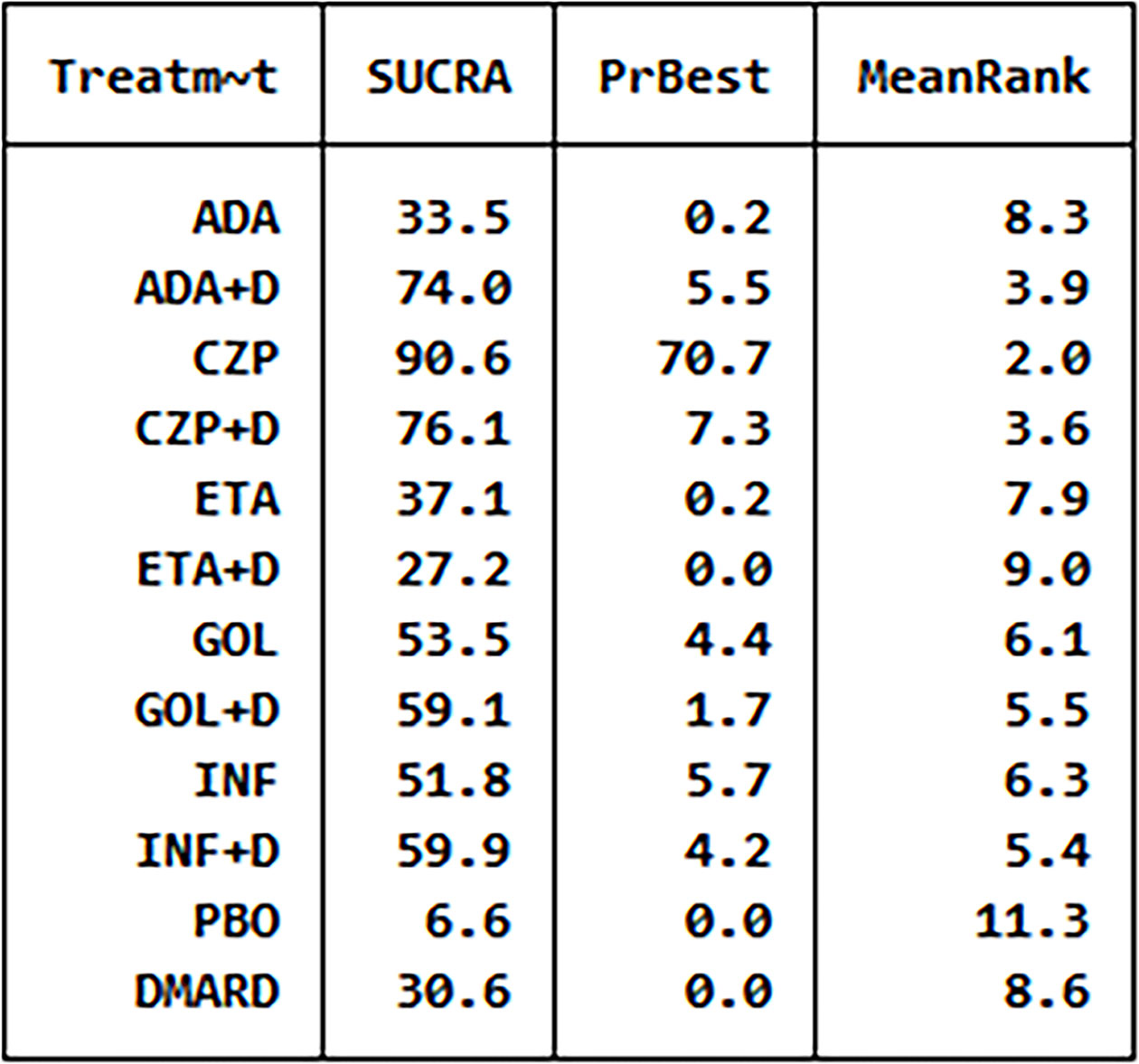
Figure 3 The analysis SUCRA of adverse events for 12 therapies. ADA, adalimumab; + D, plus DMARD; CZP, certolizumab; ETA, etanercept; GOL, golimumab; INF, infliximab; PBO, placebo; DMARD, disease-modifying anti-rheumatic drugs.
We have made global consistency. The test result p-value was 0.9095, so the consistency model could be used. We also established local consistency and the p-value of the test result exceeded 0.05, which was considered local. We analyzed SUCRA to research the probability of adverse events for each therapy. The results indicated that certolizumab had the highest probability to cause AEs (SUCRA = 0.906), while PBO had the lowest probability to cause AEs (SUCRA = 0.066) compared with the other therapies (Figure 3). There was a funnel plot with no obvious asymmetry, indicating no publication bias (Figure 4).
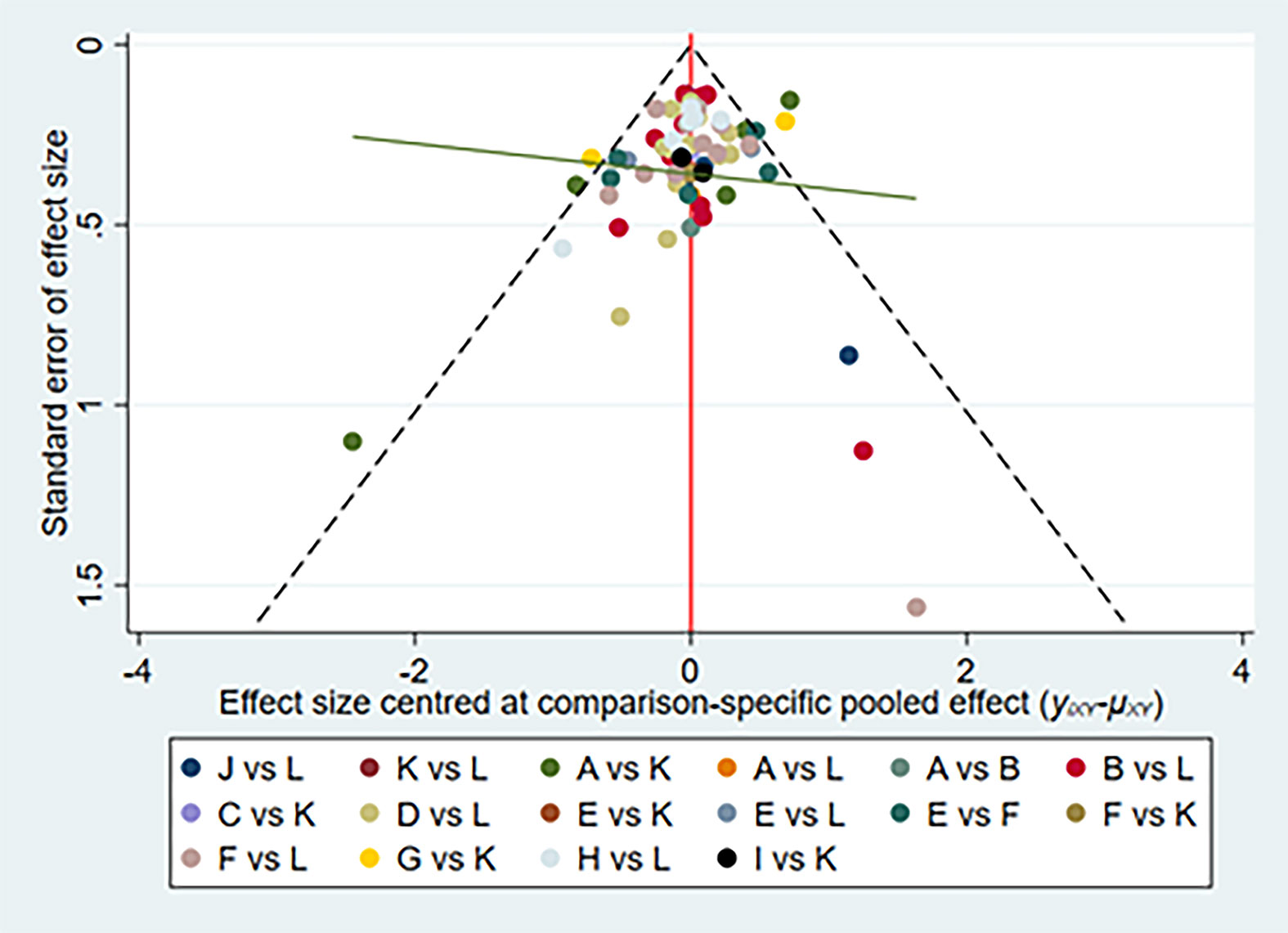
Figure 4 Network of funnel plot for adverse events. A, adalimumab; B, adalimumab + DMARD; C, certolizumab; D, certolizumab + DMARD; F, etanercept; G, etanercept + DMARD; H, golimumab; I, golimumab + DMARD; J, infliximab; K, infliximab + DMARD; L, DMARD; DMARD, disease-modifying anti-rheumatic drugs.
58 articles (12, 13, 15, 17–19, 22, 24–27, 29–32, 34–36, 38, 40–52, 54, 56–60, 62–70, 72–82) reported the occurrence of SAEs and 23805 RA patients was included. The network of eligible comparisons was shown in Figure 5. Network meta-analysis showed that golimumab combined DMARDs compared with placebo therapy statistically significantly increased the risk of SAEs by 227% (3.27, 95% CI: 1.08, 9.92); Compared with DMARDs, the risk of SAEs increased by 170% (2.70, 95% CI: 1.15, 6.32). Comparisons between treatments showed adalimumab (0.20, 95% CI: 0.07, 0.59), etanercept(0.35, 95% CI: 0.12, 1.00), etanercept combined DMARDs (0.39, 95% CI: 0.15, 0.96), golimumab (0.19, 95% CI: 0.05, 0.77) decreased the risk of SAEs compared with golimumab combined DMARDs; adalimumab (0.39, 95% CI: 0.18, 0.84) decreased the risk of SAEs compared with certolizumab combined DMARDs; golimumab combined DMARDs increased the risk of SAEs compared with infliximab (6.50, 95% CI: 1.41, 29.90) (Table 3). There was no statistically significant difference between other comparisons.
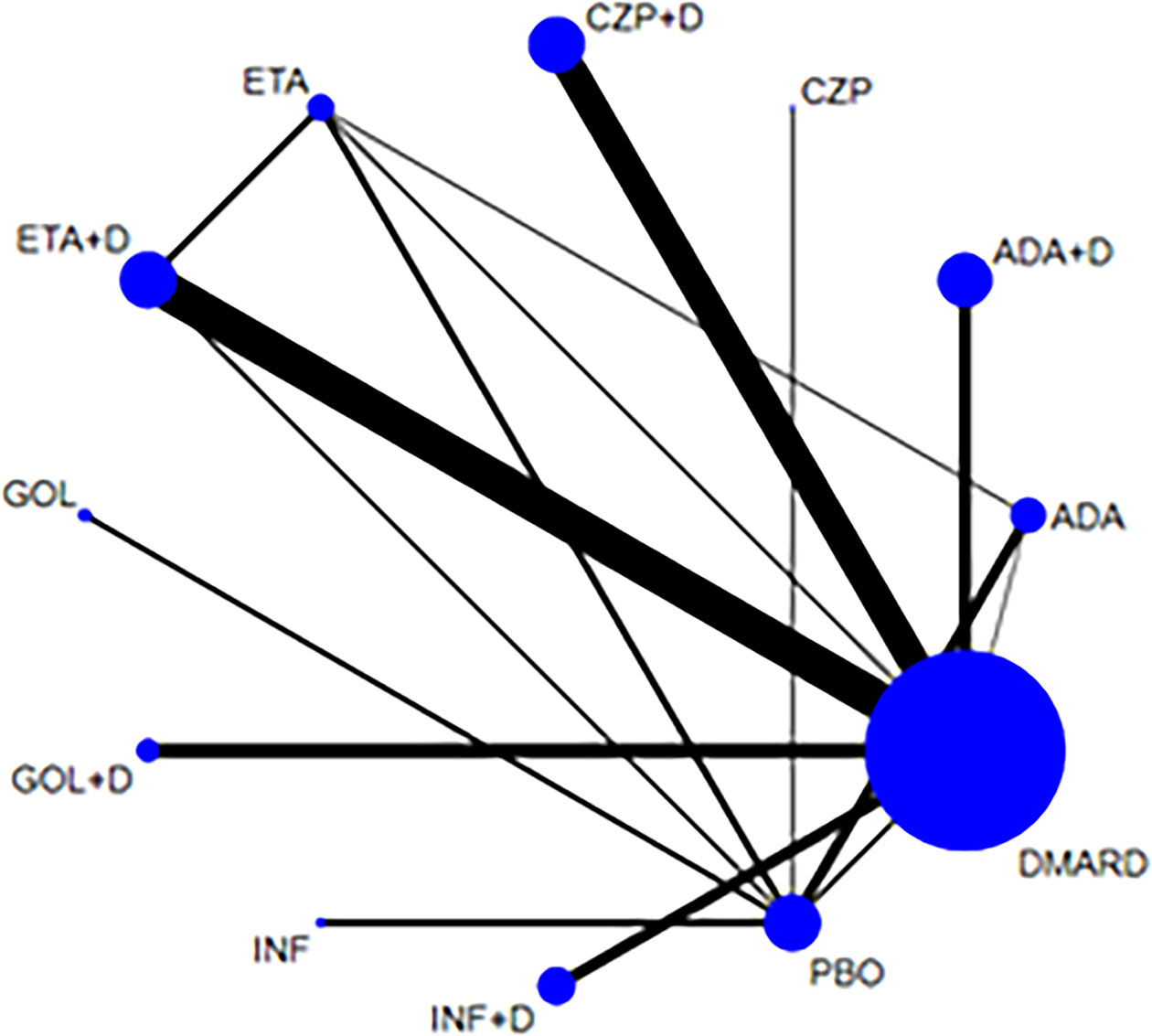
Figure 5 Network of treatment comparisons for serious adverse events. The size of the circles corresponds to the total number of people. Direct comparable treatments are connected with a line. ADA, adalimumab; + D, plus DMARD; CZP, certolizumab; ETA, etanercept; GOL, golimumab; INF, infliximab; PBO, placebo; DMARD, disease-modifying anti-rheumatic drugs.
We did the global consistency test. The test result p-value was 0.8840. We also made local consistency and the test result p-value was greater than 0.05, which was considered to be locally consistent. According to the SUCRA analysis, golimumab combined DMARDs had the highest risk to cause SAEs (SUCRA = 0.940), while adalimumab had the lowest risk to cause SAEs (SUCRA = 0.130) compared with the other 11 therapies (Figure 6). There was a funnel plot asymmetry, with the right corner of the pyramidal part of the funnel missing, which suggested a possible bias (Figure 7).
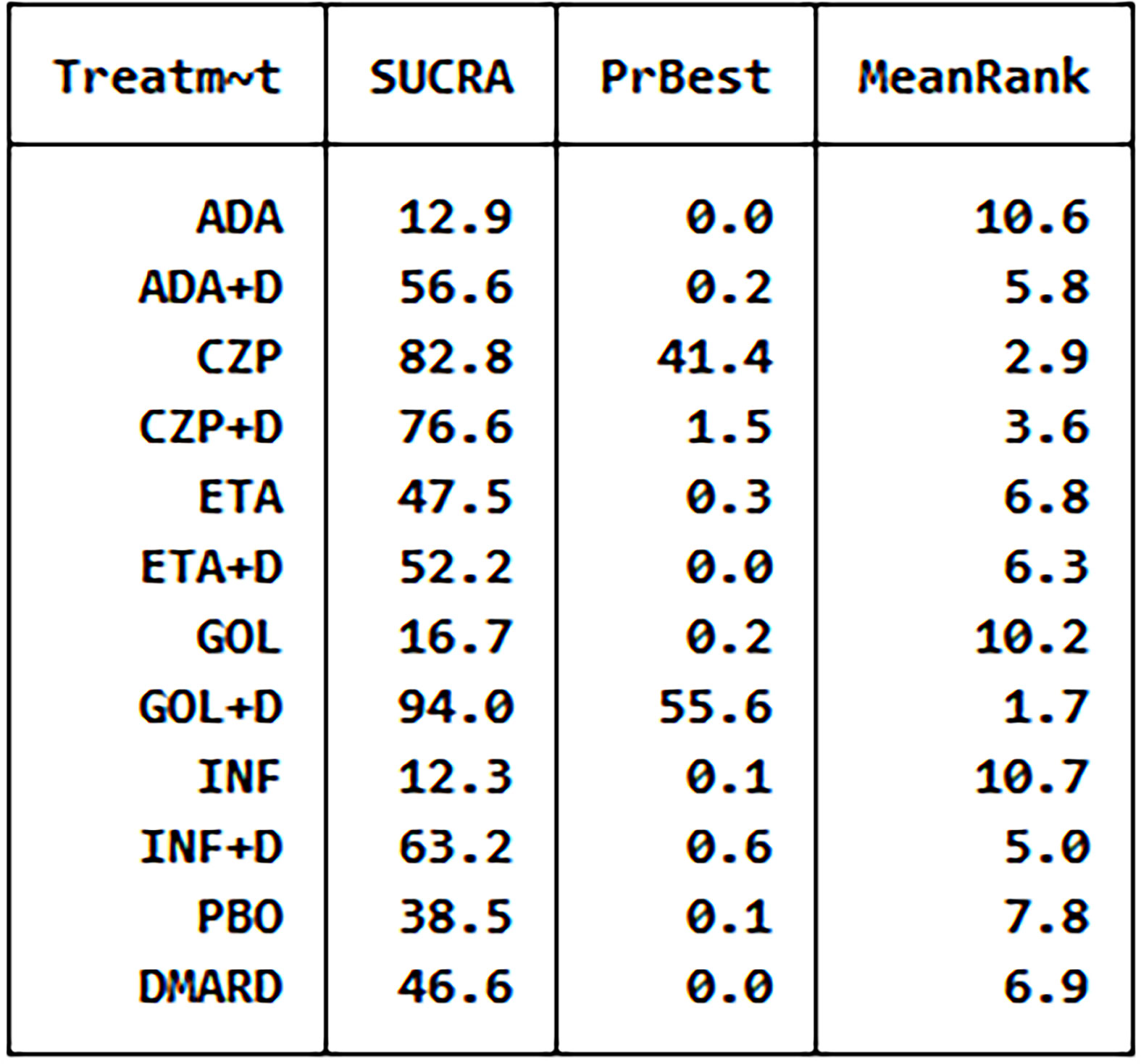
Figure 6 The analysis SUCRA of serious adverse events for 12 therapies. ADA, adalimumab; + D, plus DMARD; CZP, certolizumab; ETA, etanercept; GOL, golimumab; INF, infliximab; PBO, placebo; DMARD, disease-modifying anti-rheumatic drugs.
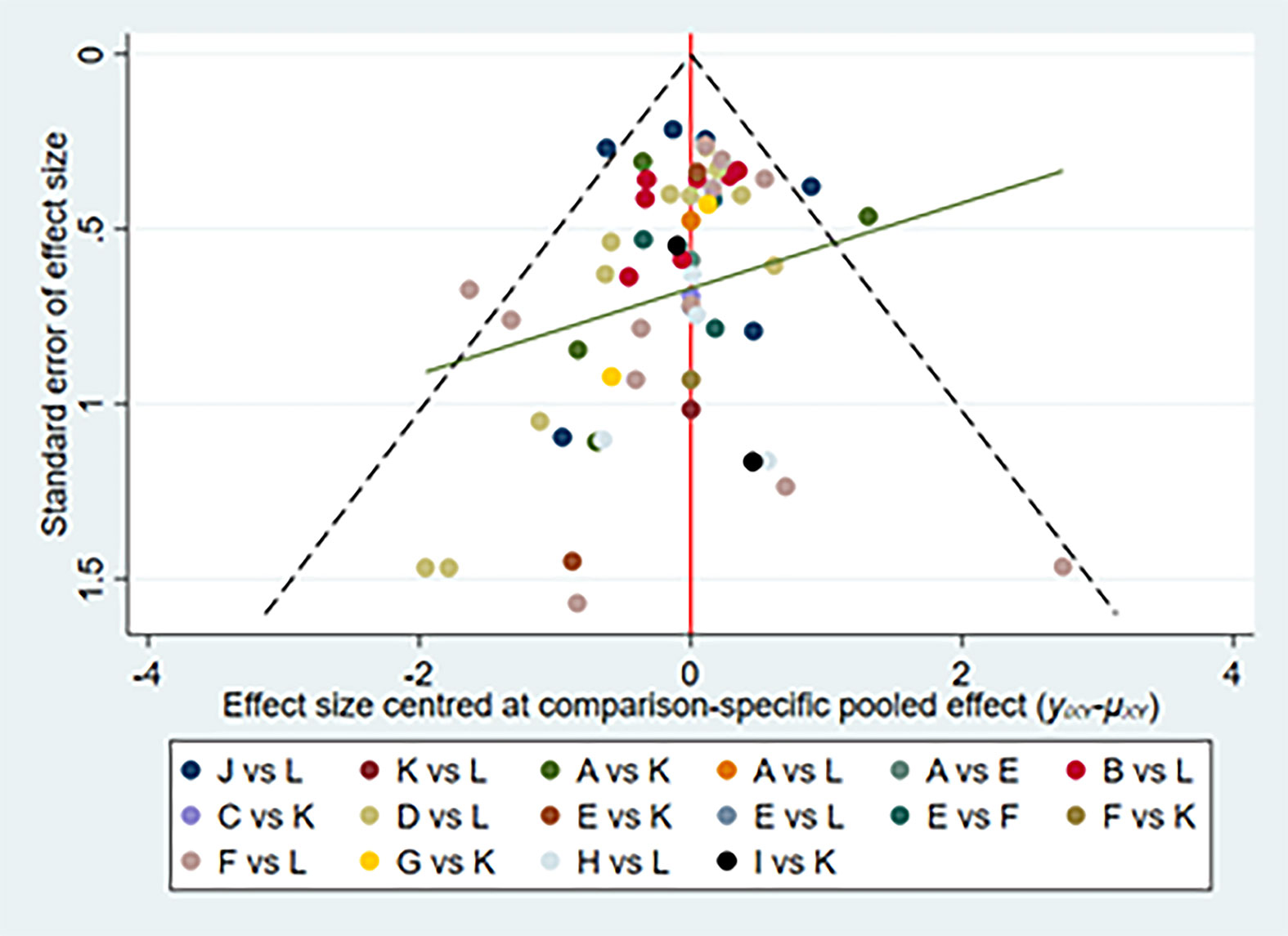
Figure 7 Network of funnel plot for serious adverse events. A, adalimumab; B, adalimumab + DMARD; C, certolizumab; D, certolizumab + DMARD; F, etanercept; G, etanercept + DMARD; H, golimumab; I, golimumab + DMARD; J, infliximab; K, infliximab + DMARD; L, DMARD; DMARD, disease-modifying anti-rheumatic drugs.
40 articles (12, 15, 17, 22, 25–28, 30, 31, 33, 34, 36, 38, 40–42, 45, 49, 54–56, 58–60, 62–66, 72–77, 79–82) reported the occurrence of AEs and 15285 RA patients was included. The network of eligible comparisons was shown in the Supplementary Figure 1. Network meta-analysis showed that golimumab combined DMARDs compared with DMARDs increased the risk of infections by 35% (1.35, 95% CI: 1.10, 1.66); infliximab combined DMARDs compared with DMARDs increased the risk of infections by 102% (2.02, 95% CI: 1.31, 3.11). Comparisons between treatments showed adalimumab combined DMARDs (0.59, 95% CI: 0.37, 0.95), etanercept(0.49, 95% CI: 0.28, 0.88), etanercept combined DMARDs (0.56, 95% CI: 0.35, 0.91), golimumab combined DMARDs (0.51, 95% CI: 0.31, 0.83) decreased the risk of infections compared with infliximab combined DMARDs (supplementary Table 1). There was no statistically significant difference between other comparisons.
We did the global consistency test. The test result p-value was 0.6713. We also established local consistency and the p-value of the test result exceeded 0.05, which was considered local. According to the SUCRA analysis, infliximab combined DMARDs had the highest risk to cause infections (SUCRA = 0.910), while DMARDs had the lowest risk to cause infections SUCRA = 0.210) compared with the other 11 therapies (Supplementary Figure 2). There was a funnel plot (Supplementary Figure 3) with no obvious asymmetry, indicating no publication bias.
55 articles (11–20, 22, 23, 26–38, 40, 42, 45, 47–49, 51, 52, 54, 56–60, 62–66, 68, 69, 72–77, 80–82) reported the occurrence of serious infections, involving a total of 24740 RA patients. The network of eligible comparisons was shown in the Supplementary Figure 4. Network meta-analysis showed that there was no statistically significant difference between 12 therapies (Supplementary Table 2).
We did the global consistency test. The resulting p-value was 0.4900. We also made local consistency and the test result p-value was greater than 0.05, which was considered to be locally consistent. According to the SUCRA analysis, certolizumab had the highest risk to cause serious infections (SUCRA =0.817), while etanercept combined DMARDs had the lowest risk to cause serious infections (SUCRA = 0.285) compared with the other 11 therapies (Supplementary Figure 5). There was a funnel plot asymmetry, with the right corner of the pyramidal part of the funnel missing, which suggested a possible bias (Supplementary Figure 6).
32 articles (14–20, 23, 26, 27, 29–32, 34–39, 43, 47–49, 52, 57, 60, 65, 74, 75, 77, 79) reported the occurrence of malignant tumors, involving 16947 RA patients. The network of eligible comparisons was shown in the Supplementary Figure 7. Mesh meta-analysis showed that there was no statistically significant difference between 12 therapies (Supplementary Table 3).
We did the global consistency test. The test result p-value was 0.6219. We also made local consistency and the test result p-value was greater than 0.05, which was considered to be locally consistent. According to the SUCRA analysis (Supplementary Figure 8), golimumab had the highest risk to cause malignant tumors (SUCRA =0.778), while golimumab combined DMARDs had the lowest risk to cause malignant tumors (SUCRA = 0.285) compared with the other 11 therapies.
Based on the data and information of included RCTs, our study aims to evaluate the risk of adverse effects of 10 anti-TNF therapies in patients with rheumatoid arthritis. All available direct and indirect evidence of various treatment options was analyzed and compared simultaneously by network meta-analysis, which has a great advantage over traditional meta-analysis and makes up for the lack of head-to-head comparisons (83). To comprehensively assess the safety of anti-TNF therapies in RA patients, we also pay attention to detailed AEs like infections, serious infections, malignant tumors. What’s more, our meta-analysis included all RCTs with medium or high quality more recent studies to August 2021, which avoided the deficiency of observational studies and low-quality studies. Therefore, our studies are much more reliable than the other meta-analyses or network meta-analyses.
After analysis of 10 therapies for patients with RA from 72 RCTs, we found golimumab monotherapy, infliximab monotherapy, etanercept monotherapy, adalimumab monotherapy, and etanercept+DMARDs therapy are the safer treatments when the efficacies are similar, they did not increase the risk of all analyzed safety indexes. A comprehensive analysis of the results of network meta-analysis and SUCRA sequencing diagram of adverse reactions showed that etanercept monotherapy is the safest therapy of the 10 therapies was etanercept monotherapy. Etanercept monotherapy was recommended as an alternative treatment due to its good safety outcomes. Certolizumab+DMARDs was considered the worst therapy, so it was necessary to avoid using this therapy. Besides, etanercept may be able to reduce the expression and production of vascular endothelial growth factor, NO, and inducible NO synthase and contribute to having a beneficial effect upon the progression of atherosclerosis, reducing the risk of acute cardiovascular and/or cerebrovascular events (84). This is further demonstrated that etanercept therapy is safer. In 2014, Murdaca et al. investigated the role of single-nucleotide polymorphisms (SNPs) at positions -238, - 308, and + 489 of the TNF-a gene in the response to TNF-a inhibitors (adalimumab, etanercept, or infliximab) and found that the SNP + 489 G allele may promote the response to etanercept. Thus, genetic polymorphisms could be performed before treatment to determine suitability for the etanercept monotherapy (85).
After head-to-head comparisons for the effects of these 10 anti-TNF therapies on the risk of serious infections, malignant tumors, we found no difference of 10 therapies. And compared with PBO therapy or DMARDs therapy, these 10 anti-TNF therapies did not affect the risk of serious infections, malignant tumors, and tuberculosis infection. This may be indicated that these 10 anti-TNF therapies are safe for serious infections, malignant tumors, and tuberculosis infection.
Interestingly, among these 10 anti-TNF therapies, five are TNF-a inhibitor monotherapies and another five are TNF-α inhibitors combinations of DMARDs. It was easy to find that in most cases the safety of TNF-α inhibitor monotherapy was superior to the corresponding TNF-α inhibitors combinations of DMARDs. For example, the SUCRAs of safety outcomes for golimumab+ DMARDs are as follows: 59.1% (AEs), 94.0% (SAEs), and 57.5% (serious infections). By contrast, golimumab monotherapy was safer with corresponding SUCRAs of 53.5%, 16.7%, and 31.8%. Previous researchers have also conducted comparisons between TNF-α inhibitor monotherapy and TNF-α inhibitor combined with MTX. For instance, Breedveld et al. demonstrated that the proportions of RA patients inducing AEs and serious infections were higher under the treatment of adalimumab + DMARDs than the adalimumab monotherapy, which was in line with our results. However, some studies published before also presented no difference between the two kinds of treatment groups (86). Patients with RA treated with etanercept and those treated with etanercept + DMARDs were similar. Thus, further research should be conducted to estimate whether TNF-α inhibitor combined with DMARDs therapy benefits TNF-α inhibitor monotherapy or not.
Although we have made the study as comprehensive as possible, there are still some limitations. Firstly, even though the included trials were all RCTs, the results of safety comparisons among 10 drug therapies still showed some statistical inconsistency. Perhaps the RCTs with contradictions between direct and indirect evidence should be reconsidered. Secondly, 22 trials only had a follow-up time of fewer than 20 weeks. A short duration was not enough to judge the safety of treatment. Thirdly, medication dose, treatment cost, patient compliance, and other influential factors also affected trial homogeneity. Last but not least, different RCTs included in our research had different definitions of safety outcomes. There was still a shortage of clear definitions of AEs and SAEs.
In conclusion, we regard etanercept monotherapy as the optimal choice for RA patients in clinical practice when the efficacy was similar. Conversely, certolizumab+DMARDs therapy was not recommended. It was necessary to conduct long-term studies on patients with RA to provide a more complete assessment of diverse treatments and make a more judicious choice in clinical practice. All efforts should be made to improve the life quality and health standards for patients with RA.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
W-xP, YL, and BH conceived this meta-analysis. YL and XC extracted data. H-rX provided statistical advice and Q-zZ did all statistical analyses. YL, BH, H-rX, and XC checked for statistical inconsistency and interpreted data. YL, BH, and W-wL contributed to data interpretation. YL, BH, and JH drafted the report. H-rX, XC, and JH critically reviewed the article. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.814429/full#supplementary-material
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid Arthritis. Lancet (2016) 388:2023–38. doi: 10.1016/S0140-6736(16)30173-8
2. Koga T, Kawakami A, Tsokos GC. Current Insights and Future Prospects for the Pathogenesis and Treatment for Rheumatoid Arthritis. Clin Immunol (2021) 225:108680. doi: 10.1016/j.clim.2021.108680
3. Bradley JR. TNF-Mediated Inflammatory Disease. J Pathol (2008) 214:149–60. doi: 10.1002/path.2287
4. Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, et al. EULAR Recommendations for the Management of Rheumatoid Arthritis With Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2016 Update. Ann Rheum Dis (2017) 76:960–77. doi: 10.1136/annrheumdis-2016-210715
5. Gottenberg JE, Morel J, Perrodeau E, Bardin T, Combe B, Dougados M, et al. Comparative Effectiveness of Rituximab, Abatacept, and Tocilizumab in Adults With Rheumatoid Arthritis and Inadequate Response to TNF Inhibitors: Prospective Cohort Study. BMJ (2019) 364:l67. doi: 10.1136/bmj.l67
6. Genovese MC, Fleischmann R, Kivitz A, Lee EB, Hoogstraten HV, Kimura T, et al. Efficacy and Safety of Sarilumab in Combination With csDMARDs or as Monotherapy in Subpopulations of Patients With Moderately to Severely Active Rheumatoid Arthritis in Three Phase III Randomized, Controlled Studies. Arthritis Res Ther (2020) 22:139. doi: 10.1186/s13075-020-02194-z
7. Dantes E, Tofolean DE, Fildan AP, Craciun L, Dumea E, Tofolean I, et al. Lethal Disseminated Tuberculosis in Patients Under Biological Treatment - Two Clinical Cases and a Short Review. J Int Med Res (2018) 46:2961–9. doi: 10.1177/0300060518771273
8. Papadopoulos CG, Gartzonikas IK, Pappa TK, Markatseli TE, Migkos MP, Voulgari PV, et al. Eight-Year Survival Study of First-Line Tumour Necrosis Factor Alpha Inhibitors in Rheumatoid Arthritis: Real-World Data From a University Centre Registry. Rheumatol Adv Pract (2019) 3:rkz007. doi: 10.1093/rap/rkz007
9. Bongartz T, Sutton AJ, Sweeting MJ. Anti-TNF Antibody Therapy in Rheumatoid Arthritis and the Risk of Serious Infections and Malignancies: Systematic Review and Meta-Analysis of Rare Harmful Effects in Randomized Controlled Trials. JAMA (2006) 295:2275–85. doi: 10.1001/jama.295.19.2275
10. Maneiro JR, Souto A, Gomez-Reino JJ. Risks of Malignancies Related to Tofacitinib and Biological Drugs in Rheumatoid Arthritis: Systematic Review, Meta-Analysis, and Network Meta-Analysis. Semin Arthritis Rheum (2017) 47:149–56. doi: 10.1016/j.semarthrit.2017.02.007
11. Den Broeder A, van de Putte L, Rau R, Schattenkirchner M, Riel PV, Sander O, et al. A Single Dose, Placebo Controlled Study of the Fully Human Anti-Tumor Necrosis Factor-α Antibody Adalimumab (D2E7) in Patients With Rheumatoid Arthritis. J Rheumatol (2002) 29:2288–98.
12. Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, et al. A Multicenter, Double-Blind, Randomized, Placebo Controlled Trial of Infliximab Combined With Low Dose Methotrexate in Japanese Patients With Rheumatoid Arthritis. J Rheumatol (2006) 33:37–44.
13. van de Putte LBA, Rau R, Breedveld FC, Kalden JR, Malaise MG, van Riel PLC M, et al. Efficacy and Safety of the Fully Human Anti-Tumour Necrosis Factor Alpha Monoclonal Antibody Adalimumab (D2E7) in DMARD Refractory Patients With Rheumatoid Arthritis: A 12 Week, Phase II Study. Ann Rheum Dis (2003) 62:1168–77. doi: 10.1136/ard.2003.009563
14. Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, A Fully Human Anti-Tumor Necrosis Factor Alpha Monoclonal Antibody, for the Treatment of Rheumatoid Arthritis in Patients Taking Concomitant Methotrexate: The ARMadalimumab Trial. Arthritis Rheum (2003) 48:35–45. doi: 10.1002/art.10697
15. Furst DE, Schiff MH, Fleischman n RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, A Fully Human Anti-Tumor Necrosis Factor-α Monoclonal Antibody, and Concomitant Standard Antirheumatic Therapy for the Treatment of Rheumatoid Arthritis: Results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J Rheumatol (2003) 30:2563–71.
16. Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh L, et al. Radiographic, Clinical, and Functional Outcomes of Treatment With Adalimumab (a Human Anti-Tumor Necrosis Factor Monoclonal Antibody) in Patients With Active Rheumatoid Arthritis Receiving Concomitant Methotrexate Therapy: A Randomized, Placebo-Controlled, 52-Week Trial. Arthritis Rheum (2004) 50:1400–11. doi: 10.1002/art.20217
17. Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained Improvement Over Two Years in Physical Function, Structural Damage, and Signs and Symptoms Among Patients With Rheumatoid Arthritis Treated With Infliximab and Methotrexate. Arthritis Rheum (2004) 50:1051–65. doi: 10.1002/art.20159
18. St.Clair EW, van der Heijde D, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of Infliximab and Methotrexate Therapy for Early Rheumatoid Arthritis: A Randomized, Controlled Trial. Arthritis Rheum (2004) 50:3432–43. doi: 10.1002/art.20568
19. van de Putte LB, Atkins C, Malaise M, Sany J, Russell AS, van Riel P, et al. Efficacy and Safety of Adalimumab as Monotherapy in Patients With Rheumatoid Arthritis for Whom Previous Disease Modifying Antirheumatic Drug Treatment Has Failed. Ann Rheum Dis (2004) 63:508–16. doi: 10.1136/ard.2003.013052
20. Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, Vollenhoven R, et al. The PREMIER Study: A Multicenter, Randomized, Double-Blind Clinical Trial of Combination Therapy With Adalimumab Plus Methotrexate Versus Methotrexate Alone or Adalimumab Alone in Patients With Early, Aggressive Rheumatoid Arthritis Who Had Not Had Previous Methotrexate Treatment. Arthritis Rheum (2006) 54:26–37. doi: 10.1002/art.21519
21. Lan JL, Chou SJ, Chen DY, Chen YH, Hsieh TY, Young M Jr. A Comparative Study of Etanercept Plus Methotrexate and Methotrexate Alone in Taiwanese Patients With Active Rheumatoid Arthritis: A 12-Week, Double-Blind, Randomized, Placebo-Controlled Study. J Formos Med Assoc (2004) 103(8):618–23.
22. van Riel PL, Taggart AJ, Sany J, Gaubitz M, Nab HW, Pedersen R, et al. Efficacy and Safety of Combination Etanercept and Methotrexate Versus Etanercept Alone in Patients With Rheumatoid Arthritis With an Inadequate Response to Methotrexate: The ADORE Study. Ann Rheum Dis (2006) 65:1478–83. doi: 10.1136/ard.2005.043299
23. Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, et al. The Safety of Infliximab, Combined With Background Treatments, Among Patients With Rheumatoid Arthritis and Various Comorbidities: A Large, Randomized, Placebo-Controlled Trial. Arthritis Rheum (2006) 54:1075–86. doi: 10.1002/art.21734
24. Zhang FC, Hou Y, Huang F, Wu DH, Bao CD, Ni LQ, et al. Infliximab Versus Placebo in Rheumatoid Arthritis Patients Receiving Concomitant Methotrexate: A Preliminary Study From China. APLAR J Rheumatol (2006) 9:127–30. doi: 10.1111/j.1479-8077.2006.00186.x
25. Kim HY, Lee SK, Song YW, Yoo DH, Koh EM, Yoo B, et al. A Randomized, Double-Blind, Placebo-Controlled, Phase III Study of the Human Anti-Tumor Necrosis Factor Antibody Adalimumab Administered as Subcutaneous Injections in Korean Rheumatoid Arthritis Patients Treated With Methotrexate. APLAR J Rheumatol (2007) 10:9–16. doi: 10.1111/j.1479-8077.2007.00248.x
26. Van Der Heijde D, Klareskog L, Landewé R, Bruyn GAW, Cantagrel A, Durez P, et al. Disease Remission and Sustained Halting of Radiographic Progression With Combination Etanercept and Methotrexate in Patients With Rheumatoid Arthritis. Arthritis Rheum (2007) 56:3928–39. doi: 10.1002/art.23141
27. Weisman MH, Paulus HE, Burch FX, Kivitz AJ, Fierer J, Dunn M, et al. A Placebo-Controlled, Randomized, Double-Blinded Study Evaluating the Safety of Etanercept in Patients With Rheumatoid Arthritis and Concomitant Comorbid Diseases. Rheumatol (Oxford) (2007) 46:1122–5. doi: 10.1093/rheumatology/kem033
28. Bejarano V, Quinn M, Conaghan PG, Reece R, Keenan A-M, Walker D, et al. Effect of the Early Use of the Anti-Tumor Necrosis Factor Adalimumab on the Prevention of Job Loss in Patients With Early Rheumatoid Arthritis. Arthritis Rheum (2008) 59:1467–74. doi: 10.1002/art.24106
29. Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of Methotrexate Monotherapy With a Combination of Methotrexate and Etanercept in Active, Early, Moderate to Severe Rheumatoid Arthritis (COMET): A Randomised, Double-Blind, Parallel Treatment Trial. Lancet (2008) 372:375–82. doi: 10.1016/s0140-6736(08)61000-4
30. Kay J, Matteson EL, Dasgupta B, Nash P, Durez PS, Hall S, et al. Golimumab in Patients With Active Rheumatoid Arthritis Despite Treatment With Methotrexate: A Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study. Arthritis Rheum (2008) 58:964–75. doi: 10.1002/art.23383
31. Miyasaka N. Clinical Investigation in Highly Disease-Affected Rheumatoid Arthritis Patients in Japan With Adalimumab Applying Standard and General Evaluation: The CHANGE Study. Mod Rheumatol (2008) 18:252–62. doi: 10.1007/s10165-008-0045-0
32. Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and Safety of Abatacept or Infliximab vs Placebo in ATTEST: A Phase III, Multi-Centre, Randomised, Double-Blind, Placebo-Controlled Study in Patients With Rheumatoid Arthritis and an Inadequate Response to Methotrexate. Ann Rheum Dis (2008) 67:1096–103. doi: 10.1136/ard.2007.080002
33. Chen DY, Chou SJ, Hsieh TY, Chen YH, Chen HH, Hsieh CW, et al. Randomized, Double-Blind, Placebo-Controlled, Comparative Study of Human Anti-TNF Antibody Adalimumab in Combination With Methotrexate and Methotrexate Alone in Taiwanese Patients With Active Rheumatoid Arthritis. J Formos Med Assoc (2009) 108:310–9. doi: 10.1016/s0929-6646(09)60071-1
34. Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, et al. Golimumab, a Human Anti-Tumor Necrosis Factor α Monoclonal Antibody, Injected Subcutaneously Every Four Weeks in Methotrexate-Naive Patients With Active Rheumatoid Arthritis: Twenty-Four-Week Results of a Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of Golimumab Before Methotrexate as First-Line Therapy for Early-Onset Rheumatoid Arthritis. Arthritis Rheum (2009) 60:2272–83. doi: 10.1002/art.24638
35. Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, et al. Efficacy and Safety of Certolizumab Pegol Monotherapy Every 4 Weeks in Patients With Rheumatoid Arthritis Failing Previous Disease-Modifying Antirheumatic Therapy: The FAST4WARD Study. Ann Rheum Dis (2009) 68:805–11. doi: 10.1136/ard.2008.099291
36. Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a Human Antibody to Tumour Necrosis Factor {Alpha} Given by Monthly Subcutaneous Injections, in Active Rheumatoid Arthritis Despite Methotrexate Therapy: The GO-FORWARD Study. Ann Rheum Dis (2009) 68:789–96. doi: 10.1136/ard.2008.099010
37. Smolen J, Landewé RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and Safety of Certolizumab Pegol Plus Methotrexate in Active Rheumatoid Arthritis: The RAPID 2 Study. A Randomised Controlled Trial. Ann Rheum Dis (2009) 68:797–804. doi: 10.1136/ard.2008.101659
38. Smolen JS, Kay J, Doyle MK, Landewé R, Matteson EL, Wollenhaupt J, et al. Golimumab in Patients With Active Rheumatoid Arthritis After Treatment With Tumour Necrosis Factor Alpha Inhibitors (GO-AFTER Study): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase III Trial. Lancet (2009) 374:210–21. doi: 10.1016/s0140-6736(09)60506-7
39. Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, et al. Etanercept Versus Methotrexate in Patients With Early Rheumatoid Arthritis: Two-Year Radiographic and Clinical Outcomes. Arthritis Rheum (2002) 46(6):1443–50. doi: 10.1002/art.10308
40. Kremer J, et al. Golimumab, a New Human Anti-Tumor Necrosis Factor α Antibody, Administered Intravenously in Patients With Active Rheumatoid Arthritis: Forty-Eight-Week Efficacy and Safety Results of a Phase III Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Rheum (2010) 62:917–28. doi: 10.1002/art.27348
41. Kameda H, Kanbe K, Sato E, Ueki Y, Saito K, Nagaoka S, et al. Continuation of Methotrexate Resulted in Better Clinical and Radiographic Outcomes Than Discontinuation Upon Starting Etanercept in Patients With Rheumatoid Arthritis: 52-Week Results From the JESMR Study. J Rheumatol (2011) 38:1585–92. doi: 10.3899/jrheum.110014
42. Choy E, McKenna F, Vencovsky J, Valente R, Goel N, VanLunen B, et al. Certolizumab Pegol Plus MTX Administered Every 4 Weeks Is Effective in Patients With RA Who Are Partial Responders to MTX. Rheumatol (Oxford) (2012) 51:1226–34. doi: 10.1093/rheumatology/ker519
43. Jobanputra P, Maggs F, Deeming A, Carruthers D, Rankin E, Jordan AC, et al. A Randomised Efficacy and Discontinuation Study of Etanercept Versus Adalimumab (RED SEA) for Rheumatoid Arthritis: A Pragmatic, Unblinded, Non-Inferiority Study of First TNF Inhibitor Use: Outcomes Over 2 Years. BMJ Open (2012) 2(6):e001395. doi: 10.1136/bmjopen-2012-001395
44. Kim HY, Hsu PN, Barba M, Sulaiman W, Robertson D, Vlahos B, et al. Randomized Comparison of Etanercept With Usual Therapy in an Asian Population With Active Rheumatoid Arthritis: The APPEAL Trial. Int J Rheum Dis (2012) 15:188–96. doi: 10.1111/j.1756-185X.2011.01680.x
45. Tanaka Y, Harigai M, Takeuchi T, Yamanaka H, Ishiguro N, Yamamoto K, et al. Golimumab in Combination With Methotrexate in Japanese Patients With Active Rheumatoid Arthritis: Results of the GO-FORTH Study. Ann Rheum Dis (2012) 71:817–24. doi: 10.1136/ard.2011.200317
46. Van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or Adalimumab Versus Placebo in Rheumatoid Arthritis. N Engl J Med (2012) 367:508–19. doi: 10.1056/NEJMoa1112072
47. Weinblatt ME, Fleischmann R WJ, Huizinga T, Emery P, Pope J, Massarotti EM, et al. Efficacy and Safety of Certolizumab Pegol in a Broad Population of Patients With Active Rheumatoid Arthritis: Results From the REALISTIC Phase IIIb Study. Rheumatol (Oxford) (2012) 51:2204–14. doi: 10.1093/rheumatology/kes150
48. Detert J, Bastian H, Listing J, Weiß A, Wassenberg S, Liebhaber A, et al. Induction Therapy With Adalimumab Plus Methotrexate for 24 Weeks Followed by Methotrexate Monotherapy Up to Week 48 Versus Methotrexate Therapy Alone for DMARD-Naïve Patients With Early Rheumatoid Arthritis: HIT HARD, Aninvestigator-Initiated Study. Ann Rheum Dis (2013) 72:844–50. doi: 10.1136/annrheumdis-2012-201612
49. Kavanaugh A, Fleischmann RM, Emery P, Kupper H, Redden L, Guerette B, et al. Clinical, Functional and Radiographic Consequences of Achieving Stable Low Disease Activity and Remission With Adalimumab Plus Methotrexate or Methotrexate Alone in Early Rheumatoid Arthritis: 26-Week Results From the Randomised, Controlled OPTIMA Study. Ann Rheum Dis (2013) 72:64–71. doi: 10.1136/annrheumdis-2011-201247
50. Kim J, Ryu H, Yoo DH, Park SH, Song GG, Park PW, et al. A Clinical Trial and Extension Study of Infliximab in Korean Patients With Active Rheumatoid Arthritis Despite Methotrexate Treatment. J Korean Med Sci (2013) 28:1716–22. doi: 10.3346/jkms.2013.28.12.1716
51. Leirisalo-Repo M, Kautiainen H, Laasonen H, Korpela M, Kauppi MJ, Kaipiainen-Seppänen O, et al. Infliximab for 6 Months Added on Combination Therapy in Early Rheumatoid Arthritis: 2-Year Results From an Investigator-Initiated, Randomised, Double-Blind, Placebo-Controlled Study (the NEO-RACo Study). Ann Rheum Dis (2013) 72:851–7. doi: 10.1136/annrheumdis-2012-201365
52. Smolen JS, Nash P, Durez P, Hall P, Ilivanova E, Irazoque-Palazuelos F, et al. Maintenance, Reduction, or Withdrawal of Etanercept After Treatment With Etanercept and Methotrexate in Patients With Moderate Rheumatoid Arthritis (PRESERVE): A Randomised Controlled Trial. Lancet (2013) 381:918–29. doi: 10.1016/s0140-6736(12)61811-x
53. Takeuchi T, Harigai M, Tanaka Y, Yamanaka H, Ishiguro N, Yamamoto K, et al. Golimumab Monotherapy in Japanese Patients With Active Rheumatoid Arthritis Despite Prior Treatment With Disease-Modifying Antirheumatic Drugs: Results of the Phase 2/3, Multicentre, Randomised, Double-Blind, Placebo-Controlled GO-MONO Study Through 24 Weeks. Ann Rheum Dis (2013) 72:1488–95. doi: 10.1136/annrheumdis-2012-201796
54. Takeuchi T, Miyasaka N, Zang C, Alvarez D, Fletcher T, Wajdula J, et al. A Phase 3 Randomized, Double-Blind, Multicenter Comparative Study Evaluating the Effect of Etanercept Versus Methotrexate on Radiographic Outcomes, Disease Activity, and Safety in Japanese Subjects With Active Rheumatoid Arthritis. Mod Rheumatol (2013) 23:623–33. doi: 10.1007/s10165-012-0742-6
55. Weinblatt ME, Bingham CO, Mendelsohn AM, Kim L, Mack M, Lu J, et al. Intravenous Golimumab Is Effective in Patients With Active Rheumatoid Arthritis Despite Methotrexate Therapy With Responses as Early as Week 2: Results of the Phase 3, Randomised, Multicentre, Double-Blind, Placebo-Controlled GO-FURTHER Trial. Ann Rheum Dis (2013) 72:381–9. doi: 10.1136/annrheumdis-2012-201411
56. Emery P, Hammoudeh M, FitzGerald O, Combe B, Martin-Mola E, H. Buch M, et al. Sustained Remission With Etanercept Tapering in Early Rheumatoid Arthritis. N Engl J Med (2014) 371:1781–92. doi: 10.1056/NEJMoa1316133
57. Hørslev-Petersen K, Hetland ML, Junker P, Pødenphant J, Ellingsen T, Ahlquist P, et al. Adalimumab Added to a Treat-to-Target Strategy With Methotrexate and Intra-Articular Triamcinolone in Early Rheumatoid Arthritis Increased Remission Rates, Function and Quality of Life. The OPERA Study: An Investigator-Initiated, Randomised, Double-Blind, Parallel-Group, Placebo-Controlled Trial. Ann Rheum Dis (2014) 73:654–61. doi: 10.1136/annrheumdis-2012-202735
58. Kennedy WP, Simon JA, Offutt C, Horn P, Herman A, Townsend MJ, et al. Efficacy and Safety of Pateclizumab (Anti-Lymphotoxin-α) Compared to Adalimumab in Rheumatoid Arthritis: A Head-to-Head Phase 2 Randomized Controlled Study (The ALTARA Study). Arthritis Res Ther (2014) 16:467. doi: 10.1186/s13075-014-0467-3
59. Machado DA, Guzman RM, Xavier RM, Simon J, Abraham, Mele L, et al. Open-Label Observation of Addition of Etanercept Versus a Conventional Disease-Modifying Antirheumatic Drug in Subjects With Active Rheumatoid Arthritis Despite Methotrexate Therapy in the Latin American Region. J Clin Rheumatol (2014) 20:25–33. doi: 10.1097/rhu.0000000000000055
60. Nam JL, Villeneuve E, Hensor EMA, Wakefield RJ, Conaghan PG, Green MJ, et al. A Randomised Controlled Trial of Etanercept and Methotrexate to Induce Remission in Early Inflammatory Arthritis: The EMPIRE Trial. Ann Rheum Dis (2014) 73:1027–36. doi: 10.1136/annrheumdis-2013-204882
61. Schiff MH, von Kempis J, Goldblum R, Tesser JR, Mueller RB. Rheumatoid Arthritis Secondary Non-Responders to TNF can Attain an Efficacious and Safe Response by Switching to Certolizumab Pegol: A Phase IV, Randomised, Multicentre, Double-Blind, 12-Week Study, Followed by a 12-Week Open-Label Phase. Ann Rheum Dis (2014) 73:2174–7. doi: 10.1136/annrheumdis-2014-205325
62. Takeuchi T, Yamanaka H, Ishiguro N, Miyasaka N, Mukai M, Matsubara T, et al. Adalimumab, a Human Anti-TNF Monoclonal Antibody, Outcome Study for the Prevention of Joint Damage in Japanese Patients With Early Rheumatoid Arthritis: The HOPEFUL 1 Study. Ann Rheum Dis (2014) 73:536–43. doi: 10.1136/annrheumdis-2012-202433
63. Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and Safety of Certolizumab Pegol Plus Methotrexate in Japanese Rheumatoid Arthritis Patients With an Inadequate Response to Methotrexate: The J-RAPID Randomized, Placebo-Controlled Trial. Mod Rheumatol (2014) 24:715–24. doi: 10.3109/14397595.2013.864224
64. Furst DE, Shaikh SA, Greenwald M, Bennett B, Davies O, Luijtens K, et al. Two Dosing Regimens of Certolizumab Pegol in Patients With Active Rheumatoid Arthritis. Arthritis Care Res (Hoboken) (2015) 67:151–60. doi: 10.1002/acr.22496
65. Smolen JS, Emery P, Ferraccioli GF, Samborski W, Berenbaum F, Davies OR, et al. Certolizumab Pegol in Rheumatoid Arthritis Patients With Low to Moderate Activity: The CERTAIN Double-Blind, Randomised, Placebo-Controlled Trial. Ann Rheum Dis (2015) 74:843–50. doi: 10.1136/annrheumdis-2013-204632
66. Atsumi T, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, et al. The First Double-Blind, Randomised, Parallel-Group Certolizumab Pegol Study in Methotrexate-Naive Early Rheumatoid Arthritis Patients With Poor Prognostic Factors, C-OPERA, Shows Inhibition of Radiographic Progression. Ann Rheum Dis (2016) 75:75–83. doi: 10.1136/annrheumdis-2015-207511
67. Keystone EC, Pope JE, Thorne JC, Poulin-Costello M, Phan-Chronis K, Vieira A, et al. Two-Year Radiographic and Clinical Outcomes From the Canadian Methotrexate and Etanercept Outcome Study in Patients With Rheumatoid Arthritis. Rheumatol (Oxford) (2016) 55:327–34. doi: 10.1093/rheumatology/kev338
68. Li Z, Zhang F, Kay J, Fei K, Han C, Zhuang Y, et al. Efficacy and Safety Results From a Phase 3, Randomized, Placebo-Controlled Trial of Subcutaneous Golimumab in Chinese Patients With Active Rheumatoid Arthritis Despite Methotrexate Therapy. Int J Rheum Dis (2016) 19:1143–56. doi: 10.1111/1756-185x.12723
69. Smolen JS, Burmester GR, Combe B, Curtis JR, Hall S, Haraoui B, et al. Head-To-Head Comparison of Certolizumab Pegol Versus Adalimumab in Rheumatoid Arthritis: 2-Year Efficacy and Safety Results From the Randomised EXXELERATE Study. Lancet (2016) 388:2763–74. doi: 10.1016/s0140-6736(16)31651-8
70. van Vollenhoven RF, Østergaard M, Leirisalo-Repo M, Uhlig T, Jansson M, Larsson E, et al. Full Dose, Reduced Dose or Discontinuation of Etanercept in Rheumatoid Arthritis. Ann Rheum Dis (2016) 75:52–8. doi: 10.1136/annrheumdis-2014-205726
71. Yamanaka H, Nagaoka S, Lee SK, Bae SC, Kasama T, Kobayashi H, et al. Discontinuation of Etanercept After Achievement of Sustained Remission in Patients With Rheumatoid Arthritis Who Initially Had Moderate Disease Activity—Results From the ENCOURAGE Study, A Prospective, International, Multicenter Randomized Study. Modern Rheumatol (2016) 26:651–61. doi: 10.3109/14397595.2015.1123349
72. Emery P, Bingham CO, Burmester GR, Bykerk VP, Furst DE, Mariette X, et al. Certolizumab Pegol in Combination With Dose-Optimised Methotrexate in DMARD-Naïve Patients With Early, Active Rheumatoid Arthritis With Poor Prognostic Factors: 1-Year Results From C-EARLY, A Randomised, Double-Blind, Placebo-Controlled Phase III Study. Ann Rheum Dis (2017) 76:96–104. doi: 10.1136/annrheumdis-2015-209057
73. Pavelka K, Akkoç N, Al- Maini M, Zerbini C, Karateev DE, Nasonov EL, et al. Maintenance of Remission With Combination Etanercept–DMARD Therapy Versus DMARDs Alone in Active Rheumatoid Arthritis: Results of an International Treat-to-Target Study Conducted in Regions With Limited Biologic Access. Rheumatol Int (2017) 37:1469–79. doi: 10.1007/s00296-017-3749-7
74. Taylor PC, Keystone FC, van der Heide D, Weinblatt ME, del Carmen Morales L, Gonzaga JR, et al. Baricitinib Versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med (2017) 376:652–62. doi: 10.1056/NEJMoa1608345
75. Kang YM, Park YE, Park W, Choe JY, Cho CS, Shim SC, et al. Rapid Onset of Efficacy Predicts Response to Therapy With Certolizumab Plus Methotrexate in Patients With Active Rheumatoid Arthritis. Korean J Intern Med (2018) 33:1224–33. doi: 10.3904/kjim.2016.213
76. Bi L, Li Y, He L, Xu H, Jiang Z, Wang Y, et al. Efficacy and Safety of Certolizumab Pegol in Combination With Methotrexate in Methotrexate-Inadequate Responder Chinese Patients With Active Rheumatoid Arthritis: 24-Week Results From a Randomised, Double-Blind, Placebo-Controlled Phase 3 Study. Clin Exp Rheumatol (2019) 37:227–34.
77. Fleischmann R, Pangan A, Song IH, Mysler E, Bessette L, Peterfy C, et al. Upadacitinib Versus Placebo or Adalimumab in Patients With Rheumatoid Arthritis and an Inadequate Response to Methotrexate: Results of a Phase III, Double-Blind, Randomized Controlled Trial. Arthritis Rheumatol (2019) 71:1788–800. doi: 10.1002/art.41032
78. Ducourau E, Rispens T, Samain M, Dernis E, Guilchard FE, Andras L, et al. Methotrexate Effect on Immunogenicity and Long-Term Maintenance of Adalimumab in Axial Spondyloarthritis: A Multicentric Randomised Trial. RMD Open (2020) 6(1):e001047. doi: 10.1136/rmdopen-2019-001047
79. Hetland ML, Haavardsholm EA, Rudin A, Nordström D, Nurmohamed M, Gudbjornsson B, et al. Active Conventional Treatment and Three Different Biological Treatments in Early Rheumatoid Arthritis: Phase IV Investigator Initiated, Randomised, Observer Blinded Clinical Trial. BMJ (Clinical Res ed.) (2020) 371:m4328. doi: 10.1136/bmj.m4328
80. Takeuchi T, Miyasaka N, Pedersen R, Sugiyama N, Hirose T. Radiographic and Clinical Outcomes Following Etanercept Monotherapy in Japanese Methotrexate-Naïve Patients With Active Rheumatoid Arthritis. Mod Rheumatol (2020) 30:259–68. doi: 10.1080/14397595.2019.1589918
81. Combe B, Kivitz A, Tanaka Y, van der Heijde D, Simon JA, Baraf HSB, et al. Filgotinib Versus Placebo or Adalimumab in Patients With Rheumatoid Arthritis and Inadequate Response to Methotrexate: A Phase III Randomised Clinical Trial. Ann Rheum Dis (2021) 80:848–58. doi: 10.1136/annrheumdis-2020-219214
82. Curtis JR, Emery P, Karis E, Haraoui B, Bykerk V, Yen PK, et al. Etanercept or Methotrexate Withdrawal in Rheumatoid Arthritis Patients in Sustained Remission. Arthritis Rheumatol (2021) 73:759–68. doi: 10.1002/art.41589
83. Jansen JP, Fleurence R, Devine B, Boersma C, Annemans L, Cappelleri JC, et al. Interpreting Indirect Treatment Comparisons and Network Meta-Analysis for Health-Care Decision Making: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 1. Value Health (2011) 14:417–28. doi: 10.1016/j.jval.2011.04.002
84. Murdaca G, Spanò F, Cagnati P, Puppo F. Free Radicals and Endothelial Dysfunction: Potential Positive Effects of TNF-α Inhibitors. Redox Rep (2013) 18:95–9. doi: 10.1179/1351000213Y.0000000046
85. Murdaca G, Gulli R, Spanò F, Lantieri F, Burlando M, Parodi A, et al. TNF-Alpha Gene Polymorphisms: Association With Disease Susceptibility and Response to Anti-TNF-Alpha Treatment in Psoriatic Arthritis. J Invest Dermatol (2014) 134:2503–9. doi: 10.1038/jid.2014.123
86. van Riel PL, Taggart AJ, Sany J, Gaubitz M, Nab HW, Pedersen R, et al. Efficacy and Safety of Combination Etanercept and Methotrexate Versus Etanercept Alone in Patients With Rheumatoid Arthritis With an Inadequate Response to Methotrexate: The ADORE Study. Ann Rheum Dis (2006) 65:1478–83. doi: 10.1136/ard.2005.043299
Keywords: adverse effects, TNF-α inhibitors, rheumatoid arthritis, network meta-analysis, serious adverse events
Citation: He B, Li Y, Luo W-w, Cheng X, Xiang H-r, Zhang Q-z, He J and Peng W-x (2022) The Risk of Adverse Effects of TNF-α Inhibitors in Patients With Rheumatoid Arthritis: A Network Meta-Analysis. Front. Immunol. 13:814429. doi: 10.3389/fimmu.2022.814429
Received: 13 November 2021; Accepted: 24 January 2022;
Published: 16 February 2022.
Edited by:
Jochen Mattner, University of Erlangen Nuremberg, GermanyCopyright © 2022 He, Li, Luo, Cheng, Xiang, Zhang, He and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-xing Peng, cHd4LmNzdUBjc3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.