- 1National Institutes for Food and Drug Control, Beijing, China
- 2NHC Key Laboratory of Research on Quality and Standardization of Biotech Products, Beijing, China
- 3NMPA Key Laboratory for Quality Research and Evaluation of Biological Products, Beijing, China
- 4Guangzhou Laboratory, Guangzhou, China
To effectively control and prevent the pandemic of coronavirus disease 2019 (COVID-19), suitable vaccines have been researched and developed rapidly. Currently, 31 COVID-19 vaccines have been approved for emergency use or authorized for conditional marketing, with more than 9.3 billion doses of vaccines being administered globally. However, the continuous emergence of variants with high transmissibility and an ability to escape the immune responses elicited by vaccines poses severe challenges to the effectiveness of approved vaccines. Hundreds of new COVID-19 vaccines based on different technology platforms are in need of a quick evaluation for their efficiencies. Selection and enrollment of a suitable sample of population for conducting these clinical trials is often challenging because the pandemic so widespread and also due to large scale vaccination. To overcome these hurdles, methods of evaluation of vaccine efficiency based on establishment of surrogate endpoints could expedite the further research and development of vaccines. In this review, we have summarized the studies on neutralizing antibody responses and effectiveness of the various COVID-19 vaccines. Using this data we have analyzed the feasibility of establishing surrogate endpoints for evaluating the efficacy of vaccines based on neutralizing antibody titers. The considerations discussed here open up new avenues for devising novel approaches and strategies for the research and develop as well as application of COVID-19 vaccines.
Introduction
The current coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS−CoV−2) is a disaster of unprecedented magnitude in modern times. On the other hand, the rapid research and development (R&D) and application of COVID-19 vaccines in response to the pandemic can be regarded as a miracle in the history of vaccine development. Over a span of less than 2 years, a total of 31 different types of COVID-19 vaccines around the world have been granted Emergency Use Authorizations (EUAs) or marketing approvals (1–3). As of January 15, 2022, more than 9.3 billion doses of COVID-19 vaccines have been administered worldwide (4), but vaccination rates remain low in many regions and countries. The current lots of vaccines are far from perfect. Reduction in immunity over a period of time and lower efficiency against variants seem to be the major concerns. However, vaccines do offer a means to combat the pandemic. The R&D and application of vaccines with superior immunogenicity and universal effectiveness against all SARS−CoV−2 variants, are of utmost priority in addition to increasing vaccination coverage and administrating of a third (booster) COVID-19 vaccine dose (5–7).
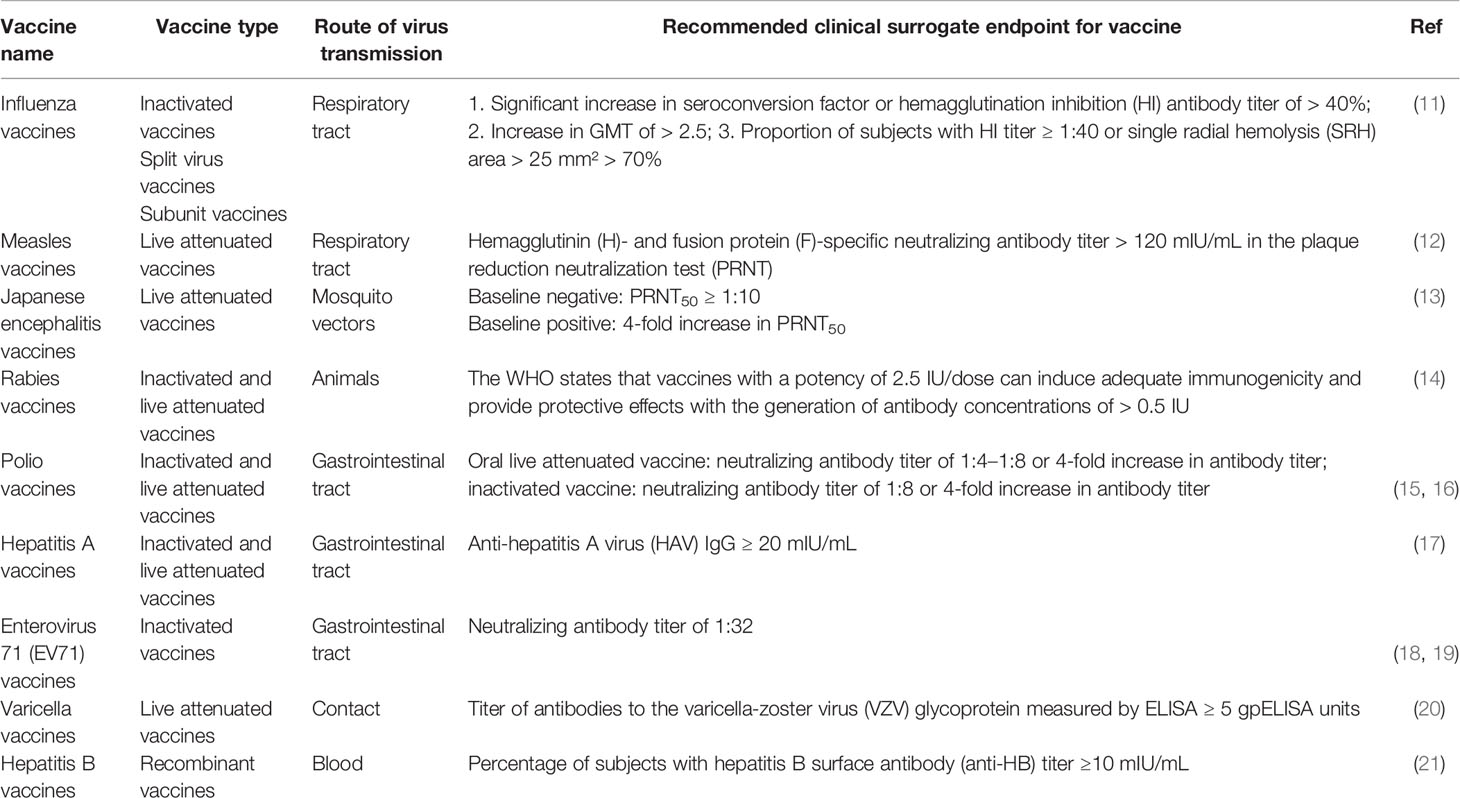
Phase III clinical trials are the main rate-limiting steps in vaccine R&D and application. In countries with high COVID-19 vaccination coverage or viral prevalence, it is difficult to conduct trials on the clinical efficacy of vaccines. The search for methods to rapidly and effectively evaluate vaccine’s effectiveness has, therefore, become a bottleneck in subsequent vaccine R&D efforts. Surrogate endpoints may effectively save clinical time and are compliant with ethical standards. There has been a successful history of using antibodies as surrogate endpoints for other licensed viral vaccines. The key to establishing surrogate endpoints relies on finding a correlationship between vaccine-induced immune responses and the level of protection (8). The cellular and humoral immunity induced by the vaccine synergistically protects the human body from viral infection (9, 10). Antibodies, especially neutralizing antibodies, are key immunological markers that signal the elicitation of defense responses for the prevention and control of viral infections and disease onset. Consequently, the respective protective antibody levels have been used as surrogate endpoints for many viral vaccines such as influenza virus vaccine, measles vaccine, Japanese encephalitis vaccine, rabies vaccine, polio virus vaccine, hepatitis A vaccine, enterovirus 71 (EV71) vaccine, varicella vaccine and hepatitis B vaccine (Table 1) (11–21). In the case of measles, a pre-exposure neutralizing antibodies level in serum samples was positively correlated with clinical protection. Based on this finding, neutralizing antibodies titers of >120 mIU/mL were considered as a reliable surrogate endpoint for measles vaccine (12, 22). Similarly, a titer of ≥ 20 mIU/mL was defined as seroconversion level for hepatitis A vaccine. This seroconversion rate showed a high level of agreement with clinical efficacy data of hepatitis A vaccine. Subsequently, in 2012, hepatitis A virus (HAV) IgG ≥ 20 mIU/mL was included as the antibody threshold level for clinical effectiveness in the World Health Organization (WHO) technical reports on hepatitis A vaccines (17, 23–25). A neutralizing antibody titer of 1:32 for EV71 vaccine has been recommended as the immunological surrogate endpoint because of its association with protection against EV71 (18, 19). Previous clinical studies have confirmed that COVID-19 vaccine-induced humoral immunity generates effective neutralization antibodies against SARS-CoV-2 (26, 27). The studies by Khoury et al. and Earle et al. demonstrated that there is a correlation between the level of neutralizing antibodies responses to SARS-CoV-2 and the protection level of the vaccine, which raises the possibility for the establishment of surrogate endpoint (28, 29). T cell response is essential in inducing high-affinity antibodies and immune memory (30). SARS-CoV-2 specific responsive T cell numbers are associated with protection against COVID-19 and accelerated viral clearance (31, 32). In fact, mRNA vaccination induced early CD4+ T cell responses have been shown to correlate well with long-term humoral immunity. Robust cellular immune memory to SARS-CoV-2 and its variants persist for at least 6 months after mRNA vaccination (33). However, the correlation between the T cell immunity and the protection level of COVID-19 vaccines is not very clear. In addition, compared to T cell immunity, antibody threshold levels are commonly used as surrogate endpoints for viral vaccines due to the ease of establishment of standardized test methods.
At present, a large number of ongoing clinical trials of COVID-19 vaccines have not reported the threshold antibody levels of protection of their respective study populations. In addition, a lack of standardized neutralizing antibody detection methods and the effects of variants on the serum neutralizing activity of vaccines also pose challenges to the establishment of surrogate endpoints (5). This present paper provides a review of the current status of research on neutralizing antibody responses and their utility in gauging the effectiveness of the various COVID-19 vaccines. An analysis of the feasibility of establishing surrogate endpoints based on neutralizing antibody levels in the hope of opening up new horizons for the R&D of an efficient and expedited application of COVID-19 vaccines.
Current Status of R&D and Application of COVID-19 Vaccines
The R&D and application of COVID-19 vaccines have progressed at an unparalleled pace in a global effort to control the ongoing pandemic. Figure 1A shows the major milestones in COVID-19 vaccine R&D and application. In December 2020, merely 1 year after the first report of COVID-19, the BNT162b2 vaccine jointly developed by Pfizer and BioNTech was granted the first-ever EUA in the United Kingdom (UK) (34). This marked the start of large-scale, rapid vaccinations around the world (Figure 1C). Currently, 31 vaccines have received EUAs or conditional marketing authorizations, including two mRNA, eleven inactivated viruses, five adenoviral vectors, twelve recombinant subunits, and one DNA vaccine (1–3). As of January 15, 2022, eight COVID-19 vaccines have been approved for emergency use by the World Health Organization; namely, BNT162b2, AZD1222, Ad26.COV2.S, mRNA-1273, BBIBP-CorV, Coronavac, NVX-CoV2373 and Covaxin (35, 36).
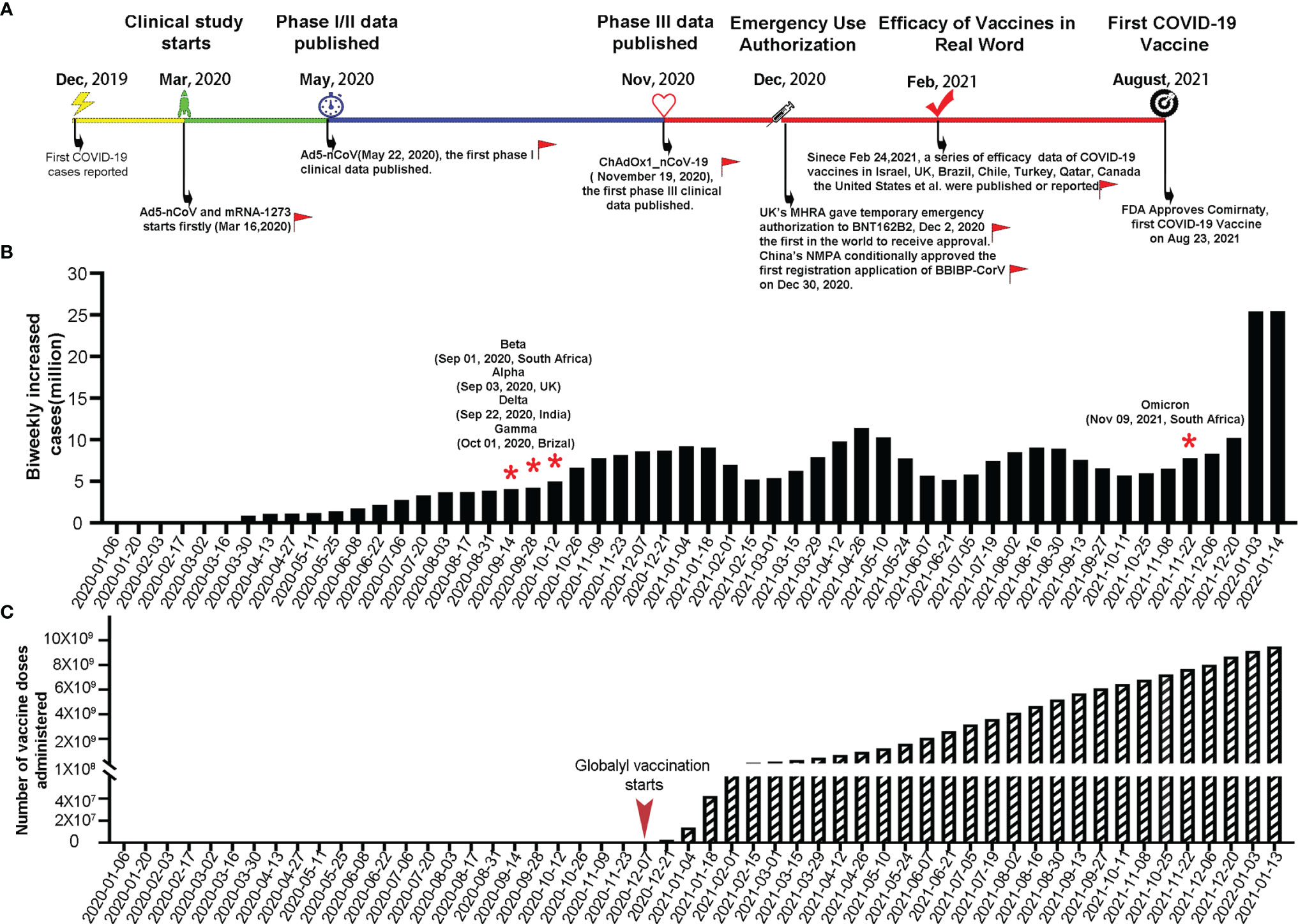
Figure 1 Key milestones in COVID-19 vaccine R&D, biweekly increases in COVID-19 cases worldwide, and total number of COVID-19 vaccine doses administered worldwide. (A) Timeline of the R&D of COVID-19 vaccines. Red flags indicate the key milestones in global COVID-19 vaccine R&D and vaccination with the corresponding dates indicated in parentheses. (B) Biweekly increases in COVID-19 cases worldwide with red stars denoting the time points of emergence of SARS−CoV−2 variants (data source: https://covid19.who.int/, https://cov-lineages.org/index.html). (C) Total number of COVID-19 vaccine doses administered worldwide (data source: https://github.com/owid/covid-19-data/blob/master/public/data/README.md).
At present, the percentage of fully vaccinated people who have received all recommended doses of a COVID-19 vaccine has exceeded 50% in the United States of America (USA), Canada, and many developed countries of the European Union. In the two most populous countries in the world (China and India), the number of COVID-19 vaccine doses administered has reached 2.93 billion and 1.56 billion, respectively, which jointly account for approximately half of the total doses administered globally (37, 38). With rapid vaccination efforts, the number of new COVID-19 cases worldwide showed a significant decrease between January and March 2021 (Figure 1B). However, the emergence of the Alpha, Beta, Gamma, Delta and Omicron variants with high transmissibility and an immune evasion ability has resulted in new waves of the pandemic, with the fourth wave caused by the Omicron variant on the rapid upswing (Figure 1B). In particular, the highly divergent Omicron variant, carrying over 30 mutations in the spike protein, has a substantial growth advantage over previous variant and has been identified in 149 countries since November 2021 (39–41). Neutralization titer against Omicron is significantly reduced in convalescent sera from previous SARS-CoV-2 patient, sera after vaccination and therapeutic monoclonal antibodies (42–44). The combined effects of the spread of the Delta and Omicron variants and decrease in neutralizing antibody titers with time after vaccination, have led to an increase in breakthrough infection rates in the real world. Therefore, current vaccines are no longer capable of effectively preventing breakthrough infections and the transmission of variants. The goal of vaccination seems to have shifted from preventing disease onset to reducing the number of critically ill patients and deaths (38, 45–47).
To cope with the aforementioned situation, booster vaccination has become the strategy of choice for certain countries. Studies have indicated that a 5–25-fold increase in neutralizing antibody titer could be achieved after the administration of a third vaccine dose, which indicates a significant increase in efficacy compared with two vaccine doses (48–51). Real-world data from Israel showed that 12 days after the booster dose, the rate of confirmed infection was lower in the booster group than in the non-booster group by a factor of 11.3 (95% confidence interval (CI): 10.4 to 12.3), and the rate of severe illness decreased by a factor of 19.5 (95% CI: 12.9 to 29.5) (9). On October 21, 2021, Pfizer and BioNTech announced the first phase 3 clinical trial data of a booster dose of their COVID-19 vaccine. When Delta is the prevalent strain, the protection rate of booster vaccine is as high as 95.6% (52). Compared with prime COVID-19 vaccination, a homologous and heterologous booster dose elicits potent neutralization titers against Omicron variant, which increasing vaccine effectiveness (28, 53–57). Currently, some countries, including Israel, the USA, the UK, Switzerland, Germany, and China, have already launched booster vaccination campaigns (58–60). However, research data on booster vaccination are relatively scarce. In particular, the duration of retention of adequate neutralizing antibody levels and mechanisms of titer decline after the booster shot remain unclear. Importantly, the safety of this approach has also not been adequately demonstrated yet (61). Considering that infectious diseases know no borders and the vaccination coverage rates worldwide remain low, the WHO has repeatedly called for developed countries to refrain from the widespread rollout of booster shots until the percentage of fully vaccinated people of other nations have been adequately increased because the vaccination of a high percentage of the world’s population may serves as the most effective pandemic prevention and control strategy (62, 63). On October 26, 2021, the WHO Emergency Committee acknowledged that the COVID-19 pandemic is far from over, calling for the development of vaccines, diagnostic tools and therapeutics for long-term control of the pandemic (7). To curb the spread of variants, institutions and companies around the world have embarked on the R&D of multi-variant COVID-19 vaccines (64–66).
Studies on the Correlation Between Neutralizing Antibody Levels and Immunological Protection
The establishment of immunological surrogate endpoints is aimed at finding relevant indicators of vaccine protection through animal or human challenge experiments, and then using clinical data to obtain the relationship between immune protection indicators and clinical protection through different data statistical analysis models. Such an exercise produces the initial surrogate endpoints. Neutralizing antibody levels are generally used as the primary surrogate endpoint of the immunological protection of viral vaccines. Results of animal challenge models, efficacy of monoclonal antibodies, data from clinical trials of different vaccines, and real-world data of COVID-19 vaccines in many countries, have demonstrated the existence of a certain correlation between neutralizing antibody levels and an vaccine’s effectiveness (5, 28, 67–71).
Immunological Protection in Nonhuman Primates
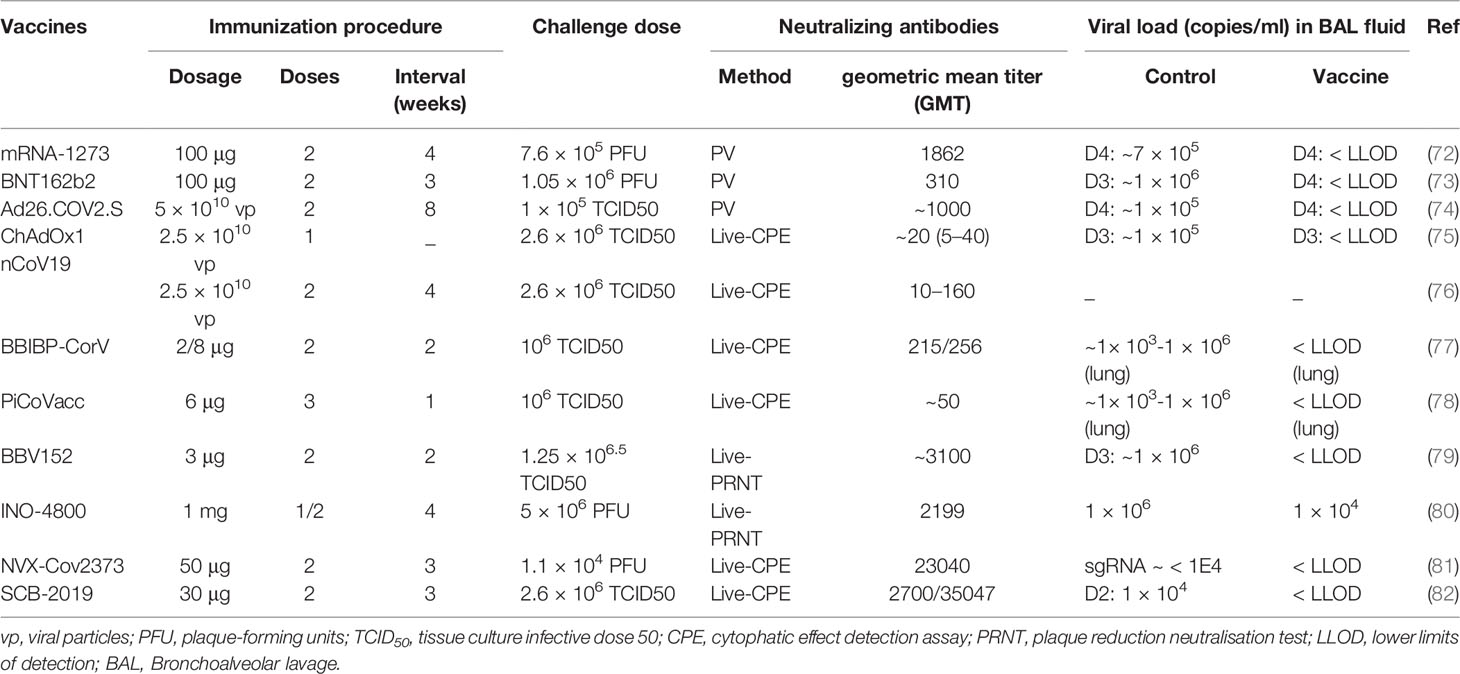
Data from preclinical nonhuman primates challenge studies investigating the correlation between neutralizing antibody levels and vaccine effectiveness have indicated that vaccines developed using various technologies are capable of inducing the production of neutralizing antibodies in nonhuman primates. The neutralizing antibody titers induced in nonhuman primates by the vast majority of vaccine candidates tested in Phase III clinical trials are within the range of 100–5000. Despite significant differences in neutralizing antibody levels, all of these vaccines are capable of reducing the pathological response and viral load in the bronchoalveolar lavage or lungs to a certain extent (Table 2). Studies on the effectiveness of adenoviral vector vaccines (Johnson & Johnson) (76) and DNA vaccines (80) have revealed that the viral load in lungs is negatively correlated with the neutralizing antibody titer (R values: −0.5714–−0.7702) and that the neutralizing antibody titer must not be lower than 100–250 for the vaccine in order to provide full protection.
Phase III Clinical Trials Data
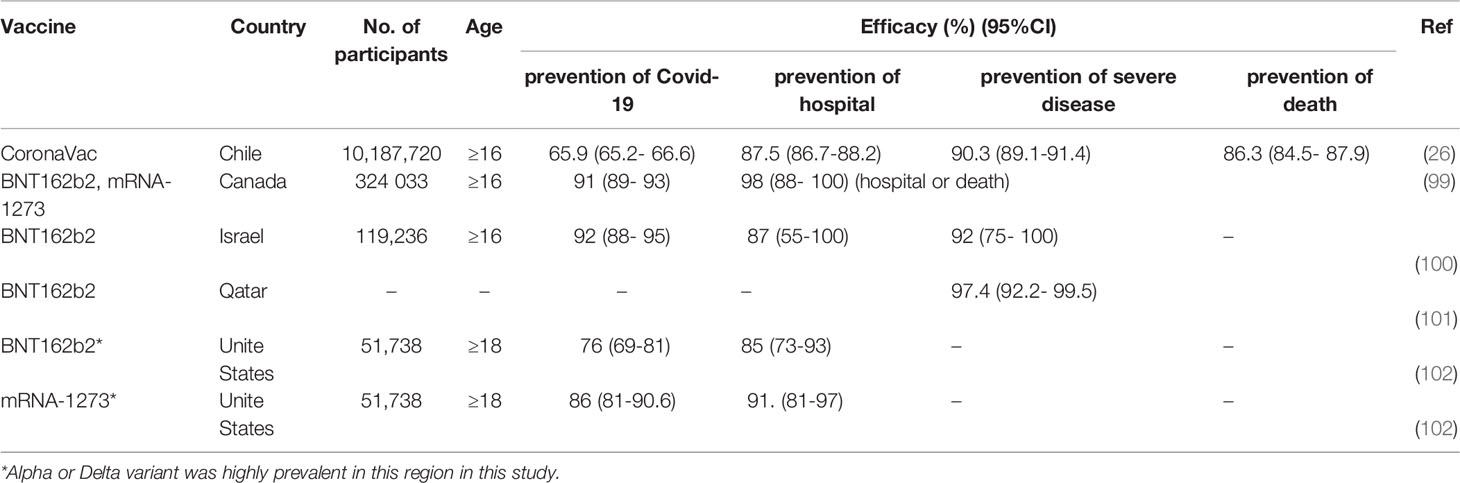
In recent months, vaccine manufacturers have published phase III COVID-19 vaccine clinical trial data, which have indicated vaccine efficacies consistent with those reported in phase I/II clinical trials. Although differences exist among specific vaccines, the various vaccines have exhibited good effects in the prevention of disease onset and critical illness (Table 3). At present, data related to the correlation between efficacy and neutralizing antibody titer in phase III clinical trials have not yet been reported. A meta-analysis that compared the correlations between efficacy and neutralization titer in recovering subjects across several phase III clinical trials revealed the presence of a strong nonlinear relationship between mean neutralization level and efficacy, which is in agreement with results obtained from animal challenge models (28). However, inconsistencies in the selected serum samples of the recovery phase may reduce the credibility of this relationship. In addition, large differences exist in the neutralizing antibody titers induced by similarly efficacious vaccines developed by different manufacturers. This may be attributed to differences in the sample population, test methods, tested variants, and dominant variant in the country of residence of the subjects, among phase III clinical trials conducted by different vaccine manufacturers (Table 3). In a recent study, Feng et al. analyzed the clinical data of the ChAdOx1 nCoV-19 (AZD1222) vaccine in the UK and found that the vaccine efficacy was associated with antibody levels (especially those of neutralizing antibodies). Measurements of neutralizing antibody titers revealed values of 938 international units (IU)/mL was associated with 90% VE against symptomatic infection at 28 days post-vaccination (71). A study on breakthrough infections in vaccinated healthcare workers at the largest medical center in Israel predicted that breakthrough infections could only be effectively prevented when the geometric mean titer (GMT) exceeded 533.7 (95% CI: 408.1 to 698.0) (98).
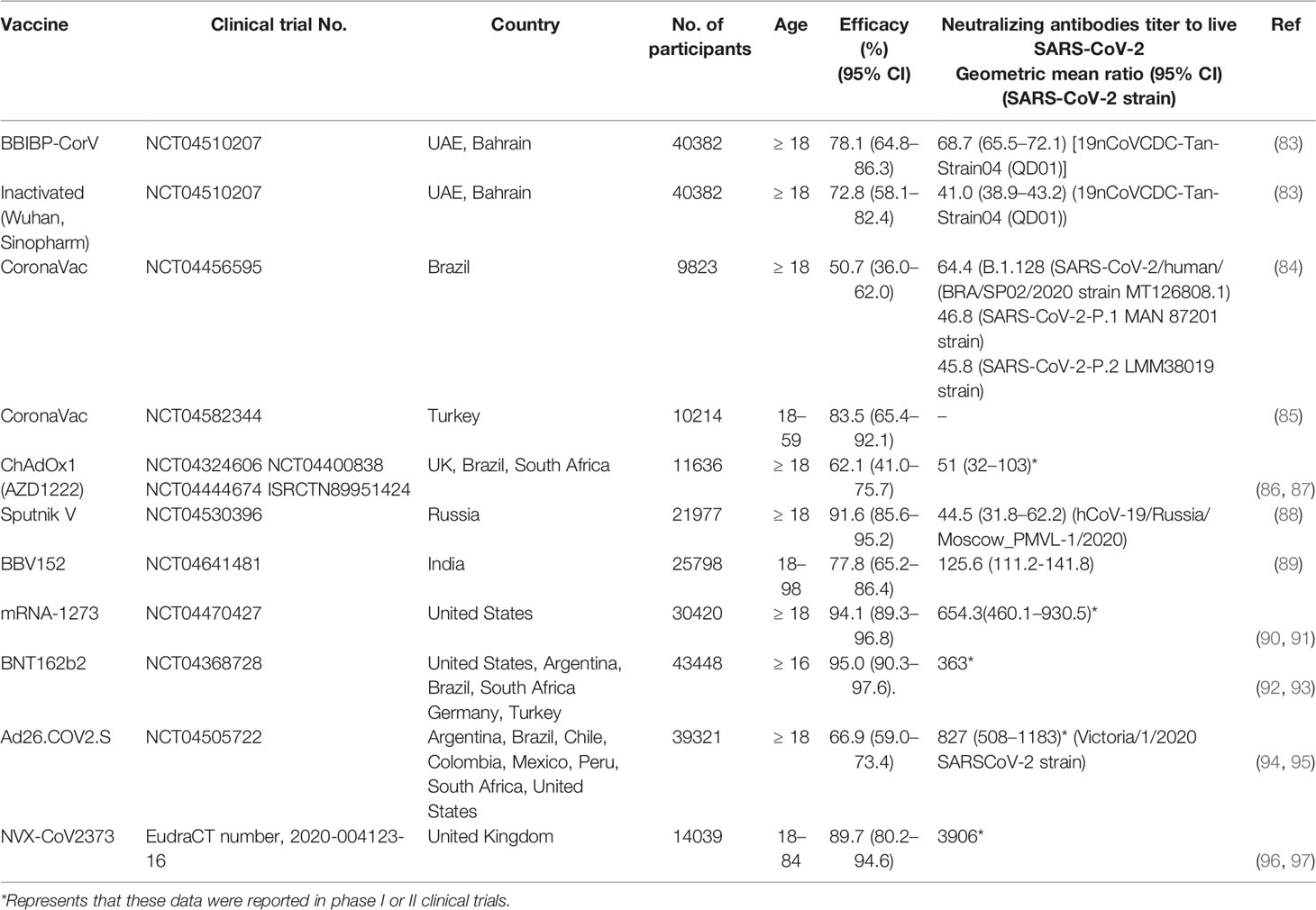
Table 3 Neutralizing antibody titer and protection efficacy of COVID-19 vaccines for emergency use in Phase III clinical trials.
Real-World Data
With the publication of real-world data, it is apparent that current COVID-19 vaccines that have been granted EUAs or conditional marketing authorizations, achieve different efficacies in individuals of different age groups, ethnicities, and countries. However, all vaccines have met the minimum efficacy requirement of 50% set by the WHO for EUAs and demonstrated high efficacies against progression towards severe disease or death (Table 4) (26, 99–102). The effectiveness of the vaccines is positively correlated with the level of neutralizing antibodies (Table 3), which is consistent with Knory’s and Earle’s studies (28, 29). Although the efficacies of current vaccines against the SARS−CoV−2 variants of concern (VOC) have decreased significantly compared to the preclinical stage, these vaccines still provide certain protective effects, especially against critical illness and disease-related death (27, 102, 103).
The decrease in the efficacy of current vaccines has mainly been caused by the following: (1) Neutralizing antibodies generated after vaccination with current vaccines providing weaker neutralizing effects against newly emerged variants, resulting in decreased vaccine efficacy (104, 105); (2) Neutralizing antibody levels starting to decrease gradually at a certain point of time after vaccination, resulting in the occurrence of breakthrough infection. Consequently, populations that have been vaccinated earlier are more susceptible to breakthrough infection than those vaccinated later (106–108). Studies have found that booster vaccination leads to increased neutralizing antibody levels against variants and enhances vaccine efficacy (49, 50, 52). Both clinical and real-world data demonstrate the presence of a positive correlation between neutralizing antibody level and vaccine effectiveness, which provides a scientific basis for further data collection and analysis, to validate the use of neutralizing antibody threshold values as surrogate endpoints.
Application of Neutralizing Antibody Immunobridging for COVID-19 Vaccine
In recent clinical studies of two COVID-19 vaccines that were performed around the world, neutralizing antibody immune bridging was used to assess vaccine’s effectiveness. The two co-primary endpoint criteria used were: 1. The neutralizing antibody titer is higher compared to active comparator vaccine AZD1222 (ChAdOx1-S); 2. The seroconversion rate of neutralizing antibody is more than the set threshold of 50% or 95% (109, 110). After full immunization schedule, seroconversion of SARS-CoV-2-specific neutralizing antibodies is defined as 4-fold increase from baseline in the phase III clinical of VLA2011 (111). The Phase II clinical results of MVC-COV1901 showed that the geometric mean serum neutralizing antibody titer at 28 days after receiving the second recommended dose (i.e. Day 57) against wild type SARS-CoV-2 virus was 408.5 IU/mL. The neutralizing antibody GMT induced by MVC-COV1901 was 3.4 times that of AZD1222, and the seroconversion rate was 99.8% (109, 112–114). On July 19, 2021, without providing clinical research data on vaccine protection, MVC-COV1901 was approved by the EUA of Taiwan, China, becoming the first approved COVID-19 vaccine based on neutralizing antibody bridging experiments to evaluate immune protection (109, 114). Phase III clinical results of the AZD1222 vaccine showed an efficacy of 62.1%. Based on the study of the relationship between the level of neutralizing antibody and vaccine immunity by Feng et al., it can be inferred that the protection rate of the MVC-COV1901 vaccine is between 80% and 90% (71, 86). On October 18, 2021, Valneva reported positive phase 3 results for VLA2001. The neutralizing antibody titer at two weeks after the second receiving the second recommended dose (i.e. Day 43) in adults aged 30 years and older of VLA2001 is 1.39 times that of AZD1222 (VLA2001 GMT 803.5, AZD1222 GMT 576.6), and the neutralizing antibody seroconversion rate is more than 95% (110, 115). The efficacies of MVC-COV1901 and VLA2001 vaccines will be evaluated based on real-world vaccination data. This will be the direct and effective verification of the feasibility of using neutralizing antibodies as surrogate endpoints for COVID-19 vaccines.
Neutralizing Antibody Test Methods
Neutralizing antibody testing can be performed using live virus, pseudovirus neutralization assays and lateral flow immunoassay (116). A report by the WHO revealed the presence of differences in the experimental methods, variants, and calculation methods used for neutralizing antibody testing among different laboratories around the world, which resulted in significant biases in the measured neutralizing antibody levels of the same sample. In the collaborative calibration of the First WHO International Standard and Reference Panel for the anti-SARS-CoV-2 antibody, live virus neutralization assays performed by 15 laboratories [including live virus plaque reduction neutralisation assay (Live-PRNT), live virus foci reduction neutralisation assay (Live-FRNT), live virus cytophatic effect detection assay (Live-CPE), and live virus microneutralization assay (Live-MN)] and pseudovirus neutralization assays performed by 12 laboratories [including pseudotyped virus - lentiviral (HIV) vector (PV-LVV) and pseudotyped virus - vesicular stomatitis virus (PV-VSV)] were adopted (117). Results indicated that with the exception of two low-titer samples and one negative sample for which the titers could not be easily calculated, the total GMTs of seven collaborative calibration samples determined by Live-PRNT, Live-FRNT, Live-CPE, and Live-MN were 317.1, 445.3, 93.9, and 239.6, respectively. When the same assay was used, the average fold (the ratio of the maximum value to the minimum value) in GMT across different laboratories were 14.7-, 12.9-, 19.5-, and 4.5-fold, respectively. Total GMTs determined by PV-LVV and PV-VSV were 371.8 and 519.2, respectively; average fold in GMT across different laboratories when the same method was used were 908.7- and 10.9-fold, respectively.
During the collaborative calibration of the first Chinese national standards for SARS-CoV-2 neutralizing antibody, live virus neutralization assays performed by four laboratories (including Live-PRNT and Live-CPE), with one laboratory adopting two types of assays for testing and the remaining three laboratories using the CPE assay, and pseudovirus neutralization assays performed by 10 laboratories (all PV-VSV) were adopted. As shown in Table 5, the GMTs of four collaborative calibration samples determined by different assays are significantly inconsistent. When the same assay was used, the fold (the ratio of the maximum value to the minimum value) in GMT across different laboratories are more than 4.7 at least. These results indicate the presence of considerable differences among different laboratories and methods (Table 5) (118).
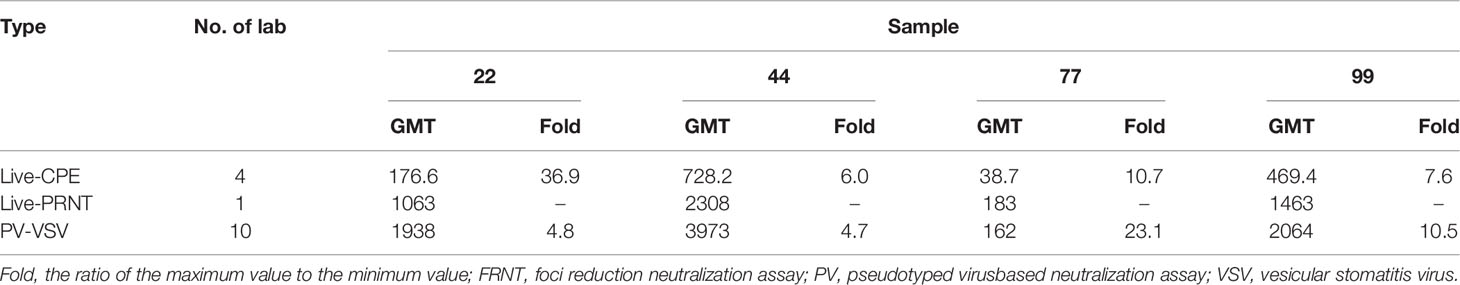
Table 5 Comparison of geometric mean of SARS-CoV-2 neutralising antibodies reported in ref. (118).
Studies have shown that differences in neutralizing antibody test results among different laboratories were significantly decreased with the use of the WHO International Standard and the Chinese National Standards (117, 118). When the International Standard was adopted, the total geometric coefficients of variation (GCVs) of five high- and medium-titer samples were reduced from 249%, 179%, 231%, 281%, and 161% to 94%, 95%, 119%, 67%, and 93%, and the upper quartile/lower quartile (UQ/LQ) values were reduced by 1.432-, 1.978-, 2.206-, 6.511-, and 2.348-fold. However, a low-titer sample did not exhibit a significant decrease in GCV and only showed a 0.97-fold decrease in UQ/LQ compared with the pre-standardization value, which may be related to the fact that the low neutralizing antibody titer was close to the threshold value (117). In the collaborative calibration of the Chinese national standards, the GCV values of three collaborative calibration samples measured by different laboratories using an authentic virus neutralization assay were 129%, 266%, and 146%. When standards with a titer of 1000 U/mL were used, the total GCVs among laboratories were reduced to 107%, 18%, and 90% (118).
The standardization of neutralizing antibody test methods directly affects the establishment of immunological surrogate endpoints and has become a major influencing factor of COVID-19 vaccine R&D and evaluation. With the establishment of the first standard pseudovirus neutralization assay by Chinese researchers (119) and WHO’s subsequent approval of the First International Standard for anti-SARS-CoV-2 immunoglobulin (human), methods for measuring neutralizing antibody titer can be standardized and the comparability of cross-platform test results can be effectively enhanced (120).
Actions to Develop Neutralizing Antibodies as Endpoint for Vaccines of WHO and More National Levels of Different Countries to Practical Application
As trials on the clinical effectiveness of vaccines constitute the main rate-limiting step in vaccine R&D, the current status of COVID-19 vaccine application and R&D urgently require the establishment of immunogenicity surrogate endpoints for testing vaccine’s effectiveness. Recently, a number of reports on clinical and real-word effectiveness of COVID-19 vaccines have been published, and two COVID19 vaccines have reported positive comparative immunogenicity trial results (109–114). We are beginning to harness the potential utility of these data for establishing surrogate endpoints. However, the current guidance documents on immunological surrogate endpoints are not systematic and comprehensive. There are obstacles to sharing and analyzing large amounts of clinical data among vaccines manufactures. In addition, the lack of a standardized neutralizing antibody test assay and continuously emerging variants further complicate the comparisons. The robustness of immunological surrogate endpoints will largely depend on how we address these issues.
Guidance Documents on Surrogate Endpoints for COVID-19 Vaccines Promptly Issued by the WHO and National Regulatory Agencies
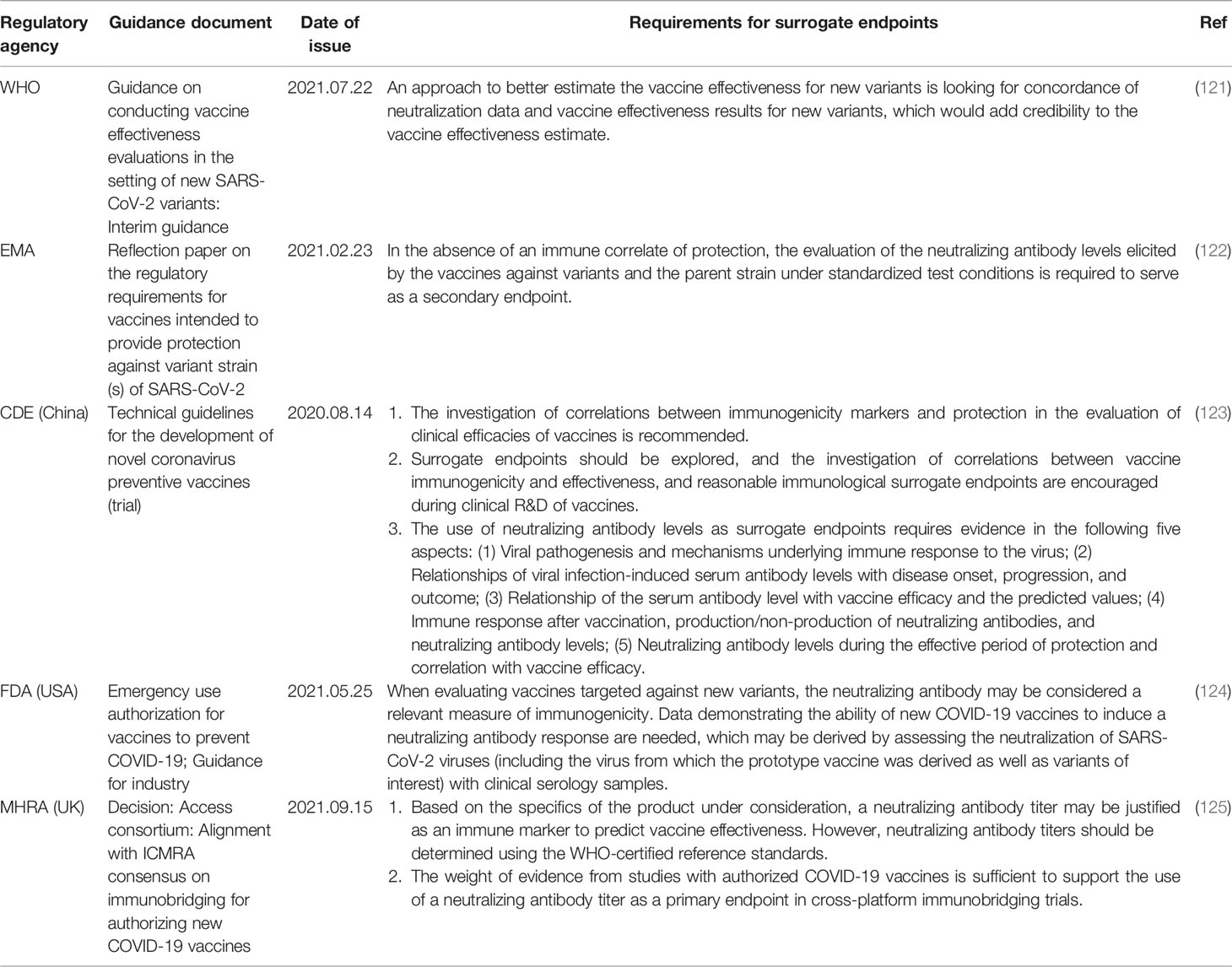
The pandemic of COVID-19 requires the WHO and national regulatory agencies to quickly assess the protective effectiveness of vaccines. Recently, the WHO and pharmaceutical regulatory agencies of the USA, UK, and China have indicated the need for studies investigating the relationships between vaccine-induced neutralizing antibody levels and vaccine’s effectiveness (Table 6). In particular, the Medicines and Healthcare products Regulatory Agency (MHRA) of the UK has announced that neutralizing antibody surrogate endpoints established using the WHO standard units can be applied to immunobridging studies in the R&D of vaccines against SARS−CoV−2 variants (121–124).
Guidelines issued by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China state that the correlation between immunological markers and protection should be investigated in COVID-19 vaccine clinical trials, and the establishment of surrogate endpoints requires the provision of evidence for five different aspects, which include the correlations of immune response mechanisms and antibody levels (particularly those of neutralizing antibodies) with protection (123). Considering the fact that the current global outbreaks are caused by SARS−CoV−2 variants, the Unite States Food and Drug Administration (USFDA) has indicated the need to assess the neutralization of the virus from which the prototype vaccine was derived, as well as variants of concern with clinical serology samples obtained from persons immunized with vaccines against variants, when investigating the effectiveness of newly developed vaccines against variants (124). Guidance provided by the WHO recommends the approach of looking for concordance of neutralization data and vaccine effectiveness results for VOCs to estimate the effectiveness of vaccines against new variants (121). The MHRA considers that the weight of evidence from studies with authorized vaccines is sufficient to support the use of neutralizing antibody titers as a primary endpoint in cross-platform immunobridging trials. Therefore, neutralizing antibody titers may be justified as an immune marker to predict vaccine effectiveness, but they should be standardized using the WHO reference standards and expressed in terms of IU (125). To guide deliberations regarding vaccines against variants, the European Medicines Agency (EMA) requires the evaluation of the neutralizing antibody levels elicited by the vaccines against variants and the parent strain under standardized conditions to serve as a secondary endpoint (122).
Analysis of the Use of Phase III Clinical and Real-World Data as Surrogate Endpoints
Currently, 31 different types of COVID-19 vaccines have received EUAs or conditional marketing authorizations, and preclinical, clinical, and real-world data on efficacy and neutralizing antibody levels are available for many of these vaccines. However, inadequacies exist in the openness and correlation analyses of the efficacy and neutralizing antibody data of current vaccines (28, 71).The clinical studies of MVC-COV1901 and VLA2001 vaccines provide the results of immune bridging of neutralizing antibodies, but there is a lack of data on the protection rate of vaccines. These data could be jointly analyzed by vaccine manufacturers and regulatory agencies, and coordination efforts could be made by international health organizations for the standardized formulation of scientific surrogate endpoints. These will be greatly beneficial to the screening of high-immunogenicity vaccines from the immense number of vaccines being subjected to preclinical and clinical testing worldwide, maximization of the protection of participants and saving various resources. On the basis of existing clinical data, statistical tools may be utilized to investigate the relationships between neutralizing antibody level and vaccine efficacy, changes in neutralizing antibody level with time, and threshold levels of neutralizing antibody protection against different variants (28, 71). However, neutralizing antibodies are likely not the only mechanism of protection (126). Further clinical and real-world data are required to determine the ability of neutralizing antibody levels to accurately evaluate the effectiveness of COVID-19 vaccines when used as surrogate endpoints.
Standardization of Laboratory Serological Test Methods and the Establishment of Secondary National Standards
There are a number of neutralizing antibody test methods available for the estimation of antibodies against SARS-CoV2 (116). Standardized laboratory serological test methods are a prerequisite for the realization of data comparability among different laboratories and platforms for surrogate endpoint establishment. The standardization of test methods has become the main rate-limiting step in the R&D of COVID-19 vaccines. On the basis of the WHO antibody standard, countries and regions are advised to expedite the establishment of secondary national standards to specify requirements for the use of IU in test methods. This will enable the comparison of neutralizing antibody test results across different studies for the accurate analysis and determination of correlations between neutralizing antibody levels and vaccine efficiencies.
New Variants Pose Challenges to the Establishment of Surrogate Endpoints
SARS−CoV−2 is highly prone to mutations. To date, several hundreds of lineages have already emerged and new lineages are still appearing on a continuous basis. In particular, lineages with immune evasion abilities exert greater effects on vaccine protection (127). Serum neutralizing capacity has been used as an efficient indicator to quickly assess the protective effect of vaccines on emerging variants. Results of these studies show that compared with the early strains, the neutralizing antibody titer against new variants has been significantly decreased (5, 128, 129). Surrogate endpoints established using current clinical data will inevitably face challenges posed by continuously emerging variants. Therefore, continuous endpoint revisions may be required based on changes in variant dominance with time and the R&D status of vaccines (130).
In addition, SARS-CoV-2 specific T cell immunity acquired by COVID-19 vaccines or previous infection still remain broadly robust and long-term protection against VOCs, including Omicron variant (131–134). A standardized measurement of T cells immunity may be served as an potential surrogate endpoint to better assess the protective effect of COVID-19 vaccines on emerging variants subsequently (135).
Conclusion
The global scale of the pandemic, high vaccination coverage rates and ethical requirements, pose challenges to the effectiveness of subsequently developed COVID-19 vaccines in clinical trials. The establishment of immunological surrogate endpoints is of great significance to the acceleration of efficacy evaluations of vaccines. Current studies have indicated that vaccine-induced neutralizing antibody levels are correlated with clinical protection, and predictable clinical progression have tried to assess clinical protection through neutralizing antibody immunobridging experiments (109, 110). Research efforts on surrogate endpoints should focus on the establishment and application of standardized test methods, and adoption of the WHO international standard to express titers in terms of IU and reduce measurement errors. In addition, it should also analyze the threshold levels of neutralization antibody protection against different variants based on current clinical data, and appropriate endpoint adjustments based on changes in variant circulation and vaccine R&D. Due to the rapid waning of neutralization tier, it is critial to assess quality and durability of the neutralization antibody in conjunction with standardization of time lines after vaccination (28). During the use of COVID-19 vaccines in clinical trials of booster and sequential vaccination, the threshold levels of new vaccines’ neutralization antibody should be significantly superior to those of the primary vaccines. Higher titers of neutralization antibody will serve as an indication of the superior effectiveness of these new vaccines.
Author Contributions
ZL and MX conceived the framework and main text of this review article. JL, QM, XW, QH, and LB wrote the draft. ZL, MX, ZW and QW reviewed the manuscript. JL, YB, and JZ searched the literature. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Emergency Key Program (NO. EKPG21-30-1) of Guangzhou Laboratory, China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. COVID-19 Vaccine Tracker and Landscape (2021). Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (Accessed January 15, 2022).
2. Shenzhen Kangtai Biological Products Co. Ltd. Indicative Announcement on the Authorization of the Inactivated COVID-19 Vaccine for Emergency Use (2021). Available at: http://www.szse.cn/disclosure/listed/bulletinDetail/index.html?d3c250ff-42f9-4a8b-87ce-41ad63495776 (Accessed May 14, 2021).
3. Zydus Cadila. Zydus Receives EUA From DCGI for ZyCoV-D, the Only Needle-Free COVID Vaccine in the World (2021). Available at: https://www.zyduscadila.com/public/pdf/pressrelease/Press%20Release-Zydus-receives-EUA-from-DCGI-for-ZyCoV-D.pdf (Accessed August 20, 2021).
4. World Health Organization. WHO Coronavirus (COVID-19) Dashboard (2021). Available at: https://covid19.who.int/ (Accessed January 15, 2022).
5. Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced Sensitivity of SARS-CoV-2 Variant Delta to Antibody Neutralization. Nature (2021) 596(7871):276–80. doi: 10.1038/s41586-021-03777-9
6. Callaway E, Ledford H. How to Redesign COVID Vaccines So They Protect Against Variants. Nature (2021) 590:15–6. doi: 10.1038/d41586-021-00241-6
7. World Health Organization. Statement on the Ninth Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Coronavirus Disease (COVID-19) Pandemic (2021). Available at: https://www.who.int/news/item/26-10-2021-statement-on-the-ninth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (Accessed October 26, 2021).
8. Krammer F. A Correlate of Protection for SARS-CoV-2 Vaccines is Urgently Needed. Nat Med (2021) 27(7):1147–8. doi: 10.1038/s41591-021-01432-4
9. Amanna IJ, Slifka MK. Contributions of Humoral and Cellular Immunity to Vaccine-Induced Protection in Humans. Virology (2011) 411(2):206–15. doi: 10.1016/j.virol.2010.12.016
10. Zellweger RM, Miller R, Eddy WE, White LJ, Johnston RE, Shresta S. Role of Humoral Versus Cellular Responses Induced by a Protective Dengue Vaccine Candidate. PloS Pathog (2013) 9(10):e1003723. doi: 10.1371/journal.ppat.1003723
11. World Health Organization. Recommendations for the Production and Control of Influenza Vaccine (Inactivated). Annex 3 of WHO TRS No. 927. (2005). Available at: https://www.who.int/publications/m/item/influenza-vaccine-inactivated-annex-3-trs-no-927. (Accessed January 1, 2005)
12. World Health Organization. WHO Position Papers on Measles (2017). Available at: https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/position-papers/measles (Accessed April 28, 2017).
13. World Health Organization. Recommendations to Assure the Quality, Safety and Efficacy of Japanese Encephalitis Vaccines (Live, Attenuated) for Human Use. Annex 7 of WHO TRS No. 980 (2014). Available at: https://www.who.int/publications/m/item/japanese-encephalitisvaccines-live-attenuated-annex-7-trs-no-980 (Accessed May 15,2014).
14. World Health Organization. Recommendations for Inactivated Rabies Vaccine for Human Use Produced in Cell Substrates and Embryonated Eggs. Annex 2. Of WHO TRS No. 941. (2007) Available at: https://www.who.int/publications/m/item/inactivated-rabies-vaccine-for-human-use-annex-2-trs-no-941 (Accessed January 1, 2007).
15. World Health Organization. Recommendations to Assure the Quality, Safety and Efficacy of Poliomyelitis Vaccines (Oral, Live, Attenuated). WHO Expert Committee on Biological Standardization. Annex 2 of WHO TRS No. 980. (2014). Available at: https://www.who.int/publications/m/item/oral-live-attenuated-poliomyelitis-vaccine-annex-2-trs-no-980 (Accessed November 6, 2014).
16. World Health Organization. Recommendations to Assure the Quality, Safety and Efficacy of Poliomyelitis Vaccines (Inactivated). Annex3 of WHO TRS No. 1024. (2020) Available at: https://www.who.int/publications/m/item/poliomyelitis-vaccines-annex-3-trs-no-1024 (Accessed November 6, 2020).
17. World Health Organization. WHO Position Paper on Hepatitis A Vaccines (2016). Available at: https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/position-papers/hepatitis-a (Accessed June 16, 2016).
18. Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, et al. Efficacy, Safety, and Immunology of an Inactivated Alum-Adjuvant Enterovirus 71 Vaccine in Children in China: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2013) 381(9882):2024–32. doi: 10.1016/s0140-6736(13)61049-1
19. Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, et al. Efficacy, Safety, and Immunogenicity of an Enterovirus 71 Vaccine in China. N Engl J Med (2014) 370(9):818–28. doi: 10.1056/NEJMoa1304923
20. Li S, Chan IS, Matthews H, Heyse JF, Chan CY, Kuter BJ, et al. Inverse Relationship Between Six Week Postvaccination Varicella Antibody Response to Vaccine and Likelihood of Long Term Breakthrough Infection. Pediatr Infect Dis J (2002) 21(4):337–42. doi: 10.1097/00006454-200204000-00014
21. World Health Organization. Recommendations to Assure the Quality, Safety and Efficacy of Recombinant Hepatitis B Vaccines. Annex 4 of WHO TRS No. 978. (2013). Available at: https://www.who.int/publications/m/item/recombinant-hep-b-A4-trs-978 (Accessed May 25, 2013).
22. Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, et al. Measles Antibody: Reevaluation of Protective Titers. J Infect Dis (1990) 162(5):1036–42. doi: 10.1093/infdis/162.5.1036
23. Lemon SM, Binn LN. Serum Neutralizing Antibody Response to Hepatitis A Virus. J Infect Dis (1983) 148(6):1033–9. doi: 10.1093/infdis/148.6.1033
24. André FE, D'Hondt E, Delem A, Safary A. Clinical Assessment of the Safety and Efficacy of an Inactivated Hepatitis A Vaccine: Rationale and Summary of Findings. Vaccine (1992) 10 Suppl 1:S160–168. doi: 10.1016/0264-410x(92)90576-6
25. Clemens R, Safary A, Hepburn A, Roche C, Stanbury WJ, André FE. Clinical Experience With an Inactivated Hepatitis A Vaccine. J Infect Dis (1995) 171 Suppl 1:S44–49. doi: 10.1093/infdis/171.supplement_1.s44
26. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med (2021) 385(10):875–84. doi: 10.1056/NEJMoa2107715
27. Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. mRNA-1273 COVID-19 Vaccine Effectiveness Against the B.1.1.7 and B.1.351 Variants and Severe COVID-19 Disease in Qatar. Nat Med (2021) 27(9):1614–21. doi: 10.1038/s41591-021-01446-y
28. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection From Symptomatic SARS-CoV-2 Infection. Nat Med (2021) 27(7):1205–11. doi: 10.1038/s41591-021-01377-8
29. Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, et al. Evidence for Antibody as a Protective Correlate for COVID-19 Vaccines. Vaccine (2021) 39(32):4423–8. doi: 10.1016/j.vaccine.2021.05.063
30. Dörner T, Radbruch A. Antibodies and B Cell Memory in Viral Immunity. Immunity (2007) 27(3):384–92. doi: 10.1016/j.immuni.2007.09.002
31. Wyllie D, Jones HE, Mulchandani R, Trickey A, Taylor-Phillips S, Brooks T, et al. SARS-CoV-2 Responsive T Cell Numbers and Anti-Spike IgG Levels are Both Associated With Protection From COVID-19: A Prospective Cohort Study in Keyworkers. medRxiv (2021) 2020.2011.2002.20222778. doi: 10.1101/2020.11.02.20222778
32. Tan AT, Linster M, Tan CW, Le Bert N, Chia WN, Kunasegaran K, et al. Early Induction of Functional SARS-CoV-2-Specific T Cells Associates With Rapid Viral Clearance and Mild Disease in COVID-19 Patients. Cell Rep (2021) 34(6):108728. doi: 10.1016/j.celrep.2021.108728
33. Goel RR, Painter MM. mRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science (2021) 374(6572):abm0829. doi: 10.1126/science.abm0829
34. Medicines & Healthcare products Regulatory Agency. Vaccine BNT162b2 – Conditions of Authorisation Under Regulation 174. (2020). Available at: https://www.gov.uk/government/publications/regulatoryapproval-of-pfizer-biontech-vaccine-for-covid-19/conditionsof-authorisation-for-pfizerbiontech-covid-19-vaccine (Accessed December 30, 2020).
35. World Health Organization. Regulation and Prequalification (2020). Available at: https://www.who.int/teams/regulation-prequalification/eul/covid-19 (Accessed January 15, 2022).
36. U.S. Food and Drug Administration. FDA Approves First COVID-19 Vaccine (2021). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (Accessed August 23, 2021).
37. Our World in Data. COVID-19 Vaccine Doses Administered (2021). Available at: https://ourworldindata.org/grapher/cumulative-covid-vaccinations (Accessed January 15, 2022).
38. National Health Commission of the Peoples’s Republic of China. Novel Coronavirus Vaccination Status (2021). Available at: http://www.nhc.gov.cn/xcs/yqfkdt/202111/5e687b673afa480da56c01be4259675a.shtml (Accessed January 15, 2022).
39. Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapid Epidemic Expansion of the SARS-CoV-2 Omicron Variant in Southern Africa. medRxiv (2021) 2012:201921268028. doi: 10.1101/2021.12.19.21268028
40. World Health Organization. Enhancing Response to Omicron SARS-CoV-2 Variant (2022). Available at: https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states (Accessed January 19, 2022).
41. U.S. Centers for Disease Control and Prevention. Potential Rapid Increase of Omicron Variant Infections in the United States (2022). Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/forecasting/mathematical-modeling-outbreak.html (Accessed January 19, 2022).
42. Ikemura N, Hoshino A, Higuchi Y, Taminishi S, Inaba T, Matoba S. SARS-CoV-2 Omicron Variant Escapes Neutralization by Vaccinated and Convalescent Sera and Therapeutic Monoclonal Antibodies. medRxiv (2021) 2021.2012.2013.21267761. doi: 10.1101/2021.12.13.21267761
43. VanBlargan LA, Errico JM, Halfmann PJ, Zost SJ, Crowe JE, Purcell LA, et al. An Infectious SARS-CoV-2 B.1.1.529 Omicron Virus Escapes Neutralization by Several Therapeutic Monoclonal Antibodies. bioRxiv (2021) 2021.2012.2015.472828. doi: 10.1101/2021.12.15.472828
44. Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, et al. Considerable Escape of SARS-CoV-2 Omicron to Antibody Neutralization. Nature (2021) 602(7898):671–5. doi: 10.1038/s41586-021-04389-z
45. Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, et al. AZD1222-Induced Neutralising Antibody Activity Against SARS-CoV-2 Delta VOC. Lancet (2021) 398:207–9. doi: 10.1016/S0140-6736(21)01462-8
46. Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, et al. Prevention and Attenuation of Covid-19 With the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med (2021) 385(4):320–9. doi: 10.1056/NEJMoa2107058
47. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Effectiveness of COVID-19 Vaccines Against the Omicron (B.1.1.529) Variant of Concern. medRxiv (2021) 2021.2012.2014.21267615. doi: 10.1101/2021.12.14.21267615
48. Wang K, Cao Y, Zhou Y, Wu J, Jia Z, Hu Y, et al. A Third Dose of Inactivated Vaccine Augments the Potency, Breadth, and Duration of Anamnestic Responses Against SARS-CoV-2. medRxiv (2021) 2021.2009.2002.21261735. doi: 10.1101/2021.09.02.21261735
49. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 Vaccine Booster Against Covid-19 in Israel. N Engl J Med (2021) 385(15):1393–400. doi: 10.1056/NEJMoa2114255
50. U.S. Centers for Disease Control and Prevention. Evidence to Recommendation Framework: Pfizer-BioNTech COVID-19 Booster Dose (2021). Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-9-23/03-COVID-Oliver.pdf (Accessed September 23, 2021).
51. Falsey AR, Frenck RW, Walsh EE, Kitchin N, Absalon J, Gurtman A, et al. SARS-CoV-2 Neutralization With BNT162b2 Vaccine Dose 3. N Engl J Med (2021) 385(17):1627–9. doi: 10.1056/NEJMc2113468
52. Pfizer. Pfizer And BioNTech Announce Phase 3 Trial Data Showing High Efficacy Of A Booster Dose Of Their COVID-19 Vaccine (2021). Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-phase-3-trial-data-showing (Accessed October 21, 2021).
53. Yu X, Wei D, Xu W, Li Y, Li X, Zhang X, et al. Pseudotyped SARS-CoV-2 Omicron Variant Exhibits Significant Escape From Neutralization Induced by a Third Booster Dose of Vaccination. medRxiv (2021) 2021.2012.2017.21267961. doi: 10.1101/2021.12.17.21267961
54. Doria-Rose NA, Shen X, Schmidt SD, O’Dell S, McDanal C, Feng W, et al. Booster of mRNA-1273 Vaccine Reduces SARS-CoV-2 Omicron Escape From Neutralizing Antibodies. medRxiv (2021) 2021.2012.2015.21267805. doi: 10.1101/2021.12.15.21267805
55. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. mRNA-Based COVID-19 Vaccine Boosters Induce Neutralizing Immunity Against SARS-CoV-2 Omicron Variant. Cell (2022) 185(3):457–66.e4. doi: 10.1016/j.cell.2021.12.033
56. Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N Engl J Med (2022) 386(5):492–4. doi: 10.1056/NEJMc2119358
57. Schmidt F, Muecksch F, Weisblum Y, Da Silva J, Bednarski E, Cho A, et al. Plasma Neutralization of the SARS-CoV-2 Omicron Variant. N Engl J Med (2022) 386(6):599–601. doi: 10.1056/NEJMc2119641
58. U.S. Food and Drug Administration. FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations (2021). Available at: https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations (Accessed September 22, 2021).
59. Our World in Data. COVID-19 Vaccine Booster Doses Administered (2021). Available at: https://ourworldindata.org/covid-vaccinations (Accessed October 02, 2021).
60. The State Council. The People's Republic of China (2021). Available at: http://www.gov.cn/xinwen/gwylflkjz165/index.htm (Accessed August 27, 2021).
61. Krause PR, Fleming TR, Peto R, Longini IM, Figueroa JP, Sterne JAC, et al. Considerations in Boosting COVID-19 Vaccine Immune Responses. Lancet. (2021) 398(10308):1377–80. doi: 10.1016/S0140-6736(21)02046-8
62. Consumer News and Business Channel. WHO Reiterates Warning Against Covid Boosters for Healthy People as U.S. Weighs Wide Distribution of Third Shots (2021). Available at: https://www.cnbc.com/2021/09/21/who-repeats-warning-against-covid-booster-shots-for-healthy-people-.html (Accessed November 02, 2021).
63. World Health Organization. WHO, UN Set Out Steps to Meet World COVID Vaccination Targets (2021). Available at: https://www.who.int/news/item/07-10-2021-who-un-set-out-steps-to-meet-world-covid-vaccination-targets (Accessed October 07, 2021).
64. Sinopharm (2021). Available at: http://www.sinopharm.com/s/1223-3769-39691.html (Accessed September 08, 2021).
65. ClinicalTrials.gov. Safety, Reactogenicity and Immunogenicity Study of ReCOV (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT04818801 (Accessed November 02, 2021).
66. Clinical Trials Arena. Gritstone Starts Phase I Dosing of Second-Gen mRNA Covid-19 Vaccine (2021). Available at: https://www.clinicaltrialsarena.com/news/gritstone-covid-vaccine-uk-phasei/ (Accessed September 21, 2021).
67. Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients With Covid-19. N Engl J Med (2020) 384(3):229–37. doi: 10.1056/NEJMoa2029849
68. Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, et al. A Systematic Review of Antibody Mediated Immunity to Coronaviruses: Kinetics, Correlates of Protection, and Association With Severity. Nat Commun (2020) 11(1):4704. doi: 10.1038/s41467-020-18450-4
69. McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of Protection Against SARS-CoV-2 in Rhesus Macaques. Nature (2021) 590(7847):630–4. doi: 10.1038/s41586-020-03041-6
70. Lilly Investors. Lilly"s Neutralizing Antibody Bamlanivimab (LY-CoV555) Prevented COVID-19 at Nursing Homes in the BLAZE-2 Trial, Reducing Risk by Up to 80 Percent for Residents (2021). Available at: https://investor.lilly.com/news-releases/news-release-details/lillys-neutralizing-antibody bamlanivimab-ly-cov555-prevented (Accessed January 21, 2021).
71. Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Correlates of Protection Against Symptomatic and Asymptomatic SARS-CoV-2 Infection. Nat Med (2021) 27(11):2032–40. doi: 10.1038/s41591-021-01540-1
72. Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, et al. Evaluation of the mRNA-1273 Vaccine Against SARS-CoV-2 in Nonhuman Primates. N Engl J Med (2020) 383(16):1544–55. doi: 10.1056/NEJMoa2024671
73. Vogel AB, Kanevsky I, Che Y, Swanson KA, Muik A, Vormehr M, et al. A Prefusion SARS-CoV-2 Spike RNA Vaccine is Highly Immunogenic and Prevents Lung Infection in non-Human Primates. bioRxiv (2020). doi: 10.1101/2020.09.08.280818
74. Solforosi L, Solforosi L, Kuipers H, Jongeneelen M, Rosendahl Huber SK, van der Lubbe JEM, et al. Immunogenicity and Efficacy of One and Two Doses of Ad26.COV2.S COVID Vaccine in Adult and Aged NHP. J Exp Med (2021) 218(7):e20202756. doi: 10.1084/jem.20202756
75. van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 Ncov-19 Vaccination Prevents SARS-CoV-2 Pneumonia in Rhesus Macaques. bioRxiv (2020) 2020.2005.2013.093195. doi: 10.1101/2020.05.13.093195
76. van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 Ncov-19 Vaccine Prevents SARS-CoV-2 Pneumonia in Rhesus Macaques. Nature (2020) 586(7830):578–82. doi: 10.1038/s41586-020-2608-y
77. Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, With Potent Protection Against SARS-CoV-2. Cell (2020) 182(3):713–21.e719. doi: 10.1016/j.cell.2020.06.008
78. Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an Inactivated Vaccine Candidate for SARS-CoV-2. Science (2020) 369(6499):77–81. doi: 10.1126/science.abc1932
79. Yadav PD, Ella R, Kumar S, Patil DR, Mohandas S, Shete AM, et al. Immunogenicity and Protective Efficacy of Inactivated SARS-CoV-2 Vaccine Candidate, BBV152 in Rhesus Macaques. Nat Commun (2021) 12(1):1386. doi: 10.1038/s41467-021-21639-w
80. Gooch KE, Smith TRF, Salguero FJ, Fotheringham SA, Watson RJ, Dennis MJ, et al. One or Two Dose Regimen of the SARS-CoV-2 Synthetic DNA Vaccine INO-4800 Protects Against Respiratory Tract Disease Burden in Nonhuman Primate Challenge Model. Vaccine (2021) 39(34):4885–94. doi: 10.1016/j.vaccine.2021.06.057
81. Guebre-Xabier M, Patel N, Tian J-H, Zhou B, Maciejewski S, Lam K, et al. NVX-CoV2373 Vaccine Protects Cynomolgus Macaque Upper and Lower Airways Against SARS-CoV-2 Challenge. Vaccine (2020) 38(50):7892–6. doi: 10.1016/j.vaccine.2020.10.064
82. Liang JG, Su D, Song T-Z, Zeng Y, Huang W, Wu J, et al. S-Trimer, a COVID-19 Subunit Vaccine Candidate, Induces Protective Immunity in Nonhuman Primates. Nat Commun (2021) 12(1):1346. doi: 10.1038/s41467-021-21634-1
83. Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA (2021) 326(1):35–45. doi: 10.1001/jama.2021.8565
84. Palacios R, Batista AP, Albuquerque CSN, Patiño EG, Santos J, do P, et al. Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study. SSRN (2021). doi: 10.2139/ssrn.3822780
85. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and Safety of an Inactivated Whole-Virion SARS-CoV-2 Vaccine (CoronaVac): Interim Results of a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial in Turkey. Lancet (2021) 398(10296):213–22. doi: 10.1016/S0140-6736(21)01429-X
86. Voysey M, Clemens S, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and Efficacy of the ChAdOx1 Ncov-19 Vaccine (AZD1222) Against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet (2021) 397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1
87. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and Immunogenicity of the ChAdOx1 Ncov-19 Vaccine Against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. Lancet (2020) 396(10249):467–78. doi: 10.1016/S0140-6736(20)31604-4
88. Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and Efficacy of an Rad26 and Rad5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine: An Interim Analysis of a Randomised Controlled Phase 3 Trial in Russia. Lancet (2021) 397(10275):671–81. doi: 10.1016/S0140-6736(21)00234-8
89. Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, Safety, and Lot to Lot Immunogenicity of an Inactivated SARS-CoV-2 Vaccine (BBV152): A Double-Blind, Randomised, Controlled Phase 3 Trial. medRxiv (2021) 2021.2006.2030.21259439. doi: 10.1101/2021.06.30.21259439
90. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med (2020) 384(5):403–16. doi: 10.1056/NEJMoa2035389
91. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA Vaccine Against SARS-CoV-2 - Preliminary Report. N Engl J Med (2020) 383(20):1920–31. doi: 10.1056/NEJMoa2022483
92. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med (2020) 383(27):2603–15. doi: 10.1056/NEJMoa2034577
93. Walsh EE, Frenck RW Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med (2020) 383(25):2439–50. doi: 10.1056/NEJMoa2027906
94. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and Efficacy of Single-Dose Ad26.Cov2.S Vaccine Against Covid-19. N Engl J Med (2021) 384(23):2187–201. doi: 10.1056/NEJMoa2101544
95. Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med (2021) 384(19):1824–35. doi: 10.1056/NEJMoa2034201
96. Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N Engl J Med (2021) 385(13):1172–83. doi: 10.1056/NEJMoa2107659
97. Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med (2020) 383(24):2320–32. doi: 10.1056/NEJMoa2026920
98. Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med (2021) 9(12):1396–406. doi: 10.1056/NEJMoa2109072
99. Chung H, He S, Nasreen S, Sundaram ME, Buchan SA, Wilson SE, et al. Effectiveness of BNT162b2 and mRNA-1273 Covid-19 Vaccines Against Symptomatic SARS-CoV-2 Infection and Severe Covid-19 Outcomes in Ontario, Canada: Test Negative Design Study. BMJ (2021) 374:n1943. doi: 10.1136/bmj.n1943
100. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med (2021) 384(15):1412–23. doi: 10.1056/NEJMoa2101765
101. Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 Vaccine Against the B.1.1.7 and B.1.351 Variants. N Engl J Med (2021) 385(2):187–9. doi: 10.1056/NEJMc2104974
102. Puranik A, Lenehan PJ, Silvert E, Niesen MJM, Corchado-Garcia J, O'Horo JC, et al. Comparison of Two Highly-Effective mRNA Vaccines for COVID-19 During Periods of Alpha and Delta Variant Prevalence. medRxiv (2021). doi: 10.1101/2021.08.06.21261707
103. Souza WM, Amorim MR, Sesti-Costa R, Coimbra LD, Brunetti NS, Toledo-Teixeira DA, et al. Neutralisation of SARS-CoV-2 Lineage P.1 by Antibodies Elicited Through Natural SARS-CoV-2 Infection or Vaccination With an Inactivated SARS-CoV-2 Vaccine: An Immunological Study. Lancet Microbe (2021) 2(10):e527–35. doi: 10.1016/S2666-5247(21)00129-4
104. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 Vaccines Against the B.1.617.2 (Delta) Variant. N Engl J Med (2021) 385(7):585–94. doi: 10.1056/NEJMoa2108891
105. Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta K-D, et al. Impact of Delta on Viral Burden and Vaccine Effectiveness Against New SARS-CoV-2 Infections in the UK. medRxiv (2021) 2021.2008.2018.21262237. doi: 10.1101/2021.08.18.21262237
106. Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of Responses After SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med (2020) 384(1):80–2. doi: 10.1056/NEJMc2032195
107. Zhang H, Jia Y, Ji Y, Cong X, Liu Y, Yang R, et al. Studies on the Level of Neutralizing Antibodies Produced by Inactivated COVID-19 Vaccines in the Real World. medRxiv (2021) 2021.2008.2018.21262214. doi: 10.1101/2021.08.18.21262214
108. Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning Immunity of the BNT162b2 Vaccine: A Nationwide Study From Israel. medRxiv (2021) 2021:2008.2024.21262423. doi: 10.1101/2021.08.24.21262423
109. Medigen Vaccine Biologics Corp. MVC COVID-19 Vaccine Obtains Taiwan EUA Approval (2021). Available at: https://www.medigenvac.com/public/en/news/detail/83?from_sort=2 (Accessed July 19, 2021).
110. Valneva. Valneva Reports Positive Phase 3 Results for Inactivated, Adjuvanted COVID-19 Vaccine Candidate Vla2001 (2021). Available at: https://valneva.com/press-release/valneva-reports-positive-phase-3-results-for-inactivated-adjuvanted-covid-19-vaccine-candidate-vla2001/ (Accessed October 18, 2021).
111. ClinicalTrials.gov. Study To Compare The Immunogenicity Against COVID-19, Of VLA2001 Vaccine To AZD1222 Vaccine (COV-COMPARE) (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT04864561 (Accessed October 29, 2021).
112. Hsieh S-M, Liu M-C, Chen Y-H, Lee W-S, Hwang S-J, Cheng S-H, et al. Safety and Immunogenicity of CpG 1018 and Aluminium Hydroxide-Adjuvanted SARS-CoV-2 S-2P Protein Vaccine MVC-COV1901: Interim Results of a Large-Scale, Double-Blind, Randomised, Placebo-Controlled Phase 2 Trial in Taiwan. Lancet Respir Med (2021) 9(12):1396-406. doi: 10.1016/S2213-2600(21)00402-1
113. Hsieh S-M, Liu W-D, Huang Y-S, Lin Y-J, Hsieh E-F, Lian W-C, et al. Safety and Immunogenicity of a Recombinant Stabilized Prefusion SARS-CoV-2 Spike Protein Vaccine (MVC COV1901) Adjuvanted With CpG 1018 and Aluminum Hydroxide in Healthy Adults: A Phase 1, Dose-Escalation Study. EClinicalMedicine (2021) 38:100989. doi: 10.1016/j.eclinm.2021.100989
114. Available at: https://geneonline.news/eua-medigen-vaccine (Accessed August 02, 2021).
115. Valneva. Valneva Reports Positive Phase 1/2 Data for Its Inactivated, Adjuvanted COVID-19 Vaccine Candidate, Vla2001 (2021). Available at: https://valneva.com/press-release/valneva-reports-positive-phase-1-2-data-for-its-inactivated-adjuvanted-covid-19-vaccine-candidate-vla2001/ (Accessed April 6, 2021).
116. Banga Ndzouboukou J-L, Zhang Y-d, Fan X-l. Recent Developments in SARS-CoV-2 Neutralizing Antibody Detection Methods. Curr Med Sci (2021) 41(6):1052–64. doi: 10.1007/s11596-021-2470-7
117. World Health Organization. WHO/BS.2020.2403 Establishment of the WHO International Standard and Reference Panel for Anti-SARS-CoV-2 Antibody (2020). Available at: https://www.who.int/publications/m/item/WHO-BS-2020.2403 (Accessed November 18, 2020).
118. Guan L, Yu Y, Wu X, Nie J, Zhang J, Wang Z, et al. The First Chinese National Standards for SARS-CoV-2 Neutralizing Antibody. Vaccine (2021) 39(28):3724–30. doi: 10.1016/j.vaccine.2021.05.047
119. Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, et al. Establishment and Validation of a Pseudovirus Neutralization Assay for SARS-CoV-2. Emerg Microbes Infect (2020) 9(1):680–6. doi: 10.1080/22221751.2020.1743767
120. Kristiansen PA, Page M, Bernasconi V, Mattiuzzo G, Dull P, Makar K, et al. WHO International Standard for Anti-SARS-CoV-2 Immunoglobulin. Lancet (2021) 397(10282):1347–8. doi: 10.1016/S0140-6736(21)00527-4
121. World Health Organization. Guidance on Conducting Vaccine Effectiveness Evaluations in the Setting of New SARS-CoV-2 Variants (2021). Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-variants-2021.1 (Accessed July 23, 2021).
122. European Medicines Agency. Regulatory Requirements for Vaccines Intended to Provide Protection Against Variant Strain(s) of SARS-CoV-2 (2021). Available at: https://www.ema.europa.eu/en/regulatory-requirements-vaccines-intended-provide-protection-against-variant-strains-sars-cov-2 (Accessed February 25, 2021).
123. National Medical Products Administration. Technical Guidelines for the Development of Novel Coronavirus Preventive Vaccines (Trial) (2020). Available at: https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20200814230916157.html (Accessed August 14, 2020).
124. U.S. Food and Drug Administration. GUIDANCE DOCUMENT Emergency Use Authorization for Vaccines to Prevent COVID-19 Guidance for Industry (2021). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-vaccines-prevent-covid-19 (Accessed May 25, 2021).
125. Medicines & Healthcare products Regulatory Agency. Decision Access Consortium: Alignment With ICMRA Consensus on Immunobridging for Authorising New COVID-19 Vaccines (2021). Available at: https://www.gov.uk/government/publications/access-consortium-alignment-with-icmra-consensus-on-immunobridging-for-authorising-new-covid-19-vaccines/access-consortium-alignment-with-icmra-consensus-on-immunobridging-for-authorising-new-covid-19-vaccines (Accessed September 15, 2021).
126. Cevik M, Grubaugh ND, Iwasaki A, Openshaw P. COVID-19 Vaccines: Keeping Pace With SARS-CoV-2 Variants. Cell (2021) 184(20):5077–81. doi: 10.1016/j.cell.2021.09.010
127. Aggarwal A, Stella AO, Walker G, Akerman A, Milogiannakis V, Brilot F, et al. SARS-CoV-2 Omicron: Evasion of Potent Humoral Responses and Resistance to Clinical Immunotherapeutics Relative to Viral Variants of Concern. medRxiv (2021) 2021.2012.2014.21267772. doi: 10.1101/2021.12.14.21267772
128. Cov-lineages.org. Lineage List (2021). Available at: https://cov-lineages.org/lineage_list.html (Accessed October 02, 2021).
129. Yadav PD, Sapkal GN, Ella R, Sahay RR, Nyayanit DA, Patil DY, et al. Neutralization of Beta and Delta Variant With Sera of COVID-19 Recovered Cases and Vaccinees of Inactivated COVID-19 Vaccine BBV152/Covaxin. J Travel Med (2021) 28(7):taab104. doi: 10.1093/jtm/taab104
130. Gómez-Carballa A, Pardo-Seco J, Bello X, Martinón-Torres F, Salas A. Superspreading in the Emergence of COVID-19 Variants. Trends Genet (2021). doi: 10.1016/j.tig.2021.09.003
131. Ahmed SF, Quadeer AA, McKay MR. SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust Against Omicron. Viruses (2022) 14(1):79. doi: 10.3390/v14010079
132. Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM, et al. Impact of SARS-CoV-2 Variants on the Total CD4(+) and CD8(+) T Cell Reactivity in Infected or Vaccinated Individuals. Cell Rep Med (2021) 2(7):100355. doi: 10.1016/j.xcrm.2021.100355
133. Adamo S, Michler J, Zurbuchen Y, Cervia C, Taeschler P, Raeber ME, et al. Signature of Long-Lived Memory CD8+ T Cells in Acute SARS-CoV-2 Infection. Nature (2021) 602(7895):148–55. doi: 10.1038/s41586-021-04280-x
134. Kundu R, Narean JS, Wang L, Fenn J, Pillay T, Fernandez ND, et al. Cross-Reactive Memory T Cells Associate With Protection Against SARS-CoV-2 Infection in COVID-19 Contacts. Nat Commun (2022) 13(1):80. doi: 10.1038/s41467-021-27674-x
135. World Health Organization. Correlates of Protection - Will Emerging Data Allow Increased Reliance on Vaccine Immune Responses for Public Health and Regulatory Decision-Making? (2022). Available at: https://www.who.int/news-room/events/detail/2021/09/03/default-calendar/save-the-date—will-emerging-data-allow-increased-reliance-on-vaccine-immune-responses-for-public-health-and-regulatory-decision-making (Accessed January 19, 2022).
Keywords: COVID-19 Vaccines, surrogate endpoints, neutralizing antibody, standard neutralization test assay, national standard
Citation: Liu J, Mao Q, Wu X, He Q, Bian L, Bai Y, Wang Z, Wang Q, Zhang J, Liang Z and Xu M (2022) Considerations for the Feasibility of Neutralizing Antibodies as a Surrogate Endpoint for COVID-19 Vaccines. Front. Immunol. 13:814365. doi: 10.3389/fimmu.2022.814365
Received: 13 November 2021; Accepted: 31 March 2022;
Published: 27 April 2022.
Edited by:
Raymund Razonable, Mayo Clinic, United StatesReviewed by:
Helen Baxendale, Royal Papworth Hospital NHS Foundation Trust, United KingdomXionglin Fan, Huazhong University of Science and Technology, China
Copyright © 2022 Liu, Mao, Wu, He, Bian, Bai, Wang, Wang, Zhang, Liang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miao Xu, eHVtaWFvYmpAMTI2LmNvbQ==; Zhenglun Liang, bHpoZW5nbHVuQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Jianyang Liu
Jianyang Liu Qunying Mao
Qunying Mao Xing Wu1,2,3
Xing Wu1,2,3 Qian He
Qian He Lianlian Bian
Lianlian Bian Jialu Zhang
Jialu Zhang Zhenglun Liang
Zhenglun Liang