- 1Department of Hematology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin, China
- 2Clinical Testing Center, Chinese Academy of Medical Sciences Blood Disease Hospital, Chinese Academy of Medical Sciences Institute of Hematology, State Key Laboratory of Experimental Hematology, National Clinical Medical Center for Blood Disease, Tianjin, China
- 3Department of Oncology, Second Hospital of Tianjin Medical University, Institute of Urology, Tianjin, China
- 4Department of Lymphoma, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center of Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, The Sino‐US Center for Lymphoma and Leukemia Research, Tianjin, China
- 5Department of Medical Oncology, Tianjin First Central Hospital, School of Medicine, Nankai University, Tianjin, China
Background: We aimed to compare the efficacy of chimeric antigen receptor T (CAR-T) cell therapy with that of autologous stem cell transplantation (auto-HSCT) in relapsed/refractory diffuse large B cell lymphoma (R/R DLBCL).
Research design and methods: We searched eligible publications up to January 31st, 2022, in PubMed, Cochrane Library, Springer, and Scopus. A total of 16 publications with 3484 patients were independently evaluated and analyzed using STATA SE software.
Results: Patients who underwent CAR-T cell therapy showed a better overall response rate (ORR) and partial response (PR) than those treated with auto-HSCT (CAR-T vs. auto-HSCT, ORR: 80% vs. 73%, HR:0.90,95%CI:0.76-1.07,P = 0.001; PR: 20% vs. 14%, HR:0.65,95%CI:0.62-0.68,P = 0.034). No significant difference was observed in 6-month overall survival (OS) (CAR-T vs. auto-HSCT, six-month OS: 81% vs. 84%, HR:1.23,95%CI:0.63-2.38, P = 0.299), while auto-HSCT showed a favorable 1 and 2-year OS (CAR-T vs. auto-HSCT, one-year OS: 64% vs. 73%, HR:2.42,95%CI:2.27-2.79, P < 0.001; two-year OS: 54% vs. 68%, HR:1.81,95%CI:1.78-1.97, P < 0.001). Auto-HSCT also had advantages in progression-free survival (PFS) (CAR-T vs. auto-HSCT, six-month PFS: 53% vs. 76%, HR:2.81,95%CI:2.53-3.11,P < 0.001; one-year PFS: 46% vs. 61%, HR:1.84,95%CI:1.72-1.97,P < 0.001; two-year PFS: 42% vs. 54%, HR:1.62,95%CI:1.53-1.71, P < 0.001). Subgroup analysis by age, prior lines of therapy, and ECOG scores was performed to compare the efficacy of both treatment modalities.
Conclusion: Although CAR-T cell therapy showed a beneficial ORR, auto-HSCT exhibited a better long-term treatment superiority in R/R DLBCL patients. Survival outcomes were consistent across different subgroups.
1 Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of lymphoma in adults worldwide. It accounts for 30–40% of newly diagnosed non-Hodgkin lymphomas (NHLs) annually, with an increasing incidence (1, 2). Although combination chemotherapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) serves as the backbone of treatment, up to 40% of patients experience treatment failure or inevitable relapse (3, 4). Patients with relapsed/refractory (R/R) DLBCL have particularly poor outcomes (5, 6).
High-dose chemotherapy with autologous stem cell transplantation (auto-HSCT) is a promising therapeutic avenue and standard of care for patients with R/R DLBCL (7). In 1995, a randomized trial of auto-HSCT (PARMA) first demonstrated the benefit of transplantation in patients with R/R DLBCL (8). In recent years, a prospective, multicenter phase II clinical trial reported that the estimated overall survival (OS) and progression-free survival (PFS) rates were 63% and 61%, respectively, three years after auto-HSCT, with a median follow-up of 31.0 months ((EudraCT) N. 2007-003198-22) (9). In addition, auto-HSCT has led to satisfactory outcomes in older adults. The European Blood and Marrow Transplantation (EBMT) registry demonstrated a three-year OS of 60% after auto-HSCT in older patients (10). The Canadian Cancer Trials Group(CCTG) LY.12 study indicated that the older group gained similar benefits from auto-HSCT compared to the younger group, with an acceptable safety profile (NCT00078949) (11).
As a newly developed cell immunotherapy, chimeric antigen receptor T (CAR-T) cell therapy continues to be a suitable treatment option and has shown remarkable achievements in B-cell malignancies (12, 13). In TRANSCEND NHL 001, CAR-T cell therapy resulted in a considerable objective response rate (ORR), with a low incidence of serious cytokine release syndrome and neurological events (NCT02631044) (14). An open-label, multicenter, international phase II study reported an overall response rate (ORR) of 52% and complete response (CR) rate of 40% after infusion of CAR-T products (NCT02445248) (15). In a phase I-II ZUMA-1 study with CAR-T cell therapy, 82% of patients had an objective response and 58% achieved CR (NCT02348216) (16). In ZUMA-7, the median event-free survival (EFS) in the CAR-T group was 8.3 months and the estimated event-free survival at 24.0 months was 41% (95% CI, 33%–48%) (NCT03391466) (17). Different anti-CD19 CAR-T cell infusion products have been used in patients with R/R DLBCL. Axicabtagene ciloleucel (axi-cel) was the first product approved in 2017, followed by tisagenlecleucel (tisa-cel) in 2018 and lisocabtagene maraleucel (liso-cel) in 2021 (18–20). CAR-T cell therapy exhibited a remarkable efficacy and acceptable safety profile. It has been successfully used for patients with R/R DLBCL and is rapidly becoming the standard of care (21).
According to the Center for International Blood & Marrow Transplant Research(CIBMTR) study, a decreasing number of patients in America are choosing auto-HSCT after salvage treatment in the CAR-T era (22, 23). The application of auto-HSCT or CAR-T cell therapy has been considered in patients with R/R DLBCL (24). According to a comparative study, CAR-T cell therapy exhibited a superior clinical outcome over auto-HSCT for efficacy (NCT03196830) (CAR-T vs. auto-HSCT, one-year OS 74.4% vs. 44.5%, P = 0.044) (25). In contrast, improved survival was reported in the auto-HSCT group vs. the CAR-T group in a large retrospective study (CAR-T vs. auto-HSCT, two-year OS 69% vs. 47%, P = 0.004) (26). Similarly, according to a BELINDA clinical trial, no apparent survival benefit was observed in CAR-T cell therapy (NCT03570892, median EFS 3.3 months vs. 3.0 months, P = 0.61). So far, no universal agreement in the option between auto-HSCT or CAR-T cell therapy in R/R DLBCL has been reached. Therefore, our study aimed to compare the efficacy of auto-HSCT with CAR-T cell therapy for R/R DLBCL.
2 Methods
2.1 Literature search
The search strategy developed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines is shown in Figure 1A. Eligible studies, with the latest update on January 31, 2022, were searched for using the PubMed, Cochrane Library, Springer, and Scopus databases. We combined the search terms DLBCL/diffuse large B-cell lymphoma, CAR-T/chimeric antigen receptor T, and ASCT/autologous stem cell transplantation/HCT to identify potential studies without language restrictions.
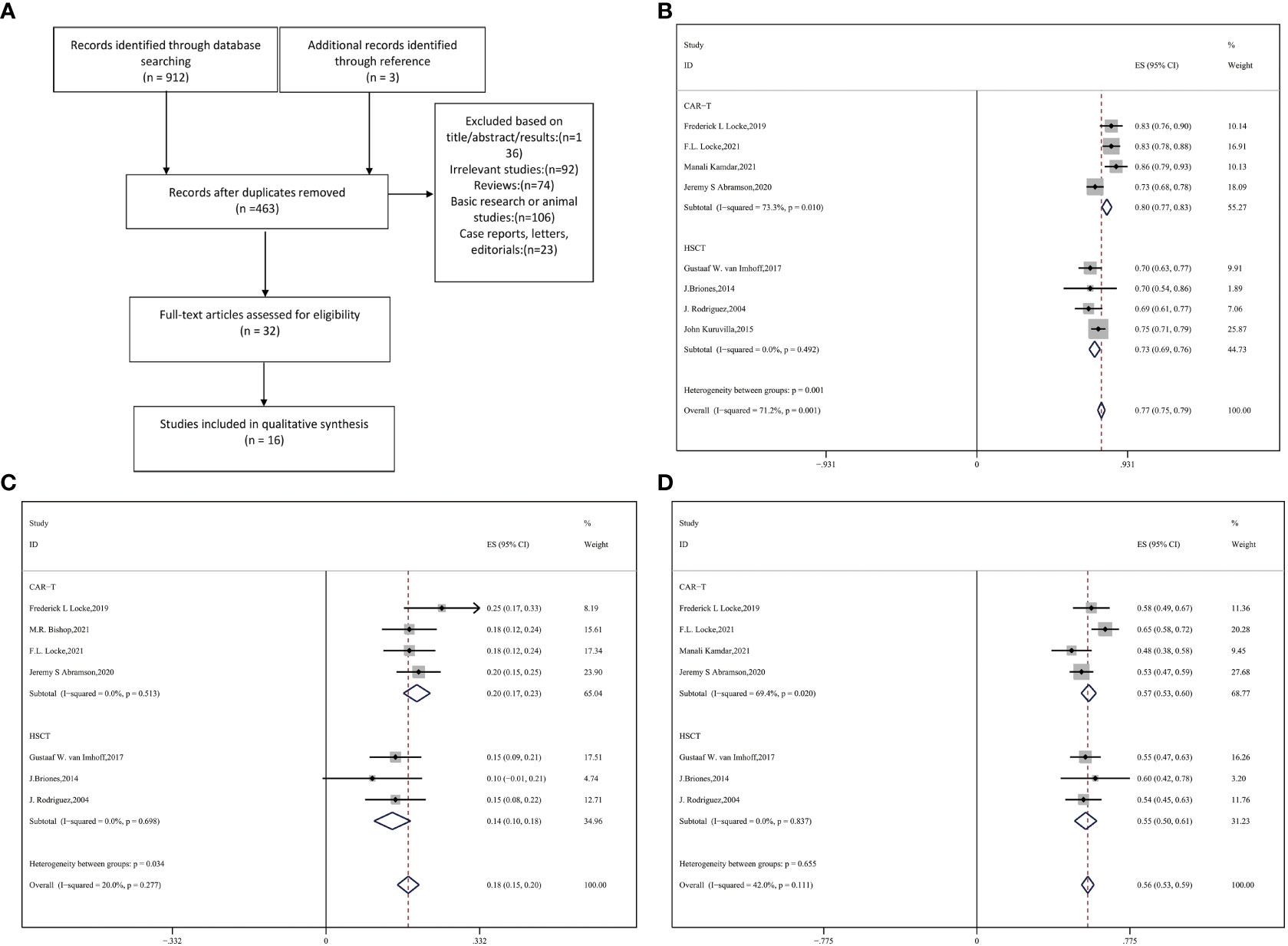
Figure 1 (A) Flow diagram of selecting eligible studies Forest plot of (B) ORR (C) PR (D) CR between CAR-T and auto-HSCT groups.
2.2 Inclusion and exclusion criteria
Studies were included if they met the following criteria (1): prospective clinical trials or retrospective studies; (2) patients with R/R DLBCL (diagnoses were rendered according to the WHO 2016 classification); (3) patients treated with CAR-T cell therapy or auto-HSCT; and (4) studies that reported at least one of the following outcome measures: ORR, CR, partial response (PR), OS, and PFS.
The exclusion criteria were as follows: (1) studies that were reviews, viewpoints, perspectives, or correspondences; (2) lack of effective data on the outcomes mentioned above; (3) basic research or animal studies; and (4) duplicate publications.
2.3 Study qualitative assessment and bias risk
As both randomized clinical trials (RCT) and nonrandomized clinical trials were involved, we adopted the Methodological Index for Non-randomized Studies (MINORS) to assess the quality of the included studies. Each item was scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate) (27). We conducted the Begg’s and Egger’s tests to evaluate publication bias.
2.4 Data collection
The following information was collected and extracted by different authors independently: first author, publication year, study design, number of enrolled patients and those who received treatment, median age, and efficacy outcomes (ORR, CR, PR, OS, and PFS). The response rate was evaluated at 6-8 weeks after CAR-T cell or auto-HSCT therapy. The ORR was defined as the combined percentage of patients who had a complete or PR according to the Lugano classification. OS was defined as the time from treatment to death from any cause. PFS was defined as the time from treatment to the first date of disease progression (28). Conventional chemotherapy regimens included rituximab (R-ICE, R-GDP), and lymphodepleting chemotherapy was administered before CAR-T cell therapy and auto-HSCT. Disputes were addressed by a third reviewer or through group discussion.
2.5 Statistical analysis
We used STATA SE software (version 12.0; StataCorp, College Station, TX, USA) to analyze the therapeutic efficacy and safety. Additionally, the I² statistic was used to test for heterogeneity. A fixed-effects model was used to calculate the pooled effects when I² < 50%. Otherwise, a random effects model was adopted. We performed stratified analysis and explored the sources of heterogeneity. Statistical significance was set at P < 0.05.
3 Results
The flowchart in Figure 1A depicts the search process. A total of 912 studies were accessible from the different databases used, of which 452 duplicates were excluded. At the same time, another three studies available in the references were considered relevant. Based on the exclusion criteria, 16 publications with 3484 total patients enrolled were ultimately included (9, 14–17, 26, 29–38). Eventually, 1067 patients with R/R DLBCL who received anti-CD19 CAR-T cells and 2417 patients who underwent auto-HSCT were evaluated.
As illustrated in Table 1, the included studies were published between 2004 and 2021. Eligible patients had R/R DLBCL (defined as a relapse or progressive or stable disease as the best response to the most recent therapy). The median age of the enrolled patients ranged from 40 to 64 years in both the groups. Most patients had stage III or IV disease and had received several previous lines of systemic therapy (Table 1). The median follow-up of the included studies ranged from 6.2 months to 55.2 months. The primary outcome was the response rate (ORR, CR, and PR), and the secondary outcomes were OS and PFS.
Overall, the included studies were reliable, according to the MINORS scale. The detailed scores are listed in Table 1. Begg’s and Egger’s tests were performed to evaluate the bias more precisely. Despite the limited number of studies involved in assessing publication bias, there was no evident publication bias for response rate(Begg’s test P =0.902; Egger’s test P =0.803), OS(Begg’s test P =0.276; Egger’s test P =0.210), and PFS(Begg’s test P =1.000; Egger’s test P =0.513) in our evaluation (Figure S1).
3.1 Response rate
Eight studies with 1402 patients in total reported ORR and response extent (CR and PR) (9, 16, 17, 25, 29, 30, 34, 36). Compared with auto-HSCT, CAR-T cell therapy performed significantly better in terms of ORR and PR (CAR-T vs. auto-HSCT, ORR: 80% vs. 73%, HR:0.90,95%CI:0.76-1.07,P = 0.001; PR: 20% vs. 14%, HR:0.65,95%CI:0.62-0.68,P = 0.034) (Figures 1B, C). However, no significant difference in CR was observed between the two groups (CAR-T vs. auto-HSCT, CR: 57% vs. 55%, HR:0.92,95%CI:0.842-0.986, P = 0.655) (Figure 1D).
3.2 OS
Twelve studies involving 2672 patients were involved in the appraisal of short- and long-term OS (9, 14, 16, 17, 26, 31–33, 35–38). We pooled all of them and analyzed the OS. The results are shown in Figures 2A-C (CAR-T vs. auto-HSCT, six-month OS: 81% vs. 84%, HR:1.23,95%CI:0.63-2.38, P=0.310 (Figure 2A); one-year OS: 64% vs. 73%, HR:2.42,95%CI:2.27-2.79, P < 0.001; two-year OS: 54% vs. 68%, HR:1.81,95%CI:1.78-1.97, P < 0.001). This finding indicates that even though no significant differences in short-term OS were observed between the CAR-T and auto-HSCT groups, patients who underwent auto-HSCT benefited from long-term OS.
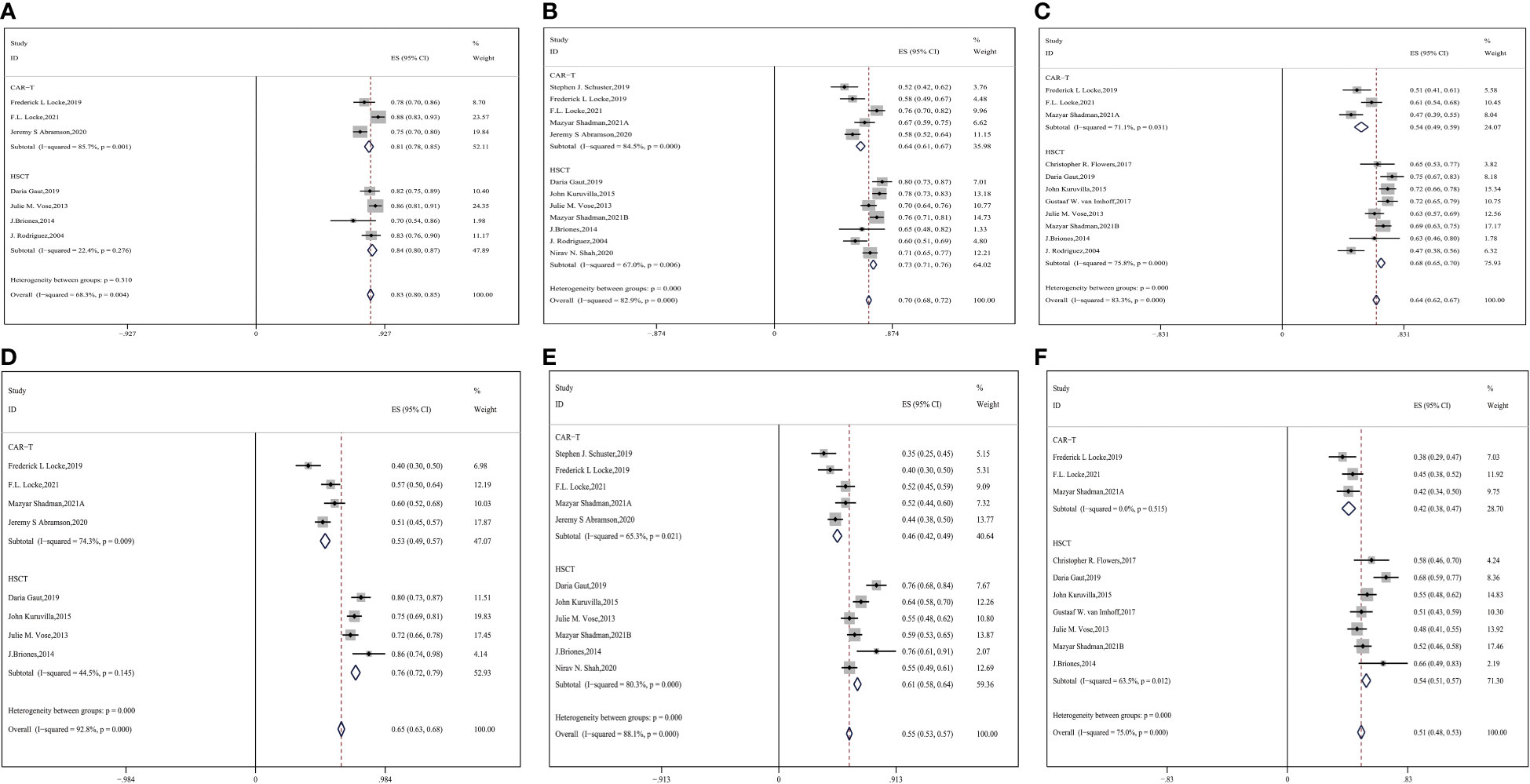
Figure 2 Forest plot of OS between CAR-T and auto-HSCT groups (A) 6-month OS (B) 1-year OS (C) 2-year OS Forest plot of PFS between CAR-T and auto-HSCT groups (D) 6-month PFS (E) 1-year PFS (F) 2-year PFS.
3.3 PFS
Twelve studies with 2632 patients were assessed for PFS, and Figure 2 shows the meta-analysis forest diagram of PFS (9, 14–17, 26, 31–35, 38). Auto-HSCT showed benefits at six months, one year, and two years of PFS compared with the CAR-T cell group (CAR-T vs. auto-HSCT, six-month PFS: 53% vs. 76%, HR:2.81,95%CI:2.53-3.11,P < 0.001; one-year PFS: 46% vs. 61%, HR:1.84,95%CI:1.72-1.97,P < 0.001; two-year PFS: 42% vs. 54%, HR:1.62,95%CI:1.53-1.71, P < 0.001) (Figures 2D–F). This finding indicates that patients who underwent auto-HSCT were more likely to have a better PFS than those who underwent CAR-T cell therapy.
3.4 Subgroup analysis
3.4.1 Subgroup analysis by age
Subgroup analysis by age showed that among the mean age of 50–60 groups, the ORR was higher in CAR-T cell groups and CR was approximately equivalent to each other (CAR-T vs. auto-HSCT, ORR: 84% vs. 70%, HR:0.45,95%CI:0.39-0.51,P < 0.001; CR: 59% vs. 56%, HR:0.88,95%CI:0.82-0.94,P = 0.448) (Figures 3A, B). Auto-HSCT showed an advantage in OS and PFS over the CAR-T cell group (CAR-T vs. auto-HSCT, two-year OS: 54% vs. 69%, HR:1.90, 95%CI:1.74-2.08, P < 0.001; two-year PFS: 42% vs. 54%, HR:1.62,95%CI:1.53-1.72, P < 0.001) (Figures 3C, D).
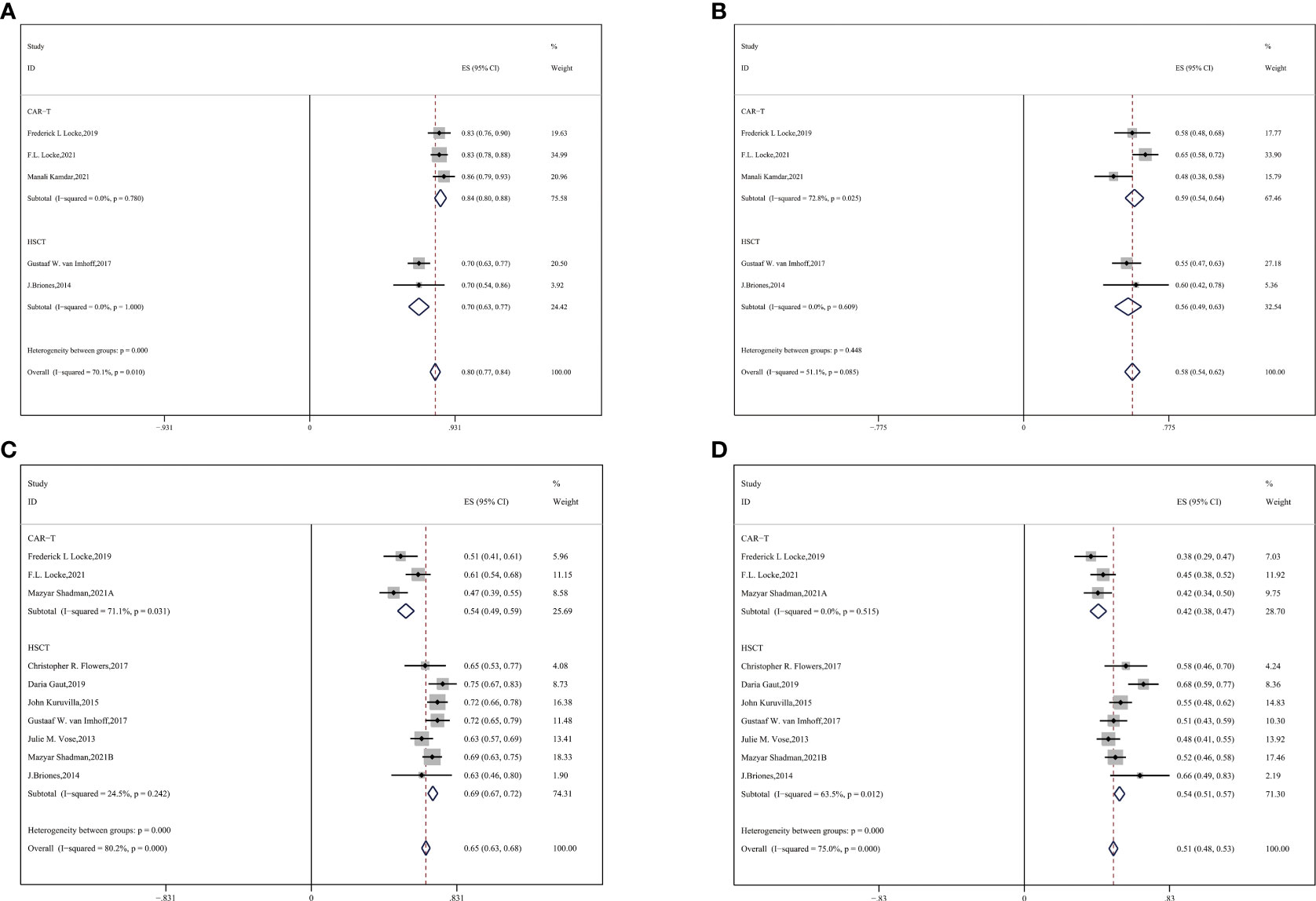
Figure 3 Subgroup analyses by age between CAR-T and auto-HSCT groups (A) ORR (B) CR (C) 2-year OS (D) 2-year PFS.
3.4.2 Subgroup analysis by previous therapeutic lines
Most patients with R/R DLBCL had previously undergone a couple of systematic therapeutic lines, so we performed a subgroup analysis and observed an advantage of two-year OS and PFS in the auto-HSCT group compared with the CAR-T group. For median previous therapies of no more than three (≤3) lines, more enrolled patients with R/R DLBCL gained benefits of survival in the auto-HSCT vs. CAR-T cell group (CAR-T vs. auto-HSCT, two-year OS: 49% vs. 70%, HR:2.43,95%CI:2.03-2.86, P < 0.001; two-year PFS: 40% vs. 54%, HR:1.76,95%CI:1.67-1.86, P < 0.001) (Figures 4A, B).
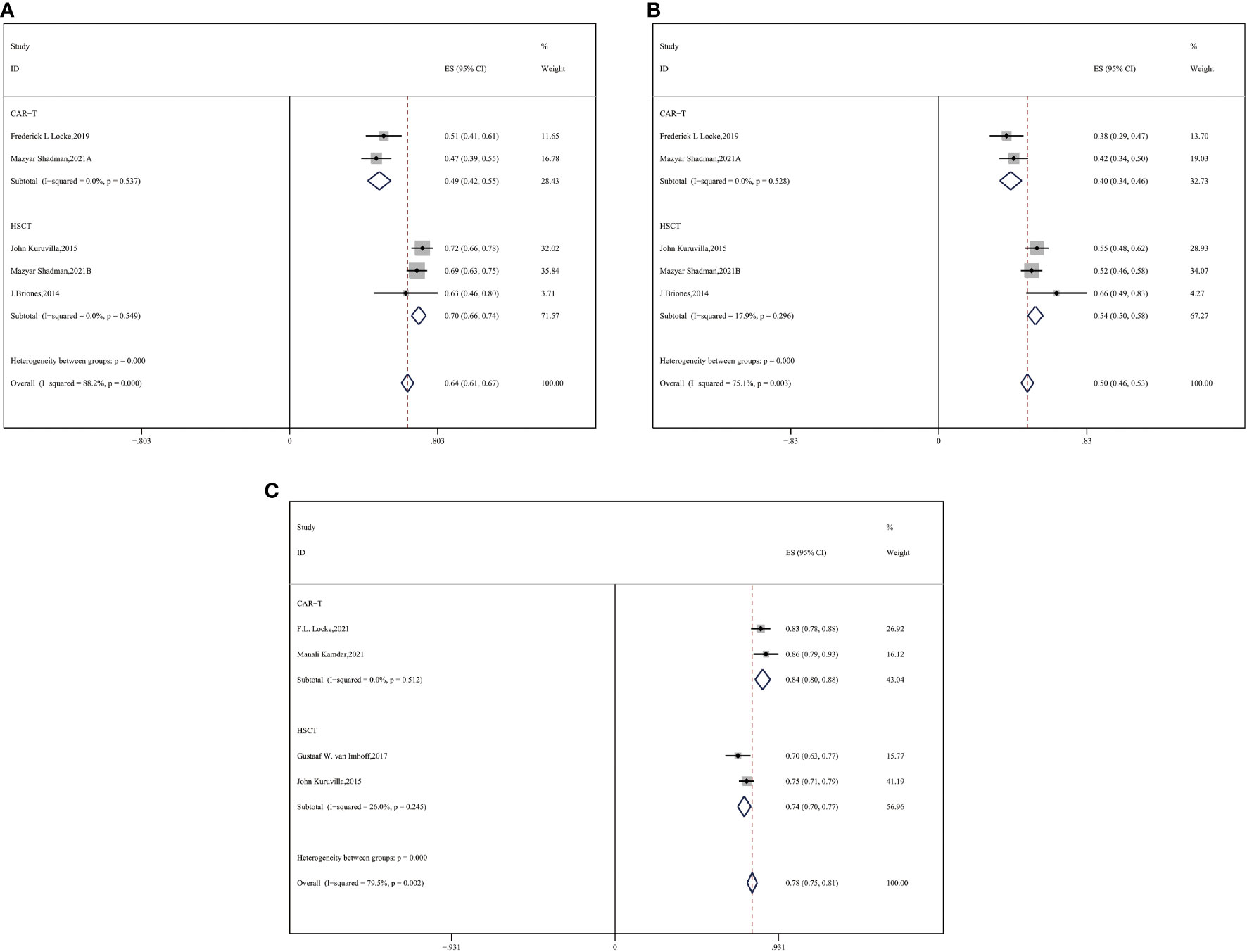
Figure 4 Subgroup analysis by previous therapeutic lines between CAR-T and auto-HSCT groups (A) 2-year OS (B) 2-year PFS (C) ORR.
We compared the ORR between the subgroups of auto-HSCT and CAR-T cell therapy as second-line treatment. CAR-T cell therapy resulted in a superior ORR for the auto-HSCT group (CAR-T vs. auto-HSCT, ORR: 84% vs. 74%, HR:0.54,95%CI:0.47-0.61, P < 0.001) (Figure 4C).
3.4.3 Subgroup analysis by ECOG scores
Subsequently, we performed subgroup analysis of patients with ECOG scores ranging from 0–2. In this subgroup analysis, a higher ORR was observed in the CAR-T cell group (CAR-T vs. auto-HSCT, ORR: 79% vs. 70%, HR:0.62,95%CI:0.54-0.70, P = 0.019) (Figure 5A). No significant difference was observed in CR between the two groups (53% vs. 56%, HR:1.13,95%CI:1.06-1.19, P = 0.531) (Figure 5B). In our study, CAR-T cell therapy showed an improved ORR and a similar PR compared to the auto-HSCT group.
Survival outcomes were consistent across the subgroups described above. CAR-T cell therapy showed a favorable response rate, whereas auto-HSCT exhibited promising long-term survival.
4 Discussion
DLBCLs are a heterogeneous group of aggressive B-cell neoplasms with different clinical prognoses (39, 40). Patients with R/R DLBCL have poor prognosis despite a variety of salvage therapies. The recent NCCN guidelines recommend high-dose chemotherapy supported by auto-HSCT for patients with R/R DLBCL eligible for transplant (41). The advent of CAR-T cell therapy has reduced the number of patients undergoing auto-HSCT. Currently, CAR-T cell therapy and auto-HSCT are recommended for patients with R/R DLBCL. We compared the efficacy of CAR-T cell therapy and auto-HSCT in patients with R/R DLBCL. In our study, although CAR-T cell therapy was associated with a higher initial remission rate, auto-HSCT showed superior long-term survival.
A comparison between pivotal clinical trials and CAR-T cell products indicated an ORR ranging from 59% to 82% in patients with R/R DLBCL (42), which was better than that of auto-HSCT, as reported previously. The ORR outcomes in our study are consistent with previous observations that CAR-T cell therapy results in a better ORR. As previously confirmed in ZUMA-1, a high CAR-T cell peak concentration in the first 28 days after infusion contributed to a remarkable ORR in the CAR-T cell group (16).
A retrospective study of the EBMT center reported a five-year OS of 63% and five-year disease free survival of 48% after auto-HSCT (43). Since CAR-T cell therapy was developed in recent years and has been recommended as a novel agent for R/R DLBCL, it has been shown to achieve satisfactory efficacy and has attracted increasing attention in this field (44). In 2021, two-year survival outcomes of randomized phase III data determined the optimal second-line therapy (BELINDA, ZUMA-7, and TRANSFORM clinical trials) (17, 29, 30). In 2022, a two-year follow-up retrospective study reported favorable survival following auto-HSCT (26). Long-term follow-ups of CAR-T cell therapy are yet to be performed. In our study, we analyzed survival for as long as two years and found that auto-HSCT was associated with long-term beneficial OS and PFS. Interestingly, it was reported in TRANSFORM that CAR-T cell therapy was superior to standard care, with a longer PFS (30). In the ZUMA-7 study, CAR-T cell therapy demonstrated a clinically meaningful improvement in EFS compared with standard care, which seemed to contradict our results (17). However, according to their studies, only 36% of patients in ZUMA-7 and 47% of patients in TRANSFORM eventually received auto-HSCT after high-dose chemotherapy (30). Over half of the assessable patients failed to undergo auto-HSCT, and we hypothesized that the low transplant rate was responsible for the inferior survival of standard care. No significant difference in survival was observed in BELINDA between CAR-T cell therapy and auto-HSCT, with a transplant rate of 32.5% (29). The best alternative for R/R DLBCL remains controversial, and further studies are warranted to explore the prognosis of patients with different transplant rates. In our study, all patients evaluated in the auto-HSCT group received auto-HSCT and exhibited superior two-year survival compared with the CAR-T group. Age, ECOG performance status, and prior lines of therapy are also factors for efficacy in key CAR-T cell therapy clinical trials (ZUMA-1, JULIET, and TRANSCEND) (45). The median age at diagnosis of DLBCL is in the sixties, and younger patients appear to have more options for treatment, given adequate bone marrow reserve, rapid drug metabolism, limited complications, or suitable physical function (46, 47). Similarly, patients with higher ECOG scores experienced higher mortality and a higher risk of a second relapse. A subgroup analysis of previous therapeutic lines was performed, where there was no difference in OS and PFS between auto-HSCT and CAR-T cell therapy, focusing on those with one or two prior lines of treatment. The auto-HCT group exhibited superior OS in patients with more therapeutic lines. Given the physical condition, adverse events, and treatment cost, patients with more than three median previous therapeutic lines tended to give up CAR-T cell therapy (45). Therefore, we performed stratified analysis by median previous therapies of no more than three lines and found that auto-HSCT was associated with promising long-term survival in each subgroup, which was consistent with overall outcomes. There appears to be a consensus on auto-HSCT for patients who achieve CR. In the CAR-T era, relapsed chemosensitive DLBCL patients not achieving CR following salvage therapy are increasingly receiving CAR-T cell therapy instead of auto-HSCT (48). Considering those with a baseline status of PR, auto-HSCT has the potential to bring about improved OS and a lower incidence of relapse compared to CAR-T cell therapy (26). Hence, we believe that patients should be prudent in choosing CAR-T cell therapy in lieu of auto-HSCT for the following reasons: first, it was still controversial to choose a proper treatment for R/R DLBCL patients to date (49); second, no other options are available in case of treatment failure or relapse after CAR-T cell therapy (50). In addition, the considerable economic burden of CAR-T cell therapy should be considered (51).
Three CAR-T cell products targeting the CD19 antigen on B cells, approved to date, were included in our study. Nevertheless, we were unable to separately analyze the efficacy of different anti-CD19 CAR-T cell infusion products because of the limited number of available clinical trials. Matching-adjusted indirect comparison showed similar ORR, CR, OS, and PFS between liso-cel and axi-cel, while tisa-cel was associated with a superior objective response, CR, and OS to axi-cel (52, 53). As the heterogeneity was acceptable and intergroup outcomes were similar in our study, we did not perform further analysis of CAR-T cell products. Due to the absence of direct head-to-head studies of various anti-CD19 CAR-T cell infusion products, future studies are warranted to further elucidate their relative efficacy.
To the best of our knowledge, this is the first systematic review and meta-analysis to integrate the available published data and compare the therapeutic effects of CAR-T and auto-HSCT in patients with R/R DLBCL. Patients were more likely to achieve a considerable remission rate with CAR-T cell therapy. Those who succeeded in receiving auto-HSCT showed promising long-term survival compared with CAR-T cell therapy. High CAR-T cell peak concentrations in the first 28 days after infusion accounted for a better ORR than auto-HSCT, but the longevity seemed to be worse (54). We were unable to discuss adverse events because of the scarcity of data in the auto-HSCT group. Most patients underwent high-dose chemotherapy before auto-HSCT, and it was difficult to determine whether the adverse events were caused by standard care or by auto-HSCT only. Nevertheless, our study offers some individual suggestions for patients with R/R DLBCL based on their physical condition as well as previous therapies, which are highly clinically relevant. We hope that this review will be thought-provoking and bring attention to this field as more head-to-head clinical trials comparing CAR-T cell therapy and auto-HSCT are needed.
5 Conclusion
CAR-T cell therapy and auto-HSCT are crucial treatment strategies for patients with R/R DLBCL. CAR-T cell therapy showed superior ORR and PR compared to auto-HSCT in patients with R/R DLBCL. However, no significant difference in CR was observed between the two groups. Auto-HSCT showed a better long-term OS and PFS than CAR-T cell therapy in patients with R/R DLBCL in different age groups, prior lines of therapy, and ECOG subgroups. In the absence of detailed information on the enrolled patients, we were unable to perform further subgroup analyses and explore additional prognostic factors; therefore, additional RCTs are needed. In any case, individual characteristics should be considered when choosing an appropriate treatment option.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ZZ and LL designed the study. LT, CL and YZ made the statistical plan. LT performed the key analyses. JW, SL, YJ and WW extracted the data. DX, YaL, JG and HX summarized the data. HS and YuL assisted in data interpretation and quality assessment. LT wrote the manuscript. CL revised the manuscript. JS offered constructive suggestions and made major revisions which improved the quality of the manuscript. All authors have read and approved the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (81870150), the National Natural Science Foundation of China(81670102), the Natural Science Foundation of Tianjin(16JCYBJC25200) and the National Natural Science Foundation of China(81572543), Tianjin Key Medical Discipline(Specialty) Construction Project(TJYXZDXK-009A).
Acknowledgments
We acknowledge the Pubmed, Cochrane Library, Springer and Scopus databases for providing data needed for our analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1041177/full#supplementary-material
Abbreviations
CAR-T, chimeric antigen receptor T (all of them are anti-CD19 CAR-T products in our study); HSCT, stem cell transplantation (autologous in our study); ORR, overall response rate; CR, complete response; PR, partial response; OS, overall survival; PFS, progression-free survival; NR, not related.
References
1. Wang L, Li LR, Young KH. New agents and regimens for diffuse large b cell lymphoma. J Hematol Oncol (2020) 13(1):175. doi: 10.1186/s13045-020-01011-z
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
3. Maurer MJ, Ghesquières H, Jais JP, Witzig TE, Haioun C, Thompson CA, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large b-cell lymphoma treated with immunochemotherapy. J Clin Oncol (2014) 32(10):1066–73. doi: 10.1200/JCO.2013.51.5866
4. Northend M, Wilson W, Osborne W, Fox CP, Davies AJ, El-Sharkawi D, et al. Results of a UK real world study of polatuzumab vedotin, bendamustine, and rituximab for relapsed/refractory large b-cell lymphoma. Blood Adv (2022) 6(9):2920–6. doi: 10.1182/bloodadvances.2021005953
5. Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, et al. Phase 1 results of ZUMA-1: A multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther (2017) 25(1):285–95. doi: 10.1016/j.ymthe.2016.10.020
6. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
7. Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large b-cell lymphoma in the rituximab era. J Clin Oncol (2010) 28(27):4184–90. doi: 10.1200/JCO.2010.28.1618
8. Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-hodgkin's lymphoma. N Engl J Med (1995) 333(23):1540–5. doi: 10.1056/NEJM199512073332305
9. Briones J, Novelli S, Garcia-Marco JA, Tomás JF, Bernal T, Grande C, et al. Autologous stem cell transplantation after conditioning with yttrium-90 ibritumomab tiuxetan plus BEAM in refractory non-Hodgkin diffuse large b-cell lymphoma: Results of a prospective, multicenter, phase II clinical trial. Haematologica (2014) 99(3):505–10. doi: 10.3324/haematol.2013.093450
10. Jantunen E, Canals C, Rambaldi A, Ossenkoppele G, Allione B, Blaise D, et al. Autologous stem cell transplantation in elderly patients (> or =60 years) with diffuse large b-cell lymphoma: An analysis based on data in the European blood and marrow transplantation registry. Haematologica (2008) 93(12):1837–42. doi: 10.3324/haematol.13273
11. Davison K, Chen BE, Kukreti V, Couban S, Benger A, Berinstein NL, et al. Treatment outcomes for older patients with relapsed/refractory aggressive lymphoma receiving salvage chemotherapy and autologous stem cell transplantation are similar to younger patients: A subgroup analysis from the phase III CCTG LY.12 trial. Ann Oncol (2017) 28(3):622–7. doi: 10.1093/annonc/mdw653
12. Zhou X, Tu S, Wang C, Huang R, Deng L, Song C, et al. Phase I trial of fourth-generation anti-CD19 chimeric antigen receptor T cells against relapsed or refractory b cell non-Hodgkin lymphomas. Front Immunol (2020) 11:564099. doi: 10.3389/fimmu.2020.564099
13. Logue JM, Zucchetti E, Bachmeier CA, Krivenko GS, Larson V, Ninh D, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large b-cell lymphoma. Haematologica (2021) 106(4):978–86. doi: 10.3324/haematol.2019.238634
14. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large b-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet (2020) 396(10254):839–52. doi: 10.1016/S0140-6736(20)31366-0
15. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse Large b-cell lymphoma. N Engl J Med (2019) 380(1):45–56. doi: 10.1056/NEJMoa1804980
16. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large b-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol (2019) 20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7
17. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for Large b-cell lymphoma. N Engl J Med (2021) 386(7):640–54. doi: 10.1056/NEJMoa2116133
18. Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-Care axicabtagene ciloleucel for relapsed or refractory Large b-cell lymphoma: Results from the US lymphoma CAR T consortium. J Clin Oncol (2020) 38(27):3119–28. doi: 10.1200/JCO.19.02104
19. Ali S, Kjeken R, Niederlaender C, Markey G, Saunders TS, Opsata M, et al. The European medicines agency review of kymriah (Tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse Large b-cell lymphoma. Oncologist (2020) 25(2):e321–7. doi: 10.1634/theoncologist.2019-0233
20. Ogasawara K, Dodds M, Mack T, Lymp J, Dell'Aringa J, Smith J. Population cellular kinetics of lisocabtagene maraleucel, an autologous CD19-directed chimeric antigen receptor T-cell product, in patients with Relapsed/Refractory Large b-cell lymphoma. Clin Pharmacokinet (2021) 60(12):1621–33. doi: 10.1007/s40262-021-01039-5
21. Oluwole OO, Bouabdallah K, Munoz J, De Guibert S, Vose JM, Bartlett NL, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large b-cell lymphoma. Br J Haematol (2021) 194(4):690–700. doi: 10.1111/bjh.17527
22. Dreger P, Fenske TS, Montoto S, Pasquini MC, Sureda A, Hamadani M, et al. Cellular immunotherapy for refractory diffuse Large b cell lymphoma in the chimeric antigen receptor-engineered T cell era: Still a role for allogeneic transplantation? Biol Blood Marrow Transplant (2020) 26(4):e77–85. doi: 10.1016/j.bbmt.2019.12.771
23. Kanate AS, Kumar A, Dreger P, Dreyling M, Le Gouill S, Corradini P, et al. Maintenance therapies for Hodgkin and non-Hodgkin lymphomas after autologous transplantation: A consensus project of ASBMT, CIBMTR, and the lymphoma working party of EBMT. JAMA Oncol (2019) 5(5):715–22. doi: 10.1001/jamaoncol.2018.6278
24. Sehn LH, Salles G. Diffuse Large b-cell lymphoma. N Engl J Med (2021) 384(9):842–58. doi: 10.1056/NEJMra2027612
25. Li C, Zhang Y, Zhang C, Chen J, Lou X, Chen X, et al. Comparation of CART19 and autologous stem-cell transplantation for refractory/relapsed non-hodgkin's lymphoma. JCI Insight 5 (2019). doi: 10.1172/jci.insight.130195
26. Shadman M, Pasquini M, Ahn KW, Chen Y, Turtle CJ, Hematti P, et al. Autologous transplant vs chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission. Blood (2022) 139(9):1330–9. doi: 10.1182/blood.2021013289
27. Du J, Yu D, Han X, Zhu L, Huang Z. Comparison of allogeneic stem cell transplant and autologous stem cell transplant in refractory or relapsed peripheral T-cell lymphoma: A systematic review and meta-analysis. JAMA Netw Open (2021) 4(5):e219807. doi: 10.1001/jamanetworkopen.2021.9807
28. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The lugano classification. J Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800
29. Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive b-cell lymphoma. N Engl J Med (2022) 386(7):629–39. doi: 10.1056/NEJMoa2116596
30. Kamdar M, Solomon SR, Arnason JE, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, versus standard of care (SOC) with salvage chemotherapy (CT) followed by autologous stem cell transplantation (ASCT) as second-line (2L) treatment in patients (Pts) with relapsed or refractory (R/R) Large b-cell lymphoma (LBCL): Results from the randomized phase 3 transform study. Blood (2021) 138(Supplement 1):91–1. doi: 10.1182/blood-2021-147913
31. Flowers CR, Costa LJ, Pasquini MC, Le-Rademacher J, Lill M, Shore TB, et al. Efficacy of pharmacokinetics-directed busulfan, cyclophosphamide, and etoposide conditioning and autologous stem cell transplantation for lymphoma: Comparison of a multicenter phase II study and CIBMTR outcomes. Biol Blood Marrow Transplant (2016) 22(7):1197–205. doi: 10.1016/j.bbmt.2016.03.018
32. Gaut D, Romero T, Oveisi D, Howell G, Schiller G. Disease characteristics of diffuse large b-cell lymphoma predicting relapse and survival after autologous stem cell transplantation: A single institution experience. Hematol Oncol (2020) 38(1):38–50. doi: 10.1002/hon.2690
33. Kuruvilla J, MacDonald DA, Kouroukis CT, Cheung M, Olney HJ, Turner AR, et al. Salvage chemotherapy and autologous stem cell transplantation for transformed indolent lymphoma: A subset analysis of NCIC CTG LY12. Blood (2015) 126(6):733–8. doi: 10.1182/blood-2015-01-622084
34. van Imhoff GW, McMillan A, Matasar MJ, Radford J, Ardeshna KM, Kuliczkowski K, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse Large b-cell lymphoma: The ORCHARRD study. J Clin Oncol (2017) 35(5):544–51. doi: 10.1200/JCO.2016.69.0198
35. Vose JM, Carter S, Burns LJ, Ayala E, Press OW, Moskowitz CH, et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large b-cell lymphoma: Results from the BMT CTN 0401 trial. J Clin Oncol (2013) 31(13):1662–8. doi: 10.1200/JCO.2012.45.9453
36. Rodriguez J, Caballero MD, Gutierrez A, Solano C, Arranz R, Lahuerta JJ, et al. Autologous stem-cell transplantation in diffuse large b-cell non-hodgkin's lymphoma not achieving complete response after induction chemotherapy: The GEL/TAMO experience. Ann Oncol (2004) 15(10):1504–9. doi: 10.1093/annonc/mdh391
37. Chihara D, Izutsu K, Kondo E, Sakai R, Mizuta S, Yokoyama K, et al. High-dose chemotherapy with autologous stem cell transplantation for elderly patients with Relapsed/Refractory diffuse Large b cell lymphoma: A nationwide retrospective study. Biol Blood Marrow Transplant (2014) 20(5):684–9. doi: 10.1016/j.bbmt.2014.01.025
38. Shah NN. CIBMTR analysis confirms ongoing role for autologous transplant in chemosensitive relapsed DLBCL. Oncologist (2020) 25 Suppl 1:S10–1.
39. Nowakowski G, Maurer MJ, Cerhan JR, Dey D, Sehn LH. Utilization of real-world data in assessing treatment effectiveness for diffuse Large b-cell lymphoma. Am J Hematol (2023) 98(1):180–92. doi: 10.1002/ajh.26767
40. Lacy SE, Barrans SL, Beer PA, Painter D, Smith AG, Roman E, et al. Targeted sequencing in DLBCL, molecular subtypes, and outcomes: A haematological malignancy research network report. Blood (2020) 135(20):1759–71. doi: 10.1182/blood.2019003535
41. Rojek AE, Smith SM. Evolution of therapy for limited stage diffuse large b-cell lymphoma. Blood Cancer J (2022) 12(2):33. doi: 10.1038/s41408-021-00596-z
42. Riedell PA, Walling C, Nastoupil LJ. A multicenter retrospective analysis of outcomes and toxicities with commercial axicabtagene ciloleucel and tisagenlecleucel for Relapsed/Refractory aggressive b-cell lymphomas. Biol Blood Marrow Transplant (2020) 26(3):S41–2. doi: 10.1016/j.bbmt.2019.12.108
43. Mounier N, Canals C, Gisselbrecht C, Cornelissen J, Foa R, Conde E, et al. High-dose therapy and autologous stem cell transplantation in first relapse for diffuse large b cell lymphoma in the rituximab era: An analysis based on data from the European blood and marrow transplantation registry. Biol Blood Marrow Transplant (2012) 18(5):788–93. doi: 10.1016/j.bbmt.2011.10.010
44. Davies K, Barth M, Armenian S, Audino AN, Barnette P, Cuglievan B, et al. Pediatric aggressive mature b-cell lymphomas, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2020) 18(8):1105–23. doi: 10.6004/jnccn.2020.0036
45. Westin JR, Kersten MJ, Salles G, Abramson JS, Schuster SJ, Locke FL, et al. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive b-cell lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am J Hematol (2021) 96(10):1295–312. doi: 10.1002/ajh.26301
46. Cerhan JR, Kricker A, Paltiel O, Flowers CR, Wang SS, Monnereau A, et al. Medical history, lifestyle, family history, and occupational risk factors for diffuse large b-cell lymphoma: The InterLymph non-Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr (2014) 2014(48):15–25. doi: 10.1093/jncimonographs/lgu010
47. Di M, Huntington SF, Olszewski AJ. Challenges and opportunities in the management of diffuse Large b-cell lymphoma in older patients. Oncologist (2021) 26(2):120–32. doi: 10.1002/onco.13610
48. Shah NN, Ahn KW, Litovich C, He Y, Sauter C, Fenske TS, et al. Is autologous transplant in relapsed DLBCL patients achieving only a PET+ PR appropriate in the CAR T-cell era? Blood (2021) 137(10):1416–23. doi: 10.1182/blood.2020007939
49. Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor T cells in refractory b-cell lymphomas. N Engl J Med (2017) 377(26):2545–54. doi: 10.1056/NEJMoa1708566
50. Chow VA, Gopal AK, Maloney DG, Turtle CJ, Smith SD, Ujjani CS, et al. Outcomes of patients with large b-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Am J Hematol (2019) 94(8):E209–13. doi: 10.1002/ajh.25505
51. Lin JK, Muffly LS, Spinner MA, Barnes JI, Owens DK, Goldhaber-Fiebert JD, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult Large b-cell lymphoma. J Clin Oncol (2019) 37(24):2105–19. doi: 10.1200/JCO.18.02079
52. Oluwole OO, Jansen JP, Lin VW, Chan K, Keeping S, Navale L, et al. Comparing efficacy, safety, and preinfusion period of axicabtagene ciloleucel versus tisagenlecleucel in Relapsed/Refractory Large b cell lymphoma. Biol Blood Marrow Transplant (2020) 26(9):1581–8. doi: 10.1016/j.bbmt.2020.06.008
53. Maloney DG, Kuruvilla J, Liu FF, Kostic A, Kim Y, Bonner A, et al. Matching-adjusted indirect treatment comparison of liso-cel versus axi-cel in relapsed or refractory large b cell lymphoma. J Hematol Oncol (2021) 14(1). doi: 10.1186/s13045-021-01144-9
Keywords: R/R DLBCL, CAR-T cell, auto-HSCT, survival, meta-analysis
Citation: Tian L, Li C, Sun J, Zhai Y, Wang J, Liu S, Jiang Y, Wu W, Xing D, Lv Y, Guo J, Xu H, Sun H, Li Y, Li L and Zhao Z (2023) Efficacy of chimeric antigen receptor T cell therapy and autologous stem cell transplant in relapsed or refractory diffuse large B-cell lymphoma: A systematic review. Front. Immunol. 13:1041177. doi: 10.3389/fimmu.2022.1041177
Received: 10 September 2022; Accepted: 21 December 2022;
Published: 17 January 2023.
Edited by:
Yang Su, Xuzhou Medical University, ChinaReviewed by:
Pouya Safarzadeh Kozani, Guilan University of Medical Sciences, IranPooria Safarzadeh Kozani, Tarbiat Modares University, Iran
Xiurui Lv, University of Rochester, United States
Copyright © 2023 Tian, Li, Sun, Zhai, Wang, Liu, Jiang, Wu, Xing, Lv, Guo, Xu, Sun, Li, Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanfang Li, bGlsYW5mYW5nbWVuZ0AxNjMuY29t; Zhigang Zhao, enpoYW8wMUB0bXUuZWR1LmNu
†These authors have contributed equally to this work
 Linyan Tian
Linyan Tian Cheng Li1†
Cheng Li1† Lanfang Li
Lanfang Li Zhigang Zhao
Zhigang Zhao