- 1Kawasaki Disease Center, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 2Department of Pediatrics, Chang Gung University College of Medicine, Kaohsiung, Taiwan
- 3School of Medicine, Stanford University, Stanford, CA, United States
- 4Center for Biomedical Industry, Department of Molecular Science and Engineering, National Taipei University of Technology, Taipei, Taiwan
- 5Pediatrics, University of California San Diego, San Diego, CA, United States
Background: Kawasaki disease (KD) is the leading cause of acquired heart disease in children. The major challenge in KD diagnosis is that it shares clinical signs with other childhood febrile control (FC) subjects. We sought to determine if our algorithmic approach applied to a Taiwan cohort.
Methods: A single center (Chang Gung Memorial Hospital in Taiwan) cohort of patients suspected with acute KD were prospectively enrolled by local KD specialists for KD analysis. Our previously single-center developed computer-based two-step algorithm was further tested by a five-center validation in US. This first blinded multi-center trial validated our approach, with sufficient sensitivity and positive predictive value, to identify most patients with KD diagnosed at centers across the US. This study involved 418 KDs and 259 FCs from the Chang Gung Memorial Hospital in Taiwan.
Findings: Our diagnostic algorithm retained sensitivity (379 of 418; 90.7%), specificity (223 of 259; 86.1%), PPV (379 of 409; 92.7%), and NPV (223 of 247; 90.3%) comparable to previous US 2016 single center and US 2020 fiver center results. Only 4.7% (15 of 418) of KD and 2.3% (6 of 259) of FC patients were identified as indeterminate. The algorithm identified 18 of 50 (36%) KD patients who presented 2 or 3 principal criteria. Of 418 KD patients, 157 were infants younger than one year and 89.2% (140 of 157) were classified correctly. Of the 44 patients with KD who had coronary artery abnormalities, our diagnostic algorithm correctly identified 43 (97.7%) including all patients with dilated coronary artery but one who found to resolve in 8 weeks.
Interpretation: This work demonstrates the applicability of our algorithmic approach and diagnostic portability in Taiwan.
Highlights
Question: What is the performance of our US validated KD diagnostic algorithm with a single center Taiwan cohort?
Findings: Our diagnostic algorithm retained sensitivity (90.7%), specificity (86.1%), PPV (92.7%), and NPV (90.3%) in Taiwan comparable to previous US 2016 single center and US 2020 fiver center results.
Meaning: This work demonstrates the applicability of our algorithmic approach and KD diagnostic portability from US to Taiwan.
Introduction
Kawasaki disease (KD) is an acute vasculitis that affects infants and children and is the leading cause of acquired pediatric heart disease in the U.S. and Japan (1). Timely and accurately diagnosis of KD is critical, yet there isn’t a gold standard diagnostic test for KD. The classic KD diagnostic criteria (2) adopted by the American Heart Association (AHA) include fever plus ≥4 of 5 principal clinical signs, which include rash, conjunctival injection, extremity changes, oropharyngeal changes, and swollen lymph nodes. However, KD shares these clinical signs with other childhood febrile control (FC, supplementary data of diseases specifically included) subjects, causing missed or delayed diagnosis in emergency departments (EDs).
Among patients ultimately diagnosed with KD, only 4.7% receive the correct diagnosis at the first medical visit (3). Delayed diagnosis causes delayed treatment (4) and thus increases the risk of developing aneurysm, and in some children leads to myocardial infarction or death (5). Delayed diagnosis is particularly problematic among patients with incomplete clinical manifestations of KD (having less than 4 principal clinical signs), despite supplementary laboratory criteria have been adopted by AHA to identify atypical KD cases. Furthermore, treatment with intravenous immunoglobulin (IVIG), which can reduce the incidence of coronary aneurysms and risk of long-term cardiovascular complications (6), is recommended to be given within 10 days of illness. There is a critical need for clinicians to timely and accurately differentiate KD from other pediatric FC subjects.
We have explored to apply an algorithmic approach to the bedside electronic health record (EHR) datasets, developing predictive analytics (7–29) to drive translational medicine for improved diagnosis of high impact diseases and prediction of clinical resource utilization. Regarding KD, in 2013, we tested the hypothesis whether statistical learning on clinical and laboratory test patterns can lead to a single-step algorithm for KD diagnosis (9). Combining both clinical and laboratory test results, the algorithm diagnosed with sensitivity of 74.3% and specificity of 62.8% with > 95% PPV and > 95% NPV. In 2016, we improved and validated a two-step algorithm to classify an individual as a patient with KD, FC or intermediate (17). In the single-center validation, the algorithm yielded a sensitivity of 96.0% and a specificity of 78.5% with > 95% PPV and > 95% NPV. We subsequently set to validate this two-step diagnostic algorithm with five pediatric hospitals in the USA (23): Boston Children’s Hospital, Boston, Massachusetts; Children’s Hospital Colorado, Aurora, Colorado; Children’s Hospital of Orange County, Orange, California; Nationwide Children’s Hospital, Columbus, Ohio; and Rady Children’s Hospital, San Diego, California. The blinded US multicenter validation validates the algorithm with a sensitivity of 91.6%, specificity of 57.8% and PPV and NPV of 95.4% and 93.1%, respectively. The algorithm also correctly identified 94.0% of KD patients with abnormal echocardiograms.
In this study, we performed a blinded, single center validation of this KD diagnostic algorithm in Taiwan. Validating the algorithmic portability from US to a single center in Taiwan, we set to evaluate this algorithm in a prospective multi-center trial in Asia.
Material and methods
Study design
We previously developed and validated, with data from a US single center, a two-step algorithm that applies a linear discriminant analysis (LDA)-based model followed by a random forest-based algorithm to differentiate patients with KD from children with other FCs (7, 9, 17, 23). Patients who are classified as indeterminate by the LDA-based model are then evaluated by the random-forest algorithm based on the number of KD clinical criteria with which they present. In line with previous approach (7, 9, 17, 23), we applied the two-step algorithm, constructed with a single-center US cohort [Rady Children’s Hospital, San Diego, California, the validation cohort in 2016 study (17)], to a single center in Taiwan. The evaluation was blinded to diagnosis during classification, and then unblinded to calculate performance.
Study population
A single center (Chang Gung Memorial Hospital (CGMH) in Taiwan) cohort of patients with acute KD were prospectively enrolled by local KD specialists, who are certified pediatricians to diagnose and treat KD in the Kawasaki Disease Center of CGMH. Signed consent or assent forms were obtained from the parents of all subjects and from all subjects >6 years of age. The study was approved by the institutional review boards of the Chang Gung Memorial Hospital and Stanford University. The diagnosis of KD was made by Taiwan KD specialists on the 2004 USA AHA guidelines (2). All FCs had unexplained fever, ≥1 of the five principal clinical criteria for KD, and laboratory evaluation. All FC diagnosis was determined by clinical features, culture, or PCR testing. No COVID-19 patients were enrolled in this study. All patients had fever (≥38.0°C) for no more than 10 days and had complete information for nine clinical or laboratory data points, including the five principal clinical criteria (illness days (ie, days of fever), total white blood cell (WBC) count, percentage of eosinophils and hemoglobin concentration), percentages of monocytes, lymphocytes, neutrophils and immature neutrophils (bands), platelet count, levels of C reactive protein (CRP), alanine aminotransferase (ALT) and erythrocyte sedimentation rate (ESR), gamma glutamyl transferase (GGT), were collected if available. The clinical parameters of bands, GGT, and ESR were not routinely collected in the Taiwan Chang Gung Hospital for the KD and FC patients, therefore, not used in the two-step algorithm. Age and sex for all patients, and coronary artery status and Z-score (SD from the mean adjusted for body surface area) for patients with KD, were also recorded when available. Coronary artery status was classified as normal (right coronary artery (RCA) and left anterior descending (LAD) Z-score always <2.5) or abnormal (RCA and/or LAD Z-score ≥2.5 within the first 6weeks after diagnosis).
Two-step algorithm for differentiation of KD and FCs
The two-step algorithm uses an LDA-based analysis followed by a random forest-based algorithm as previously described (7, 17, 23). In this study, input variables of the LDA model are illness days, the 5 principal clinical criteria and 10 laboratory test variables. One or more variables had missing values for 227 of 418 (54.3%) patients with KD and 256 of 259 (98.8%) FCs. The algorithm was less sensitive in identifying KD patients with 1 or more missing variables as compared to patients with complete data. Values were imputed with a k-nearest neighbor algorithm. The output of the LDA classifies each individual patient as KD, FC or indeterminate. The cut-off thresholds between the three categories were set in the original US single center algorithm to provide a positive predictive value (PPV) or negative positive value (NPV) of 95%. The indeterminate patients were divided into four sub cohorts based on the number of KD criteria present at the time of diagnosis, and separate random forest models were applied to the patients in each sub-cohort to further stratify these patients into KD, FC or indeterminate categories (9, 17, 23).
Algorithm performance in this Taiwan single center study
The aim of this study was to analyze the algorithm performance on a single center Taiwan cohort. We compared the classifications of patients by the algorithm with the classifications made by the clinical experts who diagnosed these patients. We calculated sensitivity, specificity, PPV, NPV and rates of indeterminate classification of the algorithm when applied to this cohort. Performance of the algorithm was evaluated according to age, illness days and coronary artery status. We also performed univariable analysis with each of the 15 variables in sub cohorts of patients manifesting ≤2, 3 or ≥4 KD principal clinical criteria. In each sub cohort, the distribution of each variable was compared between the patients with KD and FCs, and the differences were measured using Mann-Whitney U test (for continuous variables) or Fisher’s exact test (for categorical variables).
Results
Patient characteristics
As shown in Figure 1, 751 patients were enrolled with 462 KD and 289 FC subjects. 26 FC patients were excluded due to incorrect registration of days of fever. 47 patients (KD: 44; FC: 3) were excluded with days of fever greater than 10 days. One FC patient was excluded due to the absence of KD principal criteria. For this study, from the Taiwan single center cohort, 418 patients with KD and 259 FCs were used for the blind testing. Patients with KD were more likely to be male than FCs (60.5% vs 53.3%, p=0.06). The KDs had a higher median age than the patients with FC (1.3 vs 1.0 years, p=0.03). The FCs most common final diagnosis was either bacterial or viral infection.
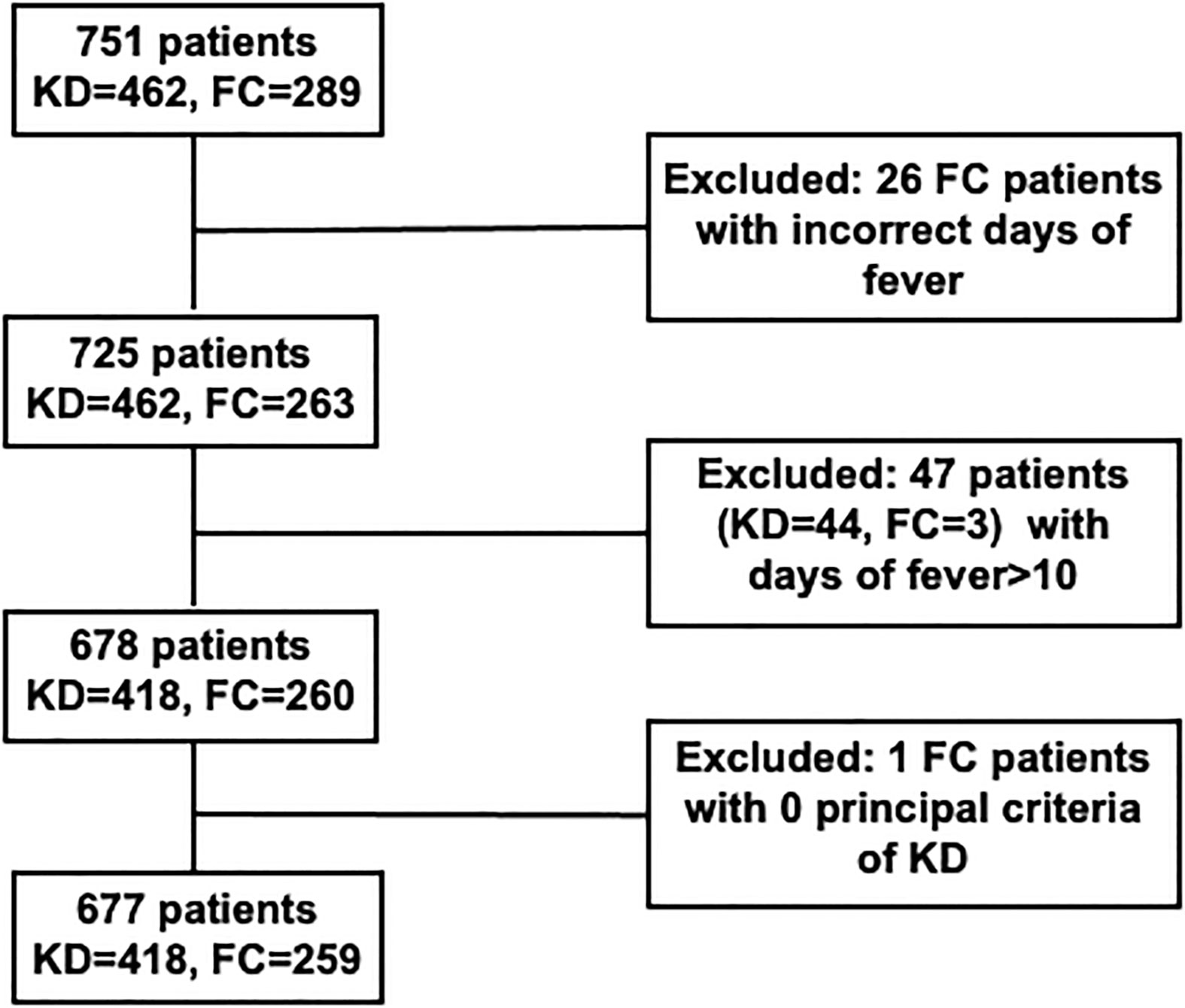
Figure 1 Cohort construction of KD and FC patients in Chang Gung Hospital. KD, Kawasaki disease; FC, febrile illness control.
Univariable analysis of clinical and laboratory test variables
The majority of the patients with KD (368 of 418, 88.0%) had 4 or 5 principal clinical criteria, and 91.3% (217 of 259, 83.7%) of FCs had 1 to 3 principal clinical criteria (Table 1). 36 KD and 59 FC patients had three clinical criteria while 14 KD and 135 FC had two clinical criteria. 157/418 KD patients were less than 1 year old while 261/418 KD patients were older than 1 year. Most of the laboratory test variables except CRP, eosinophils, hemoglobin, and platelet counts differed significantly between patients with KD and FCs in all 3 sub-cohorts.
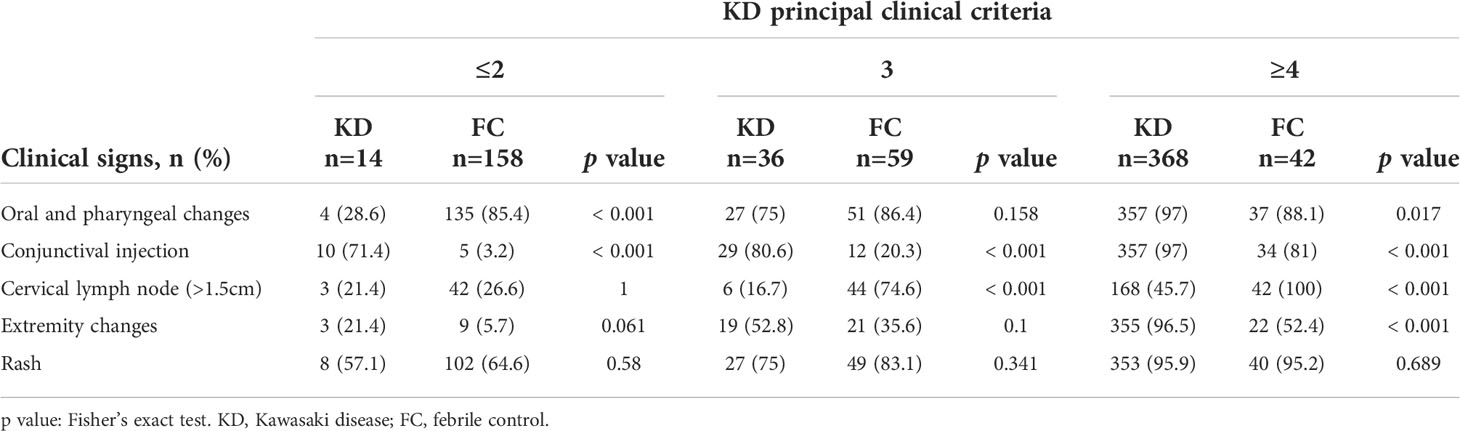
Table 1 Distribution of the clinical signs among KD and FC patients (count and percentage) in sub cohorts manifesting fewer than 2, 3 or ≥4 principal clinical criteria for KD.
Taiwan single center blind test results
In this Taiwan single center study (Figure 2), the algorithm’s testing performance is in line with our 2016 US single center (17) and US 2020 multi-center (23) analyses: sensitivity of 90.7% (379/418), specificity of 86.1% (223/259), PPV of 92.7% (379/409), and NPV of 90.3% (223/247); 3.5% (15/418) of the patients with KD and 2.3% (6/259) FCs were classified as indeterminate. Overall, 5.7% (24/418) of the patients with KD and 11.5% (30/259) of FCs were misclassified by the algorithm.
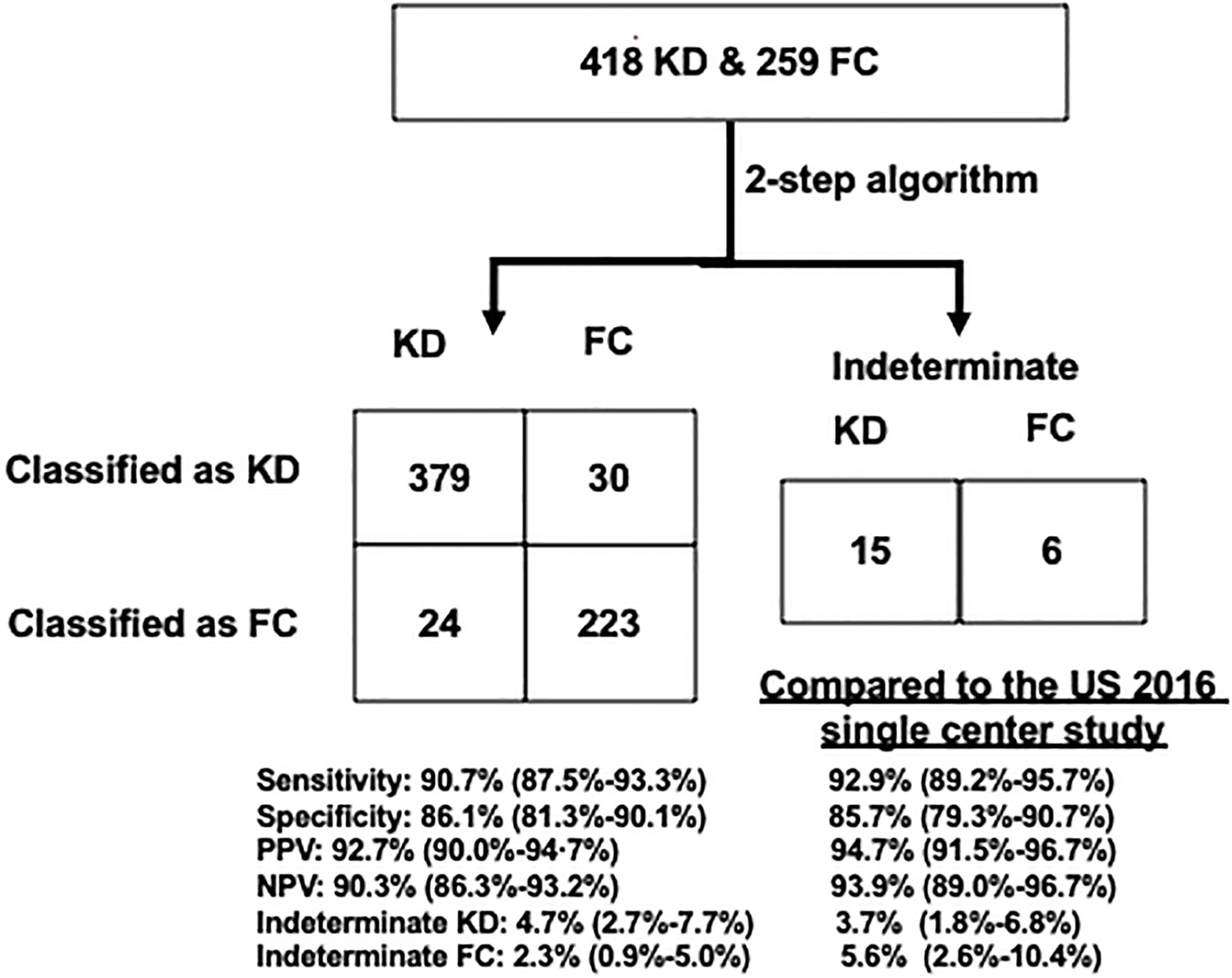
Figure 2 Performance of the US validated KD algorithm to diagnose KD from FC patients. Left: a 2×2 table compared with US single-center validation results. The US cohort was described in 2016 Hao et al. publication. Right: percentages of correctly classified, misclassified, and indeterminate patients. FCs, febrile controls; KD, Kawasaki disease; NPV, negative positive value; PPV, positive predictive value.
We stratified the patients into < 1 or ≥ 1 year old bins for the performance analysis. Of the 157/418 KD infants younger than 1 year, 89.6% were classified correctly, and 7.0% were indeterminate. Of patients with KD ≥ 1 year old, 90.3% were classified correctly, and 8.0% were indeterminate. For the 379 correctly classified KD, 140/379 were less than 1 year old and 239/379 were older than 1 year old. For the 24 KD wrongly classified into FC, 10/24 were less than 1 year old and 14/24 were older than 1 year old. For the 15 KD wrongly classified into intermediate, 7/15 were less than 1 year old and 8/15 were older than 1 year old.
We stratified the patients into < 8 or 8-10 illness day bins for the performance analysis. Of the 327/418 KD patients with < 8 illness days, 93.7% were classified correctly, and 3.7% were indeterminate. Of the 91/418 KD patients with 8-10 illness days, 95.5% were classified correctly, and 3.3% were indeterminate. Of the 252/259 FC patients with < 8 illness days, 89.1% were classified correctly, and 1.6% were indeterminate. Of the 7/259 FC patients with 8-10 illness days, 40.0% were classified correctly, and 28.6% were indeterminate. The poor performance, as previously observed in US multi-center study (23), the specificity (40.0%) and NPV (33.3%), of 8-10 illness day FCs may be due to the small sample size (n=7 FCs with 8-10 illness days). We also compared, the ratio of median laboratory test values between the KD and FC patients, of the bins of illness day less than 8 or illness day 8-10. Much more up regulated ratios, of the tests of CRP, ALT, and WBC, were observed when contrasting between the bins of illness day less than 8 and illness day 8-10 (p value < 0.01).
There were 50/418 KD patients who presented with 3 or fewer criteria and 368/418 KDs with 4 or 5 clinical criteria. Compared with its sensitivity in KD patients with 4 or 5 clinical criteria (361/368, 98.0%), the algorithm had a lower sensitivity for KD with less than or equal to 2 criteria 14.2% (2/14) and for KD with 3 criteria 44.4% (16/36) (Fisher’s exact test p < 0·0001). Compared with its specificity in FC patients with 4 or 5 clinical criteria (10/42, 23.8%), the algorithm had a higher specificity for FC with less than or equal to 2 criteria 99.4% (157/158) and for FC with 3 criteria 94.9% (56/59) (Fisher’s exact test p < 0·0001). For the total of 15 KDs and 6 FCs wrongly classified into intermediate, 1/13/1 KDs and 1/3/2 FCs were from the bins of with less than or equal to 2, with 3, or with greater than 4 clinical criteria, respectively.
Classification of KD patients with coronary artery abnormalities
Coronary artery abnormalities were documented in 44 of the 418 KD patients (10.5%) (Table 2). One patient was not classified correctly. The misclassified KD patient had a maximum Z-score of 2·8 that resolved by 8 weeks after treatment. This is still a problem, if we use the criteria for treatment. All 8 KDs with aneurysm were identified by the two-step algorithm.
Comparison of clinical and laboratory data between Taiwan single-center and US 2016 single-center and 2020 multi-center studies
The frequencies of the five KD principal criteria and illness days in this Taiwan single-center study were in line with those in the US single-center (17) and multi-center (23) validation dataset of the algorithm except for cervical lymph node (25.5%/19.5% versus 49.4%), conjunctival injection (44.1%/58.9% versus 19.7%), oropharyngeal changes (48.4%/46.8% versus 86.1%), and days of illness (5.0/5.0 versus 2.0) in FC subjects.
Discussion
It is essential to diagnose KD early in the acute phase as early administration with anti-inflammatory therapies can reduce the risk of developing coronary artery aneurysms (1). However, the diagnosis of KD remains difficult as the clinical signs overlap with those of other pediatric FCs. In this study, we tested a US multi-center validated KD diagnostic algorithm using a blinded Taiwan single-center cohort. The algorithm had a sensitivity of 90.7%, a specificity of 86.1%, a PPV of 92.7%, and a NPV of 90.3%, like that of the US single-center and multi-center validation studies (9, 17, 23). The algorithm identified 43/44 (98.0%) of patients with KD who developed coronary artery abnormalities, and only misclassified as one FC patient with Z score (RCA or LAD) ≥2.5, and resolve in 8 weeks. In this study, all 8 KD subjects with aneurysms were correctly diagnosed by the 2-step algorithm.
Given that this algorithm has been validated in US, this blinded Taiwan single center study shall provide evidence to support a multi-center prospective testing in a Taiwan setting: where the KD prevalence should be higher than US; and in which KD patients are rare and FCs are common to evaluate its potential utility as a physician support tool.
We recognize both strengths and weaknesses in this study. The strength of the study is the demonstration of the robustness and effectiveness of the algorithm and the algorithm portability from North America to Taiwan. The KD and FC subjects in the Taiwan single-center are generally younger than the US single-center and multi-center analyses, thus demonstrating its potential clinical utility across a broad range of similar appearing illnesses. However, in this Taiwan study, the clinical parameters of bands, GGT, and ESR were not routinely collected in the Taiwan Chang Gung Hospital for the KD patients, therefore, not used in the two-step algorithm. If collected in the future, the algorithm performance might be improved when prediction with Taiwan patients. The FCs were not examined by the echocardiography, which may limit our ability to completely rule out KD diagnosis. In this Taiwan single-center study, we had a larger number of KD patients than FCs as in the US multi-center study, and the frequency of KD patients in a prospective study in the ED setting will be much lower, which will undoubtedly worsen the performance of this algorithm. The actual ratio of KD to FC patients who would be screened for KD is not known and shall warrant more studies. We are planning a multi-center study in the Taiwan ED setting to assess the prevalence of KD and prospectively evaluate the utility of this algorithm.
Conclusions
We assessed the performance of our 2-step algorithm in a blinded, single-center study in Asia. This work demonstrates the algorithmic portability from North America to Asia, providing further support for moving forward with an adequately powered, multicenter study in the Taiwan ED settings to assess if this algorithm will be a useful clinical support tool to manage KD in Asia where the disease prevalence is higher than in US.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study was approved by the institutional review boards of the Chang Gung Memorial Hospital and Stanford University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
Experimental design: H-CK, XL, HJC. Data analysis: SH, BJ, CJC, ZH. Clinical data collection and curation: L-SC, Y-HH. Manuscript draft: H-CK, XL. Manuscript revision: KH, JW, KS, CR, HC, SC, JK, AT, JB, DM. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the grants CPRPG8H0051-2, CMRPG8L0011 and CMRPG8L1241 provided by Chang Gung Memorial Hospital, Taiwan; 1R41TR004351-01 provided by National Center for Advancing Translational Sciences, US.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALT, Alanine aminotransferase; AHA, American Heart Association; CRP, C-reactive protein; ED, Emergency department; EHR, Electronic health record; ESR, Erythrocyte sedimentation rate; FC, Febrile control; GGT, Gamma glutamyl transferase; IVIG, Intravenous Immunoglobulin; KD, Kawasaki disease; LAD, Left anterior descending; LDA, Linear discriminant analysis; NPV, Negative positive value; PPV, Positive predictive value; RCA, Right coronary artery; WBC, White blood cell.
References
1. Burns JC. New perspectives on Kawasaki disease. Arch Dis Child (2019) 104:616–7. doi: 10.1136/archdischild-2018-316322
2. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the committee on rheumatic fever, endocarditis and Kawasaki disease, council on cardiovascular disease in the young, American heart association. Circulation (2004) 110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78
3. Anderson MS, Todd JK, Glode MP. Delayed diagnosis of Kawasaki syndrome: An analysis of the problem. Pediatrics (2005) 115:e428–33. doi: 10.1542/peds.2004-1824
4. Daniels LB, Gordon JB, Burns JC. Kawasaki Disease: late cardiovascular sequelae. Curr Opin Cardiol (2012) 27:572–7. doi: 10.1097/HCO.0b013e3283588f06
5. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American heart association. Circulation (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
6. Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. New Engl J Med (1986) 315:341–7. doi: 10.1056/NEJM198608073150601
7. Ling XB, Lau K, Kanegaye JT, Pan Z, Peng S, Ji J, et al. A diagnostic algorithm combining clinical and molecular data distinguishes Kawasaki disease from other febrile illnesses. BMC Med (2011) 9:130. doi: 10.1186/1741-7015-9-130
8. Ling XB, Macaubas C, Alexander HC, Wen Q, Chen E, Peng S, et al. Correlation analyses of clinical and molecular findings identify candidate biological pathways in systemic juvenile idiopathic arthritis. BMC Med (2012) 10:125. doi: 10.1186/1741-7015-10-125
9. Ling XB, Kanegaye JT, Ji J, Peng S, Sato Y, Tremoulet A, et al. Point-of-care differentiation of Kawasaki disease from other febrile illnesses. J Pediatr (2013) 162:183–188 e3. doi: 10.1016/j.jpeds.2012.06.012
10. Hao S, Jin B, Shin AY, Zhao Y, Zhu C, Li Z, et al. Risk prediction of emergency department revisit 30 days post discharge: A prospective study. PloS One (2014) 9:e112944. doi: 10.1371/journal.pone.0112944
11. Hao S, Wang Y, Jin B, Shin AY, Zhu C, Huang M, et al. Development, validation and deployment of a real time 30 day hospital readmission risk assessment tool in the Maine healthcare information exchange. PloS One (2015) 10:e0140271. doi: 10.1371/journal.pone.0140271
12. Hu Z, Hao S, Jin B, Shin AY, Zhu C, Huang M, et al. Online prediction of health care utilization in the next six months based on electronic health record information: A cohort and validation study. J Med Internet Res (2015) 17:e219. doi: 10.2196/jmir.4976
13. Hu Z, Jin B, Shin AY, Zhu C, Zhao Y, Hao S, et al. Real-time web-based assessment of total population risk of future emergency department utilization: Statewide prospective active case finding study. Interactive J Med Res (2015) 4:e2. doi: 10.2196/ijmr.4022
14. Shin AY, Hu Z, Jin B, Lal S, Rosenthal DN, Efron B, et al. Exploring value in congenital heart disease: An evaluation of inpatient admissions. Congenit Heart Dis (2015) 10:E278–87. doi: 10.1111/chd.12290
15. Shin AY, Jin B, Hao S, Hu Z, Sutherland S, McCammond A, et al. Utility of clinical biomarkers to predict central line-associated bloodstream infections after congenital heart surgery. Pediatr Infect Dis J (2015) 34:251–4. doi: 10.1097/INF.0000000000000553
16. Tremoulet AH, Dutkowski J, Sato Y, Kanegaye JT, Ling XB, Burns JC. Novel data-mining approach identifies biomarkers for diagnosis of Kawasaki disease. Pediatr Res (2015) 78:547–53. doi: 10.1038/pr.2015.137
17. Hao S, Jin B, Tan Z, Li Z, Ji J, Hu G, et al. A classification tool for differentiation of Kawasaki disease from other febrile illnesses. J Pediatr (2016) 176:114–120 e8. doi: 10.1016/j.jpeds.2016.05.060
18. Jin B, Zhao Y, Hao S, Shin AY, Wang Y, Zhu C, et al. Prospective stratification of patients at risk for emergency department revisit: Resource utilization and population management strategy implications. BMC Emerg Med (2016) 16:10. doi: 10.1186/s12873-016-0074-5
19. Siehr SL, Shi S, Hao S, Hu Z, Jin B, Hanley F, et al. Exploring the role of polycythemia in patients with cyanosis after palliative congenital heart surgery. Pediatr Crit Care Med (2016) 17:216–22. doi: 10.1097/PCC.0000000000000654
20. Zheng L, Wang Y, Hao S, Shin AY, Jin B, Ngo AD, et al. Web-based real-time case finding for the population health management of patients with diabetes mellitus: A prospective validation of the natural language processing-based algorithm with statewide electronic medical records. JMIR Med Inform (2016) 4:e37. doi: 10.2196/medinform.6328
21. Hao S, Fu T, Wu Q, Jin B, Zhu C, Hu Z, et al. Estimating one-year risk of incident chronic kidney disease: Retrospective development and validation study using electronic medical record data from the state of Maine. JMIR Med Inform (2017) 5:e21. doi: 10.2196/medinform.7954
22. Jin B, Liu R, Hao S, Li Z, Zhu C, Zhou X, et al. Defining and characterizing the critical transition state prior to the type 2 diabetes disease. PloS One (2017) 12:e0180937. doi: 10.1371/journal.pone.0180937
23. Hao S, Ling XB, Kanegaye JT, Bainto E, Dominguez SR, Heizer H, et al. Multicentre validation of a computer-based tool for differentiation of acute Kawasaki disease from clinically similar febrile illnesses. Arch Dis Child (2020) 105:772–7. doi: 10.1136/archdischild-2019-317980
24. Huang J, Zheng L, Li Z, Hao S, Ye F, Chen J, et al. Kinetics of SARS-CoV-2 positivity of infected and recovered patients from a single center. Sci Rep (2020) 10:18629. doi: 10.1038/s41598-020-75629-x
25. Ye C, Li J, Hao S, Liu M, Jin H, Zheng L, et al. Identification of elders at higher risk for fall with statewide electronic health records and a machine learning algorithm. Int J Med Inform (2020) 137:104105. doi: 10.1016/j.ijmedinf.2020.104105
26. Zheng L, Wang O, Hao S, Ye C, Liu M, Xia M, et al. Development of an early-warning system for high-risk patients for suicide attempt using deep learning and electronic health records. Transl Psychiatry (2020) 10:72. doi: 10.1038/s41398-020-0684-2
27. Duong SQ, Zheng L, Xia M, Jin B, Liu M, Li Z, et al. Identification of patients at risk of new onset heart failure: Utilizing a large statewide health information exchange to train and validate a risk prediction model. PloS One (2021) 16:e0260885. doi: 10.1371/journal.pone.0260885
28. Stevenson DK, Wong RJ, Aghaeepour N, Maric I, Angst MS, Contrepois K, et al. Towards personalized medicine in maternal and child health: integrating biologic and social determinants. Pediatr Res (2021) 89:252–8. doi: 10.1038/s41390-020-0981-8
Keywords: Kawasaki disease, diagnosis, algorithm, inflammatory disease, febrile illness
Citation: Kuo H-C, Hao S, Jin B, Chou CJ, Han Z, Chang L-S, Huang Y-H, Hwa K, Whitin JC, Sylvester KG, Reddy CD, Chubb H, Ceresnak SR, Kanegaye JT, Tremoulet AH, Burns JC, McElhinney D, Cohen HJ and Ling XB (2022) Single center blind testing of a US multi-center validated diagnostic algorithm for Kawasaki disease in Taiwan. Front. Immunol. 13:1031387. doi: 10.3389/fimmu.2022.1031387
Received: 29 August 2022; Accepted: 21 September 2022;
Published: 03 October 2022.
Edited by:
James Cheng-Chung Wei, Chung Shan Medical University Hospital, TaiwanCopyright © 2022 Kuo, Hao, Jin, Chou, Han, Chang, Huang, Hwa, Whitin, Sylvester, Reddy, Chubb, Ceresnak, Kanegaye, Tremoulet, Burns, McElhinney, Cohen and Ling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuefeng B. Ling, YnhsaW5nQHN0YW5mb3JkLmVkdQ==;Ho-Chang Kuo, ZXJpY2t1bzQ4QHlhaG9vLmNvbS50dw==
†These authors have contributed equally to this work and share first authorship
 Ho-Chang Kuo
Ho-Chang Kuo Shiying Hao
Shiying Hao Bo Jin3†
Bo Jin3† Zhi Han
Zhi Han Ling-Sai Chang
Ling-Sai Chang Ying-Hsien Huang
Ying-Hsien Huang Karl G. Sylvester
Karl G. Sylvester Jane C. Burns
Jane C. Burns Harvey J. Cohen
Harvey J. Cohen Xuefeng B. Ling
Xuefeng B. Ling