- Department of Hematology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Duvelisib is the first FDA-approved oral dual inhibitor of phosphatidylinositol-3-kinase PI3K-delta (PI3K-δ) and PI3K-gamma (PI3K-γ). Although many clinical studies support the efficacy of duvelisib, the safety of duvelisib remains with great attention. This systematic review and meta-analysis aimed to evaluate the safety and efficacy of duvelisib in treating different relapsed or refractory (RR) lymphoid neoplasm types.
Methods: We searched prospective clinical trials from PUBMED, EMBASE, Cochrane Library, and ClinicalTrials.gov. For efficacy analysis, Overall response rate (ORR), complete response rate (CR), partial response rate (PR), rate of stable disease (SDR), rate of progressive disease (PDR), median progression-free survival (mPFS), 12-/24-month PFS, and 12-month overall survival (OS) were assessed. For safety analysis, the incidences of any grade and grade ≥3 adverse events (AEs), serious AEs, and treatment-related discontinuation and death were evaluated. Subgroup analysis based on the disease type was performed.
Results: We included 11 studies and 683 patients, including 305 chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), 187 B-cell indolent non-Hodgkin lymphoma (iNHL), 39 B-cell aggressive non-Hodgkin lymphoma (aNHL), and 152 T-cell non-Hodgkin lymphoma (T-NHL) patients. The pooled ORR in CLL/SLL, iNHL, aNHL and T-NHL was 70%, 70%, 28% and 47%, respectively. Additionally, the pooled ORR in CLL/SLL patients with or without TP53 mutation/17p-deletion (62% vs. 74%, p=0.45) and in follicular lymphoma (FL) or other iNHL (69% vs. 57%, p=0.38) had no significant differences. Mantle cell lymphoma (MCL) patients had higher pooled ORR than other aNHL (68% vs. 17%, p=0.04). Angioimmunoblastic TCL (AITL) patients had higher pooled ORR than other PTCL patients (67% vs. 42%, p=0.01). The pooled incidence of any grade, grade ≥3, serious AEs, treatment-related discontinuation and death was 99%, 79%, 63%, 33% and 3%, respectively. The most frequent any-grade AEs were diarrhea (47%), ALT/AST increase (39%), and neutropenia (38%). The most frequent grade ≥3 AEs were neutropenia (25%), ALT/AST increased (16%), diarrhea (12%), and anemia (12%).
Conclusion: Generally, duvelisib could offer favorable efficacy in patients with RR CLL/SLL, iNHL, MCL, and AITL. Risk and severity in duvelisib treatment may be mitigated through proper identification and management.
Introduction
Lymphoid neoplasms comprise a heterogeneous group of lymphoproliferative malignancies with a variety of clinical, morphologic, and molecular features, for which about 150,000 new cases and 40,000 deaths expected to occur in 2022 in the United States alone (1). Mature B-cell neoplasms and mature T-cell neoplasms represent the most typical lymphocytic tumors originating from cells at stages of maturation after stem cell differentiation (2). Mature B-cell neoplasms account for nearly 65% of all lymphoid neoplasms, with both aggressive and indolent subtypes (2). The latter mainly including chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), Follicular lymphoma (FL), Marginal zone lymphoma (MZL), Lymphoplasmacytic lymphoma (3).Widespread use of chemoimmunotherapy has greatly improved the survival of patients with CLL/SLL and indolent non-Hodgkin lymphomas(iNHLs). However, these diseases are currently incurable (4–9). Patients with aggressive B-cell lymphoma(aNHLs), such as diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL), are often diagnosed with advanced stage, and a substantial proportion of patients are refractory to initial chemotherapy or relapse in early years. High-dose chemotherapy with autologous stem cell transplantation may be the only curative choice for patients with relapsed/refractory (R/R) aNHLs. Radiotherapy and single-agent therapies, which play roles in the treatment of iNHL, have shown low expected response rates and duration of responses (10–16). Mature T-cell non-Hodgkin lymphomas (T-NHLs) are another heterogeneous group representing approximately 6% of all lymphoid neoplasms (2), usually including cutaneous T-cell lymphoma (CTCL) and peripheral T-cell lymphoma (PTCL). With a relatively poor prognosis, PTCL includes PTCL not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), anaplastic large-cell lymphoma (ALCL), and others (17). In contrast to mature B-cell neoplasms, T-cell lymphomas, especially PTCL, have a high failure rate of first-line chemotherapy, prone to relapse, and lacking an effective monoclonal antibody, like anti-CD20 in B-cell lymphoma (18, 19). Some agents have been approved by the FDA for relapsed PTCL, unfortunately with low response rates and short median progression-free survival (PFS) (20–22). New and more effective agents with distinct mechanisms are urgently needed for patients with relapsed or refractory mature lymphoid neoplasms, whether the tumor originates from B cells or T cells.
Duvelisib is the first FDA-approved oral dual inhibitor of phosphatidylinositol-3-kinase PI3K-delta (PI3K-δ) and PI3K-gamma (PI3K-γ) and shows great potential in many clinical trials for the treatment of relapsed or refractory lymphoid neoplasms, including CLL/SLL, iNHLs, and T-NHLs (23–33). Phosphatidylinositol 3-kinase (PI3K) is a lipid kinase involved in lots of signal transduction. Class I PI3K consists of four catalytic subunits (α, β, γ, δ) in human cells. PI3K-α and PI3K-β show a broad tissue distribution. In contrast, the PI3K-δ and PI3K-γ isoforms are primarily expressed in leukocytes, extensively regulating both innate and adaptive immune function in lymphocyte and myeloid cell function (34–39).PI3K-δ inhibition directly targets proliferation and survival of lymphoid neoplasm cells, while PI3K-γ inhibition reduces the differentiation and migration of crucial tumor support cells in the tumor microenvironment, such as Treg cells and M2 tumor-associated macrophages (33, 40–44). With dual inhibition of PI3K-δ and PIK3-g in preclinical models of CLL, B-cell lymphomas, and T-cell lymphomas, duvelisib showed more robust anti-tumor activity than inhibitors of PI3K-δ isoform alone (33, 44–46).
However, due to the heterogeneity of lymphoid neoplasms, the efficacy of PI3K inhibition in lymphoid neoplasms ranged widely. Meanwhile, the PI3K/AKT/mTOR regulates a range of cellular activities, whether in malignant or normal cells, so off-target effects and side effects are inevitable (47, 48). Some of the toxic effects reported in clinical trials of PI3K-δ inhibitors, incredibly immune dysregulation, and immune dysfunction, have raised concerns about safety (49–51). Compared with other approved PI3K-δ inhibitors, duvelisib has certain safety advantages. Compared with idelalisib, only inhibiting PI3K-δ, and based on preclinical data, duvelisib may reduce autoimmune complications through the inhibition of PI3K-γ (52, 53). Additionally, duvelisib doesn’t need infusion, without producing hyperglycemic effects mediated by PI3K-α isoform inhibition, which reduces the usage of copanlisib in older adults. A better understanding of the complexities of the adverse events and subgroup of lymphoid neoplasms patients benefitting most from duvelisib treatment could provide more precise treatment schedule. In this systematic review, we analyzed the efficacy and safety of duvelisib monotherapy in patients with relapsed or refractory lymphoid neoplasms. Besides, subgroup analysis was conducted to compare the efficacy and safety of duvelisib between different disease groups. These findings lead to offering evidence-based references for clinicians to optimize future clinical trials and treatment options.
Methods
Literature search
The study design and literature search strategy for this article followed Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The relevant studies were identified by searching Medline (PubMed), Embase, Cochrane Library, and ClinicalTrials.gov. We used a combination of terms: “(Leukemia OR Lymphoma) AND ((Duvelisib) OR (COPIKTRA) OR (IPI-145))” to search for clinical studies evaluating the safety and efficacy of duvelisib in the treatment of relapsed or refractory lymphoid neoplasms, the data cut-off was May 20, 2022. There were no date or language restrictions.
Inclusion criteria and exclusion criteria
Studies must meet the following inclusion criteria: 1) clinical trials in any phase of Duvelisib therapy for patients with relapsed/refractory CLL/SLL or relapsed/refractory NHL; 2) analyzable data on safety or efficacy available in the study; 3) drugs used in humans; 4) the patients are over 18 years old. Exclusion criteria are: (1) Studies not related to our topic; (2) studies without usable results; and (3) reviews, letters, editorials, patents, news, case reports, and retrospective or observed studies. Two authors independently searched, screened, and determined study eligibility, and any disagreements were resolved by discussion.
Data extraction and quality control
Eligible studies were reviewed, and data were extracted independently by two authors. We identified the first author, publication year, ClinicalTrials.gov number, phase, study design and treatment, disease type, patients numbers, age, gender, previous systemic therapy, overall response rate (ORR), complete response rate (CR), partial response rate (PR), rate of stable disease (SDR), rate of progressive disease (PDR), median progression-free survival (mPFS), PFS, overall survival (OS), any grade AEs, grade ≥3 AEs, serious AEs, treatment-related discontinuation and death.
Statistical analysis
The package meta version 5.2-0 Index in the R-4.1.1 was used to evaluate the results of efficacy and safety. To analyze heterogeneity between studies we included, the I-squared test (I2 test) was used. A random effects model was used when I2 >50%, and a fixed effect model was conducted when I2 ≤50%. All analyses were based on the intention-to-treat population of the studies included. The subgroup analysis by disease type or treatment was applied. Sensitivity analysis was carried out by using different effect models. No dose effect was considered. P < 0.05 suggested statistical significance.
Study qualitative assessment
Methodological Indicators for Nonrandomized Studies (MINORS) were used to assess the methodological quality of non-randomized surgical studies included. MINORS contain 12 items; The first eight items are dedicated to non-comparative research. Items include the stated purpose of the study, The inclusion of consecutive patients, prospective collection of data, endpoints suitable for study purpose, unbiased endpoints evaluation, adequate follow-up time enough for the endpoint, loss to follow-up of patients not exceeding 5%, and sample size calculated prospectively. Each item is scored from 0 to 2; 0 is not reported, 1 is insufficiently reported, and 2 is adequately reported. The Cochrane Collaboration Risk of Bias Tool (Review Manager 5.4) is used to evaluate the bias of the RCT studies involved, including six criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcomes assessment, completeness of the outcome data, and selective reporting.
Results
Study selection
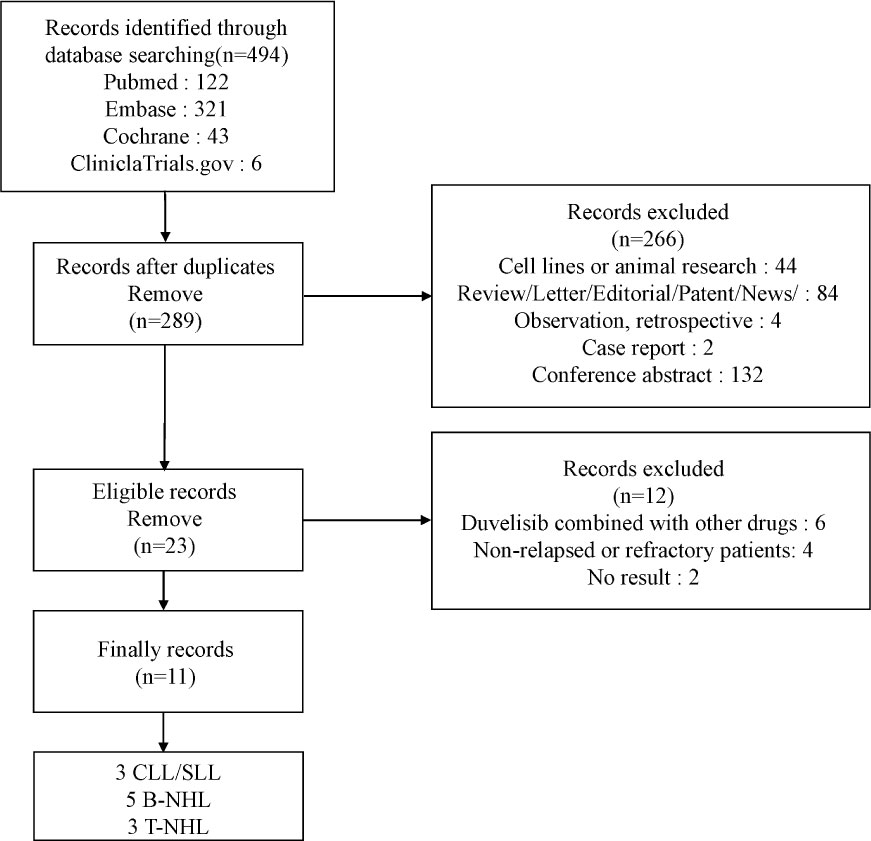
Through the above search strategy, we retrieved 494 studies. 205 were dropped after the duplication check, and 266 were excluded for the reasons we mentioned above. After Inclusion, 6 studies were removed with combined therapy, 3 studies were removed with first-line treatment, and 2 studies had no analyzable results. Figure 1 showed the details of our study selection process. Ultimately, our meta-analysis included 11 studies (23–33).
Study characteristics
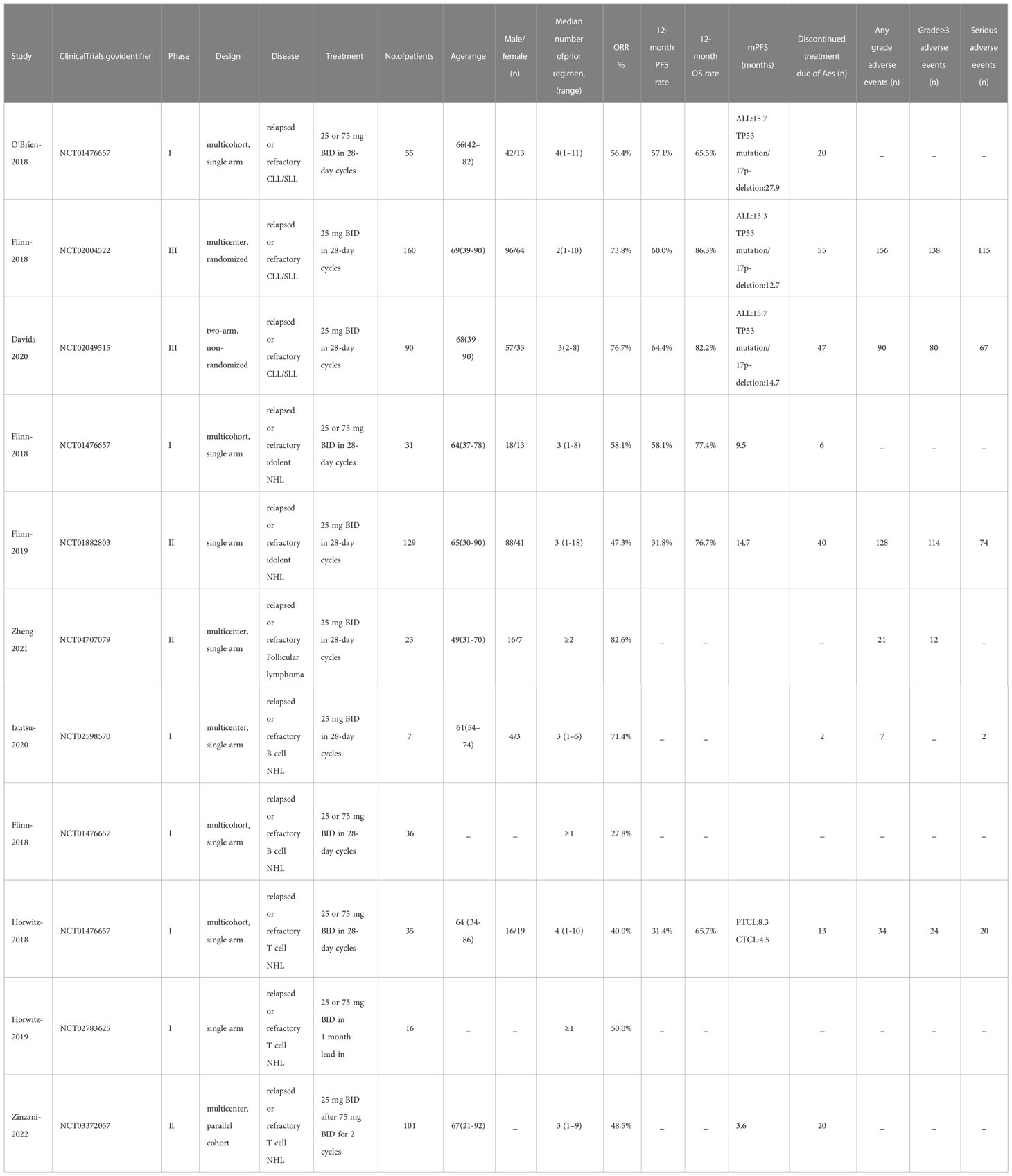
Table 1 presents the baseline characteristics of the studies included. These studies were published from 2018 to 2022. We included total of 11 studies, 683 patients, of which 305 were CLL/SLL included in 3 studies, 378 in other 8 studies were NHL [187 B-cell indolent non-Hodgkin lymphoma(iNHL), 39 B-cell aggressive non-Hodgkin lymphoma (aNHL), 152 T-cell lymphomas (T-NHL)]. There were 6 phase I studies,3 phase II studies, and 2 phase III studies. Patients in all 11 studies received duvelisib monotherapy. We assessed safety and efficacy only in patients with RR CLL/SLL or RR NHL. All studies presented information on efficacy or safety.
Assessment of study quality
The MINORS scores of 10 single arm studies ranged from 7 to 13. 3 studies without full context cannot be evaluated totally. The item of sample size calculated prospectively was not mentioned. The bias of the only 1 RCT study included was assessed by the Cochrane Collaboration Risk of Bias Tool with an acceptable quality result. Therefore, the overall quality of the 11 studies included was satisfactory. More details are shown in Supplementary Figure 1 and Supplementary Table 1.
Efficacy
All studies reported the efficacy outcomes such as overall response rate (ORR), complete response rate (CR), partial response rate (PR), stable disease rate (SDR), and progressive disease rate (PDR). The response was based on International Workshop on CLL or Revised International Working Group response criteria for CLL/SLL patients, and the International Working Group response criteria for NHL patients.
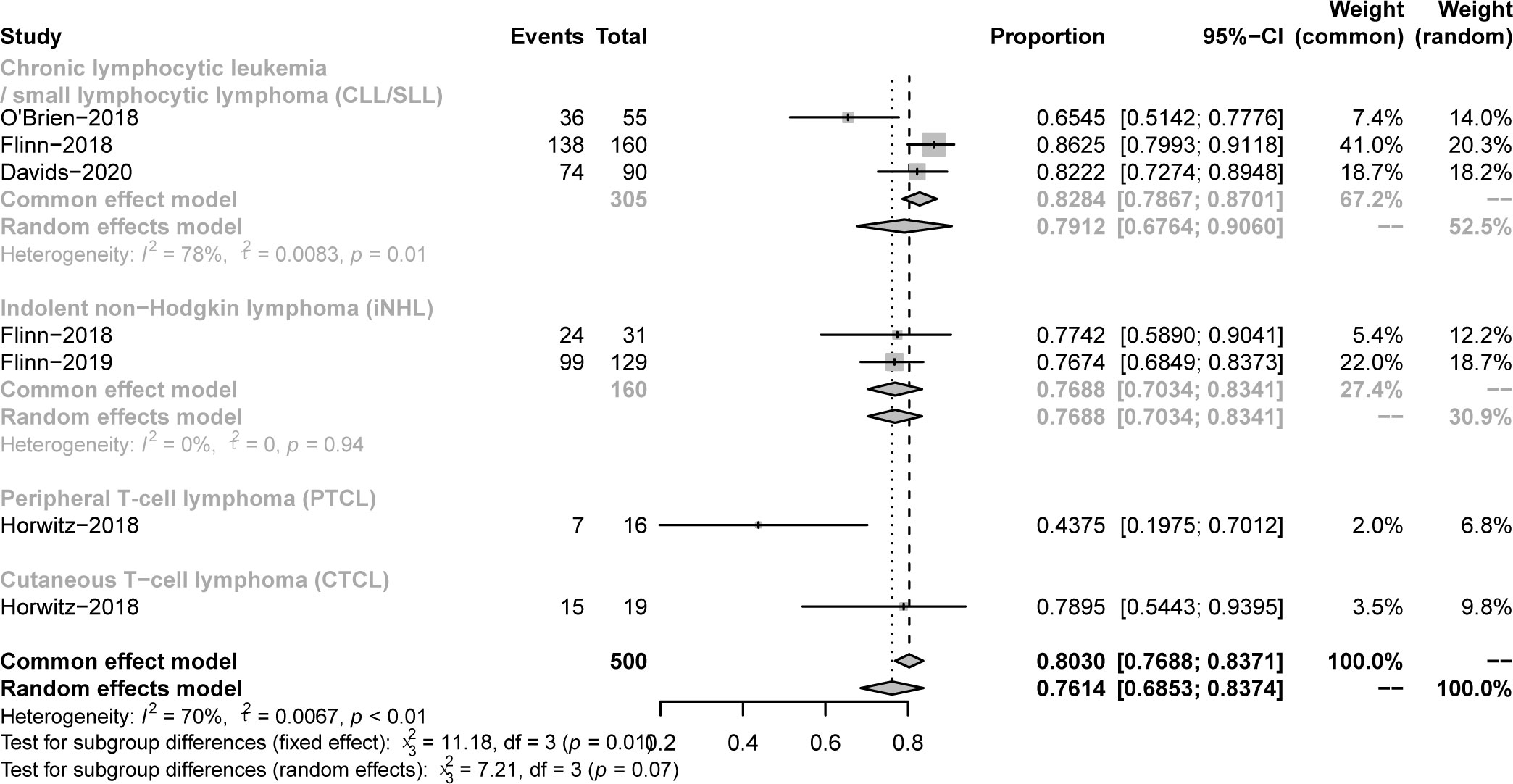
All studies focused on evaluating the ORR. The pooled ORR in CLL/SLL, iNHL, B-aNHL and T-NHL was 70% (59-81%), 70% (48-93%), 28% (14-42%) and 47% (39-55%), respectively (Figure 2). Additionally, the ORR in CLL/SLL patients with or without TP53 mutation/17p-deletion (62% vs. 74%, p=0.45) had no significant difference (Figure 3A). Follicular lymphoma (FL) had higher ORR than iNHL, yet without statistical significance (69% vs. 57%, p=0.38) (Figure 3B). Besides, Mantle cell lymphoma (MCL) patients had higher pooled ORR than other aNHL (68% vs. 17%, p=0.04) (Figure 3C). The ORR was 49% and 32% in PTCL and CTCL, respectively (Supplementary Figure 2A). In the subgroup of PTCL, Angioimmunoblastic T cell Lymphoma (AITL) patients had higher pooled ORR than other PTCL patients (67% vs. 42%, p=0.01) (Figure 3D).
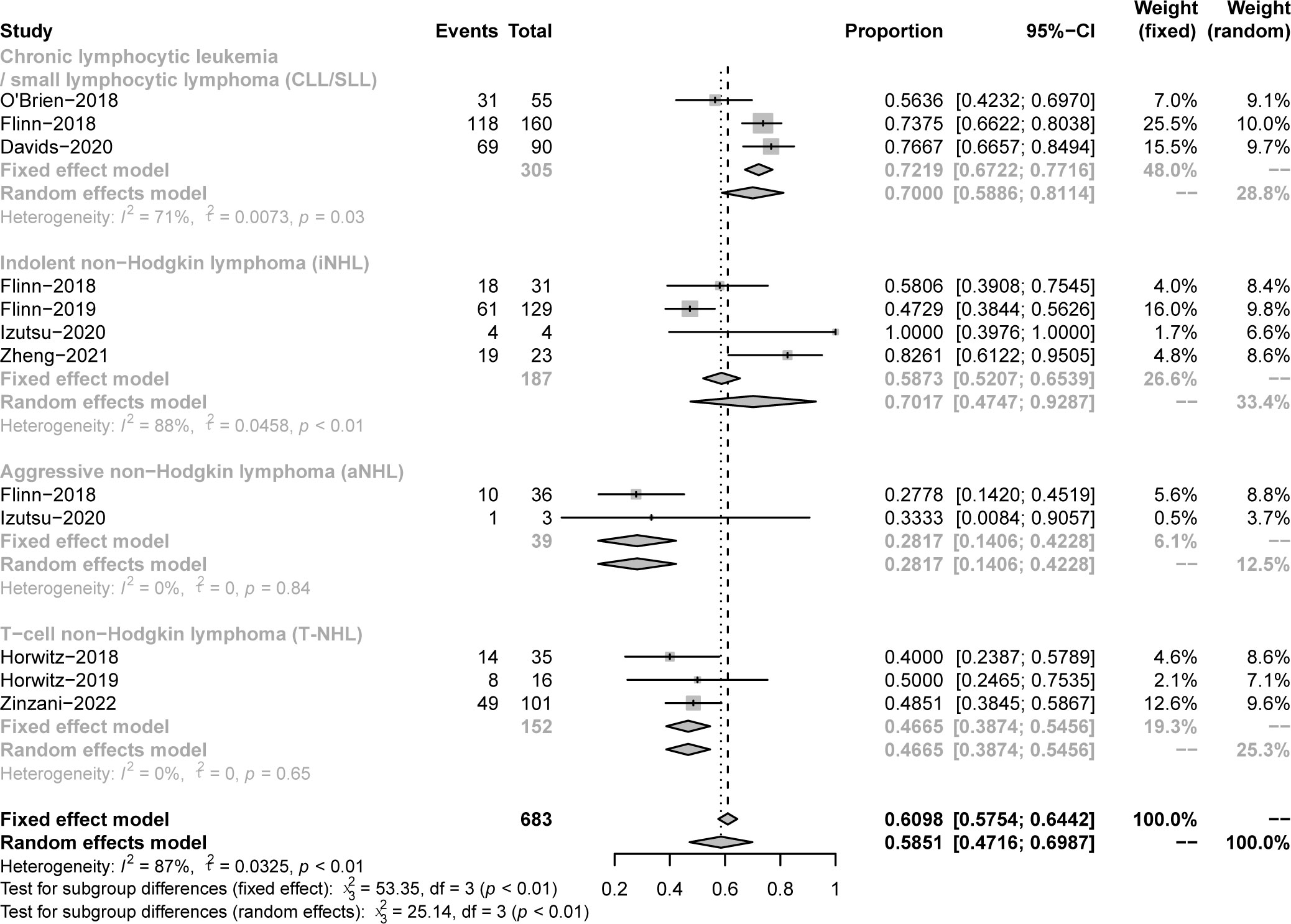
Figure 2 The forest plot of pooled ORR. Disease including chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), indolent non−Hodgkin lymphoma (iNHL), aggressive non−Hodgkin lymphoma (aNHL) and T−cell non−Hodgkin lymphoma (T-NHL).
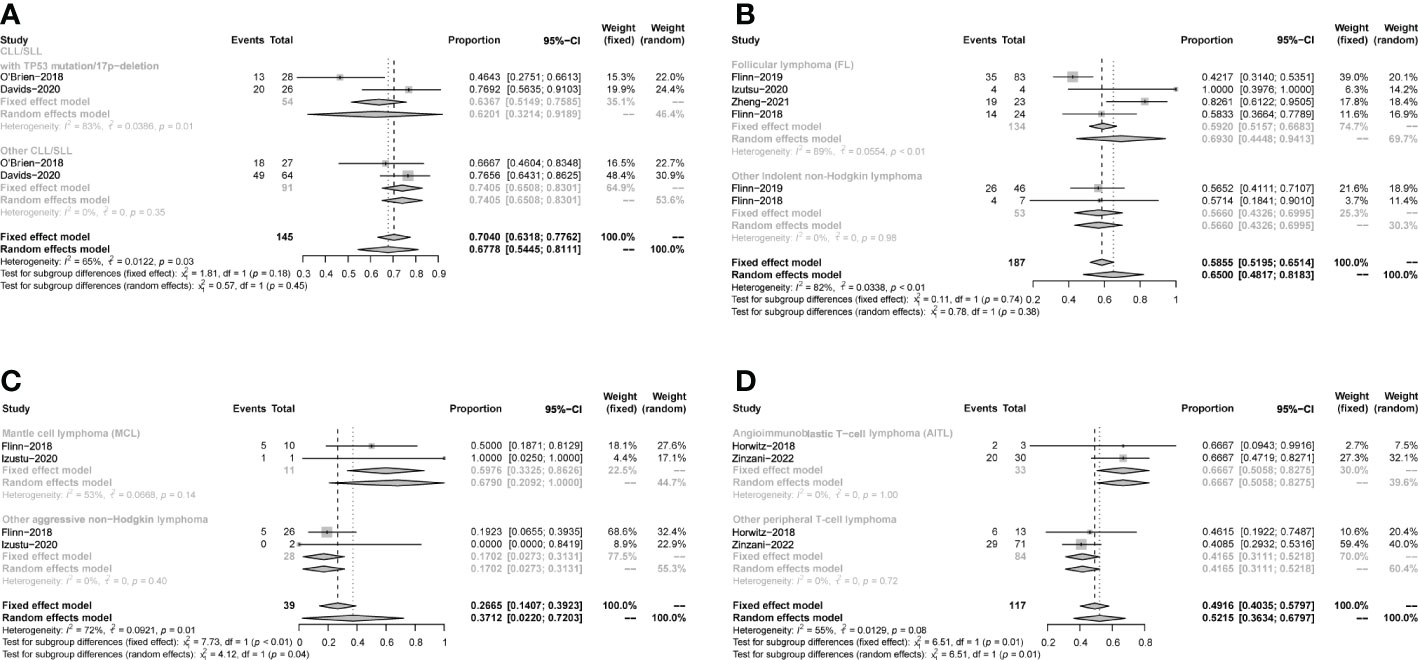
Figure 3 Subgroup analysis of the pooled ORR. (A). Subgroup of CLL/SLL, the pooled ORR of CLL/SLL with TP53 mutation/17p−deletion and others. (B). Subgroup of iNHL, the pooled ORR of Follicular lymphoma (FL) and others. (C). Subgroup of aNHL, the pooled ORR of MCL and others. (D). Subgroup of PTCL, the pooled ORR of AITL and others.
The pooled CR in CLL/SLL, iNHL, B-aNHL and T-NHL was 2% (0–1%), 16% (1–31%), 8% (0–17%) and 22% (6–38%), respectively (Figure 4). In the subgroup analysis, the CR in CLL/SLL patients with or without TP53 mutation/17p-deletion (6% vs. 1%, p=0.21) had no significant difference (Figure 5A). FL had higher CR than other iNHL, yet without statistical significance (18% vs. 2%, p=0.08) (Figure 5B). Besides, the CR in MCL patients and CR in other aNHL are similar (9% vs. 7%, p=0.87) (Figure 5C). PTCL had a higher CR than CTCL (29% vs. 0%, p<0.01) (Figure 5D).
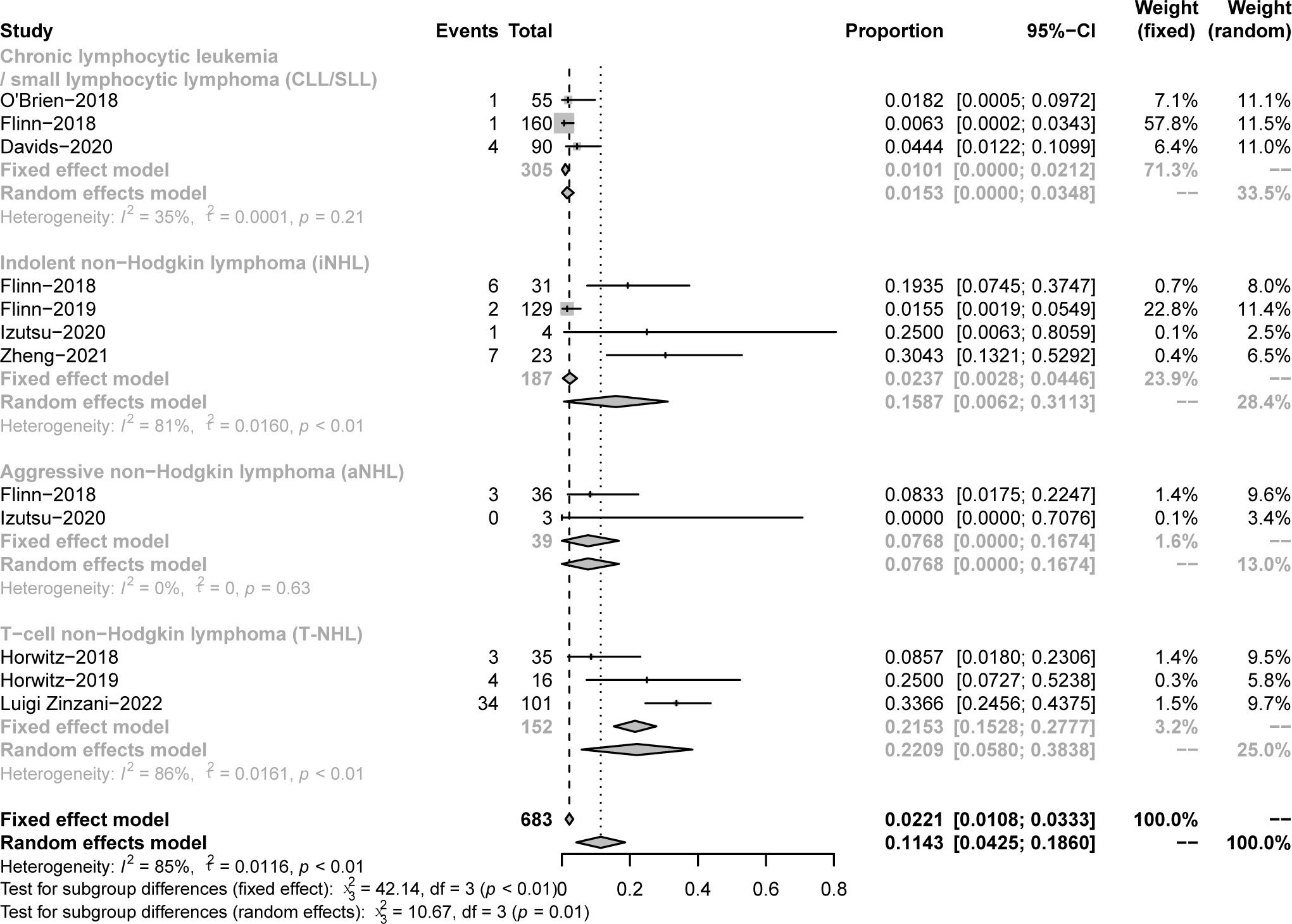
Figure 4 The forest plot of pooled CR. Disease includes chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), indolent non−Hodgkin lymphoma (iNHL), aggressive non−Hodgkin lymphoma (aNHL) and T−cell non−Hodgkin lymphoma (T-NHL).
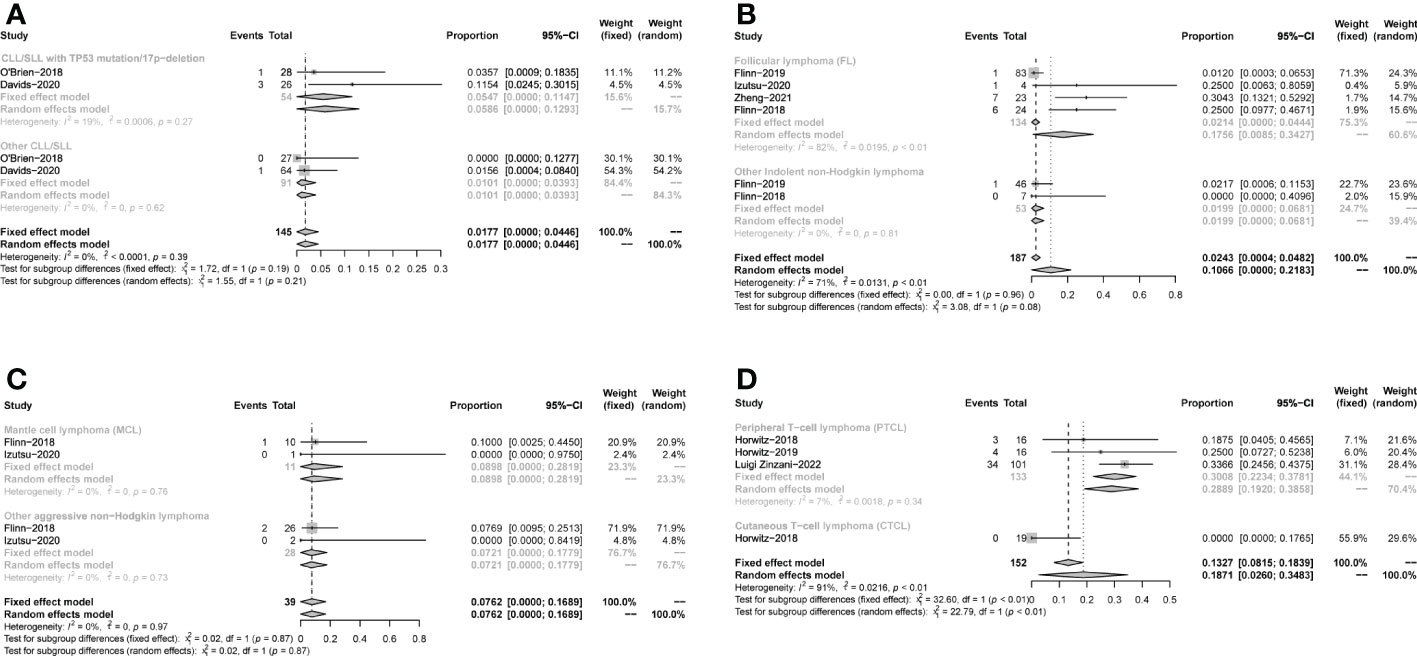
Figure 5 Subgroup analysis of the pooled CR. (A). Subgroup of CLL/SLL, the pooled CR of CLL/SLL with TP53 mutation/17p−deletion and others. (B). Subgroup of iNHL, the pooled CR of Follicular lymphoma (FL) and others. (C). Subgroup of aNHL, the pooled CR of MCL and others. (D). Subgroup of T-NHL, the pooled CR of PTCL and CTCL.
The pooled PR in CLL/SLL, iNHL, B-aNHL and T-NHL was 64% (53–74%), 46% (39–53%), 20% (8–33%) and 22% (10–33%), respectively (Figure 6). In the subgroup analysis, the PR in CLL/SLL patients with or without TP53 mutation/17p-deletion (54% vs. 73%, p=0.13) had no significant difference (Figure 7A). the PR in FL and that in other iNHL had no significant difference (43% vs. 53%, p=0.20) (Figure 7B). Besides, the PR in MCL had higher PR than other aNHL, yet without statistical significance (64% vs. 11%, p=0.08) (Figure 7C). There is no significant difference between the PR of PTCL and CTCL (19% vs. 32%, p=0.30) (Figure 7D).
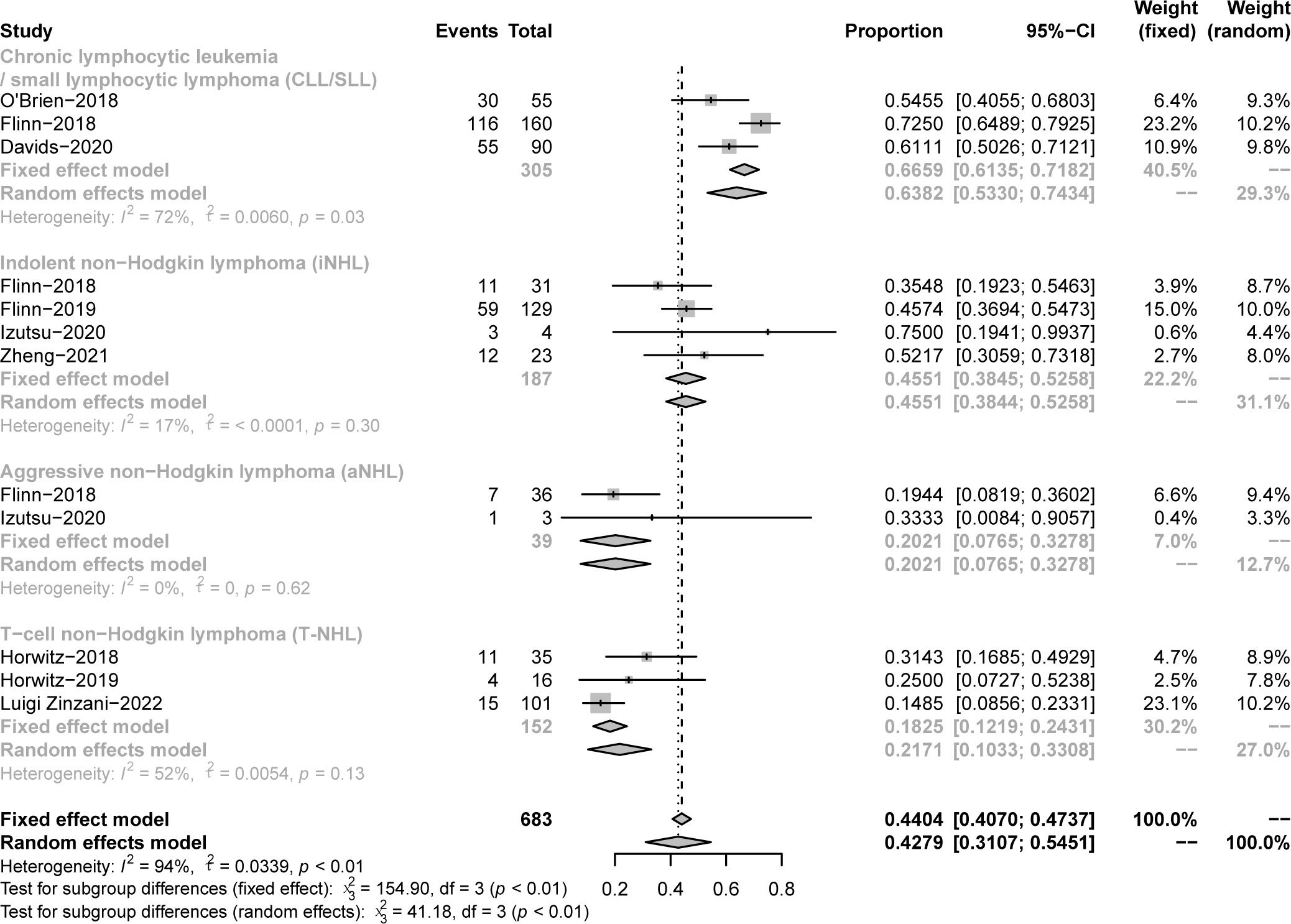
Figure 6 The forest plot of pooled PR, disease includes Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), Indolent non−Hodgkin lymphoma (iNHL), Aggressive non−Hodgkin lymphoma (aNHL) and T−cell non−Hodgkin lymphoma (T-NHL).
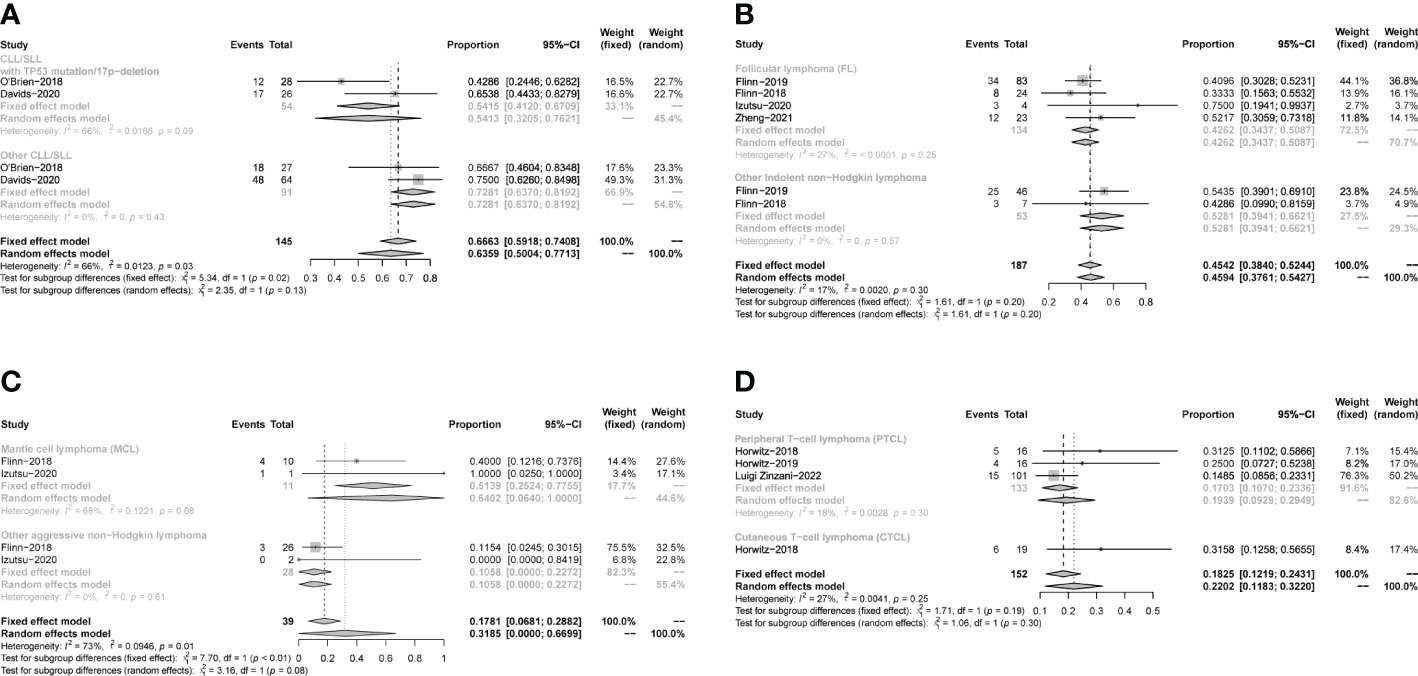
Figure 7 The forest plot of pooled PR of disease subgroup, (A). Subgroup of CLL/SLL, the pooled PR of CLL/SLL with TP53 mutation/17p−deletion and others. (B). Subgroup of iNHL, the pooled PR of Follicular lymphoma (FL) and others. (C). Subgroup of aNHL, the pooled PR of MCL and others. (D). Subgroup of T-NHL, the pooled PR of PTCL and CTCL.
The pooled SDR in CLL/SLL, iNHL, and T-NHL was 22% (12–33%), 25% (8–42%), and 10% (0–27%), respectively (Supplementary Figure 3A). The pooled PDR in CLL/SLL, iNHL, and T-NHL was 1% (0–3%), 11% (6–16%), 43% (29–57%), respectively (Supplementary Figure 3B). In the subgroup of T-NHL, the SDR was 2% (0–5%) and 32% (13–57%) in PTCL and CTCL, respectively (Supplementary Figure 2B). The PDR was 47% (38–56%) and 32% (13–57%) in PTCL and CTCL, respectively (Supplementary Figure 2C). No enough SDR or PDR data is available to analyze other subgroups.
Seven studies presented mPFS data. For all CLL/SLL patients, the mPFS reported in the three studies was 15.7, 13.3, and 15.7 months, respectively. For CLL/SLL patients with TP53 mutation/17p−deletion, the mPFS was 27.9, 12.7, and 14.7, respectively. For patients with iNHL, the mPFS reported in the two studies was 9.5 and 14.7 months, respectively. The mPFS for patients with PTCL in the reported 2 studies was 8.3, and 3.6 months, and CTCL in 1 study was 4.5 months. The mPFS data reported in studies were shown in Table1. The rate of PFS and OS was performed to assess the efficacy of duvelisib treatment in CLL/SLL and NHL. For all patients, the pooled 12-month PFS rate was 49% (37%−60%). Besides, in CLL/SLL, iNHL, and CLL/SLL with TP53 mutation/17p−deletion, the pooled 12-month PFS rate was 61% (55–66%),44% (18–69%), and 62% (52–72%), respectively (Figure 8). Only 1 T-NHL study reported the 12-month PFS of PTCL and CTCL was 38% (15–65%), 26% (9–51%), respectively. Moreover, the 24-month PFS rate was also similar to the rate between all CLL/SLL patients (27%, 22–32%) and those with TP53 mutation/17p−deletion (27%, 12–42%) (Supplementary Figure 4). For all patients, the pooled 12-month OS rate was 76% (69−84%). Besides, in CLL/SLL and iNHL, the pooled 12-month OS rate was 79% (68–66%) and 77% (70–83%), respectively (Figure 9). Only 1 T-NHL study reported the 12-month OS of PTCL and CTCL was 44% (20–70%) and 79% (54–94%), respectively.
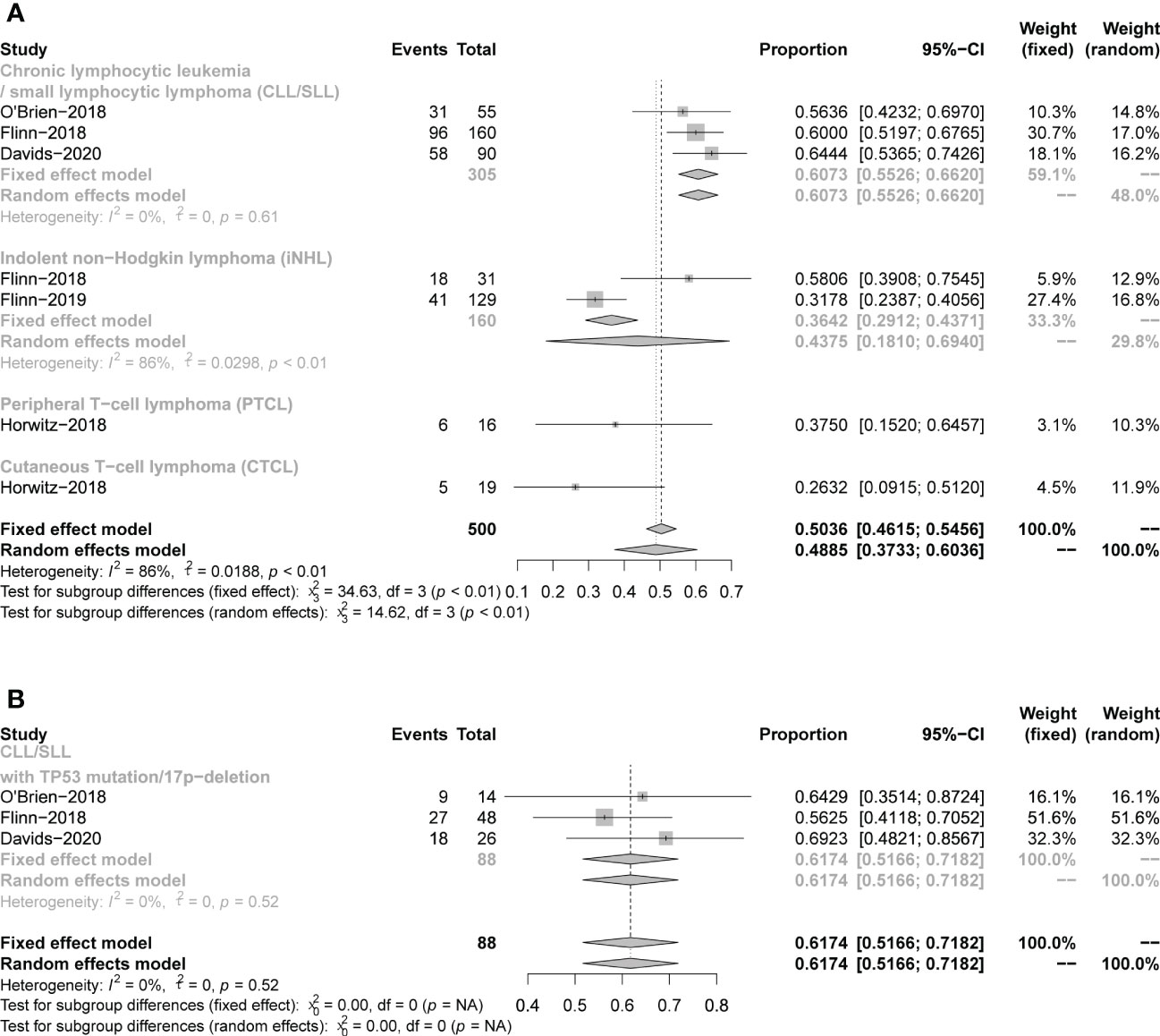
Figure 8 The forest plot of pooled 12-month PFS rate, (A). pooled 12-month PFS rate of CLL/SLL, iNHL, PTCL and CTCL. (B). Pooled 12-month PFS rate of CLL/SLL with TP53 mutation/17p-deletion.
Safety
The safety profiles in individual disease subgroups were generally consistent with those of the entire subjects. The safety assessments recorded in studies on patients were listed in Table 1. Six studies were included in the analysis of the pooled incidence of any grade AEs (99%, 98−100%), and five studies were included to evaluate the pooled incidence of grade ≥3 AEs (79%,67−91%). 5 studies were included in the analysis of pooled serious AEs (63%,53–74%). The pooled rate of treatment discontinuation due to AEs was 33% (25–41%) in 8 included studies (Figure 10). And the most frequent AEs leading to treatment discontinuation were elevated transaminases (6%, 0-11%), colitis (5%, 2-7%), diarrhea (4%, 2-6%) (Supplementary Figure 5).
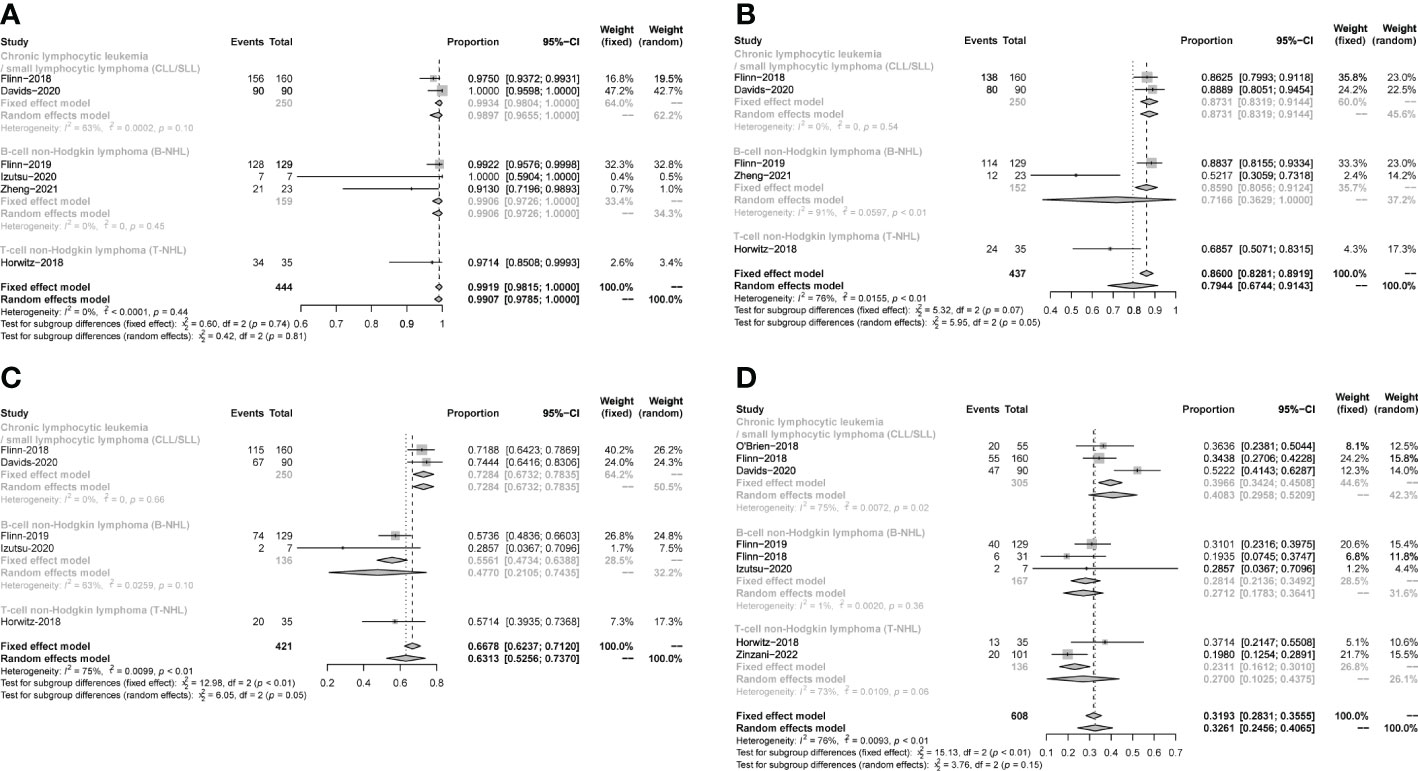
Figure 10 The forest plot of incidences of AEs. (A). AEs in any grade. (B). AEs in grade ≥3. (C). Serious AEs. (D). Treatment discontinuation due to AEs.
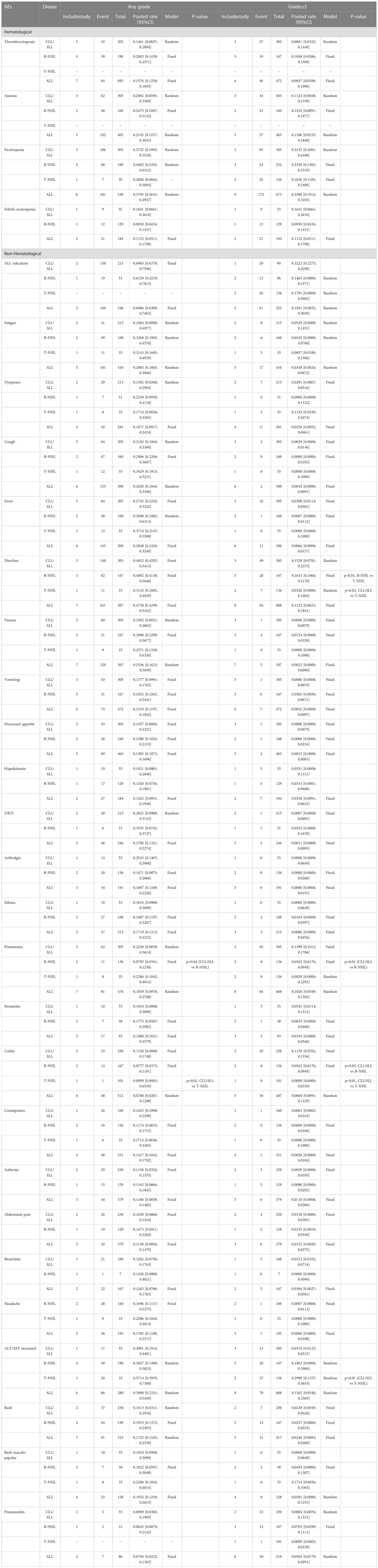
The most frequent any-grade AEs were Diarrhea (47%, 43-52%), ALT/AST increased (39%, 23-55%), neutropenia (38%, 26-50%), fatigue (29%, 18-40%), fever (29%, 25-32%) and cough (26%, 18-34%). The most frequent grade 3 or greater AEs were neutropenia (25%, 19-31%), ALT/AST increased (16%, 5-26%), diarrhea (12%, 6-18%) and anemia (12%, 5 -18%) (Table 2).
The subgroup analysis of CLL/SLL, B-NHL, and T-NHL were shown in the Table 2. The incidence of grade≥3 transaminase elevations in CLL/SLL (3%) is much lower than in B-NHL (19%, p=0.05) or T-NHL (30%, p < 0.01). And the incidence of grade≥3 diarrhea in T-NHL (3%) is much lower than in CLL/SLL (15%, p=0.02) and B-NHL (16%, p<0.01). Furthermore, the incidence of grade≥3 colitis in CLL/SLL(12%) is higher than in T-NHL(0%,p < 0.01) and B-NHL (6%, p=0.03) (Table 2). Consistent with this difference, elevated transaminases (CLL/SLL:1%, B-NHL:8%, T-NHL:15%) was a major factor for treatment discontinuation in NHL, while diarrhea (CLL/SLL:5%, B-NHL:2%) or colitis (CLL/SLL:6%, B-NHL:2%, T-NHL: 6%) were major factors for treatment discontinuation in CLL/SLL (Supplementary Figure 5). Furthermore, the CLL/SLL patients have shown higher incidence of any grade (22% vs. 8%, p=0.04) and grade≥3 pneumonia (14% vs. 6%, p<0.01) compared to B-NHL patients (Table 2).
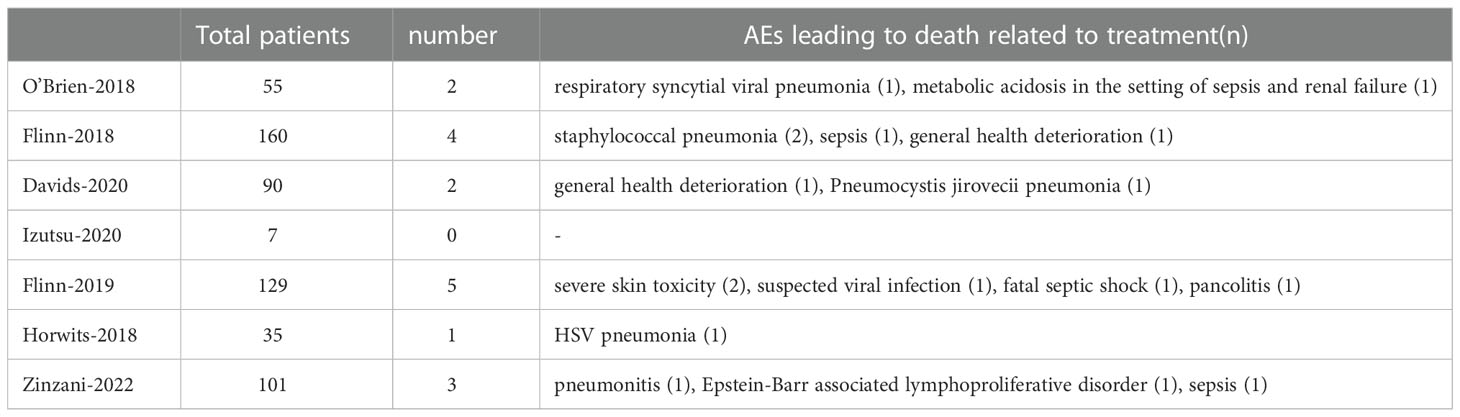
A total of 17 fatal AEs related to duvelisib application by 577 patients were reported in the 7 studies we included (pooled rate of 3%). Infectious complications are the leading causes of duvelisib-related mortality (n=12, pooled rate of 2%). Details were shown in Table 3 and Figure 11.
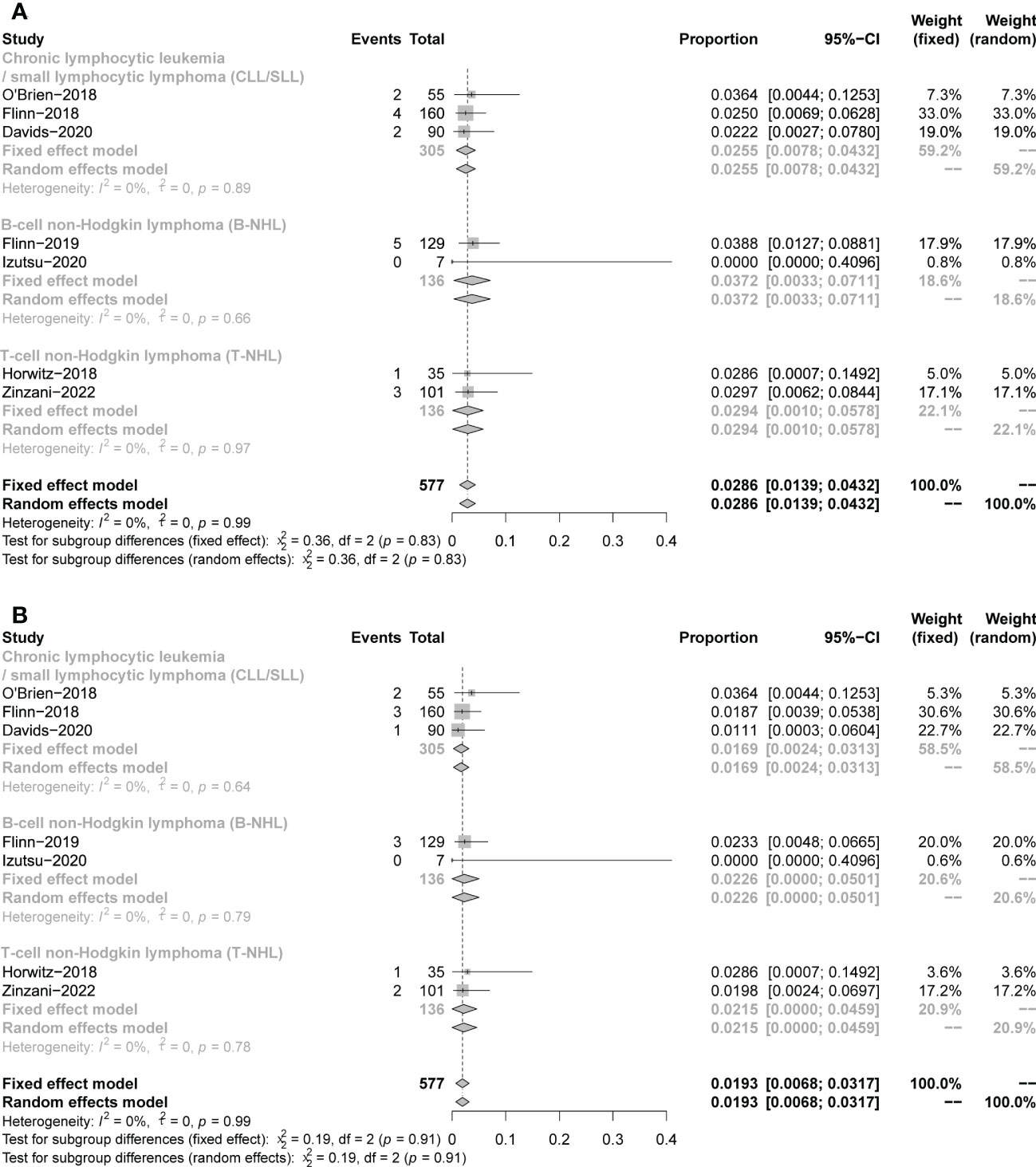
Figure 11 The forest plot of treatment-related mortality. (A). The incidence of fatal AEs related to duvelisib. (B). The incidence of fatal infectious AEs related to duvelisib.
Discussion
Duvelisib, the world’s first approved oral dual inhibitor of PI3K-δ and PI3K-γ, has been approved for treating patients with R/R CLL/SLL after at least two other treatments in the US and Europe. In Europe and China, duvelisib is indicated for treating adult patients with R/R FL after at least two prior systemic therapies. Recent clinical data on duvelisib suggest a satisfactory efficiency and acceptable safety profile in patients with advanced lymphocyte neoplasms, including R/R CLL/SLL and R/R NHL (23–29). However, the toxicity problems of PI3K inhibitor application cannot be ignored, especially immune-related and infection-related adverse events (47, 54). Unfortunately, at this point the assessments recently reaffirmed by the Panel of experts of the Oncologic Drugs Advisory Committee (ODAC) cannot be omitted, which analyzing the follow-up data of the DUO trial, among others, believes that ‘the risks associated with taking duvelisib by patients with certain blood cancers appear to outweigh the benefits “. So, it is essential to accurately identify the patients benefitting from PI3K inhibitor therapy, and to properly recognize and manage adverse events during treatment. We conducted this meta-analysis and literature review to evaluate the efficacy and safety of duvelisib in different lymphocyte neoplasms, and possible solutions to treatment-relevant toxicity were reviewed. We summarized the results of 11 prospective studies, including 683 patients, 305 patients with CLL/SLL and 378 patients with NHL [187 B-cell iNHL, 39 B-cell aNHL, 152 T-NHL], to assess the safety and efficacy.
This meta-analysis showed that duvelisib could provide satisfactory efficacy in patients with mature B-cell/T-cell Neoplasms. It is worth noting that response rates were particularly noteworthy in patients with RR CLL/SLL and RR iNHL, in whom ORR both were 70%,12-month PFS was 61% and 44%, and 12-month OS was 79% and 77%, respectively. ORR was 49% in RR PTCL patients and 67% in AITL, which are also compelling compared to the poor response rates of other approved single agents. But among the aNHL patients we included, the pooled ORR was only 17%. It’s clear that with the heterogeneity of lymphoid neoplasms, different therapeutic impacts of PI3K-δ and PI3K-γ inhibition could be derived from following aspects.
The first is tumor intracellular impact itself. A key factor is that some lymphocyte neoplasms are highly dependent on maintenance and survival signals from BCR/TCR, chemokine receptors, or co-stimulatory molecules, in which the PI3K-mTOR signaling plays an important role. It’s well known that many mature B-cell malignancies show constitutive PI3K pathway activation because of chronic BCR activation, and are susceptible to kinase inhibitors that disrupt BCR signaling (55–57). In addition, deregulation of the PI3K-AKT pathway has been shown to have a role in the pathogenesis of TCL (46, 58–61). Meanwhile, inhibitors of PI3K-mTOR signaling can effectively treat AITL in preclinical models (46, 58, 62). The second aspect of PI3Kδ inhibition is direct negative impact of the tumor microenvironment on the mitogenic and survival signaling of cells. Inhibition of PI3K-δ induces multiple effects on malignant B-cells, including the arrest of malignant B-cell proliferation and migration mediated by the tumor microenvironment, and inhibition of chemokine secretion derived from tumor cell (44, 56, 63, 64). In some PTCL subtypes, particularly AITL, molecular profiling has elucidated specific microenvironmental signatures associated with poor outcome (65). Of note, the study also shows that AITL presents significant enrichment of B-cell in the microenvironment. These mechanisms can explain why PI3Kδ inhibitors can be used to treat AITL. The third aspect focuses on the fact that inhibition of PI3K-δ, PI3K-γ, or both could activate antitumor immune responses. Indeed, PI3K-δ inhibition enhances antitumor immune response primarily due to a preferential inhibition of immunosuppressive Treg cells in the preclinical model (66–72). while PI3K-γ inhibition reduces the differentiation and migration of key Immunosuppressive cells in the tumor microenvironment, such as M2 tumor-associated macrophages, negatively regulating effector T and natural killer (NK) cells (33, 41, 42).
Despite therapy advances targeting CLL patients (73), CLL/SLL remains incurable (74). Thus, novel and effective agents for R/R CLL/SLL patients are needed. Duvelisib is currently the only FDA-approved PI3K inhibitor for the monotherapy of CLL/SLL. Our meta-analysis revealed that duvelisib could offer reasonable efficacy in patients with RR CLL/SLL without being negatively affected by del17P/TP53 mutation. The pooled ORR of duvelisib was 70%, 12- month PFS and OS were 61% and 79%, respectively. Another approved PI3K-δ inhibitor idelalisib demonstrated only 48% of ORR and 6.9-month median PFS (75). Our data show duvelisib provides improved efficacy over idelalisib. This conclusion is also consistent with previous preclinical CLL models. Dual PI3K-δ, g inhibition has shown stronger activity than blocking either isoform alone (44, 56, 76). Moreover, in a phase 3 randomized study in CLL/SLL, duvelisib demonstrated significantly improved ORR, PFS, and OS compared with ofatumumab (CD20 inhibitor) (24). In addition, duvelisib has also shown high response rates in patients with R/R CLL/SLL who progressed on ofatumumab (77). Many preclinical studies indicate that p53 could inhibit PI3K/AKT/mTOR pathway through multiple targets, mutation or deletion of p53 leading to abnormal activation of these pathways (78–83). Studies show that patients with del(17p) and/or TP53 mutations are more likely to relapse, even when treated with ibrutinib (BTK inhibitor) or venetoclax (BCL2 inhibitor) (84–86). However, duvelisib can prevent CLL/SLL tumor cell proliferation and metabolism by inhibiting abnormally activated PI3K/AKT/mTOR signaling in the context of del(17p) and/or TP53 mutations. Similarly, in our study, ORR,12-month PFS and 24-month PFS, in the subgroup of patients with del(17p) and/or TP53 mutations were similar to those of the whole population, which meant duvelisib could decrease the recurrence due to del(17p) and/or TP53 mutations. Considering the increasing use of ibrutinib and venetoclax in first-line therapy (87–90), duvelisib has been shown to effectively increase the sensitivity of CLL cells to BCL2i and BTKi (91, 92). For patients who are refractory to or intolerable to BTKi or BCL2i, duvelisib is an effective option for RR CLL/SLL with or without del(17p) and/or TP53 mutations.
Although most iNHL patients initially respond to standard chemoimmunotherapy with prolonged remission, eventually, all patients experience disease progression or relapse (93). There are currently several approved options for relapsed or refractory iNHL, but the multiple toxicities of therapies and resistance or transformation to advanced or aggressive lymphomas remain challenges. In our meta-analysis, Response rates of duvelisib were clinically meaningful in patients with RR iNHL across subtypes. In all patients, the pooled ORR was 70%, the 12-month PFS was 44%, and the 12-month OS was 77%. In FL patients, the pooled ORR was 69%. There are already several PI3K inhibitors in clinical trials for RR iNHL patients: copanlisib (intravenous inhibitor of PI3K-α, -β), idelalisib (oral inhibitor of PI3K-δ), umbralisib (oral inhibitor of PI3K-δ, CK-e). ORR in these trials ranged from 47% to 59% in all patients and 45% to 59% in FL patients (94–96). Despite heterogeneity in cross-trial patient selection and prior treatments, our data reveal that duvelisib had higher efficacy than other PI3Ki treatments. Repeating chemotherapy, even combined with different CD20 antibodies like Obinutuzumab, caused cumulative toxicities and decreased efficacy. In our study, almost all RR iNHL patients had been previously treated with rituximab (100%, 94%, 100%, and 100%, reported in 4 studies, respectively) or alkylating agent (98%, 81% reported in 2 studies, respectively). Given the increasing use of rituximab and alkylating agents for the untreated or RR iNHL, duvelisib monotherapy may provide an option for R/R patients. Additionally, PI3Kδ inhibition restores the sensitiveness of FL cells on the anti-apoptotic protein BCL-2 (63), showing a rationale potent for combined PI3Kδ and BCL-2 inhibition.
In the aggressive B-NHL, the pooled ORR was 68% in MCL and 17% in other aNHL (Mainly DLBCL), respectively, while in copanlisib, the ORR of the aggressive cohort ranged from 7% in DLBCL patients to 64% in MCL patients (97). The responses to ibrutinib have been reported at 37% in ABC DLBCL patients and 5% in GCB DLBCL patients. Although no data on DLBCL subtypes is available here, considering that ABC DLBCL often selectively acquires mutations targeting B-cell receptors (BCRs) that promote chronically active BCR signaling (98), duvelisib may be a rationale candidate for ABC DLBCL. Data from a phase 1 study have demonstrated that the combination of duvelisib with standard therapies, bendamustine, and rituximab, is well tolerated and presents a novel therapeutic option in B-NHL, including DLBCL and MCL (99).
Peripheral T cell lymphomas (PTCLs) are highly heterogenous diseases with a poor prognosis. Immunotherapy and novel chemotherapy protocols are active in B cell lymphomas, unluckily with a high failure rate and frequent relapses in T-NHL. Indeed, new treatments for peripheral T cell lymphomas (PTCLs) are developing, but patients with PTCLs still have poor survival. In our analysis, the pooled ORR was 49% in patients with RR PTCL, which is also satisfactory, for the response rates of other approved single agents for RR PTCL, like romidepsin (HDACi), belinostat (HDACi), and pralatrexate (antifolate), range from 25% to 29% (20, 100, 101). And, for another PI3K inhibitor copanlisib, the ORR of PTCL was only 21% (97). Additionally, a phase I study of duvelisib combined with romidepsin, has shown a better ORR(58%) than previous therapy using romidepsin alone (102). In the subgroup analysis of the PTCL subtype, we found that AITL patients appeared to have higher pooled ORR than other PTCL patients (67% vs. 42%, p=0.01). The similar results could be observed in phase I study of duvelisib combined with romidepsin, AITL has also shown a better ORR than other PTCL (68% vs. 53%). Meanwhile, we also noticed a high pooled PDR of 47%, also observed in copanlisib (36%) for all PTCL. However, in one of our studies included patients with AITL had a lower PDR (0 of 3) compared to other PTCLs (6 of 13) (33). These results suggested the existence of heterogeneity between diseases. Long-term outcomes of retrospective series, such as the International T-cell lymphoma project (ITCP), displayed that the 5-year failure-free survival (FFS) for the AITL patients receiving CHOP was only 18%. Therefore, more novel therapies should be explored for T-NHL. Duvelisib would be a good option for patients with RR PTCL, especially AITL. Given the phenomenon observed in our study, clinical trials with larger sample size were needed to characterize more details of differential efficacy of duvelisib in PTCL subtypes.
PI3K-δ, g dual inhibition significantly changes the cellular composition of the microenvironment by reducing Treg cell numbers and activating CD4+ and CD8+ cells, which clonally expand and display enhanced cytotoxic and cytolytic properties. Despite enhancing anti-tumor immunity, activated T cells invariably cause immune dysregulation in normal tissues. The safety profile in the individual disease subgroup was found to be generally consistent with the subjects. Nearly all patients in both subgroups experienced an AE. AEs were generally low grade and manageable, probably leading to dose reductions/interruptions, among which 33% of patients discontinued treatment, similar to observations with other PI3K-δ inhibitors (95, 103). Immune-related toxicities are common, leading cause of treatment discontinuation, including transaminase elevations, diarrhea or colitis, pneumonitis, and rash. In our meta-analysis, diarrhea and transaminase elevations were the most frequent non-hematologic AEs (47% and 39%, respectively) and the most common grade≥3 non-hematologic events (12% and 16%, respectively) in all diseases, which could be managed by adjusting dose, discontinuing and recovering treatment when monitoring hepatic function. Only 1 patient was reported to die of treatment-induced pancolitis. Similar results have been observed in other PI3K inhibitors. The incidence of diarrhea in any grade and grade ≥3 was 43% and 13% in idelalisib, and 34% and 5% in copanlisib, respectively. For transaminase elevations, the incidence in any grades and grade ≥3 were 47% and 13% in idelalisib, 28% and 2% in copanlisib, respectively (94–96). The subanalysis showed the incidence of grade≥3 spoke of potential differences among subgroup patients. This difference may be due to toxic modulation by disease-specific factors, or different prior treatments, or differences in immune cell populations. Consistent with this difference, elevated transaminases are a major factor for treatment discontinuation compared to diarrhea in NHL, whereas in CLL/SLL, the opposite could be witnessed.
Pneumonitis and rash, which are also common in the application of idelalisib and copanlisib, are the other leading cause of treatment discontinuation. Grade≥3 pneumonitis occurred in 6% of patients, including 1 fatal thought to be duvelisib relevant. Some patients experienced skin toxicity at any grade (rash 18%, rash maculo-papular 19%), and greater than grade 3 are uncommon (rash 2%, rash maculo-papular 5%) but still cause 2 casualties, which are duvelisib relevant. Duvelisib has a satisfactory hematological safety profile. The most frequent hematologic AEs were neutropenia (38%) and anemia (21%). Notably, neutropenia was the most frequent severe AEs (25%). The incidences of neutropenia and anemia in other PI3K inhibitors were similar to our results (94, 95). These events seldom require treatment modifications due to their reversibility, and rarely result in treatment discontinuations (neutropenia in 1 patient).
Grade≥3 serious infections occurred in 19% of patients treated with duvelisib. Infectious complications are major causes of mortality in duvelisib treatment, as a result of the humoral immunodepression inherent to the disease and therapy-induced immunosuppression (104, 105). A total of 17 treatment-related deaths in 577 patients (pooled rate of 3%) were reported in our included studies, 12 (pooled rate of 2%) were infection-related. These were similar in copanlisib (3/142,1 infection-related) and idelalisib (8/125, 4 infection-related). Severe pneumonia occur in 10% of patients, and few are fatal, 5 of which were assessed as related to duvelisib: staphylococcal pneumonia (n=2), pneumocystis jirovecii pneumonia (PJP), respiratory syncytial viral pneumonia, and HSV pneumonia (n=1 each). If pneumonias are suspected, appropriate and extensive evaluations should be performed for infectious etiologies of pneumonias. In clinical trials, many patients have been treated with antibiotics and corticosteroids and most recovered. Prophylaxis for PJP infections is required to mitigate the risk of these opportunistic infections, which have been reported in other B-cell receptor inhibitors (106–109). And antiviral prophylaxis also should be implemented at the consideration of the clinicians.
Generally, duvelisib is effective in treating mature lymphocyte neoplasms. Still there are significant patients requiring dose adjustments, and up to 33% of patients discontinue the treatment because they could not tolerate the AE (41% in CLL/SLL, 27% in B-NHL and T-NHL). A reasonably designed intermittent dosing regimen may help reduce the incidence of AEs without compromising efficacy. The TEMPO study (NCT04038359) evaluates the effects of duvelisib prespecified 2-week dose holidays on responses and safety/tolerability in patients with iNHL (110). Similarly, in mouse models, a modified treatment with intermittent dosing of PI3Kδi led to a decrease in tumor growth without inducing pathogenic T cells in colonic tissue, indicating that alternative dosing regimens might limit the toxicity of colits (111). What’s more, extended survival is observed in patients who had treatment interruptions with the PI3Kδ inhibitor idelalisib in FL and CLL, indicating that discontinuous PI3Kδ therapy may also achieve clinical benefit (112).
Due to the differences in study design and patient enrollment, it is difficult to compare the risk of immunotoxicity directly. between different PI3K-δ inhibitors. However, compared with idelalisib, which only inhibits PI3K-δ, duvelisib may reduce autoimmune complications through the simultaneous inhibition of PI3K-γ based on preclinical data, preventing leukocyte recruitment and reducing dextran sulfate sodium-induced colitis in mice (53). Pharmacological blockade of PI3K-γ suppresses joint inflammation in mouse models of rheumatoid arthritis and eases inflammation in a model of colitis-associated cancer (113, 114). Further studies to elucidate the effect of PI3K-γ on the immune system during duvelisib treatment are of great interest. Another intravenous PI3K-δ/α inhibitor copanlisib, has a specific AE profile, including hypertension and hyperglycemic effects mediated by PI3K-α isoform inhibition, limiting its application in elderly patients with a high prevalence of these comorbidities. And hospital visit for infusional therapies represents an essential concern for some people, who are likely to benefit significantly from oral drug treatment.
There are still some limitations in our study. Firstly, most of the studies involved were single-armed studies without double-blinded randomized controlled trials, which may lead the potential performance bias. In addition, a small number of patients received different drug doses, and some AEs may be dose-dependent. Finally, although the diseases were all relapsed or refractory, the degree of lymphoma and leukemia was dispersive, which might have caused bias in the final analysis and made comparisons with other studies difficult. No results of survival benefit were defined in the study due to the varied length of follow-up time and survival data in some studies shown incompletely.
In conclusion, our analysis shows that duvelisib is an effective monotherapy for RR mature lymphocyte neoplasms. Duvelisib could offer favorable efficacy in patients with RR CLL/SLL and is not negatively affected by del17P/TP53 mutation. Besides, duvelisib has better efficacy than other approved PI3K inhibitors in iNHL treatment, including FL. And duvelisib monotherapy shows unexpectedly good efficacy in PTCL, especially in AITL. However, the efficacy of duvelisib in aNHL was limited. Although fatal and severe toxicity occasionally exists, risk and severity in duvelisib treatment have the potential to be mitigated through identification and management properly. Based on the limits of the single-arm studies, more randomized controlled studies are needed to explore the efficacy and safety of duvelisib with or without combination with other drugs for patients with RR lymphoma and leukemia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ZW and HZ have contributed equally to this work and share first authorship. ZW and HZ participated in the study design. ZW and HZ acquired, analyzed and interpreted the data. ZW wrote the manuscript. HZ and TN revised the manuscript. JX proofread the language. JW provided methodological advice. All authors contributed to the manuscript and approved the submitted version.
Funding
This work was supported by the Incubation Program for Clinical Trials (No. 19HXFH030), Achievement Transformation Project (No. CGZH21001), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC21007), Translational Research Grant of NCRCH (No. 2021WWB03).
Acknowledgments
We thanked all of the participants enrolled in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1070660/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review. (2021). pp. 1975–2018 Bethesda, MD: National Cancer Institute. https://seer.cancer.gov/csr/1975_2018/
3. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia (2022) 36(7):1720–48. doi: 10.1038/s41375-022-01620-2
4. Schulz H, Bohlius JF, Trelle S, Skoetz N, Reiser M, Kober T, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst (2007) 99(9):706–14. doi: 10.1093/jnci/djk152
5. Bello C, Zhang L, Naghashpour M. Follicular lymphoma: current management and future directions. Cancer Control (2012) 19(3):187–95. doi: 10.1177/107327481201900303
6. Stevenson FK, Forconi F, Kipps TJ. Exploring the pathways to chronic lymphocytic leukemia. Blood (2021) 138(10):827–35. doi: 10.1182/blood.2020010029
7. Patel K, Pagel JM. Current and future treatment strategies in chronic lymphocytic leukemia. J Hematol Oncol (2021) 14(1):69. doi: 10.1186/s13045-021-01054-w
8. Lumish M, Falchi L, Imber BS, Scordo M, Keudell von G, Joffe E. How we treat mature b-cell neoplasms (indolent b-cell lymphomas). J Hematol Oncol (2021) 14(1):5. doi: 10.1186/s13045-020-01018-6
9. Carbone A, Roulland S, Gloghini A, Younes A, Keudell von G, López-Guillermo A, et al. Follicular lymphoma. Nat Rev Dis Primers (2019) 5(1):83. doi: 10.1038/s41572-019-0132-x
10. Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting b cell receptor signaling with ibrutinib in diffuse large b cell lymphoma. Nat Med (2015) 21(8):922–6. doi: 10.1038/nm.3884
11. Wiernik PH, Lossos IS, Tuscano JM, Justice G, Vose JM, Cole CE, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-hodgkin’s lymphoma. J Clin Oncol (2008) 26(30):4952–7. doi: 10.1200/JCO.2007.15.3429
12. Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, et al. Phase I first-in-Human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol (2017) 35(8):826–33. doi: 10.1200/JCO.2016.70.4320
13. Dreyling M. Mantle cell lymphoma: biology, clinical presentation, and therapeutic approaches. Am Soc Clin Oncol Educ Book (2014) p:191–8. doi: 10.14694/EdBook_AM.2014.34.191
14. Pott C, Hoster E, Delfau-Larue MH, Beldjord K, Böttcher S, Asnafi V, et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood (2010) 115(16):3215–23. doi: 10.1182/blood-2009-06-230250
15. Zinzani PL, Vose JM, Czuczman MS, Reeder CB, Haioun C, Polikoff J, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol (2013) 24(11):2892–7. doi: 10.1093/annonc/mdt366
16. Witzig TE, Geyer SM, Ghobrial I, Inwards DJ, Fonseca R, Kurtin P, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol (2005) 23(23):5347–56. doi: 10.1200/JCO.2005.13.466
17. Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood (2014) 123(17):2636–44. doi: 10.1182/blood-2013-12-516245
18. Iżykowska K, Rassek K, Korsak D, Przybylski GK. Novel targeted therapies of T cell lymphomas. J Hematol Oncol (2020) 13(1):176. doi: 10.1186/s13045-020-01006-w
19. Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol (2013) 31(16):1970–6. doi: 10.1200/JCO.2012.44.7524
20. O’Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol (2011) 29(9):1182–9. doi: 10.1200/JCO.2010.29.9024
21. O’Connor OA, Horwitz S, Masszi T, Hoof Van A, Brown P, Doorduijn J, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: Results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol (2015) 33(23):2492–9. doi: 10.1200/JCO.2014.59.2782
22. Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol (2014) 7:11. doi: 10.1186/1756-8722-7-11
23. O’Brien S, Patel M, Kahl BS, Horwitz SM, Foss FM, Porcu P, et al. Duvelisib, an oral dual PI3K-δ,γ inhibitor, shows clinical and pharmacodynamic activity in chronic lymphocytic leukemia and small lymphocytic lymphoma in a phase 1 study. Am J Hematol (2018) 93(11):1318–26. doi: 10.1002/ajh.25243
24. Flinn IW, Hillmen P, Montillo M, Nagy Z, Illés Á, Etienne G, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood (2018) 132(23):2446–55. doi: 10.1182/blood-2018-05-850461
25. Davids MS, Kuss BJ, Hillmen P, Montillo M, Moreno C, Essell J, et al. Efficacy and safety of duvelisib following disease progression on ofatumumab in patients with Relapsed/Refractory CLL or SLL in the DUO crossover extension study. Clin Cancer Res (2020) 26(9):2096–103. doi: 10.1158/1078-0432.CCR-19-3061
26. Flinn IW, Miller CB, Ardeshna KM, Tetreault S, Assouline SE, Mayer J, et al. DYNAMO: A phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol (2019) 37(11):912–22. doi: 10.1200/JCO.18.00915
27. Izutsu K, Kato K, Kiyoi H, Yamamoto G, Shimada K, Akashi K. Phase I study of duvelisib in Japanese patients with relapsed or refractory lymphoma. Int J Hematol (2020) 112(4):504–9. doi: 10.1007/s12185-020-02929-3
28. Flinn IW, Patel M, Oki Y, Horwitz S, Foss FF, Allen K, et al. Duvelisib, an oral dual PI3K-δ, γ inhibitor, shows clinical activity in indolent non-Hodgkin lymphoma in a phase 1 study. Am J Hematol (2018) 93(11):1311–7. doi: 10.1002/ajh.25228
29. Flinn IW S, Kahl B, Patel M, Oki Y, Foss FF, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood (2018) 131(8):877–87. doi: 10.1182/blood-2017-05-786566
30. Zheng Z, Gao Y, Song Y, Qian Y, Jing H, Liu T, et al. Efficacy and safety of duvelisib, a phosphoinositide 3 kinase (PI3K) δ and γ inhibitor, in Chinese patients (pts) with relapsed/refractory follicular lymphoma (R/R FL). A single-arm open-label multicenter phase Ⅱ Clin trial (2021) 39(15_suppl):e19532–2. doi: 10.1200/JCO.2021.39.15_suppl.e19532
31. Horwitz SM, Foss FM, Porcu P, Moskowitz A, Mehta-Shah N, Jacobsen E, et al. Duvelisib, an oral dual PI3K-δ,γ inhibitor, efficacy and safety in patients with relapsed or refractory (RR) peripheral T-cell lymphoma. rationale phase 2 PRIMO trial (2019) 37(S2):65–6. doi: 10.1002/hon.33_2629
32. Zinzani P, Zain J, Mead M, Casulo C, Jacobsen E, Gritti G, et al. P1172: DUVELISIB IN PATIENTS WITH RELAPSED/REFRACTORY PERIPHERAL T-CELL LYMPHOMA FROM THE PHASE 2 PRIMO TRIAL. Blood (2002) 136(Supplement 1):38–39. doi: 10.1097/01.HS9.0000847552.42271.7c
33. Horwitz SM, Koch R, Porcu P, Oki Y, Moskowitz A, Perez M, et al. Activity of the PI3K-δ,γ inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood (2018) 131(8):888–98. doi: 10.1182/blood-2017-08-802470
34. Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in b cell development and activation. J Exp Med (2002) 196(6):753–63. doi: 10.1084/jem.20020805
35. Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, et al. Impaired b and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science (2002) 297(5583):1031–4. doi: 10.1126/science.1073560
36. Perry MWD, Abdulai R, Mogemark M, Petersen J, Thomas MJ, Valastro B, et al. Evolution of PI3Kγ and δ inhibitors for inflammatory and autoimmune diseases. J Med Chem (2019) 62(10):4783–814. doi: 10.1021/acs.jmedchem.8b01298
37. Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol (2010) 11(5):329–41. doi: 10.1038/nrm2882
38. Fung-Leung W-P. Phosphoinositide 3-kinase delta (PI3Kδ) in leukocyte signaling and function. Cell signalling (2011) 23(4):603–8. doi: 10.1016/j.cellsig.2010.10.002
39. Hawkins PT, Stephens LR. PI3K signalling in inflammation. Biochim Biophys Acta (2015) 1851(6):882–97. doi: 10.1016/j.bbalip.2014.12.006
40. Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG. Cutting edge: differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J Immunol (2004) 173(4):2236–40. doi: 10.4049/jimmunol.173.4.2236
41. Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, et al. PI3Kγ is a molecular switch that controls immune suppression. Nature (2016) 539(7629):437–42. doi: 10.1038/nature19834
42. De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature (2016) 539(7629):443–7. doi: 10.1038/nature20554
43. Peluso M, Faia K, Winkler D, Patel N, Brophy E, White K, et al. Duvelisib (IPI-145) inhibits malignant b-cell proliferation and disrupts signaling from the tumor microenvironment through mechanisms that are dependent on PI3K-δ and PI3K-γ. Blood (2014) 124(21):328–8. doi: 10.1182/blood.V124.21.328.328
44. Balakrishnan K, Peluso M, Fu M, Rosin NY, Burger JA, Wierda WG, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia (2015) 29(9):1811–22. doi: 10.1038/leu.2015.105
45. Faia K, White K, Proctor J, Andrade P, Pink M, Rickles R, et al. High throughput in vitro combination sensitivity screen in hematologic malignancies with the phosphoinositide-3 kinase (PI3K)-δ,γ inhibitor, duvelisib. J Clin Oncol (2015) 33(15_suppl):8559–9. doi: 10.1200/jco.2015.33.15_suppl.8559
46. Cortes JR, Ambesi-Impiombato A, Couronné L, Quinn SA, Kim CS, da Almeida Silva AC, et al. RHOA G17V induces T follicular helper cell specification and promotes lymphomagenesis. Cancer Cell (2018) 33(2):259–273.e7. doi: 10.1016/j.ccell.2018.01.001
47. Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin Cancer Biol (2019) 59:125–32. doi: 10.1016/j.semcancer.2019.07.009
48. Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol (2013) 10(3):143–53. doi: 10.1038/nrclinonc.2013.10
49. Esposito A, Viale G, Curigliano G. Safety, tolerability, and management of toxic effects of phosphatidylinositol 3-kinase inhibitor treatment in patients with cancer: A review. JAMA Oncol (2019) 5(9):1347–54. doi: 10.1001/jamaoncol.2019.0034
50. De Santis MC, Gulluni F, Campa CC, Martini M, Hirsch E. Targeting PI3K signaling in cancer: Challenges and advances. Biochim Biophys Acta Rev Cancer (2019) 1871(2):361–6. doi: 10.1016/j.bbcan.2019.03.003
51. Richardson NC, Kasamon Y, Pazdur R, Gormley N. The saga of PI3K inhibitors in haematological malignancies: survival is the ultimate safety endpoint. Lancet Oncol (2022) 23(5):563–6. doi: 10.1016/S1470-2045(22)00200-5
52. Ladygina N, Gottipati S, Ngo K, Castro G, Ma JY, Banie H, et al. PI3Kγ kinase activity is required for optimal T-cell activation and differentiation. Eur J Immunol (2013) 43(12):3183–96. doi: 10.1002/eji.201343812
53. Barber DF, Bartolomé A, Hernandez C, Flores JM, Fernandez-Arias C, Rodríguez-Borlado L, et al. Class IB-phosphatidylinositol 3-kinase (PI3K) deficiency ameliorates IA-PI3K-induced systemic lupus but not T cell invasion. J Immunol (2006) 176(1):589–93. doi: 10.4049/jimmunol.176.1.589
54. Vanhaesebroeck B, Perry MWD, Brown JR, André F, Okkenhaug K. Author correction: PI3K inhibitors are finally coming of age. Nat Rev Drug Discovery (2021) 20(10):798. doi: 10.1038/s41573-021-00300-7
55. Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood (2010) 116(12):2078–88. doi: 10.1182/blood-2010-02-271171
56. Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3’-kinase delta inhibitor, CAL-101, inhibits b-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood (2011) 118(13):3603–12. doi: 10.1182/blood-2011-05-352492
57. Niemann CU, Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol (2013) 23(6):410–21. doi: 10.1016/j.semcancer.2013.09.001
58. Ng SY, Brown L, Stevenson K, deSouza T, Aster JC, Louissaint A Jr, et al. RhoA G17V is sufficient to induce autoimmunity and promotes T-cell lymphomagenesis in mice. Blood (2018) 132(9):935–47. doi: 10.1182/blood-2017-11-818617
59. Pechloff K, Holch J, Ferch U, Schweneker M, Brunner K, Kremer M, et al. The fusion kinase ITK-SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J Exp Med (2010) 207(5):1031–44. doi: 10.1084/jem.20092042
60. Marzec M, Kasprzycka M, Liu X, Raghunath PN, Wlodarski P, Wasik MA. Oncogenic tyrosine kinase NPM/ALK induces activation of the MEK/ERK signaling pathway independently of c-raf. Oncogene (2007) 26(6):813–21. doi: 10.1038/sj.onc.1209843
61. Heavican TB, Bouska A, Yu J, Lone W, Amador C, Gong Q, et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma. Blood (2019) 133(15):1664–76. doi: 10.1182/blood-2018-09-872549
62. Nguyen TB, Sakata-Yanagimoto M, Fujisawa M, Nuhat S.T, Miyoshi H, Nannya Y, et al. Dasatinib is an effective treatment for angioimmunoblastic T-cell lymphoma. Cancer Res (2020) 80(9):1875–84. doi: 10.1158/0008-5472.CAN-19-2787
63. Serrat N, Guerrero-Hernández M, Matas-Céspedes A, Yahiaoui A, Valero JG, Nadeu F, et al. PI3Kδ inhibition reshapes follicular lymphoma-immune microenvironment cross talk and unleashes the activity of venetoclax. Blood Adv (2020) 4(17):4217–31. doi: 10.1182/bloodadvances.2020001584
64. Aydin E, Faehling S, Saleh M, Cid Llaó L, Seiffert M, Roessner PM. Phosphoinositide 3-kinase signaling in the tumor microenvironment: What do we need to consider when treating chronic lymphocytic leukemia with PI3K inhibitors? Front Immunol (2020) 11:595818. doi: 10.3389/fimmu.2020.595818
65. Iqbal J, Weisenburger DD, Greiner TC, Vose JM, McKeithan T, Kucuk C, et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood (2010) 115(5):1026–36. doi: 10.1182/blood-2009-06-227579
66. Dong S, Harrington BK, Hu EY, Greene JT, Lehman AM, Tran M, et al. PI3K p110δ inactivation antagonizes chronic lymphocytic leukemia and reverses T cell immune suppression. J Clin Invest (2019) 129(1):122–36. doi: 10.1172/JCI99386
67. Abu-Eid R, Samara RN, Ozbun L, Abdalla MY, Berzofsky JA, Friedman KM, et al. Selective inhibition of regulatory T cells by targeting the PI3K-akt pathway. Cancer Immunol Res (2014) 2(11):1080–9. doi: 10.1158/2326-6066.CIR-14-0095
68. Abu Eid R, Ahmad S, Lin Y, Webb M, Berrong Z, Shrimali R, et al. Enhanced therapeutic efficacy and memory of tumor-specific CD8 T cells by ex vivo PI3K-δ inhibition. Cancer Res (2017) 77(15):4135–45. doi: 10.1158/0008-5472.CAN-16-1925
69. Ahmad S, Abu-Eid R, Shrimali R, Webb M, Verma V, Doroodchi A, et al. Differential PI3Kδ signaling in CD4(+) T-cell subsets enables selective targeting of T regulatory cells to enhance cancer immunotherapy. Cancer Res (2017) 77(8):1892–904. doi: 10.1158/0008-5472.CAN-16-1839
70. Ali K, Soond D.R, Pineiro R, Hagemann T, Pearce W, Lim EL, et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature (2014) 510(7505):407–11. doi: 10.1038/nature13444
71. Lauder SN, Smart K, Kersemans V, Allen D, Scott J, Pires A, et al. Enhanced antitumor immunity through sequential targeting of PI3Kδ and LAG3. J Immunother Cancer (2020) 8(2):e000693. doi: 10.1136/jitc-2020-000693
72. Gyori D, Lim EL, Grant FM, Spensberger D, Roychoudhuri R, Shuttleworth SJ, et al. Compensation between CSF1R+ macrophages and Foxp3+ treg cells drives resistance to tumor immunotherapy. JCI Insight (2018) 3(11):e120631. doi: 10.1172/jci.insight.120631
73. Patel K, Pagel JM. Current and future treatment strategies in chronic lymphocytic leukemia. J Hematol Oncol (2021) 14(1):69. doi: 10.1186/s13045-021-01054-w
74. Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med (2014) 371(3):213–23. doi: 10.1056/NEJMoa1400376
75. Sharman JP, Coutre SE, Furman RR, Cheson BD, Pagel JM, Hillmen P, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol (2019) 37(16):1391–402. doi: 10.1200/JCO.18.01460
76. Winkler DG, Faia KL, DiNitto JP, Ali JA, White KF, Brophy EE, et al. PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol (2013) 20(11):1364–74. doi: 10.1016/j.chembiol.2013.09.017
77. Zelenetz AD, Barrientos JC, Brown JR, Coiffier B, Delgado J, Egyed M, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol (2017) 18(3):297–311. doi: 10.1016/S1470-2045(16)30671-4
78. Kon N, Ou Y, Wang SJ, Li H, Rustgi AK, Gu W. mTOR inhibition acts as an unexpected checkpoint in p53-mediated tumor suppression. Genes Dev (2021) 35(1-2):59–64. doi: 10.1101/gad.340919.120
79. Coronel L, Häckes D, Schwab K, Riege K, Hoffmann S, Fischer M. p53-mediated AKT and mTOR inhibition requires RFX7 and DDIT4 and depends on nutrient abundance. Oncogene (2022) 41(7):1063–9. doi: 10.1038/s41388-021-02147-z
80. Horton LE, Bushell M, Barth-Baus D, Tilleray VJ, Clemens MJ, Hensold JO. p53 activation results in rapid dephosphorylation of the eIF4E-binding protein 4E-BP1, inhibition of ribosomal protein S6 kinase and inhibition of translation initiation. Oncogene (2002) 21(34):5325–34. doi: 10.1038/sj.onc.1205662
81. Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U.S.A. (2005) 102(23):8204–9. doi: 10.1073/pnas.0502857102
82. Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, et al. Regulation of PTEN transcription by p53. Mol Cell (2001) 8(2):317–25. doi: 10.1016/S1097-2765(01)00323-9
83. Kawase T, Ohki R, Shibata T, Tsutsumi S, Kamimura N, Inazawa J, et al. PH domain-only protein PHLDA3 is a p53-regulated repressor of akt. Cell (2009) 136(3):535–50. doi: 10.1016/j.cell.2008.12.002
84. Stilgenbauer S, Zenz T. Understanding and managing ultra high-risk chronic lymphocytic leukemia. Hematol Am Soc Hematol Educ Program 2010 (2010) p:481–8. doi: 10.1182/asheducation-2010.1.481
85. Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood (2015) 125(16):2497–506. doi: 10.1182/blood-2014-10-606038
86. Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med (2016) 374(4):311–22. doi: 10.1056/NEJMoa1513257
87. Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med (2015) 373(25):2425–37. doi: 10.1056/NEJMoa1509388
88. Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. First-line treatment of chronic lymphocytic leukemia with ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab: final analysis of the randomized, phase 3 iLLUMINATE trial. Haematologica (2022) 107(9):2108–20. doi: 10.3324/haematol.2021.279012
89. Stilgenbauer S, Eichhorst B, Schetelig J, Hillmen P, Seymour JF, Coutre S, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: Results from the full population of a phase II pivotal trial. J Clin Oncol (2018) 36(19):1973–80. doi: 10.1200/JCO.2017.76.6840
90. Blair HA. Venetoclax: A review in previously untreated chronic lymphocytic leukaemia. Drugs (2020) 80(18):1973–80. doi: 10.1007/s40265-020-01433-6
91. Patel VM, Balakrishnan K, Douglas M, Tibbitts T, Xu EY, Kutok JL, et al. Duvelisib treatment is associated with altered expression of apoptotic regulators that helps in sensitization of chronic lymphocytic leukemia cells to venetoclax (ABT-199). Leukemia (2017) 31(9):1872–81. doi: 10.1038/leu.2016.382
92. Dong S, Guinn D, Dubovsky JA, Zhong Y, Lehman A, Kutok J, et al. IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood (2014) 124(24):3583–6. doi: 10.1182/blood-2014-07-587279
93. Bargetzi M, Baumann R, Cogliatti S, Dietrich PY, Duchosal M, Goede J, et al. Diagnosis and treatment of follicular lymphoma: an update. Swiss Med Wkly (2018) 148:w14635. doi: 10.4414/smw.2018.14635
94. Gopal AK, Kahl BS, Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med (2014) 370(11):1008–18. doi: 10.1056/NEJMoa1314583
95. Dreyling M, Santoro A, Mollica L, Leppä S, Follows GA, Lenz G, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol (2017) 35(35):3898–905. doi: 10.1200/JCO.2017.75.4648
96. Fowler NH, Samaniego F, Jurczak W, Ghosh N, Derenzini E, Reeves JA, et al. Umbralisib, a dual PI3Kδ/CK1ϵ inhibitor in patients with relapsed or refractory indolent lymphoma. J Clin Oncol (2021) 39(15):1609–18. doi: 10.1200/JCO.20.03433
97. Dreyling M, Morschhauser F, Bouabdallah K, Bron D, Cunningham D, Assouline SE, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol (2017) 28(9):2169–78. doi: 10.1093/annonc/mdx289
98. Shaffer AL 3rd, Young RM, Staudt LM. Pathogenesis of human b cell lymphomas. Annu Rev Immunol (2012) 30:565–610. doi: 10.1146/annurev-immunol-020711-075027
99. Flinn IW, Cherry MA, Maris MB, Matous JV, Berdeja JG, Patel M. Combination trial of duvelisib (IPI-145) with rituximab or bendamustine/rituximab in patients with non-Hodgkin lymphoma or chronic lymphocytic leukemia. Am J Hematol (2019) 94(12):1325–34. doi: 10.1002/ajh.25634
100. O’Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma. Results Pivotal Phase II BELIEF (CLN-19) Study (2015) 33(23):2492–9. doi: 10.1200/JCO.2014.59.2782
101. Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol (2012) 30(6):631–6. doi: 10.1200/JCO.2011.37.4223
102. Horwitz SM, Moskowitz AJ, Mehta-Shah N, Jacobsen ED, Khodadoust MS, Ganesan N, et al. The combination of duvelisib and romidepsin (Dr) is highly active against Relapsed/Refractory peripheral T-cell lymphoma with low rates of transaminitis: Final Results. 16th International Conference on Malignant Lymphoma, Virtual Edition, 18–22 June, 2021(2021) 39(S2). doi: 10.1002/hon.56_2879
103. Coutré SE, Barrientos JC, Brown JR, Vos S, Furman RR, Keating MJ, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma (2015) 56(10):2779–86. doi: 10.3109/10428194.2015.1022770
104. Morrison VA. Infections in patients with leukemia and lymphoma. Cancer Treat Res (2014) 161:319–49. doi: 10.1007/978-3-319-04220-6_11
105. Nosari A. Infectious complications in chronic lymphocytic leukemia. Mediterr J Hematol Infect Dis (2012) 4(1):e2012070. doi: 10.4084/mjhid.2012.070
106. Ahn IE, Jerussi T, Farooqui M, Tian X, Wiestner A, Gea-Banacloche J. Atypical pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood (2016) 128(15):1940–3. doi: 10.1182/blood-2016-06-722991
107. Tillman BF, Pauff J.M, Satyanarayana G, Talbott M, Warner JL. Systematic review of infectious events with the bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. Eur J Haematol (2018) 100(4):325–34. doi: 10.1111/ejh.13020
108. Jones JA, Robak T, Brown JR, Awan FT, Badoux X, Coutre S, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol (2017) 4(3):e114–26. doi: 10.1016/S2352-3026(17)30019-4
109. Ghez D, Calleja A, Protin C, Baron M, Ledoux MP, Damaj G, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood (2018) 131(17):1955–9. doi: 10.1182/blood-2017-11-818286
110. Vorobyev VI, Yoon DH, Kaźmierczak M, Grosicki S, Tarella C, Genua A, et al. TEMPO: A phase 2, randomized, open-label, 2-arm study comparing 2 intermittent dosing schedules of duvelisib in subjects with indolent non Hodgkin lymphoma (iNHL). Blood (2021) 138(Supplement 1):3545–5. doi: 10.1182/blood-2021-147564
111. Eschweiler S, Ramírez-Suástegui C, Li Y, King E, Chudley L, Thomas J, et al. Intermittent PI3Kδ inhibition sustains anti-tumour immunity and curbs irAEs. Nature (2022) 605(7911):741–6. doi: 10.1038/s41586-022-04685-2
112. Ma S, Chan R.J, Gu L, Xing G, Rajakumaraswamy N, Ruzicka BB, et al. Retrospective analysis of the impact of adverse event-triggered idelalisib interruption and dose reduction on clinical outcomes in patients with Relapsed/Refractory b-cell malignancies. Clin Lymphoma Myeloma Leuk (2021) 21(5):e432–48. doi: 10.1016/j.clml.2020.12.016
113. Camps M, Rückle T, Ji H, Ardissone V, Rintelen F, Shaw J, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med (2005) 11(9):936–43. doi: 10.1038/nm1284
Keywords: dual PI3K-δ, γ inhibitor, duvelisib, lymphoid neoplasms, safety, efficacy, meta-analysis
Citation: Wang Z, Zhou H, Xu J, Wang J and Niu T (2023) Safety and efficacy of dual PI3K-δ, γ inhibitor, duvelisib in patients with relapsed or refractory lymphoid neoplasms: A systematic review and meta-analysis of prospective clinical trials. Front. Immunol. 13:1070660. doi: 10.3389/fimmu.2022.1070660
Received: 15 October 2022; Accepted: 07 December 2022;
Published: 04 January 2023.
Edited by:
Julio Delgado, Hospital Clínic/IDIBAPS, SpainReviewed by:
Marco Montillo, Niguarda Ca ‘Granda Hospital, ItalyPablo Mozas, Hospital Clinic of Barcelona, Spain
Copyright © 2023 Wang, Zhou, Xu, Wang and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Niu, bml1dGluZ0B3Y2hzY3UuY24=
†These authors have contributed equally to this work and share first authorship
 Zhongwang Wang
Zhongwang Wang Hui Zhou
Hui Zhou Jing Xu
Jing Xu Ting Niu
Ting Niu