
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 08 December 2022
Sec. Nutritional Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1061044
This article is part of the Research Topic Balanced life-diet and nutrition in cancer immunotherapy View all 5 articles
 Guang-Tan Lin1,2,3†
Guang-Tan Lin1,2,3† Jiao-Bao Huang1,2,3†
Jiao-Bao Huang1,2,3† Ju-Li Lin1,2,3†
Ju-Li Lin1,2,3† Jian-Xian Lin1,2,3
Jian-Xian Lin1,2,3 Jian-Wei Xie1,2,3
Jian-Wei Xie1,2,3 Jia-Bin Wang1,2,3
Jia-Bin Wang1,2,3 Jun Lu1,2,3
Jun Lu1,2,3 Chao-Hui Zheng1,2,3*
Chao-Hui Zheng1,2,3* Chang-Ming Huang1,2,3*
Chang-Ming Huang1,2,3* Ping Li1,2,3*
Ping Li1,2,3*Background: Immune checkpoint inhibitors are increasingly used in neoadjuvant therapy for locally advanced gastric cancer. However, the effect of body composition on the efficacy of neoadjuvant therapy has not been reported.
Methods: The computed tomography (CT) images and clinicopathological data of 101 patients with locally advanced gastric cancer who received neoadjuvant chemotherapy combined with immunotherapy (NCI) from 2019 to 2021 were collected. The CT image of L3 vertebral body section was selected, and the body composition before and after the neoadjuvant treatment was calculated using the SliceOmatic software, mainly including skeletal muscle index (SMI), subcutaneous adipose index (SAI), and visceral adipose index (VAI). The relationship between body composition and the efficacy and adverse events of NCI was analyzed.
Results: Of the 101 patients, 81 with evaluable data were included in the analysis. Of the included patients, 77.8% were male; the median age of all the patients was 62 years, and the median neoadjuvant therapy cycle was three. After the neoadjuvant therapy, 62.9% of the tumors were in remission (residual tumor cells ≤ 50%), and 37.1% of the tumors had no remission (residual tumor cells>50%). Moreover, 61.7% of the patients had treatment-related adverse events (TRAEs), and 18.5% had immune-related adverse events (irAEs). After neoadjuvant therapy, the body mass index (from 23 to 22.6 cm2/m2, p=0.042), SAI (from 34.7 to 32.9 cm2/m2, p=0.01) and VAI (from 32.4 to 26.8 cm2/m2, p=0.005) were significantly lower than those before treatment, while the SMI had no significant change (44.7 vs 42.5 cm2/m2, p=0.278). The multivariate logistics regression analysis revealed that low SMI (odds ratio [OR]: 3.23,95% confidence interval [CI]: 1.06–9.81, p=0.047), SMI attenuation (△SMI) ≥ 1.8(OR: 1.45,95%CI: 1.20–3.48, p=0.048), and clinical node positivity (OR: 6.99,95%CI: 2.35–20.82, p=0.001) were independent risk factors for non-remission. Additionally, high SAI is an independent risk factor for irAEs (OR: 14, 95%CI: 1.73–112.7, p=0.013).
Conclusion: Low SMI and △SMI≥1.8 are independent risk factors for poor tumor regression in patients with advanced gastric cancer receiving NCI. Patients with a high SAI are more likely to develop irAEs.
Gastric cancer remains one of the major malignant tumors causing cancer-related deaths, and its mortality ranks fourth among all malignant tumors worldwide (1).Even with surgery or adjuvant radiotherapy and chemotherapy, the 5-year survival rate of patients with stage II gastric cancer is 61%–63%, while that of patients with stage III decreased to 30%–35% (2). immune checkpoint blockers (ICB) therapy has made great progress in the treatment of patients with advanced gastric cancer. Preclinical studies and some phase II clinical studies have provided theoretical support and clinical evidence for neoadjuvant chemotherapy combined with immunotherapy (NCI) for locally advanced gastric cancer (3). The CheckMate-649 study has revealed that compared with chemotherapy alone, navulizumab combined with chemotherapy significantly improved overall survival (OS) and progression-free survival (PFS) in patients with metastatic gastric cancer and gastroesophageal junction (GEJ) cancer, and was recommended as the first-line treatment for subgroups with PD-L1 combined positive score ≥ 5 (4). Meanwhile, the NEONIPIGA study has demonstrated that nivolumab and ipilimumab-based neoadjuvant therapy is feasible and associated with no unexpected toxicity and a high pathologic complete response (PCR) rate in patients with mismatch repair deficient/microsatellite instability resectable GEJ adenocarcinoma (5). Previous studies in our center have confirmed that NCI has a higher gastric resection rate and better tumor regression than chemotherapy alone for locally advanced gastric cancer (6).
Weight loss and body composition change are common symptoms of patients with malignant tumors, and they are often related to poor prognosis (7), especially in gastric cancer (8). Lee et al. have reported that postoperative muscle attenuation and surgery-induced low skeletal muscle index(L-SMI) are prognostic factors for survival in patients with GC (9). Park et al. have reported that the decrease of muscle and subcutaneous adipose and visceral adipose was significantly related to the decrease of RFS and OS (10). Several recent studies have discovered no evident change in body composition of patients with locally advanced gastric cancer during neoadjuvant chemotherapy, although they have reported that L-SMI and muscle attenuation are related to the effect and postoperative complications of neoadjuvant chemotherapy (11, 12). However, whether this phenomenon exists in patients with NCI remains unknown, and the effects on body composition after immunotherapy have not been reported. Therefore, this study aimed to evaluate the changes of body composition and its effects on tumor remission and immune-related adverse events (irAEs) in patients with gastric cancer receiving NCI.
This study retrospectively analyzed the data of 101 patients with locally advanced gastric cancer who received NCI in the Department of Gastric Surgery, Fujian Medical University Union Hospital from January 2019 to April 2021. The inclusion criteria were as follows: age 18–75 years; with primary gastric adenocarcinoma confirmed by histopathology, clinical stage: cT2–4, lymph node N0~N3, and no distant metastasis (M0); received no chemotherapy (radiotherapy) or other antineoplastic therapy within 6 months; with computed tomography (CT) scans available during diagnosis and before operation; and without evidence of distant metastasis, such as liver metastasis or peritoneal implantation metastasis after laparoscopic exploratory surgery. Meanwhile, the exclusion criteria were as follows: with cancer complicated with malignant diseases of other organs; with evidence of peritoneal dissemination or distant metastasis (including intraoperative exploration after neoadjuvant therapy); and with history of gastrectomy or endoscopic submucosal dissection. In total, 11 cases without operation, six cases with incomplete CT data, and three cases with abdominal implant metastasis were excluded. Finally, 81 patients were included in the analysis (Figure S1). The study was reviewed and approved by the Ethics Committee of Fujian Medical University Union Hospital.
Body components comprise adipose and non-adipose tissues, the former including subcutaneous adipose, visceral adipose, and intermuscular adipose tissues, and the latter including the muscles, bones, and internal organs (13). A single CT image of the third lumbar vertebra (L3) was selected to quantify muscle and adipose features, as the anatomical location was closely related to body volume (13). According to the standard Hounsfield unit (HU) range, skeletal muscle cross-sectional area (SMT, −29–150 HU), visceral adipose tissue (VAT, −15–50 HU), and subcutaneous adipose tissue (SAT, −190–30 HU) were quantified. A researcher (L.J.X.) tackled how to accurately capture the image in the middle of L3 and segment muscle and adipose tissue. All CT images without any patient information were then analyzed using the SliceOmatic version 5.0 (TomoVision) (14). The measured value of each body component (square centimeter) divided by the square meter of height was converted into an index (SMI, visceral adipose index [VAI], and subcutaneous adipose index [SAI]) (15, 16),. Body mass index (BMI) was calculated by dividing the weight by height squared. In the analysis, the patients were divided into groups according to BMI as follows: low BMI (<25 kg/m2) group and high BMI (≥25 kg/m2) group. According to the results of Martin (17), the male patients with BMI < 25 kg/m2 and SMI < 43 cm/m2 (or BMI ≥ 25 kg/m2 and SMI < 53 cm/m2) were considered L-SMI, while the female patients with SMI < 41 cm/m2 were considered L-SMI regardless of BMI. According to the relationship between the SAI and incidence of irAEs, the patients were divided into two groups as follows: high subcutaneous adipose group (H-SAI) and low subcutaneous adipose group (L-SAI). Additionally, we used the median to classify the VAI because no threshold for VAI has been clinically established. The △SMI, △VAI, and △SAI represent the changes of SMI, VAI, and SAI before and after neoadjuvant therapy, respectively.
The neoadjuvant immunotherapy regimen is a fluorouracil-based chemotherapy combined with ICBs. The SOX/XELOX regimen generally comprised 2–4 cycles of SOX/XELOX regimen (18)(S-1 40–60 mg/m2 or capecitabine 1000 mg/m2, twice a day, days 1–14, and oxaliplatin 130 mg/m2 intravenous injection on the first day). The FOLFOX4 regimen comprised 2–4 cycles (19)(day 1: oxaliplatin 85 mg/m2, calcium folinate 200 mg intravenous drip for 2 h, fluorouracil 400 mg/m2 intravenous drip, 22 h intravenous drip of fluorouracil 600 mg/m2). ICBs were administered intravenously along with the chemotherapy cycle on the first day of chemotherapy (the drug dose was determined according to the patient’s body surface area, and the dose was reduced appropriately for patients with severe blood toxicity or non-blood toxicity). The next cycle of chemotherapy was repeated on the 22nd day. According to the criteria described by the Japan Gastric Cancer Association (JGCA), a whole abdominal CT scan was performed every 6–8 weeks to evaluate the response to neoadjuvant therapy, and improve the results of the relevant laboratory tests (including blood routine, liver and kidney functions, and tumor markers.) (20). The operation was performed at least 3 weeks after the completion of the neoadjuvant therapy. All surgical operations, including the extent of lymph node dissection, were performed in accordance with the guidelines of the JGCA (21), while staging was performed according to the tumor–node–metastasis classification (American Joint Committee on Cancer staging, 8th edition) (22). The Becker regression criteria were used to quantify the pathological reaction after treatment. The standard is based on the estimation of the percentage of living tumor cells relative to the tumor bed that can be recognized by the naked eye, and includes the following categories: TRG1a (no residual tumor cells), TRG1b (<10% residual tumor cells), TRG2 (10%–50% residual tumor cells), and TRG3 (>50% residual tumor cells) (23). In this study, TRG grade 1a/1b/2 was considered tumor remission (TR), and the TRG3 grade was considered non-tumor remission (non-TR).
Treatment-related adverse events (TRAEs) were assessed according to the National Cancer Institute-General terminology Standard for adverse events (AEs) version 4.0 (24). TRAEs included events reported between the first administration and the last study 30 days after treatment. For further analysis, the toxicity was classified into grades I–II and III–IV. irAEs are defined as AEs associated with immunosuppressant exposure and in accordance with immune-related phenomena (25). TRAEs include general AEs and irAEs. Any delayed dose or early cessation of treatment recorded the result of significant toxicity (III–IV), which was defined as dose limited toxicity in this study.
As in previous studies, all the patients received nutritional risk screening using the Nutritional Risk Screening 2002 (NRS 2002), as recommended by the European Society for Clinical Nutrition and Metabolism (26), and developed personalized nutritional support therapy. Patients with an NRS score ≥ 3 were routinely provided oral nutritional supplements. For patients who were unable to meet their energy needs through oral feeding, enteral tube feeding and/or parenteral nutrition were provided (27). Additionally, all patients received a nutritional assessment every 2 weeks to adjust their nutritional support treatment until 1 week preoperatively.
The main endpoint was pathological reaction. The secondary endpoints included TRAEs and irAEs. Normally distributed variables are described as the absolute number and percentage, mean, and standard deviation, and nonparametric variables are described as median and interquartile range. The classified variables were analyzed by double X2 test or Fisher’s exact test, the continuous variables were compared by Student’s t test, and the paired t-test was used before and after the comparison. The correlation among the parameters was analyzed by pearson correlation coefficient, and the cutoff point of △SMI was intercepted according to the maximum area under the receiver operating characteristic curve. According to the smooth curve, the relationship between SAI and irAEs is explored, and the potential confounding factors are adjusted (Figure 1). The experimental method was used to determine the relationship between the incidence of irAEs and SAI levels, move the test inflection point along predefined intervals, and detect the inflection point of the maximum model possibility. We further apply applied a two-stage linear regression model to test the threshold effect of SAI on irAEs according to the smoothing curve (Table S2). A logistic regression model was used for the univariate and multivariate analyses. Significance was set at p<0.05. All statistical analyses were conducted using the SPSS software version 22.0 and Empower Stats 2.0.
Altogether, 81 patients who received NCI and underwent gastrectomy in the Fujian Medical University Union Hospital from January 2019 to April 2021 were included. Among them, 63 patients were male (77.8%), and 18 were female (22.2%). The median age of the patients was 62 years (57–67). The median preoperative neoadjuvant therapy cycle was three (3–4). Total gastrectomy and distal subtotal gastrectomy were performed in 64 (79%) and 17 (21%) cases, respectively. Postoperative complications occurred in 16 cases (19.7%), including pulmonary infection in 10 cases (12.3%), abdominal infection in four cases (4.9%), and anastomotic leakage in two cases (2.5%). Postoperative pathological stages included PCR in 12 cases (14.8%), ypI stage in 11 cases (13.6%), ypII stage in 20 cases (24.7%), and ypIII stage in 38 cases (46.9%). According to the Lauren classification, the intestinal type was recorded in 68 cases (84%) and the diffuse and mixed type in 13 cases (16%). After neoadjuvant therapy, 51 cases (62.9%) had TR, and 30 cases (37.1%) had non-TR. According to the set threshold, the body components were divided into the L-SMI group with 56 cases (69.1%), high SMI (H-SMI) group with 25 cases (30.8%), L-SAI group with 47 cases (57.1%), H-SAI group with 34 cases (41.9%), low VAI (L-VAI) group with 40 cases (49.4%), and high VAI (H-VAI) group with 41 cases (50.6%) (Table 1).
Figure 2 presents a representative L3 plane CT image segmentation legend with the patient’s baseline state. Panel A depicts the representative segmentation of L-SMI, panel B indicates the representative segmentation of H-SAI, and panel C demonstrates the representative segmentation of H-VAI. After neoadjuvant therapy, the BMI (from 23 to 22.6 kg/m2, p=0.042), SAI (from 34.7 to 32.9 cm2/m2, p=0.01), and VAI (from 32.4 to 26.8 cm2/m2, p=0.005) were significantly lower than those before treatment, while the SMI had no significant change (44.7 vs 42.5 cm2/m2, p=0.278)(Figure 3). We also analyzed the relationship between the body components of patients at baseline and serum nutritional markers. The median of albumin (ALB) levels was 38g/L (33–41) before treatment and 37g/L (34–42) after treatment. The difference between the two groups before and after treatment was significant (p=0.047) (Table 2). Before neoadjuvant therapy, the SMI was positively correlated with ALB levels (Pearson’s=0.3, p=0.009), but not with SAI and VAI (SAI: Pearson’s=−0.151, p=0.626; VAI: Pearson’s=0.119, p=0.856).

Figure 2 The portal phase computed tomography image of the L3 level was used to measure the body composition. Red: SM, Skeletal muscle;Yellow:VAT, Visceral adipose tissue;Blue:SAT, Subcutaneous adipose tissue; (A) L-SMI, Low skeletal muscle index; (B) H-SAI, High subcutaneous adipose index; (C) H-VAI,High visceral adipose index.
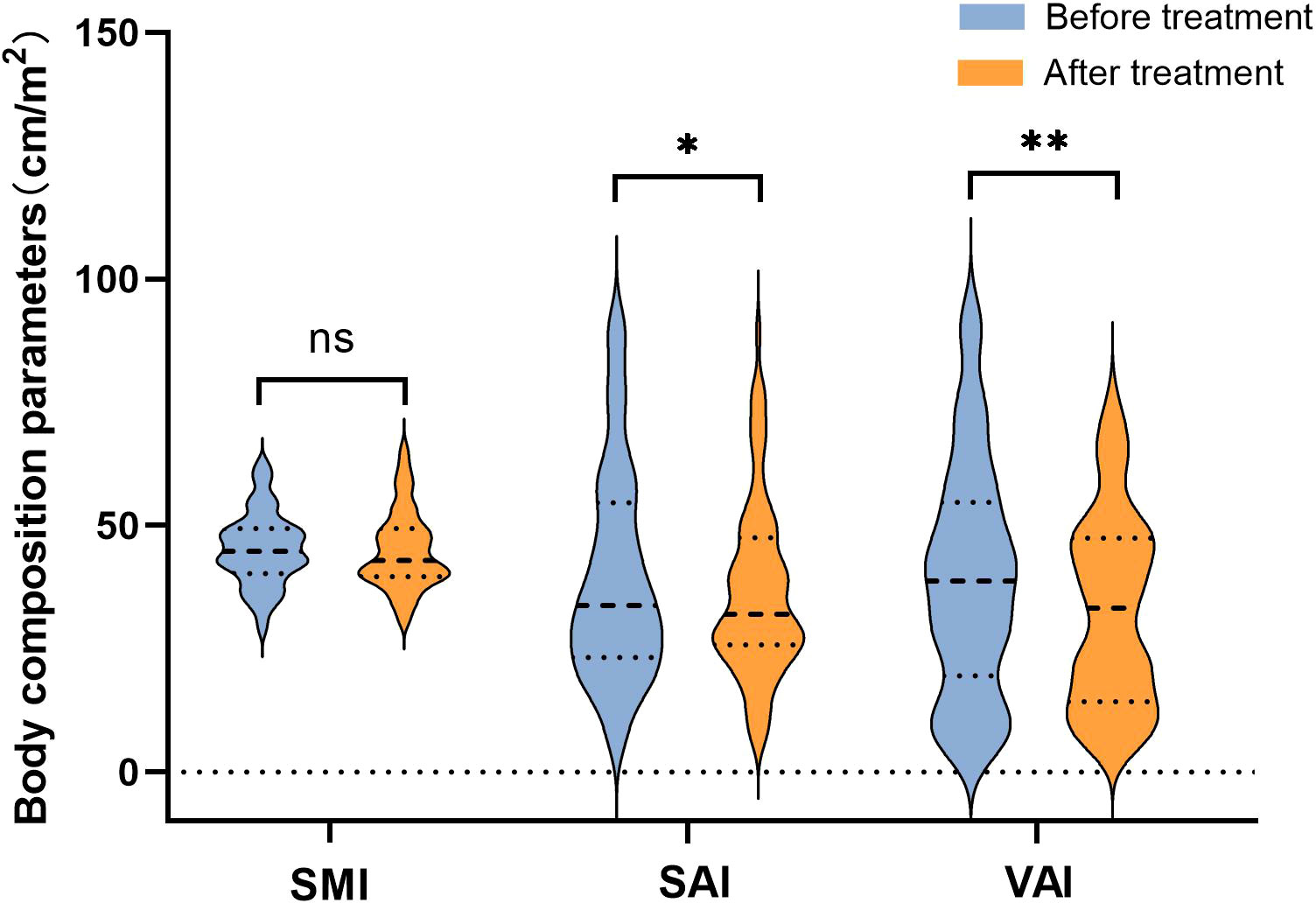
Figure 3 Changes of body composition parameters before and after neoadjuvant immunotherapy. “*”means p < 0.05; "**" means p < 0.01.
Compared with the TR group, the patients in the non-TR group were younger (63 years vs 60 years, p=0.029) and had more patients in stage III (27.5% vs 80%, p=0.001). No significant differences in sex, interval between diagnosis and operation, neoadjuvant treatment cycle, surgical methods, postoperative complications, and AEs were observed between the two groups. In terms of body composition, the non-TR group had more patients with L-SMI (60.8% vs 83.3%, p=0.046), and no significant differences in SAI, VAI, and BMI were noted between the two groups (p>0.05). During the neoadjuvant therapy, the SMI of patients in the non-TR group decreased more (△SMI: 0.5 vs −1.5, p=0.05), although the SAI and VAI did not change significantly (p>0.05) (Table 3).
Table 4 summarizes the results of the univariate and multivariate logistic analyses of body composition and their changes in patients with tumor regression before neoadjuvant therapy. Among them, the L-SMI (odds ratio [OR]: 3.45, 95% confidence interval [CI]: 2.06–6.81, p=0.041), and SMI ≥ 1.8 (OR: 1.38, 95%CI: 1.09–2.89, p=0.037) were risk factors for non-TR. After adjusting for age, Eastern Cooperative Oncology Group score, cT, cN and other clinical-related factors, the multivariate logistic analysis revealed that L-SMI (OR: 3.23, 95%CI: 1.06–9.81, p=0.047), SMI ≥ 1.8 (OR: 1.45, 95%CI: 1.20–3.48, p=0.048), and clinical node positivity (cN+) (OR: 6.99, 95%CI: 2.35–20.82, p=0.001) were independent risk factors for non-TR.
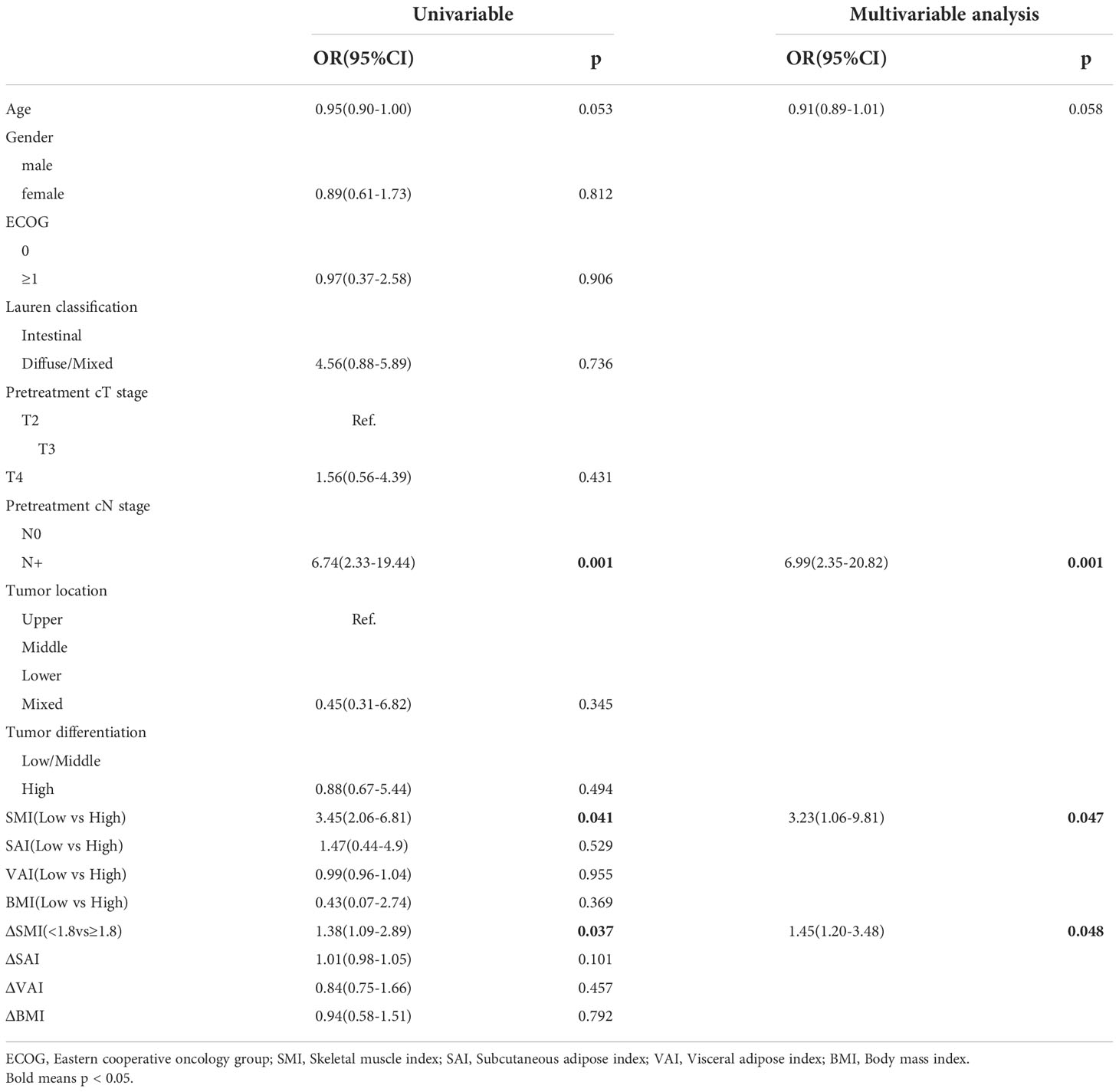
Table 4 Univariate and multivariable analysis of the relationship between body composition with non-TR.
In this study, 50 cases (61.7%) of TRAEs were recorded, of which 32 cases (39%) were grade III–IV TRAEs. Meanwhile, 15 cases (18.5%) had irAEs, of which seven cases (8.9%) were grade III–IV irAEs, including three cases of abnormal liver function, one case of interstitial pneumonia, two cases of maculopapular rash, and one case of immune colitis,irAEs are recorded separately (Table S1). No grade V AEs and drug withdrawal due to AEs were recorded.
Logistics analysis found that SMI(OR: 3.54,95%CI: 0.66-6.45,p=0.891), SAI (OR: 2.24,95%CI: 0.79-2.53, p= 0.119), VAI (OR: 0.56,95%CI: 0.46-1.64, p= 0.215) had no significant correlation with TRAEs (Table S2). However, when analyzing the relationship among SMI, SAI, VAI and irAEs, we found a nonlinear relationship between the incidence of SAI and irAEs.(Figure 1). As SAI reaches a turning point (28.5 cm2/m2), the risk of irAEs increases (Table S3). The incidence of irAEs in the H-SAI group (SAI ≥ 28.5 cm2/m2) was 29.8%. The incidence of irAEs in the L-SAI (SAI < 28.5 cm2/m2) group was 3%. Table 5 presents the results of the univariate and multivariate logistics analyses of body composition and its changes before neoadjuvant therapy. The H-SAI, H-BMI, and cN+ were related to the occurrence of irAEs. Further multivariate analysis revealed H-SAI as an independent risk factor for irAEs (OR: 14, 95%CI: 1.73–112.7; p=0.013). Among them, the incidence of abnormal liver function increased mainly (aspartate aminotransferase increased at 29.6% vs 8.9%, p=0.043; alanine aminotransferase increased at 25.9% vs 2.8%, p=0.009) (Figure S2).
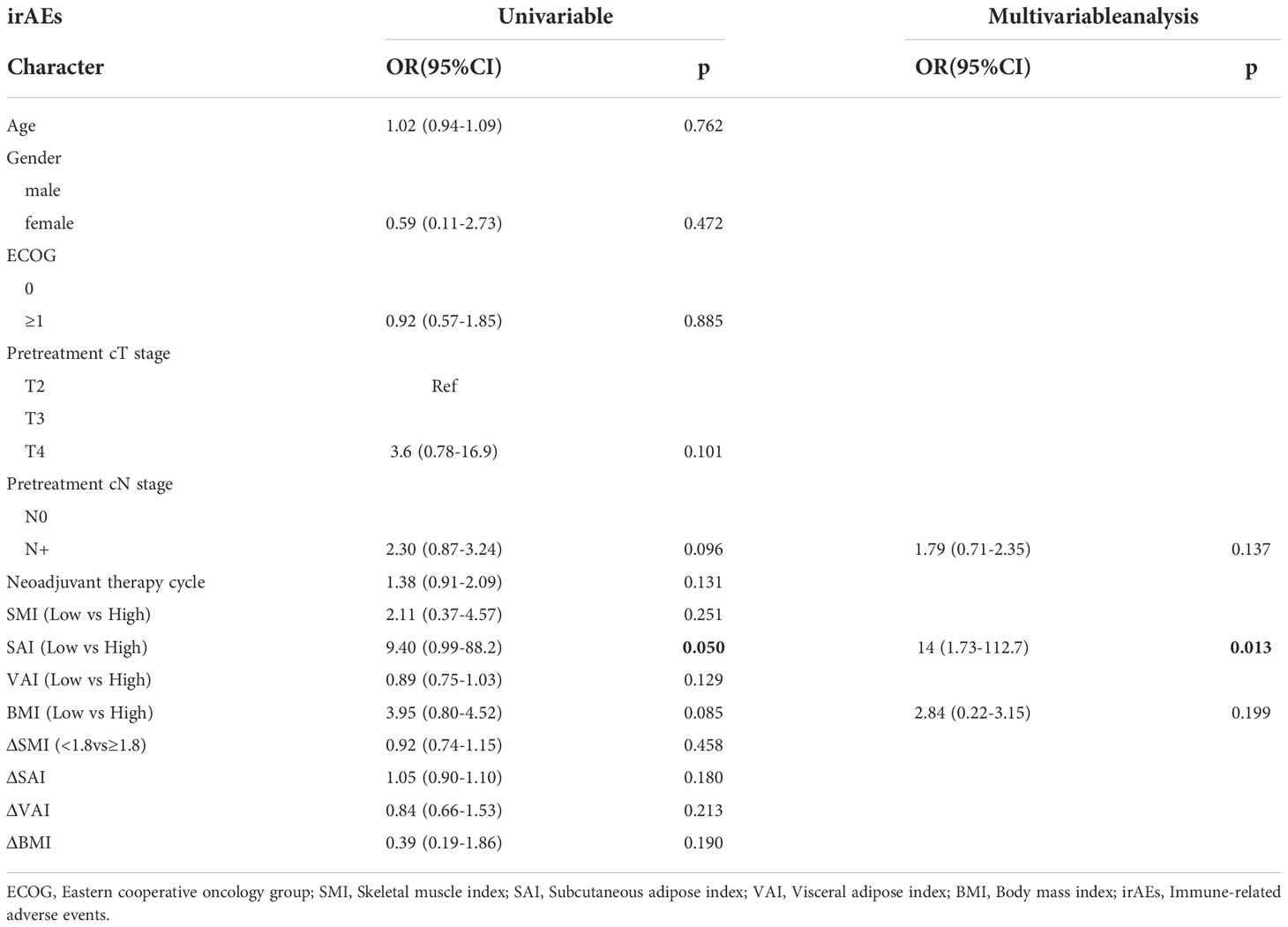
Table 5 Univariate and multivariable analysis of the relationship between body composition with irAEs.
Body composition is closely related to immunotherapy. Previous studies have reported on the relationship of body composition with the efficacy and toxicity of immunotherapy in melanoma (28, 29). However, the interaction between body composition and immunotherapy in gastric cancer remains unknown. In this study, VAI and SAI have been observed to decrease in varying degrees during NCI, which was different from the results previously reported for neoadjuvant chemotherapy alone (11, 12),. A follow-up analysis has revealed that L-SMI and SMI attenuation were independent risk factors for poor TR, and the H-SAI index was significantly correlated with irAEs.
The adverse effect of L-SMI on prognosis has been confirmed in various malignant tumors, which may be irrelevant to the mode of treatment. For example, Kudou et al. found that the survival rate of patients with low SMI after radical gastrectomy was significantly lower than that of normal SMI (30), while Kim et al. have reported that patients with L-SMI in immunotreated gastric cancer had shorter PFS (median, 1.4 months vs. 2.6 months; p = 0.026) (31).Similarity, the efficacy in patients with L-SMI was worse in this study. Sato et al. have suggested that cachexia is a manifestation of the high malignant potential of cancer, and that L-SMI is one of the characteristics of cachexia, thus making it related to poor chemotherapy response (32). However, the research of Chu et al.provides an interesting explanation in immunotherapy (29). Chu et al. believe that immunosuppressants, such as protein, are highly charged molecules. The antibody itself is extremely hydrophilic as most of the water in the human body is stored in the muscles (33). Thus, patients with the same weight but less muscle content may have lower utilization of antibodies, which eventually leads to poor efficacy.
Deshpande et al.elaborated how diet, inflammation and intestinal microbes play a role in determining the outcome of ICBs treatment (34).Malnutrition and inflammation may be the main drivers of low SMI (35, 36). Malnutrition usually leads to impaired immune response and is vulnerable to infection. Proper energy and balanced nutrition are essential for the establishment of a healthy immune system (37, 38).Inflammation is the main factor mediating skeletal muscle decomposition in cancer patients with low SMI (39, 40). Ali et al. proposed that L-SMI status induces upregulation of pro-inflammatory cytokines, including tumor necrosis factor and interleukin-1 and interleukin-6. These mediators may interfere with the immune system and tumor microenvironment, leading to adverse clinical outcomes (41).A number of studies have shown that intestinal microbiota profoundly affect the immunotherapy response of a series of malignant tumors (42). For example, in non-small cell lung cancer and renal cell carcinoma, fecal samples of patients receiving anti-PD-1 immunotherapy are rich in bacterial species (43). Therefore, malnutrition, changes in inflammatory state and disorders of intestinal microbial system in patients with low SMI may be the reasons for poor tumor regress after neoadjuvant immunotherapy.
Our results also demonstrate that SMI attenuation predicts worse efficacy. This has also been confirmed in other studies. Rutten et al. have identified that skeletal muscle loss in patients with ovarian cancer during neoadjuvant chemotherapy was significantly shorter than that in patients with unchanged OS (44). In our study, SMI attenuation was an independent factor for poor tumor response, although the cause of skeletal muscle attenuation during NCI has no exact mechanism. One possible explanation is that the strong malignant potential of the tumor leads to loss of appetite and systemic consumption during treatment. Accordingly, a high tumor malignant potential may itself be insensitive to neoadjuvant therapy.
Obesity is associated with the development of irAEs (45), which may be caused by chronic systemic inflammation caused by macrophages in adipose tissue (46). In fact, some studies have linked higher BMI to an increased risk of irAEs after immunotherapy (47).Bouchlaka et al. have reported that providing systemic irritant immunotherapy was well tolerated by mice with low fat content, although it eventually led to multiple organ pathological events and rapid death in rats with obesity (48). These results suggest that the toxic reaction is induced by a strong immune stimulation and is related to the pre-existing inflammatory environment of the patient. In this study, patients with H-SAI had a higher incidence of irAEs (29.8% vs 3.0%). Previous studies have demonstrated that the expression of leptin was positively correlated with the expression of PD-1 (49), and that the secretion of leptin in subcutaneous adipose was much higher than that in visceral adipose (50). Hence, patients with high subcutaneous adipose have higher expression of PD-1 and stronger immune stimulation, thus eventually leading to higher irAEs.
This study had some limitations. First, this was a single-center retrospective small sample study, which has unavoidable selective bias and can only represent the results from eastern countries. Nevertheless, this study is the largest report within the range of NCI for gastric cancer. Second, the treatment regimen in this study was chemotherapy combined with immunotherapy, not alone immunotherapy. At present, the best neoadjuvant therapy for locally advanced gastric cancer remains controversial. Therefore, our results can be a significant reference for patients using this regimen. Third, although immune-related adverse events were recorded separately in this study, they were not completely accurate in collection and differentiation because of the combination therapy.Finally, due to the short follow-up time, we only analyzed the short-term results of NCI. Next, we will continue to collect the long-term survival outcomes of these patients to verify the results of this study on tumor regression.
Construction of “creating double highs” of medical treatment in Fujian Province(Minwei Medical Administration (2021) No. 76) Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare.
We are grateful to the patients and his family for their participation in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1061044/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Li P, Huang CM, Zheng CH, Russo A, Kasbekar P, Brennan MF, et al. Comparison of gastric cancer survival after R0 resection in the US and China. J Surg Oncol (2018) 118:975–82. doi: 10.1002/jso.25220
3. Takei S, Kawazoe A, Shitara K. The new era of immunotherapy in gastric cancer. Cancers (2022) 14:1054. doi: 10.3390/cancers14041054
4. Yyja B, Ks C, D P, Pmg E, F PS, Pls G, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2
5. André T, Tougeron D, Piessen G, de la Fouchardière C, Louvet C, Adenis A, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch Repair/Microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: The GERCOR NEONIPIGA phase II study. J Clin Oncol (2022), JCO. 22.00686. doi: 10.1200/JCO.22.00686
6. Lin J-L, Lin J-X, Lin JP, Zheng C-H, Li P, Xie J-W, et al. Safety and efficacy of camrelizumab in combination with nab-paclitaxel plus s-1 for the treatment of gastric cancer with serosal invasion. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.783243
7. Dev R, Bruera E, Dalal S. Insulin resistance and body composition in cancer patients. Ann Oncol (2018) 29:ii18–26. doi: 10.1093/annonc/mdx815
8. Hacker UT, Hasenclever D, Linder N, Stocker G, Chung HC, Kang YK, et al. Prognostic role of body composition parameters in gastric/gastroesophageal junction cancer patients from the EXPAND trial. J cachexia sarcopenia muscle. (2020) 11:135–44. doi: 10.1002/jcsm.12484
9. Lee JK, Park YS, Lee K, Youn SI, Won Y, Min SH, et al. Prognostic significance of surgery-induced sarcopenia in the survival of gastric cancer patients: a sex-specific analysis. J Cachexia Sarcopenia Muscle (2021) 12(6):1897–907. doi: 10.1002/jcsm.12793
10. Park HS, Kim HS, Beom SH, Rha SY, Chung HC, Kim JH, et alMarked loss of muscle, visceral fat, or subcutaneous fat after gastrectomy predicts poor survival in advanced gastric cancer: Single-center study from the CLASSIC trial. Ann Surg Oncol (2018) 25(11):3222–30. doi: 10.1245/s10434-018-6624-1
11. Lin J-X, Tang Y-H, Zhou W-X, Desiderio J, Parisi A, Xie J-W, et al. Body composition parameters predict pathological response and outcomes in locally advanced gastric cancer after neoadjuvant treatment: A multicenter, international study. Clin Nutr (2021) 40:4980–7. doi: 10.1016/j.clnu.2021.06.021
12. Zhang Y, Li Z, Jiang L, Xue Z, Ma Z, Kang W, et al. Impact of body composition on clinical outcomes in people with gastric cancer undergoing radical gastrectomy after neoadjuvant treatment. Nutrition (2021) 85:111135. doi: 10.1016/j.nut.2020.111135
13. Mourtzakis M, Prado C, Lieffers JR, Reiman T, Mccargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab (2008) 33:997–1006. doi: 10.1139/H08-075
14. Feliciano E, Popuri K, Cobzas D, Baracos VE, Caan BJ. Evaluation of automated computed tomography segmentation to assess body composition and mortality associations in cancer patients. J Cachexia Sarcopenia Muscle (2020) 11(5):1258–69. doi: 10.1002/jcsm.12573
15. Kvist H, Chowdhury B, Grangrd U, Tylén U, Sjstrm L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr (1988) 48:1351–61. doi: 10.1093/ajcn/48.6.1351
16. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr (1997) 127:990S–1S. doi: 10.1093/jn/127.5.990S
17. Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol (2013) 31:1539–47. doi: 10.1200/JCO.2012.45.2722
18. Koizumi W, Takiuchi H, Yamada Y, Boku N, Fuse N, Muro K, et al. Phase II study of oxaliplatin plus s-1 as first-line treatment for advanced gastric cancer (G-SOX study). Ann Oncol (2010) 21:1001–5. doi: 10.1093/annonc/mdp464
19. De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J cancer. (2005) 92:1644–9. doi: 10.1038/sj.bjc.6602573
20. Japanese, Gastric, Cancer, Association. Japanese Classification of gastric carcinoma: 3rd English edition. Gastric Cancer (2011) 14(2):101–12. doi: 10.1007/s10120-011-0041-5
21. Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer (2017) 20:1–19. doi: 10.1007/s10120-016-0622-4
23. Becker K, Langer R, Reim D, Novotny A, zum Buschenfelde CM, Engel J, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg (2011) 253:934–9. doi: 10.1097/SLA.0b013e318216f449
24. Savarese DM. Common terminology criteria for adverse events. UpToDate Waltham, MA: UpToDate (2013) 1–9.
25. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin oncology: Off J Am Soc Clin Oncol (2018) 36:1714. doi: 10.1200/JCO.2017.77.6385
26. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr (2003) 22:415–21. doi: 10.1016/S0261-5614(03)00098-0
27. Buffart LM, Galvão DA, Brug J, Chinapaw M, Newton RU. Evidence-based physical activity guidelines for cancer survivors: Current guidelines, knowledge gaps and future research directions. Cancer Treat Rev (2014) 40:327–40. doi: 10.1016/j.ctrv.2013.06.007
28. Crombé A, Kind M, Toulmonde M, Italiano A, Cousin S. Impact of CT-based body composition parameters at baseline, their early changes and response in metastatic cancer patients treated with immune checkpoint inhibitors. Eur J Radiology. (2020) 133:109340. doi: 10.1016/j.ejrad.2020.109340
29. Chu MP, Li Y, Ghosh S, Sass S, Smylie M, Walker J, et al. Body composition is prognostic and predictive of ipilimumab activity in metastatic melanoma. J Cachexia Sarcopenia Muscle. (2020) 11:748–55. doi: 10.1002/jcsm.12538
30. Kudou K, Saeki H, Nakashima Y, Edahiro K, Korehisa S, Taniguchi D, et al. Prognostic significance of sarcopenia in patients with esophagogastric junction cancer or upper gastric cancer. Ann Surg Oncol (2017) 24:1804–10. doi: 10.1245/s10434-017-5811-9
31. Kim YY, Lee J, Jeong WK, Kim ST, Kim JH, Hong JY, et al. Prognostic significance of sarcopenia in microsatellite-stable gastric cancer patients treated with programmed death-1 inhibitors. Gastric Cancer (2020) 24. doi: 10.1007/s10120-020-01124-x
32. Sato S, Kunisaki C, Suematsu H, Tanaka Y, Endo I. Impact of sarcopenia in patients with unresectable locally advanced esophageal cancer receiving chemoradiotherapy. Vivo (2018) 32:603–10. doi: 10.21873/invivo.11282
33. Kaysen GA, Zhu F, Sarkar S, Heymsfield SB, Wong J, Kaitwatcharachai C, et al. Estimation of total-body and limb muscle mass in hemodialysis patients by using multifrequency bioimpedance spectroscopy. Am J Clin Nutr (2005) 82:988–95. doi: 10.1093/ajcn/82.5.988
34. Deshpande RP, Sharma S, Watabe K. The confounders of cancer immunotherapy: Roles of lifestyle, metabolic disorders and sociological factors. Cancers (2020) 12:2983. doi: 10.3390/cancers12102983
35. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. (2014) 14:754–62. doi: 10.1038/nrc3829
36. Papadopoulou SK, Papadimitriou K, Voulgaridou G, Georgaki E, Tsotidou E, Zantidou O, et al. Exercise and nutrition impact on osteoporosis and sarcopenia–the incidence of osteosarcopenia: A narrative review. Nutrients (2021) 13:4499. doi: 10.3390/nu13124499
37. Maggini S, Pierre A, Calder PC. Immune function and micronutrient requirements change over the life course. Nutrients (2018) 10:1531. doi: 10.3390/nu10101531
38. Calder PC. Feeding the immune system. Proc Nutr Society. (2013) 72:299–309. doi: 10.1017/S0029665113001286
39. Patel HJ, Patel BM. TNF-α and cancer cachexia: Molecular insights and clinical implications. Life Sci (2017) 170:56–63. doi: 10.1016/j.lfs.2016.11.033
40. Giordano A, Calvani M, Petillo O, Carteni' M, Melone MRAB, Peluso G. Skeletal muscle metabolism in physiology and in cancer disease. J Cell Biochem (2010) 90. doi: 10.1002/jcb.10601
41. Ali S, Garcia JM. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology (2014) 60:294–305. doi: 10.1159/000356760
42. Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J immunotherapy cancer. (2019) 7:1–15. doi: 10.1186/s40425-019-0574-4
43. Carey M, Lambert S, Smits R, Paul C, Clinton-Mcharg T. The unfulfilled promise: A systematic review of interventions to reduce the unmet supportive care needs of cancer patients. Supportive Care Cancer. (2011) 20:207–19. doi: 10.1007/s00520-011-1327-1
44. Rutten IJ, van Dijk DP, Kruitwagen RF, Beets-Tan RG, Olde Damink SW, Van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J cachexia sarcopenia muscle. (2016) 7:458–66. doi: 10.1002/jcsm.12107
45. Mirsoian A, Murphy WJ. Obesity and cancer immunotherapy toxicity. Immunotherapy (2015) 7:319–22. doi: 10.2217/imt.15.12
46. Ellulu MS, Ismail P, Huzwah K, Asmah R, Yehia A. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci Ams. (2017) 13:851–63. doi: 10.5114/aoms.2016.58928
47. Rogado J, Romero-Laorden N, Sanchez-Torres JM, Ramos-Levi AM, Pacheco-Barcia V, Ballesteros AI, et al. Effect of excess weight and immune-related adverse events on the efficacy of cancer immunotherapy with anti-PD-1 antibodies. Oncoimmunology (2020) 9:1751548. doi: 10.1080/2162402X.2020.1751548
48. Bouchlaka MN, Sckisel GD, Chen M, Mirsoian A, Zamora AE, Maverakis E, et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med (2013) 210:2223–37. doi: 10.1084/jem.20131219
49. Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med (2019) 25:141–51. doi: 10.1038/s41591-018-0221-5
Keywords: gastric cancer, neoadjuvant immunotherapy, body composition, tumor regression grade (TRG), adverse events
Citation: Lin G-T, Huang J-B, Lin J-L, Lin J-X, Xie J-W, Wang J-B, Lu J, Zheng C-H, Huang C-M and Li P (2022) Body composition parameters for predicting the efficacy of neoadjuvant chemotherapy with immunotherapy for gastric cancer. Front. Immunol. 13:1061044. doi: 10.3389/fimmu.2022.1061044
Received: 04 October 2022; Accepted: 24 November 2022;
Published: 08 December 2022.
Edited by:
Kefei Yuan, Sichuan University, ChinaReviewed by:
Likui Feng, The Rockefeller University, United StatesCopyright © 2022 Lin, Huang, Lin, Lin, Xie, Wang, Lu, Zheng, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Li, pingli811002@163.com; Chang-Ming Huang, hcmlr2002@163.com; Chao-Hui Zheng, wwkzch@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.