- 1Department of Hematology, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of Hematology, North China University of Science and Technology Affiliated Hospital, Tangshan, Hebei, China
- 3Department of Orthopedics, Kailuan General Hospital, Tangshan, Hebei, China
Myelodysplastic syndrome (MDS) is a common hematological malignant disease, characterized by malignant hematopoietic stem cell proliferation in the bone marrow (BM); clinically, it mainly manifests clinically mainly by as pathological hematopoiesis, hemocytopenia, and high-risk transformation to acute leukemia. Several studies have shown that the BM microenvironment plays a critical role in the progression of MDS. In this study, we specifically evaluated mesenchymal stromal cells (MSCs) that exert immunomodulatory effects in the BM microenvironment. This immunomodulatory effect occurs through direct cell-cell contact and the secretion of soluble cytokines or micro vesicles. Several researchers have compared MSCs derived from healthy donors to low-risk MDS-associated bone mesenchymal stem cells (BM-MSCs) and have found no significant abnormalities in the MDS-MSC phenotype; however, these cells have been observed to exhibit altered function, including a decline in osteoblastic function. This altered function may promote MDS progression. In patients with MDS, especially high-risk patients, MSCs in the BM microenvironment regulate immune cell function, such as that of T cells, B cells, natural killer cells, dendritic cells, neutrophils, myeloid-derived suppressor cells (MDSCs), macrophages, and Treg cells, thereby enabling MDS-associated malignant cells to evade immune cell surveillance. Alterations in MDS-MSC function include genomic instability, microRNA production, histone modification, DNA methylation, and abnormal signal transduction and cytokine secretion.
Introduction
Myelodysplastic syndrome (MDS) is associated with a poor clinical prognosis owing to its high risk of conversion to acute myeloid leukemia. It is, therefore, important that its pathophysiology be properly described for the development of effective treatment strategies. Mesenchymal stromal cells (MSCs) play an important role in the development and progression of the disease. This review describes the immune regulatory role played by these MSCs in patients with MDS, facilitating a wider and clearer view of the role played by these cells in the disease development and progression process, thereby identifying gaps in research on the subject and providing direction for future research. It also highlights the possibility of the use of MSC-based therapies for the treatment of MDS and other diseases.
The myelodysplastic syndrome-related bone marrow microenvironment
Myelodysplastic syndrome (MDS) is a malignant hematopoietic stem cell (HSC) disease with a poor prognosis and a high risk of acute leukemia (1). Although demethylating agents and BCL-2 inhibitors significantly improve remission rates in patients with this disease, most still relapse after 2–3 years, especially patients with high-risk MDS (HR-MDS); no clinically effective treatment is available for preventing disease progression to acute leukemia.
MDS prognostic score evolution and clinical typing have facilitated the identification of cytogenetic and epigenetic abnormalities, as well as primitive cells, associated with MDS development and prognosis. In addition, some researchers have found that in vitro, MDS-HSCs exhibit defective growth and differentiation, as well as poor transplantation capacity, in immunodeficient recipient mice (2). Cytogenetic and epigenetic abnormalities are commonly observed in several hematological disease states, and some researchers have even found that these epigenetic abnormalities can be easily detected in embryos (3–6). Thus, the mechanisms underlying MDS development and progression cannot be fully attributed to genetic and molecular changes alone. These findings suggest the existence of interactions between malignant cell clones and the bone marrow (BM) microenvironment, which includes immune cells and signaling pathways between cells, and this microenvironment may be one of the mechanisms of malignant cell cloning. Based on these findings, we can consider that the two main causative factors for MDS include stem cell abnormalities and microenvironmental disorders. MDS microenvironment abnormalities include abnormal morphology and functioning of MSCs, impaired differentiation of osteoblasts, increased number of vascular endothelial cells, abnormal number and functioning of immune cells, abnormal expression of cytokines, and abnormal activation of signaling pathways. Among these, MSCs and immune cells in the bone marrow microenvironment are the main factors in the pathogenesis of MDS.
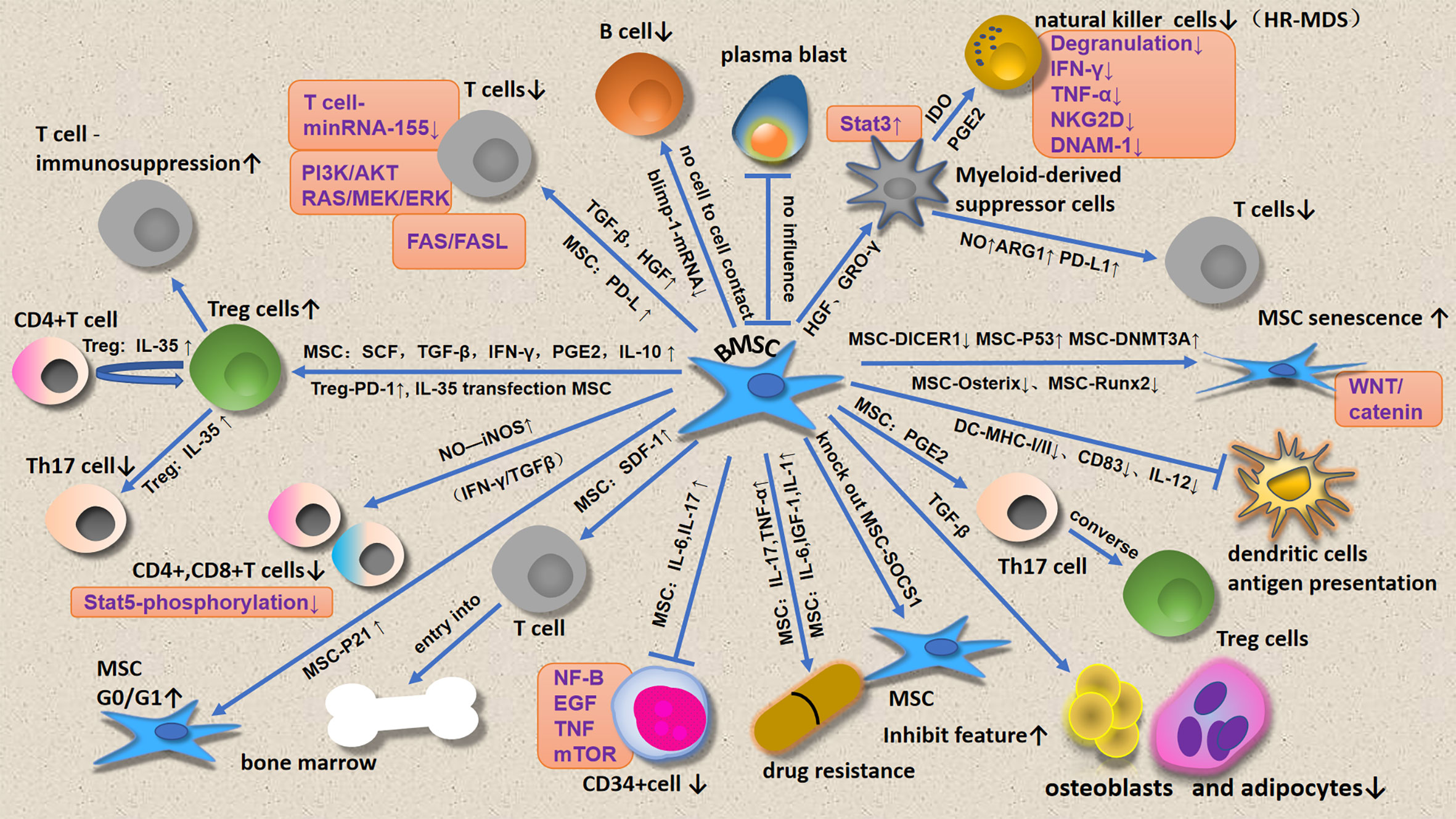
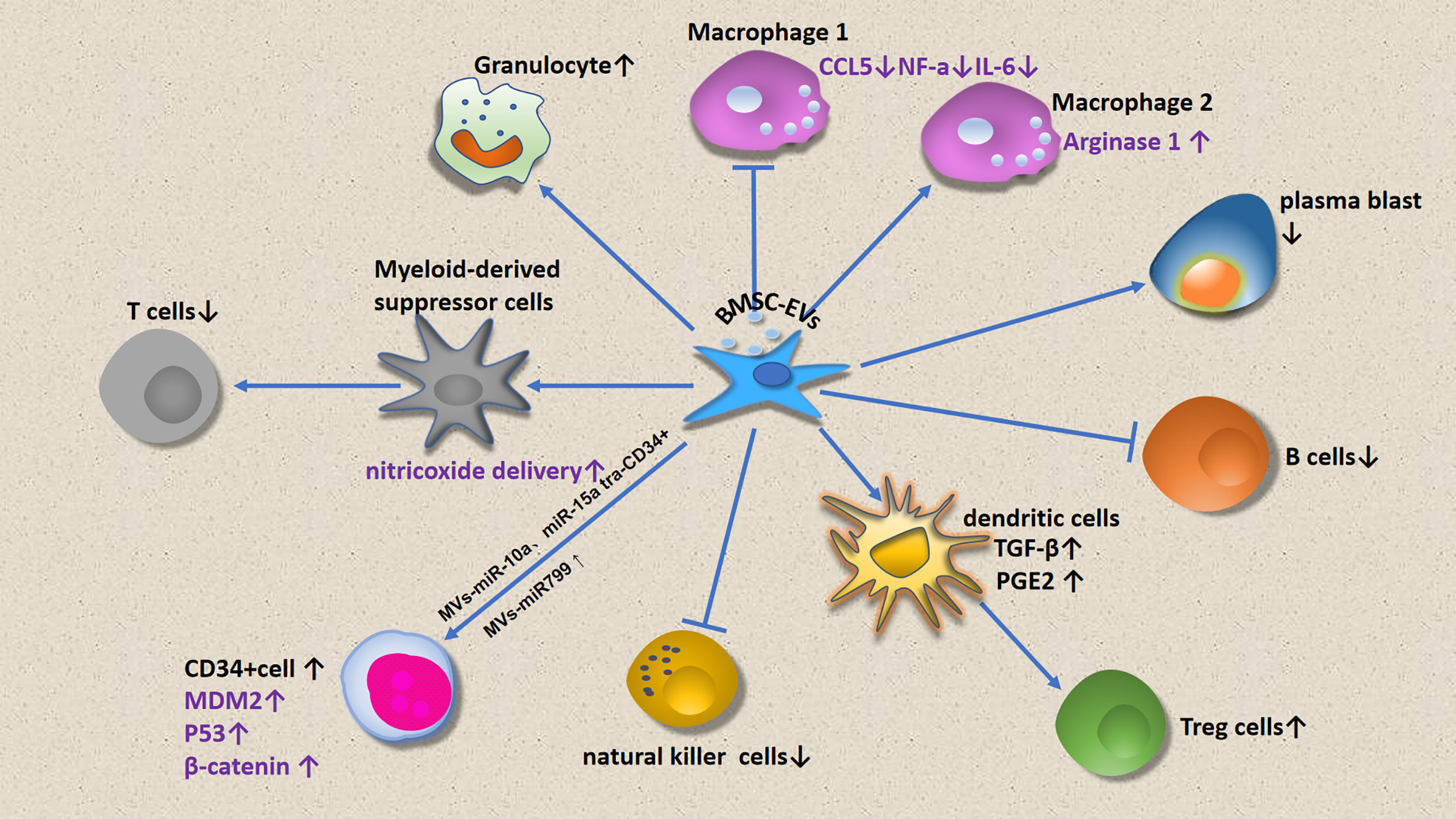
This review focuses on immune cell regulation by MSCs (Figure 1) and micro vesicles (Figure 2) secreted by MDS-associated bone mesenchymal stromal cells (MDS-MSCs), in patients with MDS. According to the findings of several studies, tumor growth, apoptosis evasion, drug resistance development, and tumor metastasis are inseparable from the tumor microenvironment (7, 8). Therefore, the abnormal BM microenvironment in MDS may serve as an umbrella to help tumor cells escape immune surveillance. The MDS BM microenvironment includes many cell types, such as MSCs, osteoblasts, fibroblasts, adipocytes, endothelial cells, immune cells, hematopoietic cells, extracellular matrix, cytokines, and MSC outer vesicles.
Bone mesenchymal stromal cells in myelodysplastic syndrome patients
Mesenchymal stromal cell properties
Friedenstein et al. were the first to discover and describe MSCs (9). The sources of MSCs are rich (10), including BM, fat, synovial membrane, bone, muscle, lung, liver, pancreas, and cord blood, among which BM-derived MSCs mainly originate from endothelial cells (11), but their number is very low (0.001–0.01%) (12).
MSC separation and expansion are easy to perform; besides, their cell properties are stable after expansion, and MSCs have homing to inflammatory sites, multidirectional differentiation ability, and immunoregulatory properties; they can also regulate the proliferation and differentiation of HSC by secreting E-selectin (13), cytochemokines, and crosstalk molecules, such as Jagged1 and CXCL12 (14–16), and this regulation also depends on the tight spatial localization of MSC and vascular endothelial cells (17).
After culture, MSCs are typically observed as a mural spindle; 95% of the cells express CD73, CD105, and CD90, and less than 2% of the cells express CD14, CD34, CD45, CD79a, and HLA-DR. These cells can differentiate into osteocytes, chondrocytes, adipocytes, tenocytes, and myocytes (9). Thus, MSCs have been clinically applied in regenerative medicine and immune disease treatment (18).
MSCs in myelodysplastic syndrome patients
Reported in vitro data have indicated the lack of difference in phenotype and growth characteristics between MSCs in all MDS subtypes (19). A few studies have shown that mesenchymal cell counts are not reduced in patients with MDS (20). However, it has been confirmed in several reports that the phenotype and function of MSCs are significantly altered in acute myelogenous leukemia (AML) and late-stage (high-risk) MDS, but not in early-stage (low-risk) MDS (21, 22), indicating that MSC damage and disease severity are correlated in MDS.
MDS-associated MSCs exhibit increased senescence rates, manifested by decreased proliferative capacity and increased expression of P53 and P21, which regulate cellular senescence; P21 was found to arrest cells in the G0/G1 phase (23). This decrease in MSC proliferative capacity may be related to chromosome methylation status, an abnormal proliferation regulation signaling pathway, and cell cycle arrest, among other factors (24, 25). As expected, a number of studies have reported increased levels of MDS-associated MSCs senescence markers, including reduced telomere lengths and increased β-galactosidase levels (26), compared with those in healthy MSCs. However, the Osterix and Runx2 genes were found to be downregulated in MDS-MSCs (27). The downregulation of DICER1, an RNase III enzyme involved in microRNA biogenesis, was found to be implicated in increased MDS mesenchymal progenitor cell senescence (28). Increased DNMT3A expression levels in MDS-derived MSCs, which leads to the hypermethylation of specific genes, is significantly associated with MSC aging (29). MDS-MSC aging results from both classical Wnt/catenin signaling and non-classical signaling. Decreased Wnt/catenin classical signaling pathway activity is significantly correlated with decreased MDS-MSC proliferation and differentiation potential (25). The activation of NF-κB mediated cellular stress and MSC proliferative damage (30).
TGF-βmiRNA is overexpressed in CD34+ cells in patients with MDS; TGF-β can inhibit MDS-MSC function (26). In addition, it can significantly reduce the MSC differentiation potential in relation to osteoblasts and adipocytes (31, 32). An increase in endogenous erythropoietin level is often observed in patients with MDS, which may downregulate the Wnt pathway and further impair MDS-MSC osteogenic differentiation (33). Some scholars have shown that the immunoregulatory function of MSCs in patients with MDS is damaged (34, 35), while other scholars have demonstrated that there is no significant difference in the immunosuppressive function between MSCs of healthy individuals and patients with MDS (36). In addition, MDS-associated MSCs exhibit their immunosuppressive effects by increasing prostaglandin production, which may reduce the destruction of leukemic cells by T cells (37). Furthermore, Zhang et al. demonstrated that treating MSCs with inflammatory cytokines could induce the production of cytokine signaling inhibitory molecule 1 (SOCS1) by MSCs, and SOCS1 knockdown was found to enhance the immunosuppressive capacity of MSCs (38).
In patients with MDS, MSCs show different genetic abnormalities compared with HSCs (39). As compared to MSCs derived from healthy individuals, MDS-associated MSCs appear to be more susceptible to acquiring mutations during culture. Some researchers have found that MDS-associated MSCs had higher levels of genotoxic stress markers, for example, the frequency of γH2AX foci phosphorylation or replication protein A (RPA) phosphorylation, than those of healthy MSCs (40). In MDS, γH2AX staining findings were found to correlate with mutation frequency. The occurrence of myelodysplastic changes and common genetic changes in AML are associated with A β-catenin-activated MSC mutations (41). The activation of β-catenin induces MSCs to express Jagged1, triggering the activation of notch signaling in hematopoietic stem progenitor cells (HSPCs) and subsequently promoting the progression of leukemia. Highly purified CD271+ MSCs isolated from low-risk MDS (LR-MDS) patients have the ability to fully activate the inflammatory response, which involves NF-κB, EGF, TGF-β, and TNF signaling (42).
The role of MSCs in the onset of MDS
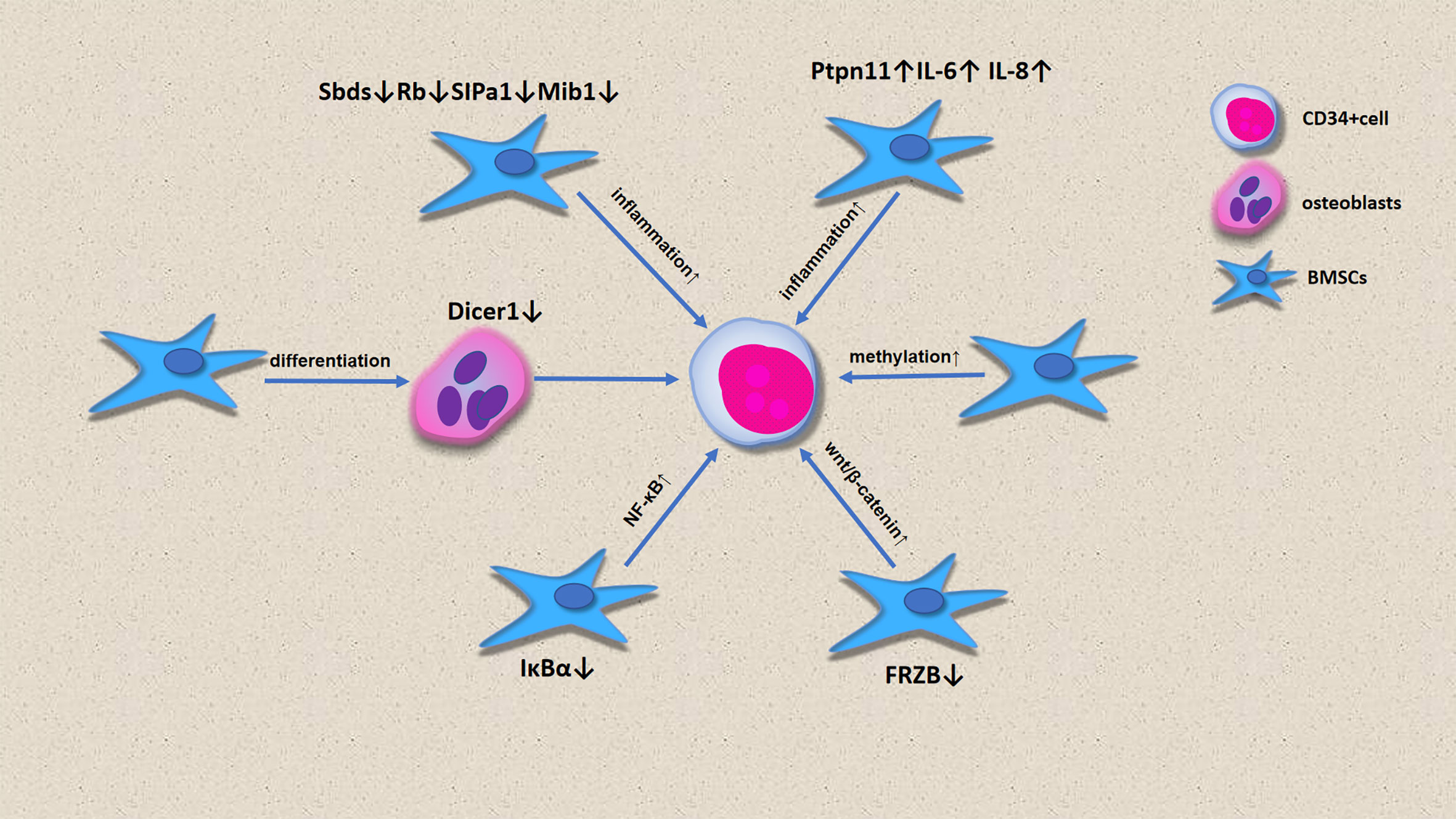
In the past, most scholars believed that MDS was caused by HSC lesions. However, increasing evidence has shown that microenvironment defects can also lead to ineffective hematopoiesis, which leads to the onset of MDS and even progression to AML (Figure 3). MSCs in microenvironments are considered to be substrates with no clear function. Bone cells differentiated by MSCs participate in the hematopoietic stem cell niche, and selective deletion of DICER1 in bone progenitor cells can induce hematopoietic disorders, which is one of the pathogenetic characteristics of MDS (43). Downregulation of DICER1 expression in MSCs was also observed in patients with MDS (28). However, the downregulation of SBDS, Rb, SIPA1 and MIB1 expression and the upregulation of PTPN11, IL-6 and IL-8 expression by BM-MSCs promotes an inflammatory environment in the bone marrow, enabling the onset of MDS (42, 44). CD34+ cells from healthy people had poor colony formation after co-culture with BM-MSCs. Contrastingly, CD34+ co-culture with BM-MSCs with 5-Aza led to an increase in the number of hematopoietic colonies, indicating that MSCs from MDS show high methylation, which is involved in the pathogenesis of MDS. FRZB is an antagonist of the Wnt pathway, and the downregulation of FRZB in MSCs leads to β-catenin activation, which promotes the onset of MDS, and this is more obvious in HR-MDS (45). lκBα is an inhibitor of NF-κB. The lack of IκBα or activation of NF-κB in hematopoietic cells is not sufficient to induce abnormal cell development, but the imbalance of NK-κB in non-hematopoietic cells leads to the onset of MDS (30, 46).
Effects of MDS-MSCs on HSC growth and survival
A study found that when healthy hematopoietic cells are exposed to MDS-associated MSCs, they will have a toxic effect on CD34+ HSPCs, and this effect is persistent, especially in high-risk patients, making this phenomenon is more evident (47). Such abnormal MDS-MSCs may not be able to support normal hematopoiesis, and may also affect the clonal proliferation of HSCs (31, 48). Other studies have found that MSC-micro vesicles (MSC-MVs) exert hematopoiesis-regulating effects. MDS-MVs are generated by a direct blossoming from the plasma membrane.
The number of pluripotent HSCs is generally low. These HSCs are at the top of hematopoietic cell development and have the ability to replicate and renew themselves to differentiate and replenish blood cells of various lineages (49). During differentiation, HSC progenitors undergo different developmental stages, including multi-potential progenitor cells and progenitor cells of various lineages (50). The BM contains niches that protect stem cells. Under normal circumstances, HSPCs are located in these niches and rarely appear in the peripheral blood (PB). MSCs derived from both early-stage (51) and late-stage patients with MDS (52) were found to exhibit decreased expression of ANGPT, which functions to maintain HSPC quiescence (48). Furthermore, we found changes in the expression levels of key molecules involved in MSC interaction with HSPCs in patients with MDS, particularly, osteopontin, Kit-ligand, Jagged1, and angiopoietin, as well as several chemokines. The change of MDS-MSCs significantly reduced the hematopoietic support ability of CD34+ HSPCs, which was related to the reduction of cell cycle activity. MSCs promote MDS development by creating an inflammatory environment. The findings of some studies have shown that IL-6, IL-17A, IFN-γ, and TNF-α produced by MSCs may contribute to HSPC dysregulation, leading to ineffective hematopoiesis (53). In patients with MDS, the inflammatory pathways of MSCs are overactivated, and these pathways include the NF-B, EGF, TGF-β, and TNF-α, which hinder hematopoiesis in LR-MDS; this is considered to be responsible for the increased apoptosis rates observed at this stage of the disease (30, 42). MDS-MSCs exhibit hypermethylation, and treatment of MDS-MSCs with demethylated drugs can improve the formation of HSC colonies (54).
However, miR-7977 upregulation in micro vesicles secreted by MSCs promote CD34+ cell proliferation. Increased secretion of miR10a- and miR15a-containing micro vesicles by MDS-MSCs increases the viability and clonogenicity of HSPCs in patients with MDS (55). Transfection of miR-10a- and miR-15a-containing micro vesicles secreted by MSCs into CD34+ cells induced an increase in their activity; in addition, these cells exhibited increased TP53 and MDM2 expression (55). Another study showed that MSC-MVs increase β-catenin expression in expanded CD34+ cells (56); furthermore, the loss of β-catenin has been shown to impair the self-renewal ability of HSCs (57). MSC-MVs possibly exert their hematopoiesis-supporting effects through the Wnt/β-catenin pathway.
HSC mainly exists in the perivascular niche. Endothelial cells (ECs) are an important component of the BM microenvironment and participate in the proliferation of HSCs. ECs in patients with MDS can be mobilized from BM to become circulating progenitors of ECs (cPECs). Patients with MDS who have increasing numbers of cPECs have increased methylation levels, which affects HSC function (58). Adipocytes, which are also components of the BM microenvironment, are negative regulators of HSC, and the number of adipocytes in BM is negatively correlated with the number of HSCs (59). Another major component of the BM microenvironment is osteoblasts, but current studies have shown no significant association between osteoblasts and HSCs. Therefore, it is not clear whether osteoblasts have a regulatory effect on HSCs (60).
MDS and MDS-MSC drug resistance
The treatment of MDS mainly includes blood transfusion support, iron removal therapy, demethylation therapy, targeted therapy for mutated genes, and BM transplantation. However, the overall therapeutic effect is not ideal, and the long-term survival rate is still less than 30%; thus, targeted BM matrix therapy is attracting researchers’ attention. New areas of research also include gene -editing MSCs (61) and nanoparticle-coated small interfering RNA (siRNA) (62).
In patients with MDS, MSCs may improve the drug resistance of CD34+ cells through two factors: endogenous factor and exogenous factor. Endogenous factor-induced drug resistance is thought to be triggered by preexisting intrinsic random gene mutations in CD34+ cells (63–65). As opposed to extrinsic factors, such as environment-mediated drug resistance (EM-DR), extrinsic factors may not only protect tumor cells with genetic mutations, but also trigger the development of other mutations. EM-DR is induced by signaling events triggered by certain factors present in the tumor microenvironment. EM-DR is divided into two categories: soluble factor-mediated drug resistance (SFM-DR) and cell adhesion-mediated drug resistance (CAM-DR) (66–68). The mechanisms of SFM-DR and CAM-DR are different. The former is mainly mediated by inducible gene transcription, while the latter is mainly mediated by non-transcriptional mechanism (68), including degradation of apoptotic activators and increased stability of apoptotic inhibitors and cell cycle regulators (69).
BM-MSCs are highly expressed in cytidine-deaminase (CDA), which can metabolize two essential drugs for MDS treatment: azacitidine and decitabine (70). BM-MSCs can also increase drug resistance by silencing CD34+ cells, thereby protecting CD34+ cells from apoptosis. In addition, MSCs can inhibit the apoptosis of CD34+ cells and promote their proliferation by activating the mTOR signaling pathway (71). In vivo, MDS-MSCs may transfer functional mitochondria to CD34+ cells, thereby increasing the survival of CD34+ cells in the presence of chemotherapeutic conditions (72, 73).
Drug-resistant malignant tumor cells highly express very late antigen4 (VLA4), which enables malignant tumor cells to attach to MSCs via VCAM1, thereby activating the NK-κB signaling pathway (74). Tumor cells with negative expression of VLA4 were more responsive to treatment, suggesting an association between the development of drug resistance and MSCs. MSCs express CXCL12. Thus, connecting CXCR4 with HSCs and blocking the CXCL12-CXCR4 axis can make tumor cells sensitive to chemotherapy drugs (75, 76).
Immunomodulation of the bone marrow microenvironment by MDS-MSCs
In patients with MDS, MSCs also exhibit immunosuppressive function; MDS-MSCs secrete high levels of TGF-β1 (77), a cytokine that exerts significant immunosuppressive effects on B, T, and natural killer (NK) cells, as well as immunostimulatory effects on regulatory T cells (Treg cells). Thus, the high TGF-β1 expression levels observed in patients with HR-MDS (77) promote the development of an immunosuppressive microenvironment, characterized by a decrease in the CD4+ T-cell population, CD8+ T-cell exhaustion, decrease in NK-activating receptor expression, and increase of non-cytotoxic NK-cell counts (CD56bright) (78). MSCs exert significant immunosuppressive and anti-inflammatory effects through several contact-dependent and contact-independent mechanisms (79).
Effects of MDS-MSCs on T lymphocytes
MSCs affect T cells in several ways, including inhibiting T-cell proliferation and cytokine secretion and cytotoxicity, as well as regulating T helper (Th)1/Th2 effector cell balance (80).
Increased SDF-1 expression in MDS-MSCs may induce circulating T-cell entry into the BM by chemotaxis (31). Zhao et al. found that the immunosuppressive effects of high-risk MDS-MSCs on T-cell proliferation were significantly higher than those of low-risk MDS-MSCs (77). The findings of in vitro experiments have confirmed that the effects of high-risk MDS-MSCs in inhibiting effector T-cell proliferation, which are mediated by the promotion of TGF-β production and secretion, are significantly higher than those of low-risk MDS-MSCs (77). Most scholars believe that MSCs exert their immunosuppressive effect only under the mediation of certain factors, which are mediated by the upregulation of T-cell suppressive pathways, until they are activated by proinflammatory cytokines, especially IFN-γ (81). Notably, in some studies, MSCs have been found to play no significant immunomodulatory role in the absence of IFN-γ or other inflammatory signaling molecules (82). MSCs induce activated T-cell anergy through the production of IL-10, which enhances regulatory T-cell development, thereby inhibiting conventional T-cell proliferation and the activities of other effector cells, MSC-based indoleamine 2,3-dioxygenase (IDO)-dependent immune modulation mechanisms (83), as well as the downregulation of costimulatory molecules, CD40, CD80, and CD86 (84), which are dominant in humans. MSCs can promote nitric oxide synthase synthesis (NOS) and inhibit STAT5 phosphorylation in T cells, thereby inhibiting their function (85). A net loss in activated T cells through an increase in cell death can lead to immunosuppression over time (86). In addition, the effect of MSCs on activated cytotoxic T cells (CTLs) is mainly manifested in the inhibition of their lytic function (87).
Besides, MSCs were also found to alter Th1, Th2, Th17, and Treg cell secretory function through the secretion of soluble cytokines, exosomes, and micro vesicles.
MSC-induced T-cell immunosuppression has been extensively studied; this MSC-induced T-cell immunosuppression, which can alter the Th1/Th2 cell ratio by secreting soluble cytokines, TGF-β, stem cell growth factor (SCF), prostaglandin E2 (PGE2), and INF-γ, induces an increase in Treg cell counts.
Aside from the soluble factors mentioned above, MSCs can also downregulate the activation and responses of T cells by interacting with T cells; this occurs by inhibiting the interaction of the receptor programmed cell death-1 (PD-1) protein with its cognate ligands PD-L1 and PD-L2 to induce apoptosis of T cells (88). miR-155 downregulation in T cells was found to inhibit T-cell proliferation. According to scholars, the reason for the immunosuppression of T cells by MSCs is not to induce T-cell apoptosis, but to arrest T cells at the G0/G1 phase of the cell cycle (89). The effects of MSCs on T cells involve changes in several signaling pathways, such as the PI3K-AKT, RAS-MEK-ERK, STAT3 phosphorylation, FAS/FASL, and WNT/-catenin pathways, which are all involved in this process. MSC-MVs were found to inhibit T lymphocyte proliferation, and these effects were more significant in the MSC-MV group than in the MSC group (56).
Effects of MDS-MSCs on B lymphocytes
The inhibition of B cells by MSCs does not require cell-cell contact, but is mediated by humoral factors secreted by MSCs; in addition, it is associated with decreased Blimp-1 mRNA expression. Unlike T cells, IL-10, TGF-β, and IDO are not involved in the inhibition of B cells by MSCs. MSCs increase the expression of IgG3 in B cells. Further, MSCs could inhibit the proliferation of B cells induced by lipopolysaccharide (LPS), but had no effect on plasma cell apoptosis. Plasmablast generation was found to be inhibited in the presence of MSC-derived extracellular vehicles (EVs) (90). In vitro, MSC-EVs preferentially select B lymphocytes cells when binding to immune cells (90).
Effects of MDS-MSCs on NK cells
NK cells exert their intrinsic antitumor activity by lysis of defective cells. However, MDS-MSCs desensitize this NK cell activity.
In HR-MDS, the number of NK cells decrease, allowing the proliferation of malignant clones. However, NK cells appear to be cytotoxic toward malignant clones, thereby slowing disease progression (91–94).
When cultured in vitro, MSCs from patients with MDS and healthy donors have similar phenotypes, and co-culture with NK cells did not affect NK cell function. However, when MDS-MSCs and HD-MSCs were co-cultured with monocytes, only MDS-MSCs could induce monocytes to possess the phenotypic and metabolic characteristics of myeloid-derived suppressor cells (MDSCs), thereby inhibiting NK cell function. In addition to inhibiting NK cell degranulation and proliferation, monocytes under the MDS-MSC condition also inhibited the production of IFN-γ and TNF-α of NK cells, which was similar to the situation of NK cells co-cultured with MDSCs in vitro (95). Carlsten et al. reported that MDS-MSCs played a role in suppressing the innate immune system by inducing monocytes, while HD-MSCs did not. The key receptors controlling human NK cell self-recognition are HLA class I-binding receptors, which include the killer immunoglobulin-like receptor (KIR) family, natural killer group 2A (NKG2A) receptors, and leukocyte immunoglobulin-like receptor subfamily B member 1 (LILRB1, also known as LIR-1) (96). Several studies have confirmed that NK cells decrease NKG2D and DNAM-1 expression, especially in patients with HR-MDS (97). MSCs impair resting NK cell cytolytic activity through the production of IDO and PGE2 (98). Carlsten et al. further reported that in vitro, NK cells lose their potent anti-tumor properties, as indicated by impaired cytotoxicity to CD34+ MDS blast cells, resulting in enhanced tumor evasion. MSC-MVs were found to inhibit NK cell proliferation, and these inhibitory effects were more significant in the MV group than in the MSC group (56).
Treg cell and MDSC regulation by MDS-MSCs
Treg cells play an immunomodulatory role by suppressing abnormal or excessive immune responses, either to self- or non-self-antigens, thereby maintaining immune homeostasis. Treg cells inhibit the antitumor immune response and participate in the occurrence and progression of tumors. They also play separate and unique roles in LR-MDS and HR-MDS. In LR-MDS, T-cell activation and apoptosis were not excessively inhibited due to the small number of Treg cells. However, the number of Treg cells is increased in HR-MDS, which inhibits the endogenous immune system ability to clear malignant clonal cells, and thus, malignant clonal cells proliferate (91). Zhao et al. reported that HR-MDS-MSCs could induce CD4+CD25 T cells to transform into CD4+CD25+Foxp3+Treg cells, and the induction rate was significantly higher than that of LR-MDS-MSCs, and the induced CD4+CD25+Foxp3+Treg cells caused T-cell inhibition (77). In late-stage MDS, anti-leukemic immunity is decreased, which may be related to Treg cell expansion at this stage (99), which is also associated with higher BM blast infiltration, higher IPSS scores, and disease progression (100). MSCs can secrete PGE2, which regulates the conversion of Th17 to Treg cells (101). MSCs can induce Treg cell expansion via IL-10 secretion. IL-35 is secreted mainly by Treg cells, and is an effector molecule through which these cells exert negative immunoregulatory effects; IL-35 can induce CD4+ T-cell differentiation into Treg cells with immunosuppressive functions. In addition, IL-35 inhibits Th17 proliferation and differentiation. Furthermore, IL-35-transfected MSCs were found to induce an increase in Treg cell counts (102). MSC-exposed Treg cells exhibit increased immunosuppressive activity; this effect may be related to the activation of PD-1 receptor on Treg cell membranes and the production of IL-10 in the MSC/Treg co-culture system (103). Moreover, MSC-mediated Treg cell function is related to PGE2 and MSC TGF-β, as well as contact-dependent mechanisms (104).
MDSCs are derived from immature myeloid cells and have the ability to inhibit immune cell response. The number of such cells is remarkably increased in patients with tumors; this accelerates tumor progression (105–107). This immunosuppression in patients with cancer is associated with a variety of factors, such as concentrations of soluble factors with immunosuppressive activity, loss of effective antigen presentation, abnormal effector cell function, and the recruitment of immunosuppressive cell populations, such as MDSCs (108, 109). The number of MDSC cells in the PB and BM of patients with HR-MDS were significantly higher than those of patients with LR-MDS (110, 111). In tumors, MDSC contributes to the formation of immune tolerance microenvironment, in which MSC promotes the expansion of MDSC (80). Moreover, the expression levels of signal transducer and activator of transcription 3 (STAT3) and C-C chemokine receptor type (CCR) 2 on MDSCs were increased in HR-MDS. Targeted inhibition of the STAT3 pathway reduced ARG1 expression in MDSCs and partially reverses the decrease in effector molecules in CD8+ T lymphocytes (112). It has been confirmed that MSCs in the BM microenvironment promote the expansion and activation of MDSC by secreting hepatocyte growth factor (HGF), which also involves the activation of the STAT3 pathway (113). Moreover, MDSCs not only inhibited T-cell activity, but also induced CD4+CD25highCD127low Treg cells, thus further improving immunosuppression (113). The growth-regulated oncogene (GRO) chemokine GRO-γ, secreted by MSCs, induced the expansion of MDSC. The transformation of monocyte-derived dendritic cells into MDSC was induced when GRO-γ was added to MSC medium (106). BM-MDSCs inhibit T-cell function without close contact between MDSCs and T cells; this involves increased expression levels of nitric oxide, ARG1, and immunosuppressive cytokines (114–116). MDSCs secrete immunosuppressive cytokines, thereby reducing effector T-cell proliferation. In addition, there is usually an increase in MDSC counts in the BM in MDS, and the magnitude of the abundance of MDSCs is a poor prognostic indicator in such patients. PD-L1 expressed on MDSC membranes binds to T-cell-expressed PD-1, thereby inhibiting T-cell function (117, 118). BM-MSC-derived exosomes were found to stimulate MDSCs in vivo, thereby increasing their nitric oxide delivery capacity, in which nitric oxide participates in T-cell blockage (106).
Effects of MDS-MSCs on macrophages
Macrophages interact with and regulate HSCs (119). They also provide critical support for erythropoiesis in the BM erythroblastic islands (120). Macrophages produce vascular endothelial growth factor (VEGF), which stimulates angiogenesis. Studies have shown increased blood vessel density in the BM in patients with MDS, and macrophages may play an indirect role in disease development (121). Some studies have found that the action of pro-apoptotic cytokines causes an increase in the number of macrophages in the BM in MDS (122). Other scholars found that macrophages were inhibited in MDS, and this was more evident in HR-MDS (123). Notably, hematopoietic precursor cell apoptosis is a hematopoietic feature of LR-MDS (124). As macrophages are responsible for engulfing apoptotic cells in the BM (125, 126), they may play a particularly important role in LR-MDS. However, the alteration of macrophage phagocytic capacity MDS has not been determined. In a mouse stem cell transplantation model, macrophages were important for successful HSC implantation and growth, suggesting their role in maintaining BM homeostasis and normal hematopoiesis (91). Further, a study showed that EVs can affect macrophage maturation, inducing lower TNF-α and higher IL-10 expression levels in these cells (127). Another study showed that MSC-EVs decrease the expression levels of several proinflammatory signaling molecules secreted by M1 macrophages, such as CCL5, TNF-α, and IL-6, while promoting the expression of the M2 macrophage-derived immunomodulatory factor, arginase 1 (128).
Effects of MDS-MSCs on dendritic cells
Dendritic cells are specialized in terms of processing and extracting various antigenic substances. They are antigen-presenting cells and are crucial for T-cell activation. MDS-associated DCs become dysfunctional, impairing immune responses (129). In addition, decreased DC counts have been observed in MDS states, with this decrease being more significant in HR-MDS than in LR-MDS (130). MSCs can impair DC antigen presentation by inhibiting the expression of MHC I and II or costimulatory proteins (such as CD83), or inhibiting the production of IL-12 (131, 132). Studies have shown that MDS-associated DCs can activate endogenous T cells; furthermore, it has been reported that the number of DCs are reduced in MDS, and DCs show defects in the activation effect of Treg cells (133). This finding is supported by data from recent in vitro studies. Analysis of DCs isolated from MDS (MDS-refractory anemia and MDS-refractory anemia with ringed sideroblasts) showed a significant reduction in the number of mature and immature DCs and an inhibition of the ability of DCs to present antigen to Treg cells (134), highlighting a significant opportunity for therapeutic intervention (130). During DC and naïve T-cell co-culture, MSC-EV-stimulated immature DCs were found to produce immunomodulatory factors, such as TGF-β and PGE2, thereby inducing Treg cell production (128).
Effects of MDS-MSCs on neutrophils
Besides inhibiting neutrophil respiratory burst, BM-MSCs can also prolong neutrophil survival and thus play an anti-inflammatory role, which involves the IL-6 and STAT-3 signaling pathways. Neutrophils in patients with MDS appear to be significantly defective, resulting in an inability to perform anti-infective functions. For example, Cao et al. demonstrated defective chemokine-dependent chemotaxis and enhanced degranulation associated with the expression of key structural proteins, such as DOCK8, Cdc42, and Rac1, in neutrophils derived from 12 patients with MDS (135). This finding confirms that in these patients, neutrophil defects prevent it from migrating effectively, and this may lead to altered microenvironment and ultimately adverse clinical outcomes. In vitro, MSC-MVs preferentially promote granulocyte expansion (56).
Conclusions
BM-MSCs are involved in immunoregulation; MDS has various pathogenetic mechanisms, among which BM-MSCs play a significant role in the immune-related pathogenesis. In addition to their immunomodulatory properties, BM-MSCs have low immunogenicity (136), rendering them useful for the treatment of immunological disorders (137). The advantages of exosomes in disease treatment include their low toxicity, biological barrier penetrability, stability, and biocompatibility (138). In addition, the advantages of using exosomes for the treatment of hematological malignancies are reflected in that, firstly, it is a non-invasive treatment method, and secondly, its mechanism can protect the exosome contents from the degradation of nucleases and proteases. Exosomes can be used as liquid biopsies to diagnose and stage disease and for monitoring disease progression and response to treatment (139). In addition, drug-loaded exosomes can bind to adjacent cell membranes to transport drugs to target cells. Another advantage of drug-loaded exosomes is that they prevent rapid clearance by the phagocytic system and increase the duration of action of the drug in vivo. As an endogenous component of the body, exosomes are expected to become biological carriers for drug transport (140–142). In addition to monoclonal antibody-based therapy against immune cells, an BM-MSC-based therapeutic strategy against immunoregulatory targets will serve as a novel option for the treatment of patients with MDS.
Author contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cazzola M. Myelodysplastic syndromes. N Engl J Med (2020) 383:1358–74. doi: 10.1056/NEJMra1904794
2. Côme C, Balhuizen A, Bonnet D, Porse BT. Myelodysplastic syndrome patient-derived xenografts: From no options to many. Haematologica (2020) 105:864–9. doi: 10.3324/haematol.2019.233320
3. Spencer Chapman M, Ranzoni AM, Myers B, Williams N, Coorens THH, Mitchell E, et al. Lineage tracing of human development through somatic mutations. Nature (2021) 595:85–90. doi: 10.1038/s41586-021-03548-6
4. Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature (2009) 462:739–44. doi: 10.1038/nature08617
5. Francine EG, Ari MM. Mutant IDH: a targetable driver of leukemic phenotypes linking metabolism, epigenetics and transcriptional regulation. Epigenomics (2016) 8:945–57. doi: 10.2217/epi-2016-0008
6. Yoshimi A, Lin K-T, Wiseman DH, Rahman MA, Pastore A, Wang B, et al. Coordinated alterations in RNA splicing and epigenetic regulation drive leukaemogenesis. Nature (2019) 574:273–7. doi: 10.1038/s41586-019-1618-0
7. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell (2000) 100:57–70. doi: 10.1016/s0092-8674(00)81683-9
8. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
9. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation (1968) 6:230–47. doi: 10.1097/00007890-196803000-00009
10. Rastegar F, Shenaq D, Huang J, Zhang W, Zhang B-Q, He B-C, et al. Mesenchymal stem cells: Molecular characteristics and clinical applications. World J Stem Cells (2010) 2:67–80. doi: 10.4252/wjsc.v2.i4.67
11. Salter AB, Meadows SK, Muramoto GG, Himburg H, Doan P, Daher P, et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood (2009) 113:2104–7. doi: 10.1182/blood-2008-06-162941
12. Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev (2006) 20:161–71. doi: 10.1016/j.blre.2005.11.002
13. Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, et al. Vascular niche e-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med (2012) 18:1651–7. doi: 10.1038/nm.2969
14. Das M, Chatterjee S, Basak P, Das P, Pereira JA, Dutta RK, et al. The bone marrow stem stromal imbalance–a key feature of disease progression in case of myelodysplastic mouse model. J Stem Cells (2010) 5:49–64.
15. Mishima S, Nagai A, Abdullah S, Matsuda C, Taketani T, Kumakura S, et al. Effective ex vivo expansion of hematopoietic stem cells using osteoblast-differentiated mesenchymal stem cells is CXCL12 dependent. Eur J Haematol (2010) 84:538–46. doi: 10.1111/j.1600-0609.2010.01419.x
16. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity (2006) 25:977–88. doi: 10.1016/j.immuni.2006.10.016
17. Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature (2012) 481:457–62. doi: 10.1038/nature10783
18. Salem HK, Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells (2010) 28:585–96. doi: 10.1002/stem.269
19. Rathnayake AJIS, Goonasekera HWW, Dissanayake VHW. Phenotypic and Cytogenetic Characterization of Mesenchymal stromal Cells in De novo myelodysplastic syndromes. Anal Cell Pathol (Amst) (2016) 2016:8012716. doi: 10.1155/2016/8012716
20. Johnson RC, Greenberg PL, Gratzinger D. CD271+ mesenchymal stromal cell density is high in poor-risk MDS and independently predicts overall survival. Blood (2013) 122:1560–0. doi: 10.1182/blood.V122.21.1560.1560
21. Fei C, Zhao YS, Guo J, Gu SC, Li X, Chang CK. Senescence of bone marrow mesenchymal stromal cells is accompanied by activation of p53/p21 pathway in myelodysplastic syndromes. Eur J Haematol (2014) 93:476–86. doi: 10.1111/ejh.12385
22. Johnson RC, Kurzer JH, Greenberg PL, Gratzinger D. Mesenchymal stromal cell density is increased in higher grade myelodysplastic syndromes and independently predicts survival. Am J Clin Pathol (2014) 142:795–802. doi: 10.1309/AJCP71OPHKOTLSUG
23. Ferrer RA, Wobus M, List C, Wehner R, Schönefeldt C, Brocard B, et al. Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide. Hematologica (2013) 98:1677–85. doi: 10.3324/haematol.2013.083972
24. Pavlaki K, Pontikoglou CG, Demetriadou A, Batsali AK, Damianaki A, Simantirakis E. Impaired proliferative potential of bone marrow mesenchymal stromal cells in patients with myelodysplastic syndromes is associated with abnormal WNT signaling pathway. Stem Cells Dev (2014) 23:1568–81. doi: 10.1089/scd.2013.0283
25. Falconi G, Fabiani E, Fianchi L, Criscuolo M, Raffaelli CS, Bellesi S, et al. Impairment of PI3K/AKT and WNT/β-catenin pathways in bone marrow mesenchymal stem cells isolated from patients with myelodysplastic syndromes. Exp Hematol (2016) 44:75–83. doi: 10.1016/j.exphem.2015.10.005
26. Geyh S, Rodríguez-Paredes M, Jäger P, Koch A, Bormann F, Gutekunst J, et al. Transforming growth factor beta1-mediated functional inhibition of mesenchymal stromal cells in myelodysplastic syndromes and acute myeloid leukemia. Haematologica (2018) 103:1462–71. doi: 10.3324/haematol.2017.186734
27. Pang YB, Deng CX, Geng SX, Weng JY, Lai PL, Liao PJ, et al. Premature exhaustion of mesenchymal stromal cells from myelodysplastic syndrome patients. Am J Transl Res (2017) 9:3462–8.
28. Santamaría C, Muntión S, Rosón B, Blanco B, López-Villar O, Carrancio S, et al. Impaired expression of DICER, DROSHA, SBDS and some microRNAs in mesenchymal stromal cells from myelodysplastic syndrome patients. Haematologica (2012) 97:1218–24. doi: 10.3324/haematol.2011.054437
29. Mattiucci D, Maurizi G, Leoni P, Poloni A. Aging - and senescence -associated changes of mesenchymal stromal cells in myelodysplastic syndromes. Cell Transplant (2018) 27:754–64. doi: 10.1177/0963689717745890
30. Ping Z, Chen S, Hermans SJF, Kenswil KJG, Feyen J, Dijk CV, et al. Activation of NF-κB driven inflammatory programs in mesenchymal elements attenuates hematopoiesis in low-risk myelodysplastic syndromes. Leukemia (2019) 33:536–41. doi: 10.1038/s41375-018-0267-x
31. Geyh S, Oz S, Cadeddu RP, Fröbel J, Brückner B, Kündgen A, et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia (2013) 27:1841–51. doi: 10.1038/leu.2013.193
32. Fei GM, Zhao YS, Gu SC, Guo J, Zhang X, Li X, et al. Impaired osteogenic differentiation of mesenchymal stem cells derived from bone marrow of patients with lower-risk myelodysplastic syndromes. Tumour Biol (2014) 35:4307–16. doi: 10.1007/s13277-013-1565-6
33. Balaian E, Wobus M, Weidner H, Baschant U, Stiehler M, Ehninger G, et al. Erythropoietin inhibits osteoblast function in myelodysplastic syndromes via the canonical wnt pathway. Haematologica (2018) 103:61–8. doi: 10.3324/haematol.2017.172726
34. Fattizzo B, Giannotta JA, Barcellini W. Mesenchymal stem cells in aplastic anemia and myelodysplastic syndromes: The “Seed and soil” crosstalk. Int J Mol Sci (2020) 21:5438. doi: 10.3390/ijms21155438
35. Han Q, Sun Z, Liu L, Chen B, Cao Y, Li KH, et al. Impairment in immuno-modulatory function of Flk1(+)CD31(–)CD34(–) MSCs from MDS-RA patients. Leuk Res (2007) 31:1469–78. doi: 10.1016/j.leukres.2006.12.016
36. Klaus M, Stavroulaki E, Kastrinaki MC, Fragioudaki P, Giannikou K, Psyllaki M, et al. Reserves, functional, immunoregulatory, and cytogenetic properties of bone marrow mesenchymal stem cells in patients with myelodysplastic syndromes. Stem Cells Dev (2010) 19:1043–54. doi: 10.1089/scd.2009.0286
37. Wu L, Amarachintha S, Xu J, Oley F Jr., Du W. Mesenchymal COX2-PG secretome engages NR4A-WNT signalling axis in haematopoietic progenitors to suppress anti-leukaemia immunity. Br J Haematol (2018) 183:445–56. doi: 10.1111/bjh.15548
38. Zhang L, Dang RJ, Li H, Li P, Yang YM, Guo XM, et al. SOCS1 regulates the immune modulatory properties of mesenchymal stem cells by inhibiting nitric oxide production. PLo S One (2014) 9:e97256. doi: 10.1371/journal.pone.0097256
39. Azuma K, Umezu T, Imanishi S, Asano M, Yoshizawa S, Katagiri S, et al. Genetic variations of bone marrow mesenchymal stromal cells derived from acute leukemia and myelodysplastic syndrome by targeted deep sequencing. Leuk Res (2017) 62:23–8. doi: 10.1016/j.leukres.2017.09.008
40. Jann JC, Mossner M, Riabov V, Altrock E, Schmitt N, Flach J, et al. Bone marrow derived stromal cells from myelodysplastic syndromes are altered but not clonally mutated in vivo. Nat Commun (2021) 12:6170. doi: 10.1038/s41467-021-26424-3
41. Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature (2014) 506:240–4. doi: 10.1038/nature12883
42. Chen S, Zambetti NA, Bindels EMJ, Kenswill K, Mylona AM, Adisty NM, et al. Massive parallel RNA sequencing of highly purified mesenchymal elements in low-risk MDS reveals tissue-Context-Dependent activation of inflammatory programs. Leukemia (2016) 30:1938–42. doi: 10.1038/leu.2016.91
43. Marc HGPR, Siddhartha M, Shangqin G, Siyi Z, Tatsuya K, Jesse AS, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukemia. Nature (2010) 8:852–7. doi: 10.1038/nature08851
44. Bella B, Rebekka KS. Mesenchymal stromal cells as a cellular target in myeloid malignancy: Chances and challenges in the genome editing of stromal alterations. Front Genome Ed (2020) 2:618308. doi: 10.3389/fgeed.2020.618308
45. Tushar DB, Si C, Matthias B A, Trevor B, Dagny VA, Gaurav SC, et al. Epigenetically aberrant stroma in MDS propagates disease Via wnt/β-catenin activation. Cancer Res (2017) 77:4846–57. doi: 10.1158/0008-5472.CAN-17-0282
46. Rudolf AR, Franziska J, Bernd R, Birgit E, Günther W, Thomas H, et al. Stroma-mediated dysregulation of myelopoiesis in mice lacking I kappa b alpha. Immunity (2005) 22:479–91. doi: 10.1016/j.immuni.2005.02.009
47. Poon ZY, Dighe N, Venkatesan SS, Cheung AMS, Fan X, Bari S, et al. Bone marrow MSCs in MDS: contribution towards dysfunctional hematopoiesis and potential targets for disease response to hypomethylating therapy. Leukemia (2019) 33:1487–500. doi: 10.1038/s41375-018-0310-y
48. Medyouf H, Mossner M, Jann JC, Nolte F, Raffel S, Herrmann C, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell (2014) 14:824–37. doi: 10.1016/j.stem.2014.02.014
49. Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: The paradigmatic tissue-specific stem cell. Am J Pathol (2006) 169:338–46. doi: 10.2353/ajpath.2006.060312
50. Pleyer L, Valent P, Greil R. Mesenchymal Stem and Progenitor Cells in Normal and Dysplastic Hematopoiesis–masters of Survival and Clonality? Int J Mol Sci (2016) 17:1009. doi: 10.3390/ijms17071009
51. Isern J, Méndez-Ferrer S. Stem cell interactions in a bone marrow niche. Curr Osteoporos Rep (2011) 9:210–8. doi: 10.1007/s11914-011-0075-y
52. Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia (2008) 22:941–50. doi: 10.1038/leu.2008.48
53. Boada M, Echarte L, Guillermo C, Diaz L, Touriño C, Grille S. 5-Azacytidine restores interleukin 6-increased production in mesenchymal stromal cells from myelodysplastic patients. Hematol Transfus Cell Ther (2021) 43:35–42. doi: 10.1016/j.htct.2019.12.002
54. Catharina W, Anne-Kathrin G, Sonja G, Christina H, Denis W, Marie W, et al. Direct modulation of the bone marrow mesenchymal stromal cell compartment by azacitidine enhances healthy hematopoiesis. Blood Adv (2018) 2:3447–61. doi: 10.1182/bloodadvances.2018022053
55. Muntión S, Ramos TL, Diez-Campelo M, Rosón B, Sánchez-Abarca LI, Misiewicz-Krzeminska I, et al. Microvesicles from mesenchymal stromal cells are involved in HPC-microenvironment crosstalk in myelodysplastic patients. PloS One (2016) 11:e0146722. doi: 10.1371/journal.pone.0146722
56. Xie H, Sun L, Zhang LM, Liu T, Chen L, Zhao AQ, et al. Mesenchymal stem cell-derived microvesicles support ex vivo expansion of cord blood-derived CD34+ cells. Stem Cells Int (2016) 2016:6493241. doi: 10.1155/2016/6493241
57. Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell (2007) 12:528–41. doi: 10.1016/j.ccr.2007.11.003
58. Teofili L, Martini M, Nuzzolo ER, Capodimonti S, Iachininoto MG, Cocomazzi A, et al. Endothelial progenitor cell dysfunction in myelodysplastic syndromes: Possible contribution of a defective vascular niche to myelodysplasia. Neoplasia (2015) 17:401–9. doi: 10.1016/j.neo.2015.04.001
59. Naveiras O, Nardi V, Wenzel PL, Fahey F, Daley GQ. Bone marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature (2009) 460:259–63. doi: 10.1038/nature08099
60. Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, et al. Quantitative imaging of hematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol (2013) 15:533–43. doi: 10.1038/ncb2730
61. Schroeder T, Geyh S, Germing U, Haas H. Mesenchymal stromal cells in myeloid malignancies. Blood Res (2016) 51:225–32. doi: 10.5045/br.2016.51.4.225
62. Krohn-Grimberghe M, Mitchell MJ, Schloss MJ, Khan OF, Courties G, Guimaraes PPG, et al. Nanoparticle-encapsulated siRNAs for gene silencing in the haematopoietic stem-cell niche. Nat Biomed Eng (2020) 4:1076–89. doi: 10.1038/s41551-020-00623-7
63. Bellamy WT, Dalton WS, Gleason MC, Grogan TM, Trentet JM. Development and characterization of a melphalan-resistant human multiple myeloma cell line. Cancer Res (1991) 51:995–1002.
64. Goldie JH, Coldman AJA. Mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep (1979) 63:1727–33.
65. Teicher BA, Herman TS, Holden SA, Wang YY, Pfeffer MR, Crawford JW, et al. Tumor resistance to alkylating agents conferred by mechanisms operative only in vivo. Science (1990) 247:1457–61. doi: 10.1126/science.247.4949.1457
66. Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Daltonet WS. Cell adhesion mediated drug resistance (CAM-DR): Role of integrins and resistance to apoptosis in human myeloma cell lines. Blood (1999) 93:1658–67. doi: 10.1182/blood.V93.5.1658
67. Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res (2008) 14:2519–26. doi: 10.1158/1078-0432.CCR-07-2223
68. Cordes N, Seidler J, Durzok R, Geinitz H, Brakebusch C. β 1-Integrin-Mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene (2006) 25:1378–90. doi: 10.1038/sj.onc.1209164
69. Lwin T, Hazlehurst LA, Dessureault S, Lai R, Bai W, Sotomayor E, et al. Cell adhesion induces P27Kip1-associated cell-cycle arrest through down-regulation of the SCFSkp2 ubiquitin ligase pathway in mantle-cell and other non-Hodgkin b-cell lymphomas. Blood (2007) 110:1631–8. doi: 10.1182/blood-2006-11-060350
70. Alonso S, Su M, Jones JW, Ganguly S, Kane MA, Jones RJ, et al. Human bone marrow niche chemoprotection mediated by cytochrome P450 enzymes. Oncotarget (2015) 6:14905–12. doi: 10.18632/oncotarget.3614
71. Brenner AK, Nepstad I, Bruserud O. Mesenchymal stem cells support survival and proliferation of primary human acute myeloid leukemia cells through heterogeneous molecular mechanisms. Front Immunol (2017) 8:106. doi: 10.3389/fimmu.2017.00106
72. Moschoi R, Imbert V, Nebout M, Chiche J, Mary D, Prebet T, et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood (2016) 128:253–64. doi: 10.1182/blood-2015-07-655860
73. Marlein CR, Zaitseva L, Piddock RE, Robinson SD, Edwards DR, Shafat MS, et al. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood (2017) 130:1649–60. doi: 10.1182/blood-2017-03-772939
74. Jacamo R, Chen Y, Wang Z, Wencai M, Zhang M, Spaeth EL, et al. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-κB mediates chemoresistance. Blood (2014) 123:2691–702. doi: 10.1182/blood-2013-06-511527
75. Greenbaum A, Hsu YMS, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. CXCL12 production by early mesenchymal progenitors is required. Nature (2013) 495:227–30. doi: 10.1038/nature11926
76. Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood (2009) 113:6206–14. doi: 10.1182/blood-2008-06-162123
77. Zhao Z, Wang Z, Li Q, Li WM, You Y, Zou P, et al. The different immunoregulatory functions of mesenchymal stem cells in patients with low-risk or high-risk myelodysplastic syndromes. PloS One (2012) 7:e45675. doi: 10.1371/journal.pone.0045675
78. Montes P, Bernal M, Campo LN, González-Ramírez AR, Jiménez P, Garrido P, et al. Tumor genetic alterations and features of the immune microenvironment drive myelodysplastic syndrome escape and progression. Cancer Immunol Immunother (2019) 68:2015–27. doi: 10.1007/s00262-019-02420-x
79. Müller L, Tunger A, Wobus M, Bonin MV, Towers R, Bornhäuser M, et al. Immunomodulatory properties of mesenchymal stromal cells: An update. Front Cell Dev Biol (2021) 9:637725. doi: 10.3389/fcell.2021.637725
80. Kapor S, Santibanez JF. Myeloid-derived suppressor cells and mesenchymal Stem/Stromal cells in myeloid malignancies. J Clin Med (2021) 10:2788. doi: 10.3390/jcm10132788
81. Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, et al. A critical role of IFN[gamma] in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res (2008) 18:846–57. doi: 10.1038/cr.2008.80
82. Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell (2018) 2:141–50. doi: 10.1016/j.stem.2007.11.014
83. Munn DH, Mellor AL. IDO in the tumor microenvironment: Inflammation, counter-regulation, and tolerance. Trends Immunol (2016) 37:193–207. doi: 10.1016/j.it.2016.01.002
84. Zhi-Gang Z, Wei-Ming L, Zhi-Chao C, Yong Y, Ping Z. Immunosuppressive properties of mesenchymal stem cells derived from bone marrow of patient with hematological malignant diseases. Leuk Lymphoma (2008) 49:2187–95. doi: 10.1080/10428190802455875
85. Sato K, Ozaki K. Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood (2007) 109:228–34. doi: 10.1182/blood-2006-02-002246
86. Chow L, Johnson V, Coy J, Regan D, Dow S. Mechanisms of immune suppression utilized by canine adipose and bone marrow-derived mesenchymal stem cells. Stem Cells Dev (2017) 26:374–89. doi: 10.1089/scd.2016.0207
87. Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation (2003) 76:1208–13. doi: 10.1097/01.TP.0000082540.43730.80
88. Davies LC, Heldring N, Kadri N, Blanc KL. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells (2017) 35:766–76. doi: 10.1002/stem.2509
89. Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood (2005) 105:2821–7. doi: 10.1182/blood-2004-09-3696
90. Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics (2018) 8:1399–410. doi: 10.7150/thno.21072
91. Lynch OF, Calvi LM. Immune dysfunction, cytokine disruption, and stromal changes in myelodysplastic syndrome: A review. Cells (2022) 11:580. doi: 10.3390/cells11030580
92. Chamuleau MED, Westers TM, Dreunen LV, Groenland J, Zevenbergen A, Eeltink CM, et al. Immune mediated autologous cytotoxicity against hematopoietic precursor cells in patients with myelodysplastic syndrome. Haematologica (2009) 94:496–506. doi: 10.3324/haematol.13612
93. Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood (2007) 109:4816–24. doi: 10.1182/blood-2006-07-035519
94. Kiladjian JJ, Bourgeois E, Lobe I, Braun T, Visentin G, Bourhis JH, et al. Cytolytic function and survival of natural killer cells are severely altered in myelodysplastic syndromes. Leukemia (2006) 20:463–70. doi: 10.1038/sj.leu.2404080
95. Sarhan D, Wang J, Arvindam US, Hallstrom C, Verneris MR, Grzywacz B, et al. Mesenchymal stromal cells shape the MDS microenvironment by inducing suppressive monocytes that dampen NK cell function. JCI Insight (2020) 5:e130155. doi: 10.1172/jci.insight.130155
96. Carlsten M, Jaras M. Natural killer cells in myeloid malignancies: Immune surveillance, NK cell dysfunction, and pharmacological opportunities to bolster the endogenous NK cells. Front Immunol (2019) 10:2357. doi: 10.3389/fimmu.2019.02357
97. Carlsten M, Baumann BC, Simonsson M, Jadersten M, Forsblom AM, Hammarstedt C, et al. Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia (2010) 24:1607–16. doi: 10.1038/leu.2010.149
98. Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood (2008) 111:1327–33. doi: 10.1182/blood-2007-02-074997
99. Kotsianidis I, Bouchliou I, Nakou E, Spanoudakis E, Margaritis D, Christophoridou AV, et al. Kinetics, function and bone marrow trafficking of CD4 + CD25 + FOXP3+ regulatory T cells in myelodysplastic syndromes (MDS). Leukemia (2009) 23:510–8. doi: 10.1038/leu.2008.333
100. Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, et al. CD4+ CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood (2007) 110:847–50. doi: 10.1182/blood-2007-01-067546
101. Obermajer N, Popp FC, Soeder Y, Haarer J, Geissler EK, Schlitt HJ, et al. Conversion of Th17 into IL-17Aneg regulatory T cells: A novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell–supported minimized immunosuppressive therapy. J Immunol (2014) 193:4988–99. doi: 10.4049/jimmunol.1401776
102. Zhao N, Li H, Yan Y, Jiang R, He X. Mesenchymal stem cells overexpressing IL-35 effectively inhibit CD4+ T cell function. Cell Immunol (2017) 312:61–6. doi: 10.1016/j.cellimm.2016.12.001
103. Yan Z, Zhuansun Y, Chen R, Li J, Ran P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp Cell Res (2014) 324:65–74. doi: 10.1016/j.yexcr.2014.03.013
104. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol (2009) 156:149–60. doi: 10.1111/j.1365-2249.2009.03874.x
105. Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother (2006) 55:237–45. doi: 10.1007/s00262-005-0048-z
106. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol (2009) 9:162–74. doi: 10.1038/nri2506
107. Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol (2021) 21:485–98. doi: 10.1038/s41577-020-00490-y
108. De Veirman K, Menu E, Maes K, De Beule N, De Smedt E, Maes A, et al. Myeloid-derived suppressor cells induce multiple myeloma cell survival by activating the AMPK pathway. Cancer Lett (2019) 442:233–41. doi: 10.1016/j.canlet.2018.11.002
109. Leone P, Solimando AG, Malerba E, Fasano R, Buonavoglia A, Pappagallo F, et al. Actors on the scene: Immune cells in the myeloma niche. Front Oncol (2020) 10:599098. doi: 10.3389/fonc.2020.599098
110. Kordasti SY, Afzali B, Lim Z, Ingram W, Hayden J, Barber L, et al. IL-17-producing CD4+ T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol (2009) 145:64–72. doi: 10.1111/j.1365-2141.2009.07593.x
111. Kittang AO, Kordasti S, Sand KE, Costantini B, Kramer AM, Perezabellan P, et al. Expansion of myeloid derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. Oncoimmunology (2015) 5:e1062208. doi: 10.1080/2162402X.2015.1062208
112. Qi X, Jiang H, Liu P, Xie N, Fu R, Wang H, et al. Increased myeloid-derived suppressor cells in patients with myelodysplastic syndromes suppress CD8+ T lymphocyte function through the STAT3-ARG1 pathway. Leuk Lymphoma (2021) 62:218–23. doi: 10.1080/10428194.2020.1817431
113. Yen BL, Yen ML, Hsu PJ, Liu KJ, Wang CJ, Bai CH, et al. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Rep (2013) 1:139–51. doi: 10.1016/j.stemcr.2013.06.006
114. Yang Y, Li C, Liu T, Dai X, Bazhin AV. Myeloid-derived suppressor cells in tumors: From mechanisms to antigen specificity and microenvironmental regulation. Front Immunol (2020) 11:1371. doi: 10.3389/fimmu.2020.01371
115. Yang Z, Guo J, Weng L, Tang W, Jin S, Ma W. Myeloid-derived suppressor cells-new and exciting players in lung cancer. J Hematol Oncol (2020) 13:10. doi: 10.1186/s13045-020-0843-1
116. De Cicco P, Ercolano G, Ianaro A. The new era of cancer immunotherapy: Targeting myeloid-derived suppressor cells to overcome immune evasion. Front Immunol (2020) 11:1680. doi: 10.3389/fimmu.2020.01680
117. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. Chouaib s. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med (2014) 211:781–90. doi: 10.1084/jem.20131916
118. Lu C, Redd PS, Lee JR, Savage N, Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology (2016) 5:e1247135.61. doi: 10.1080/2162402X.2016.1247135
119. Kaur S, Raggatt LJ, Millard SM, Wu AC, Batoon L, Jacobsen RN, et al. Self-repopulating recipient bone marrow resident macrophages promote long-term hematopoietic stem cell en-graftment. Blood (2018) 132:735–49. doi: 10.1182/blood-2018-01-829663
120. Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med (2013) 19:429–36. doi: 10.1038/nm.3057
121. Savic A, Cemerikic-Martinovic V, Dovat S, Rajic N, Urosevic I, Sekulic B, et al. Angiogenesis and survival in patients with myelodysplastic syndrome. Pathol Oncol Res (2012) 18:681–90. doi: 10.1007/s12253-012-9495-y
122. Sadahira Y, Wada H, Manabe T, Yawata Y. Immunohistochemical assessment of human bone marrow macrophages in hematologic disorders. Pathol Int (1999) 49:626–32. doi: 10.1046/j.1440-1827.1999.00913.x
123. Aggarwal S, van de Loosdrecht AA, Alhan C, Ossenkoppele GJ, Westers TM, Bontkes HJ. Role of immune responses in the pathogenesis of low-risk MDS and high-risk MDS: Implications for immunotherapy. Br J Haematol (2011) 153:568–81. doi: 10.1111/j.1365-2141.2011.08683.x
124. Banerjee T, Calvi LM, Becker MW, Liesveld JL. Flaming and fanning: The spectrum of inflammatory influences in myelodysplastic syndromes. Blood Rev (2019) 36:57–69. doi: 10.1016/j.blre.2019.04.004
125. Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, Gonzalez NA, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell (2013) 153:1025–35. doi: 10.1016/j.cell.2013.04.040
126. Frisch BJ, Hoffman CM, Latchney SE, LaMere MW, Myers J, Ashton J, et al. Aged marrow macrophages expand platelet-biased hematopoietic stem cells via Interleukin1B. JCI Insight (2019) 5:e124213. doi: 10.1172/jci.insight.124213
127. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep (2017) 7:16214. doi: 10.1038/s41598-017-15376-8
128. Seo Y, Kim HS, Hong IS. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int (2019) 2019:5126156. doi: 10.1155/2019/5126156
129. Kerkhoff N, Bontkes HJ, Westers TM, de Gruijl TD, Kordasti S, van de Loosdrecht AA. Dendritic cells in myelodys-plasticsyndromes: From pathogenesis to immunotherapy. Immunotherapy (2013) 5:621–37. doi: 10.2217/imt.13.51
130. Saft L, Björklund E, Berg E, Hellström-Lindberg E, Porwit A. Bone marrow dendritic cells are reduced in patients with high-risk myelodysplastic syndromes. Leuk Res (2013) 37:266–73. doi: 10.1016/j.leukres.2012.10.010
131. Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood (2005) 105:4120–6. doi: 10.1182/blood-2004-02-0586
132. Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood (2005) 105:2214–9. doi: 10.1182/blood-2004-07-2921
133. Ma L, Delforge M, van Duppen V, Verhoef G, Emanuel B, Boogaerts M, et al. Circulating myeloid and lymphoid precursor dendritic cells are clonally involved in myelodysplastic syndromes. Leukemia (2004) 18:1451–6. doi: 10.1038/sj.leu.2403430
134. Bento LC, Bacal NS, Rocha FA, Severino P, Marti LC. Bone marrow monocytes and derived dendritic cells from myelodysplastic patients have functional abnormalities associated with defective response to bacterial infection. J Immunol (2020) 204:2098–109. doi: 10.4049/jimmunol.1900328
135. Cao M, Shikama Y, Kimura H, Noji H, Ikeda K, Ono T, et al. Mechanisms of impaired neutrophil migration by MicroRNAs in myelodysplastic syndromes. J Immunol (2017) 198:1887–99. doi: 10.4049/jimmunol.1600622
136. Gao M, Yao H, Dong Q, Zhang H, Yang Z, Yang Y, et al. Tumourigenicity and immunogenicity of induced neural stem cell grafts versus induced pluripotent stem cell grafts in syngeneic mouse brain. Sci Rep (2016) 6:29955. doi: 10.1038/srep29955
137. Roemeling-van Rhijn M, Khairoun M, Korevaar SS, Lievers E, Leuning DG, IJzermans JN, et al. Human bone marrow- and adipose tissue-derived mesenchymal stromal cells are immunosuppressive In vitro and in a humanized allograft rejection model. J Stem Cell Res Ther (2013) Suppl 6:20780. doi: 10.4172/2157-7633.S6-001
138. Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy (2009) 11:503–15. doi: 10.1080/14653240903193806
139. Khalife J, Sanchez JF, Pichiorri F. Extracellular vesicles in hematological malignancies: From biomarkers to therapeutic tools. Diagnostics (2020) 10:1065. doi: 10.3390/diagnostics10121065
140. Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J Control Release (2014) 192:262–70. doi: 10.1016/j.jconrel.2014.07.042
141. Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, et al. Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther (2016) 24:1290–301. doi: 10.1038/mt.2016.90
Keywords: myelodysplastic syndrome, mesenchymal stromal cells, immunomodulation, mesenchymal stromal cells-micro vesicles, immune dysfunction
Citation: Zheng L, Zhang L, Guo Y, Xu X, Liu Z, Yan Z and Fu R (2022) The immunological role of mesenchymal stromal cells in patients with myelodysplastic syndrome. Front. Immunol. 13:1078421. doi: 10.3389/fimmu.2022.1078421
Received: 24 October 2022; Accepted: 24 November 2022;
Published: 07 December 2022.
Edited by:
Silvia Gregori, San Raffaele Telethon Institute for Gene Therapy (SR-Tiget), ItalyReviewed by:
Stefania Crippa, IRCCS San Raffaele Scientific Institute, ItalyYizhou Zheng, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2022 Zheng, Zhang, Guo, Xu, Liu, Yan and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Fu, ZnVyb25nODM2OUB0bXUuZWR1LmNu
†These authors share first authorship
 Likun Zheng
Likun Zheng Lei Zhang3†
Lei Zhang3† Yixuan Guo
Yixuan Guo Zhaoyun Liu
Zhaoyun Liu Rong Fu
Rong Fu