
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Immunol. , 18 January 2023
Sec. Vaccines and Molecular Therapeutics
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1104646
This article is a correction to:
rAAV immunogenicity, toxicity, and durability in 255 clinical trials: A meta-analysis
A Corrigendum on
rAAV immunogenicity, toxicity, and durability in 255 clinical trials: A meta-analysis
by Shen W, Liu S and Ou L (2022) Front. Immunol. 13:1001263. doi: 10.3389/fimmu.2022.1001263
In the published article, there was an error in Table 2 as published. Row 1, column 6 of the table should be “undisclosed” instead of “rBac-Sf9”. Furthermore, the row “Inflammatory arthritis” was removed as the death that occurred in that clinical trial was not AAV-related. The corrected Table 2 and its caption “Table 2. Patient deaths that occurred in AAV-related clinical trials” appear below.
In the published article, there was an error in the legend for Table 2 as published. We have removed one row in Table 2, so the Table legend needs to be updated accordingly. The corrected legend appears below.
“Two patient deaths were recently reported when Zolgensma is commercially used, and no detailed information was released yet (45). One patient death in a clinical trial for arthritis was excluded because it was deemed not be related with AAV (46). ALS, amyotrophic lateral sclerosis; GAN, giant axonal neuropathy; SMA, spinal muscular atrophy.”
In the published article, there was an error in Figure 6 as published. In Figure 6B, both column “patient deaths” and “deaths per trial” for rBac-Sf9 should be 0. The corrected Figure 6 and its caption “Figure 6. Manufacturing systems of 255 AAV clinical trials” appear below.
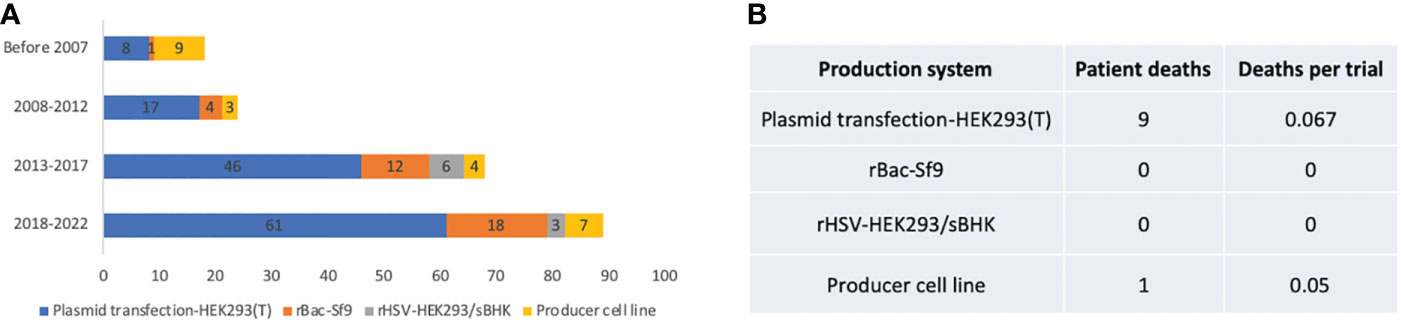
Figure 6 Manufacturing systems of 255 AAV clinical trials. (A) System usage in different time periods. (B) Patient deaths in trials using different manufacturing systems. Trials that did not report manufacturing systems are not included for analysis.
In the published article, there was an error. There was no patient death for the rBac/Sf9 system. Our information source on one trial was wrong.
A correction has been made to Results, Production system. This sentence previously stated:
“Also, a total of 9 patient deaths occurred in 134 AAV trials using the HEK293 system, while there is one individual death for both rBac/Sf9 system (n=36) and producer cell line system (n=24) (Figure 6B).”
The corrected sentence appears below:
“Also, a total of 9 patient deaths occurred in 134 AAV trials using the HEK293 system, while there is one individual death for the producer cell line system (n=24) (Figure 6B).”
The authors apologize for these errors and state that they do not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: rAAV, clinical trials, immunogenicity, toxicity, neutralizing antibodies, immunosuppressants, capsids, gene therapy
Citation: Shen W, Liu S and Ou L (2023) Corrigendum: rAAV immunogenicity, toxicity, and durability in 255 clinical trials: A meta-analysis. Front. Immunol. 13:1104646. doi: 10.3389/fimmu.2022.1104646
Received: 21 November 2022; Accepted: 29 November 2022;
Published: 18 January 2023.
Edited and reviewed by:
Arun Srivastava, University of Florida, United StatesCopyright © 2023 Shen, Liu and Ou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Ou, b3V4eHgwNDVAdW1uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.