- 1Department of Hepatology Division 2, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 2Department of Infectious Diseases, Peking University First Hospital, Beijing, China
- 3Department of Hepatology Division 2, Peking University Ditan Teaching Hospital, Beijing, China
- 4Department of Biostatistics, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Aims: Study of clinical characteristics of hepatitis B virus deoxyribonucleic acid (HBV DNA)-negative, hepatitis B surface antigen (HBsAg)-positive, hepatitis B e antigen (HBeAg)-negative patients based on liver histopathology.
Methods: We retrospectively enrolled patients with chronic HBV infection diagnosis at Beijing Ditan Hospital from May 2008 to November 2020. To study the differences between patients with significant hepatic histopathology and those without significant hepatic histopathology. And to study the independent factors of significant hepatic histopathology.
Results: 85 HBV DNA-negative and HBeAg-negative patients were 37.90 ± 10.30 years old, 23.50% of patients with grade of inflammation (G) >1, 35.30% of patients with liver fibrosis stage (S) >1, 44.70% patients were diagnosed with significant hepatic histopathology. Compared to the no significant hepatic histopathology group, another group had older age (41.70 ± 10.70 vs 34.80 ± 8.87 years, t=-3.28, P=0.002), higher total bilirubin (TBIL) [14.9(10.3, 22.4) vs 11(8.9, 14.4) μmol/L, z=-2.26, P=0.024], lower cholinesterase (CHE) (t=-2.86, P=0.005, 7388.00 ± 2156.00 vs 8988.00 ± 2823.00 U/L) and lower platelet (PLT) (t=2.75, P=0.007, 157.00 ± 61.40 vs 194.00 ± 61.00 10^9/L). Abnormal ALT patients are more likely to have significant hepatic histopathology (z=5.44, P=0.020, 66.70% vs 337.50%). G had significant correlation with CHE (P=0.008, r=-0.23), alanine aminotransferase (ALT) (P=0.041, r=0.18), aspartate aminotransferase (AST) (P=0.001, r=0.29). S had significant correlation with TBIL (P = 0.008, r = 0.23), age (P < 0.001, r = 0.32), international normalized ratio (INR) (P = 0.04, r = 0.23), CHE (P < 0.001, r = -0.30), PLT (P < 0.001, r = -0.40) and prothrombin time activity (PTA) (P = 0.046, r = -0.22). Multivariate logistic analysis indicated only age (95%CI=1.014~1.130, OR=1.069, P=0.013) was an impact factor for significant hepatic histopathology. The cutoff point of age was 34.30 years.
Conclusions: A large proportion of chronic HBV infection patients with HBeAg-negative and HBV DNA-negative still have chronic hepatitis. Age is an independent factor for significant hepatic histopathology
Introduction
As reported in the latest (2017) world health organization (WHO) global hepatitis report, in 2015, there were approximately 257 million people infected hepatitis B virus (HBV) worldwide (1). Chronic HBV infection has 5 periods of natural history, which contain hepatitis B e antigen (HBeAg)-positive chronic HBV infection, HBeAg-negative chronic HBV infection, HBeAg-positive chronic hepatitis B, HBeAg-negative chronic hepatitis B, hepatitis B surface antigen (HBsAg)-negative phase (2), and each period has its characteristics.
HBeAg-negative chronic HBV infection is characterized by HBeAb-positive, normal alanine aminotransferase (ALT), and low HBV DNA load (3). In the past, this period was termed as “inactive carriers (IC)”, but as understanding of HBV disease history and immune mechanisms has improved, scholars have replaced the term IC with HBeAg-negative chronic HBV infection (4). Although prognosis for patients in this period is said to be good, some patients may still experience fluctuations in HBV DNA and/or ALT. These fluctuations may then lead to disease progression such as liver fibrosis, liver cirrhosis or even liver cancer (5, 6). Previously, many scholars have studied the clinical features and prognosis of this stage. One limitation of those studies is that they did not use the high-sensitivity HBV DNA quantification assay. Our research was conducted to study the histopathological characteristics and clinical indicators of HBeAg-negative, HBV DNA-negative patients using a high-sensitivity assay in combination with liver biopsy.
Materials and methods
Patients
Chronic HBV-infected patients who underwent liver biopsies were obtained from Beijing Ditan Hospital affiliated to Capital Medical University between May 2008 and November 2020. Information on antiviral therapy, demographic data, HBV serological test and HBV DNA load results, renal function, liver function, blood routine examination and blood coagulation function were collected.
Admission criteria: 1) HBsAg-positive for over 6 months; 2) 18 to 65 years old; 3) no HBV treatment received; 4) complete viral load and viral serology results, coagulation, liver function, blood routine within 2 weeks after or before biopsy; 5) have clear diagnostic results of liver biopsy.
Exclusion criteria: 1) infected with other viruses (e.g., HIV, cytomegalovirus, etc.); 2) Co-infection with other viral hepatitis (such as hepatitis C, A, D and E), non-alcoholic fatty liver disease, alcoholic liver disease, autoimmune liver disease, drug-induced liver injury, cirrhosis, hepatoma; 3) patients with cardiac or renal dysfunction; 4) patients who have not obtained a clear result of inflammatory grade or liver fibrosis stage.
Study parameters
ALT, AST, HBsAg, HBeAg, HBV DNA, albumin (ALB), international normalized ratio (INR), total bilirubin (TBIL), cholinesterase (CHE), prothrombin time activity (PTA), platelet (PLT).
Liver pathology results included liver fibrosis stage S0-S4, and inflammatory grade G0-G4.
In our study, S>1 indicates significant liver fibrosis, 0<S ≤ 1 indicates slight liver fibrosis, S0 indicates no liver fibrosis. G>1 indicates significant liver inflammation, and G ≤ 1 indicates no significant liver inflammation. No significant hepatic histopathology group’s patients meet G ≤ 1 and S ≤ 1, and significant hepatic histopathology group’s patients meet G>1 and/or S>1.
Study contents
To study the correlation factors associated with the pathological grading of liver biopsies in HBeAg-negative patients with chronic HBV infection and to analyze the characteristics of their inflammatory grading and fibrosis staging. To compare the differences in clinical indicators between the two groups of patients. To study the risk factors (or protective factors) for significant liver histopathology and calculate the cutoff values of the relevant factors.
Statistical analysis
Use the mean ± standard deviation or quartiles to describe enumeration data and percentages were used to describe categorical data. Categorical data were compared using chi-square test. Student’s t-test was used to compare normally distributed data. Using Mann-Whitney test to compare non-normally distributed data. Risk factors (or protective factors) were studied using binary logistic regression analysis, and cutoff values for relevant risk factors (or protective factors) were calculated from youden’s index and receiver operating curve (ROC). The software involving figures making was Microsoft Excel 2019 and Graphpad Prism 8.0. Statistical analysis was performed in IBM SPSS 25.0. P<0.05 indicates statistical significance.
Results
Patients
A total of 4421 HBV infected people had liver puncture biopsies. After collecting HBV serologic indicators, HBV DNA load, demographic data, clinical biochemical indicators, and blood routines. We performed screening and excluded 8 patients with hepatitis C, 1870 patients with fatty liver, 219 patients whose liver pathology diagnosis did not clearly show disease grade, 226 patients who received HBV treatment, 165 patients without HBeAg results, 428 patients without HBV DNA load results, 31 patients without ALT results, 613 patients with a detectable HBV DNA load, 776 HBeAg-positive patients. Finally, a total of 85 HBeAg-negative, HBV DNA-negative patients (HBeAg-negative, HBV DNA load below the lower limit of detection) were included in this study. (Figure 1)
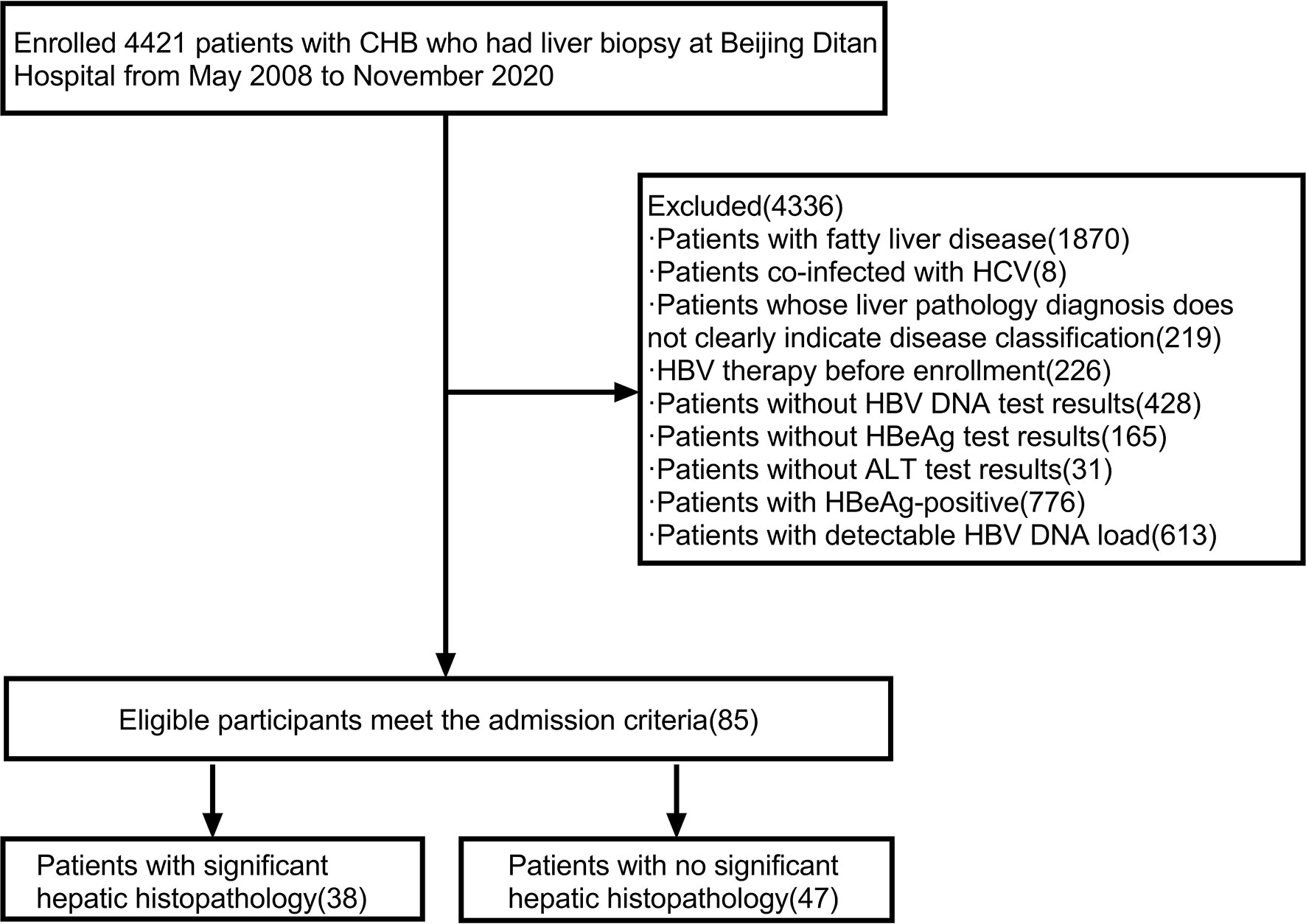
Figure 1 Flow chart for screening research patients. Abbreviations: ALT, alanine aminotransferase; CHB, chronic hepatitis B; HBV DNA, hepatitis B virus deoxyribonucleic acid; HBV, hepatitis B virus; HCV, hepatitis C virus; HBeAg, hepatitis B e antigen.
In this group of patients, 57.60% were male, age (37.90 ± 10.30) years old, significant hepatic histopathology was observed in 44.70% of patients. Compared with no significant hepatic histopathology group, the other group had older age (41.70 ± 10.70 vs 34.80 ± 8.87 years, t=-3.28, P=0.002), higher TBIL levels [14.9(10.3, 22.4) vs 11(8.9, 14.4) μmol/L, z=-2.26, P=0.024], lower CHE levels (7388.00 ± 2156.00 vs 8988.00 ± 2823.00 U/L, t=-2.86, P=0.005) and PLT counts (157.00 ± 61.40 vs 194.00 ± 61.00 10^9/L, t=2.75, P=0.007). (Table 1)
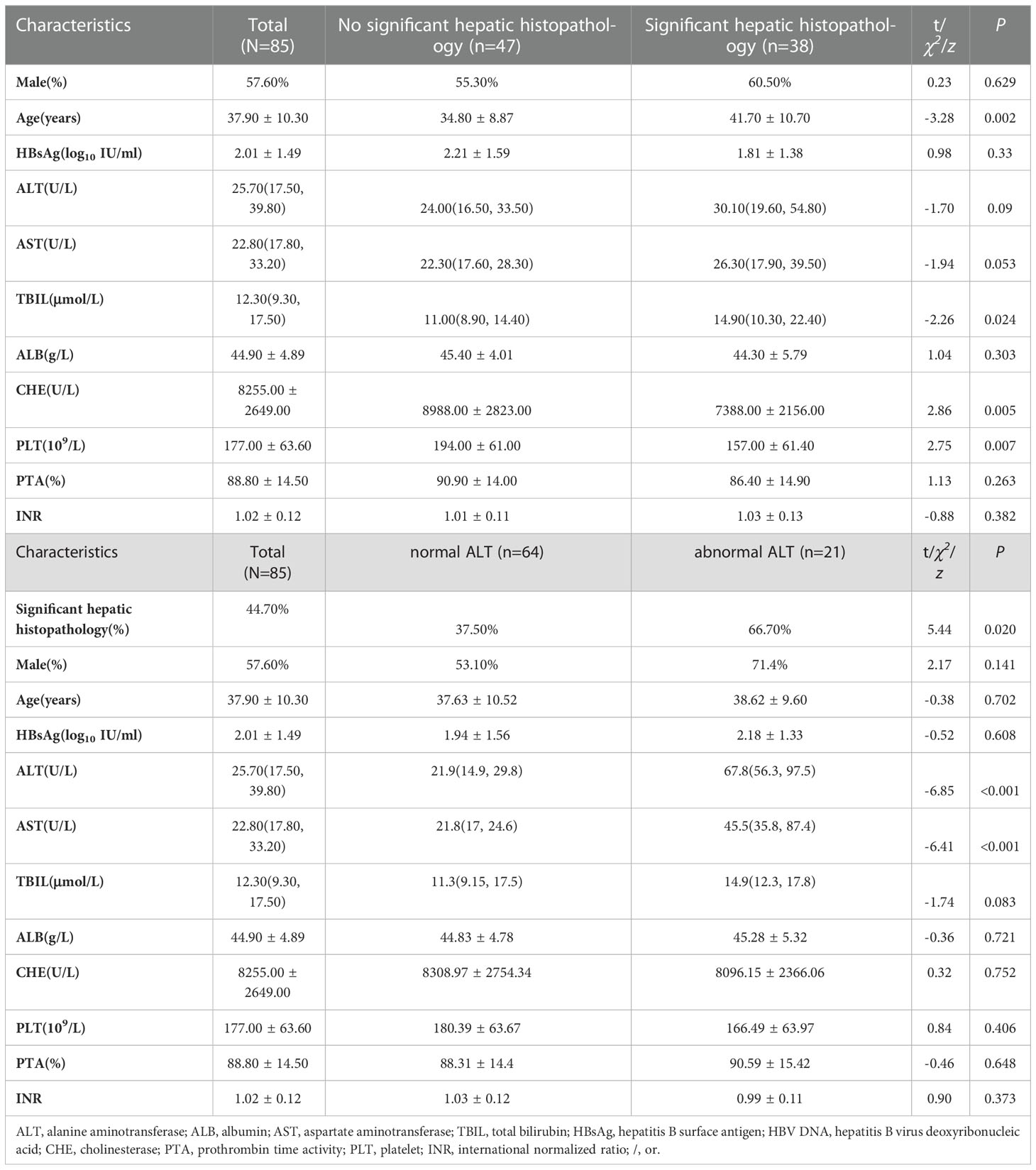
Table 1 Characteristics and comparison of clinical indicators in HBeAg-negative, HBV DNA-negative patients.
Compared with normal ALT group, abnormal ALT group had more patients with significant hepatic histopathology (66.70% vs 337.50%, z=5.44, P =0.020). (Table 1)
Liver histopathology
In liver histopathological diagnosis, liver inflammation grading: 76.50% (65 cases) of patients with G ≤ 1, 15.30% (13 cases) of patients with 1<G ≤ 2, and 8.20% (7 cases) of patients with 2<G ≤ 3. Liver fibrosis grading: S0 patients accounted for 1.20% (1 case), S ≤ 1 patients accounted for 63.50% (54 cases), 1<S ≤ 2 patients accounted for 24.70% (21 cases), 2<S ≤ 3 patients accounted for 8.20% (7 cases), and 3<S ≤ 4 patients accounted for 2.40% (2 cases) (Table 2).

Table 2 Histopathological grade distribution table for liver biopsies of HBeAg-negative and HBV DNA-negative patients.
Correlation between pathological grading and clinical indicators
The correlations of liver inflammation grade with ALT (P=0.041, r=0.18), AST (P=0.001, r=0.29), and CHE (P=0.008, r=-0.23) were significant, while the correlations with HBsAg, ALB, and PLT were not significant. The correlation between liver fibrosis stage and age (P < 0.001, r = 0.32), TBIL (P = 0.008, r = 0.23), CHE (P < 0.001, r =-0.30), PLT (P < 0.001, r =-0.40), PTA (P = 0.046, r =-0.22) and INR (P = 0.04, r = 0.23) were significant, while the correlation with HBsAg, ALT, AST, and ALB were not significant. (Table 3)
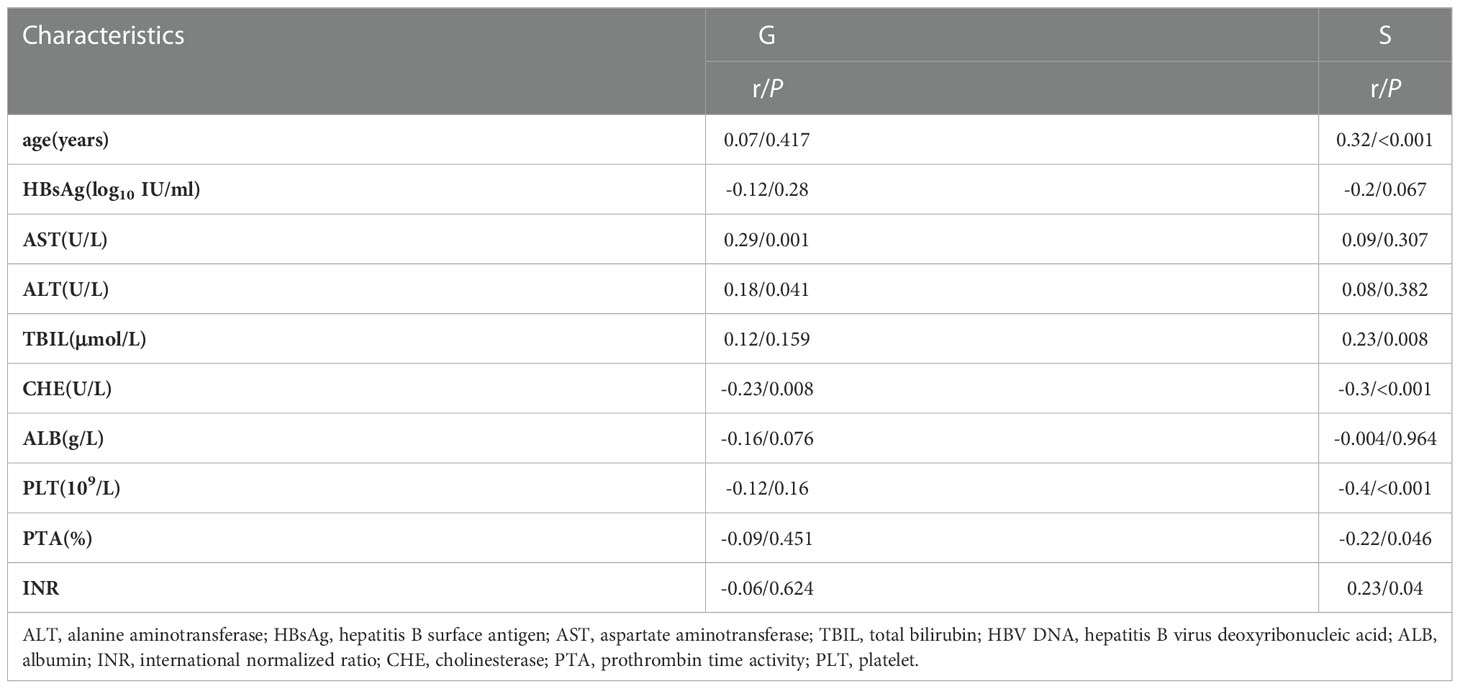
Table 3 Histopathological grade distribution of the liver biopsy of HBeAg-negative and HBV DNA-negative patients.
Logistic regression analysis
We performed logistic regression analysis on the differential indicators (age, TBIL, CHE and PLT) of two groups. The results of univariate logistic regression indicated that significant hepatic histopathology was associated with CHE level (OR = 0.018, 95% CI = 0.005 ~ 0.071, P < 0.001), age (OR = 1.077, 95% CI = 1.025 ~ 1.132, P = 0.003) and PLT count (OR = 0.988, 95% CI=0.985 ~ 0.992, P < 0.001). Multivariate logistic regression analysis indicated that only age (95% CI = 1.014 ~ 1.130, OR = 1.070, P = 0.015) was an independent factor. (Table 4)
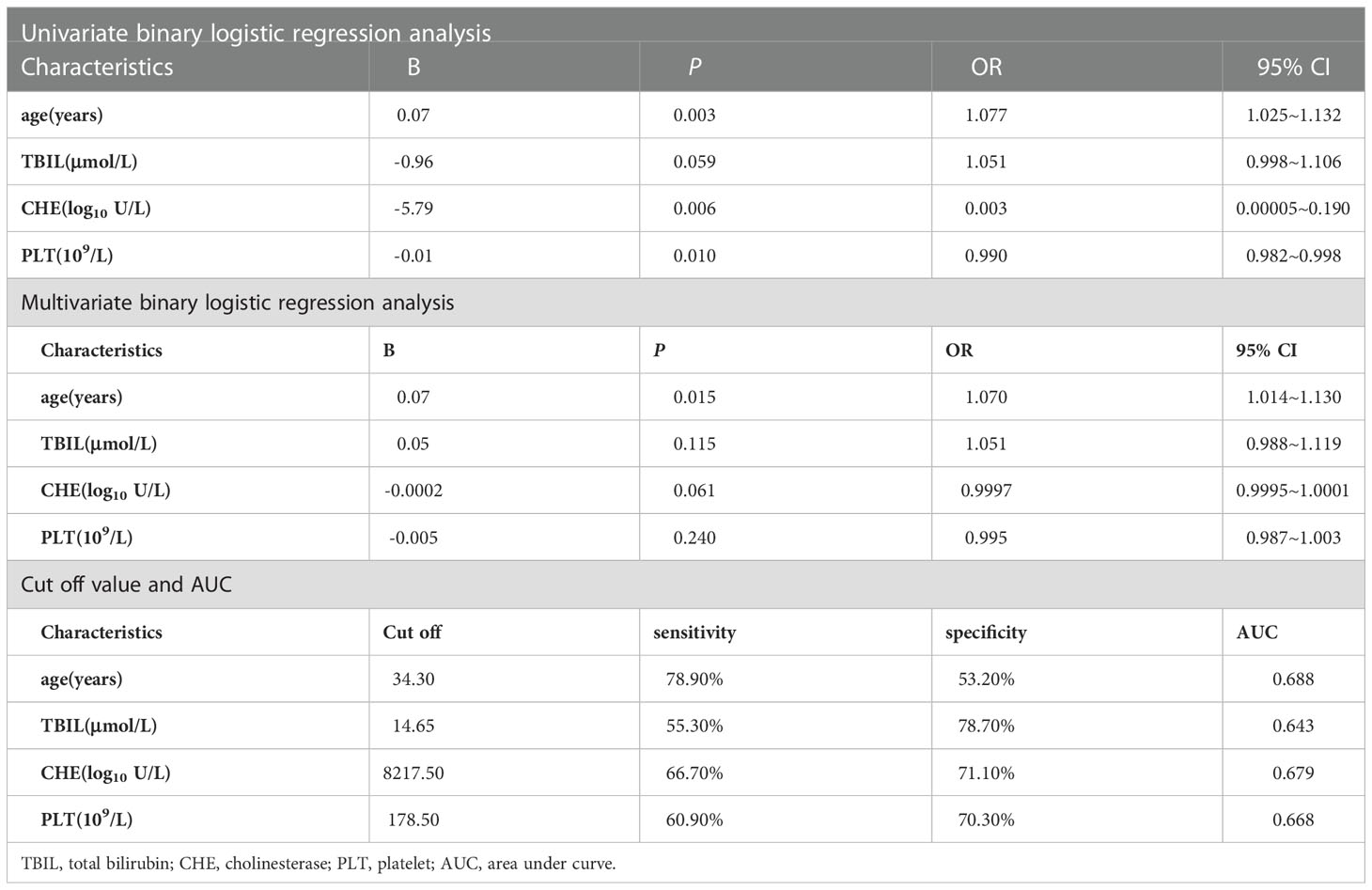
Table 4 Logistic regression analysis of HBeAg-negative, HBV DNA-negative patients with and without significant hepatic histopathology.
The cutoff value was calculated by ROC curve and Jorden index, age 34.30 years, sensitivity 78.90%, specificity 53.20%, AUC=0.688. (Table 4 and Figure 2)
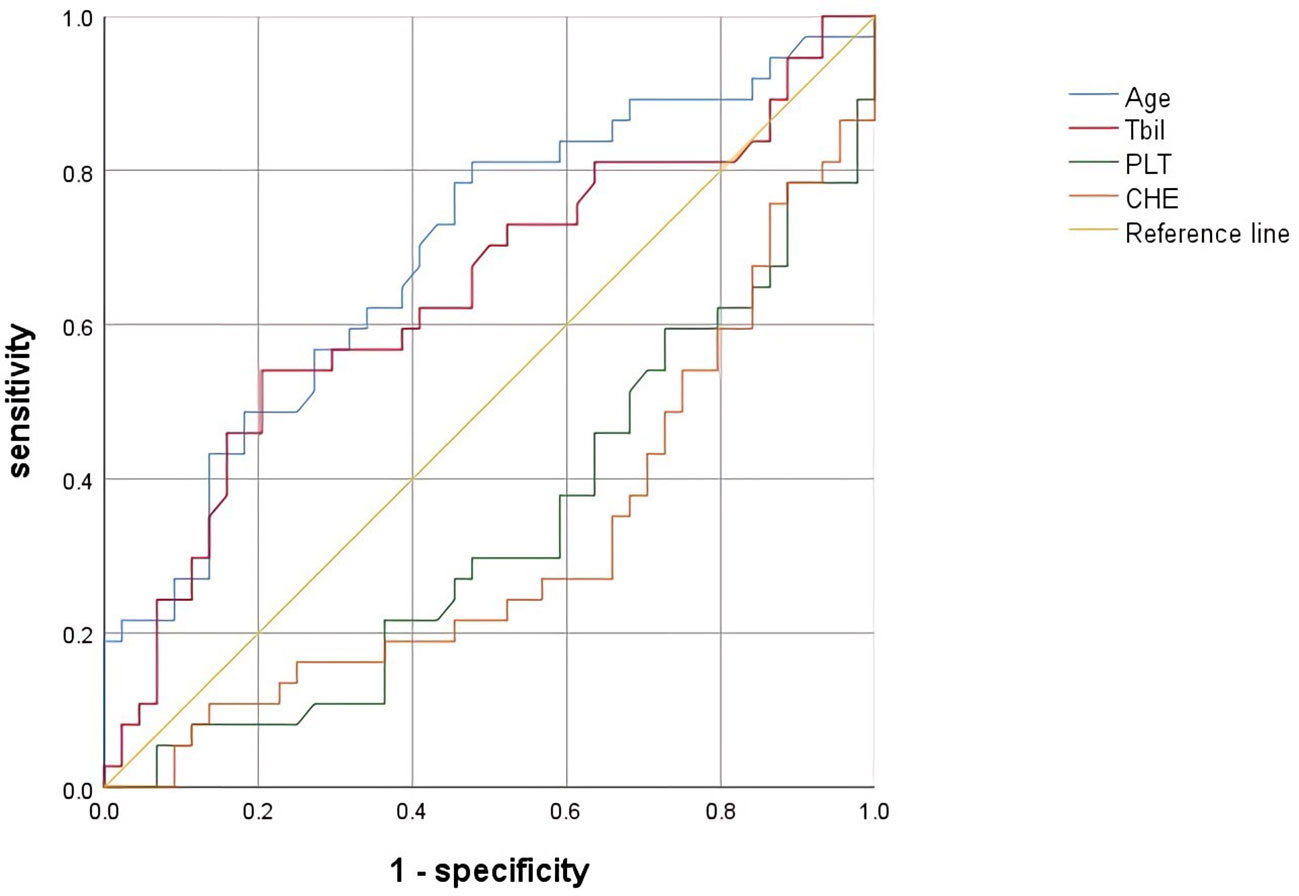
Figure 2 ROC of factors associated with significant hepatic histopathology. Abbreviations: CHE, cholinesterase; PLT, platelet; TBIL, total bilirubin; ROC, receiver operating characteristic curve.
Discussion
During the natural history of chronic HBV infection, HBeAg seroconversion is a very important point, which implies a reduction or even silencing of viral cccDNA transcription activity (7). Also, in clinical practice, HBeAg seroconversion means a better prognosis because patients with HBeAg-negative chronic HBV infection are less likely to progress to cirrhosis or liver cancer. However, if the HBeAg-negative chronic hepatitis B period develops, there is still the possibility of disease progression (3, 8–10). That is to say, effective distinction between HBeAg-negative chronic hepatitis B and HBeAg-negative chronic HBV infection plays a very important role in determining the prognosis of those patients.
Martinot, Park H, Brunett prospectively included HBeAg-negative patients in their respective studies and investigated how to identify HBeAg-negative chronic hepatitis B patients from HBeAg-negative chronic HBV infection patients (11–13). Their results showed that HBsAg could be used as a factor in determining the presence of hepatitis in HBeAg-negative patients (11–13). Martinot and Oliveri, on the other hand, found that HBV DNA could also be used as a predictive factor in their own studies (11, 14). Brunetto found in his subsequent studies that HBcrAg was highly accurate in determining the presence of hepatitis in HBeAg-negative patients (15). However, the grouping methods in all these studies were by ALT and HBV DNA, yet in fact there are times when clinical indicators and liver pathology results do not parallel each other. Liver pathology results are the gold standard for determining the progression of disease. Therefore, we designed this study to investigate the risk factors for inflammation in HBsAg-positive, HBeAg-negative, and HBV DNA-negative patients using liver pathology findings as a grouping method and a more accurate HBV DNA assay.
Chronic hepatitis B is an immune-mediated disease, and age is an important factor in disease progression. Previous studies have shown a gradual decrease in the proportion of HBeAg-positive patients with increasing age, to less than 10% in patients over 40 years old (16). In our study, after comparing the clinical characteristics of significant hepatic histopathology group and no significant hepatic histopathology group, we found that patients with significant hepatic histopathology were older, and the correlation analysis showed that growing age was significantly related to the more severe fibrosis stage. Univariate and multivariate logistic regression showed that age was the independent impact factor of significant hepatic histopathology, meanwhile, the older the patient is, the more likely the patient has significant hepatic histopathology. Hsu Y S conducted a cohort study in HBeAg seroconversion patients and found that the annual incidence of hepatitis relapse was 2.2–3.3% (9). This study proved that with the age growing, more patients would develop hepatitis B. This is consistent with our findings. We calculated the cutoff value of age and it was 34.3 years old, and the AUC was 0.688, a barely satisfactory number.
CHE is an indicator of the synthetic function of the liver, which is affected when hepatitis or fibrosis occurs, and CHE decreases. This is confirmed by our study that patients with significant hepatic histopathology have a lower level of CHE, and with the growth of G and S, CHE decreases. Coagulation is also closely related to liver function, and our study found that PLT count is lower in patients with significant hepatic histopathology. PLT is negatively correlated to S according to the correlation analysis, PTA is also negatively correlated to S and INR is positively correlated to S. While we didn’t find similar results when it came to the correlation analysis with G. The findings indicated that liver fibrosis had a greater impact on Coagulation. ALT is a sensitive indicator of liver injury, but there are many causes of liver injury. Since we only included HBV DNA-negative patients for the study, for HBV DNA-negative patients, it is reasonable to consider more whether there are other causes of liver injury, so we did not consider ALT levels at the time of enrollment. In order to find out the reasons of liver injury, we conducted liver biopsy. In the comparison of the patients with and without significant hepatic histopathology, we did not observe a significant difference between ALT. But the comparison of normal ALT patients and abnormal ALT patients showed that there were more significant hepatic histopathology patients in abnormal ALT patients.
A study has shown that the incidence of hepatic flares ranges from 6% to 33% during the 2 to 7 years of follow-up (17), and intermittent flares may result in liver fibrosis. Therefore, pursuing a better endpoint, HBsAg seroclearance, is important. HBsAg seroclearance, also called “functional cure”, can effectively improve the long-term prognosis of patients and greatly reduce the risk of cirrhosis and liver cancer (18–20). Pegylated interferon α-2a (PEG-IFN α-2a) have a unique advantage over nucleoside (acid) analogues (NAs) in achieving HBsAg seroclearance (21–23).
Our study was a retrospective cross-sectional study. The enrolled patients were grouped by liver pathology results into two groups, significant hepatic histopathology group and no significant hepatic histopathology group. We studied the characteristics of clinical indicators and independent risk factors in HBsAg-positive, HBeAg-negative, HBV DNA-negative patients under high-sensitivity test assay. And we found that age was the independent risk factor. Our study has certain flaws, firstly the retrospective cross-sectional nature makes our findings need to be validated by a more scientifically sound prospective cohort study. Secondly, the volume of patients enrolled in our study was small. In the future, we may design a large-sample prospective cohort study to validate our findings and explore if there are more factors to recognize the presence of significant hepatic histopathology.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
Author contributions
ML and YX contributed to the study design. ZZ, RL, WC, YJL, LY and XB contributed to the data analysis. GS, MC, YL, SLW, MX, XC, LH, LZ and GW contributed to the recruitment, enrolment, and assessment of participants, as well as following up with the patients. TJ, WD, FS, HL and SHW contributed to data collection. ZZ wrote the first draft of the manuscript. YX and ML revised the manuscript and were the guarantor of the article. All authors contributed to the article and approved the submitted version.
Funding
National Key R&D Program of China (2022YFC2603505). The capital health research and development of special (2022-1-2172). National Science and Technology Major Project of China(2017ZX10201201-002-006, 2017ZX10201201-001-006, 2018ZX10715-005-003-005). Project supported by Beijing science and technology commission (Z211100002921059). High-level Public Health Technical Personnel Training Program of Beijing Municipal Health Commission (2022-3-050). The Digestive Medical Coordinated Development Center of Beijing Hospitals Authority (XXZ0302 and XXT28). Beijing Hospitals Authority Clinical Medicine Development of special funding support (XMLX 202127).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor SY declared a shared affiliation with the authors at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tan M, Bhadoria AS, Cui F, Tan A, Van Holten J, Easterbrook P, et al. Estimating the proportion of people with chronic hepatitis b virus infection eligible for hepatitis b antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol (2021) 6(2):106–19. doi: 10.1016/S2468-1253(20)30307-1
2. Pollicino T, Caminiti G. HBV-integration studies in the clinic: Role in the natural history of infection. Viruses (2021) 13(3):368. doi: 10.3390/v13030368
3. European Association for the Study of the Liver. Electronic address e e e, European association for the study of the L.EASL 2017 clinical practice guidelines on the management of hepatitis b virus infection. J Hepatol (2017) 67(2):370–98. doi: 10.1016/j.jhep.2017.03.021
4. Koffas A, Kumar M, Gill US, Jindal A, Kennedy PTF, Sarin SK. Chronic hepatitis b: the demise of the 'inactive carrier' phase. Hepatol Int (2021) 15(2):290–300. doi: 10.1007/s12072-021-10137-2
5. Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis b virus infection with persistently normal ALT: a systematic review. J Hepatol (2012) 57(1):196–202. doi: 10.1016/j.jhep.2011.11.030
6. Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, et al. Virologic and histologic features of chronic hepatitis b virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology (2008) 134(5):1376–84. doi: 10.1053/j.gastro.2008.02.075
7. Suslov A, Meier MA, Ketterer S, Wang X, Wieland S, Heim MH. Transition to HBeAg-negative chronic hepatitis b virus infection is associated with reduced cccDNA transcriptional activity. J Hepatol (2021) 74(4):794–800. doi: 10.1016/j.jhep.2020.11.003
8. Liaw YF. HBeAg seroconversion as an important end point in the treatment of chronic hepatitis b. Hepatol Int (2009) 3(3):425–33. doi: 10.1007/s12072-009-9140-3
9. Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis b. Hepatology (2002) 35(6):1522–7. doi: 10.1053/jhep.2002.33638
10. Chu CM, Liaw YF. Spontaneous relapse of hepatitis in inactive HBsAg carriers. Hepatol Int (2007) 1(2):311–5. doi: 10.1007/s12072-007-9002-9
11. Martinot-Peignoux M, Lapalus M, Laouenan C, Lada O, Netto-Cardoso AC, Boyer N, et al. Prediction of disease reactivation in asymptomatic hepatitis b e antigen-negative chronic hepatitis b patients using baseline serum measurements of HBsAg and HBV-DNA. J Clin Virol (2013) 58(2):401–7. doi: 10.1016/j.jcv.2013.08.010
12. Park H, Lee JM, Seo JH, Kim HS, Ahn SH, Kim DY, et al. Predictive value of HBsAg quantification for determining the clinical course of genotype c HBeAg-negative carriers. Liver Int (2012) 32(5):796–802. doi: 10.1111/j.1478-3231.2011.02693.x
13. Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, et al. Hepatitis b surface antigen serum levels help to distinguish active from inactive hepatitis b virus genotype d carriers. Gastroenterology (2010) 139(2):483–90. doi: 10.1053/j.gastro.2010.04.052
14. Oliveri F, Surace L, Cavallone D, Colombatto P, Ricco G, Salvati N, et al. Long-term outcome of inactive and active, low viraemic HBeAg-negative-hepatitis b virus infection: Benign course towards HBsAg clearance. Liver Int (2017) 37(11):1622–31. doi: 10.1111/liv.13416
15. Brunetto MR, Carey I, Maasoumy B, Marcos-Fosch C, Boonstra A, Caviglia GP, et al. Incremental value of HBcrAg to classify 1582 HBeAg-negative individuals in chronic infection without liver disease or hepatitis. Aliment Pharmacol Ther (2021) 53(6):733–44. doi: 10.1111/apt.16258
16. Chu CM, Sheen IS, Lin SM, Liaw YF, et al. Sex difference in chronic hepatitis b virus infection: studies of serum HBeAg and alanine aminotransferase levels in 10,431 asymptomatic Chinese HBsAg carriers. Clin Infect Dis (1993) 16(5):709–13. doi: 10.1093/clind/16.5.709
17. Chang ML, Liaw YF. Hepatitis b flare in hepatitis b e antigen-negative patients: A complicated cascade of innate and adaptive immune responses. Int J Mol Sci (2022) 23(3):1552. doi: 10.3390/ijms23031552
18. Anderson RT, Choi HSJ, Lenz O, Peters MG, Janssen HLA, Mishra P, et al. Association between seroclearance of hepatitis b surface antigen and long-term clinical outcomes of patients with chronic hepatitis b virus infection: Systematic review and meta-analysis. Clin Gastroenterol Hepatol (2021) 19(3):463–72. doi: 10.1016/j.cgh.2020.05.041
19. Wu S, Yi W, Gao Y, et al. Immune mechanisms underlying hepatitis b surface antigen seroclearance in chronic hepatitis b patients with viral coinfection. Front Immunol (2022) 13:2022.893512. doi: 10.3389/fimmu.2022.893512
20. Li M, Sun F, Bi X, et al. Consolidation treatment needed for sustained HBsAg-negative response induced by interferon-alpha in HBeAg positive chronic hepatitis b patients. Virol Sin (2022) 37(3):390–7. doi: 10.1016/j.virs.2022.03.001
21. Li M, Zhang L, Lu Y, et al. Early serum HBsAg kinetics as predictor of HBsAg loss in patients with HBeAg-negative chronic hepatitis b after treatment with pegylated interferonalpha-2a. Virol Sin (2021) 36(2):311–20. doi: 10.1007/s12250-020-00290-7
22. Moucari R, Mackiewicz V, Lada O, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology (2009) 49(4):1151–7. doi: 10.1002/hep.22744
Keywords: HBV DNA, HBeAg, chronic HBV-infection, clinical indicators, liver histopathology
Citation: Zeng Z, Liu R, Cao W, Yang L, Lin Y, Bi X, Jiang T, Deng W, Wang S, Lu H, Sun F, Shen G, Chang M, Lu Y, Wu S, Hao H, Xu M, Chen X, Hu L, Zhang L, Wan G, Xie Y and Li M (2023) Study on pathological and clinical characteristics of chronic HBV infected patients with HBsAg positive, HBV DNA negative, HBeAg negative. Front. Immunol. 13:1113070. doi: 10.3389/fimmu.2022.1113070
Received: 01 December 2022; Accepted: 19 December 2022;
Published: 05 January 2023.
Edited by:
Sun Ying, Capital Medical University, ChinaReviewed by:
Jing Huang, Guangdong Provincial People’s Hospital, ChinaJunliang Fu, Fifth Medical Center of of PLA General Hospital, China
Copyright © 2023 Zeng, Liu, Cao, Yang, Lin, Bi, Jiang, Deng, Wang, Lu, Sun, Shen, Chang, Lu, Wu, Hao, Xu, Chen, Hu, Zhang, Wan, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minghui Li, d3VobTIwMDBAc2luYS5jb20=
†ORCID: Minghui Li, orcid.org/0000-0003-3233-5473
‡These authors have contributed equally to this work
 Zhan Zeng
Zhan Zeng Ruyu Liu1‡
Ruyu Liu1‡ Weihua Cao
Weihua Cao Liu Yang
Liu Yang Yanjie Lin
Yanjie Lin Xiaoyue Bi
Xiaoyue Bi Tingting Jiang
Tingting Jiang Fangfang Sun
Fangfang Sun Shuling Wu
Shuling Wu Mengjiao Xu
Mengjiao Xu Lu Zhang
Lu Zhang Yao Xie
Yao Xie Minghui Li
Minghui Li