
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 13 January 2023
Sec. Mucosal Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1049908
This article is part of the Research TopicGastrointestinal Atrophy: Between Inflammation and CancerView all 5 articles
 Yi Hu1,2†
Yi Hu1,2† Xin Xu1,2†
Xin Xu1,2† Xiao-Shun Liu1,2†
Xiao-Shun Liu1,2† Cong He1,2†
Cong He1,2† Yao-Bin Ouyang1,2
Yao-Bin Ouyang1,2 Nian-Shuang Li1,2
Nian-Shuang Li1,2 Chuan Xie1,2
Chuan Xie1,2 Chao Peng1,2
Chao Peng1,2 Zhen-Hua Zhu1,2
Zhen-Hua Zhu1,2 Yong Xie1,2
Yong Xie1,2 Xu Shu1,2*‡
Xu Shu1,2*‡ Yin Zhu1,2*‡
Yin Zhu1,2*‡ David Y. Graham3*‡
David Y. Graham3*‡ Nong-Hua Lu1,2*‡
Nong-Hua Lu1,2*‡Background and aim: We previously reported that vonoprazan-amoxicillin (VA) dual therapy for 7 or 10 days is not satisfactorily efficacious for Helicobacter pylori (H. pylori) eradication. We aimed to explore the efficacy of VA dual therapy for 14 days as a first-line treatment for H. pylori infection.
Methods: This was a single center, prospective, open-labeled, randomized non-inferiority clinical study conducted in China. Treatment naïve H. pylori infected patients were randomized into two groups: 20 mg vonoprazan (VPZ) b.i.d. in combination with low-dose (1000 mg b.i.d.) or high-dose (1000 mg t.i.d) amoxicillin for 14 days. 13C-urea breath tests were used to access the cure rate at least 4 weeks after treatment.
Results: A total of 154 patients were assessed and 110 subjects were randomized. The eradication rate of VPZ with b.i.d. amoxicillin or t.i.d. amoxicillin for 14 days was 89.1% and 87.3% by intention-to-treat analysis, respectively, and 94.1% and 95.9% by per-protocol analysis, respectively. The eradication rate and incidence of adverse events were not different between the two groups.
Conclusion: VPZ with b.i.d. or t.i.d. amoxicillin for 14 days provides satisfactory efficacy as a first-line treatment for H. pylori infection in China.
Helicobacter pylori (H. pylori) infection is considered the primary etiological and the most important risk factor for gastric cancer (GC) (1). This has resulted in worldwide efforts to eradicate the infection. Additionally, Family-based H. pylori infection control and management has been recommended as an essential part of comprehensive H. pylori infection prevention and control strategies (2). China has a high incidence of GC (3) and a high prevalence of H. pylori infection (4), and H. pylori eradication has been recommended by the Fifth Chinese National Consensus Report on the management of H. pylori infection (5). Considering the serious situation of antibiotic resistance in China, bismuth-containing quadruple therapy has been recommended as the first-line treatment of H. pylori because of its high efficacy and safety (5).
Vonoprazan (VPZ), a new potassium-competitive acid blocker, has been shown to be a more potent inhibitor of gastric acid and is able to maintain the intragastric pH above 6 more quickly and for longer than traditional proton pump inhibitors (PPI) (6). Maintaining gastric pH above 6 allows H. pylori to remain in the state of active replication, which enhances the effectiveness of amoxicillin. We previously conducted a systematic review and meta-analysis of three Japanese studies with 668 H. pylori-infected patients designed to evaluate the efficacy of vonoprazan-amoxicillin (VA) dual therapy as a first-line treatment of H. pylori infection. The crude eradication rate of VA dual therapy was 87.5% by intention-to-treat (ITT) analysis and 89.6% by per-protocol (PP) analysis (7). VA dual therapy is considered as a promising H. pylori regimen because of its high efficacy, low side effects, and avoidance of unnecessary antibiotic use (8). However, the efficacy and safety of VA dual therapy in other regions remain uncertain, thus requiring the regimen to be optimized.
Our previous study included 119 H. pylori-infected Chinese patients without previous eradication history who were randomized into low- or high-dose amoxicillin-vonoprazan regimen groups consisting of 1 g amoxicillin either b.i.d. or t.i.d plus 20 mg VPZ b.i.d for 7 or 10 days. Neither 7- or 10-day VA dual therapy with either b.i.d. or t.i.d. amoxicillin achieved satisfactory efficacy (i.e., <90%) when administered as the first-line treatment of H. pylori infection (9). This study evaluated the efficacy and safety of 14-day low- and high-dose amoxicillin-vonoprazan dual therapy as a first-line treatment of H. pylori in China.
This study was designed as a prospective open-labeled randomized non-inferiority clinical study and was conducted in accordance with the Declaration of Helsinki and the guidelines of the Consolidated Standards of Reporting Trials (10). This study was approved by the Ethics Committee of The First Affiliated Hospital of Nanchang University (2022-013) and registered in the Chinese Clinical Trial Registry (ChiCTR2000064874).
Consecutive H. pylori-infected subjects aged from 18 to 70 years old without eradication history were recruited in The First Affiliated Hospital of Nanchang University between November 2021 to February 2022. H. pylori infection was confirmed by immunohistochemistry or 13C-urea breath test. Patients were excluded if they met any of the following criteria (1): allergy to amoxicillin or VPZ (2); acute upper gastrointestinal bleeding, GC or other tumors, Zollinger–Ellison syndrome, history of gastric surgery (3); serious illness, including neurological, cardiovascular, pulmonary, hepatic, renal, metabolic, gastrointestinal, urological, endocrinological, or hematological disorders (4); pregnancy or breastfeeding (5); proton pump inhibitor and antibiotic use within 1 month (6); not willing to participate in the study. Written informed consent was obtained from all patients prior to study participation.
The 110 H. pylori-infected subjects were randomly assigned to receive either low- or high-dose amoxicillin-vonoprazan dual therapy in a 1:1 allocation ratio (a randomization list was generated using SPSS [version 25.0]). Low-dose amoxicillin-vonoprazan (L-VA) dual therapy consisted of 1000 mg amoxicillin capsules (Huabei Laboratories, Shijiazhuang, China) twice daily and 20 mg VPZ fumarate tablets (Takeda Pharmaceutical, Tokyo, Japan) twice daily for 14 days. High-dose amoxicillin-vonoprazan (H-VA) dual therapy consisted of 1000 mg amoxicillin capsules (HuaBei Laboratories, Shijiazhuang, China) three times daily and 20mg VPZ fumarate tablets (Takeda Pharmaceutical, Tokyo, Japan) twice daily for 14 days. Patients and investigators were not blinded to the allocated treatment group.
To begin with, the detailed demographics and characteristics of the subjects included in this study were recorded, including sex, age, nationality, height, weight, education status, dwelling area, history of smoking and alcohol, concomitant diseases, and medication history. In addition, physical examinations and assessments of vital signs were performed.
During (or after) treatment-emergent adverse events (TEAEs) and concomitant medication were recorded throughout the study, including bloating, nausea, vomiting, abdominal pain, diarrhea, constipation, skin rash, headache, and hunger sensation. All TEAEs were divided into mild, moderate, and severe. TEAEs leading to the discontinuation of the study drug were also recorded. The confirmation of H. pylori status was evaluated by 13C-urea breath test at least 4 weeks after treatment. H. pylori status was considered as negative or positive when the delta over baseline was below 4 or above 4 according to the manufacturer’s instructions (HCBT-01, Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd., China).
The primary outcome of the study was the eradication rate of the different regimens according to ITT and PP analyses. The ITT analysis included all randomized patients. Patients who were lost to follow-up or did not achieve >80% of drug compliance or did not undergo UBT were excluded from the PP analysis. The secondary outcomes were the frequency and severity of TEAEs and adherence.
The sample size was calculated using PASS (version 11.0.7) according to non-inferiority for two proportions. In our previous study, the eradication rate of L-VA and H-VA dual therapies for 10 days was 89.2% and 81.1%, respectively, as a first-line treatment of H. pylori infection (9), which suggested that low-dose amoxicillin-vonoprazan dual therapy might be non-inferior to high dose. We assumed that VA dual therapy for 14 days would achieve a higher efficacy than that for 10 days. Moreover, acceptable therapy was defined as achieving >90% cure rates in adherent patients with susceptible infection (11). The eradication rate of L-VA and H-VA dual therapies for 14 days was estimated as 90%. Assuming a power, an alpha, a non-inferiority margin, and a follow-up loss rate of 0.80, 0.05, −0.15, and 10%, respectively, were required for a non-inferiority clinical trial, a sample size of 110 patients (55 patients in each group) was planned.
The primary outcome was calculated for each regimen via ITT and PP analyses. The differences in eradication rate were compared using the chi-square test. The quantitative data with normal or non-normal distribution were analyzed by Student’s t-test and a non-parametric test, respectively. The categorical data among different regimens were compared using the chi-square test. All statistical analyses were performed using SPSS (version 25.0) and R (version 4.2.1). All P-values were two-sided, except for the test of non-inferiority. P<0.05 was considered statistically significant.
A total of 154 participants were assessed for eligibility between November 2021 and February 2022. Among these, 44 patients were excluded because they did not meet the inclusion criteria (n=34), declined to participate (n=1), or for other reasons (n=9). Finally, 110 patients were enrolled and randomized into two groups that received either L-VA or H-VA dual therapy. Final follow-up was completed on the 31st of August 2022. One patient in each group was non-compliant, and three patients in the L-VA group and five patients in the H-VA group did not take a 13C-urea breath test (Figure 1). Baseline characteristics (age, sex, body mass index, education status, cigarette smoking, alcohol drinking, and indications) showed no major differences between groups (Table 1).
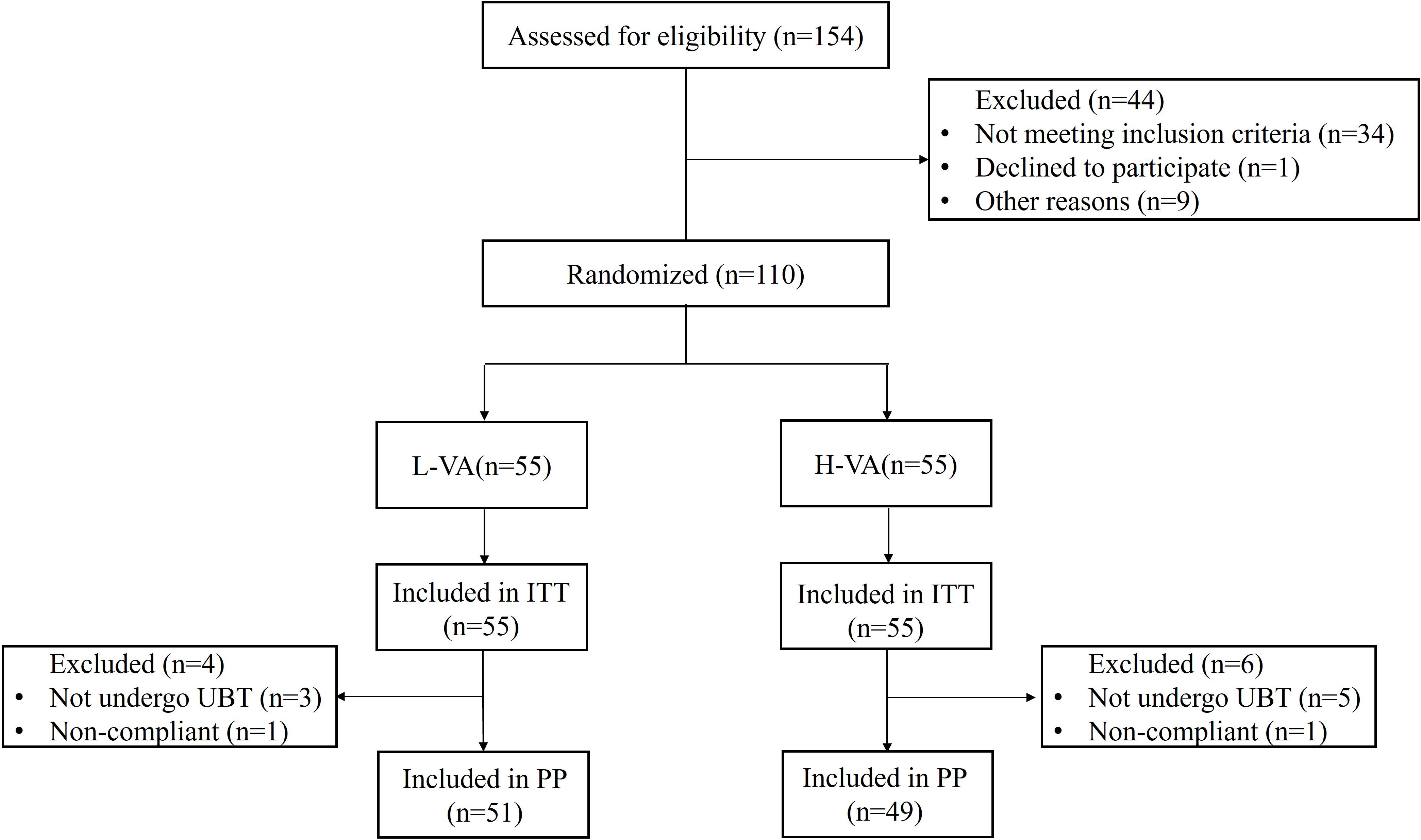
Figure 1 Flow chart of patient enrollment. ITT, intention to treat; PP, per protocol; L-VA, dual therapy consisting of low-dose amoxicillin (1000 mg b.i.d.) and vonoprazan (20 mg b.i.d.); H-VA, dual therapy consisting of high-dose amoxicillin (1000 mg t.i.d.) and vonoprazan (20 mg b.i.d.).
The H. pylori eradication rates for L-VA or H-VA dual therapy are shown in Table 2. In the ITT analysis, the H. pylori eradication rate was 89.1% (49/55, 95% CI 77.1% to 95.5%) for L-VA dual therapy and 87.3% (48/55, 95% CI 74.9% to 94.3%) for H-VA dual therapy, with the difference between each group being 1.8% (95% CI −12.1% to 15.7%). In the PP analysis, the H. pylori eradication rate was 94.1% (48/51, 95% CI 82.8% to 98.5%) for L-VA dual therapy and 95.9% (47/49, 95% CI 84.9% to 99.3%) for H-VA dual therapy, with the difference between each group being 1.8% (95% CI −10.1% to 13.6%).
The lower bound of the adjusted 95% CI for the efficacy difference between L-VA dual therapy and H-VA dual therapy was greater than the prespecified non-inferiority margin in the ITT and PP analyses. No differences in the eradication rate between the two groups were observed according to ITT and PP analysis.
TEAEs occurred in 16 patients in the L-VA dual therapy group and in 11 patients in the H-VA dual therapy group. One patient in the L-VA group presented moderate TEAEs. No patients discontinued the administration of study drugs because of TEAEs. The incidence of TEAEs between the two groups was not statistically different (29.1% vs. 20.0%, P=0.268) (Table 3). In the L-VA or H-VA dual therapy groups, 98.2% of patients took at least 80% of the study drugs. No factors were associated with the efficacy of VA dual therapy in the per-protocol population (Table 4).
To the best of our knowledge, this is the first randomized clinical trial to investigate the efficacy of VPZ in combination with low- or high-dose amoxicillin for 14 days as a first-line treatment of H. pylori infection. We found that the efficacy of L-VA (PP analysis: 94.1%) was non-inferior to H-VA (PP analysis: 95.9%) for eradicating H. pylori. L-VA and H-VA dual therapies for 14 days were defined as at least conditionally acceptable according to the clinical definitions of outcome11. Moreover, low side effects, good compliance, and safety were achieved by VA dual therapy.
VPZ is a member of a new class of acid-suppressing agents that inhibit the enzyme by reversible K+-competitive ionic binding and do not require acid activation within the parietal cell secretory canaliculus. VPZ has been widely applied to treat acid-related diseases, including H. pylori infection (12). The 24-h pH>4 and pH>5 holding time ratios were 83.4% and 73.2%, respectively, in a Japanese population when 20 mg VPZ was administered for 7 consecutive days. The holding time ratio increased to 100% and 98.6%, respectively, when 40 mg VPZ was administered once daily (13). However, the inhibition ability of gastric acid induced by VPZ was slightly decreased in the UK compared with that in Japan, which might be due to the difference in body mass index between the two regions. VPZ has been used at a dose of 20 mg twice daily in H. pylori regimens. The 24-h pH>4 and pH>5 holding time ratios were 68% and 54%, respectively, when 20 mg esomeprazole was administered twice daily, which is lower than that achieved with 20 mg VPZ b.i.d (6).
The primary and secondary resistance rates to clarithromycin are now above alarming levels (>15%) worldwide (14), and clarithromycin-resistant H. pylori was included in a high priority list of antibiotic-resistant bacteria that needed effective drugs (15). Approximately 80% of infected patients received no additive benefits from clarithromycin use in VPZ triple therapy, which has also contributed to global antimicrobial resistance (16). Suzuki et al. (17) produced one of the first studies that explored the efficacy of VPZ (20 mg b.i.d.) with low-dose amoxicillin (750 mg b.i.d.) for 7 days as a first-line treatment of H. pylori infection in Japan. VA dual therapy achieved 84.5% in ITT analysis and 87.1% in PP analysis, which was non-inferior to VPZ-based triple therapy in the same population. A similar efficacy (ITT analysis, 85.0%; PP analysis, 86.4%) was also achieved by VA dual therapy (20 mg VPZ and 750 mg amoxicillin twice daily) for 7 days in junior high school students in Japan (18). Eto et al. (19) further analyzed the factors that might influence the efficacy of VA dual therapy. A total of 163 H. pylori-infected subjects were included and the total eradication rate was 87.1%. High body surface area or body mass index have been associated with the decreased efficacy of VA dual therapy. The plasma half-life of amoxicillin is short, and an increase in the frequency of amoxicillin administered daily will theoretically improve the efficacy of amoxicillin. A retrospective study (20) was conducted in Japan to evaluate the efficacy of VPZ (20 mg b.i.d.) in combination with amoxicillin (500 mg t.i.d.) for 7 days as a first-line treatment of H. pylori infection. According to ITT analysis, VA dual therapy achieved an eradication rate of 92.9%, which was non-inferior to VPZ-based triple therapy.
China has a high resistance rate to antibiotics (21). However, the resistance rate to amoxicillin has remained relatively low (21), especially in Jiangxi province (22). VPZ was introduced in China in 2019 and the efficacy of VA dual therapy for eradicating H. pylori in China remained unclear. In our previous study, 119 H. pylori-infected patients without H. pylori eradication history were randomized into two groups: 20 mg VPZ b.i.d. with low- (1000 mg b.i.d.) or high-dose (1000 mg t.i.d.) amoxicillin for 7 or 10 days. We found that VPZ with low-or high-dose amoxicillin achieved unsatisfactory efficacy when administered for 7 or 10 days (9), suggesting that a longer duration was needed. We designed a prospective open-labeled randomized non-inferiority clinical study because VPZ in combination with low-dose amoxicillin for 10 days showed a higher eradication rate than that with a high dose, although the sample size was limited. A total of 110 H. pylori-infected subjects with no eradication history were included and randomized into VPZ (20 mg b.i.d.) with low- (1000 mg b.i.d.) or high-dose (1000 mg t.i.d.) amoxicillin groups for 14 days. According to PP analysis, the eradication rates of L-VA and H-VA were 94.1% and 95.9%, respectively, which were considered as acceptable results (11), and the difference in the eradication rates between the two groups was not statistically significant.
Furthermore, we found no factors that might influence the efficacy of VA dual therapy, including gender, age, body mass index, education status, cigarette smoking, alcohol drinking, family-based H. pylori infection, and indications. Our results showed a high efficacy of 14-day VA dual therapy as a first-line treatment of H. pylori infection in China. Gao et al. (23) conducted a real-world retrospective clinical study to analyze the efficacy of 10 mg or 20 mg VPZ twice a day and 3000 mg amoxicillin per day for 14 days as a rescue treatment of H. pylori infection. The results revealed a 92.5% eradication rate for VA dual therapy in subjects with previous treatment failure. Inconsistent with our results, Chey et al. (24) reported that 14-day VA dual therapy (20 mg VPZ b.i.d. and 1000 md amoxicillin t.i.d.) only achieved a 77.2% eradication rate in the United States and Europe, which is considered as unacceptable and remains unexplained. The amoxicillin resistance rate in the United States and Europe is relatively low (≤5%) (25). The differences in the efficacy of 14-day VA dual therapy reported in different regions might be explained by race, body mass index, or protocol differences.
In our study, only one patient in each group took <80% of the study drug; therefore, the compliance of the L-VA and H-VA groups was good. Only 29.1% of patients in the L-VA group and 20.0% of patients in the H-VA group reported TEAEs (with no serious TEAEs), indicating that 14-day VA dual therapy is associated with low adverse effects and good safety. Our previous study (9) demonstrated that the TEAE rate is 29.7% and 24.3% in patients receiving L-VA or H-LA for 10 days, respectively. Therefore, a prolonged duration of VA dual therapy from 10 days to 14 days might not lead to an increased incidence of TEAEs.
There were some limitations in this study. First, the sample size was limited to 110 patients all from one center. Further centers with larger samples, different amoxicillin resistance rates, and different races are needed. Second, the efficacy of 14-day L-VA and H-VA in H. pylori-infected subjects with previous treatment failure was not examined in this study. Third, endoscopies were conducted in a subset of the included subjects. Antibiotic resistance of amoxicillin was not analyzed so we were unable to determine whether the failure of VA dual therapy was due to amoxicillin resistance.
In conclusion, 14-day L-VA and H-VA provide high efficacy, low adverse effects, and good safety as first-line treatments of H. pylori infection in China. This supports VA dual therapy as an alternative regimen for H. pylori eradication, especially in an era of increasing antibiotic resistance.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of The First Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YH, XX, X-SL, and CH participated in recording the basic information and follow-up of patients; Y-BO, N-SL, CX, and CP provided suggestions for the study design and participated in analyzing data; Z-HZ and YX provided suggestions for the study design and revised the manuscript; YH, XS, YZ, DG, and N-HL designed the study and wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (82000531 and 82170580); the Project for Academic and Technical Leaders of Major Disciplines in Jiangxi Province (20212BCJL23065); the Key Research and Development Program of Jiangxi Province (20212BBG73018); the Youth Project of the Jiangxi Natural Science Foundation (20202BABL216006); the Key Fund of the Jiangxi Education Department (GJJ190007); the National Science and Technology Award Reserve Cultivation Project (20192AEI91008); and The First Affiliated Hospital of Nanchang University Clinical Research and Cultivation Project (YFYLCYJPY202002).
We thank Mr. Shi-Hai Chen from The First Affiliated Hospital of Nanchang University for his help regarding the statistical analysis of data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, et al. Management of helicobacter pylori infection: the maastricht VI/Florence consensus report. Gut (2022). doi: 10.1136/gutjnl-2022-327745
2. Ding SZ, Du YQ, Lu H, Wang WH, Cheng H, Chen SY, et al. Chinese Consensus report on family-based Helicobacter pylori infection control and management (2021 edition). Gut (2022) 71:238–53. doi: 10.1136/gutjnl-2021-325630
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
4. Ren S, Cai P, Liu Y, Wang T, Zhang Y, Li Q, et al. Prevalence of Helicobacter pylori infection in China: A systematic review and meta-analysis. J Gastroenterol Hepatol (2022) 37:464–70. doi: 10.1111/jgh.15751
5. Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, et al. Fifth Chinese national consensus report on the management of Helicobacter pylori infection. Helicobacter (2018) 23:e12475. doi: 10.1111/hel.12475
6. Kagami T, Sahara S, Ichikawa H, Uotani T, Yamade M, Sugimoto M, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther (2016) 43:1048–59. doi: 10.1111/apt.13588
7. Ouyang Y, Wang M, Xu YL, Zhu Y, Lu NH, Hu Y. Amoxicillin-vonoprazan dual therapy for Helicobacter pylori eradication: A systematic review and meta-analysis. J Gastroenterol Hepatol (2022) 37:1666–72. doi: 10.1111/jgh.15917
8. Hu Y, Lu NH. Letter: a promising Helicobacter pylori regimen - vonoprazan-based therapy. Aliment Pharmacol Ther (2022) 56:752–3. doi: 10.1111/apt.17114
9. Hu Y, Xu X, Ouyang YB, He C, Li NS, Xie C, et al. Optimization of vonoprazan-amoxicillin dual therapy for eradicating Helicobacter pylori infection in China: A prospective, randomized clinical pilot study. Helicobacter (2022) 27:e12896. doi: 10.1111/hel.12896
10. Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA (2012) 308:2594–604. doi: 10.1001/jama.2012.87802
11. Graham DY, Liou JM. Primer for development of guidelines for Helicobacter pylori therapy using antimicrobial stewardship. Clin Gastroenterol Hepatol (2022) 20:973–983.e1. doi: 10.1016/j.cgh.2021.03.026
12. Abadi A, Ierardi E. Vonoprazan and Helicobacter pylori treatment: A lesson from Japan or a limited geographic phenomenon? Front Pharmacol (2019) 10:316. doi: 10.3389/fphar.2019.00316
13. Jenkins H, Sakurai Y, Nishimura A, Okamoto H, Hibberd M, Jenkins R, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther (2015) 41:636–48. doi: 10.1111/apt.13121
14. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in world health organization regions. Gastroenterology (2018) 155:1372–1382. e17. doi: 10.1053/j.gastro.2018.07.007
15. Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis (2018) 18:318–27. doi: 10.1016/S1473-3099(17)30753-3
16. Graham DY, Lu H, Shiotani A. Vonoprazan-containing Helicobacter pylori triple therapies contribution to global antimicrobial resistance. J Gastroenterol Hepatol (2021) 36:1159–63. doi: 10.1111/jgh.15252
17. Suzuki S, Gotoda T, Kusano C, Ikehara H, Ichijima R, Ohyauchi M, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut (2020) 69:1019–26. doi: 10.1136/gutjnl-2019-319954
18. Gotoda T, Kusano C, Suzuki S, Horii T, Ichijima R, Ikehara H. Clinical impact of vonoprazan-based dual therapy with amoxicillin for h Pylori infection in a treatment-naive cohort of junior high school students in Japan. J Gastroenterol (2020) 55:969–76. doi: 10.1007/s00535-020-01709-4
19. Eto H, Suzuki S, Kusano C, Ikehara H, Ichijima R, Ito H, et al. Impact of body size on first-line Helicobacter pylori eradication success using vonoprazan and amoxicillin dual therapy. Helicobacter (2021) 26:e12788. doi: 10.1111/hel.12788
20. Furuta T, Yamade M, Kagami T, Uotani T, Suzuki T, Higuchi T, et al. Dual therapy with vonoprazan and amoxicillin is as effective as triple therapy with vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori. Digestion (2020) 101(6):243–70. doi: 10.1159/000502287
21. Liu DS, Wang YH, Zeng ZR, Zhang ZY, Lu H, Xu JM, et al. Primary antibiotic resistance of Helicobacter pylori in Chinese patients: a multiregion prospective 7-year study. Clin Microbiol Infect (2018) 24:780. doi: 10.1016/j.cmi.2017.11.010
22. Liu DS, Wang YH, Zhu ZH, Zhang SH, Zhu X, Wan JH, et al. Characteristics of Helicobacter pylori antibiotic resistance: data from four different populations. Antimicrob Resist Infect Control (2019) 8:192. doi: 10.1186/s13756-019-0632-1
23. Gao W, Teng G, Wang C, Xu Y, Li Y, Cheng H. Eradication rate and safety of a “simplified rescue therapy”: 14-day vonoprazan and amoxicillin dual regimen as rescue therapy on treatment of Helicobacter pylori infection previously failed in eradication: A real-world, retrospective clinical study in China. Helicobacter (2022) 27:e12918. doi: 10.1111/hel.12918
24. Chey WD, Megraud F, Laine L, Lopez LJ, Hunt BJ, Howden CW. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the united states and Europe: Randomized clinical trial. Gastroenterology (2022) 163:608–19. doi: 10.1053/j.gastro.2022.05.055
Keywords: Helicobacter pylori, vonoprazan, amoxicillin, dual therapy, efficacy
Citation: Hu Y, Xu X, Liu X-S, He C, Ouyang Y-B, Li N-S, Xie C, Peng C, Zhu Z-H, Xie Y, Shu X, Zhu Y, Graham DY and Lu N-H (2023) Fourteen-day vonoprazan and low- or high-dose amoxicillin dual therapy for eradicating Helicobacter pylori infection: A prospective, open-labeled, randomized non-inferiority clinical study. Front. Immunol. 13:1049908. doi: 10.3389/fimmu.2022.1049908
Received: 21 September 2022; Accepted: 22 December 2022;
Published: 13 January 2023.
Edited by:
Antonio Di Sabatino, University of Pavia, ItalyReviewed by:
Mitsushige Sugimoto, Tokyo Medical University Hospital, JapanCopyright © 2023 Hu, Xu, Liu, He, Ouyang, Li, Xie, Peng, Zhu, Xie, Shu, Zhu, Graham and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Shu, anhtdXNoeEAxMjYuY29t; Yin Zhu, emh1eWluMjdAc2luYS5jb20uY24=; David Y. Graham, ZGdyYWhhbUBiY20uZWR1; Nong-Hua Lu, bHVub25naHVhQG5jdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.