- 1Department of Gynecology and Obstetrics, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 2Department of Gynecology and Obstetrics, Fu Xing Hospital, Capital Medical University, Beijing, China
- 3Department of Hepatology Division 2, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 4Department of Hepatology Division 2, Peking University Ditan Teaching Hospital, Beijing, China
- 5Department of Medical Records, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Objective: To explore the correlation between postpartum hepatitis and changes of plasmacytoid dendritic cells’ (pDC) function and frequency in hepatitis B e antigen (HBeAg)-positive pregnant women with chronic hepatitis B virus (HBV) infection.
Methods: Pregnant women with chronic HBV infection receiving antiviral treatment (treated group) or not receiving antiviral treatment (untreated group) were enrolled and demographic information was collected before delivery. Clinical biochemical, virological serology, pDC frequency and functional molecular expression were tested before delivery and at 6, 12, 24 weeks after delivery.
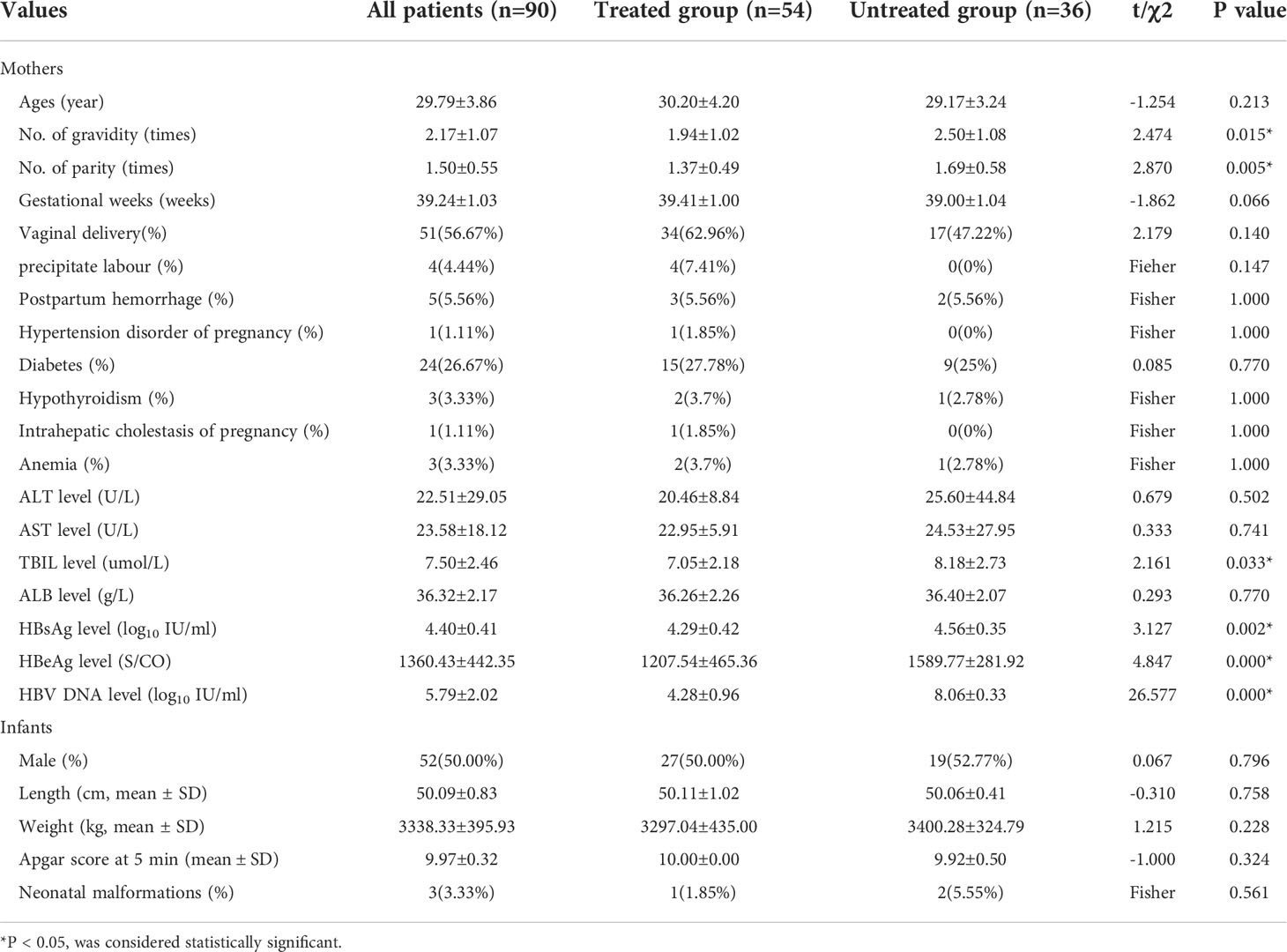
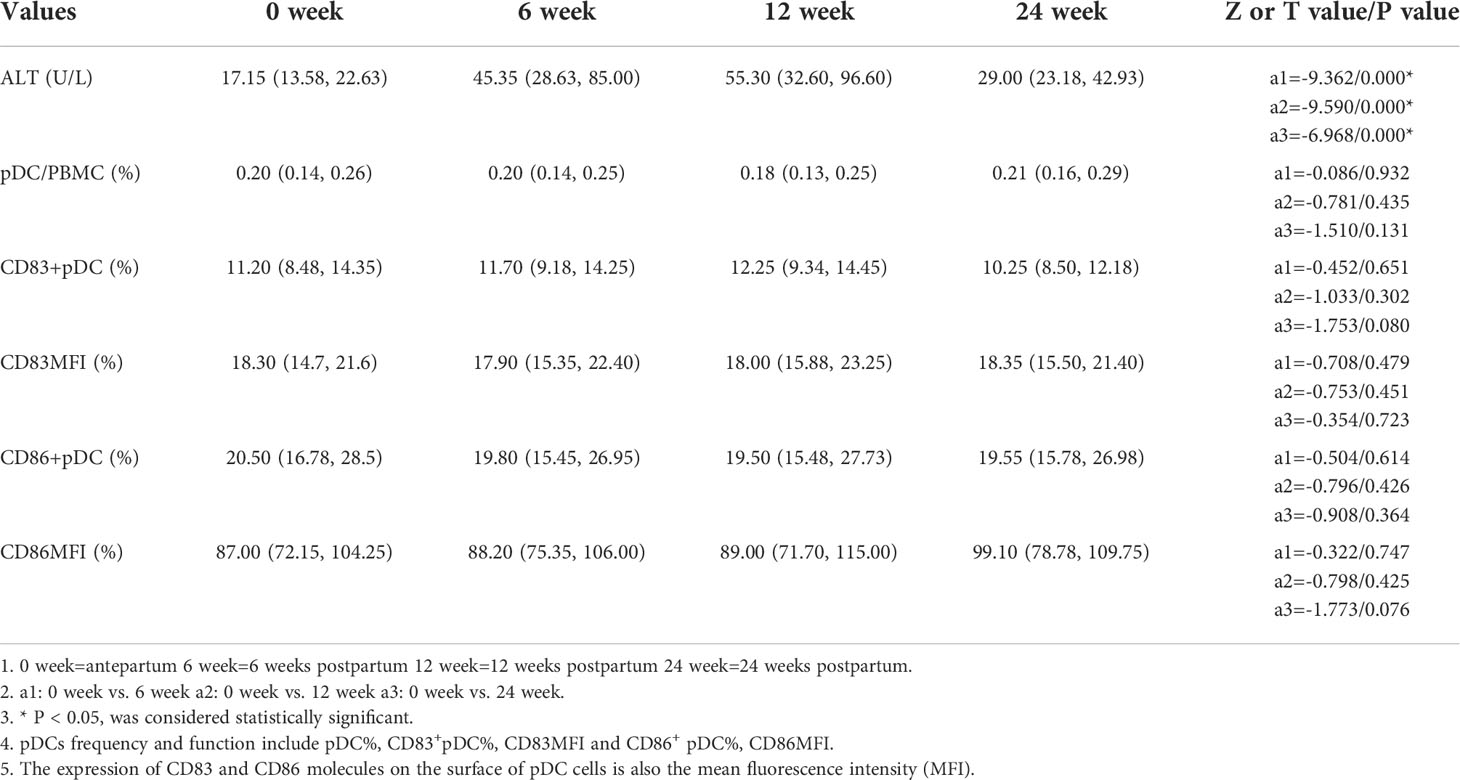
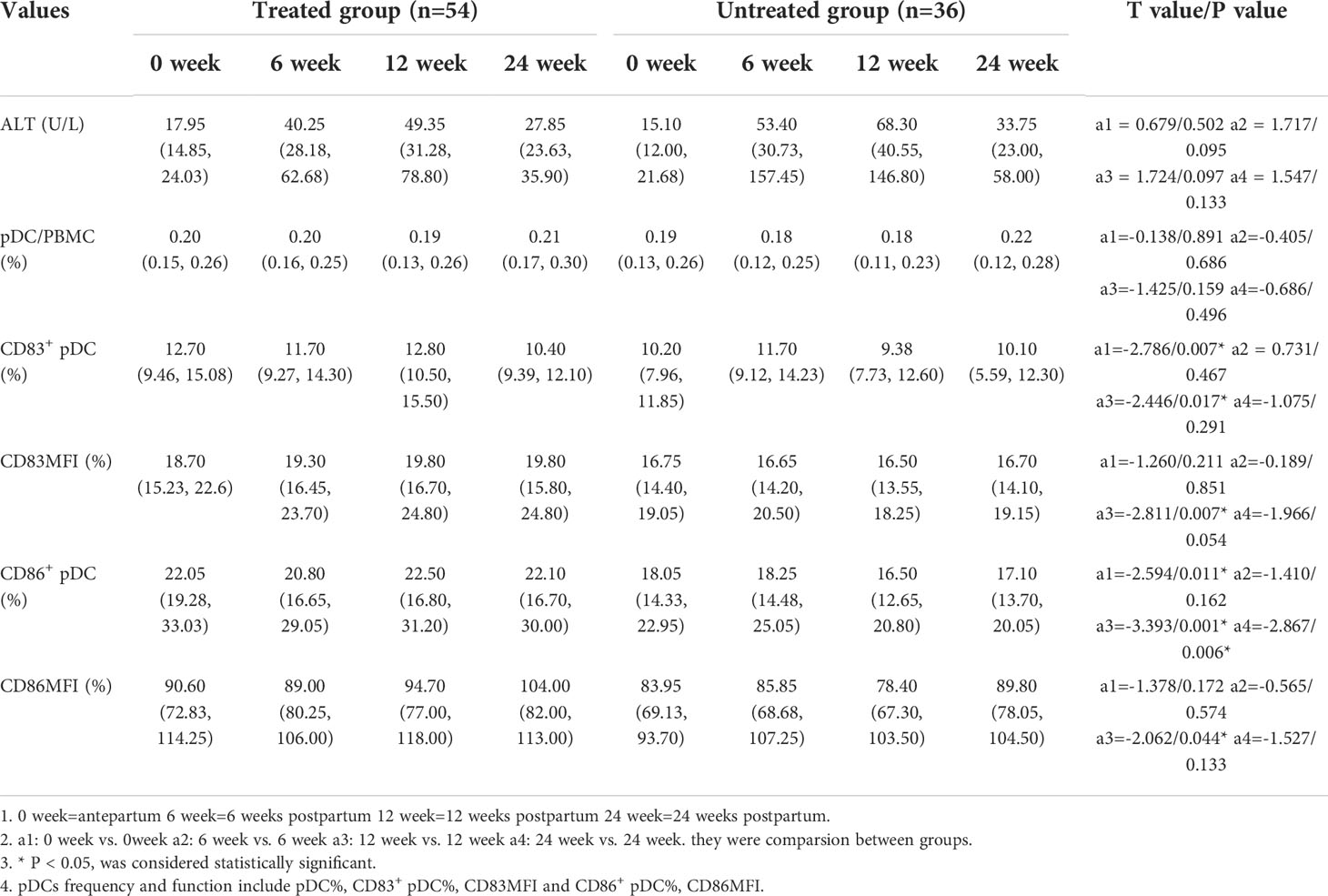
Results: 90 eligible pregnant women were enrolled, 36 in the untreated group and 54 in the treated group. 36 patients developed postpartum hepatitis, including 17 (17/36, 47.2%) in the untreated group and 19 (19/54, 35.2%) in the treated group (χ2 = 1.304 p=0.253), and 22 cases of hepatitis occurred at 6 weeks postpartum, 12 at 12 weeks postpartum, and 2 at 24 weeks postpartum. The alanine transaminase (ALT) levels at any time postpartum were significantly higher than that of the antepartum, especially at 6 weeks and 12 weeks postpartum. However, the frequencies of pDCs, CD83+ pDCs and CD86+ pDCs antepartum had no significant difference from any time postpartum. The frequencies of CD83+ pDCs, CD86+ pDCs in the treated group antepartum were significantly higher than those in the untreated group [12.70 (9.46, 15.08) vs. 10.20 (7.96, 11.85), p=0.007; 22.05 (19.28, 33.03) vs. 18.05 (14.33, 22.95), p=0.011], and the same at 12 weeks postpartum [12.80 (10.50, 15.50) vs. 9.38 (7.73, 12.60), p=0.017; 22.50 (16.80, 31.20) vs. 16.50 (12.65, 20.80), p=0.001]. The frequency of CD86+ pDCs in the treated group was significantly higher than that in the untreated group at 24 weeks postpartum [22.10 (16.70, 30.00) vs. 17.10 (13.70, 20.05), p=0.006].
Conclusions: Postpartum hepatitis in HBV infected women mainly occurs at 6-12 weeks postpartum. Antiviral treatment during pregnancy can significantly increase the frequencies of CD83+ pDCs and CD86+ pDCs in pregnant women with chronic HBV infection.
Introduction
Mother-to-child transmission (MTCT) of HBV is the main cause of chronic HBV infection in China. The rate of HBV MTCT maintained 7%-11% in newborns of HBeAg-positive mothers despite combined active-passive immunization with a hepatitis B vaccine and hepatitis B immunoglobulin (HBIG) shortly after birth. HBeAg positive and high HBV DNA level (HBV DNA > 105 IU/ml) in pregnant women are the main cause of HBV MTCT (1, 2). Antiviral treatment during the third trimester can significantly reduce the rate of MTCT in pregnant women with high HBV DNA level (3, 4).
pDCs can secrete a large amount of cytokine interferon (IFN) with pleiotropic effect during viral infection, and they have a strong ability to activate the initial immune response and activate natural killer (NK) cells and T lymphocytes to further activate and regulate the antiviral immune response (5). They play a vital role in the control and prognosis of HBV infection. Studies have shown that hepatitis B surface antigen (HBsAg) and HBeAg can inhibit the frequency and function of pDCs in chronic HBV infected patients, leading to the impairment of antigen presenting function of pDCs (6, 7).
The elevation of postpartum ALT is a common phenomenon. It is believed that due to the decrease of steroid hormone levels after delivery, the immunosuppressive state during pregnancy is rapidly relieved, triggering the immune response to HBV and mediating liver tissue injury and hepatocyte necrosis in patients with chronic hepatitis B (CHB). Hepatocyte necrosis leads to elevation of ALT and indicates that these women have entered the immune clearance period or the reactivation period (8), which is a good time to treat hepatitis (7, 9).
However, there are few studies on the changes of postpartum immune cell function of HBeAg-positive pregnant women with chronic HBV infection and its association with postpartum hepatitis, and few reports about the effects of antiviral treatment during pregnancy on postpartum immune cell function and postpartum hepatitis. Therefore, we designed a prospective case-cohort study and recruited HBeAg-positive chronic HBV infected pregnant women after informed consent. We measured the frequency of pDCs and the expression of functional molecules in peripheral blood antepartum and at 6, 12, 24 weeks postpartum to explore the frequency and functional changes of pDCs after delivery.
Materials and methods
Research objects
Pregnant women with chronic HBV infection were enrolled in the study from January 2016 to January 2018. Inclusion criteria: 1. Between the ages of 20 and 40; 2. HBsAg and HBeAg positive over 6 months, and serum HBV DNA > 2.0×105 IU/ml; 3. Liver function remained normal; 4. With or without antiviral treatment at 24-28 weeks of gestation as they wish. Exclusion criteria: 1. History of amniocentesis during pregnancy; 2. History of hereditary diseases in one or both families of the couple; 3. Fetal abnormalities detected before enrollment by imaging or other prenatal tests, including genetic testing; 4. Coinfection with hepatitis C virus, hepatitis D virus, human immunodeficiency virus, syphilis, toxoplasmosis, rubella, or cytomegalovirus; 5. History of two or more spontaneous abortions; 6. Previous delivery of a child with deformity; 7. Twin or multiple pregnancies; 8. The mother had a history of cancer or autoimmune diseases.
Pregnant women were classified into treated group (with antiviral treatment at 24-28 weeks of gestation) and untreated group (without antiviral treatment during pregnancy). Antiviral therapy was stopped immediately after delivery and all women were followed up at 6, 12 and 24 weeks postpartum.
All subjects signed an informed consent. The study was approved by the ethics committee of Beijing Ditan Hospital, Capital Medical University (JDL-2017-004-01), and registered at clinicaltrials.gov (Clinical registration No: NCT03214302).
Blood specimen collection
2 ml of peripheral venous blood of all pregnant women was collected by EDTA anticoagulant violet tube at the time of antepartum (baseline) and 6, 12, 24 weeks postpartum, cells were collected by BD FACS Calibur within 4 hours of blood collection.
Virological indexes and clinical biochemical indicators
Virological indexes (HBeAg, HBsAg and HBV DNA) and ALT, glutamic oxaloacetic transaminase (AST), total bilirubin (TBIL), albumin (ALB) were tested by the laboratory of Beijing Ditan Hospital, Capital Medical University.
pDC functional detection
pDCs were detected with Lineage1-FITC, HLA-DR-PerCP, Anti-CD123-APC, Anti-CD83-PE, Anti-CD86-PE antibodies, and CD83MFI and CD86MFI data were analyzed by FlowJo7.6 software.
Statistical analysis
Data were analyzed by SPSS 19.0 software. All data were presented as mean ± standard deviation (mean ± SD) or median, interquartile interval [median (Q1, Q3)], box-and-whisker plots or percentage. Student t-test was used for normal distribution data and Mann-Whitney test was used for skewness distribution data between groups. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
90 pregnant women were enrolled, including 54 cases with antiviral treatment (treated group) and 36 cases without antiviral treatment (untreated group). There was no difference between the two groups in age, gestational weeks, pregnancy complications, neonatal weight, length, Apgar score at 5 minutes and neonatal malformations (P>0.05). The number of gravidity and parity in the untreated group were significantly higher than those in the treated group (p=0.015 and 0.005, respectively). The level of TBIL was 7.05±2.18umol/l in the treated group and 8.18±2.73umol/l in the untreated group (P=0.033), but TBIL in both groups was within the normal range. The levels of HBsAg, HBeAg and HBV DNA antepartum in the treated group were significantly lower than those in the untreated group (P<0.05), which was related to antiviral therapy. The basic information is shown in Table 1.
Changes of pDC and ALT after delivery
In this study we use ALT≥ 2 times the upper limit of normal (40 U/L) as the diagnostic criterion for the occurrence of hepatitis according to the Asian-Pacific clinical practice guidelines on the management of hepatitis B (10). The ALT levels of all pregnant women enrolled were significantly higher at 6, 12 and 24 weeks postpartum than those of antepartum (P<0.05), postpartum hepatitis occurred in 36 cases, 22 cases occurred at 6 weeks postpartum, 12 at 12 weeks postpartum, 2 at 24 weeks postpartum. 17 cases (17/36) of abnormal ALT occurred in the untreated group and 19 (19/54) occurred in the treated group, with no significant difference (χ2 = 1.304, p=0.253). The frequencies of antepartum pDCs, CD83+ pDCs and CD86+ pDCs in all pregnant women enrolled were not significantly different from those at 6, 12 and 24 weeks postpartum (P > 0.05) (Table 2).
Frequency and expression of functional molecules of pDC cells and ALT in HBV infected pregnant women postpartum
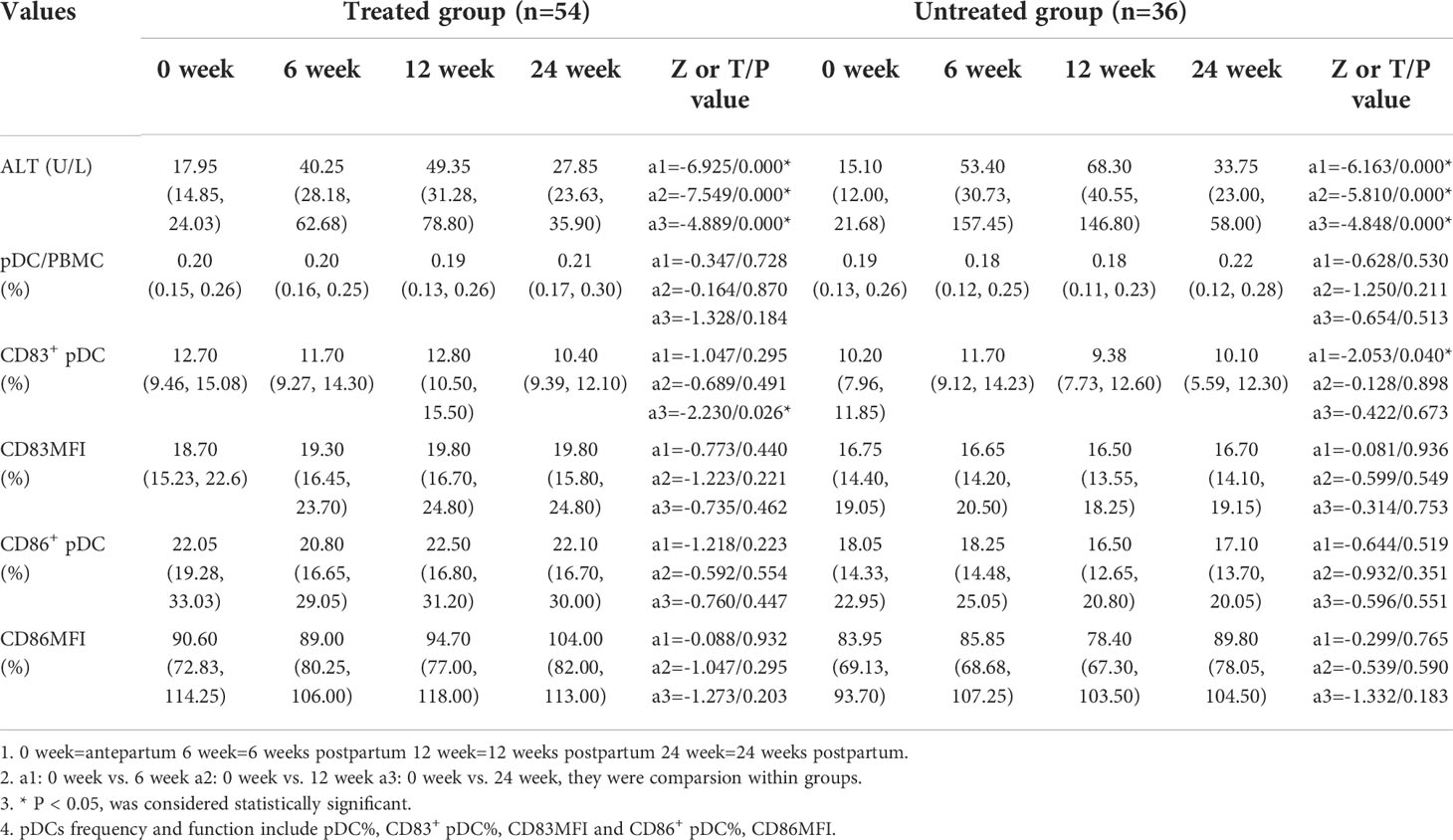
The ALT levels were significantly higher at 6, 12, and 24 weeks postpartum than those of the antepartum in both treated and untreated group (p<0.001). In the treated group, the frequency of CD83+ pDCs antepartum was significantly higher than that of the 24 weeks postpartum [12.70(9.46, 15.08) vs. 10.40(9.39, 12.10), p=0.026]. In the untreated group, the frequency of CD83+ pDCs antepartum was significantly lower than that of the 6 weeks postpartum [10.20 (7.96, 11.85) vs. 11.70 (9.12, 14.23), p=0.040] (Table 3).
Effect of antiviral therapy on the frequency and the expression of functional molecules of pDCs and ALT
The frequencies of CD83+ pDCs and CD86+ pDCs in the treated group were significantly higher than those in the untreated group before delivery [12.70 (9.46, 15.08) vs. 10.20 (7.96, 11.85), p=0.007; 22.05 (19.28, 33.03) vs. 18.05 (14.33, 22.95), p=0.011]. The frequencies of CD83+ pDCs and CD86+ pDCs in the treated group at 12 weeks postpartum were significantly higher than those in the untreated group [12.80 (10.50, 15.50) vs. 9.38 (7.73, 12.60), p=0.017; 22.50 (16.80, 31.20) vs. 16.50 (12.65, 20.80), p=0.001]. The frequency of CD86+ pDCs in the treated group at 24 weeks postpartum was significantly higher than that in the untreated group [22.10 (16.70, 30.00) vs. 17.10 (13.70, 20.05), p=0.006]. It is suggested that antiviral treatment in the third trimester of pregnancy can significantly increase the frequencies of CD83+ pDCs and CD86+ pDCs in peripheral blood antepartum and postpartum (Table 4).
Discussion
Dendritic cells (DCs) can be divided into pDCs and myeloid dendritic cells (mDCs) according to the source of precursor cells. According to specific functions, DCs can be divided into immature DCs and mature DCs, most of which are immature DCs under normal circumstances and have a strong ability to capture antigens (11, 12). However, the surface of immature DCs lacks some costimulatory molecules, which cannot provide necessary costimulatory signals for T cell activation, making T cells unable to be fully activated and in a state of loss of function, meanwhile, immature DCs also induce antigen specific regulatory T cells, block the functional response of effector T cells, and also can induce immune tolerance (12–14). Mature DCs stimulate T lymphocytes by expressing major histocompatibility complexes and costimulatory molecules, and secreting cytokines such as IFN, thus initiating the cellular immune response (14, 15). At present, research suggests that CD86 is a marker of DC activation, and CD83 is a specific marker of DC maturation. DC activation and maturation increase the expression of cell surface molecules CD86 and CD83, otherwise inhibit the differentiation and maturation of DCs, and reduce the expression of cell surface molecules CD86 and CD83 (16–19).
Recently, the function of pDCs has attracted extensive attention in the field of chronic HBV infection. As the only innate immune cells with antigen presenting ability, which can activate non-sensitized initial T cells, pDC is the main effector cell of human antiviral immunity and plays a double-acting role in antiviral treatment, which can mediate both immune response and immune tolerance (12, 14, 16). Mature pDCs can secrete a large amount of cytokines with pleiotropic effects to directly inhibit viral replication during viral infection, and can also activate mDCs, T cells, NK cells and B lymphocytes to form protective immune response, becoming a bridge connecting natural immunity and acquired immunity, with strong ability to activate initial immune response. Immature pDCs have a strong ability to capture antigen, but the ability to present antigen is weak, making T cells cannot be fully activated, and have a strong ability to induce regulatory T cells, which mediates the induction and maintenance of immune tolerance (14–18).
Studies have suggested that HBsAg and HBeAg in patients with chronic HBV infection can inhibit the function of DCs, which leads to a decrease in the frequency and function of pDCs in patients with chronic HBV infection (20, 21). Our previous study on the immune function of HBeAg positive chronic hepatitis B patients found that the frequency of pDCs was related to the incidence of CHB, the lower the frequency of pDCs in CHB patients, the more likely they were to develop hepatitis. However, we did not distinguish immature pDCs and mature pDCs frequency changes on the impact of hepatitis in this study (7, 22). Our previous retrospective study and this prospective study both suggest that postpartum liver dysfunction is common in women with chronic HBV infection, we also found that the abnormal liver function of HBV infected women mainly occurred in 6-12 weeks postpartum (23, 24), which indicated the importance of preventing postpartum hepatitis. We further studied the effect of antiviral therapy in the third trimester on postpartum hepatitis, and found that with the reduction of HBV DNA level due to the effective antiviral therapy during pregnancy, the inhibition of the virus on the pDCs function was alleviated. Based on this result, we also studied the effect of antiviral treatment in the third trimester of HBeAg-positive chronic HBV infected women on the frequency and function of postpartum peripheral blood pDCs and ALT level. Our study found that the ALT level of the pregnant women receiving antiviral treatment in the third trimester has a downward trend at 6-12 weeks postpartum, but there is no statistical significance between the two groups, which may be related to the limited number of patients in our study. Our study also found that there was no significant change in the frequency of pDCs after antiviral treatment during pregnancy, but it could significantly increase the frequencies of CD83+ pDCs and CD86+ pDCs in pregnant women with chronic HBV infection before delivery and 12 weeks postpartum. This might indicate that antiviral therapy in late pregnancy increases the proportion of mature pDCs, the immune activity and antiviral function of pDCs, which may improve the success rate of postpartum hepatitis treatment. However, this study had a small sample, and only involved the changes of pDCs in peripheral blood, without discussing the changes of the immune function in the liver, therefore, if we want to elucidate the above causal relationship, we need to further enlarge the sample and investigate the changes of the immune function in liver, as well as in peripheral blood, which would shed new light on the prevention and treatment of postpartum hepatitis in HBeAg positive chronic HBV infected pregnant women.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The ethics committee of Beijing Ditan Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
FW, GW, LZ, ML, and YX contributed to study concept and design. MS, YH, YLi, XB, YLu, TJ, WD, SyW, FS, ZZ, GS, RL, MC, SlW, YG, HH, MX, XC, and LH performed the data collection. FW, GW, LZ, ML, and YX conducted data analysis. FW, MS, YH, LY, and XB wrote the first draft. FW edited the English version. GW, LZ, ML, and YX approved the submitted version after modification. All authors contributed to the article and approved the submitted version.
Funding
Project supported by Beijing Science and Technology Commission (Z211100002921059). National Science and Technology Major Project of China (2017ZX10201201-001-006, 2017ZX10201201-002-006, 2018ZX10715-005-003-005). The Capital Health Research and Development of Special (2022–1–2172). The Digestive Medical Coordinated Development Center of Beijing Hospitals Authority (XXZ0302 and XXT28). Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (XMLX 202127). High-level Public Health Technical Personnel Training Program of Beijing Municipal Health Commission (2022–3–050).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XW declared a shared affiliation with the authors to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Huang Y, Li L, Sun X, Lu M, Liu H, Tang G, et al. Screening of pregnant women for hepatitis bvirus surface antigen (HBsAg) and subsequent management, qiandongnan prefecture, guizhou, China, 2010. Vaccine (2013) 31:J62–5. doi: 10.1016/j.vaccine.2013.05.103
2. Hu Y, Yu H. Prevention strategies of mother-to-child transmission of hepatitis b virus (HBV) infection. Pediatr Investig (2020) 4(2):133–7. doi: 10.1002/ped4.12205
3. Cryer AM, Imperial JC. Hepatitis b in pregnant women and their infants. Clinics liver Dis (2019) 23(3):451–62. doi: 10.1016/j.cld.2019.04.007
4. Quan M, Liu C, Li W, Xing H-C. Antiviral therapy for a postpartum flare in women with chronic HBV infection shortens the ALT recovery time and reduces hepatitis re-flare rates within 4 years. Can J Gastroenterol Hepatol (2022) 2022:4753267. doi: 10.1155/2022/4753267
5. Karnell JL, Wu Y, Mittereder N, Smith MA, Gunsior M, Yan Li, et al. Depleting plasmacytoid dendritic cells reduces local type I interferon responses and disease activity in patients with cutaneous lupus. Sci Trans Med (2021) 13(595):eabf8442. doi: 10.1126/scitranslmed.abf8442
6. Xu N, Yao H-P, Lv G-C, Chen Z. Down regulation of TLR7/9 leads to deficient production of IFN-α from plasmacytoid dendritic cells in chronic hepatitis b. Inflamm Res (2012) 61(9):997–1004. doi: 10.1007/s00011-012-0493-z
7. Cao WH, Li MH, Pan CQ, Lu Y, Zhang L, Ran CP, et al. Quantitation of plasmacytoid dendritic cells in chronic hepatitis b patients with HBeAg positivity during PEG-IFN and entecavir therapy. J Interferon Cytokine Res (2018) 38(5):197–205. doi: 10.1089/jir.2018.0014
8. Giles M, Visvanathan K, Lewin S, Bowden S, Locarnini S, Spelman T, et al. Clinical and virological predictors of hepatic flares in pregnant women with chronic hepatitis b. Gut (2015) 64:1810–5. doi: 10.1136/gutjnl-2014-308211
9. Sirilert S, Tongsong T. Hepatitis b virus infection in pregnancy: Immunological response, natural course and pregnancy outcomes. J Clin Med (2021) 10(13):2926. doi: 10.3390/jcm10132926
10. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis b: a 2015 update. Hepatol Int (2016) 10(1):1–98. doi: 10.1007/s12072-015-9675-4
11. van der Sluis RenéeM, Egedal JH, Jakobsen MR. Plasmacytoid dendritic cells as cell-based therapeutics: A novel immunotherapy to treat human immunodeficiency virus infection? Front Cell infection Microbiol (2020) 10:249. doi: 10.3389/fcimb.2020.00249
12. Kim MK, Kim J. Properties of immature and mature dendritic cells: phenotype, morphology, phagocytosis, and migration. RSC Adv (2019) 9(20):11230–8. doi: 10.1039/c9ra00818g
13. Santillo BT, Reis DDS, da Silva LT, Romani NT, Duarte AJDS, Oshiro TM. Phenotypic and functional profile of IFN-α-differentiated dendritic cells (IFN-DCs) from HIV-infected individuals. Hum Vaccin Immunother (2019) 15(9):2140–9. doi: 10.1080/21645515.2018.1547603
14. Alamri A, Rahman R, Zhang M, Alamri A, Gounni AS, Kung SKP. Semaphorin-3E produced by immature dendritic cells regulates activated natural killer cells migration. Front Immunol (2018) 9:1005. doi: 10.3389/fimmu.2018.01005
15. Huang XY, Jin ZK, Dou M, Zheng BX, Zhao XR, Feng Q, et al. Sinomenine promotes differentiation of induced pluripotent stem cells into immature dendritic cells with high induction of immune tolerance. World J Stem Cells (2022) 14(8):599–615. doi: 10.4252/wjsc.v14.i8.599
16. van Essen MF, Schlagwein N, van Gijlswijk-Janssen DJ, Ruben JM, van Kooten C. COMBAT consortium. properdin produced by dendritic cells contributes to the activation of T cells. Immunobiology (2022) 227(4):152246. doi: 10.1016/j.imbio.2022.152246
17. Paulikat AD, Tölken LA, Jachmann LH, Burchhardt G, Hammerschmidt S, Siemens N. Streptococcus pneumoniae impairs maturation of human dendritic cells and consequent activation of CD4+ T cells via pneumolysin. J Innate Immun (2022) 14(5):569–80. doi: 10.1159/000522339
18. Royzman D, Andreev D, Stich L, Peckert-Maier K, Wild AB, Zinser E, et al. The soluble CD83 protein prevents bone destruction by inhibiting the formation of osteoclasts and inducing resolution of inflammation in arthritis. Front Immunol (2022) 13:936995. doi: 10.3389/fimmu.2022.936995
19. Peckert-Maier K, Royzman D, Langguth P, Marosan A, Strack A, Sadeghi Shermeh A, et al. Tilting the balance: Therapeutic prospects of CD83 as a checkpoint molecule controlling resolution of inflammation. Int J Mol Sci (2022) 23(2):732. doi: 10.3390/ijms23020732
20. Renosi F, Callanan M, Lefebvre C. Genetics and epigenetics in neoplasms with plasmacytoid dendritic cells. Cancers (2022) 14(17):4132. doi: 10.3390/cancers14174132
21. Lu Y, Cao W, Chen Q, Lu H, Zhang Lu, Shen Ge, et al. Study on the pathogenesis of HBeAg positive chronic hepatitis b and plasmacytoid dendritic cells function. Chin J Exp Clin Virol (2020) 34(4):435–9. doi: 10.3760/cam.j.cn112866-20191205-00196
22. Cao W, Xie S, Zhang L, Bi X, Lin Y, Yang L, et al. Expression of functional molecule on plasmacytoid dendritic cells is associated with HBsAg loss in HBeAg-positive patients during PEG-IFN α-2a treatment. Front Immunol (2022) 13:891424. doi: 10.3389/fimmu.2022.891424
23. Yi W, Pan CQ, Li MH, Wan G, Lv YW, Liu M, et al. The characteristics and predictors of postpartum hepatitis flares in women with chronic hepatitis b. Am J Gastroenterol (2018) 113(5):686–93. doi: 10.1038/s41395-018-0010-2
Keywords: plasmacytoid dendritic cells, chronic hepatitis B, antiviral treatment, postpartum, immunity
Citation: Wang F, Song M, Hu Y, Yang L, Bi X, Lin Y, Jiang T, Deng W, Wang S, Sun F, Zeng Z, Lu Y, Shen G, Liu R, Chang M, Wu S, Gao Y, Hao H, Xu M, Chen X, Hu L, Wan G, Zhang L, Li M and Xie Y (2022) The relation of the frequency and functional molecules expression on plasmacytoid dendritic cells to postpartum hepatitis in women with HBeAg-positive chronic hepatitis B virus infection. Front. Immunol. 13:1062123. doi: 10.3389/fimmu.2022.1062123
Received: 05 October 2022; Accepted: 24 October 2022;
Published: 09 November 2022.
Edited by:
Siqing Fu, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Yan-Mei Jiao, Fifth Medical Center of the PLA General Hospital, ChinaXia Wang, Department of Infectious Diseases, Beijing Youan Hospital, Capital Medical University, China
Copyright © 2022 Wang, Song, Hu, Yang, Bi, Lin, Jiang, Deng, Wang, Sun, Zeng, Lu, Shen, Liu, Chang, Wu, Gao, Hao, Xu, Chen, Hu, Wan, Zhang, Li and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao Xie, eGlleWFvMDAxMjAxODRAc2luYS5jb20=; Minghui Li, d3VobTIwMDBAc2luYS5jb20=; Lu Zhang, emhhbmdsdTEyMThAMTI2LmNvbQ==; Gang Wan, ZHR3YW5nYW5nQDEyNi5jb20=
†These authors have contributed equally to this work
‡ORCID: Lu Zhang, orcid.org/0000-0002-3644-5306
Minghui Li, orcid.org/0000-0003-3233-5473
Yao Xie, orcid.org/0000-0003-4108-7037
 Fuchuan Wang1†
Fuchuan Wang1† Liu Yang
Liu Yang Xiaoyue Bi
Xiaoyue Bi Yanjie Lin
Yanjie Lin Tingting Jiang
Tingting Jiang Fangfang Sun
Fangfang Sun Zhan Zeng
Zhan Zeng Shuling Wu
Shuling Wu Mengjiao Xu
Mengjiao Xu Lu Zhang
Lu Zhang Minghui Li
Minghui Li Yao Xie
Yao Xie