- 1Department of Pharmacy, Tongren Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China
- 2Department of Pharmacy, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Department of Pharmacy Pharmacology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 4School of Medicine, Fudan University, Shanghai, China
- 5Department of Pharmacy, Fudan University Shanghai Cancer Center, Shanghai, China
Introduction: The adverse effects of neuromuscular junction dysfunctions caused by immune checkpoint inhibitor (ICI) drugs have not been thoroughly assessed in the clinics.
Objective: To assess the neuromuscular junction dysfunctions in cancer patients with adverse events caused by ICI therapy by searching the Food and Drug Administration Adverse Event Reporting System (FAERS) database.
Methods: The FAERS data from January 2004 to December 2020 were collected to analyze the association between neuromuscular connection dysfunction and ICI use. Disproportionate analysis and Bayesian analysis were used to quantify the association between the neuromuscular junction dysfunctions and ICIs. The onset time and outcome of neuromuscular junction dysfunctions in different ICI regimens were also compared.
Results: Out of 88,617 adverse event reports, 557 neuromuscular junction dysfunction reports (0.63%) were analyzed. Marketed ICI drugs, including ipilimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, cemiplimab, avelumab, as well as their combinations, showed positive associations with four detection methods. Most of the adverse event reports were associated with the use of nivolumab (53.32%) and pembrolizumab (31.96%). However, nivolumab-related neuromuscular junction dysfunctions were similar with pembrolizumab (33.33% vs 33.14%, p > 0.05). The onset time of neuromuscular junction dysfunctions showed no significant difference among different ICIs (p > 0.05).
Conclusions: Analysis of FAERS data identified that over 30% (32.85%) of reports of neuromuscular junction dysfunctions resulted in death. Ongoing monitoring, risk evaluations, and further comparative studies of ICIs should be considered.
Introduction
The development of immune checkpoint inhibitors (ICIs) is a revolutionary milestone in immune-oncology. ICIs revive the anti-tumor immune responses by interrupting the co-inhibitory signaling pathways and promoting immune-mediated tumor cell clearance (1). Immune-related adverse events (irAEs) from ICIs differ from toxicities caused by cytotoxic or targeted therapy agents. The onset of toxicities may be delayed and may not follow the periodic pattern observed with conventional cytotoxic drugs. The mechanism of toxicity remains to be determined and may even vary between patients receiving the same drug. The over-reactive immune response may be due to ICI exposure to low levels of autoreactive T cells, macrophage-mediated toxicity, or the elimination of tolerance caused by the production of antibodies from activated B cells (2). These irAEs have a wide range of affected organs and severity. Skin, endocrine, neurological, gastrointestinal, respiratory, and musculoskeletal toxicity may occur alone or in combination. Most events are self-limiting or can be resolved with immunosuppressive agents (such as corticosteroids) (3). Persistent irAEs that cannot be resolved with corticosteroids require tumor necrosis factor-alpha receptors antagonists, such as infliximab, an inhibitor of purine synthesis in T and B cells. Only a few irAEs don’t respond to these immune modulators (4). Numerous randomized controlled trials provide a rough overview of immune-related adverse events, including skin, gastrointestinal, lung, liver, and endocrine toxicity (5). Although most immune-related adverse events can be well controlled by supportive treatment and glucocorticoids, fatal immune-related adverse events have received increasing attention regarding the safety and patient tolerance of ICI drugs (6, 7).
ICI-related neurological adverse events are relatively infrequent. However, pooled analyses on these events show severe morbidity and mortality (8, 9). These irAEs can affect any level of the peripheral nervous system, including peripheral nerves, neuromuscular junctions, and muscles, individually or in combination (10). The adverse effects of neuromuscular junction dysfunctions caused by different ICIs have not been thoroughly assessed in the clinics. Recently, some reports updated the information on neuromuscular junction dysfunctions (11–14). Since detailed pathological mechanisms, more effective treatments, as well as rechallenge strategies remain obscure, early detection and data on the outcomes are of great importance (11, 13). Pharmacovigilance (PV) systems have been established for side effect (ADR) monitoring by countries or organizations such as the World Health Organization (WHO), the European Medicines Agency (EMA), and the US Food and Drug Administration (FDA). The FDA’s Adverse Event Reporting System (FAERS) is the world’s largest repository of reported hazardous drug events (15). Reporting all adverse events to the FDA depends on healthcare professionals, consumers, and manufacturers (16). The purpose of the system is to support the FDA safety monitoring of pharmaceuticals and therapeutic biologics on the market. In the present study, we collected, screened, and statistically analyzed FAERS data and performed signal mining analysis focusing on possible correlations for neuromuscular junction dysfunctions generated by ICI.
Materials and methods
Data source
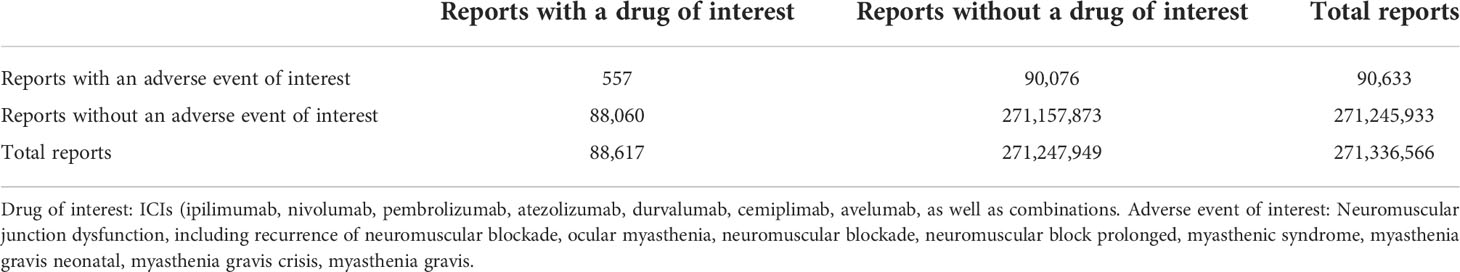
We conducted a retrospective pharmacovigilance study using data from the FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html) covering the period from January 2004 to December 2020. We conducted a retrospective pharmacovigilance survey. FAERS is a public, voluntary reporting database that provides information on adverse events and drug error reports submitted by healthcare professionals, consumers, or pharmaceutical companies. The data is not only from the United States but also from other countries. The FAERS data file contains demographic and administrative details, drug information, the Medical Dictionary for Regulatory Activities’ (MedDRA) preferred terminology for adverse event (REAC) coding, patient outcomes, reporting sources, initiation of treatment that includes date and end date reports, and indications for use. A deduplication procedure was performed according to the FDA’s recommendations of selecting the latest FDA_DT when the CASEIDs were the same and selecting the higher PRIMARYID when the CASEID and FDA_DT were the same. A total of 88,617 reports related to ICIs were mined from the FAERS database, and 557 reports (0.63%) were associated with neuromuscular junction dysfunction.
Adverse events and drug identification
Adverse events were identified using the MedDRA terms “Recurrence of neuromuscular blockade (10068408)”, “Ocular myasthenia (10049168)”, “Neuromuscular blockade (10029315)”, “Neuromuscular block prolonged (10029314)”, “Myasthenic syndrome (10028424)”, “Myasthenia gravis neonatal (10028419)”, “Myasthenia gravis crisis (10062758)”, “Myasthenia gravis (10028417)”, in the REAC file. The drugs in the FAERS database can be registered using different conventions; therefore, the MICROMEDEX® (Index Nominum) was used as a dictionary for ICIs. Reports involving the seven immunotherapies for cancer treatment (including ipilimumab, nivolumab, pembrolizumab, cemiplimab, atezolizumab, avelumab, and durvalumab) were identified using text string searches for each drug by generic names, brand names, and abbreviations. Reports listing one or more of therapies mentioned above as the primary suspect (PS) or secondary suspect (SS) agent were included. We excluded drugs reported with the eligible drug listed as interacting or concomitant, as well as those without cancer-related indications.
Data mining
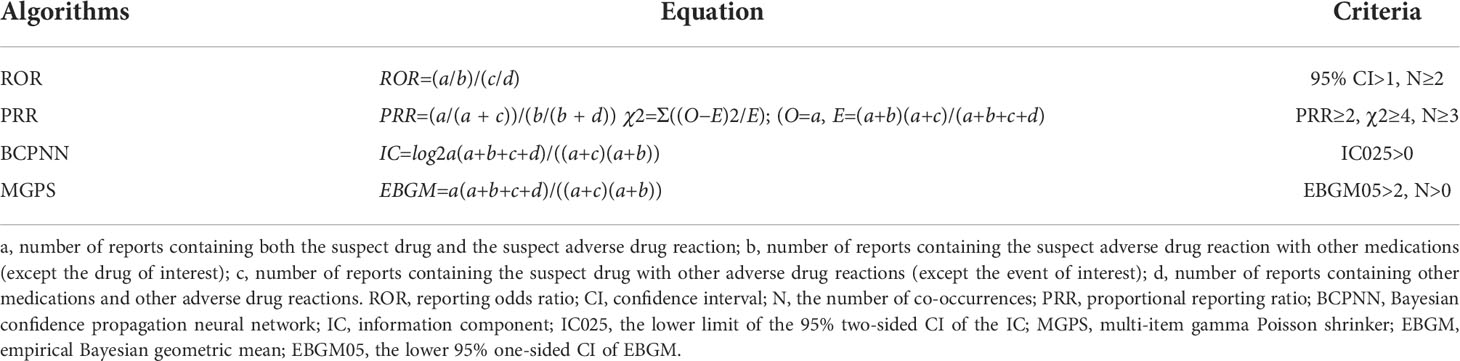
Based on disproportionality analysis and Bayesian analysis, 4 algorithms were applied: reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network, and the multi-item gamma Poisson shrinker. In combination, these algorithms were used to identify the association between drugs and adverse events. Table 1 shows the equations and criteria for the four algorithms (17–26). The use of concomitant agents is considered in the current analysis to calculate an adjusted disproportionality (adjusted ROR) which is used in the current study if not otherwise stated. We evaluated the onset time and mortality of each immune checkpoint inhibitor regimen. Onset time was defined as the interval between EVENT_DT (the date of occurrence of adverse events) and START_DT (start date of use of immune checkpoint inhibitor). It did not contain a date entry containing an input error (EVENT_DT before START_DT) and an incorrect date. Reports of fatal events due to drug toxicity were compiled, and mortality was calculated as the number of fatal events divided by the total number of neuromuscular junction dysfunction associated with each ICI regimen.
Handling missing data and removal of duplicates
A deduplication procedure was performed as described by firstly linking CASE number with relevant ISRs, secondly through the 4-key fields used in missing data handling (event_date, age, gender, reporter_country) and thirdly revising drug and event reported and the single imputation approach was performed to manage missing data (27).
Statistical analysis
Descriptive analyses were used to summarize the clinical features of the patients with neuromuscular junction dysfunctions collected from the FAERS database. Time to onset of neuromuscular junction dysfunctions between different ICI regimens was compared using nonparametric tests when the data were not normally distributed (Mann-Whitney test for dichotomous variables, the Kruskal-Wallis test when there were more than two subgroups of respondents). Pearson’s chi-square test or Fisher’s exact test was used to comparing outcome events and fatality rates between different ICI regimens. Statistical significance was determined with 95% CI and p<0.05. Data mining and statistical analyses were performed with SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
General characteristics
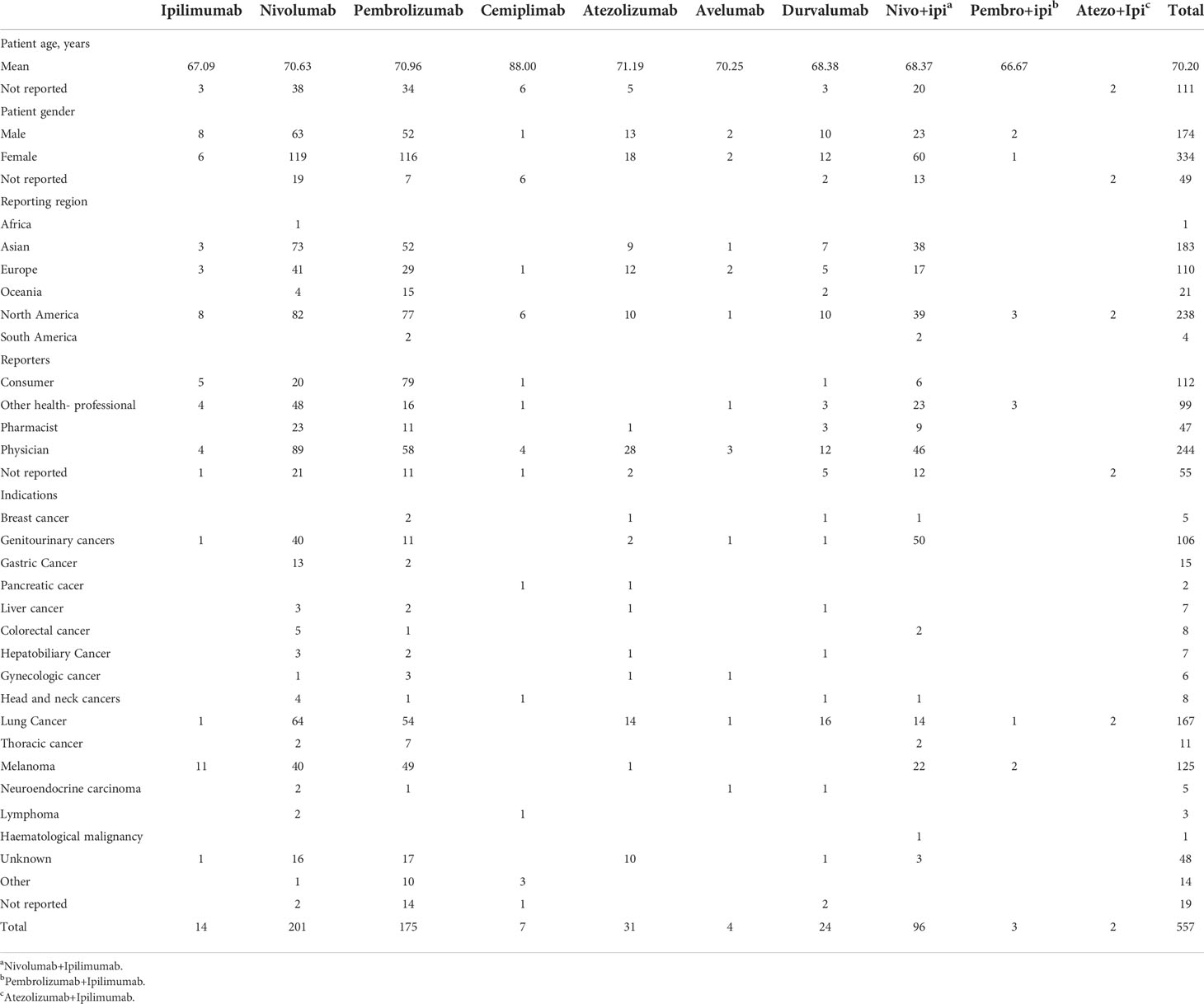
Out of 88,617 adverse reports, 557 neuromuscular junction dysfunction reports (0.63%) were documented in the FAERS database with ICIs (Table 2). Most of the 557 reports were related to nivolumab (53.32%%) and pembrolizumab (31.96%). Table 3 presents the clinical characteristics of patients.
The number of neuromuscular junction dysfunction events was the highest for nivolumab (n=201), followed by pembrolizumab (n=175). The mean age of these patients was slightly over 70 years. Most of the cases of neuromuscular junction dysfunctions involved with ICIs were reported from North America, Asia, and Europe (42.73%, 32.85%, and 19.75%, respectively). Most of the cases were submitted by physicians for nivolumab (44.28%) and by consumers for pembrolizumab (45.14%), and the cases appeared more often in females than in males (59.96% vs. 31.24%, respectively).
Signal detection
All ICI were associated with neuromuscular junction dysfunction, and signal detection was positive among all four methods (Table 4). Cemiplimab had a higher score than other ICIs in single-drug therapies. Nivolumab+Ipilimumab had a higher score than other combinations of ICIs.
Onset time of events
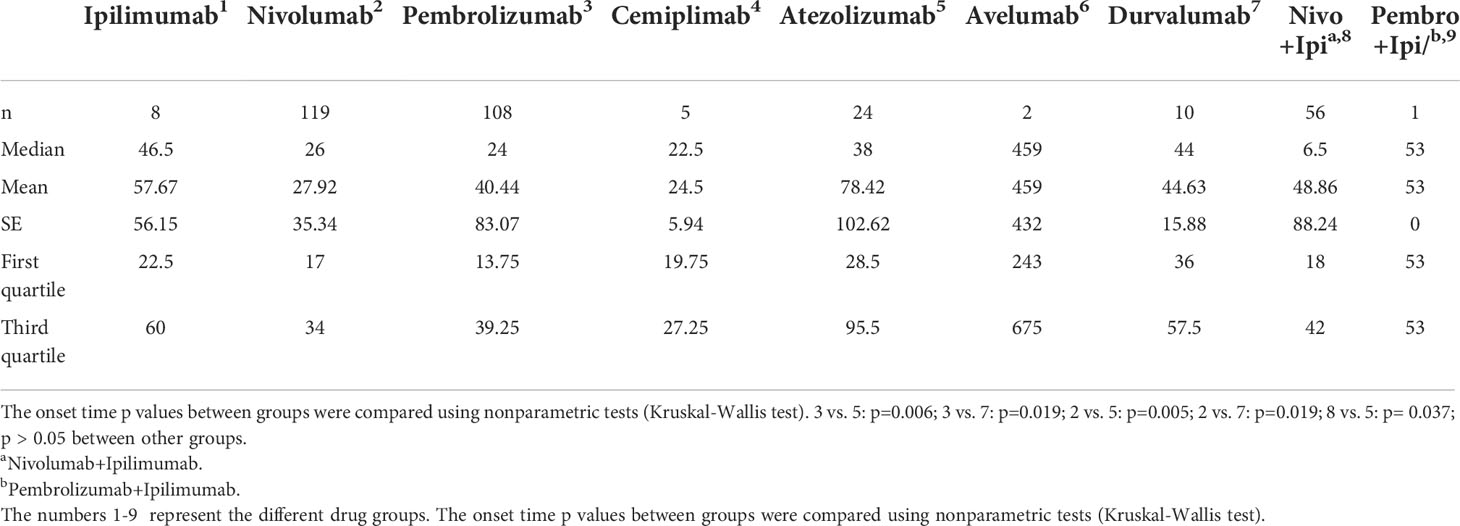
In general, the median time to event onset of ICI-associated neuromuscular junction dysfunctions was 27 days. Onset time was significantly shorter with pembrolizumab than atezolizumab (p=0.006) and durvalumab (p=0.019); similar results were found in nivolumab (p=0.005 compared to atezolizumab and p=0.019 compared to durvalumab). However, when combined with ipilimumab, nivolumab led to a significantly faster onset of events than atezolizumab (p= 0.037) (Table 5).
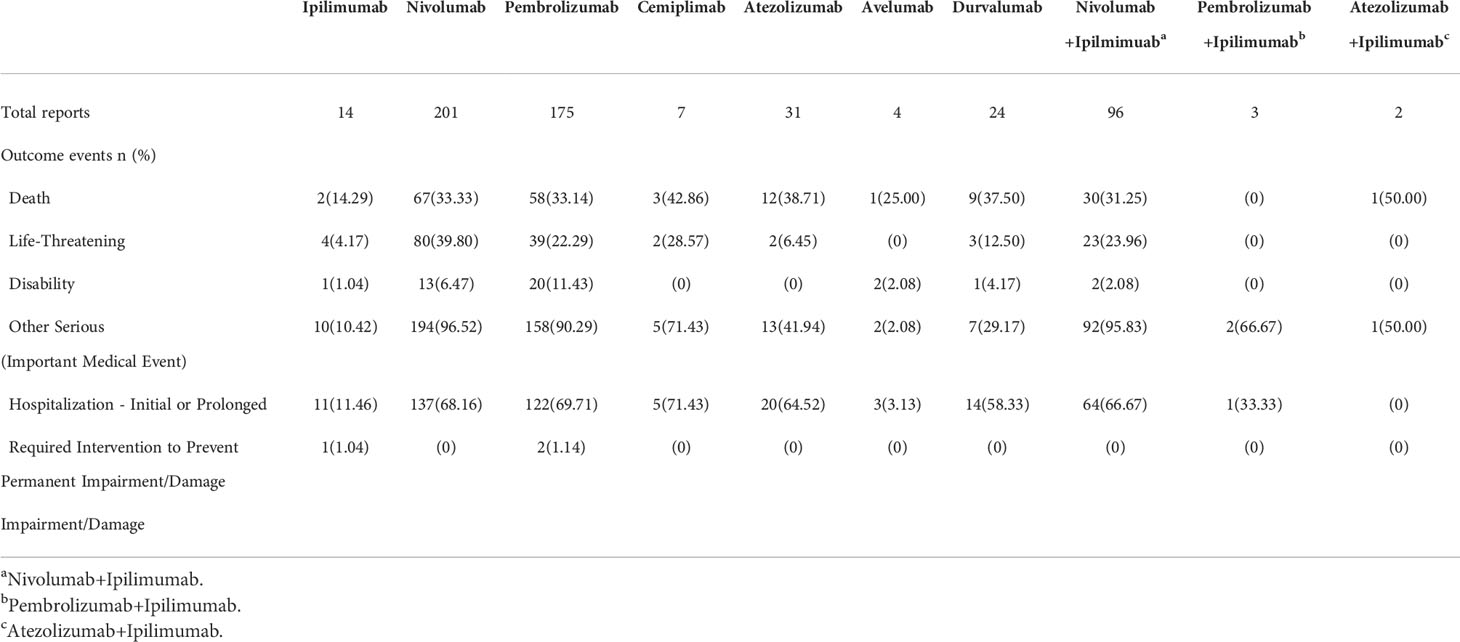
Outcome events and incidence
To determine the prognosis of neuromuscular junction dysfunctions after ICI treatments, we assessed neuromuscular junction dysfunctions following usage of ICIs (Table 6). It was observed that neuromuscular junction dysfunctions generally led to hospitalizations for nivolumab and pembrolizumab (68.16% and 69.71%, respectively). Among the 557 reports, 32.85% (183 out of 557) led to death. When comparing most frequently reported agents, nivolumab exhibits similar risks with or without ipilimumab. The outcomes of pembrolizumab shows no difference in terms of death, life-threatening disability, and other serious events compared to nivolumab.
Discussion
Our study concludes that though quite rare (0.63% of all reports), ICIs lead to severe adverse reactions (Table 6) on neuromuscular junction dysfunctions. In the current analysis, nivolumab and pembrolizumab account for most of the reports (201 + 175 = 376 vs. 557). These adverse events may be more easily discovered when ICI-drugs are prescribed in the larger patient population in the near future. However, monitoring programs must be present to help detect these rare but potentially severe side effects and provide an essential basis for future prevention.
Drugs that target the PD-L1/PD-1 pathway may exhibit a more favorable toxicity profile than CTLA4 blockade and conventional chemotherapy (28). However, our results confirm that ICIs targeting the PD-1 pathway show a higher risk of neuromuscular junction dysfunctions than ICIs targeting the CTLA4 pathway.
CTLA4 is an immune checkpoint molecule expressed on immune cells that contains both CD4+ and CD8+ T cell subsets and B cells. CTLA4 antibody (ipilimumab) can restore T cells priming and activation by tumor antigens and promote antitumor T cell response. The PD-L1/PD-1 interaction is another checkpoint for anti-cancer immunity that functions downstream of T cell priming and activation. PD-1 is expressed on the surface of activated T cells, but its specific ligand PD-L1 is present in multiple tissue types, including many other different cancer cells. When PD-L1 binds to PD-1, it inhibits T cell proliferation and activity and reduces cytokine production, helping malignant cells evade the host’s immune response. Therapeutic antibodies designed to target PD-L1/PD-1 interactions include antibodies that inhibit PD-1 (nivolumab and pembrolizumab) or PD-L1 (atezolizumab).
The clinical presentation of neuromuscular junction dysfunctions is often atypical, with considerable overlap between myasthenia gravis and myopathy, as well as cardiac/respiratory complications. However, mortality was high in these patients, despite adequate treatment strategies including corticosteroid. Several pathogenic mechanisms, including neuronal damage by T cells and autoantibodies and/or cytokine-mediated inflammation processes, have been hypothesized. However, the pathogenesis of these ICI-related complications is not completely understood (14). Clinical and experimental immune-mediated neuropathy studies have shown that autoimmune responses to peripheral nervous tissues are not limited to dense myelin along the main nerve trunk. They can also affect the nerve body, the axonal structures of the node of Ranvier, and the neuromuscular junction. Types of ICI-related neuropathy include demyelinating neuropathy, axonal sensorimotor polyneuropathy, pure sensory axonal neuropathy, and mononeuropathy multiplex based on electrophysiological studies (29).
Choosing a fast and effective signal detection method provides a valuable signal for drug risk management, which can severely impact human health and minimize harm to humans. However, risks are difficult to detect only through experimental studies with limited sample size. Therefore, this study provides a reference to confirm the occurrence of neuromuscular junction dysfunctions associated with ICIs through post-marketing surveillance studies.
Healthcare professionals treating cancer patients are advised to be aware of Guillain-Barre Syndrome (GBS) symptoms. These patients need immediate attention and should be given a low threshold for hospitalization to facilitate work-up and monitor severe or life-threatening symptoms (30). Currently, limited sources are comparing ICI-associated neuromuscular junction dysfunctions between PD-1 and PD-L1 targeting pathways. Signal detection suggested that pembrolizumab, either without or with ipilimumab, showed a higher score than other ICIs or ICI combinations. Our results also showed that ICIs targeting PD-1 (nivolumab and pembrolizumab) exhibited more neuromuscular junction dysfunctions than ICIs targeting the PD-L1 pathway (avelumab, atezolizumab, durvalumab, and semiprimal). However, there could be confounding factors such as a limited sample size since agents targeting the PD-L1 pathway entered the market later.
While the data mining techniques used in this study have many advantages as a tool for detecting signs of adverse drug reaction, it should also be noted that this technology does not solve all problems in detecting and analyzing signs of adverse drug reaction. The signals from FAERS were used only for qualitative research.
This study has certain limitations. First of all, data mining technology cannot completely reflect all clinical information from patients. Detailed information from clinical follow-ups and other studies are also needed to validate the signals detected. Secondly, data mining technology cannot address ADR reporting system issues such as inaccuracy, false reporting, incomplete reporting, underreporting, and arbitrariness. Thirdly, qualitative research makes it difficult to quantify the side effect signals of neuromuscular junction dysfunctions from a few ADRs. Finally, the restrictions of limited sample size cannot be overlooked.
Conclusion
Immune checkpoint inhibitors need to be prescribed more cautiously. Analysis of FAERS data showed that over 30% of reports of neuromuscular junction dysfunctions resulted in death, emphasizing the importance of constant monitoring, risk assessment, and more comparative studies.
Data availability statement
The original contributions presented in the study are included in the Article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
All contributors are listed as co-authors. BY and DL: conceptualization. QD and XY: methodology. BZ: data retrieving. DL: formal analysis and investigation. HC: writing original draft preparation. PZ: writing. QZ: review and editing. BY: supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Chinese Pharmaceutical Association-Servier Youth Hospital Pharmaceutical Innovation Research Funding Project and Hospital Development Center of Shanghai Municipality (SHDC2020CR3085B).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Darvin P, Toor SM, Sasidharan Nair V, Toor SM, Elkord E, et al. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp Mol Med (2018) 50(12):1–11. doi: 10.1038/s12276-018-0191-1
2. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012
3. Liu YH, Zang XY, Wang JC, Wang JC, Huang SS, Xu J, Zhang P, et al. Diagnosis and management of immune related adverse events (irAEs) in cancer immunotherapy. BioMed Pharmacother (2019) 120:109437. doi: 10.1016/j.biopha.2019.109437
4. Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol (2014) 11(2):91–9. doi: 10.1038/nrclinonc.2013.245
5. Xu C, Chen Y P, Du X J, Liu JQ, Huang CL, Chen L, et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ (2018) 363:k4226. doi: 10.1136/bmj.k4226
6. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med (2012) 366(26):2443–54. doi: 10.1056/NEJMoa1200690
7. Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-Related pneumonitis during cancer immunotherapy. N Engl J Med (2015) 373(3):288–90. doi: 10.1056/NEJMc1505197
8. Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer (2017) 73:1–8. doi: 10.1016/j.ejca.2016.12.001
9. Larkin J, Chmielowski B, Lao CD, Hodi FS, Sharfman W, Weber J, et al. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist (2017) 22(6):709–18. doi: 10.1634/theoncologist.2016-0487
10. Chen X, Haggiagi A, Tzatha E, DeAngelis LM, Santomasso B. Electrophysiological findings in immune checkpoint inhibitor-related peripheral neuropathy. Clin Neurophysiol (2019) 130(8):1440–5. doi: 10.1016/j.clinph.2019.03.035
11. Psimaras D, Velasco R, Birzu C, Tamburin S, Lustberg M, Bruna J, et al. Immune checkpoint inhibitors-induced neuromuscular toxicity: From pathogenesis to treatment. J Peripher Nerv Syst (2019) 24 Suppl 2:S74–85. doi: 10.1111/jns.12339
12. Kolb NA, Trevino CR, Waheed W, Sobhani F, Landry KK, Thomas AA, et al. Neuromuscular complications of immune checkpoint inhibitor therapy. Muscle Nerve (2018) 58(1):10–22. doi: 10.1002/mus.26070
13. Jordan B, Benesova K, Hassel JC, Wick W, Jordan K. How we identify and treat neuromuscular toxicity induced by immune checkpoint inhibitors. ESMO Open (2021) 6(6):100317. doi: 10.1016/j.esmoop.2021.100317
14. Johansen A, Christensen SJ, Scheie D, Højgaard JLS, Kondziella D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: Systematic review. Neurology (2019) 92(14):663–74. doi: 10.1212/WNL.0000000000007235
15. Rodriguez EM, Staffa JA, Graham DJ. The role of databases in drug postmarketing surveillance. Pharmacoepidemiol Drug Saf (2001) 10(5):407–10. doi: 10.1002/pds.615
16. Weiss-Smith S, Deshpande G, Chung S, Gogolak V. The FDA drug safety surveillance program: adverse event reporting trends. Arch Intern Med (2011) 171(6):591–3. doi: 10.1001/archinternmed.2011.89
17. Van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf (2002) 11(1):3–10. doi: 10.1002/pds.668
19. Ooba N, Kubota K. Selected control events and reporting odds ratio in signal detection methodology. Pharmacoepidemiol Drug Saf (2010) 19(11):1159–65. doi: 10.1002/pds.2014
20. Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf (2001) 10(6):483–6. doi: 10.1002/pds.677
21. Hauben M, Madigan D, Gerrits CM, Walsh L, Van Puijenbroek EP. The role of data mining in pharmacovigilance. Expert Opin Drug Saf (2005) 4(5):929–48. doi: 10.1517/14740338.4.5.929
22. Noren GN, Bate A, Orre R, Edwards IR. Extending the methods used to screen the WHO drug safety database towards analysis of complex associations and improved accuracy for rare events. Stat Med (2006) 25(21):3740–57. doi: 10.1002/sim.2473
23. Hauben M. A brief primer on automated signal detection. Ann Pharmacother (2003) 37(7-8):1117–23. doi: 10.1345/aph.1C515
24. Dumouchel W. Bayesian Data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Statistician (1999) 53(3):177–90. doi: 10.1080/00031305.1999.10474456
25. Szarfman A, Machado SG, O’neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf (2002) 25(6):381–92. doi: 10.2165/00002018-200225060-00001
26. Beninger P. Pharmacovigilance: An overview. Clin Ther (2018) 40(12):1991–2004. doi: 10.1016/j.clinthera.2018.07.012
27. Poluzzi E, Raschi E, Piccinni C, De Ponti F. Data mining techniques in pharmacovigilance: Analysis of the publicly accessible FDA adverse event reporting system (AERS). In: Data mining applications in engineering and medicine. London, United Kingdom: Intech Open (2012). doi: 10.5772/50095
28. Lee A, Sun S, Sandler A, Hoang T. Recent progress in therapeutic antibodies for cancer immunotherapy. Curr Opin Chem Biol (2018) 44:56–65. doi: 10.1016/j.cbpa.2018.05.006
29. Dubey D, David WS, Amato AA, Reynolds KL, Clement NF, Chute DF, et al. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology (2019) 93(11):e1093–103. doi: 10.1212/WNL.0000000000008091
Keywords: immune checkpoint inhibitor, neuromuscular junction dysfunction, adverse event, data mining, FAERS
Citation: Zhang P, Lao D, Chen H, Zhao B, Du Q, Zhai Q, Ye X and Yu B (2022) Neuromuscular junction dysfunctions due to immune checkpoint inhibitors therapy: An analysis of FAERS data in the past 15 years. Front. Immunol. 13:778635. doi: 10.3389/fimmu.2022.778635
Received: 14 October 2021; Accepted: 28 July 2022;
Published: 22 August 2022.
Edited by:
Letizia Leocani, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Nora Möhn, Hannover Medical School, GermanyRaffaele Dubbioso, Federico II University Hospital, Italy
Copyright © 2022 Zhang, Lao, Chen, Zhao, Du, Zhai, Ye and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Yu, bWlndWVsYm95dUBtc24uY24=; Xuan Ye, eWV4dWFuMTIxNkAxNjMuY29t
†These authors have contributed equally to this work
 Ping Zhang1†
Ping Zhang1† Donghui Lao
Donghui Lao Haoyan Chen
Haoyan Chen Qiong Du
Qiong Du Qing Zhai
Qing Zhai Xuan Ye
Xuan Ye