- 1Department of Hepatology Division 2, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 2Department of Infectious Diseases, Miyun Teaching Hospital, Capital Medical University, Beijing, China
- 3Department of Obstetrics and Gynecology, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Infectious Disease Department, Xuanwu Hospital, Capital Medical University, Beijing, China
- 5Department of Hepatology Division 2, Peking University Ditan Teaching Hospital, Beijing, China
Objective: To explore whether the frequencies and functional molecules expression of Natural Killer cells (NK cells) are related to hepatitis B surface antigen (HBsAg) disappearance in hepatitis B e envelope antigen (HBeAg)-positive patients with chronic hepatitis B (CHB) throughout peginterferon alpha-2a (PEG-IFN α-2a) treatment.
Methods: In this prospective research, HBeAg-positive patients with CHB received PEG-IFN α-2a treatment, completing 4-year follow-up. After PEG-IFN α-2a treatment, undetectable HBV DNA, HBsAg loss, and HBeAg disappearance were defined as functional cure. Proportions of NK, CD56dim, CD56bright, NKp46+, NKp46dim, NKp46high, and interferon alpha receptor 2 (IFNAR2)+ NK cells, and the mean fluorescence intensity (MFI) of NK cell surface receptors IFNAR2 and NKp46 were detected.
Results: 66 patients were enrolled into the study in which 17 patients obtained functional cure. At baseline, hepatitis B virus desoxyribose nucleic acid (HBV DNA) titer in patients with functional cure was remarkably lower than that in Non-functional cure group. Compared with baseline, HBV DNA levels, HBsAg levels, and HBeAg levels significantly declined at week 12 and 24 of therapy in patients with functional cure. At baseline, the negative correlation between CD56bright NK% and HBV DNA and the negative correlation between CD56dim NK% and HBV DNA was showed; CD56bright NK% and IFNAR2 MFI in patients with functional cure were remarkably higher than those in patients without functional cure. After therapy, CD56bright NK% and NKp46high NK% in patients with functional cure were higher than those in patients without functional cure. In Functional cure group, after 24 weeks of treatment NK%, CD56bright NK%, IFNAR2 MFI weakly increased, and NKp46high NK% and NKp46 MFI significantly increased, meanwhile, CD56dim NK% and NKp46dim NK% decreased. Only NKp46 MFI increased after therapy in patients without functional cure.
Conclusion: The lower HBV DNA load and the higher CD56bright NK% before therapy, and the higher the post-treatment CD56bright NK%, IFNAR2 MFI, NKp46high NK%, the easier to achieve functional cure.
Introduction
Hepatits B virus (HBV) infection is still a public health problem worldwide; especially chronic HBV infection which is very harmful to human health (1, 2). Therefore, eliminating hepatitis B has become the topical issue over recent years. Annually, the rate of spontaneous HBsAg clearance is approximately 1% in natural history of chronic HBV infection (3, 4). It is urgent to explore the optimal treatment strategies for CHB. At present, the most effective treatment to delay the progress of CHB is antiviral therapy. However, if we want to enhance living quality and lengthen the survival period to the most extent, “functional cure”, identified as undetectable HBV DNA, persistent HBsAg loss, whether or not accompanied by HBsAg serological conversion (5, 6), is pursued as the treatment end point (7, 8). Currently, by NAs therapy, CHB patients achieve HBsAg loss at a lower rate <1% per year, while HBsAg clearance rate is the highest in CHB patients with HBeAg positivity, who receive IFN therapy (9–12). Therefore, improving the hosts’ immune response through PEG-IFN optimal treatment can more effectively eliminate the virus and even achieve clinical cure (13–15).
As a crucial component in innate immunity, NK cells have a key part in eliminating viruses as well as immunologic defence (16). NK cells consist of CD56bright NK cells and CD56dim NK cells. Generally, CD56bright NK cells have a key role mainly in triggering the specific immune response by secreting immunomodulation cytokines and CD56dim NK cells have a key role in directly eliminating target cells (17, 18). The function of NK cells is induced by an array of inhibiting or active surface receptors (19). NKp46, as the activated receptor on the surface of NK cells, is a key receptor to activate the killing activity of NK cells. IFNAR2 expressed on the surface of NK cells plays an antiviral role when the expression was upregulated and can make IFN-α more easily to activate NK cells, helping to eliminate viruses. IFNAR2 deficiency results in dysregulation of NK cell functions (20). In humans, some studies showed that NK cells were activated in patients with acute HBV infection (21), with the increased expression of activation markers including NKp46, the increased CD56bright NK cells subpopulation, the cytotoxicity and IFN-γ production of NK cells (22–24). However, the activities of NK cells are significantly damaged in CHB patients. HBsAg and HBeAg directly inhibit function of NK cells by multi-signal pathways correlated to the activation of NK cells (25–27). The up-regulation of inhibition receptors and the down-regulation of activation receptors, as the phenotypic characteristics of NK cells, are commonly found in chronic infection, leading to the impairment of effector functions, which may lead to the persistence of viruses (28).
PEG-IFN α increases the inhibing effect of NK cells on regulatory T cells by IFN-γ in CHB (29). After IFN treatment, remarkable increase in expansion and activation of CD56bright NK cells, IFN-γ production, and augmentation of NK cell activating receptors (NKp46 and NKp30) expression are observed (30, 31). After PEG-IFN treatment, the recovery of CD56bright NK cells function and expansion of percentages of NKp46+ and CD56bright NK cells were revealed in inactive HBsAg carriers and CHB patients with NAs therapy (32, 33). The percentage and functional molecular expression of NK cells correlated to HBsAg clearance in treatment-naive HBeAg-positive CHB patients during PEG-IFN α-2a therapy are relatively rare. Thereby, the aim of the research is to probe in treatment-naive CHB patients with HBeAg positivity, whether proportion of NK cells and functional molecules expression on the surface of NK cells are correlated to functional cure throughout PEG-IFN α-2a treatment.
Materials and methods
Subjects
In the nonrandomized, single center, prospective cohort study, 66 HBeAg (+) CHB patients with naïve therapy were enrolled from October 2014 to November 2017 visiting the Liver Disease Center of Beijing Ditan Hospital, Capital Medical University. The inclusion criteria were detectable HBV DNA, HBsAg positive for > 24 weeks, HBeAg positive, and continuously abnormal alanine aminotransferase (ALT) for > 12 weeks, or liver puncture indicating apparent inflammation. Exclusion criteria were the followings (1): co-infected with other viruses (HDV, HIV, or HCV et al) (2); HCV, HIV or syphilis antibodies positive (3); complicated with other liver diseases consisting of fatty liver, hepatic cirrhosis, hepatocellular carcinoma, autoimmune hepatitis, metabolic liver disease, alcoholic liver disease et al (4); use of hormone or immunomodulatory medication. All patients signed written informed consent. Our study was approved by Beijing Ditan Hospital Institutional Review Board and registered at clinicaltrials.gov (ID: NCT03208998).
All patients received individualized treatment and followed up for 4 years. As follows, patients were treated with PEG-IFN α-2a alone, 180 μg subcutaneous injection weekly for 24 weeks. After 24 weeks of treatment, HBV DNA negative patients would continue PEG-IFN α-2a single-drug treatment. However, patients who didn’t achieve HBV DNA negative needed PEG-IFN α-2a combined with tenofovir or entecavir treatment, and specific need for treatment with entecavir or tenofovir depended on previous medical history and health status of those patients. In the follow-up therapy, on the basis of the decrease of HBsAg and HBeAg levels, if HBsAg and HBeAg levels continued to decrease, or even HBsAg clearance, patients were continuely treated with PEG-IFN α-2a consolidation therapy for 12 weeks-24 weeks, then drug withdrawal. Nevertheless, if HBsAg and HBeAg levels didn’t decrease continuously and remained at the platform period, PEG-IFN α-2a needed to be stopped, then the maintenance monotherapy of ETV or TDF would be continued. HBsAg/HBsAb levels, HBeAg/HBeAb levels, HBV DNA titers and ALT were tested before therapy and per 12 weeks after treatment till HBsAg disappeared. Measurements of the frequency and functional molecule expression of NK cells were performed before therapy and at 12 weeks and 24 weeks of therapy.
Functional cure was as follow: persistent HBsAg disappearance, whether or not accompanied by HBsAg serological conversion, HBeAg loss with or without seroconversion, and HBV DNA negative. Conversely, it was defined as non functional cure.
Assessment of clinical parameters
Serum HBsAg/HBsAb and HBeAg/HBeAb levels were quantitated by Abbott Architect i2000 Detection Reagent (Abbott Diagnostics, Abbott Park, IL); HBV DNA titers were detected by Roche Cobas AmpliPrep/Cobas TaqMan 96 full-automatic real-time fluorescent quantitative PCR detection reagent (Roche, Pleasanton, CA); and biochemical indexes were measured by Hitachi 7600 full-automatic biochemical analyzer (Hitachi, Japan). The detection range of HBsAg was 0.05-250 IU/ml, HBeAg ≥ 1 S/CO was positive, and HBeAb<1S/CO was positive. The lower limit of serum HBV DNA detection value was 20 IU/ml. The upper limit of normal ALT value was 40U/L.
Detection of NK cells
Peripheral venous blood of all patients was collected by EDTA anticoagulant violet tube before therapy (baseline), at the 12 and 24 weeks after PEG-IFN therapy. Cell phenotyping stained with four monoclonal Antibodies (mAbs) including Mouse anti-Human-CD3-PerCP (Becton-Dickinson, USA; clone: SP34-2), Mouse anti-Human CD56-APC (Becton-Dickinson, USA clone: B159), anti-Human CD335 (NKp46)-PE (Biolegend, USA; clone: 9E2), and Anti-Human IFNAR2-FITC (Sino Biological, Beijing, China; clone: 07), were analyzed by flow cytometry (FACS Caliburflow Cytometer, USA) within 4 hours after collection, as shown in Supplementary Figure 1. The proportions of NK, CD56dim and CD56bright, NKp46+, and IFNAR2+ NK cells, and MFI of NKp46 and IFNAR2 were analyzed by FlowJo 7.6.1 software. Expression of surface receptors NKp46 and IFNAR2 on NK cells represented NK cell function.
Statistical methods
In the research, statistical data were processed by SPSS 26 (SPSS Inc., Chicago, USA). continuous variables were represented by mean ± standard deviation or median (Q1, Q3). Repeated-measures analysis of variance was used for changes of the indexes at three observation time points, and p values for multiplicity were ajusted by Bonferroni, and p < 0.016 was considered as statistical significance. Independent sample t-test was used to analyze normal distribution data and Mann-Whitney nonparametric U test was used to analyze skewness distribution data between two groups. Spearman’s correlation was applied for the association of variables. We used binary logistic regression analysis to analyze independent factors of functional cure. P < 0.05 was considered to be statistically significant.
Results
Baseline features of various indicators in patients with CHB
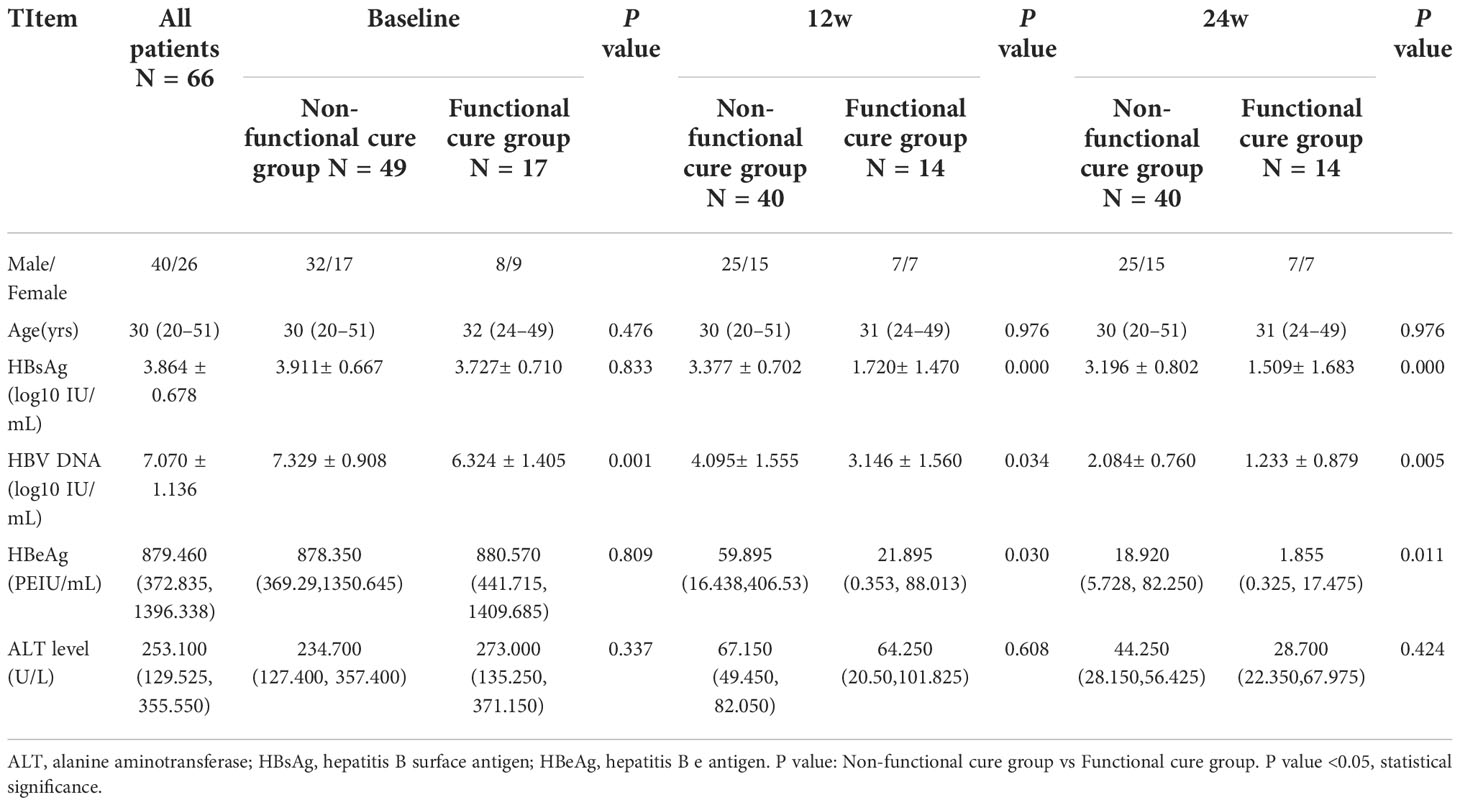
In our research, 66 HBeAg (+) patients with CHB received PEG-IFN-α-2a individually, following up for 4 years, lincluding 40 males and 26 females. Patients with undetectable HBV DNA and HBsAg disappearance were considered as functional cure, on the contrary, as non functional cure, with 17 cases obtaining functional cure. Among them, 3 patients with HBsAg clearance lost the detection of the frequency and functional molecules expression of NK cells at 12 weeks or 24 weeks of therapy, and 9 patients without HBsAg clearance lost the detection of the frequency and functional molecules expression of NK cells at 12 weeks or 24 weeks of therapy.
The baseline serological virological indicators of the two groups were compared. We found that the serum HBsAg, HBeAg and ALT had no remarkable statistical differences, respectively, between two groups (Table 1), but the HBV DNA titers were lower in functional cure group than those in non functional cure group (P = 0.001) in Table 1. As shown in Table 1, at 12 weeks and 24 weeks of therapy, HBV DNA (P = 0.034(12w), P = 0.005(24w)), HBsAg (P = 0.000(12w), P = 0.000(24w)), as well as HBeAg (P = 0.030(12w), P = 0.011(24w)) in patients without functional cure were remarkebly higher than those in patients with functional cure. Before therapy, CD56bright NK/NK% (P=0.000), CD56dim NK/NK% (P=0.000) and IFNAR2 MFI (P=0.043) had significant statistical differences between functional cure group and non functional cure group (Table 2).
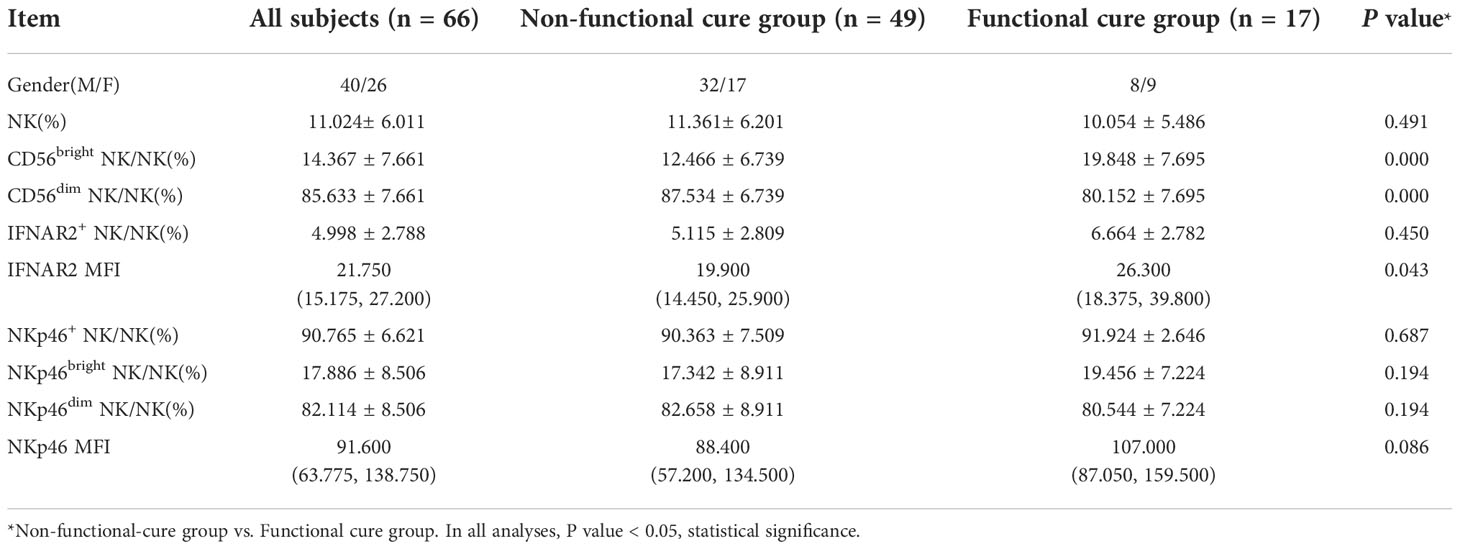
Table 2 and surface receptors NKp46 and INFAR2 expression of NK cells at baseline between functional cure group and non-functional cure group.
We used Binary logistic regression analysis to analyze baseline clinical indicators, proportions and and functional molecules expression of NK cells in two groups, but no independent influencing factors of functional cure were observed.
Correlation of the parameters of NK cells with virological indicators and ALT at baseline
At baseline, we performed the correlation analysis on the frequency of NK cells, the expression of surface functional molecules NKp46, INFAR2 and HBV virological characteristics. We found that there were no correlations between NK cell frequency or function (NK%, IFNAR2+ NK/NK%, and IFNAR2 MFI) and serological and virological indicators (HBsAg, HBeAg, and HBV DNA, ALT). (Figures 1A-F, H-J, L-T) And there were no correlations between the expression of surface functional molecules NKp46 and virological indicators.. The negative correlation between CD56bright NK% and HBV DNA (r=-0.243, P = 0.049) (Figure 1G) and the negative correlation between CD56dim NK% and HBV DNA (r=0.243, P = 0.049) (Figure 1K) was observed.
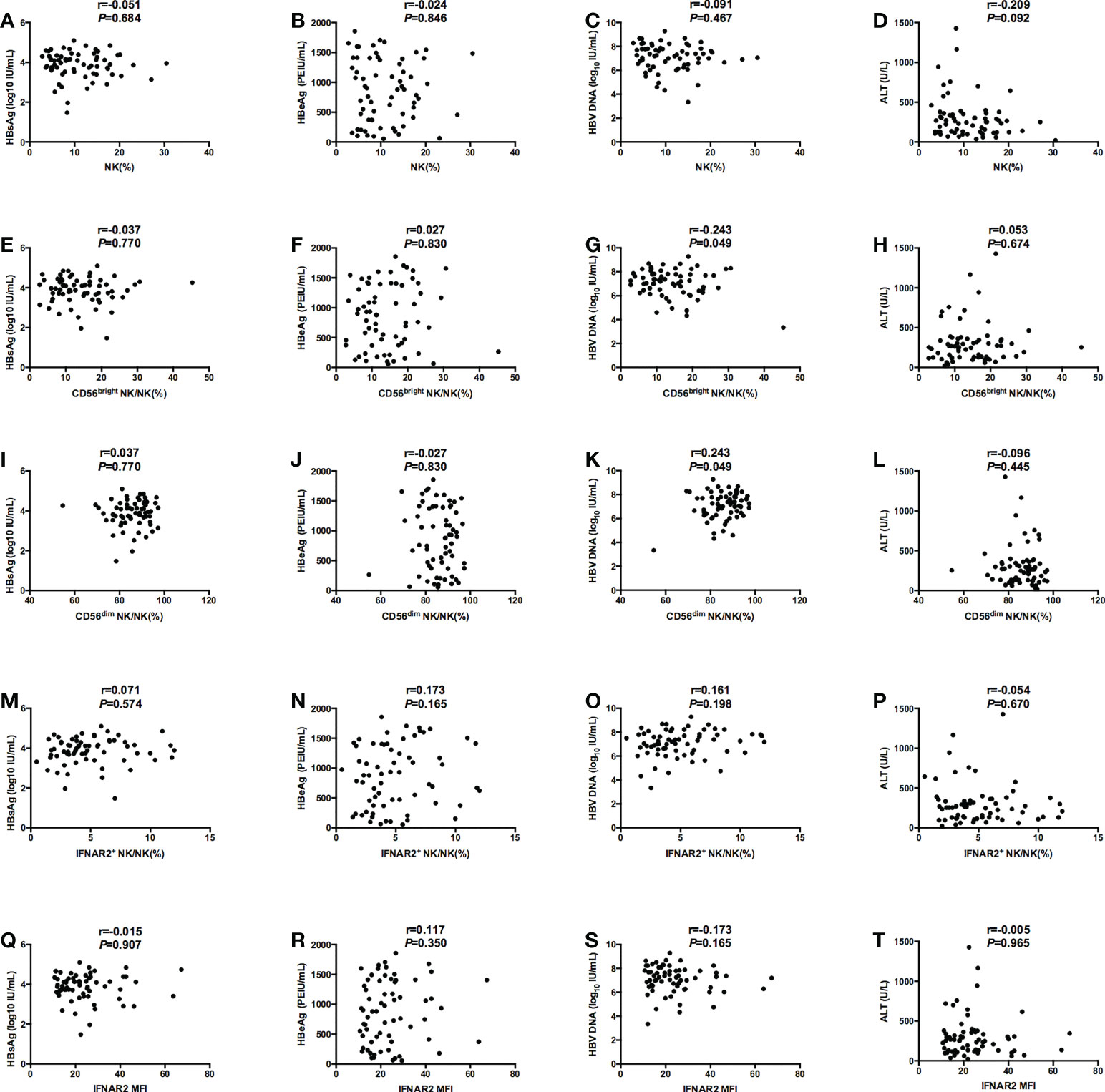
Figure 1 Correlations between NK cell frequency or function (NK%, CD56bright NK/NK%, CD56dim NK/NK%, IFNAR2+ NK/NK%, and IFNAR2 MFI) and serological and virological indicators (HBsAg, HBeAg, and HBV DNA, ALT). (A-D) Correlations between NK% and serological and virological indicators (HBsAg (A), HBeAg (B), and HBV DNA (C), ALT (D)). (E-H) Correlations between CD56bright NK/NK% and serological and virological indicators (HBsAg (E), HBeAg (F), and HBV DNA (G), ALT (H)). (I-L) Correlations between CD56dim NK/NK% and serological and virological indicators (HBsAg (I), HBeAg (J), and HBV DNA (K), ALT (L)). (M-P) Correlations between IFNAR2+ NK/NK% and serological and virological indicators (HBsAg (M), HBeAg (N), and HBV DNA (O), ALT (P)). (Q-T) Correlations between IFNAR2 MFI and serological and virological indicators (HBsAg (Q), HBeAg (R), and HBV DNA (S), ALT (T)). P < 0.05 was considered to be statistically significant.
NK cells function and CHB function cure
The baseline P values in Table 2 and Figure 2 were different, because Table 2 showed the total number of patients in the functional cure group and the non functional cure group, and at 12 and 24 weeks of treatment, 3 patients in the functional cure group and 9 patients in the non functional cure group were lost to follow-up. Figure 2 showed the statistical analysis after excluding the lost patients. At baseline and at 12 weeks and 24 weeks of therapy, CD56bright NK/NK% (P = 0.001;(0w) P = 0.012 (12w); P = 0.001(24w)), NKp46high NK/NK% (P = 0.048(0w); P = 0.004(12w); P = 0.004 (24w)) in functional cure group were markedly higher than those in non functional cure group. CD56dim NK/NK% (P = 0.001(0w); P = 0.012(12w); P = 0.0001 (24w)), NKp46dim NK/NK% (P = 0.048(0w); P = 0.004(12w); P = 0.004(24w)) in functional cure group were markbly lower than those in non functional cure group in Figure 2.
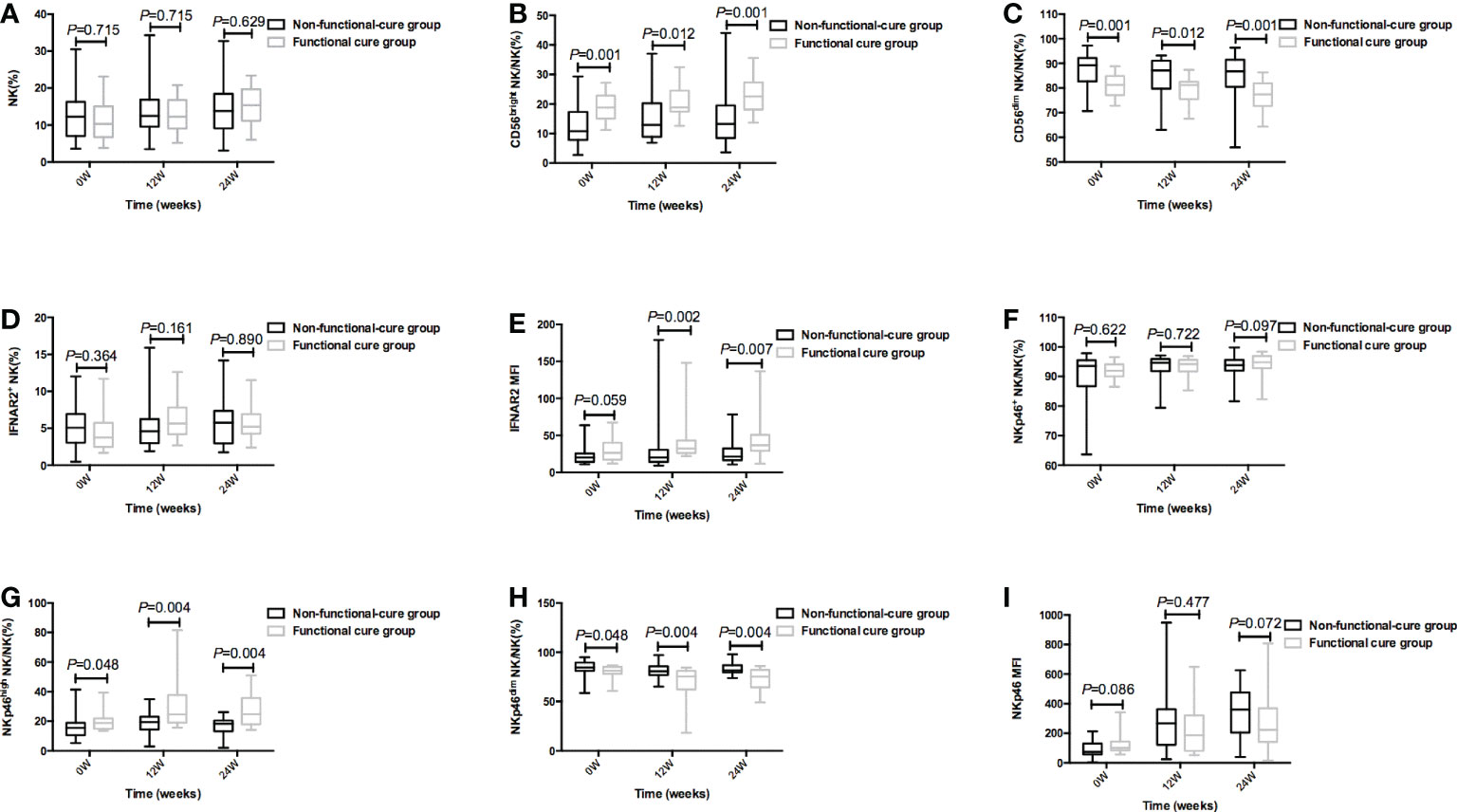
Figure 2 During PEG-IFNα-2a therapy, the frequency of NK cells and surface functional molecules [NK% (A), CD56bright NK/NK% (B), CD56dim NK/NK% (C), IFNAR2+ NK/NK% (D), IFNAR2 MFI (E), NKp46 NK/NK% (F), NKp46high NK/NK% (G), NKp46dim NK/NK% (H), and NKp46MFI (I)] between Non-functional-cure group and Functional cure group were compared before therapy and at 12 weeks and 24 weeks of therapy, respectively. P<0.05 was statistically significant.
In Figure 3, in functional cure group, our results showed NK%, CD56bright NK/NK% and IFNAR2 MFI weakly increased after 24 weeks of treatment, CD56dim NK/NK% decreased after treatment. However, p values for multiplicity were ajusted by Bonferroni, so p>0.016 has no significant statistical significance.
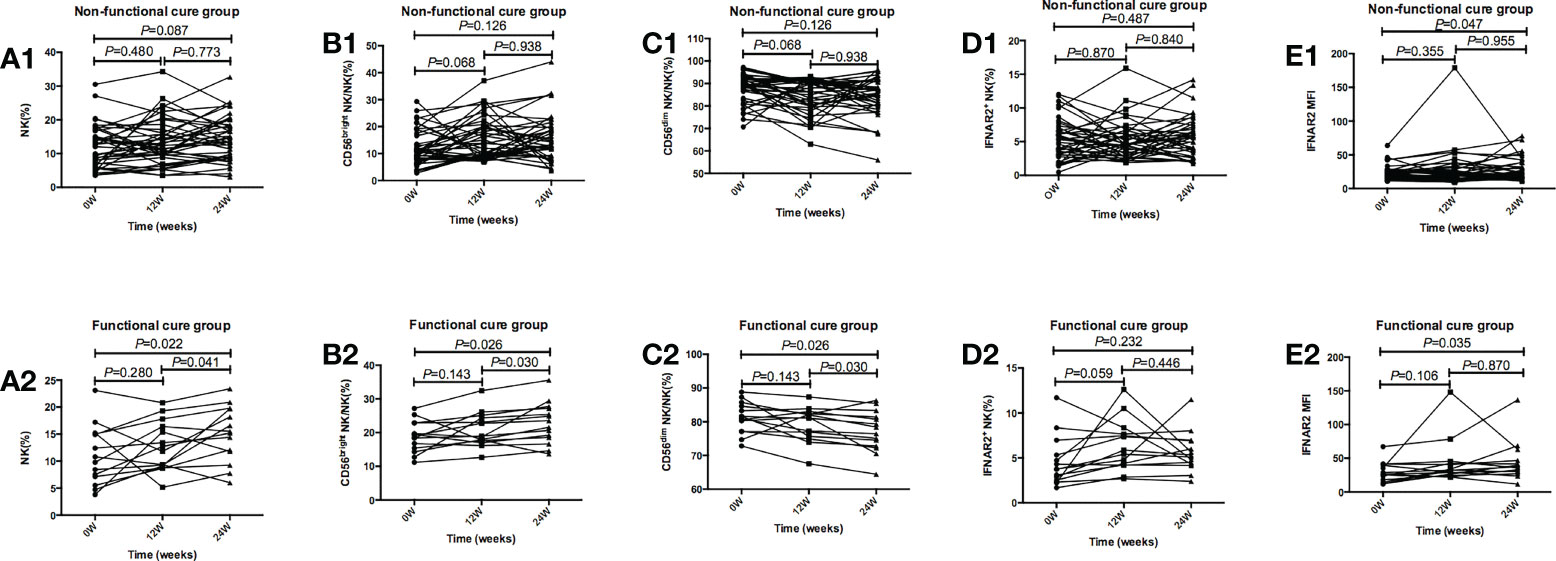
Figure 3 Tendencies of NK cells frequency and function, including NK%, CD56bright NK/NK%, CD56dim NK/NK%, IFNAR2+ NK/NK%, and IFNAR2 MFI at baseline and after 12 and 24 weeks of treatment in the Functional cure group and Non-functional-cure group. (A1-E1) Tendencies of NK% (A1), CD56bright NK/NK% (B1), CD56dim NK/NK% (C1), IFNAR2+ NK/NK% (D1), and IFNAR2 MFI (E1) at baseline and after 12 and 24 weeks of treatment in the Non-functional-cure group. (A2-E2) Tendencies of NK%(A2), CD56bright NK/NK% (B2), CD56dim NK/NK% (C2), IFNAR2+ NK/NK% (D2), and IFNAR2 MFI (E2) at baseline and after 12 and 24 weeks of treatment in the Functional cure group. p values for multiplicity were ajusted by Bonferroni, and P < 0.016 was dentifified as signifificant statistical difference.
As shown in Figure 4, in functional cure group, NKp46high NK/NK% after 12 weeks of treatment was dramatically higher than that at baseline (P=0.005), NKp46dim NK/NK% after 12 weeks of treatment was markedly lower than that before therapy (P=0.005), and NKp46 MFI after 24 weeks of treatment (P=0.005) was dramatically higher than that before treatment. In non functional cure group, NKp46 MFI was significantly higher after treatment (P=0.000) than that before treatment.
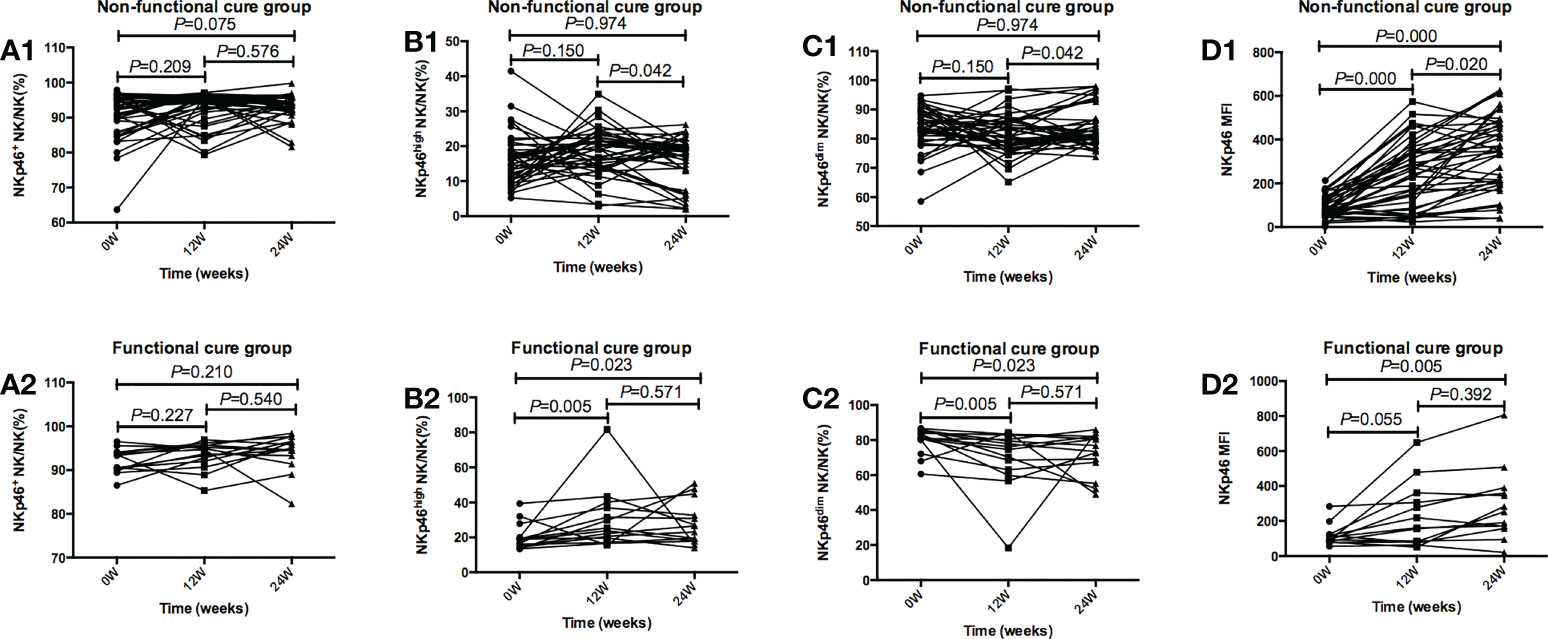
Figure 4 Tendencies of NK cells frequency and function, including NKp46 NK/NK%, NKp46high NK/NK%, NKp46dim NK/NK%, and NKp46MFI at baseline and after 12 and 24 weeks of treatment in the Functional cure group and Non-functional-cure group. (A1-D1) Tendencies of NKp46 NK/NK% (A1), NKp46high NK/NK%(B1), NKp46dim NK/NK% (C1), and NKp46MFI (D1) at baseline and after 12 and 24 weeks of treatment in the Non-functional-cure group. (A2-D2) Tendencies of NKp46 NK/NK% (A2), NKp46high NK/NK% (B2), NKp46dim NK/NK% (C2), and NKp46MFI (D2) at baseline and after 12 and 24 weeks of treatment in the Functional cure group. p values for multiplicity were ajusted by Bonferroni, and P < 0.016 was dentifified as signifificant statistical difference.
Discussion
NK cells, as the main components of innate immunity, play a crucial part in viral elimination, meanwhile, they participate in the process of liver tissue damage (34). CD56bright and CD56dim NK cells are the subsets of NK cells. The main function of CD56bright NK cells is to secrete cytokines, including TNF-α, IFN-γ and others, and play an antiviral role through non cytotoxic effects. CD56dim NK cells kill viral infected liver cells through cytotoxic effects mediated by granzyme and perforin (35). NKp46 is an activated receptor on NK cell surface. In acute hepatitis B infection, expression of activiting receptor NKp46 is up-regulated, which resulted in enhancement of NK cell activity (23); The surface of NK cells also expresses the receptor IFNAR2, and the up regulation of IFNAR2 expression can make IFN-a more easier to activate NK cells, play an antiviral role, and help to eliminate viruses. However, several studies have reported that in chronic HBV infection, dyfunction of NK cells is revealed, which is not conducive to the elimination of HBV (36). Therefore, we need to choose the optimal treatment to restore and even enhance the immune cell function, so as to enhance the hosts’ immune response to the viruses, which may lead to HBsAg loss and achieve functional cure. In a variety of treatment methods for CHB, PEG-IFN has antiviral role and immunomodulatory effect. Previous studies have shown that PEG-IFN therapy can enhance function of NK cells and facilitate virus clearance (13, 31–33). NK cells were the important part of innate immunity. Interferon triggered innate immunity, which took effect at 12 weeks of treatment and peaks at 24 weeks. The early to mid-term efficacy of interferon on natural killer cell induction determined the subsequent efficacy, so the changes in the charateristics of NK cells at 12 and 24 weeks of treatment were tested (25). In our research, we explored in CHB patients with HBeAg positivity, whether proportions and function of NK cells were related to functional cure throughout IFN treatment.
Our results demenstrated that HBV DNA titers in patients without HBsAg clearance were significantly higher than those in patients with HBsAg clearance, meanwhile, HBV DNA, HBsAg, and HBeAg dramatically declined during IFN therapy in patients with functional cure, which correspond to previous studies demonstrating the lower HBV DNA, the lower transcription and replication activities of HBV DNA, which leads to the decrease of viral protein production and even the reduction of cccDNA (37), the easier to achieve HBsAg loss and even functional cure.
Previous studies suggested that HBsAg and HBeAg could inhibit the function of NK cells resulting in insufficient immune response without the virus clearance (25–27). Negative correlation between HBsAg level and percentage of CD56bright NK cells was found in previous study (31). Even though we didn’t observe the correlation between CD56bright NK cells and HBsAg, our result demenstrated that the baseline CD56bright NK was negatively correlated with HBV DNA. Tjwa ET et al. found that the reduction of viral load improved the function of NK cells in chronic HBV infected patients (38). Some studies suggested that the decrease of viral load achieved through antiviral treatment partially reshaped phenotype and function of NK cells (13, 17), which indirectly indicated that HBV DNA had an inhibiting role on NK cells. In our study, we found that HBV DNA before therapy in patients with functional cure was dramatically lower than that in patients without non functional cure, as well as CD56bright NK increased after PEG-IFN therapy. Therefore, our results were consistent with the above conclusions. Our study suggested that the baseline CD56bright NK% at baseline and post-treatment in the functional cure group were higher than those in the non-functional cure group, meanwhile, CD56bright NK% increased, therefore, the higher baseline CD56bright NK%, the more likely to obtain functional cure. After PEG-IFN treatment, the immunity was up-regulated, and the immune response in the functional cure group was better,which made CD56bright NK increase. Thus, patients with high baseline level and good immune response after treatment were more likely to achieve functional cure. Our data also showed that CD56bright NK was negatively correlated with HBV DNA at baseline and significantly higher in patients with functional cure than those without functional cure, meanwhile it also increased in the former goup after PEG-IFN therapy, which were in line with previous studies indicating that the ability of CD56bright NK cells to secrete IFN-γ was significantly enhanced during HBeAg seroconversion, which proved the importance of IFN-γ secreted by CD56bright NK cells in controlling virus replication (39). Not only did IFN-α have direct antiviral role, but also made the up-regulation of TRAIL expression on ths surface of NK cells, activated CD56bright NK cells, and increased IFN-γ secretion (40). Activated CD56bright NK cells may be conducive to lead to HBsAg decrease or HBsAg disappearance and even obtain functional cure.
Receptors NKp46 and IFNAR2 on NK cell surface play crucial effects in chronic HBV infection. Type I interferon receptor (IFNAR) has been involved in the progression of CHB. IFNAR2 plays a vital part in initiation of type I interferon-induced JAK-STAT signaling and activation of STATs (41). IFNAR2 deficiency results in dysregulation of the function of NK cells (20). Our data showed that after PEG-IFN treatment, the expression of reporters IFNAR2 on the surface of NK cells in patients with functional cure significantly increased, which suggested that the up-regulation of IFNAR2 made NK cells more easily activated by IFN-α and contributed to clearing HBV. NKp46, as the activating receptor of NK cells, may regulate the activity of NK cells in the suppression of HBV replication and HBV-related liver injury, meanwhile, it is crucial for the activation of NK cells in chronic HBV infection (42). Our resluts showed that after therapy, the frequency of NKp46high NK cells and surface reporters IFNAR2 and NKp46 expression on NK cells in patients with HBsAg loss significantly increased, which was consist with some studies suggesting that in patient with CHB, the expression of IFNAR2 and NKp46 on the surface of NK cells elevated after PEG-IFN therapy (32, 33), NKp46high NK cells may be closely correlated to HBV clearance, and up-regulated expression of NKp46high is beneficial to the clearance of HBV.
In conclusion, this study adds to our knowledge of the relevance of functional cure and frequency and function of NK cells in CHB patients with PEG-IFN α-2a treatment. Our results demonstrate the lower HBV DNA before therapy, the higher CD56bright NK% at baseline, and the higher CD56bright NK%, IFNAR2 MFI, NKp46high NK% after therapy, the easier to achieve HBsAg clearance. Functional cure is dependent on low HBV DNA titer, the recovery and enhancment of NK cells function at the early phase of PEG-IFN α-2a therapy. These conclusions may be conducive to idnetify those easier to obtain functional cure and optimizing therapeutic strategies for CHB to pursue functional cure.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Review Committee of Beijing Ditan Hospital of Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
WC, YX, and ML made contribution to conception and design of the study. HL, LuxZ, SWang, WD, TJ, YLin, LY, GS, XB, YLu, LuZ, RL, MC, SWu, YG, MX, HH, LH, and XC organized the data. WC, YX, and ML guided statistical analysis. WC wrote the first manuscript. HL, LuxZ, SWang, WD, YX, and ML edited and modified the English manuscript. ML submitted the modified version. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by Beijing Hospitals Authority Clinical medicine Development of special funding support (XMLX 202127); The capital health research and development of special (2022–1–2172); National Science and Technology Major Project of China (2017ZX10201201-001-006, 2017ZX10201201-002-006, 2018ZX10715-005-003-005); High-level Public Health Technical Personnel Training Program of Beijing Municipal Health Commission (2022–3–050); The Digestive Medical Coordinated Development Center of Beijing Hospitals Authority (XXZ0302 and XXT28); Project supported by Beijing science and technology commission (Z211100002921059).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SR declared a shared parent affiliation with the authors to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1067362/full#supplementary-material
Supplementary Figure 1 | Using FlowJo software for CD3-CD56+NK cell image analysis to illustrative dot plots related to the FACS data.
References
1. European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis b virus infection. J Hepatol (2017) 67(2):370–98. doi: 10.1016/j.jhep.2017.03.021
2. World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, , 2021: Accountability for the global health sector strategies 2016–2021: Actions for impact. Geneva: World Health Organization (2021). Available at: https://apps.who.int/iris/handle/10665/341412. Licence: CC BY-NC-SA 3.0 IGO.
3. Chu CM, Liaw YF. Spontaneous relapse of hepatitis in inactive HBsAg carriers. Hepatol Int (2007) 1:311−5. doi: 10.1007/s12072−007−9002−9
4. Simonetti J, Bulkow L, McMahon BJ, Homan C, Snowball M, Negus S, et al. Clearance of hepatitis b surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis b virus. Hepatology (2010) 51(5):1531–7. doi: 10.1002/hep.23464
5. Lopatin U. Drugs in the pipeline for HBV. Clin Liver Dis (2019) 23:535–55. doi: 10.1016/j.cld.2019.04.006
6. Zhao Q, Guo J. Have the starting lineup of five for hepatitis b virus covalently closed circular DNA synthesis been identified? Hepatology (2020) 72:1142–44. doi: 10.1002/hep.31408
7. Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, et al. A global scientific strategy to cure hepatitis b. Lancet Gastroenterol Hepatol (2019) 4:545–58. doi: 10.1016/S2468-1253(19)30119-0
8. Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis b cure: from discovery to regulatory approval. J Hepatol (2017) 67:847–61. doi: 10.1016/j.jhep.2017.05.008
9. Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, et al. Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis b surface antigen in patients with chronic hepatitis b. Gastroenterology (2016) 150(1):134–44.e10. doi: 10.1053/j.gastro.2015.09.043
10. Lok AS, Zoulim F, Dusheiko G, Chan HL, Buti M, Ghany MG, et al. Durability of hepatitis b surface antigen loss with nucleotide analogue and peginterferon therapy in patients with chronic hepatitis b. Hepatol Commun (2019) 4(1):8–20. doi: 10.1002/hep4.1436
11. Alawad AS, Auh S, Suarez D, Ghany MG. Durability of spontaneous and treatment-related loss of hepatitis b s antigen. Clin Gastroenterol Hepatol (2020) 18(3):700–709.e3. doi: 10.1016/j.cgh.2019.07.018
12. Qiu K, Liu B, Li SY, Li H, Chen ZW, Luo AR, et al. Systematic review with meta-analysis: combination treatment of regimens based on pegylated interferon for chronic hepatitis b focusing on hepatitis b surface antigen clearance. Aliment Pharmacol Ther (2018) 47:1340–8. doi: 10.1111/apt.14629
13. Cao W, Li M, Zhang L, Lu Y, Wu S, Shen G, et al. The characteristics of natural killer cells in chronic hepatitis b patients who received PEGylated-interferon versus entecavir therapy. BioMed Res Int (2021) 2021:2178143. doi: 10.1155/2021/2178143
14. Cao W, Xie S, Zhang L, Bi X, Lin Y, Yang L, et al. Expression of functional molecule on plasmacytoid dendritic cells is associated with HBsAg loss in HBeAg-positive patients during PEG-IFN α-2a treatment. Front Immunol (2022) 13:891424. doi: 10.3389/fimmu.2022.891424
15. Zimmer CL, Rinker F, Höner zu Siederdissen C, Manns MP, Wedemeyer H, Cornberg M, et al. Increased NK cell function after cessation of long-term nucleos(t)ide analogue treatment in chronic hepatitis b is associated with liver damage and HBsAg loss. J Infect Dis (2018) 217(10):1656–66. doi: 10.1093/infdis/jiy097
16. Zheng Q, Zhu YY, Chen J, Ye YB, Li JY, Liu YR, et al. Activated natural killer cells accelerate liver damage in patients with chronic hepatitis b virus infection. Clin Exp Immunol (2015) 180(3):499–508. doi: 10.1111/cei.12597
17. Wu SF, Wang WJ and Gao YQ. Natural killer cells in hepatitis b virus infection. Braz J Infect Dis 19 (2015) 19(4):417–25. doi: 10.1016/j.bjid.2015.05.006
18. Del Zotto G, Marcenaro E, Vacca P, Sivori S, Pende D, Della Chiesa M, et al. Markers and function of human NK cells in normal and pathological conditions. Cytom Part B Clin Cytom (2017) 92(2):100–14. doi: 10.1002/cyto.b.21508
19. Sivori S, Vacca P, Munari E, Mingari MC, Moretta L. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol (2019) 16(5):430–41. doi: 10.1038/s41423-019-0206-4
20. Passarelli C, Civino A, Rossi MN, Cifaldi L, Lanari V, Moneta GM, et al. IFNAR2 deficiency causing dysregulation of NK cell functions and presenting with hemophagocytic lymphohistiocytosis. Front Genet (2020) 11:937. doi: 10.3389/fgene.2020.00937
21. Webster G, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, et al. Incubation phase of acute hepatitis b in man: dynamic of cellular immune mechanisms. Hepatology (2000) 32(5):1117–24. doi: 10.1053/jhep.2000.19324
22. Lunemann S, Malone DFG, Hengst J, Port K, Grabowski J, Deterding K, et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis (2014) 209:1362–73. doi: 10.1093/infdis/jit561
23. Zhao J, Li Y, Jin L, Zhang S, Fan R, Sun Y, et al. Natural killer cells are characterized by the concomitantly increased interferon-γ and cytotoxicity in acute resolved hepatitis b patients. PLoS One (2012) 7:e49135. doi: 10.1371/journal.pone.0049135
24. Yu WH, Cosgrove C, Berger CT, Cheney PC, Krykbaeva M, Kim AY, et al. ADCC-mediated CD56dim NK cell responses are associated with early HBsAg clearance in acute HBV infection. Pathog Immun (2018) 3:2–18. doi: 10.20411/pai.v3i1.228
25. Yang Y, Han Q, Zhang C, Xiao M, Zhang J. Hepatitis b virus antigens impair NK cell function. Int Immunopharmacol (2016) 38:291–7. doi: 10.1016/j.intimp.2016.06.015
26. Du Y, Anastasiou OE, Strunz B, Scheuten J, Bremer B, Kraft A, et al. The impact of hepatitis b surface antigen on natural killer cells in patients with chronic hepatitis b virus infection. Liver Int (2021) 41(9):2046–58. doi: 10.1111/liv.14885
27. Zheng B, Yu Y, Pan Z, Feng Y, Zhao H, Han Q, et al. HBsAg dampened STING associated activation of NK cells in HBeAg-negative CHB patients. Int J Mol Sci (2021) 22(14):7643. doi: 10.3390/ijms22147643
29. Yuan W, Huang D, Wu D, Chen Y, Ma K, Han M, et al. Pegylated interferon-α (IFN-α) enhances the inhibitory effect of natural killer cells on regulatory T cells via IFN-γ in chronic hepatitis b. J Infect Dis (2021) 224(11):1878–89. doi: 10.1093/infdis/jiab216
30. Bruder Costa J, Dufeu-Duchesne T, Leroy V, Bertucci I, Bouvier-Alias M, Pouget N, et al. Pegylated interferon α-2a triggers NK-cell functionality and specific T-cell responses in patients with chronic HBV infection without HBsAg seroconversion. PLoS One (2016) 11:e0158297. doi: 10.1371/journal.pone.0158297
31. Gill US, Peppa D, Micco L, Singh HD, Carey I, Foster GR, et al. Interferon alpha induces sustained changes in NK cell responsiveness to hepatitis b viral load suppression. in Vivo. PLoS Pathog (2016) 12:e1005788. doi: 10.1371/journal.ppat.1005788
32. Shi A, Zhang X, Xiao F, Zhu L, Yan W, Han M, et al. CD56bright natural killer cells induce HBsAg reduction via cytolysis and cccDNA decay in long-term entecavir-treated patients switching to peginterferon alfa-2a. J Viral Hepat (2018) 25(11):1352–62. doi: 10.1111/jvh.12946
33. Cao Z, Meng S, Zheng Y, Wang J, Wang R, Chen X. Contribution of NK cells to HBsAg seroconversion in inactive HBsAg carriers following pegylated IFN therapy. Innate Immun (2020) 26(7):601–8. doi: 10.1177/1753425920942580
34. Rehermann B. Natural killer cells in viral hepatitis. Cell Mol Gastroentrol Hepatol (2015) 1(6):578–88. doi: 10.1016/j.jcmgh.2015.09.004
35. Mondelli MU, Varchetta S, Oliviero B. Natural killer cells in viral hepatitis: facts and controversies. Eur J Clin Invest (2010) 40(9):851–63. doi: 10.1111/j.1365-2362.2010.02332.x
36. Li X, Zhou L, Gu L, Gu Y, Chen L, Lian Y, et al. Veritable antiviral capacity of natural killer cells in chronic HBV infection: an argument for an earlier anti-virus treatment. J Transl Med (2017) 15(1):220. doi: 10.1186/s12967-017-1318-1
37. Wang Q, Luan W, Warren L, Fiel MI, Blank S, Kadri H, et al. Serum hepatitis b surface antigen correlates with tissue covalently closed circular DNA in patients with hepatitis b-associated hepatocellular carcinoma. J Med Virol (2016) 88(2):244–51. doi: 10.1002/jmv.24326
38. Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis b. J Hepatol (2010) 54(2):209–218. doi: 10.1016/j.jhep.2010.07.009
39. Groen RA, Hou J, van Oord GW, Groothuismink ZMA, van der Heide M, de Knegt RJ, et al. NK cell phenotypic and functional shifts coincide with specific clinical phases in the natural history of chronic HBV infection. Antiviral Res (2017) 140:18–24. doi: 10.1016/j.antiviral.2017.01.00
40. Brunetto MR, Bonino F. Interferon therapy of chronic hepatitis b. Intervirology (2014) 57(3-4):163–70. doi: 10.1159/000360941
41. Shemesh M, Lochte S, Piehler J, Schreiber G. IFNAR1 and IFNAR2 play distinct roles in initiating type I interferon-induced JAK-STAT signaling and activating STATs. Sci Signal (2021) 14(710):eabe4627. doi: 10.1126/scisignal.abe4627
Keywords: nature killer cells, chronic hepatitis B, PEG-IFN α-2a, hepatitis B virus, HBsAg loss
Citation: Cao W, Lu H, Zhang L, Wang S, Deng W, Jiang T, Lin Y, Yang L, Bi X, Lu Y, Zhang L, Shen G, Liu R, Chang M, Wu S, Gao Y, Hao H, Xu M, Chen X, Hu L, Xie Y and Li M (2022) Functional molecular expression of nature killer cells correlated to HBsAg clearance in HBeAg-positive chronic hepatitis B patients during PEG-IFN α-2a therapy. Front. Immunol. 13:1067362. doi: 10.3389/fimmu.2022.1067362
Received: 11 October 2022; Accepted: 02 November 2022;
Published: 21 November 2022.
Edited by:
Siqing Fu, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
QiaoXia Tong, Union Hospital TongJi Medical College HuaZhong University of Science and Technology, ChinaShan Ren, Beijing Youan Hospital, Capital Medical University, China
Copyright © 2022 Cao, Lu, Zhang, Wang, Deng, Jiang, Lin, Yang, Bi, Lu, Zhang, Shen, Liu, Chang, Wu, Gao, Hao, Xu, Chen, Hu, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minghui Li, d3VobTIwMDBAc2luYS5jb20=; Yao Xie, eGlleWFvMDAxMjAxODRAc2luYS5jb20=
†These authors have contributed equally to this work
 Weihua Cao
Weihua Cao Huihui Lu
Huihui Lu Luxue Zhang
Luxue Zhang Shiyu Wang1†
Shiyu Wang1† Tingting Jiang
Tingting Jiang Yanjie Lin
Yanjie Lin Liu Yang
Liu Yang Xiaoyue Bi
Xiaoyue Bi Lu Zhang
Lu Zhang Shuling Wu
Shuling Wu Mengjiao Xu
Mengjiao Xu Yao Xie
Yao Xie Minghui Li
Minghui Li