- 1Department of Rheumatology and Immunology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
- 2Bengbu Medical College, Bengbu, China
- 3Department of Rheumatology, The First People’s Hospital of Hefei (Binhu Hospital), Hefei, China
- 4Department of Rheumatology, The First People’s Hospital of Hefei, Hefei, China
- 5Department of Rheumatology and Immunology, The Third People’s Hospital of Hefei, Hefei, China
- 6Department of Scientific Research, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
Objective: Numerous researches have reported the role of air pollution in the development of autoimmune diseases. However, few have evaluated the relationship between inhalable particulate matter (PM) exposure and Sjögren’s syndrome (SS). This study aimed to analyze the association between exposure to two particulate pollutants (PM2.5, PM10) and SS-related hospitalizations.
Methods: Daily data were obtained on PM2.5 and PM10, meteorological factors, and hospital hospitalizations for SS between 2016 and 2021. The daily data on PM2.5 and PM10, meteorological factors, and the number of SS hospitalizations were collected between 2016 and 2021. A distributed lag non-linear model and a generalized linear model were established to explore the association between PM2.5 and PM10 exposure and hospitalizations for SS. Stratified analyses were performed to explore possible gender-, age-, and season-related differences in PM2.5 and PM10 effects.
Results: Exposure to PM2.5 was related to the evaluated risk of hospitalizations for SS (RR=1.015, 95% CI: 1.001-1.029, lag 3 day), similarly, PM10 exposure had a statistically significant positive association with SS hospitalizations (RR =1.013, 95% CI: 1.001-1.026, lag 3 day). Stratified analyses found that exposure to PM2.5 and PM10 exhibited higher impact on SS-related hospitalizations in female patients and exposure to PM2.5 was also associated with the higher risk of SS-related hospitalizations in patients aged ≥ 65 years. In addition, exposure to PM2.5, PM10 in colder season were more likely to increase SS-related hospitalizations.
Conclusion: Our findings suggested that exposure to PM2.5 and PM10 were significantly linked to an elevated risk of hospitalizations for SS.
1 Introduction
As a common, systemic autoimmune disease, Sjögren’s syndrome (SS) is characterized by lymphocyte proliferation and progressive destruction of the exocrine glands. The major clinical manifestations of these patients include dryness of the mouth and eyes, joint pain, and fatigue, which seriously affect the life quality of SS patients (1). Moreover, these patients also exhibit other clinical manifestations involving multiple systems, such as the nervous system, musculoskeletal system, kidney, blood vessels, skin, and lung (2). However, the potential pathogenesis of SS has not been fully elucidated. Some studies have shown that host genetics and external environmental factors could regulate the pathogenesis of SS through various mechanisms. Genetic predisposition accounts for approximately 30% of autoimmune diseases; environmental factors including air pollution are also involved the development of these diseases (3, 4). Ambient air pollution levels have been reported to have acute adverse effects on population health, primarily in terms of respiratory and cardiovascular conditions (5, 6); their role in autoimmune diseases has gained attention in recent years (7). Environmental exposure to air pollution may mediate the development of autoimmune diseases by increasing inflammatory responses, oxidative stress, and immune responses. In this context, a previous study demonstrated that nitrogen dioxide (NO2) and sulfur dioxide exposure might increase the number of first admissions for systemic lupus erythematosus (8). Another study reported significative association between short-term exposure to NO2, carbon monoxide and hospitalizations for acute gout (9).
As common air pollutants, particulate matter (PM) pose an important threat to human health; the impact of PM2.5 (aerodynamic diameter ≤ 2.5 μm) and PM10 (aerodynamic diameter ≤ 10 μm) have been studied more extensively in some diseases. Studies increasingly suggest that PM2.5 exposure is related to many inflammatory dermatoses, including atopic dermatitis, acne vulgaris, and psoriasis (10–12). PM2.5 exposure has also been found to cause local or systemic inflammatory responses and regulation of bodily immune functions, thereby leading to the development of autoimmune diseases (13). One birth cohort study found that PM10 exposure in the third trimester of pregnancy could increase the levels of interleukin-1β in cord blood (14); notably, overexpression of interleukin-1β had been found to lead to the development of autoimmune diseases (15). Although these findings suggest that PM exposure may be involved in the pathogenesis of autoimmune diseases, further studies are needed for investigation and validation.
Air pollution was found to be strongly related to the severity of eye irritation, ocular surface abnormalities in SS patients, and the findings also suggested that it could influence the risk of developing SS (16–18). However, available data on the relationship between PM exposure and SS remain scarce. In addition, the lag-response effect has not been considered in several previous time-series studies. This time-series study aimed to explore the potential correlation between PM2.5 and PM10 exposure and hospitalizations for SS in the city of Hefei; it also aimed to identify the susceptible population by gender, age, and the seasonal variability.
2 Subjects and methods
2.1 Study subjects
This time-series study analyzed the data on SS-related hospitalizations in Hefei (31°52′N, 117°17′E; the capital city of Anhui Province, China) between January 1, 2016 and December 31, 2021. The total covered area of this city is 11,445.1 km2, with a population of 9.465 million in 2021, and experiences a monsoon-influenced humid subtropical climate.
We collected the daily records of hospital admissions among patients with SS from three hospitals in Hefei (the First Affiliated Hospital of University of Science and Technology of China, the First People’s Hospital of Hefei, and the Third People’s Hospital of Hefei) during the study period. The diagnosis of all SS patients was made by rheumatologic clinicians according to the 2002 American European Consensus Group (AECG) classification criteria. Data pertaining to gender, residential address, and date of hospitalizations were also obtained. Patients with a residential address outside Hefei, incomplete demographic information, and a confirmed positive COVID-19 test were all excluded from the study. In addition, the patients with SS who were admitted to other departments (besides the Department of Rheumatology and Immunology) due to other reasons were also excluded. This study was approved by the Ethics Committee of the First Affiliated Hospital of University of Science and Technology of China.
2.2 Pollutants and meteorological data
The data pertaining to particulate matter (24-h data for PM2.5 and PM10) and other air pollutants (24-h for NO2) were collected from the Hefei Environmental Monitoring Center (originally collected from 10 air quality monitoring stations). The daily concentration of PM2.5, PM10 in Hefei was calculated by the average value of the 10 monitoring stations. This study also collected the meteorological data, including mean temperature (MT, °C) and relative humidity (RH, %), from the China Meteorological Data Service Center (http://data.cma.cn/) among the same period.
2.3 Statistical analysis
The influence of PM2.5, PM10 on SS-related hospitalizations was assessed using a distributed lag non-linear model combined with a generalized linear model. These two models were used to describe the additional lag-response correlation and traditional exposure-response correlation, respectively. As daily admission was considered a minor probability event in patients with SS, the distributed lag non-linear model with quasi-Poisson distribution was employed to analyze the correlation between particulate pollution and SS hospitalizations. Spearman analysis was used to analyze the correlation between each covariate, and two variables with correlation coefficient less than 0.7 could not be included in a same model at the same time to avoid multicollinearity.
The models finally used for PM2.5 and PM10 were as follows:
The subscript t represented the day of observation, α referred to the intercept of every model and Yt, μt respectively were the actual, expected incidence of SS-related hospitalizations on day t. Take the PM2.5 Model as an example, PM2.5t,l represented the dlnm cross basis matrix of PM2.5t, l was the lag day, β1 was set as the vector of PM2.5t, and ns() denoted the natural cubic spline function. A natural cubic spline curve of time with 6 dfs/year was used to adjust for seasonality and long-term trends (17). The dummy variable DOW and the two-category variable Holidayt were used to adjust the effect of weekends and public holidays, respectively. The optimal dfs and the final model parameters for particulate pollution were confirmed based on the values of the quasi Akaike Information Criterion.
We performed stratified analyses were to determine the susceptible population according to age (Age ≤ 40 years, 41 years ≤ Age ≤ 64 years, Age ≥ 5 years), gender (The male patients were too few and this study only analyzed the female SS patients), and season (hot season: April to September and cold season: October to March). This study adopted R 3.6.1 (http://www.R-project.org) with dlnm and splines packages to conduct statistical analyses and visualization, and P<0.05 (two-sided) was considered to be statistically significant.
3 Results
3.1 Basic analysis
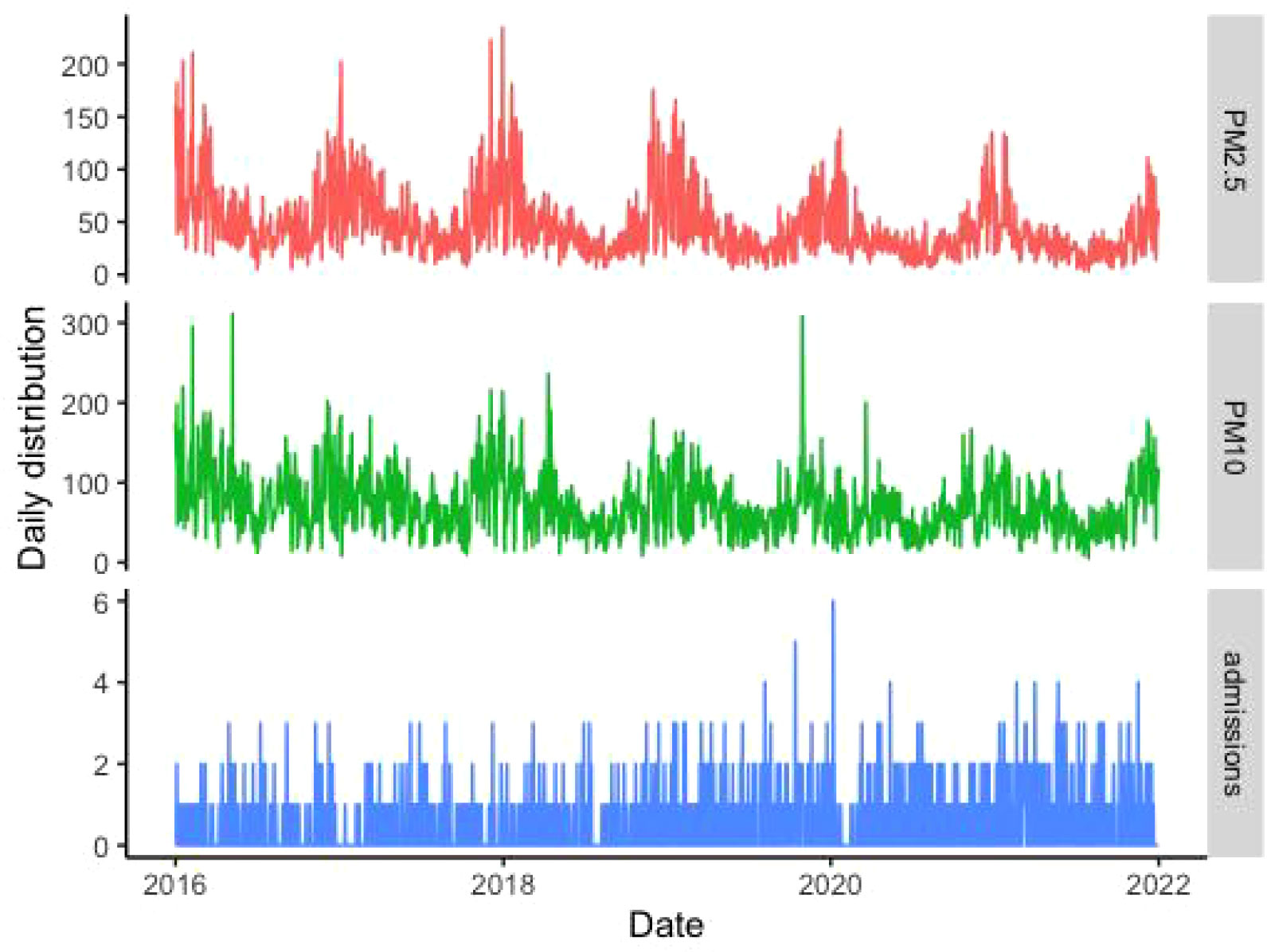
The related information pertaining to SS-related hospitalizations, particulate matter pollution, and meteorological factors are presented in Table 1. Data were obtained for a total of 1119 SS-related hospitalizations between January 1, 2016 and December 31, 2021, and the daily number of SS hospitalizations ranged 0 to 6. Among the included patients, 1061 (94.8%) were male and 350 patients (31.3%) were aged ≥ 65 years. The average daily concentrations of PM2.5 and PM10 were 45.40 μg/m3 and 71.85 μg/m3, respectively. According to correlation analysis, the correlation coefficient between PM2.5 and PM10, as well as PM10 and NO2, was greater than 0.7 (Figure 1). The temporal trends of PM2.5, PM10, SS hospitalizations from 2016 to 2021 in Hefei were showed in Figure 2.
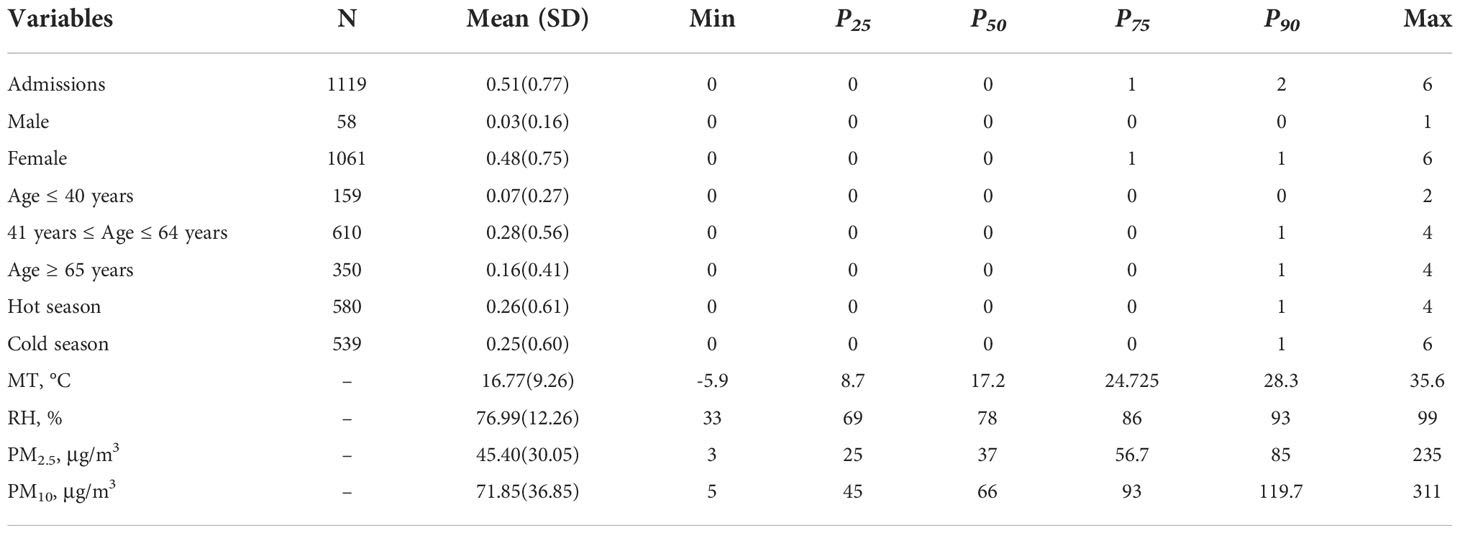
Table 1 The basic information of SS hospitalizations, meteorological data and particulate pollutants.
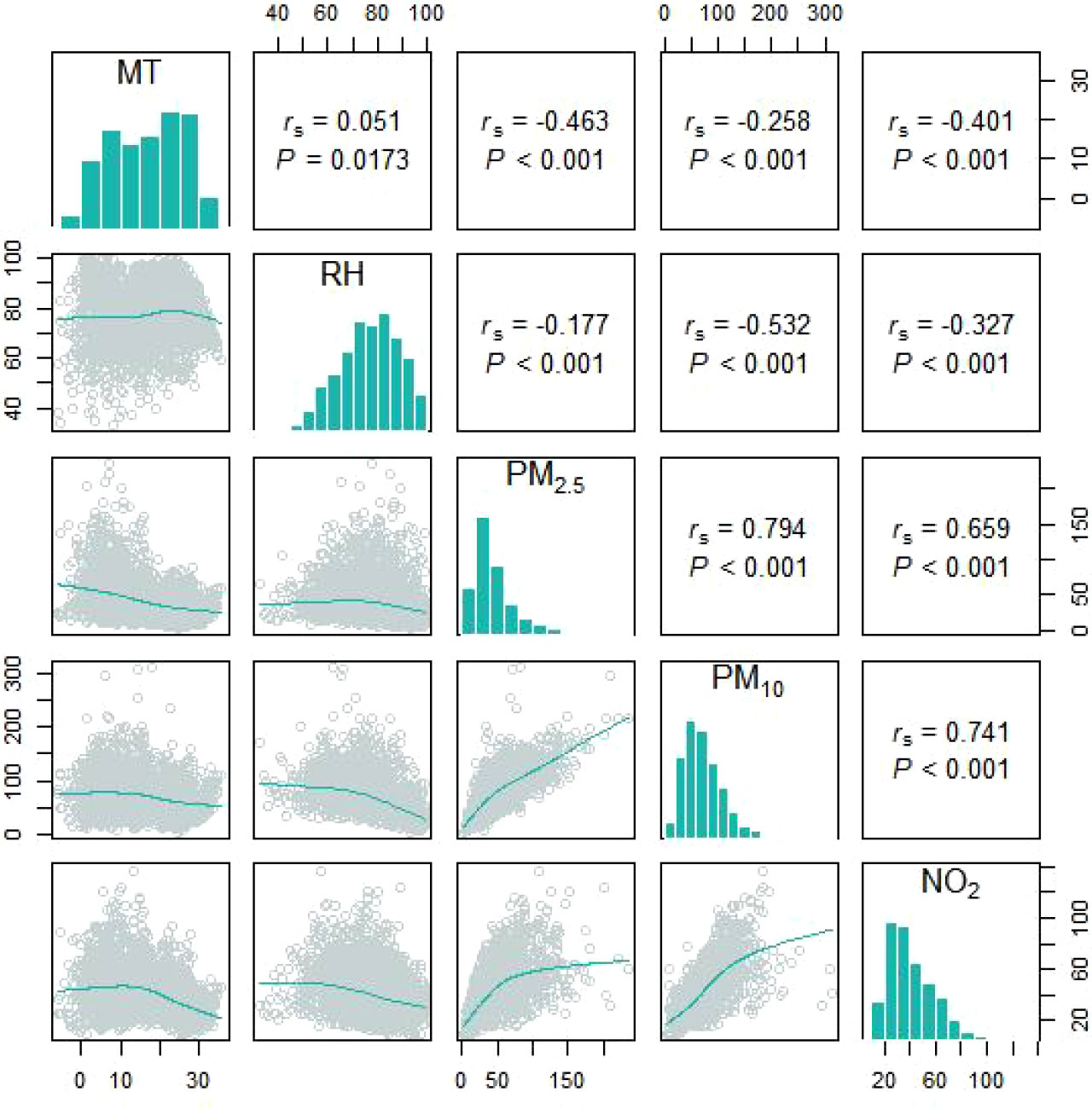
Figure 1 The spearman’s correlation coefficients between different meteorological factors and particulate pollutants.
3.2 Association between particulate pollutants and hospitalizations for SS
3.2.1 Overall effect
The exposure-response correlations between particulate pollutant (PM2.5 and PM10) exposure and SS hospitalizations on different lag days has been presented in Figure 3. Exposure to the high concentrations of PM2.5 and PM10 (reference concentrations of 37 μg/m3 and 66 μg/m3, respectively) were significantly related to the risk of SS-related hospitalizations. The concentration-response relationships between PM2.5 and PM10 concentrations and SS-related hospitalizations are presented in Figure S1.
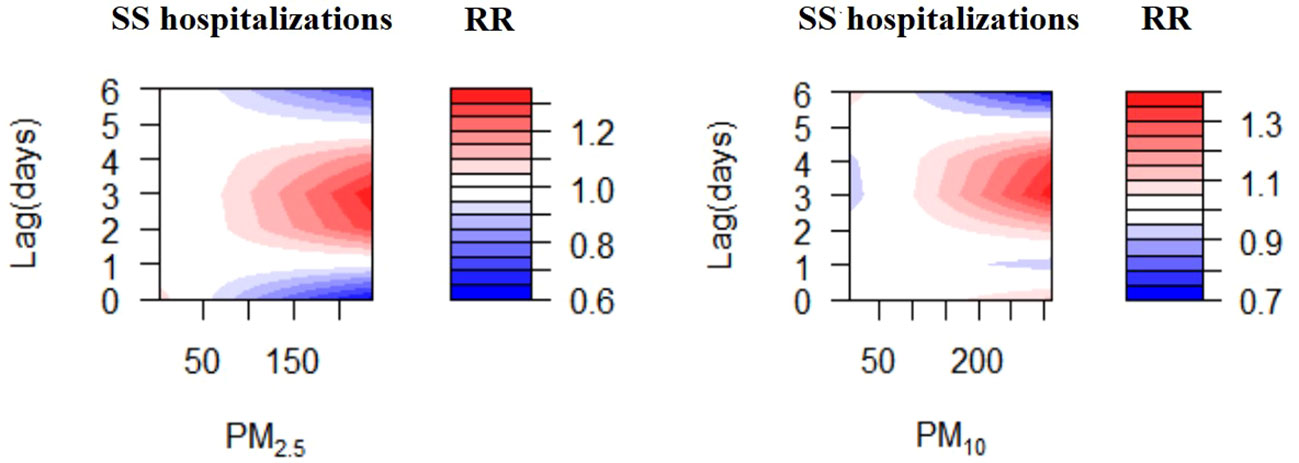
Figure 3 Contour plots for relative risk of hospitalizations for SS along PM2.5, PM10 at lag periods.
3.2.2 The effect of PM2.5 on SS hospitalizations
Increases in PM2.5 concentrations by 10 μg/m3 were found to cause significant single-day effects on the association between exposure to PM2.5 and SS-related hospitalizations at lag 3 day (relative risk [RR]: 1.015, 95% confidence interval [CI]: 1.001-1.029) (Figure 4; Table S1); no cumulative effects were observed for PM2.5 (Figure 5). On stratified analyses, PM2.5 exposure remained positively associated with SS-related hospitalizations in female patients (lag 3 day, RR: 1.015, 95% CI: 1.001-1.029), patients aged ≥ 65 years (lag 3 day, RR: 1.029, 95% CI: 1.004-1.054), and colder months (lag 3 day, RR: 1.016, 95% CI: 1.005-1.028) (Figure 6; Table S2).
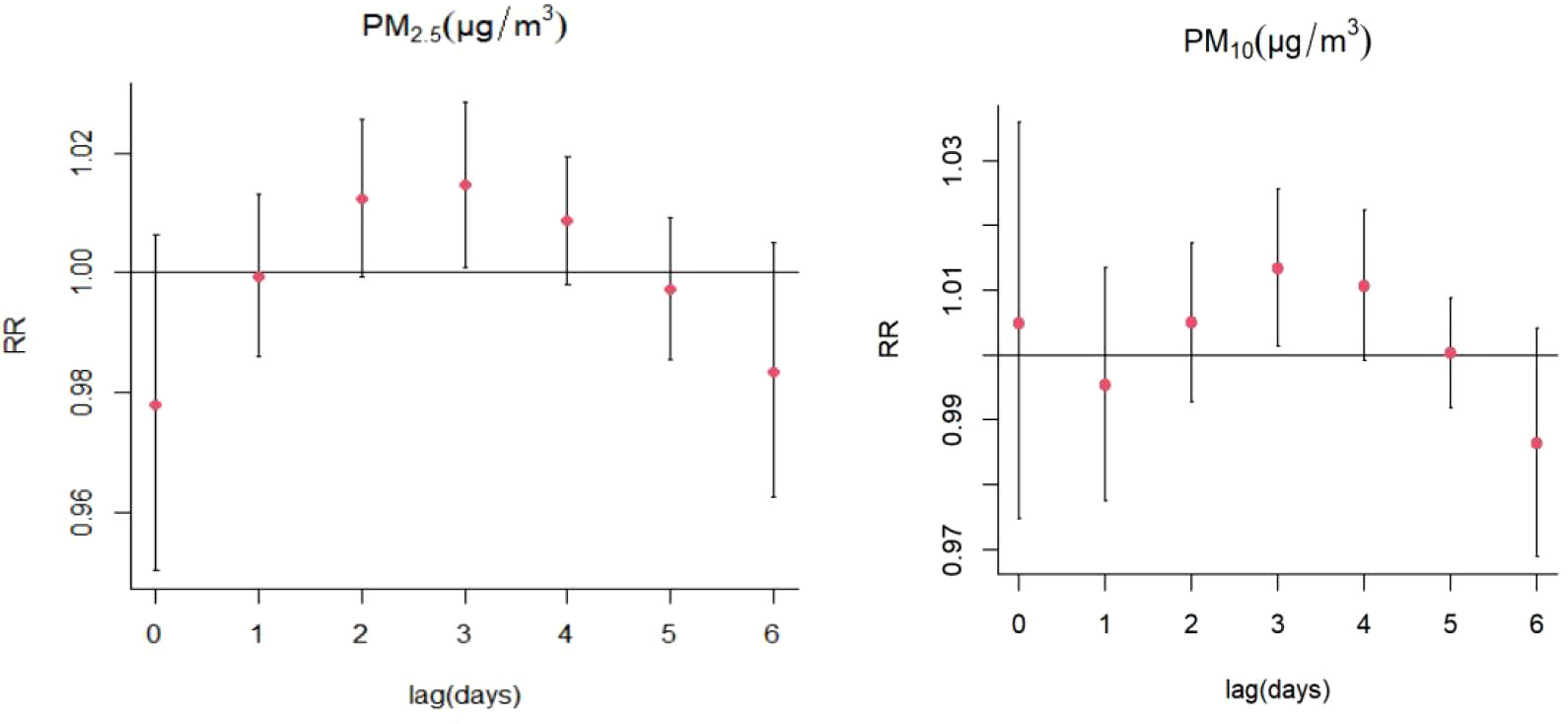
Figure 4 Lag-specific relative risks (%) in hospitalizations for SS per 10 unit increase in daily mean concentrations of PM2.5, PM10.

Figure 5 Cumulative risks (%) in hospitalizations for SS per 10 unit increase in daily mean concentrations of PM2.5, PM10.
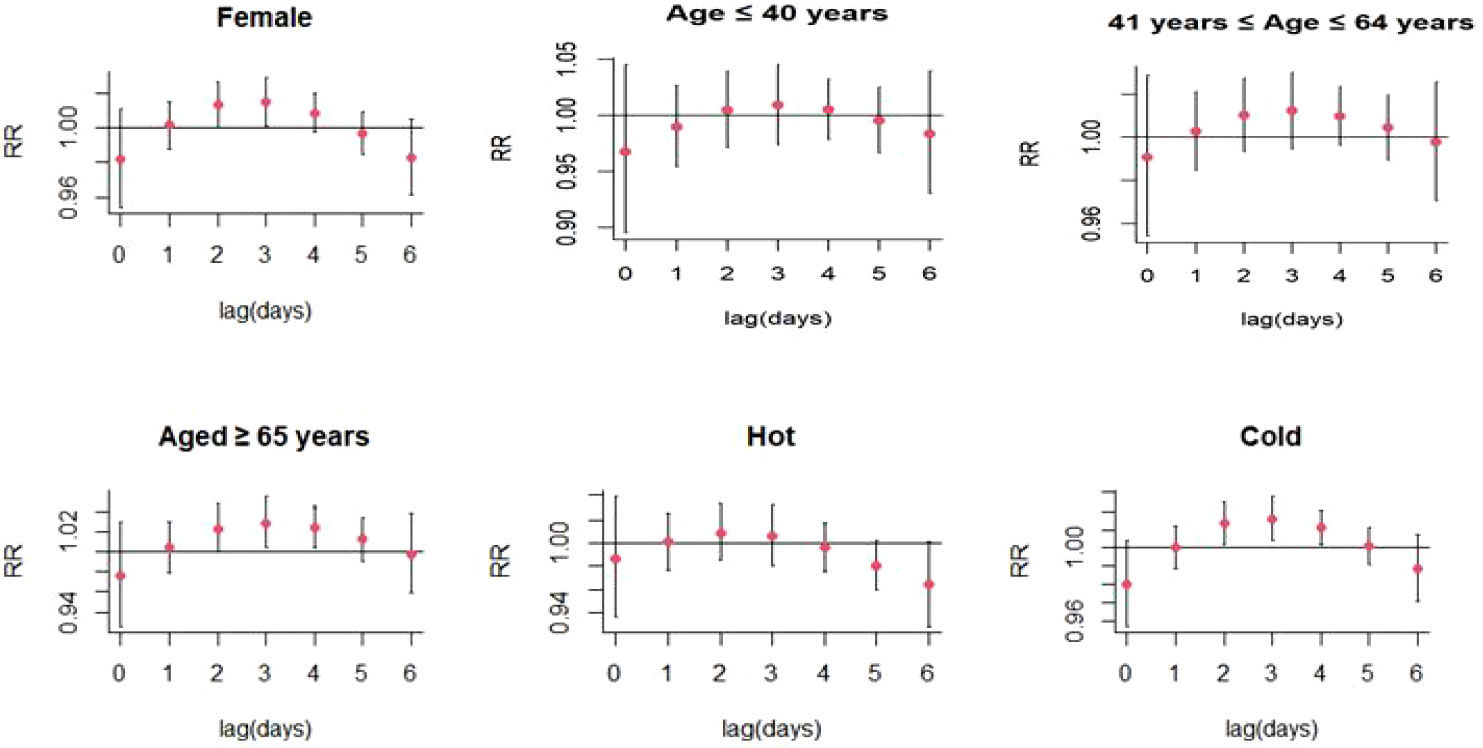
Figure 6 Lag-specific relative risks (95% CI) of SS hospitalizations per 10 unit increase in the daily concentrations of PM2.5 in model stratified by age, gender, and season.
3.2.3 The effect of PM10 on SS hospitalizations
For every 10 μg/m3 increase in PM10 concentrations, the risk of SS hospitalizations increased at lag 3 day (RR: 1.013, 95% CI: 1.001-1.026) (Figure 4; Table S3). Significant positive relationship were also existed between PM10 exposure and SS-related hospitalizations in female patients (RR: 1.014, 95% CI: 1.002-1.027; lag 3 day) and colder months (RR: 1.015, 95% CI: 1.004-1.026) (Figure 7; Table S4). The stratified analyses also suggested that PM10 exposure was both significantly associated with SS-related hospitalizations in three different age groups (Age ≤ 40 years: RR: 1.019, 95% CI: 1.001-1.039, lag 4 day; 41 years ≤ Age ≤ 64 years: RR: 1.015, 95% CI: 1.001-1.030; lag 3 day; Age ≥ 65 years: RR: 1.024, 95% CI: 1.003-1.044; lag 3 day) (Figure 7; Table S4).
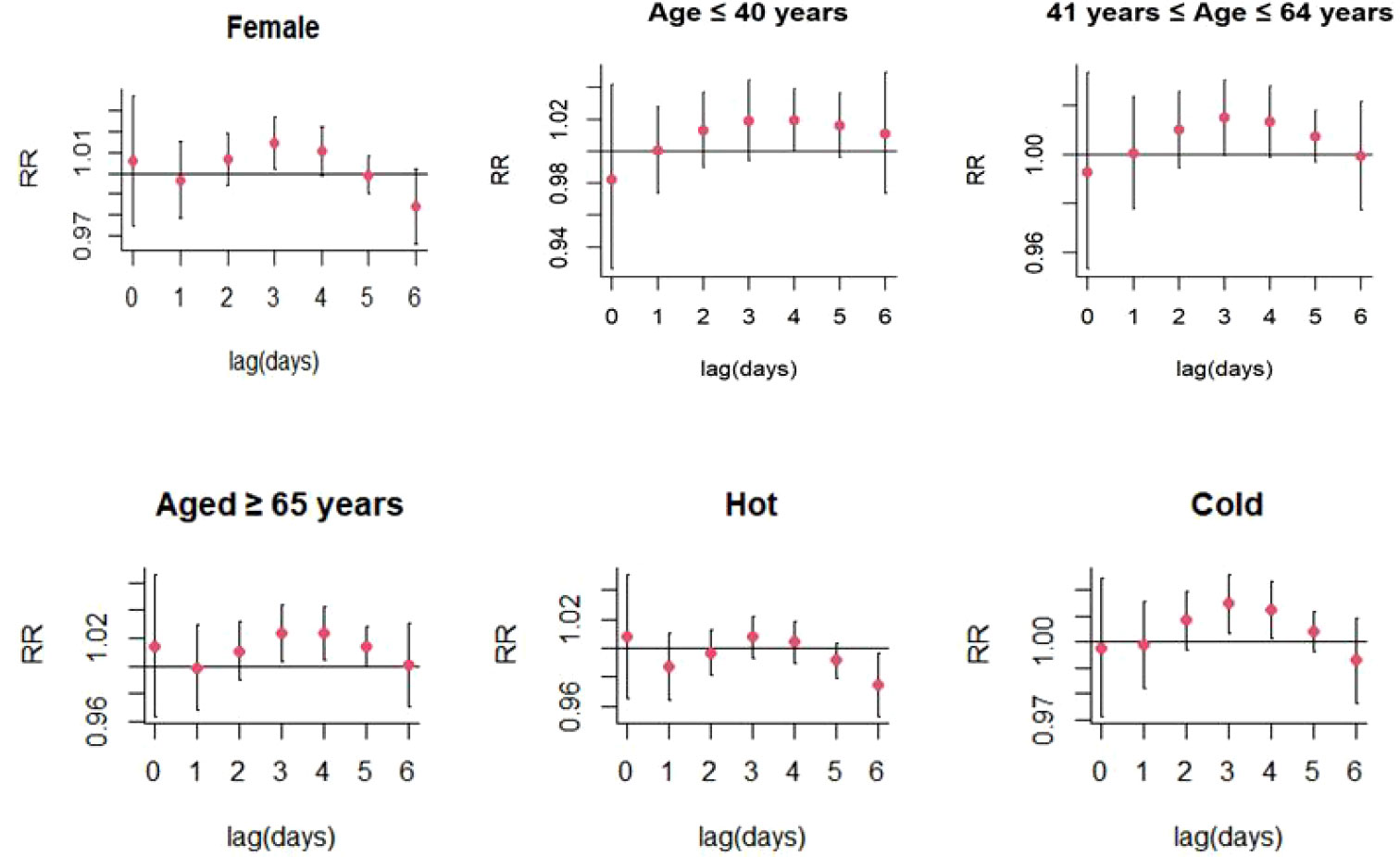
Figure 7 Lag-specific relative risks (95% CI) of SS hospitalizations per 10 unit increase in the daily concentrations of PM10 in model stratified by age, gender, and season.
3.3 Sensitivity analyses
We performed sensitivity analyses to verify the stability of the two models with the ns function after adjusting the dfs for air pollutants (3–5 dfs), meteorological factors (3–5 dfs), and time (6–8 dfs per year). For PM2.5 model, when the dfs for time was 6, we adjust the dfs for air pollution from 3 to 5 in turn (The dfs for air pollutants was the same as for meteorological factors), and get three figures (Figure S2). When the dfs for air pollutants and meteorological factors were 3, we adjusted the dfs for time from 6 to 8 in turn, and get three figures (Figure S2). For PM10 model, when the dfs for time was 6, we adjust the dfs for air pollution from 3 to 5 in turn (The dfs for air pollutants was the same as for meteorological factors), and get three figures (Figure S3). When the dfs for air pollutants and meteorological factors were 4, we adjusted the dfs for time from 6 to 8 in turn, and get three figures (Figure S3).
The results suggested that the primary results of these models were relatively stable with the adjustment of different dfs, therefore, these models established in our study could be considered reliable (Figures S2, S3).
4 Discussion
This time-series study assessed potential associations between exposure to the high concentrations of PM2.5, PM10 and the risk of SS-related hospitalizations. The findings revealed certain points of interest. First, PM2.5 and PM10 exposure was found to have a positive impact on SS-related hospitalizations in Hefei. Second, on stratified analyses by sex, age, and season, the effects of PM2.5 and PM10 on SS-related hospitalizations remained significant in female patients and cold season.
At present, some studies had evaluated the effect of PM2.5 and PM10 exposure on autoimmune disease-related hospitalizations. In their time-series study, Wu et al. showed a positive correlation between exposure to high concentrations of PM2.5 and the number of rheumatoid arthritis hospital readmissions (19). Another study suggested that exposure to PM2.5 played a significant effect on the risk of recurrence in systemic lupus erythematosus patients (8). However, another study suggested that PM2.5 exposure was not linked to the risk of gout-related hospitalizations (9). The findings from these studies suggested that the influence of particulate matter exposure on the risk of developing autoimmune diseases was not consistent; this might be attributed to the different modes of pathogenesis of autoimmune diseases. In the present study, we found that exposure to PM2.5 significantly increased the risk of SS-related hospitalizations; exposure to PM10 also had a similar effect. Consistent with one previous study, Chen et al. found that PM2.5 exposure was associated with increased risk of SS outpatient visits (20). These two studies provided strong evidence to confirm the involvement of PM2.5 in the development of SS. The effects of air pollutants on the development of autoimmune diseases may be attributed to several factors, including T-cell imbalance, proinflammatory cytokine expression, oxidative stress, and local lung inflammation (21). PM2.5 has been proven to be capable of activating nuclear factor kappa B, cyclooxygenase 2, and toll-like receptor 4 signaling pathways, thereby inducing inflammation in macrophages. PM2.5 contains high-affinity aryl hydrocarbon receptor ligands (22, 23); the particles could therefore enhance T helper 17 cell differentiation, promote regulatory T cell production, and modulate autoimmunity by targeting aryl hydrocarbon receptors (24, 25). Our study confirmed a meaningful association between PM exposure and SS hospitalizations and provided critical epidemiological evidence for investigation of the role of PM in autoimmune diseases.
Subgroup analyses were conducted according to different sex and age in order to identify vulnerable groups. Seasonal differences (hot season vs. cold season) in the effect of PM2.5 and PM10 exposure on SS-related hospitalizations were also investigated. The results of our analyses suggested that PM2.5 and PM10 exposure played significant impact on the risk of SS-related hospitalizations in female patients. Similar to the results of another research (26), the risk of hospitalizations in female patients was particularly influenced by extreme environmental factors such as extremely cold temperatures, extreme levels of humidity, and longer durations of sunlight. This might be attributed to gender-based differences in immune defenses, which could have in turn influenced the effect of PM exposure on the risk of SS-related hospitalizations. A previous study found the coefficient of variation of lipopolysaccharide-induced and lipoteichoic acid-induced cytokine responses to be higher in blood samples from females than from males; this finding was consistent for all parameters and stimuli measured (27). Because the incidence of SS in female was significantly higher than that in male, the number of male SS patients in this study was too few. Therefore, the effect of PM2.5 and PM10 on SS-related hospitalizations was not analyzed in male SS patients in this study.
Compared to younger patients, the older patients with SS were more susceptible to PM2.5 exposure in this study. This might be attributed to age-related differences in the development of immune responses, which decline in old age (28). It should be noted that the effect of PM10 exposure on SS-related hospitalizations was significant in three age groups (Age ≤ 40 years, 41 years ≤ Age ≤ 64 years, Age ≥ 65 years), suggesting that this effect might not be affected by age. The correlation between PM2.5 and PM10 exposure and increased risks of SS-related hospitalizations was stronger in the cold season; this might be explained by the low levels of humidity in these months. López-Miguel et al. suggested that exposure to dry conditions might deteriorate lacrimal gland function in patients with SS who had dry eye symptoms; this might be mediated by promotion of inflammatory activity (29).In this study, there were also several limitations. First, this study only collected some demographic data of SS patients, but did not include clinical manifestations, complications and other characteristics, so other stratified analyses could not be carried out. Second, the hospitalizations data for patients with SS were obtained from three hospitals in Hefei; however, it was not possible to include representative data for the entire population of Hefei. Third, as it had an ecological design, this study could not provide evidence for a causal relationship between PM2.5 and PM10 exposure and SS-related hospitalizations; this limitation was similar to that of certain previous time-series studies (9, 19). However, our study had certain strengths. This should be the first study to explore the association between exposure to PM2.5 and PM10 and SS-related hospitalizations in an area with a monsoon-influenced humid subtropical climate. This study also assessed the impact of gender age, and season on this association and identified the vulnerable population.
In summary, our results showed a strong association between PM2.5, PM10 exposure and the risk of SS-related hospitalizations. Moreover, female patients were more susceptible to PM2.5 and PM10 exposure, and the cold season increased the number of SS-related hospitalizations. This study provided valuable insights into the involvement of environmental factors in the pathogenesis of SS. As particulate pollutants remain a serious environmental issue, epidemiological studies based on larger sample sizes and functional studies are needed to investigate the impact of PM2.5, PM10, and other ultra-fine PM on SS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the Ethical Committee of the First Affiliated Hospital of USTC (Hefei, Anhui, China). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
X-ML, C-MY, and T-PZ designed the study. LW, SW, PW, and X-HZ participated in the data collection. T-PZ and JD conducted the data analysis, T-PZ drafted the manuscript. X-ML and C-MY contributed to manuscript revision. All the authors approved the final submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (U21A20365).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1059981/full#supplementary-material
References
1. Baldini C, Talarico R, Tzioufas AG, Bombardieri S. Classification criteria for sjogren's syndrome: a critical review. J Autoimmun (2012) 39(1-2):9–14. doi: 10.1016/j.jaut.2011.12.006
2. Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary sjögren's syndrome: a systematic review and meta-analysis. Ann Rheum Dis (2015) 74(11):1983–9. doi: 10.1136/annrheumdis-2014-205375
3. Vojdani A, Pollard KM, Campbell AW. Environmental triggers and autoimmunity. Autoimmune Dis (2014) 2014:798029. doi: 10.1155/2014/798029
4. Rosenblum MD, Remedios KA, Abbas AK. Mechanisms of human autoimmunity. J Clin Invest (2015) 125(6):2228–33. doi: 10.1172/JCI78088
5. Basith S, Manavalan B, Shin TH, Park CB, Lee WS, Kim J, et al. The impact of fine particulate matter 2.5 on the cardiovascular system: A review of the invisible killer. Nanomaterials (Basel) (2022) 12(15):2656. doi: 10.3390/nano12152656
6. Huang K, Ding K, Yang XJ, Hu CY, Jiang W, Hua XG, et al. Association between short-term exposure to ambient air pollutants and the risk of tuberculosis outpatient visits: A time-series study in hefei, China. Environ Res (2020) 184:109343. doi: 10.1016/j.envres.2020.109343
7. Celen H, Dens AC, Ronsmans S, Michiels S, De Langhe E. Airborne pollutants as potential triggers of systemic autoimmune rheumatic diseases: a narrative review. Acta Clin Belg (2021) 19:1–9. doi: 10.1080/17843286.2021.1992582
8. Zhao CN, Mei YJ, Wu GC, Mao YM, Wu Q, Dan YL, et al. Effect of air pollution on hospital admissions for systemic lupus erythematosus in bengbu, China: a time series study. Lupus (2019) 28(13):1541–8. doi: 10.1177/0961203319882503
9. He YS, Wang GH, Wu Q, Wu ZD, Chen Y, Tao JH, et al. The relationship between ambient air pollution and hospitalizations for gout in a humid subtropical region of China. J Inflammation Res (2021) 14:5827–35. doi: 10.2147/JIR.S329706
10. Oh I, Lee J, Ahn K, Kim J, Kim YM, Sun Sim C, et al. Association between particulate matter concentration and symptoms of atopic dermatitis in children living in an industrial urban area of south Korea. Environ Res (2018) 160:462–8. doi: 10.1016/j.envres.2017.10.030
11. Li X, Zhou LX, Yang LL, Huang XL, Wang N, Hu YG, et al. The relationship between short-term PM2.5 exposure and outpatient visits for acne vulgaris in chongqing, China: a time-series study. Environ Sci pollut Res Int (2022) 29(40):61502–11. doi: 10.1007/s11356-022-20236-8
12. Kim HJ, Bae IH, Son ED, Park J, Cha N, Na HW, et al. Transcriptome analysis of airborne PM2.5-induced detrimental effects on human keratinocytes. Toxicol Lett (2017) 273:26–35. doi: 10.1016/j.toxlet.2017.03.010
13. Feng S, Gao D, Liao F, Zhou F, Wang X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol Environ Saf (2016) 128:67–74. doi: 10.1016/j.ecoenv.2016.01.030
14. Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Röösli M, et al. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PloS One (2011) 6(8):e23130. doi: 10.1371/journal.pone.0023130
15. Zhao R, Zhou H, Su SB. A critical role for interleukin-1β in the progression of autoimmune diseases. Int Immunopharmacol (2013) 17(3):658–69. doi: 10.1016/j.intimp.2013.08.012
16. Galperín G, Berra M, Marquez MI, Mandaradoni M, Tau J, Berra A. Impact of environmental pollution on the ocular surface of sjögren's syndrome patients. Arq Bras Oftalmol (2018) 81(6):481–9. doi: 10.5935/0004-2749.20180091
17. Bernatsky S, Smargiassi A, Johnson M, Kaplan GG, Barnabe C, Svenson L, et al. Fine particulate air pollution, nitrogen dioxide, and systemic autoimmune rheumatic disease in Calgary, Alberta. Environ Res (2015) 140:474–8. doi: 10.1016/j.envres.2015.05.007
18. Bai YC, Wang CY, Lin CL, Lai JN, Wei JC. Association between air pollution and the risk of uveitis: A nationwide, population-based cohort study. Front Immunol (2021) 12:613893. doi: 10.3389/fimmu.2021.613893
19. Wu Q, Xu Z, Dan YL, Cheng J, Zhao CN, Mao YM, et al. Association between traffic-related air pollution and hospital readmissions for rheumatoid arthritis in hefei, China: A time-series study. Environ pollut (2021) 268(Pt A):115628. doi: 10.1016/j.envpol.2020.115628
20. Chen Y, He YS, Feng YT, Wu ZD, Wang J, Yin KJ, et al. The effect of air pollution exposure on risk of outpatient visits for sjogren's syndrome: A time-series study. Environ Res (2022) 214:114017. doi: 10.1016/j.envres.2022.114017
21. Zhao CN, Xu Z, Wu GC, Mao YM, Liu LN, Qian-Wu, et al. Emerging role of air pollution in autoimmune diseases. Autoimmun Rev (2019) 18(6):607–14. doi: 10.1016/j.autrev.2018.12.010
22. Fu H, Liu X, Li W, Zu Y, Zhou F, Shou Q, et al. PM2.5 exposure induces inflammatory response in macrophages via the TLR4/COX-2/NF-κB pathway. Inflammation (2020) 43(5):1948–58. doi: 10.1007/s10753-020-01269-y
23. Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature (2008) 453(7191):106–9. doi: 10.1038/nature06881
24. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of t(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature (2008) 453(7191):65–71. doi: 10.1038/nature06880
25. van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, et al. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PloS One (2013) 8(12):e82545. doi: 10.1371/journal.pone.0082545
26. Xin L, Zhu Y, Liu J, Fang Y, Xie J. Exposure-lag-response associations between extreme environmental conditions and primary sjögren's syndrome. Clin Rheumatol (2022) 41(2):523–32. doi: 10.1007/s10067-021-05910-5
27. Aulock SV, Deininger S, Draing C, Gueinzius K, Dehus O, Hermann C. Gender difference in cytokine secretion on immune stimulation with LPS and LTA. J Interferon Cytokine Res (2006) 26(12):887–92. doi: 10.1089/jir.2006.26.887
28. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci (2015) 282(1821):20143085. doi: 10.1098/rspb.2014.3085
Keywords: particulate pollutant, Sjögren’s syndrome, autoimmune diseases, time-series, PM2.5, PM10
Citation: Zhang T-P, Dou J, Wang L, Wang S, Wang P, Zhou X-H, Yang C-M and Li X-M (2022) Exposure to particulate pollutant increases the risk of hospitalizations for Sjögren’s syndrome. Front. Immunol. 13:1059981. doi: 10.3389/fimmu.2022.1059981
Received: 02 October 2022; Accepted: 29 November 2022;
Published: 15 December 2022.
Edited by:
James J. Pestka, Michigan State University, United StatesReviewed by:
James Cheng-Chung Wei, Chung Shan Medical University Hospital, TaiwanPaulo Hilario Nascimento Saldiva, University of São Paulo, Brazil
Copyright © 2022 Zhang, Dou, Wang, Wang, Wang, Zhou, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Mei Li, bGl4aWFvbWVpQHVzdGMuZWR1LmNu; bGl4aWFvbWVpLmZzbXlAYWxpeXVuLmNvbQ==; Chun-Mei Yang, Y2h1bm1laXlhbmdAdXN0Yy5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Tian-Ping Zhang
Tian-Ping Zhang Jing Dou2†
Jing Dou2† Chun-Mei Yang
Chun-Mei Yang Xiao-Mei Li
Xiao-Mei Li