- 1Children’s Healthcare of Atlanta/Aflac Cancer and Blood Disorders Center, Emory University, Atlanta, GA, United States
- 2Department of Pediatrics, Emory School of Medicine, Columbus, OH, United States
- 3Hematopoietic Cell Transplantation, Ohio State University, Columbus, OH, United States
Transplant-associated thrombotic microangiopathy (TA-TMA) is an increasingly recognized complication of allogeneic and autologous hematopoietic cellular therapy (HCT), associated with significant morbidity and mortality. Although the central drivers of the disease are thought to be endothelial damage and complement activation, no specific diagnostic biomarkers have been identified. TA-TMA is typically diagnosed using criteria comprised of non-specific clinical and laboratory features. Some patients will have a self-remitting course, but more than half develop multi-organ dysfunction or die, making prognostic biomarkers critical. Prevention of TA-TMA, an approach central to other HCT complications such as graft-versus-host disease, is largely untested in part due to a lack of identified early high-risk biomarkers. We conducted a systematic review to summarize the diagnostic, early risk, and prognostic biomarkers of TA-TMA. We screened the titles and abstracts of 1524 citations. After screening out duplications, we read the abstracts of 979 papers and fully reviewed 132 full-text publications. Thirty-one publications fulfilled the inclusion criteria of more than five patients with TA-TMA and a reported measure of association with diagnosis, prognosis, or risk of later development of the disease. Fourteen studies (45%) were with adults, 12 (39%) were with children <18 years old, three included both children and adults, and two did not report age. There were 53 biomarker or biomarker signature entries, and a total of 27 unique biomarkers. Only four biomarkers reported sensitivity and specificity. The single biomarker with the most robust data was sC5b-9, which conferred diagnostic, prognostic, and risk implications. Studies of combinations of biomarkers were rare. No meta-analyses were performed because of significant heterogeneity between studies. The limitations of studies included small sample size, study designs with a high risk of bias (i.e., case–control), the timing of sample collection, and the selection of controls. Furthermore, only two (6%) studies included a training and validation cohort. Cut-off points are needed to stratify groups, as most biomarkers do not have normal values, or normal values cannot be assumed in the HCT setting. In the future, multi-institutional, collaborative efforts are needed to perform rigorously designed, prospective studies with serially enrolled patients, with samples collected at the time of TA-TMA diagnosis, careful selection of controls, and validation of selected biomarkers and cut-off points in a separate cohort.
Introduction
Transplant-associated thrombotic microangiopathy (TA-TMA) is an increasingly recognized complication thought to complicate ~15%–30% of allogeneic hematopoietic cell transplantation (HCT) recipients. TA-TMA also occurs after autologous HCT, particularly in children with neuroblastoma (1–3). Multiple hits to the endothelium results in a pro-apoptotic, pro-inflammatory, and pro-thrombotic milieu and inappropriate complement activation resulting in further endothelial damage (4–7). Risk factors for TA-TMA include inherent features of underlying HCT diagnosis, age, sex, prior endothelial injury or complement genetic variations; donor-related complement genetic variations; transplant characteristics including HLA mismatch, myeloablative conditioning, radiation, and graft-versus-host disease (GVHD) prophylaxis; and transplant complications including GVHD and infection (3, 8–13).
TA-TMA shares the principal features of other TMAs—consumptive microangiopathic hemolytic anemia, platelet consumption, and organ dysfunction. Over 50% of patients with TA-TMA develop multiorgan dysfunction (MOD); once this develops mortality in untreated patients can exceed 80% (2). Clinical manifestations include diffuse alveolar hemorrhage, respiratory failure, pulmonary hypertension, kidney injury or failure, altered mental status, posterior reversible encephalopathy syndrome (PRES), seizures, bowel ischemia, abdominal pain, gastrointestinal bleeding, and pulmonary or cardiac effusions requiring drainage. Among patients who develop MOD mortality rates exceed 50% (14). Non-relapse mortality (NRM) remains significantly higher in TA-TMA patients even after adjusting for other concurrent co-morbidities (15, 16).
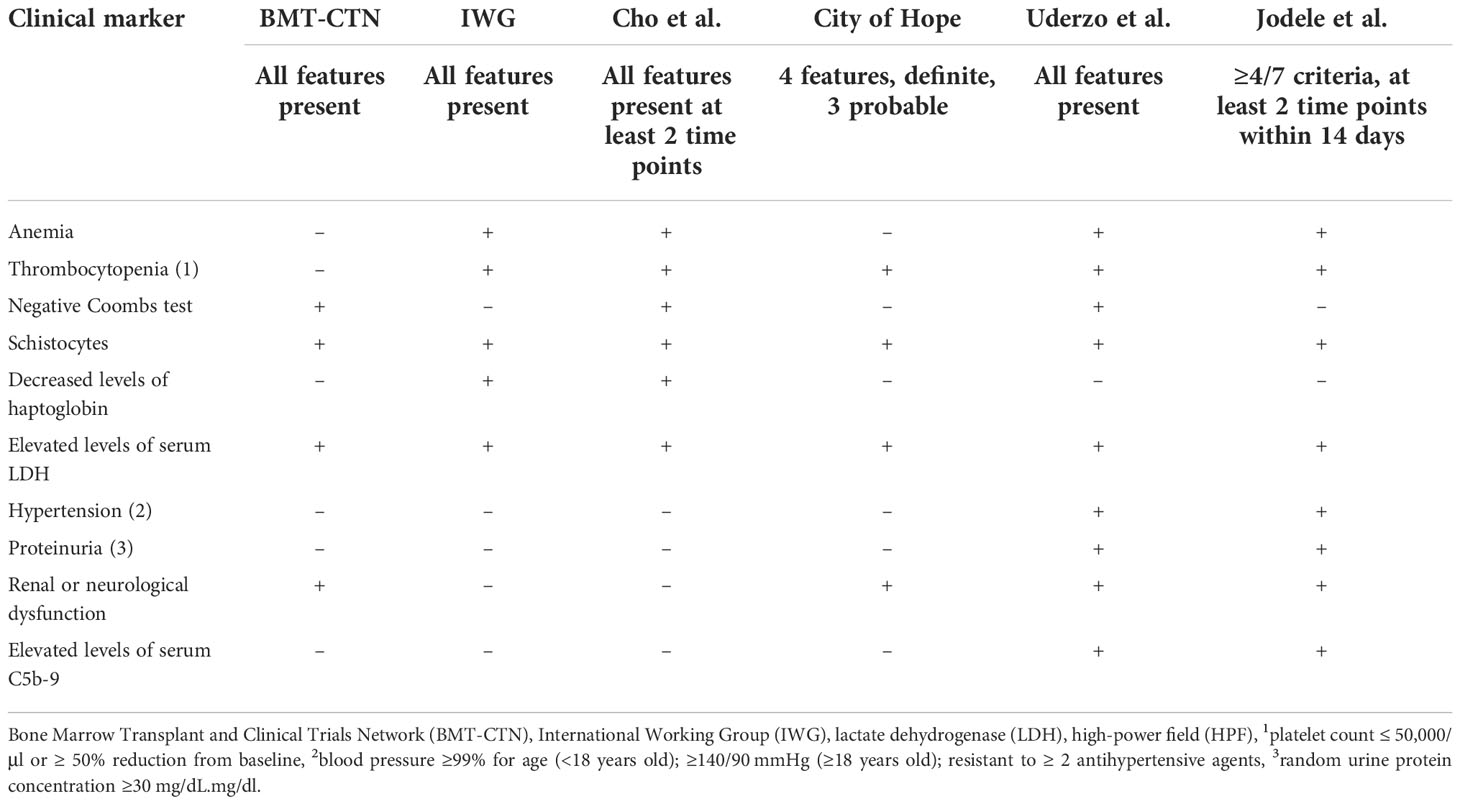
Early identification of TA-TMA is critical as there are treatments that impact survival and multiple ongoing clinical trials investigating new therapeutic agents (17–20). Although a renal biopsy is considered the gold standard for TA-TMA diagnosis, it is rarely obtained in the post-HCT setting and there are no identified specific diagnostic biomarkers of the disease. Thus, TA-TMA is primarily a clinical diagnosis. Several diagnostic criteria have been identified and all depend on a combination of non-specific features ( Table 1 ) (2, 16, 21, 22). Despite expert consensus, there is not a uniformly accepted diagnostic criterion for TA-TMA (23). In addition to identifying patients with TA-TMA, prognostic markers are critical—the goal is to identify those patients at the highest risk for developing severe disease and who will most benefit from TA-TMA-directed therapy while sparing those who are most likely to have a self-remitting course from the side effects and the costs of the drug. Subclinical biomarkers predicting the risk of developing TA-TMA could facilitate strategies to pre-emptively treat or prevent TA-TMA, akin to GVHD and sinusoidal obstructive syndrome (SOS) (24, 25). Our objective for this systemic review was to identify existing diagnostic, prognostic, and risk biomarkers for TA-TMA and to evaluate the quality and the level of evidence around them.
Methods
We conducted a systematic review of diagnostic, prognostic, and risk biomarkers in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards. All studies of genetic variants and immunohistochemical staining of tissues were excluded, and no biomarkers from other fluid sources (i.e., bronchoscopy fluid or cerebrospinal fluid) were identified.
Data sources and search strategy
A literature search was performed using Embase® (Elsevier, Amsterdam, the Netherlands) and PubMed® (National Library of Medicine, Bethesda, MD, USA) databases for relevant original studies from inception to 4 August 2022. There were no search filter, date, or language restrictions applied to the literature search to limit results. A broad search was conducted ( Supplemental Table 1 ).
Eligibility requirements
Studies were included if they were original human studies related to biomarkers in TA-TMA and included at least five patients with TA-TMA. Studies not related to TA-TMA, duplicate articles, review articles, animal studies, studies that included fewer than five patients with TA-TMA, studies with no biomarker data, abstracts that were published later, papers published only in conference proceedings, and studies of genetic variants in TA-TMA were excluded. There were no exclusion criteria regarding patient characteristics, TA-TMA diagnosis used, study design, or time.
Data extraction and quality
Two reviewers (MS and HB) independently screened all potentially relevant titles and abstracts for eligibility. Data gathered included author and publication year, type of study design (cohort, case–control, hybrid, retrospective or prospective, blinded, or not blinded), biomarkers, number of TA-TMA cases, diagnostic criteria used for TA-TMA, controls, sample approach, age group of cohorts, and measure of association such as hazard ratios (HRs), odds ratios (ORs), or information on sensitivity/specificity for biomarkers.
Assessment of quality and risk of bias of individual studies
A quality assessment of diagnostic accuracy studies (QUADAS)-2 assessment was performed independently by two authors (HB and MS). The QUADAS-2 is a tool that includes four domains designed to ascertain the risk of bias and standardize systematic reviews (26). Biomarker entries with available data on sensitivity/specificity and a post-HCT control group were included in the α-group, whereas β-group entries lacked either or both characteristics. For each group, biomarkers were categorized into four groups: markers of complement activation, endothelial activation, thrombosis, and miscellaneous. Finally, each of the biomarker groups were further divided into diagnostic, risk, and prognostic categories based on the data analysis.
Biomarker definitions
Biomarkers were defined as per the Federal Drug Association and National Institutes of Health joint leadership guidelines. Diagnostic biomarkers detect or confirm a diagnosis. Risk biomarkers detect the potential for disease in patients without a clinically evident disease. Prognostic biomarkers determine the anticipated disease course in patients with clinically evident disease (27).
Biomarker entry
Each biomarker or biomarker combination with either diagnostic, prognostic, or risk value was defined as a biomarker entry. Some manuscripts had multiple biomarker entries, resulting in several biomarker entries per paper. For example, if a single biomarker was tested in three ways—(1) predicting the development of later TA-TMA, (2) diagnostic ability, and (3) association of later non-relapsed related mortality (prognosis)—this resulted in three biomarker entries.
Results
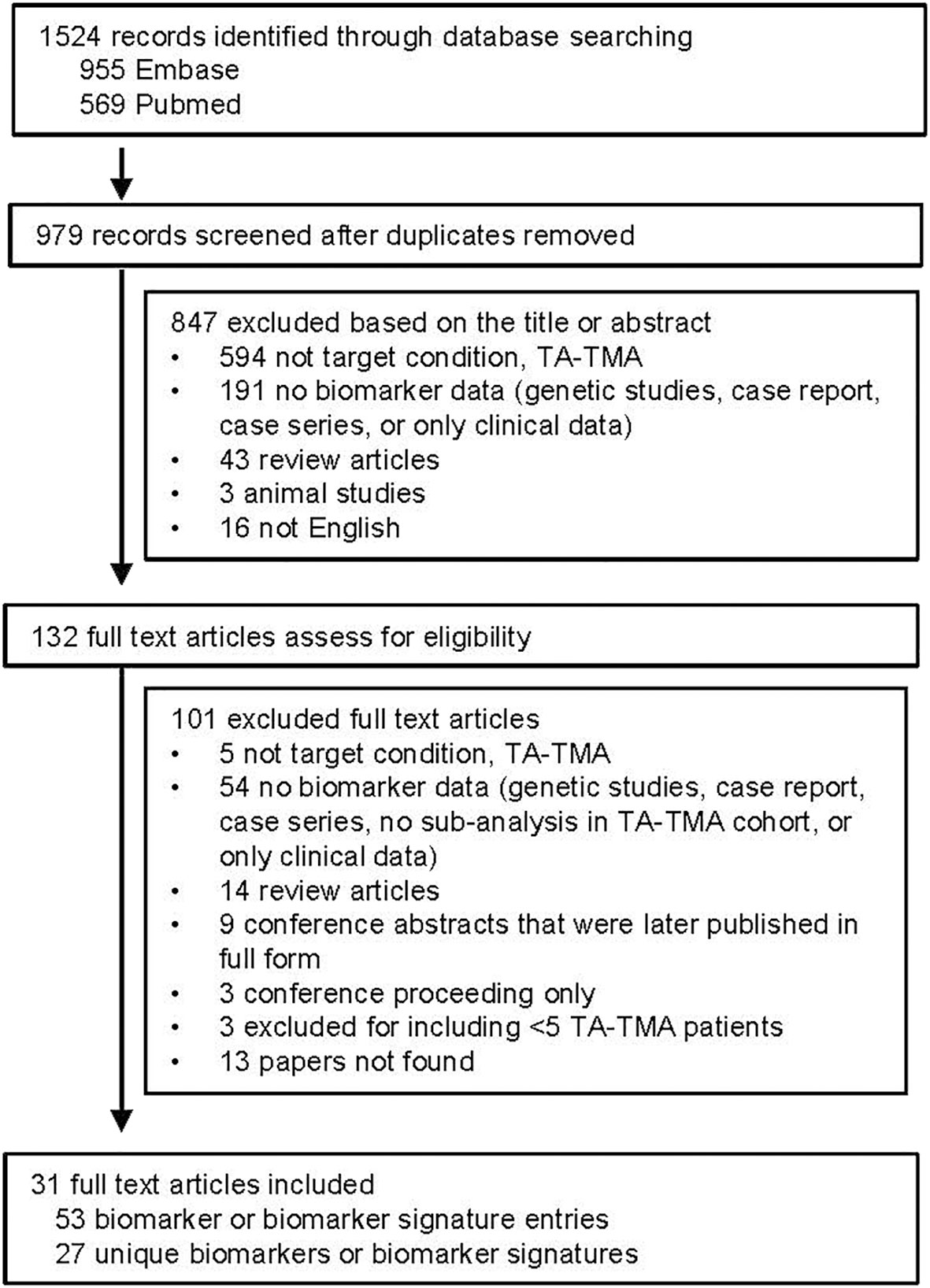
After removing duplicates, 979 publications were identified. After reviewing titles and abstracts, 132 were then manually reviewed. Most publications were excluded because they were not addressing TA-TMA or did not include biomarker data ( Figure 1 ). A total of 53 biomarker entries were identified that included 27 unique biomarkers or biomarker signatures.
Characteristics and quality of included studies
Summarized results for the quality and risk of bias assessments of the 31 studies based on QUADAS-2 are shown in Figure 2. The risk of bias was shown to be high in most studies given case–control study designs, convenience sample approaches, retrospective study design, and lack of validation in a separate cohort. No study commented on whether or not the samples were blinded to biomarker analysis.
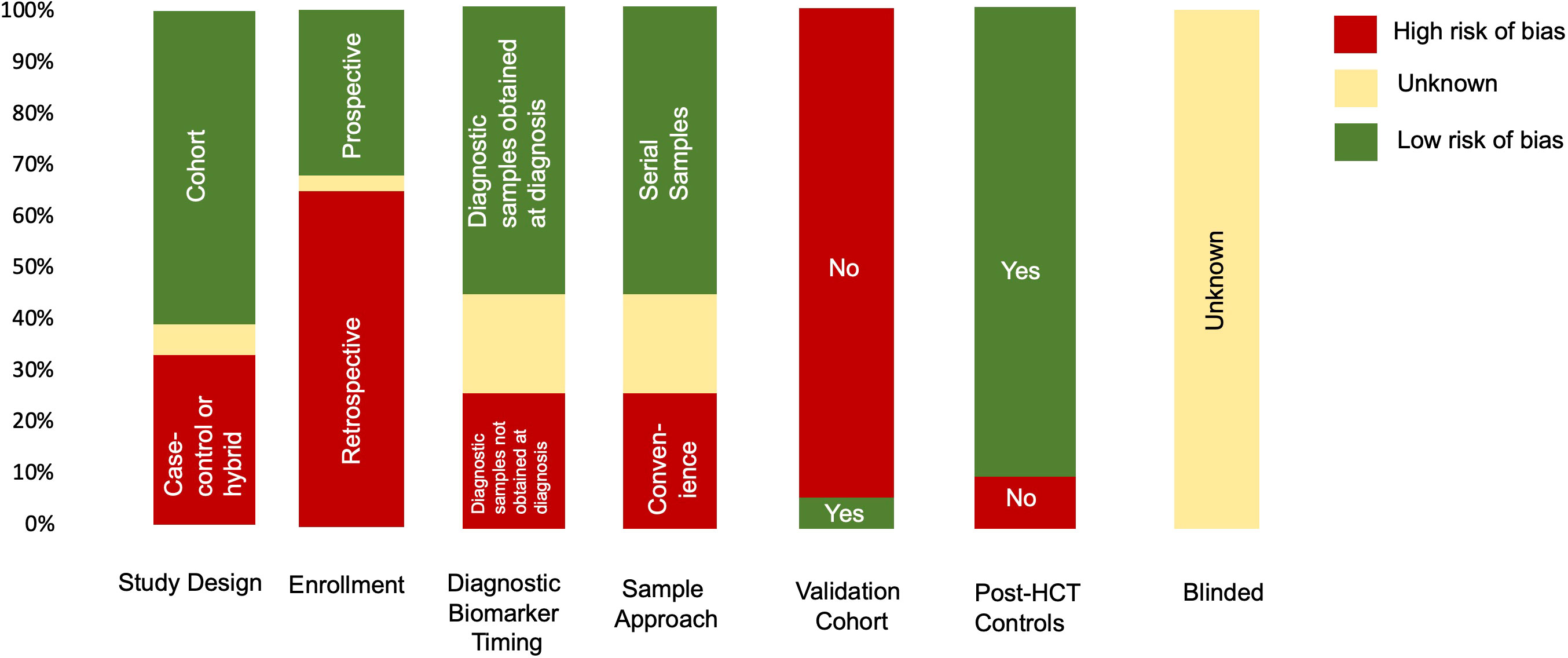
Figure 2 Summary of QUADAS-2 assessment for the risk of bias to assess study quality. Results of the QUADAS-2 questions for each study (n=31). If information was missing from the manuscript, it was indicated as unknown. In addition to typical QUADAS-2 questions, data for timing of diagnostic samples, validation cohort, and controls (whether the cohort was post HCT or healthy donors) are also indicated.
Entries per biomarker category and type
The 53 biomarker entries, from 31 studies, were classified into four predefined categories: markers of complement activation, endothelial activation, thrombosis, or other ( Figure 3 ). Markers of complement activation comprised 36% of biomarker entries. Sample sizes of cohorts varied with a median TA-TMA sample size of 15 patients (range 7–508 patients) and a median control cohort size of 26 patients(range 3–1482 patients). Multiple different diagnostic criteria were used including Cho (n= 8), Jodele (n=5), the Bone Marrow Transplant Clinical Trials Network (BMT-CTN) (n=4), the International Working Group (IWG) (n=3), City of Hope (n=1), Uderzo (n=2), multiple criteria (n=3), and other/unknown (n=5). Fourteen (45%) of the studies were with adults, 12 (39%) were with children, three (10%) included both children and adults, and two (6%) did not report age.
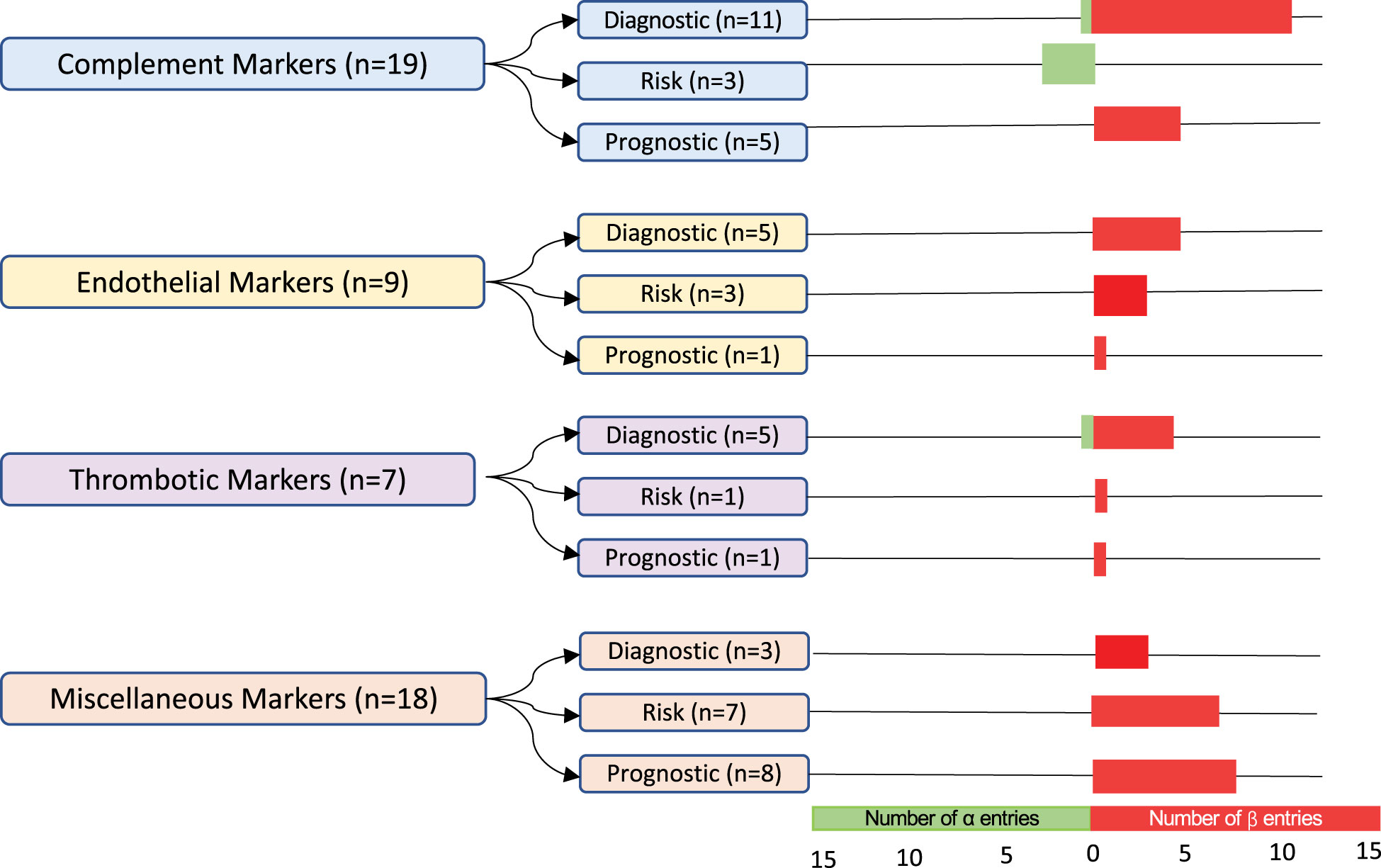
Figure 3 Classification and number of entries per biomarker category. In total, 53 biomarker entries from a set of 31 studies were included. The α-group entries included sensitivity and specificity values and post-HCT controls. The β-group entries lacked either or both of these features. Biomarkers were grouped into markers of complement activation, endothelial activation, thrombosis, and miscellaneous. They were further subdivided into diagnostic, risk, and prognostic biomarkers. The number of α-group entries is indicated in green, and the number of β-group entries is in red.
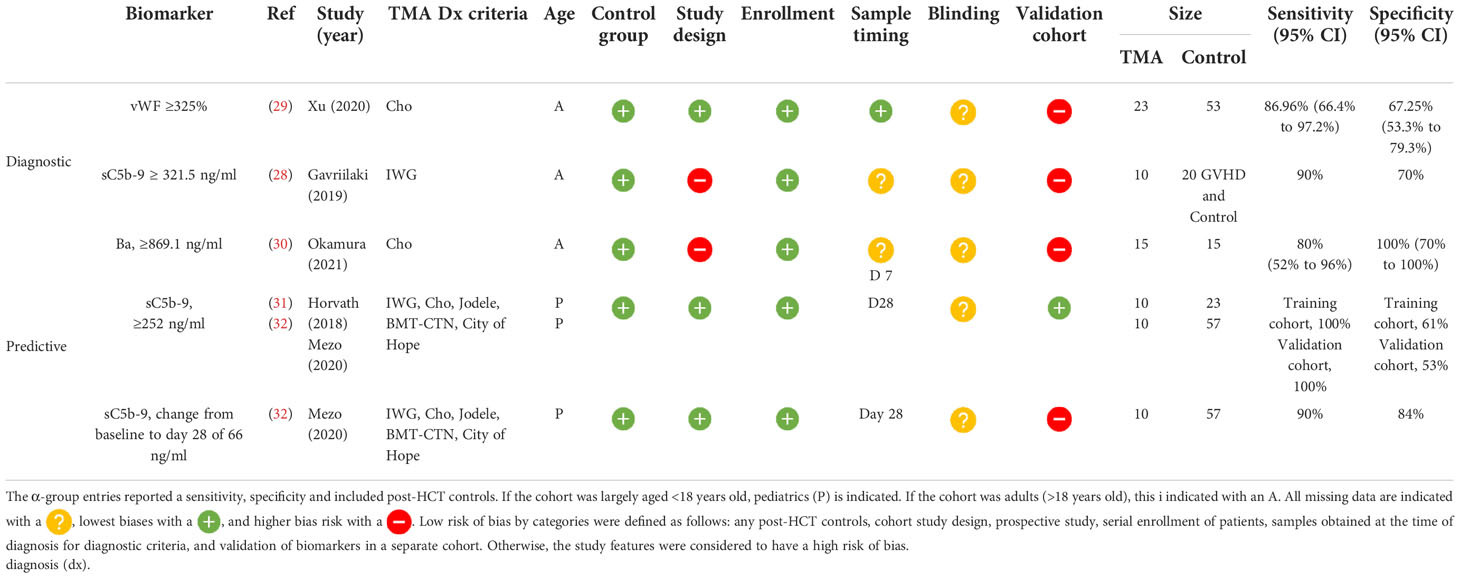
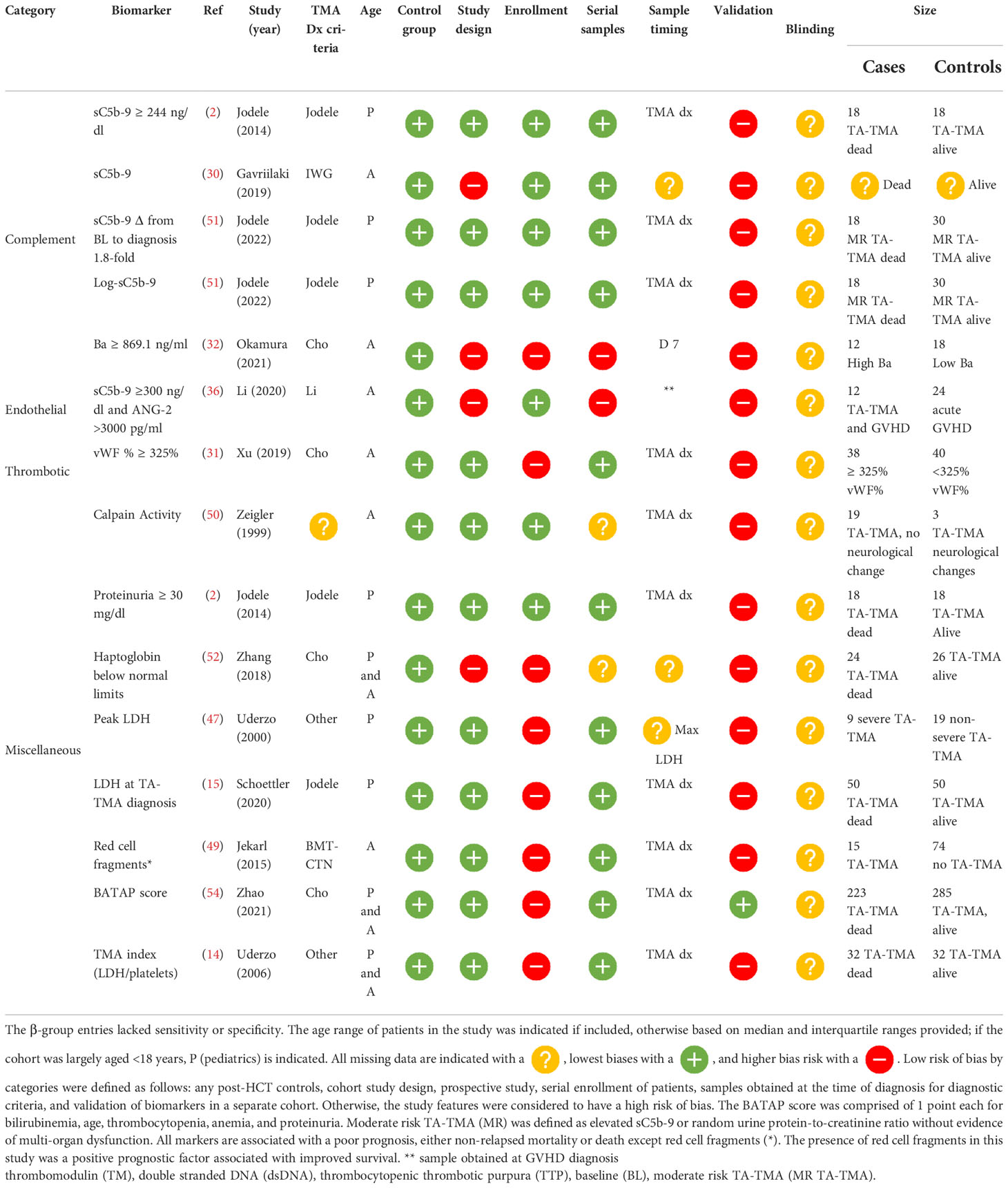
Biomarkers with reported diagnostic performance (α-group)
Of the five studies that reported a biomarker with sensitivity and specificity, two were diagnostic and three predicted later risk of TA-TMA. Levels of sC5b-9 ≥ 321.5 ng/ml in an adult cohort of 10 patients with TA-TMA and GVHD, compared with 10 patients with GVHD, only had a 90% sensitivity and a 70% specificity for TA-TMA diagnosis (28). However, this was a case–control study, limited by a small sample size, and it was not clear when samples were obtained. vWF% activity of ≥325% at the time of TA-TMA diagnosis had an 86.96% sensitivity [95% confidence interval (CI) 66.4% to 97.2%] and a specificity of 67.25% (95% CI 53.3% to 79.3%) (29). This prospective study had a larger sample size (TA-TMA, n=23; controls, n=53), but the findings were not validated in a separate cohort.
Two biomarkers were associated with the later development of TA-TMA. Levels of Ba ≥869.1 ng/ml on day 7 post HCT had a sensitivity of 80% (95% CI 52% to 96%) and a specificity of 100% (95% CI 70% to 100%) of predicting later development of TA-TMA (median day 25, range 22.5–44 days) (30). Although sensitivity and specificity were reported, this study was limited by a case–control study design and small sample size of 15 patients with TA-TMA and 15 post-HCT controls. The only biomarker that was validated in two separate cohorts was sC5b-9 level on day 28. In two pediatric cohorts, levels of sC5b-9 ≥252 ng/ml on day 28 had a sensitivity of 100% and a specificity of 61% in the first cohort, and in the validation group a sensitivity of 100% and a specificity of 53% for developing TA-TMA later in the HCT course (median day 63, range 42–85 days) (31, 32). Although validation was a strength of this study, the sample size was a limitation. Between both cohorts, only 20 patients had TA-TMA (10 in the first cohort and 10 in the second). The same group also reported that a change in 66 ng/ml from baseline to day 28 day had a sensitivity of 90% and a specificity of 84% of a later TA-TMA diagnosis (32) (Table 2).
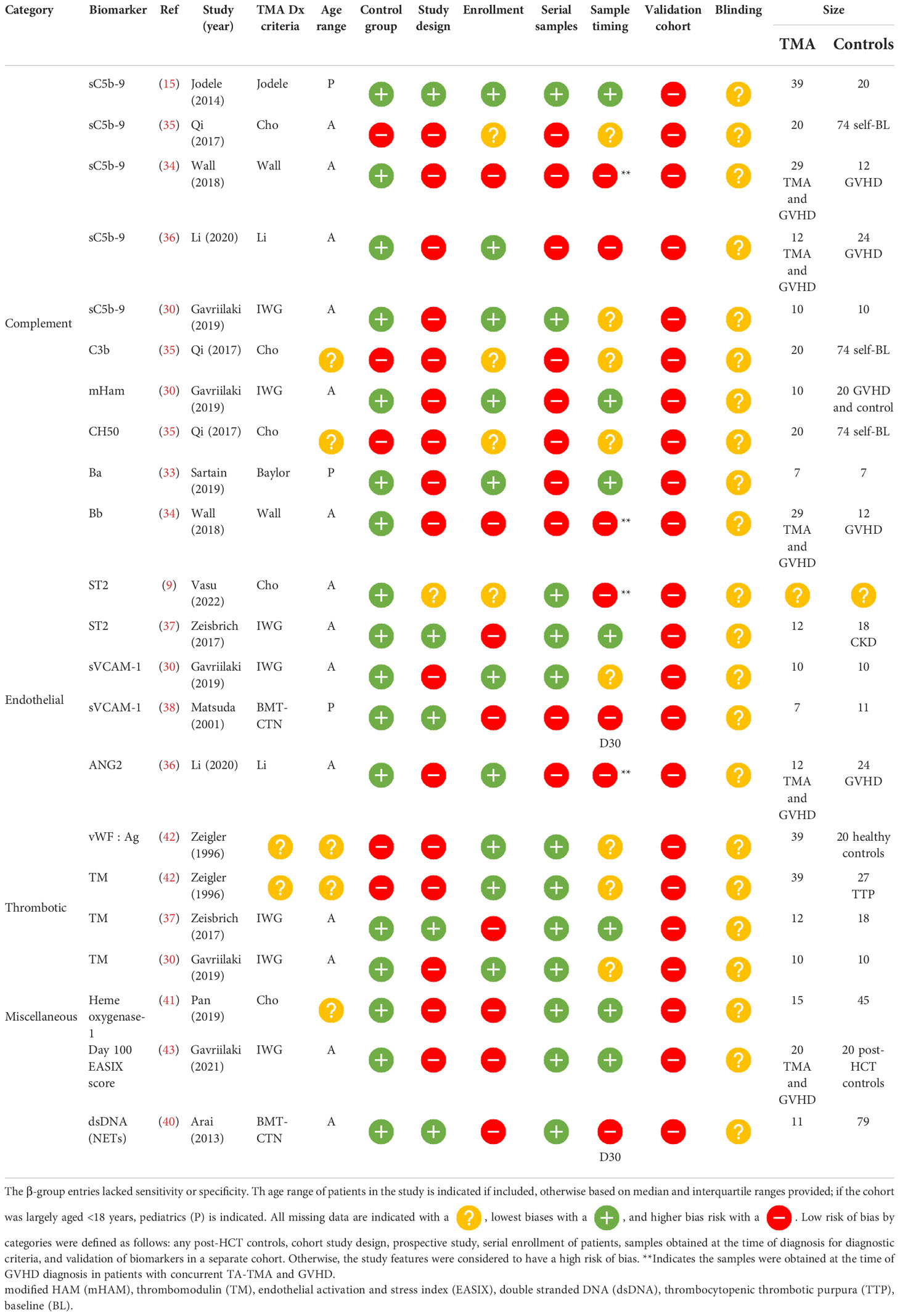
β-group diagnostic biomarkers
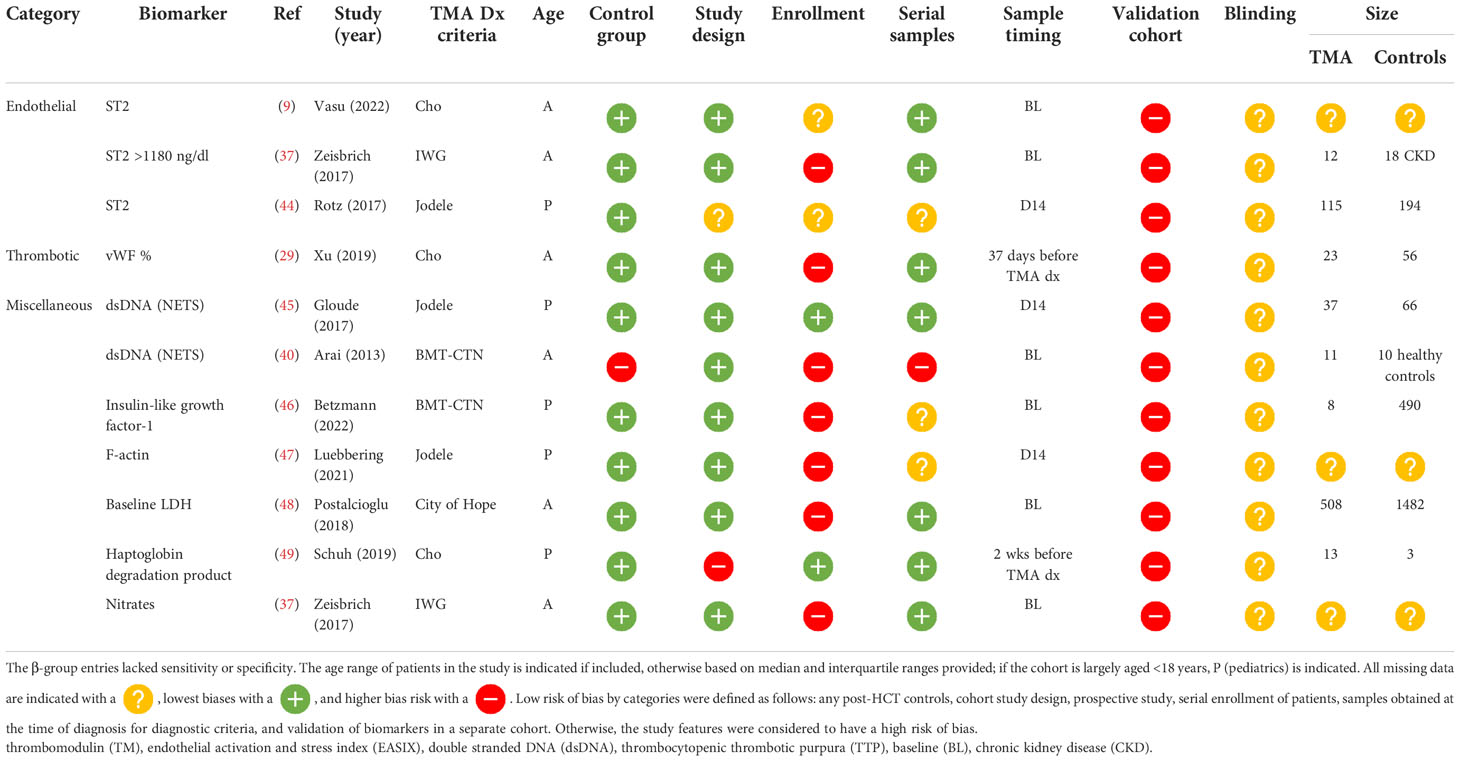
Among β-group biomarker entries (n= 22), there were 13 unique biomarkers and one scoring system incorporating multiple biomarkers. Of the 14 diagnostic biomarkers, seven (50%) were obtained at the time of clinical diagnosis, five (32%) were obtained at a standard time point available, and the timing of the sample was unclear in two (14%). Markers of complement activation were significantly higher in patients with TA-TMA and included the alternative pathway, Ba (n=1) (33) and Bb (n=1) (34), the classical pathway, CH50 (n=1) (35), terminal complement, sC5b-9 (n= 4) (2, 34–36), and anaphaylatoxin C3b (n=1). Markers of endothelial activation, including ST2 (n=2) (9, 37), sVCAM-1 (n=2) (28, 38), and ANG-2 (n=1) (36), markers of thrombosis, including thrombomodulin (n=3) (28, 37, 39), vWF : Ag (39), neutrophilic extracellular traps measured by dsDNA, and day 100 endothelial activation, and stress index (EASIX) scores, were calculated (lactate dehydrogenase (LDH) x serum creatinine)/platelets. These markers were also significantly higher in TA-TMA patients (40). Levels of heme oxygenase-1, a stress-inducible protein, which protects endothelial cells from apoptosis, were lower in patients with TA-TMA than in post-HCT controls (41) ( Table 3 ).
β-group risk biomarkers
Eleven biomarker entries from 10 studies resulted in eight unique biomarkers, which were significantly different before clinical manifestations of TA-TMA and may predict the development of the disease. Timing of samples acquisition varied significantly: six studies (55%) measured samples at baseline (BL) preconditioning, three (27%) on day 14 post HCT, one (9%) 2 weeks before clinical TA-TMA diagnosis, and one (9%) a range of days before TA-TMA diagnosis (median 37 days). There were no β-group risk markers of complement activation. ST2 was the most promising risk biomarker. It was significantly elevated in patients with TA-TMA, compared with controls, before clinical manifestations of the disease in three separate studies (9, 37, 44). Early elevation of dsDNA, thought to be from neutrophilic extracellular traps, was significantly elevated in TA-TMA patients in two studies (40, 45) (Table 4 ).
β-group prognostic biomarkers
Eleven biomarkers associated with poor prognosis were reported. Outcomes included death, non-relapse mortality, and neurological dysfunction (50). Four studies reported prognostic implications of sC5b-9 including (1) values ≥ 244 ng/dl at the time of diagnosis (2); (2) a change in level of 1.8-fold from baseline to diagnosis (51); (3) an increase of a log at the time of TA-TMA (51); and (4) levels of sC5b-9 ≥300 ng/l and ANG-2 > 3000 pg/ml in patients with concurrent TA-TMA and GVHD (36). Markers of microangiopathy including decreased levels of haptoglobin (52), elevated levels of LDH (15, 53), and scores that use laboratory values of microangiopathy, including BATAP (bilirubin, age, thrombocytopenia, anemia, proteinuria) (54) TMA index (LDH/platelets), and an EASIX score (55), all portended a poor prognosis. also portended a poor prognosis (14). BATAP scores and their association with mortality were studied in two independent cohorts and validated at the time of TA-TMA diagnosis in a large cohort. Of note is that the only identified positive prognostic biomarker was red cell fragments, which when present, compared with schistocytes without red cell fragments, was associated with decreased mortality (56) ( Table 5 ).
Discussion
This systematic review assessed the diagnostic, predictive, and prognostic performance of biomarkers of TA-TMA at multiple time points. A wide variety of biomarkers were tested, but only three biomarkers had specificity and sensitivity data and only two were validated in separate cohorts. These studies had the strongest level of evidence in this systematic review, but there were still limitations due to the small sample size and study design. The single biomarker with the most robust data is sC5b-9 levels, which has reported diagnostic, risk, and prognostic associations. Furthermore, the sC5b-9 level was one of only two biomarkers that was confirmed in a separate validation cohort. However, there is insufficient evidence that elevated levels of sC5b-9 alone are specific for TA-TMA. Furthermore, the appropriate cut-off value for the sC5b-9 level is not clear, with contradictory data in children and adults. Lastly, the sC5b-9 level is not readily available, limiting its incorporation into clinical practice. Outside of CH50, LDH, blood counts, and proteinuria, most tests are only obtained in specialty laboratories and, hence, not readily available. Furthermore, although some studies reported significant differences in sC5b-9 kinetics these have limited clinical utility at this time, as these biomarkers are not routinely assessed before the transplant or serially post transplant.
Gaps identified from review in current literature and future directions
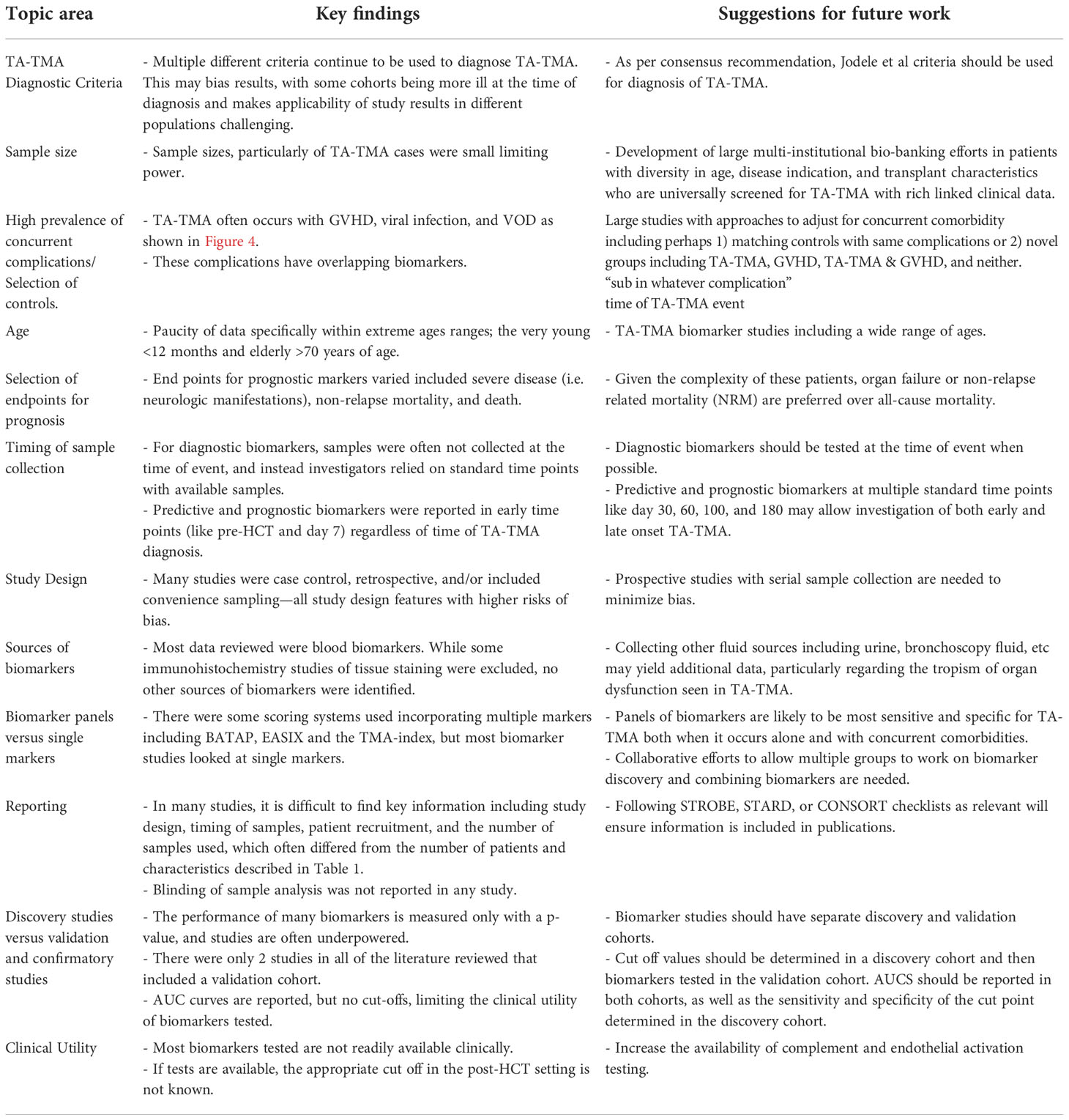
This review summarizes the biomarker landscape of TA-TMA and highlights the many unmet needs in the field. (1) There are very low-bias risk studies, and many are limited by small sample size, with a median of 15 patients with TA-TMA. (2) Many biomarkers do not have a normal range of concentration or level established. Even if a normal range is established, in the post-HCT setting the normal range can likely not be applied. Thus, a cut-off value of biomarkers is necessary for clinical utility. Only five studies reported a biomarker with a cut-off value and associated sensitivity and specificity. (3) There was significant heterogeneity in TMA diagnostic criteria used among studies. As some criteria incorporate organ dysfunction, this may bias results. (4) Among diagnostic biomarkers, only 50% were obtained at the time of clinical diagnosis. Utilizing institutional biorepositories with samples available at standard time points is often more feasible than collecting samples at the time of the event, but when investigating diagnostic biomarkers, obtaining samples at the time of the event is critical and the most rigorous approach. (5) A lack of validation in separate cohorts and demonstration of reproducibility of results. Most markers are not clinically available and, in most studies, enzyme-linked immunosorbent assay (ELISA) kits were used from multiple different companies (Supplement Table 3). We have made suggestions to address these gaps for future research (Table 6).
Another pervasive challenge of biomarker discovery in TA-TMA is concurrent comorbidities—TA-TMA often occurs with acute GVHD, hepatic sinusoidal obstructive syndrome, and/or infections ( Figure 4 ). Each of these complications has reported biomarkers, many of which overlap with the biomarkers reported in TA-TMA ( Figure 5 ). This challenge is not unique to TA-TMA and impacts most biomarker research in HCT. Because GVHD and infections, well-known risk factors for TA-TMA can directly cause endothelial damage (42, 43, 57, 58), they may play a key role in the pathophysiology of TA-TMA. Thus, adjusting for these complications in a typical multivariable biomarker analysis may not be the best approach (59). Instead, to overcome this limitation, biomarkers of TA-TMA could have three separate comparison groups: (1) post HCT without any complications, (2) TA-TMA with a concomitant complication of interest (i.e., GVHD, steroid-refractory GVHD, SOS, infections, etc.), and (3) only complication of interest without TA-TMA. This approach could help overcome this limitation and gain insight into the shared and disparate biomarkers of two diseases. However, this would require large sample sizes and multi-institutional collaboration. Furthermore, because multiple complications are often present this cannot resolve all limitations. Very large cohorts, with controls enriched for concurrent morbidities using other approaches like matching or propensity score matching, is another approach that could be taken.
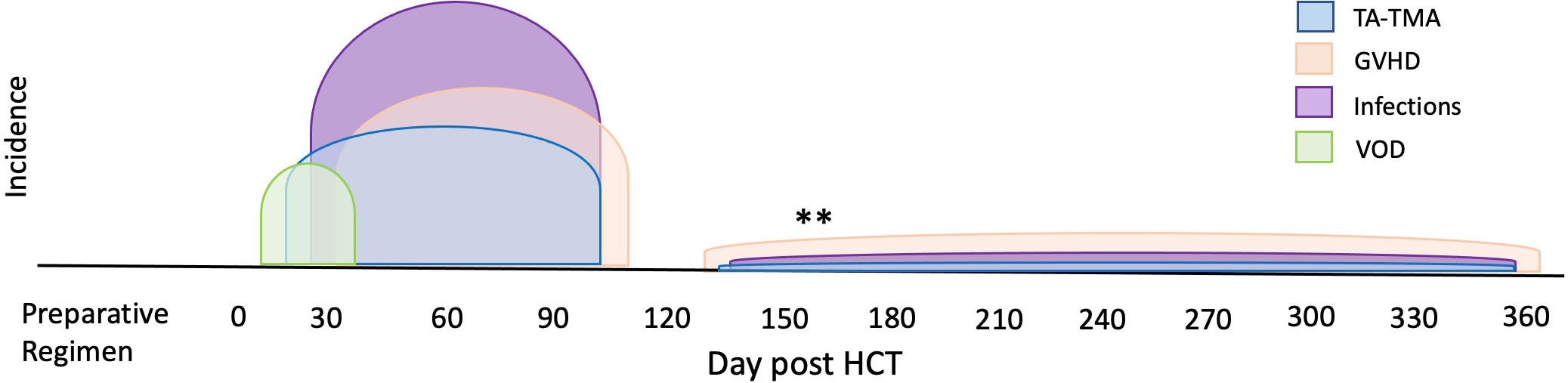
Figure 4 Overlap of TA-TMA and common post-BMT complications in time. The typical high-risk period for complications post-HCT are indicated in different colors. The high-risk period for TA-TMA overlaps with the risk period for acute GVHD, VOD, infections, and organ toxicities such as pulmonary failure. **Often in the setting of overlapping or chronic GVHD, increased risks for infections and TA-TMA can occur later in the transplant course.
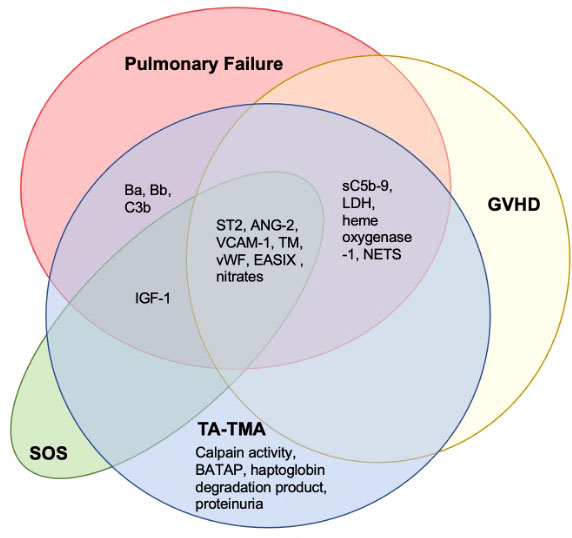
Figure 5 Overlapping biomarkers of common post-BMT complications and TA-TMA. A literature search of the TA-TMA biomarkers identified in this review and any diagnostic, prognostic, or risk implications in GVHD, SOS, and pulmonary failure (infectious and non-infectious) was completed. Most biomarkers identified in TA-TMA overlapped with other post-HCT complications. Citations provided in Supplementary Table 2. Abbreviations: lactate dehydrogenase (LDH), neutrophil extracellular traps (NETS), thrombomodulin (TM).
Based on our systemic review, while many candidate biomarkers were identified, there is insufficient sensitivity and specificity data for a single biomarker or biomarker panel to be used for the diagnosis of TA-TMA. Continued efforts to identify diagnostic, prognostic, and risk biomarkers in TA-TMA and validate them in separate cohorts is a priority for this disease. Biomarker discovery is critical for clinical care and may also provide additional insight into the pathophysiology of the disease and support novel therapeutic approaches. There are currently inhibitors that target multiple components of the complement cascade, including the lectin pathway, the alternative pathway, the classical pathway, C3, and C5 (7, 60, 61). Evidence of activation in any of these pathways could support investigating the safety and efficacy of these novel therapeutic approaches. We propose a continued investigation of biomarkers, with the most robust data to date including markers of complement activation, ST2, and vWF % and markers that could have therapeutic implications, or which are readily accessible.
Strengths and limitations of this study
This systematic review outlined how the results of 31 papers and 27 unique biomarkers have several strengths. Inclusion and exclusion criteria were defined and agreed on by authors a priori. The search strategy was validated and PRISMA guidelines were followed. Quality and bias assessment of publications were assessed using QUADAS-2, a recognized and validated tool. There were no limitations on patient populations: children and adults were included using any TA-TMA diagnostic criteria. However, the review was limited by a lack of formal assessment of publication bias. We could not perform a meta-analysis given the heterogeneity of the studies. We included only studies written in English, and may have missed studies written in other languages, though we are not aware of any such misses. Furthermore, we could have missed other biases that were not covered by the QUADAS-2 questions. Lastly, we reported only “positive” results and did not summarize contradictory studies in which there were no significant differences in biomarkers.
Conclusions
The increased interest and recognition of TA-TMA is exciting, but for the field to move forward and make significant, clinically applicable progress, multi-institutional, collaborative efforts with samples obtained at appropriate time points with well-matched controls are needed. We conclude that currently, while there is promising early data, more robust evidence with large validation cohorts are needed for diagnostic, prognostic, and predictive biomarkers in TA-TMA.
Author contributions
MS and SV designed and wrote and manuscript, MS and HB reviewed articles with the oversight of MS and SV. All authors edited the manuscript and approved the final version.
Funding
This study was supported by NIH NCI K12 (MS), K12, Award #5K12CA237806-04, Winship Pilot (MS).
Conflict of interest
SV is a consultant for Omeros.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1064203/full#supplementary-material
References
1. Gavriilaki E, Sakellari I, Batsis I, Mallouri D, Bousiou Z, Vardi A, et al. Transplant-associated thrombotic microangiopathy: Incidence, prognostic factors, morbidity, and mortality in allogeneic hematopoietic cell transplantation. Clin Transplant. (2018) 32(9):e13371. doi: 10.1111/ctr.13371
2. Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. (2014) 124(4):645 LP – 653.
3. Jodele S, Dandoy CE, Myers K, Wallace G, Lane A, Teusink-Cross A, et al. High-dose Carboplatin/Etoposide/Melphalan increases risk of thrombotic microangiopathy and organ injury after autologous stem cell transplantation in patients with neuroblastoma. Bone Marrow Transplant. (2018). doi: 10.1038/s41409-018-0159-8
4. Young JA, Pallas CR, Knovich MA. Transplant-associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. (2021) 56(8):1805–17. doi: 10.1038/s41409-021-01283-0
5. Jodele S, Sabulski A. Transplant-associated thrombotic microangiopathy: elucidating prevention strategies and identifying high-risk patients. Expert Rev Hematol (2021) 14(8):751–63. doi: 10.1080/17474086.2021.1960816
6. Dvorak CC, Higham C, Shimano KA. Transplant-associated thrombotic microangiopathy in pediatric hematopoietic cell transplant recipients: A practical approach to diagnosis and management. Front Pediatr . (2019) 7:133.
7. Schoettler M, Chonat S, Williams K, Lehmann L. Emerging therapeutic and preventive approaches to transplant-associated thrombotic microangiopathy. Curr Opin Hematol (2021) 28(6).
8. Elfeky R, Lucchini G, Lum S-H, Ottaviano G, Builes N, Nademi Z, et al. New insights into risk factors for transplant-associated thrombotic microangiopathy in pediatric HSCT. Blood Adv (2020) 4(11):2418–29. doi: 10.1182/bloodadvances.2019001315
9. xVasu S, Bostic M, Zhao Q, Sharma N, Puto M, Knight S, et al. Acute GVHD BK virus hemorrhagic cystitis and age are risk factors for transplant-associated thrombotic microangiopathy in adults. Blood Adv (2022) 6(4):1342–9. doi: 10.1182/bloodadvances.2021004933
10. Daly AS, Hasegawa WS, Lipton JH, Messner HA, Kiss TL. Transplantation-associated thrombotic microangiopathy is associated with transplantation from unrelated donors, acute graft-versus-host disease and venoocclusive disease of the liver. Transfus Apher Sci (2002) 27(1):3–12. doi: 10.1016/S1473-0502(02)00020-4
11. Schoettler M, Stenger EO, Spencer K, Lutterman D, Rumbika S, Jones JA, et al. Sickle cell disease is a risk factor for transplant-associated thrombotic microangiopathy in children. Blood Adv (2022). doi: 10.1182/bloodadvances.2022008058
12. Jodele S, Zhang K, Zou F, Laskin B, Dandoy CE, Myers KC, et al. The genetic fingerprint of susceptibility for transplant associated thrombotic microangiopathy. Blood. (2015) 127(8):989–97. doi: 10.1182/blood-2015-08-663435
13. Millicent E, Gianluigi R, Giulia A, Valentina P, Mariotti J, Verna M, et al. Gene abnormalities in transplant associated- thrombotic Microangiopathy: Comparison between recipient and donor ‘ s DNA. Thromb Haemost. (2021) 122(07):1247–50.
14. Uderzo C, Bonanomi S, Busca A, Renoldi M, Ferrari P, Iacobelli M, et al. Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation. (2006) 82(5):638–44. doi: 10.1097/01.tp.0000230373.82376.46
15. Schoettler M, Lehmann LE, Margossian S, Lee M, Kean LS, Kao P-C, et al. Risk factors for transplant-associated thrombotic microangiopathy and mortality in a pediatric cohort. Blood Adv (2020) 4(11):2536–47. doi: 10.1182/bloodadvances.2019001242
16. Li A, Wu Q, Davis C, Kirtane KS, Pham PD, Sorror ML, et al. Transplant-associated thrombotic microangiopathy is a multifactorial disease unresponsive to immunosuppressant withdrawal. Biol Blood Marrow Transplant. (2019) 25(3):570–6. doi: 10.1016/j.bbmt.2018.10.015
17. Jodele S, Fukuda T, Mizuno K, Vinks AA, Laskin BL, Goebel J, et al. Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2016) 22(2):307–15. doi: 10.1016/j.bbmt.2015.10.002
18. Jodele S, Dandoy CE, Lane A, Laskin BL, Teusink-Cross A, Myers KC, et al. Complement blockade for TA-TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. (2020) 135(13):1049–57. doi: 10.1182/blood.2019004218
19. Yeates L, Slatter MA, Bonanomi S, Lim FLWI, Ong SY, Dalissier A, et al. Use of defibrotide to treat transplant-associated thrombotic microangiopathy: A retrospective study of the paediatric diseases and inborn errors working parties of the European society of blood and marrow transplantation. Bone Marrow Transplant. (2017) 52(5):762–4. doi: 10.1038/bmt.2016.351
20. Khaled SK, Claes K, Goh YT, Kwong YL, Leung N, Mendrek W, et al. Narsoplimab, a mannan-binding lectin-associated serine protease-2 inhibitor, for the treatment of adult hematopoietic stem-cell transplantation–associated thrombotic microangiopathy. J Clin Oncol (2022) 40(22):2447–57. doi: 10.1200/JCO.21.02389
21. Ruutu T, Barosi G, Benjamin RJ, Clark RE, George JN, Gratwohl A, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an international working group. Haematologica. (2007) 92(1 SE-Decision Making and Problem Solving):95–100. doi: 10.3324/haematol.10699
22. Shayani S, Palmer J, Stiller T, Liu X, Thomas SH, Khuu T, et al. Thrombotic microangiopathy associated with sirolimus level after allogeneic hematopoietic cell transplantation with Tacrolimus/Sirolimus-based graft-versus-Host disease prophylaxis. Biol Blood Marrow Transplant. (2013) 19(2):298–304. doi: 10.1016/j.bbmt.2012.10.006
23. Uderzo C, Jodele S, El Missiry M, Cicerci F, Busca A, Bacigalupa A CS. Transplant-associated thrombotic microangiopathy (TA-TMA) and consensus based diagnostic and therapeutic recommendations: Which TA-TMA patients to treat and when? J Bone Marrow Res (2014) 02(03). doi: 10.4172/2329-8820.1000152
24. Levine JE, Harris AC, Taylor A, Braun TM, Magenau J, Ferrara JLM. A biomarker-based grading system At onset of GvHD predicts NRM better than the modified glucksberg grading system. Blood. (2013) 122(21):145. doi: 10.1182/blood.V122.21.145.145
25. Akil A, Zhang Q, Mumaw CL, Raiker N, Yu J, Velez de Mendizabal N, et al. Biomarkers for diagnosis and prognosis of sinusoidal obstruction syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2015) 21(10):1739–45. doi: 10.1016/j.bbmt.2015.07.004
26. Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
27. Bidgoli A, DePriest BP, Saatloo MV, Jiang H, Fu D, Paczesny S. Current definitions and clinical implications of biomarkers in graft-versus-Host disease. Transplant Cell Ther (2022) 28(10):657–66. doi: 10.1016/j.jtct.2022.07.008
28. Gavriilaki E, Chrysanthopoulou A, Sakellari I, Batsis I, Mallouri D, Touloumenidou T, et al. Linking complement activation, coagulation, and neutrophils in transplant-associated thrombotic microangiopathy. Thromb Haemost. (2019) 119(9):1433–40. doi: 10.1055/s-0039-1692721
29. Xu Z, Luo C, Lai P, Ling W, Wu S, Huang X, et al. Von willebrand factor as a predictor for transplant-associated thrombotic microangiopathy. Clin Appl Thromb Hemost. (2020) 26. doi: 10.1177/1076029619892684
30. Okamura H, Nakamae H, Shindo T, Ohtani K, Hidaka Y, Ohtsuka Y, et al. Early elevation of complement factor ba is a predictive biomarker for transplant-associated thrombotic microangiopathy. Front Immunol (2021) 12:695037. doi: 10.3389/fimmu.2021.695037
31. Horváth O, Kállay K, Csuka D, Mező B, Sinkovits G, Kassa C, et al. Early increase in complement terminal pathway activation marker sC5b-9 is predictive for the development of thrombotic microangiopathy after stem cell transplantation. Biol Blood Marrow Transplant. (2018) 24(5):989–96. doi: 10.1016/j.bbmt.2018.01.009
32. Mezö B, Horváth O, Sinkovits G, Veszeli N, Kriván G, Prohászka Z. Validation of early increase in complement activation marker sC5b-9 as a predictive biomarker for the development of thrombotic microangiopathy after stem cell transplantation. Front Med (2020) 7:646.
33. Sartain S, Shubert S, Wu M-F, Wang T, Martinez C. The alternative complement pathway activation product ba as a marker for transplant-associated thrombotic microangiopathy. Pediatr Blood Cancer. (2020) 67(3):e28070. doi: 10.1002/pbc.28070
34. Wall SA, Zhao Q, Yearsley M, Blower L, Agyeman A, Ranganathan P, et al. Complement-mediated thrombotic microangiopathy as a link between endothelial damage and steroid-refractory GVHD. Blood Adv (2018) 2(20):2619–28. doi: 10.1182/bloodadvances.2018020321
35. Qi J, Wang J, Chen J, Su J, Tang Y, Wu X, et al. Plasma levels of complement activation fragments C3b and sC5b-9 significantly increased in patients with thrombotic microangiopathy after allogeneic stem cell transplantation. Ann Hematol (2017) 96(11):1849–55. doi: 10.1007/s00277-017-3092-9
36. Li A, Bhatraju PK, Chen J, Chung DW, Hilton T, Houck K, et al. Prognostic biomarkers for thrombotic microangiopathy after acute graft-versus-Host disease: A nested case-control study. Transplant Cell Ther (2021) 27(4):308.e1–308.e8. doi: 10.1016/j.jtct.2020.12.010
37. Zeisbrich M, Becker N, Benner A, Radujkovic A, Schmitt K, Beimler J, et al. Transplant-associated thrombotic microangiopathy is an endothelial complication associated with refractoriness of acute GvHD. Bone Marrow Transplant. (2017) 52:1399. doi: 10.1038/bmt.2017.119
38. Matsuda Y, Hara J, Osugi Y, Tokimasa S, Fujisaki H, Takai K, et al. Serum levels of soluble adhesion molecules in stem cell transplantation-related complications. Bone Marrow Transplant. (2001) 27(9):977–82. doi: 10.1038/sj.bmt.1703026
39. Zeigler ZR, Rosenfeld CS, Andrews DF III, Nemunaitis J, Raymond JM, Shadduck RK, et al. Plasma von willebrand factor antigen (vWF:AG) and thrombomodulin (TM) levels in adult thrombotic thrombocytopenic purpura/hemolytic uremic syndromes (TTP/HUS) and bone marrow transplant-associated thrombotic microangiopathy (BMT-TM). Am J Hematol (1996) 53(4):213–20. doi: 10.1002/(SICI)1096-8652(199612)53:4<213::AID-AJH1>3.0.CO;2-0
40. Arai Y, Yamashita K, Mizugishi K, Watanabe T, Sakamoto S, Kitano T, et al. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. (2013) 19(12):1683–9. doi: 10.1016/j.bbmt.2013.09.005
41. Pan T, Qi J, You T, Han S, Yang L, Miao W, et al. Circulating heme oxygenase-1 and complement activation in transplant-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. (2019) 25(8):1486–91. doi: 10.1016/j.bbmt.2019.03.002
42. Luft T, Dreger P, Radujkovic A. Endothelial cell dysfunction: a key determinant for the outcome of allogeneic stem cell transplantation. Bone Marrow Transplant. (2021) 56(10):2326–35. doi: 10.1038/s41409-021-01390-y
43. Fosse JH, Haraldsen G, Falk K, Edelmann R. Endothelial cells in emerging viral infections. Front Cardiovasc Med (2021) 8:619690. doi: 10.3389/fcvm.2021.619690
44. Rotz SJ, Dandoy CE, Davies SM. ST2 and endothelial injury as a link between GVHD and microangiopathy. N Engl J Med (2017) 376(12):1189–90. doi: 10.1056/NEJMc1700185
45. Davies SM, Gloude NJ, Jodele S, Khandelwal P, Alder MN, Lake KE, et al. Circulating dsDNA, endothelial injury, and complement activation in thrombotic microangiopathy and GVHD. Blood. (2017) 130(10):1259–66. doi: 10.1182/blood-2017-05-782870
46. Betzmann D, Doring M, Blumenstock G, Erdmann F, Grabow D, Lang P, et al. Impact of pre-transplant serum-IGF-1 on hematopoietic stem cell transplantation outcome in pediatric cancer patients. Horm Res Paediatr (2021) 94(SUPPL 1):59.
47. Luebbering N, Abdullah S, Lounder D, Lane A, Dole N, Rubinstein J, et al. Endothelial injury, f-actin and vitamin-d binding protein after hematopoietic stem cell transplant and association with clinical outcomes. Haematologica. (2021) 106(5):1321–9. doi: 10.3324/haematol.2019.233478
48. Postalcioglu M, Kim HT, Obut F, Yilmam OA, Yang J, Byun BC, et al. Impact of thrombotic microangiopathy on renal outcomes and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2018) 24(11):2344–53. doi: 10.1016/j.bbmt.2018.05.010
49. Schuh MP, Bennett MR, Lane A, Jodele S, Laskin BL, Devarajan P. Haptoglobin degradation product as a novel serum biomarker for hematopoietic stem cell transplant-associated thrombotic microangiopathy. Pediatr Nephrol. (2019) 34(5):865–71. doi: 10.1007/s00467-018-4178-x
50. Zeigler ZR, Kelton JG, Moore JC, Shadduck RK, Andrews DF, Nath R, et al. Calpain activity in bone marrow transplant-associated thrombotic thrombocytopenic purpura. Bone Marrow Transplant. (1999) 24(6):641–5. doi: 10.1038/sj.bmt.1701928
51. Jodele S, Dandoy CE, Sabulski A, Koo J, Lane A, Myers KC, et al. TA-TMA risk stratification: is there a window of opportunity to improve outcomes? Transplant Cell Ther (2022). doi: 10.1016/j.jtct.2022.04.019
52. Zhang X, Liu X, Wang Q, He Y, Zhu X, Zhang J, et al. Thrombotic microangiopathy with concomitant GI aGVHD after allogeneic hematopoietic stem cell transplantation: Risk factors and outcome. Eur J Haematol (2018) 100(2):171–81. doi: 10.1111/ejh.12996
53. Uderzo C, Fumagalli M, De Lorenzo P, Busca A, Vassallo E, Bonanomi S, et al. Impact of thrombotic thrombocytopenic purpura on leukemic children undergoing bone marrow transplantation. Bone Marrow Transplant. (2000) 26:1005. doi: 10.1038/sj.bmt.1702648
54. Zhao P, Wu Y, He Y, Chong S, Qu Q, Deng R, et al. A prognostic model (BATAP) with external validation for patients with transplant-associated thrombotic microangiopathy. Blood Adv (2021) 5(24):5479–89. doi: 10.1182/bloodadvances.2021004530
55. Gavriilaki E, Sakellari I, Chatzikonstantinou T, Mallouri D, Batsis I, Vardi A, et al. Endothelial and complement activation as predictors of survival in adult allogeneic hematopoietic cell transplantation. HemaSphere. (2021) 5(1).
56. Jekarl DW, Kim Y, Lim J, Kim M, Han K, Cho B, et al. Fragmented red cell as a possible favorable prognostic marker of hematopoietic stem cell transplantation associated thrombotic microangiopathy. J Clin Lab Anal (2015) 29(6):444–50. doi: 10.1002/jcla.21792
57. Cordes S, Mokhtari Z, Bartosova M, Mertlitz S, Shi Y, Mengwasser J, et al. Endothelial damage and dysfunction in acute graft-versus-host disease. Haematologica. (2021) 106(8):2147–60.
58. Martinez-Sanchez J, Hamelmann H, Palomo M, Mir E, Moreno-Castaño AB, Torramade S, et al. Acute graft-vs.-Host disease-associated endothelial activation in vitro is prevented by defibrotide. Front Immunol (2019) 10:2339. doi: 10.3389/fimmu.2019.02339
59. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. (2009) 20(4):488–95. doi: 10.1097/EDE.0b013e3181a819a1
60. Ardissino G, Capone V, Tedeschi S, Porcaro L, Cugno M. Complement system as a new target for hematopoietic stem cell transplantation-related thrombotic microangiopathy. Pharmaceuticals. (2022) 15(7). doi: 10.3390/ph15070845
Keywords: transplant associated thrombotic microangiopathy, biomarkers, complement activation, endothelial activation, sC5b-9 (serum complement membrane attack complex), NETs (neutrophil extracellular traps)
Citation: Schoettler ML, Bhatt H and Vasu S (2023) A systematic review of diagnostic, prognostic, and risk blood and urine biomarkers of transplant-associated thrombotic microangiopathy. Front. Immunol. 13:1064203. doi: 10.3389/fimmu.2022.1064203
Received: 07 October 2022; Accepted: 28 November 2022;
Published: 02 February 2023.
Edited by:
Luisa Giaccone, University of Turin, ItalyReviewed by:
Eleni Gavriilaki, G. Papanikolaou General Hospital, GreeceMassimo Cugno, Università degli Studi di Milano, Italy
Copyright © 2023 Schoettler, Bhatt and Vasu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle L. Schoettler, TWljaGVsbGUuU2Nob2V0dGxlckBlbW9yeS5lZHU=
 Michelle L. Schoettler
Michelle L. Schoettler Harshil Bhatt
Harshil Bhatt Sumithira Vasu3
Sumithira Vasu3