- Department of Thoracic Surgery, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
Background: Neoadjuvant programmed death receptor-1 (PD-1) inhibitor combined with chemotherapy has been reported to improve the pathological response of locally advanced esophageal squamous cell carcinoma (ESCC), but the systematic report on survival follow-up is quite few. This study we will report the survival follow-up outcomes after a median follow-up of 21.1 months.
Methods: This was a real-world retrospective study. Locally advanced ESCC patients treated with neoadjuvant sintilimab combined with albumin-bound paclitaxel and nedaplatin followed by surgery and completed at least 1-year follow-up were reviewed. The primary outcome was disease-free survival (DFS) at 24 months. The secondary outcome was overall survival (OS) at 24 months.
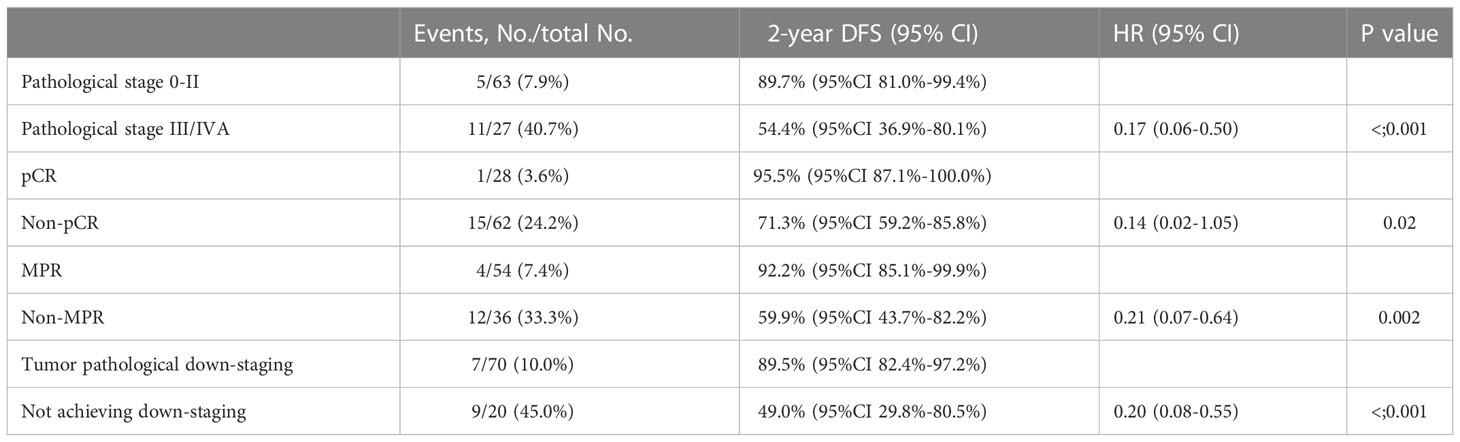
Results: Ninety eligible patients were included in the analysis between July 2019 and October 2021. The median number of neoadjuvant cycles was 3 (range 2-4). All patients achieved R0 resection. With a median follow-up of 21.1 months (range 14.0-39.0), the median DFS and median OS had not reached, 2-year DFS rate was 78.3% (95%CI 68.8%-89.1%) and 2-years OS rate was 88.0% (95%CI 80.6%-96.0%). Postoperative pathological stage, pCR, MPR, tumor down-staging were significantly correlated with favorable survival outcome. Univariable and multivariable Cox regression analysis identified cycle number of neoadjuvant treatment as independent predictor of DFS.
Conclusion: Our results preliminarily show a survival benefit of neoadjuvant sintilimab combined with chemotherapy in locally advanced ESCC.
1. Introduction
Esophageal cancer (EC) is one of the most common malignant tumors of digestive system in China (1, 2). According to the latest WHO epidemiological data, there were 324422 estimated new cases and 301135 estimated deaths of esophageal cancer in China in 2020, which were about half of the global cases (2). The predominant histological type of esophageal cancer in China is esophageal squamous cell carcinoma (ESCC), accounting for about 90% (3, 4). For locally advanced resectable ESCC, multidisciplinary comprehensive treatment strategies including neoadjuvant or perioperative treatment, and esophagectomy have been established.
Neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy have been confirmed to significantly improve the survival of patients with esophageal cancer and was recommended by the National Comprehensive Cancer Network (NCCN) guidelines and the Chinese Society of Clinical Oncology (CSCO) guidelines (5–13). Programmed cell death-1 (PD-1) inhibitor combined with chemotherapy have shown promising outcomes and approved by regulatory in the first-line therapy of advanced esophageal and gastroesophageal junction (GEJ) carcinoma (14–19). Preliminary data from several small-sample studies showed that PD-1 inhibitor combined with chemotherapy have also great application prospect in the neoadjuvant setting, with the pathological complete response (pCR) rate 21.7% to 50% (20–32). The 2022 CSCO guideline recommend PD-1 inhibitor combined with chemotherapy (category 3) as one of the neoadjuvant treatment options (13). However, the systematic report on survival follow-up of neoadjuvant PD-1 inhibitor combined with chemotherapy is quite few. In previous real-world study, we reported the encouraging pathological response and good tolerability of neoadjuvant sintilimab combined with platinum and taxanes in resectable locally advanced ESCC: the pCR rate was 30.2%, the major pathological response (MPR) rate was 62.5%, the pathological downstaging rate was 74.0% (31). In this study, we analyzed the patients who were followed up for more than one year and report the survival outcomes after a median follow-up of 21.1 months.
2. Methods
2.1. Study design and patient selection
This study was designed to be a real-world retrospective study to investigate the survival outcomes of neoadjuvant sintilimab combined with chemotherapy in patients with locally advanced ESCC at The Fourth Hospital of Hebei Medical University. The study was approved by the Ethics Committee of our hospital and conducted in accordance with the 2013 edition of the Declaration of Helsinki. Written informed consent were waived. The inclusion criteria were included: patients with histologically confirmed locally advanced resectable ESCC, aged 18 years or older, both sexes, with clinical stage II-IVA, treating with neoadjuvant sintilimab combined with albumin-bound paclitaxel and nedaplatin followed by surgery, completed at least 1-year follow-up. Exclusion criteria included: having other anti-tumor treatments before or during the neoadjuvant treatment. Pretreatment baseline staging was according to American Joint Committee on Cancer (AJCC) 8th edition TNM staging system. Chest-abdominal contrast enhanced computed tomography (CT), esophageal enhanced magnetic resonance imaging (MRI), endoscopic ultrasound (EUS), and cervical ultrasound were performed. Position emission tomography positron emission tomography computed tomography (PET-CT) was also performed when necessary.
2.2. Treatment
All eligible patients completed 2-4 cycles of neoadjuvant treatment with sintilimab (200mg, I.V, D1), albumin-bound paclitaxel (260mg/m (2), I.V, D1) and nedaplatin (80mg/m (2), I.V, D1) of each 3-week cycle. Dose reductions were not permitted for sintilimab. Sintilimab was discontinued if a grade 2 or specific grade 3 treatment-related adverse event (TRAE) occurred during treatment and resumed when the TRAE reduce to grade 1 or below. Sintilimab was permanently discontinued if a specific grade 3 or grade 4 TRAE occurred. Chemotherapy was discontinued when patients experienced a grade 3–4 TRAE and resumed when the TRAE reduce to grade 1 or below. The chemotherapy dose at resuming was reduced to 75% of the initial dose when the grade 3–4 AE occurred for the first time and continued to reduce to 50% of the initial dose when the grade 3–4 AE reoccurred. The chemotherapy was permanently discontinued if the grade 3–4 AE reoccurred despite 2 dose reductions. Radiologic responses and clinical restaging were assessed every two cycles by the same means as the baseline. McKeown esophagectomy with two- or three-field lymphadenectomy was selected according to patients’ condition and performed for all suitable patients. Pathological examination was carried out according to the standard protocols. Pathological response was assessed by tumor regression grade (TRG) referred to the Becker system (33). The adjuvant regimens include albumin-bound paclitaxel and nedaplatin, sintilimab monotherapy, or sintilimab combined with albumin-bound paclitaxel and nedaplatin. The decision was made through the multidisciplinary board according to the original response to immune-chemotherapy and postoperative clinical conditions. The adjuvant regimen was decided by selected by multidisciplinary team according to the efficacy and safety of neoadjuvant treatment and postoperative recovery, including adjuvant albumin-bound paclitaxel and nedaplatin, sintilimab monotherapy, or sintilimab combined with albumin-bound paclitaxel and nedaplatin. pCR patients may not receive adjuvant treatment. Follow-up were routinely conducted every 3 months during first 2 years after surgery, and then every 6 months after 2 years.
2.3. Outcomes
The primary outcome was disease-free survival (DFS) at 24 months. DFS was defined as the time from the date of neoadjuvant treatment to recurrence or death by any cause. Disease recurrence included locoregional recurrence (LRR) and distant recurrence (DR) that occurred after R0 resection. Locoregional recurrence was defined as the recurrence within the esophagus, anastomosis or regional lymph nodes. Distant recurrence was defined as distant organ metastases, peritoneal carcinomatosis or recurrence within nonregional lymph node. The secondary outcomes were overall survival (OS) at 24 months. OS was defined as the time from the date of neoadjuvant treatment to death by any cause. Impact of pathological response (pCR, MPR and tumor downstaging) on DFS was exploratory analyzed. pCR (corresponds with Becker TRG 1a) was defined as no evidence of residual tumor cells in the resected primary tumor and axillary lymph nodes. MPR (corresponds with Becker TRG 1a+1b) was defined as 10% or fewer residual tumor cells in the primary tumor. Tumor downstaging was defined as a decrease in T or/and N of pre-surgery stage after neoadjuvant (ypTNM) or post-surgery pathological stage (pTNM) relative to baseline clinical stage (cTNM). Upstaging was defined as an increase in T or/and N of ypTNM or pTNM stage relative to baseline cTNM.
2.4. Statistical analysis
Statistical analysis was performed using SPSS software (IBM SPSS Statistics, RRID: SCR_016479 version 26.0) and R software (RRID: SCR_001905 version 4.0.0). The continuous variables were presented as median and range. The categorical variables were presented as number and percentage. The pCR, MPR, tumor downstaging and R0 resection rate with 95% CI were calculated using the Clopper–Pearson exact method based on binomial distribution. Median follow-up time was calculated using the reverse Kaplan–Meier method. DFS and OS and corresponding 95% CIs were estimated using the Kaplan-Meier method, and a log-rank test was used for comparisons between pathological response subgroups. Univariable and multivariable Cox regression models were used to estimate the effect of neoadjuvant treatment among subgroups according to baseline characteristics. All statistical testing is two-tailed and performed at the 5% significance level.
3. Results
3.1. Baseline characteristics
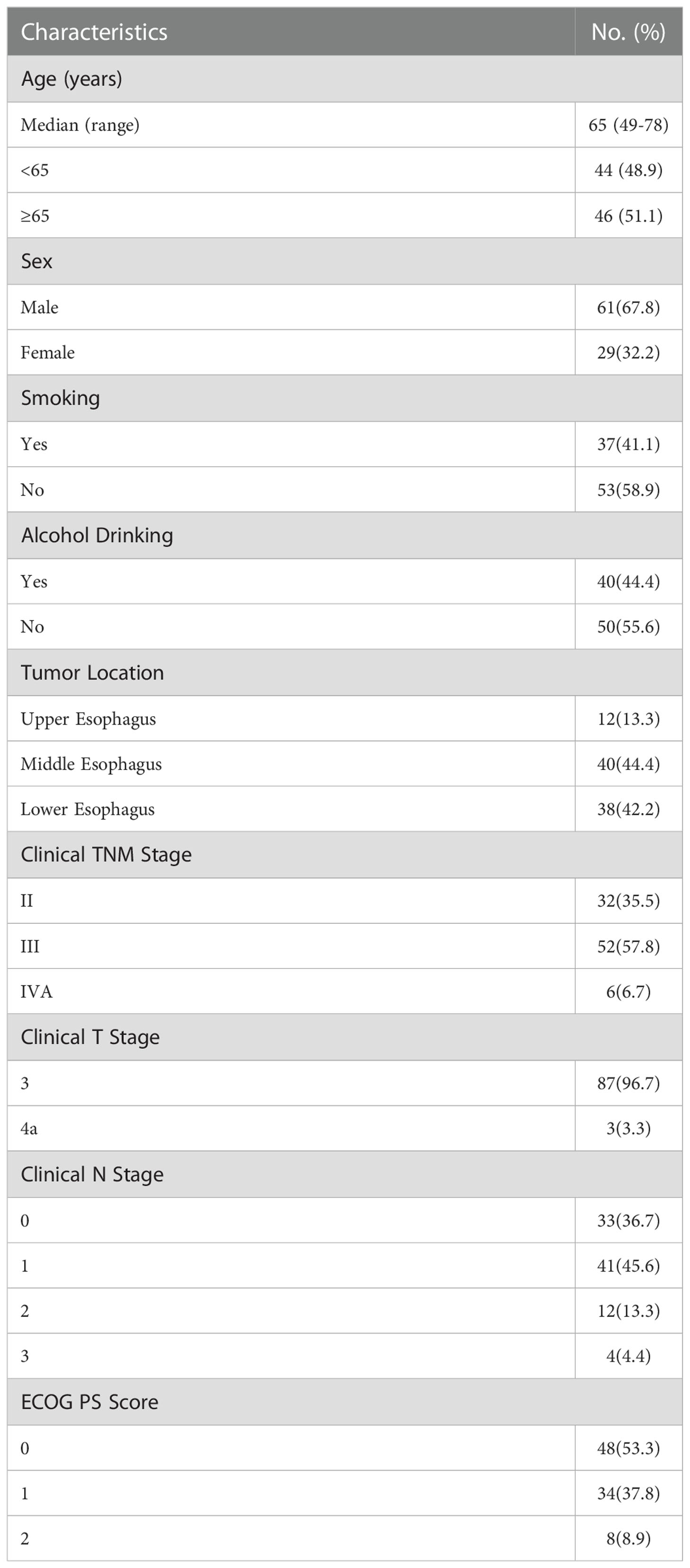
Ninety eligible patients were included in the analysis between July 2019 and October 2021. The major characteristics of the patients are shown in Table 1. Sixty-one (67.8%) were male, the median age was 65 years (range 49-78 years), most tumors were located in the middle esophagus (44.4%) and lower esophagus (42.2%), 48 (53.3%) patients had Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 and 42 (46.7%) had ECOG PS 1 or 2. Thirty-two (35.5%) patients had clinical stage II, 52 (57.8%) patients had clinical stage III, and 6 (6.7%) had clinical stage IVA.
3.2. Treatment outcomes
The median number of neoadjuvant cycles was 3 (range 2-4). Forty-two (46.7%) patients received 2 cycles of the neoadjuvant treatment, and another forty-eight (53.3%) received 3–4 cycles. Eighteen patients experienced chemotherapy dose reduction due to TRAEs. Five patients experienced treatment discontinuation due to TRAEs, including one patient because of Immune-mediated colitis, one patient because of liver Abnormalities, two patients because of hyperthyroidism, and two patients because of leukopenia and neutropenia. No treatment-related surgical delay or death was observed.
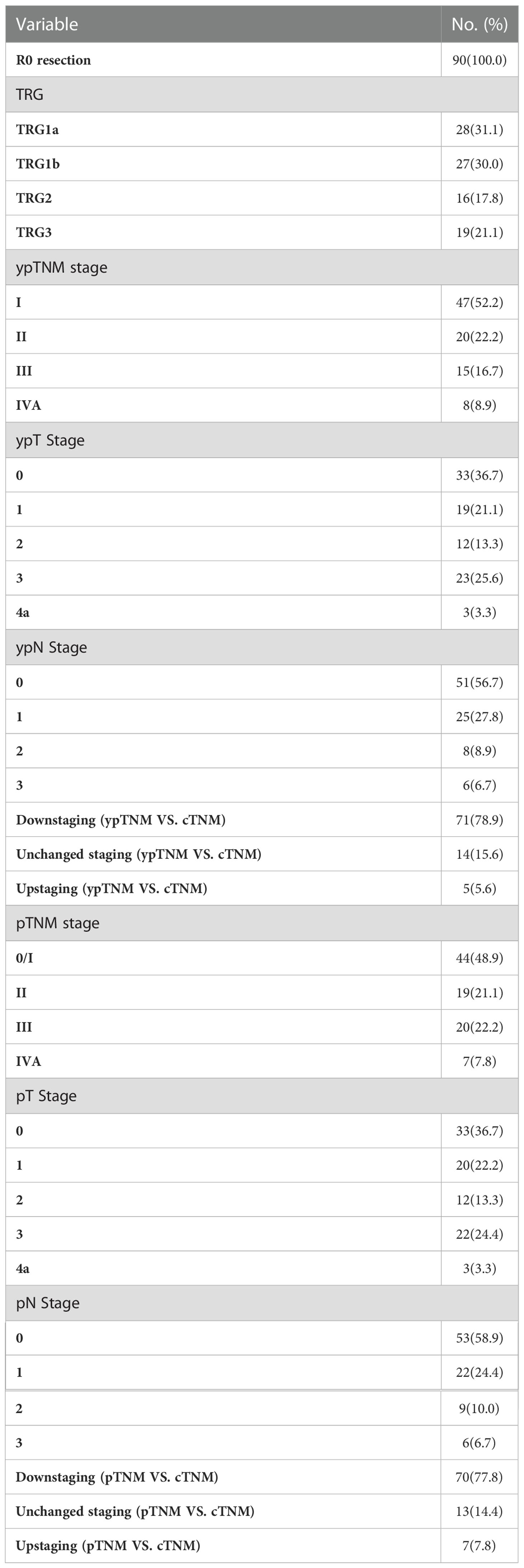
All 90 patients underwent scheduled surgery, all patients achieved R0 resection. The median interval between the end of neoadjuvant therapy and surgery was 34 days (range 21-95 days). The median number of resected lymph node was 29 (range 20-52). Seventy-five (83.3%) patients completed two field lymphadenectomy, and 15 (16.7%) completed three field lymphadenectomy. The median operation time was 303 minutes (range 142-563 minutes), and the median intraoperative blood loss was 150ml (range 100-2300 ml). The treatment responses are summarized in Table 2. Postoperative pathological analysis showed that the pCR (TRG1a) rate was 31.1% (95CI 21.8%-41.7%), MPR (TRG1a+1b) rate was 61.1% (95CI 50.3%-71.2%). The median length of hospital stay was 12 days (range 8-54 days).
Sixty (66.7%) patients received adjuvant therapy, including 50 (55.6%) patients receiving sintilimab combined with chemotherapy, 5 (5.6%) patients receiving sintilimab, and 5 (5.6%) patients receiving chemotherapy. The median cycle of adjuvant sintilimab was 8 (range, 1-17), The median cycle of adjuvant chemotherapy was 2 (range, 1-4).
3.3. Survival outcomes
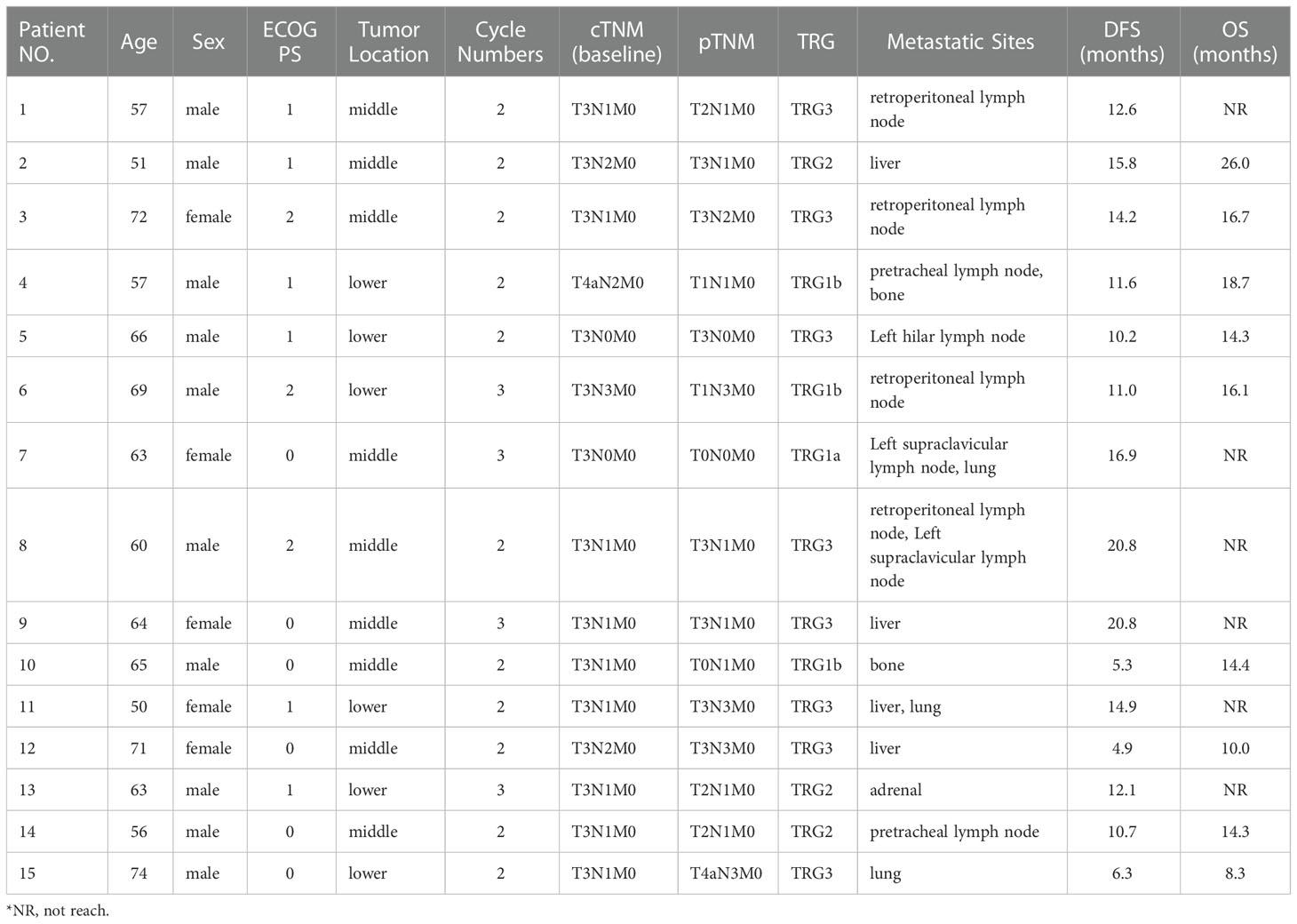
As of data cut-off, the median follow-up was 21.1 months (range 14.0-39.0). 15 (16.7%) patients had disease recurrence and 9 (10.0%) of them died, in addition, 1 (1.1%) patient died of non-cancer-related cause without recurrence. 2 (2.2%) patients experienced locoregional recurrence, 12 (13.3%) patients experienced distant recurrence, and 1 (1.1%) patient experienced concurrent locoregional recurrence and distant recurrence (Table 3). Of the 30 patients who were followed up for more than 24 months, only 3 recurred 15.8 months, 16.9 months and 20.8 months after treatment, respectively.
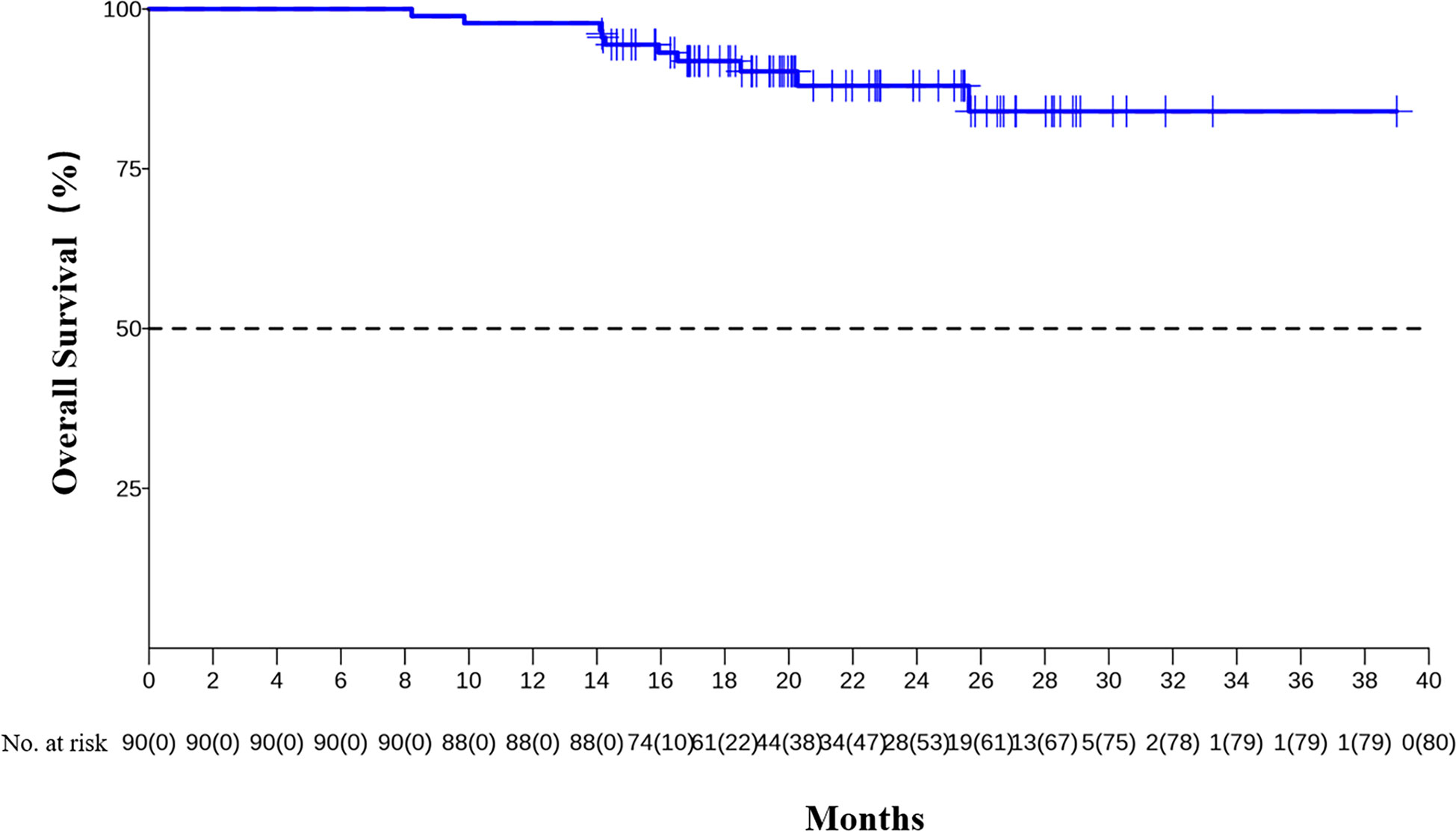
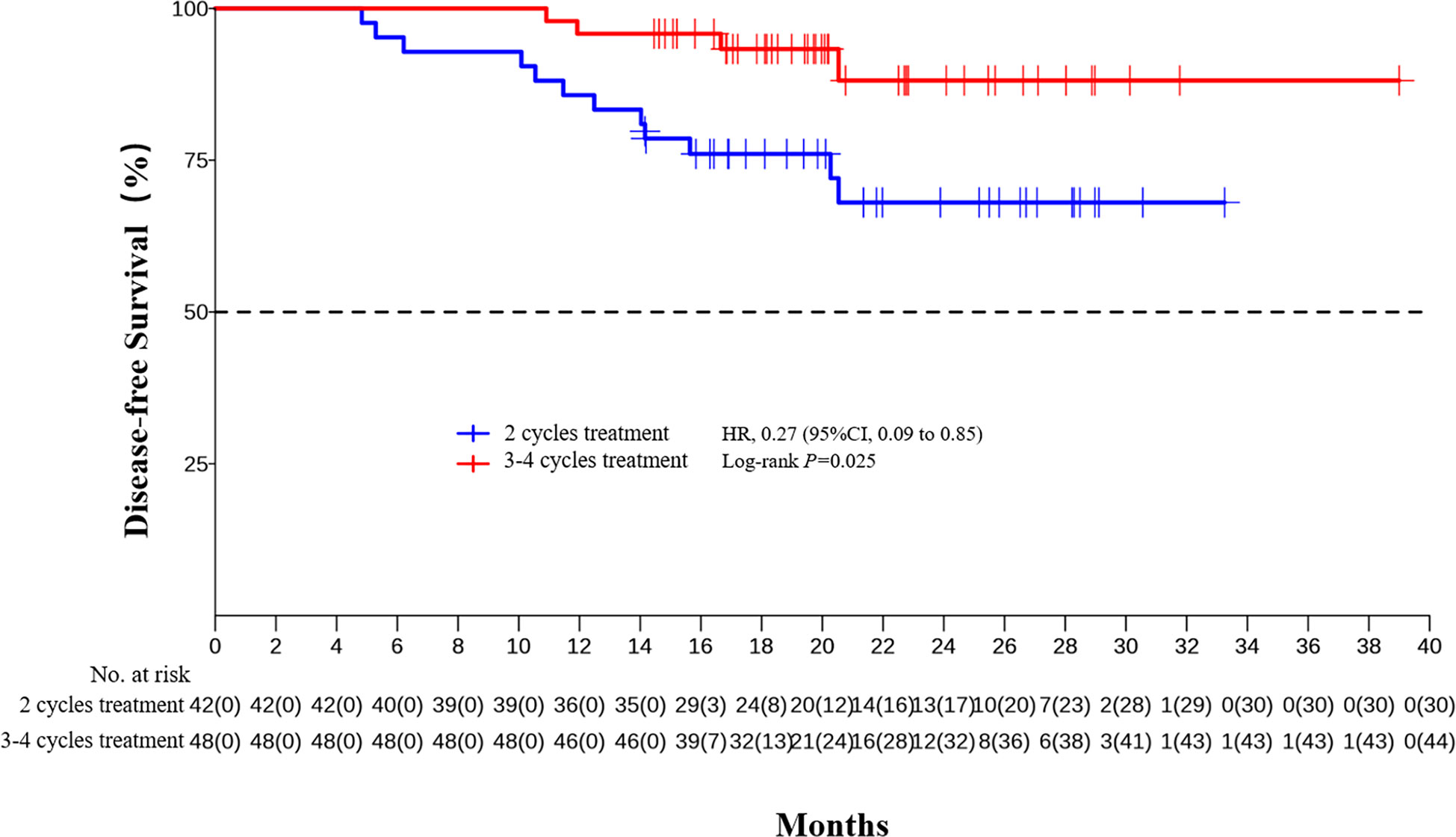
The median DFS and median OS had not been reached yet, 1-year DFS rate was 91.1% (95%CI 85.4%-97.2%), and 2-year DFS rate was 78.3% (95%CI 68.8%-89.1%). 1-years OS rate was 97.8% (95%CI 94.8%-100.0%), and 2-years OS rate was 88.0% (95%CI 80.6%-96.0%) (Figures 1, 2). Subgroup analysis showed that postoperative pathological stage, pCR, MPR, tumor pathological down-staging were significantly correlated with survival outcome (Table 4, Figure 3). Univariable and multivariable Cox regression analysis according to baseline characteristics identified cycle number of neoadjuvant treatment as independent predictor of DFS (Table 5). Patients who completed 3–4 cycles of neoadjuvant treatment increase survival compared to those received 2 cycles, 2-year DFS rate was 88.1% (95%CI 76.9%-100%) and 68.0% (95%CI 53.1%-85.6%) respectively (Figure 4).
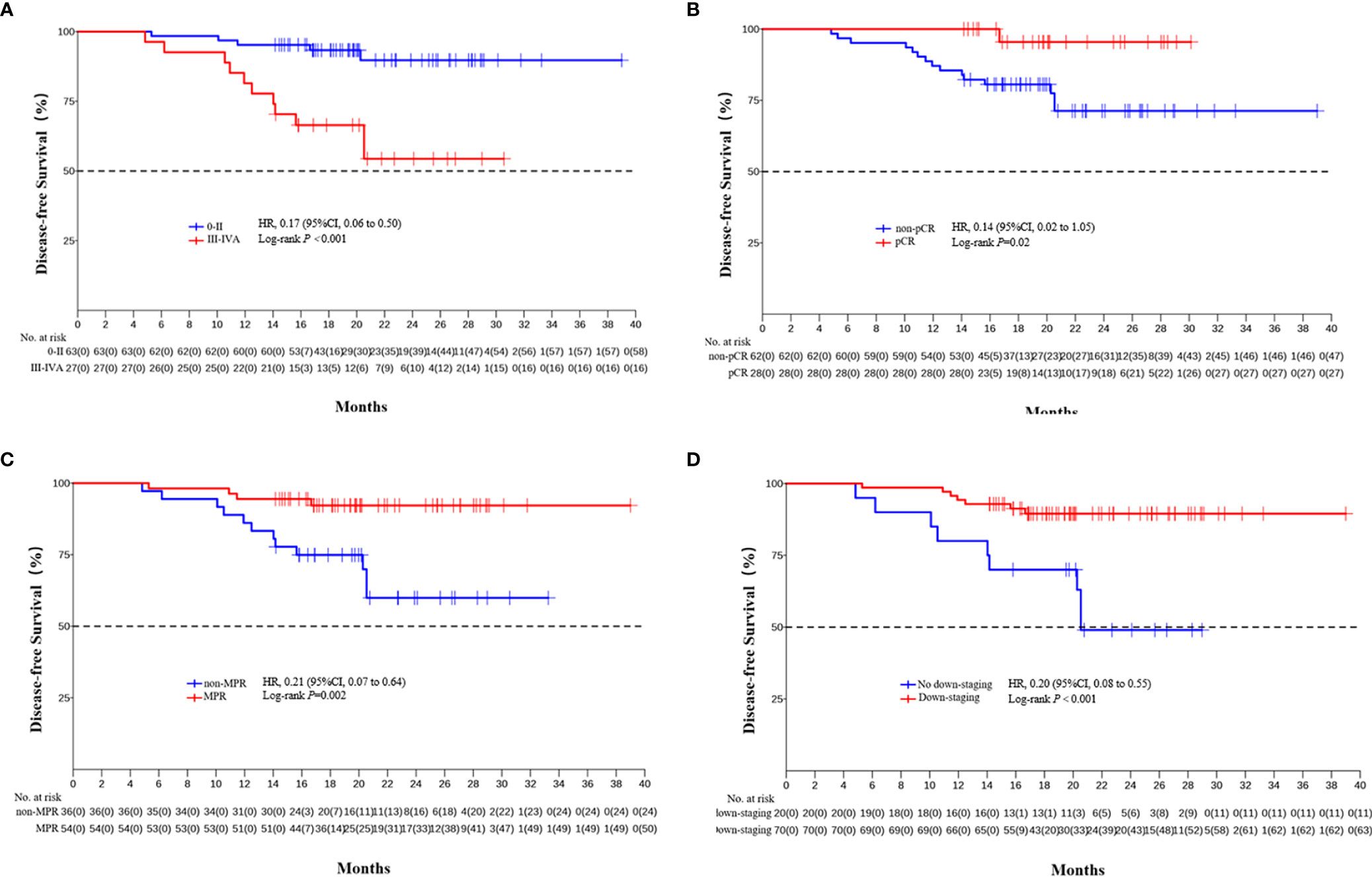
Figure 3 Kaplan-Meier estimates of DFS stratified by pathological responses. (A) DFS of the pathological stage 0-II group and the III/IVA group. (B) DFS of the pCR group and the non-PCR group. (C) DFS of the MPR group and the non-MPR group. (D) DFS of the tumor pathological down-staging group and not achieving tumor pathological down-staging group.
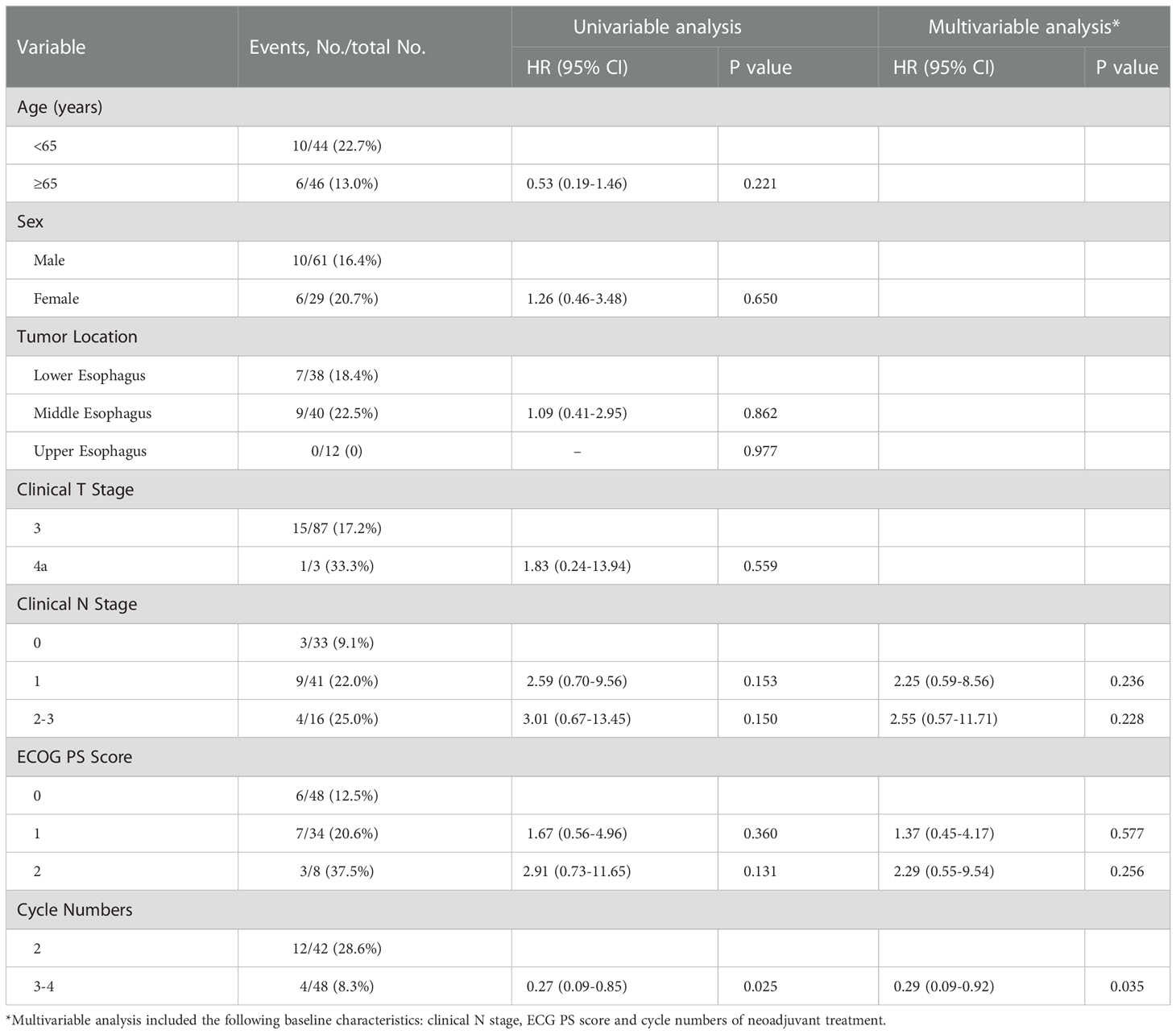
Table 5 Univariable and multivariable Cox regression analysis according to baseline characteristics.
4. Discussion
These survival Outcomes, after a median follow-up of 21.1 months, preliminarily show a survival benefit of neoadjuvant sintilimab combined with chemotherapy in locally advanced ESCC. In recent years, more and more studies have confirmed the encouraging pathological response and manageable safety of neoadjuvant PD-1 inhibitor combined with chemotherapy (20–32). Now, it is of concern whether the encouraging pathological response can be translated into survival benefit. This is a relatively large sample and systematic report on survival follow-up.
As to the survival follow-up outcomes of neoadjuvant PD-1 inhibitors and chemotherapy in the ESCC, there are also four other small sample studies. A single-arm, prospective trial of 23 enrolled patients showed after a median follow-up of 13.77 months (IQR: 9.7–17.6), 5 (25%) of the 20 patients who received surgery experienced disease recurrence or metastasis ranging from 4 to 12 months after surgery (25). Another single-arm, prospective trial of 47 enrolled patients showed after a median follow-up of 14.6 months (IQR, 11.3- 24.0 months), the 1-year OS was 90.8%, and the 1-year DFS was 68.3%, and patients who achieved MPR had improved DFS (p=0.050, HR=0.35) and OS (p=0.066, HR=0.16) (32). A retrospective study of 34 enrolled patients showed the DFS rate was 86.4% at 12 months, 70.4% at 24 months, and the OS rate was 92.8% at 12 months, 83.2% at 24 months after a median follow-up of 14.8 months (range 3.8–26.5) (29). Another retrospective study of 47 enrolled patients showed that the 1-, 2-year DFS were 95.7%, 80.7%, the 1-, 2-year OS rates were 95.7%, 83.2% (27). In our study, more patients were included and the followed-up time was nearly two years. The results showed that 1-year DFS rate of neoadjuvant sintilimab combined with chemotherapy was 91.1%, 2-year DFS rate was 78.3%, 1-years OS rate was 97.8%, 2-years OS rate was 88.0% with a median follow-up of 21.1 months. Overall, all these follow-up outcomes preliminarily show survival benefit and further confirm the prospect of neoadjuvant PD-1 inhibitor combined with chemotherapy.
Some studies reported that combined radiotherapy based on chemotherapy can mainly improve local pathological response, but may be poorly effective at controlling occult systemic metastasis (11, 34–36). The Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) showed the reduction in distant progression in the neoadjuvant chemoradiotherapy plus surgery group was significant than the surgery alone group during the first 24 months of follow-up but not thereafter (36). The NEOCRTEC5010 clinical trial also showed the reduction in distant progression was not significant after the first 24 months (11). The LRR, DR, and overall recurrence rates of neoadjuvant sintilimab combined with chemotherapy during the first 24 months decreased numerically compared with neoadjuvant chemoradiotherapy (11, 36, 37). Moreover, the presence of the whole tumor during neoadjuvant PD-1 inhibitor combined with chemotherapy allows a triggering of a broader T cell response due to a larger repertoire of tumor antigen exposure, then establish systemic immune surveillance and destruction of micrometastases (38, 39). So the better postoperative pathological response from neoadjuvant PD-1 inhibitor combined with chemotherapy may translate into a long-term survival benefit. This has been confirmed in the neoadjuvant immunotherapy of lung cancer (40–43).
In the present study, subgroup analysis showed that postoperative pathological stage, pCR, MPR, tumor down-staging were significantly correlated with survival outcome. The results preliminarily indicate that pCR and MPR can be used as alternative indicators or predictor of survival for neoadjuvant PD-1 inhibitor combined with chemotherapy, which is consistent with previous findings in neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy (44–48), and we will further confirm it through long-term follow-up.
The optimal number of neoadjuvant treatment cycles remains to be established for ESCC. For neoadjuvant chemotherapy, a randomized phase II trial showed three courses of DCF led to a relatively higher rate of pCR (15.3% vs. 9.1%, P = 0.212) compared to the two-course (49). Another randomized phase II trial showed two- and three-course of DCF have the comparable histological responses (P=0.898), and the 2-year PFS rate was also comparable between the two groups (71.4 vs. 71.1%, P = 0.669) (50). Our previous studies have shown that the pCR rate were significantly higher in patient completed 3-4 cycles of neoadjuvant therapy than those completed 2 cycles, (47.9%, 95% CI, 33.3%–62.8% vs. 12.5%, 95% CI, 4.7%–25.2%, p = 0.0003). In the present study, multivariable Cox regression analysis also identified cycle number of neoadjuvant treatment as independent predictor of DFS. Patients who completed 3–4 cycles of neoadjuvant treatment increase survival compared to those received 2 cycles (2-year DFS rate 88.1% vs. 68.0%). Long term follow-up and randomized study are still needed to further confirm the benefits of extending the treatment cycle of neoadjuvant PD-1 inhibitor combined with chemotherapy to 3-4.
There are several limitations to this study. First, this is a retrospective study which may cause biases. Second, more samples are needed to further confirm the conclusion. Third, our study only reported a short-term survival outcome, longer follow-up is necessary to evaluate the long-term clinical benefits of neoadjuvant PD-1 inhibitor combined with chemotherapy for locally advanced ESCC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Committee of The Fourth Hospital of Hebei Medical. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZT and HL designed the study. CH and SX collected the data. HL and CH analyzed and interpreted the data. CH, JL, FZ, CG, ZL, MW, and ZHL carried out the clinical treatment and management of the patients. ZT and HL prepared the final draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Natural Science Foundation of Hebei Province (Grant number: H2022206443).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) (2021) 134(7):783–91. doi: 10.1097/CM9.0000000000001474
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol (2013) 23(4):233–42. doi: 10.2188/jea.je20120162
4. He Y, Li D, Shan B, Liang D, Shi J, Chen W, et al. Incidence and mortality of esophagus cancer in China, 2008-2012. Chin J Cancer Res (2019) 31(3):426–34. doi: 10.21147/j.issn.1000-9604.2019.03.04
5. Medical research council oesophageal cancer working group. surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet (2002) 359(9319):1727–33. doi: 10.1016/S0140-6736(02)08651-8
6. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol (2009) 27(30):5062–7. doi: 10.1200/JCO.2009.22.2083
7. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol (2012) 19(1):68–74. doi: 10.1245/s10434-011-2049-9
8. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
9. Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The randomized controlled CROSS trial. J Clin Oncol (2021) 39(18):1995–2004. doi: 10.1200/JCO.20.03614
10. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol (2018) 36(27):2796–803. doi: 10.1200/JCO.2018.79.1483
11. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: The NEOCRTEC5010 randomized clinical trial. JAMA Surg (2021) 156(8):721–9. doi: 10.1001/jamasurg.2021.2373
12. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Esophageal and esophagogastric junction cancers (version 4.2022) . Available at: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
13. Chinese Society of Clinical Oncology. CSCO clinical guidelines for the diagnosis and treatment of esophageal cancer (2021). Available at: http://meeting.csco.org.cn/pdf/web/viewer.html?file=/Upload/Periodical/20211019110832.pdf.
14. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
15. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st randomized clinical trial. JAMA (2021) 326(10):916–25. doi: 10.1001/jama.2021.12836
16. Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell (2022) 40(3):277–88. doi: 10.1016/j.ccell.2022.02.007
17. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (2021) 398(10302):759–71. doi: 10.1016/S0140-6736(21)01234-4
18. Li Z, Sun Y, Ye F, Ma D, Yin X, Zhuang W, et al. First-line pembrolizumab plus chemotherapy versus chemotherapy in patients with advanced esophageal cancer: Chinese subgroup analysis of KEYNOTE-590. J Clin Oncol (2021) 39(suppl 15):4049. doi: 10.1200/JCO.2021.39.15_suppl.4049
19. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med (2022) 386(5):449–62. doi: 10.1056/NEJMoa2111380
20. Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol (2021) 12(1):1–10. doi: 10.21037/jgo-20-599
21. Yang P, Zhou X, Yang X, Wang Y, Sun T, Feng S, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol (2021) 19(1):333. doi: 10.1186/s12957-021-02446-5
22. Xing W, Zhao L, Zheng Y, Liu B, Liu X, Li T, et al. The sequence of chemotherapy and toripalimab might influence the efficacy of neoadjuvant chemoimmunotherapy in locally advanced esophageal squamous cell cancer-a phase II study. Front Immunol (2021) 12:772450. doi: 10.3389/fimmu.2021.772450
23. Zhang Z, Hong ZN, Xie S, Lin W, Lin Y, Zhu J, et al. Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: a single-arm, single-center, phase 2 trial (ESONICT-1). Ann Transl Med (2021) 9(21):1623. doi: 10.21037/atm-21-5381
24. Zhang X, Gari A, Li M, Chen J, Qu C, Zhang L, et al. Combining serum inflammation indexes at baseline and post treatment could predict pathological efficacy to anti−PD−1 combined with neoadjuvant chemotherapy in esophageal squamous cell carcinoma. J Transl Med (2022) 20(1):61. doi: 10.1186/s12967-022-03252-7
25. Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer (2022) 10(1):e003497. doi: 10.1136/jitc-2021-003497
26. Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer (2022) 10(3):e004291. doi: 10.1136/jitc-2021-004291
27. Zhang Z, Ye J, Li H, Gu D, Du M, Ai D, et al. Neoadjuvant sintilimab and chemotherapy in patients with resectable esophageal squamous cell carcinoma: A prospective, single-arm, phase 2 trial. Front Immunol (2022) 13:1031171. doi: 10.3389/fimmu.2022.1031171
28. Liu J, Li J, Lin W, Shao D, Depypere L, Zhang Z, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): A multicenter, phase 2 study. Int J Cancer (2022) 151(1):128–37. doi: 10.1002/ijc.33976
29. Yin GQ, Li ZL, Li D. The safety and efficacy of neoadjuvant camrelizumab plus chemotherapy in patients with locally advanced esophageal squamous cell carcinoma: A retrospective study. Cancer Manag Res (2022) 14:2133–41. doi: 10.2147/CMAR.S358620
30. Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surg (2022) 103:106680. doi: 10.1016/j.ijsu.2022.106680
31. Lv H, Tian Y, Li J, Huang C, Sun B, Gai C, et al. Neoadjuvant sintilimab plus chemotherapy in resectable locally advanced esophageal squamous cell carcinoma. Front Oncol (2022) 12:864533. doi: 10.3389/fonc.2022.864533
32. Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer (2003) 98(7):1521–30. doi: 10.1002/cncr.11660
33. Jing SW, Zhai C, Zhang W, He M, Liu QY, Yao JF, et al. Comparison of neoadjuvant immunotherapy plus chemotherapy versus chemotherapy alone for patients with locally advanced esophageal squamous cell carcinoma: A propensity score matching. Front Immunol (2022) 13:970534. doi: 10.3389/fimmu.2022.970534
34. Zhang G, Zhang C, Sun N, Xue L, Yang Z, Fang L, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for the treatment of esophageal squamous cell carcinoma: a propensity score-matched study from the national cancer center in China. J Cancer Res Clin Oncol (2021) 148(4):943–54. doi: 10.1007/s00432-021-03659-7
35. Pasini F, de Manzoni G, Pedrazzani C, Grandinetti A, Durante E, Gabbani M, et al. High pathological response rate in locally advanced esophageal cancer after neoadjuvant combined modality therapy: dose finding of a weekly chemotherapy schedule with protracted venous infusion of 5-fluorouracil and dose escalation of cisplatin, docetaxel and concurrent radiotherapy. Ann Oncol (2005) 16(7):1133–9. doi: 10.1093/annonc/mdi207
36. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol (2015) 16(9):1090–8. doi: 10.1016/S1470-2045(15)00040-6
37. Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: A randomized clinical trial. JAMA Surg (2021) 156(5):444–51. doi: 10.1001/jamasurg.2021.0133
38. Versluis JM, Long GV, Blank CU. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat Med (2020) 26(4):475–84. doi: 10.1038/s41591-020-0829-0
39. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science (2020) 367(6477):eaax0182. doi: 10.1126/science.aax0182
40. Zhang F, Guo W, Zhou B, Wang S, Li N, Qiu B, et al. Three-year follow-up of neoadjuvant programmed cell death protein-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol (2022) 17(7):909–20. doi: 10.1016/j.jtho.2022.04.012
41. Chaft JE, Oezkan F, Kris MG, Bunn PA, Wistuba II, Kwiatkowski DJ, et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: An open-label, single-arm phase II trial. Nat Med (2022) 28(10):2155–61. doi: 10.1038/s41591-022-01962-5
42. Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Casal Rubio J, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non-Small-Cell lung cancer (NADIM phase II trial). J Clin Oncol (2022) 40(25):2924–33. doi: 10.1200/JCO.21.02660
43. Rosner S, Reuss JE, Zahurak M, Taube JM, Broderick S, Jones DR, et al. Neoadjuvant nivolumab in early-stage non–small cell lung cancer (NSCLC): Five-year outcomes. J Clin Oncol (2022) 40(suppl 16):8537. doi: 10.1200/JCO.2022.40.16_suppl.8537
44. Blum Murphy M, Xiao L, Patel VR, Maru DM, Correa AM, G Amlashi F, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: The association with baseline variables and survival-the university of Texas MD Anderson cancer center experience. Cancer (2017) 123(21):4106–13. doi: 10.1002/cncr.30953
45. Donahue JM, Nichols FC, Li Z, Schomas DA, Allen MS, Cassivi SD, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg (2009) 87(2):392–8. doi: 10.1016/j.athoracsur.2008.11.001
46. Rizvi FH, Syed AA, Khattak S, Rizvi SS, Kazmi SA, Khan MQ. Complete pathological response after neoadjuvant treatment in locally advanced esophageal cancer predicts long term survival: A retrospective cohort study. Int J Surg (2014) 12(6):621–5. doi: 10.1016/j.ijsu.2014.04.014
47. Chiu CH, Zhang P, Chang AC, Derstine BA, Ross BE, Enchakalody B, et al. Morphomic factors associated with complete response to neoadjuvant therapy in esophageal carcinoma. Ann Thorac Surg (2020) 109(1):241–8. doi: 10.1016/j.athoracsur.2019.08.031
48. Lorenzen S, Thuss-Patience P, Al-Batran SE, Lordick F, Haller B, Schuster T, et al. Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol (2013) 24(8):2068–73. doi: 10.1093/annonc/mdt141
49. Shiraishi O, Makino T, Yamasaki M, Tanaka K, Yamashita K, Ishida T, et al. Two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for treating locally advanced esophageal cancer: Short-term outcomes of a multicenter randomized phase II trial. Esophagus (2021) 18(4):825–34. doi: 10.1007/s10388-021-00831-3
50. Makino T, Yamasaki M, Tanaka K, Yamashita K, Urakawa S, Ishida T, et al. Multicenter randomised trial of two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for locally advanced oesophageal squamous cell carcinoma. Br J Cancer (2022) 126(11):1555–62. doi: 10.1038/s41416-022-01726-5
Keywords: esophageal squamous cell carcinoma, pathological complete response, immune checkpoint inhibitors, sintilimab, survival outcomes
Citation: Lv H, Huang C, Li J, Zhang F, Gai C, Liu Z, Xu S, Wang M, Li Z and Tian Z (2023) The survival outcomes of neoadjuvant sintilimab combined with chemotherapy in patients with locally advanced esophageal squamous cell carcinoma. Front. Immunol. 13:1100750. doi: 10.3389/fimmu.2022.1100750
Received: 17 November 2022; Accepted: 28 December 2022;
Published: 19 January 2023.
Edited by:
Fanping Meng, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Qifeng Wang, Sichuan Cancer Hospital, ChinaZhentao Yu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Lv, Huang, Li, Zhang, Gai, Liu, Xu, Wang, Li and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziqiang Tian, ZXNjY25lb0AxMjYuY29t
 Huilai Lv
Huilai Lv Mingbo Wang
Mingbo Wang Ziqiang Tian
Ziqiang Tian