- 1Cancer Institute, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Dermatology, The First Hospital of Hebei Medical University, Shijiazhuang, China
- 3Candidate Branch of National Clinical Research Center for Skin Diseases, Shijiazhuang, China
Background: Coronavirus disease 2019 (COVID-19) have brought great disaster to mankind, and there is currently no globally recognized specific drug or treatment. Severe COVID-19 may trigger a cytokine storm, manifested by increased levels of cytokines including interleukin-17 (IL-17), so a new strategy to treat COVID-19 may be to use existing IL-17 inhibitors, which have demonstrated efficacy, safety and tolerability in the treatment of psoriasis. However, the use of IL-17 inhibitors in patients with psoriasis during the COVID-19 pandemic remains controversial due to reports that IL-17 inhibitors may increase the risk of respiratory tract infections.
Objectives: The systematic review and meta-analysis aimed to evaluate the effect of IL-17 inhibitors on the risk of COVID-19 infection, hospitalization, and mortality in patients with psoriasis.
Methods: Databases (including Embase, PubMed, SCI-Web of Science, Scopus, CNKI, and the Cochrane Library) were searched up to August 23, 2022, for studies exploring differences in COVID-19 outcomes between psoriasis patients using IL-17 inhibitors and those using non-biologics. Two authors independently extracted data and assessed the risk of bias in a double-blind manner. The risk ratios (RRs) and 95% confidence intervals (CIs) were calculated and heterogeneities were determined by the Q test and I2 statistic. And the numbers needed to treat (NNTs) were calculated to assess the clinical value of IL-17 inhibitors in preventing SARS-CoV-2 infection and treating COVID-19.
Results: Nine observational studies involving 7,106 participants were included. The pooled effect showed no significant differences in the rates of SARS-CoV-2 infection (P = 0.94; I2 = 19.5%), COVID-19 hospitalization (P = 0.64; I2 = 0.0%), and COVID-19 mortality (P = 0.32; I2 = 0.0%) in psoriasis patients using IL-17 inhibitors compared with using non-biologics. Subgroup analyses grouped by age and COVID-19 cases, respectively, revealed consistent results as above. Meanwhile, the pooled NNTs showed no significant differences between the two groups in the clinical value of preventing SARS-CoV-2 infection and treating COVID-19.
Conclusion: The use of IL-17 inhibitors in patients with psoriasis does not increase the risk of SARS-CoV-2 infection or worsen the course of COVID-19.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022335195.
Introduction
As of September 7, 2022, coronavirus disease 2019 (COVID-19) have caused more than 603 million confirmed cases, including more than 6.48 million deaths, and have brought great disaster to mankind (1). COVID-19 is a clinical syndrome caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for which there is currently no globally recognized specific drug or treatment. Severe COVID-19 may trigger a cytokine storm, a hyperinflammatory state triggered by viral infections, which primarily causes pneumonia followed by systemic inflammation (2–4). Among the variety of cytokines involved in the storm, interleukin-17 (IL-17) is a predominant mediator of pulmonary inflammation (5). Recently, several studies have shown that the levels of circulating IL-17 are elevated in the peripheral blood of COVID-19 patients (2, 6–8), so the use of IL-17 inhibitors may become a new treatment option for COVID-19, which directly block the IL-17 pathway (9).
As biologics, IL-17 inhibitors have been approved for the treatment of moderate-to-severe plaque psoriasis, and have demonstrated efficacy, safety and tolerability (10–14). However, IL-17 inhibitors have been reported to increase the risk of respiratory infections (15–17), so it needs to be considered whether psoriasis patients treated with IL-17 inhibitors are more susceptible to the virus during the COVID-19 pandemic. In addition, it is still unclear whether the use of IL-17 inhibitors can effectively treat COVID-19 or shorten the course of COVID-19. Although studies have compared the risk of SARS-CoV-2 infection and the course of COVID-19 in psoriasis patients receiving biologics (including IL-17 inhibitors) and those receiving non-biologics, the results are not entirely consistent. Therefore, we use a systematic review to explore the risk of COVID-19 infection, hospitalization, and mortality in psoriasis patients treated with IL-17 inhibitors during the COVID-19 pandemic.
Materials and methods
Study registration
This systematic review chose psoriasis patients using IL-17 inhibitors as the exposure group and psoriasis patients using non-biologics as the control group. And it was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (18). The research protocol was registered in the PROSPERO database (CRD42022335195).
Search strategy
To find studies exploring differences in COVID-19 outcomes between psoriasis patients using IL-17 inhibitors and those using non-biologics, we searched databases (including Embase, PubMed, SCI-Web of Science, Scopus, CNKI, and the Cochrane Library) for articles published in any language and gray literature before August 23, 2022, and manually searched the references from potentially relevant papers. The studies were screened by two reviewers (ML and LL) independently using Endnote, version X9 (Clarivate Analytics), and conflicts were resolved by a third reviewer (NW). For studies that lacked original data or unpublished, we would contact the authors by email.
MeSH (Medical Subject Headings) and free text terms for IL-17 inhibitors and COVID-19 were used, and the terms are as follows: (“COVID-19” OR “SARS-CoV-2” OR “coronavirus disease 2019” OR “severe acute respiratory syndrome coronavirus 2”) AND (“Psoriasis” OR “Psoriases”) AND (“Interleukin-17” OR “Secukinumab” OR “Ixekizumab” OR “Brodalumab” OR “Cosentyx” OR “Taltz” OR “Siliq” OR “Kyntheum” OR “Lumicef”). Supplementary Table 1 (Table S1) summarized the specific retrieval strategies and results used for each database.
Inclusion and exclusion criteria
Original studies were included if they met all of the following criteria: (1) study design: observational studies or experimental (randomized controlled trials) studies; (2) population/participants: patients with psoriasis; (3) intervention/exposure: using IL-17 inhibitors; (4) control/comparison: using non-biologics; (5) outcomes: clear indicators of COVID-19 infection, hospitalization or mortality.
Studies were excluded if they met any of the following criteria: (1) no accessible clinical data or incomplete data; (2) duplicate published studies; (3) full-text not available.
Three endpoints of the review were: (1) presence of COVID-19 cases (including confirmed cases, defined as those with positive molecular tests, and suspected cases, defined as those who have possible COVID-19 symptoms); (2) hospitalization for COVID-19; (3) death from COVID-19.
Definition of COVID-19 outcomes
The equations for SARS-CoV-2 infection rate (IR), COVID-19 hospitalization rate (HR), and COVID-19 mortality rate (MR) were as follows:
where I is the number of COVID-19 cases, R is the number of people at risk of SARS-CoV-2 infection, H is the number of those hospitalized with COVID-19, and M is the number of those died from COVID-19.
Data extraction and risk of bias assessment
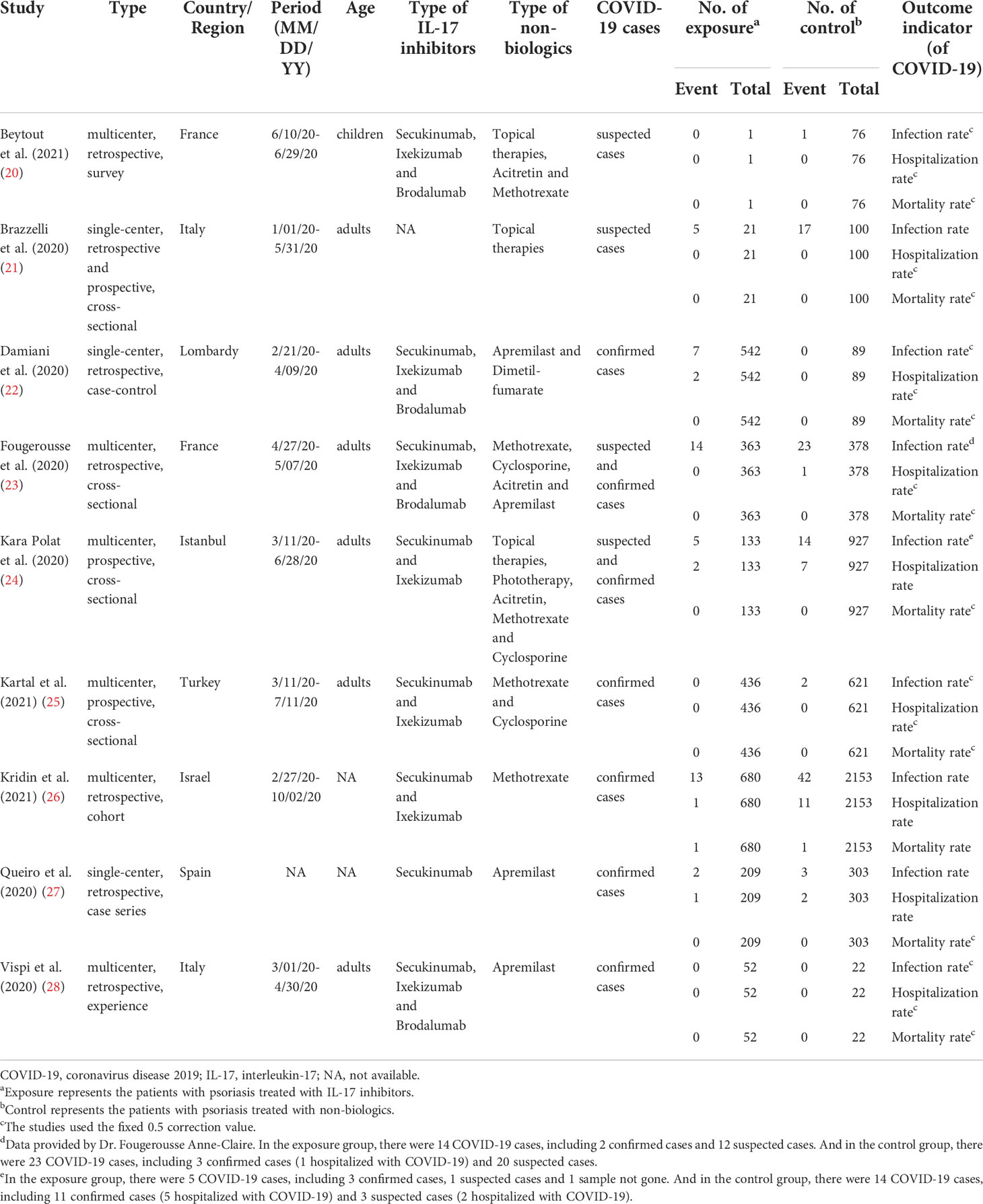
The following detailed baseline characteristics were extracted from the included studies: study, type, country/region, period, age, type of IL-17 inhibitors, type of non-biologics, COVID-19 cases, number of exposure, number of control, and outcome indicator.
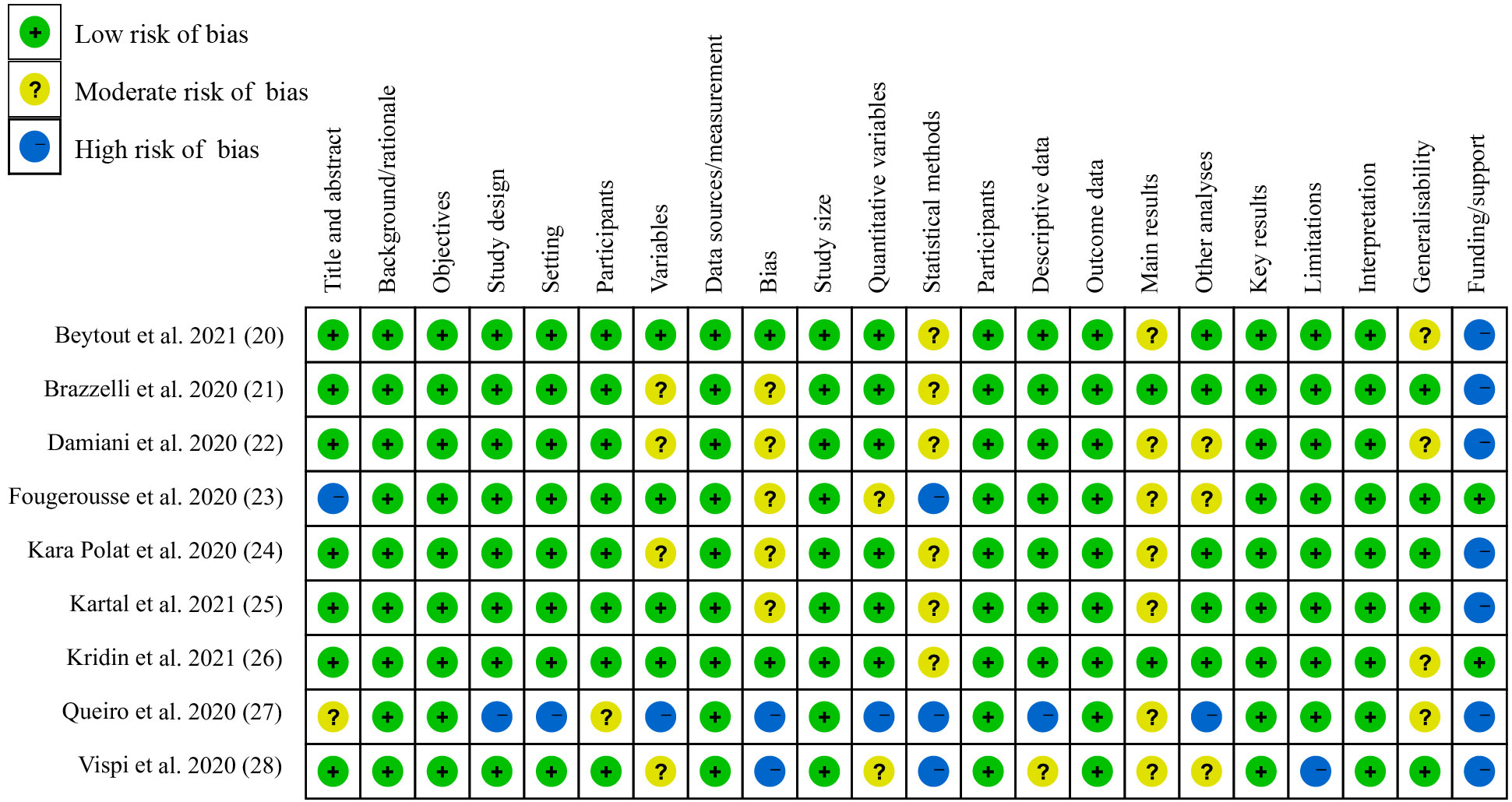
Two authors independently extracted data and assessed the risk of bias in a double-blind manner (ML and SC). Since the included studies were observational studies, the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement was used (19). The STROBE statement allows for quality assessment of 22 items. The risk of bias rating for each item was reported as low, moderate, or high, and any discrepancies in data and rating were addressed by negotiation between the two or with a third author (HW).
Statistical analysis
All statistical analyses were conducted with Stata, version 15 (StataCorp LLC) and RevMan, version 5.2 (The Nordic Cochrane Centre). All figures were generated by Stata and RevMan software, and processed by Adobe Illustrator, version CC 2018 (Adobe Systems Incorporated). The outcome measures were the risk ratios (RRs) of COVID-19 infection, hospitalization or mortality in patients with psoriasis receiving IL-17 inhibitors versus non-biologics. And the pooled numbers needed to treat (NNTs) and 95% confidence intervals (CIs) were calculated to estimate the absolute benefit or risk of using IL-17 inhibitors in preventing SARS-CoV-2 infection and treating COVID-19.
We evaluated statistical heterogeneity among studies using the X2-based Q test and the I2 statistic. The fixed-effect model with Mantel-Haenszel method was used when P > 0.05 of the Q test and I2< 50%, otherwise the random-effect model with Inverse-Variance method was used, and then generated the pooled RRs and 95% CIs for all included studies. When no events were observed in one or both groups in an individual study, we used the fixed 0.5 correction value.
The NNT was calculated using the equation:
where CER is the control event rate and EER is the experimental event rate. We used the metannt command of the Stata software to calculate the NNTs. Metannt calculates NNT by deriving an effect size, applying it to a population with a given event prevalence, and from this deriving a projected event rate if the population were to receive the intervention. In this meta-analysis, we calculated NNT by using rates of SARS-CoV-2 infection, COVID-19 hospitalization, and COVID-19 mortality in the psoriasis patients using non-biologics (control event rate), RR (the effect size) and 95% CIs.
We conducted subgroup analyses grouped by age and COVID-19 cases, respectively. Age was divided into three levels: children (age<18 years), adults (age≥18 years), and NA (not available). COVID-19 cases were divided into two levels: confirmed cases and suspected cases. Also, we performed comparative analyses with IL-17 inhibitors according to the specific types of non-biologics, respectively. We undertook sensitivity analyses for the outcome indicators, which to account for potential study limitations that could lead to spurious precision of pooled effect estimates. We generated the funnel plots to explore the possibility of small study effects, and further assessed publication bias with Egger’s and Begg’s tests. If the P value of the test was less than 0.10, there was a publication bias.
To ensure that the population was not counted several times, we would take the following measures: if the exposure population was included in multiple studies, we would appropriately include only one of them; if several studies used the same control population, we would combine the exposure populations of these studies after eliminating duplicates (if any).
Results
Characteristics of included studies
We finally included 9 studies (20–28) involving 2,437 exposure individuals and 4,669 control individuals by databases and manual search in the meta-analysis (Figure 1), and we summarized the characteristics of all included studies (Table 1).
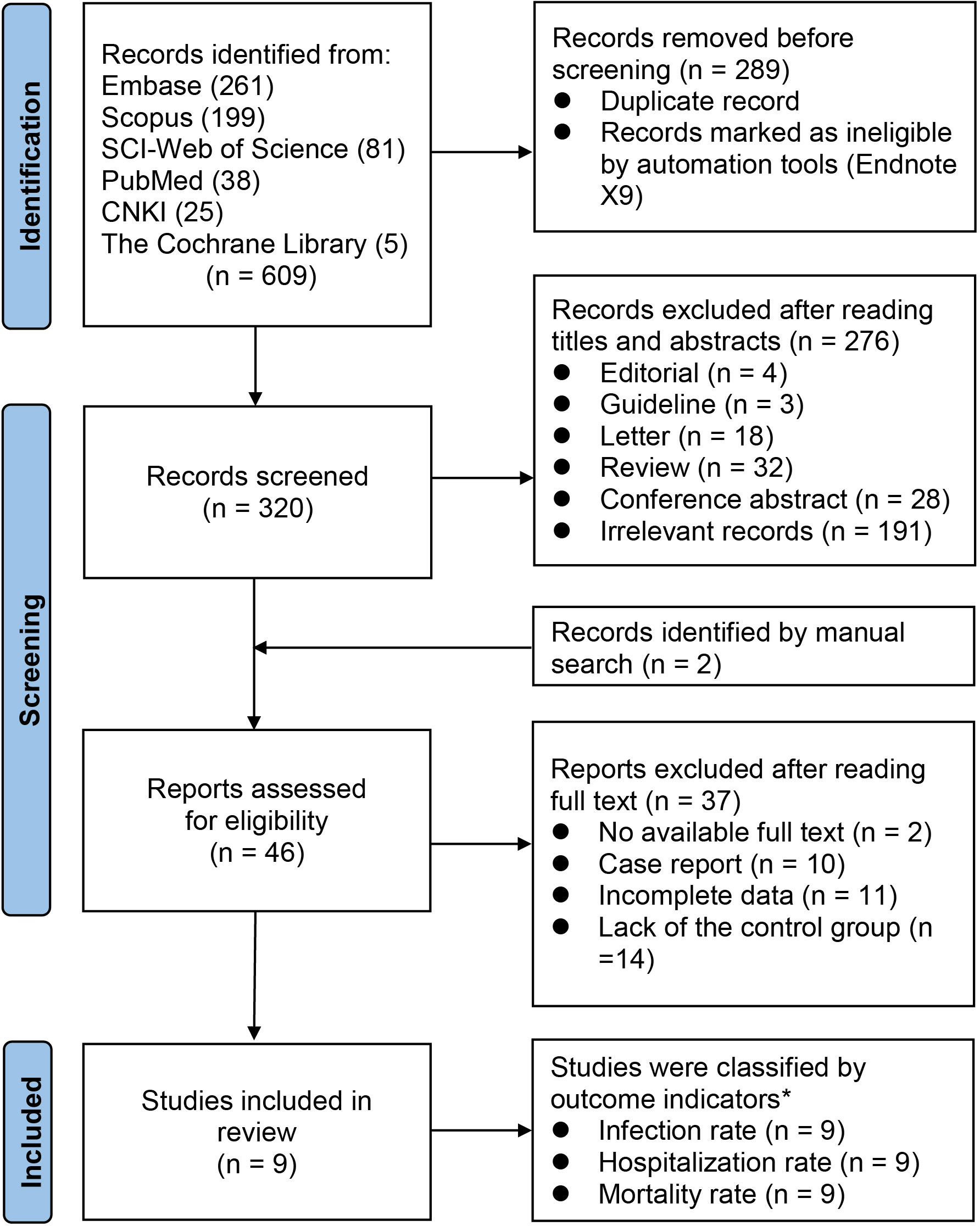
Figure 1 PRISMA flow chart for study selection. n, number; PRISMA, the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement. *All included studies contain three indicators.
Risk of bias assessment
All studies were observational, including three single-center studies and six multicenter studies. The quality of each study was assessed, and showed an overall low risk of bias rating, except for the study by Queiro et al. (27) (Figure 2). Because Queiro et al. conducted a case series study, it exhibited an overall moderate risk of bias rating.
Effect of IL-17 inhibitors on the risk of SARS-CoV-2 infection
Figure 3 showed the forest plots of including studies investigating the differences in the rate of COVID-19 infection, hospitalization and mortality between the patients with psoriasis receiving IL-17 inhibitors (secukinumab, ixekizumab, or brodalumab) and those receiving non-biologics (topical therapies, phototherapy, acitretin, methotrexate, cyclosporine, apremilast and dimetil-fumarate). The pooled effects showed no significant difference in the rate of SARS-CoV-2 infection (RR: 0.99; 95% CI: 0.70-1.40; P = 0.94) (Figure 3A) between the two groups. The low level of heterogeneity was found in the studies investigating the rate of SARS-CoV-2 infection (I2 = 19.5%; P = 0.27).
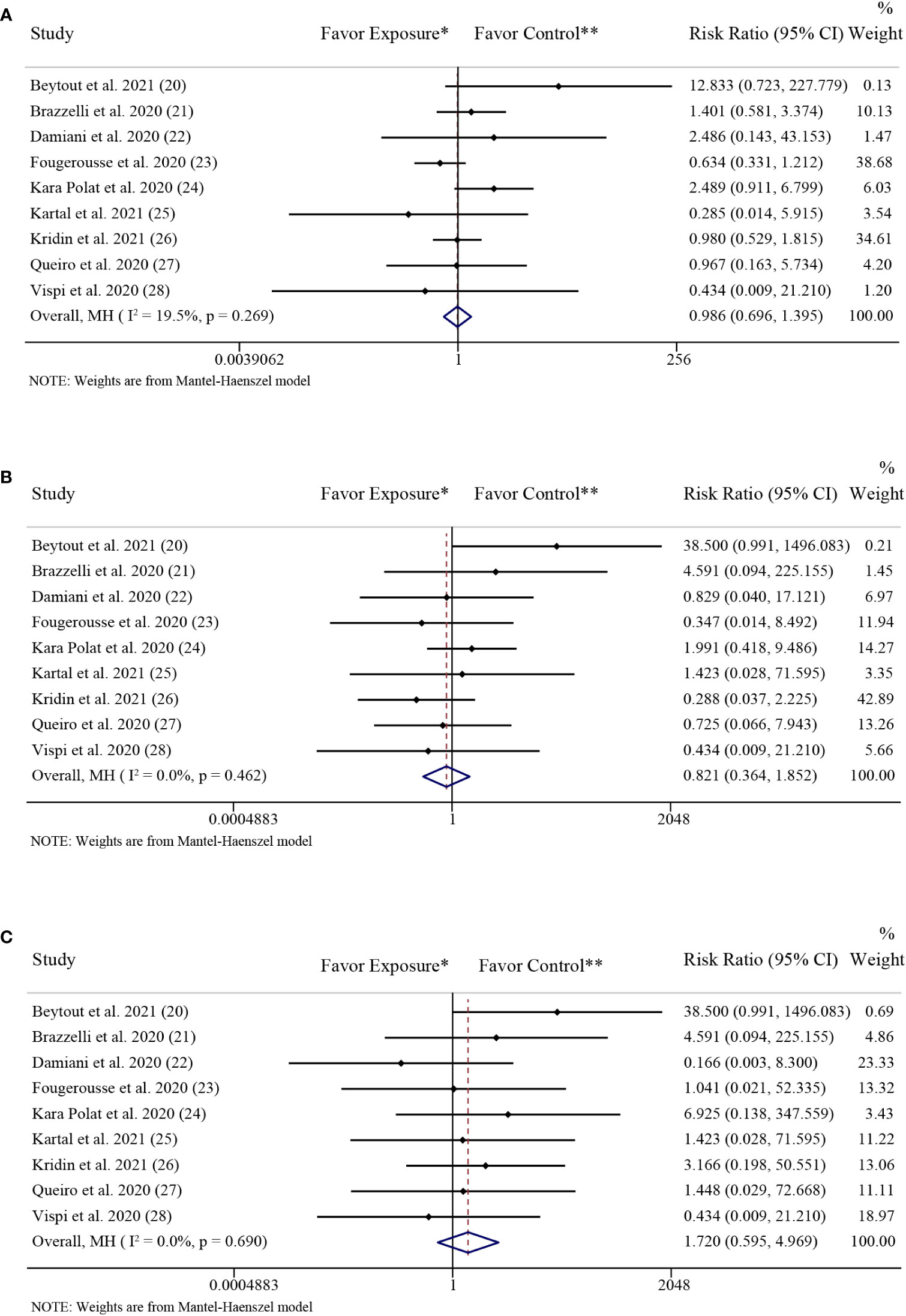
Figure 3 Forest plots of risk ratios and 95% confidence intervals. (A) Forest plot of risk ratios and 95% CIs of the SARS-CoV-2 infection rate; (B) Forest plot of risk ratios and 95% CIs of the COVID-19 hospitalization rate; (C) Forest plot of risk ratios and 95% CIs of the COVID-19 mortality rate. CI, confidence interval; COVID-19, coronavirus disease 2019; MH, Mantel-Haenszel method. *Exposure represents patients with psoriasis treated with IL-17 inhibitors. **Control represents patients with psoriasis treated with non-biologics.
The results of the subgroup analysis grouped by age were consistent with the above overall results. In the adults group, there was no significant difference in the rate of SARS-CoV-2 infection (RR: 0.97; 95% CI: 0.62-1.49; P = 0.87) between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics. There were also no significant differences in the rate of SARS-CoV-2 infection between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics in the children group (RR: 12.83; 95% CI: 0.72-227.78; P = 0.08) and the NA group (RR: 0.98; 95% CI: 0.55-1.75; P = 0.94), respectively (Figure 4A).
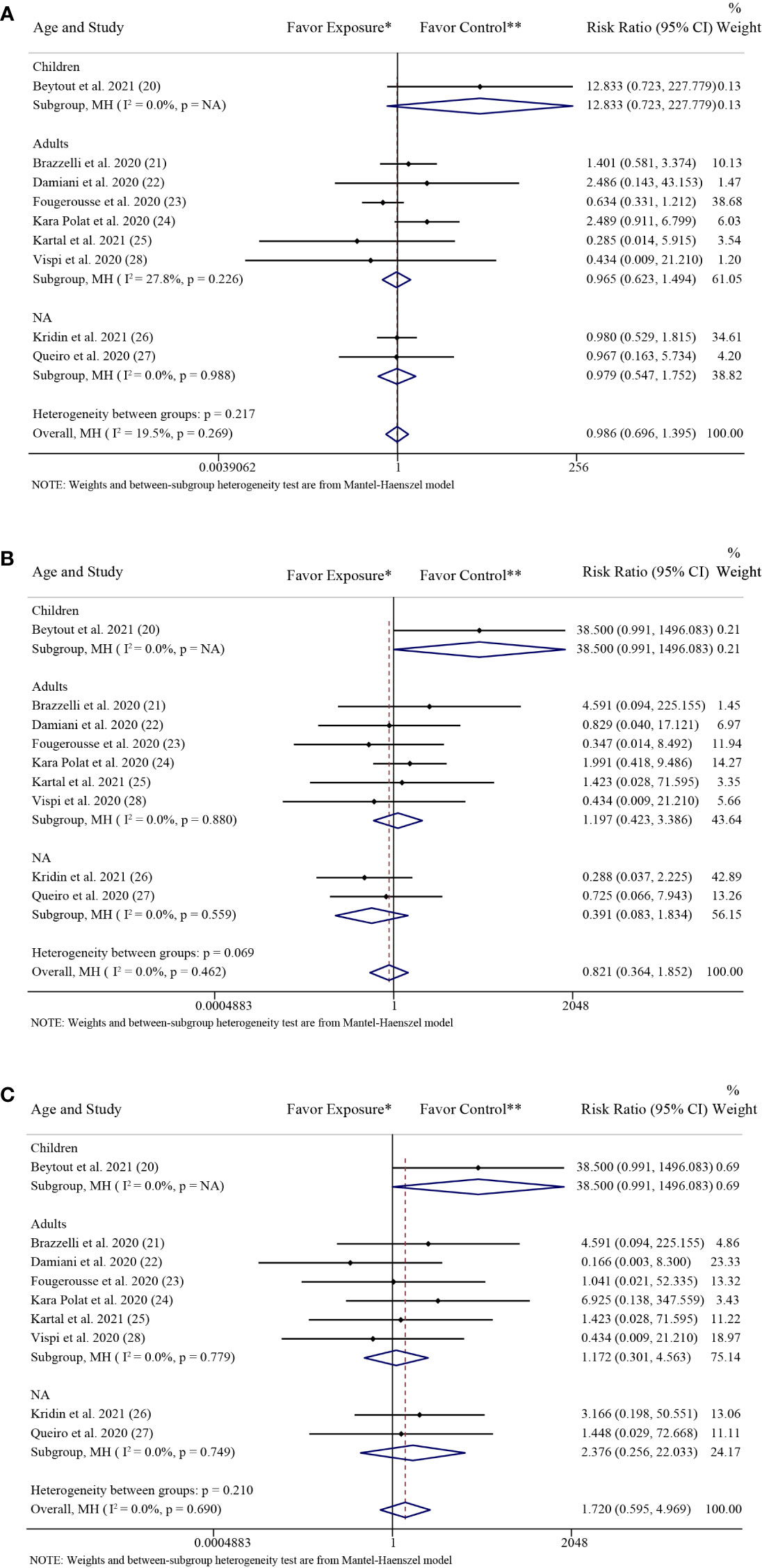
Figure 4 Subgroup analysis grouped by age. (A) Forest plot of risk ratios and 95% CIs of the SARS-CoV-2 infection rate; (B) Forest plot of risk ratios and 95% CIs of the COVID-19 hospitalization rate; (C) Forest plot of risk ratios and 95% CIs of the COVID-19 mortality rate. CI, confidence interval; COVID-19, coronavirus disease 2019; MH, Mantel-Haenszel method; NA, not available. *Exposure represents patients with psoriasis treated with IL-17 inhibitors. **Control represents patients with psoriasis treated with non-biologics.
The results of the subgroup analysis grouped by COVID-19 cases were consistent with the above overall results. There were no significant differences in the rate of SARS-CoV-2 infection between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics in the confirmed cases group (RR: 1.04; 95% CI: 0.64-1.69; P = 0.89) and in the suspected cases group (RR: 1.27; 95% CI: 0.52-3.12; P = 0.60), respectively (Figure 5A).
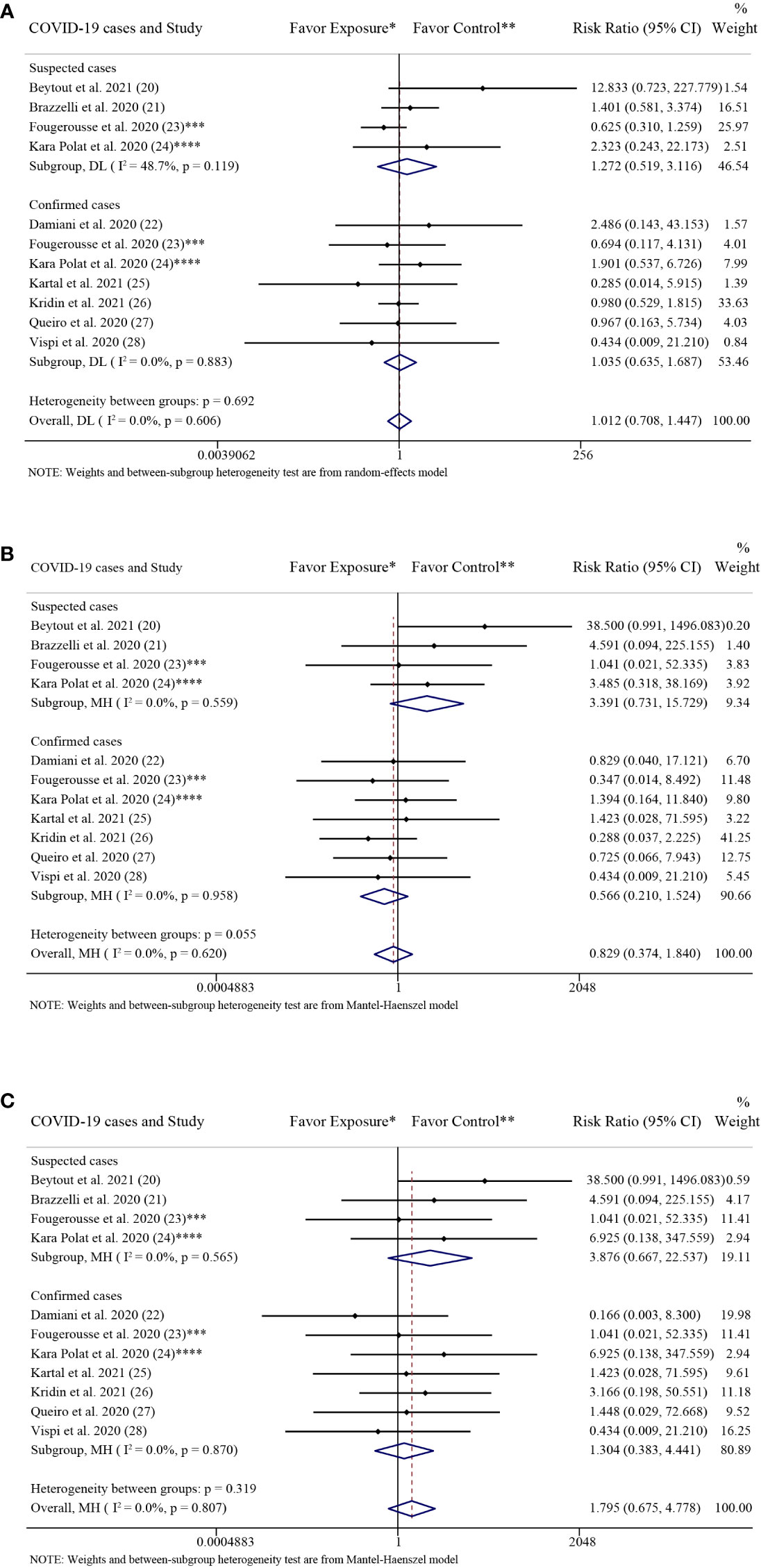
Figure 5 Subgroup analysis grouped by COVID-19 cases. (A) Forest plot of risk ratios and 95% CIs of the SARS-CoV-2 infection rate; (B) Forest plot of risk ratios and 95% CIs of the COVID-19 hospitalization rate; (C) Forest plot of risk ratios and 95% CIs of the COVID-19 mortality rate. CI, confidence interval; COVID-19, coronavirus disease 2019; DL, DerSimonian-Laird estimate of tau2; NA, not available. *Exposure represents patients with psoriasis treated with IL-17 inhibitors. **Control represents patients with psoriasis treated with non-biologics. ***In the exposure group, there were 14 COVID-19 cases, including 2 confirmed cases and 12 suspected cases. And in the control group, there were 23 COVID-19 cases, including 3 confirmed cases (1 hospitalized with COVID-19) and 20 suspected cases. ****In the exposure group, there were 5 COVID-19 cases, including 3 confirmed cases, 1 suspected cases and 1 sample not gone. And in the control group, there were 14 COVID-19 cases, including 11 confirmed cases (5 hospitalized with COVID-19) and 3 suspected cases (2 hospitalized with COVID-19).
In the separate comparative analyses, the results showed no significant differences in the rate of SARS-CoV-2 infection in psoriasis patients receiving topical therapies, phototherapy, apremilast, dimetil-fumarate, acitretin, methotrexate or cyclosporine alone compared to those receiving IL-17 inhibitors (Figure S1).
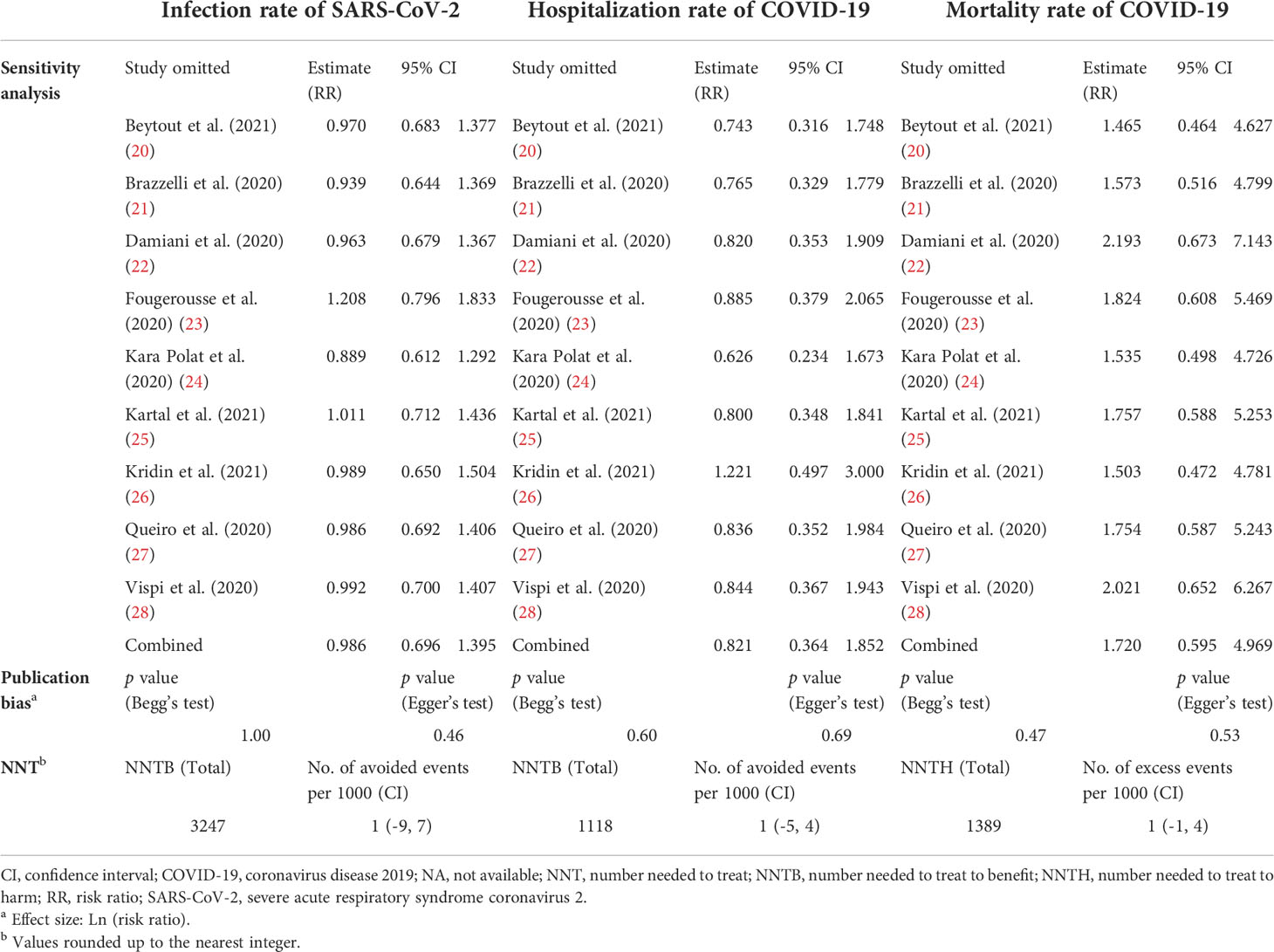
Sensitivity analysis showed the stability of the results regarding the risk of SARS-CoV-2 infection (Table 2) (Figure S4A). There was no visual asymmetry in the funnel plot (Figure S5A). Neither Egger’s test (P = 0.46) (Figure S6A) nor Begg’s test (P = 1.00) (Figure S7A) revealed publication bias (Table 2).
The pooled NNT showed no significant difference in the clinical value of preventing SARS-CoV-2 infection in the psoriasis patients receiving IL-17 inhibitors and those receiving non-biologics (Table 2). The result of NNT was consistent with the result of the RR.
Effect of IL-17 inhibitors on the risk of COVID-19 hospitalization
The pooled effects showed no significant difference in the rate of COVID-19 hospitalization (RR: 0.82; 95% CI: 0.36-1.85; P = 0.64) (Figure 3B) between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics. The low level of heterogeneity was found in the studies investigating the rate of COVID-19 hospitalization (I2 = 0.0%; P = 0.46).
The results of the subgroup analysis grouped by age were consistent with the above overall results. In the adults group, there was no significant difference in the rate of COVID-19 hospitalization (RR: 1.20; 95% CI: 0.42-3.39; P = 0.74) between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics. There were also no significant differences in the rate of COVID-19 hospitalization between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics in the children group (RR: 38.50; 95% CI: 0.99-1496.08; P = 0.05) and the NA group (RR: 0.39; 95% CI: 0.08-1.83; P = 0.23), respectively (Figure 4B).
The results of the subgroup analysis grouped by COVID-19 cases were consistent with the above overall results. There were no significant differences in the rates of COVID-19 hospitalization between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics in the confirmed cases group (RR: 0.57; 95% CI: 0.21-1.52; P = 0.26) and in the suspected cases group (RR: 3.39; 95% CI: 0.73-15.73; P = 0.12), respectively (Figure 5B).
In the separate comparative analyses, the results also showed no significant differences in the rate of COVID-19 hospitalization in psoriasis patients receiving any of the specific non-biologics alone compared to those receiving IL-17 inhibitors (Figure S2).
Sensitivity analysis showed the stability of the results regarding the risk of COVID-19 hospitalization (Table 2) (Figure S4B). There was no visual asymmetry in the funnel plot (Figure S5B). Neither Egger’s test (P = 0.69) (Figure S6B) nor Begg’s test (P = 0.60) (Figure S7B) revealed publication bias (Table 2). The pooled NNT showed no significant difference in the clinical value of alleviating the course of COVID-19 in psoriasis patients receiving IL-17 inhibitors and those receiving non-biologics (Table 2). The result of NNT was consistent with the result of the RR.
Effect of IL-17 inhibitors on the risk of COVID-19 mortality
The pooled effects showed no significant difference in the rate of COVID-19 mortality (RR: 1.72; 95% CI: 0.60-4.97; P = 0.32) (Figure 3C) between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics. The low level of heterogeneity was found in the studies investigating the rate of COVID-19 mortality (I2 = 0.0%; P = 0.69).
The results of the subgroup analysis grouped by age were consistent with the above overall results. In the adults group, there was no significant difference in the rate of COVID-19 mortality (RR: 1.17; 95% CI: 0.30-4.56; P = 0.82) between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics. There were also no significant differences in the rate of COVID-19 mortality between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics in the children group (RR: 38.50; 95% CI: 0.99-1496.08; P = 0.05) and the NA group (RR: 2.38; 95% CI: 0.26-22.03; P = 0.45), respectively (Figure 4C).
The results of the subgroup analysis grouped by COVID-19 cases were consistent with the above overall results. There were no significant differences in the rate of COVID-19 mortality between the patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics in the confirmed cases group (RR: 1.30; 95% CI: 0.38-4.44; P = 0.67) and in the suspected cases group (RR: 3.88; 95% CI: 0.67-22.54; P = 0.13), respectively (Figure 5C).
In the separate comparative analyses, the results also showed no significant difference in the rate of COVID-19 mortality in psoriasis patients receiving any of the specific non-biologics alone compared to those receiving IL-17 inhibitors (Figure S3).
Sensitivity analysis showed the stability of the results regarding the risk of COVID-19 mortality (Table 2) (Figure S4C). There was no visual asymmetry in the funnel plot (Figure S5C). Neither Egger’s test (P = 0.53) (Figure S6C) nor Begg’s test (P = 0.47) (Figure S7C) revealed publication bias (Table 2). The pooled NNT showed no significant difference in the clinical value of treating COVID-19 disease in psoriasis patients receiving IL-17 inhibitors and those receiving non-biologics (Table 2). The result of NNT was consistent with the result of the RR.
Discussion
This meta-analysis, including both single-center and multicenter observational studies, compared the risk of COVID-19 infection, hospitalization, and mortality in patients with psoriasis receiving IL-17 inhibitors and those receiving non-biologics. We found no significant differences in the rates of COVID-19 infection, hospitalization and mortality between the two groups. These findings indicated that using IL-17 inhibitors in psoriasis patients did not increase the infection risk of SARS-CoV-2 or worsen the course of COVID-19 compared with using non-biologics.
IL-17 is selectively produced by a specific subset of T helper cells–Th17, and is identified as a silent amplifier of the immunity process (29). IL-17 plays an important role in some inflammatory-based chronic diseases (30), and is involved in the process of cell activation, growth and proliferation (31, 32). IL-17 could induce the production of pro-inflammatory mediators such as IL-6 and tumor necrosis factor (TNF)-α (33). Studies showed that the levels of Th17 cells and IL-17 in the peripheral blood of patients infected with SARS-CoV-2 were elevated (2, 6–8). Also, elevated plasma levels of these pro-inflammatory mediators have also been associated with COVID-19-related pulmonary inflammation (2, 34). Study had shown that 81% of COVID-19 fatal cases were diagnosed with acute respiratory distress syndrome (ARDS) (35), which indicated ARDS was an important cause of COVID-19 death. Meanwhile, a retrospective analysis of IL-17 gene polymorphisms showed an increased 30-day survival in ARDS patients with the A-allele of rs2275913 SNP (resulting in attenuated IL-17 production) (36). Additionally, a study revealed that in COVID-19 neutrophil/T cell cocultures, neutrophils could cause a strong polarity shift to Th17, and was accompanied by a decrease of interferon (IFN)-γ-producing Th1 cells (37), which confirmed the association between Th17 and COVID-19. Moreover, a Th17 type-dominant immunophenotype could drive more severe viral myocarditis, which might further increase the risk of COVID-19 mortality (38). Together, these studies revealed the potential relation between elevated IL-17 level and the severity and progression of COVID-19, and it might be a possible effective measure for prevention of SARS-CoV-2 infection or treatment of COVID-19 to use existing IL-17 inhibitors. All currently approved IL-17 inhibitors (ixekizumab and secukinumab, both anti-IL-17A monoclonal antibody; brodalumab, anti-IL-17RA monoclonal antibody) can target IL-17 directly or indirectly. However, IL-17 inhibitors have been reported to increase the risk of respiratory infections. A study indicated that IL-17 inhibitors increased the overall risk of infections up to 11%, with much of the upper respiratory infections could be attributed to secukinumab, not ixekizumab or brodalumab (17). So there was a concern that the psoriasis patients treated with IL-17 inhibitors might be more susceptible to the viral infection or develop a more severe disease. To date, there is still a lack of sufficient evidence to elucidate the effect of IL-17 inhibitors on COVID-19 susceptibility and severity.
In this meta-analysis, we assessed the effect of IL-17 inhibitors on the risk of COVID-19 infection, hospitalization, and mortality in psoriasis patients by comparing them to psoriasis patients receiving non-biologics. These non-biologics included topical therapies, phototherapy, apremilast, dimetil-fumarate, acitretin, methotrexate and cyclosporine. Among them, methotrexate, cyclosporine and apremilast are of great attention because they are immunosuppressive agents, especially methotrexate. Comparatively, topical therapies, phototherapy, acitretin and dimetil-fumarate do not seem to cause undue concern during the COVID-19 pandemic.
Methotrexate is an inhibitor of dihydrofolate reductase that suppresses inflammation and immune infections. Previous studies have shown that methotrexate can increase infection (39), but its effect on susceptibility/severity of COVID-19 is unclear. Kridin et al. found higher rates of COVID-19 hospitalization in psoriasis patients exposed to methotrexate than to TNF inhibitors, but similar rates of SARS-CoV-2 infection (34), and that methotrexate intake independently predicted COVID-19 related hospitalizations (40). Also, Izadi et al. observed a higher risk of hospitalization or death associated with COVID-19 among patients with immune-mediated inflammatory diseases (IMIDs) treated with methotrexate compared with TNF inhibitors, but notably, patients treated with methotrexate in combination with TNF inhibitors had similar rates of hospitalization or death to those treated with TNF inhibitors alone, and importantly, methotrexate determined COVID-19 outcomes in the combination regimen (41). This suggests that in COVID-19, methotrexate acts in the same direction as TNF inhibitors. More importantly, data from a global electronic database (42) showed no statistically significant difference in hospitalization rates in COVID-19 patients exposed to methotrexate compared to those not exposed to methotrexate. Additionally, Ganjei et al. (43) found that patients with aplastic anemia taking low dose methotrexate had significantly less severe symptoms of COVID-19 than their families with normal immune systems. This demonstrates a potential protective effect of methotrexate on the progression of COVID-19. Some researchers have even speculated that methotrexate may inhibit the expression of TNF-α, IL-6 and other pro-inflammatory cytokines released with SARS-CoV-2, thereby reducing the cytokine storm associated with COVID-19 (44, 45). Taken together, these evidences suggest that methotrexate most likely does not increase the susceptibility or severity of COVID-19 and may even protect patients from severe COVID-19. Our results showed similar risks of COVID-19 infection, hospitalization and mortality in psoriasis patients under IL-17 inhibitors compared with those under methotrexate, which indirectly demonstrated the potential protective effect of IL-17 inhibitors on COVID-19 infection and progression in patients with psoriasis.
Cyclosporine is a calcineurin inhibitor and premilast is a phosphodiesterase-4 inhibitor, both of which modulate immune response. Studies showed that non-cytotoxic concentrations of cyclosporine strongly inhibited replication of several coronaviruses in vitro, including SARS-CoV, MERS-CoV and HCoV-229E (46–48), and that the psoriasis patients taking apremilast had a significantly lower rate of severe viral infection (49). Interestingly, cyclosporine and apremilast probably reduce the risk of cytokine storm associated with SARS-CoV infection by downregulating the expression of pro-inflammatory cytokines (50, 51). More importantly, several studies (27, 52, 53) have observed a favorable safety profile of apremilast on COVID-19 susceptibility/severity in patients with psoriasis. Dimetil-fumarate is a small molecule with potent, broad-spectrum antibacterial properties, and acitretin has anti-inflammatory effects and inhibits cell differentiation. Studies indicated that acitretin did not increase risk of viral or respiratory infections in psoriasis patients (49), and the rates of COVID-19 infection and hospitalization in psoriasis patients exposed to acitretin were similar to that of exposure to TNF inhibitors (34). Topical therapies and phototherapy have shown a good safety profile, and remain an important option for psoriasis patients during the COVID-19 pandemic. In conclusion, these studies strongly suggest that these non-biologics do not increase susceptibility or severity of COVID-19, which also confirm the rationality and scientific validity of using them as the control group in this meta-analysis to assess the effect of IL-17 inhibitors on the risk of COVID-19 infection, hospitalization and mortality.
Furthermore, in two studies including 119 and 346 patients with psoriasis using IL-17 inhibitors, respectively, none was infected with SARS-CoV-2 (54, 55), which an evidence that the use of IL-17 inhibitors in psoriasis could not increase the risk of SARS-CoV-2 infection. These studies were consistent with the results of our meta-analysis that the use of IL-17 inhibitors in psoriasis patients did not more prone to SARS-CoV-2. Meanwhile, a study showed that the use of biologics (including IL-17 inhibitors) in psoriasis patients did not increase susceptibility to contracting COVID-19, severe disease progression, or increased hospitalization and mortality rates (56). Moreover, psoriasis patients receiving biologics (including IL-17 inhibitors) were reported to have a lower hospitalization rate than those receiving non-biologics (acitretin, apremilast, cyclosporine, etc.) (57). Although this was not shown in our meta-analysis, it undoubtedly reinforced the viewpoint that the use of IL-17 inhibitors in psoriasis patients did not exacerbate COVID-19 conditions. At present, clinical data on IL-17 inhibitors for the treatment of COVID-19 are still scarce, with only a few case reports showing the efficacy and safety of IL-17 inhibitors in patients with COVID-19. Di Lernia et al. (58) and Balestri et al. (59) have reported favorable outcomes with secukinumab/ixekizumab in patients with COVID-19, respectively. Mugheddu et al. (60) found that secukinumab could even be safely treat severe COVID-19 patients and shorten their disease course. Additionally, other reports (61–63) also showed the similar results. Surprisingly, Carugno et al. (64) demonstrated an experience with a COVID-19 psoriatic patient treated with secukinumab who developed a late onset rash. At the onset of the rash, the patient had a nasopharyngeal positive swab, but the RT-PCR search for viruses in skin was negative, which further supported the potential therapeutic use of IL-17 inhibitors in COVID-19. Overall, these previous studies showed the safety of using IL-17 inhibitors in patients with psoriasis in the setting of COVID-19 pandemic, and further supported the positive effect of IL-17 inhibitors on alleviating the course of COVID-19. And the results of our meta-analysis largely coincided with it.
Large epidemiological studies showed that psoriasis was one of the most common dermatological diseases in patients with COVID-19 (65), and that patients with psoriasis were more susceptible to the SARS-CoV-2 and had a higher risk of dying from COVID-19 (66, 67). However, it was also reported that psoriasis alone (without its comorbidities) was not likely to be a risk factor for the severity of SARA-COV-2 infection (68). Currently, the relationship between psoriasis and COVID-19 is still not entirely clear. National Psoriasis Foundation COVID-19 Task Force (NPF COVID-19 TF) reached a high level of consensus that the likelihood of poor outcomes from COVID-19 is driven by comorbidities such as chronic heart, lung or kidney disease and metabolic disorders such as diabetes and obesity. And psoriasis patients are more prone to these comorbidities, particularly in those with more severe disease (69). Interestingly, psoriasis shares a similar immune-inflammatory mechanism with cardiovascular diseases, both involving activation of Th1 and Th17 cells and decreased T-regulatory cell function (70, 71). Studies have shown that the application of IL-17 inhibitors can improve psoriasis and cardiovascular disease (72, 73). Therefore, it further supported that IL-17 inhibitors played a significant positive role in the prevention of SARS-CoV-2 infection and COVID-19 worsening. It is well known that psoriasis is a complex disease, and it’s considered quite harmful to hastily interrupt treatment (74). On one hand, it will lead to higher pro-inflammatory states which can potentially worsen the cytokine storm and immune responses to SARS-CoV-2. On the other hand, discontinuation of biological therapies may cause increased medical costs due to the inevitable recurrence of disease, for the efficacy may be reduced if the patient uses the same biological therapy again after discontinuation (75, 76). Our study supports the continued use of IL-17 inhibitors in patients with psoriasis during the COVID-19 pandemic, which avoids potential exacerbations of psoriasis due to treatment interruption.
This research has several limitations. Firstly, due to the novelty of the research topic and the lack of randomized controlled trials data, we eventually included observational studies such as case series, cross-sectional studies, and cohort studies, which may result in a downgrade of research evidence. Secondly, individual characteristics can affect the risk of SARS-CoV-2 infection and course of COVID-19, such as gender, comorbidities, and compliance with epidemic prevention measures. And due to the lack of these elements in the included studies, our study did not address them. These limitations may be sources for heterogeneity.
Nonetheless, there are some strengths in our meta-analysis. Firstly, our study is based on observational studies that investigate psoriasis patients in their natural state, which provides certain guidance to dermatologists in their clinical management. Secondly, we included thousands of individuals, which increased the credibility of this meta-analysis. Thirdly, we performed a more comprehensive analysis, including overall analysis and subgroup analyses grouped by age and COVID-19 cases, respectively, and further confirmed that the use of IL-17 inhibitors in psoriasis did not increase the risk of SARS-CoV-2 infection or worsen the course of COVID-19 compared with the use of non-biologics.
Conclusion
Our meta-analysis study with a large sample size of 2,437 psoriasis patients using IL-17 inhibitors and 4,669 psoriasis patients using non-biologics strongly support that the use of IL-17 inhibitors in patients with psoriasis does not increase the risk of SARS-CoV-2 infection or worsen the course of COVID-19. This review provides evidence to support treatment decision making for psoriasis patients receiving IL-17 inhibitors in the COVID-19 pandemic. Well-controlled clinical trials are warranted to demonstrate the efficacy and safety of IL-17 inhibitors in the treatment of COVID-19 in the psoriasis population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
ML had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: ML, GZ, NW. Acquisition, analysis, or interpretation of data: GZ, NW, LL, SC, BW, ML. Drafting of the manuscript: ML, HW, XH, ZX, YZ. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: ML, SC, LL. Supervision: GZ, NW. All authors contributed to the article and approved the submitted version.
Acknowledgments
We sincerely thank Dr. Fougerousse Anne-Claire for her data support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1046352/full#supplementary-material
References
1. World Health Organization. WHO coronavirus (COVID-19) dashboard (2022). Available at: https://covid19.who.int/ (Accessed September 9, 2022).
2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5
3. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. Covid-19: Consider cytokine storm syndromes and immunosuppression. Lancet (2020) 395(10229):1033–4. doi: 10.1016/s0140-6736(20)30628-0
4. Maione F, Casillo GM, Raucci F, Salvatore C, Ambrosini G, Costa L, et al. Interleukin-17a (Il-17a): A silent amplifier of covid-19. BioMed Pharmacother (2021) 142:111980. doi: 10.1016/j.biopha.2021.111980
5. Shibabaw T. Inflammatory cytokine: Il-17a signaling pathway in patients present with covid-19 and current treatment strategy. J Inflammation Res (2020) 13:673–80. doi: 10.2147/jir.S278335
6. Bulat V, Situm M, Azdajic MD, Likic R. Potential role of il-17 blocking agents in the treatment of severe covid-19? Br J Clin Pharmacol (2020) 87(3):1578–81. doi: 10.1111/bcp.14437
7. Liu Y, Zhang C, Huang F, Yang Y, Wang F, Yuan J, et al. Elevated plasma levels of selective cytokines in covid-19 patients reflect viral load and lung injury. Natl Sci Rev (2020) 7(6):1003–11. doi: 10.1093/nsr/nwaa037
8. Sadeghi A, Tahmasebi S, Mahmood A, Kuznetsova M, Valizadeh H, Taghizadieh A, et al. Th17 and treg cells function in sars-Cov2 patients compared with healthy controls. J Cell Physiol (2020) 236(4):2829–39. doi: 10.1002/jcp.30047
9. Sarmiento-Monroy JC, Parra-Medina R, Garavito E, Rojas-Villarraga A. T Helper 17 response to severe acute respiratory syndrome coronavirus 2: A type of immune response with possible therapeutic implications. Viral Immunol (2021) 34(3):190–200. doi: 10.1089/vim.2020.0177
10. Erichsen CY, Jensen P, Kofoed K. Biologic therapies targeting the interleukin (Il)-23/Il-17 immune axis for the treatment of moderate-to-Severe plaque psoriasis: A systematic review and meta-analysis. J Eur Acad Dermatol Venereol (2019) 34(1):30–8. doi: 10.1111/jdv.15879
11. Ricardo JW, Lipner SR. Considerations for safety in the use of systemic medications for psoriasis and atopic dermatitis during the covid-19 pandemic. Dermatol Ther (2020) 33(5):e13687. doi: 10.1111/dth.13687
12. Bissonnette R, Luger T, Thaçi D, Toth D, Lacombe A, Xia S, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-Severe psoriasis through 5 years of treatment (Sculpture extension study). J Eur Acad Dermatol Venereol (2018) 32(9):1507–14. doi: 10.1111/jdv.14878
13. Zachariae C, Gordon K, Kimball AB, Lebwohl M, Blauvelt A, Leonardi C, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol (2018) 79(2):294–301.e6. doi: 10.1016/j.jaad.2018.03.047
14. Puig L, Lebwohl M, Bachelez H, Sobell J, Jacobson AA. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator–controlled phase 3 amagine-2 trial. J Am Acad Dermatol (2020) 82(2):352–9. doi: 10.1016/j.jaad.2019.05.095
15. Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the covid-19 pandemic era. A Review Dermatol Ther (2020) 34(1):e14498. doi: 10.1111/dth.14498
16. Wan MT, Shin DB, Winthrop KL, Gelfand JM. The risk of respiratory tract infections and symptoms in psoriasis patients treated with interleukin 17 pathway-inhibiting biologics: A meta-estimate of pivotal trials relevant to decision making during the covid-19 pandemic. J Am Acad Dermatol (2020) 83(2):677–9. doi: 10.1016/j.jaad.2020.05.035
17. Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of covid-19? J Am Acad Dermatol (2020) 82(5):1217–8. doi: 10.1016/j.jaad.2020.03.031
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
19. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (Strobe): Explanation and elaboration. PloS Med (2007) 4(10):e297. doi: 10.1371/journal.pmed.0040297
20. Beytout Q, Pepiot J, Maruani A, Devulder D, Aubert R, Beylot-Barry M, et al. Impact of the covid-19 pandemic on children with psoriasis. Annales Dermatol Venereol (2021) 148(2):106–11. doi: 10.1016/j.annder.2021.01.005
21. Brazzelli V, Isoletta E, Barak O, Barruscotti S, Vassallo C, Giorgini C, et al. Does therapy with biological drugs influencecovid-19 infection? observational monocentric prevalence study on the clinical and epidemiological data of psoriatic patients treated with biological drugs or with topical drugs alone. Dermatol Ther (2020) 33(6):e14516. doi: 10.1111/dth.14516
22. Damiani G, Pacifico A, Bragazzi NL, Malagoli P. Biologics increase the risk of sars- cov -2 infection and hospitalization, but not icu admission and death: Real-life data from a Large cohort during red-zone declaration. Dermatol Ther (2020) 33(5):e13475. doi: 10.1111/dth.13475
23. Fougerousse AC, Perrussel M, Bécherel PA, Begon E, Pallure V, Zaraa I, et al. Systemic or biologic treatment in psoriasis patients does not increase the risk of a severe form of covid-19. J Eur Acad Dermatol Venereol (2020) 34(11):e676–9. doi: 10.1111/jdv.16761
24. Kara Polat A, Oguz Topal I, Karadag AS, Aksoy H, Koku Aksu AE, Ozkur E, et al. The impact of covid-19 in patients with psoriasis: A multicenter study in Istanbul. Dermatol Ther (2020) 34(1):e14691. doi: 10.1111/dth.14691
25. Kartal SP, Celik G, Yilmaz O, Solak EO, Gul BD, Ustunbas TK, et al. The impact of covid-19 pandemic on psoriasis patients, and their immunosuppressive treatment: A cross-sectional multicenter study from Turkey. J Dermatol Treat (2021) 33(4):2137–44. doi: 10.1080/09546634.2021.1927947
26. Kridin K, Schonmann Y, Solomon A, Damiani G, Bitan DT, Onn E, et al. Risk of covid-19 infection, hospitalization, and mortality in patients with psoriasis treated by interleukin-17 inhibitors. J Dermatol Treat (2021) 33(4):2014–20. doi: 10.1080/09546634.2021.1905766
27. Queiro Silva R, Armesto S, González Vela C, Naharro Fernández C, González-Gay MA. Covid-19 patients with psoriasis and psoriatic arthritis on biologic immunosuppressant therapy vs apremilast in north Spain. Dermatol Ther (2020) 33(6):e13961. doi: 10.1111/dth.13961
28. Vispi M, Corradin T, Peccianti C, Feci L, Casini L, Pisani C, et al. Psoriasis, biological drugs and coronavirus disease 2019: Real life experience of two Italian provinces. Dermatol Rep (2020) 12(1):8642. doi: 10.4081/dr.2020.8642
29. D’Acquisto F, Maione F, Pederzoli-Ribeil M. From il-15 to il-33: The never-ending list of new players in inflammation. is it time to forget the humble aspirin and move ahead? Biochem Pharmacol (2010) 79(4):525–34. doi: 10.1016/j.bcp.2009.09.015
30. Lubberts E. The il-23-Il-17 axis in inflammatory arthritis. Nat Rev Rheumatol (2015) 11(7):415–29. doi: 10.1038/nrrheum.2015.53
31. Gaffen SL. Biology of recently discovered cytokines: Interleukin-17–a unique inflammatory cytokine with roles in bone biology and arthritis. Arthritis Res Ther (2004) 6(6):240–7. doi: 10.1186/ar1444
32. Kehlen A, Thiele K, Riemann D, Langner J. Expression, modulation and signalling of il-17 receptor in fibroblast-like synoviocytes of patients with rheumatoid arthritis. Clin Exp Immunol (2002) 127(3):539–46. doi: 10.1046/j.1365-2249.2002.01782.x
33. Wu D, Yang XO. Th17 responses in cytokine storm of covid-19: An emerging target of Jak2 inhibitor fedratinib. J Microbiol Immunol Infect (2020) 53(3):368–70. doi: 10.1016/j.jmii.2020.03.005
34. Kridin K, Schonmann Y, Damiani G, Peretz A, Onn E, Bitan DT, et al. Tumor necrosis factor inhibitors are associated with a decreased risk of covid-19-Associated hospitalization in patients with psoriasis-a population-based cohort study. Dermatol Ther (2021) 34(4):e15003. doi: 10.1111/dth.15003
35. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with sars-Cov-2 pneumonia in wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med (2020) 8(5):475–81. doi: 10.1016/s2213-2600(20)30079-5
36. Xie M, Cheng B, Ding Y, Wang C, Chen J. Correlations of il-17 and nf-κb gene polymorphisms with susceptibility and prognosis in acute respiratory distress syndrome in a Chinese population. Biosci Rep (2019) 39(2):BSR20181987. doi: 10.1042/bsr20181987
37. Parackova Z, Bloomfield M, Klocperk A, Sediva A. Neutrophils mediate Th17 promotion in covid-19 patients. J Leukoc Biol (2021) 109(1):73–6. doi: 10.1002/jlb.4covcra0820-481rrr
38. Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, et al. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight (2016) 1(9):e85851. doi: 10.1172/jci.insight.85851
39. Dávila-Seijo P, Dauden E, Descalzo MA, Carretero G, Carrascosa JM, Vanaclocha F, et al. Infections in moderate to severe psoriasis patients treated with biological drugs compared to classic systemic drugs: Findings from the biobadaderm registry. J Invest Dermatol (2017) 137(2):313–21. doi: 10.1016/j.jid.2016.08.034
40. Kridin K, Schonmann Y, Tzur Bitan D, Damiani G, Peretz A, Weinstein O, et al. Coronavirus disease 2019 (Covid-19)-Associated hospitalization and mortality in patients with psoriasis: A population-based study. Am J Clin Dermatol (2021) 22(5):709–18. doi: 10.1007/s40257-021-00605-8
41. Izadi Z, Brenner EJ, Mahil SK, Dand N, Yiu ZZN, Yates M, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and covid-19. JAMA Netw Open (2021) 4(10):e2129639. doi: 10.1001/jamanetworkopen.2021.29639
42. Yousaf A, Gayam S, Feldman S, Zinn Z, Kolodney M. Clinical outcomes of covid-19 in patients taking tumor necrosis factor inhibitors or methotrexate: A multicenter research network study. J Am Acad Dermatol (2021) 84(1):70–5. doi: 10.1016/j.jaad.2020.09.009
43. IGanjei Z, Faraji Dana H, Ebrahimi-Dehkordi S, Alidoust F, Bahmani K. Methotrexate as a safe immunosuppressive agent during the covid-19 pandemic. Int Immunopharmacol (2021) 101(Pt B):108324. doi: 10.1016/j.intimp.2021.108324
44. Frohman EM, Villemarette-Pittman NR, Cruz RA, Longmuir R, Rowe V, Rowe ES, et al. Part ii. high-dose methotrexate with leucovorin rescue for severe covid-19: An immune stabilization strategy for sars-Cov-2 induced 'Panic' attack. J Neurol Sci (2020) 415:116935. doi: 10.1016/j.jns.2020.116935
45. Safavi F, Nath A. Silencing of immune activation with methotrexate in patients with covid-19. J Neurol Sci (2020) 415:116942. doi: 10.1016/j.jns.2020.116942
46. Pfefferle S, Schöpf J, Kögl M, Friedel CC, Müller MA, Carbajo-Lozoya J, et al. The sars-Coronavirus-Host interactome: Identification of cyclophilins as target for pan-coronavirus inhibitors. PloS Pathog (2011) 7(10):e1002331. doi: 10.1371/journal.ppat.1002331
47. de Wilde AH, Zevenhoven-Dobbe JC, van der Meer Y, Thiel V, Narayanan K, Makino S, et al. Cyclosporin a inhibits the replication of diverse coronaviruses. J Gen Virol (2011) 92(Pt 11):2542–8. doi: 10.1099/vir.0.034983-0
48. de Wilde AH, Raj VS, Oudshoorn D, Bestebroer TM, van Nieuwkoop S, Limpens R, et al. Mers-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin a or interferon-α treatment. J Gen Virol (2013) 94(Pt 8):1749–60. doi: 10.1099/vir.0.052910-0
49. Dommasch ED, Kim SC, Lee MP, Gagne JJ. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol (2019) 155(10):1142–52. doi: 10.1001/jamadermatol.2019.1121
50. Russell CD, Haas J. Cyclosporine has a potential role in the treatment of sars. J Infect (2013) 67(1):84–5. doi: 10.1016/j.jinf.2013.01.004
51. Lytvyn Y, Georgakopoulos JR, Mufti A, Devani AR, Gooderham MJ, Jain V, et al. Incidence and prognosis of covid-19 in patients with psoriasis on apremilast: A multicentre retrospective cohort study. J Eur Acad Dermatol Venereol (2022) 36(2):e94–e5. doi: 10.1111/jdv.17749
52. Olisova OY, Anpilogova EM, Svistunova DA. Apremilast as a potential treatment option for covid-19: No symptoms of infection in a psoriatic patient. Dermatol Ther (2020) 33(4):e13668. doi: 10.1111/dth.13668
53. Mugheddu C, Pizzatti L, Sanna S, Atzori L, Rongioletti F. Covid-19 pulmonary infection in erythrodermic psoriatic patient with oligodendroglioma: Safety and compatibility of apremilast with critical intensive care management. J Eur Acad Dermatol Venereol (2020) 34(8):e376–8. doi: 10.1111/jdv.16625
54. Galluzzo M, Tofani L, Bianchi L, Talamonti M. Status of a real-life cohort of patients with moderate-to-Severe plaque psoriasis treated with secukinumab and considerations on the use of biological agents in the covid-19 era. Expert Opin Biol Ther (2020) 20(8):829–30. doi: 10.1080/14712598.2020.1779217
55. Potestio L, Camela E, Tajani A, Fabbrocini G, Megna M Letter to the Editor regarding the article “Oguz topal I, kara polat a, zindancı İ, et al. adherence to systemic therapy in patients with psoriasis during the covid-19 pandemic: A multicenter study. J cosmet dermatol. 2021;10.1111/Jocd.14610.” J Cosmetic Dermatol (2022) 21(3) 880–2 doi: 10.1111/jocd.14781
56. Kus MM, Ozturk P, Dogramaci AC, Guner ME, Bozkurt Y, Nazik H, et al. The effects of covid-19 on psoriasis patients using biologic treatment: Two-center retrospective study. Turkiye Klinikleri Dermatol (2022) 32(1):22–8. doi: 10.5336/dermato.2021-86216
57. Mahil SK, Dand N, Mason KJ, Yiu ZZN, Tsakok T, Meynell F, et al. Factors associated with adverse covid-19 outcomes in patients with psoriasis-insights from a global registry-based study. J Allergy Clin Immunol (2021) 147(1):60–71. doi: 10.1016/j.jaci.2020.10.007
58. Di Lernia V, Bombonato C, Motolese A. Covid-19 in an elderly patient treated with secukinumab. Dermatol Ther (2020) 33(4):e13580. doi: 10.1111/dth.13580
59. Balestri R, Rech G, Girardelli CR. Sars-Cov-2 infection in a psoriatic patient treated with il-17 inhibitor. J Eur Acad Dermatol Venereol (2020) 34(8):e357–e8. doi: 10.1111/jdv.16571
60. Mugheddu C, Sanna S, Atzori L, Rongioletti F. Safety of secukinumab treatment in covid -19 affected psoriatic patients. Dermatol Ther (2021) 34(1): e14710. doi: 10.1111/dth.14710
61. Facheris P, Valenti M, Pavia G, Gargiulo L, Narcisi A, Costanzo A, et al. Complicated coronavirus disease 2019 (Covid-19) in a psoriatic patient treated with ixekizumab. Int J Dermatol (2020) 59(8):e267–e8. doi: 10.1111/ijd.15008
62. Magnano M, Balestri R, Bardazzi F, Mazzatenta C, Girardelli CR, Rech G. Psoriasis,Covid-19, and acute respiratory distress syndrome: Focusing on the risk of concomitant biological treatment. Dermatol Ther (2020) 33(4):e13706. doi: 10.1111/dth.13706
63. Strippoli D, Barbagallo T, Prestinari F, Russo G, Fantini F. Biologic agents in psoriasis: Our experience during coronavirus infection. Int J Dermatol (2020) 59(8):e266–e7. doi: 10.1111/ijd.15002
64. Carugno A, Gambini DM, Raponi F, Vezzoli P, Robustelli Test E, Arosio MEG, et al. Coronavirus disease 2019 (Covid-19) rash in a psoriatic patient treated with secukinumab: Is there a role for interleukin 17? Dermatol Ther (2020) 33(6):e14011. doi: 10.1111/dth.14011
65. Tan EH, Sena AG, Prats-Uribe A, You SC, Ahmed WU, Kostka K, et al. Covid-19 in patients with autoimmune diseases: Characteristics and outcomes in a multinational network of cohorts across three countries. Rheumatol (Oxford) (2021) 60(Si):Si37–si50. doi: 10.1093/rheumatology/keab250
66. Patrick MT, Zhang H, Wasikowski R, Prens EP, Weidinger S, Gudjonsson JE, et al. Associations between covid-19 and skin conditions identified through epidemiology and genomic studies. J Allergy Clin Immunol (2021) 147(3):857–69.e7. doi: 10.1016/j.jaci.2021.01.006
67. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with covid-19-Related death using opensafely. Nature (2020) 584(7821):430–6. doi: 10.1038/s41586-020-2521-4
68. Yiu ZZN, Harding-Oredugba G, Griffiths CEM, Warren RB, McMullen E, Hunter HJA. Risk of covid-19 infection in adult patients with atopic eczema and psoriasis: A single-centre cross-sectional study. Br J Dermatol (2021) 185(2):441–3. doi: 10.1111/bjd.20062
69. IGelfand JM, Armstrong AW, Bell S, Anesi GL, Blauvelt A, Calabrese C, et al. National psoriasis foundation covid-19 task force guidance for management of psoriatic disease during the pandemic: Version 2-advances in psoriatic disease management, covid-19 vaccines, and covid-19 treatments. J Am Acad Dermatol (2021) 84(5):1254–68. doi: 10.1016/j.jaad.2020.12.058
70. Ahlehoff O, Gislason GH, Charlot M, Jørgensen CH, Lindhardsen J, Olesen JB, et al. Psoriasis is associated with clinically significant cardiovascular risk: A Danish nationwide cohort study. J Intern Med (2011) 270(2):147–57. doi: 10.1111/j.1365-2796.2010.02310.x
71. Alexandroff AB, Pauriah M, Camp RD, Lang CC, Struthers AD, Armstrong DJ. More than skin deep: Atherosclerosis as a systemic manifestation of psoriasis. Br J Dermatol (2009) 161(1):1–7. doi: 10.1111/j.1365-2133.2009.09281.x
72. von Stebut E, Boehncke WH, Ghoreschi K, Gori T, Kaya Z, Thaci D, et al. Il-17a in psoriasis and beyond: Cardiovascular and metabolic implications. Front Immunol (2019) 10:3096. doi: 10.3389/fimmu.2019.03096
73. Li Y, Golden JB, Camhi MI, Zhang X, Fritz Y, Diaconu D, et al. Protection from psoriasis-related thrombosis after inhibition of il-23 or il-17a. J Invest Dermatol (2018) 138(2):310–5. doi: 10.1016/j.jid.2017.09.021
74. Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, et al. Coronavirus infections and immune responses. J Med Virol (2020) 92(4):424–32. doi: 10.1002/jmv.25685
75. Kim B, Maverakis E, Raychaudhuri SP. Is it possible to discontinue tumor necrosis factor antagonists after psoriasis remission? Ann Dermatol (2019) 31(5):495–501. doi: 10.5021/ad.2019.31.5.495
Keywords: COVID-19, interleukin-17 inhibitors, psoriasis, NNT, meta-analysis, systematic review
Citation: Liu M, Wang H, Liu L, Cui S, Huo X, Xiao Z, Zhao Y, Wang B, Zhang G and Wang N (2022) Risk of COVID-19 infection, hospitalization and mortality in psoriasis patients treated with interleukin-17 inhibitors: A systematic review and meta-analysis. Front. Immunol. 13:1046352. doi: 10.3389/fimmu.2022.1046352
Received: 16 September 2022; Accepted: 10 October 2022;
Published: 21 October 2022.
Edited by:
Juan Bautista De Sanctis, Palacký University Olomouc, CzechiaReviewed by:
Undurti Narasimha Das, UND Life Sciences LLC, United StatesSelim Görgün, Samsun Training and Research Hospital, Turkey
Copyright © 2022 Liu, Wang, Liu, Cui, Huo, Xiao, Zhao, Wang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Wang, aGJ5a2R4d25AMTYzLmNvbQ==; Guoqiang Zhang, emx4MDkwNzAyQDE2My5jb20=
 Meitong Liu
Meitong Liu Huijuan Wang
Huijuan Wang Lu Liu
Lu Liu Saijin Cui
Saijin Cui Xiangran Huo
Xiangran Huo Zhuoyun Xiao
Zhuoyun Xiao Yaning Zhao
Yaning Zhao Bin Wang
Bin Wang Guoqiang Zhang
Guoqiang Zhang Na Wang
Na Wang