- 1Infectious Diseases Division, icddr, b (International Centre for Diarrhoeal Disease Research, Bangladesh), Dhaka, Bangladesh
- 2Menzies Health Institute Queensland, Griffith University, Gold Coast, Australia
- 3Department of Biology, Xavier University of Louisiana, New Orleans, AK, United States
- 4Department of Pediatrics, Harvard Medical School, Boston, MA, United States
- 5Division of Infectious Diseases, Massachusetts General Hospital, Boston, MA, United States
- 6Department of Medicine, Harvard Medical School, Boston, MA, United States
- 7Rural Health Research Institute, Charles Sturt University, Orange, New South Wales, Australia
- 8Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston, MA, United States
- 9Department of Microbiology, Harvard Medical School, Boston, MA, United States
Background: Immune responses that target sialidase occur following natural cholera and have been associated with protection against cholera. Sialidase is a neuraminidase that facilitates the binding of cholera toxin (CT) to intestinal epithelial cells. Despite this, little is known about age-related sialidase-specific immune responses and the impact of nutritional status and co-infection on sialidase-specific immunity.
Methods: We enrolled 50 culture-confirmed Vibrio cholerae O1 cholera cases presenting to the icddr,b Dhaka hospital with moderate to severe dehydration. We evaluated antibody responses out to 18 months (day 540) following cholera. We assessed immune responses targeting sialidase, lipopolysaccharide (LPS), cholera toxin B subunit (CtxB), and vibriocidal responses. We also explored the association of sialidase-specific immune responses to nutritional parameters and parasitic co-infection of cases.
Results: This longitudinal cohort study showed age-dependent differences in anti-sialidase immune response after natural cholera infection. Adult patients developed plasma anti-sialidase IgA and IgG responses after acute infection (P<0.05), which gradually decreased from day 30 on. In children, no significant anti-sialidase IgA, IgM, and IgG response was seen with the exception of a late IgG response at study day 540 (p=0.05 compared to adults). There was a correlation between anti-sialidase IgA with vibriocidal titers, as well as anti-sialidase IgA and IgG with anti-LPS and anti-CtxB antibody responses in adult patients, whereas in children, a significant positive correlation was seen only between anti-sialidase IgA and CtxB IgA responses. Stunted children showed significantly lower anti-sialidase IgA, IgG, and IgM antibody responses and higher LPS IgG and IgM antibody responses than healthy children. The anti-sialidase IgA and IgG responses were significantly higher in cases with concomitant parasitic infection.
Conclusion: Our data suggest that cholera patients develop age-distinct systemic and mucosal immune responses against sialidase. The stunted children have a lower anti-sialidase antibody response which may be associated with gut enteropathy and the neuraminidase plays an important role in augmented immune response in cholera patients infected with parasites.
Introduction
Cholera remains a major public health problem in resource-limited countries, and cholera outbreaks following natural and human-made disasters underscore the overall global health threat posed by cholera. Many epidemiological studies reveal that natural infection with V. cholerae provides long-term immunity; however, the correlation of protection afforded by an immune response to a range of V. cholerae antigens is not well understood (1). Currently available oral cholera vaccines (OCVs) provide around 65% protective efficacy (PE) for 3~5 years against cholera (2). Protective efficacy varies by age group, with protective efficacy in young children under 5 years of age approximating 40% with a short duration of protection (2, 3). Several studies in endemic settings demonstrate that vaccines and natural infection induce significant vibriocidal, cholera toxin B subunit (CtxB), toxin coregulated pilin (TcpA), and lipopolysaccharide/O-specific polysaccharide (LPS/OSP) antibody responses. Many of these immune responses fall within a few months of cholera, despite immunity afforded by previous infection lasting for years. To inform future possible vaccine development, we here describe an analysis of sialidase-specific antibodies following cholera, with analysis out to 18 months.
Vibrio cholerae O1 specific sialidase is a neuraminidase that facilitates the binding of CT to intestinal epithelial cells (4). Sialidases are enzymes that catalyze the cleavage of terminal sialic acids from oligosaccharides and glycoconjugates (5). V.cholerae sialidase has a unique essential Ca2+ ion and this sialidase can hydrolyze both 2,3- and 2,6-linked glycosidic bonds (6, 7). Sialidase plays an important role in cholera infection by removing sialic acid residues from higher-order gangliosides on gut epithelial cell membranes to generate monogangliosides (GM1), the binding receptor for CT (8), and by helping V. cholerae colonize the intestine in heavily sialylated areas like the intestinal epithelium (4).
We have recently identified and explored sialidase-specific immune responses in a number of our immune analyses in cholera patients. First, in an analysis of cloned plasmablasts in patients recovering from cholera, clones recognizing sialidase were the third most frequently identified, following clones recognizing V. cholerae OSP and CT (9). Second, we performed micro-array-based immune profiling of sera and antibody-in-lymphocyte supernatant (ALS- a marker of mucosal immune responses) in adult cholera patients and identified sialidase as a frequent target (1). Third, we analyzed short-term anti-sialidase immune responses out to 30 days following cholera in adults and children, finding that the most prominent immune responses occurred in adults, perhaps suggesting immune boosting from previous infection (4).
In our current study, we report the evolution of anti-sialidase immune responses in adults and children out to 18 months following cholera, and assess the correlation of anti-sialidase immune responses with other anti-V. cholerae immune responses, and assess the impact of nutritional and parasitic co-infection status on sialidase-specific immune responses.
Methods
Study participant and sample collection
The International Centre for Diarrhoeal Disease Research (icddr,b), Dhaka hospital serves more than 100,000 diarrhoeal patients including ~ 20,000 cholera patients annually, the majority of them living in or around Dhaka city. We enrolled participants from January 2018 to December 2020, who came to icddr,b Dhaka hospital with acute watery diarrhea (AWD), having signs of moderate to severe dehydration assessed by the World Health Organization (WHO) guidelines (10). Inclusion criteria included a stool culture positive for V. cholerae O1, aged 2 to 60 years old, and without any significant comorbid conditions.
On study day 1, we identified the patient based on inclusion and exclusion criteria and stool was sent for culture for V. cholerae. The patient was enrolled on study day 2 if stool culture was positive for V. cholerae O1. These individuals were followed up to day 540. The routine stool microscopy examination (RME) was also done for the enrolled patients. We aimed to compare the clinical features and immunological parameters between the children aged 2 to 17 years and adults aged 18 to 60 years for analysis. Blood samples were collected to measure different kinds of immunological markers such as antibodies to sialidase, LPS, CtxB, and vibriocidal titers on days 2, 7, 30, 90, 180, and 540 after enrollment of each index case.
Microbiological analysis
Stool culture for V.choleare was done to confirm the cholera cases by taurocholate-tellurite-gelatin agar (TTGA) media. The serological confirmation for V.choleare colonies was done by overnight incubation of plates, through the slide agglutination method (11). Stool parasites including protozoa and helminths were inspected using the direct microscopic method for intestinal parasites after confirmation with V.cholerae in patients. In RME, we prepared two slides for each cholera patient. For the rice watery stool, a drop of stool was examined straightly below a cover slip whereas, for the semisolid/solid stools, we prepared a thin solution mixed with ~ 2gm of stool and normal saline. The 1% Lugol’s iodine was used to make the second slide. The 22 mm cover glasses were used for the slides, and the entire film was examined under light microscopy for the detection of parasites.
Sialidase-specific IgA, IgG, and IgM antibodies in plasma
We measured anti-sialidase IgG, IgM, and IgA responses in plasma using standard enzyme-linked immunosorbent assay (ELISA) protocols. To determine anti-sialidase antibody titers, the ELISA plates were coated with sialidase (2.5 µg/mL) in carbonate buffer. Sialidase was prepared from Vibrio cholerae O1 strain N16961 that overexpressed from pXT7 cloning vector in E. coli as recombinant polyhistidine proteins (1). We added 100 µL of plasma to each well (diluted 1∶25 in 5% non-fat dry milk in phosphate-buffered saline-0.05%) and detected the presence of antigen-specific antibodies using horseradish peroxidase-conjugated goat anti-human IgG, IgM or IgA antibody (diluted 1∶1000 in 5% non-fat dry milk in phosphate buffered saline-0.05%Tween) (Southern Biotech, Birmingham, AL). After incubation at 37°C for 1 h and washing, we developed the plates with ortho-phenylenediamine (OPD; Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) and 0.012% hydrogen peroxide (H2O2) and determined the optical density at 450 nm for 5 minutes at 30s intervals with an Eon Microplate Spectrophotometer (BioTek, CA). The rate of change in optical density was measured as milli-absorbance units per minute. The rate of change in optical density of each sample was then normalized and expressed as ELISA units by calculating the ratio of the sample to a standard of pooled sera (4, 12). The maximal rate of OD change was expressed in milli absorbance units per minute, and ELISA units were standardized by calculating the ratio of the test sample to a sample of pooled convalescent stage serum from patients who had recovered from cholera, run as a positive control on each plate.
Vibriocidal antibody assay
We measured vibriocidal antibody responses in plasma by using guinea pig complement (Sigma-Aldrich Chemie GmbH) and V. cholerae O1 Ogawa (X-25049) and Inaba (T-19479) as the target organisms (10, 13). A variation of one log of the pooled sera is acceptable for vibriocidal assay over different days. Pooled convalescent stage serum from patients who had recovered from cholera, run as a positive control or standard on each plate for ensuring reproducibility. We defined the vibriocidal responses as the reciprocal of the highest dilution resulting in ≥50% reduction of the optical density compared to that of control wells without plasma. We considered those individuals as responders who showed a ≥4-fold increase in vibriocidal titer from baseline (14).
LPS, and CtxB-specific IgA, IgG, and IgM antibodies in plasma
We assessed LPS, and CtxB-specific IgA, IgG, and IgM antibody responses in plasma by using an enzyme-linked immunosorbent assay (ELISA), as previously described (13, 15, 16). The homologous LPS was measured based on specific serotypes of V.cholerae O1 or Inaba. We added 100 µl of plasma (diluted 1:50 for LPS and 1:100 for CtxB in 0.1% bovine plasma albumin in phosphate-buffered saline-0.05% Tween) per well and detected responses using horseradish peroxidase-conjugated secondary antibodies to human IgG, IgA, or IgM (Jackson Immuno Research, West Grove, PA; 1:1,000 dilution). After incubation at 37°C and washing, we developed the plates with orthophenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) and 0.012% hydrogen peroxide followed by reading the plates kinetically at 450 nm for 5 min (17, 18). We considered individuals who showed a ≥2-fold increase in LPS, and CtxB responses compared to baseline values on study day 2 to be responders (14).
Nutritional status
The nutritional status of children was measured using a z-score cut-off point according to the WHO Global Database on Child Growth and Malnutrition. The z-scores were calculated using the WHO Child Growth Standards for children aged between zero and 60 months or the WHO Growth References for school-aged children and adolescents. We used a z-score cut-off point of < −2 SD to classify low weight-for-age (underweight), low height-for-age (stunting) and low weight-for-height (wasted) (10).
Statistical analyses
The demographic and clinical characteristics of cholera patients were recorded as numbers with percentage and median with inter-quartile range (IQR). The distribution of the nutritional status of the children and clinical characteristics in children and adult cases at different time points were analyzed with the Kruskal Wallis test with a 5% level of significance. The correlation between anti-sialidase responses and other clinical characteristics in both children and adult patients was analyzed using the Pearson correlation test at a 5% level of significance using t-test.
All the analyses were done using R statistical software. For visualization, the line graph, boxplot, and line graph with error bars were generated using “ggplot2” and “ggpubr” packages and for z score calculation “zscorer” package was used.
Results
Study population
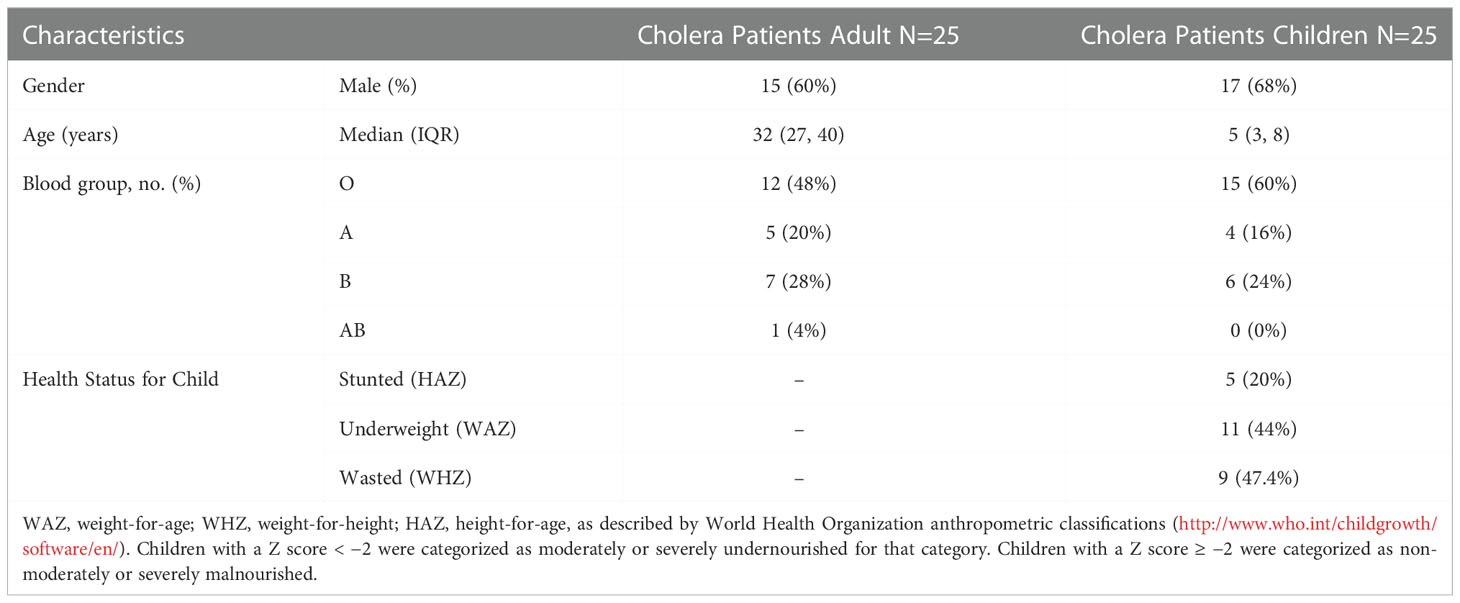
We enrolled 50 culture-confirmed cholera cases (25 adults and 25 children) with moderate to severe dehydration and the consort diagram of the study participants and their follow up at each study points have been presented in the Figure 1. Most of the cases were male (adult cases: 60% male; cases in children: 68% male). The median age of adult cases was 32 years (IQR: 27-40 years), and for cases in children 5 years (IQR: 3-8 years). Most of the cases had O-positive blood (48% for adults and 60% for children). Based on the WHO “Z Score” classification of children, 20% were stunted (height-for-age), 44% were underweight (weight-for-age) and 47% were wasted (weight-for-height) (Table 1).
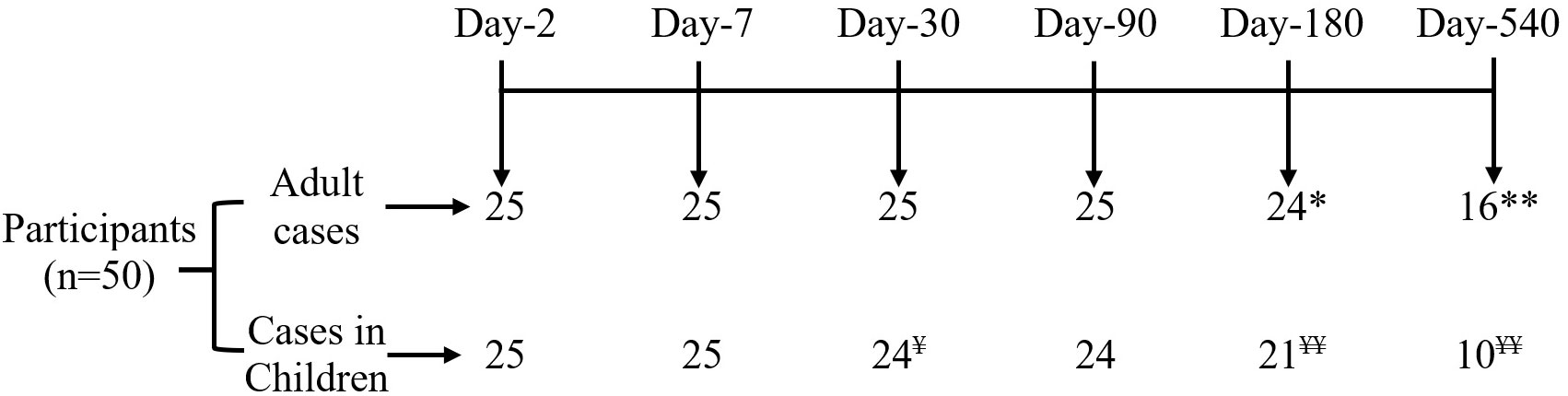
Figure 1 Consort diagram of participant disposition. *Refuse to continue study at day 180 (n=1) and **migration out at day 540 (n=8); ¥ Refuse to continue study at day 30 (n=1) and ¥ ¥ migration out on 180 (n=4), and 540 (n=10).
Anti-sialidase antibody responses among cholera patients
We measured anti-sialidase immune responses among adults and children suffering from cholera out to day 540 following infection (Figure 2). Adult patients developed significant increases in plasma anti-sialidase IgA and IgG responses on days 7 and day 30 following infection and these responses then gradually returned to baseline.
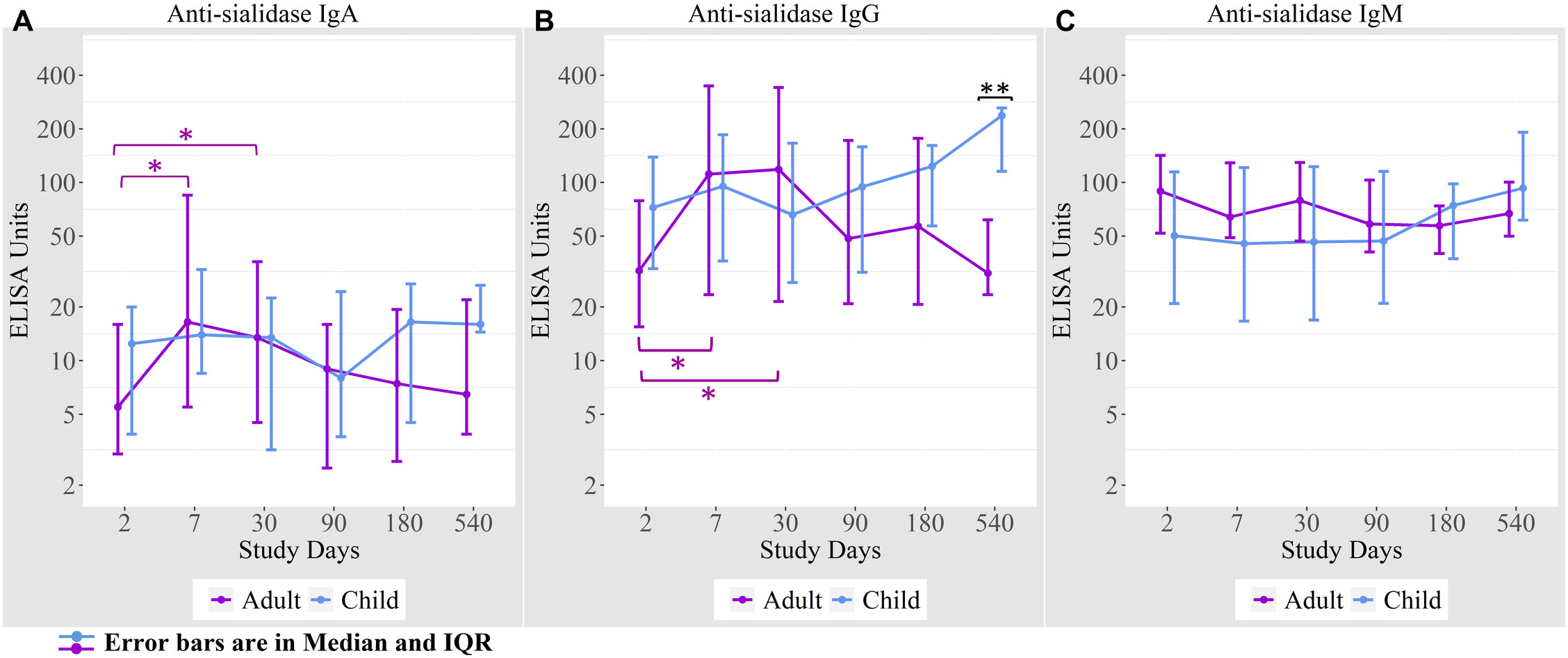
Figure 2 Anti-sialidase antibody responses in cholera cases in adults and children over the course of the study. (A) Anti-sialidase IgA responses among adults and children (B) Anti-sialidase IgG responses among adults and children (C) Anti-sialidase IgM responses among adults and children. Kruskal-Wallis test has done in order to identify the significant difference in median responses of anti-sialidase. *p=0.05- 0.01; **p=0.01-0.001. The purple asterisks denote comparisons in adult patients to day 2, and the black asterisks denote the difference between children and adult cases.
There was no the anti-sialidase IgA, IgG, and IgM significant response seen in children. From day 90 onwards, anti-sialidase IgA, IgG, and IgM immune responses increased compared to adults, but the differences were only significant for anti-sialidase IgG on day 540.
Correlation between anti-sialidase responses and other antibody responses
Anti-sialidase IgA and IgG responses in adults were associated significantly with vibriocidal responses for both the Ogawa and Inaba serotypes; and with the antibody responses to CtxB and LPS for different isotype of antibodies. In children, a significant positive association was seen only between anti-sialidase IgA and anti-CtxB IgA responses (Table 2).
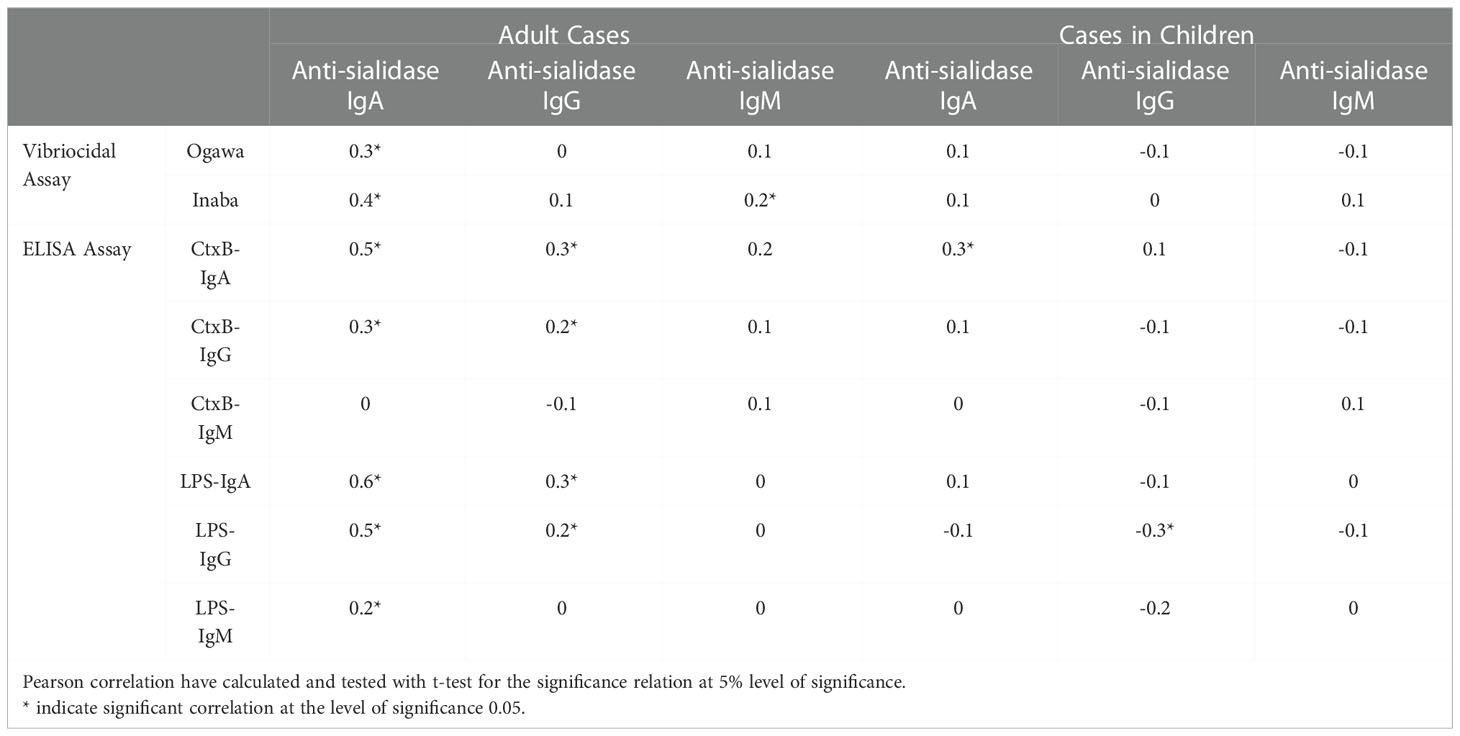
Table 2 Correlations matrix showing correlations between anti-sialidase responses and others immunological parameters.
Immunological responses among well-nourished versus malnourished children
Anti-sialidase IgA, IgG, and IgM responses were significantly lower at various time points in children who were stunted compared to well-nourished children (Figure 3).
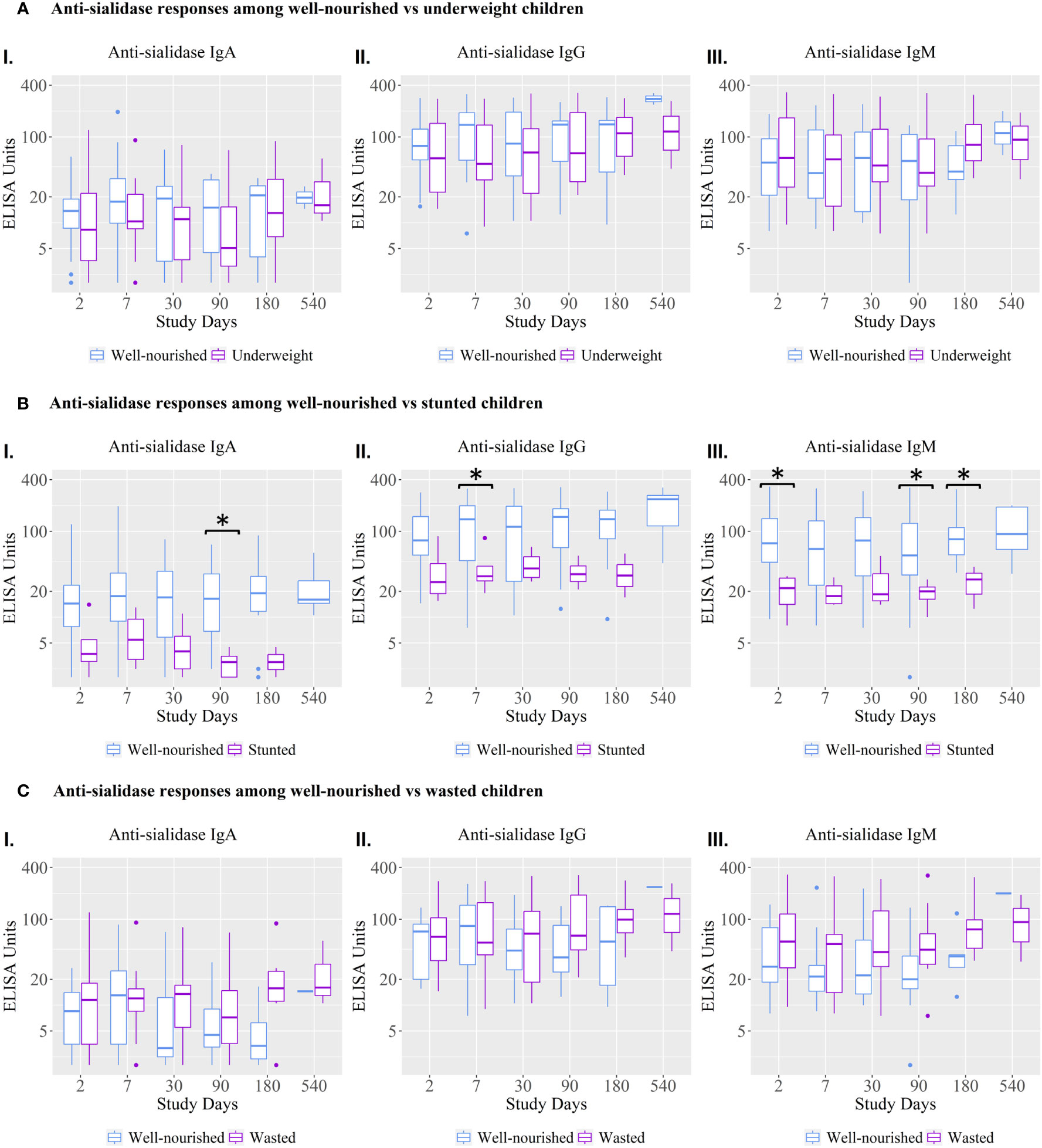
Figure 3 Anti-sialidase IgA, IgG, and IgM antibody responses among well-nourished versus malnourished children. Underweight = weight-for-age; Wasted= weight-for-height; Stunted = height-for-age. Children with a Z score < −2 were categorized as moderately or severely undernourished for that category. Children with a Z score ≥ −2 were categorized as non-moderately or severely malnourished. Kruskal-Wallis test has done in order to identify the significant difference in median responses of anti-sialidase. Asterisks denote the level of significance; *p=0.05- 0.01 and ** denotes p=0.01-0.001. The black asterisks denote the difference between well-nourished and malnourished children.
The vibriocidal, anti-LPS IgA and anti-CtxB IgA, IgG, and IgM antibody responses were not statistically significant between stunted and well-nourished children (Figure 4), but the stunted children showed significantly higher responses for anti-LPS IgG on day 90 and LPS IgM antibody responses on day 30.

Figure 4 Vibriocidal antibody responses and anti-LPS and anti-CtxB IgA, IgG, and IgM immune responses among well-nourished versus stunted children. Kruskal-Wallis test has done in order to identify the significant difference in median responses of different antibody. Asterisks denote the level of significance; *p=0.05- 0.01. The black asterisks denote the difference between Well-nourished and Stunted Children.
Anti-sialidase immune responses among cholera patients with concomitant parasitic infection
We found that 16% of cholera cases were co-infected with intestinal parasites (Ascaris lumbricoides, Trichuris trichiura, Giardia lamblia, and Endolimax nana). Anti-sialidase IgA antibody responses were higher among the cholera cases with a concomitant parasitic infection on days 2, 7, and 180 compared to cholera cases with no parasitic co-infection (Figure 5).
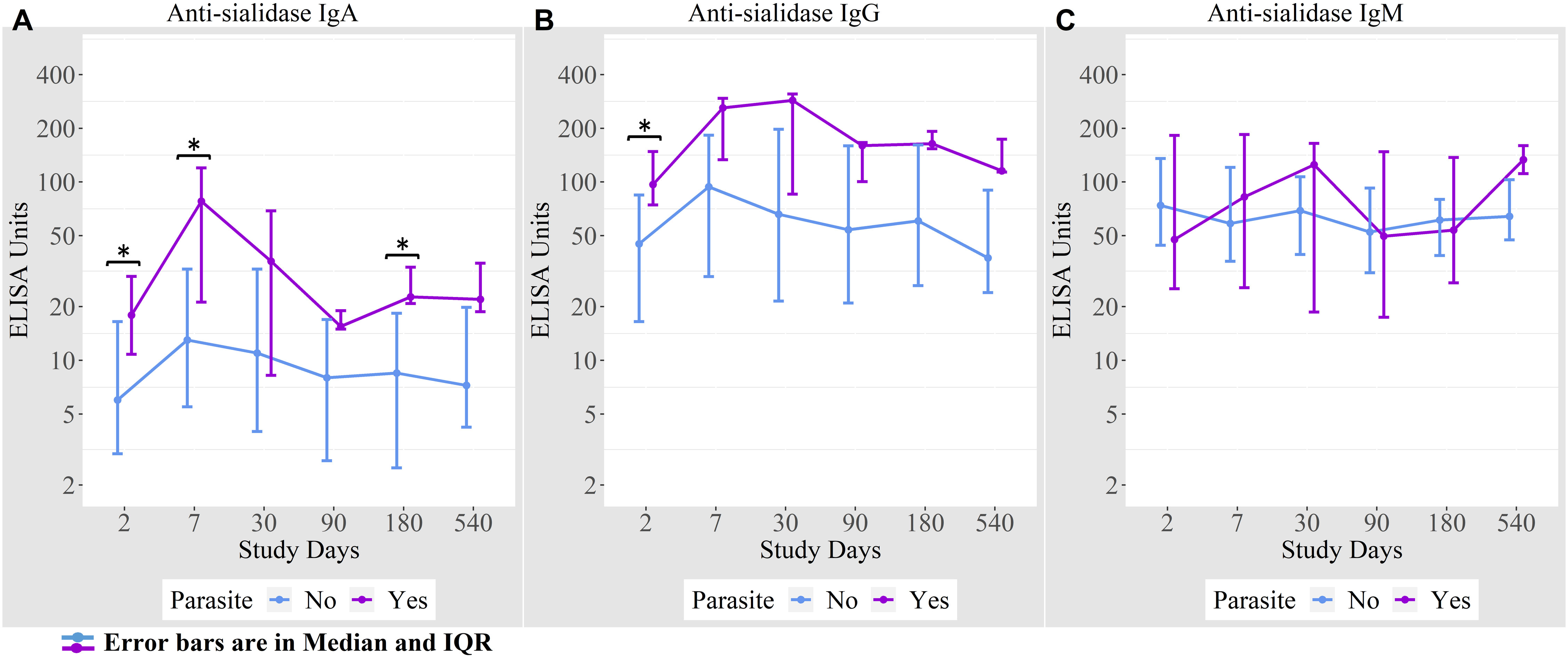
Figure 5 (A) Anti-sialidase IgA responses among patients with and without intestinal parasite co-infection (B) Anti-sialidase IgG responses among patients with and without intestinal parasite co-infection (C) Anti-sialidase IgM responses among patients with and without intestinal parasite co-infection.
We also looked at the anti-sialidase antibody levels in household contacts of the cholera index cases but did not find any significant anti-sialidase antibody responses.
Discussion
Sialidase is enzyme, acting on sialic acid residues on carbohydrates. Following cholera, patients develop antibody responses to the sialidase produced by Vibrio cholerae. Here, we measured anti-sialidase IgA, IgG, and IgM immune responses in children and adults after natural cholera infection out to 540 days following infection and their association with other immunological parameters, nutritional status, and parasitic co-infection. The anti-sialidase IgA and IgG responses were more robust within the first month after infection in adult cholera cases and then gradually decreased from day 30 to day 540. The immediate enhanced anti-sialidase immune responses to V. cholerae infection in adults may be associated with a decrease in susceptibility to cholera infection as we have seen in other studies that the immediate rise of vibriocidal and other antibody titers protect individuals from cholera infection (19, 20).
Our previous studies of acute immune responses following Vibrio cholerae infection in adults showed that vibriocidal, anti-CtxB, anti-TcpA, anti-LPS, and anti-OSP antibody responses are increased by day 7 after infection; while some of these antibody responses last longer than others, all fall back to baseline levels in the blood within 3-12 months after infection (14, 21, 22). Vibriocidal antibody responses increase sharply by 7 days after infection in symptomatic cases but rapidly wane within 30 days. Anti-CtxB, anti-TcpA, anti-LPS, and anti-OSP antibodies persist for 3-12 months after infection. We find a similar pattern of antibody responses to sialidase in adults in the current study and prior shorter-term study (4), with IgG and IgA responses peaking by day 7 to 30 and then gradually decreasing out to day 540. We have also previously shown that antibodies to CtxB, TcpA, LPS, and OSP provide protection against infection in contacts exposed to cholera in a household if still elevated on re-exposure (19). In a recent study, we showed that anti-sialidase IgG, IgA, and IgM anti-sialidase antibodies are associated with similar protection on household exposure to cholera (4). In addition to differentiation and maturation of B cells to antibody-secreting plasma cells following infection, a subset of B cells differentiates into longer-lasting memory B cells recognizing specific antigens (18, 22, 23). Memory B-cells recognizing CtxB, TcpA, LPS, and OSP develop following cholera and are detectable in circulation from 90 days to 1 year (14, 21, 22). Memory B cell responses to the O antigen of LPS diminish well before one year after infection, while memory B cells recognizing the protein antigens CtxB and TcpA remained elevated to one year and beyond, suggesting that T-cell-dependent antigens may provide longer-lasting memory B cell responses (4). In household contacts exposed to cholera, the level of memory Bcells recognizing CtxB, TcpA, LPS, and OSP antigens detectablein the circulation correlate with protection against subsequentinfection, suggesting that these memory B cells may produce an anamnestic response for longer-term protection. Anti-sialidase-specific memory B cells also develop following infection (1, 4), however, the duration and whether these also correlate with protection on exposure in the household has not yet been evaluated. This would add value to the current seroepidemiological studies based on other immunological markers following V. cholerae O1 infection (24).
The anti-sialidase responses were different between children and adults; overall, children lacked significant anti-sialidase immune responses following infection, as also seen in our previous shorter-term study (4). Children, however, do mount robust vibriocidal and LPS and CtxB antibody responses. This discordance can explain the weak to absent associations in children of anti-sialidase responses to other cholera-specific antibody responses (i.e. LPS, CtxB, vibriocidal), in contrast to adults who do mount significant anti-sialidase IgG and IgA and other cholera-specific antibody responses. It is possible that adults develop significant anti-sialidase immune responses after infection because they have been previously exposed to the organism and mount an anamnestic immune response, while children are more likely to be naïve to past infection, and may not mount a significant immune response on primary exposure. Differences in host factors between adults and children, such as malnutrition, enteric enteropathy, intestinal parasite burden, gut microbiome, etc. may also be playing a role (25). The unexpected finding of an increased IgG anti-sialidase response on day 540 in children raised the possibility that children were infected more commonly than adults during the follow-up period, but we considered this unlikely as there were no increases in other immune responses that follow infection, such as vibriocidal or anti-LPS or CtxB responses, in the same children over the same follow-up period. So, we are not certain of the significance or cause of the increased IgG anti-sialidase response seen in children compared to adults on day 540.
During measuring the correlation of antibody responses, we usually correlate the homologous antibody isotypes of antibody-secreting cells (ASC) (26). In this analysis we found some correlation among different antibody isotypes especially in adult patients and this different isotype correlation may have a privileged capacity to generate protective immune responses against cholera; a future study is needed to explain these exceptional findings.
Malnutrition is common in cholera endemic areas and malnutrition is a known factor influencing susceptibility to cholera (8). We found that stunted children (HAZ score <-2) showed significantly lower anti-sialidase IgA, IgG, and IgM antibody responses than well-nourished children; this finding was not seen for children who were malnourished or wasted by WAZ and WHZ scores. This result may be due to our small sample size used to stratify children by “WHO Z Score”. Furthermore, these two markers of malnutrition (WAZ and WHZ scores) represent more recent states of under nutrition than stunting and so their effects may not yet be evident (26). Previous studies have shown that well-nourished children with dehydrating cholera had higher vibriocidal and anti-CtxB responses than adult cases, suggesting that at least well-nourished children can respond satisfactorily to these antigens (27). Very few studies have been conducted to examine immune responses to V. cholerae O1 antigens in malnourished children; thus, the complex with different cholera antigens is not yet clear. The study by Falkard et al. found that stunting was associated with an increased response to CtxB (26) but in this study we did not find an increased response to CtxB in stunted compared to well-nourished children, although we did find increased IgM and IgG antibody responses to LPS. It has been reported that LPS-specific antibodies may be higher in LMICs in comparison to the developed world related to gut leakiness (28, 29). This could reflect prior exposure since cholera incidence rates are quite high in young children in LMICs compared to non-endemic-developed settings (20). Environmental enteric dysfunction (EED) in developing countries is a causal factor for stunting (30). A separate cohort of Bangladeshi children from urban slums showed that enteropathy biomarkers specially myeloperoxidase (MPO), and soluble CD14 were positively associated with increased LPS antibody responses among the OCV recipients in children, implying that microbial translocation may augment immune responses to the oral vaccine antigens (31). Moreover, based on the evidence, we may explain that higher LPS antibody responses following cholera in malnourished children may be associated with EED though we did not analyze the biomarkers in stunted children, highlighting the complexity of these interactions.
We also found significantly higher anti-sialidase IgA and IgG responses at various time points in cases of cholera with parasitic co-infection compared to cases of cholera without parasitic co-infection. Immune responses in cholera patients with concomitant parasitic co-infection have not been systematically evaluated. Harris et al. showed that following cholera, IgA immune responses to CtxB were decreased in children with parasitic co-infection, in contrast to other immunological markers like LPS and vibriocidal antibody responses (11). Another study by Araujo et al. analyzed anti-sialidase activity that developed in sera of mice and humans after Trypanosoma cruzi parasitic infection, suggesting that the neuraminidase activity of the parasite might play a significant role in the invasion of host cells (32). Here we may explain that neuraminidase in cholera patients infected with parasite plays an augmented role for higher anti-sialidase immune response. Moreover, parasites modulate the host immune response, especially T cell-dependent pathways (33, 34), and its effects on LPS antibody levels may not be similarly impacted as the LPS is a T cell-independent antigen (11).
This study has some limitations. We included relatively small sample size and did not analyze stool samples for mucosal immune responses and excluded demonstration of antibody functionality. Furthermore, we did not evaluate the duration of memory B-cell or development of T-cell responses against sialidase among study participants, and did not evaluate whether long-term anti-sialidase antibody responses were induced by currently available oral cholera vaccines. No healthy participant was included in this analysis to compare the baseline response. Future study related to these issues may show new insight to more knowledge regarding anti-sialidase responses.
Notwithstanding these limitations, we have conducted a longitudinal cohort study in an endemic setting for cholera and followed anti-sialidase antibody responses in adults and children over an extended period of time. This cohort compared the immune responses in stunted vs well-nourished children and also studied of the cholera cases who were co-infected with intestinal parasites.
Future studies are needed to examine anti-sialidase memory B and T cell development and duration following natural infection and vaccination, as well as the role these cells may play in protection from subsequent infection.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was reviewed and approved by the Institutional Review Board, icddr,b. Written informed consent was taken to participate in this study, which was provided by the participant or participant's legal guardian/next of kin.
Author contributions
FC, FQ, and TB designed and supervised the study. FC, TB, AA, IT, and RB helped to collect the specimens, performed the laboratory work and immunological analyses. FC, AA, TB, and MF analyzed the data. FQ, FC, RC, and ER provided key reagents. FC, and AA drafted the manuscript. FQ, FC, SC, AA, TB, RB, JH, RL, AGR, NM, RC, ER and IT reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the icddr,b and extramural grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases-NIAID (R01 AI106878 [E.T.R.,F.Q.], U01 AI058935 [S.B.C., E.T.R.], R01 AI137164 [J.B.H, R.C.C.], R01 AI130378 [T.R.B.] and the Fogarty International Center (E.T.R, F.Q., F.C.) and NIAID, Training Grant in Vaccine Development and Public Health (D43 TW005572 [F.Q., F.C., T.R.B., R.B.]), Global Emerging Leader Award (K43 TW010362 [T.R.B.]).
Acknowledgments
The authors would like to express their sincere gratitude to the donors for their support. The authors would also like to thanks to the staff members of icddr,b for their dedicated work in the hospital, field and laboratory. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden, and the UK for providing core/unrestricted support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Charles RC, Nakajima R, Liang L, Jasinskas A, Berger A, Leung DT, et al. Plasma and mucosal immunoglobulin m, immunoglobulin a, and immunoglobulin G responses to the vibrio cholerae O1 protein immunome in adults with cholera in Bangladesh. J Infect Dis (2017) 216(1):125–34. doi: 10.1093/infdis/jix253
2. Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, et al. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: Results from 3 years of follow-up of a randomized, controlled trial. PloS Negl Trop Dis (2011) 5(10):e1289. doi: 10.1371/journal.pntd.0001289
3. Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, et al. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in kolkata, India: A cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis (2013) 13(12):1050–6. doi: 10.1016/S1473-3099(13)70273-1
4. Kaisar MH, Bhuiyan MS, Akter A, Saleem D, Iyer AS, Dash P, et al. Vibrio cholerae sialidase-specific immune responses are associated with protection against cholera. Msphere (2021) 6(2):e01232–20. doi: 10.1128/mSphere.01232-20
5. Slack TJ, Li W, Shi D, McArthur JB, Zhao G, Li Y, et al. Triazole-linked transition state analogs as selective inhibitors against v. cholerae sialidase. Bioorganic medicinal Chem (2018) 26(21):5751–7. doi: 10.1016/j.bmc.2018.10.028
6. Corfield AP, Higa H, Paulson JC, Schauer R. The specificity of viral and bacterial sialidases for α (2–3)-and α (2–6)-linked sialic acids in glycoproteins. Biochim Biophys Acta (BBA)-Protein Structure Mol Enzymol (1983) 744(2):121–6. doi: 10.1016/0167-4838(83)90080-8
7. Crennell S, Garman E, Laver G, Vimr E, Taylor G. Crystal structure of vibrio cholerae neuraminidase reveals dual lectin-like domains in addition to the catalytic domain. Structure (1994) 2(6):535–44. doi: 10.1016/S0969-2126(00)00053-8
8. Galen JE, Ketley J, Fasano A, Richardson S, Wasserman S, Kaper J. Role of vibrio cholerae neuraminidase in the function of cholera toxin. Infection Immun (1992) 60(2):406–15. doi: 10.1128/iai.60.2.406-415.1992
9. Kauffman RC, Bhuiyan TR, Nakajima R, Mayo-Smith LM, Rashu R, Hoq MR, et al. Single-cell analysis of the plasmablast response to vibrio cholerae demonstrates expansion of cross-reactive memory b cells. MBio (2016) 7(6):e02021–16. doi: 10.1128/mBio.02021-16
10. Weil AA, Begum Y, Chowdhury F, Khan AI, Leung DT, LaRocque RC, et al. Bacterial shedding in household contacts of cholera patients in Dhaka, Bangladesh. Am J Trop Med Hygiene (2014) 91(4):738. doi: 10.4269/ajtmh.14-0095
11. Harris JB, Podolsky MJ, Bhuiyan TR, Chowdhury F, Khan AI, LaRocque RC, et al. Immunologic responses to vibrio cholerae in patients co-infected with intestinal parasites in Bangladesh. PloS Negl Trop Dis (2009) 3(3):e403. doi: 10.1371/journal.pntd.0000403
12. Alam MM, Bufano MK, Xu P, Kalsy A, Yu Y, Freeman YW, et al. Evaluation in mice of a conjugate vaccine for cholera made from vibrio cholerae O1 (Ogawa) O-specific polysaccharide. PloS Negl Trop Dis (2014) 8(2):e2683. doi: 10.1371/journal.pntd.0002683
13. Qadri F, Wennerås C, Albert MJ, Hossain J, Mannoor K, Begum YA, et al. Comparison of immune responses in patients infected with vibrio cholerae O139 and O1. Infection Immun (1997) 65(9):3571–6. doi: 10.1128/iai.65.9.3571-3576.1997
14. Aktar A, Rahman MA, Afrin S, Faruk MO, Uddin T, Akter A, et al. O-Specific polysaccharide-specific memory b cell responses in young children, older children, and adults infected with vibrio cholerae O1 ogawa in Bangladesh. Clin Vaccine Immunol (2016) 23(5):427–35. doi: 10.1128/CVI.00647-15
15. Qadri F, Ahmed F, Karim MM, Wenneras C, Begum YA, Salam MA, et al. Lipopolysaccharide-and cholera toxin-specific subclass distribution of b-cell responses in cholera. Clin Diagn Lab Immunol (1999) 6(6):812–8. doi: 10.1128/CDLI.6.6.812-818.1999
16. Johnson RA, Uddin T, Aktar A, Mohasin M, Alam MM, Chowdhury F, et al. Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of vibrio cholerae O1 in Bangladeshi adult patients with cholera. Clin Vaccine Immunol (2012) 19(11):1712–21. doi: 10.1128/CVI.00321-12
17. Uddin T, Aktar A, Xu P, Johnson RA, Rahman MA, Leung DT, et al. Immune responses to O-specific polysaccharide and lipopolysaccharide of vibrio cholerae O1 ogawa in adult Bangladeshi recipients of an oral killed cholera vaccine and comparison to responses in patients with cholera. Am J Trop Med Hygiene (2014) 90(5):873. doi: 10.4269/ajtmh.13-0498
18. Jayasekera CR, Harris JB, Bhuiyan S, Chowdhury F, Khan AI, Faruque AS, et al. Cholera toxin-specific memory b cell responses are included in patients with dehydrating diarrhea caused by vibrio cholerae O1. J Infect Dis (2008) 198(7):1055–61. doi: 10.1086/591500
19. Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque AS, et al. Susceptibility to vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PloS Negl Trop Dis (2008) 2(4):e221. doi: 10.1371/journal.pntd.0000221
20. Chowdhury F, Khan AI, Harris JB, LaRocque RC, Chowdhury MI, Ryan ET, et al. A comparison of clinical and immunologic features in children and older patients hospitalized with severe cholera in Bangladesh. Pediatr Infect Dis J (2008) 27(11):986. doi: 10.1097/INF.0b013e3181783adf
21. Chac D, Bhuiyan TR, Saha A, Alam MM, Salma U, Jahan N, et al. Gut microbiota and development of vibrio cholerae-specific long-term memory b cells in adults after whole-cell killed oral cholera vaccine. Infection Immun (2021) 89(9):e00217–21. doi: 10.1128/IAI.00217-21
22. Harris AM, Bhuiyan MS, Chowdhury F, Khan AI, Hossain A, Kendall EA, et al. Antigen-specific memory b-cell responses to vibrio cholerae O1 infection in Bangladesh. Infection Immun (2009) 77(9):3850–6. doi: 10.1128/IAI.00369-09
23. Akter A, Dash P, Aktar A, Jahan SR, Afrin S, Basher SR, et al. Induction of systemic, mucosal and memory antibody responses targeting vibrio cholerae O1 O-specific polysaccharide (OSP) in adults following oral vaccination with an oral killed whole cell cholera vaccine in Bangladesh. PloS Negl Trop Dis (2019) 13(8):e0007634. doi: 10.1371/journal.pntd.0007634
24. Jones FK, Bhuiyan TR, Muise RE, Khan AI, Slater DM, Hutt Vater KR, et al. Identifying recent cholera infections using a multiplex bead serological assay. mBio (2022) 2022:e0190022. doi: 10.1101/2022.06.27.22276845
25. Qadri F, Bhuiyan TR, Sack DA, Svennerholm A-M. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine (2013) 31(3):452–60. doi: 10.1016/j.vaccine.2012.11.012
26. Falkard B, Uddin T, Rahman MA, Franke MF, Aktar A, Uddin MI, et al. Plasma leptin levels in children hospitalized with cholera in Bangladesh. Am J Trop Med Hygiene (2015) 93(2):244. doi: 10.4269/ajtmh.15-0172
27. Leung DT, Chowdhury F, Calderwood SB, Qadri F, Ryan ET. Immune responses to cholera in children. Expert Rev Anti-infective Ther (2012) 10(4):435–44. doi: 10.1586/eri.12.23
28. Hoque SS, Ghosh S, Poxton IR. Differences in intestinal humoral immunity between healthy volunteers from UK and Bangladesh. Eur J Gastroenterol Hepatol (2000) 12(11):1185–93. doi: 10.1097/00042737-200012110-00004
29. Provenzano D, Kováč P, Wade WF. The ABCs (Antibody, b cells, and carbohydrate epitopes) of cholera immunity: Considerations for an improved vaccine. Microbiol Immunol (2006) 50(12):899–927. doi: 10.1111/j.1348-0421.2006.tb03866.x
30. Uddin MI, Hossain M, Islam S, Akter A, Nishat NS, Nila TA, et al. An assessment of potential biomarkers of environment enteropathy and its association with age and microbial infections among children in Bangladesh. PloS One (2021) 16(4):e0250446. doi: 10.1371/journal.pone.0250446
31. Uddin MI, Islam S, Nishat NS, Hossain M, Rafique TA, Rashu R, et al. Biomarkers of environmental enteropathy are positively associated with immune responses to an oral cholera vaccine in Bangladeshi children. PloS Negl Trop Dis (2016) 10(11):e0005039. doi: 10.1371/journal.pntd.0005039
32. Araújo-Jorge, T.C.dThe biology of trypanosoma cruzi-macrophage interaction. Memórias do Instituto Oswaldo Cruz (1989) 84:441–62. doi: 10.1590/S0074-02761989000400001
33. Harn DA, McDonald J, Atochina O, Da’dara AA. Modulation of host immune responses by helminth glycans. Immunol Rev (2009) 230(1):247–57. doi: 10.1111/j.1600-065X.2009.00799.x
Keywords: sialidase, cholera, V. cholerae, immunity, nutrition
Citation: Chowdhury F, Akter A, Bhuiyan TR, Biswas R, Firoj MG, Tauheed I, Harris JB, Larocque RC, Ross AG, McMillan NAJ, Charles RC, Ryan ET, Calderwood SB and Qadri F (2022) Long-term sialidase-specific immune responses after natural infection with cholera: Findings from a longitudinal cohort study in Bangladesh. Front. Immunol. 13:1067737. doi: 10.3389/fimmu.2022.1067737
Received: 12 October 2022; Accepted: 02 December 2022;
Published: 22 December 2022.
Edited by:
Oscar Medina-Contreras, Mexico Children’s Hospital, MexicoReviewed by:
Nicholas J. Mantis, Wadsworth Center, United StatesJayaum S. Booth, University of Maryland, United States
Copyright © 2022 Chowdhury, Akter, Bhuiyan, Biswas, Firoj, Tauheed, Harris, Larocque, Ross, McMillan, Charles, Ryan, Calderwood and Qadri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahima Chowdhury, ZmNob3dkaHVyeUBpY2RkcmIub3Jn
†These authors share senior authorship
 Fahima Chowdhury
Fahima Chowdhury Afroza Akter1
Afroza Akter1 Taufiqur Rahman Bhuiyan
Taufiqur Rahman Bhuiyan Nigel A. J. McMillan
Nigel A. J. McMillan Richelle C. Charles
Richelle C. Charles Edward T. Ryan
Edward T. Ryan Firdausi Qadri
Firdausi Qadri