- 1Department of Biochemistry, All India Institute of Medical Sciences, New Delhi, India
- 2Department of Anatomy, All India Institute of Medical Sciences, New Delhi, India
- 3Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India
- 4Department of Gastroenterology, All India Institute of Medical Sciences, New Delhi, India
Background: Acute kidney injury (AKI) considerably increases the risk of short-term mortality in acute-on-chronic liver failure (ACLF) but predicting AKI is not possible with existing tools. Our study aimed at de novo discovery of AKI biomarkers in ACLF.
Methods: This observational study had two phases- (A) Discovery phase in which quantitative proteomics was carried-out with day-of-admission plasma from ACLF patients who initially had no-AKI but either progressed to AKI (n=10) or did not (n=9) within 7 days of admission and, (B) Validation phase in which selected biomarkers from the discovery phase were validated by ELISA in a larger set of ACLF plasma samples (n=93) followed by sub-group analyses.
Results: Plasma proteomics revealed 56 differentially expressed proteins in ACLF patients who progressed to AKI vs those who did not. The metallothionein protein-family was upregulated in patients who progressed to AKI and was validated by ELISA as significantly elevated in both- (i) ACLF-AKI vs no-AKI (p-value ≤ 0.0001) and (ii) progression to AKI vs no-progression to AKI (p-value ≤ 0.001). AUROC for AKI vs no-AKI was 0.786 (p-value ≤0.001) and for progression to AKI vs no-progression to AKI was 0.7888 (p-value ≤0.001). Kaplan-Meier analysis revealed that ACLF patients with plasma MT concentration >5.83 ng/mL had a high probability of developing AKI by day 7 (p-value ≤0.0001). High expression of metallothionein genes was found in post-mortem liver biopsies of ACLF patients.
Conclusion: Day-of-admission measurements of plasma metallothionein can act as predictive biomarkers of AKI in ACLF.
Introduction
Acute-on-Chronic Liver Failure (ACLF) is a complication of cirrhosis, with a very high short-term mortality of ~50% (1, 2). The hallmarks of ACLF are- systemic inflammation, innate immune dysfunction and extra-hepatic multiple organ failure (3, 4). The chief reason for mortality in ACLF is multiple organ failure (2). Among the organs affected, acute kidney injury (AKI) is the central event that changes the course of the disease leading to poor patient outcomes (5, 6).
Kidney dysfunction that occurs in the context of liver disease, particularly liver cirrhosis, is usually classified as hepato-renal syndrome (HRS) which occurs due to hypovolemia (7, 8). Recent evidence suggests that the mechanism of ACLF associated AKI is different from HRS and involves an increase in inflammatory markers such as TLR4 and caspase 3 in ACLF patient derived kidney biopsies (5, 9, 10). Reported observations show that ACLF-AKI is associated with acute tubular necrosis and a greater reversibility, along with rapid progression. However, at the same time, ACLF-AKI is relatively less responsive to the vasoconstrictor drug terlipressin which is prescribed for the correction of HRS, thus indicating that a different therapeutic approach is required for ACLF-AKI (11). Therefore early prediction and prevention of AKI in ACLF are top priorities that need to be addressed in order to prolong the window of recovery for ACLF patients, during which reversal of AKI can take place and risk of mortality can reduce (9, 10).
The human plasma is an ideal source of biomarkers due to two reasons- (i) it is the source of the most comprehensive human proteome representative of plasma proteins, tissue leakage markers, as well as markers emanating out of diseased cells and, (ii) the ease of plasma collection and preparation using methods that can be uniformly followed all over the world. Our study aimed to identify predictive changes in the plasma proteome of ACLF patients prior to the development of clinically defined AKI. Towards this, the objectives of our study were- (i) To identify plasma proteome changes in day-of-admission samples derived from ACLF patients who did not present with AKI initially but who progressed to AKI within 7 days of admission as compared to those who did not and, (ii) to validate selected plasma protein markers in a larger cohort of ACLF patients and carry out further sub-group analyses to understand the relevance of the biomarkers to patient outcomes.
We found that quantitative changes in plasma proteome were apparent even on day-of-admission, prior to the development of clinically defined AKI and some of these changes could be validated in a larger cohort of ACLF patients. Our study provides insights into the dynamic changes that occur in the plasma proteome of ACLF patients prior to the onset of AKI thereby providing a potential predictive biomarker for AKI in ACLF.
Study design, patient recruitment and methodology
Study design
An overall workflow of the study has been provided in Figure 1A. This was an observational study which was divided into 2 phases- (A) Discovery phase and, (B) Validation phase (Figure 1A). The discovery phase aimed at a de novo identification of plasma proteins which were significantly altered (up- or down-regulated) in ACLF patients who did not have clinically defined AKI at admission but progressed to AKI within 7 days. Therefore, day-of-admission (Day 0) plasma samples were collected from ACLF patients without AKI at admission (n=19). These patients were followed up over a 7 day period for the development of AKI as defined by the criteria given below. At the end of 7 days, patients were classified as either those who progressed to AKI (ACLF progression to AKI; n=10) or those who did not progress to AKI (ACLF no-progression to AKI, n=9). The Day 0 plasma samples of these patients were then subjected to label free quantitative proteomics and differentially expressed proteins were identified. Candidate biomarker proteins were selected on the basis of their relative fold up regulation in ACLF patients who progressed to AKI. The second phase of the study was the validation phase, where the identified biomarker proteins were quantitatively measured using ELISA in a larger cohort of 93 ACLF patients (n=33 ACLF-AKI at admission and n=60 ACLF no-AKI) (Figure 1A). Among the 60 ACLF patients without AKI (ACLF no-AKI), 20 progressed to AKI within 7 days (progression to AKI) whereas 40 did not progress to AKI (no-progression to AKI). Day 0 plasma samples were collected from all the study participants which was used for biomarker validation by ELISA. ELISA were performed in a blinded manner by two investigators- investigator 1 labeled patient samples with laboratory IDs and assigned a code to each of the samples in order to blind investigator 2 who performed the experimental ELISA measurements. Investigator 2 then provided the absorbance values to investigator 1 who decoded the samples and performed calculations to arrive at plasma metallothionein concentrations for each sample and carried out sub-group analyses. Biomarker performance statistics were then carried out by biostatisticians.
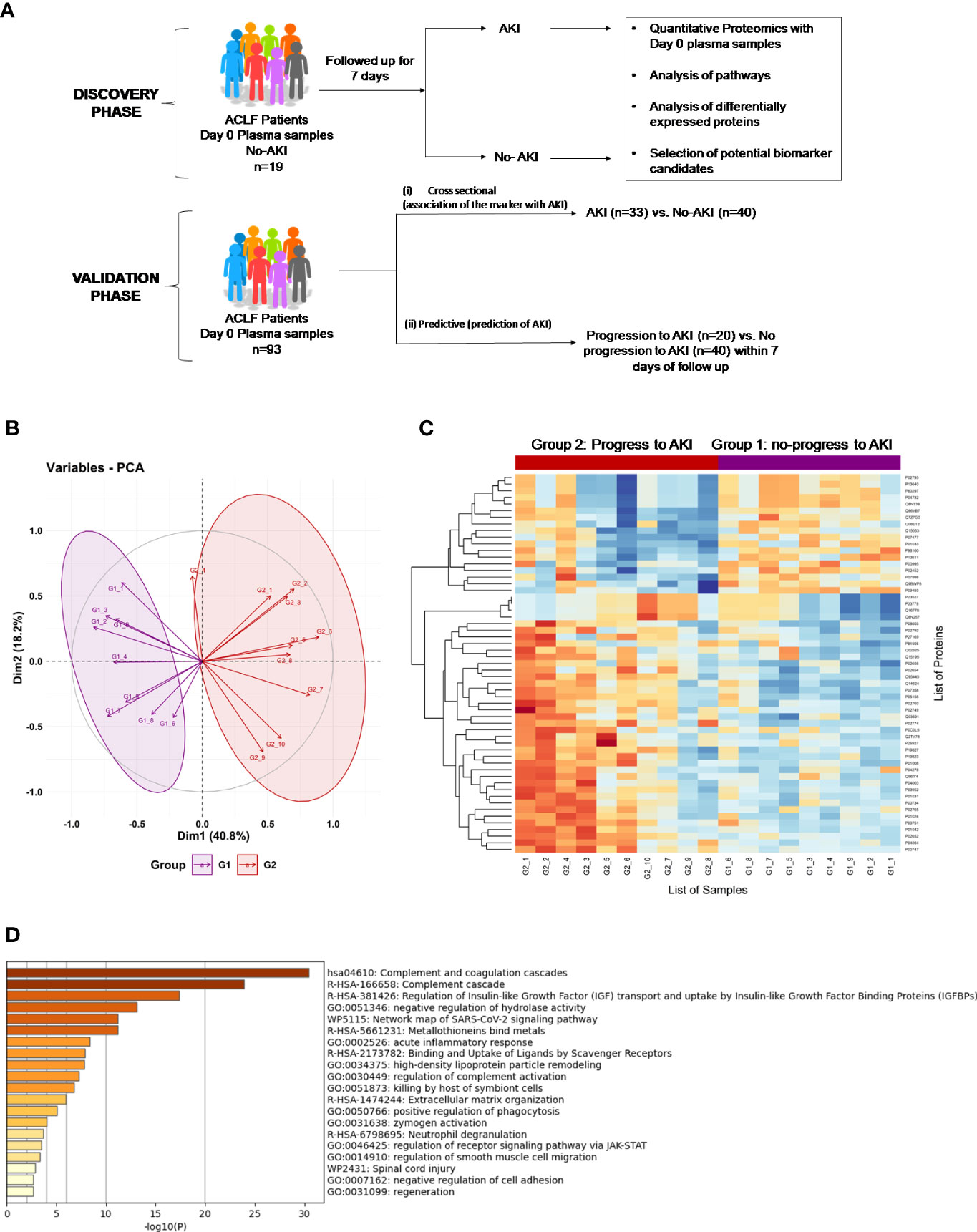
Figure 1 Proteomics Analysis of ACLF patients who progress to AKI vs ACLF patients who do not progress to AKI. (A) Study design of proteomics analysis. (B) PCA analysis clusters patient population into two distinct groups based on plasma proteome profiles, (C) Heatmap of protein expression in the two different groups (G1: ACLF patients who do not progress to AKI and G2: ACLF patients who progress to AKI) and (D) Pathways analysis using Metascape suggests an upregulation of coagulation, acute inflammatory pathways and metal binding proteins metallothioneins as major pathways upregulated in the plasma of ACLF patients who progress to AKI.
All patients were managed as per standard uniform protocol described earlier from our center (12).
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Recruitment of patients
Consecutive patients diagnosed with acute-on-chronic liver failure (ACLF) and admitted in the Department of Gastroenterology, All India Institute of Medical Sciences, New Delhi, India were included. The patient recruitment period was between April 2017 and November 2021. A diseased control group (patients with compensated Cirrhosis, chronic liver disease (CLD) who presented to the Gastroenterology OPD were included in the study to identify the baseline biomarker levels in patients with compensated cirrhosis of liver.
Diagnostic and exclusion criteria
ACLF was diagnosed as per the EASL (European Association for the Study of Liver) criteria as an acute deterioration of pre-existing chronic liver disease, usually related to a precipitating event and associated with increased mortality at 3 months due to multisystem organ failure and ACLF grades were defined as per EASL-CLIF consortium criteria (13).
Compensated cirrhosis was diagnosed based on conventional clinical, biochemical, endoscopic and imaging as well liver histological criteria and these patients were recruited from the Department of Gastroenterology out patients department. Such patients were within the age group ≥ 18 years and ≤ 75 years, treatment naïve, ambulatory, without any extra-hepatic complications, and no overt symptoms (14).
AKI was defined as per the consensus recommendations of the International Club of Ascites i.e., increase in sCr ≥ 0.3 mg/dL (≥26.5 mmol/L) from base line within 48 h; or a percentage increase sCr ≥ 50% from baseline which is known, or presumed, to have occurred within the prior 7 days (7).
Exclusion criteria were hepatocellular carcinoma or portal vein thrombosis, age ≤18 years and ≥ 75 years, presence of prior renal, respiratory and/or cardiovascular disease.
Plasma preparation for proteomics analysis
Whole blood was collected in Becton Dickinson (BD) vacutainer EDTA vials within 24 hours of admission for plasma preparation. Processing of whole blood was done within 2 hours of sample collection. Briefly, the blood vial was centrifuged at 400 x g for 10 minutes at room temperature. Plasma was separated and aliquoted in cryo-vials and stored at -800C until further use.
Protein isolation and sample preparation
Total protein concentration of each of the plasma samples was estimated using a commercially available BCA kit (Puregene Cat no. GX6410AR). Plasma corresponding to 600 µg of protein were subjected to depletion using Thermo Scientific Top 12 abundant protein depletion column. Depleted plasma samples were submitted for trypsin digestion (Trypsin Gold, Promega) and clean-up using C18 columns (NEST group microspin silica column). 50 µg of the Gn-HCL protein lysate were first reduced with 5 mM TCEP and further alkylated with 50 mM iodoacetamide. Alkylated proteins were further diluted using 50mM ammonium bicarbonate to bring final Gn-HCL concentration to 0.6M and then digested with trypsin (1:50, trypsin: lysate ratio, Promega) for 16 h at 37°C. The overnight digests were clarified with brief spin and the supernatant pH was adjusted around pH 2 using 10% TFA. Digests were cleaned using a C18 silica cartridge to remove the salt and dried using a speed vac. The dried pellet was resuspended in buffer A (5% acetonitrile, 0.1% formic acid).
Mass spectrometric analysis of peptide mixtures
Mass spectrometric analysis of the plasma samples was performed at vProteomics (New Delhi). The trypsin-digested sample (1 µg) was resolved on a 50-cm long PicoFrit column (360 µm outer diameter, 75 µm inner diameter &10 µm tip) filled with 2.0 m-C18 resin on nano1000 chromatography system (Proxeon, Thermo) attached to QExactive mass spectrometer. The peptides were eluted with a 5- 15% gradient of the Buffer-B (95% acetonitrile, 0.1% formic acid) for 85 min, 15-40% gradient for 80min, followed by 95% gradient for 6 min at a flow rate of 300 nl/min for the total run time of 100 min. The QExactive spray voltage was set at 2.5 kV, S lens RF level at 50 and ITC heated capillary temperature at 275°C. The MS data were acquired in positive polarity using a data-dependent method choosing 10 most intense peaks with charge state +2 to+5, with exclude isotope option enabled and dynamic exclusion of time of 12 sec. The MS1 (mass range 150-2000 m/z) and MS2 scans were acquired in Orbitrap Mass analyzer with resolution of 70,000 and 17500 at m/z 200 respectively with lockmass (445.12003) option enabled. The MS1 or Full scan target was 1×106 with a maximum fill time of 100 ms with mass range set to 350−1700. Target value for MS2 or fragment scans was set at 1×105, and intensity threshold was set at 5×103. Isolation window of parent ion of interest was set at 2 m/z. Normalized collision energy for Higher-energy collisional dissociation (HCD) was set at 27. Peptide match option was set to preferred mode along with activation of isotope exclusion option.
Data processing and differential proteome analysis
All samples were processed and the RAW files generated were analyzed with Proteome Discoverer (v2.4) against the Uniprot reference proteome database as provided. For Sequest and Amanda search, the precursor and fragment mass tolerances were set at 10 ppm and 0.5 Da, respectively. The protease used to generate peptides, i.e., enzyme specificity was set for trypsin/P (cleavage at the C terminus of “K/R: unless followed by “P”) along with maximum missed cleavages value of two. Carbamidomethyl on cysteine as fixed modification and oxidation of methionine and N-terminal acetylation were considered as Variable modifications for database search. Both peptide spectrum match and protein false discovery rate were set to 0.01 FDR.
Abundance values of each sample were used for differential statistical analysis. Protein abundance values were filtered on the basis of valid values. Filtered values were Log2 transformed followed by Z-score standardization. Student T-Test was used and a p-value≤ 0.05 was considered statistically significant for the difference in abundance of proteins between groups. Z score abundance values of the significant proteins were then used for bioinformatics data visualization using in-house R programming Scripts (vProteomics, New Delhi). Heatmap for differentially expressed proteins was drawn using R programming. Group legends were used to categorize samples (reference G1- no progression to AKI and test G2- progression to AKI). Principle components analysis (PCA) was performed to see the dimensional spread of data variability. A Biplot (using PC1 and PC2) was used to visualize the spread of samples. Pathways analysis were carried out using Metascape and information on protein expression were retrieved from human protein atlas (proteinatlas.org) (15–17).
Preparation of RNA, cDNA and PCR from post mortem liver tissue biopsies
Tru-cut liver and kidney biopsies were retrieved from ACLF patients within 30 minutes of death with informed consent, and immediately stored in RNA Later in the AB2 ward of the Department of Gastroenterology, AIIMS New Delhi. The biopsied tissue were transferred to the Department of Biochemistry within 1-12 hours of collection and transferred to Trizol for long term storage, and stored at -800C until further use. These patients were Grade 3 ACLF with AKI. This part of the study received a separate ethical clearance from the Institute Ethics Committee (Ref. No.IEC/687/8/2019).
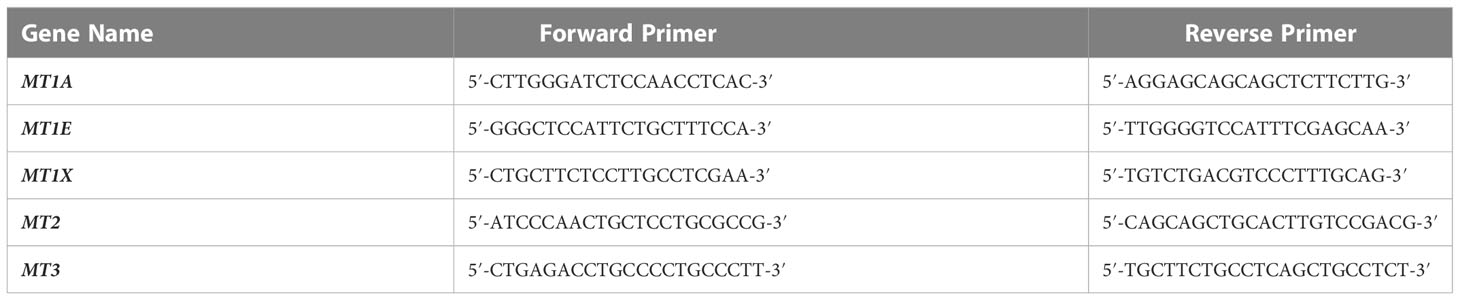
Prior to use, RNA was prepared from the tissue samples using a Qiagen RNeasy miniprep kit (Cat. No. 74106) as per the manufacturer’s instructions. RNA quantity was measured using a nanodrop machine and 500 ng of RNA per tissue sample was subjected to cDNA preparation using the Thermofisher verso cDNA preparation kit (Cat. No.AB1453B). Primers for MT1 and MT2 were designed on the basis of a previously published study (18). The published primers were subjected to a nucleotide BLAST analysis in order to ensure that they were specific to human MT genes as expected. The primer sequences used in this study are listed in Table 1.
cDNA obtained from the post-mortem liver biopsies of deceased ACLF patients were subjected to Reverse Transcriptase-PCR analysis and the PCR products were analyzed on a 1% agarose gel along with molecular weight markers.
ICP-MS for elemental analysis
Collected plasma samples were diluted in acid digestion matrix containing ultra-high pure n-butanol, EDTA, hydrogen peroxide, HNO3 and Triton-x solution. The blood samples were diluted 100 times (specimen:digestion matrix ratio was 1:99). The specimens were allowed to be digested in the digestion matrix for 1 hour. After matrix digestion, specimens were vortexed for 5 minutes. The matrix was prepared using supra-pure metal free double distilled water. The same digestion matrix was used for calibration standard preparation. The multi calibration standards containing As, Cu, Mn, Se, Cd, Pb and Hg (Agilent Technologies, USA) were used to prepare multiple calibration matrix having different elemental concentrations (blank matrix, 0.1 ppb, 0.5 ppb, 1 ppb, 5 ppb, 10 ppb and 50 ppb). After warming-up and start-up sequence calibration, we calibrated the analytical batches using these standards.The internal standard (Agilent Technologies, USA) containing Lithium, Bismuth, Germanium, Scandium and Indium were diluted in the same matrix in which standard and samples were prepared. The internal standard was run with calibration standards and samples were analysed for the standard stability and accuracy of the concentration measurement.The certified standards of blood (Recipe, Germany) were used for quality control (QC). QC samples were prepared in the same matrix used for standard preparation and were analysed after every 6 analytical runs. The working standards were freshly prepared before analysis to avoid any interference and contamination. A standard analytical run included three replicates for each specimen and for each replicate the machine recorded 50 readings. We compared the quality of the analysis and concentration measurements with the internationally certified blood standards containing As, Cu, Mn, Se, Cd, Pb and Hg.
Validation of selected differentially expressed plasma proteins
Metallothionein (MT) proteins were selected from the differentially expressed protein list for further validation in ACLF and CLD patient plasma through ELISA. Protocols were followed as per manufacturer’s suggestions for the Human Metallothionein (MT) ELISA kit (CUSABIO, Cat no. CSB-E09060h). All plasma samples were used at a dilution of 1:200. ELISA results were read on a MicroTek multimode reader. ELISA results were analyzed on MS Excel and statistical analysis was carried out using GraphPad Prism9.0.
Statistical analysis
Data were analysed by statistical software Stata 14.0. Variables were checked for approximate normal distribution. Quantitative data were expressed as Mean ± SD and Median (25th percentile-75th percentile). Categorical data was expressed as frequency (%). Chi-squared test/Fisher’s exact test was used to compare categorical variables between the groups. Independent t-test was used to compare quantitative variables btween the groups. Those variables that did not follow normal distribution were compared by ranksum test. One way ANOVA/Kruskal Wallis test, followed by Bonferroni/Dunn test correction were used to compare quantitative variables among different list of variables. The diagnostic ability of MT to discriminate between ACLF patient with and without AKI, and progression to AKI was determined based on AUROC. The optimal cut-off was determined on the basis of minimum difference between sensitivity and specificity. For the AUROC analysis, control group was selected as ACLF no-AKI or no-progression to AKI, and the test group was selected as ACLF-AKI or progression to AKI respectively. In order to determine the superiority of MT over the traditional severity scores, AUROC analysis was also performed for MELD, MELD-Na, CLIF-OF, and CTP. An overall graphical representation of AUROC is provided in Figures 2C, D. Logistic regression was used to estimate the odds of AKI adjusted for covariates. Time to event analysis (Kaplan- Meier curve) was used to compare probability of an event (no-progression to AKI) at a certain time interval. All statistical tests used were two-sided and p-value ≤0.05 was used to indicate a statistically significant difference.
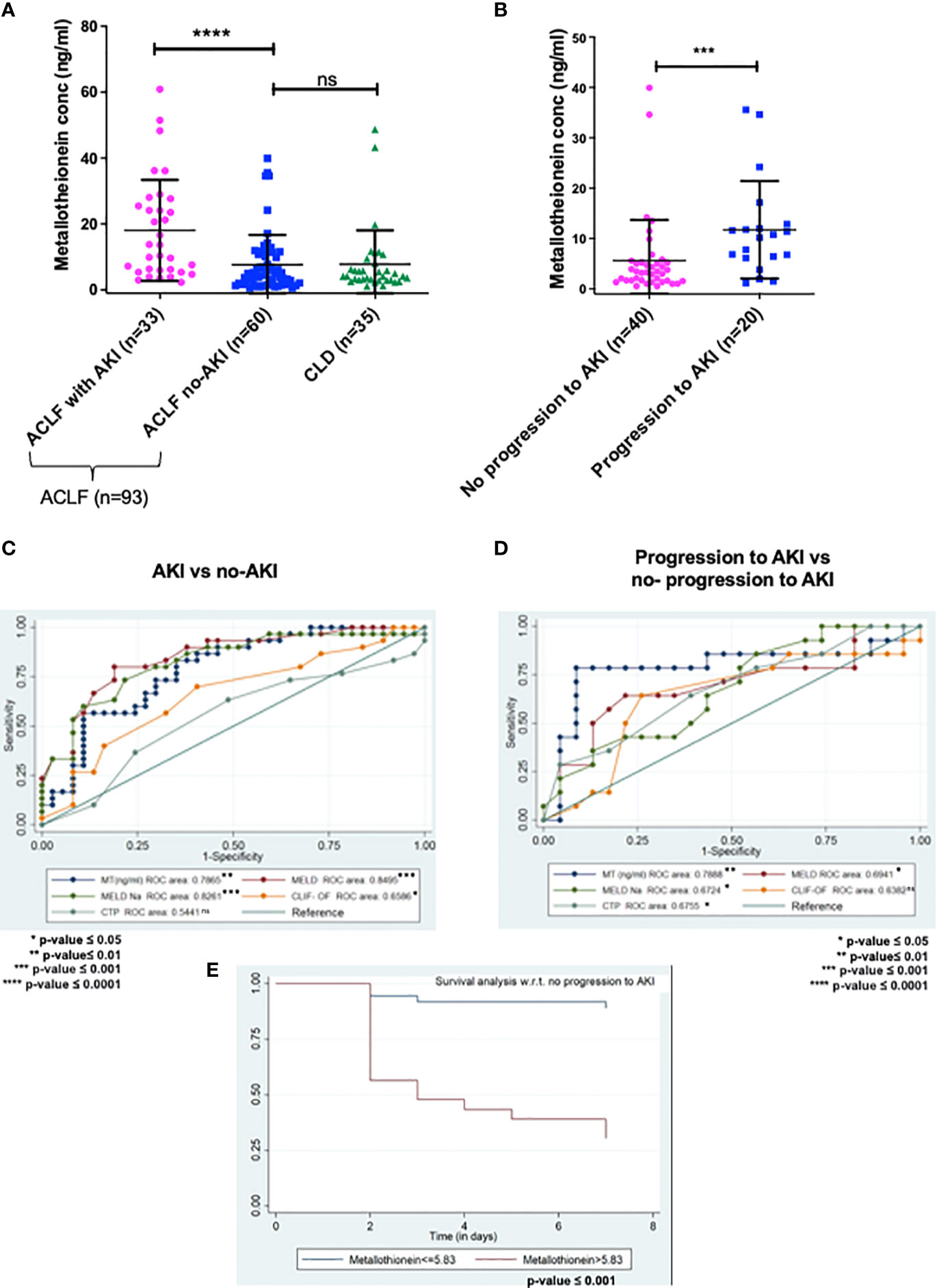
Figure 2 ELISA validation of plasma metallothionein (MT) levels in day-of-admission plasma from ACLF and CLD patients. (A,C)MT levels are significantly higher in ACLF-AKI patient plasma as compared to ACLF no-AKI or CLD with an AUROC of ~ 0.7865 (p- value ≤ 0.0001); (B, D) MT levels are significantly higher in plasma of ACLF patients who progress to AKI vs those who do not with an AUROC of ~0.7888 (***p-value ≤ 0.001; ****p-value ≤ 0.0001). (E) Kaplan-Meier analysis for progression/no-progression to AKI for ACLF patients with plasma MT concentrations > 5.83 ng/mL (p-value <0.0001). ns = non-significant.
Study approval
The patients gave written informed consent for participation in the study and the study was approved by the All India Institute of Medical Sciences, New Delhi ethics committee [Reference No. IEC/473/9/2016 and, IEC/369/7/2016], which is in agreement with the declaration of Helsinki (19).
Results
Baseline characteristics of patients
93 patients were included in the study. The study had 2 phases- (i) The discovery phase and (ii) The validation phase (Figure 1A).
The discovery phase
Plasma proteome analysis in order to identify differentially expressed proteins in ACLF patients who progressed to AKI vs those who did not. This part of the study included the following patient groups:
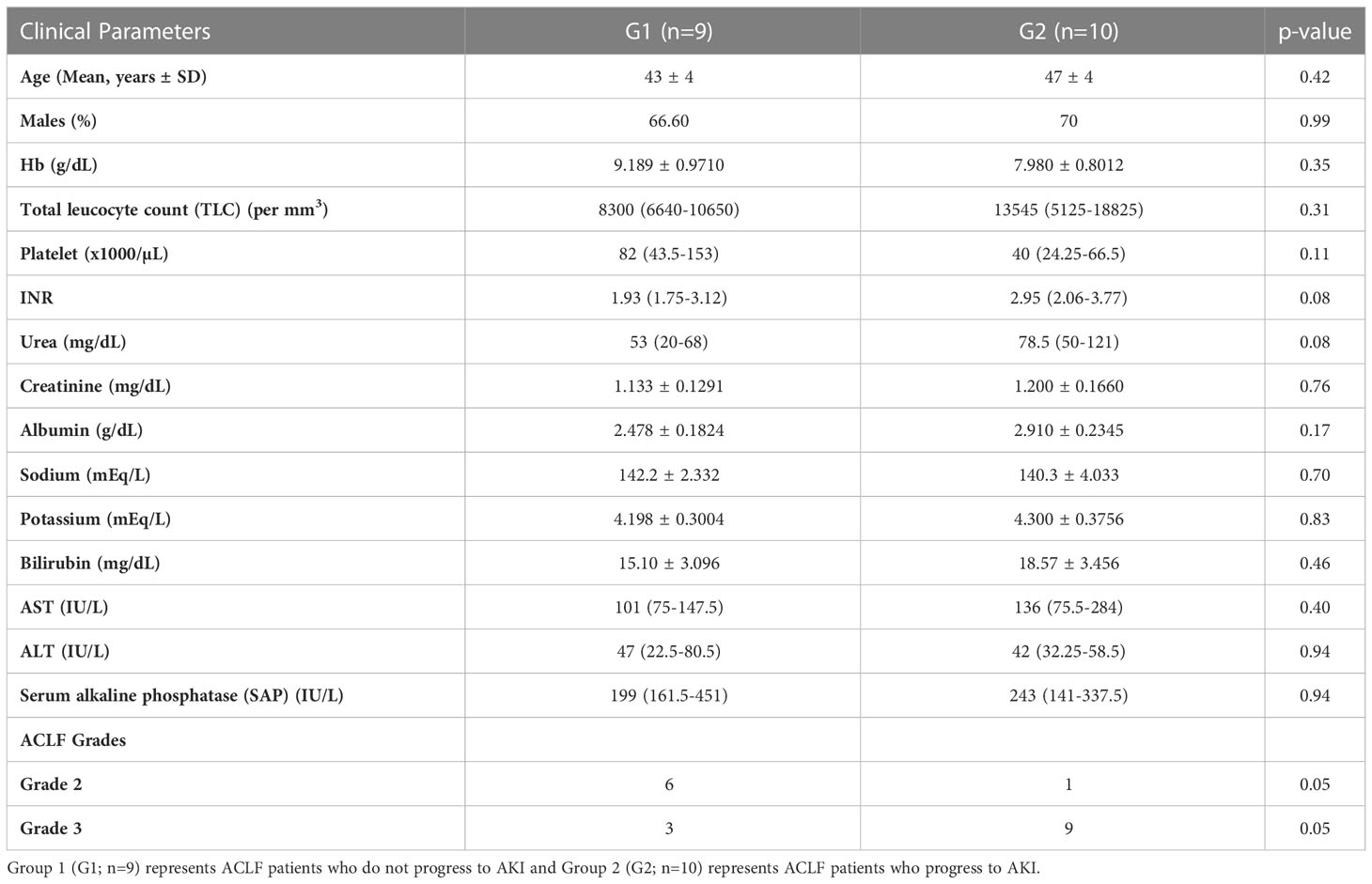
The study groups included in the proteomics analysis were- (i) ACLF patients who did not progress to AKI (Group1; n = 9) and, (ii) ACLF patients who progressed to AKI (Group 2; n=10) (Table 2). The mean ages in Group 1 and Group 2 were 43 ± 4 years and 47 ± 4 years respectively. There were no differences in the laboratory parameters between ACLF patients who progressed to AKI vs those who did not progress to AKI (Table 2).
The validation phase
ELISA based validation of identified biomarkers in the discovery phase. This included the following study groups:
ACLF AKI vs ACLF no-AKI
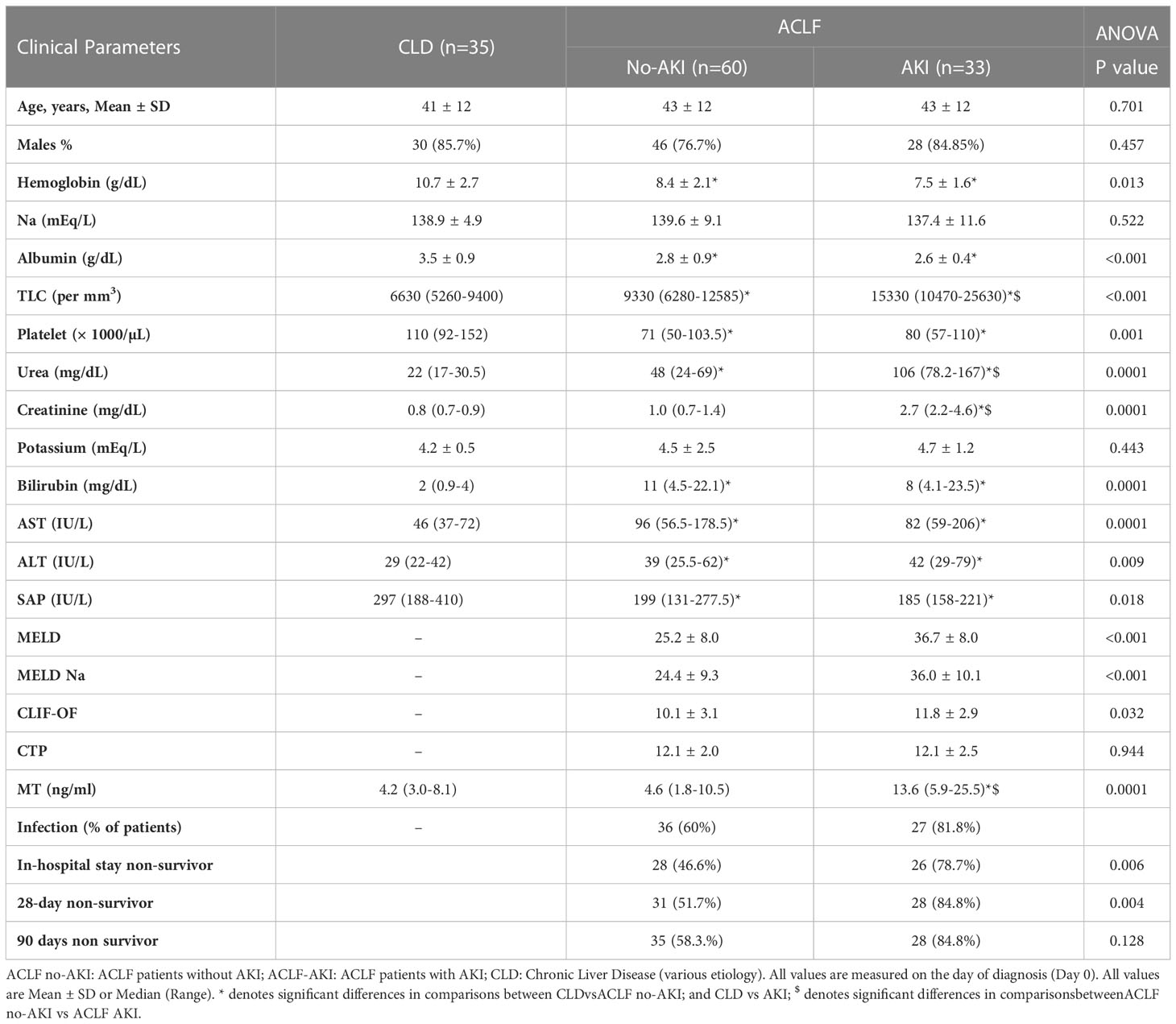
For the validation studies, n=93 ACLF patients were recruited and n=36 CLD patients were recruited (Tables 3, 4). Plasma samples were collected on the day of admission (Day 0) from all study participants and prepared within 2 hours of sample collection. ACLF patients were further classified in two different ways- (i) ACLF AKI and ACLF no-AKI, based on the presence or absence of AKI on the day of admission (Table 3) or (ii) into ACLF-progression to AKI and ACLF no-progression to AKI. The latter classification was done with only those patients who were ACLF no-AKI on the day-of-admission and were followed up for the appearance of AKI within 7 days of admission (Table 4). Baseline comparison between ACLF-AKI, ACLF no-AKI and CLD showed that the mean age of patients in all the three groups were comparable (mean ages were 43 ± 12 years, 43 ± 12 years and 41 ± 12 years respectively; Table 3). A majority of the patients in all three groups were males. Urea and creatinine levels were significantly higher in ACLF-AKI vs ACLF no-AKI, as expected (median creatinine levels were 2.7 mg/dL and 1.0 mg/dL respectively, p-value ≤0.001; median urea levels were 106mg/dL and 48 mg/dL respectively, p-value ≤0.001; Table 3). TLC values were also higher in ACLF-AKI vs ACLF No-AKI (median values 15330/mm3 and 9330/mm3, respectively, p-value ≤0.001; Table 3).
ACLF progression to AKI vs ACLF no-progression to AKI
All the ACLF no-AKI patients were followed up for 7 days to evaluate the development of AKI and were retrospectively classified into ACLF no-progression to AKI (Group1) and ACLF progression to AKI (Group 2). Platelet counts (89 x103 and 50 x103 per μL, p-value ≤0.01), urea levels (31.5 mg/dL and 69 mg/dL, p-value ≤ 0.001) and creatinine levels (0.8 and 1.3 mg/dL, p-value ≤ 0.05) were found to be significantly different in ACLF progression to AKI vs ACLF no-progression to AKI respectively (Table 4).
Plasma proteomics of ACLF-progression to AKI patients vs ACLF no progression to AKI patients
The major aim of the proteomics experiment was to discover plasma proteome changes that occur prior to the onset of clinically defined AKI and can act as predictive biomarkers of AKI in ACLF. Therefore, from the cohort of ACLF patients who were enrolled into the study, we retrospectively selected 19 ACLF patients with no-AKI on the day of admission; as per the ICA-AKI criteria. Of these 19 patients (ACLF No-AKI), 9 patients did not develop AKI over the next 28 days of follow up (Group 1- ACLF no-progression to AKI) and 10 patients developed AKI within the next 7 days (Group 2- ACLF progression to AKI). The day-of-admission (Day 0) plasma of these patients were collected and subjected to label free quantitative mass spectrometry as described in methods (Figure 1A). The proteome profiles of the two groups were found to be significantly different from each other (Figures 1B, C). The proteome profiles of Groups 1 and 2 were able to separately cluster the two groups in PCA analysis (Figure 1B). About 56 differentially expressed proteins were found in Group 2 (ACLF- Progression to AKI) as compared to Group 1 (No-progression to AKI) (Figure 1C and Table 5). Pathways analysis using Metascape revealed signatures of activation of the complement system and coagulation cascade (circulatory failure), activation of metal binding pathway (novel pathway to be detected) and acute inflammatory response (Figure 1D). Among the highest upregulated plasma proteins, metallothioneins formed a prominent group; metallothionein isoforms MT1E, MT1M, MT1X and MT2 were upregulated in those ACLF patients who progressed to AKI (Tables 4, 5; Figure 1D).
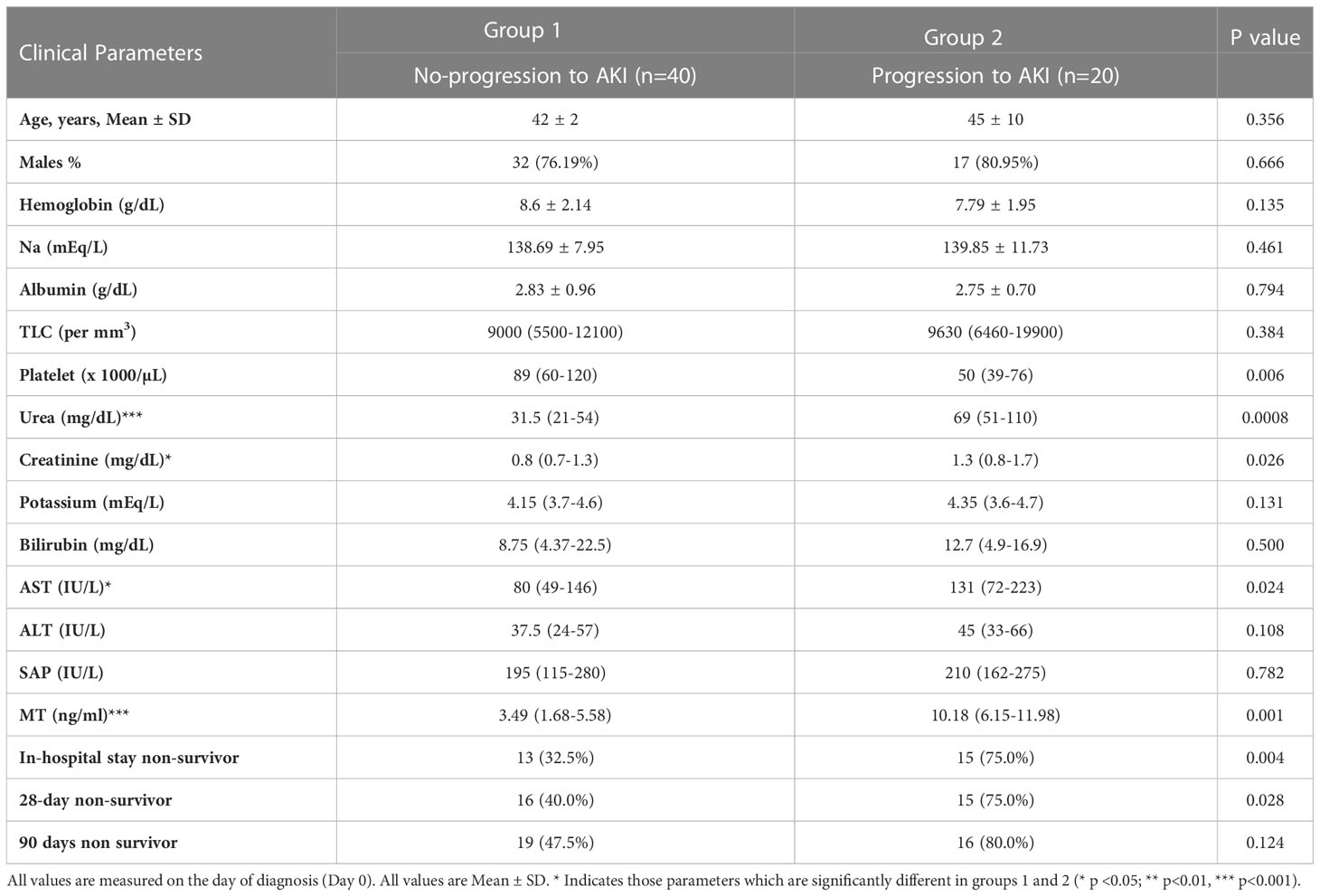
Table 4 Baseline characteristics of ACLF patients who progress to AKI and who ACLF patients who do not progress to AKI.
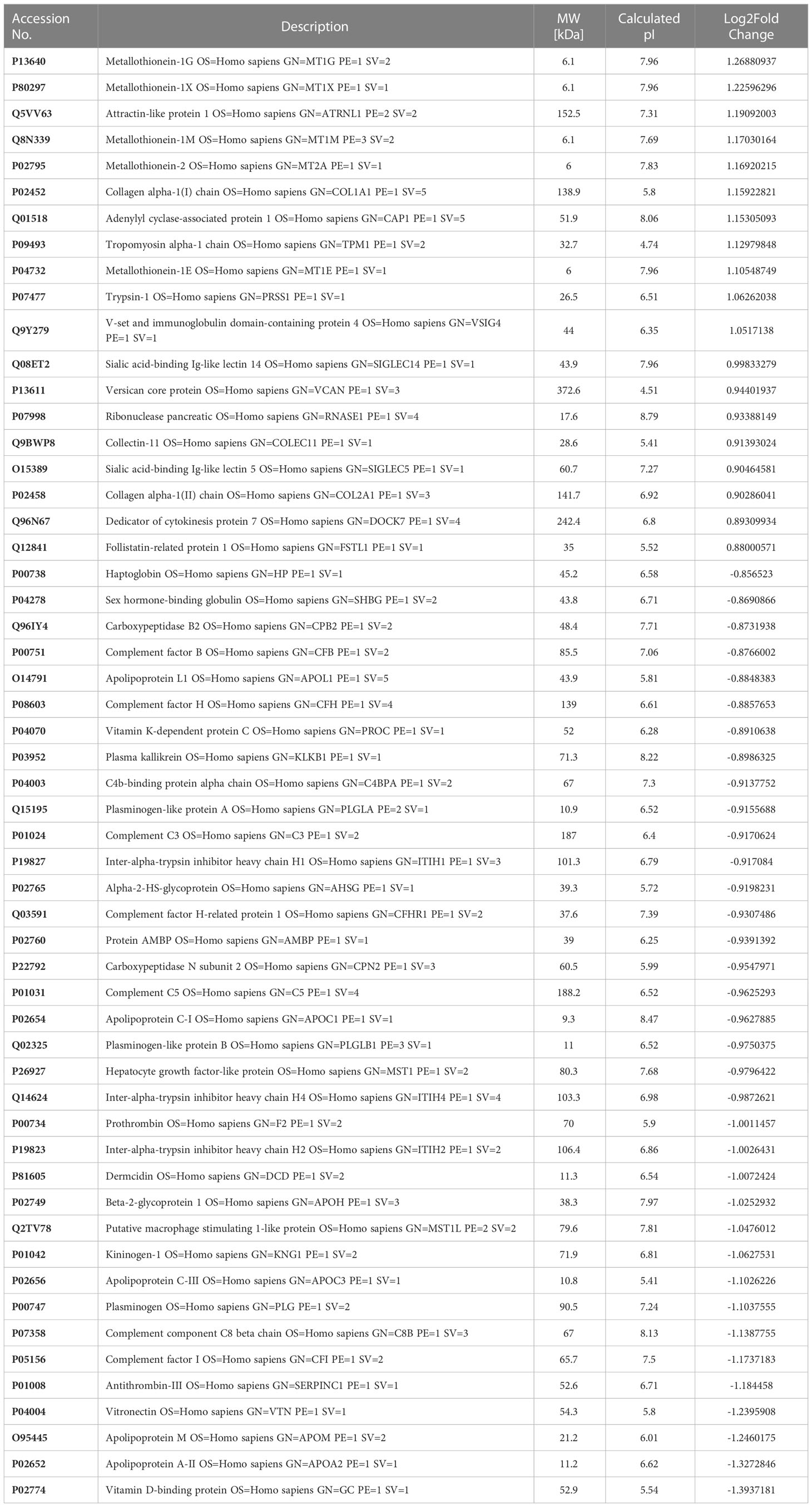
Table 5 Differentially expressed proteins in Group 2 (ACLFprogression to AKI; n=10) vs Group 1 (ACLF no-progression to AKI; n=9).
Cell type enrichment analysis revealed the presence of liver-enriched proteomic signature in the plasma proteome of ACLF patients who progressed to AKI (Supplementary Figure 1).Protein expression data from the human protein atlas (proteinatlas.org) showed that MT proteins are liver enriched genes (Figure 4B; Supplementary Figure 3, proteinatlas.org). In addition, the plasma proteomic signature for ACLF patients who progress to AKI revealed a close resemblance with DisGeNet signatures associated with acute kidney injury (AKI, Supplementary Figure 2).
Plasma metallothionein levels in ACLF patients who progress to AKI
ELISA based quantification confirmed that in ACLF-AKI, plasma MT levels (MT1 + MT2) were higher than in CLD patients (p-value ≤ 0.0001; Figure 2A). Further, plasma MT levels were higher in ACLF -AKI vs ACLF No-AKI (p-value≤ 0.0001) and in ACLF progression to AKI vs ACLF no-progression to AKI (p-value≤ 0.001; Figures 2A, B). A Spearman’s rank correlation matrix revealed that plasma MT levels correlated significantly with blood total leukocyte count TLC (coefficient: 0.296, p-value = 0.004), serum creatinine (coefficient: 0.412, p-value <0.0001), serum urea (coefficient: 0.359, p-value <0.0001), serum alkaline phosphatase SAP (coefficient: 0.243, p-value = 0.019) and estimated GFR (coefficient: -0.359, p-value <0.0001) (Supplementary Table 2). The eGFR was calculated by the CKD-EPI formula (18). The area under the receiver operating characteristics curves (AUROC) for ACLF-AKI vs ACLF no-AKI was 0.786 (95% C.I. 0.66 - 0.85; p-value≤ 0.001) and for ACLF progression to AKI vs ACLF no-progression to AKI was 0.7888 (95% C.I. 0.64-0.91, p-value≤ 0.001) (Figures 2C, D and Table 6). It was observed that MT and Urea (AUROC 0.78; 95% C.I. 0.65 to 0.91, p-value=0.0004) had similar predictive power for AKI progression whereas, platelet (AUROC 0.727, 95% C.I. 0.588 to 0.866, p-value = 0.004) and creatinine (AUROC 0.69, 95% C.I. 0.53 to 0.84, p-value=0.02) had comparatively lower predictive power (Supplementary Figure 4).The AUROC for other clinical scores were- (a) for AKI vs no-AKI: MELD {0.8495; 95% C.I(0.76087- 0.92408) p-value ≤0.0001}; MELD-Na {0.8261; 95% C.I. (0.73746-0.91771) p-value≤ 0.0001}; CLIF-OF {0.6586; 95% C.I. (0.52561-0.79151); p-value =0.0265}; CTP {0.5441; 95% C.I. (0.38865-0.66407) p-value =0.7032} (Figure 2C) and, (b) for progression to AKI vs no-progression to AKI: MELD {0.6941; 95% C.I. (0.59038- 0.87673) p-value =0.0037}; MELD-Na {0.6724; 95% C.I. (0.6141 to 0.8675) p-value =0.0028}; CLIF-OF {0.6382; 95% C.I. (0.44083-0.83557) p-value 0.16}; CTP {0.6755; 95% C.I. (0.52519-0.86455) p-value 0.0397} (Figure 2D). A discriminatory cut-off of 5.4 ng/mL (78.8% sensitivity and 58% specificity) was determined for ACLF-AKI vs no-AKI; and a discriminatory cut-off of 5.83 ng/mL (80% specificity and 80% sensitivity) was determined for ACLF progression to AKI vs no-progression to AKI (Table 6). Univariate and multivariate analysis (adjusted for age, gender, ACLF Grade and infection) were carried out in order to calculate the odds ratio (OR) of having AKI and of progression to AKI with these cut-offs. The OR of having AKI, based on a cut-off of 5.4 ng/mL was 4.38 (univariate; 95% C.I. 1.7-11.3) and (adjusted; C.I. 5.3 (1.3-21.3) (Table 7). The OR of ACLF patients progressing to AKI within 7 days of admission, based on a cut-off of 5.83 ng/mL was 18.9 (univariate; C.I. 4.8-73.9) and 76.0 (adjusted, C.I. 1.67-3459) (Table 7).
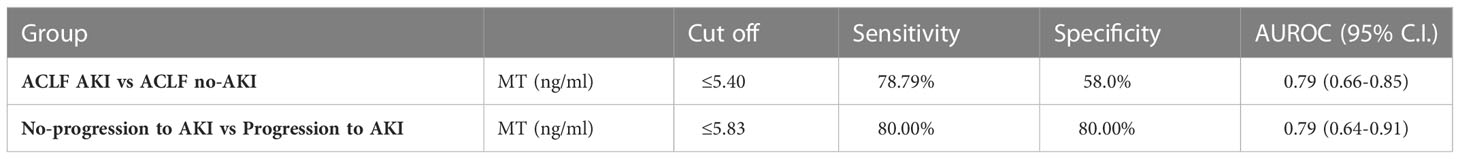
Table 6 AUROC analysis results of (a) ACLF AKI vs ACLF no-AKI and, (b) Progression to AKI vs No-progression to AKI.
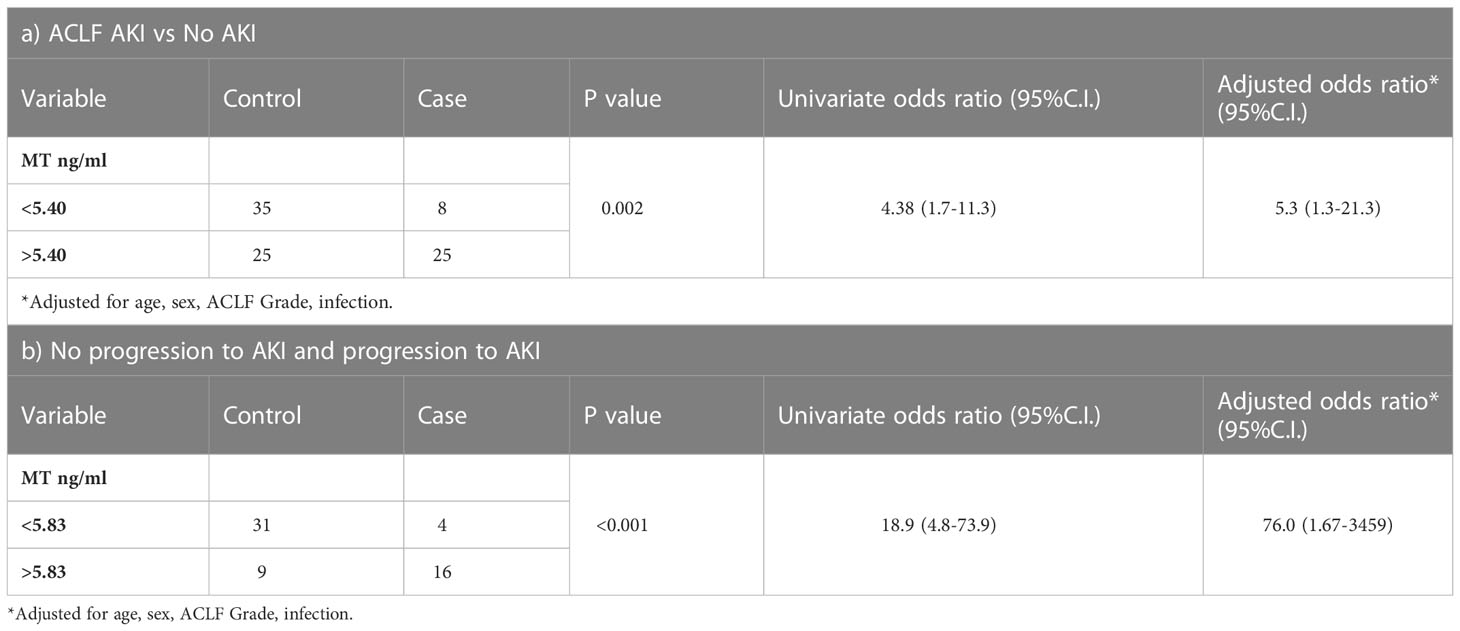
Table 7 Multiple logistic regression analysis to discriminate between (a) AKI vs no AKI and (b) progression of AKI in ACLF patients without AKI, based MT levels (ng/ml) cut-off.
A Kaplan-Meier survival analysis was carried out for metallothionein levels (MT) in 20 ACLF patients who progressed to AKI within a follow up period of 7 days from admission (Table 8). The cut off of 5.83 ng/ml was obtained from AUROC analysis for ACLF patients who progress to AKI v/s patients with no progression to AKI. The survivor function (no-progression to AKI) was more likely for ACLF patients with MT levels < 5.83 ng/ml, whereas ACLF patients with MT levels >5.83 had a higher chance of developing AKI by day 7 (p-value <0.0001) (Table 8).
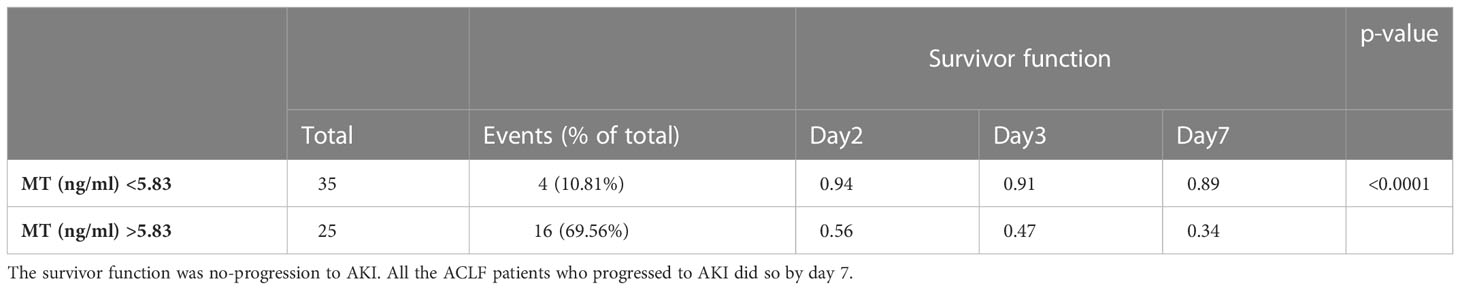
Table 8 Kaplan Meier analysis for metallothionein levels (MT) (cut-off 5.83 ng/mL) in the 20 ACLF patients who progress to AKI within a follow up period of 7 days.
In order to evaluate whether the induction of MT was due to an excess of metal ions, plasma elemental analysis was carried out by ICP-MS, as described in the methods. A small proportion of ACLF-AKI patients showed elevated Ni levels in the plasma as compared to ACLF no-AKI and CLD (p-value= 0.06), whereas Fe levels were found to be highest in CLD patients, and lowest in ACLF-AKI (p-value = 0.018); (Figure 3A and Supplementary Figure 5). None of the other metals estimated showed significant differences between ACLF AKI and ACLF no-AKI or in ACLF vs CLD (Figures 3B-D). Plasma Fe levels in ACLF no-AKI and ACLF-AKI patients neither correlate with any of the blood parameters (Supplementary Table 3).
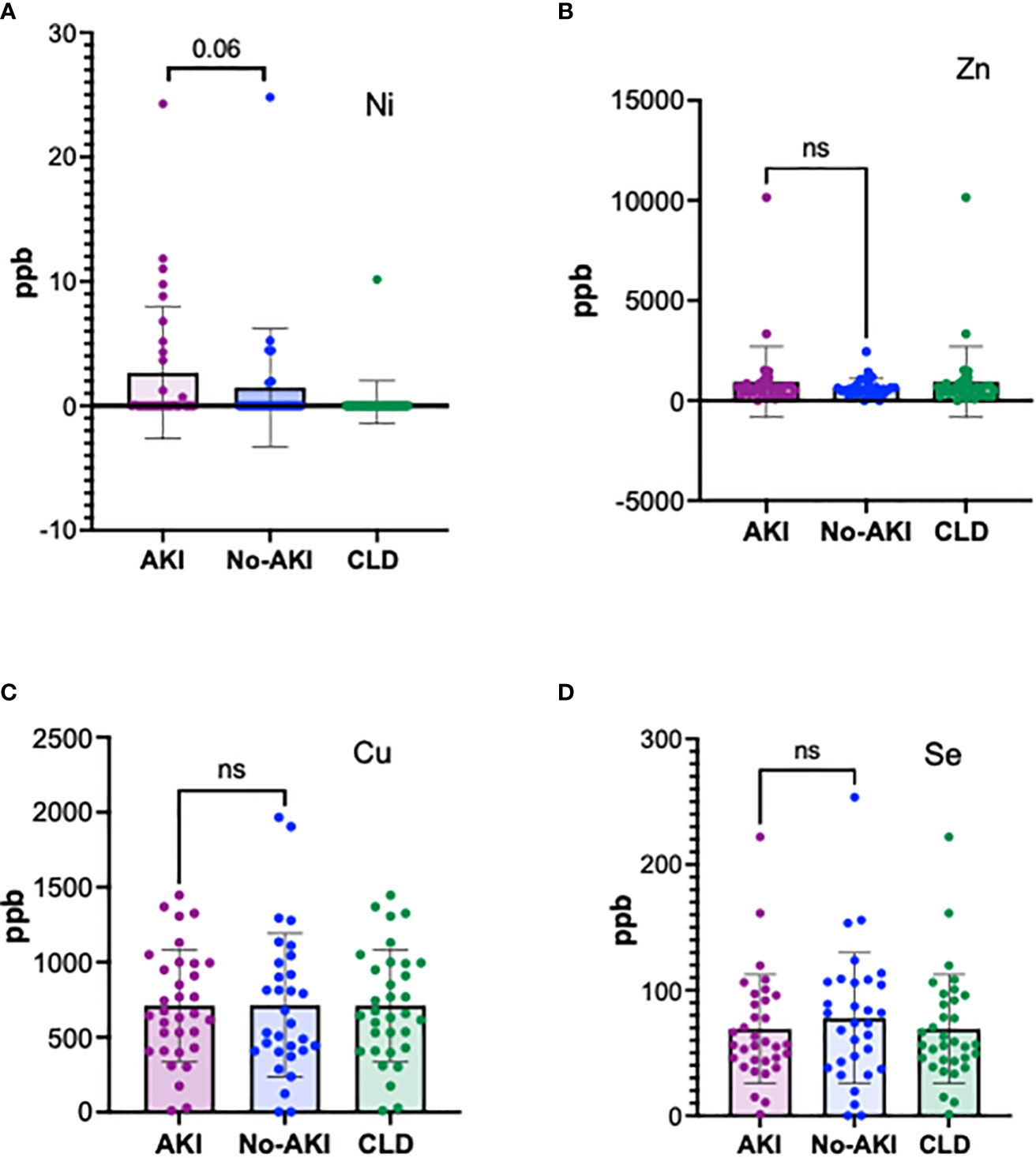
Figure 3 Plasma elemental analysis by ICP-MS for (A) Ni, (B) Zn, (C) Cu and (D) Se. The levels of the metal ions are not significantly different in ACLF patients with and without AKI showing that heavy metal toxicity is not the cause for MT induction in ACLF. A fraction of ACLF-AKI patients had elevated levels of Ni (A). ns = non-significant.
MT2 structure (downloaded from PDB) has 4 cysteine residues that coordinate with metal ions (https://www.rcsb.org/structure/1MHU; Figure 4A) (18, 20). The tissue protein expression graphs for all the isoforms of human MT were downloaded from human protein atlas (https://www.proteinatlas.org/api/search_download.php?search=metallothionein&columns=g,gd,pe&compress=no&format=tsv) and all MT were found to be liver enriched, although many were also expressed by other tissues (representative graphs Supplementary Figure 3 and Figure 4B, proteinatlas.org) (16, 17). The protein atlas derived expression data for MT receptor megalin showed enrichment of megalin in the human proximal convoluted tubule epithelial cells (RPTECS) (Figure 4B, inset) (17). In order to examine if the ACLF liver or kidney could be potential sources of MT, we carried out PCR analysis for MT1 and MT2 genes with cDNA derived from post-mortem unmatched liver biopsy (n=7) and kidney biopsy (n=8) ACLF patients (Supplementary Table 1 and Supplementary Figure 6). Among all the isoforms, low levels of expression of MT1A, MT1E, and MT1X were observed in both liver and kidney. Robust expression of the MT2 gene was observed in ACLF liver as well as kidney biopsies and MT3 gene expression was detectable in some of the liver and kidney samples from post mortem biopsies of liver and kidney samples. However, an analysis of MT peptides detected in the plasma proteome suggested the presence of MT2 as the major circulating isoform (Supplementary Figure 7). The scavenger receptor megalin binds several plasma proteins and a major ligand is albumin. As expected, in our study groups, serum albumin (Alb) levels were found to be significantly lower in ACLF vs CLD plasma (p-value ≤ 0.0001; Figure 4C). Ratio of MT to Alb (MT/Alb) concentrations were calculated in order to analyze the relative proportions of the two megalin ligands in circulation. MT/Alb was found to be significantly higher in ACLF vs CLD (p-value ≤0.01, Figure 4D), in ACLF-AKI vs ACLF no-AKI; (p-value ≤ 0.0001; Figure 4E) as well as in ACLF progression to AKI vs no progression to AKI, p-value≤ 0.0001; Figure 4F).
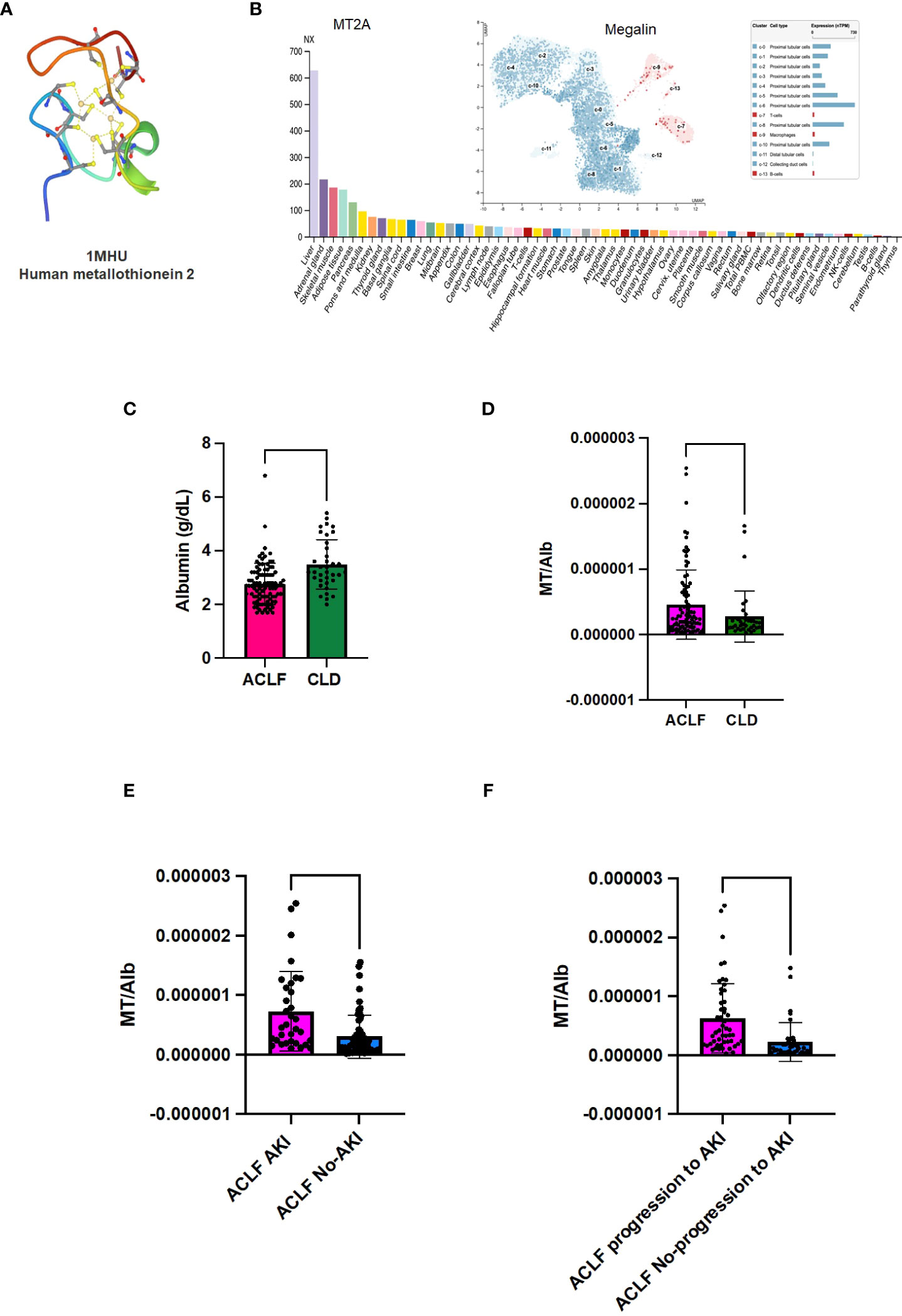
Figure 4 Metallothionein (MT) to Albumin (Alb) ratio in ACLF patients. Metallothioneins and Albumin are both ligands for the renal proximal convoluted epithelial cell (RPTEC) receptor Megalin. (A) PDB structure of MT2A; (B) MT2A expression in various tissues shows that it is a liver enriched gene; (B, inset) Expression data for megalin, the scavenger receptor that binds MT, shows that it is expressed at high levels in renal PCT cells; (C) Serum albumin (Alb) levels are significantly lower in ACLF-AKI patients compared to ACLF no-AKI; MT/Alb is significantly higher in (D) ACLF vs CLD; (E) ACLF AKI vs ACLF no-AKI and (F) ACLF patients who progress to AKI vs ACLF patients who do not progress to AKI. (**** p-value ≤ 0.0001, ** p-value ≤ 0.01).
Discussion
Acute kidney injury (AKI) is a defining feature of ACLF and significantly increases the risk of short-term mortality in ACLF patients (4, 21). Currently there are no biomarkers for the prediction of AKI in ACLF patients, and the pathogenesis of AKI in ACLF is poorly characterized. The gold standard for renal function measurement is the quantitative estimation of serum urea and creatinine levels (22). However these are neither very reliable nor sensitive biomarkers for renal function since their levels are influenced by multiple factors such as muscle mass, muscle injury, fluid therapy, and GFR (23).Using quantitative proteomics approaches combined with ELISA based validation, our study demonstrated that the metallothionein (MT) family of proteins are highly upregulated in day-of-admission plasma samples of ACLF patients at risk of developing AKI, prior to clinical onset of AKI. In addition, plasma MT concentrations were also significantly higher in ACLF patients who had AKI on the day-of-admission (ACLF-AKI) as compared to those without AKI on the day-of-admission (ACLF no-AKI).Further, we defined a cut-off of 5.83 ng/mL for plasma MT concentration, above which the odds of progression to AKI was 18.9 (C.I. 4.8-73.9) times in univariate analyses and ~70 (C.I. 1.67-3459) times in multivariate analysis adjusted for age, sex and the presence of sepsis, which is a common occurrence in ACLF. While the p-value of the OR calculations were significant (p≤ 0.001), the large C.I. suggested a larger spread of data points. An increase in sample size is likely to reduce the C.I.AUROC was also calculated for other clinical scores commonly used in evaluating patient outcomes. While MELD and MELD-Na performed well in discriminating AKI at admission vs no-AKI, their performance in the prediction of AKI was poorer than plasma metallothionein levels (Figures 2C, D and Tables 6, 7). This suggested that existing clinical scores may be good at diagnosing AKI but may not be good predictors of AKI whereas plasma metallothionein levels may be useful for the prediction as well as discrimination of AKI. Further, in our analysis, the performance of urea in predicting progression to AKI was comparable to MT (Supplementary Figure 4). But keeping in mind, the many variables influencing the levels of urea in blood (23) as mentioned above, MT may be a better predicitive parameter.Plasma MT levels also correlated weakly with urea, creatinine and eGFR, but not with liver function parameters, suggesting that MT may be a marker of kidney injury (Supplementary Table 2). In Kaplan-Meier analysis for time-to-AKI with the cut-off of 5.83 ng/mL plasma MT concentration, patients with MT values higher than the cut-off had a higher probability of progression to AKI by day 7 (p-value ≤0.0001). This suggested that plasma MT concentrations measured on day-of-admission might be a useful indicator of a progression to AKI in ACLF patients (Table 8).
MT are small (~6 kDa) thiol-rich pleiotropic proteins that associate with multiple other proteins and participate in various processes that affect cellular stress and cell death (24, 25). In humans, the MT gene family is comprised of 14 genes located on chromosome 16q13-22 which can be broadly classified in 4 groups- MT1, MT2, MT3 and MT4 (26). The MT family of proteins is expressed by several tissues but is enriched in the liver tissue, as evidenced by the expression data extracted from human protein atlas. Necrosis or necroptosis of hepatocytes or hepatocytes under inflammatory stress have been shown to release MT into the circulation (24). We found expression of the MT1 and MT2 genes in post-mortem liver and kidney biopsies from ACLF patients, suggesting that potential sources of the elevated plasma MT in ACLF could be either liver, kidney or even other cells and tissues that may be under inflammatory stress in ACLF (Supplementary Figure 6). However, proteome analysis suggested that the major circulating isoform in ACLF was MT2 (Supplementary Figure 7).The relevance of elevated plasma MT in ACLF-AKI patients, interpreted in the context of their reported functions in immunomodulation and renal tissue physiology, are discussed below.
Regulation of MT expression
MT gene expression can be regulated by several factors such as toxic heavy metals, reactive oxygen species, different types of stress and cell type specific transcription factors (24, 25).Since heavy metal toxicity has been previously reported to be a cause of renal injury (27, 28), we assessed the levels of heavy metal ions in the plasma of ACLF patients. Published studies show that metallothioneins bind different divalent heavy metals with differing affinities in which higher affinity metals can replace lower affinity metal ions. The metal binding affinity for divalent metal ions has been shown to follow a hierarchy e.g., Hg(II) >Ag(I) ≫ Cu(I) > Cd(II) >Zn(II) (29). Analyses with human liver show that adult human liver MT predominantly contains Zn, with lower amounts of Cu and Cd whereas human fetal liver derived MT mainly bound Cu (30–32). Human MT isoforms derived from other tissues have been found to be bound to Cu, Zn or Cd, Co and Ni bound MT have also been described (33).
Detailed plasma elemental analysis for 7 different heavy metals (Cu, Zn, Ni, Cd, Hg, As, Se) ruled out the role of heavy metal poisoning as a cause for MT induction and consequent nephrotoxicity in our cohort of ACLF patients. Metal ion levels in the plasma of ACLF-AKI vs ACLF- no AKI were not found to be significantly different. However, plasma Ni levels were more frequent in ACLF as compared to CLD (12 of 93 ACLF patients tested has elevated plasma Ni levels) suggesting that at least in a small proportion of patients, elevated Ni levels may be a possible cause for nephrotoxicity. Most of the genes encoding for MT isoforms are under the control of metal response element (MRE)-binding transcription factor-1(MTF-1) which is a Zn finger binding protein (34). Similarly most of the other transcription factors that control MT gene expression are also Zn finger proteins (35). Over a period of time it has become clear that MT genes are regulated by both- Zn concentration and cellular redox stress (36). In fact, MT have been shown to bind to hydroxyl free radicals with a 340-fold higher rate constant as compared to glutathione (37, 38). In fact, it has been shown that three of the major neutrophil derived oxidative species hydrochlorous acid, superoxide, and peroxide, cause the release of Zn(II) from MT (38). Several recently published studies show that neutrophil responses are a major component and driver of ACLF related pathogenesis (3, 39). In ACLF, neutrophils have been shown to be highly expanded as reflected by their heightened neutrophil-to-lymphocyte ratio (NLR) which also has a prognostic significance (40). Therefore, we think that the induction of MT in ACLF patients, particularly in those that develop ACLF-AKI is a consequence of increased oxidative stress resulting from innate immune activation, particularly neutrophils.
MT and effect on reactive oxygen species
MT modulate the activity of cytotoxic ROS in two different ways- (i) intracellular MT acts as a ROS scavenger, thereby preventing cellular damage due to free radicals whereas, (ii) extracellular MT, such as the elevated plasma MT observed in our study, stimulates ROS production by innate immune cells like macrophages (24, 25). This suggests that elevated plasma MT could be promoting the innate immune pathogenesis driving ACLF, and this process may be more efficient or higher in magnitude in the fraction of ACLF patients who develop AKI. This is corroborated by several independent studies that show that ACLF patients have elevated levels of oxidative stress and ROS markers in the plasma and macrophages and neutrophils derived from ACLF patients have higher ROS generation (41, 42).Independent studies also show that ROS accumulation is a risk factor for mortality in AKI in general (43).This suggests that extracellular MT-induced ROS generation by macrophages and neutrophils in ACLF might be a potential mechanism by which cellular damage occurs in ACLF patients, thereby increasing the risk of AKI.
MT-Megalin interaction in RPTEC
MT can also act as a ligand for the scavenger endocytic receptor megalin (44, 45). Human protein atlas derived expression data shows that megalin expression is maximum in renal proximal convoluted tubular epithelial cells (RPTEC) (Figure 4B; proteinatlas.org) (16, 17,).Under normal circumstances, megalin binds to several different protein ligands in addition to MT, such as albumin, folate binding protein and vitamin D binding protein, to facilitate their re-uptake by the RPTEC (46, 47). All megalin ligands compete with each other for binding (48). Excessive ligand binding that leads to megalin overload in RPTEC can lead to cytotoxicity and cell death (49). In ACLF, serum albumin levels decrease due to liver failure, as reported in literature (50). Serum albumin levels were lower in ACLF patients as compared to CLD (p-value ≤0.0001) in our study,as expected. We calculated MT/Albumin ratio (MT/Alb) in order to investigate the relationship between the two liver-derived megalin ligands. In our study the MT/Alb was significantly elevated in ACLF patients as compared to CLD patients (p-value ≤0.01). ACLF-AKI patients had significantly higher MT/Alb ratio compared to ACLF no-AKI patients (p-value ≤ 0.0001) as well as in ACLF patients who progressed to AKI vs those who did not (p-value ≤ 0.0001). These data suggest that in ACLF, the decrease in albumin concentrations is accompanied by several folds increase in MT concentrations. Therefore, MT is a major ligand for megalin binding on RPTEC in ACLF. MT has been shown to be a chemotactic factor, causing entry of leukocytes at sites of tissue stress or injury (51). Therefore,the elevation of plasma MT and, subsequent increase in its concentration at RPTECs due to megalin binding, may lead to localized tissue inflammation leading to upregulation of inflammatory markers such as TLR4 and IL-6 as reported in literature.
While further studies are needed to elucidate the specific molecular interactions that govern AKI development in ACLF, the more immediate application of our results lie in the fact that plasma MT levels might enable the prediction of AKI in ACLF patients on the day-of-admission and understanding the dynamic changes that occur in ACLF patients prior to the onset of clinical AKI might be the key to understanding the pathogenesis of AKI in ACLF.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by 1. All India Institute of Medical Sciences, New Delhi ethics committee [Reference No. IEC/473/9/2016 and, IEC/369/7/2016], 2. All India Institute of Medical Sciences, New Delhi ethics committee the Institute Ethics Committee (Ref. No.IEC/687/8/2019). The patients/participants provided their written informed consent to participate in this study.
Author contributions
PA: Conceptualization, data curation, formal analysis, experimental investigation, project administration, resources, validation, supervision, writing of manuscript, funding acquisition. RS, JQ, SaS: data curation, formal analysis, experimental investigation, project administration, writing of manuscript. MK, HS: formal analysis. NG: investigation. AS: critical inputs into manuscript. ShS, PP, S: data curation, formal analysis, project administration, resources. All authors contributed to the article and approved the submitted version.
Funding
The work carried out in this study was funded by the BioCARE grant from the Department of Biotechnology, Government of India [Grant no.BT/PR18574/BIC/101/151]. RS was supported by a fellowship from the Indian Council of Medical Research [Fellowship no. JRF-2016/HRD/LS/98/4080].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1041230/full#supplementary-material
References
1. Amin A, Mookerjee RP. Acute-on-chronic liver failure: definition, prognosis and management. Frontline Gastroenterol (2020) 11(6):458–67. doi: 10.1136/flgastro-2018-101103
2. Kumar D, Vadiraja PK, Nayak B, Thakur B, Das P, Datta Gupta S, et al. Acute on chronic liver failure because of acute hepatic insults: etiologies, course, extrahepatic organ failure and predictors of mortality. J Gastroenterol Hepatol (2016) 31(4):856–64. doi: 10.1111/jgh.13213
3. Saha R, Pradhan SS, Das P, Mishra P, Singh R, Sivaramakrishnan V, et al. Inflammatory signature in acute-on-chronic liver failure includes increased expression of granulocyte genes ELANE, MPO and CD177. Sci Rep (2021) 11(1):1–6. doi: 10.1038/s41598-021-98086-6
4. Davenport A, Sheikh MF, Lamb E, Agarwal B, Jalan R. Acute kidney injury in acute-on-chronic liver failure: where does hepatorenal syndrome fit? Kidney Int (2017) 92(5):1058–70. doi: 10.1016/j.kint.2017.04.048
5. Shah N, Montes de Oca M, Jover-Cobos M, Tanamoto KI, Muroi M, Sugiyama KI, et al. Role of toll-like receptor 4 in mediating multiorgan dysfunction in mice with acetaminophen induced acute liver failure. Liver Transplant (2013) 19(7):751–61. doi: 10.1002/lt.23655
6. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology (2013) 144(7):1426–37. doi: 10.1053/j.gastro.2013.02.042
7. Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the international club of ascites. Gut (2015) 64(4):531–7. doi: 10.1136/gutjnl-2014-308874
8. Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. bmj (2020), 370. doi: 10.1136/bmj.m2687
9. Jiang QQ, Han MF, Ma K, Chen G, Wan XY, Kilonzo SB, et al. Acute kidney injury in acute-on-chronic liver failure is different from in decompensated cirrhosis. World J Gastroenterol (2018) 24(21):2300. doi: 10.3748/wjg.v24.i21.2300
10. Maiwall R, Kumar S, Chandel SS, Kumar G, Rastogi A, Bihari C, et al. AKI in patients with acute on chronic liver failure is different from acute decompensation of cirrhosis. Hepatol Int (2015) 9(4):627–39. doi: 10.1007/s12072-015-9653-x
11. Piano S, Schmidt HH, Ariza X, Amoros A, Romano A, Hüsing-Kabar A, et al. Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin Gastroenterol Hepatol (2018) 16(11):1792–800. doi: 10.1016/j.cgh.2018.01.035
12. Kedia S, Mahapatra SJ, Nayak B, Gunjan D, Thakur B, Acharya SK. Severity and outcome of acute-on-Chronic liver failure is dependent on the etiology of acute hepatic insults. J Clin Gastroenterol (2017) 51(8):734–41. doi: 10.1097/MCG.0000000000000823
13. Gustot T, Moreau R. Acute-on-chronic liver failure vs. traditional acute decompensation of cirrhosis. J Hepatol (2018) 69(6):1384–93. doi: 10.1016/j.jhep.2018.08.024
14. D’Amico G, Morabito A, D’Amico M, Pasta L, Malizia G, Rebora P, et al. Clinical states of cirrhosis and competing risks. J Hepatol (2018) 68(3):563–76. doi: 10.1016/j.jhep.2017.10.020
15. Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun (2019) 10(1):1–0. doi: 10.1038/s41467-019-09234-6
16. Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science (2015) 347(6220):1260419. doi: 10.1126/science.1260419
17. Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based human protein atlas. Nat Biotechnol (2010) 28(12):1248–50. doi: 10.1038/nbt1210-1248
18. Levey AS, Coresh J. Chronic kidney disease. Lancet (2012) 379(9811):165–80. doi: 10.1016/S0140-6736(11)60178-5
19. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. Jama (2013) 310(20):2191–4. doi: 10.1001/jama.2013.281053
20. Messerle BA, Schäffer A, Vašák M, Kägi JH, Wüthrich K. Three-dimensional structure of human [113Cd7] metallothionein-2 in solution determined by nuclear magnetic resonance spectroscopy. J Mol Biol (1990) 214(3):765–79. doi: 10.1016/0022-2836(90)90291-S
21. Napoleone L, Solé C, Juanola A, Ma AT, Carol M, Pérez-Guasch M, et al. Patterns of kidney dysfunction in acute-on-chronic liver failure: Relationship with kidney and patients’ outcome. Hepatol Commun (2022). doi: 10.1002/hep4.1963
22. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet (2019) 394(10212):1949–64. doi: 10.1016/S0140-6736(19)32563-2
23. Salazar JH. Overview of urea and creatinine. Lab Med (2014) 45(1):e19–20. doi: 10.1309/LM920SBNZPJRJGUT
24. Lynes MA, Zaffuto K, Unfricht DW, Marusov G, Samson JS, Yin X. The physiological roles of extracellular metallothionein. Exp Biol Med (2006) 231(9):1548–54. doi: 10.1177/153537020623100915
25. Dai H, Wang L, Li L, Huang Z, Ye L. Metallothionein 1: A new spotlight on inflammatory diseases. Front Immunol (2021) 12:739918. doi: 10.3389/fimmu.2021.739918
26. Cornelis R, Nordberg M. General chemistry, sampling, analytical methods, and speciation. In: Handbook on the toxicology of metals. Elsevier (2007). p. 11–38.
27. Fischer RS, Unrine JM, Vangala C, Sanderson WT, Mandayam S, Murray KO. Evidence of nickel and other trace elements and their relationship to clinical findings in acute mesoamerican nephropathy: A case-control analysis. PloS One (2020) 15(11):e0240988. doi: 10.1371/journal.pone.0240988
28. Wills MR, Brown CS, Bertholf RL, Ross R, Savory J. Serum and lymphocyte, aluminum and nickel in chronic renal failure. Clin Chim Acta (1985) 145(2):193–6. doi: 10.1016/0009-8981(85)90286-4
29. IH DH. Metallothionein. Annu Rev Biochem (1986) 55:9–13. doi: 10.1146/annurev.bi.55.070186.004405
30. Bühler RH, Kägi JH. Human hepatic metallothioneins. FEBS Lett (1974) 39(2):229–34. doi: 10.1016/0014-5793(74)80057-8
31. Porter H. The particulate half-cystine-rich copper protein of newborn liver. relationship to metallothionein and subcellular localization in non-mitochondrial particles possibly representing heavy lysosomes. Biochem Biophys Res Commun (1974) 56(3):661–8. doi: 10.1016/0006-291x(74)90656-1
32. Ryden L, Deutsch HF. Preparation and properties of the major copper-binding component in human fetal liver. Its Identification as Metallothionein J Biol Chem (1978) 253(2):519–24. doi: 10.1016/S0021-9258(17)38240-6
33. Vasak M, Kaegi JH, Holmquist B, Vallee BL. Spectral studies of cobalt (II)-and nickel (II)-metallothionein. Biochemistry (1981) 20(23):6659–64. doi: 10.1021/bi00526a021
34. Westin G, Schaffner W. A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J (1988) 7(12):3763–70. doi: 10.1002/j.1460-2075.1988.tb03260.x
35. Krezel A, Maret W. The bioinorganic chemistry of mammalian metallothioneins. Chem Rev (2021) 121(23):14594–648. doi: 10.1021/acs.chemrev.1c00371
36. Thornalley PJ, Vašák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta (BBA)-Protein Structure Mol Enzymol (1985) 827(1):36–44. doi: 10.1016/0167-4838(85)90098-6
37. Sato M, Bremner I. Oxygen free radicals and metallothionein. Free Radical Biol Med (1993) 14(3):325–37. doi: 10.1016/0891-5849(93)90029-T
38. Fliss H, Ménard M. Oxidant-induced mobilization of zinc from metallothionein. Arch Biochem Biophys (1992) 293(1):195–9. doi: 10.1016/0003-9861(92)90384-9
39. Weiss E, de la Grange P, Defaye M, Lozano JJ, Aguilar F, Hegde P, et al. Characterization of blood immune cells in patients with decompensated cirrhosis including ACLF. Front Immunol (2021) 11:619039. doi: 10.3389/fimmu.2020.619039
40. Sun J, Guo H, Yu X, Zhu H, Zhang X, Yang J, et al. A neutrophil-to-lymphocyte ratio-based prognostic model to predict mortality in patients with HBV-related acute-on-chronic liver failure. BMC Gastroenterol (2021) 21(1):1–4. doi: 10.1186/s12876-021-02007-w
41. Tian Z, Yao N, Wu Y, Wang F, Zhao Y. Association between plasma level of superoxide dismutase and survival of patients with acute-on-chronic liver failure. BMC Gastroenterol (2022) 22(1):1–0. doi: 10.1186/s12876-022-02126-y
42. Khanam A, Trehanpati N, Riese P, Rastogi A, Guzman CA, Sarin SK. Blockade of neutrophil’s chemokine receptors CXCR1/2 abrogate liver damage in acute-on-chronic liver failure. Front Immunol (2017) 8:464. doi: 10.3389/fimmu.2017.00464
43. Dennis JM, Witting PK. Protective role for antioxidants in acute kidney disease. Nutrients (2017) 9(7):718. doi: 10.3390/nu9070718
44. Klassen RB, Crenshaw K, Kozyraki R, Verroust PJ, Tio L, Atrian S, et al. Megalin mediates renal uptake of heavy metal metallothionein complexes. Am J Physiology-Renal Physiol (2004) 287(3):F393–403. doi: 10.1152/ajprenal.00233.2003
45. Onodera A, Tani M, Michigami T, Yamagata M, Min KS, Tanaka K, et al. Role of megalin and the soluble form of its ligand RAP in cd-metallothionein endocytosis and cd-metallothionein-induced nephrotoxicity in vivo. Toxicol Lett (2012) 212(2):91–6. doi: 10.1016/j.toxlet.2012.05.012
46. Nielsen R, Christensen EI, Birn H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int (2016) 89(1):58–67. doi: 10.1016/j.kint.2015.11.007
47. Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol (2002) 3(4):258–67. doi: 10.1038/nrm778
48. Caruso-Neves C, Pinheiro AA, Cai H, Souza-Menezes J, Guggino WB. PKB and megalin determine the survival or death of renal proximal tubule cells. Proc Natl Acad Sci (2006) 103(49):18810–5. doi: 10.1073/pnas.0605029103
49. Motoyoshi Y, Matsusaka T, Saito A, Pastan I, Willnow TE, Mizutani S, et al. Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney Int (2008) 74(10):1262–9. doi: 10.1038/ki.2008.405
50. Bernardi M, Ricci CS, Zaccherini G. Role of human albumin in the management of complications of liver cirrhosis. J Clin Exp Hepatol (2014) 4(4):302–11. doi: 10.1016/j.jceh.2014.08.007
Keywords: ACLF, AKI, proteomics, biomarkers, metallothionein, inflammation
Citation: Acharya P, Saha R, Quadri JA, Sarwar S, Khan MA, Sati HC, Gauniyal N, Shariff A, Swaroop S, Pathak P and Shalimar (2023) Quantitative plasma proteomics identifies metallothioneins as a marker of acute-on-chronic liver failure associated acute kidney injury. Front. Immunol. 13:1041230. doi: 10.3389/fimmu.2022.1041230
Received: 10 September 2022; Accepted: 30 December 2022;
Published: 26 January 2023.
Edited by:
Nirupma Trehanpati, The Institute of Liver and Biliary Sciences (ILBS), IndiaReviewed by:
Albrecht Piiper, University Hospital Frankfurt, GermanyZhao Huang, Sichuan University, China
Copyright © 2023 Acharya, Saha, Quadri, Sarwar, Khan, Sati, Gauniyal, Shariff, Swaroop, Pathak and Shalimar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pragyan Acharya, cHJhZ3lhbi5hY2hhcnlhQGFpaW1zLmVkdQ==
†These authors have contributed equally to this work
 Pragyan Acharya
Pragyan Acharya Rohini Saha
Rohini Saha Javed Ahsan Quadri
Javed Ahsan Quadri Saba Sarwar2
Saba Sarwar2 Shalimar
Shalimar