
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 25 January 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1073971
This article is part of the Research Topic The Role of Negative Immune Checkpoints in the Treatment of Systemic Lupus Erythematosus and Rheumatoid Arthritis View all 5 articles
Hypoxia-inducible factor-1α (HIF-1α) is a primary metabolic sensor, and is expressed in different immune cells, such as macrophage, dendritic cell, neutrophil, T cell, and non-immune cells, for instance, synovial fibroblast, and islet β cell. HIF-1α signaling regulates cellular metabolism, triggering the release of inflammatory cytokines and inflammatory cells proliferation. It is known that microenvironment hypoxia, vascular proliferation, and impaired immunological balance are present in autoimmune diseases. To date, HIF-1α is recognized to be overexpressed in several inflammatory autoimmune diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and function of HIF-1α is dysregulated in these diseases. In this review, we narrate the signaling pathway of HIF-1α and the possible immunopathological roles of HIF-1α in autoimmune diseases. The collected information will provide a theoretical basis for the familiarization and development of new clinical trials and treatment based on HIF-1α and inflammatory autoimmune disorders in the future.
HIF-1α involves in metabolic pathway, which regulates immune cell function and inflammation (1, 2). The gene encoding HIF-1α is located at chromosome 14q21-q24 (3). Under a normal oxygen condition, proline hydroxylase (PHD) binds to HIF-1α and then combines with E3 ubiquitin ligase (VHL), triggering proline hydroxylation-ubiquitination and proteasomal degradation of HIF-1α (4). In the absence of oxygen, effects of the metabolic pathway is inhibited, and much HIF-1α accumulated in the nucleus, forming the active HIF-1α heterodimer with HIF-1β. Then, the activated HIF-1 binds to hypoxia response element (HRE) in the DNA and regulates expression of target genes related to angiogenesis, apoptosis, and cell migration (5). Expression of HIF-1α was up-regulated in response to tumor necrosis factor-α (TNF-α), interleukin-17A (IL-17A), phosphatidylinositol-3-kinase (PI3K) stimulation, which regulates the homeostasis of immune cells. HIF-1α also plays an anti-infection role in innate immune cells when they sense microorganisms (6). For example, after infecting with mycobacterium tuberculosis, HIF-1α in macrophages increase phagocytosis and accelerate glucose metabolism (7). Hypoxia alters the phenotype of dendritic cells, allowing naive T cells to differentiate into Th2 cells (8). To date, overexpression of HIF-1α was detected in the serum, skin tissue, and urine of different inflammatory autoimmune diseases, such as systemic lupus erythematosus (SLE) (9), rheumatoid arthritis (RA) (10), systemic sclerosis (SSc) (11), and psoriasis (12). In addition, functional studies in vivo and in vitro suggested an important role of HIF-1α in the pathogenesis of these diseases. Interestingly, targeting HIF-1α makes a potential for alleviating inflammatory disorders (13). Therefore, this review summarized the molecular mechanism of HIF-1α and discussed the function of HIF-1α in immune cells, particularly the relationship between HIF-1α and inflammatory autoimmune diseases.
As a nuclear transcription factor, HIF-1α enters the nucleus after binding to HIF-1β, and then activates downstream signaling pathways, inducing generation of inflammatory components, vascularization and cell proliferation (14). Reactive oxygen species (ROS) reflects oxidative stress and cellular inflammatory metabolism, and expression of ROS is elevated under hypoxic condition. Substantial ROS stimulates NF-κB and inhibits activity of PHD and HIF asparaginyl hydroxylase (FIH), allowing HIF-1α to accumulate in the cytoplasm (15, 16). G protein-coupled receptor kinase 2 (GRK2)/HIF-1α are highly expressed after ROS stimulation and then affect expression of nucleotide-binding oligomerization structure-like receptor family Pyrin domain protein 3 (NLRP3) (17). NLRP3 contributes to pro-IL-1β and pro-IL-18 maturation with the aid of enzymes, which will activate IL-1β, IL-18 and lead to cytolytic death. Conversely, low levels of HIF-1α reduce expression of target genes, including phosphoinositide-dependent protein kinase-1 (Pdk1) and glucose transporter type 1 (Glut1), which in turn affect the glycolytic pathway and cellular ATP supply (18). Epidermal growth factor (EGF) and insulin-like growth factor-1 (IGF-1) activate PI3K. Activated PI3K then activates protein kinase B (Akt) on the cell membrane (19). In addition, mammalian target of rapamycin (mTOR) was activated by PI3K/Akt, which then increases HIF-1α expression. Elevated expression of HIF-1α up-regulates expression of VEGF, promoting vascular expansion (20). IL-23-induced glycolysis is diminished after inhibiting the Akt/mTOR/HIF-1α pathway (21). (AMP)-activated protein kinase (AMPK) is activated by SIRT3 stimulation, which then inhibits mTOR/HIF-1α pathway and induces less cell growth and more apoptosis (22–24).
IL-6 binds to gp160, then activates STAT3/HIF-1α, which promotes the proliferation of Foxp3+ regulatory T (Treg) cells and reduces activity and migration of hemangioma-derived stem cells (25, 26). IL-17 induces defective autophagy through interacting with STAT3/HIF-1α and causes inflammatory death of keloid fibroblasts (27). In addition, Janus kinase (JAK) signaling, an important upstream activator of STAT3, directly promotes NLRP3 expression and IL-1β secretion to aggravate inflammation (28). The classical mitogen-activated protein kinases (MAPK) and regulated extracellular protein kinases (ERK) pathways adapt to hypoxia, and then activate HIF-1α, thereby protecting cell development and avoiding oxidative damage (29, 30).
Under hypoxia, high mobility group proteins 1 (HMGB1) accelerated the c-Jun N-terminal kinase (JNK) pathway to stimulate HIF-1α/vascular endothelial growth factor (VEGF) axis, which is conducive to angiogenesis (17). In addition, TNF-α interacts with transforming growth factor-activated kinase 1 (TAK1), and promotes HIF-1α expression and cell glycolytic (31). MicroRNA-210 (miR-210), a marker of hypoxia, is regulated by HIF-1α. Since the 3’UTR of HIF-1α contains a non-canonical miR-210 target site, miR-210 also negatively inhibits HIF-1α expression by binding to this target (2, 32). Elevated miR-210 suppressed expression of HIF-1α target genes Glut1, p53 and fas, therefore protecting cell against hypoxia-induced apoptosis. Succinic acid, SIRT1/6 are two upstream signals for HIF-1α activation. After transporting from mitochondria to cytoplasm, succinic acid inhibits PHD activity, and activates HIF-α, leading to increased expression of IL-1β and inflammation (33). SIRT1 binds to the HIF-1α inhibitory domain (ID) and protects HIF-1α from deacetylation (34). Overexpression of SIRT6 inhibits the ubiquitination-protease system and favors HIF-1α accumulation, resulting in increased expression of VEGF, Ang1, Ang2, endothelin-1 (EF-1), and platelet-derived growth factor-BB (PDGF-BB), and promoting migration, invasion, and proliferation of human umbilical vein endothelial cells (35). When retinoic acid related orphan nuclear receptor γt (ROR γt) was subjected to HIF-1α, a trimer composed of P300, ROR γt, and HIF-1α fosters Th17 cells differentiation and IL-17 secretion (2). All these revealed that HIF-1α may involve in cytokines secretion and regulation of cellular function through downstream signaling pathways (Figure 1).
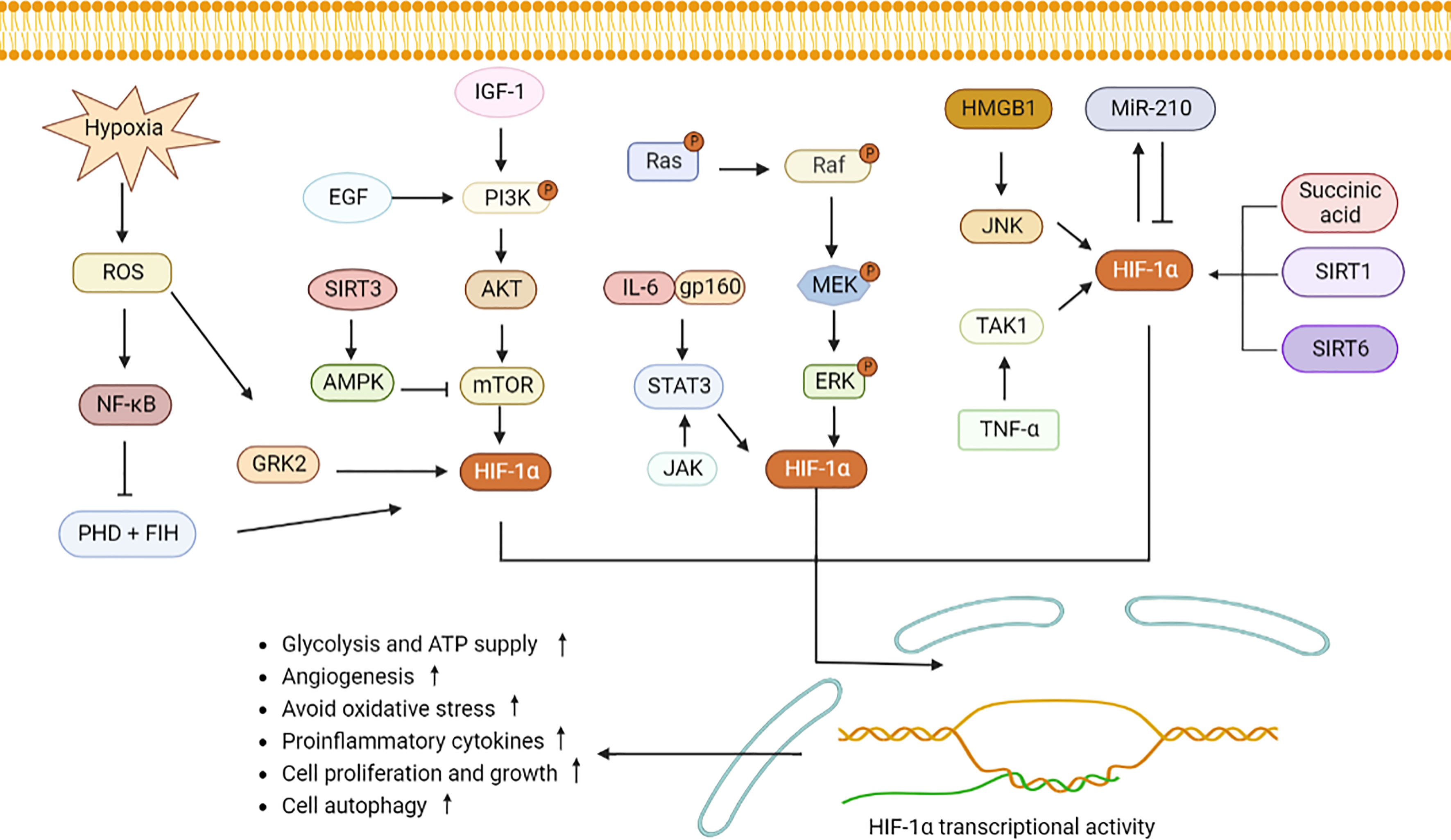
Figure 1 HIF-1α signaling pathway induces inflammatory response and metabolic changes. Hypoxia induces GRK2 and NF-κB expression by stimulating ROS and increases HIF-1α expression. PHD and FIH can be inhabited by NF-κB, leading to accumulation of HIF-1α. PI3K stimulated by EGF and IGF-1 induces Akt and mTOR to elevate HIF-1α secretion. SIRT3 activates AMPK to decrease mTOR expression. IL-6 binds to gp160, JAK, and ERK, and then stimulates STAT3/HIF-1α signaling. Similarly, succinic acid, SIRT1 and SIRT6 increase the intracellular expression of HIF-1α. MiR-210 interacts with HIF-1α. High level of HIF-1α promotes angiogenesis, cell migration and invasion, increases pro-inflammatory cells differentiation and cytokines production. ROS, reactive oxygen species; GRK2, G protein-coupled receptor kinase 2; PHD, proline hydroxylase; FIH, HIF asparaginyl hydroxylase; STAT3, signal transducer and activator of transcription 3; PI3K, phosphatidylinositol-3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin; IL-6, interleukin-6; NF-κB, nuclear factor-κB; JAK, Janus kinase; MAPK, mitogen-activated protein kinases; ERK, regulated extracellular protein kinases; HMGB1, high mobility group protein 1; JNK, c-Jun N-terminal kinase; TNF-ɑ, tumor necrosis factor-alpha; TAK1, transforming growth factor-activated kinase 1; miR-210, microRNA-210; AMPK, adenosine monophosphate activated protein kinase; SIRT1, sirtuin 1.
HIF-1α accumulates in nucleus due to hypoxia or external stimulation, which then induces proliferation, and metabolic changes in innate immune cells, such as macrophages, neutrophils, dendritic cells, natural killer cells, and mast cells. As for adaptive immunity, a few studies have focused on the relationship between T, B cells and HIF-1α. Nevertheless, available evidence showed that HIF-1α regulates inflammatory cytokines secretion, leading to imbalance of Th1, Th2, Th17, Treg cells, and CD8+ T cells that are participating in autoimmune disorders.
Macrophages have strong deformation movement and phagocytosis ability, and are involved in antigen presentation. When monocytes differentiate into macrophages, expression pattern of HIF-1α changes (36). Localization of HIF-1α shifts from the cytoplasm of monocytes to the nucleus of macrophages (36, 37). More monocytes were differentiated into macrophages under low oxygen condition, and there was higher expression of HIF-1α in differentiated macrophages (38). Therefore, the increase of glycolysis may be an inevitable result for monocytes-derived macrophages in a hypoxic microenvironment. Since then, increased HIF-1α will up-regulate function of macrophages, such as antigen presentation and inflammatory cytokines secretion (39). Elevated HIF-1α in macrophages also resists infection, which are able to kill and clear pathogens, such as mycobacterium tuberculosis, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and some fungi (7, 40–42). There are two types of macrophages, M1 and M2 macrophages. HIF-1α is activated in both cells (43, 44).
Neutrophils are one of the most abundant leukocytes in peripheral blood with chemotaxis and phagocytosis. HIF-1α regulates neutrophils survival under hypoxic condition, which depends on NF-kappa B (NF-κB) activation and ROS production (45, 46). Elevated HIF-1α causes neutrophils to exhibit antimicrobial activity. High level of HIF-1α in neutrophils before infection enhances reactive nitrogen species (RNS) production, which will lessen the mycobacterial burden (47). HIF-1α-deficient (HIF-1α-/-) mice are susceptible to bacterial infection and have negative response to vaccine (6). Activation of HIF-1α in zebrafish reduced apoptosis of neutrophils, delayed the improvement of inflammation, exhibiting pro-inflammatory properties (48). A dual host defense mechanism known as neutrophil traps (NETs) can resist germs, harm tissue, and blood vessels as a result of inflammation (49, 50). Blocking HIF-1α inhibits the extracellular bactericidal impact of NETs (50). Treatment of neutrophils with IL-4 inhibited HIF-1α-dependent hypoxic survival, which then limited production of pro-inflammatory components such as CCL2, CCL3, and TNF-α (51). Lipopolysaccharides (LPS) stimulation triggered lactate release by up-regulating glycolysis, NADPH-oxidase-mediated ROS and HIF-1α levels in bone marrow neutrophils (52). There was decreased glycolysis and lactate accumulation in bone marrow neutrophils from HIF-1α-/- mice (52). Lactate induced mobilization of bone marrow neutrophils into peripheral blood and recruitment to the liver, leading to bone marrow neutropenia (52). Activating transcription factor 3 (ATF3) deficient (ATF3-/-) mice showed increased percentage of intrahepatic neutrophil trafficking, elevated expression of pro-inflammatory mediators IL-17A, CCL1, CCL2, and increased HIF-1α activity. Silencing of HIF-1α in ATF3-/- mice inhibited neutrophil trafficking and production of IL-17A, CCL1, CCL2 in liver (53). In conclusion, HIF-1α is a global regulator of neutrophil inflammation and makes a role for anti-bacterial infection (54).
Dendritic cells (DCs) are the most effective antigen-presenting cells and act as a bridge between innate and adaptive immunity (55). DCs in anoxic tissues showed high expression of HIF-1α (56). Hypoxia and LPS stimulation led to HIF-1α accumulation in DCs, along with reduced biological activity of proline hydroxylase (57, 58). HIF-1α alone, or interacts with target gene Glut1, glycolytic enzymes enhance glycolysis and ATP production (59). HIF-1α-/- mice showed increased IL-22 secretion under hypoxia (56). Moreover, HIF-1α interacts with PI3K/Akt pathway to enhance migration ability of DCs (60). HIF-1α binding to p38 MAPK or long noncoding RNA Dpf3 (Lnc-Dpf3) will inhibit the reprogramming of glycolytic metabolism of DCs (61). HIF-1α-/- immature DCs showed low expression of surface molecules MHC-II, CD80, CD86 (62). Coculturing HIF-1α-/- immature DCs with CD4+ T cells or coculturing HIF-1α-/- immature DCs with CD8+ T cells in the presence of LPS, TNF-α led to less proliferation of CD4+ T cells, CD8+ T cells (62). Similarly, HIF-1α-/- mice had low titers of IgG antibody after vaccination, suggesting that HIF-1α deficiency may impair antigen presentation ability of DCs (62). Furthermore, maturation of DCs is negatively regulated by HIF-1α/NOS axis during mycobacterium tuberculosis infection (61). Silencing HIF-1α in DCs down-regulates the anti-fungal effect of DCs (63). Collectively, HIF-1α plays a role in antigen presentation, glycolysis reprogramming, and antimicrobial resistance of DCs.
Natural killer (NK) cells mainly maintain anti-tumor and anti-infection effects in innate immune response (64). In human cardiomyocytes (HCMS), HIF-1α up-regulates expression of major histocompatibility complexes I-related molecule A/B (MICA/B), and then enhances the cytotoxicity of NK cells during hypoxia-reoxygenation (65). HIF-1α induces MICA expression to amplify the killing ability of NK cells. Loss of HIF-1α in NK cells leads to nonproductive angiogenesis to suppress tumors (66). HIF-1α-/- NK cells fail to control cytomegalovirus viral load, resulting in increased morbidity (64). In addition, IL-15 activates STAT3 pathway and IL-2 activates PI3K/mTOR signaling, which then stabilize HIF-1α expression, and maintain natural defense against microbial infection and tumor development (67, 68). Thus, HIF-1α plays a role in regulating NK cell glucose metabolism, anti-tumor, and anti-infection during hypoxia.
Mast cells are involved in inflammation and type I hypersensitivity. HIF-1α is expressed in mast cells of human and animal melanoma tissues (69). In LAD2 mast cells, HIF-1α knockdown attenuates IL-6 release after Toll-like receptor 4 (TLR4) stimulation (70). Similarly, silencing HIF-1α reduces mast cells degranulation and down-regulates expression of TGF-β, and VEGF (71). In ovalbumin (OVA) vaccination-treated mice, administration of HIF-1α increases vascular permeability and plasma exudation through the PI3K/VEGF signaling axis (6). Desferrioxamine treatment leads to elevated expression of HIF-1α in human mast cell 1 (HMC-1), and promotes IL-6, IL-8, TNF-α production in mast cells by activating HIF-1α or NF-κB signaling (72). Lactic acid interrupts miR-155-activated HIF-1α, leading to diminished IL-33 secretion in mast cells (73). Treatment of melanoma mice with H1-receptor antagonist blocks HIF-1α expression and suppresses tumor growth and mast cells infiltration, suggesting that mast cell-derived HIF-1α accelerates melanoma growth (74). MC extracellular traps (MCETs) are formed as a result of phagocytosis of MCs, which produce antimicrobial peptides (74). Enhancement of HIF-1α activity leads to elevated anti-bacterial activity of MCs by inducing MCETs. Conversely, mice lacking HIF-1α are more susceptible to bacterial infection (75) (Figure 2).
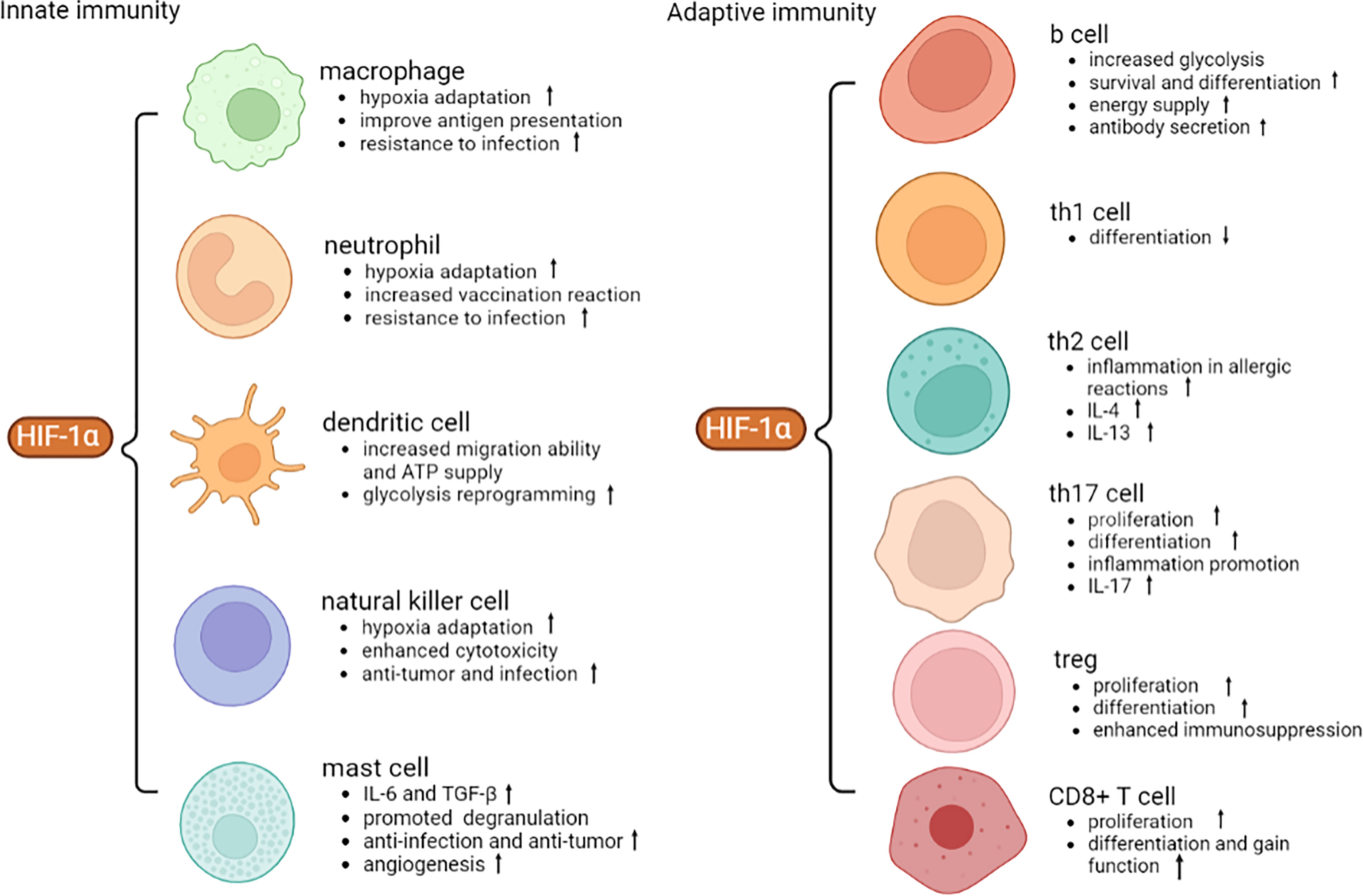
Figure 2 Role of HIF-1α in different immune cells. HIF-1α facilitates anti-infection, anti-tumor and hypoxic adaptation of innate immunity. HIF-1α promotes the proliferation and differentiation of adaptive immune cells and secretion of inflammatory cytokines. VEGF, vascular endothelial growth factor; ATP, adenosine triphosphate; IL-4, interleukin-4; TGF-β, transforming growth factor-β.
HIF-1α involves the glycolytic process of B cells and affects their differentiation, maturation, antibody secretion and viability. Following LPS and IL-4 stimulation, HIF-1α-/- germinal center (GC) B cells have reduced expression of glycolytic genes and glycolytic rate-limiting enzymes, including GAPDH, M2-type pyruvate kinase (PKM2) (76). Similarly, loss of von Hippel-Lindau tumor suppressor protein (VHL) in B cells allows excessive stabilization of HIF-1α in B cells, thereby interfering with the balance of glycolysis and aerobic metabolism (77). HIF-1α-/- B220+ bone marrow cells have lower glycolytic capacity than wild-type cells. This process is due to restricted expression of genes encoding glucose transporters, including phosphofructokinase 2 and fructose-2,6-bisphosphate kinase (78). HIF-1α-regulated glycolysis is important for early pre-B cells and IgM+ B cells, however, blocking glycolysis using 2-DOG does not slow down pre-B cells differentiation into immature B cells, suggesting that HIF-1α is required for different stages of B cells (78). Similarly, HIF-1α activity is higher in bone marrow pro-B cells and pre-B cells, and is lower in immature B cells (79). HIF-1α limits pyruvate entry into tricarboxylic acid cycle (TCA), and B cells with HIF-1α deficiency can transport more pyruvate and generate energy in the respiratory chain (78). In addition, binding of HIF-1α to HRE of IL-10 gene promoter increases IL-10 secretion in B cells (80), and regulates innate-like B cells and B10 differentiation, resulting in decreased IgM secretion (81). Splenic B cells from HIF-α-/- mice were cultured with hypoxia condition, showing increased expression of IL-10 in B cells as compared to that in normoxia (80). When naive CD4+ T cells were co-cultured with CD1dhiCD5+ B cells from HIF-ɑ-/- mice, there were high percentage of CD4+IFN-γ+, CD4+IL-17A+ T cells, and increased expression of IFN-γ, IL-17A (80). Overexpression of HIF-1α in RA synovial fibroblasts (RASFs) promoted expression of IL-6, IL-8, TNF-α, and IL-1β (82), and co-culturing HIF-1α-/- RASFs with allogenic CD19+ B cells down-regulated expression of stromal cell-derived factor (SDF)-1, vascular cell adhesion molecule (VCAM)-1, IgG and up-regulated percentage of CD19+CD24hiCD27+ B10 cells, CD19+CD27+IgD+ innate-like B cells, expression of natural IgM (82). ROS activates tyrosine kinase and promotes nuclear factor (erythroid-derived 2) like 2 (Nrf2), HIF-1α to improve B cells survival (83). In Wil2-NS B cells under hypoxia, Nrf2 and HIF-1α promote expression of C-X-C chemokine receptor type 4 (CXCR4) and increase viability of B cells (84). HIF-1α was highly expressed in GC B cells. Knockout HIF-1α in B cells impaired GC reaction, leading to defective class-switch recombination and production of high-affinity plasma cells (76).
Coculturing HIF-1α-/- antigen-presenting cells (APCs) with Th1 cells does not induce Th1 cells expansion (85). HIF-1α selectively induced secretion of IL-12p40 to interrupt differentiation of naive T helper cells into Th1 cells, limiting mucosal inflammation (86). Under hypoxia, increased phosphorylation of STAT3 in Th1 cells contributes to transcription of HIF-1α, which in reversely inhibits transcription of cell signal transduction inhibitor 3. Consequently, this positive feedback enhances STAT3 activation and down-regulates Th1 response. Furthermore, Th1 cells under hypoxia lost the ability to secrete IFN-γ. HIF-1α limits Th1 cells differentiation through inhibiting production of IL-12 under hypoxia (87, 88). In addition, treatment with miR-182, an inhibitor of HIF-1α, accelerates Th1 cells differentiation (89).
Th2 cells involve anti-infection, asthma, and other hypersensitivity responses. More Th2 cells differentiation and increased VEGF expression were observed in OVA-induced asthma mice model, and there was high expression of HIF-1α in the mice lung tissue (90). In HIF-1α-/- mice exposed to cobalt, expression of IgE, leukotriene C4 (LTC4), eosinophil cationic protein (ECP) was decreased in alveolar lavage fluid and lung tissue (91). In mice with HIF-1α-/- DCs, secretion of Th2 cytokines, such as IL-5, IL-10, and IL-13 was reduced (91). Under hypoxic condition, expression of membrane binding protein CD44 on DCs is increased, which then promotes Th2 cells polarization, accompanied by increased IL-4 secretion (8). Usage of anthraquinone, a HIF-1α inhibitor, is able to restrain HIF-1α expression, and inhibits differentiation of Th2 cells and expression of IL-4, IL-13 (92–94). During infection with pathogens, HIF-1α expression is increased in Th2 cells, leading to Th2 cells proliferation (95).
Evidence suggests that HIF-1α is a key molecule regulates activities of Th17 cells and expression of IL-17 (1, 96). It is known that RORγt is the transcription factor for Th17 cell. HIF-1α deficiency inhibits Th0 cells developing into Th17 cells and down-regulates RORγt expression (97). Escherichia coli infection increases the amount of HIF-1α in the liver, which then induces Th17 cells differentiation by increasing IL-6 expression (98). At condition of 5% O2, HIF-1α is activated (99), and there are elevated percentages of Th17 cells and expression of IL-6 (100). Treatment with metformin and epigallocatechin-3-gallate (EFCG) inhibits the mTOR signaling, thereby inhibiting HIF-1α expression and Th17 cells differentiation (101, 102). In Adipor1-/-CD4+ T cells, there was reduced glycolysis metabolism and Th17 cells polarization, which is due to disturbance of HIF-1α (103). In addition, HIF-1α is a target gene of miR-210. MiR-210 directly reduces the transcription of HIF-1α to delay differentiation of Th17 cells (32).
Treg cells, including natural regulatory T cells and inducible regulatory T cells, are a class of cells with inhibitory effects in immune response. During different Th cells metabolism and differentiation, Th1 and Th17 utilize high levels of glycolytic metabolism to provide capacity for their proliferation, whereas Treg cells require aerobic metabolism to enhance their inhibitory function (104). HIF-1α promotes CD73 expression in Treg cells and binds to CD73 to expand Treg cells to convert ATP into immunosuppressive adenosine (105). Increased expression of O2 at the tumor site down-regulates HIF-1α to affect tumor cell metabolism and negatively regulates Treg cells differentiation (106). Under hypoxia, transfection of CD4+CD25+ T cells with lentiviral vector containing low expression of HIF-1α increases expression of Foxp3, which induces Treg cells differentiation and immunosuppressive function (107). IL-1β up-regulates HIF-1α expression to inhibit Treg cells polarization in response to inflammatory stimuli (108).
As HIF-1α-/-CD8+ T cells were differentiated into effector cytotoxic T lymphocytes (CTLs), there was reduced expression of genes regulating glycolytic metabolism, such as Hk2, Pdk1, Mct4 and PgK, and less glucose uptake and lactate production (109). HIF-1α-/- effector CD8+ T cells did not down-regulate surface expression of CD62L, but down-regulated expression of IFN-γ, TNF-α. Hypoxia increased expression of the cytolytic molecule granzyme B, activation-related costimulatory molecules CD137, OX40, GITR, and checkpoint receptors PD-1, TIM3, VEGF-A and LAG3 (109), which was obtained in HIF-1α-/-CD8+ T cells in response to IL-2 stimulation as well. HIF-1α-/- effector CD8+ T cells showed a reduced ability to kill target cells (109). Deficiency in NIX-dependent mitophagy results in metabolic defects in effector memory CD8+ T cells, and NIX deficiency promoted HIF-1α accumulation, altering cellular metabolism from long-chain fatty acid to short/branched-chain fatty acid oxidation, thereby compromising ATP synthesis (110). Inhibiting HIF-1α accumulation restored long-chain fatty acid metabolism and effector memory CD8+ T cells formation, suggesting that HIF-1α regulates effector memory CD8+ T cells formation by NIX-mediated mitophagy (110). High activity of HIF-1α in tumor microenvironment down-regulated infiltration and activity of CD8+ T cells (111). There was elevated T cells infiltration at early stage of tumorigenesis in the tumor site along with up-regulated percentage of memory CD4+, CD8+ T cells (112). Inhibition of HIF-1α down-regulated expression of pro-inflammatory factors IL-10, IL-12, PGE2, S-180, TNF-α, and abrogated memory CD4+, CD8+ T cells-mediated suppression of tumor-associated macrophages (TAM) (112). Knocking down HIF-1α negative regulator von Hippel-Lindau (VHL) in CD8+ T cells led to differentiation of tissue-resident memory-like (Trm-like) tumor-infiltrating lymphocyte (TIL), by which VHL-/- TILs accumulated in tumors and showed a core Trm signature, indicating that HIF-1α activity in CD8+ TILs up-regulates accumulation and antitumor activity (113). Similarly, VHL-/-CD8+ effector T cells did not express KLRG1, a marker of T cell terminal differentiation, suggesting a positive effect of HIF-1α on CD8+ T cells differentiation (114).
SLE is a typical inflammatory autoimmune disease characterized by production of autoantibodies and damage to multiple tissues and organs, such as skin, joints, and kidneys. Lupus nephritis (LN) is the mostly complicated disease in SLE, which is also the major cause of incidence and mortality in lupus patients (115).
Urinary HIF-1α levels are higher in LN patients compared with that in healthy controls, and were associated with histologic chronicity indexes and the estimated glomerular filtration rate (eGFR) in LN patients (116). In LN patients and MRL/lpr lupus mice, expression of HIF-1α in both glomerular and tubulointerstitial areas was increased and percentage of intraglomerular HIF-1α+ cells was increased (9). The levels of intraglomerular HIF-1α were related to renal pathology activity index and clinical manifestations in LN patients. In SLE patients’ CD4+ T cells, HIF-1α was overexpressed (2). Regarding gene single-nucleotide polymorphism (SNP) and SLE risk, a study showed that there are no significant differences in genotypes frequencies between the patients with SLE and the controls (rs11549465, rs12434438, rs1957757, rs1951795, rs7143164) (117). Silencing HIF-1α in MRL/lpr mice can inhibit serum levels of IL-17, anti-nucleosome antibody, proteinuria, IgG and C3 depositions in kidney (1). Inhibition of glutaminase in MRL/lpr mice affects the glycolysis pathway by reducing HIF-1α expression and decreases percentage of CD3+CD4-CD8- T cells, urine albumin, and glomerular renal pathology scores (118). Thus, HIF-1α may be a promising target for treatment of lupus.
RA is a chronic disease with symmetry arthritis as its main clinical manifestation, which is characterized by synovial hyperplasia and osteoarticular destruction (119). HIF-1α expression was increased in serum, sublining layer in synovial membrane from RA patients (10, 120, 121). The number of HIF-1α+ cells in RA synovial tissue is correlated with blood vessels, inflammatory endothelial cells infiltration, proliferation, and synovial score (119). Moreover, expression of HIF-1α was reinforced in collagen-induced arthritis (CIA) mice (122–125).
In CIA mice treated with hyperbaric oxygen, there was elevated percentage of Treg cells accompanied by lower expression of HIF-1α. Pannus formation represents a distinctive pathological feature of RA, and VEGF mediates arthropathic proliferative angiogenesis in arthritis. In adjuvant-induced arthritis (AA) rats and RA patients, expression of HIF-1α was positively related to expression of VEGF, and increased HIF-1α accelerated synovial angiogenesis and resulted in joint inflammation (126, 127). On the contrary, inhibition of HIF-1α expression in AA rats showed opposite effects (128). It is known that erosion and destruction of articular cartilage is a prominent pathological feature of RA. Under hypoxic condition, fibroblast-like synovial cells in RA (RA-FLSs) transformed into epithelial mesenchyme, and HIF-1α promoted migration and invasion of the cells via STAT3/HIF-1α/fascin-1 axis (129, 130). NF-κB interacts with HIF-1α to promote the enzymatic activity of matrix metalloproteinases 2 (MMP2) and MMP9, and then disrupts histological barrier and destroys bone material (131). When CD14+ monocytes differentiate into osteoclasts, there was elevated expression of HIF-1α in osteoclasts (132). HIF-1α increases osteoclasts-mediated bone resorption (133). In RASFs, HIF-1α overexpression induces Th1 and Th17 cells expansion and increases expression of INF-γ and IL-17 (82, 134). HIF-1α inhibitor, Pyridine formamide compound AMSP-30m, facilitated synovial cells apoptosis (125). Citrullinated proteins are considered as a biomarker of RA. Knocking out HIF-1α in RASFs decreased citrulline protein (135). CIA mice treated with IL-34, succinate, and sinomenine up-regulated Ang-1 expression via the HIF-1α/VEGF axis (128, 136, 137). IL-38, an inflammatory related cytokine, exerts angiopoietin-inhibiting and anti-inflammatory function in CIA mice (138). Activation of PI3K/Akt/HIF-1α and NK-κB/HIF-1α signaling pathways augmented migration and invasion of RA-FLSs (129, 131). HIF-1α is capable of up-regulating osteoclasts-mediated bone resorption (139), whereas IL-38 contributed to secretion of osteogenic factors through SIRT1/HIF-1α signallings (129). Therefore, expression of HIF-1α was increased in arthritis and may promote arthritis development by downstream signals.
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are a class of chronic intestinal inflammatory diseases characterized by intestinal barrier dysfunction and intestinal mucosal hypoxia. Compared with controls, higher expression of HIF-1α exists in intestinal cells and M1-type macrophages of CD patients (140, 141). In terms of population susceptibility, HIF-1α gene rs11549467 polymorphism did not correlate with IBD risk in Moroccan population (142).
The HIF-1α/glycolytic pathway disrupts balance of M1/M2 macrophages and the secretion function of neutrophils to affect the pathological state of colitis (143–145). Succinate is an intermediate product of the tricarboxylic acid cycle that drives HIF-1α to stimulate IL-1β production and aerobic glycolysis in M1 macrophages, favoring the M1 phenotype (146). M2 macrophages, on the other hand, acquire energy mainly from fatty acid metabolism and oxidative metabolism (146). Tiliroside attenuates disease activity in mice with colitis, where it promotes HIF-1α enzyme degradation (143). In clinical trials with cyclosporine from UC patients, cyclosporine increased HIF-1α expression and glycolysis in neutrophils, accompanied by release of antimicrobial peptides, ROS, and myeloperoxidase (MPO) (145). CD-associated Escherichia coli activated VEFG in intestinal epithelial cells, triggering angiogenesis (147). HIF-1α interacted with IL-33 at the promoter region and is able to stabilize IL-33-induced mucosal homeostasis (148). Inhibition of PHD1 stabilizes HIF-1α levels, and then protects the intestinal mucosa (149). Furthermore, treatment of Bifidobacterium IL-10 inhibited inflammation in colitis mice by restoring Treg/Th17 balance (150). Dimethyloxalylglycine (DMOG) is a hydroxylase inhibitor that stabilizes HIF-1α, and DMOG improved chronic intestinal inflammation (151). However, a study revealed that mice with HIF-1α deficiency in DCs lost much weight and exhibited severe intestinal inflammation after dextran sodium sulfate (DSS) treatment. HIF-1α plays a protective role in DCs (152), T cells (153), and epithelial cells (154) in murine colitis. Inhibition of HIF-1ɑ in myeloid cells exacerbated infiltration of neutrophils and Ly6+ monocytes in lesion tissues, and HIF-1α-/- colonic macrophages had a reduced pro-resolving profile (155). Therefore, HIF-1α signaling contributes to colitis resolution.
Systemic sclerosis (SSc) is an autoimmune disease featured by autoimmunity, vascular lesions and interstitial fibrosis. Chronic hypoxia is a prominent feature in SSc, which can lead to vasculopathy and tissue fibrosis (11). It has been shown that expression of HIF-1α in human microvascular endothelial cell line-1 (HMEC-1) was up-regulated under hypoxia (11), and the skin tissue had much HIF-1α+ cells in patients with SSc (156). According to a study in French Caucasian population, HIF-1α gene polymorphism was associated with SSc risk. The frequencies of genotypes AG, GG in rs12434438 were higher in SSc patients than in controls (157). Another study in Japanese SSc patients obtained that AA genotype in rs12434438 was associated with SSc patients with severe pulmonary arterial hypertension (PAH), suggesting that rs12434438 polymorphism may relate to occurrence of SSc combined with PAH (158).
HIF-1α expression was closely related to VEGF expression in SSc patients (11). HIF-1α/VEGF axis induced vascular endothelial transformation into interstitial under hypoxia, leading to tissue fibrosis and vasculopathy (159, 160). Expression of connective tissue growth factor (CTGF) and HIF-1α was both rised in the skin of SSc patients, by which HIF-1α facilitated CTGF expression, and then resulted in skin fibrosis (161, 162). On the contrary, treatment with 2-methylestradiol diminished HIF-1α expression, reduced collagen synthesis, fibrocyte proliferation in fibroblasts, suggesting that targeting HIF-1α may give potential for treatment of SSc (161).
Psoriasis is a chronic inflammatory disease characterized by excessive angiogenesis, proliferation of keratin-forming cells (163). Expression of HIF-1α was increased in both skin lesions, and serum from patients with psoriasis as compared to those in controls (12, 164–167). Ang-1, Ang-2, and Tie-2 are overexpressed in the papillary dermis of psoriatic skin, which are induced by HIF-1α. Expression of insulin-like growth factor-II (IGF-II) and VEGF in human keratinocytes cells (HaCat cells) was regulated by HIF-1α. Expression of HIF-1α positively correlated with microvessel density (164). MiR-150 restrains HaCat cells proliferation by binding to promoter of HIF-1α (168). It is accepted that increased proliferation and reduced differentiation of keratinocytes are characteristics of psoriasis. Stimulation of the cells with bone morphogenic protein 6 (BMP6) inhibited proliferation and promoted differentiation of keratinocytes. HIF-1α inhibited expression of BMP6 by binding to the HRE of promoter of BMP6, thereby aggravating the pathological features in psoriasis (168). Furthermore, HIF-1α bound to miR-210, suppressed expression of target genes STAT6 and LYN, leading to Th17 cells differentiation in psoriasis mice (169).
Multiple sclerosis (MS) is a central nervous system disease caused by autoimmune inflammation, accompanied by demyelination, blood-brain barrier damage. Experimental autoimmune encephalomyelitis (EAE) mouse model is the classic animal model of MS.
In MS patients, white matters had hypoxia and high expression of HIF-1α (170). Similarly, EAE mice had elevated HIF-1α expression in mice tissues, and was related to the neurological defect (171). A case-control study discussed association between MS and HIF-1α polymorphism, showing no association of HIF-1α polymorphism and MS risk (172). In EAE mice, inhibiting HIF-1α expression leads to reduced intermittent hypoxia and promotes Treg cells differentiation and IL-10, TGF-β production (101). Treatment of MS patients with fumarate caused accumulation of HIF-1α, lowered the risk of MS recurrence (171). IL-1β induced expression of HIF-1α in astrocytes, changing the permeability of the blood-brain barrier in brain (173).
Type 1 diabetes mellitus (T1DM) is characterized by hyperglycemia, in which islet β-cell damage is mainly caused by autoimmunity. High expression of HIF-1α attenuated β-cell death and β-cell loss in islet (174). Induction of hypoxia in islet β-cell with CoCl (cobalt chloride) improved β-cell survival and relieved proteinuria and tubulointerstitial damage in diabetic rats, mediated by increased transcription of HIF-1α (175, 176). Thus, HIF-1α may protect against the hypoxia stress. Inhibiting expression of HIF-1α increased the infectivity of β cells to viruses, especially coxsackie viruses (177). Peripheral nerve damage, diabetic heart disease and diabetic nephropathy are some severe complications of diabetes. HIF-1α protects against peripheral nerves damage caused by hyperglycemia via inhibiting ROS, VEGF expression (178). P53 reduces cardiomyocyte apoptosis by increasing HIF-1α stabilization and ameliorating defects in glycolysis and angiogenesis. Similarly, a carbohydrate restriction diet (CR) can up-regulate HIF-1α expression and improve nephropathy in T1DM rats (179). Therefore, HIF-1α may suppress T1DM pathogenesis (Table 1).
HIF-1α regulates angiogenesis and secretion of inflammatory cytokines by adapting to a hypoxic environment. In recent years, growing evidence has indicated that HIF-1α worked in several inflammatory autoimmune diseases. Functional studies suggest the effects of HIF-1α in the pathology of these disorders. For example, HIF-1α mediates excessive activation of innate immunity, leading to dysregulated biological function of innate immune cells, such as antigen presentation and anti-infection. Similarly, HIF-1α impacts cell proliferation and differentiation, pro-inflammatory cytokines release in adaptive immunity. However, some points need to be clarified in the future. Firstly, limited studies discussed polymorphisms in the HIF-1α gene and lupus, IBD. Gene polymorphism studies may provide basic data for the treatment and prevention of autoimmune disorders by revealing the risk of HIF-1α in autoimmune diseases, disease phenotype, and responsiveness to drug treatment. Thus, the above disorders require more studies with large samples and multiple races. Secondly, since HIF-1α is closely related to cell metabolism and energy supply, the relationship between HIF-1α and non-immune cells involved in the process of autoimmune diseases should be paid attention like cancer cells (180), renal tubular epithelial cells (9), and synovial cells (121). Thirdly, When Treg cells were subjected to hypoxia, high levels of HIF-1α stimulated proliferation of Treg cells and promoted the immunosuppressive effect. For instance, activation of the Akt/mTORC1 signaling pathway and subsequent activation of HIF-1α induces glucose transporter and glycolytic enzyme expression. HIF-1α increases the levels of pyruvate dehydrogenase kinase (PDK) and lactate dehydrogenase (LDH), inhibits the conversion of pyruvate to acetyl-CoA and promotes lactate production. The metabolic shift of Treg cells to aerobic glycolysis facilitates immunosuppressive function (181). However, in the presence of high levels of mTOR stimulator, Treg cells prefer aerobic glycolytic reprogramming accompanied by elevation of HIF-1α expression, thereby inhibiting Treg cells’ function. For Treg cells, the same pathway that inhibits their development may be necessary in functionally mature Treg cells (182). Therefore, when exploring the mechanism of HIF-1α in regulating Treg cells, different proliferation and differentiation stages, different metabolic patterns of Treg cells, and expression of mTOR signaling should be considered. Fourthly, in T1DM, HIF-1α protects pancreatic islet β-cell, and reduces the complications related to T1DM. Overexpressed HIF-1α protects against intestinal inflammation, and low expression of HIF-1α aggravates IBD. Interestingly, inhibition of HIF-1α expression in bone marrow cells and myeloid cells exacerbates intestinal inflammation, which contradicts its function in other diseases. Thus, the clear molecular mechanism for HIF-1α in different inflammatory autoimmune diseases needs specific discussion in the future.
Although some of the above limitations remain to be discussed to date, it is undeniable that HIF-1α performs significantly in inflammatory autoimmune diseases. This review can provide a theoretical basis for the development and application of HIF-1α as a disease marker and targeted drugs in the future.
Study conception and design: Y-YT, W-DX. Acquisition of data, analysis and interpretation of data: D-CW, Y-QW, A-FH. Drafting the article: Y-YT, W-DX. Final approval of the version of the article to be published: all authors, and that all authors agree to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Sichuan Provincial Natural Science Foundation (2022NSFSC0697, 2022NSFSC0694).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhao W, Wu C, Li LJ, Fan YG, Pan HF, Tao JH, et al. RNAi silencing of HIF-1α ameliorates lupus development in MRL/lpr mice. Inflammation (2018) 41(5):1717–30. doi: 10.1007/s10753-018-0815-6
2. Garchow B, Maque Acosta Y, Kiriakidou M. HIF-1α and miR-210 differential and lineage-specific expression in systemic lupus erythematosus. Mol Immunol (2021) 133:128–34. doi: 10.1016/j.molimm.2021.02.019
3. Yang ZC, Liu Y. Hypoxia-inducible factor-1α and autoimmune lupus, arthritis. Inflammation (2016) 39(3):1268–73. doi: 10.1007/s10753-016-0337-z
4. Kim YI, Yi EJ, Kim YD, Lee AR, Chung J, Ha HC, et al. Local stabilization of hypoxia-inducible factor-1α controls intestinal inflammation via enhanced gut barrier function and immune regulation. Front Immunol (2021) 14:609689. doi: 10.3389/fimmu.2020.609689
5. Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem (1995) 270(3):1230–7. doi: 10.1074/jbc.270.3.1230
6. Bhandari T, Olson J, Johnson RS, Nizet V. HIF-1α influences myeloid cell antigen presentation and response to subcutaneous OVA vaccination. J Mol Med (Berl) (2013) 91(10):1199–205. doi: 10.1007/s00109-013-1052-y
7. Li F, Luo J, Xu H, Wang Y, Jiang W, Chang K, et al. Early secreted antigenic target 6-kDa from mycobacterium tuberculosis enhanced the protective innate immunity of macrophages partially via HIF1α. Biochem Biophys Res Commun (2020) 522(1):26–32. doi: 10.1016/j.bbrc.2019.11.045
8. Yang M, Liu Y, Ren G, Shao Q, Gao W, Sun J, et al. Increased expression of surface CD44 in hypoxia-DCs skews helper T cells toward a Th2 polarization. Sci Rep (2015) 1:5. doi: 10.1038/srep13674
9. Deng W, Ren Y, Feng X, Yao G, Chen W, Sun Y, et al. Hypoxia inducible factor-1 alpha promotes mesangial cell proliferation in lupus nephritis. Am J Nephrol (2014) 40(6):507–15. doi: 10.1159/000369564
10. Yang R, Zhang Y, Wang L, Hu J, Wen J, Xue L, et al. Increased autophagy in fibroblast-like synoviocytes leads to immune enhancement potential in rheumatoid arthritis. Oncotarget (2017) 8(9):15420–30. doi: 10.18632/oncotarget.14331
11. Mao J, Liu J, Zhou M, Wang G, Xiong X, Deng Y. Hypoxia-induced interstitial transformation of microvascular endothelial cells by mediating HIF-1α/VEGF signaling in systemic sclerosis. PloS One (2022) 17(3):e0263369. doi: 10.1371/journal.pone.0263369
12. Tang W, Long T, Li F, Peng C, Zhao S, Chen X, et al. HIF-1α may promote glycolysis in psoriasis vulgaris via upregulation of CD147 and GLUT1. Zhong Nan Da Xue Xue Bao Yi Xue Ban (2021) 46(4):333–44. doi: 10.11817/j.issn.1672-7347
13. Wang T, Jiao Y, Zhang X. Immunometabolic pathways and its therapeutic implication in autoimmune diseases. Clin Rev Allergy Immunol (2021) 60(1):55–67. doi: 10.1007/s12016-020-08821-6
14. Le Rossignol S, Ketheesan N, Haleagrahara N. Redox-sensitive transcription factors play a significant role in the development of rheumatoid arthritis. Int Rev Immunol (2018) 37(3):129–43. doi: 10.1080/08830185.2017.1363198
15. Masson N, Singleton RS, Sekirnik R, Trudgian DC, Ambrose LJ, Miranda MX, et al. The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity. EMBO Rep (2012) 13(3):251–7. doi: 10.1038/embor.2012.9
16. Köhl R, Zhou J, Brüne B. Reactive oxygen species attenuate nitric-oxide-mediated hypoxia-inducible factor-1alpha stabilization. Free Radic Biol Med (2006) 40(8):1430–42. doi: 10.1016/j.freeradbiomed
17. Hong Z, Zhang X, Zhang T, Hu L, Liu R, Wang P, et al. The ROS/GRK2/HIF-1α/NLRP3 pathway mediates pyroptosis of fibroblast-like synoviocytes and the regulation of monomer derivatives of paeoniflorin. Oxid Med Cell Longev (2022) 29:2022. doi: 10.1155/2022/4566851
18. Zhang T, Guo S, Zhu X, Qiu J, Deng G, Qiu C. Alpinetin inhibits breast cancer growth by ROS/NF-κB/HIF-1α axis. J Cell Mol Med (2020) 24(15):8430–40. doi: 10.1111/jcmm.15371
19. King D, Yeomanson D, Bryant HE. PI3King the lock: Targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol (2015) 37(4):245–51. doi: 10.1097/MPH.0000000000000329
20. Park ST, Kim BR, Park SH, Lee JH, Lee EJ, Lee SH, et al. Suppression of VEGF expression through interruption of the HIF−1α and akt signaling cascade modulates the anti−angiogenic activity of DAPK in ovarian carcinoma cells. Oncol Rep (2014) 31(2):1021–9. doi: 10.3892/or.2013.2928
21. Lin Y, Xue K, Li Q, Liu Z, Zhu Z, Chen J, et al. Cyclin-dependent kinase 7 promotes Th17/Th1 cell differentiation in psoriasis by modulating glycolytic metabolism. J Invest Dermatol (2021) 141(11):2656–2667.e11. doi: 10.1016/j.jid.2021.04.018
22. Wu WD, Hu ZM, Shang MJ, Zhao DJ, Zhang CW, Hong DF, et al. Cordycepin down-regulates multiple drug resistant (MDR)/HIF-1α through regulating AMPK/mTORC1 signaling in GBC-SD gallbladder cancer cells. Int J Mol Sci (2014) 15(7):12778–90. doi: 10.3390/ijms150712778
23. Woo YM, Shin Y, Lee EJ, Lee S, Jeong SH, Kong HK, et al. Inhibition of aerobic glycolysis represses Akt/mTOR/HIF-1α axis and restores tamoxifen sensitivity in antiestrogen-resistant breast cancer cells. PloS One (2015) 10(7):e0132285. doi: 10.1371/journal.pone.0132285
24. Zhang J, Han L, Yu J, Li H, Li Q. miR-224 aggravates cancer-associated fibroblast-induced progression of non-small cell lung cancer by modulating a positive loop of the SIRT3/AMPK/mTOR/HIF-1α axis. Aging (2021) 13(7):10431–49. doi: 10.18632/aging.202803
25. Maimaiti A, Aierken Y, Zhou L, He J, Abudureyimu A, Li SX. Inhibiting interleukin-6/Signal transducers and activators of transduction-3/Hypoxia-Inducible factor-1α signaling pathway suppressed the growth of infantile hemangioma. Eur J Pediatr Surg (2022) 12. doi: 10.1055/s-0042-1749436
26. Yeh H, Moore DJ, Markmann JF, Kim JI. Mechanisms of regulatory T cell counter-regulation by innate immunity. Transplant Rev (Orlando) (2013) 27(2):61–4. doi: 10.1016/j.trre.2013.02.001
27. Lee SY, Lee AR, Choi JW, Lee CR, Cho KH, Lee JH, et al. IL-17 induces autophagy dysfunction to promote inflammatory cell death and fibrosis in keloid fibroblasts via the STAT3 and HIF-1α dependent signaling pathways. Front Immunol (2022) 10:888719. doi: 10.3389/fimmu.2022.888719
28. Zhang M, Wu D, Xu J, Liu L, Jiao W, Yu J, et al. Suppression of NLRP3 inflammasome by dihydroarteannuin via the HIF-1α and JAK3/STAT3 signaling pathway contributes to attenuation of collagen-induced arthritis in mice. Front Pharmacol (2022) 27:884881. doi: 10.3389/fphar.2022.884881
29. Xu X, You K, Bu R. Proximal tubular development is impaired with downregulation of MAPK/ERK signaling, HIF-1α, and catalase by hyperoxia exposure in neonatal rats. Oxid Med Cell Longev (2019) 28:2019. doi: 10.1155/2019/9219847
30. Domènech M, Hernández A, Plaja A, Martínez-Balibrea E, Balañà C. Hypoxia: The cornerstone of glioblastoma. Int J Mol Sci (2021) 22(22):12608. doi: 10.3390/ijms222212608
31. Koedderitzsch K, Zezina E, Li L, Herrmann M, Biesemann N. TNF induces glycolytic shift in fibroblast like synoviocytes via GLUT1 and HIF1A. Sci Rep (2021) 11(1):19385. doi: 10.1038/s41598-021-98651-z
32. Wang H, Flach H, Onizawa M, Wei L, McManus MT, Weiss A. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol (2014) 15(4):393–401. doi: 10.1038/ni.2846
33. Liu P, Wang J, Wen W, Pan T, Chen H, Fu Y, et al. Cinnamaldehyde suppresses NLRP3 derived IL-1β via activating succinate/HIF-1 in rheumatoid arthritis rats. Int Immunopharmacol (2020) 84:106570. doi: 10.1016/j.intimp.2020.106570
34. Joo HY, Yun M, Jeong J, Park ER, Shin HJ, Woo SR, et al. SIRT1 deacetylates and stabilizes hypoxia-inducible factor-1α (HIF-1α) via direct interactions during hypoxia. Biochem Biophys Res Commun (2015) 462(4):294–300. doi: 10.1016/j.bbrc.2015.04.119
35. Yang Z, Huang Y, Zhu L, Yang K, Liang K, Tan J, et al. SIRT6 promotes angiogenesis and hemorrhage of carotid plaque via regulating HIF-1α and reactive oxygen species. Cell Death Dis (2021) 12(1):77. doi: 10.1038/s41419-020-03372-2
36. Fangradt M, Hahne M, Gaber T, Strehl C, Rauch R, Hoff P, et al. Human monocytes and macrophages differ in their mechanisms of adaptation to hypoxia. Arthritis Res Ther (2012) 14(4):R181. doi: 10.1186/ar4011
37. Elbarghati L, Murdoch C, Lewis CE. Effects of hypoxia on transcription factor expression in human monocytes and macrophages. Immunobiology (2008) 213(9-10):899–908. doi: 10.1016/j.imbio.2008.07.016
38. Staples KJ, Sotoodehnejadnematalahi F, Pearson H, Frankenberger M, Francescut L, Ziegler-Heitbrock L, et al. Monocyte-derived macrophages matured under prolonged hypoxia transcriptionally up-regulate HIF-1α mRNA. Immunobiology (2011) 216(7):832–9. doi: 10.1016/j.imbio.2010.12.005
39. Chang Y, Li X, Cheng Q, Hu Y, Chen X, Hua X, et al. Single-cell transcriptomic identified HIF1A as a target for attenuating acute rejection after heart transplantation. Basic Res Cardiol (2021) 116(1):64. doi: 10.1007/s00395-021-00904-5
40. Li Q, Xie Y, Cui Z, Huang H, Yang C, Yuan B, et al. Activation of hypoxia-inducible factor 1 (Hif-1) enhanced bactericidal effects of macrophages to mycobacterium tuberculosis. Tuberculosis (Edinb) (2021) 126:102044. doi: 10.1016/j.tube.2020.102044
41. Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-Dependent axis. Cell Metab (2020) 32(3):498–9. doi: 10.1016/j.cmet.2020.07.015
42. Friedrich D, Zapf D, Lohse B, Fecher RA, Deepe GS Jr, Rupp J. The HIF-1α/LC3-II axis impacts fungal immunity in human macrophages. Infect Immun (2019) 87(7):e00125-19. doi: 10.1128/IAI.00125-19
43. Christoph M, Pfluecke C, Mensch M, Augstein A, Jellinghaus S, Ende G, et al. Myeloid PHD2 deficiency accelerates neointima formation via hif-1α. Mol Immunol (2022) 149:48–58. doi: 10.1016/j.molimm.2022.06.003
44. Hammami A, Abidin BM, Charpentier T, Fabié A, Duguay AP, Heinonen KM, et al. HIF-1α is a key regulator in potentiating suppressor activity and limiting the microbicidal capacity of MDSC-like cells during visceral leishmaniasis. PloS Pathog (2017) 13(9):e1006616. doi: 10.1371/journal.ppat.1006616
45. Walmsley SR, Print C, Farahi N, Peyssonnaux C, RS J, Cramer T, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med (2005) 201(1):105–15. doi: 10.1084/jem.20040624
46. Willson JA, Arienti S, Sadiku P, Reyes L, Coelho P, Morrison T, et al. Neutrophil HIF-1α stabilization is augmented by mitochondrial ROS produced via the glycerol 3-phosphate shuttle. Blood (2022) 139(2):281–6. doi: 10.1182/blood.2021011010
47. Fritzenwanger M, Jung C, Goebel B, Lauten A, Figulla HR. Impact of short-term systemic hypoxia on phagocytosis, cytokine production, and transcription factor activation in peripheral blood cells. Mediators Inflammation (2011) 2011:429501. doi: 10.1155/2011/429501
48. Elks PM, van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, et al. Activation of hypoxia-inducible factor-1α (Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood (2011) 118(3):712–22. doi: 10.1182/blood-2010-12-324186
49. Henneck T, Mergani A, Clever S, Seidler AE, Brogden G, Runft S, et al. Formation of neutrophil extracellular traps by reduction of cellular cholesterol is independent of oxygen and HIF-1α. Int J Mol Sci (2022) 23(6):3195. doi: 10.3390/ijms23063195
50. McInturff AM, Cody MJ, Elliott EA, Glenn JW, Rowley JW, Rondina MT, et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 α. Blood (2012) 120(15):3118–25. doi: 10.1182/blood-2012-01-405993
51. Harris AJ, Mirchandani AS, Lynch RW, Murphy F, Delaney L, Small D, et al. IL4Rα signaling abrogates hypoxic neutrophil survival and limits acute lung injury responses in vivo. Am J Respir Crit Care Med. (2019) 200(2):235–46. doi: 10.1164/rccm.201808-1599OC
52. Khatib-Massalha E, Bhattacharya S, Massalha H, Biram A, Golan K, Kollet O, et al. Lactate released by inflammatory bone marrow neutrophils induces their mobilization via endothelial GPR81 signaling. Nat Commun (2020) 11(1):3547. doi: 10.1038/s41467-020-17402-2
53. Zhu Q, Wang H, Jiang B, Ni X, Jiang L, Li C, et al. Loss of ATF3 exacerbates liver damage through the activation of mTOR/p70S6K/ HIF-1α signaling pathway in liver inflammatory injury. Cell Death Dis (2018) 9(9):910. doi: 10.1038/s41419-018-0894-1
54. Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med (Berl) (2007) 85(12):1339–46. doi: 10.1007/s00109-007-0282-2
55. Naldini A, Morena E, Pucci A, Miglietta D, Riboldi E, Sozzani S, et al. Hypoxia affects dendritic cell survival: Role of the hypoxia-inducible factor-1α and lipopolysaccharide. J Cell Physiol (2012) 227(2):587–95. doi: 10.1002/jcp.22761
56. Köhler T, Reizis B, Johnson RS, Weighardt H, Förster I. Influence of hypoxia-inducible factor 1α on dendritic cell differentiation and migration. Eur J Immunol (2012) 42(5):1226–36. doi: 10.1002/eji.201142053
57. Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, et al. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol (2008) 180(7):4697–705. doi: 10.4049/jimmunol.180.7.4697
58. Siegert I, Schödel J, Nairz M, Schatz V, Dettmer K, Dick C, et al. Ferritin-mediated iron sequestration stabilizes hypoxia-inducible factor-1α upon LPS activation in the presence of ample oxygen. Cell Rep (2015) 13(10):2048–55. doi: 10.1016/j.celrep.2015.11.005
59. Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras hypoxia. J Biol Chem (2001) 276(12):9519–25. doi: 10.1074/jbc.M010144200
60. Filippi I, Morena E, Aldinucci C, Carraro F, Sozzani S, Naldini A. Short-term hypoxia enhances the migratory capability of dendritic cell through HIF-1α and PI3K/Akt pathway. J Cell Physiol (2014) 229(12):2067–76. doi: 10.1002/jcp.24666
61. Liu J, Zhang X, Chen K, Cheng Y, Liu S, Xia M, et al. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1α-Mediated glycolysis. Immunity (2019) 50(3):600–615.e15. doi: 10.1016/j.immuni.2019.01.021
62. Bhandari T, Olson J, Johnson RS, Nizet V. HIF-1 influences myeloid cell antigen presentation and response to subcutaneous OVA vaccination. J Mol Med (Berl). (2013) 91(10):1199–205. doi: 10.1007/s00109-013-1052-y
63. Fliesser M, Morton CO, Bonin M, Ebel F, Hünniger K, Kurzai O, et al. Hypoxia-inducible factor 1α modulates metabolic activity and cytokine release in anti-aspergillus fumigatus immune responses initiated by human dendritic cells. Int J Med Microbiol (2015) 305(8):865–73. doi: 10.1016/j.ijmm.2015.08.036
64. Victorino F, Bigley TM, Park E, CH Y, Benoit J, LP Y, et al. HIF1α is required for NK cell metabolic adaptation during virus infection. Elife (2021) 16:e68484. doi: 10.7554/eLife.68484
65. Wei L, Lu J, Feng L, Long D, Shan J, Li S, et al. HIF-1alpha accumulation upregulates MICA and MICB expression on human cardiomyocytes and enhances NK cell cytotoxicity during hypoxia-reoxygenation. Life Sci (2010) 87(3-4):111–9. doi: 10.1016/j.lfs.2010.05.012
66. Krzywinska E, Kantari-Mimoun C, Kerdiles Y, Sobecki M, Isagawa T, Gotthardt D, et al. Loss of HIF-1α in natural killer cells inhibits tumour growth by stimulating non-productive angiogenesis. Nat Commun (2017) 8(1):1597. doi: 10.1038/s41467-017-01599-w
67. Cluff E, Magdaleno CC, Fernandez E, House T, Swaminathan S, Varadaraj A, et al. Hypoxia-inducible factor-1 alpha expression is induced by IL-2 via the PI3K/mTOR pathway in hypoxic NK cells and supports effector functions in NKL cells and ex vivo expanded NK cells. Cancer Immunol Immunother (2022) 71(8):1989–2005. doi: 10.1007/s00262-021-03126-9
68. Coulibaly A, Velásquez SY, Kassner N, Schulte J, Barbarossa MV, Lindner HA. STAT3 governs the HIF-1α response in IL-15 primed human NK cells. Sci Rep (2021) 11(1):7023. doi: 10.1038/s41598-021-84916-0
69. Jeong HJ, Oh HA, Nam SY, Han NR, Kim YS, Kim JH, et al. The critical role of mast cell-derived hypoxia-inducible factor-1α in human and mice melanoma growth. Int J Cancer (2013) 132(11):2492–501. doi: 10.1002/ijc.27937
70. Sumbayev VV, Yasinska I, Oniku AE, Streatfield CL, Gibbs BF. Involvement of hypoxia-inducible factor-1 in the inflammatory responses of human LAD2 mast cells and basophils. PloS One (2012) 7(3):e34259. doi: 10.1371/journal.pone.0034259
71. Liang X, Yin G, Ma Y, Xu K, Liu J, Li J. The critical role of mast cell-derived hypoxia-inducible factor-1α in regulating mast cell function. J Pharm Pharmacol (2016) 68(11):1409–16. doi: 10.1111/jphp
72. Jeong HJ, Chung HS, Lee BR, Kim SJ, Yoo SJ, Hong SH, et al. Expression of proinflammatory cytokines via HIF-1alpha and NF-kappaB activation on desferrioxamine-stimulated HMC-1 cells. Biochem Biophys Res Commun (2003) 306(4):805–11. doi: 10.1016/s0006-291x(03)01073-8
73. Abebayehu D, Spence AJ, Qayum AA, Taruselli MT, McLeod JJ, Caslin HL, et al. Lactic acid suppresses IL-33-Mediated mast cell inflammatory responses via hypoxia-inducible factor-1α-Dependent miR-155 suppression. J Immunol (2016) 197(7):2909–17. doi: 10.4049/jimmunol.1600651
74. Di Nardo A, Vitiello A, Gallo RL. Cutting edge: Mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol (2003) 170(5):2274–8. doi: 10.4049/jimmunol.170.5.2274
75. Branitzki-Heinemann K, Okumura CY, Völlger L, Kawakami Y, Kawakami T, Naim HY, et al. A novel role for the transcription factor HIF-1α in the formation of mast cell extracellular traps. Biochem J (2012) 446(1):159–63. doi: 10.1042/BJ20120658
76. Li L, Feng C, Qin J, Li D, Liu M, Han S, et al. Regulation of humoral immune response by HIF-1α-dependent metabolic reprogramming of the germinal center reaction. Cell Immunol (2021) 367:104409. doi: 10.1016/j.cellimm.2021.104409
77. Xu S, Huo J, Huang Y, Aw M, Chen S, Mak S, et al. Von hippel-lindau protein maintains metabolic balance to regulate the survival of naive b lymphocytes. iScience (2019) 26:17. doi: 10.1016/j.isci.2019.07.002
78. Kojima H, Kobayashi A, Sakurai D, Kanno Y, Hase H, Takahashi R, et al. Differentiation stage-specific requirement in hypoxia-inducible factor-1alpha-regulated glycolytic pathway during murine b cell development in bone marrow. J Immunol (2010) 184(1):154–63. doi: 10.4049/jimmunol.0800167
79. Burrows N, Bashford-Rogers RJM, Bhute VJ, Peñalver A, Ferdinand JR, Stewart BJ, et al. Dynamic regulation of hypoxia-inducible factor-1α activity is essential for normal b cell development. Nat Immunol (2020) 21(11):1408–20. doi: 10.1038/s41590-020-0772-8
80. Meng X, Grötsch B, Luo Y, Knaup KX, Wiesener MS, Chen XX, et al. Hypoxia-inducible factor-1α is a critical transcription factor for IL-10-producing b cells in autoimmune disease. Nat Commun (2018) 9(1):251. doi: 10.1038/s41467-017-02683-x
81. Cho SH, Raybuck AL, Stengel K, Wei M, Beck TC, Volanakis E, et al. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature (2016) 537(7619):234–8. doi: 10.1038/nature19334
82. Hu F, Liu H, Xu L, Li Y, Liu X, Shi L, et al. Hypoxia-inducible factor-1α perpetuates synovial fibroblast interactions with T cells and b cells in rheumatoid arthritis. Eur J Immunol (2016) 46(3):742–51. doi: 10.1002/eji.201545784
83. Jang JW, Park S, Moon EY. Spleen tyrosine kinase regulates crosstalk of hypoxia-inducible factor-1α and nuclear factor (erythroid-derived2)-like 2 for b cell survival. Int Immunopharmacol (2021) 95:107509. doi: 10.1016/j.intimp.2021.107509
84. Jang JW, Thuy PX, Lee JW, Moon EY. CXCR4 promotes b cell viability by the cooperation of nuclear factor (erythroid-derived 2)-like 2 and hypoxia-inducible factor-1α under hypoxic conditions. Cell Death Dis (2021) 12(4):330. doi: 10.1038/s41419-021-03615-w
85. Chaudhari SM, Sluimer JC, Koch M, Theelen TL, Manthey HD, Busch M, et al. Deficiency of HIF1α in antigen-presenting cells aggravates atherosclerosis and type 1 T-helper cell responses in mice. Arterioscler Thromb Vasc Biol (2015) 35(11):2316–25. doi: 10.1161/ATVBAHA.115.306171
86. Marks E, Naudin C, Nolan G, Goggins BJ, Burns G, Mateer SW, et al. Regulation of IL-12p40 by HIF controls Th1/Th17 responses to prevent mucosal inflammation. Mucosal Immunol (2017) 10(5):1224–36. doi: 10.1038/mi.2016.135
87. Westendorf AM, Skibbe K, Adamczyk A, Buer J, Geffers R, Hansen W, et al. Hypoxia enhances immunosuppression by inhibiting CD4+ effector T cell function and promoting treg activity. Cell Physiol Biochem (2017) 41(4):1271–84. doi: 10.1159/000464429
88. Hammami A, Abidin BM, Heinonen KM, Stäger S. HIF-1α hampers dendritic cell function and Th1 generation during chronic visceral leishmaniasis. Sci Rep (2018) 8(1):3500. doi: 10.1038/s41598-018-21891-z
89. Wan C, Bi W, Lin P, Zhang Y, Tian J, Fang S, et al. MicroRNA 182 promotes T helper 1 cell by repressing hypoxia induced factor 1 alpha in experimental autoimmune encephalomyelitis. Eur J Immunol (2019) 49(12):2184–94. doi: 10.1002/eji.201948111
90. Lee KS, Kim SR, Park SJ, Park HS, Min KH, Jin SM, et al. Peroxisome proliferator activated receptor-gamma modulates reactive oxygen species generation and activation of nuclear factor-kappaB and hypoxia-inducible factor 1alpha in allergic airway disease of mice. J Allergy Clin Immunol (2006) 118(1):120–7. doi: 10.1016/j.jaci.2006.03.021
91. Niu Y, Wang J, Li Z, Yao K, Wang L, Song J. HIF1α deficiency in dendritic cells attenuates symptoms and inflammatory indicators of allergic rhinitis in a SIRT1-dependent manner. Int Arch Allergy Immunol (2020) 181(8):585–93. doi: 10.1159/000506862
92. Jang TY, Park CS, Kim KS, Heo MJ, Kim YH. Benzaldehyde suppresses murine allergic asthma and rhinitis. Int Immunopharmacol (2014) 22(2):444–50. doi: 10.1016/j.intimp.2014.07.029
93. Mo JH, Kim JH, Lim DJ, Kim EH. The role of hypoxia-inducible factor 1α in allergic rhinitis. Am J Rhinol Allergy (2014) 28(2):e100–6. doi: 10.2500/ajra.2014.28.4025
94. de Souza Alves CC, Collison A, Hatchwell L, Plank M, Morten M, Foster PS, et al. Inhibiting AKT phosphorylation employing non-cytotoxic anthraquinones ameliorates TH2 mediated allergic airways disease and rhinovirus exacerbation. PloS One (2013) 8(11):e79565. doi: 10.1371/journal.pone.0079565
95. PXiong Y, Lingrel JB, Wüthrich M, Klein BS, Vasudevan NT, Jain MK, et al. Transcription factor KLF2 in dendritic cells downregulates Th2 programming via the HIF-1α/Jagged2/Notch axis. mBio (2016) 7(3):e00436–16. doi: 10.1128/mBio.00436-16
96. Yao Y, Wang L, Zhou J, Zhang X. HIF-1α inhibitor echinomycin reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. J Transl Med (2017) 15(1):28. doi: 10.1186/s12967-017-1132-9
97. Zhou W, Yang J, Saren G, Zhao H, Cao K, Fu S, et al. HDAC6-specific inhibitor suppresses Th17 cell function via the HIF-1α pathway in acute lung allograft rejection in mice. Theranostics (2020) 10(15):6790–805. doi: 10.7150/thno.44961
98. Groneberg M, Hoenow S, Marggraff C, Fehling H, Metwally NG, Hansen C, et al. HIF-1α modulates sex-specific Th17/Treg responses during hepatic amoebiasis. J Hepatol (2022) 76(1):160–73. doi: 10.1016/j.jhep.2021.09.020
99. kejiri A, Nagai S, Goda N, Kurebayashi Y, Osada-Oka M, Takubo K, et al. Dynamic regulation of Th17 differentiation by oxygen concentrations. Int Immunol (2012) 24(3):137–46. doi: 10.1093/intimm/dxr111
100. Liu J, Li W, Zhu W, He W, Zhao H, Xiang Y, et al. Chronic intermittent hypoxia promotes the development of experimental non-alcoholic steatohepatitis by modulating Treg/Th17 differentiation. Acta Biochim Biophys Sin (Shanghai) (2018) 50(12):1200–10. doi: 10.1093/abbs/gmy131
101. Sun Y, Tian T, Gao J, Liu X, Hou H, Cao R, et al. Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J Neuroimmunol (2016) 15:58–67. doi: 10.1016/j.jneuroim.2016.01.014
102. Yang EJ, Lee J, Lee SY, Kim EK, Moon YM, Jung YO, et al. EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF-1α with Th17/Treg control. PloS One (2014) 9(2):e86062. doi: 10.1371/journal.pone.0086062
103. Zhang Q, Wang L, Jiang J, Lin S, Luo A, Zhao P, et al. Critical role of AdipoR1 in regulating Th17 cell differentiation through modulation of HIF-1α-Dependent glycolysis. Front Immunol (2020) 18:2040. doi: 10.3389/fimmu.2020.02040
104. Gerriets VA, Danzaki K, Kishton RJ, Eisner W, Nichols AG, Saucillo DC, et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol (2016) 46(8):1970–83. doi: 10.1002/eji.201545861
105. Jarvis LB, Rainbow DB, Coppard V, Howlett SK, Georgieva Z, Davies JL, et al. Therapeutically expanded human regulatory T-cells are super-suppressive due to HIF1A induced expression of CD73. Commun Biol (2021) 4(1):1186. doi: 10.1038/s42003-021-02721-x
106. Pei P, Shen W, Zhang Y, Zhang Y, Qi Z, Zhou H, et al. Radioactive nano-oxygen generator enhance anti-tumor radio-immunotherapy by regulating tumor microenvironment and reducing proliferation. Biomaterials (2022) 280:121326. doi: 10.1016/j.biomaterials.2021.121326
107. Bollinger T, Gies S, Naujoks J, Feldhoff L, Bollinger A, Solbach W, et al. HIF-1α- and hypoxia-dependent immune responses in human CD4+CD25high T cells and T helper 17 cells. J Leukoc Biol (2014) 96(2):305–12. doi: 10.1189/jlb.3A0813-426RR
108. Feldhoff LM, Rueda CM, Moreno-Fernandez ME, Sauer J, Jackson CM, Chougnet CA, et al. IL-1β induced HIF-1α inhibits the differentiation of human FOXP3+ T cells. Sci Rep (2017) 7(1):465. doi: 10.1038/s41598-017-00508-x
109. Palazon A, Tyrakis PA, Macias D, Veliça P, Rundqvist H, Fitzpatrick S, et al. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell (2017) 32(5):669–683.e5. doi: 10.1016/j.ccell.2017.10.003
110. Gupta SS, Sharp R, Hofferek C, Kuai L, Dorn GW 2nd, Wang J, et al. NIX-mediated mitophagy promotes effector memory formation in antigen-specific CD8+ T cells. Cell Rep (2019) 29(7):1862–77. doi: 10.1016/j.celrep.2019.10.032
111. Mortezaee K, Majidpoor J. The impact of hypoxia on immune state in cancer. Life Sci (2021) 286:120057. doi: 10.1016/j.lfs.2021.120057
112. Saha J, Sarkar D, Pramanik A, Mahanti K, Adhikary A, Bhattacharyya S. PGE2-HIF1α reciprocal induction regulates migration, phenotypic alteration and immunosuppressive capacity of macrophages in tumor microenvironment. Life Sci (2020) 253:117731. doi: 10.1016/j.lfs.2020.117731
113. Liikanen I, Lauhan C, Quon S, Omilusik K, AT P, LB Bartrolí, et al. Hypoxia-inducible factor activity promotes antitumor effector function and tissue residency by CD8+ T cells. J Clin Invest. (2021) 131(7):e143729. doi: 10.1172/JCI143729
114. Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol (2013) 14(11):1173–82. doi: 10.1038/ni.2714
115. Kang Y, Zuo Y, He M, Duo L, Chen X, Tang W. Clinical predictive model to estimate probability of remission in patients with lupus nephritis. Int Immunopharmacol (2022) 110:108966. doi: 10.1016/j.intimp.2022.108966
116. Ma C, Wei J, Zhan F, Wang R, Fu K, Wan X, et al. Urinary hypoxia-inducible factor-1alpha levels are associated with histologic chronicity changes and renal function in patients with lupus nephritis. Yonsei Med J (2012) 53(3):587–92. doi: 10.3349/ymj.2012.53.3.587
117. Feng CC, QL Ye, Zhu Y, Leng RX, Chen GM, Yang J, et al. Lack of association between the polymorphisms of hypoxia-inducible factor 1A (HIF1A) gene and SLE susceptibility in a Chinese population. Immunogenetics (2014) 66(1):9–13. doi: 10.1007/s00251-013-0743-4
118. Kono M, Yoshida N, Maeda K, Suárez-Fueyo A, Kyttaris VC, Tsokos GC. Glutaminase 1 inhibition reduces glycolysis and ameliorates lupus-like disease in MRL/lpr mice and experimental autoimmune encephalomyelitis. Arthritis Rheumatol (2019) 71(11):1869–78. doi: 10.1002/art.41019
119. Ahn JK, Koh EM, Cha HS, Lee YS, Kim J, Bae EK, et al. Role of hypoxia-inducible factor-1alpha in hypoxia-induced expressions of IL-8, MMP-1 and MMP-3 in rheumatoid fibroblast-like synoviocytes. Rheumatol (Oxford) (2008) 47(6):834–9. doi: 10.1093/rheumatology/ken086
120. Brouwer E, Gouw AS, Posthumus MD, van Leeuwen MA, Boerboom AL, Bijzet J, et al. Hypoxia inducible factor-1-alpha (HIF-1alpha) is related to both angiogenesis and inflammation in rheumatoid arthritis. Clin Exp Rheumatol (2009) 27(6):945–51.
121. Chen J, Cheng W, Li J, Wang Y, Chen J, Shen X, et al. Notch-1 and notch-3 mediate hypoxia-induced activation of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheumatol (2021) 73(10):1810–9. doi: 10.1002/art.41748
122. Bai L, Chen SZ, Liang S, SS Li, Wang W. [The influence of tripterygium hypoglaucum hutch on the HIF-1α expression in the CIA rat models]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi (2011) 27(10):1061–4.
123. Lei Z, Ouyang L, Gong Y, Wang Z, Yu B. Effect of eriodictyol on collagen-induced arthritis in rats by Akt/HIF-1α pathway. Drug Des Devel Ther (2020) 29:1633–9. doi: 10.2147/DDDT.S239662
124. Zhang XJ, Liu J, Wan L, Sun Y, Wang F, YJ Qi, et al. [Relations of synovial angiogenesis and PTEN/PI3K/AKT signaling pathway in rats with adjuvant arthritis]. Zhongguo Gu Shang (2015) 28(1):71–4.
125. Meng B, Liu FY, Liu MM, Yu LC, Zhang WT, Zhou MY, et al. AMSP-30 m as a novel HIF-1α inhibitor attenuates the development and severity of adjuvant-induced arthritis in rats: Impacts on synovial apoptosis, synovial angiogenesis and sonic hedgehog signaling pathway. Int Immunopharmacol (2022) 103:108467. doi: 10.1016/j.intimp.2021.108467
126. Jiang TT, CF Ji, Cheng XP, Gu SF, Wang R, Li Y, et al. α-mangostin alleviated HIF-1α-Mediated angiogenesis in rats with adjuvant-induced arthritis by suppressing aerobic glycolysis. Front Pharmacol (2021) 20:785586. doi: 10.3389/fphar.2021.785586
127. Westra J, Brouwer E, Bouwman E, Doornbos-van der Meer B, Posthumus MD, van Leeuwen MA, et al. Role for CaMKII inhibition in rheumatoid arthritis: Effects on HIF-1-induced VEGF production by rheumatoid synovial fibroblasts. Ann N Y Acad Sci (2009) 1173:706–11. doi: 10.1111/j.1749-6632.2009.04736.x
128. Feng ZT, Yang T, Hou XQ, Wu HY, Feng JT, Ou BJ, et al. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. BioMed Pharmacother (2019) 113:108759. doi: 10.1016/j.biopha.2019.108759
129. Li GQ, Zhang Y, Liu D, Qian YY, Zhang H, Guo SY, et al. PI3 kinase/Akt/HIF-1α pathway is associated with hypoxia-induced epithelial-mesenchymal transition in fibroblast-like synoviocytes of rheumatoid arthritis. Mol Cell Biochem (2013) 372(1-2):221–31. doi: 10.1007/s11010-012-1463-z
130. Yang W, Wei X, Jiao Y, Bai Y, Sam WN, Yan Q, et al. STAT3/HIF-1α/fascin-1 axis promotes RA FLSs migration and invasion ability under hypoxia. Mol Immunol (2022) 142:83–94. doi: 10.1016/j.molimm.2021.12.004
131. Li G, Zhang Y, Qian Y, Zhang H, Guo S, Sunagawa M, et al. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-κB/HIF-1α pathway. Mol Immunol (2013) 53(3):227–36. doi: 10.1016/j.molimm.2012.08.018
132. Hulley PA, Bishop T, Vernet A. Hypoxia-inducible factor 1-alpha does not regulate osteoclastogenesis but enhances bone resorption activity via prolyl-4-hydroxylase 2. J Pathol (2017) 242(3):322–33. doi: 10.1002/path.4906
133. Knowles HJ. Distinct roles for the hypoxia-inducible transcription factors HIF-1α and HIF-2α in human osteoclast formation and function. Sci Rep (2020) 10(1):21072. doi: 10.1038/s41598-020-78003-z
134. Hu F, Mu R, Zhu J, Shi L, Li Y, Liu X, et al. Hypoxia and hypoxia-inducible factor-1α provoke toll-like receptor signalling-induced inflammation in rheumatoid arthritis. Ann Rheum Dis (2014) 73(5):928–36. doi: 10.1136/annrheumdis-2012-202444
135. Yu R, Li C, Sun L, Jian L, Ma Z, Zhao J, et al. Hypoxia induces production of citrullinated proteins in human fibroblast-like synoviocytes through regulating HIF1α. Scand J Immunol (2018) 87(4):e12654. doi: 10.1111/sji.12654
136. Li Y, Liu Y, Wang C, WR X, Zheng JY, Yang J, et al. Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1α/VEGF axis. Free Radic Biol Med (2018) 126:1–14. doi: 10.1016/j.freeradbiomed.2018.07.009
137. Ding LL, Li X, Lei YM, Xia LP, Lu J, Shen H. Effect of interleukin-34 on secretion of angiogenesis cytokines by peripheral blood mononuclear cells of rheumatoid arthritis. Immunol Invest (2020) 49(1-2):81–7. doi: 10.1080/08820139.2019.1649281
138. Pei B, Chen K, Zhou S, Min D, Xiao W. IL-38 restrains inflammatory response of collagen-induced arthritis in rats via SIRT1/HIF-1α signaling pathway. Biosci Rep (2020) 40(5). doi: 10.1042/BSR20182431
139. Knowles HJ, Cleton-Jansen AM, Korsching E, Athanasou NA. Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: Role of angiopoietin-like 4. FASEB J (2010) 24(12):4648–59. doi: 10.1096/fj.10-162230
140. Ortiz-Masiá D, Cosín-Roger J, Calatayud S, Hernández C, Alós R, Hinojosa J, et al. M1 macrophages activate notch signalling in epithelial cells: Relevance in crohn's disease. J Crohns Colitis (2016) 10(5):582–92. doi: 10.1093/ecco-jcc/jjw009
141. Hume GE, Doecke JD, Huang N, Fowler EV, Brown IS, Simms LA, et al. Altered expression of angiotensinogen and mediators of angiogenesis in ileal crohn's disease. J Gastrointestin Liver Dis (2016) 25(1):39–48. doi: 10.15403/jgld.2014.1121.251.chr
142. Senhaji N, Nadifi S, Zaid Y, Serrano A, Rodriguez DAL, Serbati N, et al. Polymorphisms in oxidative pathway related genes and susceptibility to inflammatory bowel disease. World J Gastroenterol (2017) 23(47):8300–7. doi: 10.3748/wjg.v23.i47.8300
143. Wu MM, Wang QM, Huang BY, Mai CT, Wang CL, Wang TT, et al. Dioscin ameliorates murine ulcerative colitis by regulating macrophage polarization. Pharmacol Res (2021) 172:105796. doi: 10.1016/j.phrs.2021.105796
144. Zhuang H, Lv Q, Zhong C, Cui Y, He L, Zhang C, et al. Tiliroside ameliorates ulcerative colitis by restoring the M1/M2 macrophage balance via the HIF-1α/glycolysis pathway. Front Immunol (2021) 31:649463. doi: 10.3389/fimmu.2021.649463
145. Lu H, Lin J, Xu C, Sun M, Zuo K, Zhang X, et al. Cyclosporine modulates neutrophil functions via the SIRT6-HIF-1α-glycolysis axis to alleviate severe ulcerative colitis. Clin Transl Med (2021) 11(2):e334. doi: 10.1002/ctm2.334
146. Galván-Peña S, O'Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol (2014) 2:420. doi: 10.3389/fimmu.2014.00420
147. Mimouna S, Gonçalvès D, Barnich N, Darfeuille-Michaud A, Hofman P, Vouret-Craviari V. Crohn disease-associated escherichia coli promote gastrointestinal inflammatory disorders by activation of HIF-dependent responses. Gut Microbes (2011) 2(6):335–46. doi: 10.4161/gmic.18771
148. Sun M, He C, Wu W, Zhou G, Liu F, Cong Y, et al. Hypoxia inducible factor-1α-induced interleukin-33 expression in intestinal epithelia contributes to mucosal homeostasis in inflammatory bowel disease. Clin Exp Immunol (2017) 187(3):428–40. doi: 10.1111/cei.12896
149. Brown E, Rowan C, Strowitzki MJ, Fagundes RR, Faber KN, Güntsch A, et al. Mucosal inflammation downregulates PHD1 expression promoting a barrier-protective HIF-1α response in ulcerative colitis patients. FASEB J (2020) 34(3):3732–42. doi: 10.1096/fj.201902103R
150. Zhang D, Wei C, Yao J, Cai X, Wang L. Interleukin-10 gene-carrying bifidobacteria ameliorate murine ulcerative colitis by regulating regulatory T cell/T helper 17 cell pathway. Exp Biol Med (Maywood) (2015) 240(12):1622–9. doi: 10.1177/1535370215584901
151. Hindryckx P, De Vos M, Jacques P, Ferdinande L, Peeters H, Olievier K, et al. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. J Immunol (2010) 185(10):6306–16. doi: 10.4049/jimmunol.1002541
152. Flück K, Breves G, Fandrey J, Winning S. Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol (2016) 9(2):379–90. doi: 10.1038/mi.2015.67
153. Higashiyama M, Hokari R, Hozumi H, Kurihara C, Ueda T, Watanabe C, et al. HIF-1 in T cells ameliorated dextran sodium sulfate-induced murine colitis. J Leukoc Biol (2012) 91(6):901–9. doi: 10.1189/jlb.1011518
154. Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest (2004) 114(8):1098–106. doi: 10.1172/JCI21086
155. Lin N, Shay JES, Xie H, Lee DSM, Skuli N, Tang Q, et al. Myeloid cell hypoxia-inducible factors promote resolution of inflammation in experimental colitis. Front Immunol (2018) 5:2565. doi: 10.3389/fimmu.2018.02565
156. Ioannou M, Pyrpasopoulou A, Simos G, Paraskeva E, Nikolaidou C, Venizelos I, et al. Upregulation of VEGF expression is associated with accumulation of HIF-1α in the skin of naïve scleroderma patients. Mod Rheumatol (2013) 23(6):1245–8. doi: 10.1007/s10165-012-0787-6
157. Wipff J, Dieude P, Avouac J, Tiev K, Hachulla E, Granel B, et al. Association of hypoxia-inducible factor 1A (HIF1A) gene polymorphisms with systemic sclerosis in a French European Caucasian population. Scand J Rheumatol (2009) 38(4):291–4. doi: 10.1080/03009740802629432
158. Takagi K, Kawamoto M, Higuchi T, Tochimoto A, Harigai M, Kawaguchi Y. Single nucleotide polymorphisms of the HIF1A gene are associated with susceptibility to pulmonary arterial hypertension in systemic sclerosis and contribute to SSc-PAH disease severity. Int J Rheum Dis (2020) 23(5):674–80. doi: 10.1111/1756-185X.13822
159. Nicolosi PA, Tombetti E, Maugeri N, Rovere-Querini P, Brunelli S, Manfredi AA. Vascular remodelling and mesenchymal transition in systemic sclerosis. Stem Cells Int (2016) 2016:4636859. doi: 10.1155/2016/4636859
160. Cutolo M, Soldano S, Smith V. Pathophysiology of systemic sclerosis: Current understanding and new insights. Expert Rev Clin Immunol (2019) 15(7):753–64. doi: 10.1080/1744666X.2019.1614915
161. Zhou X, Liu C, Lu J, Zhu L, Li M. 2-methoxyestradiol inhibits hypoxia-induced scleroderma fibroblast collagen synthesis by phosphatidylinositol 3-kinase/Akt/mTOR signalling. Rheumatology (2018) 57(9):1675–84. doi: 10.1093/rheumatology/key166
162. Hong KH, Yoo SA, Kang SS, Choi JJ, Kim WU, Cho CS. Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts. Clin Exp Immunol (2006) 146(2):362–70. doi: 10.1111/j.1365-2249.2006.03199.x
163. Zhu WJ, Li P, Wang L, Xu YC. Hypoxia-inducible factor-1: A potential pharmacological target to manage psoriasis. Int Immunopharmacol (2020) 86:106689. doi: 10.1016/j.intimp.2020.106689
164. Li Y, Zhang G, Xiao R, Chen H, Wen H. [Expression of iNOS and HIF-1α with angiogenesis in affected skin biopsies from patients with psoriasis]. Zhong Nan Da Xue Xue Bao Yi Xue Ban (2010) 35(9):952–7. doi: 10.3969/j.issn.1672-7347.2010.09.009
165. Rosenberger C, Solovan C, AD R, Jinping L, Treudler R, Frei U, et al. Upregulation of hypoxia-inducible factors in normal and psoriatic skin. J Invest Dermatol (2007) 127(10):2445–52. doi: 10.1038/sj.jid.5700874
166. Tovar-Castillo LE, Cancino-Díaz JC, García-Vázquez F, Cancino-Gómez FG, León-Dorantes G, Blancas-González F, et al. Under-expression of VHL and over-expression of HDAC-1, HIF-1alpha, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int J Dermatol (2007) 46(3):239–46. doi: 10.1111/j.1365-4632.2006.02962.x
167. Armstrong AW, SV V, EJ A, EN F, Rutledge JC. Angiogenesis and oxidative stress: Common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci (2011) 63(1):1–9. doi: 10.1016/j.jdermsci.2011.04.007
168. Li Y, Su J, Li F, Chen X, Zhang G. MiR-150 regulates human keratinocyte proliferation in hypoxic conditions through targeting HIF-1α and VEGFA: Implications for psoriasis treatment. PloS One (2017) 12(4):e0175459. doi: 10.1371/journal.pone.0175459
169. Wu R, Zeng J, Yuan J, Deng X, Huang Y, Chen L, et al. MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J Clin Invest (2018) 128(6):2551–68. doi: 10.1172/JCI97426
170. Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Brück W, et al. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol (2003) 62(1):25–33. doi: 10.1093/jnen/62.1.25
171. Wiesner D, Merdian I, Lewerenz J, Ludolph AC, Dupuis L, Witting A. Fumaric acid esters stimulate astrocytic VEGF expression through HIF-1α and Nrf2. PloS One (2013) 8(10):e76670. doi: 10.1371/journal.pone.0076670
172. Saravani M, Rokni M, Mehrbani M, Amirkhosravi A, Faramarz S, Fatemi I, et al. The evaluation of VEGF and HIF-1α gene polymorphisms and multiple sclerosis susceptibility. J Gene Med (2019) 21(12):e3132. doi: 10.1002/jgm.3132
173. Argaw AT, Zhang Y, BJ S, ML Z, Kopp N, SC L, et al. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol (2006) 177(8):5574–84. doi: 10.4049/jimmunol.177.8.5574
174. Nomoto H, Pei L, Montemurro C, Rosenberger M, Furterer A, Coppola G, et al. Activation of the HIF1α/PFKFB3 stress response pathway in beta cells in type 1 diabetes. Diabetologia (2020) 63(1):149–61. doi: 10.1007/s00125-019-05030-5
175. Sankar KS, Altamentova SM, Rocheleau JV. Hypoxia induction in cultured pancreatic islets enhances endothelial cell morphology and survival while maintaining beta-cell function. PloS One (2019) 14(10):e0222424. doi: 10.1371/journal.pone.0222424
176. Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, et al. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol (2015) 26(2):328–38. doi: 10.1681/ASN.2013090990
177. Lalwani A, Warren J, Liuwantara D, Hawthorne WJ, O'Connell PJ, Gonzalez FJ, et al. β cell hypoxia-inducible factor-1α is required for the prevention of type 1 diabetes. Cell Rep (2019) 27(8):2370–2384.e6. doi: 10.1016/j.celrep.2019.04.086
178. Rojas DR, Tegeder I, Kuner R, Agarwal N. Hypoxia-inducible factor 1α protects peripheral sensory neurons from diabetic peripheral neuropathy by suppressing accumulation of reactive oxygen species. J Mol Med (Berl) (2018) 96(12):1395–405. doi: 10.1007/s00109-018-1707-9
179. Krishan P, Singh G, Bedi O. Carbohydrate restriction ameliorates nephropathy by reducing oxidative stress and upregulating HIF-1α levels in type-1 diabetic rats. J Diabetes Metab Disord (2017) 19:16. doi: 10.1186/s40200-017-0331-5
180. Wu Q, You L, Nepovimova E, Heger Z, Wu W, Kuca K, et al. Hypoxia-inducible factors: Master regulators of hypoxic tumor immune escape. J Hematol Oncol (2022) 15(1):77. doi: 10.1186/s13045-022-01292-6
181. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science (2014) 345(6204):1250684. doi: 10.1126/science.1250684
Keywords: immune cell, inflammation, autoimmune diseases, HIF-1α, signaling pathway
Citation: Tang Y-Y, Wang D-C, Wang Y-Q, Huang A-F and Xu W-D (2023) Emerging role of hypoxia-inducible factor-1α in inflammatory autoimmune diseases: A comprehensive review. Front. Immunol. 13:1073971. doi: 10.3389/fimmu.2022.1073971
Received: 19 October 2022; Accepted: 13 December 2022;
Published: 25 January 2023.
Edited by:
Lifei Hou, Boston Children’s Hospital and Harvard Medical School, United StatesReviewed by:
Chengxian Xu, Boston Children’s Hospital and Harvard Medical School, United StatesCopyright © 2023 Tang, Wang, Wang, Huang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang-Dong Xu, bG91dGNoMTIzQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.