- 1Division of Rheumatology, Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria
- 2Thermo Fisher Scientific, Freiburg, Germany
- 3Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria
- 4Ludwig Boltzmann Institute for Arthritis and Rehabilitation, Vienna, Austria
Objectives: Anti-citrullinated peptide antibodies (ACPA) are specific markers for rheumatoid arthritis (RA) and typically measured by assays employing a cyclic citrullinated peptide (CCP) as antigen. This study was aimed at investigating the diagnostic performance of anti-CCP2 and anti-CCP3 IgG and IgA assays in patients with early RA with a particular focus on the potential prognostic value of IgA ACPA.
Methods: The anti-CCP3.1 assay (Inova Diagnostics) measuring IgG and IgA antibodies simultaneously was compared to anti-CCP2 IgG and IgA assays (Thermo Fisher Scientific) employing sera of 184 early RA patients, 360 disease controls and 98 healthy subjects.
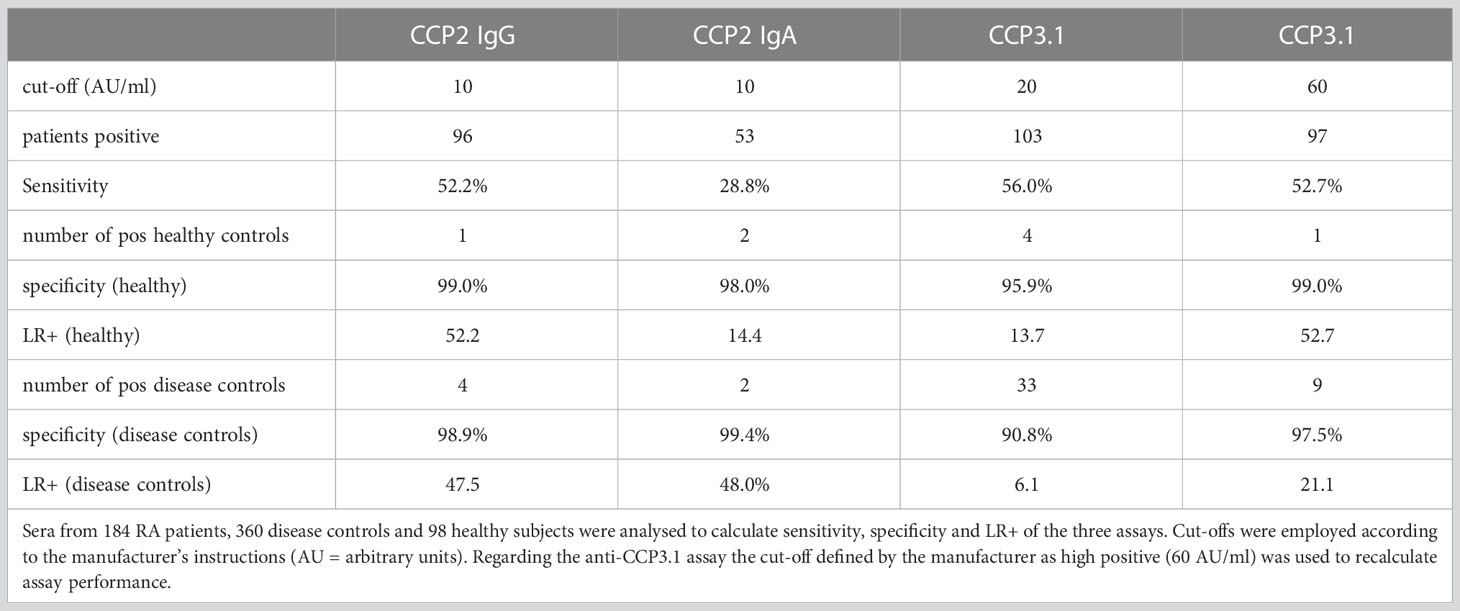
Results: Anti-CCP2 IgG and IgA assays showed high specificity versus disease controls (98.9%; 99.4%). Sensitivity was 52.2% (IgG) and 28.8% (IgA), resulting in positive likelihood ratios (LR+) of 47.5 (IgG) and 48.0 (IgA). The anti-CCP3.1 assay proved slightly more sensitive than the anti-CCP2 IgG assay (56%) but specificity was markedly lower (90.8% versus disease controls). However, when using a threefold higher cut-off specificity of the anti-CCP3.1 assay increased (97.5%) while sensitivity (52.7%) became comparable to the anti-CCP2 IgG assay resulting in a LR+ of 21.5. Anti-CCP2 IgA antibodies did not increase the diagnostic sensitivity of ACPA testing, but IgA positive patients showed diminished responses to treatment with anti-TNF biologicals compared to patients who had only IgG antibodies.
Conclusion: Specificity of ACPA assays should be adjusted to reduce the risk of misclassification and a false positive diagnosis. Determination of ACPA IgA might provide important prognostic information concerning therapeutic responses.
Highlights
● Anti-CCP2 and anti-CCP3 assays show equivalent diagnostic performance in early RA patients when adjusted for high specificity. Determination of IgA anti-citrullinated protein antibodies might provide additional prognostic information with respect to therapeutic responses.
Introduction
Besides rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA) are the most important serological markers for rheumatoid arthritis (RA). ACPA are predominantly of the immunoglobulin (Ig)G isotype and are more specific than IgM-RF. They are commonly determined by assays using a cyclic citrullinated peptide (CCP) as antigen. Since their first description (1), different generations of CCP based assays (CCP1-CCP3) have been developed which show some differences regarding sensitivities and specificities (2–4). Serological testing for ACPA and RF is particularly important in early disease stages and has been incorporated in the 2010 ACR/EULAR classification criteria for RA (5). This may have considerable impact on clinical decision making because patients with high titer antibodies would be more readily diagnosed as RA (6, 7). However, ACPA show only moderate sensitivity in early RA which is comparable to sensitivity of RF. Thus, about one third of early RA patients are seronegative for ACPA and/or RF. Therefore reducing the serological gap is an important issue in RA sero-diagnostics and several studies have tried to achieve this by determining additionally IgA isotypes of RF and ACPA (8–11). However, the overall sensitivity of ACPA testing could not be substantially increased and therefore routine diagnostics still rely on anti-CCP IgG assays.
Nevertheless, an assay has been developed (anti-CCP3.1) that detects IgG and IgA ACPA isotypes simultaneously and may have superior sensitivity compared to anti-CCP2 assays that measure only the IgG isotype (12, 13). However, it is unclear if this increased sensitivity is based on inclusion of IgA antibodies or rather on the nature of the CCP3 peptides which might be more sensitive for ACPA detection than the peptides contained in the CCP2 assay (14, 15).
Therefore this study aimed to investigate the diagnostic performance of the anti-CCP3.1 assay in comparison to anti-CCP2 IgG and IgA assays in patients with early RA and an appropriate number of disease controls. Moreover, we addressed the potential prognostic value of ACPA IgA determination, especially with respect to therapeutic responses to disease modifying anti-rheumatic drugs.
Methods
Patients
Sera were routinely obtained from patients with early RA, classified according to the 2010 ACR/EULAR criteria (3), recruited during their regular visits at the outpatients clinic at the Division of Rheumatology of the Medical University of Vienna. Patients presented with a median symptom duration of 0.2 years. The demographic data of the 184 patients selected retrospectively for this study are shown in Supplementary Table 1. All clinical information was stored in a database including >4.000 patients with RA. Treatment information was available for all patients and used to calculate drug survival times to methotrexate (MTX) (n=144) and the first anti-TNF biological therapy (n=142): 78 patients were treated with Adalimumab, 38 with Etanercept, 14 with Golimumab and 12 patients received Infliximab; out of these 142 patients 22 received an anti-TNF monotherapy. Treatment responses after 3 months were calculated using the simplified disease activity index (SDAI)50, referring to a 50% improvement in SDAI. In addition, x-rays from hands and feet of 140 RA patients were analysed for radiographic progression using the Sharp/van der Heijde (SvdH) score. A mean annual progression rate was calculated out of multiple timepoints and was compared within groups; 32% of patients showed an erosive disease course with >5 mean annual progression rate.
Sera from 360 patients with other rheumatic diseases were collected from 95 patients with osteoarthritis (OA), 92 patients with systemic lupus erythematosus (SLE), 48 patients with ankylosing spondylitis (SpA), 45 patients with systemic sclerosis, 13 patients with reactive arthritis, 19 patients with primary Sjögren´s syndrome, 15 patients with autoimmune inflammatory myopathies, 14 patients with granulomatosis with polyangiitis and 19 patients with a diagnosis of osteoporosis. Sera from 98 healthy subjects were collected during voluntary health examination offered by the Austrian social insurance. Disease controls had a median age of 55 (43–64) years and 68.8% were females. Healthy controls had a median age of 50 (42.5–55) years and 72% were females. The study was approved by the ethics committee of the Medical University of Vienna (ethics vote numbers: 559/2005 and 2002/2014). Biomaterial was processed and stored until analysis according to standard operating procedures by the Medical University Vienna biobank, a central facility included in a certified quality management system (16).
Antibody detection
Sera were analysed for the presence of anti-CCP antibodies by anti-CCP2 IgG and IgA assays (EliA™ CCP, Thermo Fisher Scientific) and the combined IgG/IgA anti-CCP3.1 assay (Quanta LiteRCCP3.1, Inova Diagnostics). Cut-offs recommended by the manufacturers were 10 arbitrary units (AU)/ml for anti-CCP2 IgG and IgA and 20 AU/ml for anti-CCP3.1. Regarding the anti-CCP3.1 assay, a cut-off of 60 AU/ml (high positive according to the manufacturer) was additionally employed.
Statistical analysis
Data are shown as median and interquartile ranges (IQR). Nonparametric statistical methods were used for comparisons. Kruskal-Wallis test for comparing continuous variables between groups and Fisher’s exact test for differences in dichotomous variables. Specificities as well as positive likelihood ratios (LR+) were calculated either against healthy controls or disease controls. MTX and anti-TNF retention rates were calculated and presented using Kaplan-Meier curves.
All statistical analysis was performed using SPSS (version 28). A P value of less than 0.05 was considered to indicate statistical significance.
Results
Sensitivity and specificity
As expected, anti-CCP2 IgG and IgA assays showed very high specificities ≥ 98% both versus healthy subjects and disease controls. Sensitivities were 52.2% for IgG and 28.8% for IgA antibodies, respectively. This resulted in high positive likelihood ratios (LR+) versus disease controls of 47.5 for the IgG and 48.0 for the IgA assay (Table 1). However, IgA antibodies did not show an added diagnostic value in our early RA cohort as all IgA positive patients were also IgG positive. The anti-CCP3.1 assay was found to be slightly more sensitive than the anti-CCP2 IgG assay with 56% of early RA patients testing positive. However, specificity was lower amounting to 95.9% versus healthy subjects and 90.8% versus disease controls which resulted in a relatively low LR+ of 13.7 vs. healthy subjects and 6.1 vs. disease controls, respectively (Table 1).
Thus, out of 360 disease controls 33 patients (9.2%) were found to be positive for anti-CCP3.1 whereas only 4 control patients (1.1%) were anti-CCP2 IgG positive; of these 2 patients (0.6%) had also IgA antibodies (Table 2). Among anti-CCP3.1 positive disease controls the most common diagnoses were osteoarthritis (n=12), ankylosing spondylitis (n=6) and SLE (n=5); 6 patients suffered from other autoimmune rheumatic diseases, two patients were diagnosed with reactive arthritis and two had osteoporosis. Furthermore, 4 healthy controls were positive in the CCP3.1 assay of which only one was also positive in the CCP2 assays (Table 2).
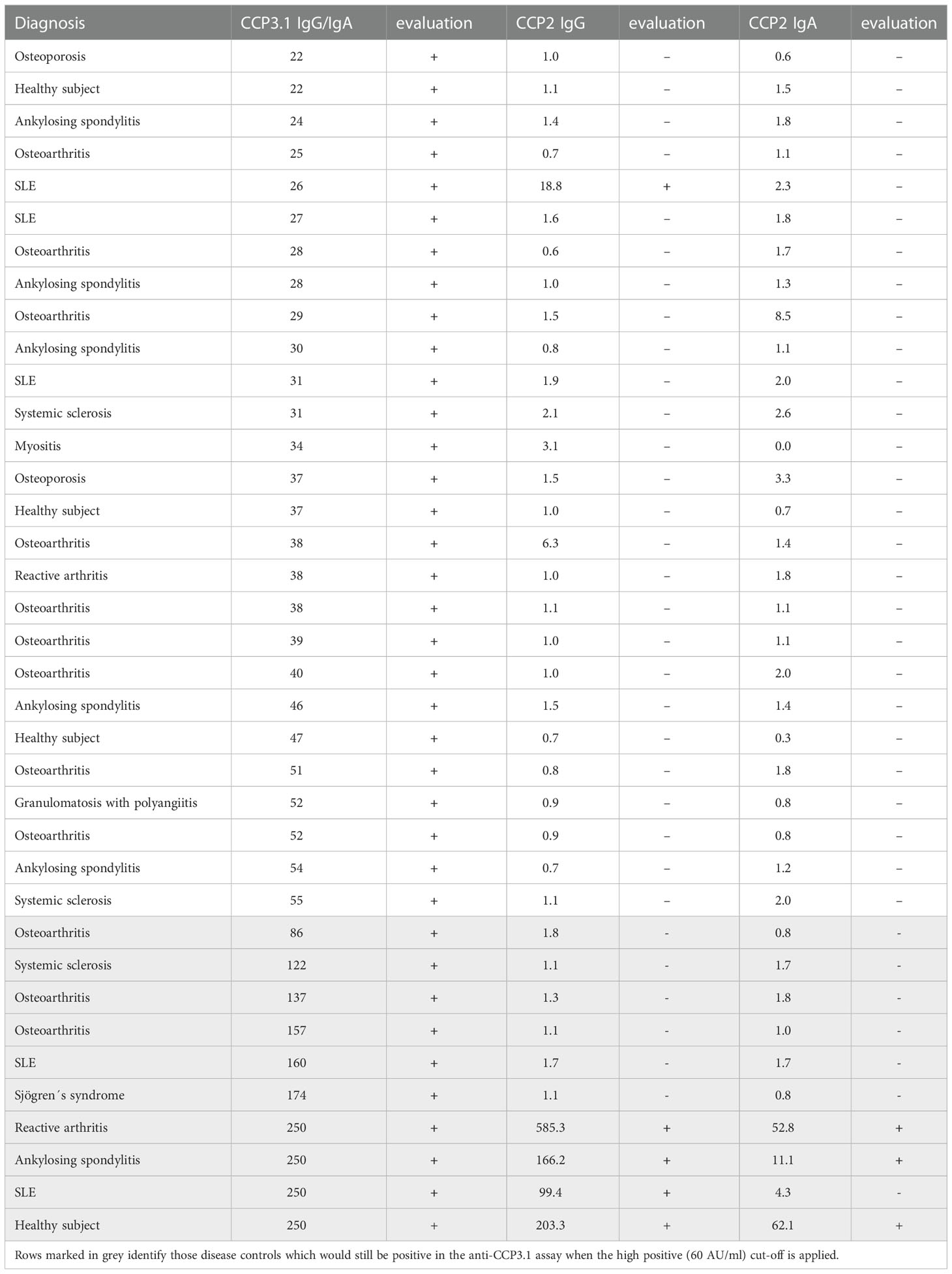
Table 2 Non-RA patients and healthy subjects showing a positive result in anti-CCP3.1 IgG/IgA and/or anti-CCP2 IgG and IgA testing.
Since the majority of false positive patients had low level anti-CCP3.1 antibodies we re-analysed the data using a cut-off value of 60 AU/ml (high positive as defined by the manufacturer, see Methods). This resulted in an increased specificity of 99% versus healthy subjects and of 97.5% versus disease controls with a LR+ of 52.7 versus healthy subjects and 21.1 versus disease controls. Only 9 out of 33 control patients remained positive (3 osteoarthritis, 1 ankylosing spondylitis, 1 reactive arthritis and 4 with other autoimmune inflammatory diseases). In contrast, two thirds out of the 12 anti-CCP3.1 positive early RA patients testing negative in the anti-CCP2 assay showed levels above 60 AU/ml (Figure 1A). Thus, at this elevated cut-off sensitivity was only slightly reduced (and virtually identical to the anti-CCP2 IgG assay) while the LR+ increased 3.5-fold versus disease controls and 3.8-fold versus healthy controls.
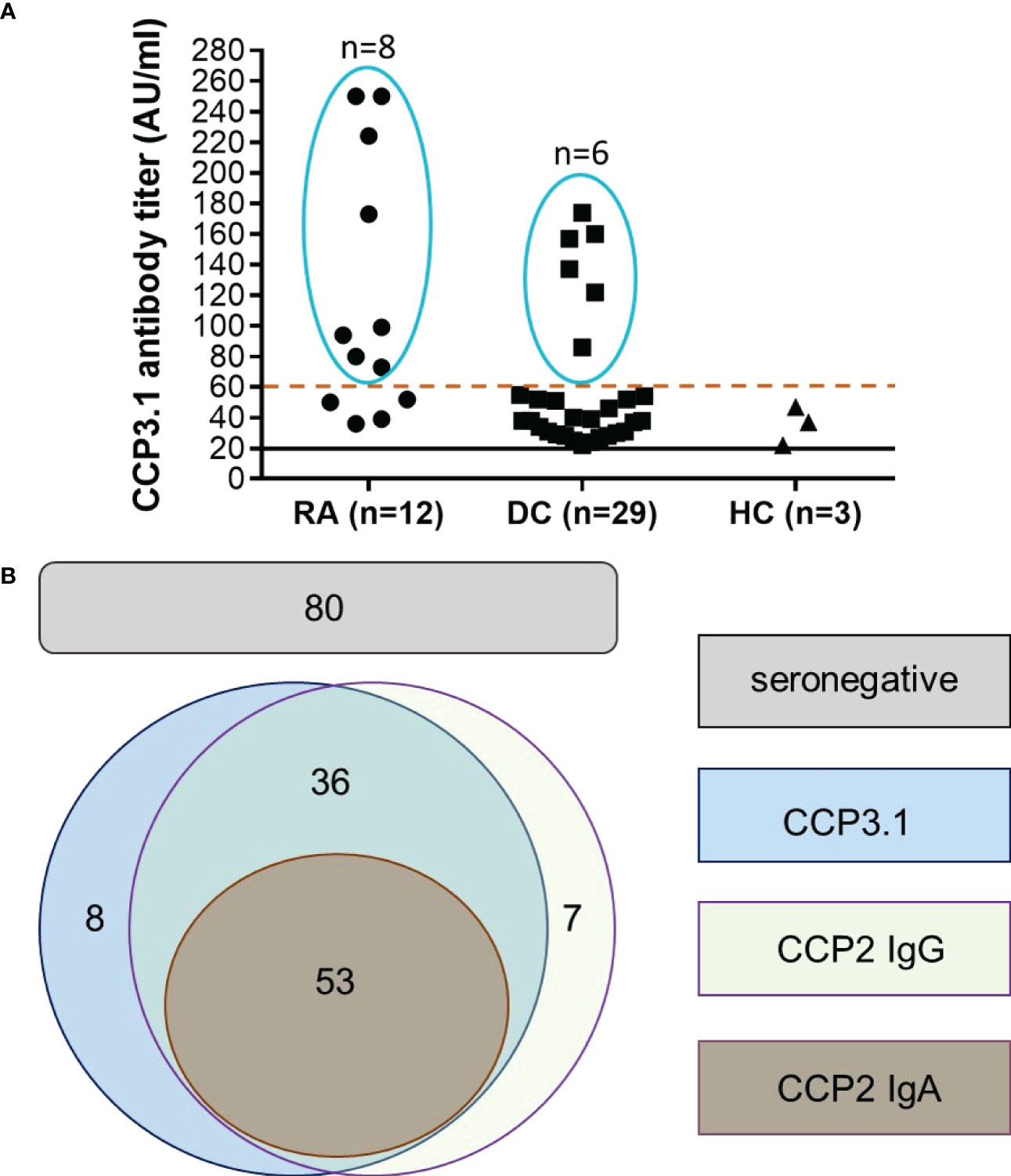
Figure 1 Anti-CCP2 IgG, CCP2 IgA and CCP3.1 IgG/IgA antibodies in early RA patients and disease controls. (A) Anti-CCP3.1 antibody levels in anti-CCP3.1 positive/anti-CCP2 IgG negative early RA patients, disease controls (DC) and healthy controls (HC). When applying a cut-off level of 60 AU/ml (high positive), the number of positive disease controls and healthy controls was reduced from 29 to 6 and from 3 to 0, respectively, while 8 out of the 12 anti-CCP2 negative early RA patients remained positive. (B) Schematic representation of the overlap of anti-CCP2 IgG, anti-CCP2 IgA and anti-CCP3.1 IgG/IgA positivity in the early RA cohort when applying the high positive cut-off (60 AU/ml) for the anti-CCP3.1 assay. Out of 184 patients, 53 were found being triple positive for anti-CCP2 IgG, IgA and anti-CCP3.1, 36 patients were double positive for anti-CCP2 IgG and anti-CCP3.1; 7 patients were solely positive for anti-CCP2 IgG and 8 patients for anti-CCP3.1. Eighty patients were negative in all assays (seronegative).
Taken together, when applying the high cut-off, 53 early RA patients were triple positive for anti-CCP2 IgG, anti-CCP2 IgA and anti-CCP3.1; 36 patients were double positive for anti-CCP2 IgG and anti-CCP3.1; 7 patients were solely positive for anti-CCP2 IgG and 8 patients solely for anti-CCP3.1. Overall sensitivity of the two IgG assays was 56.5% with 80 patients remaining seronegative for anti-CCP antibodies (Figure 1B).
Prognostic value of ACPA IgA
Although anti-CCP2 IgA antibodies did not increase the diagnostic specificity of ACPA testing, they might have some prognostic value as has been proposed for RF IgA (17). To address this issue we calculated drug retention rates for MTX and anti-TNF biologicals. These analyses revealed decreased drug survival times for anti-TNF biologicals in patients with anti-CCP2 IgA antibodies (Figure 2A). The difference between anti-CCP2 IgG single positive and IgG/IgA double positive patients was statistically significant at 18 months and persisted until the end of the observation period (60 months). After one year, 65% of anti-CCP2 IgG single positive patients were on anti-TNF treatment as compared to 50% of patients with anti-CCP2 IgG/IgA antibodies. After three years 44% of the IgG single positive patients but only 23% of IgG/IgA double positive patients were still under anti-TNF treatment. A reduced retention rate was also observed in seronegative patients of which only 19% remained on anti-TNF therapy. Five years after treatment initiation still approximately 30% of anti-CCP2 IgG single positive patients were taking anti-TNF biologicals whereas almost 95% of patients with anti-CCP2 IgG/IgA antibodies had discontinued their first anti-TNF biological, similar to seronegative patients. As expected, such prognostic value could not be attributed to the CCP3.1 assay which measures IgG and IgA antibodies simultaneously (Supplementary Figure 1).
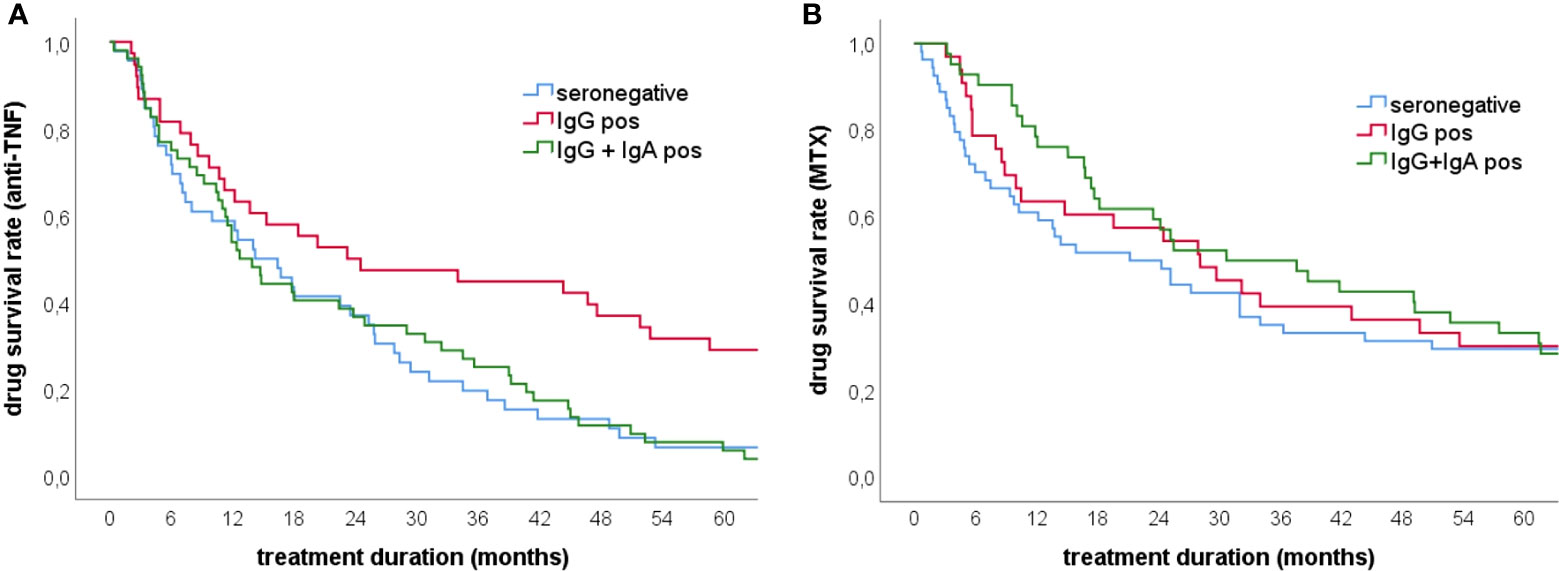
Figure 2 Drug survival rates for anti-TNF and methotrexate treatment in early RA patients. (A) Anti-TNF survival rates in patients showing anti-CCP2 IgG/IgA antibodies (n=53), anti-CCP2 IgG antibodies (n=43) and seronegative patients (n=46) (B) Methotrexate survival rates in patients showing anti-CCP2 IgG/IgA antibodies (n=47), anti-CCP2 IgG antibodies (n=43) and seronegative patients (n=54). The presence of IgA antibodies was significantly associated with reduced anti-TNF retention rates while for MTX retention no significant difference was found between the groups.
Regarding the response to anti-TNF treatment after 3 months there was no significant difference in SDAI50 between patients with anti-CCP2 IgG and those with IgG/IgA antibodies. In addition, there was also no significant difference between IgG single positive patients and IgG/IgA double positive patients regarding disease activity at baseline (Supplementary Table 1) as well as during the disease course.
Concerning baseline treatment with MTX, no statistically significant differences between the three patient groups were observed although during the first year MTX survival rate appeared to be higher in patients with anti-CCP2 IgA antibodies (Figure 2B).
Mean radiographic progression rate was higher in anti-CCP positive patients compared to seronegative patients. Interestingly however, the difference in progression rates reached the level of statistical significance only for IgG/IgA positive patients although no significant difference was seen between anti-CCP2 IgG single positive and anti-CCP2 IgG/IgA double positive patients (Supplementary Figure 2).
Discussion
ACPA are undoubtedly the most specific serological markers of RA and according to the classification criteria would be strongly indicative of RA, particularly in case of high titer (5). However, the prevalence of ACPA in early RA does usually not exceed 55% and therefore manufacturers of diagnostic assays have attempted to increase the sensitivity of ACPA assays. Since most commercial assays are employing the CCP2 peptides as antigen (and therefore show similar performance) efforts have been undertaken to increase sensitivity of ACPA testing by using other antigens such as mutated citrullinated vimentin (18, 19), citrullinated viral peptides (20) or another artificial peptide mixture such as CCP3 (13–15). Moreover, additional determination of ACPA IgA isotypes has been suggested but proven to only marginally increase overall sensitivity of ACPA testing (8–10).
In line with some previous reports (6, 21) we confirmed that in our cohort of early RA patients the anti-CCP3.1 assay showed indeed a slightly higher sensitivity than the anti-CCP2 IgG assay. However, this was not due to the simultaneous measurement of IgG and IgA isotypes but rather due to the low cut-off of the assay. Thus, the moderate gain in sensitivity was obtained at the expense of specificity which was significantly reduced both versus disease controls and healthy subjects confirming observations made also by other researchers (3, 6). This issue has been addressed in some detail in a recent study by van Hoovels et al. in which commercial assays for ACPA and RF were compared, particularly in respect of their impact on RA classification (7).
When employing a three-fold higher cut-off (as suggested by the manufacturer), diagnostic performance of the anti-CCP3.1 assay became comparable to the anti-CCP2 assay since 24 disease controls (but only 6 early RA patients) became negative. Therefore, anti-CCP3.1 levels below 60 AU/ml did not appear to be meaningful or could even be misleading because the majority of patients with low antibody levels (33 out of 38) did not have a diagnosis of RA (Table 2). This was obviously also the case for the four anti-CCP3.1 positive healthy controls among which only one had high titer antibodies. Remarkably, the serum showed also high levels of anti-CCP2 IgG and IgA antibodies and therefore this healthy subject might be at risk for developing RA (6).
Although at the higher cut-off the agreement between the two assays was very good, 8 patients were solely positive for anti-CCP3.1 while 7 patients were solely positive for anti-CCP2 (Figure 1B). This may reflect the different nature of the two peptides and/or the technical differences between both test methods (4) as the anti-CCP2 IgG assay is an automated and random access fluoroenzyme immunoassay (FEIA) compared to the manual enzyme-linked immunosorbent assay (ELISA) used to detect anti-CCP3.1 antibodies.
Clearly, no single assay will detect all ACPA positive patients but users should be aware of specificity and adjust cut-off values accordingly to obtain at least 98% specificity vs healthy controls. Our data also confirm that measuring IgA antibodies does not increase the diagnostic sensitivity of anti-CCP testing. However, ACPA IgA may further increase the diagnostic specificity because in accordance with previous investigations only two disease controls and one healthy subject showed this type of dual IgG/IgA reactivity (10). Interestingly, in a recent study a broad autoantibody profile at baseline including also ACPA IgA and IgM isotypes was associated with an increased early response to treatment with MTX but not with long-term outcome (22). This appeared also to be the case in our cohort of early RA patients although statistical significance was not obtained (Figure 2B). In contrast, with respect to anti-TNF biologicals patients with anti-CCP2 IgG/IgA antibodies showed a significantly reduced anti-TNF retention rate as compared to patients who had only IgG antibodies or seronegative patients. Interestingly, in seronegative patients a reduced retention rate has been reported for Abatacept suggesting that the presence of ACPA and/or RF could be used as an indicator for a more long-lasting response to biological drugs (23). Thus, it will be interesting to see if Abatacept retention rates are also affected by IgA antibodies, a matter that would deserve further investigations. However, it should be borne in mind that an association of ACPA (IgG) or RF (IgM) with the clinical treatment response to TNF inhibitors has not been observed (24) and also in our cohort no significant difference was seen between the three patient groups with respect to anti-TNF treatment responses as measured by SDAI.
The prognostic value of IgA anti-CCP2 appeared to be long lasting because 5 years after treatment initiation, only 5% of IgG/IgA positive patients were still under anti-TNF treatment as compared to 30% of IgG positive patients. This might have some impact on clinical decision making and favour treatment of ACPA and/or RF IgA positive patients with a B- or T cell targeting therapy. Our finding is also in line with a previous study in which the presence of RF IgA was associated with a blunted response to treatment with TNF inhibitors (17). Of note, the vast majority of our anti-CCP2 IgG/IgA positive early RA patients was also positive for RF IgA (Supplementary Table 1). Furthermore, in a recently published study ACPA IgA was identified as a risk factor for disease flare during drug tapering in patients with RA (25) and there is now emerging eveidence that IgA autoantibodies, and particularly the IgA2 subclass, contribute to the inflammatory processes of RA by activating macrophages and neutrophils (26). Taken together, these data further support the arguments for testing separately for RF and ACPA IgA isotypes because these assays can provide valuable information which could allow further stratification of RA patients.
In summary, the two ACPA assays under investigation showed comparable performance in patients with early RA when the cut-off of the CCP3.1 assay was adjusted to ensure high specificity. Of note, in our early RA cohort overall sensitivity of the CCP2 IgG and CCP3.1 assay amounted to 56.5% as compared to approximately 52% of the individual assays. Therefore, use of both assays might be considered to increase overall sensitivity of ACPA testing without affecting specificity. Moreover, the use of both assays could also improve diagnostic accuracy, particularly in patients at risk for developing RA (6, 13, 27). Although this would increase the costs of ACPA testing the gain of identifying additional RA patients in the earliest stages of disease would justify such strategy because the costs of a delayed diagnosis and treatment in a false negative patient would by far outmatch the relatively low costs of one additional assay that needs to be done only at baseline (28).
Since approximately 10% of anti-CCP2/CCP3.1 negative patients tested positive for RF IgM (and most of them also for RF IgA) overall sensitivity of ACPA and RF testing in our cohort amounted to approximately 67%. While compared to ACPA specificity of RF IgM is moderate, especially in the absence of ACPA, combined presence of RF IgM/IgA has been found to be more specific for RA arguing again for including also RF IgA into routine diagnostics (10, 11). Interestingly, the seven CCP2 IgG positive/CCP3.1 negative sera were all positive for RF IgM as compared to only four of the eight CCP3.1 positive/CCP2 negative sera. Even though this may be pure coincidence such observation would deserve further investigation in larger cohorts of RA patients.
For the future, assays containing a mixture of citrullinated, carbamylated and acetylated peptides might indeed reduce the serological gap (29) and further increase specificity of ACPA testing because the presence of multiple reactivities has been reported to be highly specific for RA (10, 30). Furthermore, determination of IgA isotypes should be taken into serious consideration because their presence might have considerable impact on therapeutic decision making which needs to be addressed in more elaborate multi-centre studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of the Medical University of Vienna. The patients/participants provided their written informed consent to participate in this study.
Author contributions
GS: Study design, data interpretation, manuscript writing. DS: Study design, data acquisition, data interpretation, preparation of figures, manuscript writing. CK and SS: Study design, data acquisition, data interpretation. HH: Data acquisition. DA: Data interpretation, manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Innovative Medicines Initiative Joint Undertaking under grant agreement no 777357 (RTCure).
Conflict of interest
DS and GS received speaker fees from Thermo Fisher Scientific, CK and SS are employees of Thermo Fisher Scientific.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1096866/full#supplementary-material
References
1. Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheumatol (2000) 43(1):155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3
2. Coenen D, Verschueren P, Westhovens R, Bossuyt X. Technical and diagnostic performance of 6 assays for the measurement of citrullinated protein/peptide antibodies in the diagnosis of rheumatoid arthritis. Clin Chem (2007) 53(3):498–504. doi: 10.1373/clinchem.2006.078063
3. Bizzaro N, Tonutti E, Tozzoli R, Villalta D. Analytical and diagnostic characteristics of 11 2nd- and 3rd-generation immunoenzymatic methods for the detection of antibodies to citrullinated proteins. Clin Chem (2007) 53(8):1527–33. doi: 10.1373/clinchem.2007.087569
4. Van der Cruyssen B, Nogueira L, Van Praet J, Deforce D, Elewaut D, Serre G, et al. Do all anti-citrullinated protein/peptide antibody tests measure the same? Evaluation of discrepancy between anti-citrullinated protein/peptide antibody tests in patients with and without rheumatoid arthritis. Ann Rheum Dis (2008) 67(4):542–6. doi: 10.1136/ard.2007.071654
5. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: An American college of Rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis (2010) 69(9):1580–8. doi: 10.1136/ard.2010.138461
6. Demoruelle MK, Parish MC, Derber LA, Kolfenbach JR, Hughes-Austin JM, Weisman MH, et al. Performance of anti-cyclic citrullinated peptide assays differs in subjects at increased risk of rheumatoid arthritis and subjects with established disease. Arthritis Rheumatol (2013) 65(9):2243–52. doi: 10.1002/art.38017
7. Van Hoovels L, Jacobs J, Vander Cruyssen B, Van den Bremt S, Verschueren P, Bossuyt X. Performance characteristics of rheumatoid factor and anti-cyclic citrullinated peptide antibody assays may impact ACR/EULAR classification of rheumatoid arthritis. Ann Rheum Dis (2018) 77(5):667–77. doi: 10.1136/annrheumdis-2017-212365
8. Svard A, Kastbom A, Reckner-Olsson A, Skogh T. Presence and utility of IgA-class antibodies to cyclic citrullinated peptides in early rheumatoid arthritis: the Swedish TIRA project. Arthritis Res Ther (2008) 10(4):R75. doi: 10.1186/ar2449
9. dos Anjos LM, Pereira IA, d 'Orsi E, Seaman AP, Burlingame RW, Morato EF. A comparative study of IgG second- and third-generation anti-cyclic citrullinated peptide (CCP) ELISAs and their combination with IgA third-generation CCP ELISA for the diagnosis of rheumatoid arthritis. Clin Rheumatol (2009) 28(2):153–8. doi: 10.1007/s10067-008-0999-5
10. Sieghart D, Platzer A, Studenic P, Alasti F, Grundhuber M, Swiniarski S, et al. Determination of autoantibody isotypes increases the sensitivity of serodiagnostics in rheumatoid arthritis. Front Immunol (2018) 9:876. doi: 10.3389/fimmu.2018.00876
11. Van Hoovels L, Vander Cruyssen B, Sieghart D, Bonroy C, Nagy E, Pullerits R, et al. IgA rheumatoid factor in rheumatoid arthritis. Clin Chem Lab Med (2022) 60(10):1617–26. doi: 10.1515/cclm-2022-0244
12. QuantaLite CCP3.1 IgG/IgA ELISA, in: Inova diagnostics (2009). Available at: https://www.yumpu.com/en/document/read/42214812/ccp-31-igg-iga-elisa-704550-inova (Accessed 1st December 2022).
13. Vos I, Van Mol C, Trouw LA, Mahler M, Bakker JA, Van Offel J, et al. Anti-citrullinated protein antibodies in the diagnosis of rheumatoid arthritis (RA): diagnostic performance of automated anti-CCP-2 and anti-CCP-3 antibodies assays. Clin Rheumatol (2017) 36(7):1487–92. doi: 10.1007/s10067-017-3684-8
14. Swart A, Burlingame RW, Gürtler I, Mahler M. Third generation anti-citrullinated peptide antibody assay is a sensitive marker in rheumatoid factor negative rheumatoid arthritis. Clin Chim Acta (2012) 414:266–72. doi: 10.1016/j.cca.2012.09.015
15. van Venrooij WJ, van Beers JJBC, Pruijn GJ. Anti-CCP antibodies: the past, the presence and the future. Nat Rev Rheumatol (2011) 7:391–8. doi: 10.1038/nrrheum.2011.76
16. Haslacher H, Gerner M, Hofer P, Jurkowitsch A, Hainfellner J, Kain R, et al. Usage data and scientific impact of the prospectively established fluid bioresources at the hospital-based MedUni wien biobank. Biopreserv Biobank (2018) 16(6):471–82. doi: 10.1089/bio.2018.0032
17. Bobbio-Pallavicini F, Caporali R, Alpini C, Moratti R, Montecucco C. Predictive value of antibodies to citrullinated peptides and rheumatoid factors in anti-TNF-alpha treated patients. Ann N Y Acad Sci (2007) 1109:287–95. doi: 10.1196/annals.1398.034
18. Innala L, Kokkonen H, Eriksson C, Jidell E, Berglin E, Dahlqvst SR. Antibodies against mutated citrullinated vimentin are a better predictor of disease activity at 24 months in early rheumatoid arthritis than antibodies against cyclic citrullinated peptides. J Rheumatol (2008) 35(6):1002–8.
19. Damjanovska L, Thabet MM, Levarth EW, Stoeken-Rijsbergen G, van der Voort EI, Toes RE, et al. Diagnostic value of anti-MCV antibodies in differentiating early inflammatory arthritis. Ann Rheum Dis (2010) 69(4):730–2. doi: 10.1136/ard.2009.108456
20. Bizzaro N, Allegri F, Alpini C, Doria A, Gerli R, Lotzniker M, et al. Multicentric evaluation of a second generation assay to detect antiviral citrullinated peptide antibodies: a collaborative study by the forum interdisciplinare per la ricerca nelle malattie autoimmuni. J Clin Pathol (2011) 64(12):1139–41. doi: 10.1136/jclinpath-2011-200308
21. Szekanecz Z, Szabó Z, Zeher M, Soós L, Dankó K, Horváth I, et al. Superior performance of the CCP3.1 test compared to CCP2 and MCV in the rheumatoid factor-negative RA population. Immunol Res (2013) 56(2-3):439–43. doi: 10.1007/s12026-013-8425-8
22. de Moel EC, Derksen VFAM, Stoeken G, Trouw LA, Bang H, Goekoop RJ, et al. Baseline autoantibody profile in rheumatoid arthritis is associated with early treatment response but not long-term outcomes. Arthritis Res Ther (2018) 20(1):33. doi: 10.1186/s13075-018-1520-4
23. Gottenberg JE, Courvoisier DS, Hernandez MV, Iannone F, Lie E, Canhão H, et al. Brief report: Association of rheumatoid factor and anti-citrullinated protein antibody positivity with better effectiveness of abatacept: Results from the pan-European registry analysis. Arthritis Rheumatol (2016) 68(6):1346–52. doi: 10.1002/art.39595
24. Lv Q, Yin Y, Li X, Shan G, Wu X, Liang D, et al. The status of rheumatoid factor and anti-cyclic citrullinated peptide antibody are not associated with the effect of anti-TNFα agent treatment in patients with rheumatoid arthritis: a meta-analysis. PloS One (2014) 9(2):e89442. doi: 10.1371/journal.pone.0089442
25. Sokolova MV, Hagen M, Bang H, Schett G, Rech J, Steffen U, et al. IgA anti-citrullinated protein antibodies are associated with flares during DMARD tapering in rheumatoid arthritis. Rheumatol (Oxford) (2022) 61(5):2124–213. doi: 10.1093/rheumatology/keab585
26. Steffen U, Koeleman CA, Sokolova MV, Bang H, Kleyer A, Rech J, et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun (2020) 11(1):120. doi: 10.1038/s41467-019-13992-8
27. Di Matteo A, Mankia K, Duquenne L, Mahler M, Corscadden D, Mbara K, et al. Third-generation anti-cyclic citrullinated peptide antibodies improve prediction of clinical arthritis in individuals at risk of rheumatoid arthritis. Arthritis Rheumatol (2020) 72(11):1820–8. doi: 10.1002/art.41402
28. Nell VP, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatol (Oxford) (2004) 43(7):906–14. doi: 10.1093/rheumatology/keh199
29. Grönwall C, Liljefors L, Bang H, Hensvold AH, Hansson M, Mathsson-Alm L, et al. A comprehensive evaluation of the relationship between different IgG and IgA anti-modified protein autoantibodies in rheumatoid arthritis. Front Immunol (2021) 12:627986. doi: 10.3389/fimmu.2021.627986
30. Verheul MK, Böhringer S, van Delft MAM, Jones JD, Rigby WFC, Gan RW, et al. Triple positivity for anti-citrullinated protein autoantibodies, rheumatoid factor, and anti-carbamylated protein antibodies conferring high specificity for rheumatoid arthritis: Implications for very early identification of At-risk individuals. Arthritis Rheumatol (2018) 70(11):1721–1. doi: 10.1002/art.40562
Keywords: anti-citrullinated protein autoantibodies, cyclic citrullinated peptide, IgA autoantibodies, diagnostic performance, rheumatoid arthritis
Citation: Sieghart D, Konrad C, Swiniarski S, Haslacher H, Aletaha D and Steiner G (2023) The diagnostic and prognostic value of IgG and IgA anti-citrullinated protein antibodies in patients with early rheumatoid arthritis. Front. Immunol. 13:1096866. doi: 10.3389/fimmu.2022.1096866
Received: 12 November 2022; Accepted: 09 December 2022;
Published: 05 January 2023.
Edited by:
Kutty Selva Nandakumar, Halmstad University, SwedenReviewed by:
Raimon Sanmarti, Hospital Clinic of Barcelona, SpainJan Damoiseaux, Maastricht University Medical Centre, Netherlands
Copyright © 2023 Sieghart, Konrad, Swiniarski, Haslacher, Aletaha and Steiner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Günter Steiner, Z3VlbnRlci5zdGVpbmVyQG1lZHVuaXdpZW4uYWMuYXQ=
 Daniela Sieghart
Daniela Sieghart Christian Konrad
Christian Konrad Sascha Swiniarski2
Sascha Swiniarski2 Helmuth Haslacher
Helmuth Haslacher Günter Steiner
Günter Steiner