- 1School of Forensic Medicine, Hebei Key Laboratory of Forensic Medicine, Hebei Medical University, Shijiazhuang, China
- 2Hebei Food Inspection and Research Institute, Hebei Food Safety Key Laboratory, Key Laboratory of Special Food Supervision Technology for State Market Regulation, Hebei Engineering Research Center for Special Food Safety and Health, Shijiazhuang, Hebei, China
- 3Department of Cell Biology, Cardiovascular Medical Science Center, Key Laboratory of Neural and Vascular Biology of Ministry of Education, Hebei Medical University, Shijiazhuang, China
Since ancient times, Tremella aurantialba has been proposed to have medicinal and food benefits. Modern phytochemistry and pharmacological studies have demonstrated that polysaccharides, the main components from T. aurantialba appear to be an all-round talent resisting a variety of chronic inflammatory diseases and protecting against different types of tumors, diabetes and cardiovascular diseases. These health and pharmacological benefits have gained much attention from scholars around the world. Further, more and more methods for polysaccharides extraction, purification, structure identification have been proposed. Significantly, the bioactivity of fungus polysaccharides is affected by many factors such as extraction and purification conditions and chemical structure. This paper provides an overview of recent advances in the isolation, structural features and biological effects of polysaccharides derived from T. aurantialba, covers recent advances in the field and outlines future research and applications of these polysaccharides.
1 Introduction
Tremella aurantialba, belonging to the genus Naematelia Fr, is a well-known medicinal and edible fungus widely distributed in Asia, Europe, North America and Oceania (1–3). It is now found all over the world due to artificial cultivation. The color is golden yellow, the taste is delicate and sticky, and the nutritional value are better than those of Tremella Fuciformis and auricularia auricula (4). Relevant pictures of T. aurantialba are shown in Figure 1. Wild T. aurantialba have been consumed as food and medicine in countries such as Asia and Europe for thousands of years (2, 5). It was once considered to have the functions of “benefiting the mind and body”, “turning the weak into the strong” and “prolonging life” (6). Besides, the Chinese medicine book “Compendium of Materia Medica” records that T. aurantialba can be used to treat a variety of diseases, especially moistening the lung, relieving cough, protecting the liver and tonifying the kidney. Xizang Common Chinese Herbal Medicine also records the effects of T. aurantialba on asthenia tuberculosis cough, hemoptysis, tuberculosis, asthma, hypertension and chronic bronchitis in the elderly (7).
In recent decades, the fungus polysaccharides as ideal natural resources for supplements and pharmaceuticals have attracted a lot of attention due to their abundant bioactivities (8–12). Modern phytochemistry and pharmacological studies have been proved that polysaccharides are major bioactive constituents of T. aurantialba, accounting for 37.8%-40.55% (13). Presently, crude polysaccharides or their purified fractions from T. aurantialba are attracting growing attention due to their remarkable immune-stimulating activity (4, 14) and the ability to resist various chronic inflammatory diseases (15–18). However, there are few published reviews concerning extraction, purification, structure and biological activities of polysaccharides from T. aurantialba. Based on current available studies, it is speculated that T. aurantialba may be used as more promising functional food supplements or therapeutic agents than some other fungi such as Auricularia auricula (4, 19). Therefore, this paper aims to systematically summarize and update some of the latest research achievements of T. aurantialba polysaccharides, including extraction, purification, structural characterization, biological activity and the relationship between its structure and activity, so as to provide detailed reference for the future utilization of T. aurantialba polysaccharides.
2 Procedure of extraction and purification
Polysaccharides are the structural components of the fungus cell wall. Therefore, it is critical to select an efficient extraction method that can destroy the cell wall to release fungus polysaccharides (20). At present, the methods used for extraction of T. aurantialba polysaccharides include hot water extraction, alkali extraction, enzyme-assisted extraction and ultrasonic-assisted extraction.
2.1 Extraction
2.1.1 Hot water extraction
Hot water extraction is the most classical and convenient method in the laboratory and industrial extraction of fungus polysaccharides (2, 18, 21). It is often used in combination with other methods such as hydrolysis (alkali (22–25) and enzyme (26–30)), chemical derivatization (31–36), or physical treatment (37–41) to obtain polysaccharides with specific structural features and high bioactivity. For example, for some acidic or high molecular weight polysaccharides, especially those containing uronic acids, their solubility in dilute alkali solution is generally greater than that in hot water, so alkali extraction of such polysaccharides is common. Dong compared the effects of water extraction and alkaline extraction on the polysaccharides from T. aurantialba mycelium, and the results showed that when the latter method was used, the extracted mycelium polysaccharides content increased by 9.4% (42). Liu and Yu compared the polysaccharides yield obtained by hot water extraction and alkali extraction, and found that the polysaccharides yield of alkali extraction reached 14.9% at 4 h after treatment, and then showed a downward trend. The yield of hot water extraction was 10.3% at 4 h, and the yield reached the maximum at 10 h, which was 15.5%. The results showed that under suitable conditions, alkaline extraction could increase the yield of polysaccharides, but might also cause polysaccharides degradation (43).
2.1.2 Enzymatic extraction
Enzymatic extraction is also commonly used to increase the yield of polysaccharides. Wang compared the effects of water extraction, enzymatic extraction, microwave extraction and ultrasonic assisted extraction on polysaccharides yield of T. aurantialba fruiting bodies. The results showed that under the optimal extraction conditions, enzymatic extraction obtained the highest polysaccharides yield, up to 25.96%, with the highest antioxidant activity (44).
2.1.3 Ultrasonic assisted extraction
Ultrasonic assisted extraction method has gradually become a popular method for the extraction of various fungus polysaccharides in recent years. Ultrasound can destroy the structure of fungus cell walls and make polysaccharides flow out of cells faster, thereby effectively shortening the extraction time of polysaccharides and improving the yield of polysaccharides. Huang et al. compared the effects of water extraction and ultrasonic assisted extraction on the yield of polysaccharides from T. aurantialba mycelium, and found that compared with water extraction, ultrasonic assisted extraction had a higher polysaccharides yield (45). However, when the ultrasonic power exceeded 600 W, the polysaccharides yield would decrease, which might be related to the ultrasonic-induced polysaccharides degradation.
2.1.4 Others
Several new polysaccharides extraction methods, including microwave extraction (41, 46–49), pressurized liquid extraction (49–52), ultrasound-enhanced subcritical water extraction (53–56), continuous fractionation by supercritical fluid extraction (57) and induced or pulsed electric fields extraction (58, 59) have been proposed for efficient extraction of polysaccharides. These deserve further attention in the extraction process of T. aurantialba polysaccharides.
In addition, extraction conditions, including extraction solvent, the ratio of raw material to solvent, time, temperature, etc., all have a great influence on the yield of polysaccharides. In this regard, Huang used a single factor test to improve the extraction rate of T. aurantialba polysaccharides. The optimized conditions were as follows: the ratio of water to mycelium powder was 120: 1 (V/W), 100 °C, ultrasonic power 600 W, extraction for 20 min and extraction twice. Under these conditions, the maximum yield of T. aurantialba polysaccharides was 11.16% (45). Jiang et al. adopted the Box-Benhnken method to investigate the ultrasonic-assisted extraction parameters of T. aurantialba polysaccharides. It was concluded that under the best conditions of ultrasonic power of 518 W, ultrasonic time of 16 min and ultrasonic temperature of 50 °C, the extraction amount of T. aurantialba polysaccharides could reach 2.85 g/L (60).
2.2 Purification
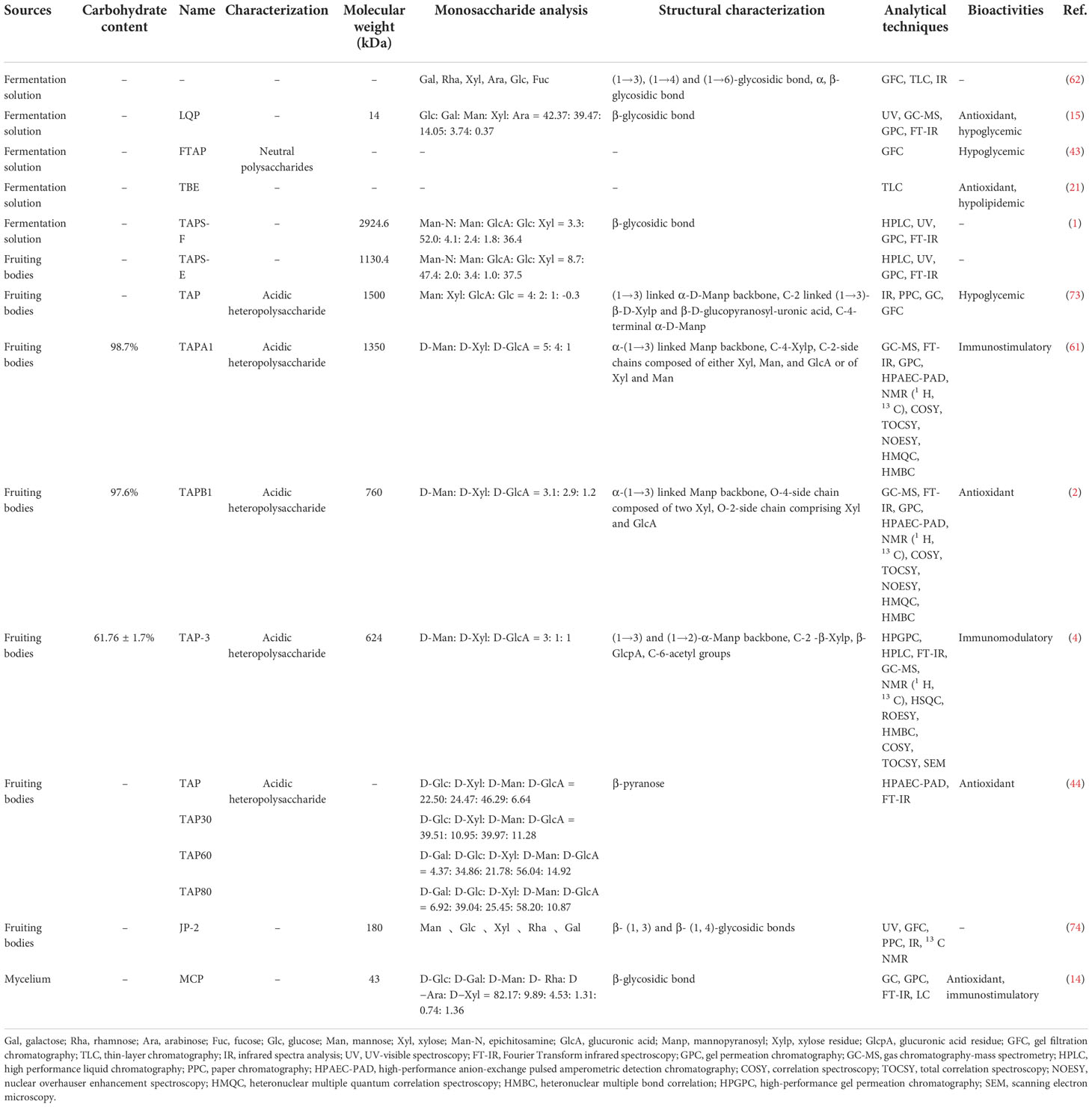
The primary extracted crude polysaccharides usually contain many impurities. In order to fully explore the pharmacological activities of T. aurantialba polysaccharides, the crude polysaccharides extracted initially are usually further collected and purified to obtain pure polysaccharides. At present, the purification methods for T. aurantialba polysaccharides include ethanol fractional precipitation, fiber membrane filtration, gel chromatography, anion exchange column chromatography and gel permeation chromatography. Among them, hydro-alcohol precipitation method is a routine method for isolating and purifying fungus polysaccharides. Besides, some studies have combined the various methods mentioned above to prepare pure polysaccharides from T. aurantialba in recent years. For example, Du et al. used ultrafiltration, anion exchange column chromatography and gel permeation column chromatography to separate and purify the crude polysaccharides from T. aurantialba and finally obtained two acidic heteropolysaccharides, TAPA1 and TAPB1 (2, 61). Deng et al. used microfiltration and gel permeation column chromatography to separate and purify polysaccharides from T. aurantialba fermentation broth (62). The table below gives a summary of the methods for extracting and purifying polysaccharides from T. aurantialba (Table 1). It is worth noting that T. aurantialba also contains high levels of proteins, pigments and other molecules, which may affect the extraction and activity of polysaccharides. Thus, some organic solvents such as petroleum ether (69) and ethanol at different concentrations (2, 61, 64) are commonly used to remove interfering components such as pigment, fat, monosaccharide and disaccharide before extraction. Additionally, savage reagent is often used to remove proteins from extracts prior to characterization to obtain crude polysaccharides (70). Unfortunately, this method is inefficient, complex, and time-consuming, and the use of organic solvents (n-butanol and chloroform) also adversely affects the purity of the synthetic products and the environment (71). Therefore, proteolytic enzymes have been proposed to remove protein components during polysaccharides purification (72).
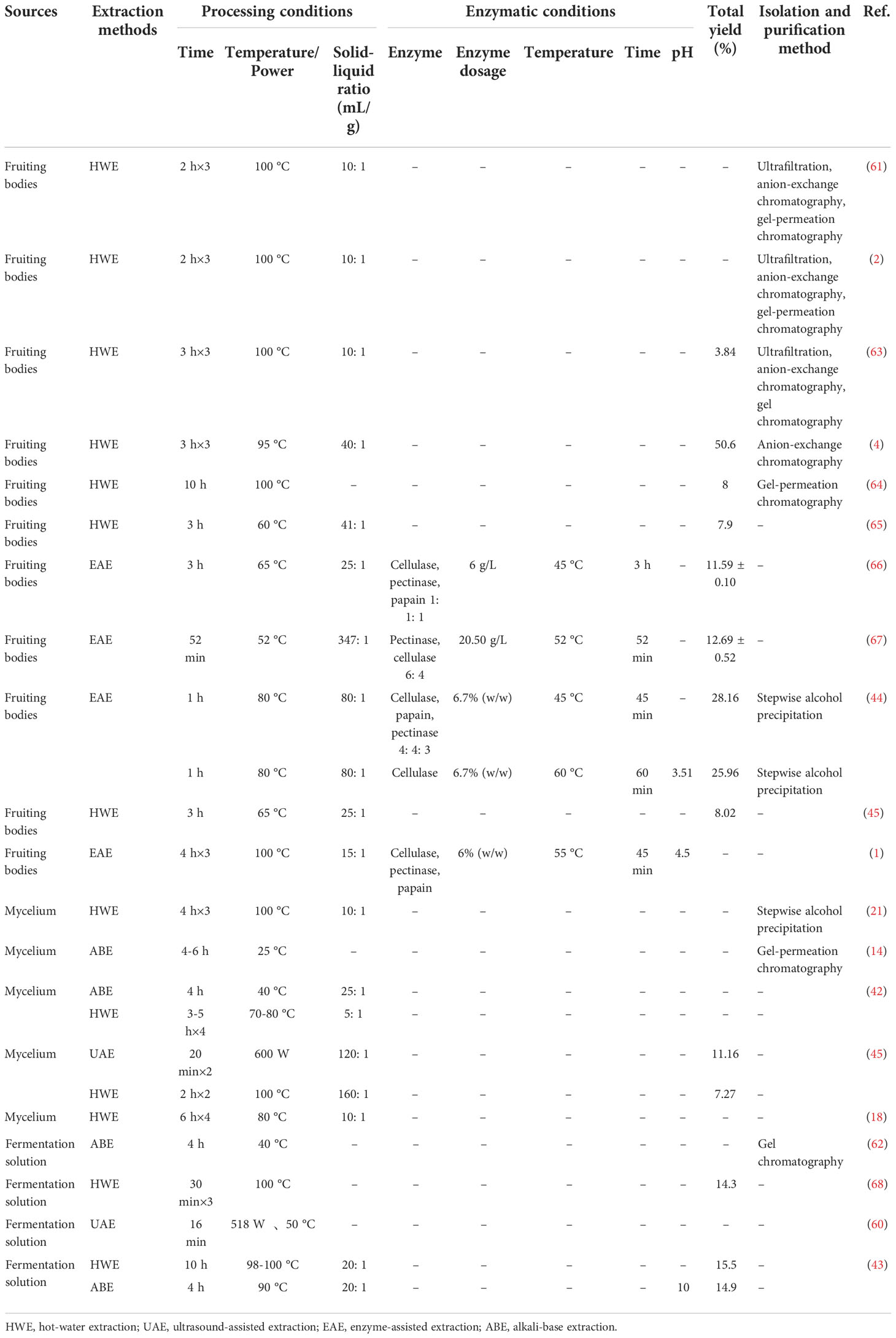
Table 1 A summary of the methods of extraction and purification, conditions and total yield of polysaccharides from T. aurantialba.
3 Physiochemical and structural features
A large number of studies have shown that the biological activities of fungus polysaccharides depend on their complex physiochemical properties. The molecular weight, chain length, type and number of functional groups, type of glycosidic bond and triple helix structure of polysaccharides all have significant effects on their biological activities. Therefore, structural identification of polysaccharides and subsequent structure-activity relationship analysis are very important, but also a challenge. To date, the identification of T. aurantialba polysaccharides has focused on their physicochemical properties (purity, molecular mass and distribution) and structural characterization (composition of monosaccharides and ratios, characteristics of glycosidic bonds). Herein, the reported T. aurantialba polysaccharides in the past few years are listed and integrated information about their source, molecular weight, monosaccharide composition and structures are comprehensively introduced in Table 2. Many technologies including infrared (IR) spectroscopy, ultraviolet (UV)-visible spectroscopy, high-performance anion-exchange pulsed amperometric detection chromatography (HPAEC-PAD), gas chromatography-mass spectrometry (GC-MS), nuclear magnetic resonance (NMR) spectroscopy, high-performance liquid chromatography (HPLC), gas chromatography (GC), etc. have been used for determining the primary structures of T. aurantialba polysaccharides and scanning electron microscopy have been used to analyze the advanced structure of T. aurantialba polysaccharides. It is worth noting that gel permeation chromatography technology (GPC) is widely applied to evaluate the molecular weight of T. aurantialba polysaccharides and GC, PMP pre-column derivatization HPLC or high-performance anion-exchange pulsed amperometric detection chromatography is used for the monosaccharide composition analysis. Since polysaccharides generally do not have UV or fluorescent chromophore groups, derivatization is necessary for structural identification of T. aurantialba polysaccharides by GC or HPLC. The complex structure and derivatization of polysaccharides may lead to the loss of bonds or the emergence of isomers. In the future, it is still necessary to continuously improve the structure identification technology of polysaccharides. Furthermore, although many polysaccharides with different structural characteristics have been obtained from T. aurantialba, only a small amount of structural information has been published. Further research should be carried out in combination of advanced techniques to clarify the primary structure of T. aurantialba polysaccharides.
Diverse T. aurantialba polysaccharides were obtained because of different raw materials, extraction and purification methods. The molecular weight of T. aurantialba polysaccharides obtained shows diverse distribution, ranging from 14.0 kDa to 2924.6 kDa. Most of these polysaccharides are composed of more glucose (Glc), mannose (Man), xylose (Xyl), rhamnose (Rha) and galactose (Gal) with different molar fractions of the individual components. Remarkably, most fruit-body polysaccharides also contain uronic acid, which is considered to be highly correlated with polysaccharides activity. However, it is worth noting that most of the current studies use gas chromatography-mass spectrometry (GC-MS) to detect the uronic acid content in T. aurantialba (2, 4, 61, 73). To our best knowledge, this method is not suitable for the analysis of acidic sugars due to its low sensitivity to uronic acids. Moreover, the pre-column derivatization of PMP is unstable and prone to produce by-products. In fact, some new liquid chromatography-mass spectrometry techniques have been proposed to comprehensively characterize the composition of various monosaccharides such as uronic acid in polysaccharides, although most of these methods require derivatization, but with higher accuracy and sensitivity (75–78). In the future, these new technologies mentioned above can be applied to the study of T. aurantialba polysaccharides, so as to help us better analyze the structure-activity relationship of T. aurantialba polysaccharides and promote the application of T. aurantialba polysaccharides in medical and health care.
4 Biological activities
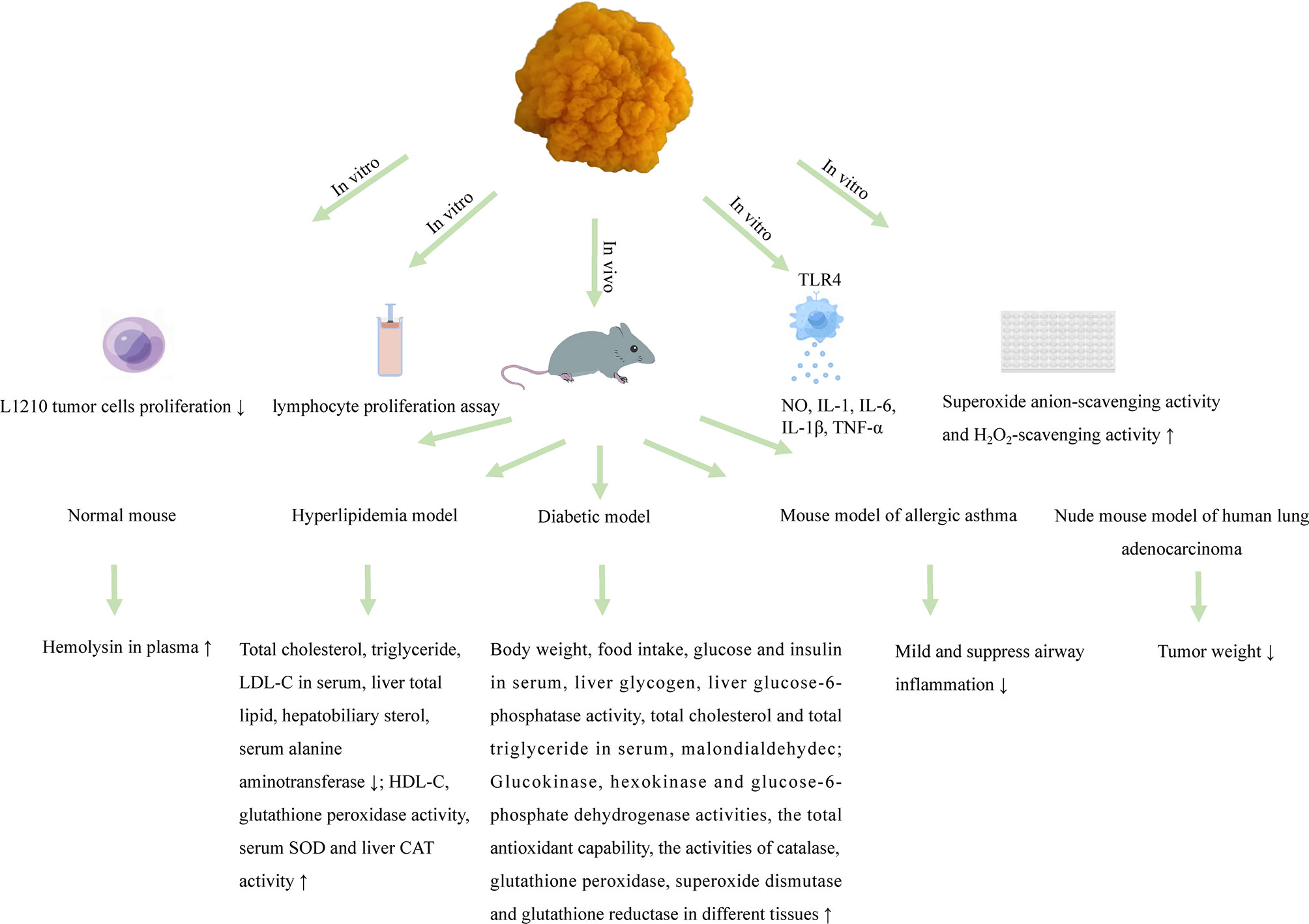
Polysaccharides obtained from T. aurantialba appear to be an all-round talent resisting a variety of chronic inflammatory diseases and protecting against different types of tumors, diabetes and cardiovascular diseases. Below is the summary of all the biological activities reported to date from the polysaccharides from this plant (Figure 2).
4.1 Immunostimulatory activity
The immunomodulatory activity of T. aurantialba polysaccharides is an exciting area that may yield new therapeutic avenues to maintain and improve human health. Macrophages are important members of the mononuclear macrophage system and exhibit various functions in immune response. A glucuronoxylomannan named TAP-3 extracted from the fruiting bodies of T. aurantialba could significantly stimulate the production of nitric oxide (NO), interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α) in RAW 264.7 macrophages, and anti-TLR4 (anti-toll-like receptor 4) treatment of TAP-3 stimulated macrophages could significantly reduce the levels of NO, IL-1β and TNF-α, suggesting that T. aurantialba polysaccharides might exert immunostimulatory activity through toll-like receptor 4 (TLR4) on macrophage surface (4). Du et al. used mouse splenic lymphocyte proliferation experiment to compare the immunostimulatory activity of crude polysaccharides, semi-purified polysaccharides and purified polysaccharides extracted from the fruiting bodies of T. aurantialba, and found that purified polysaccharide TAPA1 had the strongest immunostimulatory activity (64). Polysaccharide MCP extracted from T. aurantialba mycelium also had significant immunostimulatory activity and could promote the proliferation of macrophages, and stimulate macrophages to produce NO, interleukin-1 (IL-1), interleukin-6 (IL-6) and TNF-α (14).
4.2 Antitumor activity
So far, some studies have tried to use T. aurantialba polysaccharides in anti-tumor experiments. Yuan et al. found that oral or injection of T. aurantialba polysaccharides had inhibitory effect on transplanted human lung adenocarcinoma, and the antitumor activity of polysaccharides is stronger when injected (64). Choi confirmed that T. aurantialba polysaccharide, TAP2, had stronger anticancer activity by in vitro and in vivo experiments. Intraperitoneal injection of TAP could effectively inhibit the growth of malignant sarcoma cells (Sacroma 180) in BALB/C mice, with an inhibition rate of 73.3% (79). Lee et al. showed that intraperitoneal injection of crude polysaccharides from T. aurantialba could prolong the life span of mice with malignant sarcoma cell (Sacroma 180) cancer by 11.1% to 66.7%, which was consistent with Choi Pui-Yu’s research results (80). Zhang et al. reported that T. aurantialba polysaccharides could also inhibit the proliferation of L1210 tumor cells in vitro (17). These studies have only tentatively demonstrated the anti-tumor activity of T. aurantialba polysaccharides, and the specific molecular mechanism has not been reported. There is increasing evidence that complex signals from the tumor immune microenvironment have the potential to eliminate or promote malignancy. In addition, tumor immune microenvironment has become a new target for polysaccharides adjuvant therapy. However, whether T. aurantialba polysaccharides can reverse the tumor immune microenvironment by stimulating the immune system or enhancing the immune function of the body is worthy of further investigation.
4.3 Anti-diabetic activity
Long-term hyperglycemia will make the body in a long-term chronic inflammation state, directly leading to low immunity or immune dysregulation. Impaired immune function will in turn aggravate the occurrence and development of diabetes and its complications (81). Kiho et al. found that immune-stimulating agent T. aurantialba polysaccharide, TAP, had hypoglycemic effects on insulin-dependent diabetes mellitus (IDDM) model mice, non-insulin-dependent diabetes mellitus (NIDDM) model mice and glucose-loaded mice (73). In-depth study found that TAP could increase the activities of glucokinase and hexokinase involved in glucose uptake in the liver. At the same time, glucose-6-phosphatase activity, which was responsible for removing glucose from the liver, was decreased, suggesting that the hypoglycemic mechanism of TAP might be related to the accelerated glucose metabolism in the liver of diabetic mice (82). In addition, Kiho et al. also found that both TAP and acid hydrolysate TAP-H could reduce the plasma insulin level in diabetic mice, and the food intake and weight gain of mice fed TAP-H were less than those fed TAP, suggesting that TAP and TAP-H might have the effect of improving insulin resistance, and TAP-H was stronger than TAP. This might be related to the decreased viscosity and molecular weight of TAP-H or the enhanced activity after acid hydrolysis (83). Zhang et al. also reported that TMP, a polysaccharide from T. aurantialba mycelium, could significantly reduce blood glucose, serum triglyceride and total cholesterol levels and enhance insulin sensitivity index in rats with experimental type 、 diabetes mellitus, which might be related to the way that TMP increased insulin sensitivity and regulates lipid metabolism disorder (84). In addition, Deng et al. showed that exopolysaccharide of T. aurantialba also has hypoglycemic effects (15). However, it has not been reported whether T. aurantialba polysaccharides can affect the occurrence and progression of diabetes by intervening the immune-metabolic dialogue.
4.4 Hypolipidemic activity
The hypolipidemic effect of natural polysaccharides has been confirmed by many studies (85, 86), and a few studies have shown that T. aurantialba polysaccharides also have the potential to treat hyperlipidemia. TBE could increase the activities of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase, and reduced the content of malondialdehyde in the tissue of T. aurantialba fermentation broth extract, which indicated that TBE might play a role in correcting abnormal lipid metabolism, reducing oxidative stress and controlling diabetic complications (21). Wang et al. found that TMP, the mycelium polysaccharides of T. aurantialba, significantly increased HDL-C and HDL-C/TC ratio while reducing blood lipids, and effectively reduced LDL-C levels in the process of treatment, indicating that TMP had a certain role in the prevention and treatment of hyperlipidemia (18). Zhang et al. reported that mycelial polysaccharide TMP significantly reduced serum triglyceride and total cholesterol levels in type 、 diabetic rats, indicating that TMP effectively improved the lipid metabolism disorder in model animals and alleviated the toxic effects of hyperlipidemia (84). Kiho et al. showed that TAP and its acidolysis product TAP-H could significantly reduce plasma levels of total cholesterol and triglyceride. However, the potential lipid-lowering mechanism of T. aurantialba polysaccharides was still unclear (83).
4.5 Anti-asthmatic activity
Chinese medicine books mention that T. aurantialba can treat lung fever, phlegm, cold, cough, asthma, etc. (87). Jiang and Xiong showed that polysaccharides extracted from the fruiting bodies of T. aurantialba, TP, could reduce and inhibit airway inflammation in asthmatic rats, and had a significant protective effect on the delayed phase of allergic asthma model in rats (88). Besides, the significant protective effect of T. aurantialba polysaccharides on the rapid onset of allergic asthma was also found in the guinea pig allergic asthma model (89).
4.6 Hepatoprotective activity
After feeding polysaccharides extracted from the fruiting bodies or mycelium of T. aurantialba to rats with excess liver lipids for 8 weeks, total liver lipids and hepatobiliary sterols decreased by 55% and 50%, respectively. Besides, it can significantly reduce the serum alanine aminotransferase, protect and restore the acute and chronic liver poisoning caused by carbon tetrachloride, which indicated that T. aurantialba had a good effect on lowering lipids and protecting liver (90).
5 Structure-activity relationship analysis
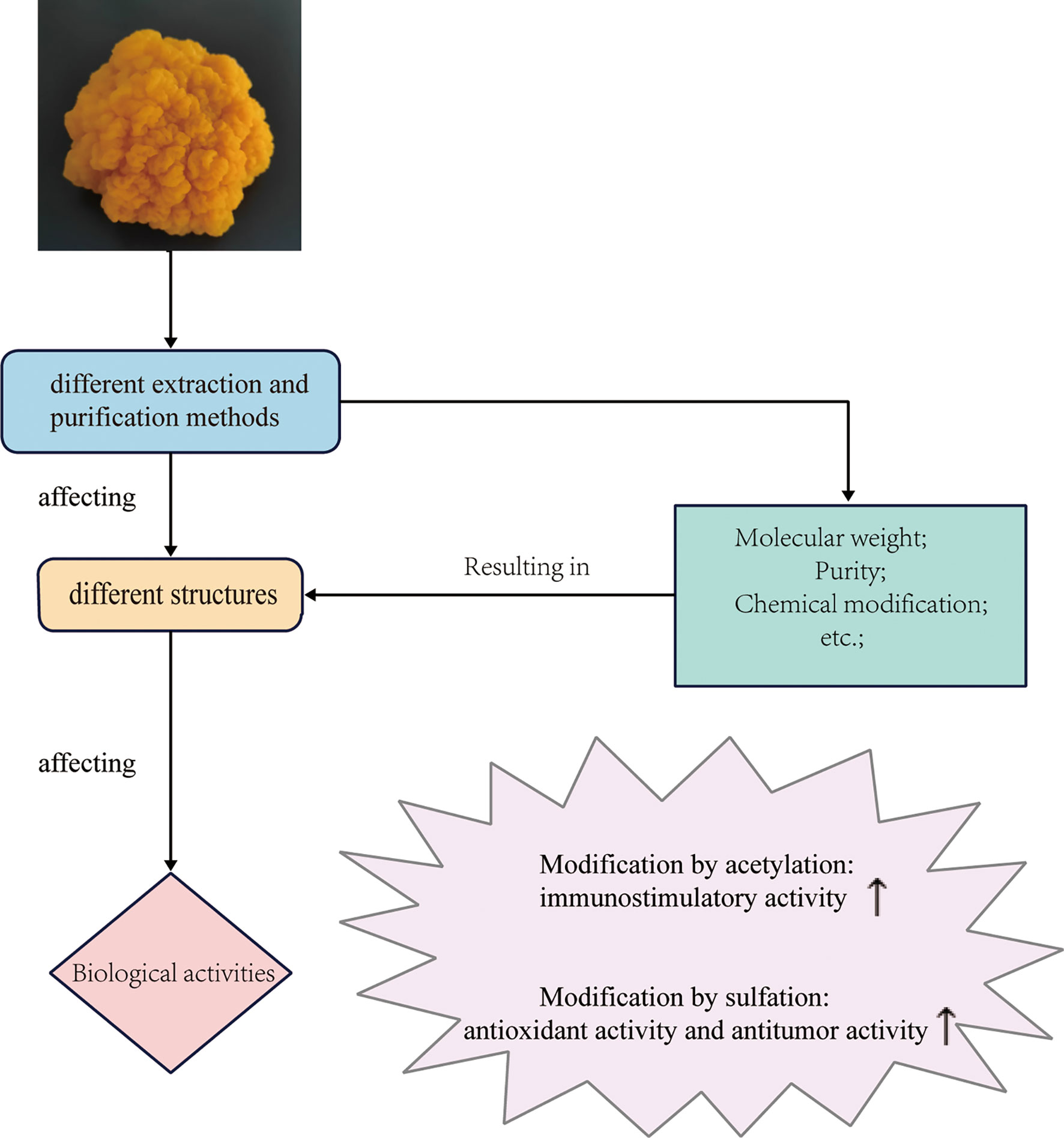
It is generally believed that polysaccharides with different biological activities should have different structures (91). However, the studies on the structure-activity relationships of polysaccharides are limited due to their low purity and high heterogeneity. At present, only a few studies on the structure-activity relationship of T. aurantialba polysaccharides have been reported and some relationships can be inferred as follows (Figure 3). Yuan et al. used the purified polysaccharide TAP-3 from the fruiting bodies of T. aurantialba and its depolymerized small molecule fragments DTAP-3a and DTAP-3b to stimulate macrophages and found that the polysaccharide fragment DTAP-3b with the lowest molecular weight stimulated macrophages to produce the lowest levels of NO, IL-1β and TNF-α. This suggested that the immunostimulatory activity of polysaccharide TAP-3 was related to its molecular weight (4). Purified polysaccharide MCP from T. aurantialba mycelium had stronger immunostimulatory and antioxidant activities than crude polysaccharide CMCP before purification, which indicated that polysaccharide MCP activity might be related to purity (14). Zhang et al. reported that both TBE, the extract of T. aurantialba fermentation broth, and TMP, T. aurantialba mycelium extract, could regulate lipid metabolism and reduce oxidative stress, but the activity of TBE was significantly stronger than that of TMP, which might be related to the saponins in TBE (21). Zhao et al. demonstrated that the free radical scavenging activity of purified polysaccharide TAP was related to the pressure crushing time of T. aurantialba powder. The longer the crushing time, the more hydroxyl number of polysaccharides exposed, the stronger the DPPH and ·OH free radical scavenging activity (92). Chemical modification of polysaccharides such as acetylation and sulfation are an effective way to enhance the activity of natural polysaccharides (93–95). Du et al. compared in vitro immunostimulating activity of acid polysaccharide TAPA1 extracted from the fruiting bodies of T. aurantialba and its acetylated derivative TAPA1-ac and deacetylated derivative TAPA1-deac using the mouse spleen lymphocytes (MSLs) proliferation assay and nitric oxide (NO) production assay. The results showed that the stimulatory potency of MSLs of TAPA1-ac was higher than that of TAPA1, while the stimulatory potency of MSLs of TAPA1-deac was significantly lower than that of TAPA1 (96). Besides, Du et al. also found that TAPA1-s, a sulfated derivative of TAPA1, had more obvious immunostimulating activity than TAPA1 in the mouse spleen lymphocyte proliferation assay, indicating that sulfonation was an effective way to enhance the immunostimulating activity of polysaccharides (64). Zhang et al. compared the anti-tumor activity and antioxidant activity of T. aurantialba polysaccharide sulfated with different derivatization reagents, and the results showed that with the increase of the sulfate group substitution degree of T. aurantialba, its antioxidant activity and the activity of inhibiting tumor cell proliferation in vitro were enhanced (17). These studies indicated that the immunostimulatory activity and antitumor activity of T. aurantialba polysaccharides were affected by the types of functional groups and the degree of side chain branching. Further chemical modifications should be investigated to explore the effects of chemical modifications on other biological activities of T. aurantialba polysaccharides. Understanding the clear structure-activity relationship is the premise for the industrial production of active polysaccharides from T. aurantialba and the development of safe and effective new drugs. In the future, different extraction, separation and purification methods should be used to obtain more polysaccharides with different structures, and the application of various derivatization methods should be strengthened to carry out more studies on the relationship between structure and activity.
6 Application
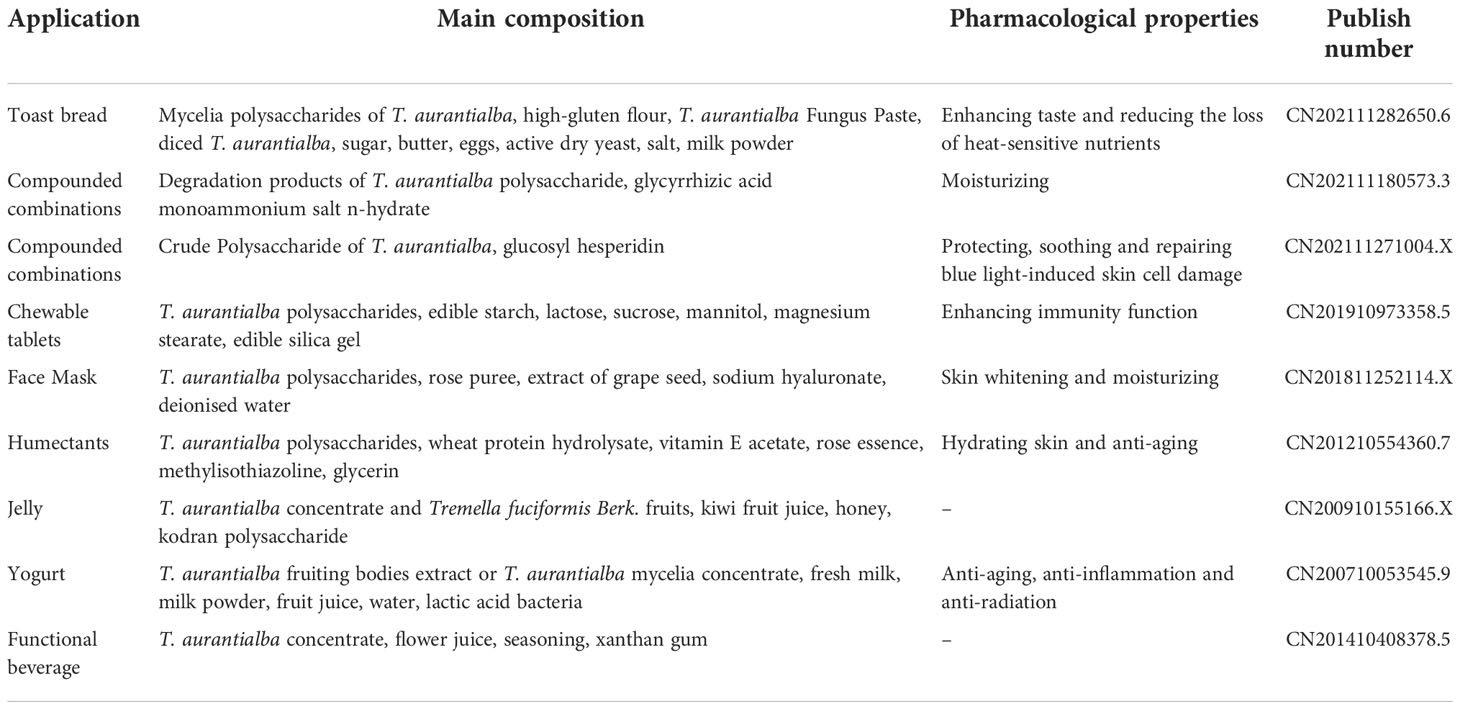
Due to its diverse biological activity and special taste, T. aurantialba polysaccharides has been widely used in medicine, food, skin care and other industries. T. aurantialba glycopeptide capsules made from T. aurantialba polysaccharides have shown clear hepatoprotective effects in preliminary clinical trials and phase II clinical trials, without side effects, and can assist the treatment of liver diseases (97, 98). Pharmacological studies have showed that T. aurantialba glycopeptide capsules may mainly prevent hepatitis and other diseases by protecting hepatocytes, regulating or stabilizing cell functional activities, and improving immunity (99). Polysaccharides from T. aurantialba also can be used as food additives in bread production. For example, when 1% to 3% T. aurantialba crude polysaccharides is added to the flour, the colloid property of the dough can be greatly improved, and the bread taste is soft and delicate, with regular large holes and uniform organization (100). The nutritional liquid made from T. aurantialba polysaccharides is a kind of health drink with novel taste and bright color, which not only has good immune-promoting effect, but also has no toxic and side effects (101). The drink made from the fermentation broth of Tremella aurantialba is also a health drink with unique taste and immune and anti-inflammatory effects (102). In addition, T. aurantialba polysaccharides have lubricity, film-forming property, moisturizing property and anti-radiation activity (103, 104), thus it has been used to make facial masks, facial masks, moisturizers and other high-end skin care and beauty cosmetics (104–106). Patents list of products containing T. aurantialba polysaccharides is shown in Table 3. Figure 4 shows the methods of extraction, purification, biological activities and applications of the polysaccharides obtained from T. aurantialba.
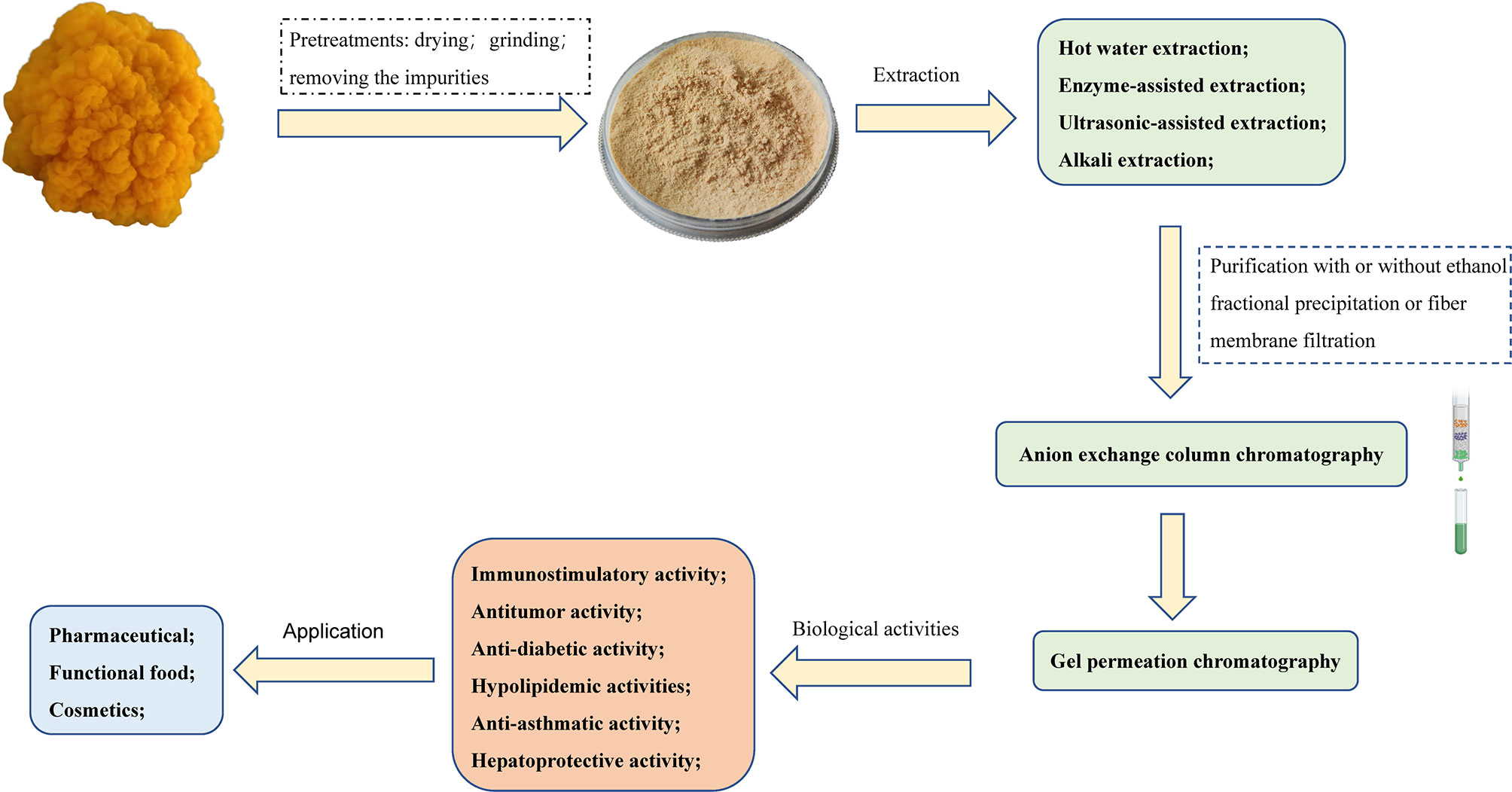
Figure 4 Schematic representation of the extraction, purification, biological activities and applications of T. aurantialba polysaccharides.
7 Conclusion and perspectives
According to the traditional Chinese medicine and modern pharmacological research, T. aurantialba has high medicinal value and nutritional value. Polysaccharide is one of the major active components of T. aurantialba, which is abundant in T. aurantialba, and has significant immune-stimulating activity and the ability to resist various inflammatory diseases. In recent years, the isolation methods, structural properties, biological activities and applications of T. aurantialba polysaccharides have been widely reported. However, much remains to be explored in the field of T. aurantialba polysaccharides. Firstly, efficient enzyme extraction and ultrasound-assisted extraction methods have been proposed by a few studies for the extraction of T. aurantialba polysaccharides, but there is a lack of comprehensive and detailed comparison with hot water extraction. New separation techniques such as microwave-assisted extraction, high pressure pulse extraction, supercritical fluid extraction can be further used in the extraction process of T. aurantialba polysaccharides, thereby improving the yield and activity of polysaccharides. Second, the structure of polysaccharides is affected by the conditions of separation and purification, the source of samples and other factors. At present, there are few studies on the structure of T. aurantialba polysaccharides, especially the advanced structure. Future research should be combined with advanced and convenient structure characterization technology to deeply explore the structural information of T. aurantialba polysaccharides. Third, as the main active component of T. aurantialba, the bioactivities of T. aurantialba polysaccharides is not fully explore, especially the specific molecular mechanism such as immunostimulating activity is rarely reported. Various in vitro and in vivo experiments and clinical trials should be supplemented to improve the research results. Fourth, there are few studies on structure-activity relationship of T. aurantialba polysaccharides and structural modification techniques such as hydroxymethylation, selenylation and phosphorylation can be further applied to the activity study of T. aurantialba polysaccharides. In the future, it is hoped that the research on T. aurantialba polysaccharides can be more comprehensive and thorough, and the standardized preparation technology of T. aurantialba polysaccharides will be established as soon as possible, so as to realize the industrial production and application of T. aurantialba polysaccharides.
Author contributions
YY: Ideals, Conceptualization, Writing-original draft, Supervision. MW: Investigation, Data curation and Writing-original draft. NC: Literature collection and Writing-original draft. XW, CF, YL and XG: Literature collection. PL: Conceptualization, Funding acquisition. YZ: Resources, Investigation, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Key Research Project of China (2022YFF1100300), Major Public Welfare Projects in Henan Province (201300110200), National Key Research Project of Hebei Province (20375502D).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun T, Wang R, Sun DF, Li S, Xu H, Qiu YB, et al. High-efficiency production of Tremella aurantialba polysaccharide through basidiospore fermentation. Bioresour Technol (2020) 318:124268. doi: 10.1016/j.biortech.2020.124268
2. Du Xj, Zhang Y, Mu HM, Lv ZW, Yang Y, Zhang JS. Structural elucidation and antioxidant activity of a novel polysaccharide (Tapb1) from. Tremella Aurantialba. Food Hydrocolloids (2015) 43:459–64. doi: 10.1016/j.foodhyd.2014.07.004
3. Yang LL, Li RC, Cao Y, Li MJ, Lou XY, Yang XJ, et al. Research on the scientific name and taxonomic status of Tremella aurantialba. Edible medicinal mushrooms (2020) 28(04):252–255+276. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=7102495382.
4. Yuan QX, Zhang XD, Ma MY, Long T, Xiao CL, Zhang J, et al. Immunoenhancing glucuronoxylomannan from Tremella aurantialba bandoni et zang and its low-Molecular-Weight fractions by radical depolymerization: Properties, structures and effects on macrophages. Carbohydr Polymers (2020) 238:116184. doi: 10.1016/j.carbpol.2020.116184
5. Dai CX, Huang XY, Lv RQ, Zhang ZC, Sun J, Aheto JH. Analysis of volatile compounds of Tremella aurantialba fermentation Via electronic nose and HS-SPME-GC-MS. J Food Saf (2018) 38(6):e12555. doi: 10.1111/jfs.12555
6. Niu SK. Studies on the anticoagulation active fraction of tremella aurantialba mycelium. Shanxi university (2007). [Master]. Available at: https://cdmd.cnki.com.cn/Article/CDMD-10269-2007083152.htm.
7. Liu CH, Xie H, Su BN, Han JX, Liu YP. Anti-thrombus effect on the fermented products of mycelium from Tremella aurantialba. Natural Product Res Dev (2003) 04):289–92. doi: 10.16333/j.1001-6880.2003.04.003
8. Yin ZH, Liang ZH, Li CQ, Wang JM, Ma CY, Kang WY. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci Hum Well (2021) 10(4):393–400. doi: 10.1016/j.fshw.2021.04.001
9. Fang DL, Wang D, Ma GX, Ji Y, Zheng HH, Chen H, et al. Auricularia polytricha noodles prevent hyperlipemia and modulate gut microbiota in high-fat diet fed mice. Food Sci Hum Well (2021) 10(4):431–41. doi: 10.1016/j.fshw.2021.04.005
10. Zhang YR, Wang DW, Chen YT, Liu TT, Zhang SS, Fan HX, et al. Healthy function and high valued utilization of edible fungi. Food Sci Hum Well (2021) 10(4):408–20. doi: 10.1016/j.fshw.2021.04.003
11. Zhao SZ, Lei M, Xu H, He H, Suvorov A, Wang J, et al. The normal cell proliferation and wound healing effect of polysaccharides from Ganoderma amboinense. Food Sci Hum Well (2021) 10(4):508–13. doi: 10.1016/j.fshw.2021.04.013
12. Yin ZH, Sun-Waterhouse DX, Wang JM, Ma CY, Waterhouse GIN, Kang WY. Polysaccharides from edible fungi pleurotus spp.: Advances and perspectives. J Future Foods (2021) 1(2):128–40. doi: 10.1016/j.jfutfo.2022.01.002
13. Zhou S, Tang QJ, Zhang Z, Li CH, Cao H, Yang Y, et al. Nutritional composition of three domesticated culinary-medicinal mushrooms: Oudemansiella sudmusida, Lentinus squarrosulus, and Tremella aurantialba. Int J Medicinal Mushrooms (2015) 17(1):43–9. doi: 10.1615/intjmedmushrooms.v17.i1.50
14. Deng C, Sun YY, Fu HT, Zhang SX, Chen JH, Xu X. Antioxidant and immunostimulatory activities of polysaccharides extracted from Tremella aurantialba mycelia. Mol Med Rep (2016) 14(5):4857–64. doi: 10.3892/mmr.2016.5794
15. Deng C, Fu HT, Shang JY, Qiao ZQ, Zhang C, Chen JH. Preparation, chemical analysis and biological activity of polysaccharides from fermented Tremella aurantialba. J Food Sci Biotechnol (2017) 36(01):67–73. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=671990370.
16. Du XJ, Wang XD, Chen Y, Tian SY, Lu SF. Antioxidant activity and oxidative injury rehabilitation of chemically modified polysaccharide (TAPA1) from Tremella aurantialba. Macromol Res (2018) 26(6):479–83. doi: 10.1007/s13233-018-6078-0
17. Zhang Z, Liu YF, Zhou S, Tang QJ, Yang Y, Feng N, et al. Sulphated modification of the polysaccharides of Tremella aurantialba fruit bodies. Acta Edulis Fungi (2018) 25(01):67–73. doi: 10.16488/j.cnki.1005-9873.2018.01.011
18. Wang H, Qu WJ, Chu SD, Li MJ, Tian CP. Studies on the preventive and therapeutic effects of the polysaccharide of Tremella aurantiabla mycelia on diet-induced hyperlipidemia in mice. Acta Nutrimenta Sin (2002) 53(8):431–0. doi: 10.1055/s-0035-1559491
19. Chen NN, Zhang H, Zong X, Li SY, Wang JJ, Wang YZ, et al. Polysaccharides from Auricularia auricula: Preparation, structural features and biological activities. Carbohyd Polym (2020) 247:116750. doi: 10.1016/j.carbpol.2020.116750
20. Wang ZQ, Zhu CX, Dai AR, Chen L, You CP, Zhang BB. Chemical characterization and antioxidant properties of cell wall polysaccharides from Antrodia cinnamomea mycelia. Food Biosci (2021) 41:100932. doi: 10.1016/j.fbio.2021.100932
21. Zhang ZC, Lian B, Huang DM, Cui FJ. Compare activities on regulating lipid-metabolism and reducing oxidative stress of diabetic rats of Tremella aurantialba broth's extract (TBE) with its mycelia polysaccharides (TMP). J Food Sci (2009) 74(1):H15–21. doi: 10.1111/j.1750-3841.2008.00989.x
22. Sun YJ, Wang F, Liu Y, An YY, Chang DW, Wang JK, et al. Comparison of water- and alkali-extracted polysaccharides from fuzhuan brick tea and their immunomodulatory effects in vitro and in vivo. Food Funct (2022) 13(2):806–24. doi: 10.1039/d1fo02944d
23. Teng C, Qin PY, Shi ZX, Zhang WY, Yang XS, Yao Y, et al. Structural characterization and antioxidant activity of alkali-extracted polysaccharides from quinoa. Food Hydrocolloids (2021) 113:106392. doi: 10.1016/j.foodhyd.2020.106392
24. Zhang NN, Ma H, Zhang ZF, Zhang WN, Chen L, Pan WJ, et al. Characterization and immunomodulatory effect of an alkali-extracted galactomannan from Morchella esculenta. Carbohydr Polymers (2022) 278:118960. doi: 10.1016/j.carbpol.2021.118960
25. Chang XN, Chen XF, Gong P, Yang WJ, Wang L, Liu N, et al. Anti-oxidant and anti-fatigue properties of Apple pomace polysaccharides by acid or alkali extraction. Int J Food Sci Technol (2022) 57(1):78–91. doi: 10.1111/ijfs.15200
26. Gao S, Yan S, Zhou Y, Feng Y, Xie XY, Guo W, et al. Optimisation of enzyme-assisted extraction of Erythronium sibiricum bulb polysaccharide and its effects on immunomodulation. Glycoconjugate J (2022) 39(3):357–68. doi: 10.1007/s10719-021-10038-4
27. Olawuyi IF, Kim SR, Hahn D, Lee WY. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of Okra polysaccharides. Food Hydrocolloids (2020) 100:105396. doi: 10.1016/j.foodhyd.2019.105396
28. Ji YB, Wang FL. Optimization of trypsin extraction technology of Allium cepa l. polysaccharide by response surface methodology and the antitumor effects through immunomodulation. Bioengineered (2021) 12(1):382–91. doi: 10.1080/21655979.2020.1870320
29. Chen X, Zhang HB, Du WQ, Qian LY, Xu Y, Huang YG, et al. Comparison of different extraction methods for polysaccharides from Crataegus pinnatifida bunge. Int J Biol Macromol (2020) 150:1011–9. doi: 10.1016/j.ijbiomac.2019.11.056
30. Hu J, Liu Y, Cheng L, Shi RJ, Qayum A, Bilawal A, et al. Comparison in bioactivity and characteristics of Ginkgo biloba seed polysaccharides from four extract pathways. Int J Biol Macromol (2020) 159:1156–64. doi: 10.1016/j.ijbiomac.2020.05.129
31. Zhang Y, Liu PH, Wang CY, Zhang FM, Linhardt RJ, Eliezer D, et al. Homogalacturonan from squash: Characterization and tau-binding pattern of a sulfated derivative. Carbohydr Polymers (2022) 285:119250. doi: 10.1016/j.carbpol.2022.119250
32. Deng QF, Wang X, Chen H, Zhao CJ, Gong X, Zhou X. Structural characterization, modification and hepatoprotective effects of polysaccharide from Mori fructus. Int J Biol Macromol (2020) 153:357–63. doi: 10.1016/j.ijbiomac.2020.02.300
33. Rizkyana AD, Ho TC, Roy VC, Park JS, Kiddane AT, Kim G-D, et al. Sulfation and characterization of polysaccharides from oyster mushroom (Pleurotus ostreatus) extracted using subcritical water. J Supercritical Fluids (2022) 179:105412. doi: 10.1016/j.supflu.2021.105412
34. Zhang JJ, Li ZG, Zhou LN, Bao JH, Xu J. The modifications of a fructan from Anemarrhena asphodeloides bunge and their antioxidant activities. Int J Biol Macromol (2020) 164:4435–43. doi: 10.1016/j.ijbiomac.2020.09.024
35. Ren YY, Sun PP, Ji YP, Wang XT, Dai SH, Zhu ZY. Carboxymethylation and acetylation of the polysaccharide from Cordyceps militaris and their alpha-glucosidase inhibitory activities. Natural Product Res (2020) 34(3):369–77. doi: 10.1080/14786419.2018.1533830
36. Huang Z, Zong MH, Lou WY. Effect of acetylation modification on the emulsifying and antioxidant properties of polysaccharide from Millettia speciosa champ. Food Hydrocolloids (2022) 124:107217. doi: 10.1016/j.foodhyd.2021.107217
37. Liu M, Lu W, Ku KM, Zhang LH, Lei LL, Zong W. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides from Eucommia ulmoides leaf. Pakistan J Pharm Sci (2020) 33(2):581–8. doi: 10.36721/pjps.2020.33.2.Reg.581-588.1
38. Surin S, You SG, Seesuriyachan P, Muangrat R, Wangtueai S, Jambrak AR, et al. Optimization of ultrasonic-assisted extraction of polysaccharides from purple glutinous rice bran (Oryza sativa l.) and their antioxidant activities. Sci Rep (2020) 10(1):10410. doi: 10.1038/s41598-020-67266-1
39. Zhao HK, Wei XY, Xie YM. Optimization of extraction technology, structure, and antioxidant activity of polysaccharide from grifola frondosa. Starch-Starke (2021) 73:9–10. doi: 10.1002/star.202000200
40. Meng HH, Wu JJ, Shen L, Chen GW, Jin L, Yan MX, et al. Microwave assisted extraction, characterization of a polysaccharide from Salvia miltiorrhiza bunge and its antioxidant effects Via ferroptosis-mediated activation of the Nrf2/HO-1 pathway. Int J Biol Macromol (2022) 215:398–412. doi: 10.1016/j.ijbiomac.2022.06.064
41. Liu H, Fan H, Zhang J, Zhang S, Zhao W, Liu T, et al. Isolation, purification, structural characteristic and antioxidative property of polysaccharides from a. cepa l. var. agrogatum don. Food Sci Hum Well (2020) 9(1):71–9. doi: 10.1016/j.fshw.2019.12.006
42. Dong CH. Study on the liquid-submerged fermentation and polysaccharide extraction of Tremella aurantialba mycelia. J Anhui Agri Sci (2010) 38(01):342–4. doi: 10.13989/j.cnki.0517-6611.2010.01.101
43. Liu CH, Yu JG. Isolation and purification of polysaccharides from fermented products of Tremella aurantialba and hypoglycemic effect. Chin Agric Sci Bull (2009) 25(05):63–7. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=29716813.
44. Wang XD. Study on extraction, isolation and purification of polysaccharide from tremella aurantialba and its antioxidant activity. Liaocheng University (2018). Available at: https://cdmd.cnki.com.cn/Article/CDMD-10447-1018824384.htm.
45. Huang LR, Li YL, Duan YQ, Zhang Y, Ma HL. Ultrasound-assisted extraction of polysaccharides from Tremella aurantialba and its kinetic model. Chem Industry For Products (2011) 31(02):7–11. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=37607293.
46. Wang WX, Liu JJ. Efficient extraction, antioxidant activities and anti-inflammation of polysaccharides from Notopterygium franchetii boiss. Carbohydr Polymers (2020) 248:116783. doi: 10.1016/j.carbpol.2020.116783
47. Shen SW, Zhou C, Zeng YB, Zhang HT, Hossen MA, Dai JW, et al. Structures, physicochemical and bioactive properties of polysaccharides extracted from Panax notoginseng using ultrasonic/ microwave-assisted extraction. LWT (2022) 154:112446. doi: 10.1016/j.lwt.2021.112446
48. Garcia-Vaquero M, Ummat V, Tiwari B, Rajauria G. Exploring ultrasound, microwave and ultrasound-microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar Drugs (2020) 18(3):172. doi: 10.3390/md18030172
49. Dobrincic A, Pedisic S, Zoric Z, Jurin M, Roje M, Coz-Rakovac R, et al. Microwave assisted extraction and pressurized liquid extraction of sulfated polysaccharides from Fucus virsoides and Cystoseira barbata. Foods (2021) 10(7):1481. doi: 10.3390/foods10071481
50. Truc Cong H, Kiddane AT, Sivagnanam SP, Park JS, Cho YJ, Getachew AT, et al. Green extraction of polyphenolic-polysaccharide conjugates from Pseuderanthemum palatiferum (Nees) radlk.: Chemical profile and anticoagulant activity. Int J Biol Macromol (2020) 157:484–93. doi: 10.1016/j.ijbiomac.2020.04.113
51. Luft L, Confortin TC, Todero I, Chaves Neto JR, Tres MV, Zabot GL, et al. Extraction and characterization of polysaccharide-enriched fractions from Phoma dimorpha mycelial biomass. Bioproc Biosyst Eng (2021) 44(4):769–83. doi: 10.1007/s00449-020-02486-3
52. Hao W, Wang SF, Zhao J, Li SP. Effects of extraction methods on immunology activity and chemical profiles of Lycium barbarum polysaccharides. J Pharm Biomed Anal (2020) 185:113219. doi: 10.1016/j.jpba.2020.113219
53. Getachew AT, Cho YJ, Chun BS. Effect of pretreatments on isolation of bioactive polysaccharides from spent coffee grounds using subcritical water. Int J Biol Macromol (2018) 109:711–9. doi: 10.1016/j.ijbiomac.2017.12.120
54. Zhang JX, Wen CT, Qin W, Qin PY, Zhang HH, Duan YQ. Ultrasonic-enhanced subcritical water extraction of polysaccharides by two steps and its characterization from Lentinus edodes. Int J Biol Macromol (2018) 118:2269–77. doi: 10.1016/j.ijbiomac.2018.07.098
55. Chen HM, Fu X, Luo ZG. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem (2015) 168:302–10. doi: 10.1016/j.foodchem.2014.07.078
56. Yang RF, Zhao C, Chen X, Chan SW, Wu JY. Chemical properties and bioactivities of goji (Lycium barbarum) polysaccharides extracted by different methods. J Funct Foods (2015) 17:903–9. doi: 10.1016/j.jff.2015.06.045
57. Yu IL, Yu ZR, Koo M, Wang BJ. A continuous fractionation of ginsenosides and polysaccharides from Panax ginseng using supercritical carbon dioxide technology. J Food Process Preservat (2016) 40(4):743–8. doi: 10.1111/jfpp.12655
58. Yang N, Zhang NN, Jin YM, Jin ZY, Xu XM. Development of a fluidic system for efficient extraction of mulberry leaves polysaccharide using induced electric fields. Sep Purif Technol (2017) 172:318–25. doi: 10.1016/j.seppur.2016.08.025
59. Liu C, Sun YH, Mao Q, Guo XL, Li P, Liu Y, et al. Characteristics and antitumor activity of morchella esculenta polysaccharide extracted by pulsed electric field. Int J Mol Sci (2016) 17(6):986. doi: 10.3390/ijms17060986
60. Jiang Y, Zheng JH, Liu GJ, Xue J, Ji HG, Zhang L. Response surface optimization of ultrasonic-assisted extraction of Tremella aurantialba polysaccharides. Edible Fungi (2019) 41(06):60–4. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=7100245872.
61. Du XJ, Zhang JS, Yang Y, Ye L, Tang QJ, Jia W, et al. Structural elucidation and immuno-stimulating activity of an acidic heteropolysaccharide (TAPA1) from Tremella aurantialba. Carbohydr Res (2009) 344(5):672–8. doi: 10.1016/j.carres.2009.01.021
62. Deng YX, Qu WJ, Cao QH, Niu W, Pan YF, Gu Y, et al. Structural analysis of the extracellular polysaccharides of Tremella aurantialba. Chin Traditional Herbal Drugs (2005) 04):497–8. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=15384903.
63. Du XJ, Zhang JS, Yang Y, Tang QJ, Jia W, Pan YJ. Purification, chemical modification and immunostimulating activity of polysaccharides from Tremella aurantialba fruit bodies. J Zhejiang Univ Sci B (2010) 11(6):437–42. doi: 10.1631/jzus
64. Yuan XL, Zhang PD, Chen SG. Preliminary study on chemical properties of a soluble polysaccharide from the mushroom Tremella aurantia, and its oncosuppressive activity. J Fudan Univ (Natural Science) (1996) 06):703–6. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=2360463.
65. Zhang JC, Qin LY, Liu Y, Jia FC, Liu ZD, Xue B. Study on optimization of polysaccharide extraction from Tremella aurantialba in Tibet by response surface methodology. Food Res Dev (2020) 41(11):113–7. doi: 10.12161/j.issn.1005-6521.2020.11.019
66. Zha L, Liu ZD, Wang B, Xue B. Study on the extraction of crude polysaccharide from Tremella aurantialba by complex enzymatic method. Light Industry Sci Technol (2018) 34(01):23–5. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=71888171504849564849484949.
67. You JK, Zhu Y, Gao GS, Wang TT, Wu SR. Optimization of aqueous enzymatic extraction of polysaccharide from Tremella aurantialba. Sci Technol Food Industry (2019) 40(04):189–94. doi: 10.13386/j.issn1002-0306.2019.04.031
68. Cai HM, Zhao YC, Li SH, Zhong MH. Study on the polysaccharide production from yeast- like conidia of Tremella aurantiabla. Edible Fungi China (2008) 03):39–40. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=27197436.
69. Li YW. Studies on chemical composition and antioxidant activity in vitro from tremella aurantialba fruiting bodies. Jilin Agricultural University (2016). [Master]. Available at: https://cdmd.cnki.com.cn/Article/CDMD-10193-1016732613.htm.
70. Xiang H, Sun-Waterhouse D, Cui C. Hypoglycemic polysaccharides from Auricularia auricula and Auricularia polytricha inhibit oxidative stress, NF-κB signaling and proinflammatory cytokine production in streptozotocin-induced diabetic mice. Food Sci Hum Well (2021) 10(1):87–93. doi: 10.1016/j.fshw.2020.06.001
71. Zhao J, Deng Y, Li SP. Advanced analysis of polysaccharides, novel functional components in food and medicine dual purposes Chinese herbs. Trac-Trend Anal Chem (2017) 96:138–50. doi: 10.1016/j.trac.2017.06.006
72. Wu JN, Chen XT, Qiao K, Su YC, Liu ZY. Purification, structural elucidation, and in vitro antitumor effects of novel polysaccharides from Bangia fuscopurpurea. Food Sci Hum Well (2021) 10(1):63–71. doi: 10.1016/j.fshw.2020.05.003
73. Kiho T, Morimoto H, Sakushima M, Usui S, Ukai S. Polysaccharides in fungi. XXXV. Anti-diabetic activity of an acidic polysaccharide from the fruiting bodies of Tremella aurantia. Biol Pharm Bull (1995) 18(12):1627–9. doi: 10.1248/bpb.18.1627
74. Li WQ, He GQ, Li ZA. Study on separation and chemical structure of water-soluble polysaccharide JP-2 in Tremella aurantialba. J Chin Inst Food Sci Techol (2003) 03):13–7. doi: 10.16429/j.1009-7848.2003.03.004
75. Li Y, Liang J, Shen Y, Kuang HX, Xia YG. A new application of acetylation for analysis of acidic heteropolysaccharides by liquid chromatography-electrospray mass spectrometry. Carbohydr Polym (2020) 245:116439. doi: 10.1016/j.carbpol.2020.116439
76. Jiang Q, Wang Y, Li HL, Chen DDY. Combining online size exclusion chromatography and electrospray ionization mass spectrometry to characterize plant polysaccharides. Carbohydr Polym (2020) 246:116591. doi: 10.1016/j.carbpol.2020.116591
77. Li Y, Liang J, Gao JN, Shen Y, Kuang HX, Xia YG. A novel LC-MS/MS method for complete composition analysis of polysaccharides by aldononitrile acetate and multiple reaction monitoring. Carbohydr Polym (2021) 272:118478. doi: 10.1016/j.carbpol.2021.118478
78. Wang TL, Li YC, Lin CS, Zou YP. Comprehensive analysis of natural polysaccharides from TCMS: A generic approach based on UPLC-MS/MS. Carbohydr Polym (2022) 277:118877. doi: 10.1016/j.carbpol.2021.118877
79. Pui-yu C. Antitumor Activities of Tremella Aurantialba Polysaccharides [Master]: Chinese University of Hong Kong Graduate School (2001). Available at: https://repository.lib.cuhk.edu.hk/en/item/cuhk-324069.
80. Lee GW, Hur H, Shim MJ, Lee TS. Antitumor and immune-modulatory effect of drude polysaccharides from fruiting body of Tremella aurantialba against mouse sarcoma 180. Kor J Mycol (2008) 36(1):66–74. doi: 10.4489/KJM.2008.36.1.066
81. Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, et al. Inflammation and the incidence of type 2 diabetes the multi-ethnic study of atherosclerosis (Mesa). Diabetes Care (2010) 33(4):804–10. doi: 10.2337/dc09-1679
82. Kiho T, Morimoto H, Kobayashi T, Usui S, Ukai S, Aizawa K, et al. Effect of a polysaccharide (TAP) from the fruiting bodies of Tremella aurantia on glucose metabolism in mouse liver. Biosci Biotechnol Biochem (2000) 64(2):417–9. doi: 10.1271/bbb.64.417
83. Kiho T, Kochi M, Usui S, Hirano K, Aizawa K, Inakuma T. Antidiabetic effect of an acidic polysaccharide (TAP) from Tremella aurantia and its degradation product (TAP-h). Biol Pharm Bull (2001) 24(12):1400–3. doi: 10.1248/bpb.24.1400
84. Zhang W, Zhao JJ, Wang JS, Zhu XL, Zhang M, Qu WJ. Hypoglycemic activity of polysaccharides extracted from Tremella aurantialba mycelia in experimental type 2 diabetic rats. Natural Product Res Dev (2010) 22(01):49–53. doi: 10.16333/j.1001-6880.2010.01.027
85. Pan YX, Wang C, Chen ZQ, Li WW, Yuan GQ, Chen HX. Physicochemical properties and antidiabetic effects of a polysaccharide from corn silk in high-fat diet and streptozotocin-induced diabetic mice. Carbohydr Polymers (2017) 164:370–8. doi: 10.1016/j.carbpol.2017.01.092
86. Yang ZW, Wang J, Li JE, Xiong L, Chen H, Liu X, et al. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohydr Polymers (2018) 183:11–20. doi: 10.1016/j.carbpol.2017.11.033
87. Deng L, Li XX, Lv L, Liu SH, Wu YJ, Wang Y, et al. Overview of edible and medicinal value of Tremella aurantialba and its application in food industry. Edible medicinal mushrooms (2019) 27(02):112–6. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=7001713754.
88. Jiang JP, Xiong YK. Effect of Tremella aurantialba polysaccharide on the inflammatory response in the airways of asthmatic rats. Chin Traditional Herbal Drugs (2009) 40(10):1623–6. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=31793741.
89. Jiang JP, Xiong YK, Yu B, Zhang SL, Zhang CN. Effect of Tremella mesenterica polysacchrides on pulmonary function in asthmatic model of Guinea pigs. China J Traditional Chin Med Pharm (2013) 28(01):206–9. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=44426168.
90. Xie H, Liu CH, Su BN, Chen LM, Qin FS, Rong FX. Nutritive value and pharmacologic research on Tremella aurantialba 8254. Edible Fungi China (2000) 06):39–41. doi: 10.13629/j.cnki.53-1054.2000.06.026
91. Yang M, Zhang ZL, He Y, Li CL, Wang JM, Ma X. Study on the structure characterization and moisturizing effect of Tremella polysaccharide fermented from Gcmcc5.39. Food Sci Hum Well (2021) 10(4):471–9. doi: 10.1016/j.fshw.2021.04.009
92. Zhao XY, Liu HK, Zhang XW, Ao Q. Effect of pressure grinding technology on the physicochemical and antioxidant properties of Tremella aurantialba powder. J Food Process Preservat (2018) 42(12):e13833. doi: 10.1111/jfpp.13833
93. Kumari N, Kumar M, Radha, Lorenzo JM, Sharma D, Puri S, et al. Onion and garlic polysaccharides: A review on extraction, characterization, bioactivity, and modifications. Int J Biol Macromol (2022) 219:1047–61. doi: 10.1016/j.ijbiomac.2022.07.163
94. Li SJ, Xiong QP, Lai XP, Li X, Wan MJ, Zhang JN, et al. Molecular modification of polysaccharides and resulting bioactivities. Compr Rev Food Sci Food Saf (2016) 15(2):237–50. doi: 10.1111/1541-4337.12161
95. Xie LM, Shen MY, Hong YZ, Ye HD, Huang LX, Xie JH. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr Polymers (2020) 229:115436. doi: 10.1016/j.carbpol.2019.115436
96. Du XJ, Zhang JS, Lv ZW, Ye L, Yang Y, Tang QJ. Chemical modification of an acidic polysaccharide (TAPA1) from Tremella aurantialba and potential biological activities. Food Chem (2014) 143:336–40. doi: 10.1016/j.foodchem.2013.07.137
97. Meng LJ, Liu JH, Pan PP. Phase clinical observation of Tremella aurantialba polysaccharide-peptide capsule. Edible Fungi China (2002) 04):40–1. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=6542978.
98. Wang N, Yang LP, Wang ZS. 31 cases of chronic hepatitis and liver cirrhosis treated with Tremella aurantialba polysaccharide-peptide capsule. Shanxi J Traditional Chin Med (1994) 05):11–2. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=1460154.
99. Meng LJ, Zhao YM, Liu JH, Pan PP, Liu GZ, Feng ML, et al. Basic pharmacological studies on Tremella aurantialba polysaccharide-peptide capsule. Acta Edulis Fungi (2000) 03):31–6. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=4955208.
100. He R, Sun DF, Zhou P, Lou XL, Cao JJ, Zhang SS, et al. A tremella aurantialba mycelium polysaccharide toast and its preparation method. China: CN113973866A. (2022). Available at: https://s1.qizhidao.com/zZWTHt.
101. Wu SR, Tai LM, Gui MY, Gao GS, Hou K, Hou B. A tremella aurantialba health drink and its preparation method. China: CN104207276B. (2016). Available at: https://s1.qizhidao.com/ZLbrzV.
102. Li YQ. Study of tremella aurantialba advanced nutritional health drink - II development of Tremella aurantialba drink and nutritional composition analysis. J Shanxi Agric Univ (1997) 01:46–9+108-9. Available at: http://qikan.cqvip.com/Qikan/Article/Detail?id=2780978.
103. Feng B, Tang QJ, Ma LJ. Study of moisturizing efficacy of acidolysis products of Tremella aurantialba polysaccharides. Detergent Cosmet (2015) 38(09):19–21. doi: 10.13222/j.cnki.dc.2015.09.005
104. Cao CJ, Yao L, Cheng SJ. A composition with protective effect against blue light-induced skin cell damage and its application in cosmetics. China: CN113876626A. (2022). Available at: https://s1.qizhidao.com/FuKGFr.
105. Huang CM, Huang CJ, Huang CJA. Tremella aurantialba polysaccharide extract and its application in cosmetics. China: CN106496343A. (2017). Available at: https://s1.qizhidao.com/afjNSV.
106. Yang XJ, Li RC. A method for the preparation of tremella aurantialba polysaccharide mask. China: CN109157455A. (2019). Available at: https://s1.qizhidao.com/sgQmfY.
Glossary
Keywords: tremella aurantialba, polysaccharides, isolation, structures, bioactivities, application
Citation: Yan Y, Wang M, Chen N, Wang X, Fu C, Li Y, Gan X, Lv P and Zhang Y (2022) Isolation, structures, bioactivities, application and future prospective for polysaccharides from Tremella aurantialba: A review. Front. Immunol. 13:1091210. doi: 10.3389/fimmu.2022.1091210
Received: 06 November 2022; Accepted: 24 November 2022;
Published: 08 December 2022.
Edited by:
Shiming Li, Rutgers, The State University of New Jersey, United StatesReviewed by:
Bin Du, Hebei Normal University of Science and Technology, ChinaLanzhou Li, Jilin Agricultural University, China
Copyright © 2022 Yan, Wang, Chen, Wang, Fu, Li, Gan, Lv and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pin Lv, bHZwaW5AaGVibXUuZWR1LmNu; Yan Zhang, c25vd3dpbmdsdkAxMjYuY29t
 Yonghuan Yan
Yonghuan Yan Mengtian Wang1,2
Mengtian Wang1,2 Pin Lv
Pin Lv Yan Zhang
Yan Zhang