- 1Department of Health Management, The Third Xiangya Hospital, Central South University, Changsha, China
- 2Department of Neurology, The Second Xiangya Hospital, Central South University, Changsha, China
- 3Department of Neurology, The Affiliated Changsha Hospital of Xiangya School of Medicine, Central South University, Changsha, China
The presence of anti-GQ1b antibodies in serum or cerebrospinal fluid is a diagnostic indicator of the Miller–Fisher variant of Guillain–Barré syndrome (GBS), whereas anti-GQ1b antibody syndrome is rarely presented as acute bilateral pain in the cheeks and masticatory muscle fatigue without ophthalmoplegia, ataxia, or limb weakness. Here, we report a case of a female patient diagnosed with GBS characterized only by the involvement of the facial and trigeminal nerves who was positive for serum anti-GQ1b antibodies secondary to Mycoplasma pneumoniae infection. The patient was treated with macrolide antibiotics and neurotrophic drugs, and her symptoms were significantly alleviated after 1 month. This case indicates a new clinical presentation of GBS and anti-GQ1b antibody syndrome with a differential diagnosis of multiple cranial nerve damage of which neurological physicians should be aware. Positive anti-GQ1b antibodies secondary to infection were observed in this case, and antibiotic treatment resulted in a favorable prognosis. The specific underlying mechanism requires further investigation.
Introduction
GQ1b gangliosides are expressed in the extraocular neuromuscular junctions, extraocular muscle spindles, and paranodal regions of the extraocular cranial nerves (1, 2). Anti-GQ1b antibodies targeted to these regions develop in response to similar antigens from invading pathogens, such as Campylobacter jejuni, Cytomegalovirus, Epstein–Barr virus, or Mycoplasma pneumoniae (3). Anti-GQ1b antibody syndrome has overlapping clinical manifestations, including Miller–Fisher syndrome (MFS), Guillain-Barré syndrome (GBS) with ophthalmoplegia, Bickerstaff’s brain stem encephalitis, and acute ophthalmoparesis without ataxia (4).
Isolated cranial nerve damage without ataxia reported previously includes cranial nerves III, IV, VI, VII, VIII (5), and IX (6, 7); however, it is less reported in patients with anti-GQ1b antibodies in general. Herein, we report a case of a female patient diagnosed with GBS characterized only by the involvement of the facial and trigeminal nerves, who was positive for serum anti-GQ1b antibody secondary to M. pneumoniae infection. Diagnosis was challenging due to the atypical presentation, which supplemented the further understanding of anti-GQ1b antibody syndrome. The patient made a full recovery without the use of immunotherapy. Patients with positive anti-GQ1b antibodies do not present the full complement of MFS, which has been reported previously (3, 8). However, involvement of the facial and trigeminal nerves presenting as facial pain and limited mouth opening has not been previously reported. This report further expands the phenotypic spectrum of anti-GQ1b syndrome. The patient provided written informed consent for the publication of this report.
Case description
A 14-year-old girl was admitted to our department because of mouth opening limitation and facial pain for 3 days. She developed a cough after a cold, accompanied by white sticky sputum. The symptoms gradually aggravated, and on day 6, she was diagnosed with pulmonary infection in the local county hospital and treated with fosfomycin sodium and ribavirin. During the pneumonia treatment, she experienced a sudden onset of mouth opening limitation and complained of bilateral pain in the cheeks with facial paralysis. Magnetic resonance image (MRI) scan of the head and electromyogram were normal. Treatment with fosfomycin sodium and ribavirin lasted for 6 days, alleviating the cough while the intracranial nerve dysfunction was more severe. She was admitted to our department 13 days after the onset of the disease. The patient had no previous medical history, including fever, tetanus, and botulism infection, and her family history was similar.
Upon admission to our hospital, physical examinations showed dysfunction of the motor branch of the facial and trigeminal nerves (Figures 1A, B). The bilateral temporal and masseter muscles presented with tenderness but no atrophy, and the patient was unable to bite or open her mouth. There was no mandibular deviation, and the bilateral corneal reflex was present. The patient was unable to wrinkle her brows, bilateral forehead wrinkles disappeared, and the orbicularis oculi muscle was weakened. The patient presented shallow bilateral nasolabial muscles and an inability to show the teeth, gills drums, or whistle. The rest of the physical examination was unremarkable. She was afebrile and systemically well with no evidence of meningismus. Routine blood biochemical results were normal. Laboratory tests for infectious agents were positive for antibodies against M. pneumoniae (1:160), whereas cytomegalovirus, Epstein–Barr, herpes simplex virus (HSV), rubella, coxsackie, hepatitis B and C viruses, HIV, Toxoplasma gondii, and Borrelia burgdorferi were all negative. An extensive immunological investigation included autoimmune markers (ANA, APCA, ASMA, P-ANCA, C-ANCA, anti-DNA, and RA), which were normal. Cerebrospinal fluid (CSF) showed albuminocytological dissociation (pressure 140 mm H2O, the liquid was colorless and transparent, white cell concentration 2 ×106/L, protein concentration 1338.8 mg/L, and normal glucose). CSF culture and PCR for rubella, Borrelia, morbilli, varicella-zoster virus, HSV type 1, and HSV type 2 were negative. The CSF was negative for ganglioside antibody, but the serum was positive for GQ1b IgG antibody.

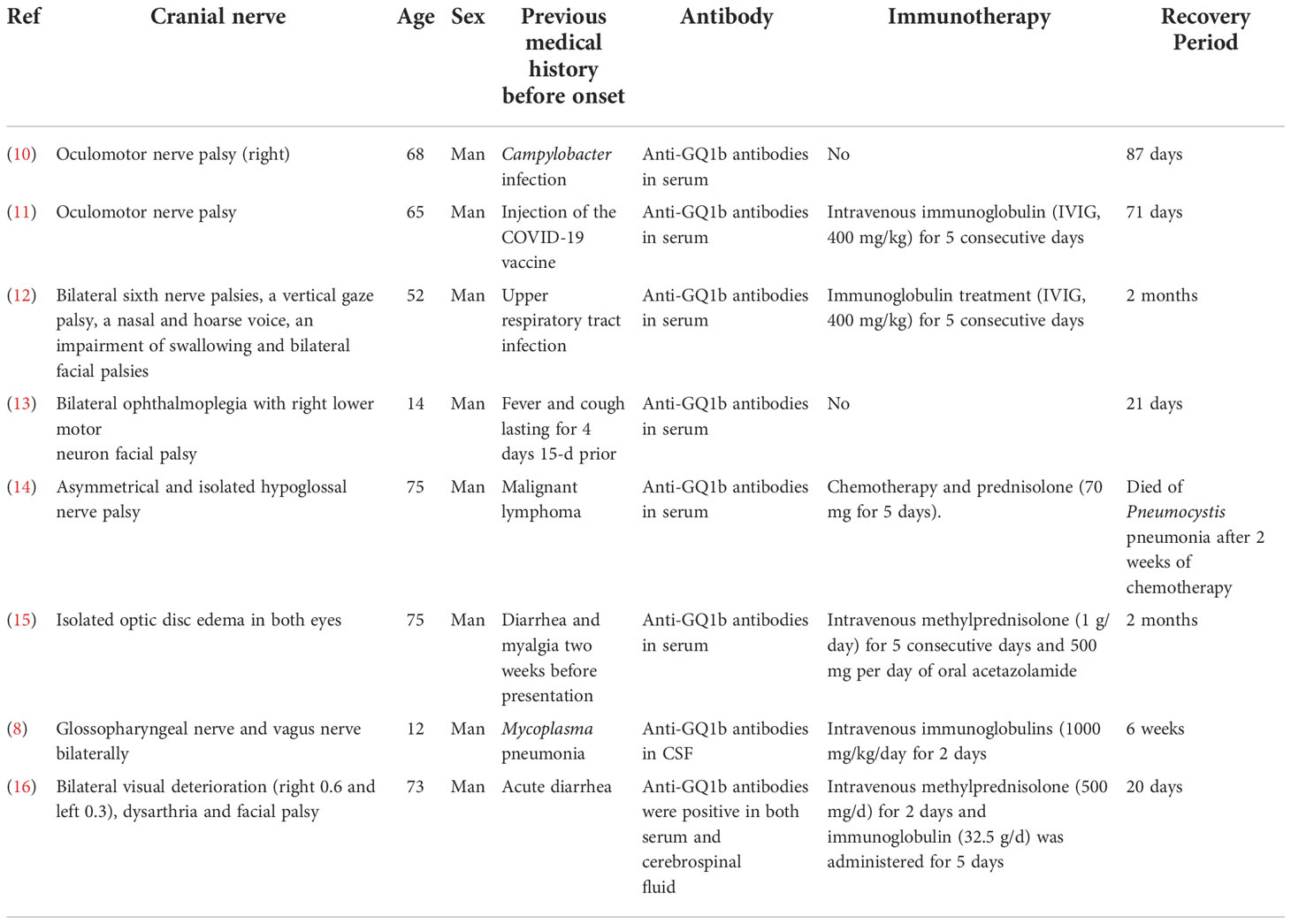
Figure 1 Clinical examination reveals dysfunction of facial nerve and the motor branch of the trigeminal nerve. (A) Bilateral facial weakness and Bell’s sign, (B) Limited mouth opening (tried the most effort to open her mouth); (C-E). Multiple lesions in the lower lobe of the right lung; (F-J). Head magnetic resonance imaging was normal.
A lung CT scan revealed multiple lesions in the lower lobe of the right lung, suggesting lung infection (Figures 1C–E). Combining these results with laboratory tests, the patient was diagnosed with M. pneumoniae infection. An X-ray of the mandibular joint and head MRI were normal (Figures 1F–J). Nerve conduction velocity and electromyography (EMG) were performed on day 15. Motor nerve conduction of the bilateral facial nerves (temporal, zygomatic, and buccal branches) were prolonged and accompanied by potential amplitude decreases. Needle EMG of the bilateral buccinator and orbicularis muscles and the right musculus dormitator showed reduced recruitment of motor units with obvious spontaneous activity, indicating demyelination and axonal loss. Blink reflex R1 and R2 latency was prolonged with a discrete waveform. The examination revealed that the bilateral blink reflex and facial nerve presented with remarkable demyelination and axonal loss (Figure 2). These findings, in combination with the clinical history, highly suggested a GBS variant with facial nerve dysfunction.
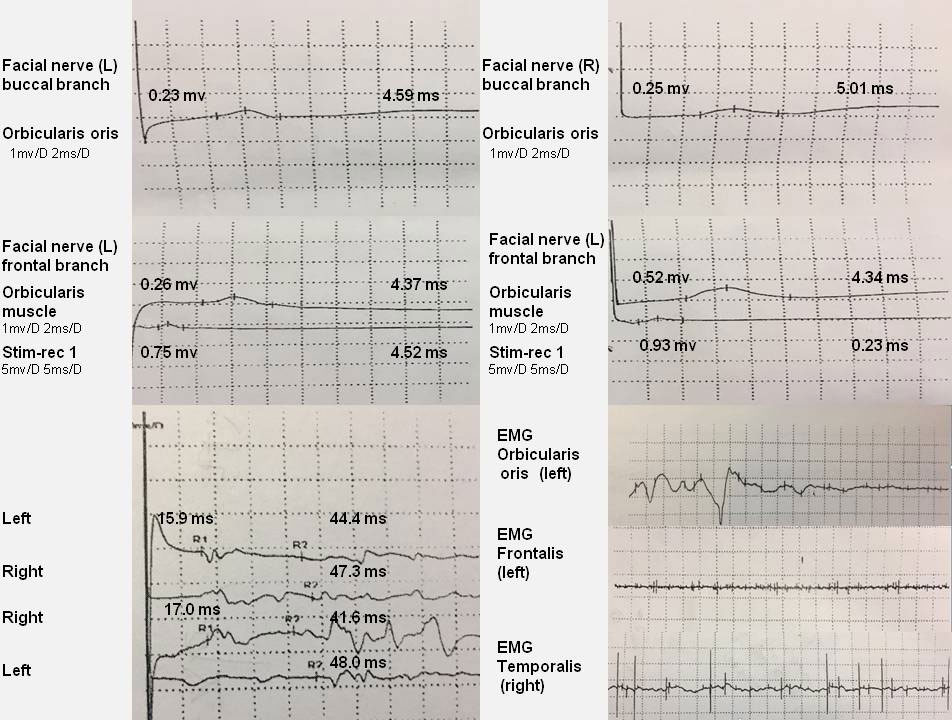
Figure 2 EMG reveals that the facial nerve has obvious demyelination and axonal loss. Motor nerve conduction of the bilateral facial nerve (temporal, zygomatic, and buccal branches) is prolonged, and amplitude potentials are decreased. EMG of the bilateral buccinator and orbicularis muscle and the right musculus dormitator show reduced recruitment of motor units with obvious spontaneous activity. Blink reflex R1 and R2 latency is prolonged with a discrete waveform.
Given the physical exam, it was not important to diagnose viral, traumatic, and neoplastic encephalitis. The patient was diagnosed with a GBS variant involving the fifth and eighth cranial nerves, which may be related to anti-GQ1b antibodies after M. pneumoniae infection. In addition to the albuminocytologic dissociation in the CSF and anti-GQ1b antibodies in the serum, the findings of M. pneumoniae infection history, clinical manifestation, and EMG showed that the bilateral blink reflex and facial nerve presented with notable demyelination and axonal loss, all supporting a GBS diagnosis. Considering that nervous dysfunction does not involve breath and the medical economic burden, the patient was only treated with macrolide antibiotics and neurotrophic drugs. The cough and expectoration were much alleviated, and the bilateral buccal muscle pain and mouth opening restriction were also significantly improved after 1 month of treatment. At the third month of follow-up, the patient’s symptoms of facial palsy completely resolved.
Discussion
To diagnose this case, the pathological changes in the bilateral masticator and facial nucleus were determined without involving other brain nuclei groups and pyramidal or extrapyramidal systems. The lesion involving only the bilateral trigeminal nerve motor branch and facial nerve, excluding the masticator and facial nucleus, was considered. The examination of brain MRI and EMG results demonstrated a peripheral lesion of the bilateral trigeminal nerve motor branch and facial nerve. To make an etiological diagnosis, tetanus was excluded based on the lack of exposure to tetanus toxin or Clostridium tetani. However, the patient was diagnosed with pneumonia before the neurological deficit appeared, subsequently anti-GQ1b and anti-M. pneumoniae IgG were detected in serum, and albuminocytologic dissociation was observed in the CSF. Therefore, the etiologic diagnosis was focused on inflammation and an autoimmune reaction, indicating a diagnosis of anti-GQ1b antibody syndrome with an MFS variant. A diagnosis of anti-GQ1b antibody syndrome with MFS depends on the clinical manifestation of bilateral ophthalmoplegia, decreased reflexes, and ataxia together with the absence of limb weakness and CNS involvement (9). Albuminocytological dissociation in the CSF or electrodiagnostic evidence of neuropathy are considered supplementary to support the diagnosis (9).
The patient complained mainly of facial pain and limited mouth opening, which is a new type of GBS variant that has not been reported before. The patient was first misdiagnosed as having mandibular joint disorder. However, examination of temporomandibular joint and brain MRI were conducted repeatedly and were normal, suggesting a different diagnosis. Subsequently, albuminocytologic dissociation in the CSF and M. pneumoniae antibodies (1:160) were identified in the serum. This case featured limited involvement of the facial and trigeminal nerves with positive serum anti-GQ1b antibody titers secondary to M. pneumoniae infection.
Until now, isolated cranial nerve damage without ataxia reported previously included only cranial nerves III, IV, VI, VII, VIII, and IX in anti-GQ1b antibody syndrome (Table 1). Respiratory infections more frequently precede the neurological onset of patients that are anti-GQ1b positive, whereas C. jejuni infection is a preceding infection in a smaller proportion of patients. In a clinical study including 213 patients with GBS, 58% of pediatric patients with GBS had M. pneumoniae-specific IgG antibodies in the serum compared with 19% of healthy children, whereas these percentages were 34% and 12%, respectively, in adults (17). The most common gangliosides antibody secondary to M. pneumoniae infection is anti-GalC antibodies (18). M. pneumoniae infection is the most important inducement of GBS (19). However, generation of anti-GQ1b antibodies secondary to M. pneumoniae infection is not common (19). A possible relationship between anti-GQ1b antibodies and M. pneumoniae infection was intriguing. Potential mechanisms involved in the generation of anti-GQ1b antibodies secondary to M. pneumoniae infection are presented as follows: (і) molecular mimicry—the M. pneumoniae cytoplasm contains potent immunogenic substances (e.g., glycolipids and glycoproteins) that elicit autoimmunity through molecular mimicry of various human cellular components, such as those of brain tissue (2, 20). However, the molecular mimicry between GQ1b and M. pneumoniae has not been confirmed. (ii) Abnormal antigen expression of GQ1b—we hypothesized that M. pneumoniae may enter the body and change the antigenicity of cells not expressing GQ1b, inducing the production of antibodies and T-cell reactions, resulting in antigen–antibody combinations leading to neurological manifestation.
M. pneumoniae–related anti-GQ1b antibody syndrome presents as Bickerstaff brainstem encephalitis (21), MFS, GBS with ophthalmoplegia (22), or isolated bulbar involvement (8). The ophthalmoplegia and oropharyngeal symptoms frequently observed in various anti-GQ1b syndromes are due to the abundant expression of ganglioside GQ1b on the cranial nerves, including the oculomotor (III), trochlear (IV), abducens nerves (VI), glossopharyngeal (XI), and vagal nerves (XI) (23). As far as we know, no reports indicate the binding of anti-GQ1b autoantibodies to the trigeminal nerve (V). This is the first case of anti-GQ1b antibody syndrome presenting as bilateral cheek pain and masticatory muscle fatigue without ophthalmoplegia, bulbar paralysis, limb weakness, ataxia, and sensory disturbance. However, whether ganglioside GQ1b is abundant on cranial nerve V and how anti-GQ1b antibodies result in damage requires further investigation. We hypothesized that antibody binding to antigen produced antigen–antibody complexes, followed by activated, soluble complement protein deposition forming a membrane-attack complex on nearby non-GQ1b expressing cells, resulting in “bystander injury” and causing damage to cells not expressing GQ1b (24, 31). In addition, antigen–antibody complex formation may recruit T cells and phagocytic cells to engulf damaged cells, including nearby non-GQ1b expressing cells. Therefore, the bystander injury is derived from complement-dependent cytotoxicity or antibody-dependent cell-mediated cytotoxicity (24, 31) (Figure 3). The specific mechanism requires further investigation.
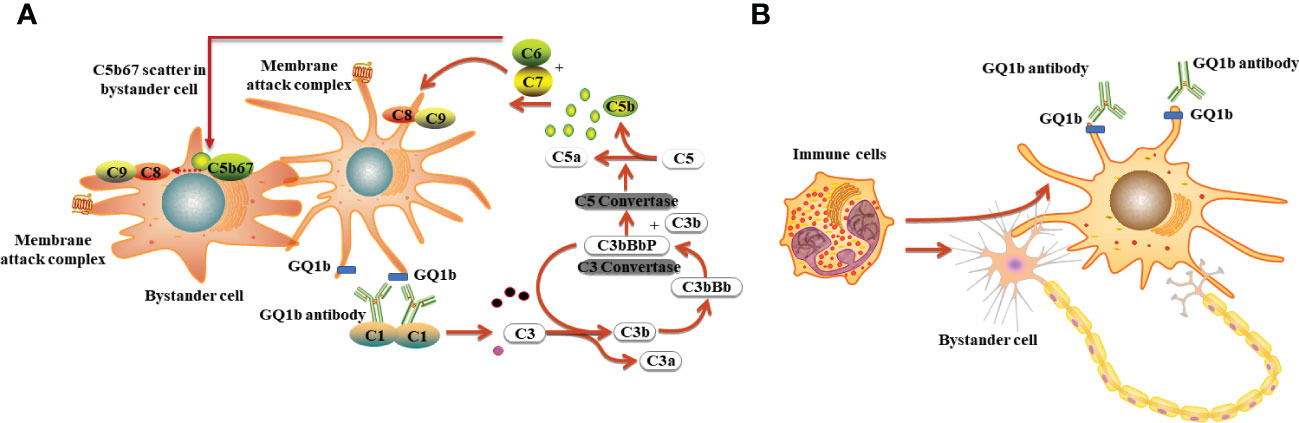
Figure 3 The possible mechanisms underlying anti-GQ1b antibody syndrome following M. pneumoniae infection. (A) Complement dependent cytotoxicity: antibody binding to antigen produced antigen–antibody complexes, followed by activated, soluble complement protein deposition forming a membrane-attack complex on nearby non-GQ1b expressing cells, resulting in bystander injury and causing damage to cells not expressing GQ1b; (B) Antibody-dependent cell-mediated cytotoxicity: antigen–antibody complex formation recruiting T cells or other immune cells to engulf damaged cells including nearby non-GQ1b expressing cells.
Treatment of MFS is controversial because MFS is a self-limited disease (25). Mori et al. have found that intravenous immunoglobulin or plasmapheresis therapy does not seem to influence patient outcomes though it slightly hastens the alleviation of ophthalmoplegia and ataxia (32, 26). In line with previous studies, the case reported here was treated with only macrolide antibiotics and neurotrophic drugs and had a full recovery after long-term follow-up. However, some cases suggest the possible efficacy of immunomodulating therapy in MFS, where the choice of immunotherapy is based on the severity of clinical symptoms, such as profound ataxia, severe bulbar palsy, respiratory and motor impairment, or during pregnancy (27–29). Therefore, clinicians should not treat pure MFS using immunomodulating therapy until there is enough supporting evidence (30).
Conclusions
Patients presenting with only bilateral paralysis, facial muscle pain, and masticatory dysfunction may be assessed for a variant of GBS. Besides CSF examination, including cell count and biochemical analysis, ganglioside antibody spectrum detection and careful EMG examination should be performed. Once diagnosis of a rare type of GBS is suspected, treatment should be administered immediately. Active determination of inducing factors is indispensable for rare cases of GBS because they are both helpful for treatment and target the prevention of critical GBS presented as dyspnea. Finally, a different treatment may be administered depending on the severity of GBS. In this case, treatment with macrolide antibiotics successfully treated the pneumonia as well as the neurological deficit, without immunoregulatory treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SD drafted the manuscript. SO collected the data. LY and WL carried out the literature review. WY finalized the draft. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant 82102549), Natural Science Foundation of Hunan Province China P.R (Grant 2022JJ40724 ; 2022JJ30061), Science and Technology Innovation Guidance Project of Hunan Province (Grant Number: 2020SK53009), and Changsha Municipal Natural Science Foundation (Grant Number: kq2007037; kq2202402).
Acknowledgments
We thank the patient for allowing us to upload her medical and imaging data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Murakami K, Miyamoto K, Terayama A, Yoshikawa K, Kuwahara M, Ito H. Acute eye movement-retained internal ophthalmoplegia in atypical miller Fisher syndrome variants are associated with IgG anti-GQ1b antibodies. J Neuroimmunol (2022) 368:577880. doi: 10.1016/j.jneuroim.2022.577880
2. Lee SY, Lee YH, Chun BY, Lee SY, Cha SI, Kim CH, et al. An adult case of Fisher syndrome subsequent to mycoplasma pneumoniae infection. J Korean Med Sci (2013) 28:152–5. doi: 10.3346/jkms.2013.28.1.152
3. Jebaraj AP, Swiston CJ, Vegunta S, Warner JEA. Isolated bilateral internal ophthalmoplegia as an atypical initial presentation of anti-GQ1b antibody syndrome. J Neuroophthalmol (2022) 42:e389–e90. doi: 10.1097/WNO.0000000000001314
4. Yvon C, Nee D, Chan D, Malhotra R. Ophthalmoplegia associated with anti-GQ1b antibodies: case report and review. Orbit (2021), 1–4. doi: 10.1080/01676830.2021.1974495
5. Psillas G, Arnaoutoglou M, Gatsios T, Rizos D, Koutsouraki E, Vital V. Autoimmune recurrent facial palsy and bilateral sudden sensorineural hearing loss following Ramsay hunt-like syndrome. Auris Nasus Larynx (2012) 39:229–32. doi: 10.1016/j.anl.2011.03.007
6. Murakami K, Kajimoto Y, Ito H. Acute oropharyngeal palsy following bilateral adie's tonic pupils associated with anti-GT1a and GQ1b IgG antibodies. Intern Med (2022) 61(20):3121–24. doi: 10.2169/internalmedicine.8416-21
7. O'Leary CP, Veitch J, Durward WF, Thomas AM, Rees JH, Willison HJ. Acute oropharyngeal palsy is associated with antibodies to GQ1b and GT1a gangliosides. J Neurol Neurosurg Psychiatry (1996) 61:649–51. doi: 10.1136/jnnp.61.6.649
8. Verhelst H, Maes M, Deblaere K, Van Coster R. Palatal insufficiency as isolated sign of GQ1b antibody syndrome. Pediatr Neurol (2011) 44:292–4. doi: 10.1016/j.pediatrneurol.2010.11.010
9. Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barré Syndrome. Lancet (2021) 397:1214–28. doi: 10.1016/S0140-6736(21)00517-1
10. Ueno T, Kon T, Kurihara AI, Tomiyama M. Unilateral oculomotor nerve palsy following campylobacter infection: A mild form of miller Fisher syndrome without ataxia. Intern Med (2017) 56:2929–32. doi: 10.2169/internalmedicine.8824-17
11. Kubota T, Hasegawa T, Ikeda K, Aoki M. Case report: Isolated, unilateral oculomotor palsy with anti-GQ1b antibody following COVID-19 vaccination. F1000Res (2021) 10:1142. doi: 10.12688/f1000research.74299.1
12. Edvardsson B, Persson S. Polyneuritis cranialis presenting with anti-GQ1b IgG antibody. J Neurol Sci (2009) 281:125–6. doi: 10.1016/j.jns.2009.02.340
13. Kauser H, Jain P, Sharma S, Aneja S. Complete bilateral ophthalmoplegia with unilateral facial palsy in a child with anti-GQ1b syndrome. Indian J Pediatr (2015) 82:192–4. doi: 10.1007/s12098-014-1514-4
14. Ohta Y, Kawahara Y, Tadokoro K, Sato K, Sasaki R, Takahashi Y, et al. Asymmetrical and isolated hypoglossal nerve palsy accompanied by a new subset of anti-ganglioside antibodies in a patient with diffuse Large b cell lymphoma. Intern Med (2019) 58:283–86. doi: 10.2169/internalmedicine.1269-18
15. Kim SH, Baek SH, Lee SU, Yu S, Kim JS. Optic disc edema as a sole manifestation of anti-GQ1b antibody syndrome. J Neurol (2021) 268:2263–66. doi: 10.1007/s00415-021-10518-5
16. Zhao T, Deng Y, Ding Y, Zhang R, Zhou C, Lin W. Anti-GQ1b antibody syndrome presenting with visual deterioration as the initial symptom: A case report. Med (Baltimore) (2020) 99:e18805. doi: 10.1097/MD.0000000000018805
17. Meyer Sauteur PM, Huizinga R, Tio-Gillen AP, Roodbol J, Hoogenboezem T, Jacobs E, et al. Mycoplasma pneumoniae triggering the Guillain-barre syndrome: A case-control study. Ann Neurol (2016) 80:566–80. doi: 10.1002/ana.24755
18. de Groot RCA, Meyer Sauteur PM, Unger WWJ, van Rossum AMC. Things that could be mycoplasma pneumoniae. J Infect (2017) 74 Suppl 1:S95–s100. doi: 10.1016/S0163-4453(17)30198-6
19. Kuwahara M, Samukawa M, Ikeda T, Morikawa M, Ueno R, Hamada Y, et al. Characterization of the neurological diseases associated with mycoplasma pneumoniae infection and anti-glycolipid antibodies. J Neurol (2017) 264:467–75. doi: 10.1007/s00415-016-8371-1
20. Koga M, Gilbert M, Li J, Koike S, Takahashi M, Furukawa K, et al. Antecedent infections in Fisher syndrome: a common pathogenesis of molecular mimicry. Neurology (2005) 64:1605–11. doi: 10.1212/01.WNL.0000160399.08456.7C
21. Kikuchi M, Tagawa Y, Iwamoto H, Hoshino H, Yuki N. Bickerstaff's brainstem encephalitis associated with IgG anti-GQ1b antibody subsequent to mycoplasma pneumoniae infection: favorable response to immunoadsorption therapy. J Child Neurol (1997) 12:403–5. doi: 10.1177/088307389701200612
22. Hishida R, Baba M, Ozaki I, Matsunaga M, Saitoh T. A case of Guillain-barre syndrome with ophthalmoplegia showing high titers of anti-GQ1b and -GD1b antibodies in the serum. Rinsho Shinkeigaku (1995) 35:306–8.
23. Liu JX, Willison HJ, Pedrosa-Domellöf F. Immunolocalization of GQ1b and related gangliosides in human extraocular neuromuscular junctions and muscle spindles. Invest Ophthalmol Vis Sci (2009) 50:3226–32. doi: 10.1167/iovs.08-3333
24. Duan T, Smith AJ, Verkman AS. Complement-dependent bystander injury to neurons in AQP4-IgG seropositive neuromyelitis optica. J Neuroinflamm (2018) 15:294. doi: 10.1186/s12974-018-1333-z
26. Mori M, Kuwabara S, Fukutake T, Hattori T. Plasmapheresis and miller Fisher syndrome: analysis of 50 consecutive cases. J Neurol Neurosurg Psychiatry (2002) 72:680. doi: 10.1136/jnnp.72.5.680
27. Ángel-Páez JA, Hurtado-Bugna S, Aragón-Mendoza RL, Altman-Restrepo M, Díaz-Yamal IJ, Centanaro-Meza GA. Miller Fisher syndrome treated with plasmapheresis during pregnancy: Case report and review of the literature. Rev Colomb Obstet Ginecol (2021) 72:210–18. doi: 10.18597/rcog.3611
28. Ejma M, Waliszewska-Prosół M, Hofman A, Budrewicz S, Podemski R, Bilińska M, et al. Progressive subacute miller-Fisher syndrome successfully treated with plasmapheresis. Neurol Neurochir Pol (2015) 49:137–8. doi: 10.1016/j.pjnns.2015.03.002
29. Truong J, Conley J, Ashurst J. Miller-Fisher syndrome: A case report and review of the literature. Clin Pract cases Emerg Med (2020) 4:653–55. doi: 10.5811/cpcem.2020.7.48507
30. Bai HX, Wang ZL, Tan LM, Xiao B, Goldstein JM, Yang L. The effectiveness of immunomodulating treatment on miller Fisher syndrome: a retrospective analysis of 65 Chinese patients. J Peripher Nerv Syst (2013) 18:195–6. doi: 10.1111/jns5.12030
31. Duan T, Smith AJ, Verkman AS. Complement-independent bystander injury in AQP4-IgG seropositive neuromyelitis optica produced by antibody-dependent cellular cytotoxicity. Acta Neuropathol Commun (2019) 7:112. doi: 10.1186/s40478-019-0766-7
Keywords: Guillain-Barré syndrome, anti-GQ1b antibody syndrome, Mycoplasma pneumoniae, facial nerve, trigeminal nerve
Citation: Deng S, Yin L, Lu W, Ouyang S and Yin W (2022) Case Report: Isolated facial and trigeminal nerve palsy without ataxia in anti-GQ1b antibody syndrome secondary to Mycoplasma pneumonia. Front. Immunol. 13:1062567. doi: 10.3389/fimmu.2022.1062567
Received: 07 October 2022; Accepted: 28 November 2022;
Published: 14 December 2022.
Edited by:
Robert Weissert, University of Regensburg, GermanyReviewed by:
Luis Del Carpio-Orantes, Instituto Mexicano del Seguro Social, Delegación Veracruz Norte, MexicoAlvin Oliver Payus, Universiti Malaysia Sabah, Malaysia
Yuji Tomizawa, Juntendo University, Japan
Copyright © 2022 Deng, Yin, Lu, Ouyang and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifan Yin, eWlud2VpZmFuMTk4NUBjc3UuZWR1LmNu
 Shuwen Deng
Shuwen Deng Lihong Yin2
Lihong Yin2 Wei Lu
Wei Lu Song Ouyang
Song Ouyang Weifan Yin
Weifan Yin