
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 10 January 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1058759
This article is part of the Research Topic Environmental Factors in Autoimmunity View all 14 articles
Pemphigus vulgaris (PV) is a potentially life-threatening blistering disorder characterized by autoantibodies directed against cell-cell adhesion molecules that serves as an excellent model to study human autoimmune development. Numerous studies have identified specific Human Leukocyte Antigen (HLA) genes, in particular DRB1*0402 and DQB1*0503, that confer disease risk. Although HLA is required, it is not sufficient for the initiation of disease. As with all autoimmune diseases, the etio-pathogenesis of PV is complex, meaning it is multifactorial. Susceptibility is polygenic, and the search for non-HLA disease-linked genes continues. Moreover, twin studies across autoimmune conditions indicate that non-genetic environmental and lifestyle factors, which can be collectively grouped under the term “exposome”, are also major contributors to disease development. The literature presents evidence for the potential role of multiple triggers such as medications, infections, stress, diet, immunizations, and sleep to influence the etiology, pathophysiology, and prognosis of PV. However, a clear understanding of the degree to which specific factors impact PV is lacking. In this investigation, we comprehensively review the environmental elements listed above and consider the strength of evidence for these factors. The overall goals of this work are to provide greater insights into the factors that influence disease susceptibility, disease development and disease course and ultimately help to better guide clinicians and inform patients in the management of PV.
Pemphigus vulgaris (PV) is the most common subtype of a group of rare autoimmune blistering disorders (AIBD) in which autoantibodies primarily directed against the cell-cell adhesion molecules desmoglein 3 (Dsg3) and desmoglein 1 (Dsg1) lead to characteristic epidermal blistering. PV is classified into the major subtypes of mucosal-dominant, and mucocutaneous. The existence of a cutaneous phenotype of PV has also been documented in several case reports and recent studies (1–4). The etiology of PV is clearly multifactorial involving both genetic and environmental factors. Although key Human Leukocyte Antigen (HLA) genes, in particular DRB1*0402 and DQB1*0503, have been identified, we have little to no information on the broader genetic architecture that undergirds disease susceptibility. Beyond the holes in our information on polygenic risk elements, we similarly have a weak understanding of environmental factors that trigger or influence disease development and clinical expression in PV. Here, we undertook a comprehensive review by searching PubMed/Medline databases to determine the level of existing support for the relationship between PV and multiple environmental and lifestyle factors that contribute to the broader “exposome” including: medications, infections, stress, diet, immunizations, and sleep.
In this investigation, we comprehensively evaluated the evidence for, and the level of support associated with each of the factors listed above. We sought to illuminate the relationship more clearly between these factors and the onset, recurrence, or exacerbation of PV. This information deepens our understanding of disease risk as well as the basis of phenotypic variation and disease heterogeneity; and provides a step forward towards a more detailed framework to support disease relevant decision-making by physicians and patients.
To accomplish these goals, we performed a comprehensive literature review by utilizing the PubMed/Medline database to identify key articles related to the onset and/or exacerbation of pemphigus vulgaris. We conducted a search within the parameter of a 20-year submitted literature period from January 2001 to December 2021. Search strings were compiled per each factor and combined to encompass specific keywords (Table 1). The inclusion criteria involved articles that reported or reviewed cases relating to PV induction, trigger and or exacerbation by environmental and or lifestyle factors under infection, medication, stress, immunization, diet, and stress. The exclusion criteria involved articles that reported cases of pemphigus foliaceus, or other pemphigus subtypes, and articles that were not in English. Initial results of the literature search based on the inclusion and exclusion criteria outlined yielded n = 2093 papers. A total of n =1460 duplicates were identified. All duplicates were excluded from this study. n= 39 met additional exclusion criteria, resulting in n = 672 papers that met inclusion criteria. All articles meeting inclusion criteria were then further screened in a two-step process:
Step 1: Screening of title & abstract on PV induction/exacerbation, which yielded n = 186 papers of relevance to overall study goals.
Step 2: Screening of content relevant in Step 1 that provides evidence for the potential role of environmental and lifestyle “exposome” factors on PV pathophysiology, yielded n = 157 papers (Figure 1).
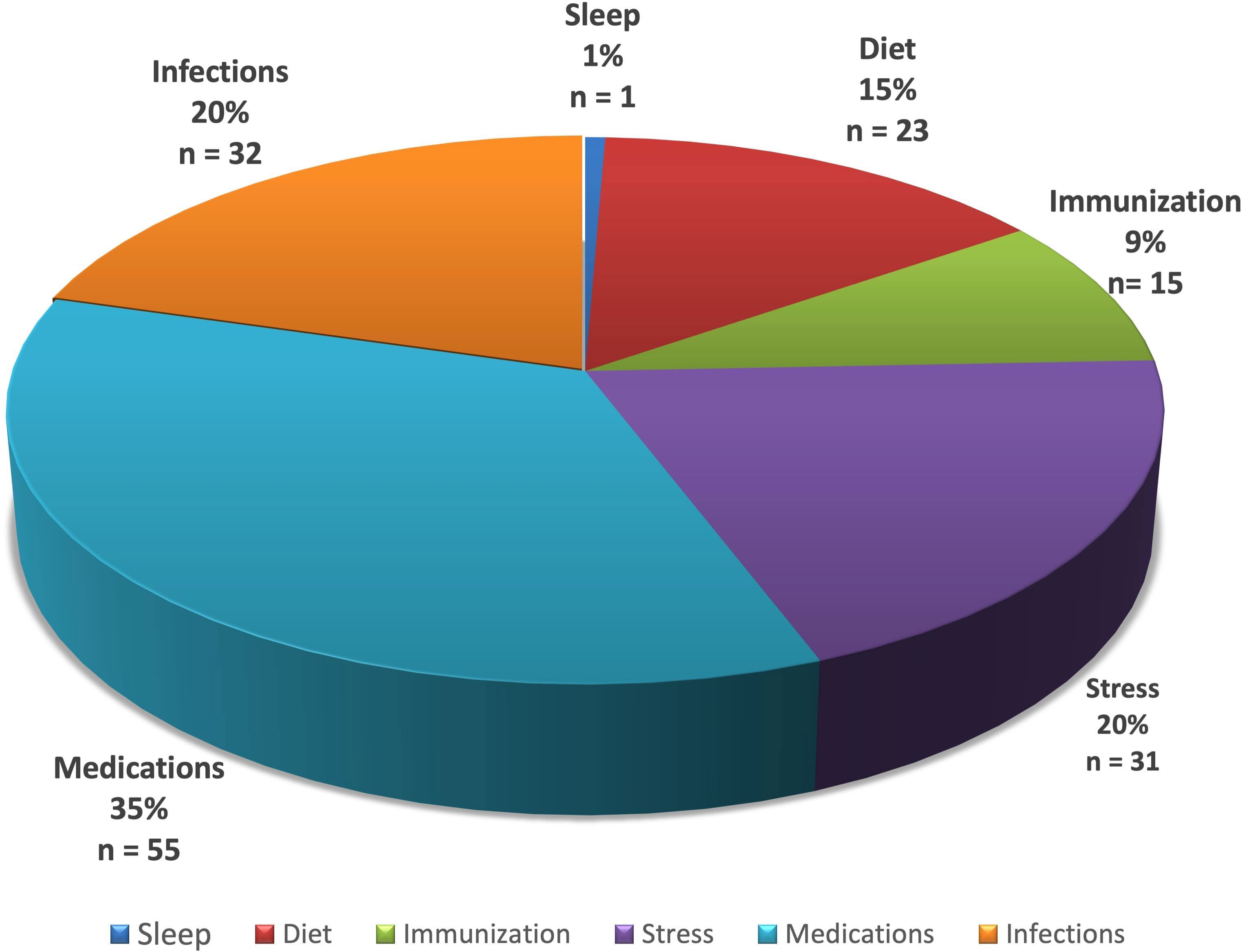
Figure 1 Distribution of literature results after screening for PV induction or exacerbation as linked to specific lifestyle/environmental factors. Subgroups identified are represented as a percentage (%) and number (n) of all studies meeting criteria.
After the 2-step screening process, we undertook a detailed manual assessment to determine the relationship of each one of the environmental/lifestyle exposome factors listed above to the etio-pathogenesis of PV.
Support for the role of medications in pemphigus was found to be the most highly represented in the literature relative to the other factors studies (Figure 1). The role of sleep was least reported. It should be noted that the underlying disease relevance of each factor studied may not be reflected only by the number of associated articles in the literature, which may simply be the result of investigative biases and access at this point in time. More extensive data in larger patient populations and future mechanistic studies will be required to better establish the relative rank of exposome linked factors in terms of contributing to disease development and clinical modulation.
Medications known to induce or exacerbate PV flares have been studied extensively in the literature (see Table 2). Drug induced pemphigus (DIP) has been recognized as a distinct subtype of pemphigus distinguishing this known etiology from the idiopathic form. DIP makes diagnosis challenging due to the similarities in clinical, histological and immunochemistry features shared with idiopathic PV. The earliest reports of DIP were first described by Degos et al. in 1969 (51). Isolated case studies have provided clarity regarding the specific drugs that may cause pemphigus foliaceus (PF) and/or pemphigus vulgaris and those that have been reported to induce and/or exacerbate PV flares.
Various case reports and in vitro experiments since the earliest findings of DIP report similar findings and have identified drugs with certain chemical properties as the most common triggers of induced PV. The concept of drug induced PV is by far the most common environmental factor linked to the induction of pemphigus (see Figure 1). The literature provides evidence of reported cases of DIP following therapy for a non-related pemphigus diagnosis and the eventual rapid remission of PV upon discontinuation of the culprit agent (12, 20, 25, 52). The most common clinical variant associated with drug exposure is PF, however, PV has also been described. The drugs linked to DIP vulgaris are categorized into 3 main categories: thiol drugs, phenols, and non-thiols/non-phenols (16).
Thiol drugs contain a sulfhydryl group (-SH) and are responsible for the stimulation of antibody production through interactions that increase antigenicity of desmoglein proteins. Experiments done by Ruccco et al. demonstrated the induction of acantholysis without antibody mediation (5). The main thiol containing PV inducing drugs include penicillamine, bucillamine, and captopril (6, 7, 9, 10, 12, 14, 16, 18–20, 22, 53). Other known thiol drugs listed in Table 2 have been identified in isolated in case reports, longitudinal studies, and reviews (6, 8, 11, 14–17, 20–24, 54).
The most reported phenol containing medications known to induce PV include Rifampin, Cefadroxil, Aspirin, Levodopa, Pentachlorophenol and phenobarbital (11, 16, 17, 20, 26). The mechanisms of induction proposed by Brenner et al. are similar to those with that of the thiol drugs (25). The proposed mechanism of acantholysis caused by thiol and phenol drugs is as follows: inhibition of enzymes responsible for keratinocyte aggregation, disruption of thiol-cysteine binding epithelial cells, activation of plasminogen activators which disaggregate keratinocytes and increase synthesis of cytokines (13, 16, 53).
Interestingly, some studies have highlighted an increased incidence of PV induction by non-thiols rather than thiols. This difference goes beyond the chemical nature of the causative agent; differences regarding the mechanism of induction is also key to understanding the pathophysiology of PV (9, 11, 14, 30, 31, 42, 43, 53). It is important to know what specific chemical group, and the pemphigus subtype being induced by said drug. Non-thiol drugs are mainly identified by sulfur and amide components. Most of the commonly prescribed diuretics fall under this category. Known drugs include Cilazapril, fosinopril, lisinopril, nifedipine, imiquimod, carbamazepine, chloroquine/hydroxychloroquine, dipyrone, glibenclaimde, ingenol mebutate, acetazolamide, candesartan, ciprofloxacin, cocaine, diazinon, hydrochlorothiazide, irbesartan, methylisothiazolinone, metoprolol, phenytoin (11, 20, 27–29, 32–41, 44, 45). These groups (sulfur and amides) are postulated to induce PV via immunological mechanisms that stimulate keratinocytes to produce proinflammatory cytokines such as TNF and IL-1 (8, 55–57), leading to the activation of proteases and complement that contribute to acantholysis.
To our knowledge, reports of PV induction following radiotherapy and immunotherapy are rarely reported in the literature. Isolated case-control studies identify an elevated risk of developing PV with previous radiotherapy (RT) and ultraviolet light (42, 48–50, 55, 58–60). These studies report an accompanying malignancy, most commonly breast cancer or lymphomas, with an associated eruption of pemphigus lesions that range from 1 week to 1 year following irradiation. A case-report revealed the induction of PV 1 month following radiation for hypopharynx carcinoma and other related neoplastic RT-induced pemphigus (61). The mechanisms responsible for RT induced pemphigus share some similarities to that of DIP. Specifically, ionizing radiation is said to increase the antigenicity of keratinocytic surface molecules via peptidyl sulfhydryl disruptive mechanisms (42, 48, 49, 59). There are only two reported cases of DIP developed in a patient after treatment with the immunotherapeutic agent nivolumab (46, 47).
Several studies have looked at the Herpesviridae family of viruses (HSV) that produce various skin/oral lesions or ulcerations including “cold sores”. Transmission of this viral family is usually via skin-to-skin contact or contact with bodily fluids of an infected individual. Potentially, lesions caused by HSV could lead to the exposure of intraepidermal epitopes to the immune system causing intercellular adhesion molecule antibody production, and thus the development of PV (62–64). In a paper by Senger et al., the link between PV and the various Herpesviridae was explored by evaluating levels of antiviral antibodies. Anti-HSV1 antibody levels were found to be higher in active PV patients than in remittent patients and controls, supporting a potential role of HSV1 in disease expression and clinical activity. However, there was no way to establish causality in this retrospective evaluation, and further studies would be required to follow anti-HSV levels in patients longitudinally. Alternatively, HSV could simply mimic PV like erosions and/or contribute to the growth of preexisting lesions rather than initiating autoimmunity. This same paper examined the literature in terms of relations between Cytomegalovirus (CMV) and Varicella Zoster virus (VZV) and PV; there were no substantial relationships found (65).
Bacterial infections such as Legionella pneumophila, Staphylococcus aureus, Proteus Vulgaris, and Pseudomonas Aeruginosa have also been studied for potential associations with PV (see Table 3). Staphylococcus aureus is a gram-positive bacillus that can cause both toxin mediated and systemic infections in a host. One of the presentations of Staph aureus is Scalded Skin Syndrome that is mediated by the exotoxins A and B. It has been identified that exotoxin A targets and cleaves the Dsg-1 protein which leads to loss of cell-cell adhesion, mimicking the autoimmune condition seen in PF (82, 90). Legionella pneumophila is a gram-negative bacterium that is known to cause Legionnaires’ disease. In a study by Tirado et al., the relationship between the prevalence of legionella specific antibodies and PV patients was examined. This study concluded that the antibodies themselves may be the trigger for autoimmunity (84). This is in opposition to other studies that tend to equate the infection being secondary to immunosuppressive therapy. Examples of bacteria that support the latter include Pseudomonas aeruginosa and Proteus Vulgaris (79, 91).
Fungal infection such as oral Candidiasis, Aspergillus, and Pneumocystis jiroveci may play a role in the exacerbation of Pemphigus vulgaris due to their opportunistic tendency in patients on immunosuppressive therapy (90), but there is scant literature to establish their role they play in autoimmunity.
More recently several case reports on COVID-19 and PV have appeared. Ghalamkarpour et al. reported a patient who was previously diagnosed with Pemphigus vulgaris went into remission but had exacerbation following a Covid-19 infection (87). Interestingly, De Medeiros VLS et al., presented a case of a previously healthy patient who after contracting COVID-19 presented with bullae on his chest that was determined to be PV (88). There have also been reports that SARS-CoV-2 can lead to autoimmunity and hence the induction of cutaneous diseases. Further research must be done to determine the actual role that COVID may play in the development and/or exacerbation of PV.
Another recent interesting study shed a light on a potential link between roseolovirus and autoimmunity. In a study done by Bigley et al., a mouse model was infected with murine roseolovirus, which is related to human roseolovirus, and it was hypothesized that murine roseolovirus (MRV) impacted central tolerance by disrupting medullary thymic epithelial cells (mTECs) and CD11c+ thymic dendritic cells (tDCs) in the thymus. The authors were able to determine that neonatal MRV infection leads to a variety of autoantibodies in adult mice (92). This mechanism of action has not been previously proposed in the context of pemphigus vulgaris development but lends support to extend the investigation into the viral induction of autoimmunity.
Stress and stressful life events have long been postulated as potential triggering factors for skin disease. Stress can be defined as a state of emotional or physical tension that induces the release of stress hormones, such as adrenaline and cortisol. This initial hormonal response is referred to as “fight or flight” and it serves as a survival mechanism to react quickly to a perceived threat. While acute stress can be beneficial to the individual in the context of dangerous situations, chronic stress where hormonal levels remain elevated far longer than is necessary for survival can have deleterious consequences for health.
The skin and the central nervous system are both derived from the embryonic ectoderm, which may explain the existence of a relationship between psychological factors and dermatologic diseases (93). In addition, there has been growing evidence of a unique neuroimmunocutaneous system. The skin, nervous system, and immune system all share hormones, cytokines, and neurotransmitters as a way to communicate, which can account for a pathogenic link between stress and the onset or worsening of autoimmune skin diseases, such as pemphigus vulgaris (PV) (14).
Some of the notable stressful life events that are most associated to PV include environmental disasters, war, terrorism, partner’s or near relative’s death, separation from partner, physical trauma, sexual aggression, or sex-related disturbance (14). The first two cases of PV occurring after a stressful event were reported by Brenner and Bar-Nathan in 1984 (94). Since this initial observation, a limited number of studies have also pointed to stress as an inducing and triggering factor in the etiology of PV. In a clinical investigation involving 13 pemphigus patients with personality disorders, it was revealed that 12 out of 13 patients had experienced a stressful event during the year preceding the onset of the autoimmune disorder (14). A combined retrospective and prospective epidemiological study evaluated all cases of pemphigus from 2000 to 2004. It was concluded that all 10 patients that participated in the study had recordable stressful life events less than 6 months before their first clinical symptoms or worsening of pemphigus (94). In an isolated study, two exogenous factors were found to trigger PV in a 56 year-old Jewish woman of Ashkenazi origin. This woman experienced the Holocaust and the Persian Gulf War, and it was concluded that both emotional stress and the drug rifampin led to her first clinical symptoms of PV (26).
Another study had the aim to establish incidence of acquired bullous dermatitis (BD) among hospitalized patients in Eastern Croatia before and after war. There was a higher incidence of acquired BD during the years of war and the period immediately after compared to before the war. It was concluded that prolonged exposure to stressful conditions influenced the incidence of disease (95). A case-control study had the objective to estimate the initial serum levels of TNF-alpha in pemphigus patients and compare them with history of stress, body surface area affected, disease severity, and disease outcome. Significantly higher serum levels of TNF-alpha were found in PV patients compared to healthy patients. 30% of the PV patients reported severe emotional stress within a month prior to the onset of disease. Those patients had high initial levels of serum TNF-alpha and showed poor response to treatment, resulting in poor prognosis (96).
A bidirectional relationship between PV and psychological stress has also been reported. There have been several reports of PV impairing patients’ quality of life (97–103), which can then impact one’s stress levels and mental health (see Table 4). Some forms of psychological distress could also be in part due to one’s perception of his/her own body image (104). In multiple investigations, there was a significant association between pemphigus and an increased risk of depression and/or anxiety (106). In a case-control study, 30 PV patients and 30 healthy patients were interviewed for their health-related quality of life (HR-QoL) and psychological profile. Anxiety and depression were found in 60% and 50% of the PV patients. The persistence of a poor HR-QoL and higher levels of anxiety and depression is also considered as a risk factor for a relapse of the disease because the onset of anxiety and depression has been associated with immune system dysregulation (105). Thus, it is possible that stress could both play a role in triggering disease, and also that the disease state itself can generate stress that not only impacts quality of life, but potentially perpetuates stress linked autoimmune mechanisms to propagate disease activity and clinical flares. It is commonly recommended that PV patients receive consistent psychiatric assessment and intervention as part of a treatment plan to prevent an exacerbation of the disease.
In addition to psychological stress, there are studies that report on the impact that physical stress has on the exacerbation of pemphigus vulgaris as well. In a case series, a total of 36 PV patients had a history of physical trauma before the onset of lesions (118). In a retrospective study of 15 PV patients, sites of dental trauma-induced lesions were detected in 13 patients (125). A case report of a 49-year old woman described the development of PV after a Mohs surgical excision of squamous cell carcinoma. The pre-operative lesion did not reveal PV, however, the postoperative lesion revealed PV without any residual squamous cell carcinoma. The report concluded that Mohs surgery, and perhaps other surgical interventions, may activate PV (121). UV radiation can also induce or exacerbate the clinical manifestation of PV, in addition to physical factors, such as x-ray radiotherapy, burns, major surgery, and cosmetic procedures (117). A retrospective case-control study revealed that approximately 40% of patients had been continuously exposed to UV radiation in their work 5 years prior to developing the disease. The distribution of PV lesions on sun-exposed areas also supports these findings (120). The exposure to the sun and other UV sources suggests special caution for PV patients because of the risk for photo-induced relapses (112). Taken together, there is literature to indicate that various forms of physical trauma to the skin or mucosa can trigger PV, therefore, unnecessary operations should also be avoided or postponed (118).
There appears to be strong support for the essential role of stress in the etiology of pemphigus vulgaris via psychosomatic mechanisms. However, it remains difficult to draw significant conclusions due to the relatively limited number of research studies, small sample sizes, and lack of controlled studies. Additional studies are needed to clarify whether psychological stress is an inducing factor of PV, a complication of the disease, or an adverse effect of its therapy.
Nutrition has been well documented as an exogenous factor influencing several disease states. There is growing research on the relation between nutrition and specific skin diseases, such as atopic dermatitis and urticaria (several foods), dermatitis herpetiformis (gluten), and porphyria cutanea tarda (alcohol) (126). Due to the complexity of autoimmune skin disease, its clinical course and the variability of human nutrition, dietary factors in relation to PV have remained elusive. However, recent epidemiological, clinical, and experimental data collected have allowed the inclusion of nutrition as an agent that can impact PV.
Many studies have reported on the possible induction of PV by dietary ingredients rich in thiols, phenols, and tannins (14, 16, 20, 112, 114, 117, 120, 127–132). Similar to medications that contain thiol groups, dietary sources high in similar compounds have been reported as triggering factors. Some of these foods and drinks include many fruits, garlic, onions, leeks, spices, legumes, nuts, tea, red wine, and beer (16, 128). Some of these ingredients are also widely consumed in India and Brazil, which might, at least partially, explain the high incidence of pemphigus in Indian and Brazilian patients (16). In addition, the high incidence of PV in Russian Jews has been linked to the frequent use of spices in this ethnic group (14). In an isolated study, a woman from Naples had an abrupt outbreak of pemphigus following a meal heavily seasoned in garlic (thiol-containing) after years in remission. A 49 year old man who consumed large amounts of garlic developed superficial pemphigus and the lesions dissipated while on a garlic-free diet. Another woman from Poland had remission of the disease after withdrawing large amounts of leeks from her diet (16). These reports highlight individual cases of onset or exacerbation, however, in order to better understand the potential role of these compounds in the pathogenesis of pemphigus, controlled studies are needed.
In one study, 40 volunteers were divided into four groups to measure the presence of tannic acid in the skin of different populations. The group that had a high dietary tannin intake correlated with higher levels of tannins in the skin (127, 128). In another study conducted by Brenner, five skin explants were cultured with tannic acid at different concentration levels and the most constant and specific induced effect was marked by acantholytic changes (128). PV patients living in Amazonian, Mediterranean, and Indian subcontinent areas where the diet is rich in tannins should be informed about tannins as a possible trigger (130). The suggested mechanisms for thiol-induced acantholysis include the direct biochemical impairment of cell adhesion, protease activation, and immunological reaction with the formation of neo-antigens. The suggested mechanisms for phenol-induced PV include the release of IL-1a and TNF-a from keratinocytes by phenol molecules, which can trigger cutaneous inflammation. These two cytokines enhance the synthesis and regulation of complement and proteases, such as C3 and plasminogen activator, which have been associated to the pathogenesis of acantholysis in PV (127, 128).
There have also been reports of other dietary sources that may induce PV (see Table 5). In one investigation, the findings suggest that the intake of a dietary walnut antigen through gastrointestinal epithelial cells can activate naive B cells in subjects genetically predisposed to PV through a “hit-and-run” mechanism. According to this mechanism, the cross-reactivity between an infectious antigen and autoantigen can lead to a long-lasting immune response, even once the pathogen is cleared, because the continued presence of the autoantigen would perpetually drive subsequent autoantibody generation and the development (and perpetuation and/or exacerbation of) disease (144). In a case report, it was proposed that the immune-enhancing effects of herbal supplements, specifically Echinacea and the alga Spirulina platensis, contributed to flares of pemphigus vulgaris in two patients. It was suggested that increased production of TNF-alpha may be playing a role in disease exacerbation, although additional research is required to confirm this mechanism (145).

Table 5 Studies in support of the hypothesis that PV is triggered or exacerbated by a nutritional element.
In regards to micronutrients, one study found that serum vitamin D levels are significantly lower in newly diagnosed PV patients compared to healthy controls. There was also a negative correlation between the vitamin D level and the severity of disease. Vitamin D is known as an important immunomodulatory agent and it was suggested that the insufficient vitamin D level could be considered as an environmental factor that contributes to the pathophysiology of disease (141). Recently, the possible beneficial role of retinoic acid has also been discussed (147). In another case-control study, the results show that PV causes depletion of some trace elements including zinc, selenium, and copper. These may have important roles in the functioning of the immune system, wound healing, and antioxidant defense, so supplementation could potentially alleviate disease severity and mortality. But again, clinical trials are needed to confirm this theory (138).
Another study found that copper concentrations in Iranian patients with PV were less than in controls (140). An investigation on the trace element profile of pemphigus patients in Southeastern Brazil showed that PV patients had higher lead (Pb) values as compared to the controls. Pemphigus is endemic in Southeastern Brazil and Pb is known to play an immunomodulatory role that favors Th2 proliferation and consequent production of Th2 cytokines. Pb contamination in chronic doses may constitute a trigger factor for PV pathogenesis (139).
Taken together, the relevance of nutritional factors seems to be underestimated in the induction of PV. Avoiding exposure of genetically predisposed individuals to ingredients high in thiols, phenols, and tannins may be beneficial in the prevention and management of PV. However, there are numerous dietary factors that need to be more fully investigated. In the future, modulation of nutrient and micronutrient levels in PV patients may be part of a viable management strategy (20).
Recent attention has been given to COVID-19 vaccination and Pemphigus vulgaris (see Table 6). There were two cases of patients who received the Pfizer COVID-19 vaccine. Both patients, who were previously healthy, later developed oral lesions and blistering on their trunks and had demonstrated Dsg3 and Dsg1 autoantibodies (148, 149). Damiani et al. report multiple cases of patients forming bullae on their back, trunk, arms, and legs after the first shot of the Pfizer and Moderna COVID-19 vaccines. They proposed that both mRNA vaccines may trigger relapses in PV patients (150).
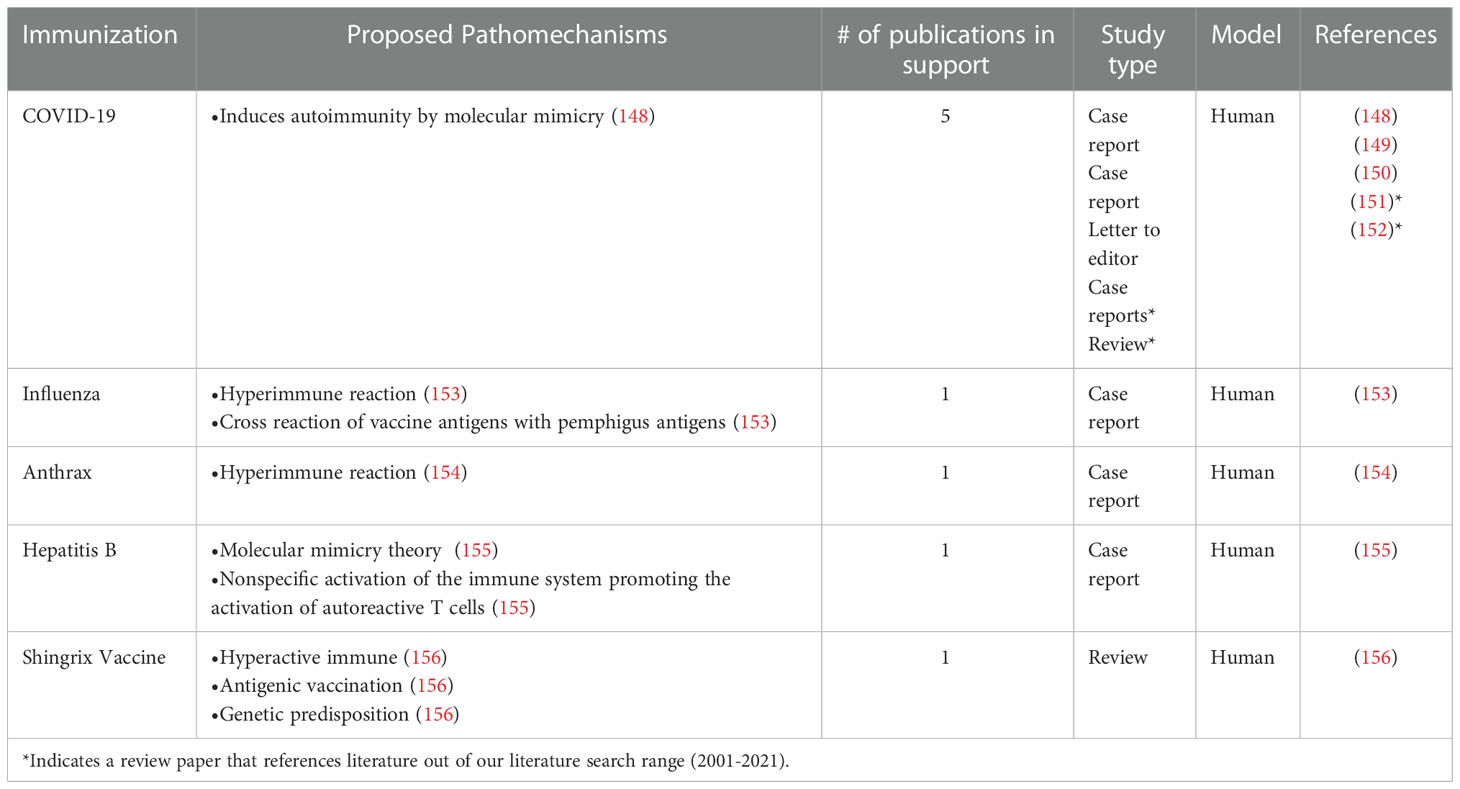
Table 6 Studies in support of the hypothesis that PV is triggered or exacerbated by immunizations and vaccinations.
Another well-known vaccine that may have led to the reactivation of Pemphigus Vulgaris is the influenza vaccine, which is a killed virus vaccine. In most cases, the influenza vaccine causes little to no side effects, but there was a case of a patient who was previously diagnosed with PV and in remission, who later had a flare up following the influenza vaccine on two different occasions (153).
The killed virus anthrax vaccine also had a known case of causing the development of Pemphigus. In this case, the patient received three parts of a six-part vaccine and after each dose, the patient developed new and worsening lesions on the skin and oral cavity (154). In a case report by Berkun et al., a 43-year-old patient who was previously healthy and had no family history of autoimmune disease received the first dose of the recombinant Engerix-B hepatitis B vaccine and three months following the vaccine, was diagnosed with Pemphigus vulgaris (155). It has been proposed that mechanisms by which various vaccines lead to autoimmunity may have common threads. One possibility involves molecular mimicry - vaccine antigens are similar in structure to self-antigens leading to (auto)antibody cross-reactivity. A second proposed mechanism invokes that there is a hyper-immune response to vaccination in certain predisposed individuals that spills into autoimmunity due to an over-exuberant immune state (148, 153).
Sleep can be defined in part by a rapid reversible state of immobility and greatly reduced sensory response that is homeostatically regulated (157). The disruption of the circadian rhythm has been conducted in various mammals and sleep has been proven to be vital for survival. Of note, severe deprivation of sleep can lead to a debilitating appearance, increased food intake and/or weight loss, increased energy expenditure, decreased body temperature (158). Sleep is also vital to combat infection.
Sleep architecture has been uncovered with the use of electroencephalographic recordings that trace the electrical patterns of brain activity. The duality of sleep has been explained and divided into non-rapid eye-movement (NREM) sleep and rapid eye-movement (REM) sleep. The two types of sleep have specific characterizations in brain wave variation patterns, eye movements, muscle tone, architecture of sleep and varying effects by sleep regulatory substances (SRS) (159, 160). Cytokines, interleukin 1 β (IL-1) and tumor necrosis factor (TNF)α, growth hormone releasing hormone (GHRH), prolactin and nitric oxide (NO) have been noted for their roles in sleep regulation. The criterion for SRS is as follows: 1) the substance and/or its receptor oscillates with sleep propensity; 2) sleep is increased or decreased with administration of the substance; 3) blocking the action or inhibiting the production of the substance changes sleep; 4) disease states, e.g., infection, associated with altered sleep also change levels of the putative SRS; and finally 5) the substance acts on known sleep regulatory circuits (161–163).
Cytokines are known immunomodulators secreted by specific immune cell types that direct communication between cells. The humoral regulation of sleep by the pro-inflammatory cytokines IL-1 and TNF has been studied by many groups and has linked alterations in sleep to cytokine levels (163–171). A few of these studies support the notion that IL-6 possesses sleep regulatory properties but may not be involved in regulation of spontaneous sleep in healthy animals due to the lack of sleep altering criteria upon antagonization of IL-6 in animals. The effect of IL-1, TNF and IL-6 sleep has been studied in two ways: documented increased expression of regulatory cytokines under physiological and inflammatory conditions versus low-dose and high dose exogenous injection regulatory cytokines. IL-6, TNF and IL-1 are reported to increase NREM sleep in a dose-dependent manner, whereas doses that have shown to maximally increase NREM sleep can suppress REM sleep (164, 165). Of note, in healthy men, IL-6 injections significantly reduce the time spent in REM sleep compared to controls and affect self-reported measures of mood. The alterations in mood and increase in fatigue after injections of IL-6 mirrors symptoms often reported during an infection (166). In addition, in a study of healthy women deprived of sleep for 42 hours, there was a marked increase of TNF, IL-1 and natural killer cell function but no difference in plasma levels of IL-10 (167).
The skin, as well as sleep, both hold unique roles towards combating infection. The skin is the body’s barrier to the external environment and can be greatly affected by changes in sleep. Jang et al. revealed the effects of sleep deprivation on the barrier function of the skin. The restriction of sleep from an average of 8 to 4 hours over 6 consecutive nights significantly decreased the elasticity of the skin (168). In an animal model of psoriasis Hirotsu et al., found that cytokine and humoral levels of proinflammatory cytokines (IL-1 β, IL-6 and IL-12) were significantly increased after sleep deprivation and returned to normal levels after 48h of sleep rebound (169). Disturbances in sleep are known to have profound effects on pain sensitivity, resulting in tenderness and fatigue in healthy individuals. The painful nature of blistering lesions in addition to the cytokine profile in PV point towards a possible bidirectional relationship established between the cytokine-related SRS of sleep and the quality of sleep. While poor sleep quality has been an associated risk factor for various medical disorders that involve immunity and/or autoimmunity (172), the exact relationship of poor sleep quality in patients with pemphigus to the onset and/or exacerbation of disease remains to be elucidated (Table 7).
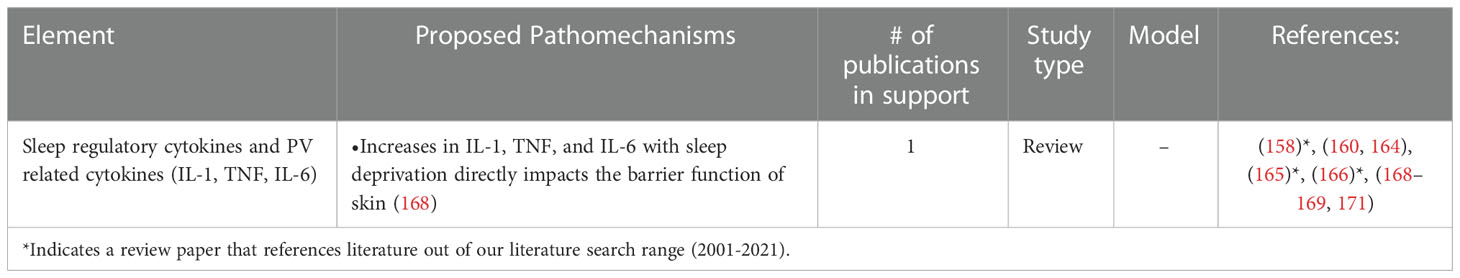
Table 7 Studies in support of the hypothesis that PV is triggered or exacerbated by alterations in sleep.
There is a substantial amount of data supporting the role of a wide range of environmental and lifestyle factors in the induction and exacerbation of autoimmune diseases including pemphigus vulgaris. In this review, we sought to comprehensively and critically examine the literature to better determine and define the associations between PV and various components constituting the “exposome” such as medications, infections, stress, diet, immunizations, and sleep. It is evident that there is no consensus to what degree each factor can contribute to the onset and course of disease. A myriad of factors may be individually, additively, or synergistically influential.
Our investigation highlights the need for new assessment methods to better track the exposome in real-time in patients across global populations. In particular, longitudinal and prospective data are needed to better understand the shared effects and relationships between multiple genetic and non-genetic (environmental) factors in disease onset or exacerbation. Detailed diaries designed to document large-scale information on patient encounters with environmental elements can then be linked to fluctuating disease activity, response to treatment, and health outcomes, and further linked to genetic variations and immunologic dysregulations to help unravel the intertwined multi-complex factors that conspire to cause and regulate autoimmunity. With the advance and availability of digital medicine devices and services there is the opportunity for app-based platforms, perhaps paired with sensors that monitor environmental as well as biological information, to yield individually curated data collected over time to more critically investigate the interplay of genetics and environment relevant to the autoimmune state. Going further, the impact of socioeconomic class and racial disparities on environment and lifestyle needs to be weighted into the calculus of autoimmune disease risk and prognosis.
Overall, we need to arrive at a more detailed mechanistic framework of the multifactorial inputs that determine the set points for immunological self-nonself discrimination at the cellular level. The development of autoimmune disease involves an interplay of both internal genetic factors comprised of HLA genes and non-HLA genes, as well as external environmental factors that collectively can be considered as an individual’s umwelt that is compromised of exposome factors (including medications, diet, sleep, immunization, infections, stress) and key social determinants of health (SDOH, including health care access and quality, social and community factors, education access and equality, neighborhood and local physical environment, economic stability), see Figure 2. Internal and external factors are inextricably linked and together determine that thresholds and criteria for autoimmune susceptibility, disease induction and disease course. The exact balance of factors are likely to vary across various autoimmune conditions, phenotypic subtypes of disease and the evolution of clinical expression within individuals. Models constructed based on a greater appreciation of the interwoven gene-environment fabric from which disease is formulated could provide investigators, providers, as well as patients with actionable insights and a scientifically rooted rationale to envision increasingly precision based, and ideally, personalized approaches to the prevention and control of autoimmunity.
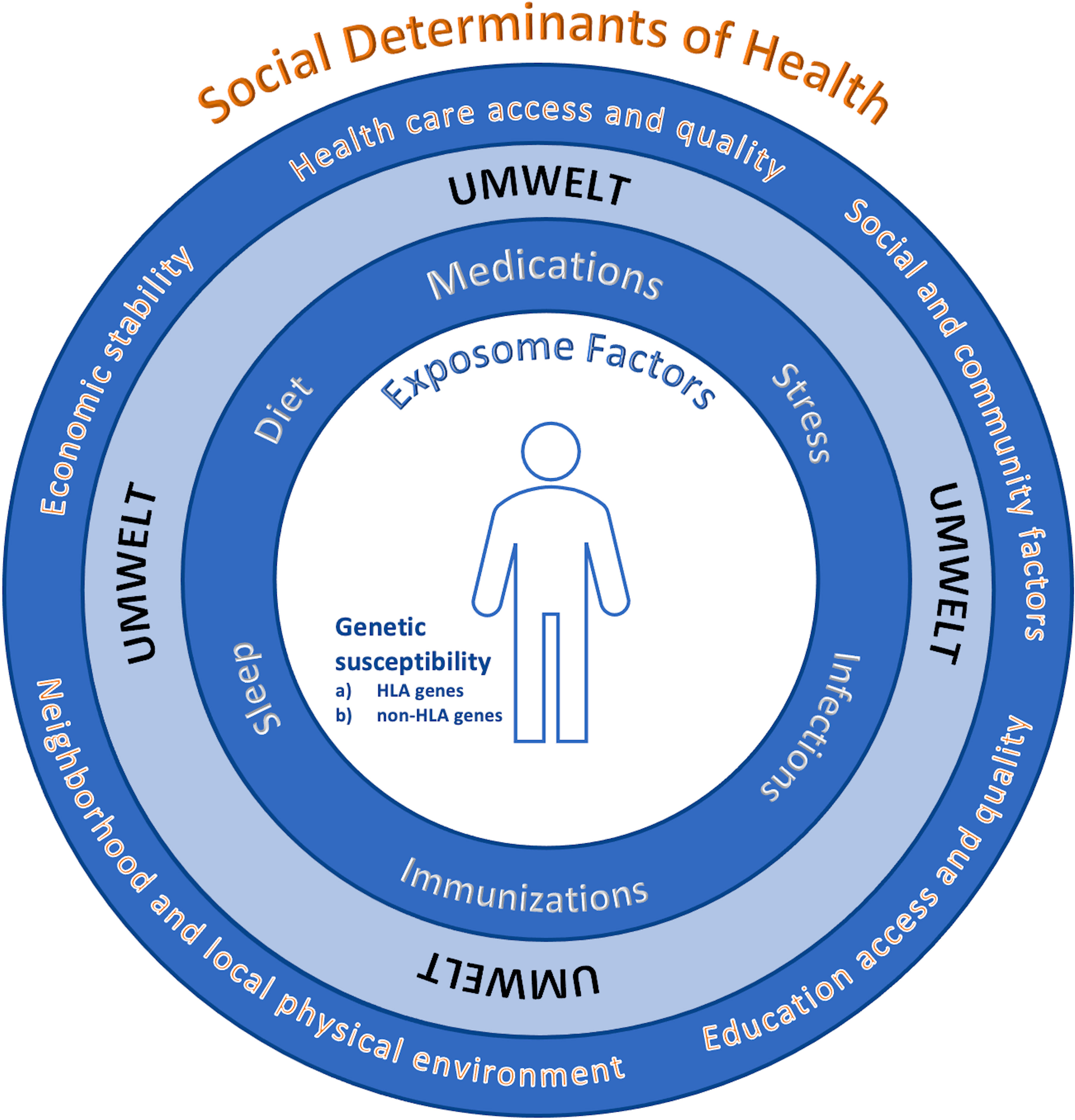
Figure 2 Multifactorial complexities of disease. There is an interplay of: 1) internal genetic factors including (A) HLA genes and (B) non-HLA genes, and 2) external environmental factors (an individual’s umwelt) that is compromised of both a) exposome factors (including medications, diet, sleep, immunization, infections, stress), and key social determinants of health (SDOH*, including health care access and quality, social and community factors, education access and equality, neighborhood and local physical environment, economic stability) that are inextricably linked and interwoven in a dynamic fashion to set the thresholds for autoimmune susceptibility, disease induction, the severity and course of disease and treatment response. The overall balance and factors is likely to vary across various autoimmune conditions, phenotypic subtypes of disease and the evolution of clinical expression within individuals. *https://health.gov/healthypeople/priority-areas/social-determinants-health.
OA contributed equally to this work and shares first authorship. DG contributed equally to this work. MA contributed equally to this work. AS conceived of and designed the study. AS contributed equally to this work and shares senior authorship. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sielski L, Baker J, DePasquale MC, Attwood K, Seiffert-Sinha K, Sinha AA. Desmoglein compensation hypothesis fidelity assessment in pemphigus. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.969278
2. Baker J, Seiffert-Sinha K, Sinha AA. Case report: Documentation of cutaneous only pemphigus vulgaris without history of mucosal lesions in north America. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.969279
3. Yoshida K, Takae Y, Saito H, Oka H, Tanikawa A, Amagai M, et al. Cutaneous type pemphigus vulgaris: A rare clinical phenotype of pemphigus. J Am Acad Dermatol (2005) 52(5):839–45. doi: 10.1016/j.jaad.2005.01.106
4. Shinkuma S, Nishie W, Shibaki A, Sawamura D, Ito K, Tsuji-Abe Y, et al. Cutaneous pemphigus vulgaris with skin features similar to the classic mucocutaneous type: A case report and review of the literature. Clin Exp Dermatol (2008) 33(6):724–8. doi: 10.1111/j.1365-2230.2008.02871.x
5. Ruocco V, De Angelis E, Lombardi ML. Drug-induced pemphigus. II. pathomechanisms and experimental investigations. Clin Dermatol (1993) 11(4):507–13. doi: 10.1016/0738-081x(93)90158-9
6. Ruocco V, Pisani M, de Angelis E, Lombardi ML. Biochemical acantholysis provoked by thiol drugs. Arch Dermatol (1990) 126(7):965–6. doi: 10.1001/archderm.1990.01670310129023
7. Baroni A, Buommino E, Ruocco E, Petrazzuolo M, De Filippis A, Satriano RA, et al. Captopril modulates acetylcholinesterase in human keratinocytes. Arch Dermatol Res (2011) 303(7):491–7. doi: 10.1007/s00403-011-1124-1
8. Brenner S, Bialy-Golan A, Ruocco V. Drug-induced pemphigus. Clin Dermatol (1998) 16(3):393–7. doi: 10.1016/S0738-081X(98)00010-8
9. Yanagishita T, Tamada Y, Watanabe D. Bucillamine-induced pemphigus vulgaris. J Eur Acad Dermatol Venereol (2015) 29(6):1242–3. doi: 10.1111/jdv.12472
10. Hur JW, Lee CW, Yoo DH. Bucillamine-induced pemphigus vulgaris in a patient with rheumatoid arthritis and polymyositis overlap syndrome. J Korean Med Sci (2006) 21(3):585–7. doi: 10.3346/jkms.2006.21.3.585
11. Brenner S, Goldberg I. Drug-induced pemphigus. Clin Dermatol (2011) 29(4):455–7. doi: 10.1016/j.clindermatol.2011.01.016
12. Gornowicz-Porowska J, Dmochowski M, Pietkiewicz P, Bowszyc-Dmochowska M. Mucosal-dominant pemphigus vulgaris in a captopril-taking woman with angioedema. Bras Dermatol (2015) 90(5):748–51. doi: 10.1590/abd1806-4841.20153390
13. Cozzani E, Rosa GM, Drosera M, Intra C, Barsotti A, Parodi A. ACE inhibitors can induce circulating antibodies directed to antigens of the superficial epidermal cells. Arch Dermatol Res (2011) 303(5):327–32. doi: 10.1007/s00403-010-1060-5
14. Ruocco V, Ruocco E, Lo Schiavo A, Brunetti G, Guerrera LP, Wolf R. Pemphigus: Etiology, pathogenesis, and inducing or triggering factors: Facts and controversies. Clin Dermatol (2013) 31(4):374–81. doi: 10.1016/j.clindermatol.2013.01.004
15. Heymann AD, Chodick G, Kramer E, Green M, Shalev V. Pemphigus variant associated with penicillin use: A case-cohort study of 363 patients from Israel. Arch Dermatol (2007) 143(6):704–7. doi: 10.1001/archderm.143.6.704
16. Brenner S, Srebrnik A, Goldberg I. Pemphigus can be induced by topical phenol as well as by foods and drugs that contain phenols or thiols. J Cosmet Dermatol (2003) 2(3-4):161–5. doi: 10.1111/j.1473-2130.2004.00098.x
17. Goldberg I, Kashman Y, Brenner S. The induction of pemphigus by phenol drugs. Int J Dermatol (1999) 38(12):888–92. doi: 10.1046/j.1365-4362.1999.00836.x
18. Szegedi A, Suranyi P, Szucs G, Kiss M, Hunyadi J, Gaal J. D-penicillamine-induced pemphigus vulgaris in a patient with scleroderma-rheumatoid arthritis overlap syndrome. Acta Derm Venereol (2004) 84(4):318–9. doi: 10.1080/00015550410026399
19. Teoh L, Moses G, McCullough MJ. A review and guide to drug-associated oral adverse effects-oral mucosal and lichenoid reactions. part 2. J Oral Pathol Med (2019) 48(7):637–46. doi: 10.1111/jop.12910
20. Tavakolpour S. Pemphigus trigger factors: Special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res (2018) 310(2):95–106. doi: 10.1007/s00403-017-1790-8
21. Brenner S, Wolf R, Ruocco V. Drug-induced pemphigus. i. a survey. Clin Dermatol (1993) 11(4):501–5. doi: 10.1016/0738-081x(93)90157-8
22. De Dobbeleer G, Godfrine S, Gourdain JM, De Graef C, Heenen M. In vitro acantholysis induced by d-penicillamine, captopril, and piroxicam on dead de-epidermized dermis. J Cutan Pathol (1992) 19(3):181–6. doi: 10.1111/j.1600-0560.1992.tb01656.x
23. Wolf R, Tamir A, Brenner S. Drug-induced versus drug-triggered pemphigus. Dermatologica (1991) 182(4):207–10. doi: 10.1159/000247795
24. Baroni A, Russo T, Faccenda F, Piccolo V. Amoxicillin/clavulanic acid-induced pemphigus vulgaris: case report. Acta Dermatovenerol Croat (2012) 20(2):108–11.
25. Ruocco V, Pisani M. Induced pemphigus. Arch Dermatol Res (1982) 274(1-2):123–40. doi: 10.1007/BF00510366
26. Goldberg I, Ingher A, Brenner S. Pemphigus vulgaris triggered by rifampin and emotional stress. Skinmed (2004) 3(5):294. doi: 10.1111/j.1540-9740.2004.03343.x
27. Goldberg I, Sasson A, Gat A, Srebrnik A, Brenner S. Pemphigus vulgaris triggered by glibenclamide and cilazapril. Acta Dermatovenerol Croat (2005) 13(3):153–5.
28. Palleria C, Bennardo L, Dastoli S, Iannone LF, Silvestri M, Manti A, et al. Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers induced pemphigus: A case series and literature review. Dermatol Ther (2019) 32(1):e12748. doi: 10.1111/dth.12748
29. Gallo R, Gariazzo L, Gasparini G, Basso D, Torino A, Cozzani E, et al. Contact allergy caused by methylisothiazolinone in a mouthwash as the likely trigger of oral pemphigus vulgaris. Contact Dermatitis (2018) 79(2):104–5. doi: 10.1111/cod.13001
30. Paterson AJ, Lamey PJ, Lewis MA, Nolan A, Rademaker M. Pemphigus vulgaris precipitated by glibenclamide therapy. J Oral Pathol Med (1993) 22(2):92–5. doi: 10.1111/j.1600-0714.1993.tb00051.x
31. Patterson CR, Davies MG. Carbamazepine-induced pemphigus. Clin Exp Dermatol (2003) 28(1):98–9. doi: 10.1046/j.1365-2230.2003.01156_6.x
32. Tang X, Zhang X. Drug-induced pemphigus after six years of treatment with phenytoin and carbamazepine. Int J Dermatol (2012) 51(4):485–6. doi: 10.1111/j.1365-4632.2010.04706.x
33. Ghaffarpour G, Jalali MH, Yaghmaii B, Mazloomi S, Soltani-Arabshahi R. Chloroquine/hydroxychloroquine-induced pemphigus. Int J Dermatol (2006) 45(10):1261–3. doi: 10.1111/j.1365-4632.2006.03075.x
34. Parodi A, Cozzani E, Milesi G, Drosera M, Rebora A. Fosinopril as a possible pemphigus-inducing drug. Dermatology (2002) 204(2):139–41. doi: 10.1159/000051833
35. Campagne G, Roca M, Martínez A. Successful treatment of a high-grade intraepithelial neoplasia with imiquimod, with vulvar pemphigus as a side effect. Eur J Obstetrics Gynecol Reprod Biol (2003) 109(2):224–7. doi: 10.1016/S0301-2115(02)00482-7
36. Bauza A, Del Pozo LJ, Saus C, Martin A. Pemphigus-like lesions induced by imiquimod. Clin Exp Dermatol (2009) 34(5):e60–2. doi: 10.1111/j.1365-2230.2008.03181.x
37. Russo I, Ferrazzi A, Alaibac M. Relapse of pemphigus vulgaris after topical application of ingenol mebutate. Clin Exp Dermatol (2016) 41(6):664–6. doi: 10.1111/ced.12875
38. Lo Schiavo A, Sangiuliano S, Puca RV, Brunetti G, Ruocco E. Pemphigus relapse and acetazolamide, a drug with an active amide group: A casual or causal relationship? J Eur Acad Dermatol Venereol (2009) 23(6):716–7. doi: 10.1111/j.1468-3083.2009.03170.x
39. Saito Y, Hayashi S, Yamauchi A, Okamoto M, Kaminaga T, Hamasaki Y, et al. Tracing the origins of active amide group-positive drug-induced pemphigus vulgaris along the silk road: A case report of candesartan-induced pemphigus vulgaris and review of nonthiol drug-induced pemphigus. Int J Dermatol (2018) 57(11):e131–e4. doi: 10.1111/ijd.14108
40. Anadolu RY, Birol A, Bostanci S, Boyvatt A. A case of pemphigus vulgaris possibly triggered by quinolones. J Eur Acad Dermatol Venereol (2002) 16(2):152–3. doi: 10.1046/j.1468-3083.2002.00393.x
41. Jimenez-Zarazua O, Guzman-Ramirez A, Velez-Ramirez LN, Lopez-Garcia JA, Casimiro-Guzman L, Mondragon JD. A case of acute pemphigus vulgaris relapses associated with cocaine use and review of the literature. Dermatol Ther (Heidelb) (2018) 8(4):653–63. doi: 10.1007/s13555-018-0271-0
42. Orion E, Matz H, Wolf R. Pemphigus vulgaris induced by radiotherapy. J Eur Acad Dermatol Venereol (2004) 18(4):508–9. doi: 10.1111/j.1468-3083.2004.00952.x
43. Brenner S, Bialy-Golan A, Crost N. Dipyrone in the induction of pemphigus. J Am Acad Dermatol (1997) 36(3 Pt 1):488–90. doi: 10.1016/S0190-9622(97)80238-1
44. Tariq U, Nasrullah A, Guha A, Mitre M. Oroesophageal pemphigus vulgaris secondary to lisinopril use: A new side effect. Cureus (2021) 13(4):e14333. doi: 10.7759/cureus.14333
45. Patel S, Kim S, Allen C. Metoprolol-induced pemphigus-like reaction. Clin Adv Periodontics (2019) 9(1):24–8. doi: 10.1002/cap.10044
46. Ito M, Hoashi T, Endo Y, Kimura G, Kondo Y, Ishii N, et al. Atypical pemphigus developed in a patient with urothelial carcinoma treated with nivolumab. J Dermatol (2019) 46(3):e90–e2. doi: 10.1111/1346-8138.14601
47. Krammer S, Krammer C, Salzer S, Bağci IS, French LE, Hartmann D. Recurrence of pemphigus vulgaris under nivolumab therapy. Front Med (Lausanne) (2019) 6:262. doi: 10.3389/fmed.2019.00262
48. Robbins AC, Lazarova Z, Janson MM, Fairley JA. Pemphigus vulgaris presenting in a radiation portal. J Am Acad Dermatol (2007) 56(5 Suppl):S82–5. doi: 10.1016/j.jaad.2006.10.956
49. Vigna-Taglianti R, Russi EG, Denaro N, Numico G, Brizio R. Radiation-induced pemphigus vulgaris of the breast. Cancer Radiother (2011) 15(4):334–7. doi: 10.1016/j.canrad.2011.01.006
50. Badri T, Hammami H, Lachkham A, Benmously-Mlika R, Mokhtar I, Fenniche S. Radiotherapy-induced pemphigus vulgaris with autoantibodies targeting a 110 kDa epidermal antigen. Int J Dermatol (2011) 50(12):1475–9. doi: 10.1111/j.1365-4632.2011.04889.x
51. Degos R, Touraine R, Belaich S, Revuz J. [Pemphigus in a patient treated with penicillamine for wilson's disease]. Bull Soc Fr Dermatol Syphiligr (1969) 76(6):751–3.
52. Yuan PY, Qiu M, Wan ZX, Jiang L. Experience in the diagnosis and treatment of a drug-induced pemphigus. Hua Xi Kou Qiang Yi Xue Za Zhi (2021) 39(6):724–7. doi: 10.7518/hxkq.2021.06.016
53. Ghaedi F, Etesami I, Aryanian Z, Kalantari Y, Goodarzi A, Teymourpour A, et al. Drug-induced pemphigus: A systematic review of 170 patients. Int Immunopharmacol (2021) 92:107299. doi: 10.1016/j.intimp.2020.107299
54. Ruocco E, Lo Schiavo A, Baroni A, Sangiuliano S, Puca RV, Brunetti G, et al. Pemphigus vulgaris after coxsackievirus infection and cephalosporin treatment: A paraviral eruption? Dermatology (2008) 216(4):317–9. doi: 10.1159/000113944
55. Golberg O, Harman K. Drug-induced pemphigus. European Handbook of Dermatological Treatments (2015) 3rd Edition:725–30. doi: 10.1007/978-3-662-45139-7_72
56. Pietkiewicz P, Gornowicz-Porowska J, Bowszyc-Dmochowska M, Dmochowski M. A retrospective study of antihypertensives in pemphigus: A still unchartered odyssey particularly between thiols, amides and phenols. Arch Med Sci (2015) 11(5):1021–7. doi: 10.5114/aoms.2015.54857
57. Pile HD, Yarrarapu SNS, Crane JS. Drug induced pemphigus. Treasure Island (FL: StatPearls Publishing (2022).
58. Hung TL, Chen YL, Lin KT, Chiang CP, Chung CH, Hung CT, et al. Risk of radiotherapy-associated autoimmune bullous disease among Taiwanese patients with breast cancer: A case-control study. Arch Dermatol Res (2020) 312(1):69–75. doi: 10.1007/s00403-019-01985-y
59. Schauer F, Ishii N, Mockenhaupt M, Bruckner-Tuderman L, Hashimoto T, Kiritsi D. Radiation-associated pemphigus vulgaris in a patient with preceding malignancy: Treatment with rituximab as a valuable option. Front Immunol (2019) 10:3116. doi: 10.3389/fimmu.2019.03116
60. Thimon S, Machet L, Machet MC, Samimi M, Maruani A. Pemphigus induced by radiotherapy for breast cancer: Clinical, immunological and histological features of one case with antidesmoglein immunostaining. Eur J Dermatol (2014) 24(1):119–20. doi: 10.1684/ejd.2013.2239
61. Baricevic M, Mravak Stipetic M, Situm M, Marinovic B, Seiwerth S, Baricevic D, et al. Oral bullous eruption after taking lisinopril–case report and literature review. Wien Klin Wochenschr (2013) 125(13-14):408–11. doi: 10.1007/s00508-013-0382-7
62. Douard PA, Delaumenie S, Pittoni J, Assikar S, Matei I, Prudhomme R, et al. Reactivation of pemphigus by varicella zoster virus after anti-CD20 treatment. Int J Dermatol (2020) 59(3):e52–e3. doi: 10.1111/ijd.14711
63. Esmaili N, Hallaji Z, Abedini R, Soori T, Mortazavi H, Chams-Davatchi C. Pemphigus vulgaris and herpesviruses: is there any relationship? Int J Dermatol (2010) 49(11):1261–5. doi: 10.1111/j.1365-4632.2010.04515.x
64. Yang GQQ, Yap T, Martyres R, Varigos GA, Scardamaglia L. The impact of human herpesvirus detection in pemphigus vulgaris. Australas J Dermatol (2019) 60(3):e259–e61. doi: 10.1111/ajd.12977
65. Senger P, Sinha AA. Exploring the link between herpes viruses and pemphigus vulgaris: literature review and commentary. Eur J Dermatol (2012) 22(6):728–35. doi: 10.1684/ejd.2012.1836
66. Machado A, La Serra L, Turatti A, Machado AM, Roselino AM. Herpes simplex virus 1 and cytomegalovirus are associated with pemphigus vulgaris but not with pemphigus foliaceus disease. Exp Dermatol (2017) 26(10):966–8. doi: 10.1111/exd.13342
67. Oliveira-Batista DP, Janini ME, Fernandes NC, Santos N. Laboratory diagnosis of herpesvirus infections in patients with pemphigus vulgaris lesions. Intervirology (2013) 56(4):231–6. doi: 10.1159/000349889
68. Sirka CS, Pradhan S. Clinical diagnosis of herpes simplex-infected pemphigus erosions. Skinmed (2019) 17(2):96–8.
69. Mejri K, Kallel Sellami M, Tombari W, Laadhar L, Zitouni M, Makni S, et al. Human herpesvirus-8 and hepatitis b and c virus infections in pemphigus. Int J Dermatol (2014) 53(10):e4757. doi: 10.1111/ijd.12583
70. Kurata M, Mizukawa Y, Aoyama Y, Shiohara T. Herpes simplex virus reactivation as a trigger of mucous lesions in pemphigus vulgaris. Br J Dermatol (2014) 171(3):554–60. doi: 10.1111/bjd.12961
71. Barzilai O, Sherer Y, Ram M, Izhaky D, Anaya JM, Shoenfeld Y. Epstein-Barr Virus and cytomegalovirus in autoimmune diseases: Are they truly notorious? a preliminary report. Ann N Y Acad Sci (2007) 1108:567–77. doi: 10.1196/annals.1422.059
72. Kalra A, Ratho RK, Kaur I, Kumar B. Role of herpes simplex and cytomegalo viruses in recalcitrant oral lesions of pemphigus vulgaris. Int J Dermatol (2005) 44(3):259–60. doi: 10.1111/j.1365-4632.2004.02370.x
73. Wang GQ, Xu H, Wang YK, Gao XH, Zhao Y, He C, et al. Higher prevalence of human herpesvirus 8 DNA sequence and specific IgG antibodies in patients with pemphigus in China. J Am Acad Dermatol (2005) 52(3 Pt 1):460–7. doi: 10.1016/j.jaad.2004.10.882
74. Chiu HY, Chang CY, Hsiao CH, Wang LF. Concurrent cytomegalovirus and herpes simplex virus infection in pemphigus vulgaris treated with rituximab and prednisolone. Acta Derm Venereol (2013) 93(2):200–1. doi: 10.2340/00015555-1429
75. Goon AT, Tay YK, Tan SH. Pemphigus vulgaris following varicella infection. Clin Exp Dermatol (2001) 26(8):661–3. doi: 10.1046/j.1365-2230.2001.00912.x
76. Brandao ML, Fernandes NC, Batista DP, Santos N. Refractory pemphigus vulgaris associated with herpes infection: Case report and review. Rev Inst Med Trop Sao Paulo (2011) 53(2):113–7. doi: 10.1590/S0036-46652011000200010
77. Brown P, Taylor B. Herpes simplex infection associated with pemphigus vulgaris. case report and literature review. J Am Acad Dermatol (1989) 21(5 Pt 2):1126–8. doi: 10.1016/S0190-9622(89)70312-1
78. Meibodi NT, Nahidi Y, Mahmoudi M, Javidi Z, Rastin M, Sheikh A, et al. Evaluation of coexistence of the human herpesvirus type 8 (HHV-8) infection and pemphigus. Int J Dermatol (2010) 49(7):780–3. doi: 10.1111/j.1365-4632.2009.04398.x
79. Asilian A, Yoosefi A, Faghihi G. Cutaneous and pulmonary nocardiosis in pemphigus vulgaris: A rare complication of immunosuppressive therapy. Int J Dermatol (2006) 45(10):1204–6. doi: 10.1111/j.1365-4632.2006.02738.x
80. Ali RA, Elsherif RH, Saleh MA, Ismail MH. Evaluation of exposure of pemphigus vulgaris patients to mycobacterium tuberculosis and aspergillus fumigatus. Eur J Clin Microbiol Infect Dis (2016) 35(11):1749–52. doi: 10.1007/s10096-016-2721-x
81. Mortazavi H, Hejazi P, Khamesipour A, Mohebali M, Ehsani AH, Mohammadi Y, et al. Frequency of seropositivity against infectious agents amongst pemphigus vulgaris patients: A case-control study on strongyloides stercoralis, helicobacter pylori, toxoplasma gondii, leishmania major, and Epstein-Barr virus. Int J Dermatol (2015) 54(11):e458–65. doi: 10.1111/ijd.12869
82. Amagai M, Matsuyoshi N, Wang ZH, Andl C, Stanley JR. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat Med (2000) 6(11):1275–7. doi: 10.1038/81385
83. Li F, Wu Y, Bian W, Huang L, Zhu X, Chen X, et al. Features and associated factors of bacterial skin infections in hospitalized patients with pemphigus: A single-center retrospective study. Ann Clin Microbiol Antimicrob (2020) 19(1):46. doi: 10.1186/s12941-020-00388-6
84. Tirado-Sanchez A, Bonifaz A. Presence of antibodies against legionella pneumophila in patients with pemphigus vulgaris. Int J Dermatol (2017) 56(4):e87–e8. doi: 10.1111/ijd.13505
85. Pakshir K, Ghasemi N, Zomorodian K, Jowkar F, Nouraei H, Dastgheib L. Identification and antifungal activity profile of candida species isolated from patients with pemphigus vulgaris with oral lesions. Acta Dermatovenerol Croat (2019) 27(3):137–41.
86. Zou H, Daveluy S. Pemphigus vulgaris after COVID-19 infection and vaccination. J Am Acad Dermatol (2022) 87(3):709–10. doi: 10.1007/s00403-022-02497-y
87. Ghalamkarpour F, Pourani MR. Aggressive course of pemphigus vulgaris following COVID-19 infection. Dermatol Ther (2020) 33(6):e14398. doi: 10.1111/dth.14398
88. De Medeiros VLS, Monteiro-Neto AU, Franca DDT, Castelo Branco R, de Miranda Coelho EO, Takano DM. Pemphigus vulgaris after COVID-19: A case of induced autoimmunity. SN Compr Clin Med (2021) 3(8):1768–72. doi: 10.1007/s42399-021-00971-8
89. Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol (2020) 16(8):413–4. doi: 10.1038/s41584-020-0448-7
90. Aragues M, Gonzalez-Arriba A. [Primary cutaneous infections due to staphylococcus and streptococcus]. Actas Dermosifiliogr (2007) 98(Suppl 1):4–14. doi: 10.1016/s0001-7310(07)70176-x
91. Brenner S, Sasson A, Sharon O. Pemphigus and infections. Clin Dermatol (2002) 20(2):114–8. doi: 10.1016/S0738-081X(01)00254-1
92. Bigley TM, Yang L, Kang LI, Saenz JB, Victorino F, Yokoyama WM. Disruption of thymic central tolerance by infection with murine roseolovirus induces autoimmune gastritis. J Exp Med (2022) 219(3):1–2. doi: 10.1084/jem.20211403
93. Picardi A, Abeni D. Stressful life events and skin diseases: Disentangling evidence from myth. Psychother Psychosom (2001) 70(3):118–36. doi: 10.1159/000056237
94. Tamir A, Ophir J, Brenner S. Pemphigus vulgaris triggered by emotional stress. Dermatology (1994) 189(2):210. doi: 10.1159/000246837
95. Sustić N, Rucević I, Barisić-Drusko V. Epidemiology of acquired bullous diseases in Eastern Croatia: A retrospective prewar to postwar study. Acta Dermatovenerol Croat (2005) 13(4):228–32.
96. Ragab N, Abdallah M, El-Gohary E, Elewa R. Stress and serum TNF-alpha levels may predict disease outcome in patients with pemphigus: A preliminary study. Cutis (2011) 87(4):189–94.
97. Paradisi A, Cianchini G, Lupi F, Di Pietro C, Sampogna F, Didona B, et al. Quality of life in patients with pemphigus receiving adjuvant therapy. Clin Exp Dermatol (2012) 37(6):626–30. doi: 10.1111/j.1365-2230.2011.04282.x
98. Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol (2015) 27(5):492–8. doi: 10.5021/ad.2015.27.5.492
99. Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, Valikhani M, Esmaili N. Quality of life and psychological status of patients with pemphigus vulgaris using dermatology life quality index and general health questionnaires. J Dermatol (2012) 39(2):141–4. doi: 10.1111/j.1346-8138.2011.01382.x
100. Metwally D, Fawzy M, ElKalioby M, Hegazy R, Hay RA, Abd-Elreheem H, et al. Assessment of the quality of life, prevalence of depression, and the level of interleukin 6 in patients with pemphigus vulgaris. Acta Dermatovenerol Croat (2020) 28(2):57–62.
101. Segal O, Goldzweig G, Tako E, Barzilai A, Lyakhovitsky A, Baum S. Illness perception, perceived social support and quality of life in patients with pemphigus vulgaris: What should dermatologists know? Acta Derm Venereol (2021) 101(4):adv00441. doi: 10.2340/00015555-3785
102. Okcin F, Ugur O. What does it mean to be pemphigus patient? a qualitative study. Clin Nurs Res (2021) 30(6):790–8. doi: 10.1177/10547738211002354
103. Viti G, Forcella C, Feliciani C, Murrell DF. Beyond the skin: disease parameters in pemphigus. Ital J Dermatol Venerol (2021) 156(2):147–50. doi: 10.23736/S2784-8671.21.06678-5
104. Mazzotti E, Mozzetta A, Antinone V, Alfani S, Cianchini G, Abeni D. Psychological distress and investment in one's appearance in patients with pemphigus. J Eur Acad Dermatol Venereol (2011) 25(3):285–9. doi: 10.1111/j.1468-3083.2010.03780.x
105. Calabria E, Adamo D, Leuci S, Pecoraro G, Coppola N, Aria M, et al. The health-related quality of life and psychological profile in patients with oropharyngeal pemphigus vulgaris in complete clinical remission: A case-control study. J Oral Pathol Med (2021) 50(5):510–9. doi: 10.1111/jop.13150
106. Hsu YM, Fang HY, Lin CL, Shieh SH. The risk of depression in patients with pemphigus: A nationwide cohort study in Taiwan. Int J Environ Res Public Health (2020) 17(6):3–4. doi: 10.3390/ijerph17061983
107. Wohl Y, Mashiah J, Kutz A, Hadj-Rabia S, Cohen AD. Pemphigus and depression comorbidity: a case control study. Eur J Dermatol (2015) 25(6):602–5. doi: 10.1684/ejd.2015.2649
108. Layegh P, Mokhber N, Javidi Z, Mashhadi MP, Moghiman T. Depression in patients with pemphigus: is it a major concern? J Dermatol (2013) 40(6):434–7. doi: 10.1111/1346-8138.12067
109. Tabolli S, Mozzetta A, Antinone V, Alfani S, Cianchini G, Abeni D. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the medical outcomes study 36-item short form health survey questionnaire. Br J Dermatol (2008) 158(5):1029–34. doi: 10.1111/j.1365-2133.2008.08481.x
110. Tabolli S, Pagliarello C, Paradisi A, Cianchini G, Giannantoni P, Abeni D. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol (2014) 170(5):1087–91. doi: 10.1111/bjd.12836
111. Caccavale S, Lo Schiavo A. Psychiatric morbidity and pemphigus: A review of theories and literature on pathogenesis. G Ital Dermatol Venereol (2016) 151(2):198–202.
112. Ruocco E, Wolf R, Ruocco V, Brunetti G, Romano F, Lo Schiavo A. Pemphigus: Associations and management guidelines: Facts and controversies. Clin Dermatol (2013) 31(4):382–90. doi: 10.1016/j.clindermatol.2013.01.005
113. Cremniter D, Baudin M, Roujeau JC, Prost C, Consoli SG, Francés C, et al. Stressful life events as potential triggers of pemphigus. Arch Dermatol (1998) 134(11):1486–7. doi: 10.1001/archderm.134.11.1486
114. Ruocco E, Aurilia A, Ruocco V. Precautions and suggestions for pemphigus patients. Dermatology (2001) 203(3):201–7. doi: 10.1159/000051749
115. Morell-Dubois S, Carpentier O, Cottencin O, Queyrel V, Hachulla E, Hatron PY, et al. Stressful life events and pemphigus. Dermatology (2008) 216(2):104–8. doi: 10.1159/000111506
116. Brenner S, Tur E, Shapiro J, Ruocco V, D'Avino M, Ruocco E, et al. Pemphigus vulgaris: environmental factors. occupational, behavioral, medical, and qualitative food frequency questionnaire. Int J Dermatol (2001) 40(9):562–9. doi: 10.1046/j.1365-4362.2001.01266.x
117. Brenner S, Mashiah J, Tamir E, Goldberg I, Wohl Y. PEMPHIGUS: An acronym for a disease with multiple etiologies. Skinmed (2003) 2(3):163–7. doi: 10.1111/j.1540-9740.2003.02040.x
118. Daneshpazhooh M, Fatehnejad M, Rahbar Z, Balighi K, Ghandi N, Ghiasi M, et al. Trauma-induced pemphigus: A case series of 36 patients. J Dtsch Dermatol Ges (2016) 14(2):166–71. doi: 10.1111/ddg.12738
119. Jin X, Rosenbohm J, Kim E, Esfahani AM, Seiffert-Sinha K, Wahl JK 3rd, et al. Modulation of mechanical stress mitigates anti-Dsg3 antibody-induced dissociation of cell-cell adhesion. Adv Biol (Weinh) (2021) 5(1):e2000159. doi: 10.1002/adbi.202000159
120. Wohl Y, Brenner S. Pemphigus in Israel–an epidemiologic analysis of cases in search of risk factors. Isr Med Assoc J (2003) 5(6):410–2.
121. Duick MG, Zaks B, Moy RL, Kaplan RP. Mohs micrographic surgery-induced pemphigus. Dermatol Surg (2001) 27(10):895–7. doi: 10.1046/j.1524-4725.2001.00247.x
122. David M, Feuerman EJ. Induction of pemphigus by X-ray irradiation. Clin Exp Dermatol (1987) 12(3):197–9. doi: 10.1111/j.1365-2230.1987.tb01894.x
123. Chorzelski T, Jablonska S, Beutner EH, Kowalska M. Can pemphigus be provoked by a burn? Br J Dermatol (1971) 85(4):320–5. doi: 10.1111/j.1365-2133.1971.tb14025.x
124. Kaplan RP, Detwiler SP, Saperstein HW. Physically induced pemphigus after cosmetic procedures. Int J Dermatol (1993) 32(2):100–3. doi: 10.1111/j.1365-4362.1993.tb01445.x
125. Karagöz G, Bektaş-Kayhan K, Ünür M. Evaluation of pemphigus cases involving oral mucosa. Oral Health Dent Manage (2014) 13(3):605–9.
126. Brenner S, Wolf R. Possible nutritional factors in induced pemphigus. Dermatology (1994) 189(4):337–9. doi: 10.1159/000246874
127. Feliciani C, Ruocco E, Zampetti A, Toto P, Amerio P, Tulli A, et al. Tannic acid induces in vitro acantholysis of keratinocytes via IL-1alpha and TNF-alpha. Int J Immunopathol Pharmacol (2007) 20(2):289–99. doi: 10.1177/039463200702000209
128. Caldarola G, Feliciani C. A glass of red wine to keep vascular disease at bay, but what about pemphigus vulgaris? Expert Rev Clin Immunol (2011) 7(2):187–91. doi: 10.1586/eci.10.94
129. Kneiber D, Kowalski EH, Kridin K, Yale ML, Grando SA, Amber KT. Gastrointestinal symptoms, gastrointestinal bleeding and the role of diet in patients with autoimmune blistering disease: A survey of the international pemphigus and pemphigoid foundation. J Eur Acad Dermatol Venereol (2019) 33(10):1935–40. doi: 10.1111/jdv.15731
130. Ruocco V, Brenner S, Ruocco E. Pemphigus and diet: Does a link exist? Int J Dermatol (2001) 40(3):161–3. doi: 10.1046/j.1365-4362.2001.01099.x
131. Czerninski R, Zadik Y, Kartin-Gabbay T, Zini A, Touger-Decker R. Dietary alterations in patients with oral vesiculoulcerative diseases. Oral Surg Oral Med Oral Pathol Oral Radiol (2014) 117(3):319–23. doi: 10.1016/j.oooo.2013.08.006
132. Atarzadeh F, Daneshfard B, Dastgheib L, Jaladat AM, Amin G. Early description of diet-induced blistering skin diseases in medieval Persia: Avicenna's point of view. Skinmed (2016) 14(5):367–70.
133. Brenner S, Ruocco V, Wolf R, de Angelis E, Lombardi ML. Pemphigus and dietary factors. In vitro acantholysis by allyl compounds of the genus allium. Dermatology (1995) 190(3):197–202. doi: 10.1159/000246684
134. Ruocco V, Brenner S, Lombardi ML. A case of diet-related pemphigus. Dermatology (1996) 192(4):373–4. doi: 10.1159/000246417
135. Tur E, Brenner S. The role of the water system as an exogenous factor in pemphigus. Int J Dermatol (1997) 36(11):810–6. doi: 10.1046/j.1365-4362.1997.00350.x
136. Tur E, Brenner S. Contributing exogenous factors in pemphigus. Int J Dermatol (1997) 36(12):888–93. doi: 10.1046/j.1365-4362.1997.00334.x
137. Tur E, Brenner S. Diet and pemphigus. in pursuit of exogenous factors in pemphigus and fogo selvagem. Arch Dermatol (1998) 134(11):1406–10. doi: 10.1001/archderm.134.11.1406
138. Javanbakht M, Daneshpazhooh M, Chams-Davatchi C, Eshraghian M, Zarei M, Chamari M, et al. Serum selenium, zinc, and copper in early diagnosed patients with pemphigus vulgaris. Iran J Public Health (2012) 41(5):105–9.
139. La Serra L, Salathiel AM, Trevilato TMB, Alves RIS, Segura-Muñoz SI, de Oliveira Souza VC, et al. Trace element profile in pemphigus foliaceus and in pemphigus vulgaris patients from southeastern Brazil. J Trace Elem Med Biol (2019) 51:31–5. doi: 10.1016/j.jtemb.2018.09.005
140. Torkian S, Khanjani N, Mahmoodi MR, Khosravi V. A review of copper concentrations in Iranian populations. Environ Monit Assess (2019) 191(9):537. doi: 10.1007/s10661-019-7633-7
141. Zarei M, Javanbakht MH, Chams-Davatchi C, Daneshpazhooh M, Eshraghian MR, DE-Rakhshanian H, et al. Evaluation of vitamin d status in newly diagnosed pemphigus vulgaris patients. Iran J Public Health (2014) 43(11):1544–9.
142. Yazdanpanah MJ, Ghayour-Mobarhan M, Taji A, Javidi Z, Pezeshkpoor F, Tavallaie S, et al. Serum zinc and copper status in Iranian patients with pemphigus vulgaris. Int J Dermatol (2011) 50(11):1343–6. doi: 10.1111/j.1365-4632.2011.04968.x
143. El-Komy MH, Samir N, Shaker OG. Estimation of vitamin d levels in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol (2014) 28(7):859–63. doi: 10.1111/jdv.12179
144. Lin L, Moran TP, Peng B, Yang J, Culton DA, Che H, et al. Walnut antigens can trigger autoantibody development in patients with pemphigus vulgaris through a "hit-and-run" mechanism. J Allergy Clin Immunol (2019) 144(3):720–8.e4. doi: 10.1016/j.jaci.2019.04.020
145. Lee AN, Werth VP. Activation of autoimmunity following use of immunostimulatory herbal supplements. Arch Dermatol (2004) 140(6):723–7. doi: 10.1001/archderm.140.6.723
146. Bakhshi M, Manifar S, Azizi N, Asayesh H, Mansouri P, Nasiri S, et al. Risk factors in patients with oral pemphigus vulgaris: A case-control study. Gen Dent (2016) 64(3):e10–3.
147. Tavakolpour S, Daneshpazhooh M, Mahmoudi HR, Balighi K. The dual nature of retinoic acid in pemphigus and its therapeutic potential: Special focus on all-trans retinoic acid. Int Immunopharmacol (2016) 36:180–6. doi: 10.1016/j.intimp.2016.04.031
148. Knechtl GV, Seyed Jafari SM, Berger T, Rammlmair A, Feldmeyer L, Borradori L. Development of pemphigus vulgaris following mRNA SARS-CoV-19 BNT162b2 vaccination in an 89-year-old patient. J Eur Acad Dermatol Venereol (2022) 36(4):e251–e3. doi: 10.1111/jdv.17868
149. Solimani F, Mansour Y, Didona D, Dilling A, Ghoreschi K, Meier K. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol (2021) 35(10):e649–e51. doi: 10.1111/jdv.17480
150. Damiani G, Pacifico A, Pelloni F, Iorizzo M. The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: Is the second dose therefore contraindicated? J Eur Acad Dermatol Venereol (2021) 35(10):e645–e7. doi: 10.1111/jdv.17472
151. Hali F, Araqi L Jr., Marnissi F, Meftah A, Chiheb S. Autoimmune bullous dermatosis following COVID-19 vaccination: A series of five cases. Cureus (2022) 14(3):e23127. doi: 10.7759/cureus.23127
152. Calabria E, Canfora F, Mascolo M, Varricchio S, Mignogna MD, Adamo D. Autoimmune mucocutaneous blistering diseases after SARS-Cov-2 vaccination: A case report of pemphigus vulgaris and a literature review. Pathol Res Pract (2022) 232:153834. doi: 10.1016/j.prp.2022.153834
153. De Simone C, Caldarola G, D'Agostino M, Zampetti A, Amerio P, Feliciani C. Exacerbation of pemphigus after influenza vaccination. Clin Exp Dermatol (2008) 33(6):718–20. doi: 10.1111/j.1365-2230.2008.02835.x
154. Muellenhoff M, Cukrowski T, Morgan M, Dorton D. Oral pemphigus vulgaris after anthrax vaccine administration: Association or coincidence? J Am Acad Dermatol (2004) 50(1):136–9. doi: 10.1016/S0190-9622(03)00407-9
155. Berkun Y, Mimouni D, Shoenfeld Y. Pemphigus following hepatitis b vaccination–coincidence or causality? Autoimmunity (2005) 38(2):117–9. doi: 10.1080/08916930400027078
156. Bell H, Kamal N, Wong U. Blistering autoimmune skin reaction following SHINGRIX vaccination in an ulcerative colitis patient: Case report and literature review. Vaccine (2020) 38(47):7455–7. doi: 10.1016/j.vaccine.2020.09.073
157. Siegel JM. Do all animals sleep? Trends Neurosci (2008) 31(4):208–13. doi: 10.1016/j.tins.2008.02.001
158. Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep (1989) 25(1):68–87.
159. Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: An unmet public health problem. In: The national academies collection: Reports. Washington (DC: National Institutes of Health (2006).
160. Davis CJ, Krueger JM. Sleep and cytokines. Sleep Med Clin (2012) 7(3):517–27. doi: 10.1016/j.jsmc.2012.06.006
161. Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des (2008) 14(32):3408–16. doi: 10.2174/138161208786549281
162. Clinton JM, Davis CJ, Zielinski MR, Jewett KA, Krueger JM. Biochemical regulation of sleep and sleep biomarkers. J Clin Sleep Med (2011) 7(5 Suppl):S38–42. doi: 10.5664/JCSM.1360
164. Hogan D, Morrow JD, Smith EM, Opp MR. Interleukin-6 alters sleep of rats. J Neuroimmunol (2003) 137(1-2):59–66. doi: 10.1016/S0165-5728(03)00038-9
165. Opp MR, Obal F Jr., Krueger JM. Interleukin 1 alters rat sleep: temporal and dose-related effects. Am J Physiol (1991) 260(1 Pt 2):R52–8. doi: 10.1152/ajpregu.1991.260.1.R52
166. Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab (1998) 83(5):1573–9. doi: 10.1210/jc.83.5.1573
167. Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol (2001) 117(2):309–17. doi: 10.1046/j.1523-1747.2001.01373.x
168. Jang SI, Lee M, Han J, Kim J, Kim AR, An JS, et al. A study of skin characteristics with long-term sleep restriction in Korean women in their 40s. Skin Res Technol (2020) 26(2):193–9. doi: 10.1111/srt.12797
169. Hirotsu C, Rydlewski M, Araujo MS, Tufik S, Andersen ML. Sleep loss and cytokines levels in an experimental model of psoriasis. PloS One (2012) 7(11):e51183. doi: 10.1371/journal.pone.0051183
170. D'Auria L, Bonifati C, Mussi A, D'Agosto G, De Simone C, Giacalone B, et al. Cytokines in the sera of patients with pemphigus vulgaris: Interleukin-6 and tumour necrosis factor-alpha levels are significantly increased as compared to healthy subjects and correlate with disease activity. Eur Cytokine Netw (1997) 8(4):383–7.
171. Pedroni MN, Hirotsu C, Porro AM, Tufik S, Andersen ML. The role of sleep in pemphigus: a review of mechanisms and perspectives. Arch Dermatol Res (2017) 309(8):659–64. doi: 10.1007/s00403-017-1765-9
Keywords: pemphigus vulgaris (PV), induction, trigger, environmental factors, exacerbation, lifestyle factors
Citation: Adebiyi OT, Galloway DF, Augustin MS and Sinha AA (2023) The multifactorial complexities of autoimmune development in Pemphigus vulgaris: Critical evaluation of the role of environmental and lifestyle “exposome” factors. Front. Immunol. 13:1058759. doi: 10.3389/fimmu.2022.1058759
Received: 30 September 2022; Accepted: 19 December 2022;
Published: 10 January 2023.
Edited by:
Shannon E. Dunn, St Michael’s Hospital, CanadaReviewed by:
Roberta Lotti, DermoLAB, Azienda Ospedaliero-Universitaria Modena, ItalyCopyright © 2023 Adebiyi, Galloway, Augustin and Sinha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Animesh A. Sinha, YWFzaW5oYUBidWZmYWxvLmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.