- 1Department of Emergency, Min-Sheng General Hospital, Taoyuan, Taiwan
- 2Graduate Institute of Injury Prevention and Control, College of Public Health, Taipei Medical University, Taipei, Taiwan
- 3Division of Neurosurgery, Department of Surgery, Kuang Tien General Hospital, Taichung, Taiwan
- 4Department of Biotechnology and Animal Science, College of Bioresources, National Ilan University, Yilan, Taiwan
- 5Department of Biotechnology, College of Medical and Health Care, Hung Kuang University, Taichung, Taiwan
- 6Department of Health Business Administration, College of Medical and Health Care, Hung Kuang University, Taichung, Taiwan
- 7Department of Surgery, Division of Neurosurgery, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan
- 8Department of Healthcare Information and Management, School of Health Technology, Ming Chuan University, Taipei, Taiwan
- 9Division of Neurosurgery, Department of Surgery, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan
- 10Department of Exercise and Health Promotion, College of Kinesiology and Health, Chinese Culture University, Taipei, Taiwan
Background and aims: Epidemiological studies have been conducted on the relationship between systemic rheumatic diseases (SRDs) and dementia. Therefore, we focused on determining the extent of alliances bounded by SRDs, along with the risk of dementia.
Materials and methods: Two independent reviewers assessed all studies retrieved from the PubMed, EMBASE, Scopus, and Web of Science databases between January 1, 2000 and November 30, 2021. Only observational studies that estimated the possibility of dementia in participants with SRD were considered. The random-effects model was applied to forecast pooled risk ratios (RRs) and 95% confidence intervals (CI). Heterogeneity among the studies was evaluated using the Q and I2 statistics. The quality of the included studies was assessed using the Newcastle-Ottawa Scale. Funnel plots were used to calculate the risk of bias.
Results: Seventeen observational studies with 17,717,473 participants were recruited. Our findings showed that among the participants with SRDs, those with osteoarthritis, systemic lupus erythematosus, and Sjogren’s syndrome were highly related to an elevated risk of dementia (pooled RR: 1.31; 95% CI: 1.15–1.49, p<0.001; pooled RR: 1.43; 95% CI: 1.19–1.73, p<0.001; and pooled RR: 1.26; 95% CI: 1.14–1.39, p<0.001, respectively). However, participants with rheumatoid arthritis (RA) were not associated with an increased risk of dementia (pooled RR: 0.98; 95% CI: 0.90–1.07, p<0.001).
Conclusion: This systematic review and meta-analysis demonstrated an increased dementia risk among SRDs participants, except for RA.
Introduction
Dementia is a common neurological disorder associated with impaired performance in daily activities (1, 2). The total number of dementia victims is forecast to increase to 78,000,000 by 2030 and 139,000,000 by 2050 (3, 4). Alzheimer’s disease (AD) is the most commonly seen dementia in the aging population, contributing roughly 60–70% of all dementia cases (5, 6). In contrast, the remaining dementia categories consist of dementia with Lewy bodies, vascular dementia, and frontotemporal dementia (7). Previous studies, mainly based on histopathological examination, have shown that these two biomarkers (neuritic plaques and neurofibrillary tangles caused by glial activation) are associated with dementia (8, 9). Multiple evidence-based medicine studies have reported that inflammation plays an important role in AD pathogenesis (10, 11). However, no medication is available to reverse or prevent the progression of AD (12).
Systemic rheumatic diseases (SRDs) such as osteoarthritis (OA), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and Sjogren’s syndrome (SS) are chronic inflammatory conditions that are common causes of disability (13). Over the decades, the prevalence of SRDs has increased and evidence shows that SRDs are associated with substantial morbidity and mortality (14, 15). Several epidemiological investigations have found a relationship between SRDs and the risk of dementia. However, the findings from previous studies remain controversial, and some methodological limitations have been observed. Previous biological studies have demonstrated that these two diseases share similar inflammatory mechanisms. Indeed, pro-inflammatory cytokines [interleukin (IL)-1b, IL-6, and tumor necrosis factor (TNF)-α] are linked to an increased possibility of dementia and are also associated with the pathogenesis (16–18).
Regarding the current evidence of SRDs, little information can be gathered if SRDs are related to dementia. Thus, we examined the relationship between SRDs and dementia risk through an updated systematic review of evidence (19).
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (20) guidelines were used to prepare this meta-analysis (2, 20).
Data sources and literature search
An extensive search was conducted in the PubMed, MEDLINE, Embase, and Web of Science databases for studies evaluating the association of dementia with SRDs and dementia risk. A search strategy was developed based on the PICO [Patient, Intervention/Exposure, Comparison, Outcome] questions, and two expert authors (Y.-C.W., M.-S.L.) assisted in developing the search strategies, which are provided in Supplementary Table S1. Articles published in English were considered in this study, although geographic location was not imposed. In addition, the citation directories of the included references and relevant review articles on this topic were screened for additional studies.
Study selection and outcomes
To analyze the possibility of dementia incidence and diminish potential bias, our primary target included only randomized controlled trials (RCTs). However, no RCTs have been published on this topic. Therefore, we included observational studies (e.g., case-control and cohort studies) to reduce the recall bias. Our inclusion criteria were as follows: (1) all observational studies that evaluated the association between dementia and SRDs, (2) provided accurate information about their methodology, and (3) reported the risk estimates of dementia. We considered studies with proven diagnoses of dementia and SRDs, based on clinical guidelines or standard diagnostic codes. If studies were published as cross-sectional, case reports, editorials, review articles, or non-human studies, they were excluded. The same two expert authors (Y.-C.W. and M.-S.L.) independently examined all the topics and abstracts of the retrieved manuscripts using predefined criteria. Subsequently, the entire content of possible articles was screened for acceptability. Any differences during the study screening were resolved through discussion with the principal investigator until a final decision was made.
Data extraction and risk of bias assessment
The same expert authors (Y.-C.W., M.-S.L.) separately extracted the following data: author name (first author only), publication year, origin, database, population characteristics, study period, participant selection process, adjusting factors (drugs, diseases etc.), number of participants, and outcome of interest (dementia). We collected only data on adjusted risk estimates, including risk ratios (RRs), odds ratios (ORs), and hazard ratios (HRs) with 95% confidence intervals (CIs). To examine the risk of bias for the included articles, the Newcastle-Ottawa Scale was applied by the same expert authors. All controversies within the period of data extraction and the possibility of bias calculation were finalized through a dissertation with other authors.
Statistical analyses
We conducted a comprehensive meta-analysis (CMA) of our study. We calculated the overall RRs of dementia and utilized the HRs and ORs from the included studies. Pooled RRs with corresponding CIs were analyzed using a random-effects model to reduce considerable clinical heterogeneity. The I2 statistic was used to evaluate appropriate heterogeneity between studies. I2 statistics ranging from 0–25%, 25–50%, 50–75%, and 75–100% were expressed as very low, low, medium, and high heterogeneity, respectively (21, 22). We also performed subgroup analyses based on study design, location, and disease to determine whether other factors affected the findings. We also presented funnel plots to calculate publication bias. A p-value of <0.05 was considered significant.
Results
Study selection
The PRISMA selection flowchart is presented in Figure 1. Initially, 1,285 articles were selected from the database. Subsequent screening of all titles and abstracts revealed 24 full-text articles for the initial study qualification. Finally, 17 studies were included in the current research (23–39).

Figure 1 Preferred reporting items for systematic reviews and meta-analyses based search strategy flow chart.
Characteristics of included studies
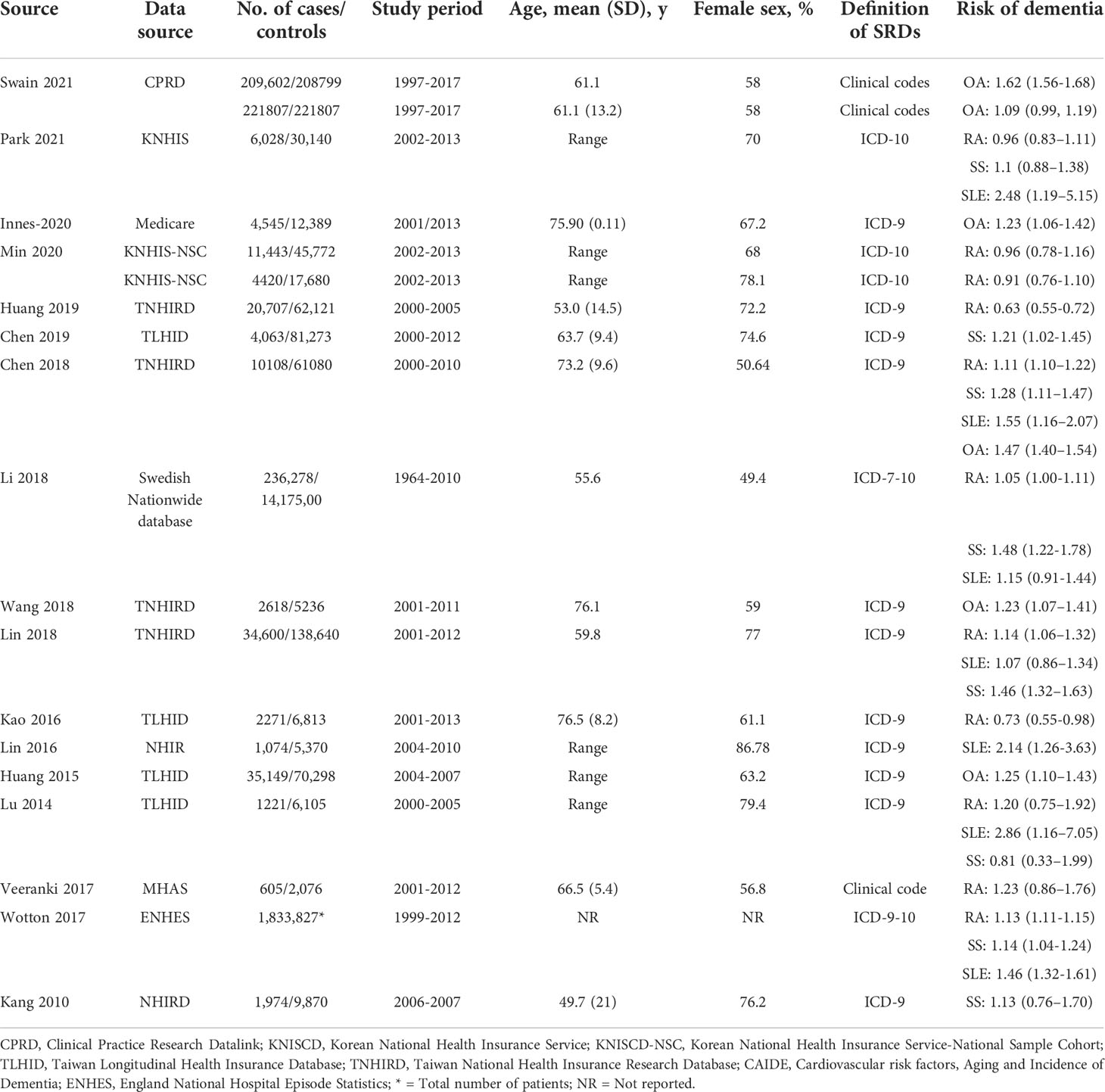
The basic characteristics of the results and important outcomes are outlined in Table 1. Seventeen observational studies (case-control and cohort) with 17,717,473 participants were included. In total, 12 studies were conducted in Asia, three in Europe, and two in North America. The publication period ranged from 2010 (37) to 2021 (24). SRDs and dementia were illustrated by the International Classification of Diseases (ICD) Ninth and Tenth Revision. The modified covariates of all encompassed studies are presented in Supplementary Table S2.
Risk of bias assessment
NOS assessment was used to examine risk bias in the selected studies. The methodological study quality was divided into two categories (high, >7; low, ≤7) according to the NOS value. In total, 11 studies had low risk bias, five had moderate risk bias, and one had high risk bias. However, the general quality of evidence was high in the meta-analysis (Supplementary Table S2).
Risk for dementia
Sum of 17 researches with 17,717,473 participants examined the association between dementia risk and SRDs. However, five studies assessed dementia risk within OA participants. As shown in Figure 2, participants with OA had an elevated dementia risk (pooled RR: 1.31; 95% CI: 1.15–1.49) compared to those without OA. The I2 statistic of these studies was 94.10%, indicating a high risk of heterogeneity.
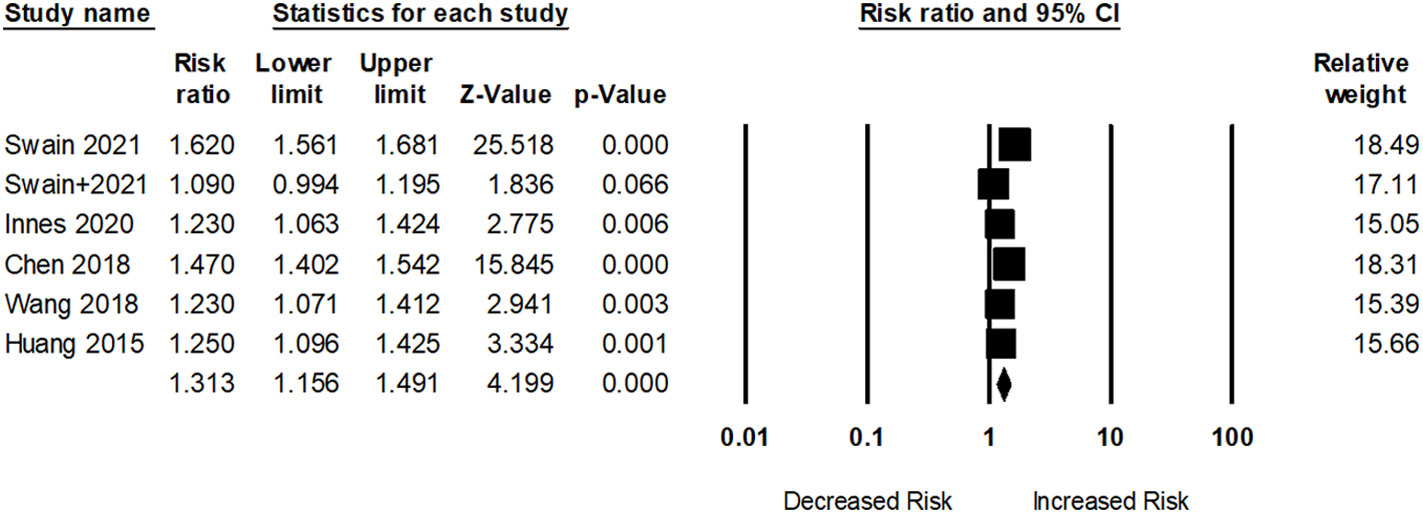
Figure 2 Association between osteoarthritis (OA) and dementia risk. + indicates that the same author has 2 studies.
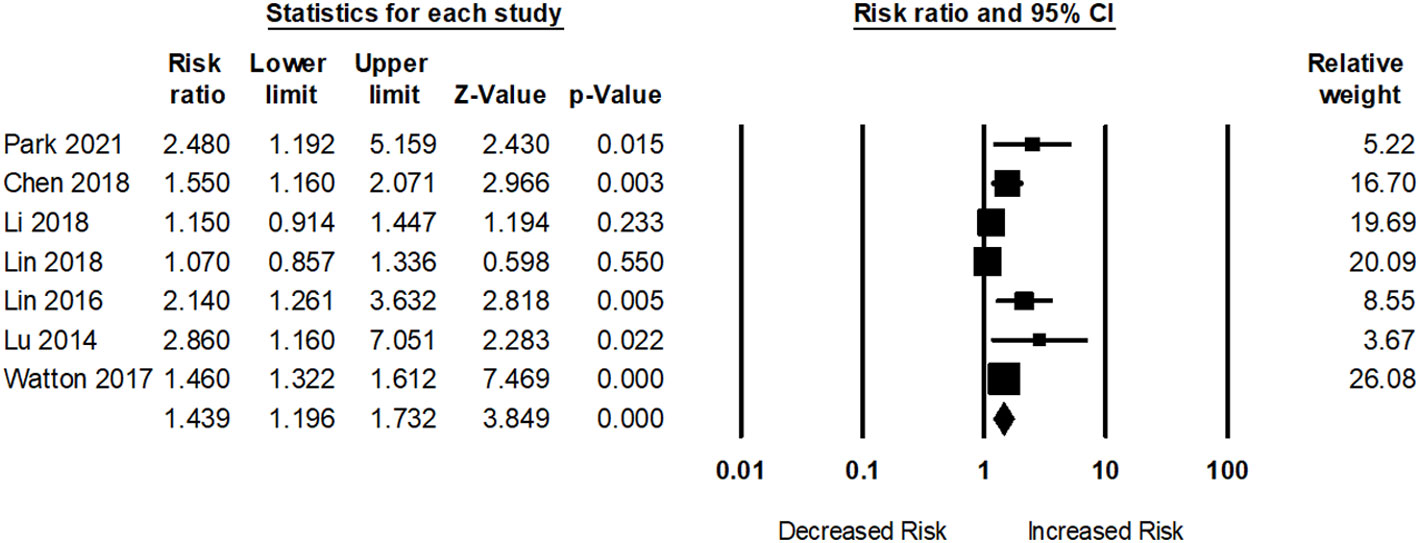
Seven studies assessed dementia risk in participants with SLE. As presented in Figure 3, participants with SLE had an elevated dementia risk (pooled RR: 1.43; 95% CI: 1.19–1.73) compared to those without SLE. The I2 statistic among these studies was 64.38%, indicating a moderate risk of heterogeneity.
Eight studies evaluated dementia risk among participants with SS. Participants with SS were associated with a significantly greater risk of dementia (pooled RR: 1.26; 95% CI: 1.14–1.39) compared to those without SS (Figure 4). The I2 statistics of these studies were 61.36%, indicating a moderate heterogeneity risk.
In total, ten studies examined the risk of dementia among participants with RA. Participants with RA were not related to any dementia risk (pooled RR: 0.98; 95% CI: 0.90–1.07) compared to those without RA (Figure 5). The I2 statistic among these studies was 89.61%, indicating a high risk of heterogeneity.
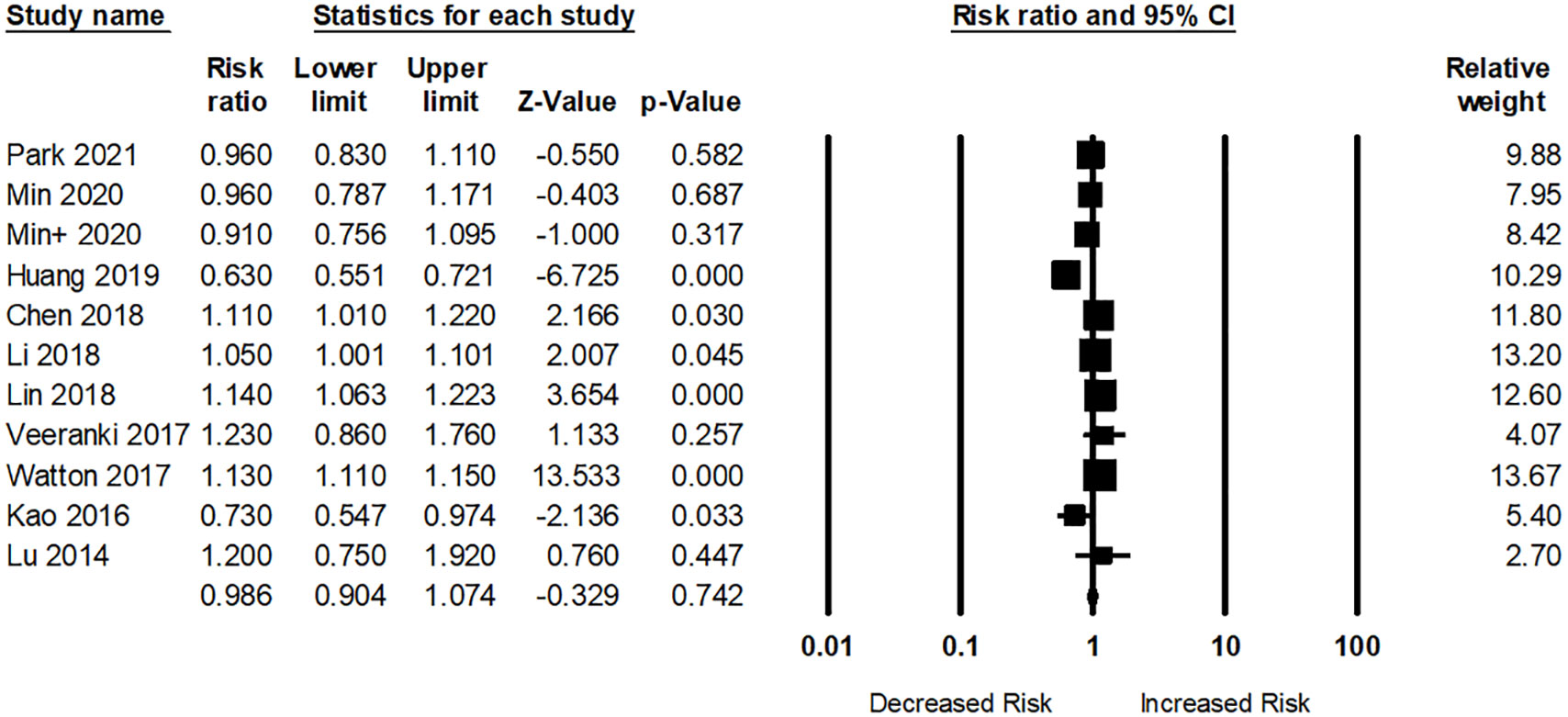
Figure 5 Association between rheumatoid arthritis (RA) and dementia risk. + indicates that the same author has 2 studies.
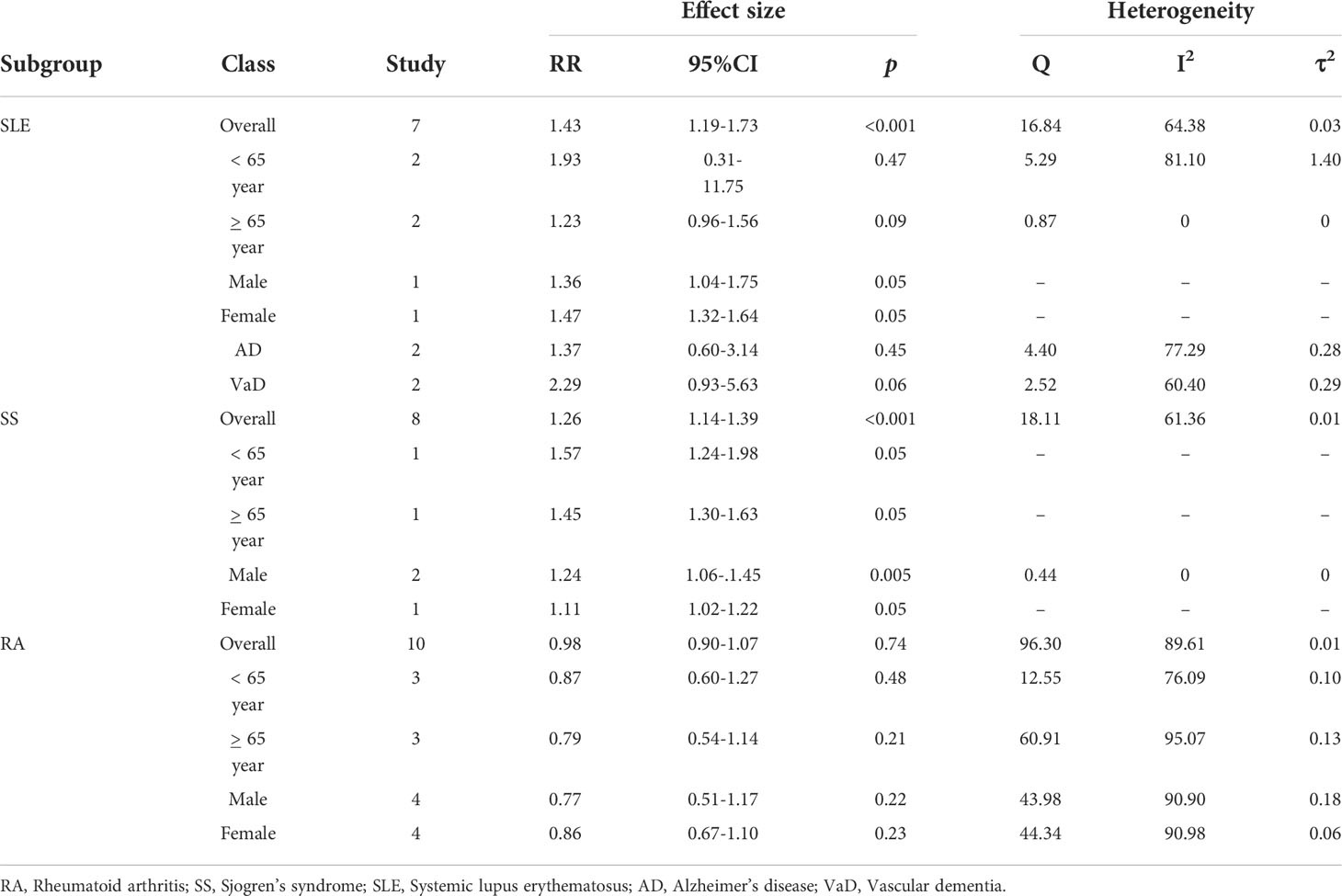
Subgroup analyses
Table 2 shows the results of subgroup analyses. Two studies assessed the risk of dementia in participants aged >65 years with SLE. For participants aged <65 years, the adjusted pooled RR was 1.93; 95% CI: 0.31–11.75 and the heterogeneity of the studies was high (I2 = 81.10, Q = 5.29, tau2 = 1.10). For participants aged <65 years, the adjusted pooled RR was 1.23; 95% CI: 0.96–1.56 and the heterogeneity in these studies was very low (I2 = 0, Q = 0.87, tau2 = 0). However, the risk of dementia among SLE patients was higher in female in comparison with male (RR: 1.47; 95% CI: 1.32–1.64 vs RR: 1.36; 95% CI: 1.04–1.75.
The dementia risk was slightly higher in female than male participants with RA (RR: 0.86 vs RR: 0.77). However, the risk of excessive dementia was significantly higher in male than females among SS participants (RR: 1.24 vs RR: 1.11).
Risk of bias
Figure 6 illustrates the funnel plots for publication bias. Figure 6A shows a funnel plot that demonstrates the publication bias of the studies. However, Figures 6B–D showed no publication bias. Egger’s regression test was used to calculate funnel asymmetry, which indicated publication bias (p<0.03).

Figure 6 Funnel plots for the association between dementia risk and (A) OA, (B) SLE, (C) SS, and (D) RA.
Figure 7 illustrates funnel plots for publication bias after applying the trim-and-fill method. Figures 7A–D shows funnel plots with missing research inserted using the trim-and-fill method. There was no missing data imputed in the plots. The overall log risk ratios of Figures 7A-D were: 1.31; 95% CI: 1.15–1.49, 1.36; 95% CI: 1.12–1.64, 1.26; 95% CI: 1.14–1.39, and 0.98; 95% CI: 0.90–1.07, respectively.
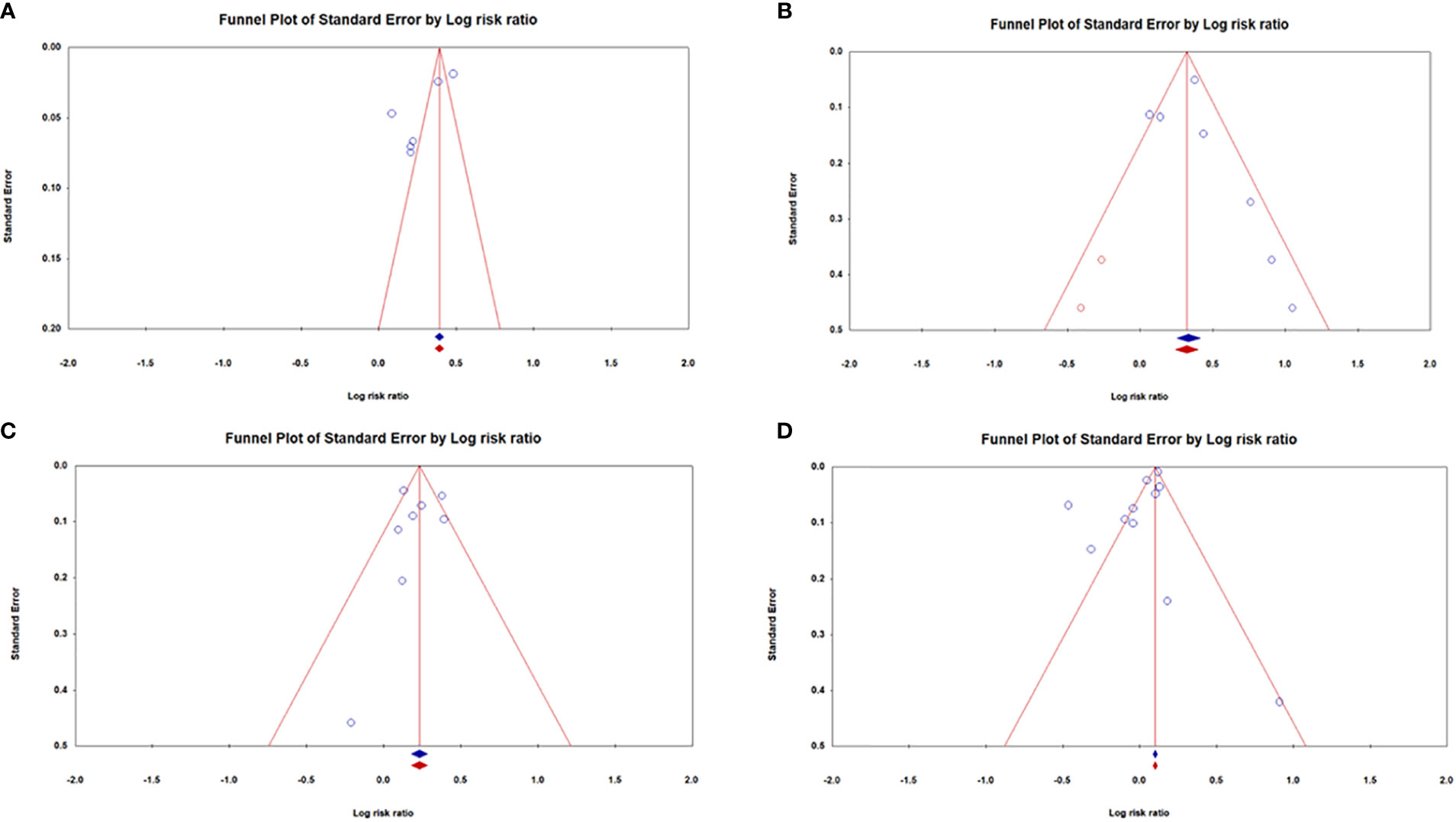
Figure 7 Funnel plots for the association between dementia risk and (A) OA, (B) SLE, (C) SS, and (D) RA after using a trim-and-fill method to diminish the consequences of existing publication bias.
Discussion
Main findings
To the best of our knowledge, this is the first meta-analysis examining the association between SRDs and dementia. An analysis based on 17 observational studies showed that SRDs, such as OA, SLE, and SS, were associated with an increased risk of dementia. However, no relationship was found between RA and the risk of dementia.
The relationship between chronic inflammation and dementia is well established. SRDs such as OA, SLE, and SS, which trigger systemic or organ-specific inflammation, can induce cognitive impairment (30, 36). There is no clear evidence of a pertinent mechanism between them. Several possibilities may help explain the biological mechanism because SRDs and dementia share common risk factors. Previous biological studies have reported that neuroinflammation, a prominent hallmark of dementia, is triggered by microglial cell activation (40–43). Activated microglial cells are further divided into M1 (classical phenotype) and M2 (alternatively activated phenotype) phenotypes. The M1 phenotype releases overwhelming amounts of inflammatory cytokines such as IL-1β, IL-6, IL-11, IL-12, and TNF-α, which may cause cell apoptosis and ultimately lead to loss of neurons (44–46). In addition, the M2 phenotype increases the production of pro-inflammatory cytokines [IL-4, interferon-gamma-inducible protein (IP)-10] and reactive oxygen species (ROS) (47). Recently, the M1/M2 paradigm of microglial cell activation has been evaluated in some neurodegenerative diseases to discover some of the explicit biological mechanisms of immunopathogenesis (48).
Previous studies have also shown that the level of serum IL-1b is extremely high in dementia participants compared to controls (48, 49). Increasing evidence has shown an essential factor for IL-1b overexpression and the deterioration of tau phosphorylation function and tangle formation (50). Moreover, a higher level of IL-1b can hamper the amyloid-beta (Aβ) clearance functions of microglia (51) and increase blood-brain barrier permeability, which is mainly responsible for the accumulation of Aβ in the brain (19, 52, 53). Pro-inflammatory cytokines, such as TNF, IL-1, IL-6, and IL-17 in SRDs may increase the risk of dementia through cell apoptosis and neuronal damage (54). Another reason is the use of anti-inflammatory drugs that are linked to an elevated risk of hypertension and cardiovascular disease, both of which are thought to be the main hallmarks of dementia and cognitive impairment (55). Moreover, the use of glucocorticoids can decrease the volume of the hippocampus and accelerate dementia (56). Recently, an investigation has demonstrated that the use of disease-modifying anti-rheumatic drugs (DMARDs) can increase the risk of dementia (57) because methotrexate may reduce the level of folic acid in the brain (58) and alter the amino acids of the hippocampus (59). Nevertheless, our results showed that RA was not associated with an increased risk of dementia. Meanwhile, earlier researchers have also reported that the use of anti-inflammatory drugs or granulocyte macrophage colony-stimulating factors (GM-CSFs) can decrease the risk of dementia in RA participants (60, 61). An alternative mechanism may affect cognitive function or the risk of dementia in patients with lupus. Several studies have highlighted the association between antiphospholipid autoantibodies and cognitive problems or dementia, including patients with antiphospholipid autoantibodies and lupus (62, 63). RA pathogenesis of RA is the production of anti-cyclic citrullinated peptide antibody (anti-CCP Ab), and periodontitis is a widely known environmental trigger. Moreover, epidemiological studies also showed an association between periodontitis and dementia (40, 64, 65). A previous study reported that non-steroidal anti-inflammatory drugs (NSAIDs) reduced the risk of dementia in collagen-induced arthritis (CIA) by normalizing the activated mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) pathways (66). More biological studies are warranted to determine the relationship between RA and reduction in dementia.
The primary advantage of the present study is the utilization of an extensive search strategy to identify and select studies and to evaluate the risk bias using standard guidelines, thereby minimizing selection bias. An expert researcher (with more than five years of experience in systematic reviews and meta-analyses) and an epidemiologist developed the search strategy, which was further validated by three senior faculty members. Our study had some limitations. First, all the studies used diagnostic codes. Therefore, selection bias might exist. Second, the majority of these studies were published in Asian countries, which might have introduced some geographical bias. Third, no study has reported the duration of SRDs or the risk of dementia. Therefore, we were unable to calculate the effect of time on the risk of dementia. Fourth, our analysis could not categorize dementia subtypes (e.g., AD and vascular dementia) with SRDs due to a lack of data. Fifth, a significant heterogeneity was observed in the meta-analysis. Different study designs, geographical regions, and confounding factors may have contributed to this heterogeneity. Therefore, a random effects model was utilized because it could estimate the mean value of the distribution of effect sizes for heterogeneous populations. We also conducted subgroup analyses based on the study design and methodological quality (Supplementary Table S3). Finally, a small number of studies assessed OA and dementia risks that prevented the application of funnel plots to measure actual publication bias. Furthermore, we applied the trim-and-fill method to check the variance of the observed and adjusted values (67).
Conclusion
This systematic review and meta-analysis found that SRDs, such as OA, SLE, and SS, were associated with an increased risk of dementia. However, no significant association was observed between RA and dementia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Y-CW and W-MK proposed the research idea and wrote the draft. M-SL and C-CW performed the analysis. AP-HH supported and contributed the literature review. Y-CW, M-SL, and W-MK helped revise the manuscript. M-SL and AP-HH provided clinical suggestions. Y-CW and W-MK supported data analysis and prepared the manuscript for submission. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1054246/full#supplementary-material
References
1. Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: A meta-analysis. Neurology (2010) 74(12):995–1002. doi: 10.1212/WNL.0b013e3181d5a4a3
2. Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen P-A, et al. Benzodiazepine use and risk of dementia in the elderly population: A systematic review and meta-analysis. Neuroepidemiology (2016) 47(3-4):181–91. doi: 10.1159/000454881
3. Hashimoto V, Jacinto AF, Araújo LMQ, Cendoroglo MS. Almada filho CdM: Cognitive impairment and metabolic syndrome in a population of Brazilian oldest-old. Rev da Associação Méd Bras (2021) 67:496–9. doi: 10.1590/1806-9282.20200940
4. Organization WHO. Global status report on the public health response to dementia. (2021). Available at: https://digitalcommons.fiu.edu/srhreports/health/health/65/.
5. Poly TN, Islam MM, Walther BA, Yang H-C, Wu C-C, Lin M-C, et al. Association between use of statin and risk of dementia: A meta-analysis of observational studies. Neuroepidemiology (2020) 54(3):214–26. doi: 10.1159/000503105
6. Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat Rev Neurol (2021) 17(3):157–72. doi: 10.1038/s41582-020-00435-y
7. Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with parkinson’s disease and alzheimer’s disease: Similarities and differences. J Alzheimer’s Dis (2007) 11(4):509–19. doi: 10.3233/JAD-2007-11412
8. Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun (1984) 120(3):885–90. doi: 10.1016/S0006-291X(84)80190-4
9. Sheng M, Sabatini BL, Südhof TC. Synapses and alzheimer’s disease. Cold Spring Harbor Perspect Biol (2012) 4(5):a005777. doi: 10.1101/cshperspect.a005777
10. Latta CH, Brothers HM, Wilcock DM. Neuroinflammation in alzheimer’s disease; a source of heterogeneity and target for personalized therapy. Neuroscience (2015) 302:103–11. doi: 10.1016/j.neuroscience.2014.09.061
11. Tao Q, Ang TFA, DeCarli C, Auerbach SH, Devine S, Stein TD, et al. Association of chronic low-grade inflammation with risk of Alzheimer disease in ApoE4 carriers. JAMA netw Open (2018) 1(6):e183597. doi: 10.1001/jamanetworkopen.2018.3597
12. Luchsinger JA, Mayeux R. Dietary factors and alzheimer’s disease. Lancet Neurol (2004) 3(10):579–87. doi: 10.1016/S1474-4422(04)00878-6
13. Yu KH, See LC, Kuo CF, Chou IJ, Chou MJ. Prevalence and incidence in patients with autoimmune rheumatic diseases: A nationwide population-based study in Taiwan. Arthritis Care Res (2013) 65(2):244–50. doi: 10.1002/acr.21820
14. Toledano E, Candelas G, Rosales Z, Prada CM, León L, Abásolo L, et al. A meta-analysis of mortality in rheumatic diseases. Reumatol clin (2012) 8(6):334–41. doi: 10.1016/j.reuma.2012.05.006
15. Bournia V-K, Fragoulis GE, Mitrou P, Mathioudakis K, Tsolakidis A, Konstantonis G, et al. All-cause mortality in systemic rheumatic diseases under treatment compared with the general population, 2015–2019. RMD Open (2021) 7(3):e001694. doi: 10.1136/rmdopen-2021-001694
16. Tan Z, Beiser A, Vasan R, Roubenoff R, Dinarello C, Harris T, et al. Inflammatory markers and the risk of Alzheimer disease: The framingham study. Neurology (2007) 68(22):1902–8. doi: 10.1212/01.wnl.0000263217.36439.da
17. Tobinick EL. Inflammatory markers and the risk of Alzheimer disease: The framingham study. Neurology (2008) 70(14):1222–3. doi: 10.1212/01.wnl.0000307660.86647.7b
18. Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Su K, et al. Perry V: Systemic inflammation and disease progression in Alzheimer disease. Neurology (2009) 73(10):768–74. doi: 10.1212/WNL.0b013e3181b6bb95
19. Hanly JG, Legge A, Kamintsky L, Friedman A, Hashmi JA, Beyea SD, et al. Role of autoantibodies and blood–brain barrier leakage in cognitive impairment in systemic lupus erythematosus. Lupus Sci Med (2022) 9(1):e000668. doi: 10.1136/lupus-2022-000668
20. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
21. Poly TN, Islam MM, Yang HC, Lin MC, Jian W-S, Hsu M-H, et al. Obesity and mortality among patients diagnosed with COVID-19: a systematic review and meta-analysis. Front Med (2021) 8. doi: 10.3389/fmed.2021.620044
22. Poly T, Islam M, Yang H-C, Wu C, Li Y-C. Proton pump inhibitors and risk of hip fracture: A meta-analysis of observational studies. Osteoporos Int (2019) 30(1):103–14. doi: 10.1007/s00198-018-4788-y
23. Huang S-W, Wang W-T, Chou L-C, Liao C-D, Liou T-H, Lin H-W. Osteoarthritis increases the risk of dementia: A nationwide cohort study in Taiwan. Sci Rep (2015) 5(1):1–7. doi: 10.1038/srep10145
24. Swain S, Coupland C, Mallen C, Kuo CF, Sarmanova A, Bierma-Zeinstra S, et al. Temporal relationship between osteoarthritis and comorbidities: a combined case control and cohort study in the UK primary care setting. Rheumatology (2021) 60(9):4327–39. doi: 10.1093/rheumatology/keab067
25. Park H, D-h Y, Ochirpurev B, Eom S-Y, Choi IA, Ju G. Kim JH: Association between dementia and systemic rheumatic disease: A nationwide population-based study. PloS One (2021) 16(3):e0248395. doi: 10.1371/journal.pone.0248395
26. Innes KE, Sambamoorthi U. The association of osteoarthritis and related pain burden to incident alzheimer’s disease and related dementias: A retrospective cohort study of US Medicare beneficiaries. J Alzheimer’s Dis (2020) 75(3):789–805. doi: 10.3233/JAD-191311
27. Min C, Bang WJ, Kim M, Oh DJ, Choi HG. Rheumatoid arthritis and neurodegenerative dementia: a nested case-control study and a follow-up study using a national sample cohort. Clin Rheumatol (2020) 39(1):159–66. doi: 10.1007/s10067-019-04769-x
28. Huang L-C, Chang Y-H, Yang Y-H. Can disease-modifying anti-rheumatic drugs reduce the risk of developing dementia in patients with rheumatoid arthritis? Neurotherapeutics (2019) 16(3):703–9. doi: 10.1007/s13311-019-00715-6
29. Chen H-H, Perng W-T, Chiou J-Y, Wang Y-H, Huang J-Y, Wei JC-C. Risk of dementia among patients with sjogren’s syndrome: A nationwide population-based cohort study in Taiwan. In: Seminars in arthritis and rheumatism, Elsevier (2019). 48(5):895–9.
30. Chen KT, Chen YC, Fan YH, Lin WX, Lin WC, Wang YH, et al. Rheumatic diseases are associated with a higher risk of dementia: A nation-wide, population-based, case-control study. Int J rheum Dis (2018) 21(2):373–80. doi: 10.1111/1756-185X.13246
31. Li X, Sundquist J, Zöller B, Sundquist K. Dementia and alzheimer’s disease risks in patients with autoimmune disorders. Geriatr gerontol Int (2018) 18(9):1350–5. doi: 10.1111/ggi.13488
32. Wang J-H, Wu Y-J, Tee BL, Lo RY. Medical comorbidity in alzheimer’s disease: A nested case-control study. J Alzheimer’s Dis (2018) 63(2):773–81. doi: 10.3233/JAD-170786
33. Lin T-M, Chen W-S, Sheu J-J, Chen Y-H, Chen J-H, Chang C-C. Autoimmune rheumatic diseases increase dementia risk in middle-aged patients: A nationwide cohort study. PloS One (2018) 13(1):e0186475. doi: 10.1371/journal.pone.0186475
34. Kao L-T, Kang J-H, Lin H-C, Huang C-C, Lee H-C, Chung S-D. Rheumatoid arthritis was negatively associated with alzheimer’s disease: A population-based case-control study. PloS One (2016) 11(12):e0168106. doi: 10.1371/journal.pone.0168106
35. Lin YR, Chou LC, Chen HC, Liou TH, Huang SW, Lin HW. Increased risk of dementia in patients with systemic lupus erythematosus: A nationwide population-based cohort study. Arthritis Care Res (2016) 68(12):1774–9. doi: 10.1002/acr.22914
36. Lu K, Wang H-K, Yeh C-C, Huang C-Y, Sung P-S, Wang L-C, et al. Association between autoimmune rheumatic diseases and the risk of dementia. BioMed Res Int (2014) 2014:861812. doi: 10.1155/2014/861812
37. Kang J-H, Lin H-C. Comorbidities in patients with primary sjögren’s syndrome: A registry-based case-control study. J Rheumatol (2010) 37(6):1188–94. doi: 10.3899/jrheum.090942
38. Wotton CJ, Goldacre MJ. Associations between specific autoimmune diseases and subsequent dementia: retrospective record-linkage cohort study, UK. J Epidemiol Community Health (2017) 71(6):576–83. doi: 10.1136/jech-2016-207809
39. Veeranki SP, Downer B, Jupiter D, Wong R. Arthritis and risk of cognitive and functional impairment in older Mexican adults. J Aging Health (2017) 29(3):454–73. doi: 10.1177/0898264316636838
40. Leira Y, Dominguez C, Seoane J, Seoane-Romero J, Pías-Peleteiro JM, Takkouche B, et al. Is periodontal disease associated with alzheimer’s disease? A systematic review with meta-analysis. Neuroepidemiology (2017) 48(1-2):21–31. doi: 10.1159/000458411
41. Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in alzheimer’s disease. Lancet Neurol (2015) 14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5
42. Morales I, Guzmán-Martínez L, Cerda-Troncoso C, Farías GA, Maccioni RB. Neuroinflammation in the pathogenesis of alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci (2014) 8:112. doi: 10.3389/fncel.2014.00112
43. Zhang F, Jiang L. Neuroinflammation in alzheimer’s disease. Neuropsychiatr Dis Treat (2015) 11:243. doi: 10.2147/NDT.S75546
44. Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci (2007) 8(1):57–69. doi: 10.1038/nrn2038
45. Kumar M, Verma S, Nerurkar VR. Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death. J Neuroinflamm (2010) 7(1):1–14. doi: 10.1186/1742-2094-7-73
46. Brabers N, Nottet H. Role of the pro-inflammatory cytokines TNF-α and IL-1β in HIV-associated dementia. Eur J Clin Invest (2006) 36(7):447–58. doi: 10.1111/j.1365-2362.2006.01657.x
47. Ghosh M, Xu Y, Pearse DD. Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines. J Neuroinflamm (2016) 13(1):1–14. doi: 10.1186/s12974-015-0463-9
48. Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol (2016) 53(2):1181–94. doi: 10.1007/s12035-014-9070-5
49. Zuliani G, Guerra G, Ranzini M, Rossi L, Munari M, Zurlo A, et al. High interleukin-6 plasma levels are associated with functional impairment in older patients with vascular dementia. Int J Geriatr Psychiatry (2007) 22(4):305–11. doi: 10.1002/gps.1674
50. Sheng J, Zhu S, Jones R, Griffin W, Mrak R. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp Neurol (2000) 163(2):388–91. doi: 10.1006/exnr.2000.7393
51. Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, et al. Locus ceruleus controls alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci (2010) 107(13):6058–63. doi: 10.1073/pnas.0909586107
52. Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, et al. Interleukin-1β induces blood–brain barrier disruption by downregulating sonic hedgehog in astrocytes. PloS One (2014) 9(10):e110024. doi: 10.1371/journal.pone.0110024
53. Kamintsky L, Beyea SD, Fisk JD, Hashmi JA, Omisade A, Calkin C, et al. Response to:’Correspondence on ‘Blood-brain barrier leakage in systemic lupus erythematosus is associated with gray matter loss and cognitive impairment’’by pamuk and hasni. Ann Rheum Dis (2021). doi: 10.1136/annrheumdis-2021-220057
54. Shaw AT, Gravallese EM. Mediators of inflammation and bone remodeling in rheumatic disease. In: Seminars in cell & developmental biology, Elsevier (2016). 49:2–10.
55. Gorelick PB, Counts SE, Nyenhuis D. Vascular cognitive impairment and dementia. Biochim Biophys Acta (BBA)-Mol Basis Dis (2016) 1862(5):860–8. doi: 10.1016/j.bbadis.2015.12.015
56. Kenna HA, Poon AW, de los Angeles CP, Koran LM. Psychiatric complications of treatment with corticosteroids: Review with case report. Psychiatry Clin Neurosci (2011) 65(6):549–60. doi: 10.1111/j.1440-1819.2011.02260.x
57. Chou M-H, Wang J-Y, Lin C-L, Chung W-S. DMARD use is associated with a higher risk of dementia in patients with rheumatoid arthritis: a propensity score-matched case–control study. Toxicol Appl Pharmacol (2017) 334:217–22. doi: 10.1016/j.taap.2017.09.014
58. Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, et al. Low folate status is associated with impaired cognitive function and dementia in the Sacramento area Latino study on aging. Am J Clin Nutr (2005) 82(6):1346–52. doi: 10.1093/ajcn/82.6.1346
59. Madhyastha S, Somayaji S, Rao M, Nalini K, Bairy KL. Hippocampal brain amines in methotrexate-induced learning and memory deficit. Can J Physiol Pharmacol (2002) 80(11):1076–84. doi: 10.1139/y02-135
60. Andersen K, Launer L, Ott A, Hoes A, Breteler M, Hofman A. Do nonsteroidal anti-inflammatory drugs decrease the risk for alzheimer’s disease?: The Rotterdam study. Neurology (1995) 45(8):1441–5. doi: 10.1212/WNL.45.8.1441
61. McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for alzheimer’s disease: A review of 17 epidemiologic studies. Neurology (1996) 47(2):425–32. doi: 10.1212/WNL.47.2.425
62. Donnellan C, Cohen H, Werring DJ. Cognitive dysfunction and associated neuroimaging biomarkers in antiphospholipid syndrome: A systematic review. Rheumatology (2022) 61(1):24–41. doi: 10.1093/rheumatology/keab452
63. Hassan F, Naffaa ME, Saab A, Putterman C. Cognitive impairment in anti-phospholipid syndrome and anti-phospholipid antibody carriers. Brain Sci (2022) 12(2):222. doi: 10.3390/brainsci12020222
64. Lee YL, Hu HY, Huang LY, Chou P, Chu D. Periodontal disease associated with higher risk of dementia: population-based cohort study in Taiwan. J Am Geriatr Soc (2017) 65(9):1975–80. doi: 10.1111/jgs.14944
65. Ma K, Hasturk H, Carreras I, Dedeoglu A, Veeravalli J, Huang J, et al. Dementia and the risk of periodontitis: A population-based cohort study. J Dental Res (2022) 101(3):270–7. doi: 10.1177/00220345211037220
66. Zhang N-Y, Wang T-H, Chou C-H, Wu K-C, Yang C-R, Kung F-L, et al. Ibuprofen treatment ameliorates memory deficits in rats with collagen-induced arthritis by normalizing aberrant MAPK/NF-κB and glutamatergic pathways. Eur J Pharmacol (2022) 2022:175256. doi: 10.1016/j.ejphar.2022.175256
Keywords: systemic rheumatic diseases, osteoarthritis, rheumatoid arthritis, systemic lupus erythematosus, Sjogren’s syndrome, dementia
Citation: Wang Y-C, Lin M-S, Huang AP-H, Wu C-C and Kung W-M (2022) Association between systemic rheumatic diseases and dementia risk: A meta-analysis. Front. Immunol. 13:1054246. doi: 10.3389/fimmu.2022.1054246
Received: 26 September 2022; Accepted: 17 October 2022;
Published: 09 November 2022.
Edited by:
James Cheng-Chung Wei, Chung Shan Medical University Hospital, TaiwanReviewed by:
Po Cheng Shih, Changhua Christian Hospital, TaiwanKevin Sheng-Kai Ma, University of Pennsylvania, United States
Copyright © 2022 Wang, Lin, Huang, Wu and Kung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Woon-Man Kung, bnNrdW5nd21AeWFob28uY29tLnR3
 Yao-Chin Wang
Yao-Chin Wang Muh-Shi Lin
Muh-Shi Lin Abel Po-Hao Huang
Abel Po-Hao Huang Chieh-Chen Wu
Chieh-Chen Wu Woon-Man Kung
Woon-Man Kung