
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 15 December 2022
Sec. Molecular Innate Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1058862
This article is part of the Research Topic A year in review: Discussions in Molecular Innate Immunity View all 8 articles
 Kellen J. Cavagnero
Kellen J. Cavagnero Richard L. Gallo*
Richard L. Gallo*The term fibroblast has been used generally to describe spindle-shaped stromal cells of mesenchymal origin that produce extracellular matrix, establish tissue structure, and form scar. Current evidence has found that cells with this morphology are highly heterogeneous with some fibroblastic cells actively participating in both innate and adaptive immune defense. Detailed analysis of barrier tissues such as skin, gut, and lung now show that some fibroblasts directly sense pathogens and other danger signals to elicit host defense functions including antimicrobial activity, leukocyte recruitment, and production of cytokines and lipid mediators relevant to inflammation and immunosuppression. This review will synthesize current literature focused on the innate immune functions performed by fibroblasts at barrier tissues to highlight the previously unappreciated importance of these cells in immunity.
The idea of functionally distinct fibroblasts subpopulations was suggested in the literature over 40 years ago (1). The heterogeneity of fibroblasts has since been confirmed through multiple approaches including genetic lineage-tracing and next-generation sequencing. Focusing first on their classical role in wound repair and development, discrete lineages of fibroblasts with unique functions were identified in the skin (2, 3). Fibroblast phenotypic diversity across murine tissues was later revealed using bulk transcriptomic and epigenetic sequencing approaches (4). More recently, single-cell RNA sequencing (scRNAseq) has enabled unbiased investigation into intra- and inter-tissue fibroblast heterogeneity. For example, scRNAseq analysis of fibroblasts from naïve and Cutibacterium acnes infected skin revealed a mixture of eight distinct fibroblast subtypes and states, four of which were unique to infected skin (5). Three of these populations expressed low levels of genes related to classical fibroblast matrix organization function while others expressed high levels of genes related to immunity. Furthermore, integration of scRNAseq datasets from several inflamed tissues in mice and humans showed that while tissue-specific characteristics can be defined for some fibroblastic cells, all tissues are composed of three broad categories of fibroblasts: progenitor fibroblasts, steady-state fibroblasts, and inflammation-associated fibroblasts (6). These observations suggest that some subsets of fibroblasts perform immune functions that are somewhat specific to the tissue and type of perturbation. Overall, current information from analysis of gene expression by fibroblasts suggest they can exist in several functional states with a range of activities in organism development, scar formation, and immunity (Figure 1).
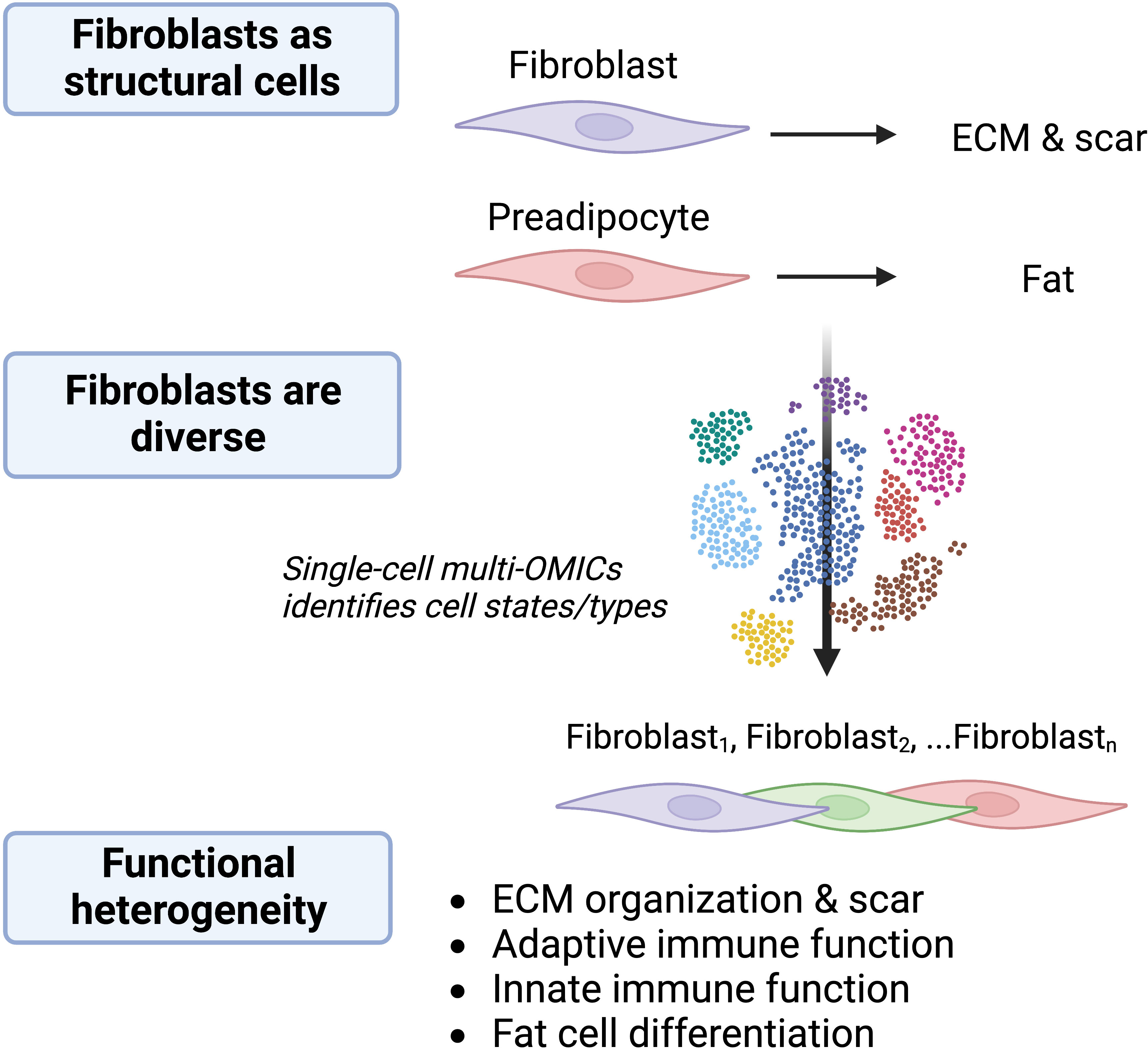
Figure 1 Fibroblasts are diverse and multifunctional. The understanding of cells with fibroblastic morphology is evolving. Fibroblasts were previously considered to be primarily structural cells responsible for production of ECM, acting in wound healing and fibrosis, and serving as stem cells for differentiation into structures such as fat. Single-cell multi-OMICS technology including scRNAseq has identified several fibroblast subtypes and activation states within and between tissues and disease states and revealed numerous potential distinct functions for these cells. Determining the functional significance of fibroblasts is an ongoing area of research. The choice of colors for fibroblasts illustrates that there are different functional subtypes/activation states.
In the following review, we will discuss the emerging and somewhat unexpected revelations that some fibroblasts are direct participants in innate host defense and inflammation. To illustrate this, we will synthesize in vitro work done over the last several decades that has demonstrated that barrier tissue fibroblasts act in pattern recognition, respond to cytokines, and elicit innate immune effector functions. The latter half of this review will then focus on work that has begun to unveil the functional significance of fibroblast immune activity during infections of skin, gut, and lung, as well as in inflammatory diseases and cancer. The capacity of fibroblasts to educate lymphoid cells will not be discussed in detail as these indirect activities of fibroblasts in adaptive immunity were the subject of another excellent recent review (7).
A key feature of innate immunity is the ability to rapidly detect pathogen/microbial/danger-associated molecular patterns (PAMPs, MAMPs, and DAMPs) using pattern recognition receptors (PRRs) that initialize host defense against bacterial, viral, and fungal pathogens (8). This function has been generally attributed to front-line epithelial cells and tissue-resident classical immunocytes such as macrophages and dendritic cells. Numerous studies have now demonstrated that fibroblasts in the skin, gut, and lung express functional PRRs including membrane and endosomal toll-like receptors (TLRs) and cytoplasmic retinoic acid-inducible gene I (RIG-I)-like receptors and NOD-like receptors (NLRs) (Figure 2) (9–12).
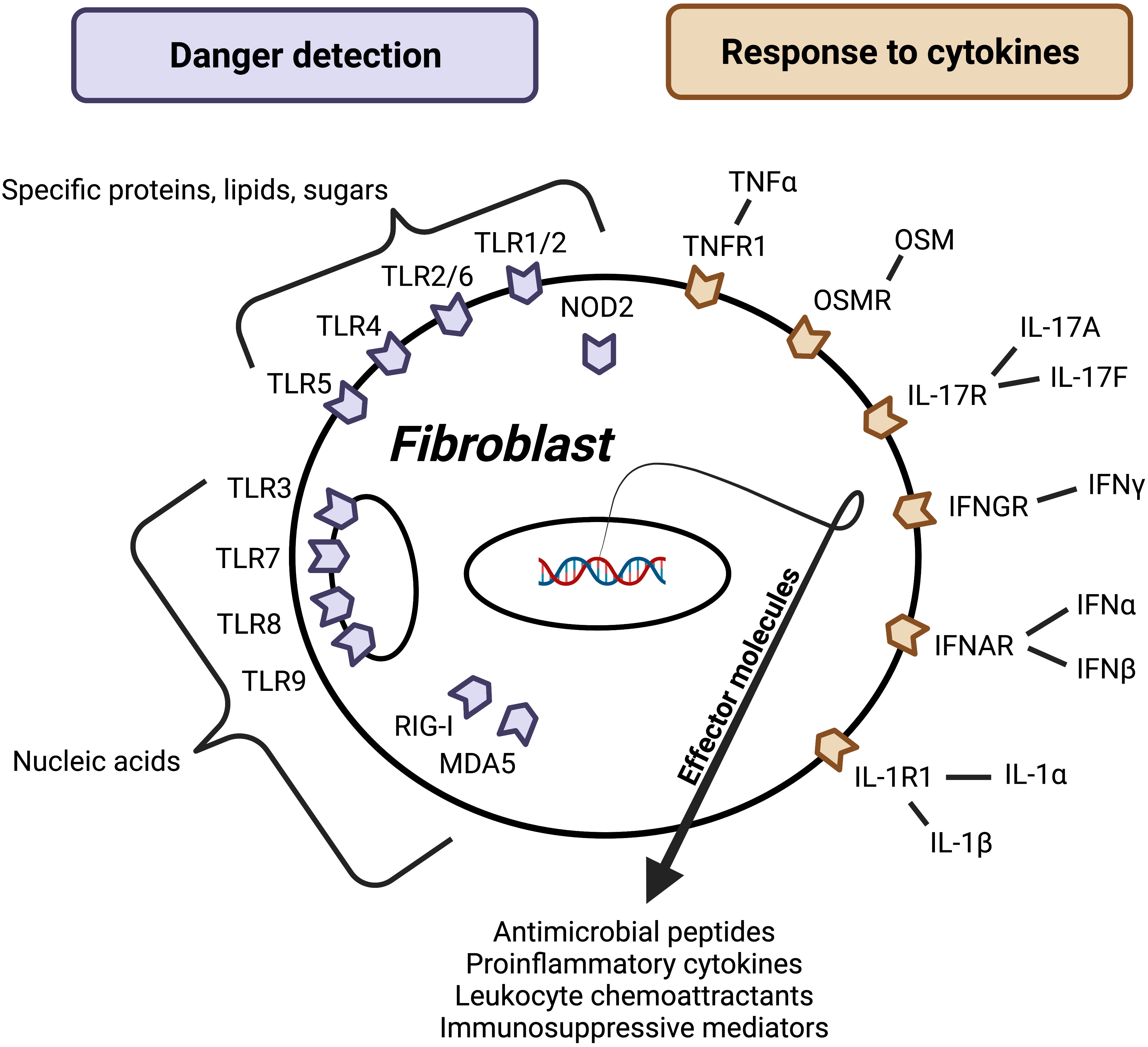
Figure 2 Factors regulating fibroblast effector function. Barrier tissue fibroblasts sense danger and respond to cytokines, leading to activation and release of effector molecules. Danger signals may be microbe- or host-derived and detected using surface, cytosolic, or endosomal receptors (purple). While these receptors can broadly be divided into a group that recognizes nucleic acids and group that recognizes certain molecules composed of proteins, lipids, and/or sugars, each receptor has evolved to recognize a unique set of ligands. Host-derived soluble protein signals called cytokines activate surface receptors (orange). Importantly, this figure represents a synthesis of barrier tissue fibroblast activating signals; more research is needed to understand how fibroblast activation varies between different fibroblast populations. Additionally, more work is needed to identify negative regulators and understand how other signals like neuronal factors, lipid mediators, and hormones impact barrier tissue fibroblast effector function.
One outcome of fibroblast pattern recognition is the secretion of inflammation mediators, and this response appears to depend on the molecule being detected, the tissue of origin, and the host of origin. Danger detection systems must be able to elicit a diversity of responses in order to promote defense against a broad array of pathogens. Stimulation of skin fibroblasts with ligands to TLR1/2, TLR2/6, TLR3, TLR4, TLR5, TLR7, TLR8, and TLR9 has been shown to promote secretion of the proinflammatory cytokine IL-6 and neutrophil chemokine CXCL8 (13, 14), while the TLR3 ligand Poly(I:C), but not ligands to other TLRs, was shown to induce expression of the leukocyte chemokines CXCL9 and CXCL10 by fibroblasts in the skin (15). Commensal and pathogenic microbes are typically unique to the epithelial organ and therefore the danger detections system must be specific to the location. Contrary to skin fibroblasts, ligands for TLR1/2, TLR2/6, and TLR4—but not TLR3, TLR5, TLR7, and TLR9—led to IL-6 secretion by intestinal fibroblasts (10, 16). Microbiota composition is also unique to the host, necessitating somewhat individualized pattern recognition capabilities. It has been observed that skin fibroblasts from different donors display a large range of responses to the TLR4 ligand lipopolysaccharide (LPS) independent of age and sex (4-7x difference between low and high responders) (17, 18).
Fibroblast PRR signaling is also important for cell-intrinsic antiviral immunity. Human skin fibroblast expression of TLR3 is necessary for mounting cell-autonomous antiviral responses to Zika virus infection (19). Intriguingly, the mechanisms underlying cell-intrinsic antiviral immunity in fibroblasts appear to differ from those employed by classical immunocytes. One study showed that skin fibroblasts from an individual with an autosomal recessive TLR3 deficiency demonstrated an impaired response to herpes simplex virus (HSV)-1 infection, while the individual’s peripheral blood mononuclear cells responded normally (20).
Taken together, these reports demonstrate that barrier tissue fibroblasts are poised to detect a variety of danger signals and initiate defense responses tailored to the environment and threat encountered. That fibroblasts in the skin, gut, and lung subepithelial stroma have functional pattern recognition activity further illuminates the multilayered defense system of barrier tissues and fills an important gap in knowledge that is currently not typically considered when modeling inflammatory pathways in these organs.
Effective innate immune cells must also be able to detect inflammatory mediators sent from other immune cells and propagate immune responses. Like conventional innate immunocytes, fibroblasts in the skin, gut, and lung have been shown to respond to a wide variety of proinflammatory cytokines known to be important for host defense (Figure 2).
IFNγ is produced largely by T helper 1 (Th1) cells and group 1 innate lymphoid cells (ILC1s) for defense against intracellular bacteria, viruses, and parasites (21). In 1984, Pfefferkorn demonstrated that in vitro stimulation of skin fibroblasts with IFNγ induces cell-intrinsic defense against the intracellular pathogen Toxoplasma gondii (22). IFNγ has also been shown to elicit skin fibroblast expression of the CXCR3 ligands CXCL9 and CXCL10 that are important for Th1 recruitment and maturation (14, 15). Moreover, a recent study found that a given fibroblast’s response to IFNγ is dependent upon its anatomic location. Specifically, murine skin fibroblast expression of CXCR3 ligands following IFNγ stimulation is decreased in cells isolated from paw skin compared to those from dorsal or ventral skin (23). Intriguingly, IFNγ and TLR ligands synergistically promote skin fibroblast expression of CXCL8 and CXCL10 (14), suggesting that fibroblasts are sentinel cells that integrate danger signals from immune cells and microbes.
TNFα is a proinflammatory cytokine that is critical for host defense and is clinically relevant to several barrier tissue chronic inflammatory diseases including psoriasis and inflammatory bowel disease (IBD) (24). Biologic TNFα inhibitors effectively treat patients with these conditions, though treatment increases risk of certain infections. Despite the clinical success of TNFα inhibitors, the precise mechanisms by which these therapeutics regulate immunity are unclear, but they may act in part by interfering in the capacity of fibroblasts to propagate inflammatory signals. In support of this, TNFα was initially found to induce IL-6 and CXCL8 in skin fibroblasts (25). Currently, a long list of inflammatory mediators are known to be expressed by TNFα-activated fibroblasts from the skin, gut, and lung and these include a vast array of proinflammatory cytokines and chemokines relevant to innate immunity (14, 26–28). Interestingly, one study found that Poly(I:C) primes lung fibroblasts to produce more IL-6 following a later TNFα exposure (29), suggesting that fibroblasts could potentially drive post-viral inflammatory conditions.
IL-17A is a proinflammatory cytokine produced by subsets of lymphocytes including T helper 17 (Th17) cells, γδT cells, and group 3 innate lymphoid cells (ILC3s), and it is important for defense against extracellular pathogens (30, 31). As with TNFα, IL-17A is associated with barrier tissue chronic inflammatory diseases including psoriasis and IBD, and drugs inhibiting IL-17A signaling are currently used to treat patients. While IL-17A is widely appreciated as a potent, albeit indirect, driver of neutrophil recruitment, the exact mechanisms underlying IL-17A-mediated immune responses are unclear but could potentially be in part through fibroblasts. In 1996, Fossiez and colleagues first cloned and purified recombinant human IL-17A and found that it induces IL-6, CXCL8, granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), and prostaglandin E2 (PGE2) production in skin fibroblasts (32). Since this early discovery, skin, gut, and lung fibroblasts stimulated with IL-17A have been shown to produce additional inflammatory mediators including IL-1α and IL-1β (26, 28, 33–36). IL-17A is well-known for synergizing with proinflammatory cytokines by stabilizing mRNA and promoting translation (30). In barrier tissue fibroblasts, IL-17A has been found to synergize with TNFα, IL-1β, and Poly(I:C) (26, 33, 36–38). Intriguingly, conditioned medium from IL-17A- and TNFα-stimulated skin fibroblasts promotes γδT cell production of IL-17A, suggesting that fibroblasts contribute to the propagation of immune responses through bi-directional communication with IL-17A-producing lymphocytes (26).
IL-1R1 ligands, IL-1α and IL-1β, are proinflammatory cytokines secreted primarily by epithelial cells and macrophages, respectively. Like IL-17A and TNFα, IL-1α and IL-1β are associated with barrier tissue chronic inflammatory diseases and targeted in treatment, and inhibition of these mediators also increases risk of infection. Pioneering work in the 1980s revealed that IL-1, alone and in synergy with TNFα, induces lung fibroblast production of inflammatory mediators (28, 39–42). Others have since shown that IL-1β activates skin and gut fibroblasts (43, 44) and that IL-1β together with Poly(I:C) synergistically induces IL-6 and CXCL8 expression in lung fibroblasts (11). Elegant in vitro experiments have demonstrated the importance of IL-1-induced fibroblast activation in physiologically relevant settings. Lysates from damaged intestinal and lung epithelial cells drive IL-1α-dependent intestinal and lung fibroblast inflammatory mediator production, respectively, and conditioned media from IBD patient tissue explants elicits IL-1-dependent intestinal fibroblast secretion of neutrophil chemoattractants including CXCL1 and CXCL8 (11, 45, 46). Importantly, the effect of IL-1α on skin fibroblasts is lineage-specific. Skin fibroblasts arise from at least two distinct lineages (2). One lineage (Lrig1+ and Blimp1+) gives rise to the upper dermis (proximal to the epithelium) and includes papillary fibroblasts. The other lineage (Dlk1+) gives rise to the lower dermis (distal to the epithelium) and includes reticular fibroblasts and preadipocytes. IL-1α-stimulated papillary fibroblasts produce more GM-CSF than IL-1α-stimulated reticular fibroblasts (47), indicating that cells derived from the upper dermal lineage are primed to interact with local keratinocyte epithelial cells.
Oncostatin M (OSM), a member of the IL-6 family of cytokines, has been linked to inflammatory barrier tissue diseases including psoriasis, atopic dermatitis, IBD, and allergic airway disease but has no defined role in host defense (48). Nevertheless, OSM has also been shown to promote skin, gut, and lung fibroblast immune activation and synergize with TNFα (49–51).
Collectively, these studies demonstrate that barrier tissue fibroblasts are equipped with the machinery necessary to propagate innate immune responses initiated by other tissue-resident cells for defense against several classes of pathogens.
That some fibroblasts can sense danger and respond to a variety of inflammatory mediators has gained new relevance as there now is abundant evidence that fibroblasts can elicit antimicrobial activity, promote leukocyte recruitment, and secrete cytokines and bioactive lipids. These functions have relevance to organism immune system development and homeostasis, inflammation, and immunosuppression (Figure 3), thus demonstrating how fibroblasts are a key element of innate immune defense. Fibroblasts may also influence immunity through antigen presentation and matrix regulation; however, discussion of these mechanisms is beyond the scope of this review and have been reviewed elsewhere (52, 53). In this section, we will review the emerging innate immune effector functions of barrier tissue fibroblasts.
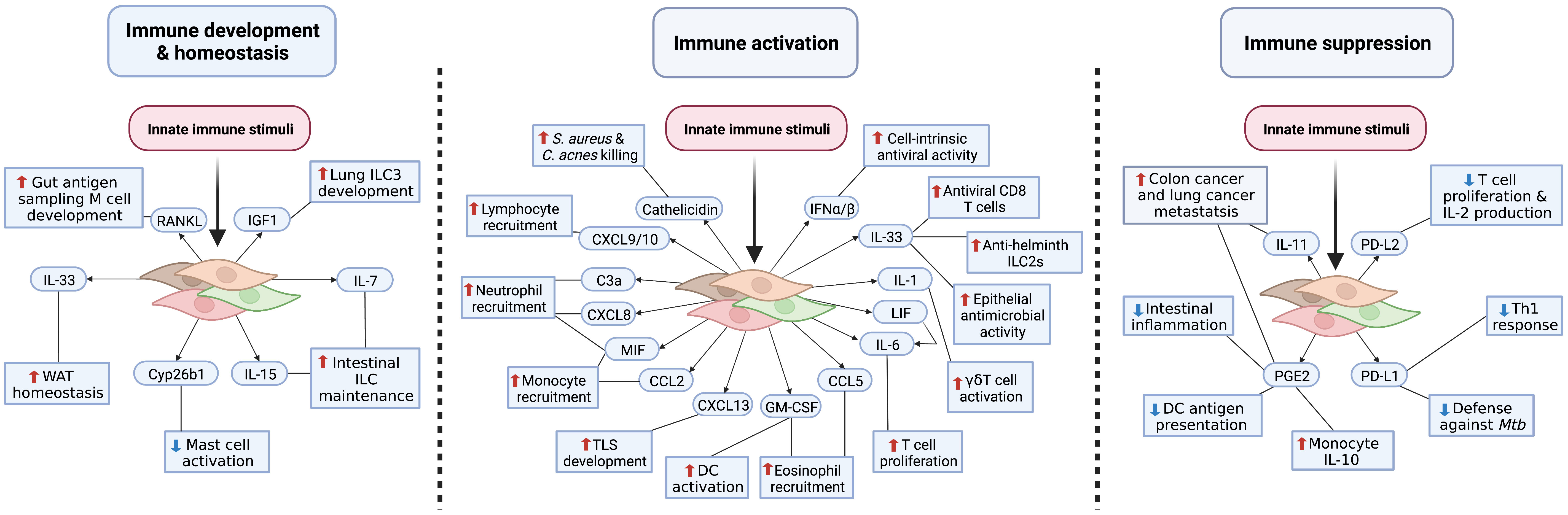
Figure 3 Fibroblast innate immune effector functions. Barrier tissue immune acting fibroblasts elicit innate immune functions required for development and homeostasis, immune activation, and immunosuppression. During steady-state conditions, fibroblasts produce cytokines and growth factors necessary for immunological priming and homeostasis (left panel). To potentially eliminate pathogens, fibroblasts release antimicrobial peptides, chemokines, and proinflammatory cytokines (middle panel). Left unchecked, this defense response could contribute to chronic inflammatory conditions. To limit excessive and protracted immune responses and prevent inflammatory disease pathogenesis, fibroblasts upregulate immune checkpoint molecules, suppressive bioactive lipid mediators, and anti-inflammatory cytokines; however, inappropriate fibroblast immunosuppression may increase risk of infection and cancer (right panel). This figure represents an amalgamation of proposed barrier tissue fibroblast innate immune effector functions. It is unclear whether each fibroblast subtype/state across tissues is capable of achieving an activation state that will enable these functions.
Arguably the most critical aspect of host defense is the elimination of microbial threats through production of endogenous antibiotics. While direct antimicrobial activity is largely attributed to epithelial cells, neutrophils, and macrophages, there is a growing body of evidence demonstrating that fibroblasts are a key source of antimicrobials. The essential role of the cathelicidin antimicrobial peptide in the skin was demonstrated in 2001 (54). Subsequent in vitro studies suggested that microbes may induce fibroblast expression of cathelicidin as well as defensin antimicrobial peptides (55–58). In 2015, dermal fibroblasts belonging to a preadipocyte lineage (Dlk1+) were shown to secrete large amounts of cathelicidin while undergoing adipogenesis induced by Staphylococcus aureus infection (59). Importantly, the antimicrobial function of these cells was necessary for the skin to resist infection. Production of cathelicidin by preadipocytes has more recently been shown to be potentiated by TLR2 and TLR4 ligands and retinoic acid (5, 60, 61). Further, the profibrotic cytokine TGFβ has been found to inhibit the host defense function of this fibroblast subset, and this mechanism may contribute to increased infections observed during ageing and obesity (states of elevated TGFβ production) (62, 63).
Subsets of fibroblasts have also been shown to display phagocytic capacity, hinting that some fibroblasts also possess intracellular killing mechanisms (64–66). Indeed, a recent investigation found that Sca-1+ lung fibroblasts produce reactive oxygen species and antimicrobial proteins and potently inhibit the growth of intracellular Klebsiella pneumoniae (66). Notably, several chemokines known to be produced by fibroblasts demonstrate direct antimicrobial activity in vitro—particularly CXCL9 and CXCL10—and therefore may be relevant to the ability of fibroblasts to kill bacteria (reviewed by (67)). In sum, these reports reveal that some fibroblasts are potent antimicrobial cells.
Another key aspect of host defense is the propagation of immune responses through chemokine and proinflammatory cytokine production. In response to innate immune stimulation, barrier tissue fibroblasts have been shown to secrete several proinflammatory cytokines and chemokines.
In 1996, Noso and colleagues published one of the earliest studies identifying barrier tissue fibroblasts as important regulators of immune cell recruitment (68). In this study, skin fibroblasts stimulated with TNFα were shown to promote eosinophil chemotaxis through GM-CSF and CCL5. A more recent study demonstrated that Ng2+ skin pericytes (an endothelial-adjacent type of fibroblast) colocalize with neutrophils and monocytes following subcutaneous injection of TNFα, and in vitro transwell migration assays with umbilical pericytes indicated that these cells promote macrophage migration inhibitory factor (MIF)- and CXCL8-dependent neutrophil migration and MIF- and CCL2-dependent monocyte migration (69). Other studies showed that lung fibroblasts promote complement C3a-dependent neutrophil migration in an in vitro transwell migration assay (70), and that skin fibroblasts activated by IFNγ promote CXCL9- and CXCL10-dependent lymphocyte migration (23). Beyond leukocyte recruitment, chemokine production by fibroblasts is known to be important for the development of lymphoid structures (reviewed by (71)). Of note, innate immune stimuli—including IL-1, TNFα, IL-17A, and type 1 IFN—induce barrier tissue fibroblast production of CXCL13 required for lymphoid tissue inducer cell clustering and germinal center formation (72–78).
Some fibroblasts from barrier tissues have been found to express the IL-1 family members IL-33, IL-1α, and IL-1β. IL-33 and IL-1α are unique in that they function as alarmins—pre-formed cytokines stored in the nucleus. Following cellular injury, alarmins are rapidly released into the extracellular space to initiate innate immune responses. IL-33 is widely regarded for its critical role in anti-parasitic immunity and allergic disease pathogenesis through activation of type 2 cytokine producing cells including group 2 innate lymphoid cells (ILC2s) and T helper 2 (Th2) cells. Ultraviolet radiation, which may worsen outcomes in patients with the allergic skin disease atopic dermatitis, was shown to induce skin fibroblast IL-33 (79). In terms of IL-1α and IL-1β, in vitro studies using skin, gut, and lung fibroblasts have demonstrated that fibroblasts are not only activated by IL-1 (as discussed in the previous section) but also that innate immune stimuli induce fibroblast secretion of IL-1α and IL-1β (26, 41). Functionally, IL-1 secreted by skin fibroblasts was shown to activate IL-17A producing γδT cells (26).
IL-6 is a pleiotropic cytokine essential for innate immune responses to bacteria, viruses, and parasites (48, 80). While IL-6 was one of the earliest barrier tissue fibroblast cytokines discovered (42), there is limited evidence demonstrating the importance of fibroblast-derived IL-6 in host defense beyond its initially described role in promoting T cell proliferation (42). Intriguingly, however, a recent study found that a human genetic polymorphism in the IL6 gene has a profound effect on IL-6 production by skin fibroblasts but not by other cell types (81). This result supports previously mentioned studies that identified inter-individual differences in fibroblast activation following pattern recognition (17, 20).
G-CSF and GM-CSF are glycoprotein inflammatory mediators that support myeloid cell function (82–84). A wide variety of innate immune stimuli have been found to induce skin, gut, and lung fibroblast secretion of these mediators (32, 64, 85). Fibroblast-derived GM-CSF may be important not only for myeloid cell chemotaxis (as described above) but also for dendritic cell maturation as production of GM-CSF from an intestinal fibroblast subset was shown to be necessary for local dendritic cell education (86). Future work should investigate whether a similar mechanism applies to skin fibroblasts where location-specific fibroblast GM-CSF production has also been observed (47).
Type 1 interferons (IFN), IFNα and IFNβ, are pleiotropic antiviral cytokines produced by many cell types rapidly following PRR ligation. Barrier tissue fibroblasts are beginning to be appreciated for their ability to both produce and respond to type 1 IFN. The innate immune function of this defense system was demonstrated early on in a study using Dengue virus infected skin fibroblasts which showed that viral infection induces IFNβ that protects neighboring uninfected fibroblasts from infection (85). More recently, infection of skin fibroblasts with Zika virus was shown to induce RIG-I-dependent expression of IFN-stimulated genes and suppression of virus (9).
Innate immune stimuli also induce fibroblast expression of immunosuppressive mediators. In the 1980s, Dayer and Elias discovered that TNFα and IL-1 activate barrier tissue fibroblasts to secrete the cyclooxygenase (COX) metabolite PGE2 (39, 87). The role of this bioactive lipid mediator in host defense is complex; generally, however, PGE2 increases susceptibility to infection and does so by inhibiting neutrophils, macrophages, and Th1 cells (88). Indeed, lung fibroblast-derived PGE2 promotes monocyte production of anti-inflammatory IL-10 (89), and intestinal fibroblast-derived PGE2 inhibits the development of inflammatory macrophages (90). More recently, a study investigating PGE2 production in fibroblasts across tissues (including intestine but not skin) found that lung fibroblasts secrete significantly more PGE2 compared to fibroblasts from to other tissues (91). Further, this report demonstrated that lung fibroblasts use PGE2 to suppress dendritic cell antigen presentation. Additional in vitro studies have suggested that fibroblasts can tamp down immune responses through expression of the immune checkpoint transmembrane proteins PD-L1 and PD-L2 and suppression of T cell IL-2 production and proliferation (92). TLR4 activation has been shown to further induce fibroblast PD-L1 expression and Th1 suppression (93). Notably, a multipotent and immunosuppressive subset of fibroblasts was discovered in the early 1970s and has since been referred to as mesenchymal stem/stromal cells (MSCs) (94). The immunosuppressive function of fibroblasts known as MSCs is currently being leveraged in adoptive transfer-based clinical trials for inflammatory diseases including IBD. Most of the preclinical and clinical work related to MSC immunosuppression has relied on cells derived from tissues other than skin, gut, and lung, and is thus beyond the scope of this review but has been reviewed elsewhere (95).
Collectively, these reports demonstrate that barrier tissue fibroblasts exhibit innate immune effector functions generally attributed to classical immunocytes. Immune acting fibroblasts kill bacteria and recruit and activate other cells important for defense. Further, the work highlighted above paints a picture of fibroblasts as immunological governors, equipped with the machinery to ramp up or down immune responses to best defend the host. Precisely which factors dictate whether a given fibroblast will take on more of a pro- versus anti-inflammatory state is an exciting area for future investigation. Will it be due to specific innate immune stimuli, chronic stimulation, or something else entirely? Critically, studies going forward should aim to directly compare the immune function of fibroblasts derived from different anatomical regions to better understand fibroblast heterogeneity and tissue-specificity.
Residing in the tissue and armed with sentinel immune capabilities, barrier tissue fibroblasts are positioned to be key players in innate immunity (Figure 4). Importantly, however, many of the immune functions described for fibroblasts could presumably drive the development of inflammatory disease if left unchecked. In this section, we will describe evidence that is now emerging from in vivo systems that demonstrates the importance of fibroblast immune function in the skin, gut, and lung. Much of this evidence has been gleaned from the use of fibroblast-specific conditional knockout mice (Table 1).
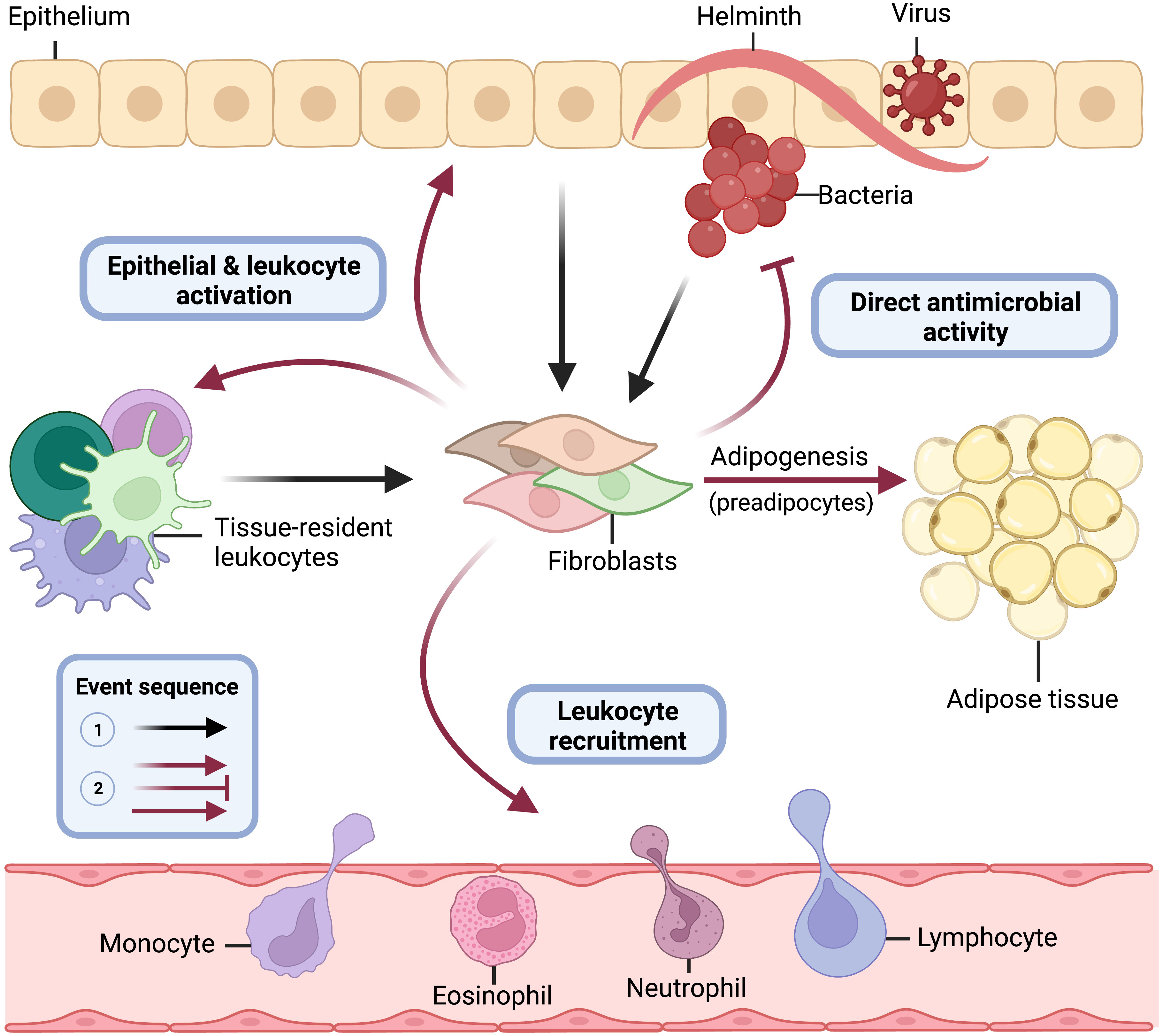
Figure 4 Central role of immune acting fibroblasts in innate immunity. Barrier tissue fibroblasts are tissue-resident sentinel cells, and some orchestrate innate immune responses. Following epithelial damage and breach by bacterial, viral, or parasitic pathogens, fibroblasts recognize threats directly through pattern recognition receptors (i.e., TLRs) and receive proinflammatory cytokine danger signals (i.e., TNFα) from epithelial cells and tissue-resident lymphoid and myeloid cells (black lines). Upon activation by these innate immune stimuli, committed preadipocyte fibroblasts differentiate into adipocytes (solid red line) and elicit critical immune effector functions for pathogen elimination (faded red lines) including direct antimicrobial activity, leukocyte recruitment, and activation of epithelial cells and tissue-resident leukocytes. This figure models the role fibroblasts play in innate immunity at barrier tissue sites and is not meant to suggest that each fibroblast subtype and state across tissues is known to perform all functions depicted.
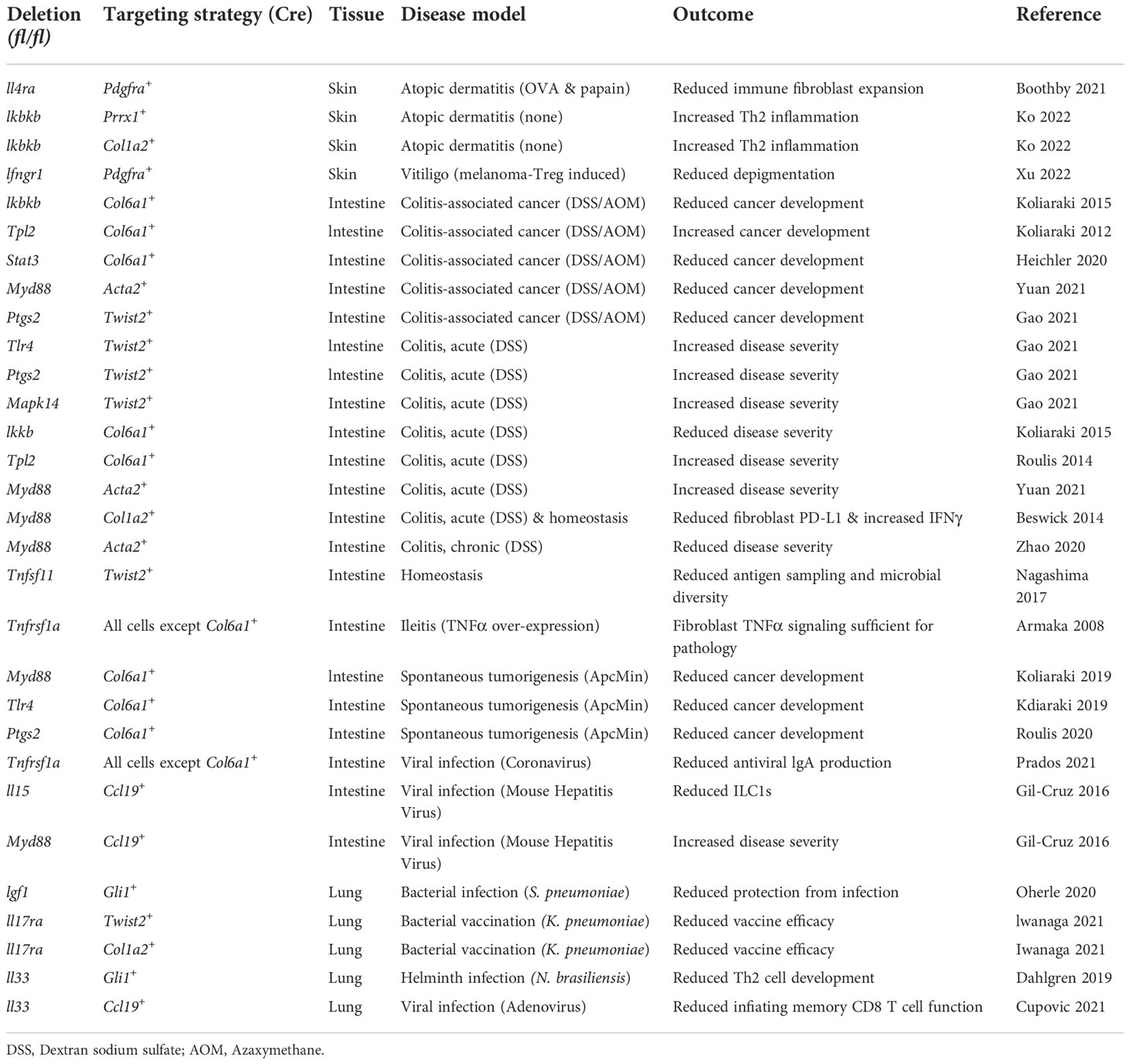
Table 1 Conditional knockout mice demonstrate critical role of fibroblast immune function in barrier tissue disease.
Subsets of fibroblasts in the skin have recently been shown to be important for defense against bacterial infection and contribute to type 1 and type 2 inflammation. S. aureus is responsible for most skin and soft tissue infections in humans (96). In 2015, our group observed that intradermal S. aureus infection causes dermal Dlk+ preadipocyte fibroblasts to differentiate into adipocytes (59). We refer to this process as “reactive adipogenesis”, and we found that fibroblasts undergoing reactive adipogenesis secrete large amounts of the antimicrobial peptide cathelicidin. Genetic or pharmacological inhibition of adipogenesis demonstrated that cathelicidin production by this process is necessary for adequate host defense against S. aureus. We later showed that TGFβ-mediated suppression of adipogenic fibroblast antimicrobial activity underlies the increased susceptibility to S. aureus infection seen in obese and elderly individuals (62, 63). C. acnes, another skin dwelling bacterium, is associated with acne—one of the most prevalent human skin conditions (97). Intradermal C. acnes infection induces perifollicular reactive adipogenesis, and experiments with human acne biopsies indicated that isotretinoin, a mainstay treatment for severe acne, likely works by amplifying fibroblast cathelicidin production (5).
There is increasing in vivo evidence that the immune functions of fibroblasts are important in inflammatory skin disease. Boothby and colleagues found that type 2 inflammatory events (Treg depletion, helminth infection, and allergen challenge) early in life drive inflammatory crosstalk between Th2 cells and a novel eosinophilic fasciitis-associated Pdgfra+ (a pan-fibroblast marker) fibroblast population that responds to Th2 cytokines and produces IL-33 (98). Paradoxically, Ko and colleagues discovered that mice harboring a ventral fibroblast-specific (Prrx1+) deletion of Ikbkb—a key gene in the NF-κB signaling pathway that is generally proinflammatory—develop spontaneous atopic dermatitis-like Th2 inflammation (99). Fibroblasts have also been shown to play a role in Th1-skewed inflammatory disease. By performing scRNAseq on vitiligo patient biopsies and experimental vitiligo mouse models with Ifngr1-deficient Pdgfra+ fibroblasts, Xu and colleagues found that fibroblasts activated by IFNγ mediate CD8 T cell recruitment underlying autoimmune depigmentation (23). Other scRNAseq studies have suggested that fibroblasts may also contribute to type 1 inflammation in cutaneous lupus erythematosus as well as type 17 inflammation during wound healing and psoriasis (100–102). Finally, it is worth reiterating that the mechanisms by which fibroblasts act as immune cells to regulate inflammatory disease pathogenesis are somewhat tissue-specific. In terms of type 2 inflammatory disease, skin fibroblasts, unlike lung and gut fibroblasts, are primed to prevent the activation of mast cells—an important cell type for the development of atopic dermatitis (103).
Fibroblasts in the gut are beginning to be appreciated for their vital roles in immune development and homeostasis, host defense, and immunosuppression. During steady-state conditions, intestinal Twist2+ (also known as Dermo1) fibroblasts located in the subepithelial dome of gut-associated lymphoid tissues (GALT) secrete RANKL necessary for epithelial sampling of luminal antigens, plasma cell IgA production, and maintenance of gut microbiome diversity (104, 105), and Ccl19+ fibroblasts in the Peyer’s patches (a type of GALT) generate IL-7 and IL-15 required for homeostasis of ILC1s (106, 107).
Recent evidence suggests that immune acting fibroblasts also play important roles during infection. In response to Salmonella infection, pericryptal fibroblasts release IL-33 that drives epithelial cell antimicrobial activity (108). For defense against enteropathogenic viral infection, Ccl19+ fibroblasts activated by TNFα induce plasma cell production of antiviral IgA (109). Following Citrobacter rodentium infection, CCL2 is upregulated, and stromal cells are the major source. In the absence of Ccl2, mice demonstrate reduced monocyte recruitment and pathogen clearance (12).
Because pathogens are heterogenous in terms of their susceptibility to specific immune responses, fibroblast host defense responses to one pathogen may increase the risk of infection by another. For example, when Myd88 is deleted in Ccl19+ fibroblasts, these cells ramp up IL-15 production and activation of antiviral ILC1s, thereby protecting mice against mouse hepatitis virus; however, increased ILC1 activation also drives post-viral dysbiosis, inflammatory disease, and sensitivity to bacterial infection (106).
Fibroblasts may also be important in IBD, which represents a collection of intestinal inflammatory conditions, including Crohn’s disease (CD) and ulcerative colitis (UC), wherein a damaged and permeable intestinal epithelium facilitates microbial translocation. Intriguingly, patients with CD often present during resection surgery with mesenteric adipose tissue projections onto inflamed intestinal tissue called “creeping fat” (110). Our group found that intestinal injury drives reactive adipogenesis and cathelicidin production and that inhibition of fat cell differentiation limits microbial translocation (111). Thus, creeping fat appears to represent the aftermath of extensive fibroblast-mediated host defense.
The innate immune role of fibroblasts in IBD extends beyond cathelicidin production. Using experimental colitis models and IBD patient samples, studies have shown that fibroblasts in the intestine produce an array of proinflammatory cytokines and chemokines (112, 113). Specifically, intestinal fibroblasts may exert direct antimicrobial activity through production of the antimicrobial lipocalin 2 and promote epithelial cell-mediated antimicrobial activity though production of IL-33. To put the brakes on protracted inflammatory responses, specific intestinal fibroblast subsets (Twist2+ but not Acta2+) produce PGE2 and express PD-L1 and PD-L2 (90, 92, 93, 114). Intriguingly, fibroblasts in CD express more PD-L1 than healthy control fibroblasts, while fibroblasts in UC express less PD-L1 than healthy controls (115–117).
Understanding how fibroblasts are turned on in IBD is an area of active investigation. Emerging data suggests that the mechanisms underlying fibroblast immune activation in intestinal inflammatory disease are complex and disease- and fibroblast subset-specific. For example, acute colitis is ameliorated in mice with a Col6a1+ (a pan-fibroblast marker) fibroblast-specific deletion of Ikbkb (a positive regulator of proinflammatory NF-κB signaling) (118). However, other studies using mice with an Acta2+ (basement membrane adjacent) fibroblast-specific deletion of Myd88 (also a positive regulator of NF-κB signaling) found that acute colitis was exacerbated but that chronic colitis was ameliorated (119, 120).
In terms of upstream innate immune stimuli, it has been observed that mice with the TNFα receptor 1 (TNFR1) restricted to Col6a1+ fibroblasts develop pathology in a TNFα-induced model of Crohn’s disease (121). This finding further suggests that biologic TNFα inhibitors, a mainstay treatment in the IBD pharmacological armamentarium, may work in part by inhibiting proinflammatory fibroblast activity. Unfortunately, 30% of patients do not respond to anti-TNFα treatment. In these patients, OSM and IL-1 have been shown to be dominant drivers of intestinal fibroblast production of proinflammatory cytokines and chemokines (46, 50). These findings, among others, have generated large interest in OSM and IL-1 inhibitors as potential therapeutic strategies for IBD.
Emerging evidence indicates that immune acting fibroblasts also participate in intestinal tumorigenesis. Colon cancer may arise spontaneously or due to unresolved inflammation. In spontaneous models of tumorigenesis, mice with Col6a1+ fibroblast-specific deletions of Tlr4, Myd88, or Ptgs2 (encodes an enzyme important for PGE2 production) exhibit reduced cancer development (10, 118, 122). In colitis-associated cancer (CAC) models, mice with Col6a1+ fibroblast-specific deletion of Stat3 or Ikbkb (but not Tlr4 or Myd88) demonstrate reduced cancer development (10, 118, 123, 124). Interestingly, when Myd88 is deleted specifically in Acta2+ fibroblasts, mice exhibit reduced CAC development (120). Twist2+ fibroblasts have also been targeted in CAC models. When Ptgs2 is deleted in this fibroblast subset, mice demonstrate reduced CAC (90). This finding, coupled with the results from the Col6a1+ fibroblast-specific Ptgs2 knockout mouse mentioned above, paints a picture of fibroblast-derived PGE2 as a powerful tumor promoter. Another fibroblast-derived immune mediator that plays a role in cancer development is the IL-6 family member IL-11. A recent study showed that IL-11 is upregulated in CAC and that a subpopulation of fibroblasts lacking αSMA is the major source (125). Further, in the absence of IL-11 (or its receptor) mice were protected from cancer development. Collectively, these reports suggest that fibroblast immunosuppressive activity generates an environment permissive for cancer growth, though the precise mechanisms underlying fibroblast immune activity in cancer are, as in IBD, complex.
While most of the work regarding gut fibroblast immune function has focused on the intestines, there is a growing body of work surrounding the role of immune acting fibroblasts in the esophageal and oral mucosae. In the allergic disease eosinophilic esophagitis, the overexpressed TNF superfamily member LIGHT has been shown to drive proinflammatory fibroblast responses (126–128), and a recent scRNAseq study of the oral mucosa suggested that fibroblasts may drive neutrophil infiltration in health and periodontal disease (129).
Taken together, this large body of work on gut fibroblasts demonstrates that specific populations are important for development of the immune system, defense against pathogens, and timely immunosuppression; however, these cells must be tightly regulated to not inadvertently contribute to pathogenesis.
Fibroblasts in the lung are emerging as important cells in pulmonary host defense against bacteria, viruses, and parasites. Using mice with IL-17R genes specifically deleted in Col1a2 + (a pan-fibroblast marker) or Twist2+ fibroblasts, IL-17 signaling in fibroblasts was shown to be important for vaccine efficacy against K. pneumoniae (130). Another study found that adoptive transfer of Sca-1+ fibroblasts in a mouse model of K. pneumoniae-induced pneumonia suppresses bacterial growth and improves survival (66). Lung fibroblasts also function upstream of Th17 responses. Alveolar Gli1+ fibroblasts produce IGF1 critical for the development of ILC3s and protection against Streptococcus pneumoniae (131). For defense against metazoan parasites and viruses, perivascular Gli1+ fibroblast-derived IL-33 is required for the development of helminth-induced Th2 cells (132), and perivascular Ccl19+ fibroblasts (in the lung but not lymphoid tissue) produce IL-33 necessary for adenovirus-induced inflating memory CD8 T cell development and function (133). Notably, some lung pathogens have evolved mechanisms to subvert fibroblast immune responses for survival. For example, a recent study showed that Mycobacterium tuberculosis invades Sca-1+CD73+ lung fibroblasts and drives up fibroblast PGE2 production, thus protecting itself from immune- and drug-mediated killing (134).
Like gut fibroblasts, specific lung fibroblast subsets have been shown to play roles in cancer and immune suppression. Ccl19+ perivascular lung fibroblasts were found to be essential for leukocyte chemotaxis and protection against cancer (135). Other studies have discovered detrimental roles for fibroblasts in cancer. Specifically, a Sca-1+CD44+ fibroblast subset recruit and activate neutrophils using C3a, thereby creating a niche for metastasis (70). Pdgfra+ fibroblast Ptgs2 also promotes metastasis (91). In terms of immunosuppression, it was recently observed that mice with a Twist2+ fibroblast-specific deletion of Ryk (co-receptor for Wnt) develop spontaneous lung inflammation (136).
Collectively, these studies illustrate that specific populations of lung fibroblasts can act as immune cells and are important for the development of several branches of the immune system, defense against a diverse array of clinically relevant pathogens, and regulation of inflammation and tumorigenesis.
Fibroblasts were once thought to represent a homogenous group of structural cells but are now appreciated as a diverse and multifunctional class of cells. Immune functions have been clearly detected in subsets of fibroblasts, and these activities appear to be important for host defense. Fibroblasts located in the subepithelial layers of the skin, gut, and lung likely serve as sentinel immune cells that respond to pathogens and elicit innate immune effector functions including direct antimicrobial activity, leukocyte recruitment, and cytokine production. Importantly, the precise outcome of immune acting fibroblasts varies depending on several factors including the inflammatory insult, the fibroblast subset, and its environment.
Historically, most studies revealing fibroblast function have done so using populations of cells expanded in vitro without a complete understanding of the diversity and multifunctionality of cells called ‘fibroblasts’. Future work should build on recent single-cell analysis efforts to characterize markers that identify subsets of fibroblasts and define their lineages, activation states, and spatial locations. Such studies will facilitate discussion of immune acting fibroblasts and guide future research. Because some innate immune functions are conserved between fibroblasts from different barrier tissues, future work should address whether findings related to fibroblasts in one tissue apply to another. For example, do fibroblasts in the lung undergo reactive adipogenesis like fibroblasts in the skin and gut? And do skin and gut fibroblasts exhibit features of trained immunity like fibroblasts in the lung? Moreover, ongoing efforts should elucidate whether fibroblast immune functions that have been identified in barrier tissues also apply to non-barrier tissue diseases, such as rheumatoid and psoriatic arthritis. For example, does lung fibroblast trained immunity contribute to chronic inflammatory disease exacerbation and recurrence as synovial fibroblast trained immunity does in rheumatoid arthritis (137)? Finally, while the list of mediators discovered to activate fibroblasts grows long, our understanding of inhibitory molecules is severely lacking. Overall, with a better understanding of fibroblast biology, immune acting fibroblasts may ultimately be specifically targeted or used in adoptive transfer cell-based therapies for treatment of infection, inflammatory disease, and cancer.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
KC and RG wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
KC is supported by NSF GRFP2038238 and previously by NIH T32DK007202, and RG is supported by NIH R01DK121760, NIH R01AR076082, NIHR01AI153185, U01AI152038, P50AR080594, and NIH R37AI052453.
Figures created using BioRender.com.
RG is a consultant for and has equity interest in MatriSys bioscience and Sente Inc.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Harper RA, Grove G. Human skin fibroblasts derived from papillary and reticular dermis: differences in growth potential in vitro. Science (1979) 204:526–7. doi: 10.1126/science.432659
2. Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature (2013) 504:277–81. doi: 10.1038/nature12783
3. Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol (2018) 138:811–25. doi: 10.1016/j.jid.2018.01.016
4. Krausgruber T, Fortelny N, Fife-Gernedl V, Senekowitsch M, Schuster LC, Lercher A, et al. Structural cells are key regulators of organ-specific immune responses. Nature (2020) 583:296–302. doi: 10.1038/s41586-020-2424-4
5. O'Neill AM, Liggins MC, Seidman JS, Do TH, Li F, Cavagnero KJ, et al. Antimicrobial production by perifollicular dermal preadipocytes is essential to the pathophysiology of acne. Sci Transl Med (2022) 14:eabh1478. doi: 10.1126/scitranslmed.abh1478
6. Buechler MB, Pradhan RN, Krishnamurty AT, Cox C, Calviello AK, Wang AW, et al. Cross-tissue organization of the fibroblast lineage. Nature (2021) 593:575–9. doi: 10.1038/s41586-021-03549-5
7. Onder L, Cheng HW, Ludewig B. Visualization and functional characterization of lymphoid organ fibroblasts. Immunol Rev (2022) 306:108–22. doi: 10.1111/imr.13051
8. Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol (1994) 12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015
9. Kim JA, Seong RK, Son SW, Shin OS. Insights into ZIKV-mediated innate immune responses in human dermal fibroblasts and epidermal keratinocytes. J Invest Dermatol (2019) 139:391–9. doi: 10.1016/j.jid.2018.07.038
10. Koliaraki V, Chalkidi N, Henriques A, Tzaferis C, Polykratis A, Waisman A, et al. Innate sensing through mesenchymal TLR4/MyD88 signals promotes spontaneous intestinal tumorigenesis. Cell Rep (2019) 26:536–45.e4. doi: 10.1016/j.celrep.2018.12.072
11. Suwara MI, Green NJ, Borthwick LA, Mann J, Mayer-BARBER KD, Barron L, et al. IL-1α released from damaged epithelial cells is sufficient and essential to trigger inflammatory responses in human lung fibroblasts. Mucosal Immunol (2014) 7:684–93. doi: 10.1038/mi.2013.87
12. Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity (2011) 34:769–80. doi: 10.1016/j.immuni.2011.04.013
13. Yao C, Oh JH, Lee DH, Bae JS, Jin CL, Park CH, et al. Toll-like receptor family members in skin fibroblasts are functional and have a higher expression compared to skin keratinocytes. Int J Mol Med (2015) 35:1443–50. doi: 10.3892/ijmm.2015.2146
14. Proost P, Vynckier AK, Mahieu F, Put W, Grillet B, Struyf S, et al. Microbial toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-gamma and provide a mechanism for enhanced synovial chemokine levels in septic arthritis. Eur J Immunol (2003) 33:3146–53. doi: 10.1002/eji.200324136
15. Farina GA, York MR, Di Marzio M, Collins CA, Meller S, Homey B, et al. Poly(I:C) drives type I IFN- and TGFβ-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J Invest Dermatol (2010) 130:2583–93. doi: 10.1038/jid.2010.200
16. Walton KL, Holt L, Sartor RB. Lipopolysaccharide activates innate immune responses in murine intestinal myofibroblasts through multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol (2009) 296:G601–11. doi: 10.1152/ajpgi.00022.2008
17. Chansard A, Dubrulle N, Poujol De Molliens M, Falanga PB, Stephen T, Hasan M, et al. Unveiling interindividual variability of human fibroblast innate immune response using robust cell-based protocols. Front Immunol (2020) 11:569331. doi: 10.3389/fimmu.2020.569331
18. Kandasamy S, Green BB, Benjamin AL, Kerr DE. Between-cow variation in dermal fibroblast response to lipopolysaccharide reflected in resolution of inflammation during escherichia coli mastitis. J Dairy Sci (2011) 94:5963–75. doi: 10.3168/jds.2011-4288
19. Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, et al. Biology of zika virus infection in human skin cells. J Virol (2015) 89:8880–96. doi: 10.1128/JVI.00354-15
20. Guo Y, Audry M, Ciancanelli M, ALSINA L, Azevedo J, Herman M, et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med (2011) 208:2083–98. doi: 10.1084/jem.20101568
21. Thakur A, Mikkelsen H, Jungersen G. Intracellular pathogens: Host immunity and microbial persistence strategies. J Immunol Res (2019) 2019:1356540. doi: 10.1155/2019/1356540
22. Pfefferkorn ER. Interferon gamma blocks the growth of toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A (1984) 81:908–12. doi: 10.1073/pnas.81.3.908
23. Xu Z, Chen D, Hu Y, Jiang K, Huang H, Du Y, et al. Anatomically distinct fibroblast subsets determine skin autoimmune patterns. Nature (2021) 601: 118–124. doi: 10.1038/s41586-021-04221-8
24. Li X, Körner H, Liu X. Susceptibility to intracellular infections: Contributions of TNF to immune defense. Front Microbiol (2020) 11:1643. doi: 10.3389/fmicb.2020.01643
25. Kadono T, Kikuchi K, Ihn H, Takehara K, Tamaki K. Increased production of interleukin 6 and interleukin 8 in scleroderma fibroblasts. J Rheumatol (1998) 25:296–301. doi: 10.1016/S0923-1811(98)84143-1
26. Ha HL, Wang H, Pisitkun P, Kim JC, Tassi I, Tang W, et al. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc Natl Acad Sci U S A (2014) 111:E3422–31. doi: 10.1073/pnas.1400513111
27. Nguyen HN, Noss EH, Mizoguchi F, Huppertz C, Wei KS, Watts GFM, et al. Autocrine loop involving IL-6 family member LIF, LIF receptor, and STAT4 drives sustained fibroblast production of inflammatory mediators. Immunity (2017) 46:220–32. doi: 10.1016/j.immuni.2017.01.004
28. Xu X, Prens E, Florencia E, Leenen P, Boon L, Asmawidjaja P, et al. Interleukin-17A drives IL-19 and IL-24 expression in skin stromal cells regulating keratinocyte proliferation. Front Immunol (2021) 12:719562. doi: 10.3389/fimmu.2021.719562
29. Yap JMG, UEDA T, Kanemitsu Y, Takeda N, Fukumitsu K, Fukuda S, et al. Human lung fibroblasts exhibit induced inflammation memory via increased IL6 gene expression and release. Front Immunol (2022) 13:921728. doi: 10.3389/fimmu.2022.921728
30. Li X, Bechara R, Zhao J, Mcgeachy MJ, Gaffen SL. IL-17 receptor-based signaling and implications for disease. Nat Immunol (2019) 20:1594–602. doi: 10.1038/s41590-019-0514-y
31. Mcgeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity (2019) 50:892–906. doi: 10.1016/j.immuni.2019.03.021
32. Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T Cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med (1996) 183:2593–603. doi: 10.1084/jem.183.6.2593
33. Noack M, Beringer A, Miossec P. Additive or synergistic interactions between IL-17A or IL-17F and TNF or IL-1β depend on the cell type. Front Immunol (2019) 10:1726. doi: 10.3389/fimmu.2019.01726
34. Kerami Z, Duijvis NW, Vogels EW, Van Dooren FH, Moerland PD, Te Velde AA. Effect of interleukin-17 on gene expression profile of fibroblasts from crohn's disease patients. J Crohns Colitis (2014) 8:1208–16. doi: 10.1016/j.crohns.2014.02.009
35. Lei L, Zhao C, Qin F, He ZY, Wang X, Zhong XN. Th17 cells and IL-17 promote the skin and lung inflammation and fibrosis process in a bleomycin-induced murine model of systemic sclerosis. Clin Exp Rheumatol (2016) 34 Suppl 100:14–22.
36. Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol (2002) 282:G1035–44. doi: 10.1152/ajpgi.00494.2001
37. Park MJ, Moon SJ, Lee EJ, Jung KA, Kim EK, Kim DS, et al. IL-1-IL-17 signaling axis contributes to fibrosis and inflammation in two different murine models of systemic sclerosis. Front Immunol (2018) 9:1611. doi: 10.3389/fimmu.2018.01611
38. Ryzhakov G, Lai CC, Blazek K, To KW, Hussell T, Udalova I. IL-17 boosts proinflammatory outcome of antiviral response in human cells. J Immunol (2011) 187:5357–62. doi: 10.4049/jimmunol.1100917
39. Elias JA, Gustilo K, Baeder W, Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol (1987) 138:3812–6.
40. Elias JA, Reynolds MM. Interleukin-1 and tumor necrosis factor synergistically stimulate lung fibroblast interleukin-1 alpha production. Am J Respir Cell Mol Biol (1990) 3:13–20. doi: 10.1165/ajrcmb/3.1.13
41. Elias JA, Reynolds MM, Kotloff RM, Kern JA. Fibroblast interleukin 1 beta: synergistic stimulation by recombinant interleukin 1 and tumor necrosis factor and posttranscriptional regulation. Proc Natl Acad Sci U S A (1989) 86:6171–5. doi: 10.1073/pnas.86.16.6171
42. Elias JA, Trinchieri G, Beck JM, Simon PL, Sehgal PB, May LT, et al. A synergistic interaction of IL-6 and IL-1 mediates the thymocyte-stimulating activity produced by recombinant IL-1-stimulated fibroblasts. J Immunol (1989) 142:509–14.
43. Witte-Händel E, Wolk K, Tsaousi A, Irmer ML, Mössne R, Shomroni O, et al. The IL-1 pathway is hyperactive in hidradenitis suppurativa and contributes to skin infiltration and destruction. J Invest Dermatol (2019) 139:1294–305. doi: 10.1016/j.jid.2018.11.018
44. Wolk K, Brembach TC, Šimaitė D, Bartnik E, Cucinotta S, Pokrywka A, et al. Activity and components of the granulocyte colony-stimulating factor pathway in hidradenitis suppurativa. Br J Dermatol (2021) 185:164–76. doi: 10.1111/bjd.19795
45. Scarpa M, Kessler S, Sadler T, West G, Homer C, Mcdonald C, et al. The epithelial danger signal IL-1α is a potent activator of fibroblasts and reactivator of intestinal inflammation. Am J Pathol (2015) 185:1624–37. doi: 10.1016/j.ajpath.2015.02.018
46. Friedrich M, Pohin M, Jackson MA, Korsunsky I, Bullers SJ, Rue-Albrecht K, et al. IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat Med (2021) 27: 1970–1981 doi: 10.1038/s41591-021-01520-5
47. Sorrell JM, Baber MA, Caplan AI. Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J Cell Physiol (2004) 200:134–45. doi: 10.1002/jcp.10474
48. West NR. Coordination of immune-stroma crosstalk by IL-6 family cytokines. Front Immunol (2019) 10:1093. doi: 10.3389/fimmu.2019.01093
49. Hergovits S, Mais C, Haan C, Costa-Pereira AP, Hermanns HM. Oncostatin m induces RIG-I and MDA5 expression and enhances the double-stranded RNA response in fibroblasts. J Cell Mol Med (2017) 21:3087–99. doi: 10.1111/jcmm.13221
50. West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, et al. Oncostatin m drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med (2017) 23:579–89. doi: 10.1038/nm.4307
51. Fritz DK, Kerr C, Tong L, Smyth D, Richards CD. Oncostatin-m up-regulates VCAM-1 and synergizes with IL-4 in eotaxin expression: involvement of STAT6. J Immunol (2006) 176:4352–60. doi: 10.4049/jimmunol.176.7.4352
52. Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature (2020) 587:555–66. doi: 10.1038/s41586-020-2938-9
53. Owens BM. Inflammation, innate immunity, and the intestinal stromal cell niche: Opportunities and challenges. Front Immunol (2015) 6:319. doi: 10.3389/fimmu.2015.00319
54. Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature (2001) 414:454–7. doi: 10.1038/35106587
55. Castañeda-Sánchez JI, García-Pérez BE, Muñoz-Duarte AR, Baltierra-Uribe SL, Mejia-López H, López-López C, et al. Defensin production by human limbo-corneal fibroblasts infected with mycobacteria. Pathogens (2013) 2:13–32. doi: 10.3390/pathogens2010013
56. Rizzo A, Paolillo R, Buommino E, Lanza AG, Guida L, Annunziata M, et al. Modulation of cytokine and beta-defensin 2 expressions in human gingival fibroblasts infected with chlamydia pneumoniae. Int Immunopharmacol (2008) 8:1239–47. doi: 10.1016/j.intimp.2008.04.015
57. Rodriguez-Martinez S, Cancino-Diaz ME, Cancino-Diaz JC. Expression of CRAMP via PGN-TLR-2 and of alpha-defensin-3 via CpG-ODN-TLR-9 in corneal fibroblasts. Br J Ophthalmol (2006) 90:378–82. doi: 10.1136/bjo.2005.082289
58. Bustos-Arriaga J, García-Machorro J, León-Juárez M, García-Cordero J, Santos-Argumedo L, Flores-Romo L, et al. Activation of the innate immune response against DENV in normal non-transformed human fibroblasts. PLoS Negl Trop Dis (2011) 5:e1420. doi: 10.1371/journal.pntd.0001420
59. Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, et al. Innate immunity. dermal adipocytes protect against invasive staphylococcus aureus skin infection. Science (2015) 347:67–71. doi: 10.1126/science.1260972
60. Liggins MC, Li F, Zhang LJ, Dokoshi T, Gallo RL. Retinoids enhance the expression of cathelicidin antimicrobial peptide during reactive dermal adipogenesis. J Immunol (2019) 203:1589–97. doi: 10.4049/jimmunol.1900520
61. Dokoshi T, Seidman JS, Cavagnero KJ, Li F, Liggins MC, Taylor BC, et al. Skin inflammation activates intestinal stromal fibroblasts and promotes colitis. J Clin Invest (2021) 131. doi: 10.1172/JCI147614
62. Zhang LJ, Guerrero-Juarez CF, Chen SX, Zhang X, Yin M, LI F, et al. Diet-induced obesity promotes infection by impairment of the innate antimicrobial defense function of dermal adipocyte progenitors. Sci Transl Med (2021) 13. doi: 10.1126/scitranslmed.abb5280
63. Zhang LJ, Chen SX, Guerrero-Juarez CF, Li F, Tong Y, Liang Y, et al. Age-related loss of innate immune antimicrobial function of dermal fat is mediated by transforming growth factor beta. Immunity (2019) 50:121–136.e5. doi: 10.1016/j.immuni.2018.11.003
64. Owens BM, Steevels TA, Dudek M, Walcott D, Sun MY, Mayer A, et al. CD90(+) stromal cells are non-professional innate immune effectors of the human colonic mucosa. Front Immunol (2013) 4:307. doi: 10.3389/fimmu.2013.00307
65. Vázquez A, Fernández-Sevilla LM, Jiménez E, Pérez-Cabrera D, Yañez R, Subiza JL, et al. Involvement of mesenchymal stem cells in oral mucosal bacterial immunotherapy. Front Immunol (2020) 11:567391. doi: 10.3389/fimmu.2020.567391
66. Rangasamy T, Ghimire L, Jin L, Le J, Periasamy S, Paudel S, et al. Host defense against klebsiella pneumoniae pneumonia is augmented by lung-derived mesenchymal stem cells. J Immunol (2021) 207:1112–27. doi: 10.4049/jimmunol.2000688
67. Yung SC, Murphy PM. Antimicrobial chemokines. Front Immunol (2012) 3:276. doi: 10.3389/fimmu.2012.00276
68. Noso N, Sticherling M, Bartels J, Mallet AI, Christophers E, Schröder JM. Identification of an n-terminally truncated form of the chemokine RANTES and granulocyte-macrophage colony-stimulating factor as major eosinophil attractants released by cytokine-stimulated dermal fibroblasts. J Immunol (1996) 156:1946–53.
69. Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, Von Brühl ML, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs. Nat Immunol (2013) 14:41–51. doi: 10.1038/ni.2477
70. Zheng Z, Li YN, Jia S, Zhu M, Cao L, Tao M, et al. Lung mesenchymal stromal cells influenced by Th2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nat Commun (2021) 12:6202. doi: 10.1038/s41467-021-26460-z
71. Asam S, Nayar S, Gardner D, Barone F. Stromal cells in tertiary lymphoid structures: Architects of autoimmunity. Immunol Rev (2021) 302:184–95. doi: 10.1111/imr.12987
72. Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol (2011) 12:639–46. doi: 10.1038/ni.2053
73. Denton AE, Innocentin S, Carr EJ, Bradford BM, Lafouresse F, Mabbott NA, et al. Type I interferon induces CXCL13 to support ectopic germinal center formation. J Exp Med (2019) 216:621–37. doi: 10.1084/jem.20181216
74. Neyt K, Geurtsvankessel CH, Deswarte K, Hammad H, Lambrecht BN. Early IL-1 signaling promotes iBALT induction after influenza virus infection. Front Immunol (2016) 7:312. doi: 10.3389/fimmu.2016.00312
75. Kuroda E, Ozasa K, Temizoz B, Ohata K, Koo CX, Kanuma T, et al. Inhaled fine particles induce alveolar macrophage death and interleukin-1α release to promote inducible bronchus-associated lymphoid tissue formation. Immunity (2016) 45:1299–310. doi: 10.1016/j.immuni.2016.11.010
76. Rodriguez AB, Peske JD, Woods AN, Leick KM, Mauldin IS, Meneveau MO, et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep (2021) 36:109422. doi: 10.1016/j.celrep.2021.109422
77. Barone F, Nayar S, Campos J, Cloake T, Withers DR, Toellner KM, et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc Natl Acad Sci U.S.A. (2015) 112:11024–9. doi: 10.1073/pnas.1503315112
78. Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Mourcin F, et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc Natl Acad Sci U S A (2019) 116:13490–7. doi: 10.1073/pnas.1905301116
79. Byrne SN, Beaugie C, O'sullivan C, Leighton S, Halliday GM. The immune-modulating cytokine and endogenous alarmin interleukin-33 is upregulated in skin exposed to inflammatory UVB radiation. Am J Pathol (2011) 179:211–22. doi: 10.1016/j.ajpath.2011.03.010
80. Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol (2017) 13:399–409. doi: 10.1038/nrrheum.2017.83
81. Noss EH, Nguyen HN, Chang SK, Watts GF, Brenner MB. Genetic polymorphism directs IL-6 expression in fibroblasts but not selected other cell types. Proc Natl Acad Sci U S A (2015) 112:14948–53. doi: 10.1073/pnas.1520861112
82. Achuthan AA, Lee KMC, Hamilton JA. Targeting GM-CSF in inflammatory and autoimmune disorders. Semin Immunol (2021) 54:101523. doi: 10.1016/j.smim.2021.101523
83. Ingelfinger F, De Feo D, Becher B. GM-CSF: Master regulator of the T cell-phagocyte interface during inflammation. Semin Immunol (2021) 54:101518. doi: 10.1016/j.smim.2021.101518
84. Mehta HM, Corey SJ. G-CSF, the guardian of granulopoiesis. Semin Immunol (2021) 54:101515. doi: 10.1016/j.smim.2021.101515
85. Kurane I, Janus J, Ennis FA. Dengue virus infection of human skin fibroblasts in vitro production of IFN-beta, IL-6 and GM-CSF. Arch Virol (1992) 124:21–30. doi: 10.1007/BF01314622
86. Vicente-Suarez I, Larange A, Reardon C, Matho M, Feau S, Chodaczek G, et al. Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal Immunol (2015) 8:141–51. doi: 10.1038/mi.2014.51
87. Dayer JM, Zavadil-Grob C, Ucla C, Mach B. Induction of human interleukin 1 mRNA measured by collagenase- and prostaglandin E2-stimulating activity in rheumatoid synovial cells. Eur J Immunol (1984) 14:898–901. doi: 10.1002/eji.1830141007
88. Martínez-Colón GJ, Moore BB. Prostaglandin E(2) as a regulator of immunity to pathogens. Pharmacol Ther (2018) 185:135–46. doi: 10.1016/j.pharmthera.2017.12.008
89. Vancheri C, Mastruzzo C, Tomaselli V, Sortino MA, D'amico L, Bellistrí G, et al. Normal human lung fibroblasts differently modulate interleukin-10 and interleukin-12 production by monocytes: implications for an altered immune response in pulmonary chronic inflammation. Am J Respir Cell Mol Biol (2001) 25:592–9. doi: 10.1165/ajrcmb.25.5.4609
90. Gao L, Yu Q, Zhang H, Wang Z, Zhang T, Xiang J, et al. A resident stromal cell population actively restrains innate immune response in the propagation phase of colitis pathogenesis in mice. Sci Transl Med (2021) 13. doi: 10.1126/scitranslmed.abb5071
91. Gong Z, Li Q, Shi J, Wei J, Li P, Chang CH, et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity (2022) 55:1483–500.e9. doi: 10.1016/j.immuni.2022.07.001
92. Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, Mifflin RC, et al. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology (2008) 135:1228–37–1237.e1–2. doi: 10.1053/j.gastro.2008.07.016
93. Beswick EJ, Johnson JR, Saada JI, Humen M, House J, Dann S, et al. TLR4 activation enhances the PD-L1-mediated tolerogenic capacity of colonic CD90+ stromal cells. J Immunol (2014) 193:2218–29. doi: 10.4049/jimmunol.1203441
94. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet (1970) 3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x
95. Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol (2018) 14:493–507. doi: 10.1038/s41581-018-0023-5
96. Miller LS, Cho JS. Immunity against staphylococcus aureus cutaneous infections. Nat Rev Immunol (2011) 11:505–18. doi: 10.1038/nri3010
97. O'Neill AM, Gallo RL. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome (2018) 6:177. doi: 10.1186/s40168-018-0558-5
98. Boothby IC, Kinet MJ, Boda DP, Kwan EY, Clancy S, Cohen JN, et al. Early-life inflammation primes a T helper 2 cell-fibroblast niche in skin. Nature (2021) 599: 667–672. doi: 10.1038/s41586-021-04044-7
99. Ko KI, Merlet JJ, Dergarabedian BP, Zhen H, Suzuki-Horiuchi Y, Hedberg ML, et al. NF-κB perturbation reveals unique immunomodulatory functions in Prx1(+) fibroblasts that promote development of atopic dermatitis. Sci Transl Med (2022) 14:eabj0324. doi: 10.1126/scitranslmed.abj0324
100. Gao Y, Yao X, Zhai Y, Li L, Li H, Sun X, et al. Single cell transcriptional zonation of human psoriasis skin identifies an alternative immunoregulatory axis conducted by skin resident cells. Cell Death Dis (2021) 12:450. doi: 10.1038/s41419-021-03724-6
101. Theocharidis G, Thomas BE, Sarkar D, Mumme HL, Pilcher WJR, Dwivedi B, et al. Single cell transcriptomic landscape of diabetic foot ulcers. Nat Commun (2022) 13:181. doi: 10.1038/s41467-021-27801-8
102. Billi AC, Ma F, Plazyo O, Gharaee-Kermani M, Wasikowski R, Hile GA, et al. Nonlesional lupus skin contributes to inflammatory education of myeloid cells and primes for cutaneous inflammation. Sci Transl Med (2022) 14:eabn2263. doi: 10.1126/scitranslmed.abn2263
103. Kurashima Y, Amiya T, Fujisawa K, Shibata N, Suzuki Y, Kogure Y, et al. The enzyme Cyp26b1 mediates inhibition of mast cell activation by fibroblasts to maintain skin-barrier homeostasis. Immunity (2014) 40:530–41. doi: 10.1016/j.immuni.2014.01.014
104. Nagashima K, Sawa S, Nitta T, Tsutsumi M, Okamura T, Penninger JM, et al. Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat Immunol (2017) 18:675–82. doi: 10.1038/ni.3732
105. Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling m cells in the intestinal epithelium. J Immunol (2009) 183:5738–47. doi: 10.4049/jimmunol.0901563
106. Gil-Cruz C, Perez-Shibayama C, Onder L, Chai Q, Cupovic J, Cheng HW, et al. Fibroblastic reticular cells regulate intestinal inflammation via IL-15-mediated control of group 1 ILCs. Nat Immunol (2016) 17:1388–96. doi: 10.1038/ni.3566
107. Cheng HW, Mörbe U, Lütge M, Engetschwiler C, Onder L, Novkovic M, et al. Intestinal fibroblastic reticular cell niches control innate lymphoid cell homeostasis and function. Nat Commun (2022) 13:2027. doi: 10.1038/s41467-022-29734-2
108. Mahapatro M, Foersch S, Hefele M, He GW, Giner-Ventura E, Mchedlidze T, et al. Programming of intestinal epithelial differentiation by IL-33 derived from pericryptal fibroblasts in response to systemic infection. Cell Rep (2016) 15:1743–56. doi: 10.1016/j.celrep.2016.04.049
109. Prados A, Onder L, Cheng HW, Mörbe U, Lütge M, Gil-Cruz C, et al. Fibroblastic reticular cell lineage convergence in peyer's patches governs intestinal immunity. Nat Immunol (2021) 22:510–9. doi: 10.1038/s41590-021-00894-5
110. Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front Immunol (2014) 5:462. doi: 10.3389/fimmu.2014.00462
111. Dokoshi T, Zhang LJ, Nakatsuji T, Adase CA, Sanford JA, Paladini RD, et al. Hyaluronidase inhibits reactive adipogenesis and inflammation of colon and skin. JCI Insight (2018) 3. doi: 10.1172/jci.insight.123072
112. Stzepourginski I, Nigro G, Jacob JM, Dulauroy S, Sansonetti PJ, Eberl G, et al. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci U S A (2017) 114:E506–e513. doi: 10.1073/pnas.1620059114
113. Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell (2018) 175:372–86.e17. doi: 10.1016/j.cell.2018.08.067
114. Roulis M, Nikolaou C, Kotsaki E, Kaffe E, Karagianni N, Koliaraki V, et al. Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proc Natl Acad Sci U S A (2014) 111:E4658–67. doi: 10.1073/pnas.1415762111
115. Grim C, Noble R, Uribe G, Khanipov K, Johnson P, Koltun WA, et al. Impairment of tissue-resident mesenchymal stem cells in chronic ulcerative colitis and crohn's disease. J Crohns Colitis (2021) 15:1362–75. doi: 10.1093/ecco-jcc/jjab001
116. Aguirre JE, Beswick EJ, Grim C, Uribe G, Tafoya M, Chacon Palma G, et al. Matrix metalloproteinases cleave membrane-bound PD-L1 on CD90+ (myo-)fibroblasts in crohn's disease and regulate Th1/Th17 cell responses. Int Immunol (2020) 32:57–68. doi: 10.1093/intimm/dxz060
117. Beswick EJ, Grim C, Singh A, Aguirre JE, Tafoya M, Qiu S, et al. Expression of programmed death-ligand 1 by human colonic CD90(+) stromal cells differs between ulcerative colitis and crohn's disease and determines their capacity to suppress Th1 cells. Front Immunol (2018) 9:1125. doi: 10.3389/fimmu.2018.01125
118. Koliaraki V, Pasparakis M, Kollias G. IKKβ in intestinal mesenchymal cells promotes initiation of colitis-associated cancer. J Exp Med (2015) 212:2235–51. doi: 10.1084/jem.20150542
119. Zhao S, Dejanovic D, Yao P, Bhilocha S, Sadler T, Schirbel A, et al. Selective deletion of MyD88 signaling in α-SMA positive cells ameliorates experimental intestinal fibrosis via post-transcriptional regulation. Mucosal Immunol (2020) 13:665–78. doi: 10.1038/s41385-020-0259-9
120. Yuan Q, GU J, Zhang J, Liu S, Wang Q, Tian T, et al. MyD88 in myofibroblasts enhances colitis-associated tumorigenesis via promoting macrophage M2 polarization. Cell Rep (2021) 34:108724. doi: 10.1016/j.celrep.2021.108724
121. Armaka M, Apostolaki M, Jacques P, Kontoyiannis DL, Elewaut D, Kollias G. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med (2008) 205:331–7. doi: 10.1084/jem.20070906
122. Roulis M, Kaklamanos A, Schernthanner M, Bielecki P, Zhao J, Kaffe E, et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature (2020) 580:524–9. doi: 10.1038/s41586-020-2166-3
123. Heichler C, Scheibe K, Schmied A, Geppert CI, Schmid B, Wirtz S, et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut (2020) 69:1269–82. doi: 10.1136/gutjnl-2019-319200
124. Koliaraki V, Roulis M, Kollias G. Tpl2 regulates intestinal myofibroblast HGF release to suppress colitis-associated tumorigenesis. J Clin Invest (2012) 122:4231–42. doi: 10.1172/JCI63917
125. Nishina T, Deguchi Y, Ohshima D, Takeda W, Ohtsuka M, Shichino S, et al. Interleukin-11-expressing fibroblasts have a unique gene signature correlated with poor prognosis of colorectal cancer. Nat Commun (2021) 12:2281. doi: 10.1038/s41467-021-22450-3
126. Manresa MC, Chiang AWT, Kurten RC, Dohil R, Brickner H, Dohil L, et al. Increased production of LIGHT by T cells in eosinophilic esophagitis promotes differentiation of esophageal fibroblasts toward an inflammatory phenotype. Gastroenterology (2020) 159:1778–92.e13. doi: 10.1053/j.gastro.2020.07.035
127. Manresa MC, Wu A, Nhu QM, Chiang AWT, Okamoto K, Miki H, et al. LIGHT controls distinct homeostatic and inflammatory gene expression profiles in esophageal fibroblasts via differential HVEM and LTβR-mediated mechanisms. Mucosal Immunol (2021) 15: 327–337. doi: 10.1038/s41385-021-00472-w
128. Manresa MC, Miki H, Miller J, Okamoto K, Dobaczewska K, Herro R, et al. A deficiency in the cytokine TNFSF14/LIGHT limits inflammation and remodeling in murine eosinophilic esophagitis. J Immunol (2022). doi: 10.4049/jimmunol.2200326
129. Williams DW, Greenwell-Wild T, Brenchley L, Dutzan N, Overmiller A, Sawaya AP, et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell (2021) 184:4090–104.e15. doi: 10.1016/j.cell.2021.05.013
130. Iwanaga N, Chen K, Yang H, Lu S, Hoffmann JP, Wanek A, et al. Vaccine-driven lung TRM cells provide immunity against klebsiella via fibroblast IL-17R signaling. Sci Immunol (2021) 6:eabf1198. doi: 10.1126/sciimmunol.abf1198
131. Oherle K, Acker E, Bonfield M, Wang T, Gray J, Lang I, et al. Insulin-like growth factor 1 supports a pulmonary niche that promotes type 3 innate lymphoid cell development in newborn lungs. Immunity (2020) 52:275–94.e9. doi: 10.1016/j.immuni.2020.01.005
132. Dahlgren MW, Jones SW, Cautivo KM, Dubinin A, Ortiz-Carpena JF, Farhat S, et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity (2019) 50:707–22.e6. doi: 10.1016/j.immuni.2019.02.002
133. Cupovic J, Ring SS, Onder L, Colston JM, Lütge M, Cheng HW, et al. Adenovirus vector vaccination reprograms pulmonary fibroblastic niches to support protective inflating memory CD8(+) T cells. Nat Immunol (2021) 22:1042–51. doi: 10.1038/s41590-021-00969-3
134. Jain N, Kalam H, Singh L, Sharma V, Kedia S, Das P, et al. Mesenchymal stem cells offer a drug-tolerant and immune-privileged niche to mycobacterium tuberculosis. Nat Commun (2020) 11:3062. doi: 10.1038/s41467-020-16877-3
135. Cheng HW, Onder L, Cupovic J, Boesch M, Novkovic M, Pikor N, et al. CCL19-producing fibroblastic stromal cells restrain lung carcinoma growth by promoting local antitumor T-cell responses. J Allergy Clin Immunol (2018) 142:1257–71.e4.
136. Kim HT, Panza P, Kikhi K, Nakamichi Y, Atzberger A, Guenther S, et al. WNT/RYK signaling functions as an antiinflammatory modulator in the lung mesenchyme. Proc Natl Acad Sci U S A (2022) 119:e2201707119. doi: 10.1073/pnas.2201707119
Keywords: fibroblast, mesenchymal, stromal, innate, immunity, barrier, defense, inflammation
Citation: Cavagnero KJ and Gallo RL (2022) Essential immune functions of fibroblasts in innate host defense. Front. Immunol. 13:1058862. doi: 10.3389/fimmu.2022.1058862
Received: 30 September 2022; Accepted: 09 November 2022;
Published: 15 December 2022.
Edited by:
Francesca Granucci, University of Milano-Bicocca, ItalyReviewed by:
Xiaofeng Yang, Temple University, United StatesCopyright © 2022 Cavagnero and Gallo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard L. Gallo, cmdhbGxvQGhlYWx0aC51Y3NkLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.