
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 12 January 2023
Sec. B Cell Biology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1047297
This article is part of the Research Topic B Cell Contributions to Innate Immunity View all 4 articles
Introduction: Natural antibody (NAb) derived from CD5+ B-1 cells maintains tissue homeostasis, controls inflammation, aids in establishing long-term protective responses against pathogens, and provides immediate protection from infection. CD5+ B-1 cell NAbs recognize evolutionarily fixed epitopes, such as phosphatidylcholine (PtC), found on bacteria and senescent red blood cells. Anti-PtC antibodies are essential in protection against bacterial sepsis. CD5+ B-1 cell-derived NAbs have a unique germline-like structure that lacks N-additions, a feature critical for providing protection against infection. Previously, we demonstrated the repertoire and germline status of PtC+CD5+ B-1 cell IgM obtained from male mice changes with age depending on the anatomical location of the B-1 cells. More recently, we demonstrated serum antibody from aged female mice maintains protection against pneumococcal infection, whereas serum antibody from male mice does not provide protection.
Results: Here, we show that aged female mice have significantly more splenic PtC+CD5+ B-1 cells and more PtC specific serum IgM than aged male mice. Furthermore, we find both age and biological sex related repertoire differences when comparing B cell receptor (BCR) sequencing results of PtC+CD5+ B-1 cells. While BCR germline status of PtC+CD5+ B-1 cells from aged male and female mice is similar in the peritoneal cavity, it differs significantly in the spleen, where aged females retain germline configuration and aged males do not. Nucleic acid sensing toll-like receptors are critical in the maintenance of PtC+ B-1 cells; therefore, to begin to understand the mechanism of differences observed between the male and female PtC+CD5+ B-1 cell repertoire, we analyzed levels of cell-free nucleic acids and found increases in aged females.
Conclusion: Our results suggest the antigenic milieu differs between aged males and females, leading to differential selection of antigen-specific B-1 cells over time. Further elucidation of how biological sex differences influence the maintenance of B-1 cells within the aging environment will be essential to understand sex and age-related disparities in the susceptibility to bacterial infection and will aid in the development of more effective vaccination and/or therapeutic strategies specific for males and females.
Natural antibodies are polyreactive, low affinity immunoglobulins of varying isotypes found in both humans and mice (1–3). Specifically, IgM specific natural antibodies provide several essential functions including protection from infection (1), regulation of B cell development (4–6), selection of the B cell repertoire (5–7), clearance of apoptotic debris (1), protection against atherosclerosis (8, 9), and allergic suppression (10). In many diseases associated with aging including atherosclerosis (11–13), cancer (14), stroke (15), Alzheimer’s disease (16), influenza (17), and pneumococcal infection (18) natural antibodies have been demonstrated to be protective. In mice, 80-90% of natural IgM is produced by B-1 cells (2–4).
Phenotypic and functional analyses have identified different subsets of B cells, which are broadly categorized as B-1 and conventional B2 cells. While B2 cells are generally understood to play a major role in adaptive immunity, B-1 cells seem to exist in the margin between adaptive and innate immunity. Most circulating natural IgM is derived from B-1 cells (2–4), which arise early in life and persist into adulthood via self-renewal (19). B-1 cells were originally identified by expression of CD5 and were further characterized by surface expression of IgMhigh, IgDlow, CD19high, B220low, CD23-, and CD43+ (20), which contrasts with the surface phenotype of follicular B-2 cells: CD5-, IgMlow, IgDhigh, CD19+, B220+, CD23+, and CD43-. Later, an additional population of B-1 cells was identified sharing the characteristics of CD5+ B-1 but lacking CD5 expression (21). Recent literature indicates that CD5+ cells lose CD5- expression upon multiple rounds of cell division (22). Given that CD5- B-1 cells appear to derive from CD5+ B-1 cells (22), examination of antigen specific CD5+ B-1 cells is representative of the available natural antibody repertoire. Herein, we examine CD5+ antigen specific B-1 cells to understand how age and biological sex influences antigen specific natural antibody.
The B-1 cell compartment changes with increasing age (23, 24), and, as recently demonstrated, is also influenced by biological sex (25). Examination of the B-1 cell repertoire has shown that unlike conventional B2 cells, the structure of natural IgM is germline-like due to minimal insertion of non-template-encoded N nucleotides (N-region additions) along with little evidence of somatic hypermutation (26, 27). This germline-like nature of B-1 cell derived natural IgM is required for protection against Streptococcus pneumoniae infection (28), and is lost in aged males (23, 25). In contrast to aged males, B-1 cell derived natural IgM obtained from aged females retains germline-like status as well as the ability to protect against S. pneumoniae infection (25). In males, age-related changes seen in natural antibodies are dependent upon antibody specificity and anatomical location (24), however, it is unknown if such changes occur similarly in aged females.
Approximately 5-15% of peritoneal CD5+ B-1 cells are specific for phosphatidylcholine (PtC), an antigen found on senescent red blood cells as well as bacterial cell membranes (29, 30). Anti-PtC antibodies have been shown to be essential in protection from bacterial sepsis (31). Importantly, biological sex differences are observed in the mortality of patients with sepsis (32–35). Considering our previous studies and the extensive literature demonstrating the major role selection plays in shaping the CD5+ B-1 cell pool, we questioned how PtC specific (PtC+) CD5+ B-1 cells would be affected during aging in female mice. To examine this, we performed single-cell BCR sequencing on PtC+CD5+ B-1 cells from young and aged female BALB/c-ByJ mice. Here, we find differences in the selection of PtC+CD5+ B-1 cells in females versus males over time, demonstrating that aged males utilize different BCR specificities than aged females. The sex-related differences in the natural antibody repertoire observed in our studies have implications for susceptibility to infection and disparities between men and women in the incidence and/or death rate of many diseases of the aged (36).
Male and female BALB/cByJ mice were obtained from The Jackson Laboratory at 6–8 weeks of age and aged in our vivarium until the age indicated. The mice were housed at 5 mice per cage with a 12-hour light/12-hour dark cycle and ad libitum access to water and food. Mice were cared for and handled in accordance with the Guide for the Care and Use of Laboratory Animals, National Institutes of Health, and institutional guidelines. All animal studies were approved by the Western Michigan University Homer Stryker M.D. School of Medicine IACUC committee.
Peritoneal lavage and spleen removals were performed on all euthanized mice. Spleens were homogenized using the rough ends of glass slides or the Miltenyi gentleMACS dissociator and then passed through a 70-μm cell strainer. All samples were treated with RBC lysis buffer for 2 minutes (Lonza), subsequently diluted with HBSS with 2.5% FBS, and then centrifuged at 1200rpm for 10 minutes. The cells were resuspended in HBSS with 2.5% FBS, stained with immunofluorescent antibodies, and then analyzed on a LSR Fortessa flow cytometer or Influx cell sorter (BD Biosciences) with gating on live cells by forward side scatter and/or Aqua Live/Dead stain (Invitrogen). Images were constructed with FlowJo™ v10.6.2 Software (BD Life Sciences). The following antibodies were obtained from BD Pharmingen: CD19 (clone ID3), CD43 (clone S7), B220/CD45 (clone RA3-6B2), CD23 (clone B3B4), CD5 (clone 53-7.3). FITC labeled PtC liposomes (purchased from Dr. Aaron Kantor), diluted at 1:30,000, were used to detect PtC+ B cells, as previously described (5, 24). The composition of the PtC liposomes used is DSPC : DSPG : Chol (Molar ratio: 45:5:50).
Peritoneal washout cells and splenocytes were obtained from BALB/c-ByJ mice at the indicated age and stained with fluorescence-labeled antibodies. PtC+CD5+ B-1 cell populations were single-cell sorted using an Influx cell sorter (BD Biosciences) into a 96-well plate containing lysis buffer (RNaseOut, 5X Buffer, DTT, IgePAL, carrier RNA, Invitrogen). Post-sort re-analysis of CD5+ B-1 cell populations showed them to be ≥;98% pure. To obtain cDNA, a 20μl reverse transcription reaction was run per well using the SuperScript III enzyme and random hexamers (Invitrogen). Qiagen’s HotStart Taq Plus master mix kit was used to perform the first round of PCR (25μl reaction) using 2.5μl of cDNA diluted 1:2 and the following primers: MsVHE and MsCμE each at 0.6μM, as previously described (24, 27). Each 25μl reaction was run as follows: 95°C for 5 minutes; 35 cycles at 94°C for 30 seconds, 50°CCfor 30 seconds, 72°C for 30 seconds; and then a final extention at 72°C for 10 minutes. The product from this first reaction was then diluted at 1:100 in dH2O and 2μl was used in the second semi-nested 25μl reaction using the following primers: MsVHE and MsCμN each at 0.6μM, as previously described (24, 27). The second reaction was run as follows: 95°C for 5 minutes; 40 cycles at 94°C for 30 seconds, 53°C for 30 seconds, 72°C for 30 seconds; and then a final extension at 72°C for 10 minutes. The products were run on the Qiagen Qiaxcel. PCR products were sequenced (Genewiz) using the MsVHE primer. Sequences were analyzed using an online sequence analysis tool, IMGT/HighV-Quest (37).
Serum was collected from individual female BALB/c-ByJ naïve mice at the time of euthanasia at the ages indicated. The serum was analyzed for antibody against DMPC by ELISA. ELISA strips were obtained from Avanti Polar Lipids pre-coated with 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC). Wells were blocked with 200μl of 3% fatty acid free bovine serum albumin in PBS for one hour at room temperature with gentle shaking and then washed three times with 1X PBS. Diluted serum was added at 50μl per well and incubated for one hour at room temperature with gentle shaking. The wells were then washed three times with 1X PBS. Bound antibody was measured using HRP-conjugated goat anti-mouse IgM (Bethyl Labs) at 1:20,000. NC-17, kindly provided by Dr. Gregg Silverman, was used as a standard and included on each plate.
Serum was collected from individual young (3- to 4-mo) and old (18- to 24-mo) male and female mice and processed with the Quick-cfDNA/cfRNA Serum & Plasma kit (Zymo Research) according to the manufacturer’s directions. Briefly, serums were centrifuged at 16,000 g for 10 minutes to remove any cell debris and precipitates before undergoing Proteinase K digestions. Nucleic acids were extracted from 200 μL of serum. Samples were run through the Quick-cfDNA/cfRNA columns and cfDNA/cfRNA was collected through the co-purification method. Extracted cfDNA/RNA was stored at -80°C prior to further analysis.
Extracted cell-free nucleic acids from each individual serum sample were quantified using the dsDNA high sensitivity assay, ssDNA assay, and RNA high sensitivity assay for the Qubit 4 (ThermoFisher) as well as on the 2100 Bioananalzyer (Agilent Technologies) with HS DNA chips for assessment of sample purity, concentration, and fragment size distribution according to the manufacturer’s instructions. The average fragment size was determined with the Agilent 2100 Bioanalzyer Expert software and calculated across the first three major peaks 50 – 650 base pairs (bp) corresponding to the length of nucleosomal footprints derived from apoptotic cells while high molecular weight cfDNA was calculated between 2000 – 3000 bp corresponding to cfDNA derived from necrotic cells (38–40). The final serum cfDNA/RNA concentration were calculated by adjusting for the initial serum and final elution volumes and quantified with the Qubit 4.
Statistical analyses were performed using Prism (Version 9.0). All statistical analyses used are indicated in each figure legend. The outlier test was performed on all data sets using Prism’s ROUT method of identifying outliers. Outliers were removed when detected by Prism’s ROUT method using the coefficient Q set at 1%. Error reported as standard error of the mean.
Using fluorescently labeled PtC-liposomes (41), we assessed the female peritoneal (PerC) and splenic CD5+ B-1 cell pools for PtC specificity (representative plots in Figures 1A, B). While the frequency of PerC CD5+ B-1 cells that bound PtC-liposomes did not change significantly in age-grouped female mice (mean of 3.8%±0.8 in aged vs. 6.2%±0.8 in young) the frequency of PerC PtC+CD5+ B-1 cells is significantly higher in both young and aged males as compared to young and aged females, respectively (Figure 1C). The absolute number of PerC PtC+CD5+ B-1 cells shows no significant difference (Figure 1D). In the spleen, the frequency of PtC+CD5+ B-1 cells is significantly higher in aged males as compared to aged females (Figure 1E); however, the absolute number of splenic PtC+CD5+ B-1 cells is significantly higher in aged females as compared to aged males (Figure 1F). The differences in percent but not total number of PtC specific B-1 cells is due to differences in total CD5+ B-1 cell numbers in the peritoneal cavity and spleen (Supplemental Figure 1).
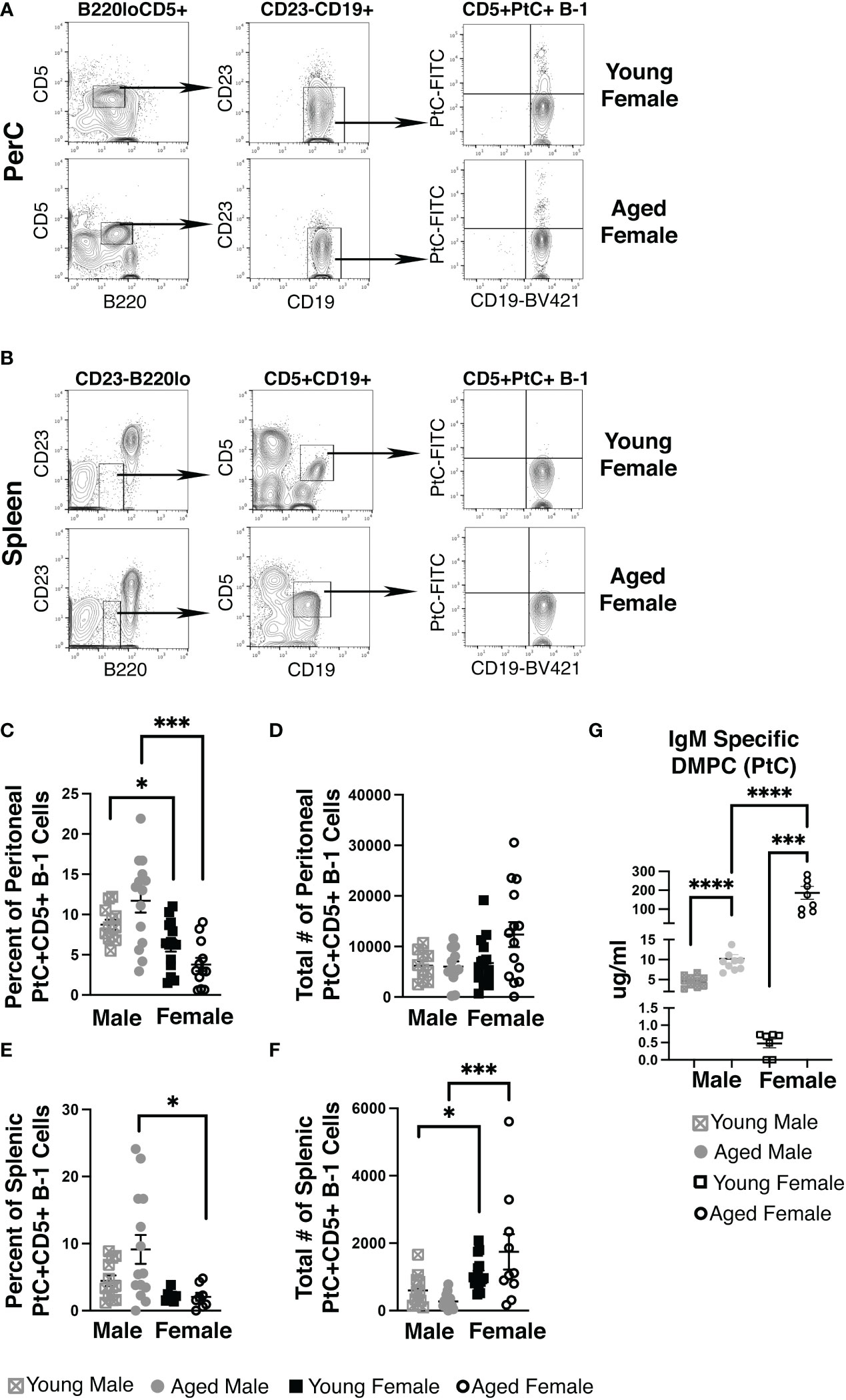
Figure 1 Female mice have an increase in PtC-specific splenic CD5+ B-1 cells and serum anti-DMPC antibody. Representative gating strategy for (A) peritoneal cavity and (B) splenic PtC+CD5+ B-1 cells. The frequency (C, E) and absolute number (D, F) of PtC+CD5+ B-1 cells were assessed in young (3-month-old) and aged (18-22-month-old) male and female BALB/c-ByJ mice (young male n=12, aged male n=14, young female n=15, aged female n=14). (C) The percent of live peritoneal lymphocytes staining positive for PtC+CD5+ B-1 cells (B220loCD5+CD19hiCD23-PtC+), (D) total number of peritoneal PtC+CD5+ B-1 cells, (E) the the percent of live splenocytes staining positive for PtC+CD5+ B-1 cells (B220loCD5+CD19hiCD23-PtC+), and (F) total number of splenic PtC+CD5+ B-1 cells. (G) Serum levels of DMPC-specific IgM from young (3-month old) and aged (22-26-month old) mice (young male n=10, aged male n=10, young female n=7, aged female n=9). Grey squares represent young male mice, grey circles represent aged male mice, open black squares represent young female mice, and open black circles represent aged female mice. All results are based on 3 independent experiments. Values are displayed as the mean ( ± SEM) of individual mouse serum samples. Statistics used: Mann-Whitney test. Asterisks for p values: *p<0.05, ***p<0.001.
To test serum levels of PtC-specific antibody in aged females, we analyzed serum from young and aged mice by ELISA for IgM antibody that recognizes PtC (DMPC, 1,2-dimyristoyl-sn-glycero-3-phosphocholine is the phosphatidylcholine used to coat the ELISA plate). Interestingly, we observed significantly more DMPC specific IgM in serum from aged female mice as compared to young female mice (p=0.0079) (Figure 1G). Furthermore, young female mice have significantly less serum DMPC specific IgM levels than young male mice (0.80 μg/ml ±0.2 vs. 4.58 μg/ml ±0.43, p=0.0007). Aged female mice have significantly more serum DMPC specific IgM levels than aged male mice (189 μg/ml ±40.1 vs. 10.2 μg/ml ±1.06, p=0.0007) (Figure 1G). These results demonstrate in both male and female mice, serum PtC-specific antibody increases with age; however, aged female mice have significantly more serum PtC-specificIgM than aged males.
Considering aged females display considerably higher levels of PtC-specific serum antibody, we hypothesized that the aged female repertoire of PtC+CD5+ B-1 cells might be distinct from that of young females and aged males. Examination of the variable (VH), diversity (DH), and joining (JH), gene segments of the immunoglobulin heavy chain as well as germline status of PtC+CD5+ B-1 cells obtained from either the peritoneal cavity (PerC) or spleen of young and aged female mice revealed differences in 1) the germline status and 2) the VH, DH, and JH gene usage between young and aged females. Aged PerC PtC+CD5+ B-1 cells utilized VH12 (58% vs. 39%) more frequently than young. In contrast, the young PerC PtC+CD5+ B-1 cells used VH5 (3% vs. 1%) and VH11 (31% vs. 15%) more frequently than aged. Aged splenic PtC+CD5+ B-1 cells used VH1 (8% vs. 4%), VH10 (3% vs. 0%), VH12 (58% vs. 40%), and VH14 (6% vs. 1%) more frequently than young whereas, the young splenic PtC+CD5+ B-1 cells used VH11 more frequently than aged (37% vs. 9%) (Figure 2A).
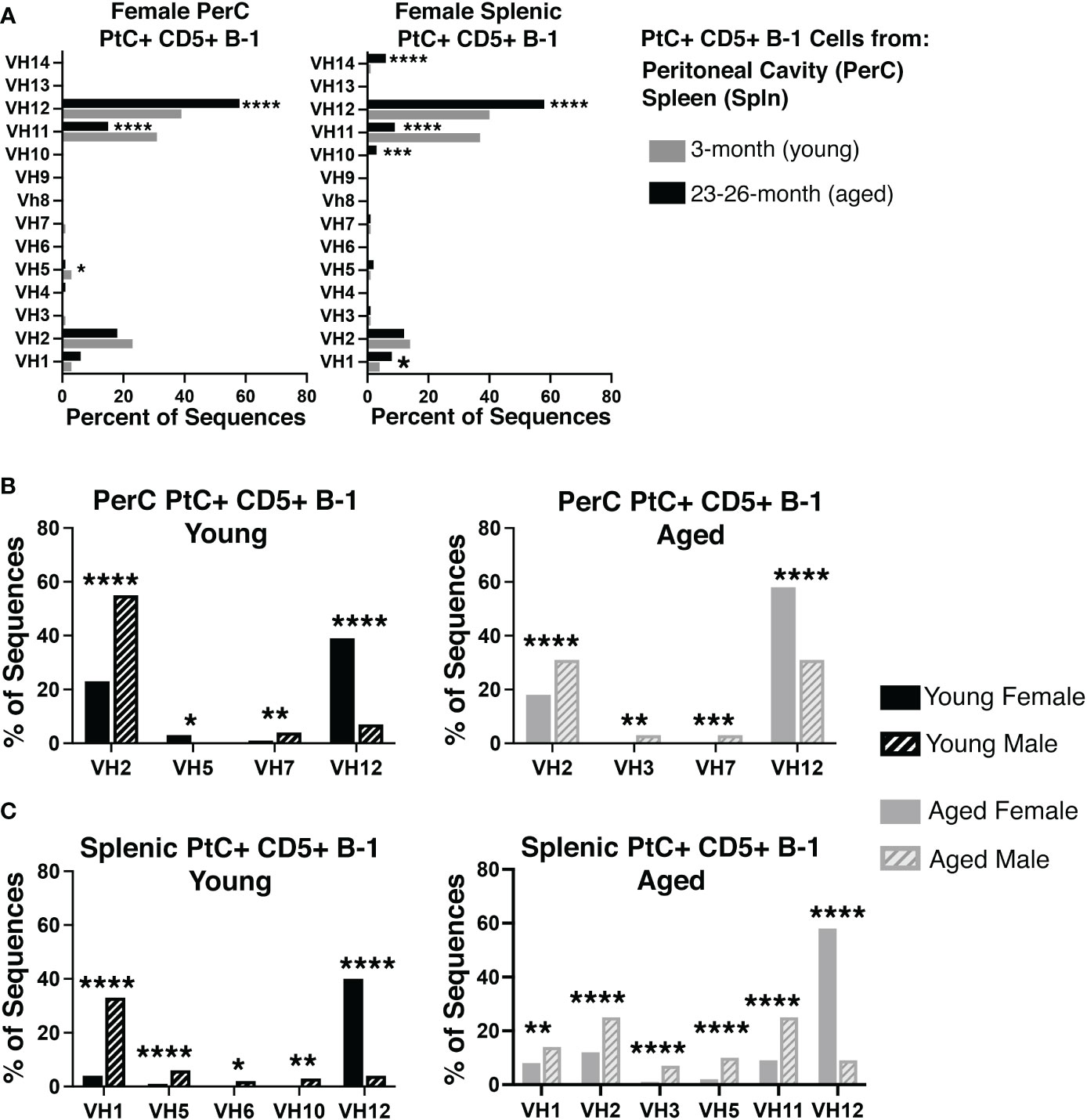
Figure 2 Repertoire analysis of natural IgM from peritoneal and splenic PtC+ CD5+ B-1 cells in adult female young and aged mice. PtC+CD5+ B-1 cells were single-cell sorted from the peritoneal cavity or spleen of 3- and 23-26-month-old female BALB/c-ByJ mice. The VH region was amplified and sequenced as detailed in Materials and Methods. (A) The percent of VH gene segment usage (young in grey bars, aged in black bars). (B, C) For direct comparison of females and males,previously published (24) repertoire analyses of young and aged male PtC+CD5+ B-1 cells is included. Results are based on 4 independent experiments with sequences combined from each independent experiment (n=11 for 3-month-old mice, n=15 for 23-26-month-old female mice). Statistics used: 2x2 chi-square test. Asterisks for p values: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
When female PtC+CD5+ B-1 cell repertoire data is compared to our previously published male PtC+CD5+ B-1 cell data, numerous significant differences were observed between male and female VH utilization (Figure 2B, C). Young female PerC PtC+ CD5+ B-1 cells utilized VH12 and VH5 more frequently than young males whereas, young males utilized VH2 and VH7 more frequently than young females (Figure 2B). Aged female PerC PtC+CD5+ B-1 cells utilized VH12 more frequently than aged males, whereas aged males used VH2, VH3, and VH7 more frequently (Figure 2B). In the spleen, young and aged female PtC+CD5+ B-1 cells utilized VH12 more frequently than young and aged males (Figure 2C). However, young male splenic PtC+CD5+ B-1 cells used VH1, VH5, VH6, and VH10 more frequently than young females. In the aged spleen, male PtC+CD5+ B-1 cells utilized VH1, VH2, VH3, VH5, and VH11 more frequently than aged females. Together these results demonstrate significant differences in PtC+CD5+ B-1 cell VH usage associated with biological sex and age.
While heavy chain sequencing of complementary determining region 3 (CDR-H3) alone is not sufficient to establish clonal expansion, a higher proportion of replicate sequences (sequences with the exact same CDR-H3 sequence) suggests the presence of clonally divided cells. Sequences from young PerC PtC+CD5+ B-1 cells were replicated at 67% (237/354), while PerC PtC+CD5+ B-1 cells from aged mice were replicated at 85% (412/484) (Figure 3A). Similar results were found in the spleen, 69% (287/417) replicates from young vs. 85% (412/484) from aged (Figure 3A). Of the replicates observed in aged PerC and splenic PtC+CD5+ B-1 cells, VH12 was the most abundantly used VH gene segment (Figure 3A). Furthermore, we observed a decrease in diversity, defined as the proportion of unique CDR-H3 sequences, within replicate sequences from aged female PerC and splenic PtC+CD5+ B-1 cells (Figure 3B). Quantification of the most utilized CDR-H3 sequences found the PtC/PC (phosphorylcholine) cross-reactive CDR-H3 (MRYGNYWYFDV, VH11) as the most frequently utilized replicate in both young female and male PerC and splenic PtC+CD5+ B-1 cells (Figures 3C, D). However, aged female PerC and splenic PtC+CD5+ B-1 cells utilize VH12 more frequently in replicates whereas, aged males utilize VH11 (Figures 3C, D). Splenic PtC+CD5+ B-1 cells from aged female mice are more diverse in their CDR-H3 replicate sequences (Figure 3) than splenic PtC+CD5+ B-1 cells from aged males (24); however, PerC PtC+CD5+ B-1 cells from both aged females and males are similar in replicate diversity.
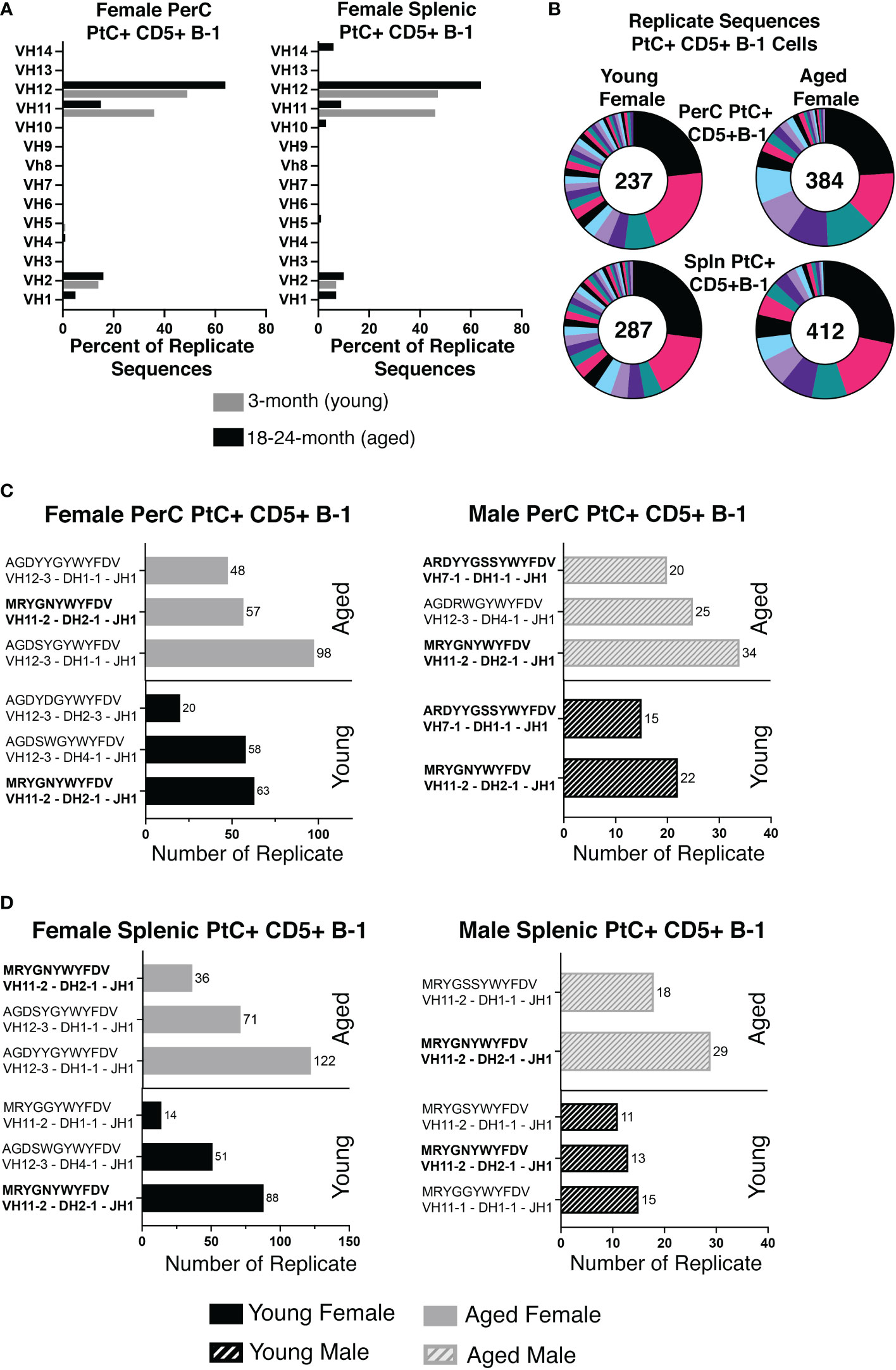
Figure 3 Significant differences in female versus male peritoneal and splenic PtC+CD5+ B-1 cell CDR-H3 use. PtC+CD5+ B-1 cells were single-cell sorted from the peritoneal cavity or spleen of young and aged female BALB/c-ByJ mice (as presented in Figure 2). (A) The percent of VH gene segment usage within the replicate sequences. (B) Distribution of replicate CDR-H3 sequences in the young and aged (number in the middle represents the number of replicates within the population). Each color represents a unique CDR-H3 amino acid sequence. (C, D) For direct comparison of females and males, previously published (24) CDR-H3 analysis of young and aged malePtC+CD5+ B-1 cells is included. (C) Comparison of the most frequently utilized CDR-H3 sequences of peritoneal PtC+CD5+ B-1 cells from young and aged male and female mice. (D) Comparison of the most frequently utilized CDR-H3 sequences of splenic PtC+CD5+ B-1 cells from young and aged male and female mice. Results are based on 4 independent experiments with sequences combined from each independent experiment (n=11 for 3-month-old mice, n=15 for 23-26-month-old female mice). Statistics used: 2x2 chi-square tests.
Examination of DH and JH genes show significant differences in utilization in the aged versus young female PerC and splenic PtC+ populations (Figures 4A, B), the details of which are summarized in Supplemental Figure 2. Of note, aged female PerC and splenic PtC+CD5+ B-1 cells utilized DFL16.1 (DH1-1) more frequently than young PtC+CD5+ B-1 (Figure 4A). The germline sequence of DFL16.1 has been shown to be essential in protection against pneumococcal infection (42).
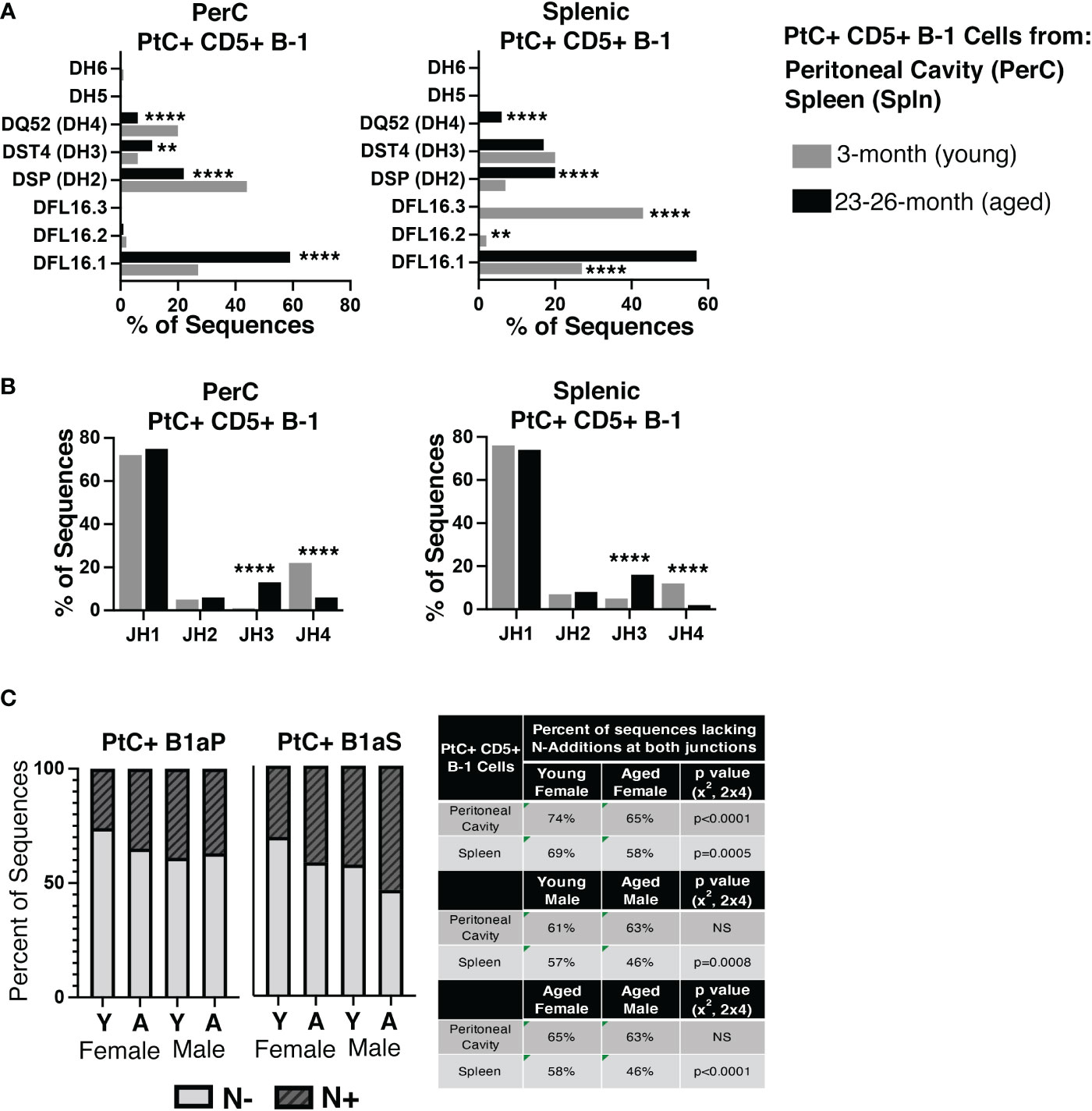
Figure 4 Analysis of DH use, JH use, and germline status of young and aged PtC+CD5+ B-1 cells. PtC+CD5+ B-1 cells were single-cell sorted from the peritoneal cavity or spleen of young and aged female BALB/c-ByJ mice (as presented in Figure 2). (A) The percent of DH gene segment usage. (B) The percent of JH gene segment usage. (C) The percent of sequences containing zero N-additions (light grey bars) or 1 or more N-additions (dark grey hashed bars) at both junctions is shown with replicate sequences included in the analysis. For direct comparison of females and males, previously published (24) N- addition analysis of young and aged male PtC+ CD5+ B-1 cells is included. Results are based on 4 independent experiments with sequences combined fromeach independent experiment (n=11 for 3-month-old mice, n=15 for 23-26-month-old female mice). Statistics used: 2x2 and 2x4 chi-square test. Asterisks for p values: **p<0.01, ****p<0.0001.
The number of BCR sequences lacking N-additions at both female junctions (germline-like structure) changes with age in both female PerC PtC+CD5+ B-1 cells (65% in aged vs. 74% in young, p<0.0001, x2, 2x4) and splenic PtC+CD5+ B-1 cells (58% in aged vs. 0 69% in young, p=0.0005, x2, 2x4) (Figure 4C). Interestingly, there is no significant difference in the number of PerC PtC+CD5+ B-1 cell sequences lacking N-additions between aged female (65%) and aged male mice (63%); however, there is a significant difference in the number of splenic PtC+CD5+ B-1 cell sequences lacking N-additions between aged female mice (58%) as compared to aged male mice (46%) (p<0.0001, x2, 2x4) (Figure 4C). Together, these results demonstrate significant changes in the repertoire of both peritoneal and splenic PtC specific natural IgM obtained from aged female mice as compared to young female mice. Furthermore, these differences observed in female mice differ from previously published results examining PtC-specific natural IgM from aged male mice as compared to young male mice (24).
Of the 6 complementary determining regions (CDRs) in antibodies, CDR-H3 is the central point for antigen contact and the most variable in nucleotide/amino acid sequence. Hydrophobicity is a measurable characteristic of the CDR-H3. Hydrophobic CDR-H3 loops have been shown to be important for certain broadly neutralizing antibodies against HIV (43), whereas antibodies with highly charged CDR-H3 loops are often autoreactive (44, 45). We calculated the average hydrophobicity of each CDR-H3 loop using the Kyte-Doolittle scale. Our results demonstrate the CDR-H3 loop of peritoneal (PerC) PtC+CD5+ B-1 cell IgM increases in charge (decreases in hydrophobicity) with age (-0.247 ±0.010 in young vs. -0.300 ±0.009 in aged, p<0.0001) (Figure 5A). Furthermore, females have more highly charged CDR-H3 loops than males in both the PerC and splenic compartments (Figure 5B).
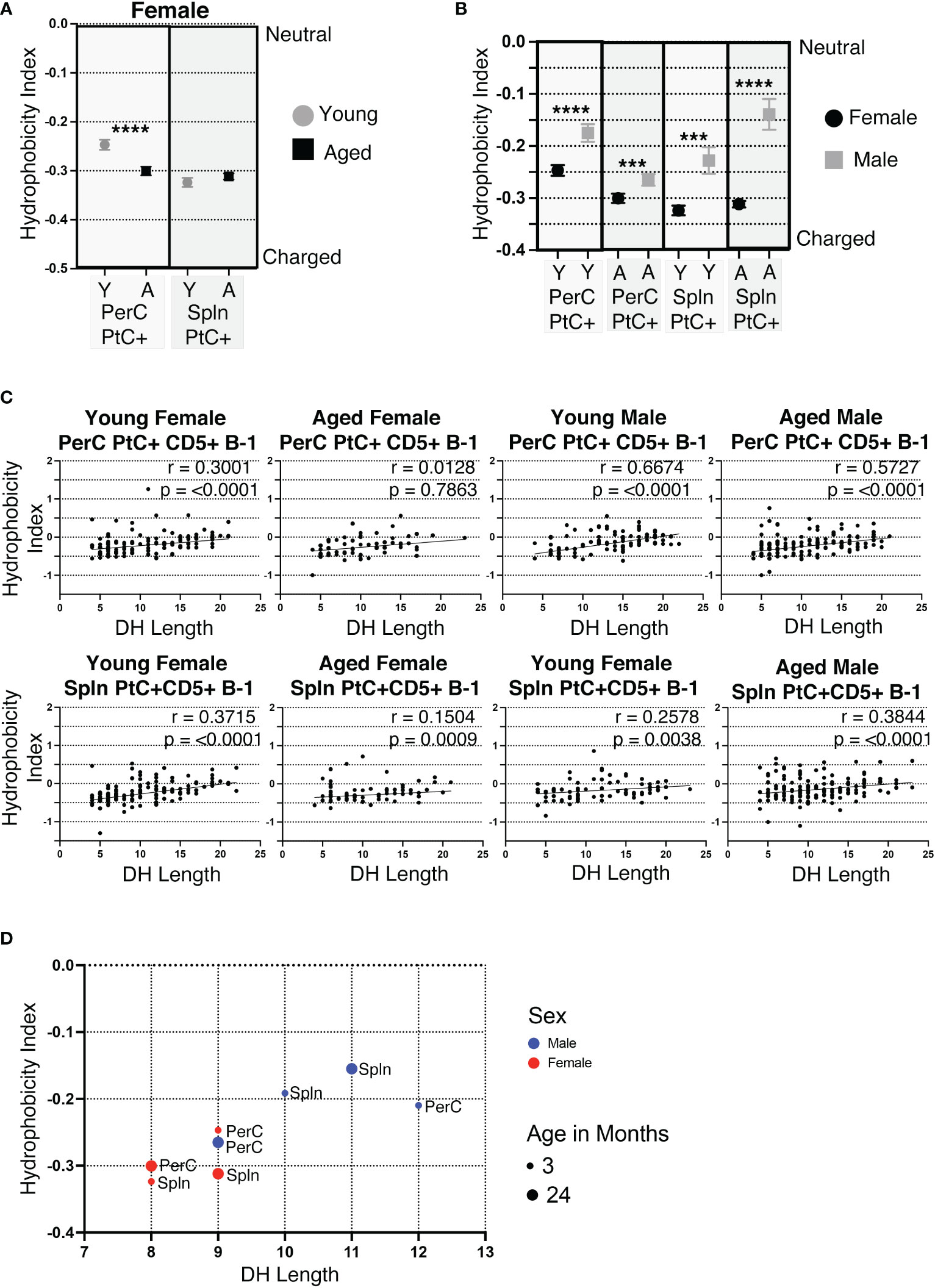
Figure 5 CDR-H3 hydrophobicity of PtC+CD5+ B-1 cell IgM changes with age and sex. PtC+CD5+ B-1 cells were single-cell sorted from the peritoneal cavity or spleen of young and aged female BALB/c-ByJ mice (as presented in Figure 2). “Y” indicates young mice (3-month-old) and “A” indicates aged (23-26-month-old) mice. (A) The average charge of the CDR-H3 loop region of IgM from the peritoneal or splenic PtC+CD5+ B-1 cells. (B-D) For direct comparison of females and males, previously published (24) hydrophobicity analysis of young and aged male PtC+CD5+ B-1 cells is included. (B) Female and male comparison of the average charge of the CDR-H3 loop region of IgM from peritoneal or splenic PtC+CD5+ B-1 cells. (C, D) Correlation of average charge of the CDR-H3 loop region with DH length. Results are based on 4 independent experiments with sequences combined from each independent experiment (n=11 for 3-month-old mice, n=15 for 23-26-month-old female mice). Statistics used: Mann-Whitney test for (A, B) and Spearman’s rank correlation coefficient (C). Asterisks for p values: ***p<0.001, ****p<0.0001.
The CDR-H3 loop of B-1 cells is characteristically hydrophobic (46). DH use has been shown to be primarily responsible, with DH length positively correlating with increased hydrophobicity of CDR-H3 (46). In concurrence, the hydrophobicity of PerC and splenic PtC+CD5+ B-1 cell CDR-H3 loops positively correlate with the length of the respective DH gene (Figure 5C, D). Young and aged male mice tend to utilize longer DH gene segments than females, which correlates with the more hydrophobic CDR-H3 loops seen in males as compared to the more charged CDR-H3 loops in females (Figures 5C, D).
Previous studies have shown PtC specific B-1 cells most frequently utilize VH2, VH11, and VH12 (47, 48). Examination of CDR-H3 hydrophobicity of PtC specific B-1 cells utilizing VH2, VH11, or VH12 reveals sex and age-related differences. In female PerC and splenic PtC+CD5+ B-1 cells utilizing VH11 and VH2, there is a significant increase in charge with age (Figure 6A). Young female cells utilizing VH2 were more hydrophobic than those utilizing VH11 and VH12. The hydrophobicity of cells using VH12 did not change with age (Figure 6A). There were no significant differences in the hydrophobicity of PtC specific B-1 cells utilizing VH2, VH11, or VH12 between young female and male mice (Figure 6B). However, aged females displayed significantly higher charged CDR-H3 loops than aged males for VH2, VH11, and VH12 with one exception; PerC PtC+ B-1 cells utilizing VH12 from aged males were significantly increased in charge as compared to aged females (Figure 6B).
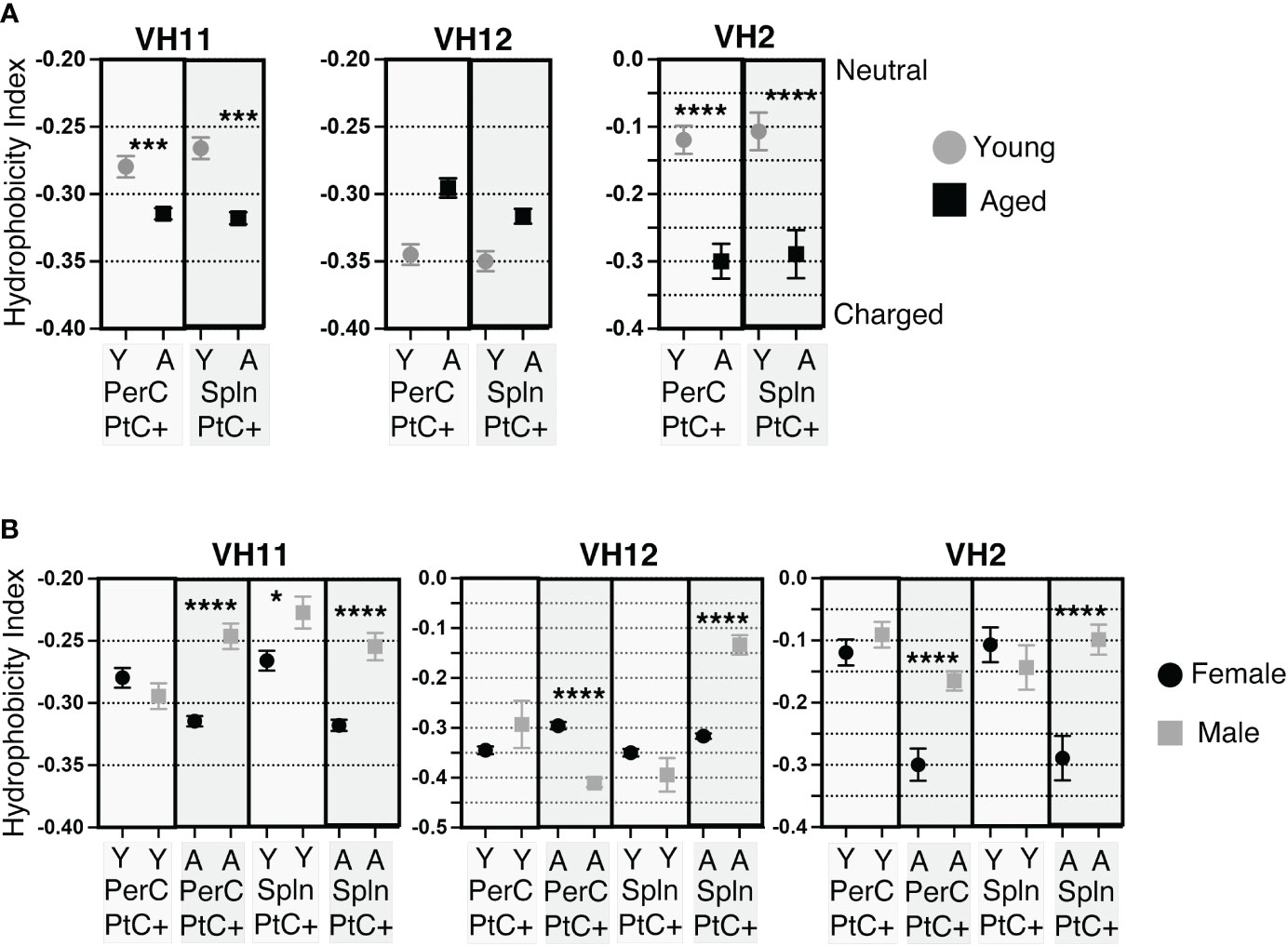
Figure 6 CDR-H3 hydrophobicity of PtC+CD5+ B-1 cell IgM utilizing VH11, VH12, or VH2. PtC+CD5+ B-1 cells were single-cell sorted from the peritoneal cavity or spleen of young and aged female BALB/c-ByJ mice (as presented in Figure 2). “Y” indicates young mice (3-month-old) and “A” indicates aged (23-26-month-old) mice. (A) The average charge of the CDR-H3 loop region of IgM utilizing either VH11, VH12, or VH2 from peritoneal or splenic PtC+CD5+ B-1 cells. (B) For direct comparisonof females and males, previously published (24) hydrophobicity analysis of young and aged male PtC+CD5+ B-1 cells is included. Female and male comparison of the average charge of the CDR-H3 loop region of IgM utilizing either VH11, VH12, or VH2 from peritoneal or splenic PtC+CD5+ B-1 cells. Results are based on 4 independent experiments with sequences combined from each independent experiment (n=11 for 3-month-old mice, n=15 for 23-26-month-old female mice). Statistics used: Mann-Whitney test. Asterisks for p values: *p<0.05, ***p<0.001, ****p<0.0001.
Together, these results demonstrate differences in hydrophobicity of the CDR-H3 loop in female PtC specific CD5+ B-1 cell populations with age as well as differences between aged male and female mice. Overall, female mice display more highly charged CDR-H3 loops than males.
We examined the amino acid content of the CDR-H3 regions of PtC+CD5+ B-1 cells and found a predominance of tyrosine and glycine in cells from both the peritoneal cavity (PerC) and spleen (Figure 7). We observed many significant differences in amino acid content of PerC and splenic PtC+CD5+ B-1 cells between young and aged female mice (Figure 7A), the details of which are summarized in Supplemental Figure 3. When comparing these results to our previously published study of males, aged males displayed significantly fewer differences in amino acid content with age (24) as compared to aged females (Figures 7A, B). This distinction is even more apparent when examining sequences utilizing VH11, VH12, and VH2 (Figure 7B and (24)). Female sequences utilizing VH12 and VH2 had many significant changes in amino acid content with age (summarized in Supplemental Figure 4), which contrasts with the few changes observed in aged male mice (24). These results demonstrate differential selection of the PtC+CD5+ B-1 cell repertoire in males versus females over time.
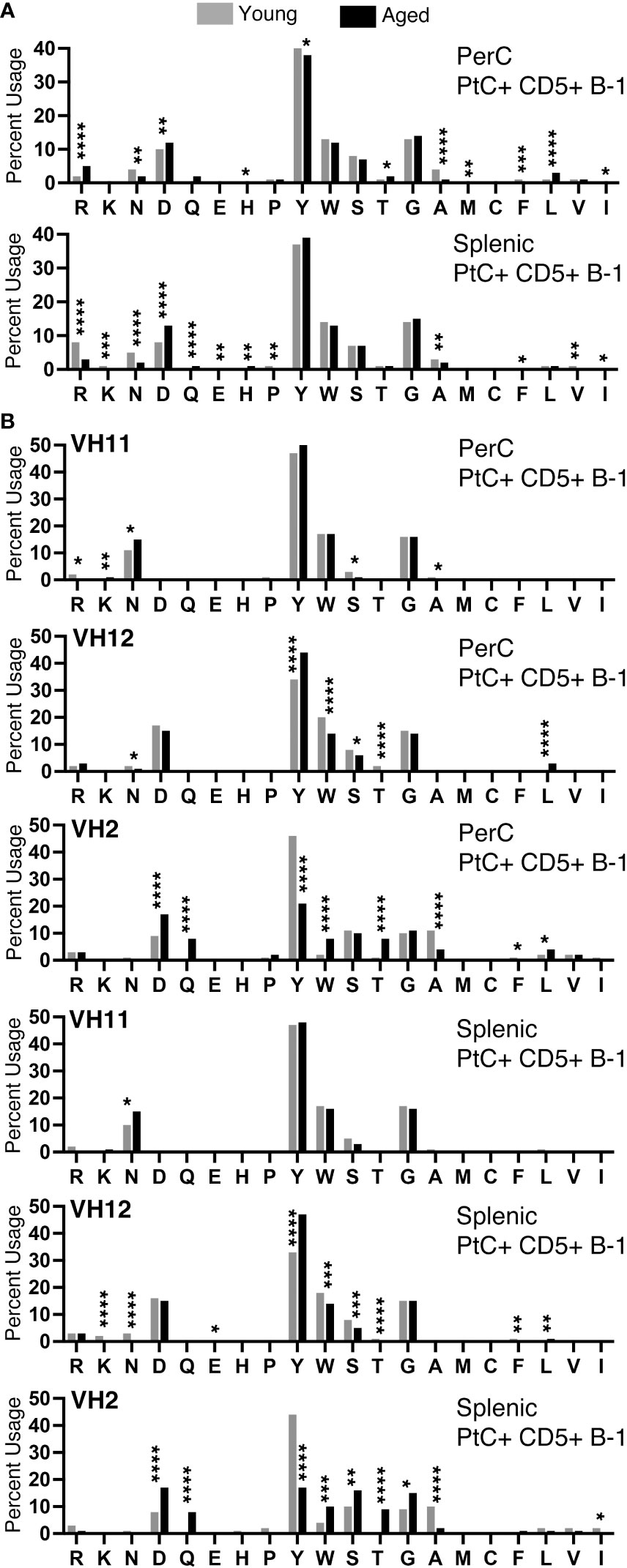
Figure 7 CDR-H3 amino acid distribution within the CDR-H3 changes with age. The percent of each amino acid used within the CDR-H3 was determined for each PtC+CD5+ B-1 cell subset as indicated. (A) Peritoneal and splenic PtC+CD5+ B-1 cells. (B) Peritoneal and splenic PtC+CD5+ B-1 cells utilizing VH11, VH12, or VH2. These results are based on sequences obtained from experiments performed in Figure 2. Results are based on 4 independent experiments with sequences combined from each independent experiment (n=11 for 3-month-old mice, n=15 for 23-26-month-old female mice). Statistics used: Chi-square test. Asterisks for p values: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
We observed an overall preference for less hydrophobic and less charged amino acids in the CDR-H3 from both young and aged female PtC+CD5+ B-1 cells (Figure 7A). When examining sequences utilizing VH11, asparagine is preferentially used, whereas in sequences utilizing VH12 and VH2 aspartic acid usage is preferred (Figure 7B). These findings reflect previous studies showing DSP gene segments utilizing RF1 contain either asparagine or aspartic acid (49). Both splenic and PerC aged PtC+CD5+ B-1 cell sequences utilizing VH12 display an increase in tyrosine, whereas sequences utilizing VH2 display a decrease in tyrosine with age. These changes in CDR-H3 amino acid content could have implications for CD5+ B-1 cell function in the aged.
Considering our results demonstrating large differences in the serum and cellular repertoire of PtC-specific CD5+ B-1 cells in aged females as compared to young females and aged males, we hypothesized differences in antigenic load may be a driving factor. Recently, it was shown that Toll-like receptors (TLR) play a role in shaping the CD5+ B-1 cell repertoire, and TLRs sensing nucleic acids were specifically tied to anti-PtC responses (50). Cell-free nucleic acids (cfNCs) found in circulation are mainly the result of apoptosis (51). The most well characterized cell-free nucleic acid is DNA (cfDNA) (52), but many other types of cfNCs have been described including RNAs and mitochondrial DNA (53). After isolation of cfNC from the serum, we found a significant increase in double stranded cfDNA with age (Figure 8A). Levels of serum cfDNA increased from an average of 1.272 ng/μL in young males to 4.640 ng/μL in aged males. The increase in cfDNA in aged female serum was greater with young females having an average of 1.252 ng/μL and aged females 22.74 ng/μL of cfDNA (Figure 8A). Levels of single stranded cfDNA was significantly increased in aged male mice as compared to young male mice, and a non-statistically significant trend toward increase was observed in aged female mice (Figure 8B). When examining cfRNA, aged female mice displayed significantly increased levels of serum cfRNA as compared to both young females and aged males (Figure 8C).
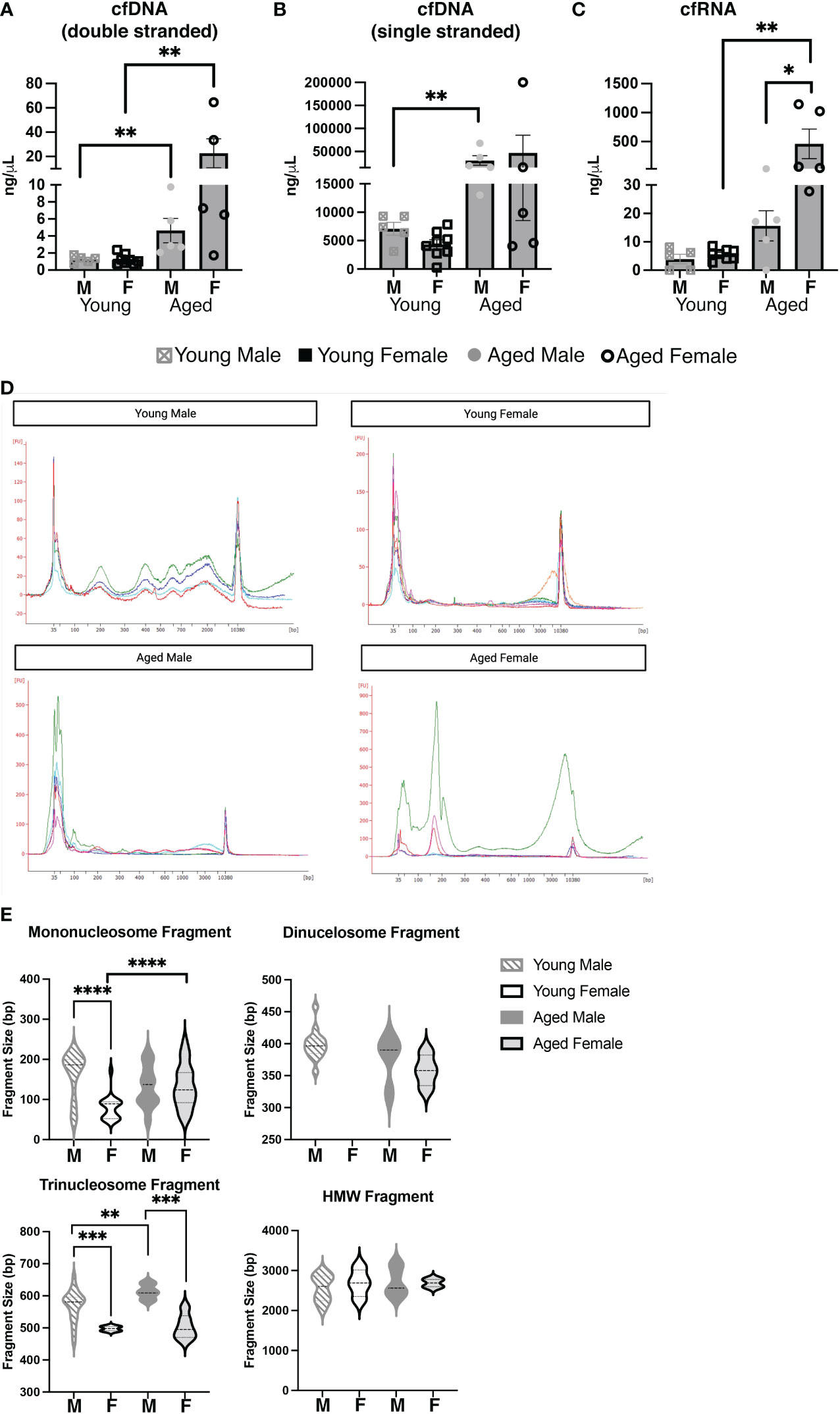
Figure 8 Aging and biological sex influences the levels and composition of serum cell-free nucleic acids. Serum was obtained from young (3-month-old) or aged (18-25-month-old) male and female BALB/c-ByJ mice (young male mice n=10, aged male mice n=10, young female mice n=7, aged female mice n=9). Cell-free (cf) nucleic acids were isolated from 200ul of each individual serum sample. (A) Double-stranded cfDNA, (B) single-stranded cfDNA, and (C) cfRNA were quantified using the Qubit 4. (D) cfDNA was measured on the Agilent 2100 Bioanalyzer using the HS DNA assay, and individual electropherograms for each sample were combined for each group (young and old males and females). (E) cfDNA fragment sizes for each of the three major cfDNA peaks (50–250, 250–450, 450–650) and the HMWcfDNA (~2300bp) from each trace were mapped with violin plots. Statistics used: Mann-Whitney test and Welch’s t-test (violin plots). Asterisks for p values: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
We further explored the cell-free nucleic acid composition of these serum samples using a high sensitivity DNA assay for the Bioanalzyer (Figures 8D, E). Interestingly, young females had relatively low amounts of smaller (50-650bp) cfDNA but larger HMW cfDNA peaks as compared to young males (Figure 8D). The small cfDNA peaks were strong in aged females, as seen in both young and old males; however, in agreement with the quantitation from the Qubit (Figures 8A-C), the total amount of cfDNA is drastically increased in aged females as compared to the other groups (Supplemental Figure 4). Importantly, smaller (50-650bp) fragments have been shown to stem from apoptosis while larger fragments are thought to originate from necrosis (38, 54–56). To better explore the possible source of cfDNA from our samples, the fragment size was analyzed for the following three groups: 50-250 bp (corresponding to mononucleosome fragments), 250-450 bp fragments (corresponding to dinucleosome fragments), 450-650 bp fragments (corresponding to trinucleosome fragments) and high molecular weight (HMW) cfDNA fragments which were measured at 2,000-3,000 bp (Figure 8E). Total distribution and the average size of the fragment shows that the cfDNA products are different with both sex and age. In the 50-250bp fragment size, the most significant change is seen between young males and females: the average bp size of young males mononucleosome fragment is 161.5±12.27 bp while the young female is 79.26±6.895 bp (p<0.0001). This difference was not observed in aged males and females (132.5 bp vs. 133.3 bp, respectively, p=0.9542). These data suggest the origin of circulating cfDNA is primarily apoptosis of cells, but there are differences between females (of both age groups) as compared to males (Figures 8D, E). In total, the quantity and composition of cfDNA is significantly different between young and aged males and females. Together these results demonstrate the amount of cell-free nucleic acids in the serum of mice changes with age and/or biological sex.
Our previous studies have demonstrated that protection against pneumococcal infection afforded by natural IgM is influenced by biological sex and age. In contrast to aged males, B-1 cell derived natural IgM obtained from aged females retains the ability to protect against S. pneumoniae infection (25). To explore how females are able to retain protective natural antibodies (NAbs) into old age we examined antigen specific NAbs in young and aged female mice. Specifically, we sought to determine whether sex and age influence CD5+ B-1 cells producing natural antibody specific for the phospholipid phosphatidylcholine (PtC), which is protective against sepsis (31).
We find an increase in the number of splenic PtC+CD5+ B-1 cells in aged females as compared to aged males, in correlation with 18 times more serum anti-PtC (DMPC) specific IgM in aged female vs. aged male mice. BCR repertoire analysis reflects this change as well. We found splenic PtC+CD5+ B-1 cell IgM maintains germline-like status in aged females more than aged males, suggesting that retention of protection in aged females is in part due to sex-related amino acid structural differences in the B cell receptor and corresponding antibody. Furthermore, we observed differences in the amino acid use within the antigen binding region of the antibody (CDR-H3 region) based on both sex and age. Changes in amino acid use within the CDR-H3 are indicative of selection over time. Increase in arginine use is associated with an increase in autoreactive antibodies (44, 57, 58). Herein, we demonstrate female peritoneal PtC+CD5+ B-1 cells have an increase in arginine use with age, suggesting this population could contribute to a corresponding increase in autoantibodies during aging. Together, our results demonstrate significant differences in the actual (serum PtC specific antibody) and available (cellular PtC specific BCRs) PtC repertoire in aged males and females.
It has been previously shown that changes in B cell repertoire such as DH usage, JH usage, N-region additions (germline status), and/or amino acid content within the CDR-H3 greatly influences the hydrophobicity of the CDR-H3 (45). Hydrophobicity is a characteristic of the CDR-H3, which plays a role in the interaction of the antibody and antigen. Hydrophobic CDR-H3 loops have been shown to be important for certain broadly neutralizing antibodies against HIV (43). The CDR-H3 loops of peritoneal cavity B-1 cell antibodies are characteristically hydrophobic (44). We found that as males and females age, the CDR-H3 regions change in hydrophobicity based on their location: splenic PtC+CD5+ B-1 cells increase remain hydrophobic while those in the peritoneal cavity increase in charge. While the trends in hydrophobicity with age are similar for aged males and females, we find female PtC+CD5+ B-1 cell IgM is more charged than male PtC+CD5+ B-1 cell IgM in both the spleen and peritoneal cavity. Importantly, the CDR-H3 of autoreactive antibodies is more charged than the CDR-H3 of non-autoreactive antibodies (44, 45). The results presented here are intriguing and consistent considering the higher prevalence of autoimmunity seen in females (59).
When comparing the repertoire of healthy aged mice to those with disease, there are differences in the utilization of specific CDR-H3 sequences (summarized in Supplemental Figure 6). Herein, we find peritoneal B-1 cells from aged females utilize AGDSYGYWYFDV (VH12/DH1-1/JH1) most frequently. In female B6.Sle1.Sle2.Sle3 lupus-prone mice, peritoneal B-1 cells utilize AGDYDGYWYFDV (VH12/DH2/JH1) and MRYGNYWYFDV (VH11/DH2/JH1) most frequently (60), which are distinct from the CDR-H3 utilization we observe in the healthy aged female mice herein. In CD5+ B-1 cells obtained from aged male ApoE knockout mice, the most frequently utilized CDR-H3 is AGDYDGYWYFDV (VH12/DH2/JH1) (61), which is the same frequently utilized CDR-H3 seen in the lupus-prone mice. Interestingly, the frequently utilized CDR-H3 sequences observed in the aged utilize VH12, which has been previously shown to expand with age in an antigen-dependent manner (47). VH12 is known to recognize the phospholipid PtC (48). It is possible PtC could play a role in selection of the B cell repertoire over time. Interestingly, phospholipids have been shown to have direct effects on B cell function (62–64). Alterations in the regulation of PtC have been shown to be influenced by both age (65, 66) and sex (67, 68). Our data illustrating sex and age-related differences in VH12 utilization warrant further investigation regarding differences within the aging environment that influence the selection of B-1 cells and the natural antibodies they produce over time.
Overall, our BCR repertoire results suggest the antigenic environment of aged females may differ from that of aged male, and our analysis of cell-free DNA (cfDNA) supports that conclusion. The maintenance of CD5+ B-1 cells recognizing the phospholipid PtC is dependent on nucleic acid-sensing Toll-like receptors (TLR) (50). Aging is associated with increased cell senescence (69), decreased clearance of apoptotic cells (70), and higher levels of circulating cell-free nucleic acids (such as DNA, RNA, mtDNA, self and non-self-nucleic acids) (71, 72), which could be ligands for such nucleic acid sensing TLRs in the aged. Here, we report that cell-free nucleic acid levels differ in males and females during aging. In healthy individuals, cell-free nucleic acids are mainly derived from hematopoietic cells (39, 51), whereas, in disease states, an accumulation of cell-free nucleic acids results from the diseased tissue (40). cfDNA circulating in plasma has been an intensively investigated biomarker for the diagnosis of cancer, with specific cfDNA fragment sizes and sequences associated with specific forms of cancer (73–76). Here, we found that the size of the cfDNA fragment is associated with sex and age. We demonstrated a drastically different mononucleosome fragment size of cfDNA obtained from the serum of young male mice (162 bp) as compared to young female mice (79 bp), which changes with age to the same average length in both aged males and females (133 bp); however, aged females have much more cfDNA as compared to the other age and sex groups. Importantly, accumulation of cell-free nucleic acids might be one type of antigenic load that could play a role in the selection of PtC+ B-1 cells over time through nucleic acid sensing TLRs.
Our study highlights the need to further our understanding of how both the male and female aging environments impact the function and repertoire of B cells over time. As shown here, both age and biological sex influence PtC-specific antibodies, which provide protection against sepsis (31). Importantly, sex differences have been shown in patients with sepsis over the age of 50, where women have lower mortality than men (32). As such, it will be essential to fully grasp the influence of sex in the context of age to design effective vaccines and treat diseases prevalent in the aging population.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, 2620705, 2620704, 2620702, and 2020696.
The animal study was reviewed and approved by Western Michigan University Homer Stryker M.D. School of Medicine institutional IACUC committee.
SW performed experiments, analyzed data, interpreted data, and was a major contributor to writing the manuscript. NT performed experiments. MC aided in data interpretation, provided technical expertise, and edited the manuscript. NH performed experiments, analyzed data, interpreted data, and wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI154539. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported in part by funds received from Western Michigan University Homer Stryker M.D. School of Medicine.
We would like to thank Dr. Thomas L. Rothstein for his thoughtful and insightful review of the manuscript. This study was supported in part by the WMU Homer Stryker M.D. School of Medicine Flow Cytometry and Imaging Core.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1047297/full#supplementary-material
1. Ehrenstein MR, Notley CA. The importance of natural IgM: Scavenger, protector and regulator. Nat Rev Immunol (2010) 10:778–86. doi: 10.1038/nri2849
2. Blandino R, Baumgarth N. Secreted IgM: New tricks for an old molecule. J Leukocyte Biol (2019) 106:1021–34. doi: 10.1002/jlb.3ri0519-161r
3. Holodick NE, Rodríguez-Zhurbenko N, Hernández AM. Defining natural antibodies. Front Immunol (2017) 8:872. doi: 10.3389/fimmu.2017.00872
4. Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced b-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol Baltim Md 1950 (1998) 160:4776–87. doi: 10.4049/jimmunol.160.10.4776
5. Nguyen TTT, Elsner RA, Baumgarth N. Natural IgM prevents autoimmunity by enforcing b cell central tolerance induction. J Immunol (2015) 194:1489–502. doi: 10.4049/jimmunol.1401880
6. Tsiantoulas D, Kiss M, Bartolini-Gritti B, Bergthaler A, Mallat Z, Jumaa H, et al. Secreted IgM deficiency leads to increased BCR signaling that results in abnormal splenic b cell development. Sci Rep-uk (2017) 7:3540. doi: 10.1038/s41598-017-03688-8
7. Ehrenstein MR, Cook HT, Neuberger MS. Deficiency in serum immunoglobulin (Ig)m predisposes to development of igg autoantibodies. J Exp Med (2000) 191:1253–8. doi: 10.1084/jem.191.7.1253
8. Weksler ME, Pawelec G, Franceschi C. Immune therapy for age-related diseases. Trends Immunol (2009) 30:344–50. doi: 10.1016/j.it.2009.03.011
9. Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immun (2005) 26:385–404. doi: 10.1007/s00281-004-0185-z
10. Kearney JF, Patel P, Stefanov EK, King RG. Natural antibody repertoires: Development and functional role in inhibiting allergic airway disease. Annu Rev Immunol (2013) 33:1–30. doi: 10.1146/annurev-immunol-032713-120140
11. Gruber S, Hendrikx T, Tsiantoulas D, Ozsvar-Kozma M, Göderle L, Mallat Z, et al. Sialic acid-binding immunoglobulin-like lectin G promotes atherosclerosis and liver inflammation by suppressing the protective functions of b-1 cells. Cell Rep (2016) 14:2348–61. doi: 10.1016/j.celrep.2016.02.027
12. Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, et al. B1a b lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res (2011) 109:830–40. doi: 10.1161/circresaha.111.248542
13. Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, et al. B-1b cells secrete atheroprotective IgM and attenuate atherosclerosis. Circ Res (2015) 117:e28–39. doi: 10.1161/circresaha.117.306044
14. Rawat K, Tewari A, Morrisson MJ, Wager TD, Jakubzick CV. Redefining innate natural antibodies as important contributors to anti-tumor immunity. Elife (2021) 10:e69713. doi: 10.7554/elife.69713
15. Zbesko JC, Frye JB, Becktel DA, Gerardo DK, Stokes J, Calderon K, et al. IgA natural antibodies are produced following T-cell independent b-cell activation following stroke. Brain Behav Immun (2021) 91:578–86. doi: 10.1016/j.bbi.2020.09.014
16. Liu Y-H, Wang J, Li Q-X, Fowler CJ, Zeng F, Deng J, et al. Association of naturally occurring antibodies to β-amyloid with cognitive decline and cerebral amyloidosis in alzheimer’s disease. Sci Adv (2021) 7:eabb0457. doi: 10.1126/sciadv.abb0457
17. Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and b-2 cell–derived immunoglobulin m antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med (2000) 192:271–80. doi: 10.1084/jem.192.2.271
18. Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and b-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to s. pneumoniae. Immunity (2005) 23:7–18. doi: 10.1016/j.immuni.2005.04.011
19. Lalor PA, Herzenberg LA, Adams S, Stall AM. Feedback regulation of murine ly-1 b cell development. Eur J Immunol (1989) 19:507–13. doi: 10.1002/eji.1830190315
20. Yenson V, Baumgarth N. Purification and Immune Phenotyping of B-1 Cells from Body Cavities of Mice. In: Vitale G., Mion F. (eds) Regulatory B Cells. Methods in Molecular Biology, vol 1190. Humana Press, New York, NY. doi: 10.1007/978-1-4939-1161-5_2
21. Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three b-cell lineages. Proc Natl Acad Sci (1992) 89:3320–4. doi: 10.1073/pnas.89.8.3320
22. Savage HP, Kläsener K, Smith FL, Luo Z, Reth M, Baumgarth N. TLR induces reorganization of the IgM-BCR complex regulating murine b-1 cell responses to infections. Elife (2019) 8:e46997. doi: 10.7554/elife.46997
23. Holodick NE, Vizconde T, Hopkins TJ, Rothstein TL. Age-related decline in natural IgM function: Diversification and selection of the b-1a cell pool with age. J Immunol (2016) 196:4348–57. doi: 10.4049/jimmunol.1600073
24. Tsuji N, Rothstein TL, Holodick NE. Antigen receptor specificity and cell location influence the diversification and selection of the b-1a cell pool with age. J Immunol (2020) 205(3):741–59. doi: 10.4049/jimmunol.1901302
25. Webster SE, Ryali B, Clemente MJ, Tsuji NL, Holodick NE. Sex influences age-related changes in natural antibodies and CD5 + b-1 cells. J Immunol (2022) 208:1755–71. doi: 10.4049/jimmunol.2101150
26. Feeney AJ. Lack of n regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med (1990) 172:1377–90. doi: 10.1084/jem.172.5.1377
27. Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from b-1a, b-1b, and conventional b cells. J Immunol Baltim Md 1950 (1997) 158:1175–86. doi: 10.4049/jimmunol.158.3.1175
28. Benedict CL, Kearney JF. Increased junctional diversity in fetal b cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity (1999) 10:607–17. doi: 10.1016/s1074-7613(00)80060-6
29. Mercolino TJ, Arnold LW, Hawkins LA, Haughton G. Normal mouse peritoneum contains a large population of ly-1+ (CD5) b cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J Exp Med (1988) 168:687–98. doi: 10.1084/jem.168.2.687
30. Hardy RR, Hayakawa K. Development of b cells producingnatural autoantibodies to thymocytes and senescent erythrocytes. Springer Semin Immun (2005) 26:363–75. doi: 10.1007/s00281-004-0183-1
31. Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin m in immediate defense against systemic bacterial infection. J Exp Med (1998) 188:2381–6. doi: 10.1084/jem.188.12.2381
32. Adrie C, Azoulay E, Francais A, Clec’h C, Darques L, Schwebel C, et al. Influence of gender on the outcome of severe sepsis a reappraisal. Chest (2007) 132:1786–93. doi: 10.1378/chest.07-0420
33. Nasir N, Jamil B, Siddiqui S, Iqbal NT, Khan F, Hussain R. Mortality in sepsis and its relationship with gender. Pak J Med Sci (2014) 31:1201–6. doi: 10.12669/pjms.315.6925
34. Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis. Virulence (2013) 5:12–9. doi: 10.4161/viru.26982
35. Kondo Y, Miyazato A, Okamoto K, Tanaka H. Impact of sex differences on mortality in patients with sepsis after trauma: A nationwide cohort study. Front Immunol (2021) 12:678156. doi: 10.3389/fimmu.2021.678156
36. Hägg S, Jylhävä J. Sex differences in biological aging with a focus on human studies. Elife (2021) 10:e63425. doi: 10.7554/elife.63425
37. Alamyar E, Duroux P, Lefranc MP, Giudicelli. and V. IMGT((R)) tools for the nucleotide analysis of immunoglobulin (IG) and T cell receptor (TR) V-(D)-J repertoires, polymorphisms, and IG mutations: IMGT/V-QUEST and IMGT/HighV-QUEST for NGS. Methods Mol Biol (2012) 882:569–604. doi: 10.1007/978-1-61779-842-9_32
38. Lapin M, Oltedal S, Tjensvoll K, Buhl T, Smaaland R, Garresori H, et al. Fragment size and level of cell-free DNA provide prognostic information in patients with advanced pancreatic cancer. J Transl Med (2018) 16:300. doi: 10.1186/s12967-018-1677-2
39. Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J. Cell-free DNA comprises an In vivo nucleosome footprint that informs its tissues-Of-Origin. Cell (2016) 164:57–68. doi: 10.1016/j.cell.2015.11.050
40. Ulz P, Thallinger GG, Auer M, Graf R, Kashofer K, Jahn SW, et al. Inferring expressed genes by whole-genome sequencing of plasma DNA. Nat Genet (2016) 48:1273–8. doi: 10.1038/ng.3648
41. Mercolino TJ, Arnold LW, Haughton G. Phosphatidyl choline is recognized by a series of ly-1+ murine b cell lymphomas specific for erythrocyte membranes. J Exp Med (1986) 163:155–65. doi: 10.1084/jem.163.1.155
42. Vale AM, Kapoor P, Skibinski GA, Elgavish A, Mahmoud TI, Zemlin C, et al. The link between antibodies to OxLDL and natural protection against pneumococci depends on DH gene conservationRole of DH amino acid sequence in immunity. J Exp Med (2013) 210:875–90. doi: 10.1084/jem.20121861
43. Ofek G, McKee K, Yang Y, Yang Z-Y, Skinner J, Guenaga FJ, et al. Relationship between antibody 2F5 neutralization of HIV-1 and hydrophobicity of its heavy chain third complementarity-determining region. J Virol (2010) 84:2955–62. doi: 10.1128/jvi.02257-09
44. Krishnan MR, Jou NT, Marion TN. Correlation between the amino acid position of arginine in VH-CDR3 and specificity for native DNA among autoimmune antibodies. J Immunol Baltim Md 1950 (1996) 157:2430–9. doi: 10.4049/jimmunol.157.6.2430
45. Khass M, Vale AM, Burrows PD, Schroeder HW. The sequences encoded by immunoglobulin diversity (DH) gene segments play key roles in controlling b-cell development, antigen-binding site diversity, and antibody production. Immunol Rev (2018) 284:106–19. doi: 10.1111/imr.12669
46. Vale AM, Nobrega A, Schroeder HW. The role of evolutionarily conserved germ-line DH sequence in b-1 cell development and natural antibody production. Ann Ny Acad Sci (2015) 1362:48–56. doi: 10.1111/nyas.12808
47. Herzenberg LA, Baumgarth N, Wilshire JA. B-1 cell origins and VH repertoire determination. Curr Top Microbiol (2000) 252:3–13. doi: 10.1007/978-3-642-57284-5_1
48. Seidl KJ, Wilshire JA, MacKenzie JD, Kantor AB, Herzenberg LA, Herzenberg LA. Predominant VH genes expressed in innate antibodies are associated with distinctive antigen-binding sites. Proc Natl Acad Sci (1999) 96:2262–7. doi: 10.1073/pnas.96.5.2262
49. Schroeder HW Jr, Zemlin M, Khass M, Nguyen HH, Schelonka RL. Genetic control of DH reading frame and its effect on b-cell development and antigen-specifc antibody production. Crit Rev Immunol (2010) 30:327–44. doi: 10.1615/critrevimmunol.v30.i4.20
50. Kreuk LS, Koch MA, Slayden LC, Lind NA, Chu S, Savage HP, et al. B cell receptor and toll-like receptor signaling coordinate to control distinct b-1 responses to both self and the microbiota. Elife (2019) 8:e47015. doi: 10.7554/elife.47015
51. Lui YY, Chik K-W, Chiu RW, Ho C-Y, Lam CW, Lo YD. Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin Chem (2020) 48:421–7. doi: 10.1093/clinchem/48.3.421
52. Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and death of circulating cell-free DNA. Cancer Biol Ther (2019) 20:1057–67. doi: 10.1080/15384047.2019.1598759
53. Pös O, Biró O, Szemes T, Nagy B. Circulating cell-free nucleic acids: Characteristics and applications. Eur J Hum Genet (2018) 26:937–45. doi: 10.1038/s41431-018-0132-4
54. Wagner JT, Kim HJ, Johnson-Camacho KC, Kelley T, Newell LF, Spellman PT, et al. Diurnal stability of cell-free DNA and cell-free RNA in human plasma samples. Sci Rep-uk (2020) 10:16456. doi: 10.1038/s41598-020-73350-3
55. Grabuschnig S, Bronkhorst AJ, Holdenrieder S, Rodriguez IR, Schliep KP, Schwendenwein D, et al. Putative origins of cell-free DNA in humans: A review of active and passive nucleic acid release mechanisms. Int J Mol Sci (2020) 21:8062. doi: 10.3390/ijms21218062
56. Ungerer V, Bronkhorst AJ, den Ackerveken PV, Herzog M, Holdenrieder S. Serial profiling of cell-free DNA and nucleosome histone modifications in cell cultures. Sci Rep-uk (2021) 11:9460. doi: 10.1038/s41598-021-88866-5
57. Radic MZ, Mackle J, Erikson J, Mol C, Anderson WF, Weigert M. Residues that mediate DNA binding of autoimmune antibodies. J Immunol Baltim Md 1950 (1993) 150:4966–77. doi: 10.4049/jimmunol.150.11.4966
58. Silva-Sanchez A, Liu CR, Vale AM, Khass M, Kapoor P, Elgavish A, et al. Violation of an evolutionarily conserved immunoglobulin diversity gene sequence preference promotes production of dsDNA-specific IgG antibodies. PloS One (2015) 10:e0118171. doi: 10.1371/journal.pone.0118171
59. Sohn E. Why autoimmunity is most common in women. Nature (2021) 595:S51–3. doi: 10.1038/d41586-021-01836-9
60. Holodick NE, Zeumer L, Rothstein TL, Morel L. Expansion of b-1a cells with germline heavy chain sequence in lupus mice. Front Immunol (2016) 7:108. doi: 10.3389/fimmu.2016.00108
61. Upadhye A, Srikakulapu P, Gonen A, Hendrikx S, Perry HM, Nguyen A, et al. Diversification and CXCR4-dependent establishment of the bone marrow b-1a cell pool governs atheroprotective IgM production linked to human coronary atherosclerosis. Circ Res (2019) 125(10):e55–e70. doi: 10.1161/circresaha.119.315786
62. Brewer JW, Solodushko V, Aragon I, Barrington RA. Phosphatidylcholine as a metabolic cue for determining b cell fate and function. Cell Immunol (2016) 310:78–88. doi: 10.1016/j.cellimm.2016.08.002
63. Whelan J, Gowdy KM, Shaikh SR. N-3 polyunsaturated fatty acids modulate b cell activity in pre-clinical models: Implications for the immune response to infections. Eur J Pharmacol (2016) 785:10–7. doi: 10.1016/j.ejphar.2015.03.100
64. Teague H, Harris M, Fenton J, Lallemand P, Shewchuk BM, Shaikh SR. Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance b-cell activity in murine obesity[S]. J Lipid Res (2014) 55:1420–33. doi: 10.1194/jlr.m049809
65. Kim S, Cheon H-S, Song J-C, Yun S-M, Park SI, Jeon J-P. Aging-related changes in mouse serum glycerophospholipid profiles. Osong Public Heal Res Perspect (2014) 5:345–50. doi: 10.1016/j.phrp.2014.10.002
66. Collino S, Montoliu I, Martin F-PJ, Scherer M, Mari D, Salvioli S, et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PloS One (2013) 8:e56564. doi: 10.1371/journal.pone.0056564
67. Young DL. Estradiol- and testosterone-induced alterations in phosphatidylcholine and triglyceride synthesis in hepatic endoplasmic reticulum. J Lipid Res (1971) 12:590–5. doi: 10.1016/s0022-2275(20)39478-5
68. Rauschert S, Uhl O, Koletzko B, Mori TA, Beilin LJ, Oddy WH, et al. Sex differences in the association of phospholipids with components of the metabolic syndrome in young adults. Biol Sex Diff (2017) 8:10. doi: 10.1186/s13293-017-0131-0
69. McHugh D, Gil J. Senescence and aging: Causes, consequences, and therapeutic avenues. J Cell Biol (2018) 217:65–77. doi: 10.1083/jcb.201708092
70. Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol (2008) 152:448–55. doi: 10.1111/j.1365-2249.2008.03658.x
71. Yuwono NL, Warton K, Ford CE. The influence of biological and lifestyle factors on circulating cell-free DNA in blood plasma. Elife (2021) 10:e69679. doi: 10.7554/elife.69679
72. Alghofaili L, Almubarak H, Gassem K, Islam SS, Coskun S, Kaya N, et al. Cell-free DNA levels of twins and sibling pairs indicate individuality and possible use as a personalized biomarker. PloS One (2019) 14:e0223470. doi: 10.1371/journal.pone.0223470
73. Chen E, Cario CL, Leong L, Lopez K, Márquez CP, Chu C, et al. Cell-free DNA concentration and fragment size as a biomarker for prostate cancer. Sci Rep-uk (2021) 11:5040. doi: 10.1038/s41598-021-84507-z
74. Thierry AR, Messaoudi SE, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metast Rev (2016) 35:347–76. doi: 10.1007/s10555-016-9629-x
75. Aucamp J, Bronkhorst AJ, Badenhorst CPS, Pretorius PJ. The diverse origins of circulating cell-free DNA in the human body: A critical re-evaluation of the literature. Biol Rev (2018) 93:1649–83. doi: 10.1111/brv.12413
Keywords: B-1 cell, natural antibody, phosphatidylcholine, aging, biological sex
Citation: Webster SE, Tsuji NL, Clemente MJ and Holodick NE (2023) Age-related changes in antigen-specific natural antibodies are influenced by sex. Front. Immunol. 13:1047297. doi: 10.3389/fimmu.2022.1047297
Received: 17 September 2022; Accepted: 23 December 2022;
Published: 12 January 2023.
Edited by:
Erin Janssen, Michigan Medicine, University of Michigan, United StatesReviewed by:
Inger Gjertsson, University of Gothenburg, SwedenCopyright © 2023 Webster, Tsuji, Clemente and Holodick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nichol E. Holodick, bmljaG9sLmhvbG9kaWNrQG1lZC53bWljaC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.