
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 12 December 2022
Sec. Immunological Tolerance and Regulation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1080980
This article is part of the Research Topic Immune Responses and Immunotolerance in the Process of Reproduction View all 16 articles
 Dingyi Zhang1,2†
Dingyi Zhang1,2† Ying Hu1,3†
Ying Hu1,3† Weijie Guo1
Weijie Guo1 Yang Song4
Yang Song4 Lin Yang5
Lin Yang5 Shuhan Yang1,2
Shuhan Yang1,2 Taoaixin Ou1,2
Taoaixin Ou1,2 Yanxu Liu1,2
Yanxu Liu1,2 Yaoyao Zhang1*
Yaoyao Zhang1*Background: Epidemiological observational studies have investigated the relationship between rheumatoid arthritis(RA) and pre-eclampsia, but no consistent conclusions were obtained due to various limitations. Hence, we conducted a two-sample mendelian randomization analysis to evaluate the potential causal effect of RA on pre-eclampsia.
Methods: Summary-level statistics for RA were derived from a large-scale meta-analysis of datasets of genome-wide association studies(GWAS) which involved 14,361 cases and 43,923 controls. Moreover, summary statistics for pre-eclampsia or eclampsia were sourced from the Finn biobank which contained 3,903 cases and 114,735 controls. The inverse variance weighting (IVW) as well as other four effective methods including MR-Egger, weighted median, weighted mode, and simple mode were applied to deduce the potential causal relationships between RA and pre-eclampsia comprehensively.
Results: The two-sample MR analysis suggested a strong causal relationship between RA and pre-eclampsia[OR,1.05;95%CI, 1.01-1.09;p<0.05]. The OR estimates obtained from the weighted mode[OR,1.09;95%CI,1.03-1.15;p<0.01] and weighted median[OR,1.07;95%CI, 1.01-1.14;p<0.05] were similar to those from the IVW method, but there was no significant association observed in MR Egger and simple mode analysis.
Conclusion: This MR analysis provides evidence of a positive causal association between RA and pre-eclampsia genetically. Our findings highlight the importance of more intensive prenatal care and early intervention among pregnant women with RA to prevent potential adverse obstetric outcomes. Moreover, our study provides clues for risk factor identification and early prediction of pre-eclampsia.
Hypertensive disorders of pregnancy(HDP) are common pregnancy complications, which are comprised of gestational hypertension, pre-eclampsia or eclampsia, pre-eclampsia superimposed on chronic hypertension, and chronic hypertension. HDP, especially pre-eclampsia, complicate 2-8% of pregnancies and account for a substantial proportion of maternal and perinatal mortality (1, 2). Worldwide, more than 50,000 pregnant women and 500,000 fetuses have died from pre-eclampsia (3). The multifactorial pathogenesis of HDP is complex, and it can be influenced by the environment during pregnancy as well as the underlying pathological and immunological conditions of patients. Pre-eclampsia as a type of HDP, results from heterogeneous causes (4).Some potential risk factors for pre-eclampsia include history of pre-eclampsia, chronic hypertension, pre-gestational diabetes, multifetal pregnancy, obstetric complications in a previous pregnancy such as fetal growth restriction, stillbirth, abruption, and autoimmune diseases including antiphospholipid syndrome and systemic lupus erythematosus (5, 6). However, most of factors that have been identified lack accuracy in predicting its onset and preventative therapies only moderately reduce a woman’s risk of pre-eclampsia. Therefore, it is crucial to identify more reliable risk factors for the identification and early prediction of pre-eclampsia.
Rheumatoid arthritis(RA) is a systemic autoimmune disease with a chronic inflammatory process, which has a female predominance (7). Several studies have investigated the potential relationship between RA and pre-eclampsia and provided conflicting results, possibly due to residual confounding. A cohort study of 312,081 women has reported that women with RA were significantly more likely to have hypertensive disorders in pregnancy [OR,1.51;95% CI, 1.16-1.97;p<0.01] (8). Particularly for pre-eclampsia, a nationwide population-based study in Taiwan showed that the odds of women with RA suffering from pre-eclampsia was 2.22 times that of women without RA[OR,2.22; 95%CI, 1.59-3.11] (9). However, another research showed that although the confidence intervals approached significance for outcomes, no greater risk existed among women with RA than non-RA women of suffering from pre-eclampsia[aRR,1.08;95% CI, 0.97- 2.50] (10). Owing to the complication of pregnancy, and study deficiencies such as geographical and ethnic differences, reverse causation, selection bias, and other potential biases (11), these observational studies do not adequately and directly reflect the causal association between RA and pre-eclampsia.
Mendelian randomization(MR) is an emerging analysis method that uses genetic variants as instrumental variables (IVs) to evaluate the causal effect of modifiable exposures on outcomes (12). Because of the randomization of genetic variant inheritance, MR analysis can reduce the possibility of being influenced by potential confounders and reverse causation bias, inferring the correlation between exposure and outcome genetically, thus providing more reliable results (13). Therefore, we conducted a two-sample MR analysis to explore the causal effect of RA on pre-eclampsia.
In this study, we conducted a two-sample MR to evaluate the causal association between RA and pre-eclampsia. SNPs were used as instrumental variables (11). To maximize the accuracy of results, three important hypotheses must be confirmed during the whole process (14). First, the selected IVs should be directly associated with RA. Second, the IVs are independent of any potential confounders that impact exposure and outcome. Third, the IVs influence pre-eclampsia only through RA. All original studies acquired ethical review approval and informed consent (Figure 1).
Datasets of genome-wide association studies(GWAS) provide reliable instruments for MR analyses. In our study, summary-level statistics for RA were derived from a large-scale meta-analysis of GWAS which involved 14,361 cases and 43,923 controls (15). And summary statistics for pre-eclampsia or eclampsia were sourced from the Finn biobank which contained 3,903 cases and 114,735 controls. The threshold for SNP selection was P < 5e–8 and all SNPs and related data were sourced from studies that separately analyzed only populations of European ancestry to eliminate demographic stratification bias.
The linkage disequilibrium (LD) in selected SNPs was tested to ensure the data were valid. We conducted the clumping procedure to filter independent SNPs within a window size of, 5000kb and r2<0.01 threshold. Then, the F-statistics were calculated to evaluate the strength of each IV and exclude weak instruments. The following rigorous mathematical formula was adopted:
where R2 indicates exposure variance explained by each IV, N refers to the sample size of the GWAS, and K denotes the number of SNP for MR analysis. F>10 indicated sufficient strength of the instruments, which meant the IVs had enough estimated effect for the subsequent MR analysis without weak-tool bias. In this study, we used 65 SNPs as eligible IVs after eliminating ten SNPs with minor allele frequency (MAF) less than the threshold of 0.01. Finally, according to alleles and allele frequencies, we harmonized the SNPs of exposure and outcome by removing or adjusting SNPs with inconsistent alleles to ensure they have corresponding alleles.
In this study, we used the inverse variance weighting (IVW) method as the main approach to deduce the potential causal relationships between RA and pre-eclampsia. Other four effective methods including MR-Egger, weighted median, weighted mode, and Simple mode were also applied to evaluate the possible relationship comprehensively.
Characterized by using the inverse of outcome variance as weight and not taking the intercept into account, the IVW method can provide unbiased causality estimates in an ideal state where all selected genetic variations are assumed to be valid IVs without pleiotropy (16). In terms of MR-Egger, although the significant influence of outlying genetic variables may contribute to its low statistical ability, it can infer the corrected causal effect and provide estimates without bias, even if all selected IVs are not valid (16, 17). If at least 50% of the information from valid instruments is accessible, the weighted median method can offer accurate and robust effect estimates (18). And as for the weighted mode, it is reliable on the condition that the largest subset of instruments with similar causal effects is valid.
Firstly, several sensitivity analyses were conducted to assess pleiotropy. We performed the mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) to detect potential outlier variants (19). Furthermore, the MR-Egger regression was used to evaluate the bias generated by gene pleiotropy, of which the intercept is an indicator (17). Secondly, the Cochrane Q statistic was applied to quantify the heterogeneity between SNPs, where the p-value greater than 0.05 indicated no heterogeneity. Thirdly, we utilized the leave‐one‐out analysis to verify where there exist outliers affecting the result strongly by eliminating each SNP in turn and then performing the IVW method on the rest.
At last, scatter and forest plots were provided to visualize the result of the MR analysis. We did all the analyses on the R software (version 4.2.1.; R Foundation for Statistical Computing, 2021) and RStudio(version, 2022.07.0 + 548), and R package TwoSampleMR and MR-PRESSO were used.
Sixty-five independent (LD R2< 0.01) and genome-wide significant (p< 5e-08) SNPs were identified as qualified IVs with all F-statistics above 10. Detailed information is available in Supplementary Data Sheet 1.
Notably, the two-sample MR analyses showed a strong causal relationship between RA and pre-eclampsia or eclampsia[OR,1.05;95%CI, 1.01-1.09;p<0.05]. The OR estimates obtained from the weighted mode[OR,1.09;95%CI,1.03-1.15;p<0.01] and weighted median[OR,1.07;95%CI, 1.01-1.14;p<0.05] were similar to those from IVW method, but there was no significant association observed in MR Egger and Simple mode analysis. The results of the five MR analysis methods are shown and visualized in Figure 2.
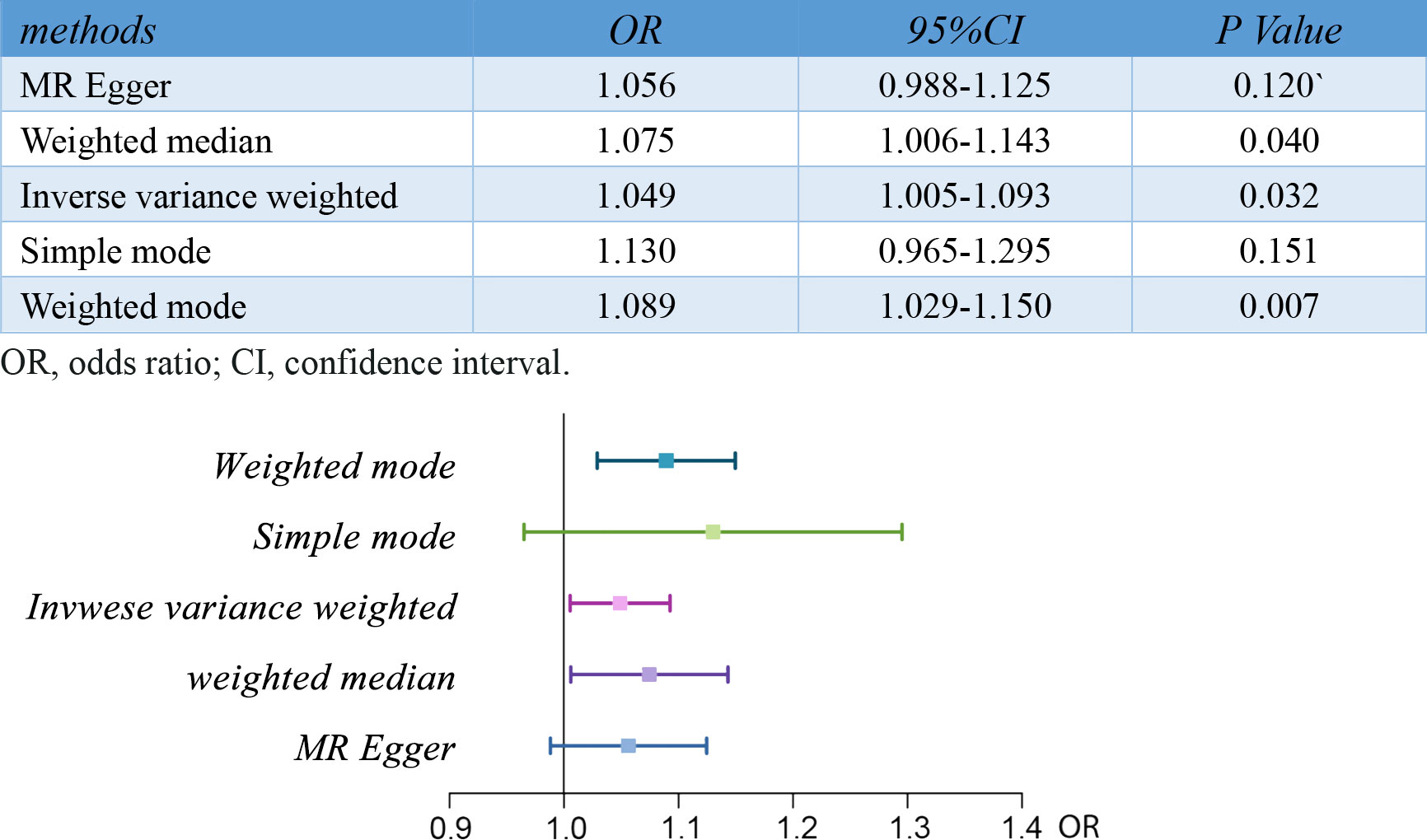
Figure 2 Results of MR analyses conducted to estimate potential associations between RA and risk of pre-eclampsia. MR, mendelian randomization; RA, rheumatoid arthritis.
In the sensitivity analyses, no evidence for directional pleiotropy was obtained when we performed MR-Egger regression to reanalyze the result[p=0.79]. And no outlier SNPs were identified by using MR-PRESSO in our study. Heterogeneity was evaluated by Cochrane’s Q test, and the results of both IVW and MR Egger analysis were not significant[p>0.05](Figure 3). Furthermore, leave-one-out plots suggested the causal estimates were unlikely to be influenced by certain SNPs (Figure 4). In addition, SNP effects individually and jointly from each MR method were displayed in scatter plots (Figure 5).
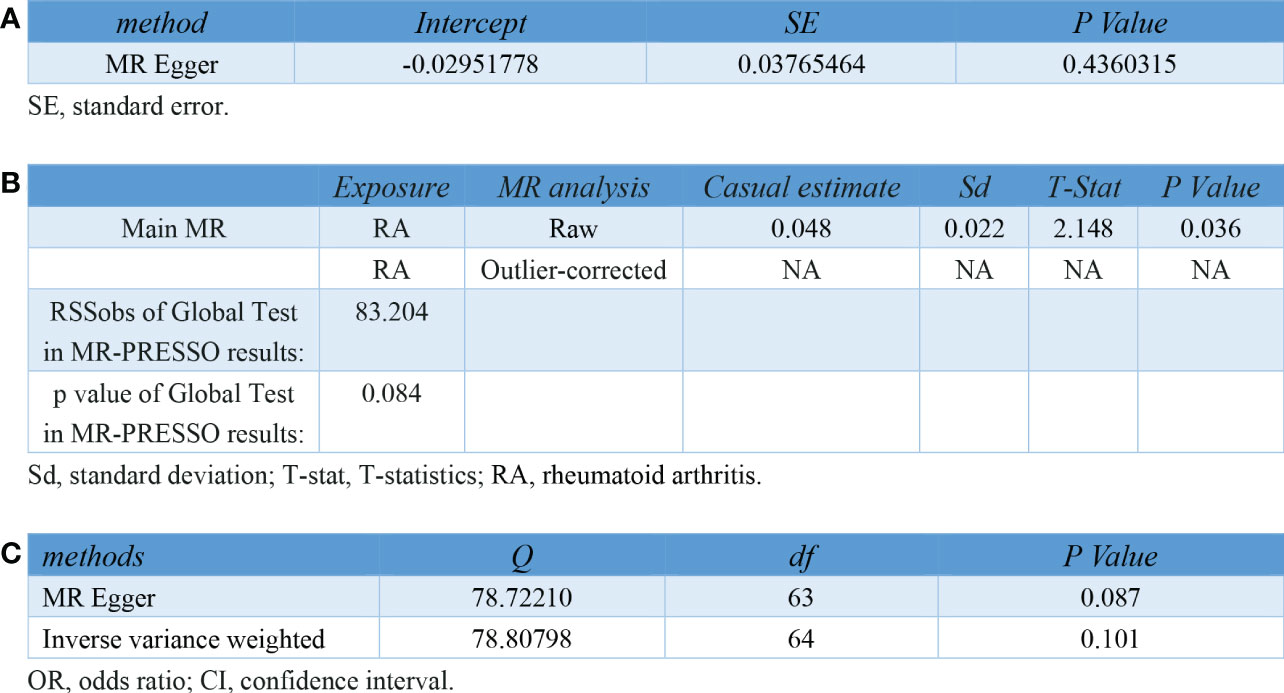
Figure 3 Pleiotropy and heterogeneity testing in sensitivity analyses of IVs for RA. (A) Pleiotropy testing using MR Egger regression (B) Pleiotropy testing using MR-PRESSO. (C) Heterogeneity testing using the Cochrane Q statistic. IV, instrument variant; RA, rheumatoid arthritis; MR, mendelian randomization; MR-PRESSO, mendelian randomization pleiotropy residual sum and outlier.
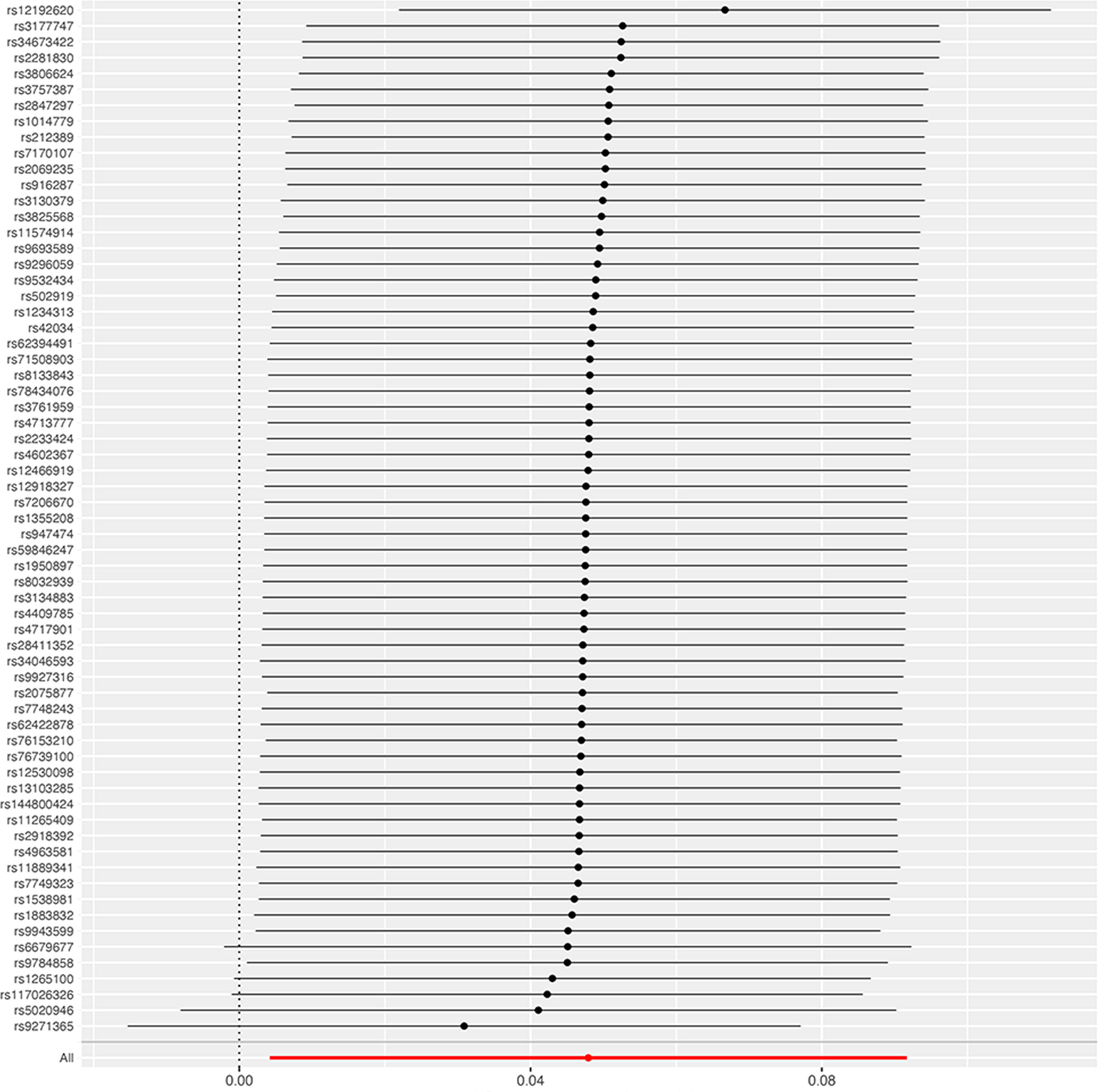
Figure 4 Leave-one-out sensitivity analysis of the causal effect of RA on pre-eclampsia. The red line indicates reliable estimations from the IVW and MR Egger methods. RA, rheumatoid arthritis; IVW, inverse variance weighting.
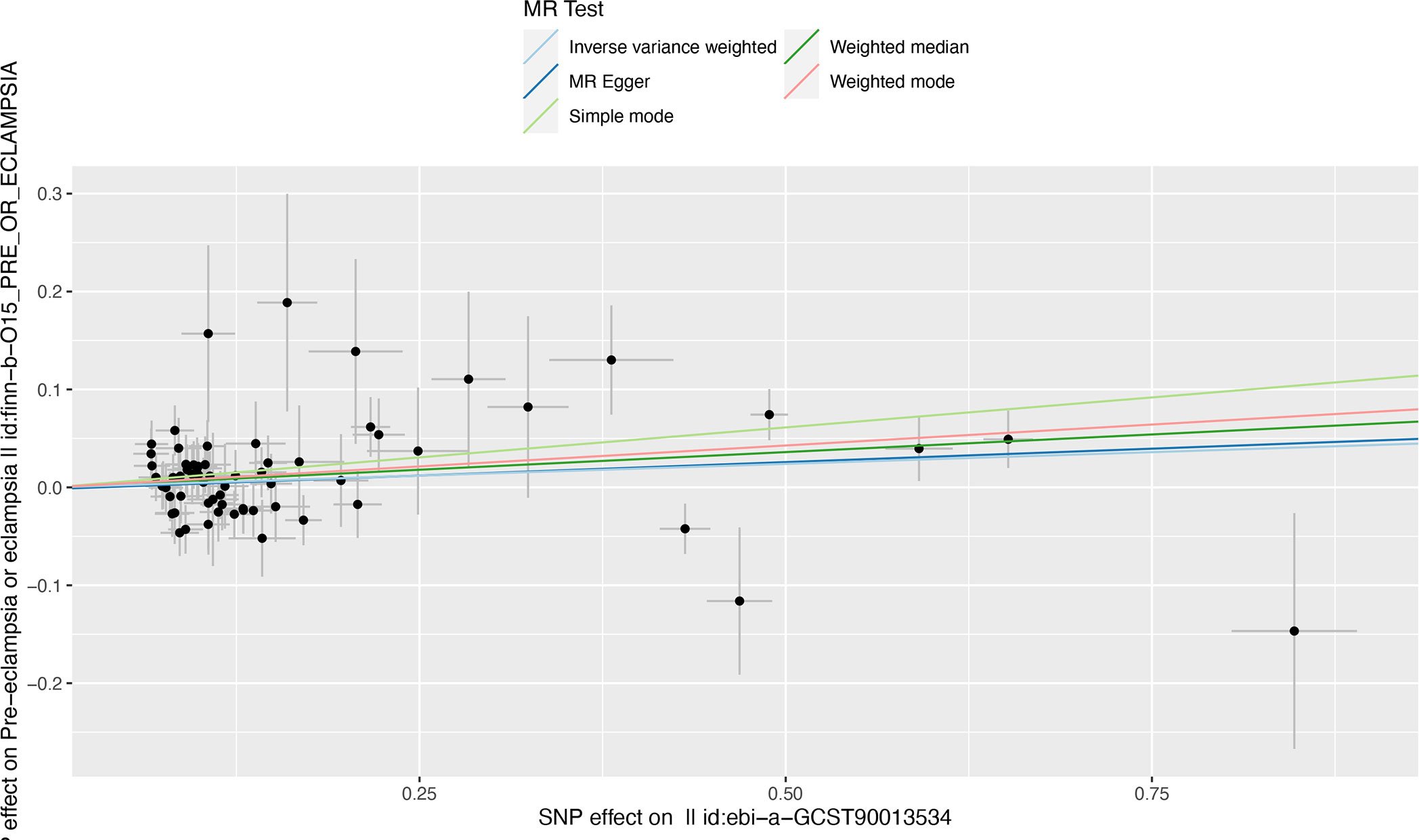
Figure 5 Scatter plot illustrating the distribution of individual ratio estimates of RA with pre-eclampsia as the outcome. Trend lines derived from five different MR methods indicate cause and effect. Inverse variance weighted(light blue), Weighed median(dark green), MR Egger(dark blue), weighted mode(red), Simple mode(light green); SNP, single-nucleotide polymorphism; RA, rheumatoid arthritis; MR, mendelian randomization.
For the first time, we conducted a two-sample MR analysis to investigate the causal effect of RA on pre-eclampsia, which suggested a strong positive association. The results were reliable and robust in sensitivity analyses. We chose the largest genome-wide meta-analysis result for exposure with the advantage of analyzing two distinct ancestral populations originally. Results in the European population could be validated by the other one, which especially provided more reliable GWAS data for MR analysis (15).
Several previous observational studies have been conducted but the conclusions were not consistent. Contradictory to our results, a study with a sample size of 2,802 investigated the risk of pregnancy complications in women with RA in Washington State and showed no significant difference in pre-eclampsia (10). A similar result was revealed when Norwegian researchers conducted a study of 631 women diagnosed with RA before the age of 45, but the exposure of this study included women diagnosed with RA as well as other types of chronic inflammatory arthritides, to some degree, reducing the accuracy of the association inference (20). Due to the limitation of sample size, ethnic homogeneity, and insufficient information, these observational studies with negative results may carry various biases and cannot provide very accurate inferences. On the other hand, our positive causality is in accordance with several previous studies that concluded pregnant women with RA suffered a higher risk of pre-eclampsia. In a retrospective population-based study in the United States, women with RA had a greater likelihood to develop eclampsia or pre-eclampsia. It emphasized the awareness of these risks and suggested women with RA be closely monitored for high-risk pregnancy (21). Not only pre-eclampsia but also other pregnancy complications had a higher frequency to occur when RA patients were pregnant, so doctors should pay more attention to evaluating and detecting complications during the entire pregnancy process (22). In a word, whether RA will increase the risk of pre-eclampsia during pregnancy or not is inclusive, and more reliable analyses should be conducted to provide more solid evidence and give suggestions for clinical practice.
The positive correlation between RA and pre-eclampsia has been presumed for multiple reasons (Figure 6). Firstly, data from animal studies conducted by Babbette LaMarca et al. supported that endogenous TNF-alpha was vital in mediating endothelial cell activation and pre-eclampsia (23). Meanwhile, Zhongbin Lai et al. and Julia M Orshal et al. respectively examined the crucial role of interleukin-6(IL-6) in the pathogenesis of eclampsia by knocking out the IL-10 gene and treating pregnant rats with IL-6 (24, 25). It is well known that IL-6 and TNF-α are confirmed to mediate many chronic inflammatory diseases, especially rheumatoid arthritis (26, 27). Therefore, it is reasonably assumed that increased production of IL-6 and TNF-α in RA patients might be associated with a higher risk of pre-eclampsia. Secondly, from the molecular perspective, research respectively confirmed that cysteine–cysteine chemokine receptor type 5 (CCR5) delta 32 polymorphism conferred susceptibility to both RA in Europe with a significant negative association and pre-eclampsia in the population consisting of Caucasians with the same allele (28, 29). These two studies provided evidence for the assumption that patients with RA may possess less mutation of CCR5 delta32, which increases the incidence of pre-eclampsia. Notably, animal experiments and further studies are required to confirm this inference. Thirdly, another hypothesis focuses on the role of vascular endothelial growth factor(VEGF). Yusuke Murakami et al. found that exogenous VEGF can induce preeclampsia-like symptoms in pregnant mice (30). Meanwhile, a systematic review and meta-analysis concluded that compared with that in the healthy population, the level of circulating VEGF in patients with RA was significantly elevated (31). So, we presumed that the upregulated expression of VEGF in RA patients might induce pre-eclampsia during pregnancy.
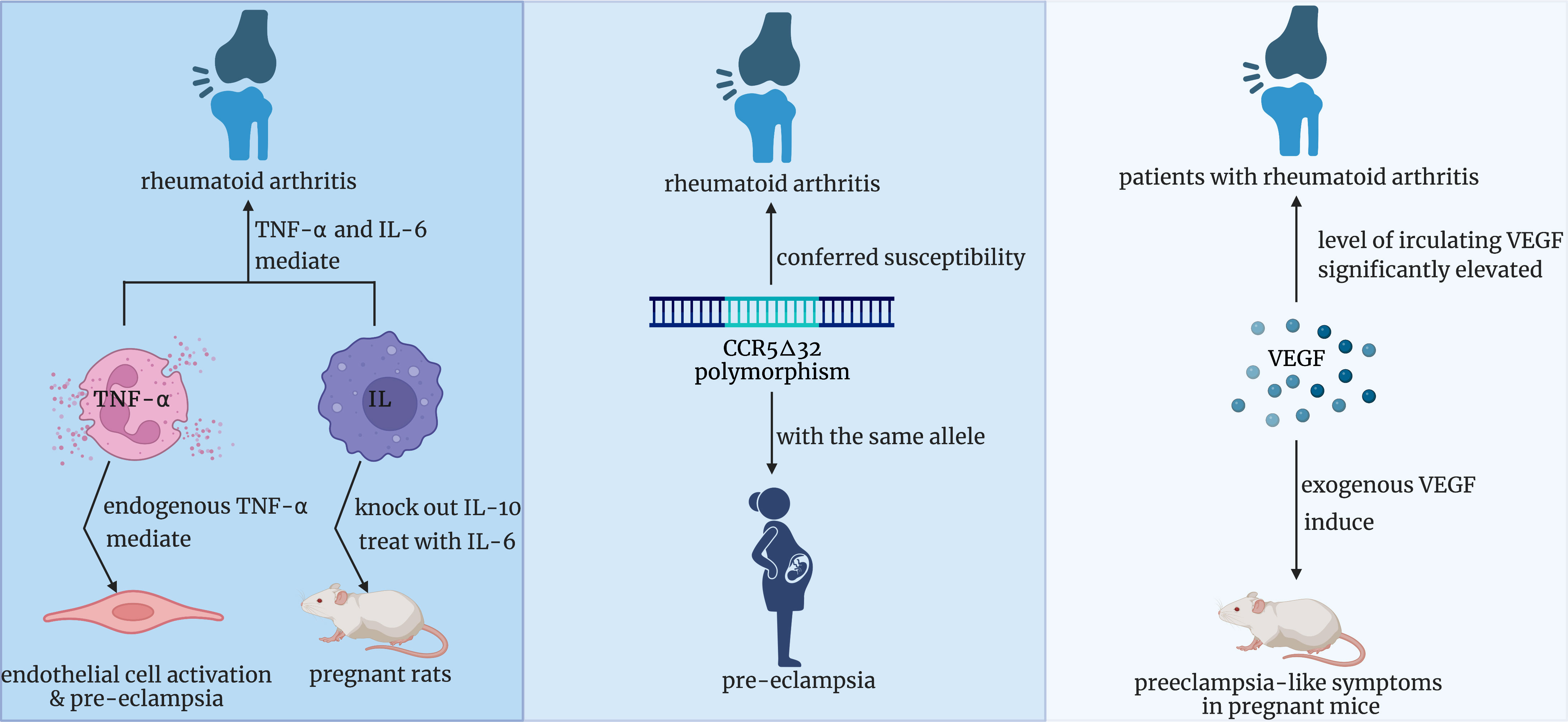
Figure 6 Three potential underlying mechanisms of the positive correlation between RA and pre-eclampsia. RA, rheumatoid arthritis.
The results of several related research should be considered. Bandoli, G. et al. found that pregnancy complications, particularly pre-eclampsia, can mediate some excess risk for adverse pregnancy outcomes(APOs). Based on the calculation, 20.4% of the excess preterm birth that happened to women with RA was due to pre-eclampsia/hypertension and it also accounted for more than 10% of the excess cesarean delivery (32). Interestingly, according to a systematic review of the risk of APOs prior to the onset of an autoimmune rheumatic disease, the incidence of APOs in pre-RA pregnancies was similar to that in the general population, which suggests a reassuring pregnant condition for pre-RA women (33).
A chief strength of our study is the two-sample MR design which allowed us to evaluate the potential causal effect based on large-scale GWAS with credible sample sizes(14,361 RA cases and 43,923 controls; 3,903 pre-eclampsia/eclampsia cases and 114,735 controls). In the meanwhile, because SNPs are randomly distributed at conception, biases caused by potential confounders and reverse causation can be reduced significantly in this design. It is worth mentioning that our result can present a lifelong pregnant risk for women with RA since genetic variants could not be changed from the time you were born.
The limitations of our study should be noted as well. Firstly, we only enrolled European ancestry populations in our study which means all related data were sourced from studies that separately analyzed only populations of European ancestry. The uniformity of participants eliminates demographic stratification bias and ensures the accuracy of MR analysis results, but whether our findings can be applied to other populations or not remains to be validated. Therefore, further analyses involving other populations require being conducted to confirm whether our findings can be generalized or not. Secondly, the data on outcome was collected from the population with pre-eclampsia or eclampsia and no more specific information was given to describe the severe degree of disease. Further studies are warranted to research the association between RA and subtypes of pre-eclampsia.
This is the first MR analysis conducted to explore the causality of RA on pre-eclampsia. We find a positive association between RA and elevated risk of pre-eclampsia. Our findings highlight the importance of more intensive prenatal care and early intervention among pregnant women with RA to prevent potential adverse obstetric outcomes. Moreover, our study provides clues for risk factor identification and early prediction of pre-eclampsia.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
DZ and SY designed the research. WG and DZ collected and analyzed the data. LY, YL, TO, SY, YZ, and YH performed the literature search. DZ and YH drafted the article. YS, WG, YZ, and YH corrected and edit the article. All authors contributed to the article and approved the submitted version.
Our study was supported by funding from projects of Chengdu Science and Technology Bureau, (YZ, Grant No., 2021-YF05-02110-SN), China Postdoctoral Science Foundation (YZ, Grant No., 2020M680149, 2020T130087ZX), Sichuan Natural Science Foundation for Youth (China, NO., 2022NSFSC1281) and Sichuan Medical Association Foundation(China, NO. S21006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1080980/full#supplementary-material
1. Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. (2006) 367(9516):1066–74. doi: 10.1016/S0140-6736(06)68397-9
2. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. (2010) 376(9741):631–44. doi: 10.1016/S0140-6736(10)60279-6
3. Karrar SA, Hong PL. Preeclampsia. StatPearls. Treasure Island (FL: StatPearls PublishingCopyright © 2022, StatPearls Publishing LLC (2022).
4. Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. (1996) 175(5):1365–70. doi: 10.1016/S0002-9378(96)70056-X
5. Ding X, Yang Z, Han Y, Yu H. Long-chain fatty acid oxidation changes in a β2 glycoprotein I-induced preeclampsia-like mouse model. Placenta. (2014) 35(6):392–7. doi: 10.1016/j.placenta.2014.03.013
6. Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. (2021) 398(10297):341–54. doi: 10.1016/S0140-6736(20)32335-7
7. Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis (2014) 73(7):1316–22. doi: 10.1136/annrheumdis-2013-204627
8. Keeling SO, Bowker SL, Savu A, Kaul P. A population-level analysis of the differing effects of rheumatoid arthritis and spondyloarthritis on peripartum outcomes. J Rheumatol (2020) 47(2):197–203. doi: 10.3899/jrheum.181320
9. Lin HC, Chen SF, Lin HC, Chen YH. Increased risk of adverse pregnancy outcomes in women with rheumatoid arthritis: a nationwide population-based study. Ann Rheum Dis (2010) 69(4):715–7. doi: 10.1136/ard.2008.105262
10. Reed SD, Vollan TA, Svec MA. Pregnancy outcomes in women with rheumatoid arthritis in Washington state. Matern Child Health J (2006) 10(4):361–6. doi: 10.1007/s10995-006-0073-3
11. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med (2008) 27(8):1133–63. doi: 10.1002/sim.3034
12. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet (2014) 23(R1):R89–98. doi: 10.1093/hmg/ddu328
13. Richmond RC, Davey Smith G. Mendelian randomization: Concepts and scope. Cold Spring Harb Perspect Med (2022) 12(1). doi: 10.1101/cshperspect.a040501
14. Davies NM, Holmes MV, Davey Smith G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj (2018) 362:k601. doi: 10.1136/bmj.k601
15. Ha E, Bae S, Kim K. Large-Scale meta-analysis across East Asian and European populations updated genetic architecture and variant-driven biology of rheumatoid arthritis, identifying 11 novel susceptibility loci. Ann rheumatic diseases. (2021) 80(5):558–65. doi: 10.1136/annrheumdis-2020-219065
16. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data mendelian randomization. Stat Med (2017) 36(11):1783–802. doi: 10.1002/sim.7221
17. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
18. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40(4):304–14. doi: 10.1002/gepi.21965
19. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
20. Wallenius M, Skomsvoll JF, Irgens LM, Salvesen K, Nordvåg BY, Koldingsnes W, et al. Pregnancy and delivery in women with chronic inflammatory arthritides with a specific focus on first birth. Arthritis Rheumatol (2011) 63(6):1534–42. doi: 10.1002/art.30210
21. Aljary H, Czuzoj-Shulman N, Spence AR, Abenhaim HA. Pregnancy outcomes in women with rheumatoid arthritis: a retrospective population-based cohort study. J Matern Fetal Neonatal Med (2020) 33(4):618–24. doi: 10.1080/14767058.2018.1498835
22. Zbinden A, van den Brandt S, Østensen M, Villiger PM, Förger F. Risk for adverse pregnancy outcome in axial spondyloarthritis and rheumatoid arthritis: disease activity matters. Rheumatol (Oxford). (2018) 57(7):1235–42. doi: 10.1093/rheumatology/key053
23. LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. (2008) 52(6):1161–7. doi: 10.1161/HYPERTENSIONAHA.108.120881
24. Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. (2011) 57(3):505–14. doi: 10.1161/HYPERTENSIONAHA.110.163329
25. Orshal JM, Khalil RA. Interleukin-6 impairs endothelium-dependent NO-cGMP-mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol (2004) 286(6):R1013–23. doi: 10.1152/ajpregu.00729.2003
26. Hamilton K, Clair EW. Tumour necrosis factor-alpha blockade: a new era for effective management of rheumatoid arthritis. Expert Opin Pharmacother. (2000) 1(5):1041–52. doi: 10.1517/14656566.1.5.1041
27. An Q, Yan W, Zhao Y, Yu K. Enhanced neutrophil autophagy and increased concentrations of IL-6, IL-8, IL-10 and MCP-1 in rheumatoid arthritis. Int Immunopharmacol. (2018) 65:119–28. doi: 10.1016/j.intimp.2018.09.011
28. Lee YH, Bae SC, Song GG. Association between the chemokine receptor 5 delta32 polymorphism and rheumatoid arthritis: a meta-analysis. Mod Rheumatol (2013) 23(2):304–10. doi: 10.3109/s10165-012-0665-2
29. Gurdol F, Yurdum LM, Ozturk U, Isbilen E, Cakmakoglu B. Association of the CC chemokine receptor 5 (CCR5) polymorphisms with preeclampsia in Turkish women. Arch Gynecol Obstet. (2012) 286(1):51–4. doi: 10.1007/s00404-012-2244-3
30. Murakami Y, Kobayashi T, Omatsu K, Suzuki M, Ohashi R, Matsuura T, et al. Exogenous vascular endothelial growth factor can induce preeclampsia-like symptoms in pregnant mice. Semin Thromb Hemost. (2005) 31(3):307–13. doi: 10.1055/s-2005-872437
31. Zhan H, Li H, Liu C, Cheng L, Yan S, Li Y. Association of circulating vascular endothelial growth factor levels with autoimmune diseases: A systematic review and meta-analysis. Front Immunol (2021) 12:674343. doi: 10.3389/fimmu.2021.674343
32. Bandoli G, Singh N, Strouse J, Baer RJ, Donovan BM, Feuer SK, et al. Mediation of adverse pregnancy outcomes in autoimmune conditions by pregnancy complications: A mediation analysis of autoimmune conditions and adverse pregnancy outcomes. Arthritis Care Res (Hoboken). (2020) 72(2):256–64. doi: 10.1002/acr.24037
Keywords: rheumatoid arthritis, pre-eclampsia, pregnancy, causal effect, mendelian randomization, genome-wide association studies
Citation: Zhang D, Hu Y, Guo W, Song Y, Yang L, Yang S, Ou T, Liu Y and Zhang Y (2022) Mendelian randomization study reveals a causal relationship between rheumatoid arthritis and risk for pre-eclampsia. Front. Immunol. 13:1080980. doi: 10.3389/fimmu.2022.1080980
Received: 26 October 2022; Accepted: 28 November 2022;
Published: 12 December 2022.
Edited by:
Yin Tailang, Reproductive Medicine Center, Wuhan University, ChinaReviewed by:
Bo Zhang, Huazhong University of Science and Technology, ChinaCopyright © 2022 Zhang, Hu, Guo, Song, Yang, Yang, Ou, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaoyao Zhang, eWFveWFvemhhbmdAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.