
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Immunol. , 20 December 2022
Sec. Alloimmunity and Transplantation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1112027
This article is a correction to:
Mettl14-mediated m6A modification enhances the function of Foxp3+ regulatory T cells and promotes allograft acceptance
 Yanzhuo Liu1,2
Yanzhuo Liu1,2 Yinglin Yuan1,2
Yinglin Yuan1,2 Zili Zhou1,2
Zili Zhou1,2 Yuanyuan Cui1,2
Yuanyuan Cui1,2 Yan Teng3
Yan Teng3 Hao Huang1
Hao Huang1 Hao Yuan1
Hao Yuan1 Yanling Zhang1
Yanling Zhang1 Lu Yang3*
Lu Yang3* Gaoping Zhao1,2*
Gaoping Zhao1,2*A Corrigendum on
Mettl14-mediated m6A modification enhances the function of Foxp3+ regulatory T cells and promotes allograft acceptance
by Liu Y, Yuan Y, Zhou Z, Cui Y, Teng Y, Huang H, Yuan H, Zhang Y, Yang L and Zhao G (2022) Front. Immunol. 13:1022015. doi: 10.3389/fimmu.2022.1022015
In the published article, there was an error in Figure 4B as published. In the original publication of this article, the same GraphPad file was accidentally linked in Figures 4B, D, which is a mistake. The corrected Figure 4B and its caption appear below.
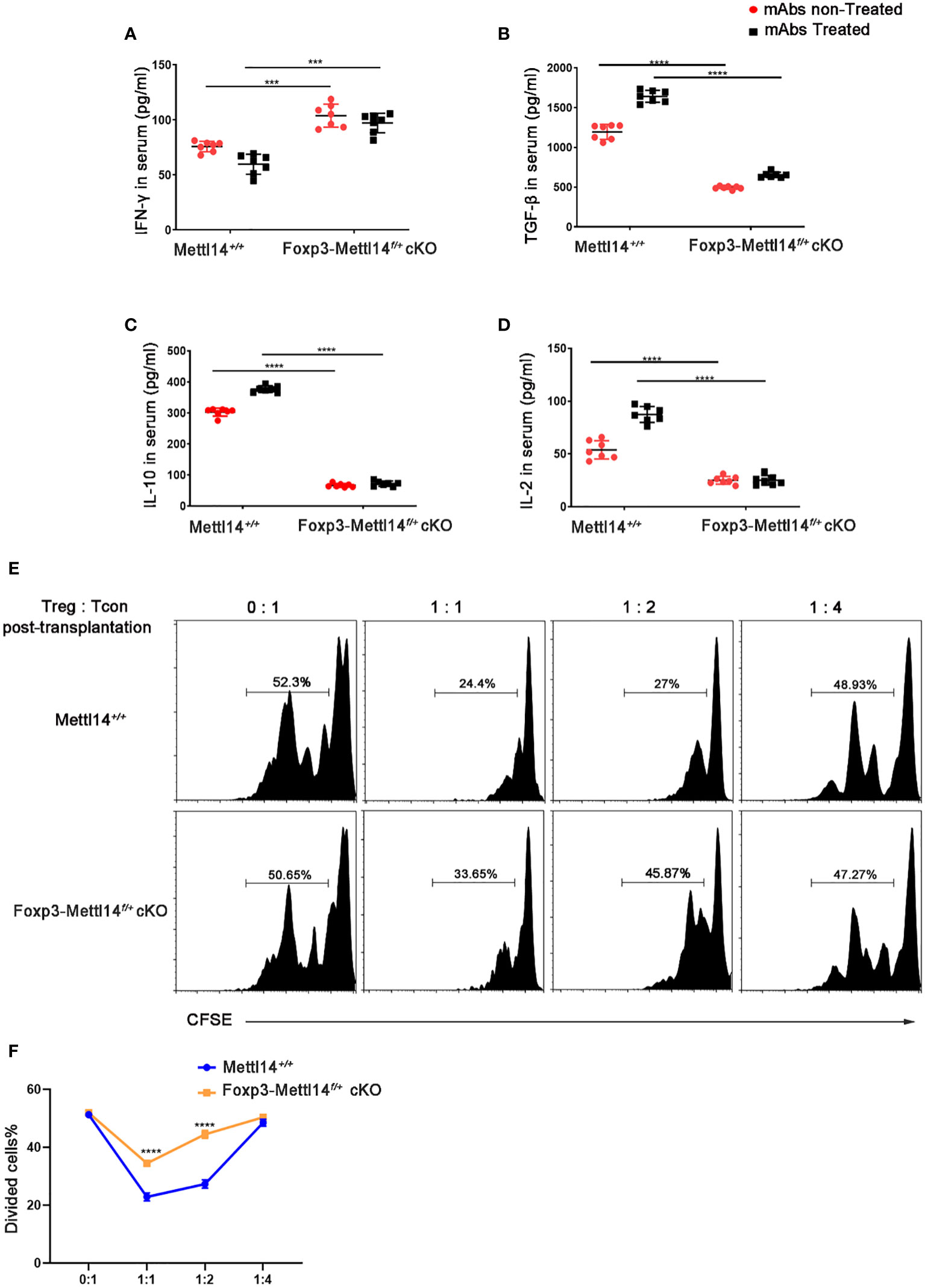
Figure 4 METTL14 deficiency Treg cells resulted in the loss of suppressive function after transplantation. (A–D) Serum was collected on day 7 after transplantation and cytokine levels were measured using ELISA assay. In the presence or absence of mAbs treatment, the scatter plots show the mean serum cytokine concentration in picograms per milliliter of IFN-γ (A), TGF-β (B), IL-10 (C) and IL-2 (D) from serum in Foxp3-Mettl14f/+ cKO mice and littermate controls (n=7). (E) The islets were isolated from BALB/c mice and transplanted them under the kidney capsule of littermate controls or Foxp3-Mettl14f/+ cKO mice with STZ-induced diabetes. These mice were treated with mAbs as described above. Treg cells were isolated on day 7 post-transplantation which were co-cultured with CFSE-labeled CD4+ naïve T cells from C57BL/6J mice, in round-bottom 96-well plates containing anti-CD3 (3 μg/ml) and anti-CD28 (5 μg/ml) monoclonal antibodies, at various ratios for 5 days. The suppressive effect on T cell expansion was detected using flow cytometry. (F) The suppressive function of Treg cells was quantified. The data are presented as the means ± SD. The data in (A-D) depict the mean values measured from seven separate experiments, while the data in (D) are representative of at least three independent experiments. The statistical analysis was performed with an unpaired Student’s t-test (two-tailed). ****P<0.0001 and ***P<0.001.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: N6-methyladenosine, Mettl14, Treg function, transplantation, allograft acceptance
Citation: Liu Y, Yuan Y, Zhou Z, Cui Y, Teng Y, Huang H, Yuan H, Zhang Y, Yang L and Zhao G (2022) Corrigendum: Mettl14-mediated m6A modification enhances the function of Foxp3+ regulatory T cells and promotes allograft acceptance. Front. Immunol. 13:1112027. doi: 10.3389/fimmu.2022.1112027
Received: 30 November 2022; Accepted: 06 December 2022;
Published: 20 December 2022.
Edited and Reviewed by:
Sina NASERIAN, Hôpital Paul Brousse, FranceCopyright © 2022 Liu, Yuan, Zhou, Cui, Teng, Huang, Yuan, Zhang, Yang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Yang, bHlhbmdAdWVzdGMuZWR1LmNu; Gaoping Zhao, Z3poYW9AdWVzdGMuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.