- 1The Department of Pediatrics, The First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, China
- 2The Outpatient Department, Shantou Longhu People’s Hospital, Shantou, Guangdong, China
- 3The Clinical Research Unit, Shantou University Medical College, Shantou, Guangdong, China
Background: As a form of severe combined immunodeficiency (SCID), Janus kinase 3 (JAK3) deficiency can be fatal during severe infections in children, especially after inoculation of live-attenuated vaccines. We report a unique case of JAK3 deficiency with two compound heterozygous JAK3 mutations complicated by disseminated Bacille Calmette–Guérin (BCG) disease and Pneumocystis pneumonia.
Case description: A 5-month-old Chinese girl presented with recurring fever and productive cough after BCG vaccination and ineffective antibiotic treatment. Chest CT demonstrated bilateral infiltrations, enlarged mediastinal and axillary lymph nodes, and hypoplasia of the thymus. Mycobacterium tuberculosis and Pneumocystis jirovecii were detected from blood samples by sequencing. Acid-fast bacilli were also found from the sputum aspirate and gastric aspirate. Lymphocyte subset analyses indicated T-B+NK- immunodeficiency, and gene sequencing identified two heterozygous missense mutations (one unreported globally) in the Janus homology 7 (JH7) domain of JAK3. The patient received rifampicin, isoniazid, ethambutol, and trimethoprim/sulfamethoxazole and was discharged after improvements but against advice.
Outcome: The patient died at 13 months of age due to severe infections and hepatic damage.
Discussion: SCID should be recognized before inoculation of live-attenuated vaccines in children. Newborn screening for SCID is advocated. Further investigations are needed to better understand the pathogenicity of the variants and molecular mechanism of the JH7 domain of JAK3.
Introduction
Severe combined immunodeficiency (SCID) is a heterogeneous group of inherited disorder characterized by profound impairment in cellular and humoral immunity that can lead to death from severe infection(s) in infancy (1, 2). The incidence of SCID has been recorded as 1 in 58,000 live births based on a US newborn screening data (3, 4). According to immunologic phenotype classification, SCID can be grouped into four distinct categories: T-B+NK+ [T cell-deficient, normal B cells and natural killer (NK) cells], T-B+NK-, T-B-NK+, and T-B-NK- (1, 2). T-B+NK± SCIDs, accounting for 67%~74% of all SCIDs, are mostly triggered by pathogenic genetic variants affecting the common gamma chain (γc) of interleukin 2 receptor (IL-2R), the associated downstream signaling enzyme Janus kinase 3 (JAK3), or IL-7R alpha chain gene (2, 5). As an unusual subtype comprising about 5% of all SCIDs (4, 6), JAK3 deficiency with various JAK3 mutations has been sporadically reported globally (6–8). Unlike X-linked γc SCID that generally affects male patients, JAK3 deficiency is autosomal recessive inheritance and affects both male and female patients (2). The patients with SCID, including JAK3 deficiency, have a noticeably high risk for severe, disseminated, and even fatal infections after inoculation of live-attenuated vaccines (5, 8–11), particularly Bacille Calmette–Guérin (BCG), being an active attenuated Mycobacterium bovis vaccine (5, 8, 10, 11). The incidence of disseminated BCG disease is approximately two cases per 1 million BCG-vaccinated children with a mortality rate of 80%, while the incidence of disseminated BCG disease is as high as one in every two BCG-vaccinated SCID patients (5, 11). JAK3 deficiency complicated by BCG disease after BCG vaccination has been rarely reported worldwide (8, 12, 13). Herein, we describe a unique case of JAK3 deficiency with two compound heterozygous JAK3 mutations complicated by disseminated BCG disease and Pneumocystis jirovecii pneumonia (PJP).
Case presentation
A 5-month-old female infant was admitted to the First Affiliated Hospital of Shantou University Medical College due to recurring fever and productive cough for 2 weeks unresponsive to cephalosporin and penicillin in a local hospital. She was born full-term with a weight of 2.7 kg (3 < P < 10). BCG vaccine, routinely administered at birth in China, was given at 2 months of age because of meconium aspiration pneumonia after birth. She had been hospitalized locally for 7 days for generalized small papules and pustules at 4 months and recovered gradually after supportive treatment. No tuberculosis contact was noted. Her non-consanguineous parents and only older brother were all healthy. There was no family history of similar or genetic diseases. Her total white blood cell count, neutrophil count percentage, and C-reactive protein were apparently elevated along with a low to normal lymphocyte count; chest radiograph showed bilateral infiltrations (thymus shadow not mentioned) before her current admission.
On physical examination, her weight was 5.2 kg (<P1). Several enlarged axillary lymph nodes were palpable bilaterally. Her oral mucosa was intact. Coarse breath sounds and rhonchi were audible bilaterally. Throat swab was positive for parainfluenza virus (PIV) and respiratory syncytial virus (RSV) by PCR assays, while routine cerebrospinal fluid analysis/culture and blood HIV testing were all negative. Chest CT revealed bilateral parenchymal and interstitial infiltrations, multiple enlarged lymph nodes in the mediastinum and right axilla, and hypoplasia of the thymus.
The patient was treated sequentially with intravenous ceftriaxone, piperacillin/tazobactam, and vancomycin, but her symptoms were not relieved.
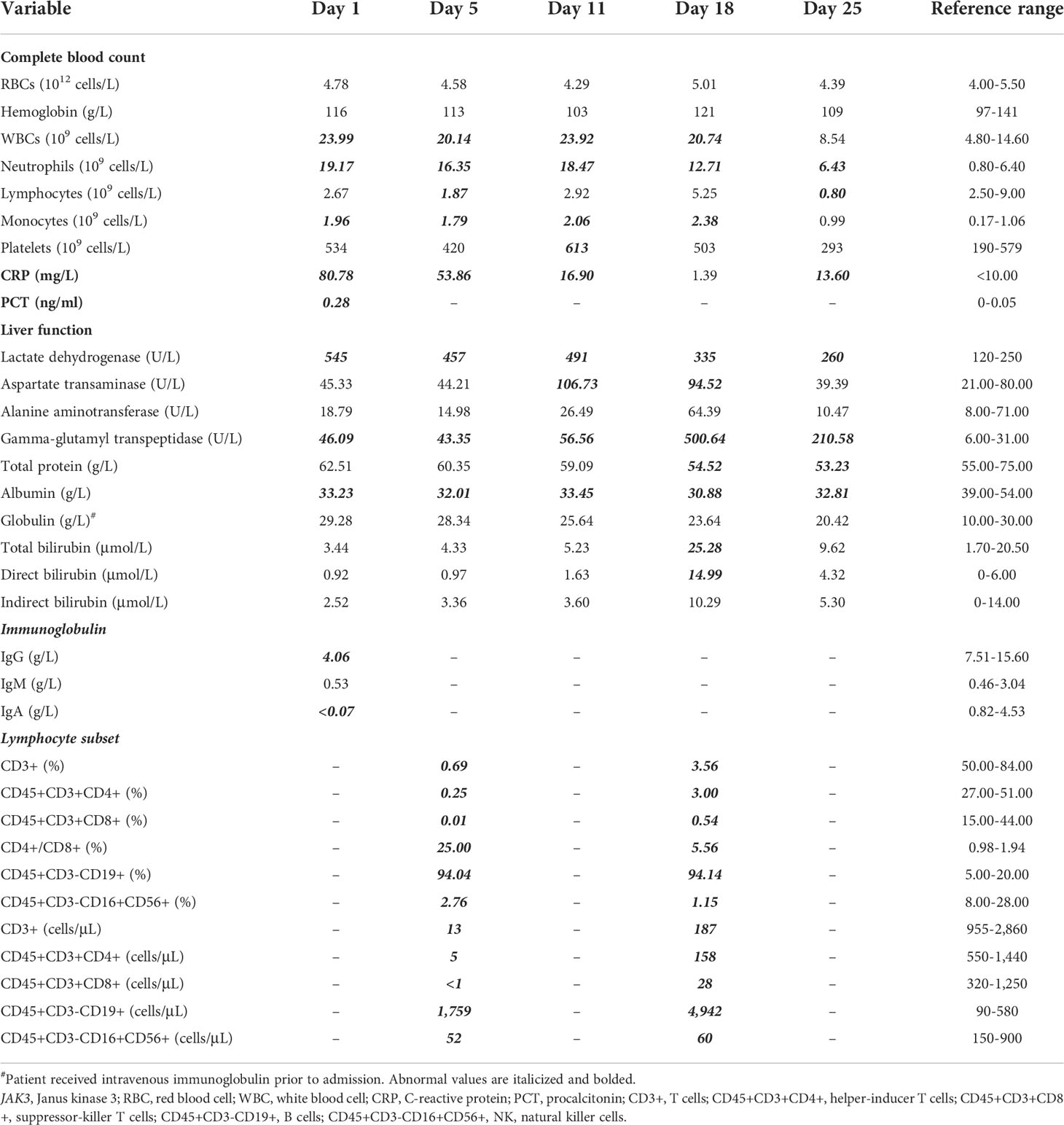
From her blood samples, the sequences of M. tuberculosis and P. jirovecii were mapped through next-generation sequencing. Acid-fast bacilli were found in the concentrated smears of the sputum aspirate and gastric aspirate; M. tuberculosis and P. jirovecii were not isolated after culturing. The subtypes of M. tuberculosis failed to be identified prior to antituberculosis therapy. To clarify the source of tuberculosis infection, her older brother, parents, and grandparents underwent chest X-ray examinations and purified protein derivative (PPD) skin tests, which were all negative. In addition, low numbers of T cells and natural killer (NK) cells and increased B lymphocytes from lymphocyte subset analyses confirmed T-B+NK- immunodeficiency (Table 1). Subsequent gene sequencing identified two heterozygous missense mutations in the Janus homology 7 (JH7) domain of the JAK3 gene of the patient, each inherited from her parents (Figure 1). The paternal mutation C.301A>G at nucleotide 301 encoded by exon 3 changed amino acid 101 from arginine to glycine (i.e., p.Arg101Gly), and the maternal mutation C.331G>A at nucleotide 331 encoded by exon 4 altered amino acid 111 from glycine to arginine (i.e., p.Gly111Arg). These two mutations were predicted to be pathogenic by Sorting intolerant from tolerant (SIFT) (both <0.05, deleterious), PolyPhen2_HVAR (paternal: 0.997, maternal: 0.992; both probably damaging), PolyPhen2_HDIV (both >0.957, probably damaging), and Rare exome variant ensemble learner (REVEL) (paternal: 0.809, maternal: 0.655; both pathogenic).
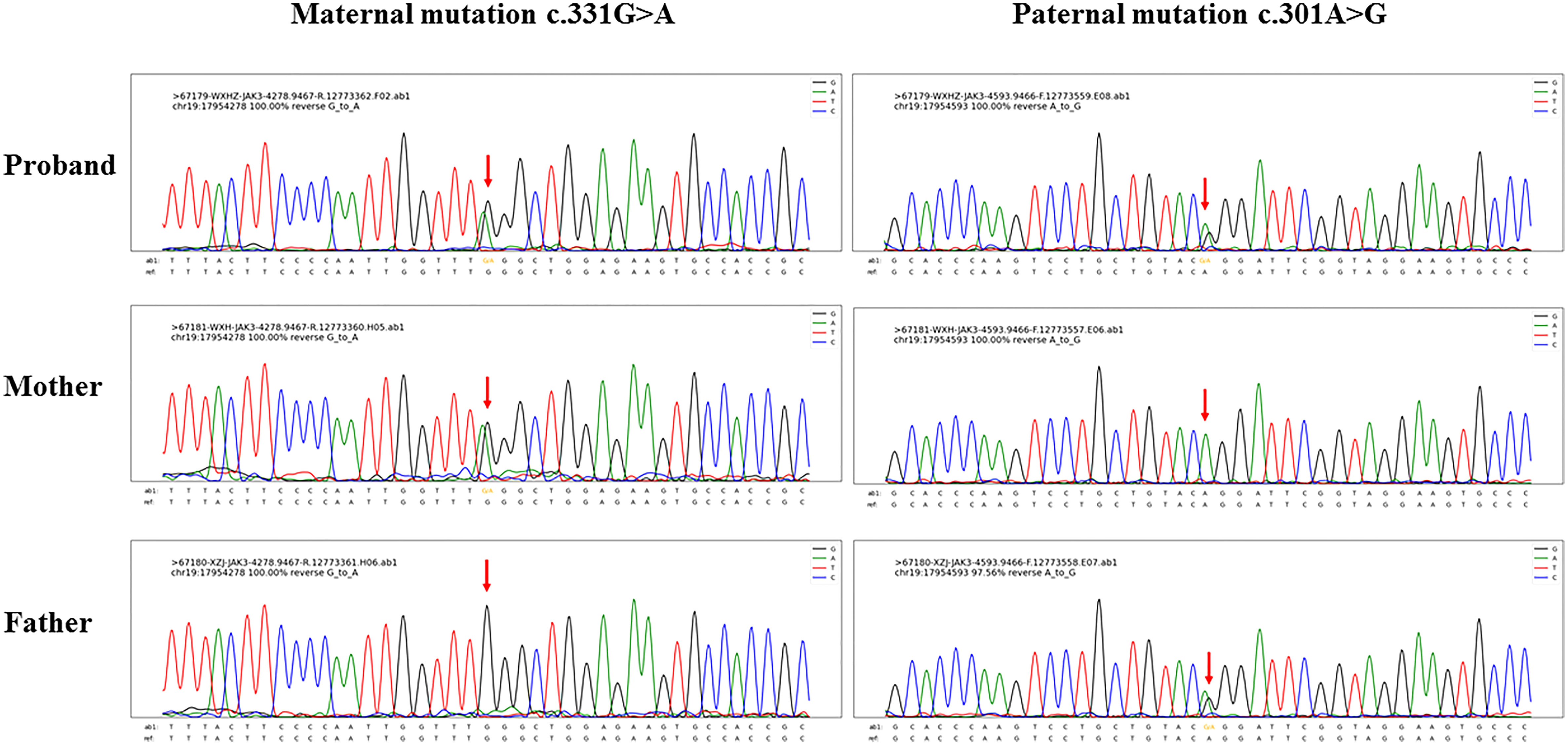
Figure 1 Gene sequencing results of the index patient of Janus kinase 3 (JAK3) deficiency and her parents. Sequencing detected two missense mutations both located in the Janus homology 7 (JH7) domain of the JAK3 gene, as maternal mutation c.331G>A and paternal mutation c.301A>G.
Consequently, her treatment strategy was switched to rifampicin, isoniazid, and ethambutol for tuberculosis, intravenous immunoglobulin to enhance immune functions, and further trimethoprim/sulfamethoxazole for PJP, after which improvements in symptoms (cough and fever) and lab results (e.g., decreased serum lactate dehydrogenase) were noted. After being hospitalized for 28 days, the patient improved but was discharged upon request of her parents who were satisfied with the timely diagnosis and treatment. During our follow-up, the patient continued antituberculosis treatment routinely at the assigned local tuberculosis prevention clinic and died without hematopoietic stem cell transplantation (HSCT) or autopsy at 13 months of age mainly because of severe infections in addition to hepatic injury (autopsy is culturally unacceptable and therefore uncommon in China). Her other laboratory results are shown in Table 1. Written informed consent for laboratory examinations and submission/publications of this work was obtained from her parents. This study was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College.
Discussion
The human JAK3 gene is predominantly expressed in lymphoid and myeloid cells and located in chromosome 19p12-13.1, consisting of 23 exons and an open reading frame of 3,372 nucleotides (1, 2, 14–17). Tyrosine kinase JAK3 binds specially to the γc subunit, an essential component of the cytokine (i.e., IL-2, IL-4, IL-7, IL-9, IL-10, IL-11, IL-15, and IL-21) receptor complexes (Figure 2) (1, 7, 15, 18). Intracellular activation signal through cytokine-γc-JAK3 binding induces JAK3 to phosphorylate signal transducer and activator of transcription (STAT) proteins (i.e., STAT1, STAT3, STAT5, and STAT6) (18, 19), which therefore dimerize and translocate to the nucleus to initiate the transcription program (1, 7). While IL-2, IL-4, and IL-9 play crucial roles in the development of B cells and T cells (1, 14, 20), IL-7 and IL-15 mainly regulate the differentiation and activation of T cells and NK cells (1, 2, 6, 14, 21). In consequence, JAK3 deficiency can lead to serious defects of T cells and NK cells with a normal or an increased number of poorly effective B cells (i.e., T-B+NK-) and a clinical phenotype nearly identical to that of γc deficiency (2, 6, 16). In our case, the profiles of lymphocyte subsets and the obviously decreased levels of immunoglobulins A and G are concordant with the manifestations of JAK3 deficiency.
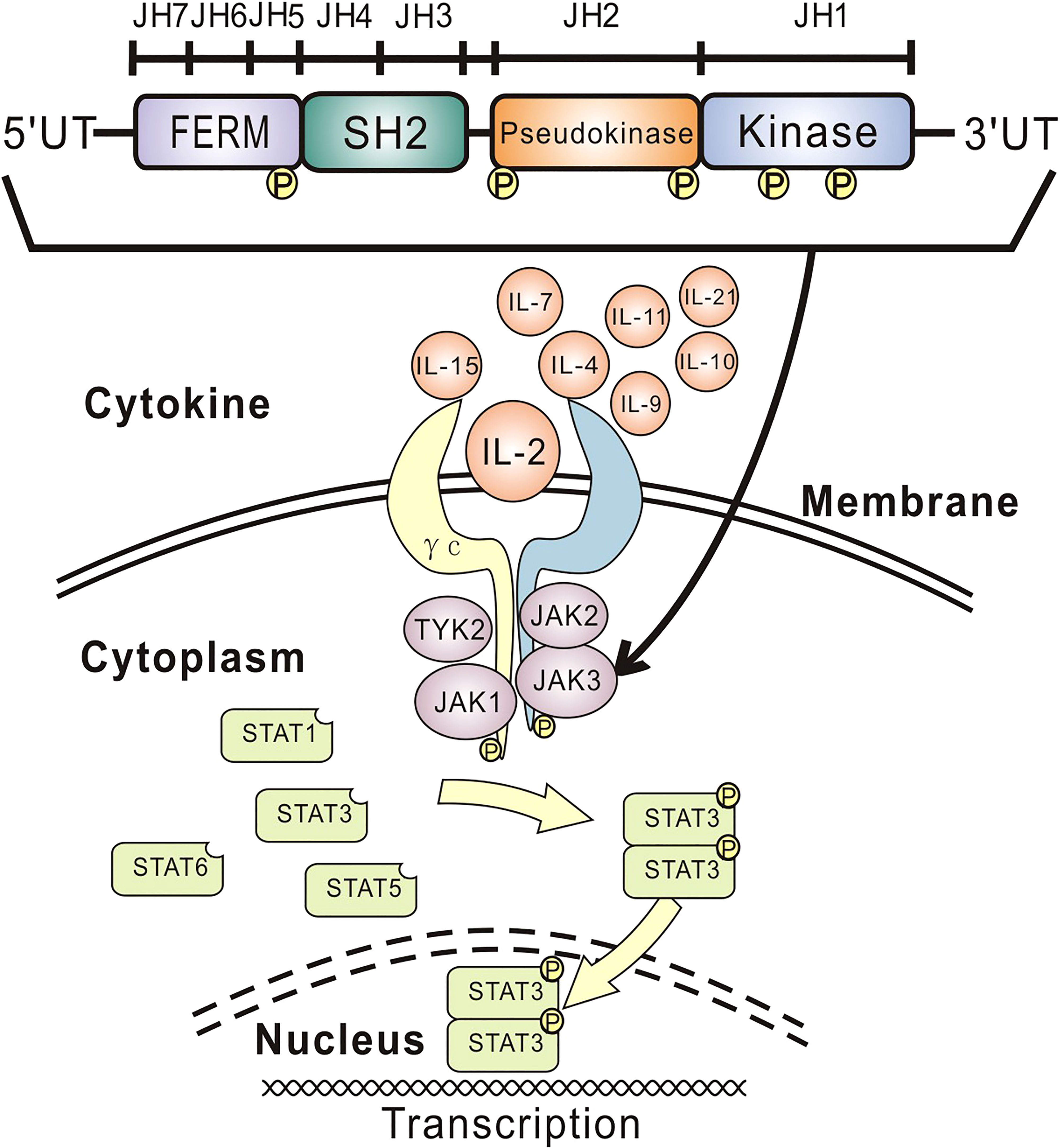
Figure 2 Overview of cytokine signaling and JAK3 structure. Intracellular activation signal through cytokine-γc-JAK3 binding induces JAK3 to phosphorylate STAT proteins, which therefore dimerize and translocate to the nucleus to initiate the transcription program. The JAK family includes four homologs (i.e., JAK1, JAK2, JAK3, and TYK2), each containing seven conserved JH domains. The JH1 (kinase) domain regulates kinase activity, and the JH2 (pseudokinase) domain is essential for normal JAK3 functions, including catalytic activity and autophosphorylation. The SH2-like JH3, JH4, and JH5 domains are considered imperative for the in vivo assembly of the JAKs. The JH5, JH6, and JH7 domains (namely, FERM) are required for binding to γc for signal transduction and regulating catalytic activity. JAK, Janus kinase; γc, gamma chain; STAT, signal transducer and activator of transcription; JH, Janus homology; IL, interleukin; FERM, four-point-one, ezrin, radixin, moesin; SH2, Src homology-2; TYK2, tyrosine kinase 2.
The JAK family consists of four mammalian homologs (i.e., JAK1, JAK2, JAK3, and TYK2) (17, 22), each containing 1,100–1,200 amino acids arranged in seven conserved JH domains according to sequence similarity (Figure 2) (15, 17). The C-terminal JH1 (kinase) domain plays a key role in regulating kinase activity (7). The JH2 (pseudokinase) domain, despite its lack of kinase activity, binds to the JH1 domain and thereby is essential for normal JAK3 functions, including catalytic activity and autophosphorylation (6, 7, 23). The majority of reported pathogenic mutations leading to SCID, including JAK3 deficiency, occur in the JH1 and JH2 domains, since they constitute nearly half of the whole JAK sequence (6, 7, 14, 24). The JH3, JH4, and JH5 domains are considered imperative for the in vivo assembly of the JAKs (6, 14). The N-terminal JH5, JH6, and JH7 domains (namely, four-point-one, ezrin, radixin, moesin (FERM)) are required for binding to γc for signal transduction and regulating catalytic activity (6, 14), of which JH7 and the head of JH6 (i.e., amino acids 1–193) of JAK3 are sufficient for binding to the BOX1 (a highly conserved motif) proline residues 266 and 269 of γc specifically in response to IL-2 (22, 25). In our case, the maternal mutation C.331G>A has not been reported globally, while the paternal mutation C.301A>G has been listed at low frequency (i.e., 0.00006157) in the Genome Aggregation Database (gnomAD; http://www.gnomad-sg.org/). These mutations were predicted to be pathogenic by different predicting tools. Single/multiple amino acid substitutions in the JH7 domain naturally occurring in JAK3 deficiency patients or from JAK3-JAK1 or JAK3-JAK2 chimeras can prevent kinase–receptor interaction and IL-2-induced signaling (6, 7, 22, 24, 25). Similarly, the two missense mutations in the JH7 domain of our patient may affect IL-2-γc-JAK3 binding and thus the DNA transcriptions of B cells and T cells. Point mutations only in the JH7 domain of JAK3 observed in our patient are speculated to affect the binding of JAK3 to γc and other upstream cytokines, probably IL-7 and/or IL-15, therefore contributing to the reduced number of NK cells. Considering HSCT, the best remedy for JAK3 deficiency, is still not completely successful for restoring the functions of B cells and NK cells (6); our findings call for further investigations to better understand the role/mechanism of the JH7 domain of the JAK3 gene in regulating the functions of NK cells, which would be helpful for improving HSCT and gene therapy in the future.
The manifestations and clinical outcomes of JAK3 deficiency due to different mutations in the JH1–7 domains are nearly identical (6, 8, 26). Similar to other SCIDs, JAK3 deficiency patients normally present with recurrent severe respiratory infections, refractory diarrhea, thrush, and/or retarded growth, characterized by irrecoverable infections mostly caused by opportunistic and/or multiple pathogens (2, 5, 6, 8, 14) or live-attenuated vaccination (5, 6, 8–11). Although we were unable to further identify the M. tuberculosis subspecies, the bloodstream/multisite tuberculosis infection following BCG vaccination and a lack of tuberculosis contact in the infant support a diagnosis of disseminated BCG disease, consistent with the definition of disseminated BCG disease (10–12). Along with other reports (8, 12, 13), our case highlights the severe consequences of SCID and disseminated BCG disease.
The inoculation of BCG vaccine has been implemented as an essential and generally safe means to prevent tuberculosis infection, mostly given at birth in developing countries, including China (http://www.bcgatlas.org/). However, primary immunodeficiency diseases (PIDs) should be ruled out before BCG vaccination, as disseminated BCG disease is usually severe and life-threatening for PID patients (5). M. bovis is the cause of BCG disease, naturally resistant to pyrazinamide (11). Hence, anti-M. bovis therapy basically comprises isoniazid, rifampicin, ethambutol, and streptomycin (5, 11, 12). The compromised liver function of our patient led to a final choice of triad therapy being isoniazid, rifampicin, and ethambutol based on the literature (5, 11, 12). Indeed, early screening for PIDs in children will be definitely better to avoid inoculation of live-attenuated vaccines and consequently reduce vaccine-related mortality (5). Neonatal PID screening programs, such as T cell receptor excision circles (TREC)-based newborn screening (2), have been deployed in some developed countries/regions (8) but unfortunately not in mainland China. However, absolute lymphopenia (i.e., absolute lymphocyte count <3,000/mm3) in a newborn complete blood count should alert us to screen for and exclude SCID (27).
The fungus P. jirovecii, commonly existing in ambient environments, can cause PJP as a serious and fatal opportunistic infection more commonly in HIV patients than in SCID patients (26). The symptoms of PJP usually last for several days/weeks, including fever, cough, difficulty breathing, chest pain, chills, and fatigue. Elevated serum lactate dehydrogenase is one of the most remarkable laboratory findings for PJP and other fungal infections (28). Chest radiograph can show diffuse bilateral interstitial infiltrates, while chest CT may reveal ground-glass attenuation or cystic lesions (28). As Pneumocystis is extremely difficult to culture, conclusive diagnosis basically needs detection/identification of the organism by PCR, dye staining, or fluorescein antibody staining of respiratory specimens (28). Trimethoprim/sulfamethoxazole is the drug of choice for preventing/treating PJP in immunocompromised patients. In our case, Pneumocystis as an air-borne transmitted pathogen, recurring fever and cough, P. jirovecii detected in blood, serum lactate dehydrogenase changes during the course, bilateral parenchymal and interstitial infiltrations shown by chest CT (despite the influence of simultaneous infection of M. tuberculosis), and effective response to trimethoprim/sulfamethoxazole treatment support a diagnosis of PJP. This is the second global report of JAK3 deficiency and PJP, aside from a Japanese case (26). PIV and RSV detected from the throat specimen by PCR alone in our patient could not be confirmed as current or past infections or just colonization.
Mainstream therapy regimen for SCID includes antimicrobial drugs, immunoglobulin replacement, enzyme replacement, retroviral gene therapy, and finally HSCT as a life-saving but not perfect modality (6, 14, 24). The outcome of gene therapy for JAK3 deficiency is still unsatisfactory (6). Nearly all SCID patients die before 2 years of age, unless effective therapies are utilized to reconstruct their immune systems (2). Our patient died without HSCT at 13 months of age as a consequence of severe infections and hepatic damage.
There are several limitations in this study. M. bovis as the cause of the disseminated BCG disease failed to be identified in advance of antituberculosis treatment. After ruling out the possibilities of tuberculosis contact/infection in all of her family members, M. bovis was highly likely to be the only cause of the infection. Although the observed mutations were predicted to be pathogenic, further basic research (6, 7, 25, 29) is still needed to examine the actual pathogenicity of the variants.
Conclusions
This report has elucidated an exceptional case of JAK3 deficiency with two JAK3 mutations complicated by disseminated BCG disease, Pneumocystis pneumonia, and probable respiratory viral infections. If possible, SCID should be identified before inoculation of live-attenuated vaccines in children. Screening for SCID in neonatal lymphopenia is suggested for developing countries such as China. Further basic/clinical studies are required to understand the pathogenicity of the variants and molecular mechanism of the JH7 domain of the JAK3 protein for better gene therapy and HSCT.
Data availability statement
The raw sequence data (GSA-Human: HRA002763) for this study have been deposited in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, and Chinese Academy of Sciences (https://ngdc.cncb.ac.cn/gsa-human) 30, 31).
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital of Shantou University Medical College. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
YP, HP, and BC wrote the manuscript, performed analyses, and revised the manuscript. YP, CL, BW, JL, GH, and BC provided clinical information. CL, BW, JL, and GH provided critical discussion. BC conceptualized the study and revised the manuscript. All authors contributed to the work and approved the final version of the manuscript.
Funding
This study was funded by the 2020 Guangdong Provincial Funds (grant no. 2020A111129021).
Acknowledgments
We are grateful to Dr. Frieda Law (Li Ka-Shing Foundation, Consultant of Shantou University Medical College, Shantou, Guangdong, 515041, P.R. China) for invaluable suggestions on revising the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gaspar HB, Gilmour KC, Jones AM. Severe combined immunodeficiency–molecular pathogenesis and diagnosis. Arch Dis Childhood (2001) 84(2):169–73. doi: 10.1136/adc.84.2.169
2. Cirillo E, Giardino G, Gallo V, D'Assante R, Grasso F, Romano R, et al. Severe combined immunodeficiency–an update. Ann NY Acad Sci (2015) 1356:90–106. doi: 10.1111/nyas.12849
3. Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the united states. Jama (2014) 312(7):729–38. doi: 10.1001/jama.2014.9132
4. Dvorak CC, Haddad E, Buckley RH, Cowan MJ, Logan B, Griffith LM, et al. The genetic landscape of severe combined immunodeficiency in the united states and Canada in the current era (2010-2018). J Allergy Clin Immunol (2019) 143(1):405–7. doi: 10.1016/j.jaci.2018.08.027
5. Marciano BE, Huang CY, Joshi G, Rezaei N, Carvalho BC, Allwood Z, et al. BCG Vaccination in patients with severe combined immunodeficiency: Complications, risks, and vaccination policies. J Allergy Clin Immunol (2014) 133(4):1134–41. doi: 10.1016/j.jaci.2014.02.028
6. Roberts JL, Lengi A, Brown SM, Chen M, Zhou YJ, O'Shea JJ, et al. Janus kinase 3 (JAK3) deficiency: Clinical, immunologic, and molecular analyses of 10 patients and outcomes of stem cell transplantation. Blood (2004) 103(6):2009–18. doi: 10.1182/blood-2003-06-2104
7. Vihinen M, Villa A, Mella P, Schumacher RF, Savoldi G, O'Shea JJ, et al. Molecular modeling of the JAK3 kinase domains and structural basis for severe combined immunodeficiency. Clin Immunol (2000) 96(2):108–18. doi: 10.1006/clim.2000.4880
8. Li M, Chen Z, Zhu Y, Chen J. Disseminated bacille calmette-guerin infection in a patient with severe combined immunodeficiency caused by JAK3 gene mutation. Pediatr Dermatol (2019) 36(5):672–6. doi: 10.1111/pde.13884
9. Patel NC, Hertel PM, Estes MK, de la Morena M, Petru AM, Noroski LM, et al. Vaccine-acquired rotavirus in infants with severe combined immunodeficiency. N Engl J Med (2010) 362(4):314–9. doi: 10.1056/NEJMoa0904485
10. Bukhari E, Alaklobi F, Bakheet H, Alrabiaah A, Alotibi F, Aljobair F, et al. Disseminated bacille calmette-guerin disease in Saudi children: clinical profile, microbiology, immunology evaluation and outcome. Eur Rev Med Pharmacol Sci (2016) 20(17):3696–702.
11. Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacille calmette-guerin disease after vaccination: case report and review. Clin Infect Dis (1997) 24(6):1139–46. doi: 10.1086/513642
12. Ong RYL, Chan SB, Chew SJ, Liew WK, Thoon KC, Chong CY, et al. Disseminated bacillus-Calmette-Guerin infections and primary immunodeficiency disorders in Singapore: A single center 15-year retrospective review. Int J Infect Dis (2020) 97:117–25. doi: 10.1016/j.ijid.2020.05.117
13. Barreiros LA, Segundo GRS, Grumach AS, Roxo-Junior P, Torgerson TR, Ochs HD, et al. A novel homozygous JAK3 mutation leading to T-B+NK- SCID in two Brazilian patients. Front Pediatr (2018) 6:230. doi: 10.3389/fped.2018.00230
14. Vihinen M, Arredondo-Vega FX, Casanova JL, Etzioni A, Giliani S, Hammarstrom L, et al. Primary immunodeficiency mutation databases. Adv Genet (2001) 43:103–88. doi: 10.1016/S0065-2660(01)43005-7
15. Riedy MC, Dutra AS, Blake TB, Modi W, Lal BK, Davis J, et al. Genomic sequence, organization, and chromosomal localization of human JAK3. Genomics (1996) 37(1):57–61. doi: 10.1006/geno.1996.0520
16. Schumacher RF, Mella P, Badolato R, Fiorini M, Savoldi G, Giliani S, et al. Complete genomic organization of the human JAK3 gene and mutation analysis in severe combined immunodeficiency by single-strand conformation polymorphism. Hum Genet (2000) 106(1):73–9. doi: 10.1007/s004399900200
17. Kawamura M, McVicar DW, Johnston JA, Blake TB, Chen YQ, Lal BK, et al. Molecular cloning of l-JAK, a janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci USA (1994) 91(14):6374–8. doi: 10.1073/pnas.91.14.6374
18. Eskiizmir G, Seda Vatansever H, Ozgur E, Aslan A, Tanyeri G, Gozuacik D, et al. Jak-stat signaling pathway may play a role in the pathogenesis of cholesteatoma. Am J Otolaryngol (2014) 35(2):130–6. doi: 10.1016/j.amjoto.2013.10.005
19. Tibbles HE, Vassilev A, Wendorf H, Schonhoff D, Zhu D, Lorenz D, et al. Role of a JAK3-dependent biochemical signaling pathway in platelet activation and aggregation. J Biol Chem (2001) 276(21):17815–22. doi: 10.1074/jbc.M011405200
20. Goswami R, Kaplan MH. A brief history of IL-9. J Immunol (2011) 186(6):3283–8. doi: 10.4049/jimmunol.1003049
21. ElKassar N, Gress RE. An overview of IL-7 biology and its use in immunotherapy. J Immunotoxicol (2010) 7(1):1–7. doi: 10.3109/15476910903453296
22. Cacalano NA, Migone TS, Bazan F, Hanson EP, Chen M, Candotti F, et al. Autosomal SCID caused by a point mutation in the n-terminus of JAK3: Mapping of the JAK3-receptor interaction domain. EMBO J (1999) 18(6):1549–58. doi: 10.1093/emboj/18.6.1549
23. Chen M, Cheng A, Candotti F, Zhou YJ, Hymel A, Fasth A, et al. Complex effects of naturally occurring mutations in the JAK3 pseudokinase domain: evidence for interactions between the kinase and pseudokinase domains. Mol Cell Biol (2000) 20(3):947–56. doi: 10.1128/MCB.20.3.947-956.2000
24. Notarangelo LD, Mella P, Jones A, de Saint Basile G, Savoldi G, Cranston T, et al. Mutations in severe combined immune deficiency (SCID) due to JAK3 deficiency. Hum mutat (2001) 18(4):255–63. doi: 10.1002/humu.1188
25. Chen M, Cheng A, Chen YQ, Hymel A, Hanson EP, Kimmel L, et al. The amino terminus of JAK3 is necessary and sufficient for binding to the common gamma chain and confers the ability to transmit interleukin 2-mediated signals. Proc Natl Acad Sci USA (1997) 94(13):6910–5. doi: 10.1073/pnas.94.13.6910
26. Sato T, Okano T, Tanaka-Kubota M, Kimura S, Miyamoto S, Ono S, et al. Novel compound heterozygous mutations in a Japanese girl with janus kinase 3 deficiency. Pediatr Int Off J Japan Pediatr Soc (2016) 58(10):1076–80. doi: 10.1111/ped.13070
27. Poyraz A, Cansever M, Muderris I, Patiroglu T. Neonatal lymphopenia screening is important for early diagnosis of severe combined immunodeficiency. Am J perinatol (2021). doi: 10.1055/s-0041-1731044
29. Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, et al. Mutations of jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature (1995) 377(6544):65–8. doi: 10.1038/377065a0
30. Chen T, Chen X, Zhang S, Zhu J, Tang B, Wang A, et al. The genome sequence archive family: Toward explosive data growth and diverse data types. Genomics Proteomics Bioinf (2021) 19(4):578–83. doi: 10.1016/j.gpb.2021.08.001
Keywords: JAK3 deficiency, severe combined immunodeficiency, Mycobacterium tuberculosis, Pneumocystis jirovecii, case report
Citation: Pan Y, Pan H, Lian C, Wu B, Lin J, Huang G and Cui B (2022) Case Report: Mutations in JAK3 causing severe combined immunodeficiency complicated by disseminated Bacille Calmette–Guérin disease and Pneumocystis pneumonia. Front. Immunol. 13:1055607. doi: 10.3389/fimmu.2022.1055607
Received: 28 September 2022; Accepted: 26 October 2022;
Published: 17 November 2022.
Edited by:
Anders Fasth, University of Gothenburg, SwedenReviewed by:
Matthias Griese, LMU Munich University Hospital, GermanyTri Wibawa, Faculty of Medicine Public Health and Nursing Universitas Gadjah Mada, Indonesia
Copyright © 2022 Pan, Pan, Lian, Wu, Lin, Huang and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binglin Cui, YmluZ2xpbmN1aUAxMzkuY29t
†These authors have contributed equally to this work and share first authorship
 Ying Pan1†
Ying Pan1† Beiyan Wu
Beiyan Wu Binglin Cui
Binglin Cui