
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Immunol. , 29 December 2022
Sec. Molecular Innate Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1114275
This article is part of the Research Topic Innate Immunity and Severe Asthma: From Microbiome to Target Therapy View all 8 articles
Editorial on the Research Topic
Innate immunity and severe asthma: From microbiome to target therapy
An increasing amount of evidence supports the role of microbiome as a major player in the pathobiology of several conditions. Most of the research on the topic has so far investigated gut microbiome, but a number of studies have recently focused on other districts including the respiratory tract. Although it has been considered for a long time a sterile organ without any commensal population, according to the latest evidence the lung microbiome seems to be primarily involved in the immunological background of respiratory tract diseases (1).
The increasing interest in microbiome has been supported by some methodological innovations in the field, in terms of omics sciences, combining the more traditional culture and incubation on media with the newer protein chain reaction (PCR). Relying on those techniques large databases including genomes and metagenomes of the human microbiome have been developed, enabling the investigation of the functional effects of the human microbiome through the detection of functional genes encoded by the microbial community and their products (proteins, metabolites, etc.) (2).
Although the permanent cross talking between the environment and the skin, gut and respiratory tract surfaces exerts an inevitably dynamic effect on microbiome (3), the first two months after birth remain critical for the growth of the commensal microorganisms pool in the respiratory tract (4). The development of the immune tolerance to environmental agents, including allergens, also takes place in the first few weeks of life, and seems to be heavily affected by the microbiome composition (5).
Under that perspective, the relevance of environmental determinants characterizing the early life “exposome” of each individual, including hygienic conditions but also feeding habits, is quite expected.
Breastfeeding, for example, has been associated with a reduced likelihood of developing asthma later in life, compared with infants who were not naturally breastfed (6). It has been described that alterations in the pulmonary micro biota, starting at birth, lead to the development of a variety of chronic respiratory diseases such as asthma (7). Of note, the specific composition of lung and respiratory tract microbiome seems to substantially contribute to different inflammatory patterns and consequently to the definition of the asthma endotype. According to some evidence coming from murine models reproducing chronic lung inflammation the enrichment of pseudomonas and lactobacillus, derived from affected human subjects, stimulates a TH 17-driven response, while pathogens such as proteobacteria induces severe airways inflammation mediated by the activation of a TLR2-independent pathway (4).
The relevance of microbiome has been explored also in lung carcinoma, specifically adenocarcinoma, whose pathobiology seems to be related to the presence of specific commensals facilitating the production of interleukin (IL) - 17 through the stimulation of T γδ cells (8).
Regarding allergic asthma, some authors have postulated a link between the presence and diversity of dust bacterial content and susceptibility to allergic sensitization to mites; however, a clear-cut association between pulmonary bacterial dysbiosis and type 2 (T2) inflammation has not been demonstrated so far (9). Some authors speculated about a major role of fungi more than bacteria in the induction of a T2 response., but there is still little evidence on that (10, 11). A recent study compared the microbiome of two subpopulations, namely patients with mild asthma and atopic subjects without asthma, with a control group of healthy nonatopic patients; the authors observed a specific pattern of dysbiosis strongly which was strongly associated with atopy alone, and a different one associated with asthma (12). Although the results showed that a particular microbiome might favor one type of disease over another, the differentiating factors are not known in details. On the opposite, in the case of neutrophilic asthma, an altered composition of the bacterial microbiota has been observed to be present, when compared with T2 endotype (13). Haemophilus, Klebsiella, Moraxella, and Pseudomonas, represent the most frequently detected bacteria, although in several studies (1, 14, 15) severe asthma patients with neutrophilic inflammation showed a wide diversity of the microorganisms composing the airway bacterial flora, compared with a more limited variability characterizing eosinophilic or pauci-granulocytic patients. Other studies have also confirmed a direct relationship between the abundance of microorganisms from the proteobacterial family and IL-17 (13), as well as a correlation between the above-described bacteria with increased cytokine levels and worse disease control. Under that perspective, the role of staphylococcus aureus and its enterotoxins (SEs) in inducing T2 inflammation in subjects with severe asthma, recently confirmed and further investigated by studies in Real Life (16, 17) another example of the cross-talking between microorganisms and the immunological background underlying bronchial inflammation.
Although not yet fully understood, the relationship between commensal and non-commensal microorganisms and the immune system and its relevance in the development of asthma represents a new perspective to look at asthma pathobiology (Figure 1). At the same time it paves the way to potential primary prevention strategies, in terms of early life or interventions, as well as to innovative targeted therapeutic approaches in affected individuals, especially those suffering from difficult to treat asthma phenotyopes. It implies further development of dedicated tools, such as omics sciences, in terms of translational research and their implementation in clinical practice.
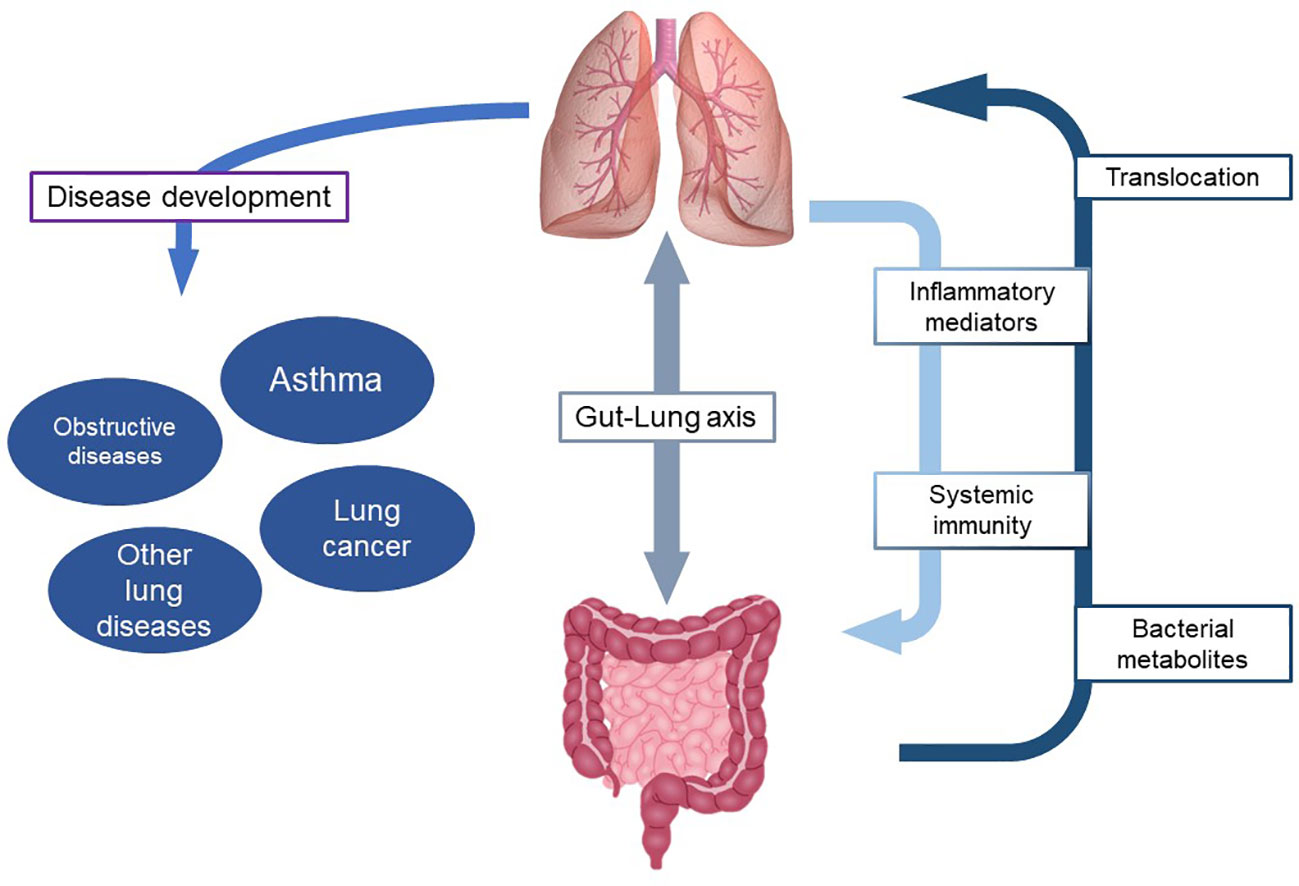
Figure 1 Role of microbiome in the interaction between gut and lung and development of respiratory diseases.
DB and MC equally contribute to the development and writing of manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kozik AJ, Huang YJ. The microbiome in asthma: Role in pathogenesis, phenotype, and response to treatment. Ann Allergy Asthma Immunol (2019) 122:270–5. doi: 10.1016/j.anai.2018.12.005
2. Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe (2015) 17:592–602. doi: 10.1016/j.chom.2015.04.007
3. Pattaroni C, Watzenboeck ML, Schneidegger S, Kieser S, Wong NC, Bernasconi E, et al. Early-life formation of the microbial and immunological environment of the human airways. Cell Host Microbe (2018) 24:857–865.e4. doi: 10.1016/j.chom.2018.10.019
4. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res (2020) 30(6):492–506. doi: 10.1038/s41422-020-0332-7
5. Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med (2014) 20:642–7. doi: 10.1038/nm.3568
6. Hou W, Guan F, Xia L, Xu Y, Huang S, Zeng P. Investigating the influence of breastfeeding on asthma in children under 12 years old in the UK biobank. Front Immunol (2022) 13:5792. doi: 10.3389/fimmu.2022.967101
7. Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet (2014) 384:691–702. doi: 10.1016/S0140-6736(14)61136-3
8. Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell (2019) 176:998–1013.e16. doi: 10.1016/j.cell.2018.12.040
9. Kheradmand F, Kiss A, Xu J, Kolattukudy EP, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol (2002) 169:5904–11. doi: 10.4049/jimmunol.169.10.5904
10. Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM, et al. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J (2006) 27:615–26. doi: 10.1183/09031936.06.00074705
11. Porter PC, Lim DJ, Maskatia ZK, Mak G, Tsai C-L, Citardi JM, et al. Airway surface mycosis in chronic TH2-associated airway disease. J Allergy Clin Immunol (2014) 134:325–11. doi: 10.1016/j.jaci.2014.04.028
12. Durack J, Huang YJ, Nariya S, Christian LS, Ansel KM, Beigelman A, et al. Bacterial biogeography of adult airways in atopic asthma. Microbiome (2018) 6(1):104. doi: 10.1186/s40168-018-0487-3
13. Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, et al. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J Allergy Clin Immunol (2015) 136:874–84. doi: 10.1016/j.jaci.2015.05.044
14. Simpson JL, Daly J, Baines KJ, Yang IA, Upham JW, Reynolds PN, et al. Airway dysbiosis: Haemophilus influenzae and tropheryma in poorly controlled asthma. Eur Respir J (2016) 47:792–800. doi: 10.1183/13993003.00405-2015
15. Taylor SL, Leong LEX, Choo JM, Wesselingh S, Yang IA, Upham JW, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol (2018) 141:94–103.e15. doi: 10.1016/j.jaci.2017.03.044
16. Caruso C, Stefania C, Ciasca G, Basile, Di Santo R, Bagnasco D, et al. Different aspects of severe asthma in real life: Role of staphylococcus aureus enterotoxins and correlation to comorbidities and disease severity. Allergy (2022). doi: 10.1111/all.15466
Keywords: microbiome, asthma, immunity, T2 inflammation, enterotoxins
Citation: Bagnasco D and Caminati M (2022) Editorial: Innate immunity and severe asthma: From microbiome to target therapy. Front. Immunol. 13:1114275. doi: 10.3389/fimmu.2022.1114275
Received: 02 December 2022; Accepted: 19 December 2022;
Published: 29 December 2022.
Edited and Reviewed by:
Francesca Granucci, University of Milano-Bicocca, ItalyCopyright © 2022 Bagnasco and Caminati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diego Bagnasco, ZGllZ28uYmFnbmFzY29AZGltaS51bmlnZS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.