- 1Fifth Department of Medicine (Nephrology/Endocrinology/Rheumatology/Pneumology), University Medical Centre Mannheim, University of Heidelberg, Mannheim, Germany
- 2Clinical Research Center for Reproduction and Genetics in Hunan Province, Reproductive and Genetic Hospital of CITIC-Xiangya, Changsha, Hunan, China
- 3Institute of Pharmacy, Freie Universität Berlin, Berlin, Germany
- 4Institute of Reproductive and Stem Cell Engineering, NHC Key Laboratory of Human Stem Cell and Reproductive Engineering, School of Basic Medical Science, Central South University, Changsha, Hunan, China
- 5Key Laboratory of Stem Cells and Reproductive Engineering, Ministry of Health, Changsha, China
- 6Institute of Medical Diagnostics, IMD, Berlin, Germany
Background: It was suggested that vaccination in general might affect reproductive health. Safety of COVID-19 vaccination in women undergoing assisted reproductive techniques (ART) treatment is not well established.
Methods: We performed a retrospective study including 536 women undergoing fresh embryo transfer after IVF/ICSI treatment in a huge IVF center in southern China to investigate the effect of COVID-19 vaccination on oocyte maturation, fertilization rate, blastulation rate, implantation rate, clinical pregnancy rate and miscarriage rate. In addition, we performed a systematic review of existing studies on the safety of COVID-19 vaccination in women undergoing ART treatment.
Results: In our study, 268 women received inactivated or recombinant COVID-19 vaccination and 268 controls were enrolled based on propensity score matching. We observed a decreased fertilization rate and signs for impaired oocyte maturation in vaccinated women. Besides our study, there were 15 studies analyzing the safety of COVID-19 vaccination in women undergoing ART treatment. For the mRNA vaccines, no adverse signals were reported concerning oocyte maturation, fertilization rate, blastulation rate, implantation rate, clinical pregnancy rate and miscarriage rate. In women being vaccinated with an inactivated vaccine, implantation rate, clinical pregnancy rate and miscarriage rate were not affected, whereas oocyte maturation and fertilization rate were impaired.
Conclusions: Vaccination against COVID-19 in women undergoing ART treatment seems to be safe especially for women getting mRNA vaccines. The effects on oocyte maturation and fertilization rate of inactivated and recombinant COVID-19 vaccinations might be a safety signal and need further investigation and independent confirmation.
Introduction
The appearance and rapid spread of the novel coronavirus disease 2019 (COVID-19) in 2020 have caused loss of life and poorer health outcomes also in pregnant women and their newborns. The SARS-CoV-2 virus belongs to the β-corona virus family as the other human pathogenic corona viruses. In patients with a more severe upper respiratory tract infection, the SARS CoV-2 infection often causes a mild and/or severe acute respiratory syndrome (SARS) with subsequent release of cytokines/mediators such as: interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8 (CXCL8), IL-10, IP10, IL-12, IL-13, IL-17, IL-33, IL-25, IL-37, IL-38, GCSF, GM-CSF, HGF, IP-10, MCP-1, MIP-1α, IFN-γ, IFN-α, TRAIL, MCSF, and TNF-α (1). There is clear evidence suggesting that SARS-CoV-2 may infect the ovary, uterus and vagina because these organs do express angiotensin converting enzyme 2 (ACE2), the key host factor for SARS-CoV2 infection (2, 3). SARS-CoV-2 infection was shown to adversely affect female reproductive functions (luteal angiogenesis and degeneration, regular changes in endometrial tissue, and embryo development) and increased the risk of infertility and menstrual cycle disorder (4–6). Infection with SARS-CoV-2 results in a down-regulation of ACE-2 expression, subsequentially leading to reduced conversion of angiotensin (ANG)-II to Ang 1–7 and excessive accumulation of ANG-II which causes for example reactive oxygen species (ROS) production. This alters ovarian steroidogenesis, follicular development, oocyte maturation, ovulation, and atresia (7). Moreover, SARS-CoV-2 infection, often causing an excessive production of proinflammatory cytokines, including IL6, IL1β, TNFα, and MCP-1/CCL2, impairs reproductive physiology, such as ovulation and endometrial receptivity (7). It was suggested that Ang II mediated excessive ROS production might reduce female fertility (8). Moreover, pregnant women with symptomatic COVID-19 were more likely to experience ICU admission, intubation, and death (9). SARS CoV-2 infection seems to be associated with oligohydramnios, fetal growth restriction, preterm birth, and stillbirth (10–14) and potential long-term adverse health outcomes (15). Since there is no precise cure for the disease in pregnant women approved so far, mass vaccination is a key method by which countries are aiming to control the pandemic for this special population (16).
Due to safety reasons, however, pregnant and breastfeeding women were not included in the initial Phase III clinical trials of the COVID-19 vaccine developmental programs, resulting in limited data on the efficacy and more important safety of the COVID-19 vaccine in pregnant and breastfeeding women (17). Since the end of 2020, key national and international health institutions like the WHO as well as academic associations guided the COVID-19 vaccine, including women who are pregnant, breastfeeding, or planning pregnancy (naturally or with the help of assisted reproductive technology) (18). Antibody titers after vaccination are similar in pregnant and non-pregnant women (19). A large prospective cohort study including 7809 pregnant women found that COVID-19 vaccines were well tolerated in pregnant and lactating women (20). A systematic review including 26 studies analyzing perinatal outcomes after COVID-19 vaccination in pregnancy showed that the overall rates of adverse perinatal outcomes were not increased after maternal vaccination (21). However, there is still an ongoing controversial debate on the safety and efficacy of COVID-19 vaccination in this population (22).
Bentov et al. assessed the follicular steroidogenesis and oocyte quality in women undergoing mRNA vaccination and did not see any difference when compared with unvaccinated women (23). For in-vitro fertilization (IVF) treatment, a study reported that COVID-19 infection could lead to a significantly lower proportion of top-quality embryos while no difference in the number of oocytes and mature oocytes retrieved, and fertilization rate was seen. However, the sample size in this study was small and for sure beyond the power to seriously address safety concerns (24).
For the prevention of COVID-19, several vaccine types have been developed and approved so far (inactivated virus vaccine, mRNA vaccine, live attenuated vaccine, recombinant protein subunit vaccine, and vector vaccine) (25). Whether a different type of vaccine affects reproduction outcomes is unknown. Since the IVF procedure is a unique controlled process of human reproduction, the effects of vaccination on the different steps of human reproduction (oocyte maturation, fertilization, embryo development, implantation, biochemical pregnancy, clinical pregnancy, early and late abortion, and finally life birth rate) can be distinguished. We thus compared in a retrospective study the effects of two main types of vaccination on pregnancy outcomes. We compared inactivated and recombinant COVID-19 vaccines:
A) Inactivated vaccine, which used Vero cells to culture and grow COVID-19 virus and β-propionolactone to inactivate the virus while retaining antigenic components that induce immune responses (26).
B) Recombinant protein vaccine, which is based on recombined coronavirus S protein receptor-binding region (RBD) gene expression in Chinese hamster ovary (CHO) cells. The CHO cells make RBD protein dimers that are purified and concentrated for use as a vaccine. Aluminum hydroxide adjuvant is added to improve immunogenicity (26).
In addition, we performed a systematic review of all available information’s on the safety of the different COVID-19 vaccines during pregnancy with a focus on reproductive medicine safety endpoints.
Materials and methods
Study design and setting
This is a retrospective analysis of women who received ART treatment from June 2021 to September 2021 in the Reproductive and Genetic Hospital of CITIC-Xiangya. 536 women were enrolled in the analysis by propensity score matching (PSM): women received COVID-19 vaccination (n = 268) and control group (n = 268). (for more details see: Supplementary Figure S1). The vaccines used are described in detail in Supplementary Table S1. Based on the vaccine type we build two vaccination groups and a control group without any COVID-19 vaccination.
The study was approved by the Ethics Committee of the Reproductive and Genetic Hospital of CITIC-Xiangya (approval number: LL-SC-2022).
Participants
The participant’s inclusion and exclusion criteria were as follows:
Inclusion criteria: (1) age: 20-45 years, (2) women received ovarian stimulation and oocyte retrieval due to any type of infertility. Exclusive criteria: (1) women receiving oocyte donation, (2) women undergoing frozen embryo transfer cycles, and (3) prior SARS-CoV-2 infection. (4) male partners received any COVID-19 vaccination or had SARS-CoV2 infection.
Outcome measurement
The primary study endpoints were: normal fertilization rate and clinical pregnancy rate, For the primary study endpoints multivariant linear regression analysis considering confounding factors were done.
All other study outcomes such as laboratory values, oocyte and embryo quality data were analyzed as described recently (27). Normal fertilization rate was defined as
Normal fertilization rate was defined as the presence of two pronuclei (2PN) 16-18 hours after fertilization, basically one from sperm and the other from oocyte. Clinical pregnancy was defined as the existence of gestational sac(s) with fetal heart activity by ultrasound at week 4 after ET. Implantation rate was defined as the total number of embryos transferred divided by the number of sacs. Miscarriage was defined as intrauterine pregnancy loss after confirmation of gestational sacs.
Data analysis
We applied Statistical Package for Social Sciences for Windows, version 25.0 (SPSS Inc, Chicago, IL, USA) to perform data analyses. Homogeneity of variance and normality of data was estimated using the Levene and Kolmogorov-Smirnov tests, respectively. Values were expressed as means ± standard deviation or frequency (%). A comparison of quantitative variables between groups was performed using the Mann-Whitney U test or T-test. Qualitative variables were compared by the Chi-square test or Fisher’s exact test. Multivariate logistic regression analysis (enter model) and multiple linear regression analysis (kick-out model) were performed to figure out the impact factor on pregnancy and fertilization rate. Baseline clinical factors related to fertility were considered confounding factors. Statistically significant data was indicated with p < 0.05.
Systematic review
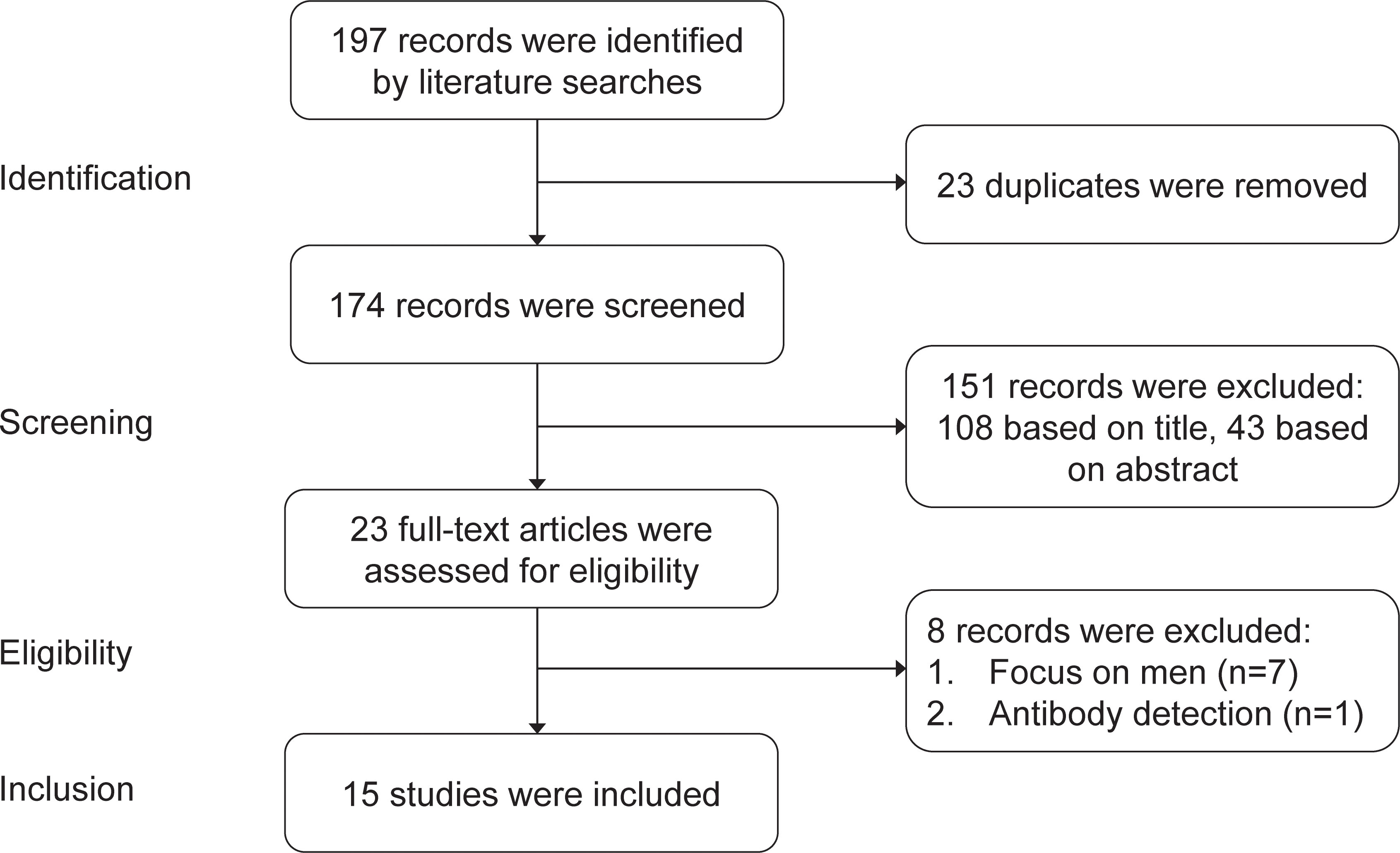
The systematic review of the safety of COVID-19 vaccines in embryo transfer included studies published from January 1st, 2021 to December 1st, 2022. We focused on studies published in English. We used the following sources: PubMed, Google scholar, Embase and Scopus. The search terms were ‘COVID-19 vaccine’ OR ‘COVID-19 mRNA vaccine’ OR ‘COVID-19 inactivated vaccine’ AND ‘embryo transfer’ OR ‘IVF outcomes’ OR ‘ICSI outcomes’ OR ‘pregnancy outcomes’. Our literature searches yielded 197 studies, from which 23 duplicates were removed. After a review of the titles and abstracts, 23 studies were identified as potentially eligible for inclusion. 7 studies focused on men instead of women and 1 study focused on the antibody after COVID-19 vaccination. Finally, 15 studies were included in the systematic review. See details in Figure 1.
Results
Clinical findings
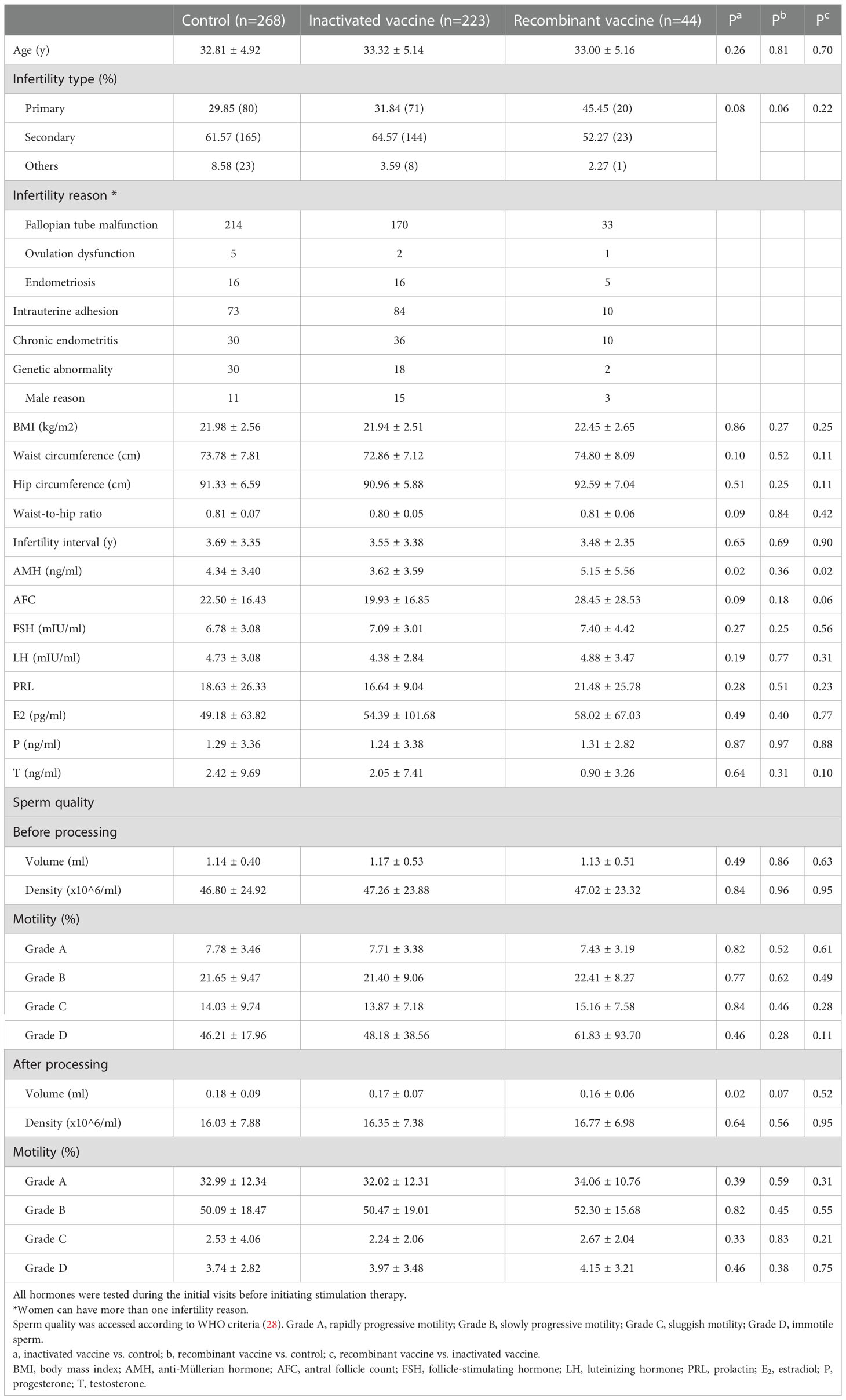
We matched the vaccinated women and controls by PSM (propensity score matching) (Supplementary Figure S1). Next, we analyzed the different types of vaccines separately. In the present study, most of our participants (n=223) received an inactivated vaccine, 44 women received a recombinant vaccine and only 1 woman received an adenovirus vector vaccine, which was excluded from further analysis. Information’s of the used vaccine is shown in Supplementary Table S1. For women who received the inactivated vaccine, baseline anti-Müllerian hormone (AMH) concentrations were lower as compared to the controls and those who received the recombinant vaccine (Table 1). No other differences were observed in other baseline characteristics among groups, see Table 1.
The primary study endpoints of our single center study were normal fertilization rate and clinical pregnancy rate.
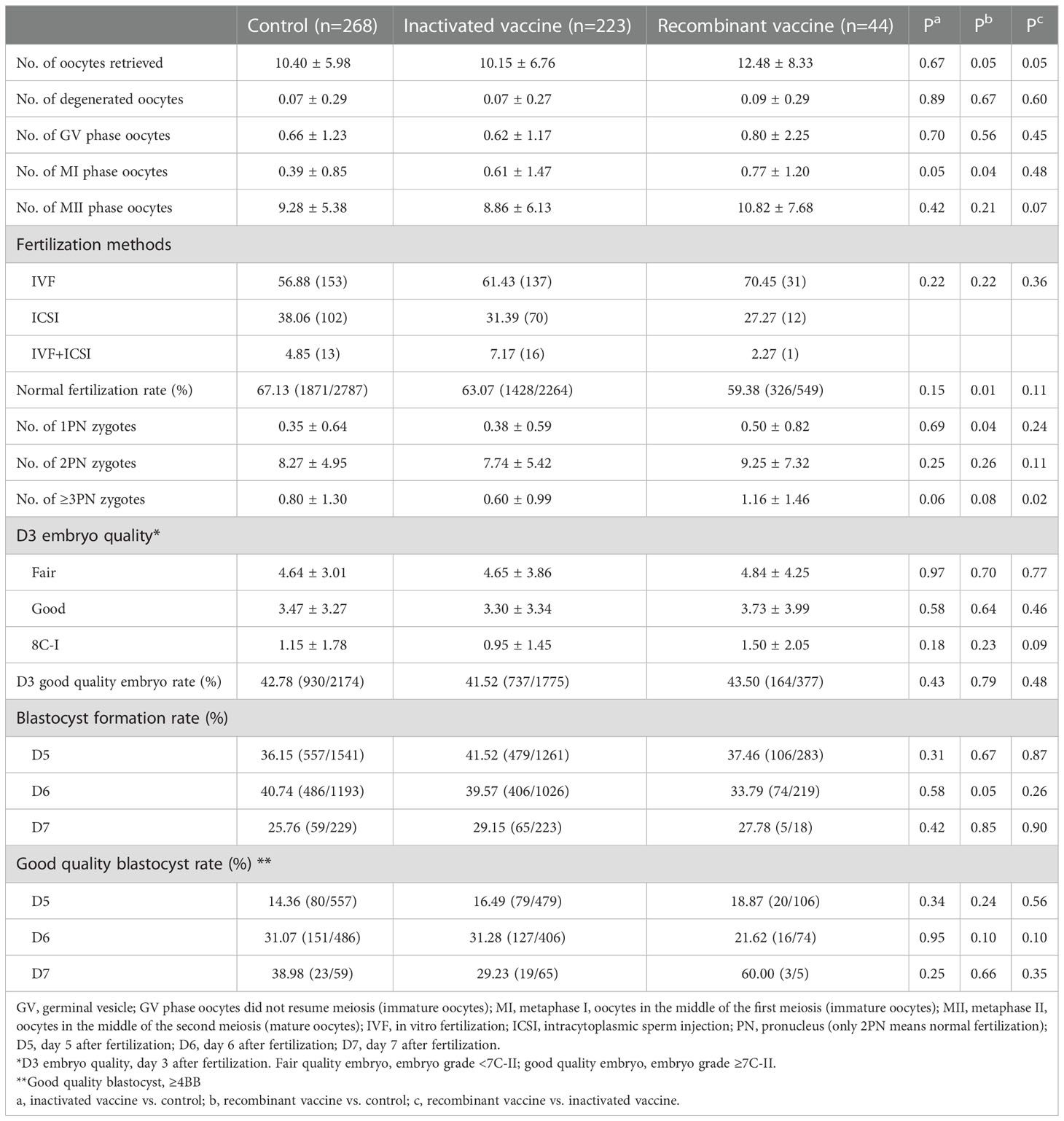
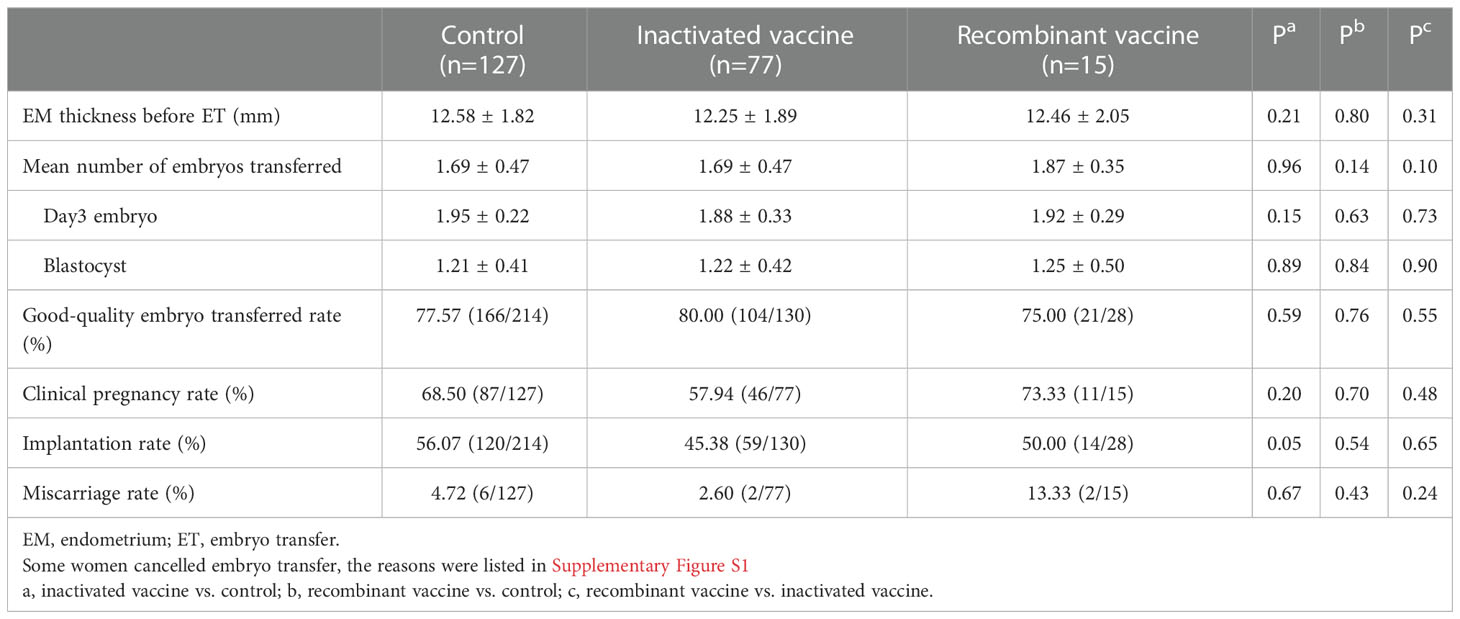
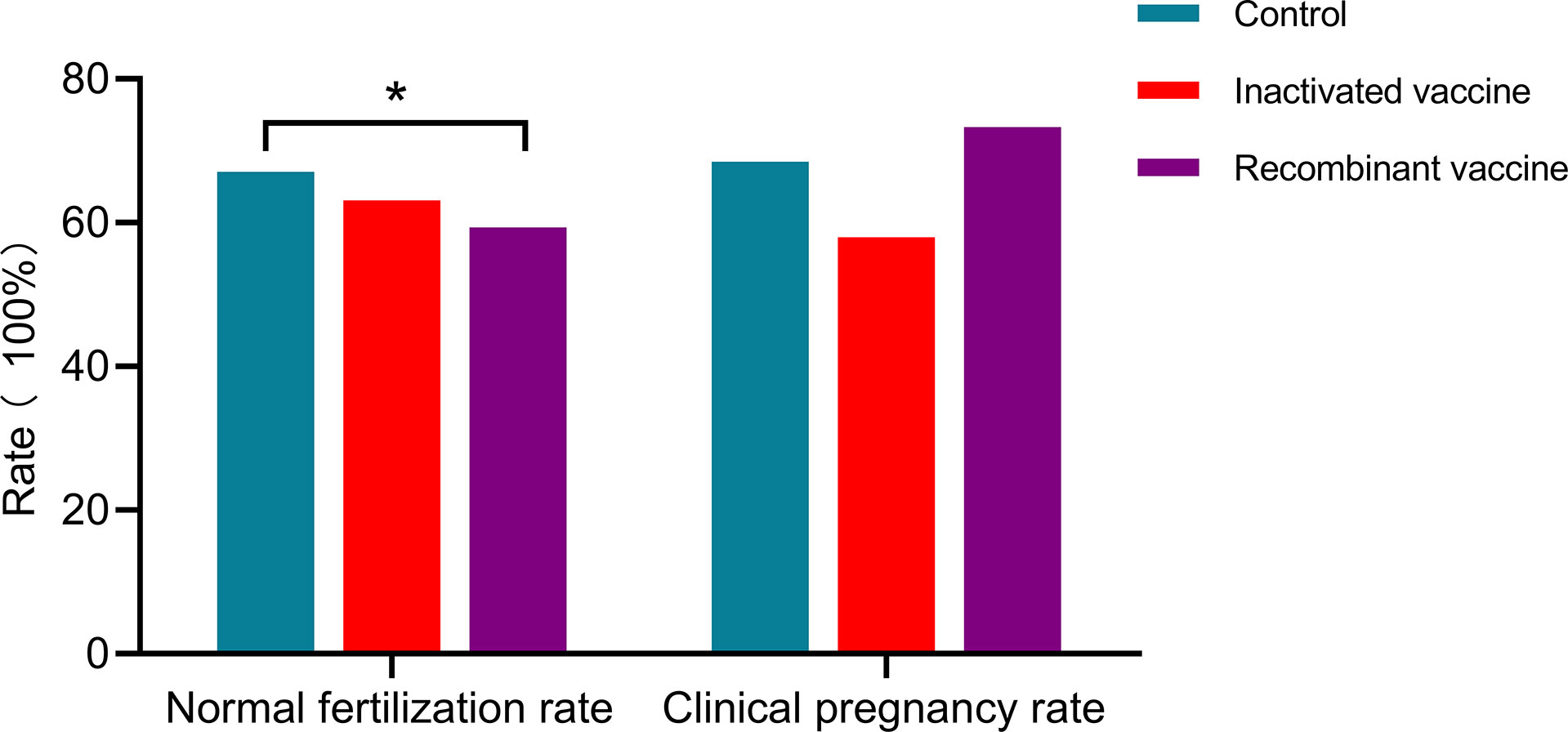
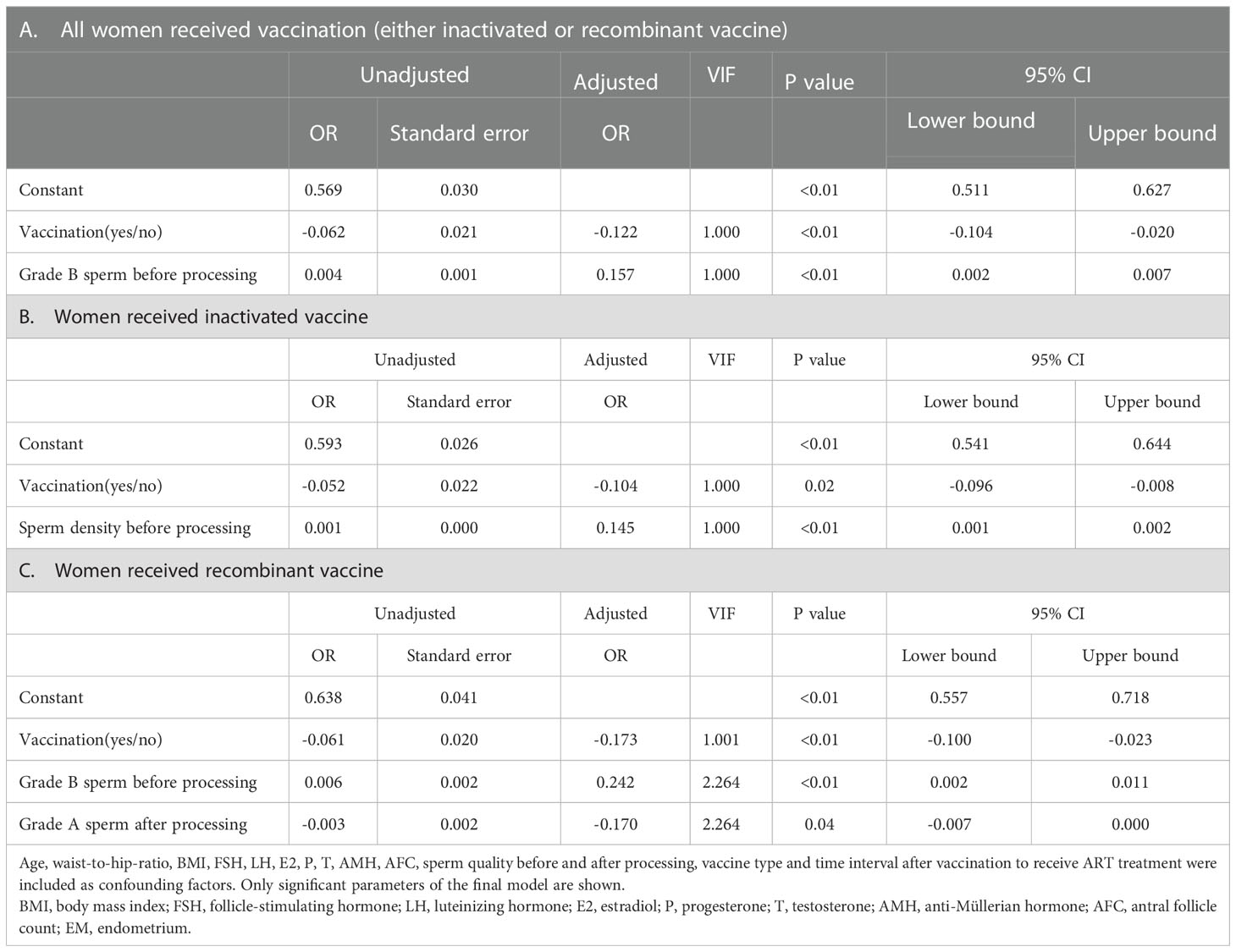
In women received inactivated vaccine, there was no significant difference in both normal fertilization rate and clinical pregnancy rate compared to controls (Tables 2, 3; Figure 2). Concerning the secondary outcomes, only the number of MI phase oocytes (immature oocytes) was higher than controls (Table 2). Since there is no consensus on the time interval after vaccination to receive ART treatment, we regrouped the participants according to a time cut-off determined by Receiver Operating Characteristic (ROC) analysis. The time cut-off value was six weeks. Hence, we grouped our participants into two groups: <six weeks and ≥ six weeks. Analysis of time-dependency (<six weeks versus ≥ six weeks) of potential effects of inactivated vaccine revealed no difference with regard to oocyte-, zygote- and embryo-quality and pregnancy outcome (Supplementary Table S2, S4). Multivariant linear regression analysis (normal fertilization rate) and logistic regression analysis (clinical pregnancy rate) considering confounding factors were performed. The results showed that inactivated vaccination had a negative impact on the normal fertilization rate (Table 4) but not on the clinical pregnancy rate (Table 5). There was no time dependency of the adverse effects of inactivated vaccination on normal fertilization rate and clinical pregnancy rate (Tables 4, 5).
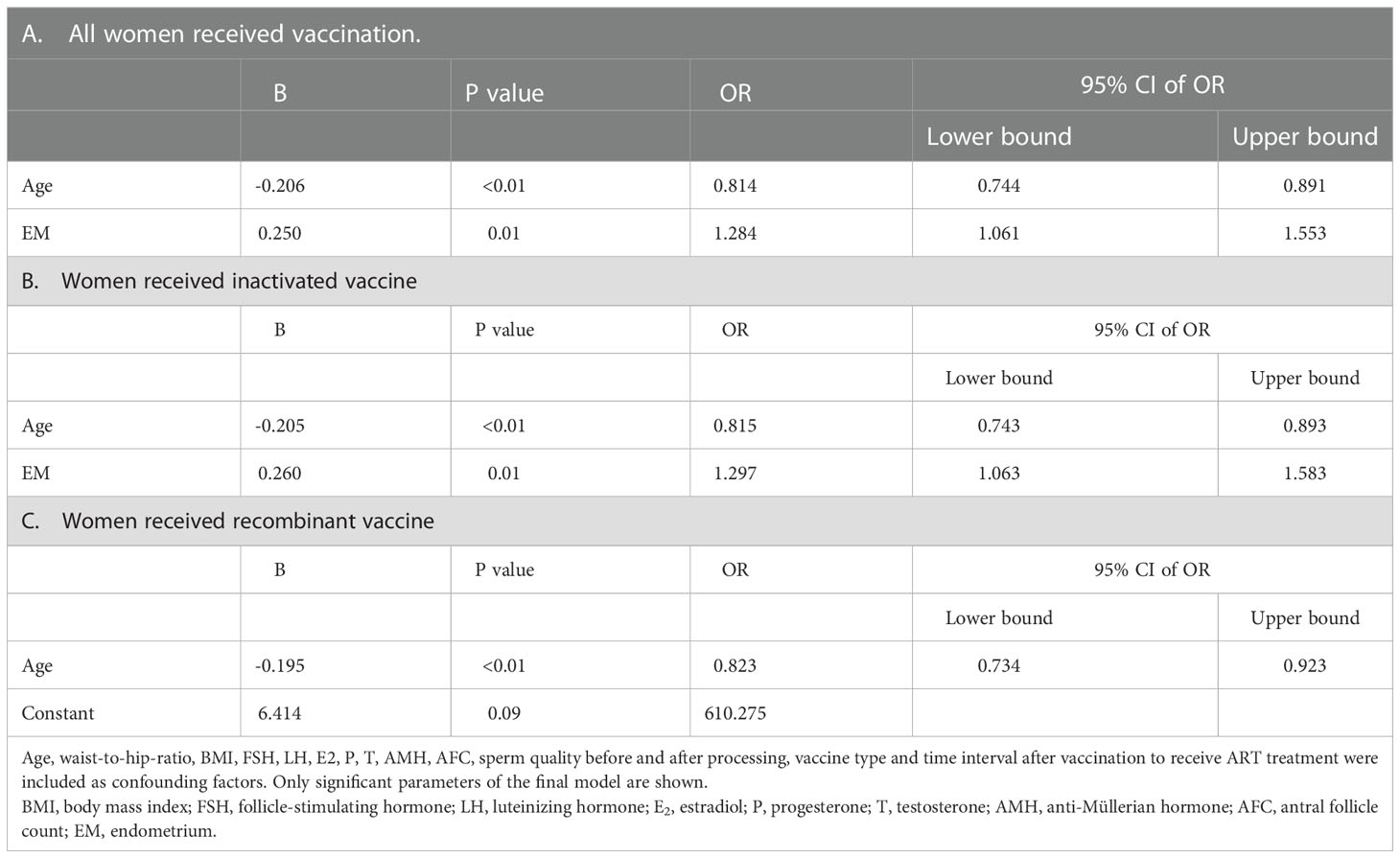
Table 5 Multivariate regression analysis on clinical pregnancy rate (only women received embryo transfer).
In women receiving recombinant vaccine, normal fertilization rate was lower as compared to controls (Table 2; Figure 2). Concerning the secondary outcomes, Women with recombinant vaccination received more retrieved oocytes, MI phase oocytes (immature oocytes) and one pronucleus (1PN) than controls (Table 2). Analysis of time-dependency (<six weeks versus ≥ six weeks) of potential effects of recombinant vaccine showed that the number of 1PN zygotes was significantly higher while the day five (D5) blastocyst formation rate and the day six (D6) good quality blastocyst rate were lower in women who received ART treatment less than six weeks after recombinant vaccination (Supplementary Table S3). Univariant not adjusted analysis showed that clinical pregnancy rate was higher in women received ART treatment more than six weeks after recombinant vaccination (Supplementary Table S5). Multivariant regression analysis showed that recombinant vaccination had a negative impact on the normal fertilization rate (Table 4) but had no impact on clinical pregnancy rate (Table 5). Time interval after recombinant vaccination to receive ART treatment had no effects on normal fertilization rate and clinical pregnancy rate (Tables 4, 5).
Taken together, it appears that the inactivated vaccine might have adverse effects on oocyte maturation. This, however, does not substantially affect the primary study endpoints of our single center study: normal fertilization rate and clinical pregnancy rate
Systematic review
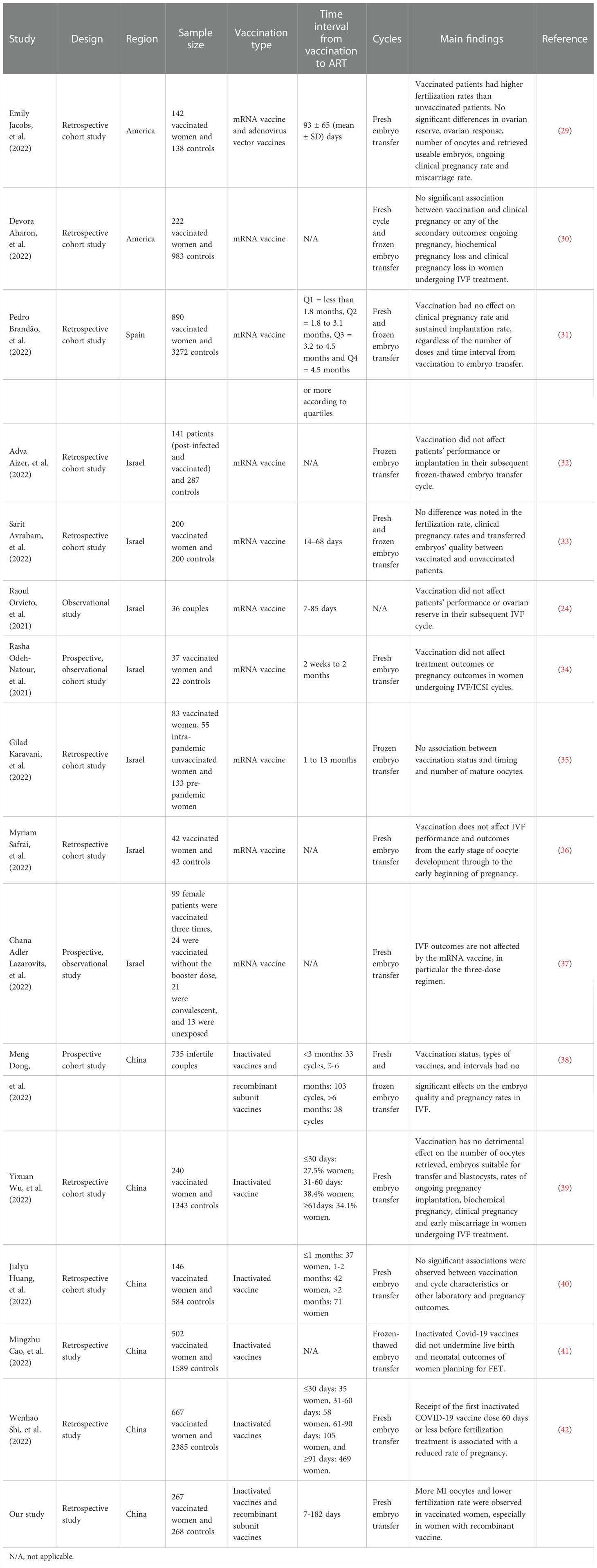
We compared existing studies on the safety of COVID-19 vaccines in embryo transfer published from January 1st, 2021 to December 1st, 2022. According to the searching and screening criteria described in the method section, 15 studies were taken into account finally.
In these 15 studies, 2 studies from America reported the safety of mRNA vaccination and one of which involved adenovirus vector vaccines (29, 30), 1 study from Spain (31) and 7 studies from Israel (24, 32–37) mainly reported the safety of mRNA vaccination and 5 studies from China (38–42) reported the safety of inactivated vaccination and one of which also involved adenovirus vector vaccines and recombinant subunit vaccines.
Oocyte maturation
Nine studies analyzed oocyte maturation and there was no difference in oocyte maturation in all these studies (24, 30, 33–37, 40, 42). However, there are more MI oocytes in vaccinated women, especially in those who received the recombinant vaccine in our study.
Fertilization rate
Twelve studies analyzed the fertilization rate. One study (29) reported a higher fertilization rate in vaccinated women while other studies found no difference (24, 30, 32–36, 38–40, 42). In our present study, a lower fertilization rate was observed in vaccinated women, especially in those who received recombinant vaccine
Blastulation rate
Nine studies reported blastulation rate, no difference was observed in all the studies (29, 30, 33, 36, 38–42) and also in our study.
Implantation rate
Five studies reported implantation rate, no difference was observed in all the studies (31, 32, 39, 40, 42) and also in our study.
Clinical pregnancy rate
Twelve studies analyzed clinical pregnancy rate, no difference was observed in all the studies (30–34, 36–42) and also in our study.
Ongoing pregnancy rate
Six studies analyzed ongoing pregnancy rate. One study showed that ongoing pregnancy rate was significantly lower in women who received the first inactivated COVID-19 vaccine dose 60 days or less before fertilization treatment. However, no reduced risk for ongoing pregnancy in the 91 days or more subgroup was observed (42). No difference was observed in the other studies (29, 30, 32, 39, 41) and also in our study.
Miscarriage rate
Four studies analyzed miscarriage rate, no difference was observed in all the studies (29, 30, 39, 41) and also in our study.
In summary, almost all of these studies stated that vaccination had no detrimental effect on cycle characteristics or other laboratory and pregnancy outcomes in women undergoing ART treatment except that one study observed that vaccinated patients had higher fertilization rates than unvaccinated patients. However, in the current study, more MI oocytes and lower fertilization rate were observed in vaccinated women, especially in those who received recombinant vaccine. See more details in Table 6.
Discussion
The safety of vaccines against COVID-19 in women undergoing IVF/ICSI treatment is not well established. There were no placebo-controlled studies in women being vaccinated against COVID-19 in women undergoing IVF/ICSI treatment with regard to relevant safety endpoints for human reproduction. Only observational studies were reported. In addition, a comparison of the different vaccine types (mRNA vaccine, Adeno-viral based vaccine, inactivated vaccine types) is missing. We thus systematically analyzed safety data from our center and data coming from so far published observational studies. Oocyte maturation, fertilization rate, blastulation rate, implantation rate, clinical pregnancy rate, and miscarriage rate were the criteria to evaluate reproductive safety. Two types of vaccines against COVID-19 were mainly analyzed. For the mRNA vaccine no adverse signals were reported concerning fertilization rate, blastulation rate, implantation rate, clinical pregnancy rate, and miscarriage rate. Data on life birth rate – probably the most important hard clinical endpoint – and oocyte maturation, however, after mRNA vaccination were not reported so far. Thus, as of today, mRNA vaccines seem to be safe for women undergoing IVF/ICSI treatment, see Table 6.
In women being vaccinated with the Chinese inactivated vaccine, implantation rate, clinical pregnancy rate, and miscarriage rate were not affected. However, we saw a decreased fertilization rate and signs for impaired oocyte maturation in vaccinated women in women with recombinant and inactivated vaccine. Until now, the effect of COVID-19 vaccination on oocyte maturation was reported in nine studies testing mRNA vaccination and there was no difference in oocyte maturation between vaccinated and unvaccinated patients in all these studies (24, 30, 33–37, 40, 42). Safety data on adenovirus vector vaccines and recombinant subunit vaccines of COVID-19 in women undergoing ART treatment were limited. Only our study observed a sign of impaired oocyte maturation in women with recombinant vaccine especially, hence, further confirmation on this safety endpoint is required. The folliculogenesis process is complex and dynamic, which involves multiple endocrine cells and numerous signals that have been estimated to span > three months (43). Follicular cytokines in folliculogenesis are keys to reproductive success. They induce an immune-permissive, embryotropic environment that supports gametogenesis, fertilization, implantation, embryo development, and fetal growth (44). Anti-Müllerian hormone (AMH), produced by the granulosa cells of the ovarian follicles, is an indicator of a patient’s ability to respond quickly and efficiently to ovarian reserve (45). Being a member of transforming growth factor b (TGF-b) superfamily, AMH is involved in cytokine and growth factor network in the human body and responsible for the intercellular communications and immunological responses including inflammation. AMH may protect the ovary from inflammation by decreasing its own production in response to a rise in cytokines (46). The COVID-19 infection could activate a pro-inflammatory response and lead to cytokine storm (such as interferons, interleukins, chemokines, colony-stimulating factors, and TNF-alpha) and systemic inflammation (47). Vaccination might cause some of these inflammatory responses, which might also interfere with folliculogenesis, resulting in abnormal oocytes and fertilization (24). Clear evidence shows that after vaccination (for example, influenza vaccine), the “innate immune system”/”innate immunity” is activated and the vaccine is processed by antigen-presenting cells, which include macrophages and dendritic cells. Macrophages can produce some inflammatory molecules (cytokines), which is one of the important reasons why there is a local reaction after the vaccine at the site of injection. Mature dendritic cells migrate into regional lymph nodes, where they induce activation and clonal expansion of naïve CD4+ T-helper and CD8+ cytotoxic T cells. The activation and differentiation of naïve B cells are induced by antigen and CD4+ T-helper cells. Naïve B cells differentiate into memory B cells and antibody-secreting B cells. Long-term immunity is assured by memory B and T cells in the blood and lymph nodes as well as by long-living plasma cells and memory T cells in the bone marrow (48). These early inflammatory processes after vaccination might have an effect on folliculogenesis in the stimulated ovaries. Basic science studies are needed to confirm this hypothesis coming from our clinical data.
Vaccinating women in the third trimester of pregnancy offers the opportunity to provide early protection to infants through the transplacental transfer of maternal antibodies (49). Many studies have demonstrated the efficient transfer of pertussis antibodies across the placenta (50, 51). The WHO has recommended influenza vaccination during influenza season for all pregnant women since 2005 (3). Min Seo Kim et al. compared the safety of mRNA COVID-19 vaccines to influenza vaccines and demonstrated that the overall safety profile pattern suggests a lower risk of serious adverse events following immunization by mRNA vaccines compared to influenza vaccines (52).
However, limitations also exist in our single center observational study as well as in all other vaccination studies. We cannot fully exclude potential bias even though we made PSM before the study. Moreover, all studies only did first-term trimester follow-ups, whether long-term pregnancy complications and neonatal outcomes are affected needs to be investigated. Since all studies in the field are just observational studies, none of the studies can control for yet unknown confounding factors.
Conclusion
Vaccination against COVID-19 in women undergoing ART treatment seems to be safe especially for women getting mRNA vaccines. The effects on oocyte maturation and fertilization rate of inactivated and recombinant COVID-19 vaccinations need further investigation and independent confirmation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Reproductive and Genetic Hospital of CITIC-Xiangya (approval number: LL-SC-2022). The patients/participants provided their written informed consent to participate in this study.
Author contributions
BH and HC designed the study. HC collected the data. HC and XZ analyzed the data. HC, XZ, and BH wrote the manuscript. HC, GL, FG, and BH revised the manuscript. All authors contributed to the article and approved the submitted version
Funding
China Scholarship Council supported HC and XZ.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1054273/full#supplementary-material
References
1. Yalcin AD, Yalcin AN. Future perspective: biologic agents in patients with severe COVID-19. Immunopharmacol Immunotoxicol (2021) 43:1–7. doi: 10.1080/08923973.2020.1818770
2. Chu C, Zeng S, Hasan AA, Hocher CF, Krämer BK, Hocher B. Comparison of infection risks and clinical outcomes in patients with and without SARS-CoV-2 lung infection under renin-angiotensin-aldosterone system blockade: Systematic review and meta-analysis. Br J Clin Pharmacol (2021) 87:2475–92. doi: 10.1111/bcp.14660
3. World Health Organisation. Influenza vaccines: WHO position paper (2020). Available at: https://www.who.int/wer/2005/wer8033.pdf?ua=1.
4. Jing Y, Run-Qian L, Hao-Ran W, Hao-Ran C, Ya-Bin L, Yang G, et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod (2020) 26:367–73. doi: 10.1093/molehr/gaaa030
5. Lee W, Mok A, Chung JP. Potential effects of COVID-19 on reproductive systems and fertility; assisted reproductive technology guidelines and considerations: a review. Hong Kong Med J (2021) 27:118. doi: 10.12809/hkmj209078
6. Li F, Lu H, Zhang Q, Li X, Liu Q, Yang Q, et al. Impact of COVID-19 on female fertility: a systematic review and meta-analysis protocol. BMJ Open (2021) 11:e045524. doi: 10.1136/bmjopen-2020-045524
7. Rajak P, Roy S, Dutta M, Podder S, Sarkar S, Ganguly A, et al. Understanding the cross-talk between mediators of infertility and COVID-19. Reprod Biol (2021) 21:100559. doi: 10.1016/j.repbio.2021.100559
8. Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal (2013) 19:1085–94. doi: 10.1089/ars.2012.4604
9. Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–united states, January 22–October 3, 2020. Morbidity Mortality Weekly Rep (2020) 69:1641. doi: 10.15585/mmwr.mm6944e3
10. Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of new York city hospitals. Am J obstetrics gynecology MFM (2020) 2:100118. doi: 10.1016/j.ajogmf.2020.100118
11. Chen L, Li Q, Zheng D, Jiang H, Wei Y, Zou L, et al. Clinical characteristics of pregnant women with covid-19 in wuhan, China. New Engl J Med (2020) 382:e100. doi: 10.1056/NEJMc2009226
12. Pereira A, Cruz-Melguizo S, Adrien M, Fuentes L, Marin E and Perez-Medina T. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstetricia Gynecologica Scandinavica (2020) 99:839–47. doi: 10.1111/aogs.13921
13. Qiu Q, Huang J, Li Y, Chen X, Lin H, Li L, et al. Does an FSH surge at the time of hCG trigger improve IVF/ICSI outcomes? a randomized, double-blinded, placebo-controlled study. Hum Reprod (2020) 35:1411–20. doi: 10.1093/humrep/deaa087
14. Wu X, Sun R, Chen J, Xie Y, Zhang S and Wang X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int J Gynecology Obstetrics (2020) 150:58–63. doi: 10.1002/ijgo.13165
15. Tian M, Reichetzeder C, Li J and Hocher B. Low birth weight, a risk factor for diseases in later life, is a surrogate of insulin resistance at birth. J hypertension (2019) 37:2123–34. doi: 10.1097/HJH.0000000000002156
16. Machingaidze S, Wiysonge CS. Understanding COVID-19 vaccine hesitancy. Nat Med (2021) 27:1338–9. doi: 10.1038/s41591-021-01459-7
17. Sebghati M, Khalil A. Uptake of vaccination in pregnancy. Best Pract Res Clin Obstetrics Gynaecology (2021) 76:53–65. doi: 10.1016/j.bpobgyn.2021.03.007
18. Brillo E, Tosto V, Gerli S and Buonomo E. COVID-19 vaccination in pregnancy and postpartum. J Maternal-Fetal Neonatal Med (2021) 35:7890–910. doi: 10.1080/14767058.2021.1937991
19. Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol (2021) 225:303 e1–303 e17. doi: 10.1016/j.ajog.2021.03.023
20. Kachikis A, Englund JA, Singleton M, Covelli I, Drake AL, Eckert LO. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open (2021) 4:e2121310. doi: 10.1001/jamanetworkopen.2021.21310
21. Badell ML, Dude CM, Rasmussen SA, Jamieson DJ. Covid-19 vaccination in pregnancy. BMJ (2022) 378:e069741. doi: 10.1136/bmj-2021-069741
22. Wang EW, Parchem JG, Atmar RL, Clark EH. SARS-CoV-2 vaccination during pregnancy: a complex decision. Open Forum Infect Dis (2021) 8:180. doi: 10.1093/ofid/ofab180
23. Bentov Y, Beharier O, Moav-Zafrir A, Kabessa M, Godin M, Greenfield CS, et al. Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum Reprod (2021) 36:2506–13. doi: 10.1093/humrep/deab182
24. Orvieto R, Noach-Hirsh M, Segev-Zahav A, Haas J, Nahum R and Aizer A. Does mRNA SARS-CoV-2 vaccine influence patients' performance during IVF-ET cycle? Reprod Biol Endocrinol (2021) 19:69. doi: 10.1186/s12958-021-00757-6
25. Zhao J, Zhao S, Ou J, Zhang J, Lan W, Guan W, et al. COVID-19: coronavirus vaccine development updates. Front Immunol (2020) 11:602256. doi: 10.3389/fimmu.2020.602256
26. Group C-V T W. Technical vaccination recommendations for COVID-19 vaccines in China. China CDC Weekly (2021) 3:459. doi: 10.46234/ccdcw2021.083
27. Chen H, Li J, Cai S, Tang S, Zeng S, Chu C, et al. Blastocyst transfer: A risk factor for gestational diabetes mellitus in women undergoing In vitro fertilization. J Clin Endocrinol Metab (2022) 107:e143–52. doi: 10.1210/clinem/dgab594
28. Kandil H, Agarwal A, Saleh R, Boitrelle F, Arafa M, Vogiatzi P, et al. Editorial commentary on draft of world health organization sixth edition laboratory manual for the examination and processing of human semen. World J Men's Health (2021) 39:577–80. doi: 10.5534/wjmh.210074
29. Jacobs E, Summers K, Sparks A and Mejia R. Fresh embryo transfer cycle characteristics and outcomes following In vitro fertilization via intracytoplasmic sperm injection among patients with and without COVID-19 vaccination. JAMA Netw Open (2022) 5:e228625. doi: 10.1001/jamanetworkopen.2022.8625
30. Aharon D, Lederman M, Ghofranian A, Hernandez-Nieto C, Canon C, Hanley W, et al. In vitro fertilization and early pregnancy outcomes after coronavirus disease 2019 (COVID-19) vaccination. Obstet Gynecol (2022) 139:490–7. doi: 10.1097/AOG.0000000000004713
31. Brandao P, Pellicer A, Meseguer M, Remohi J. Garrido n and Garcia-velasco J a. COVID-19 mRNA Vaccines have no effect endometrial receptivity after euploid embryo transfer. Reprod BioMed Online (2022) 45:688–95. doi: 10.1016/j.rbmo.2022.05.017
32. Aizer A, Noach-Hirsh M, Dratviman-Storobinsky O, Nahum R, Machtinger R, Yung Y, et al. The effect of coronavirus disease 2019 immunity on frozen-thawed embryo transfer cycles outcome. Fertil Steril (2022) 117:974–9. doi: 10.1016/j.fertnstert.2022.01.009
33. Avraham S, Kedem A, Zur H, Youngster M, Yaakov O, Yerushalmi GM, et al. Coronavirus disease 2019 vaccination and infertility treatment outcomes. Fertil Steril (2022) 117:1291–9. doi: 10.1016/j.fertnstert.2022.02.025
34. Odeh-Natour R, Shapira M, Estrada D, Freimann S, Tal Y, Atzmon Y, et al. Does mRNA SARS-CoV-2 vaccine in the follicular fluid impact follicle and oocyte performance in IVF treatments? Am J Reprod Immunol (2022) 87:e13530. doi: 10.1111/aji.13530
35. Karavani G, Chill HH, Dick A, Meirman C, Gutman-Ido E, Herzberg S, et al. Pfizer SARS-CoV-2 BNT162b2 mRNA vaccination SARS-CoV-2 mRNA vaccination (BNT162b2) has no adverse effect on elective oocyte cryopreservation outcomes. Reprod BioMed Online (2022) 45:987–94. doi: 10.1016/j.rbmo.2022.06.001
36. Safrai M, Kremer E, Atias E and Ben-Meir A. BNT162b2 covid-19 vaccine does not affect fertility as explored in a pilot study of women undergoing IVF treatment. Minerva Obstet Gynecol (2022). doi: 10.23736/S2724-606X.22.05148-X
37. Adler Lazarovits C, Smadja A, Kabessa M, Allouche Kam H, Nevo L, Godin M, et al. Boosting dose of pfizer-BioNtech mRNA vaccine against SARS-CoV-2 does not affect reproductive outcomes in in-vitro fertilization patients: A cohort study. J Womens Health (Larchmt) (2022). doi: 10.1089/jwh.2022.0163
38. Dong M, Wu S, Zhang X, Zhao N, Qi J, Zhao D, et al. Effects of COVID-19 vaccination status, vaccine type, and vaccination interval on IVF pregnancy outcomes in infertile couples. J Assist Reprod Genet (2022) 39:1849–59. doi: 10.1007/s10815-022-02543-8
39. Wu Y, Cao M, Lin Y, Xu Z, Liang Z, Huang Q, et al. Inactivated COVID-19 vaccination does not affect in vitro fertilization outcomes in women. Hum Reprod (2022) 37:2054–62. doi: 10.1093/humrep/deac160
40. Huang J, Xia L, Lin J, Liu B, Zhao Y, Xin C, et al. No effect of inactivated SARS-CoV-2 vaccination on in vitro fertilization outcomes: A propensity score-matched study. J Inflammation Res (2022) 15:839–49. doi: 10.2147/JIR.S347729
41. Cao M, Wu Y, Lin Y, Xu Z, Liang Z, Huang Q, et al. Inactivated covid-19 vaccine did not undermine live birth and neonatal outcomes of women with frozen-thawed embryo transfer. Hum Reprod (2022) 37:2942–51. doi: 10.1093/humrep/deac220
42. Shi W, Wang M, Xue X, Li N, Chen L and Shi J. Association between time interval from COVID-19 vaccination to In vitro fertilization and pregnancy rate after fresh embryo transfer. JAMA Netw Open (2022) 5:e2236609. doi: 10.1001/jamanetworkopen.2022.36609
43. Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod (1986) 1:81–7. doi: 10.1093/oxfordjournals.humrep.a136365
44. Hong KJ, Lin JJ, Lin LH, Lai TH. The intrafollicular concentration of leptin as a potential biomarker to predict oocyte maturity in in-vitro fertilization. Sci Rep (2022) 12:19573. doi: 10.1038/s41598-022-23737-1
45. Kuang H, Duan Y, Li D, Xu Y, Ai W, Li W, et al. The role of serum inflammatory cytokines and berberine in the insulin signaling pathway among women with polycystic ovary syndrome. PloS One (2020) 15:e0235404. doi: 10.1371/journal.pone.0235404
46. Erel CT, Ozcivit IB. Anti-mullerian hormone and ovarian aging. Gynecol Endocrinol (2021) 37:867–8. doi: 10.1080/09513590.2021.1977276
47. Coperchini F, Chiovato L, Croce L, Magri F and Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev (2020) 53:25–32. doi: 10.1016/j.cytogfr.2020.05.003
48. Lang PO, Samaras D. Aging adults and seasonal influenza: Does the vitamin d status (H)Arm the body? J Aging Res (2012) 2012:806198. doi: 10.1155/2012/806198
49. Amirthalingam G. Strategies to control pertussis in infants. Arch Dis Child (2013) 98:552–5. doi: 10.1136/archdischild-2012-302968
50. Gall SA, Myers J and Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstetrics Gynecology (2011) 204:334. doi: 10.1016/j.ajog.2010.11.024
51. Leuridan E, Hens N, Peeters N, de Witte L, van der Meeren O, Van Damme P. Effect of a prepregnancy pertussis booster dose on maternal antibody titers in young infants. Pediatr Infect Dis J (2011) 30:608–10. doi: 10.1097/INF.0b013e3182093814
Keywords: COVID-19, vaccine, IVF/ICSI, fertilization rate, pregnancy outcomes
Citation: Chen H, Zhang X, Lin G, Gong F and Hocher B (2023) Safety of COVID-19 vaccination in women undergoing IVF/ICSI treatment - Clinical study and systematic review. Front. Immunol. 13:1054273. doi: 10.3389/fimmu.2022.1054273
Received: 26 September 2022; Accepted: 22 December 2022;
Published: 11 January 2023.
Edited by:
Thomas G. Egwang, Med Biotech Laboratories, UgandaCopyright © 2023 Chen, Zhang, Lin, Gong and Hocher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Berthold Hocher, YmVydGhvbGQuaG9jaGVyQG1lZG1hLnVuaS1oZWlkZWxiZXJnLmRl; Fei Gong, Z29uZ2ZlaTIwMTgxMjI0QDE2My5jb20=
†These authors contributed equally to this work and share first authorship
‡These authors contributed equally to this work and share senior authorship
§ORCID: Fei Gong, orcid.org/0000-0002-7474-0680
Berthold Hocher, orcid.org/0000-0001-8143-0579
 Huijun Chen
Huijun Chen Xiaoli Zhang
Xiaoli Zhang Ge Lin2,4,5
Ge Lin2,4,5 Berthold Hocher
Berthold Hocher