- Department of Dermatology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, China
Objective: This study aimed to investigate the clinical features of biologics-induced bullous pemphigoid (BP) and the therapeutic effects of those agents for BP, exploring the underlying pathophysiological mechanisms.
Methods: We searched PubMed, Web of Science, and Elsevier for studies involving pemphigoid patients treated with or induced by identical biologics published in English from January 2009 to April 2022.
Results: Seventeen cases of drug-induced BP associated with anti-tumor necrosis factor (aTNF)-α therapies, one with interleukin (IL)-17 inhibitors, and seven with IL-12/IL-23 or IL-23 inhibitors were enrolled. Time to cutaneous toxicity varied among different types of agents, and the characteristics of clinical examinations were similar to idiopathic BP. Discontinuation of the culprit drugs and initiation of topical or systemic corticosteroids were adequate in most cases. Several monoclonal antibodies above have also been reported for the treatment of refractory or recurrent BP, especially concurrent with psoriasis.
Conclusion: Biologics for immune-related diseases, including TNF-α, IL-17, and IL-12/IL-23 or IL-23 inhibitors, can both induce and treat BP, which might be associated with a helper T cells Th1/Th2 imbalance, complicated inflammatory networks, and a specific individual microenvironment, suggestive of a new perspective on the therapeutic algorithms of BP. There have been numerous reports about biologics inducing or treating BP. We have taken note of this phenomenon and focused on biologics with both pathogenetic and therapeutic effects on BP. Our review summarized the clinical characteristics of associated cases, trying to figure out the underlying mechanisms of this paradoxical phenomenon and to provide an integrated perspective and new therapeutic alternatives for BP.
Introduction
Bullous pemphigoid (BP) is a common autoimmune blistering disease characterized by intense bullae on normal or erythematous skin with prominent pruritus, subepidermal blisters on histological examination, and immunopathological findings showing immunoglobulin (Ig) G and complement (C)3 deposition at the basement membrane zone (BMZ). Autoantibodies targeting BP180 and BP230 lead to the degradation of the BMZ and bulla formation, which can be detected in the sera of most patients (1).
The pathogenesis of BP is quite unclarified, and lots of triggering and predisposing factors, such as central neurological disorders, old age, infections, and medications, were reported to be associated with its occurrence. Administration of specific topical or systemic drugs can be identified as a causal factor of BP (2). Noteworthy, with biologics increasingly utilized in treating a broader spectrum of immune-related diseases, such as psoriasis, rheumatoid arthritis, and inflammatory bowel diseases, biologics-induced BP has been reported more and was highlighted in our review. In addition to BP, other autoimmune blistering diseases (AIBDs), such as pemphigus, pemphigoid nodularis, and linear IgA bullous dermatosis, induced by biologics have also been reported. There may be an underlying etiologic relationship between biological agents and the onset of AIBDs.
Dramatically, biologics-induced BP has also shown an unexpected treatment effect in some reports. Programmed cell death protein-1 (PD-1)/programmed death ligand-1 (PD-L1) inhibitors, the mainstay of biologics causing BP, have been reported in many cases and reviews (3, 4), but to our knowledge, there were no reports to illustrate their roles in the treatment of BP. Hence, in this paradoxical phenomenon, we mainly focused on biologics including tumor necrosis factor (TNF)-α, interleukin (IL)-17, and IL-12/IL-23 or IL-23 inhibitors that have effects of both inducing and treating BP.
Tumor Necrosis Factor-α blockers
TNF-α blockers inducing BP
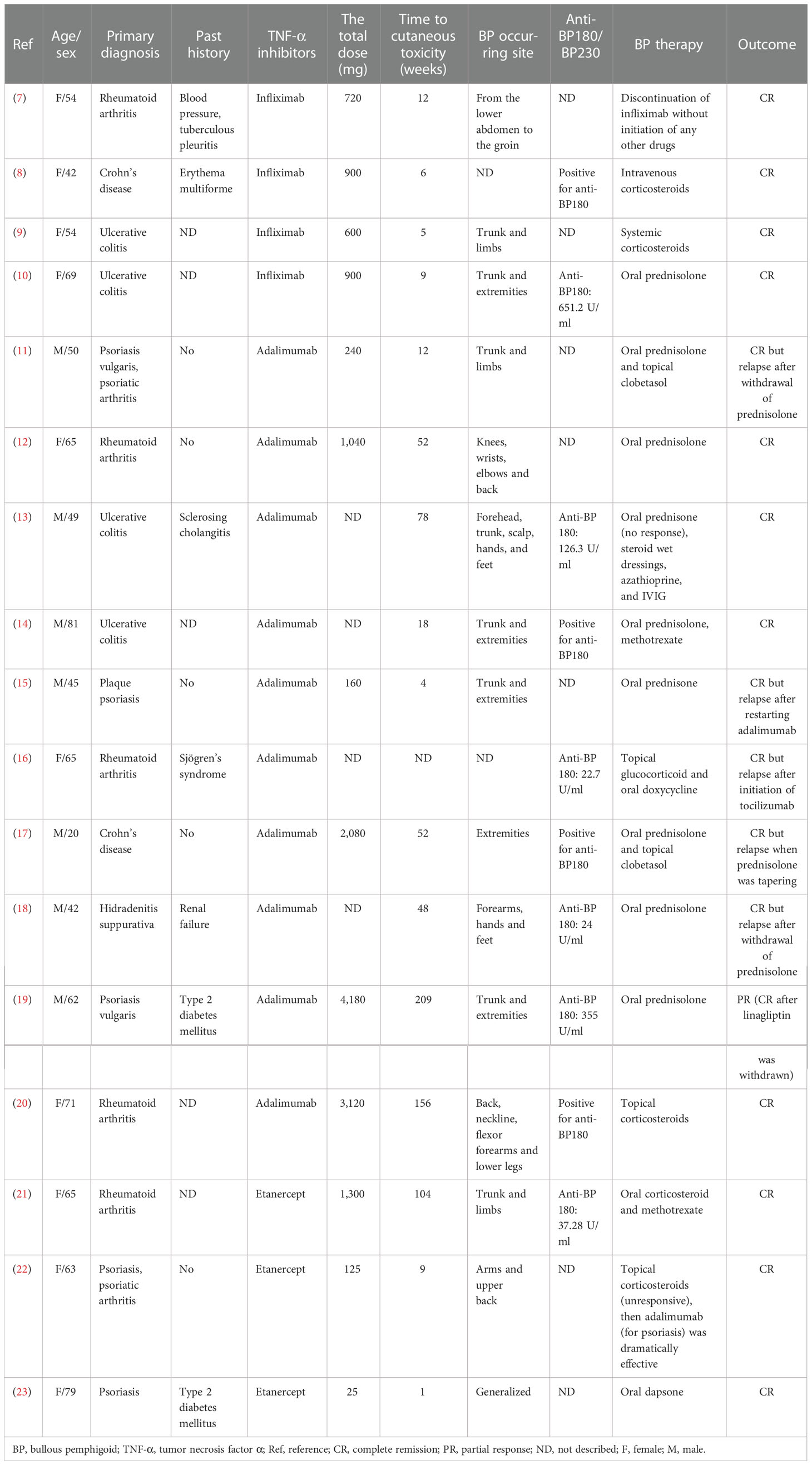
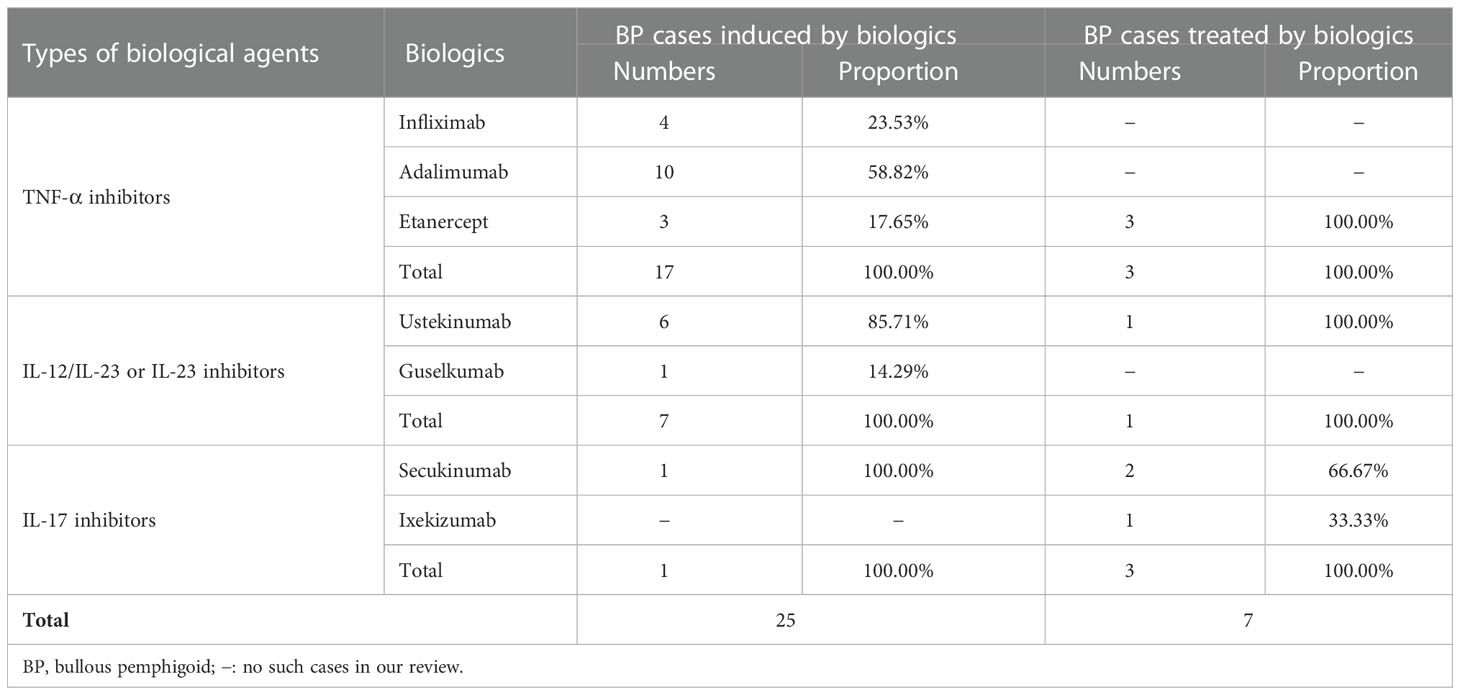
TNF-α blockers, or anti-TNF-α (aTNF-α), have been involved in the treatment of psoriasis vulgaris, rheumatoid arthritis, and inflammatory bowel disease and were recently approved for hidradenitis suppurativa. In the past decades, a growing number of cases of adverse effects, especially autoimmune diseases, have been reported, such as cutaneous vasculitis, lupus-like syndrome, systemic lupus erythematosus, and BP (5, 6). Reviewing the published literature from January 2009 to April 2022, we collected 17 cases (7–23) of aTNF-α-induced BP and analyzed the clinical characteristics (Tables 1, 2).
Demographic characteristics
Of the 17 cases, 10 women (58.8%) and seven men (41.2%), only one was a 20-year-old young man (17), and the rest were middle-aged and elderly people, who had almost comparable spontaneous BP. The median age of the patients was 57 (mean = 57; range 20–81) years. The primary diseases consisted of five cases of rheumatoid arthritis (29.4%), two cases of Crohn’s disease (11.8%), four cases of ulcerative colitis (23.5%), five cases of psoriasis including psoriatic arthritis (29.4%), and one case of hidradenitis suppurativa (5.9%). Four cases were treated with infliximab (23.5%), 10 with adalimumab (58.8%), and three with etanercept (17.6%) (Table 1).
Sixteen (94.1%) of the 17 patients developed blistering lesions in their entire clinical courses. The duration between the initiation of aTNF-α therapy and the onset of BP was variable. After excluding two unspecified cases, the median time from aTNF-α treatment to the onset of BP was 33 weeks (mean = 54 weeks; range 1–209 weeks). One patient, who developed BP within just 3 days of receiving etanercept (23), had the shortest time to bulla on our record. Most of the occurring locations were the trunks and extremities, while only in one case (23) was the initial BP localized on the perianal, perineal, and perivaginal areas. Only two (11.8%) subjects had BP with oral mucosal involvement.
Laboratory examinations
Skin biopsies were taken in 12 of the 17 cases for histological examination. Nine patients (75%, 9/12) presented with subepidermal blisters or clefts and three (25%, 3/12) with only inflammatory cell infiltration. A classification of cell infiltration has been described in 11 of the 12 cases. It was manifested in nine cases (81.8%, 9/11) as eosinophil infiltration, three (27.3%, 3/11) as lymphocyte infiltration, and one (9.1%, 1/11) as neutrophil infiltration.
Direct immunofluorescence (DIF) was performed in 12 of the 17 cases, of which 11 (91.7%, 11/12) cases were positive for linear deposition of IgG at the BMZ, 10 (83.3%,10/12) for C3, two (16.7%, 2/12) for IgA and IgM, respectively. Indirect immunofluorescence (IIF) using salt-split normal human skin as a substrate was detected in five patients. Four cases were positive for IgG deposits on the epidermal side, one of which demonstrated C3 deposition as well, and one patient was negative.
Ten (58.8%) of the 17 cases detailed the serum autoantibodies as positive. Apart from one case with unspecified types of BP antibodies, the remaining nine cases were positive for anti-BP180 at different concentrations.
Therapy and outcome
Fifteen of the 17 cases were treated with topical and/or systemic corticosteroids (Table 1). Systemic corticosteroids were applied in 12 cases intravenously or orally and were efficacious in most cases. Of these 12 cases, one patient (19), whose BP was induced by adalimumab and linagliptin, was in partial response with prednisolone but in complete remission after withdrawing linagliptin. In two cases, corticosteroids were combined with methotrexate for the treatment of BP due to a poor effect of the original medication in one patient.
Six (35.3%, 6/17) cases relapsed, and the others were in complete clinical remission. The cessation and tapering of corticosteroids induced recurrences in four cases, and BP was controlled with an increased dose of prednisolone. A reoccurrence in one patient was due to reintroducing adalimumab, and symptoms were relieved after ceasing the drug (15). In one case, the initiation of tocilizumab for rheumatoid arthritis induced a recurrence of BP, and switching from topical corticosteroids to oral achieved remission of the disease successfully (16).
TNF-α blockers treating BP
Little information is known regarding the therapeutic use of aTNF-α agents for BP. To our knowledge, there were three reported cases (24–26) of TNF-α antagonists (etanercept) successfully treating BP concomitant with psoriasis (Tables 2, 3).
Etanercept was started due to the poor effect of the original drugs (steroids or immunosuppressants), a worry about the potential relapse of psoriasis or converting to pustular psoriasis, and the recurrence or worsening of BP when corticosteroids were tapered. In those cases, all showed significant efficacy for both BP and psoriasis. Coincidentally, in one of our cases, BP triggered by etanercept was unresponsive to topical corticosteroids but settled dramatically after adalimumab administration (22). Interestingly, her psoriasis continued to deteriorate after the switch. Apart from BP, TNF-α inhibitors were found to be effective in the treatment of mucous membrane pemphigoid (31–33) and anti-laminin gamma-1 pemphigoid (34).
Pathogenesis of TNF-α blockers inducing or treating BP
The pathogenesis of BP induced by TNF-α blockers remains unclear and is considered to be linked to the interaction of various immune cascades. Three hypotheses have been put forward to explain the underlying mechanism. Firstly, increased cell apoptosis was found in patients receiving aTNF-α therapies due to which the autoantigen exposure increased and subsequently led to the formation of autoantibodies (35), consistent with one case in our records that reported coexisting anti-desmoglein (Dsg)3 and anti-BP180 autoantibodies (10). Secondly, these individuals have an unbalanced cytotoxic T-cell response, which relieves the suppression of autoresponsive B cells and results in increased autoantibody production (5, 35, 36). Thirdly, TNF-α blockers may have the ability to act as antigenic haptens to bind and modify protein molecules in the BMZ and make them susceptible to an immune attack (37). Autoantibodies targeting BP180 can lead to a decreased adhesion of BP180 and recruitment of inflammatory cells, including mast cells, eosinophils, and neutrophils, activating the inflammatory network consistent with various cytokines and proteases (38). This cascade, in turn, increases the production of TNF-α (Figure 1).
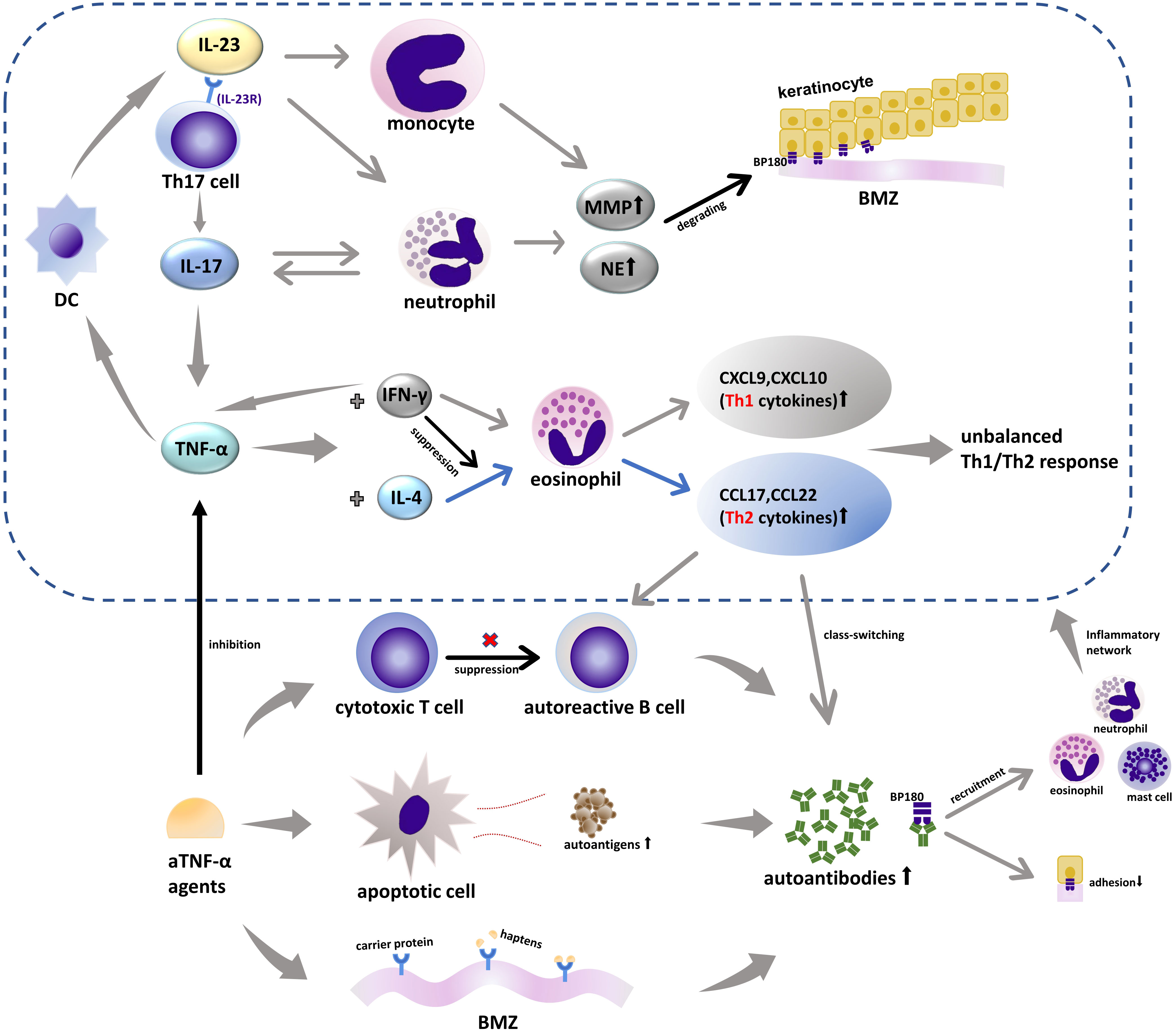
Figure 1 TNF-α-, IL-17-, and IL-23-associated inflammatory networks in BP and biologics. IL-23 acts as an upstream regulatory cytokine for the secretion of IL-17 from Th17 through IL-23R and can induce MMP-9 expression by monocytes alone. IL-17 can promote the production of a series of inflammatory molecules including TNF-α and increase the secretion of MMP-9 and NE from neutrophils that are involved in the formation of subepidermal blisters. TNF-α, which is considered as an upstream product of IL-23, can act on DCs that can secrete IL-23. TNF-α can also induce different immune responses dominated by Th1 or Th2 depending on the micro immune profile (the levels of IL-4 and IFN-γ). IFN-γ reduces the response of eosinophil to IL-4 plus TNF-α and can increase the production of endogenous TNF-α of different cell types in a specific microenvironment. aTNF-α agents increase the production of autoantibodies in three ways: increasing cell apoptosis and espousing autoantigens, inhibiting the cytotoxic T-cell response that was involved in suppressing autoreactive B cells, and acting as antigenic haptens to bind or modify proteins in the BMZ. TNF-α, tumor necrosis factor α; IL-17, interleukin 17; IL-23, interleukin 23; IL-23R, interleukin 23 receptor; MMP-9, matrix metalloproteinase 9; NE, neutrophil elastase; DC, dendritic cell; IL-4, interleukin 4; Th, helper T cells; IFN-γ, interferon γ; BMZ, basement membrane zone; CCL, chemokine (C-C motif) ligand; CXCL, C-X-C motif ligand.
TNF-α antagonists can also treat BP, and this paradoxical phenomenon has been noticed. Increased levels of TNF-α have been identified in the serum and blister fluid of BP patients (39, 40), and the serum levels of TNF-α are correlated with BP severity (41). TNF-α is pivotal in the secretion of Th1 [C-X-C motif ligand (CXCL)9, CXCL10]- and Th2 [chemokine (C-C motif) ligand (CCL)17, CCL22]-associated cytokines, which depends on the individual microenvironment, more specifically the level of IL-4 and interferon (IFN)-γ (2, 37, 42). The process of secreting CCL12 and CCL22 is regulated by IL-4 and significantly enhanced in combination with TNF-α, which is associated with a Th2-type response, whereas TNF-α and IFN-γ have a synergistic effect on the generation of CXCL9, CXCL10, and Th1 cytokines. Th2 cells and relevant cytokines can promote B cell proliferation and differentiation into autoreactive ones and the class-switching of autoantibodies, which is considered to be a dominant immune type in the development of BP (43, 44). Various inflammatory factors in the complicated network interact with each other, and individuals in different immune microenvironments will show different immune effects (Figure 1). Therefore, we postulate that aTNF-α can both treat Th1-driven diseases such as psoriasis and induce Th2-driven diseases like BP depending on the micro immune profile (the levels of IL-4 and IFN-γ). Namely, TNF-α blockers may potentially suppress the Th2 response, thus becoming an alternative therapy for BP, especially when accompanied by psoriasis.
Interleukin-12/Interleukin-23 or Interleukin-23 inhibitors
IL-12/IL-23 or IL-23 inhibitors inducing BP
Anti-IL-12/IL-23 or IL-23 therapies, inhibiting the activity of IL-23 and IL-12, have been approved for the treatment of psoriasis and Crohn’s disease. Observed adverse effects regarding dermatological manifestations include atopic dermatitis, cutaneous lupus erythematosus, BP, and so on. Collecting data from the published literature, we found seven cases (45–51) of BP associated with IL-12/IL-23 or IL-23 inhibitors (Table 4).
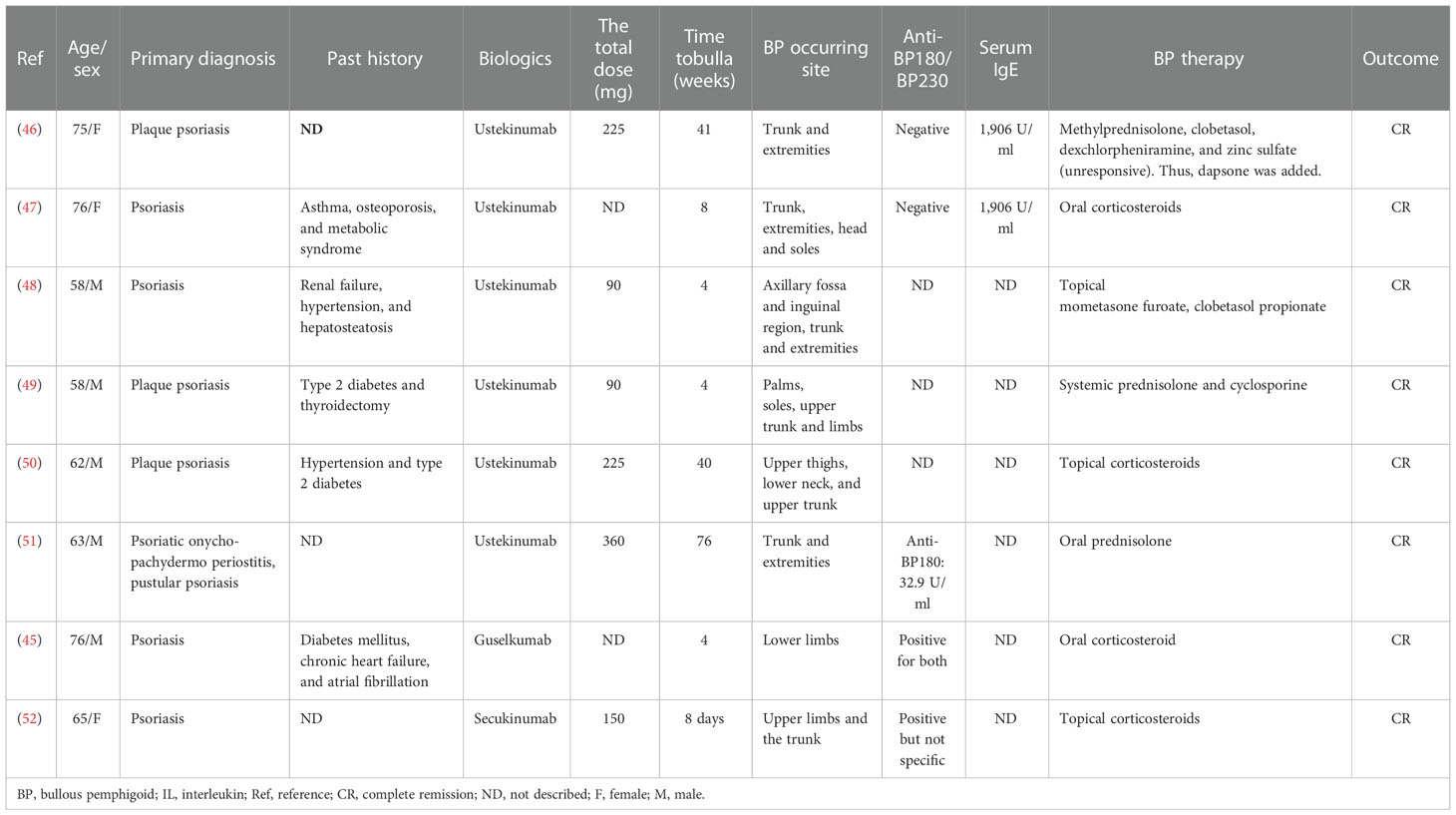
Table 4 Clinical characteristics of BP cases associated with IL-12/IL-23, IL-23, or IL-17 inhibitors.
Demographic characteristics
There were two women (28.6%) and five men (71.4%) with a median age of 63 (mean = 66; range 58–76) years. Guselkumab, an antibody inhibiting IL-23p19, was introduced in one case (14.3%) and ustekinumab, an antagonist of the p40 subunit of IL-12 and IL-23, in the remaining six patients (85.7%) for psoriasis treatment (Table 2). Anti-IL-12/IL-23 or IL-23 agents were administered at standard doses and induced blistering lesions during the treatment. The median time to bulla was 8 weeks (mean = 25 weeks; range 4–76 weeks). The trunks and extremities were the most frequently involved sites. Oral mucosal involvement was noticed in one subject (14.3%).
Laboratory examination
Histopathologic examinations in all cases showed a subepidermal blister (100%), with eosinophil infiltration in seven cases (100%, 7/7) and neutrophil and lymphocyte infiltration in one case (14.3%, 1/7), respectively.
DIF was performed in all cases, of which seven (100%, 7/7) cases were positive for IgG and C3, two (28.6%, 2/7) for IgM, one (14.3%, 1/7) for C4 and IgA linear deposits along the BMZ, respectively. IIF was documented as positive in five cases (71.4%, 5/7).
Surprisingly, autoantibodies were detected as positive in only two cases (28.6%, 2/7), for anti-BP180NC16a in one and both anti-BP180 and anti-BP230 antibodies in the other one. Eosinophilia and increased serum IgE were observed in two cases (28.6%, 2/7), respectively.
Therapy and outcome
All seven patients were given corticosteroids orally and/or topically. Systemic corticosteroids were applied as monotherapy in three cases and with a combination of cyclosporine in another. Systemic and topical steroids were administered in only one case, and dapsone was added to the regimen due to the unsuccessful control (46). Topical corticosteroid monotherapy was prescribed in the remaining two cases. Remission was achieved with the medications above, and no recurrences were observed in the seven patients in the follow-up.
IL-12/IL-23 or IL-23 inhibitors treating BP
From our knowledge, there was just one case illustrating the therapeutic use of IL-12/IL-23 inhibitors in treating typical BP (27) (Tables 2, 3). In this case, the patient with a long history of psoriasis preceding BP was given ustekinumab due to the recurrence and persistent clinical activity of both diseases, and improvements were rapidly achieved clinically and serologically. Also, cases of anti-laminin-1 pemphigoid that had difficulty in corticosteroid tapering (34) and refractory lichen planus pemphigoids (53, 54) that were successfully treated by IL-12/IL-23 blockers had been reported.
Pathogenesis of IL-12/IL-23 or IL-23 inhibitors inducing or treating BP
IL-23 is associated with the terminal differentiation of Th17 cells, the primary IL-17-producing cells in BP (55). The biological cascade of IL-17 is performed by an elevated production of a series of inflammatory molecules, including TNF-α (43, 55). Thus, IL-17 and IL-12/IL-23 or IL-23 inhibitors may share a similar mechanism to trigger BP with TNF-α blockers, modifying the immune response of Th1/Th2 and disinhibiting the autoreactive B cells, with TNF-α being a downstream proinflammatory cytokine (56). Additionally, there is such a cycle that dendritic cells (DCs) can secrete IL-23, and TNF-α, as an upstream product of IL-23, can also act on DCs, which makes the regulatory relationship among IL-23, IL-17, and TNF-α a small network (57) (Figure 1). Beyond the Th1/Th2 paradigm, other pathways for IL-17 and IL-12/IL-23 or IL-23 inhibitors are involved in the onset of BP. To our knowledge, there was only one case of IL-17 inhibitors inducing BP, and the author postulated that the preexisting low titer of anti-BP180 antibodies, secukinumab-related eosinophil activation, and increased level of Th17 cells during the initial secukinumab administration might contribute to the formation of BP (52). For IL-12/IL-23 inhibitors, however, it was also reported that the balance of Th1/Th2 was not altered during ustekinumab treatment, which indicates that there might be other unknown pathways independent of TNF-α (58, 59). Noteworthy, all of the reported BP cases induced by IL-12/IL-23 or IL-23 inhibitors had a previous history of aTNF-α treatment, increasing susceptibility to BP.
It was reported that there were increased levels of IL-17 and IL-23 in the lesional skin and serum of BP patients (60, 61). IL-17 can upregulate matrix metalloproteinase (MMP)-9 and neutrophil elastase expression from neutrophils, which were involved in the blistering formation and significant in the extension of the disease (60, 62). In addition to being an upstream regulator of IL-17, IL-23 alone can also enhance MMP-9 levels from monocytes (60) (Figure 1). That may explain the paradoxical phenomenon of why there were cases of BP successfully treated by IL-17 and IL-12/IL-23 blockers. Except for what is mentioned above, these biological agents may improve the manifestations of BP by controlling its triggers, as psoriasis in our study. However, the mechanism is not yet understood, and further investigations are warranted.
Interleukin-17 inhibitors
IL-17 inhibitors inducing BP
IL-17 inhibitors, including secukinumab, brodalumab, and ixekizumab, have been approved for treating moderate-to-severe psoriasis and assessed for non-psoriatic uses, such as hidradenitis suppurativa and alopecia areata (63). Likewise, adverse effects began to manifest, like AIBDs. There were reports about pemphigus caused by secukinumab and brodalumab. But for BP, we found only one case caused by IL-17 inhibitor administration (52).
A 65-year-old woman receiving two doses of 150 mg of secukinumab weekly for psoriasis developed bullae on the upper limbs and trunk 1 day after the second dose. Histological examination was consistent with BP. DIF showed linear deposits of IgG and C3 at the BMZ and a mixed deposition pattern (roof and floor) of both IgG and C3 on salt-split skin immunofluorescence. The serum autoantibodies of BP were found positive, but the type of antibody was not specified. The diagnosis of BP was made, and topical corticosteroids were initiated in addition to the cessation of secukinumab. The BP improved rapidly and was kept in remission even after the reintroduction of secukinumab (Tables 2, 4).
Except for the case described above, secukinumab-caused BP was documented as an adverse effect in some clinical trials but not detailed (64, 65).
IL-17 inhibitors treating BP
In contrast with inducing BP, IL-17 antagonists have been reported more frequently in treating BP. To our knowledge, the therapeutic use of IL-17 inhibitors for BP coexisting with psoriasis has been described in three cases (Table 3), one by ixekizumab (28) and the remaining two by secukinumab (Table 2) (29, 30).
Lu et al. (28) reported that a patient with a 20-year history of psoriasis concurrent with BP was treated with ixekizumab at a standard dose for psoriasis due to the patient’s rejection of methotrexate. Clinical remissions of both diseases were rapidly achieved within 2 weeks and still in remission after 1 year of initiating the drug (28).
Secukinumab was applied as an adjuvant therapy combined with oral and topical corticosteroids in another patient and showed a promising efficacy. But the therapeutic effect of secukinumab for BP was not particularly specific due to the combination (30). Yun et al. (29) recently reported the first case describing the clinical improvement of autoantibody-negative BP coexisting with refractory psoriasis treated with secukinumab. Both were still in remission at follow-up, and unexpected better response for BP (29).
Interestingly, one patient was given secukinumab for psoriasis resulting in an original rising of anti-BP180 autoantibody to a peak and decline soon. No clinical improvement of BP was demonstrated, as the patient had no blisters prior to and during the whole duration of secukinumab administration. But as a gradual increase of anti-BP180 antibodies without blister formation was common in patients with relapses, we speculate that secukinumab prevented the recurrence of BP, indicating its underlying therapeutic use (66).
Pathogenesis of IL-17 inhibitors inducing or treating BP
As aforementioned in the IL-12/IL-23 inhibitor section, IL-17 inhibitors can cause BP by regulating TNF-α, thereby modulating its downstream pathways. There might be unknown pathways apart from T-cell imbalance, and the mechanism remains to be elucidated (56). In addition to Th17 cells, the main sources of IL-17, recruited inflammatory cells including eosinophils and mast cells can also produce IL-17 (38). IL-17 can increase MMP-9 production from eosinophils and neutrophil elastase release from neutrophils, which were associated with the degrading of BP180 and the formation of the subepidermal cleft, thus blocking it could have a therapeutic effect (62). In the meantime, neutrophils affected by IL-17 can, in turn, promote IL-17 production, turning into a cascade loop (67) (Figure 1).
Other biologics inducing or treating BP
In addition, some biologics can only cause or treat BP. In terms of inducing BP, immune checkpoint inhibitors targeting PD-1/PD-L1, which are used to treat advanced solid malignancies, can cause immune-related adverse effects including BP. There were many cases and reviews relevant to BP cases caused by PD-1/PD-L1 inhibitors including pembrolizumab, nivolumab, and cemiplimab, but no reports about treating BP to date. Nonspecific activation of the immunity and the shared antigens between tumor cells and the BMZ have been involved in the development of PD-1/PD-L1 blockers-inducing BP (3, 4, 68–70). In the treatment of BP, omalizumab, an antibody targeting IgE, and dupilumab, an antibody binding IL-4 receptor subunit α (IL-4Rα), are now increasingly utilized for the treatment of BP as novel therapeutic approaches (71–73).
Conclusion and further perspectives
With the broader indications and more extensive clinical practice of biologics, the incidence of adverse effects like BP will be much more common. As the early manifestations of drug-induced BP are not specific, biologics-induced BP might be underreported, and diagnoses are challenging. Dermatologists should keep this rare but life-threatening adverse effect in mind and be more cautious on identifying the disease and initiating treatment as early as possible.
Reviewing all of the 25 cases of BP induced by biological agents collected in our study, we would like to know if there are some common threads for the patients who developed BP. Although the number of cases was limited and the recorded data were uneven, we can still distill out some possible common threads. Patients are usually younger. The average age of all 25 patients was 60 years, which is younger than that of spontaneous BP (74). And in our recorded cases, the positive rate of anti-BP230 was low, with only one out of 25 cases being positive (Tables 1, 4).
The findings raised above may not have a high degree of confidence, but they provide us with new perspectives and future research directions that may indicate underlying mechanisms other than those mentioned in this article.
In addition to the direct effects of those culprit drugs, the primary diseases and polypharmacy in patients may act as predisposing factors and have an underlying relationship with BP occurrence (75). Patients involved in our study were usually elderly and fragile and had a long history of psoriasis and other diseases. Psoriasis has been associated with a higher incidence of BP. It is hypothesized that epigenetic changes altered by psoriasis lesions may trigger or accelerate autoreactive response to specific antigens, resulting in autoantibody production to induce BP. Neutrophil elastase may also be involved in subepidermal blister formation (76). For other involved diseases treated with biologics, there are also reports about epitope spreading, such as the plectin hypothesis, which indicates that the inflammation present in inflammatory bowel disease exposes plectin within the bowel. Plectin exposures can stimulate the production of autoantibodies to cross-react with the skin of certain susceptible individuals, provoking a secondary immune response resulting in BP occurrence (77).
We also tried to refine and summarize the features of a particular type of BP patient responding to biologics, which may have significant clinical implications. Noteworthy, six of the seven cases were comorbid with psoriasis, and multiple medications for psoriasis were prescribed, indicating that those biologics might be effective in treating BP concomitant with psoriasis. We can also assume that they can also be used for treating BP concurrent with other immune diseases except for psoriasis. To our knowledge, etanercept is the most commonly used aTNF-α in the treatment of BP (Table 2), and clinical trials evaluating the effect of IL-17 inhibitors on BP are ongoing as well (78). Interestingly, a case of etanercept-induced BP improved after switching to adalimumab (22), suggesting that biologics with different preparation processes may have a distinct effect even focusing on the same target.
The optimal therapeutic recommendations for biologics-induced BP have yet to be well-established (78), and questions about the mechanism of the paradoxical phenomena that these agents can both cause and treat BP still need to be fully elucidated. Further investigations concerning these issues are warranted to improve our understanding of this phenomenon, and we believe that it would be significant for future optimized treatments of BP.
Author contributions
The manuscript was written by JZ and S-HW with significant contributions from Y-GZ. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (grant number 81972944), National High Level Hospital Clinical Research Funding (2022-PUMCH-B-092).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miyamoto D, Santi CG, Aoki V, Maruta CW. Bullous pemphigoid. Bras Dermatol (2019) 94(2):133–46. doi: 10.1590/abd1806-4841.20199007
2. Moro F, Fania L, Sinagra JLM, Salemme A, Di Zenzo G. Bullous pemphigoid: Trigger and predisposing factors. Biomolecules (2020) 10(10):14321–28. doi: 10.3390/biom10101432
3. Lopez AT, Khanna T, Antonov N, Audrey-Bayan C, Geskin L. A review of bullous pemphigoid associated with pd-1 and pd-L1 inhibitors. Int J Dermatol (2018) 57(6):664–9. doi: 10.1111/ijd.13984
4. Sadik CD, Langan EA, Gutzmer R, Fleischer MI, Loquai C, Reinhardt L, et al. Retrospective analysis of checkpoint inhibitor therapy-associated cases of bullous pemphigoid from six German dermatology centers. Front Immunol (2020) 11:588582. doi: 10.3389/fimmu.2020.588582
5. Ramos-Casals M, Brito-Zerón P, Soto MJ, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by tnf-targeted therapies. Best Pract Res Clin Rheumatol (2008) 22(5):847–61. doi: 10.1016/j.berh.2008.09.008
6. Husein-ElAhmed H, Steinhoff M. Bullous pemphigoid induced by biologic drugs in psoriasis: A systematic review. J Dermatolog Treat (2022) 33(7):2886–93. doi: 10.1080/09546634.2022.2089331
7. Park SO, Lee JH. A case of bullous pemphigoid arising after infliximab therapy in a patient with rheumatoid arthritis. J Korean Rheum Assoc (2010) 17(4):4221–4. doi: 10.4078/jkra.2010.17.4.422
8. Hall B, Kiely C, Tobin AM, McNamara D. Haha antibodies – not such a funny story. J Crohns Colitis (2014) 8(5):439–40. doi: 10.1016/j.crohns.2013.11.018
9. Ricci M, Zauli S, Zelante A, Trevisani L, Virgili A, Bettoli V. Bullous pemphigoid occurring under anti-tumor necrosis factor-alpha therapy. Int J Colorectal Dis (2014) 29(12):1573–4. doi: 10.1007/s00384-014-1924-9
10. Kozaru T, Fukumoto T, Shirai T, Takasawa N, Kameoka JI, Oka M. Infliximab-induced bullous pemphigoid and anti-desmoglein 3 and anti-Bp180 autoantibodies in a patient with ulcerative colitis. Eur J Dermatol (2019) 29(1):87–8. doi: 10.1684/ejd.2018.3439
11. Stausbøl-Grøn B, Deleuran M, Sommer Hansen E, Kragballe K. Development of bullous pemphigoid during treatment of psoriasis with adalimumab. Clin Exp Dermatol (2009) 34(7):e285–e6. doi: 10.1111/j.1365-2230.2008.03204.x
12. Altindag O, Aydeniz A, Gursoy S. A case with anti tnf- alpha induced bullous pemhigoid. Turkish J Rheumatol (2010) 25(4):214–6. doi: 10.5152/tjr.2010.31
13. Wessman LL, Blixt EK, Wetter DA, Miest RY. Adalimumab-associated bullous pemphigoid in a patient with ulcerative colitis. JAAD Case Rep (2017) 3(4):339–41. doi: 10.1016/j.jdcr.2017.03.008
14. Hoffmann S, Berneburg M, Schreml S. Bullous pemphigoid associated with adalimumab therapy in a patient with ulcerative colitis. Case Rep Dermatol (2018) 10(2):145–8. doi: 10.1159/000489163
15. Tirado-Sánchez A, Bonifaz A. Simultaneous bullous pemphigoid and vitiligo associated with adalimumab therapy in a patient with psoriasis vulgaris. Indian Dermatol Online J (2020) 11(2):229–31. doi: 10.4103/idoj.IDOJ_53_19
16. Yoshikawa N, Matsubara E, Yamamoto M, Yamazaki H, Uehara M, Kamata M, et al. Drug-induced bullous pemphigoid and lupus erythematosus occurring under anti-Tnf-α and il-6 therapy in a patient with rheumatoid arthritis. Intern Med (2020) 59(20):2611–8. doi: 10.2169/internalmedicine.4646-20
17. van Ammers M, Lai FYX, Abrahams T, Simpson I, Mar A. Bullous pemphigoid in a young Male during treatment with adalimumab. Australas J Dermatol (2021) 62(1):e155–e6. doi: 10.1111/ajd.13466
18. Sugaya M, Ishii M, Takahashi-Shishido N, Ichimura Y, Morimura S. Case of bullous pemphigoid under treatment with adalimumab for hidradenitis suppurativa. J Dermatol (2021) 48(4):e163–e4. doi: 10.1111/1346-8138.15770
19. Yamada K, Noto M, Yamakawa T, Manabe M, Osada SI. Superimposed effects of adalimumab and linagliptin on the development of bullous pemphigoid in a psoriatic patient: A case report. Indian J Dermatol (2021) 66(2):208–10. doi: 10.4103/ijd.IJD_794_19
20. Boussemart L, Jacobelli S, Batteux F, Goulvestre C, Grange P, Carlotti A, et al. Autoimmune bullous skin diseases occurring under anti-tumor necrosis factor therapy: Two case reports. Dermatology (2010) 221(3):201–5. doi: 10.1159/000318008
21. Bordignon M, Belloni-Fortina A, Pigozzi B, Tarantello M, Alaibac M. Bullous pemphigoid during long-term tnf-alpha blocker therapy. Dermatology (2009) 219(4):357–8. doi: 10.1159/000243805
22. Kluk J, Goulding JM, Bhat J, Finch TM. Drug-induced bullous pemphigoid: Cases triggered by intravenous iodine and etanercept. Clin Exp Dermatol (2011) 36(8):871–3. doi: 10.1111/j.1365-2230.2011.04102.x
23. Wilmer EN, Becker N, Chen A, Kroumpouzos G. Etanercept-induced generalization of chronic, localized, anogenital bullous pemphigoid in a psoriatic patient. JAAD Case Rep (2016) 2(1):25–7. doi: 10.1016/j.jdcr.2015.12.006
24. Nin M, Tokunaga D, Ishii N, Komai A, Hashimoto T, Katoh N. Case of coexisting psoriatic arthritis and bullous pemphigoid improved by etanercept. J Dermatol (2013) 40(1):55–6. doi: 10.1111/j.1346-8138.2012.01659.x
25. Cusano F, Iannazzone SS, Riccio G, Piccirillo F. Coexisting bullous pemphigoid and psoriasis successfully treated with etanercept. Eur J Dermatol (2010) 20(4):520. doi: 10.1684/ejd.2010.0970
26. Yamauchi PS, Lowe NJ, Gindi V. Treatment of coexisting bullous pemphigoid and psoriasis with the tumor necrosis factor antagonist etanercept. J Am Acad Dermatol (2006) 54(3 Suppl 2):S121–2. doi: 10.1016/j.jaad.2005.10.055
27. Loget J, Plée J, Antonicelli F, Bernard P. A successful treatment with ustekinumab in a case of relapsing bullous pemphigoid associated with psoriasis. J Eur Acad Dermatol Venereol (2017) 31(5):e228–e30. doi: 10.1111/jdv.14002
28. Lu L, Yu Y, Zhang J, Fan X, Qi Y, Lin B. Incidental amelioration of bullous pemphigoid during ixekizumab treatment for psoriasis. J Dermatol (2022) 49(1):e13–e5. doi: 10.1111/1346-8138.16189
29. Yun JS, Scardamaglia L, Tan CG, McCormack CJ. Successful secukinumab treatment of active bullous pemphigoid and chronic severe psoriasis: A case report. Australas J Dermatol (2022) 63(2):e155–e8. doi: 10.1111/ajd.13803
30. Holtsche MM, Hammers CM, Chakievska L, Ludwig RJ, Thaci D, Zillikens D, et al. Adjuvante therapie mit secukinumab induziert langzeitremission bei einer patientin mit schwerem bullösen pemphigoid. J Dtsch Dermatol Ges (2020) 18(12):1478–80. doi: 10.1111/ddg.14291_g
31. Canizares MJ, Smith DI, Conners MS, Maverick KJ, Heffernan MP. Successful treatment of mucous membrane pemphigoid with etanercept in 3 patients. Arch Dermatol (2006) 142(11):1457–61. doi: 10.1001/archderm.142.11.1457
32. Kennedy JS, Devillez RL, Henning JS. Recalcitrant cicatricial pemphigoid treated with the anti-Tnf-Alpha agent etanercept. J Drugs Dermatol (2010) 9(1):68–70.
33. Heffernan MP, Bentley DD. Successful treatment of mucous membrane pemphigoid with infliximab. Arch Dermatol (2006) 142(10):1268–70. doi: 10.1001/archderm.142.10.1268
34. Majima Y, Yagi H, Tateishi C, Groth S, Schmidt E, Zillikens D, et al. A successful treatment with ustekinumab in a case of antilaminin-Gamma1 pemphigoid associated with psoriasis. Br J Dermatol (2013) 168(6):1367–9. doi: 10.1111/bjd.12163
35. De Bandt M, Sibilia J, Le Loët X, Prouzeau S, Fautrel B, Marcelli C, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: A French national survey. Arthritis Res Ther (2005) 7(3):R545–51. doi: 10.1186/ar1715
36. Genovese G, Di Zenzo G, Cozzani E, Berti E, Cugno M, Marzano AV. New insights into the pathogenesis of bullous pemphigoid: 2019 update. Front Immunol (2019) 10:1506. doi: 10.3389/fimmu.2019.01506
37. Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: A review of the literature. J Eur Acad Dermatol Venereol (2014) 28(9):1133–40. doi: 10.1111/jdv.12366
38. Liu Y, Li L, Xia Y. Bp180 is critical in the autoimmunity of bullous pemphigoid. Front Immunol (2017) 8:1752. doi: 10.3389/fimmu.2017.01752
39. D'Auria L, Cordiali Fei P, Ameglio F. Cytokines and bullous pemphigoid. Eur Cytokine Netw (1999) 10(2):123–34.
40. Ameglio F, D'Auria L, Bonifati C, Ferraro C, Mastroianni A, Giacalone B. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: Relationships with disease intensity. Br J Dermatol (1998) 138(4):611–4. doi: 10.1046/j.1365-2133.1998.02169.x
41. Ameglio F, D'Auria L, Cordiali-Fei P, Mussi A, Valenzano L, D'Agosto G, et al. Bullous pemphigoid and pemphigus vulgaris: Correlated behaviour of serum vegf, Se-selectin and tnf-alpha levels. J Biol Regul Homeost Agents (1997) 11(4):148–53.
42. Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: Evidence for a critical role of tnf-alpha. J Immunol (2007) 179(7):4840–8. doi: 10.4049/jimmunol.179.7.4840
43. Fang H, Li Q, Wang G. The role of T cells in pemphigus vulgaris and bullous pemphigoid. Autoimmun Rev (2020) 19(11):102661. doi: 10.1016/j.autrev.2020.102661
44. Zhang J, Fang H, Shen S, Dang E, Li Q, Qiao P, et al. Identification of immunodominant Th2-cell epitopes in Chinese patients with bullous pemphigoid. J Invest Dermatol (2018) 138(9):1917–24. doi: 10.1016/j.jid.2018.03.1515
45. Burlando M, Capurro N, Herzum A, Cozzani E, Parodi A. Guselkumab-associated bullous pemphigoid in a psoriasis patient: A case report and review of the literature. Dermatol Ther (2022) 35(1):e15207. doi: 10.1111/dth.15207
46. Marin M, Alzueta N, Castresana M, Gascón A, Pío M. Bullous pemphigoid induced by ustekinumab: A case report. Eur J Hosp Pharm (2021) 28(1):47–9. doi: 10.1136/ejhpharm-2018-001849
47. Querol-Cisneros E, Moreno-Artero E, Rodríguez-Garijo N, Tomás-Velázquez A, Querol I, Ishii N, et al. Bullous pemphigoid without detection of autoantibodies in a patient with psoriasis under ustekinumab. J Dtsch Dermatol Ges (2021) 19(2):265–7. doi: 10.1111/ddg.14199
48. Vural S, Gundogdu M, Ertop P, Sanli H, Korkmaz P, Heper AO, et al. Ustekinumab associated bullous pemphigoid in a psoriasis patient and a review of the literature. Turkderm-Turkish Arch Dermatol Venerol (2019) 53(1):32–5. doi: 10.4274/turkderm.galenos.2018.11354
49. Onsun N, Sallahoglu K, Dizman D, Su Ö, Tosuner Z. Bullous pemphigoid during ustekinumab therapy in a psoriatic patient. Eur J Dermatol (2017) 27(1):81–2. doi: 10.1684/ejd.2016.2888
50. Le Guern A, Alkeraye S, Vermersch-Langlin A, Coupe P, Vonarx M. Bullous pemphigoid during ustekinumab therapy. JAAD Case Rep (2015) 1(6):359–60. doi: 10.1016/j.jdcr.2015.07.014
51. Nakayama C, Fujita Y, Watanabe M, Shimizu H. Development of bullous pemphigoid during treatment of psoriatic onycho-pachydermo periostitis with ustekinumab. J Dermatol (2015) 42(10):996–8. doi: 10.1111/1346-8138.12943
52. Ho PH, Tsai TF. Development of bullous pemphigoid during secukinumab treatment for psoriasis. J Dermatol (2017) 44(9):e220–e1. doi: 10.1111/1346-8138.13909
53. Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis (2017) 100(6):415–8.
54. Kerkemeyer KL, Pinczewski J, Sinclair R. Successful treatment of recalcitrant lichen planus pemphigoides with tildrakizumab. Australas J Dermatol (2020) 61(3):e366–e8. doi: 10.1111/ajd.13263
55. Plée J, Le Jan S, Giustiniani J, Barbe C, Joly P, Bedane C, et al. Integrating longitudinal serum il-17 and il-23 follow-up, along with autoantibodies variation, contributes to predict bullous pemphigoid outcome. Sci Rep (2015) 5:18001. doi: 10.1038/srep18001
56. Mease PJ. Inhibition of interleukin-17, interleukin-23 and the Th17 cell pathway in the treatment of psoriatic arthritis and psoriasis. Curr Opin Rheumatol (2015) 27(2):127–33. doi: 10.1097/bor.0000000000000147
57. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol (2017) 140(3):645–53. doi: 10.1016/j.jaci.2017.07.004
58. Tsuda K, Yamanaka K, Kondo M, Matsubara K, Sasaki R, Tomimoto H, et al. Ustekinumab improves psoriasis without altering T cell cytokine production, differentiation, and T cell receptor repertoire diversity. PloS One (2012) 7(12):e51819. doi: 10.1371/journal.pone.0051819
59. Reddy M, Torres G, McCormick T, Marano C, Cooper K, Yeilding N, et al. Positive treatment effects of ustekinumab in psoriasis: Analysis of lesional and systemic parameters. J Dermatol (2010) 37(5):413–25. doi: 10.1111/j.1346-8138.2010.00802.x
60. Le Jan S, Plée J, Vallerand D, Dupont A, Delanez E, Durlach A, et al. Innate immune cell-produced il-17 sustains inflammation in bullous pemphigoid. J Invest Dermatol (2014) 134(12):2908–17. doi: 10.1038/jid.2014.263
61. Tabatabaei-Panah PS, Moravvej H, Aghaei S, Akbari M, Rajabi S, Kia A, et al. Th17/Il23 cytokine gene polymorphisms in bullous pemphigoid. Mol Genet Genomic Med (2020) 8(12):e1519. doi: 10.1002/mgg3.1519
62. Giusti D, Bini E, Terryn C, Didier K, Le Jan S, Gatouillat G, et al. Net formation in bullous pemphigoid patients with relapse is modulated by il-17 and il-23 interplay. Front Immunol (2019) 10:701. doi: 10.3389/fimmu.2019.00701
63. Wu KK, Dao H Jr. Off-label dermatologic uses of il-17 inhibitors. J Dermatolog Treat (2022) 33(1):41–7 doi: 10.1080/09546634.2020.1737638
64. Morishima-Koyano M, Nobeyama Y, Fukasawa-Momose M, Kikuchi S, Asahina A. Case of pemphigus foliaceus misdiagnosed as a single condition of erythrodermic psoriasis and modified by brodalumab. J Dermatol (2020) 47(5):e201–e2. doi: 10.1111/1346-8138.15295
65. Hayashida MZ, Pinheiro JRS, Enokihara M, Vasconcellos MRA. Biologic therapy-induced pemphigus. Bras Dermatol (2017) 92(4):591–3. doi: 10.1590/abd1806-4841.20176481
66. Kamata M, Asano Y, Shida R, Maeda N, Yoshizaki A, Miyagaki T, et al. Secukinumab decreased circulating anti-Bp180-Nc16a autoantibodies in a patient with coexisting psoriasis vulgaris and bullous pemphigoid. J Dermatol (2019) 46(6):e216–e7. doi: 10.1111/1346-8138.14760
67. Taylor PR, Roy S, Leal SM Jr., Sun Y, Howell SJ, Cobb BA, et al. Activation of neutrophils by autocrine il-17a-Il-17rc interactions during fungal infection is regulated by il-6, il-23, rorγt and dectin-2. Nat Immunol (2014) 15(2):143–51. doi: 10.1038/ni.2797
68. Chatterjee T, Rashid TF, Syed SB, Roy M. Bullous pemphigoid associated with pembrolizumab therapy for non-Small-Cell lung cancer: A case report. Cureus (2022) 14(1):e21770. doi: 10.7759/cureus.21770
69. Klepper EM, Robinson HN. Dupilumab for the treatment of nivolumab-induced bullous pemphigoid: A case report and review of the literature. Dermatol Online J (2021) 27(9):1–6. doi: 10.5070/d327955136
70. Grünig H, Skawran SM, Nägeli M, Kamarachev J, Huellner MW. Immunotherapy (Cemiplimab)-induced bullous pemphigoid: A possible pitfall in 18f-fdg Pet/Ct. Clin Nucl Med (2022) 47(2):185–6. doi: 10.1097/rlu.0000000000003894
71. Castel M, Alexandre M, Jelti L, Pham-Ledard A, Viguier M, Bédane C, et al. Updated French guidelines for the therapeutic management of bullous pemphigoid. Ann Dermatol Venereol (2022) 149(2):81–91. doi: 10.1016/j.annder.2021.08.005
72. Wang SH, Zuo YG. Commentary: Efficacy and safety of dupilumab in moderate-to-Severe bullous pemphigoid. Front Immunol (2021) 12:800609. doi: 10.3389/fimmu.2021.800609
73. Zhang Y, Xu Q, Chen L, Chen J, Zhang J, Zou Y, et al. Efficacy and safety of dupilumab in moderate-to-Severe bullous pemphigoid. Front Immunol (2021) 12:738907. doi: 10.3389/fimmu.2021.738907
74. Marazza G, Pham HC, Schärer L, Pedrazzetti PP, Hunziker T, Trüeb RM, et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: A 2-year prospective study. Br J Dermatol (2009) 161(4):861–8. doi: 10.1111/j.1365-2133.2009.09300.x
75. Patsatsi A, Vyzantiadis TA, Chrysomallis F, Devliotou-Panagiotidou D, Sotiriadis D. Medication history of a series of patients with bullous pemphigoid from northern Greece - observations and discussion. Int J Dermatol (2009) 48(2):132–5. doi: 10.1111/j.1365-4632.2009.03839.x
76. Toh JJ, Lim YL, Yew YW. Characteristics and management of autoimmune bullous disease in psoriasis patients. Ann Acad Med Singap (2019) 48(9):301–5. doi: 10.47102/annals-acadmedsg.V48N9p301
77. Cobb CBC, Caravaglio JV, Qureshi AA, Robinson-Bostom L. Concominant bullous pemphigoid and cutaneous crohn disease. J Cutan Pathol (2022) 49(6):579–83. doi: 10.1111/cup.14206
Keywords: bullous pemphigoid, TNF-α inhibitor, IL-17 inhibitor, IL12/IL23 inhibitor, paradoxical phenomena
Citation: Zhang J, Wang S-H and Zuo Y-G (2023) Paradoxical phenomena of bullous pemphigoid induced and treated by identical biologics. Front. Immunol. 13:1050373. doi: 10.3389/fimmu.2022.1050373
Received: 21 September 2022; Accepted: 07 December 2022;
Published: 05 January 2023.
Edited by:
Ciro Romano, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Kelly Nordyke Messingham, The University of Iowa, United StatesDaisuke Tsuruta, Osaka City University, Japan
Christoph M. Hammers, University of Lübeck, Germany
Copyright © 2023 Zhang, Wang and Zuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ya-Gang Zuo, enVveWFnYW5nQDI2My5uZXQ=
 Jie Zhang
Jie Zhang Si-Hang Wang
Si-Hang Wang Ya-Gang Zuo
Ya-Gang Zuo