
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 11 November 2022
Sec. Molecular Innate Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1054457
This article is part of the Research Topic Innate Immunity: Platelets and their Interaction with other Cellular Elements in Host Defense and Disease Pathogenesis View all 10 articles
Introduction: Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a potentially life-threatening systemic small-vessel vasculitis that is characterized by pauci-immune glomerulonephritis, depicting in turn a major denominator of AAV mortality. It is well established that AAV patients feature an increased risk of developing thrombotic events, and platelets are activated in AAV patients being triggered by the alternative complement pathway. Platelets guard vessels integrity and initiate thrombus formation in response to endothelial damage, further constituting a triangular interconnection with the activation of neutrophils and the complement system. We here aimed to systematically assess the relevance of platelet counts and systemic complement system activation regarding distinct histopathological lesions in ANCA-associated renal vasculitis.
Methods: A cohort of 53 biopsy-proven cases of ANCA-associated renal vasculitis were retrospectively enrolled in a single-center observational study. Univariate and multivariate regression analysis was performed to identify parameters associated with platelet counts in ANCA-associated renal vasculitis compared to disease controls. Finally, the relevance of platelets for disease course and recovery was assessed by survival analysis.
Results: Lower platelet counts correlated with markers of kidney injury including eGFR loss (p=0.0004) and lower complement C3 levels (p=0.0037). Multivariate and subgroup analysis revealed that this association was only present in the subgroup with MPO-ANCA seropositivity (eGFR loss: p=0.0009, lower C3: p=0.0032). While lower platelet counts correlated with kidney injury in the PR3-ANCA subgroup (eGFR loss: p=0.0272), we did not observe an independent association with complement C3 levels (p=0.4497). Independent of any glomerular lesion, lower platelet counts correlated with interstitial fibrosis (p=0.0313), tubular atrophy (p=0.0073), and tubulitis in areas of interstitial fibrosis and tubular atrophy (p=0.0033). Finally, we observed significant differences with increased requirement of kidney replacement therapy (KRT) or death in the subgroup below median platelet counts (HR: 4.1, 95% CI: 1.6-10, p=0.0047), associated with a lower probability of discharge and prolonged hospitalization in this subgroup (HR: 0.5, 95% CI: 0.3-0.9, p=0.0113).
Conclusion: Based on our observation that an association between platelets and complement system activation is only observed in the MPO-ANCA subgroup, this could implicate that platelets and complement C3 link innate immunity to tubulointerstitial injury in the presence of MPO-ANCA autoantibodies.
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a potentially life-threatening systemic small-vessel vasculitis that is characterized by pauci-immune glomerulonephritis in case of kidney involvement, depicting in turn a major denominator of AAV mortality (1–4). Two principal antigens on neutrophils, namely proteinase 3 (PR3) and myeloperoxidase (MPO), provide epitopes for ANCA binding, thus promoting neutrophil activation and neutrophil extracellular traps (NETs) formation (“NETosis”), which consists of the extrusion of lattice-like chromatin fibers harboring cytokines and antimicrobial proteins that contribute to host defense under physiological conditions and promote endothelial damage and vascular inflammation culminating in necrotizing vasculitis in the context of autoimmunity (5–7). ANCA-stimulated neutrophils have been shown to induce the formation of NETs, which contain the ANCA target antigens MPO and PR3 (8). In ANCA-associated renal vasculitis, NETs are located in close proximity to neutrophil infiltrates in affected glomeruli and tubulointerstitium (8). NETs are capable to activate the classical complement pathway due to the interaction with C1q (9). Neutrophils itself contain various components of the alternative but not the classical complement pathway (10). Particularly in AAV with activated neutrophils by ANCA autoantibodies, the alternative dominates over the classical complement pathway (11). Therefore, the involvement of the alternative pathway of the complement system captures a pathophysiological key role in NETosis and more generally in AAV, which is impressively constrained by the efficacy of avacopan, a novel drug targeting the C5a receptor (12–14). As part of innate immunity, the complement system is composed of approximately more than thirty serum proteins, whose pathways are either classical-, alternative- or lectin-categorized (15, 16). Depending on different induction modes, a self-reinforcing domino-effect-like cascade is initiated that disembarks in the common formation of the so-called membrane attack complex (MAC), wherein also inhibitory regulatory mechanisms are interposed, such as factor H suppressing the activation of the alternative pathway by preventing C3b opsonization, which in turn was shown to affect NETosis (17, 18).
Being influenced by both components, complement system activation and neutrophils/NETosis, the coagulation system is another relevant pathophysiological factor acting on the injured endothelium as the primary side of inflammation (18, 19). It is well established that AAV patients feature a two- to three-fold increased risk of developing thrombotic events, and platelets are activated in AAV patients being triggered by the alternative complement pathway (20–26). Platelets guard vessels integrity and initiate thrombus formation in response to endothelial damage, further constituting a triangular interconnection with the activation of neutrophils, formation of NETs and the complement system (26–28). Although ANCA autoantibody activity is an established inducer of NETs, its presence is not always consistent with disease activity (29). Regarding additional mechanisms of neutrophil activation, recent reports suggested that platelets itself can also regulate formation of NETs in AAV (30). However, the implication of platelets regarding vasculitis manifestation and complement system activation in ANCA-associated renal vasculitis has not been described yet. Therefore, we here aimed to systematically assess the relevance of platelet counts and systemic complement system activation regarding distinct histopathological lesions in ANCA-associated renal vasculitis.
A well characterized cohort of 53 biopsy-proven cases of ANCA-associated renal vasculitis were retrospectively enrolled between 2015 till 2020 in a single-center observational study at the University Medical Center Göttingen, Göttingen, Germany (Supplementary Table 1) (31–35). In addition, 18 cases with IgA nephropathy, 20 with diabetic kidney disease, and 27 with acute interstitial nephritis were included as disease controls (Supplementary Table 2). While no formal approval was required for the use of routine clinical data, a favorable ethical opinion was granted by the local Ethics committee (no. 22/2/14 and 28/09/17). All participants provided their written informed consent for the utilization of routinely collected data for research purposes as part of their regular medical care. Medical records were used to collect data on age, sex, medication, comorbidities, laboratory findings at admission (creatinine, estimated glomerular filtration rate/eGFR, blood urea nitrogen/BUN, potassium, albumin, aspartate-amino transferase/AST, alkaline phosphatase/AP, gamma glutamyl transferase/γGT, bilirubin, complement C3 and C4), at the time of kidney biopsy (platelets, hemoglobin, white blood cells/WBC, C-reactive protein/CRP), dates of admission and discharge from hospital. The Birmingham Vasculitis Activity Score (BVAS) was assessed as previously described (36).
Renal pathologists evaluated all kidney biopsies and was blinded to clinical data analysis. Based on the current version of the Banff scoring system for renal allograft pathology, tubulointerstitial lesions were scored as previously reported: arteriolar hyalinosis (ah), arteritis (v), glomerulitis (g), inflammation in areas of interstitial fibrosis and tubular atrophy (i-IFTA), interstitial fibrosis (ci), interstitial inflammation (i), peritubular capillaritis (ptc), total inflammation (ti), tubular atrophy (ct), tubulitis (t), and tubulitis in areas of interstitial fibrosis and tubular atrophy (t-IFTA) (37, 38). Tubular injury lesions were systematically assessed as recently described (39). Briefly, tubular dilation, tubular necrosis, epithelial simplification, non-isometric cell vacuolization, red blood cell (RBC) and necrotic casts were scored with a range from 0 to 4 depending on the fraction of affected cortical area of renal biopsy (score 0: <1%, 1: ≥1-10%, 2: ≥10-25%, 3: ≥25-50%, 4: >50%). Moreover, all injured glomeruli (crescentic or/and necrotic) were screened for the presence of a Bowman’s capsule rupture, whose extent was further quantified as previously described (40–42).
Steroids were administered either as intravenous pulse therapy or orally with a tapering schedule. At time of platelet measurement and kidney biopsy, all patients received steroids and further remission induction therapy was initiated thereafter based on histopathological confirmation of ANCA GN. Plasma exchange (PEX) was administered during the induction period at the discretion of treating physicians. Rituximab (RTX) was administered as four intravenous doses at 375 mg/m2 every week; RTX was not administered within 48 hours before PEX treatment. Cyclophosphamide (CYC) was administered as three intravenous doses up to 15 mg/kg every 2 weeks and every 3 weeks thereafter, adjusted for age and renal function. Combination therapy was administered as four intravenous doses at 375 mg/m2 RTX every week and two intravenous doses at 15 mg/kg CYC every 2 weeks. On the discretion of treating physicians, choice of remission induction therapy was dependent on previous regimens and individual patients, more likely to choose RTX in younger patients with toxicity being the main reason for this choice (Supplementary Table 1) (43). Prophylaxis to prevent pneumocystis (carinii) jiroveci infection was administered according to local practice.
Normally distributed values are presented as mean ± standard deviation (SD), while non-normally distributed parameters are shown as median and interquartile range (IQR). Normal distribution was evaluated by Shapiro-Wilk testing. Categorical variables as percentages of total. Statistical comparisons were not formally powered or prespecified. Probability values (p value) below 0.05 were considered statistically significant. For normally distributed values, mean comparisons were performed with unpaired student’s t-test, while estimation plots were used for data visualization. For non-normally distributed values, median comparisons were performed with the Mann-Whitney-U-test. Heatmaps reflect the mean values of Spearman’s ρ in the univariate linear regression analysis, circle size represents significance level. Survival-curve analyses were performed using the Kaplan-Meier method, wherein log rank (Mantel-Cox) testing was conducted for curve comparison. Time-to-event was registered and discharge from hospital was defined as event. Results are shown as hazard ratio (HR) and 95% confidence interval (CI). For stepwise multiple linear regression, covariates were retained to significant differences in the linear regression model to avoid model over-fit. Data analyses were performed with GraphPad Prism (version 9.4 for MacOS, GraphPad Software, San Diego, California, USA) and IBM SPSS Statistics (version 28 for MacOS, IBM Corporation, Armonk, NY, USA).
The study conduction is summarized in Supplementary Figure 1, the baseline characteristics of the total cohort are shown in Supplementary Table 1. Median (IQR) platelet counts were 300,000/µL (207,000-438,000/µL), therefore most patients were within or above the normal range (150,000-350,000/µL, Figure 1A). Univariate analysis revealed that lower platelet counts correlated with markers of kidney injury, including serum creatinine levels (ρ=-0.51, p<0.0001), eGFR loss (ρ=0.47, p=0.0004), and BUN (ρ=-0.44, p=0.0025, Figure 1B). Furthermore, lower platelet counts were also associated with reduced levels of serum albumin (ρ=0.59, p=0.0020) and complement C3 (ρ=0.46, p=0.0037, Figure 1B). By contrast, we observed no association between platelet counts and ANCA autoantibody levels, coagulation parameters, other markers of systemic inflammation, or parameters indicative for liver injury (Figure 1B). Multivariate analysis confirmed an independent association between platelet counts, kidney injury (eGFR loss: p=0.0005) and lower levels of complement C3 (p=0.0416) in the total cohort of ANCA-associated renal vasculitis (Table 1). Moreover, BUN itself was not the main denominator associated with lower platelet counts (p=0.4011, Table 1), implicating distinct mechanisms independent of uremia in ANCA-associated renal vasculitis. This association was equally detectable in the subgroup with MPO-ANCA seropositivity (eGFR loss: p=0.0009, lower complement C3: p=0.0032, Table 1). While lower platelet counts correlated with kidney injury in renal vasculitis with PR3-ANCA seropositivity (eGFR loss: p=0.0272), we did not observe an independent association with complement C3 levels in this subgroup (p=0.4497, Table 1). To validate that these findings are specific for ANCA-associated renal vasculitis, we next analyzed platelet counts, markers of kidney injury and complement levels in disease controls including IgA nephropathy, diabetic kidney disease, and acute interstitial nephritis (Supplementary Table 2). We did not observe any significant association between platelet counts and serum creatinine levels (ρ=-0.23, p>0.05), eGFR (ρ=0.24, p>0.05), complement C3 (ρ=0.07, p>0.05) or C4 (ρ=0.06, p>0.05, Supplementary Figure 2). In summary, lower platelet counts associated with kidney injury in ANCA-associated renal vasculitis. Furthermore, lower platelet counts were independently correlated with lower complement C3 levels specifically in patients with MPO-ANCA seropositivity that was not confirmed in the PR3-ANCA subgroup. Finally, there was no association with unspecific markers of systemic inflammation in ANCA-associated renal vasculitis.
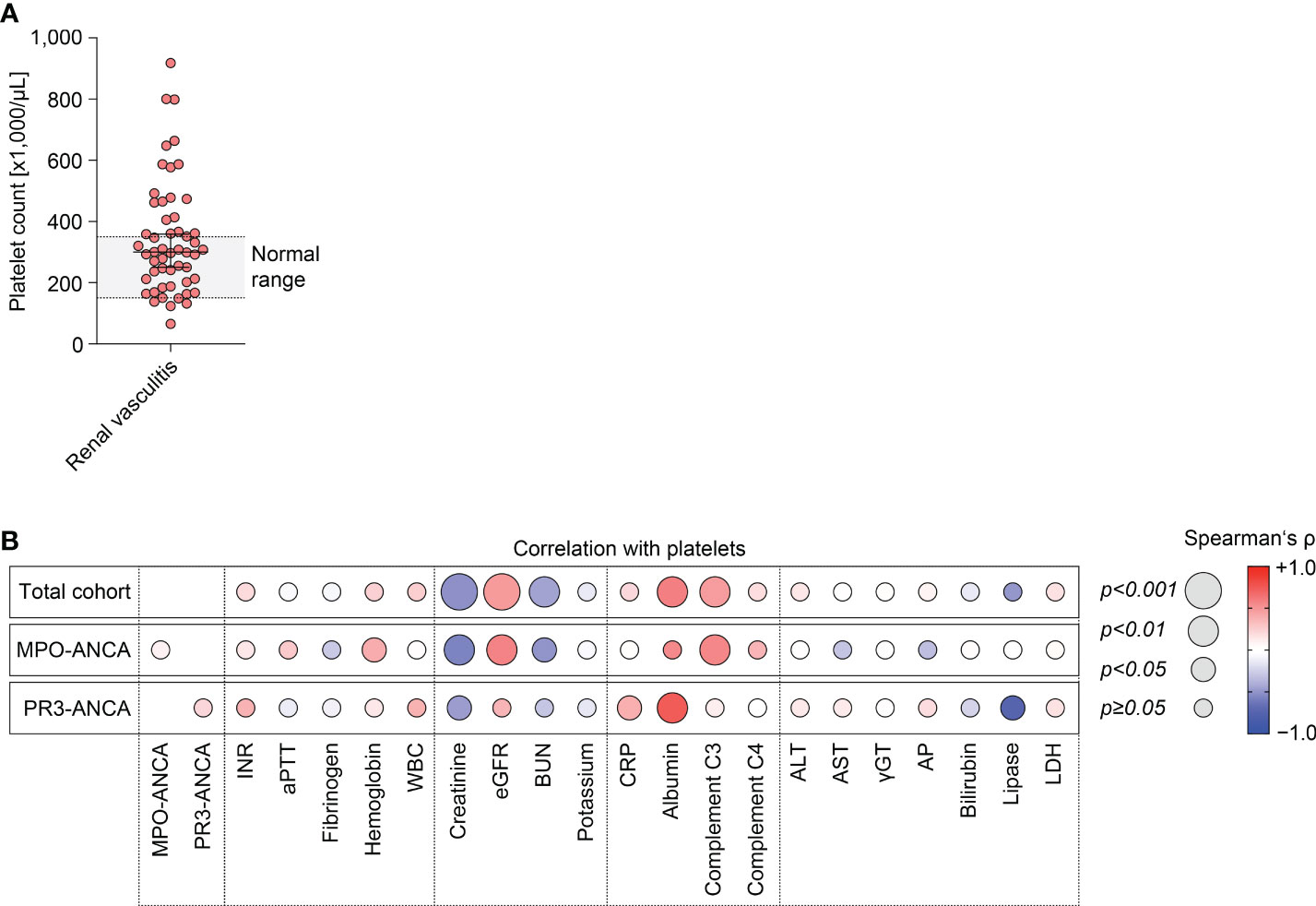
Figure 1 Lower platelet counts associate with kidney injury and lower complement C3 levels in ANCA-associated renal vasculitis. (A) Distribution of platelet counts in the total cohort of ANCA-associated renal vasculitis (normal range: 150,000-350,000/µL). (B) Correlations between platelet counts and laboratory parameters in ANCA-associated renal vasculitis are shown by heatmap reflecting mean values of Spearman’s ρ, circle size represents significance level. ALT, alanine aminotransferase; ANCA, antineutrophil cytoplasmic antibody; AP, alkaline phosphatase; aPTT, activated partial thromboplastin time; AST, aspartate amino transferase; BUN, blood urea nitrogen; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate (CKD-EPI); INR, international normalized ratio; LDH, lactate dehydrogenase; MPO, myeloperoxidase; PR3, proteinase 3; WBC, white blood cells; γGT, gamma glutamyl transferase.
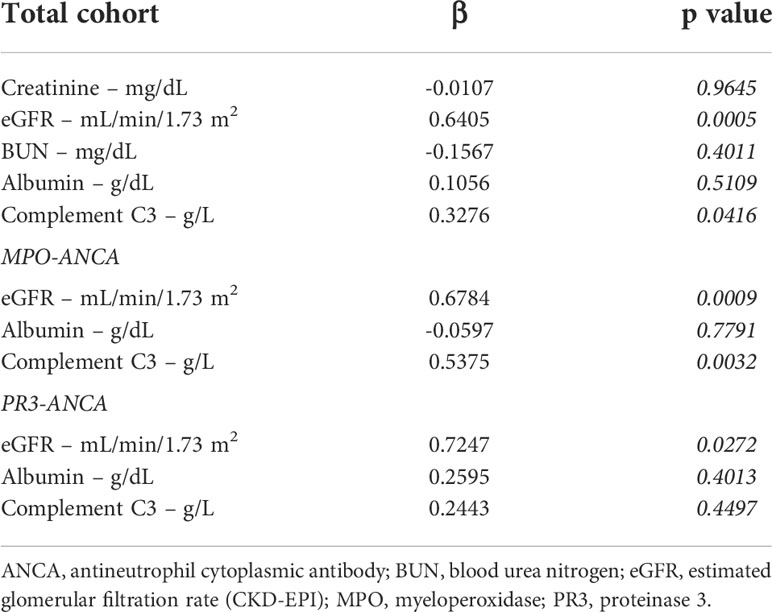
Table 1 Stepwise multiple linear regression analyses with platelet counts as the dependent variable.
Because we have observed an association between lower platelet counts and laboratory markers of kidney injury, we next analyzed the association between platelet counts and histopathological lesions in ANCA-associated renal vasculitis. Interestingly, there was no association between platelet counts and any glomerular lesion in ANCA-associated renal vasculitis (Figure 2). By contrast, lower platelet counts correlated with interstitial fibrosis (ci, p=0.0313), tubular atrophy (ct, p=0.0073), and tubulitis in areas of interstitial fibrosis and tubular atrophy (t-IFTA, p=0.0033, Figure 2). Detailed morphological analysis of tubular injury indicated that lower platelet counts correlated specifically with tubular dilatation in renal vasculitis with MPO-ANCA seropositivity (p=0.0347), while an association with cellular casts was observed in the PR3-ANCA subgroup (p=0.0352, Figure 2). By contrast, we did not observe any association with inflammatory lesions including intrarenal immune cell infiltration (Figure 2). In summary, lower platelet counts correlated specifically with tubulointerstitial injury in ANCA-associated renal vasculitis. Moreover, we again observed differences between ANCA subtypes regarding distinct tubular injury patterns.
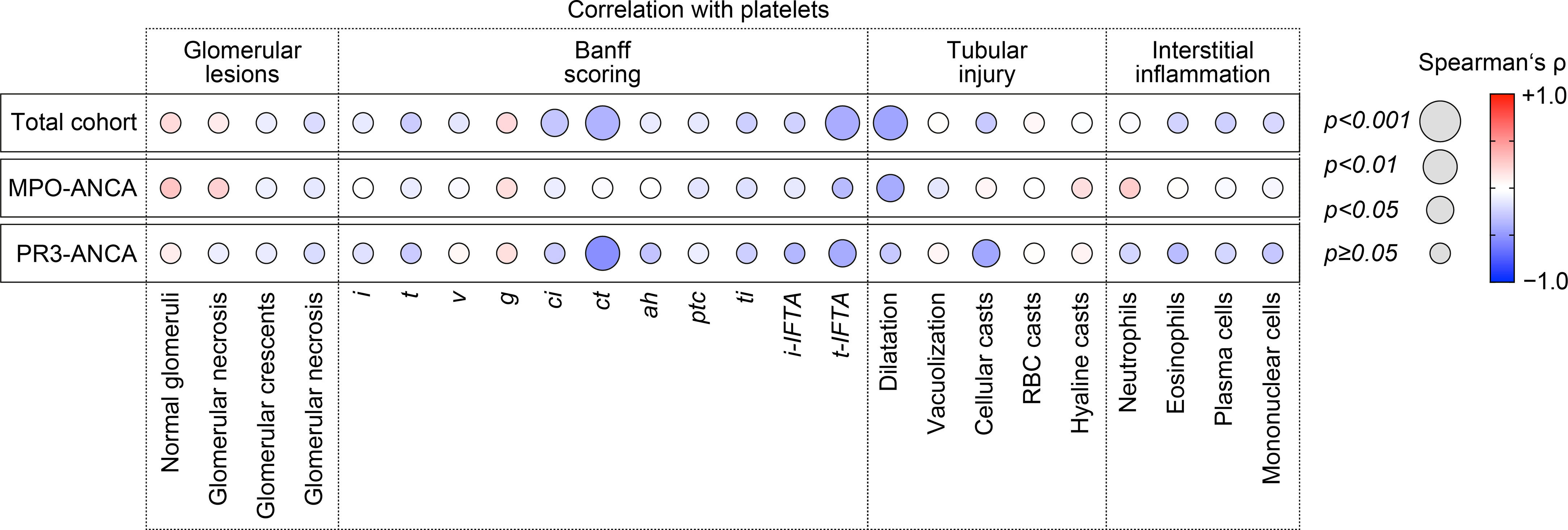
Figure 2 Lower platelet counts associate specifically with tubulointerstitial injury in ANCA-associated renal vasculitis. Correlations between platelet counts and histopathological lesions in ANCA-associated renal vasculitis are shown by heatmap reflecting mean values of Spearman’s ρ, circle size represents significance level. ah, arteriolar hyalinosis; ANCA, antineutrophil cytoplasmic antibody; ci, interstitial fibrosis; ct, tubular atrophy; g, glomerulitis; i, interstitial inflammation; i-IFTA, inflammation in areas of interstitial fibrosis and tubular atrophy; MPO, myeloperoxidase; PR3, proteinase 3; ptc, peritubular capillaritis; RBC, red blood cell; t, tubulitis; ti, total inflammation; t-IFTA, tubulitis in areas of interstitial fibrosis and tubular atrophy; v, intimal arteritis.
Finally, we performed cohort dichotomization by using median platelet counts (300,000/µL) to separate groups for survival-curve analyses using the Kaplan-Meier method and log-rank testing (Figure 3A). As previously observed, group separation resulted in significant differences were again observed for markers of kidney injury, including serum creatinine levels (p=0.0007), eGFR (p=0.0029), and BUN (p=0.0061), and complement C3 levels (p=0.0313, Table 2). This also correlated with disease course reflected by significant differences in requirement of kidney replacement therapy (KRT) or death in the subgroup below median platelet counts (HR: 4.1, 95% CI: 1.6-10, p=0.0047, Figure 3B). Furthermore, we observed a lower probability of discharge with prolonged hospitalization in this subgroup (HR: 0.5, 95% CI: 0.3-0.9, p=0.0113, Figure 3C), indicating disease course and recovery in ANCA-associated renal vasculitis differed according to platelet counts.
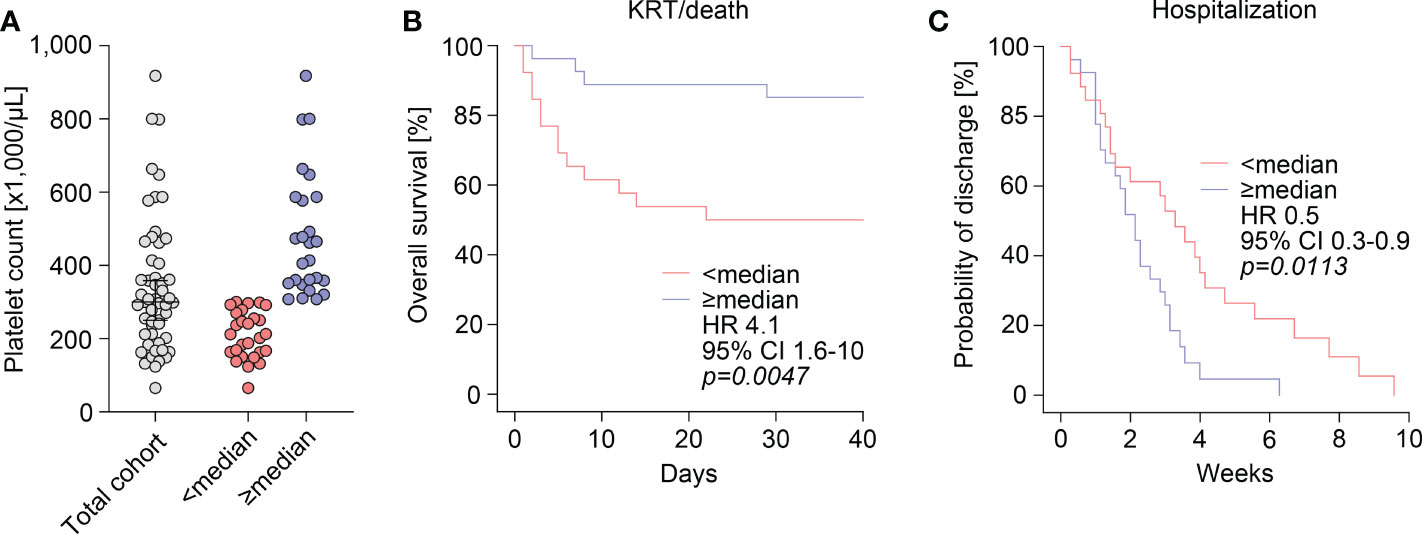
Figure 3 Disease course and recovery in ANCA-associated renal vasculitis differs according to platelet counts. (A) The scatter dot plots show platelet counts with median ± IQR, group separation was performed according to median platelet count (300,000/µL). (B) Overall survival (KRT/death) within 40 days after admission according to group separation by median platelet count (300,000/µL). Comparison of survival curves was performed with log rank (Mantel-Cox) testing. (C) Probability of discharge from hospital after admission according to group separation by median platelet count (300,000/µL). Comparison of survival curves was performed with log rank (Mantel-Cox) testing. ANCA, antineutrophil cytoplasmic antibody; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; KRT, kidney replacement therapy.
Innate immunity refers to non-specific recognition of exogenous or endogenous components, leading to an activation of the immune system, and resulting in clearance of these molecules. Megakaryocyte-derived platelets are a key component of the blood, playing a critical role in immune responses by secretion of active mediators. The ability of enucleated platelets to crosstalk with immune cells contributing to innate immune responses is attributed to the expression of membrane proteins, storage granules, and a variety of megakaryocyte-derived molecules. Platelets are a key component of innate immunity that trap immunogenic molecules by necessitating the process of clotting, preventing them from being accessible and to disseminate. Moreover, it is also well established that the platelets are the primary mediators of hemostasis and thrombosis. Therefore, it is increasingly recognized that a hypercoagulable state is persistently active in patients with ANCA-associated renal vasculitis, and studies investigating the impact of tailored anticoagulation are needed to reduce the burden of thromboembolism (24).
We here observed that most patients with active ANCA-associated renal vasculitis had platelet counts within or above the normal range. This is in line with previous reports that platelet counts were significantly higher in patients in active disease stage compared with those in remission (44). Within our cohort of active ANCA-associated renal vasculitis, we observed an association between lower platelet counts and kidney injury, disease course and recovery in ANCA-associated renal vasculitis. These results implicate that platelets in active ANCA-associated renal vasculitis result from a reactive increase, and that a consumptive decrease may indicate accelerated kidney injury. Interestingly, this concept is confirmatory to previous reports that hypercoagulability contributes to kidney injury in vascular diseases, including ANCA-associated renal vasculitis (45–48). In addition, we here observed that lower platelet counts associated specifically with tubulointerstitial injury in ANCA-associated renal vasculitis independent of any glomerular lesion. In this context, experimental studies have already shown that platelets are main contributors to microvascular injury and rarefaction of tubulointerstitial capillaries (49). In ANCA-associated renal vasculitis, the occurrence of tubulointerstitial injury independent of glomerular lesions has already been described (50–53). Our observations might indicate that the tubulointerstitial compartment is particularly susceptible to a hypercoagulable state mediated by platelets. The concept that loss of kidney function is more affected by tubulointerstitial rather than glomerular injury has been described more than 50 years ago in glomerulonephritis (54). In response to tubular damage, different cell death modes (namely apoptosis, necroptosis, and ferroptosis) feature different degrees of immunogenicity, wherein the two latter-mentioned constitute different pathways that are implied in acute kidney injury (AKI), transition from AKI to CKD, sepsis-associated AKI, but also autoimmune vasculitis affecting the kidneys (55).
Moreover, lower platelet counts associated with complement system activation reflected by lower serum C3 levels in ANCA-associated renal vasculitis. Complement system activation with reduced complement C3 levels and intrarenal complement deposits are important denominators of poor outcome in ANCA-associated renal vasculitis (56–60). Our observation that an association between platelets, kidney injury and complement C3 is not present in disease controls (IgA nephropathy, diabetic kidney disease, and acute interstitial nephritis) and specifically observed in the MPO-ANCA subgroup implicates distinct pathomechanisms dependent on seropositivity for MPO-ANCA or PR3-ANCA. This is especially relevant since two complement system inhibitors are currently in clinical development for AAV. Thus, our results may contribute to a more personalized treatment approach of AAV depending on the ANCA serotype and the relevance of complement system activation.
Finally, we here provide evidence that platelets and complement system activation is especially relevant in the subgroup with MPO-ANCA seropositivity. Detailed morphological analysis of tubular injury indicated that lower platelet counts correlated specifically with tubular dilatation in renal vasculitis with MPO-ANCA seropositivity, while an association with cellular casts was observed in the PR3-ANCA subgroup. Although tubular dilatation and cellular casts are both observed in injured kidneys indicating tubular injury and cell death, the association with either ANCA subtype again could also implicate distinct pathomechanisms of tubulointerstitial injury dependent on seropositivity for MPO-ANCA or PR3-ANCA (61, 62). Regarding potential mechanisms, it has been suggested that MPO can directly interact and induce priming of platelets that can contribute to the development of vascular inflammation (63). Therefore, binding of MPO-ANCA autoantibodies could further aggravate platelet activation and vascular injury. These results require confirmation but may contribute to a personalized treatment approach of AAV.
Our study has several limitations, such as the small patient number and the retrospective study design. Furthermore, our observations are associative and do not proof causality requiring mechanistic studies that also include platelet activation and function. Nevertheless, we provide a clinically approached link between platelets, complement system activation and tubulointerstitial injury patterns specifically in MPO-ANCA-associated renal vasculitis, thus broadening our current pathophysiological understanding.
Based on our observation that an association between platelets and complement system activation is only observed in the MPO-ANCA subgroup, this could implicate that platelets and complement C3 link innate immunity to tubulointerstitial injury in the presence of MPO-ANCA autoantibodies. These results require confirmation but may contribute to a personalized treatment approach of AAV.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the local Ethics committee (no. 22/2/14 and 28/09/17). The patients/participants provided their written informed consent to participate in this study.
BT conceived the study and edited the manuscript. EB collected and analyzed data, and wrote the first draft. DT collected and analyzed data, IK and SH evaluated kidney biopsies. All authors contributed to the article and approved the submitted version.
This study was supported by the Else-Kröner research program entitled “molecular therapy and prediction of gastrointestinal malignancies”, grant number 7-67-1840876. We also acknowledge support by the Open Access Publication Funds of the Göttingen University. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author SH was employed by company SYNLAB Pathology Hannover, SYNLAB Holding Germany.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1054457/full#supplementary-material
1. Jennette JC, Nachman PH. Anca glomerulonephritis and vasculitis. Clin J Am Soc Nephrol (2017) 12(10):1680–91. doi: 10.2215/CJN.02500317
2. Lee T, Gasim A, Derebail VK, Chung Y, McGregor JG, Lionaki S, et al. Predictors of treatment outcomes in anca-associated vasculitis with severe kidney failure. Clin J Am Soc Nephrol (2014) 9(5):905–13. doi: 10.2215/CJN.08290813
3. Jennette JC, Wilkman AS, Falk RJ. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol (1989) 135(5):921–30.
4. Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. Long-term patient survival in anca-associated vasculitis. Ann Rheum Dis (2011) 70(3):488–94. doi: 10.1136/ard.2010.137778
5. Lee KH, Kronbichler A, Park DD, Park Y, Moon H, Kim H, et al. Neutrophil extracellular traps (Nets) in autoimmune diseases: A comprehensive review. Autoimmun Rev (2017) 16(11):1160–73. doi: 10.1016/j.autrev.2017.09.012
6. Berthelot JM, Le Goff B, Neel A, Maugars Y, Hamidou M. Netosis: At the crossroads of rheumatoid arthritis, lupus, and vasculitis. Joint Bone Spine (2017) 84(3):255–62. doi: 10.1016/j.jbspin.2016.05.013
7. Frangou E, Vassilopoulos D, Boletis J, Boumpas DT. An emerging role of neutrophils and netosis in chronic inflammation and fibrosis in systemic lupus erythematosus (Sle) and anca-associated vasculitides (Aav): Implications for the pathogenesis and treatment. Autoimmun Rev (2019) 18(8):751–60. doi: 10.1016/j.autrev.2019.06.011
8. Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med (2009) 15(6):623–5. doi: 10.1038/nm.1959
9. Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol (2012) 188(7):3522–31. doi: 10.4049/jimmunol.1102404
10. Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun (2010) 34(3):J276–86. doi: 10.1016/j.jaut.2009.11.014
11. Van Timmeren MM, Chen M, Heeringa P. Review article: Pathogenic role of complement activation in anti-neutrophil cytoplasmic auto-Antibody-Associated vasculitis. Nephrol (Carlton) (2009) 14(1):16–25. doi: 10.1111/j.1440-1797.2009.01086.x
12. Jayne D. Complement inhibition in anca vasculitis. Nephrol Ther (2019) 15(6):409–12. doi: 10.1016/j.nephro.2019.04.001
13. Andrighetto S, Leventhal J, Zaza G, Cravedi P. Complement and complement targeting therapies in glomerular diseases. Int J Mol Sci (2019) 20(24):6336. doi: 10.3390/ijms20246336
14. Chen M, Jayne DRW, Zhao MH. Complement in anca-associated vasculitis: Mechanisms and implications for management. Nat Rev Nephrol (2017) 13(6):359–67. doi: 10.1038/nrneph.2017.37
15. Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I - molecular mechanisms of activation and regulation. Front Immunol (2015) 6:262. doi: 10.3389/fimmu.2015.00262
16. Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement system part ii: Role in immunity. Front Immunol (2015) 6:257. doi: 10.3389/fimmu.2015.00257
17. Moore SR, Menon SS, Cortes C, Ferreira VP. Hijacking factor h for complement immune evasion. Front Immunol (2021) 12:602277. doi: 10.3389/fimmu.2021.602277
18. de Bont CM, Boelens WC, Pruijn GJM. Netosis, complement, and coagulation: A triangular relationship. Cell Mol Immunol (2019) 16(1):19–27. doi: 10.1038/s41423-018-0024-0
19. Maugeri N, Rovere-Querini P, Baldini M, Sabbadini MG, Manfredi AA. Translational mini-review series on immunology of vascular disease: Mechanisms of vascular inflammation and remodelling in systemic vasculitis. Clin Exp Immunol (2009) 156(3):395–404. doi: 10.1111/j.1365-2249.2009.03921.x
20. Miao D, Li DY, Chen M, Zhao MH. Platelets are activated in anca-associated vasculitis Via thrombin-pars pathway and can activate the alternative complement pathway. Arthritis Res Ther (2017) 19(1):252. doi: 10.1186/s13075-017-1458-y
21. Nygaard L, Polcwiartek C, Nelveg-Kristensen KE, Carlson N, Kristensen S, Torp-Pedersen C, et al. Long-term cardiovascular outcomes and temporal trends in patients diagnosed with anca-associated vasculitis: A Danish nationwide registry study. Rheumatol (Oxford) (2022). doi: 10.1093/rheumatology/keac386
22. Kronbichler A, Leierer J, Leierer G, Mayer G, Casian A, Hoglund P, et al. Clinical associations with venous thromboembolism in anti-neutrophil cytoplasm antibody-associated vasculitides. Rheumatol (Oxford) (2017) 56(5):704–8. doi: 10.1093/rheumatology/kew465
23. Kronbichler A, Leierer J, Shin JI, Merkel PA, Spiera R, Seo P, et al. Association of pulmonary hemorrhage, positive proteinase 3, and urinary red blood cell casts with venous thromboembolism in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol (2019) 71(11):1888–93. doi: 10.1002/art.41017
24. Kronbichler A, Leierer J, Gauckler P, Shin JI. Comorbidities in anca-associated vasculitis. Rheumatol (Oxford) (2020) 59(Suppl 3):iii79–83. doi: 10.1093/rheumatology/kez617
25. Moiseev S, Kronbichler A, Makarov E, Bulanov N, Crnogorac M, Direskeneli H, et al. Association of venous thromboembolic events with skin, pulmonary and kidney involvement in anca-associated vasculitis: A multinational study. Rheumatol (Oxford) (2021) 60(10):4654–61. doi: 10.1093/rheumatology/keab071
26. Misra DP, Thomas KN, Gasparyan AY, Zimba O. Mechanisms of thrombosis in anca-associated vasculitis. Clin Rheumatol (2021) 40(12):4807–15. doi: 10.1007/s10067-021-05790-9
27. Hamad OA, Back J, Nilsson PH, Nilsson B, Ekdahl KN. Platelets, complement, and contact activation: Partners in inflammation and thrombosis. Adv Exp Med Biol (2012) 946:185–205. doi: 10.1007/978-1-4614-0106-3_11
28. Mezger M, Nording H, Sauter R, Graf T, Heim C, von Bubnoff N, et al. Platelets and immune responses during thromboinflammation. Front Immunol (2019) 10:1731. doi: 10.3389/fimmu.2019.01731
29. Kraaij T, Kamerling SWA, van Dam LS, Bakker JA, Bajema IM, Page T, et al. Excessive neutrophil extracellular trap formation in anca-associated vasculitis is independent of anca. Kidney Int (2018) 94(1):139–49. doi: 10.1016/j.kint.2018.01.013
30. Matsumoto K, Yasuoka H, Yoshimoto K, Suzuki K, Takeuchi T. Platelet Cxcl4 mediates neutrophil extracellular traps formation in anca-associated vasculitis. Sci Rep (2021) 11(1):222. doi: 10.1038/s41598-020-80685-4
31. Hakroush S, Kluge IA, Strobel P, Korsten P, Tampe D, Tampe B. Systematic histological scoring reveals more prominent interstitial inflammation in myeloperoxidase-anca compared to proteinase 3-anca glomerulonephritis. J Clin Med (2021) 10(6):1231. doi: 10.3390/jcm10061231
32. Hakroush S, Tampe D, Korsten P, Strobel P, Tampe B. Complement components C3 and C4 indicate vasculitis manifestations to distinct renal compartments in anca-associated glomerulonephritis. Int J Mol Sci (2021) 22(12):6588. doi: 10.3390/ijms22126588
33. Tampe D, Strobel P, Korsten P, Hakroush S, Tampe B. Consideration of therapeutic plasma exchange in association with inflammatory lesions in anca-associated glomerulonephritis: A real-world retrospective study from a single center. Front Immunol (2021) 12:645483. doi: 10.3389/fimmu.2021.645483
34. Tampe D, Korsten P, Strobel P, Hakroush S, Tampe B. Comprehensive analysis of sex differences at disease manifestation in anca-associated glomerulonephritis. Front Immunol (2021) 12:736638. doi: 10.3389/fimmu.2021.736638
35. Hakroush S, Tampe D, Strobel P, Korsten P, Tampe B. Comparative histological subtyping of immune cell infiltrates in mpo-anca and Pr3-anca glomerulonephritis. Front Immunol (2021) 12:737708. doi: 10.3389/fimmu.2021.737708
36. Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham vasculitis activity score (Version 3). Ann Rheum Dis (2009) 68(12):1827–32. doi: 10.1136/ard.2008.101279
37. Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, et al. A 2018 reference guide to the banff classification of renal allograft pathology. Transplantation (2018) 102(11):1795–814. doi: 10.1097/TP.0000000000002366
38. Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, et al. The banff 2019 kidney meeting report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant (2020) 20(9):2318–31. doi: 10.1111/ajt.15898
39. Hakroush S, Tampe D, Korsten P, Strobel P, Tampe B. Systematic scoring of tubular injury patterns reveals interplay between distinct tubular and glomerular lesions in anca-associated glomerulonephritis. J Clin Med (2021) 10(12):2682. doi: 10.3390/jcm10122682
40. Hakroush S, Tampe D, Korsten P, Strobel P, Tampe B. Bowman’s capsule rupture links glomerular damage to tubulointerstitial inflammation in anca-associated glomerulonephritis. Clin Exp Rheumatol (2021) 39 Suppl 129(2):27–31. doi: 10.55563/clinexprheumatol/7eol6d
41. Hakroush S, Tampe B. Neutrophils associate with bowman’s capsule rupture specifically in Pr3-anca glomerulonephritis. J Nephrol (2022) 35(4):1177–83. doi: 10.1007/s40620-021-01208-6
42. Hakroush S, Tampe B. Correspondence on ‘Bowman’s capsule rupture on renal biopsy improves the outcome prediction of anca-associated glomerulonephritis classifications’. Ann Rheum Dis (2021). doi: 10.1136/annrheumdis-2021-219970
43. Forbess LJ, Griffin KW, Spiera RF. Practice patterns of anca-associated vasculitis: Exploring differences among subspecialties at a single academic medical centre. Clin Exp Rheumatol (2014) 32(3 Suppl 82):S48–50.
44. Ma TT, Huang YM, Wang C, Zhao MH, Chen M. Coagulation and fibrinolysis index profile in patients with anca-associated vasculitis. PLoS One (2014) 9(5):e97843. doi: 10.1371/journal.pone.0097843
45. Sebastian JK, Voetsch B, Stone JH, Romay-Penabad Z, Lo GH, Allen NB, et al. The frequency of anticardiolipin antibodies and genetic mutations associated with hypercoagulability among patients with wegener’s granulomatosis with and without history of a thrombotic event. J Rheumatol (2007) 34(12):2446–50.
46. Berden AE, Nolan SL, Morris HL, Bertina RM, Erasmus DD, Hagen EC, et al. Anti-plasminogen antibodies compromise fibrinolysis and associate with renal histology in anca-associated vasculitis. J Am Soc Nephrol (2010) 21(12):2169–79. doi: 10.1681/ASN.2010030274
47. Hertig A, Rondeau E. Role of the Coagulation/Fibrinolysis system in fibrin-associated glomerular injury. J Am Soc Nephrol (2004) 15(4):844–53. doi: 10.1097/01.asn.0000115400.52705.83
48. Kumar SV, Kulkarni OP, Mulay SR, Darisipudi MN, Romoli S, Thomasova D, et al. Neutrophil extracellular trap-related extracellular histones cause vascular necrosis in severe gn. J Am Soc Nephrol (2015) 26(10):2399–413. doi: 10.1681/ASN.2014070673
49. Schwarzenberger C, Sradnick J, Lerea KM, Goligorsky MS, Nieswandt B, Hugo CP, et al. Platelets are relevant mediators of renal injury induced by primary endothelial lesions. Am J Physiol Renal Physiol (2015) 308(11):F1238–46. doi: 10.1152/ajprenal.00535.2014
50. Plafkin C, Zhong W, Singh T. Anca vasculitis presenting with acute interstitial nephritis without glomerular involvement. Clin Nephrol Case Stud (2019) 7:46–50. doi: 10.5414/CNCS109805
51. Kasahara H, Hiroyuki N, Shinohara M, Koike T. Ap-vas 2012 case report: An atypical case of microscopic polyangiitis presenting with acute tubulointerstitial nephritis without glomerular change. CEN Case Rep (2014) 3(1):1–4. doi: 10.1007/s13730-013-0103-0
52. Nakabayashi K, Sumiishi A, Sano K, Fujioka Y, Yamada A, Karube M, et al. Tubulointerstitial nephritis without glomerular lesions in three patients with myeloperoxidase-Anca-Associated vasculitis. Clin Exp Nephrol (2009) 13(6):605–13. doi: 10.1007/s10157-009-0200-8
53. Morimoto K, Kanzaki G, Niikura T, Koike K, Matsuo N, Maruyama Y, et al. Acute tubulointerstitial nephritis associated with antineutrophil cytoplasmic antibody following cimetidine treatment: A case report. BMC Nephrol (2021) 22(1):294. doi: 10.1186/s12882-021-02502-y
54. Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet (1968) 2(7564):363–6. doi: 10.1016/s0140-6736(68)90589-8
55. Maremonti F, Meyer C, Linkermann A. Mechanisms and models of kidney tubular necrosis and nephron loss. J Am Soc Nephrol (2022) 33(3):472–86. doi: 10.1681/ASN.2021101293
56. Augusto JF, Langs V, Demiselle J, Lavigne C, Brilland B, Duveau A, et al. Low serum complement C3 levels at diagnosis of renal anca-associated vasculitis is associated with poor prognosis. PLoS One (2016) 11(7):e0158871. doi: 10.1371/journal.pone.0158871
57. Lionaki S, Marinaki S, Liapis G, Kalaitzakis E, Fragkioudaki S, Kalogeropoulos P, et al. Hypocomplementemia at diagnosis of pauci-immune glomerulonephritis is associated with advanced histopathological activity index and high probability of treatment resistance. Kidney Int Rep (2021) 6(9):2425–35. doi: 10.1016/j.ekir.2021.05.043
58. Tampe D, Baier E, Hakroush S, Tampe B. Low serum levels of complement C3c at diagnosis indicate poor outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis. Kidney Int Rep (2022) 7(3):660–1. doi: 10.1016/j.ekir.2021.12.038
59. Tampe D, Baier E, Hakroush S, Tampe B. Comparative analysis of complement C3 and C4 serum levels for outcome prediction in anca-associated renal vasculitis. J Nephrol (2022). doi: 10.1007/s40620-022-01414-w
60. Hakroush S, Tampe D, Baier E, Kluge IA, Strobel P, Tampe B. Intrarenal synthesis of complement C3 localized to distinct vascular compartments in anca-associated renal vasculitis. J Autoimmun (2022) 133:102924. doi: 10.1016/j.jaut.2022.102924
61. Lusco MA, Fogo AB, Najafian B, Alpers CE. Ajkd atlas of renal pathology: Tubular atrophy. Am J Kidney Dis (2016) 67(6):e33–4. doi: 10.1053/j.ajkd.2016.04.007
62. Fogo AB, Lusco MA, Najafian B, Alpers CE. Ajkd atlas of renal pathology: Ischemic acute tubular injury. Am J Kidney Dis (2016) 67(5):e25. doi: 10.1053/j.ajkd.2016.03.003
Keywords: innate immunity, platelets, complement system, complement C3, ANCA-associated renal vasculitis, MPO-ANCA, PR3-ANCA, tubulointerstitial injury
Citation: Baier E, Tampe D, Kluge IA, Hakroush S and Tampe B (2022) Implication of platelets and complement C3 as link between innate immunity and tubulointerstitial injury in renal vasculitis with MPO-ANCA seropositivity. Front. Immunol. 13:1054457. doi: 10.3389/fimmu.2022.1054457
Received: 26 September 2022; Accepted: 31 October 2022;
Published: 11 November 2022.
Edited by:
Meganathan Kannan, Central University of Tamil Nadu, IndiaReviewed by:
Chen Wang, Peking University, ChinaCopyright © 2022 Baier, Tampe, Kluge, Hakroush and Tampe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Björn Tampe, YmpvZXJuLnRhbXBlQG1lZC51bmktZ29ldHRpbmdlbi5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.