- 1Department of Epidemiology, School of Public Health, Air Force Medical University, Xi’an, China
- 2Ministry of Education Key Lab of Hazard Assessment and Control in Special Operational Environment, School of Public Health, Air Force Medical University, Xi’an, China
- 3Faculty of Health and Medicine, Division of Health Research, Lancaster University, Lancaster, United Kingdom
- 4Clinical Drug Experiment Institution, Gansu Wuwei Tumor Hospital, Wuwei, China
- 5Hepatobiliary Center, Gansu Wuwei Tumor Hospital, Wuwei, China
Background: Hepatitis B surface antigen (HBsAg) loss is considered a functional cure for chronic hepatitis B (CHB), however, several factors influence HBsAg loss.
Methods: 29 CHB patients who had achieved HBsAg loss, were selected and 58 CHB patients with persistent HBsAg were matched, according to gender and age (+/- 3 years). Logistic regression and restricted cubic spline (RCS) modelling were performed.
Results: Multivariate-adjusted logistic regression, based on stepwise selection, showed that baseline HBsAg levels negatively correlated with HBsAg loss (odds ratio [OR] = 0.99, 95% confidence interval [CI] = 0.98-0.99). Interferon treatment positively related with HBsAg loss (OR = 7.99, 95%CI = 1.62-44.88). After adjusting for age, HBsAg level, ALT level, HBeAg status and interferon treatment, MMP-1 (OR = 0.66, 95%CI = 0.44-0.97), CXCL9 (OR = 0.96, 95%CI = 0.93-0.99) and TNF-R1 (OR = 0.97, 95%CI = 0.94-0.99) baseline levels all negatively correlated with HBsAg loss. Our multivariate-adjusted RCS model showed that baseline CXCL10 was associated with HBsAg loss although the relationship was “U-shaped”.
Conclusions: Cytokines such as MMP-1, CXCL9, CXCL10 and TNF-R1 are important factors which influence HBsAg loss. It may be possible to develop a nomogram which intercalates these factors; however, further research should consider immune processes involved in HBsAg loss.
Introduction
Approximately 316 million people worldwide suffer with chronic hepatitis B virus infections (HBV) (1) although, each country has different prevalence rates. According to a relatively recent meta-analysis, the pooled estimated prevalence of HBV infections in the Chinese mainland population, between 2013 to 2017, was thought to be 6.89%. Anything between 5.00-7.99% is officially classified as a higher intermediate prevalence (2) although again prevalence rates vary regionally. Wuwei city, which is in the north-west of mainland China, is one area in China considered to have a particularly high prevalence of chronic hepatitis B (CHB), with 7.2% (95% CI: 6.3-8.1%) in 2010 (3). As such, Wuwei city has been targeted by Chinese public health organisations as a location for pilot HBV prevention and treatment programmes.
CHB patients are at a substantially higher risk of developing cirrhosis and hepatocellular carcinoma (HCC). These diseases are responsible for approximately 1.45 million deaths worldwide, each year (4) and therefore must be prevented. Hepatitis B surface antigen (HBsAg) seroclearance can occur spontaneously in CHB patients but can also be induced with anti-viral treatments. HBsAg loss is associated with improvements in liver histologies and a decreased risk of cirrhosis, HCC development and death (5). Therefore, HBsAg seroclearance is often regarded as the optimal endpoint for treatments according to clinical guidelines (6, 7). However, HBsAg seroclearance is rare (8, 9) and therefore, we must learn to understand HBsAg seroclearance as well as the factors that influence HBsAg loss.
Previous studies have identified several factors related to HBsAg loss e.g. gender, HBV DNA levels, Hepatitis B e antigen (HBeAg) status and ALT level at baseline (10–12). Apart from the direct action of immune cells, cytokines are also thought to mediate HBsAg loss (13). At present, Interferon (IFN)-α is the main antiviral therapy for CHB and Interleukins (IL) -4, IL-6, IL-17, and IL-28 act as key “coordinators” of inflammatory responses involved in HBsAg seroclearance (13). Serum interferon-inducible protein (IP) 10, also known as C-X-C motif ligand (CXCL) 10, and CXCL13 levels could also play an important role in predicting HBsAg seroclearance (14, 15). However, the established knowledge base does not include a clear description of associations between cytokines, SNPs related to cytokines and HBsAg loss.
Materials and methods
Participants
From August 2018 until January 2021, 4,000 individuals, aged 30–65 years with previous HBsAg seropositive results (for at least six months), were screened in Wuwei City, China. Potential participants were asked for formal consent to participate in this study which involved a survey, a physical examination and a direct consultation with a clinician at Gansu Wuwei Tumor Hospital. After excluding duplicate data (n = 16) and those who did not complete examinations (n = 22), 3,962 participants were recruited and engaged in routine examinations every six to twelve months until 31st October 2021.
All research was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee in the Air Force Medical University. All participants were fully informed of the purpose and details of this study, and participants (or their legal guardians) were required to provide informed consent, before participating.
The baseline for this study was defined according to the time of recruitment. The events of interest during follow-up were HBsAg loss and HBsAg seroclearance. HBsAg loss was defined as serum HBsAg which reverted to negative from positive for CHB cases with (or without) treatment. HBsAg seroclearance was defined as maintaining a HBsAg negative status on two separate occasions over six months until the endpoint. 29 participants with HBsAg loss were initially assessed using blood samples at baseline. 58 participants with persistent HBsAg(+) status were matched, according to gender and age (+/- 3 years). A flow chart of the selection and assignment process is presented in Supplementary Figure 1.
Data collection
A questionnaire was specifically designed for Prevention and Control of Infectious Diseases studies in China. Trained investigators collected information through face-to-face interviews. Physical examinations were performed locally in Gansu Wuwei Tumor Hospital and results were collated. Details of examinations have been described in Supplementary Materials, Methods.
Quantitative examination of HBsAg
Blood samples were obtained through standard forearm venipunctures and samples were processed within 1 h of collection. Blood samples were centrifuged at 12,000 g for 10 mins at 4°C and then supernatants were transferred into microcentrifuge tubes, which were stored at −80°C. Chemiluminescence analysis reagents (Snibe Ltd. Shenzhen, China) were used for quantitative HBsAg examinations in the Tianbo Medical Laboratory. The range was 0.02-1000 IU/mL.
Cytokines
An RD systems Luminex® Discovery Assay Human Premixed Multi-Analyte Kit (Kit Catalog Number: LXSAHM-17, Lit Lot Number: L141528) was used to detect 17 types of cytokines, including IL-6, IL-8, IL-21, IL-23, IL-33, matrix metalloproteinases (MMP)-1, MMP-2, MMP-3, C-X-C motif ligand (CXCL) 9, CXCL10, CXCL11, CXCL13, C-C motif ligand (CCL2), tumor necrosis factor (TNF) -α TNF-R1, IFN-γ, and B cell activating factor (BAFF) using a Luminex xPONENT® for FLEXMAP3D® analyzer. These 17 cytokines were chosen according to an associated pilot study we conducted focusing on CHB patients compared to healthy controls (in press).
A standard curve was created for each analyte through data reduction, using milliplex analyst (5.1) which is capable of generating a five-parameter logistic (5-PL) curve-fit. Cytokines below the lower limit were recorded as lower limit values. Replacement numbers for each cytokine were listed as follows: IL-8 (1/87), IL-23 (12/87), IL-33 (8/87), MMP-3 (1/87), CXCL9 (3/87), CXCL11 (6/87), TNF-α (1/87) and IFN-γ (7/87). Considering the magnitude of cytokines, we transformed MMP-1, MMP-2 and MMP-3 from pg/mL to 1000 pg/mL, and CXCL9, CXCL10, CXCL11, CXCL13, TNF-α, TNF- R1, IFN-γ, BAFF and CCL2 from pg/mL to 10 pg/mL. This enabled us to perform more sophisticated logistic regression and RCS modelling.
Human SNPs
Genomic DNA was isolated from whole blood samples using Human Genome Whole Blood Extraction Kit (Tianlong Technology, Xi’an, China). 14 single nucleotide polymorphisms (SNP) related to cytokines (rs1061624, rs1143634, rs12979860, rs1799724, rs1799964, rs1800469, rs1800795, rs1800872, rs1800896, rs2055979, rs2069762, rs2227306, rs3025058 and rs3806798) were assessed with TaqMan® MGB SNP Genotyping Kit (Fuyuan Biotechnology Ltd, Shanghai, China) using a real-time polymerase chain reaction allelic discrimination system (Tianlong Technology, Xi’an, China). Shannon entropy (SE) for human SNPs were calculated according to the following formula (16), where i means genotypes and pi means the percentage of each genotype.
Statistical analysis
Variables are presented as means with corresponding standard deviations (SD), or as medians with quartiles, or just as simple numbers with percentages. These data points were compared using Student’s T test, Wilcoxon’s test, or by way of a standard Chi-square tests (or Fisher’s Exact tests), according to HBsAg status. Person-time at follow-up was calculated for each participant from the date of the first survey to the end of follow-up on the 31th October, 2021.
Unadjusted and multivariable-adjusted (i.e., adjusting for age, HBsAg level, ALT level, HBeAg status, and interferon treatment) logistic regression models were used to analyze associations between variables and HBsAg loss. Odds ratios (OR) with 95% confidence intervals (CI) were estimated. Restricted cubic spline (RCS) methods with three knots (p5, p50 and p95) were applied to assess the linearity of relationships using “%RCS_REG” microprogram developed by Desquilbet (17). Details of RCS modelling process are described in the Supplementary Materials, Method Section. RCS plots were performed with proportion distribution.
Correlations between cytokines were assessed using Spearman’s correlation analysis. To consider relations among cytokines, we established each dataset as independent and integral. Standardized cytokines were calculated according to normalized Z-scores. Partial Least Squares Discriminant Analysis (PLS-DA) and Permutational Multivariate Analysis of Variance (PERMANOVA) with Euclidean distance were also performed to compare fundamental differences between the two groups using “mixOmics” R package (http://mixomics.org/,v6.8.5) (18).
Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc, Cary, NC) and with R software, version 2.13.2 (http://cran.r-project.org/). A p value of 0.05 was established as the threshold for statistical significance.
Results
Baseline characteristics
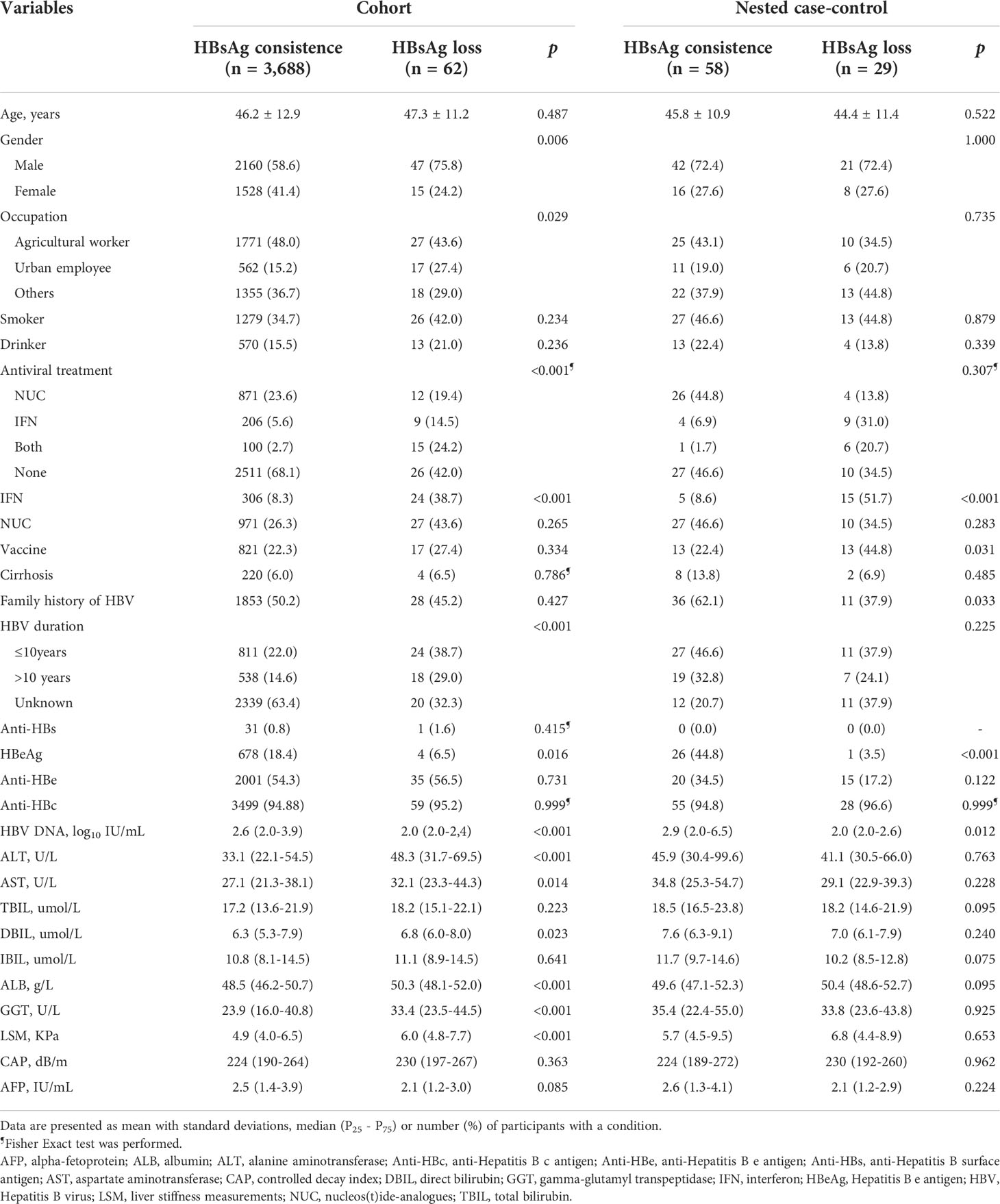
3,962 participants were initially included at baseline, of whom 212 were HBsAg(–) and 3,750 were HBsAg(+). Among the 3,750 CHB cases with HBsAg(+), 1,694 were followed-up for a median of 1.5 years. 62 participants achieved HBsAg loss, corresponding to an annual loss rate of 2.41 per 100 person-years and with a cumulative incidence of 3.66%. Those with HBsAg loss were predominantly male, urban employees, taking antiviral treatments (specifically IFN), had a longer HBV duration since infection, and had higher levels of ALT, AST, DBIL, ALB, GGT and LSM. However, there was a small proportion of participants with HBsAg loss who were HBeAg(+) and who had lower HBV DNA loads (Table 1).
According to a strict definition of HBsAg seroclearance, we observed 21 participants who achieved HBsAg seroclearance, corresponding to an annual incidence of 0.82 per 100 person-years. The majority of participants who achieved HBsAg seroclearance were also taking antivirals (specifically IFN), had longer HBV duration since infection, and had the highest levels of ALT, AST, DBIL, ALB and GGT, but lower level of HBV DNA (Supplementary Table 3).
Characteristics of the nested case-control groups
29 participants who achieved HBsAg loss were selected and matched with 58 who had not, according to gender and age (+/- 3 years). The HBsAg loss group also had a lower proportion of HBeAg(+) cases and a lower HBV DNA load, overall. Additionally, the HBsAg loss group had a higher proportion of people who were prescribed a IFN treatment and who had an HBV vaccine history. Within this group there was also a smaller proportion who had a family history of HBV infection (Table 1).
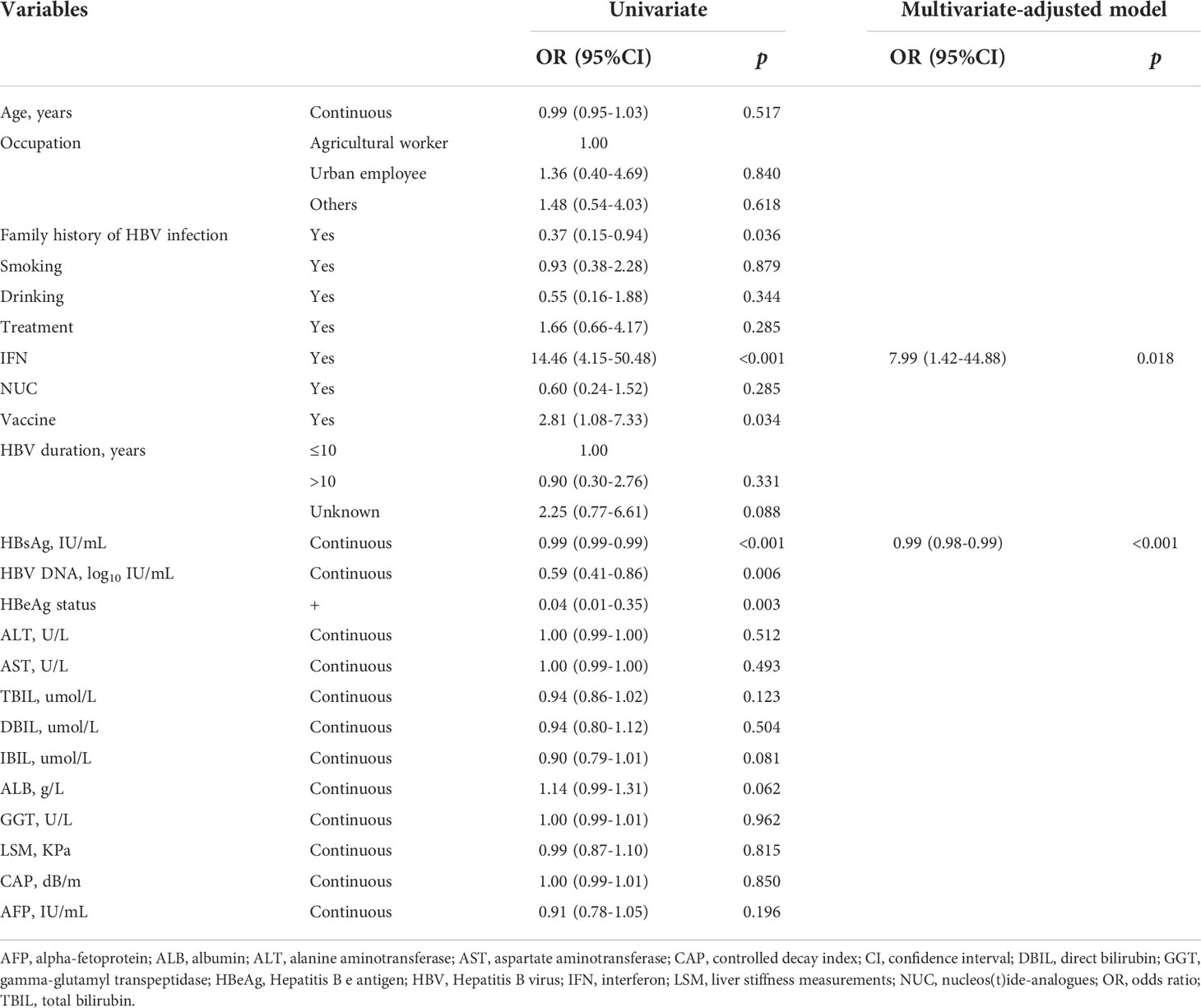
Univariate logistic modelling showed that having a family history of HBV infection, taking IFN treatment, an HBV vaccine history, HBsAg level, HBV DNA level, and HBeAg status significantly associated with HBsAg loss. Multivariate-adjusted modelling with stepwise selection showed that baseline HBsAg levels negatively correlated with HBsAg loss (OR = 0.99, 95%CI = 0.98-0.99). However, IFN treatments also positively related with HBsAg loss (OR = 7.99, 95%CI = 1.62-44.88) under multivariate-adjusted modelling. Please see Table 2 for further details.
In order to explore non-linear relationships, we performed RCS with three knots, adjusting for age, ALT level, HBeAg status, and IFN treatment intake. Results showed that baseline HBsAg levels negatively correlated with HBsAg loss in a linear manner (overall: p < 0.001; non-linear: p = 0.766, Figure 1A).
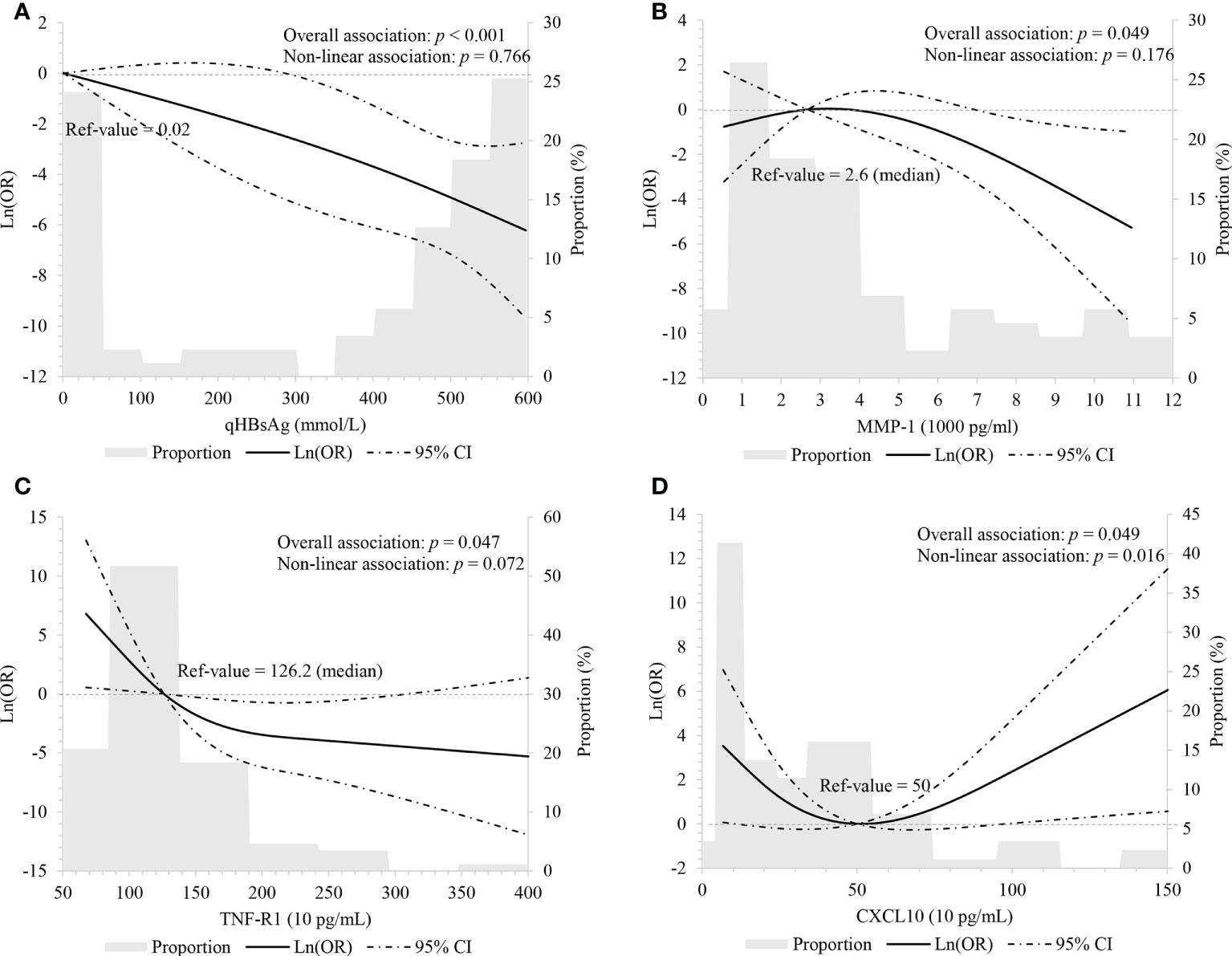
Figure 1 Linear association between HBsAg, cytokines and HBsAg loss using restricted cubic spline with three knots (p5, p50 and p95) (A) HBsAg, adjusting for age, ALT level, HBeAg status and interferon treatment; (B-D) MMP-1, TNF-R1, and CXCL10, adjusting for age, HBsAg level, ALT level, HBeAg status and interferon treatment.
Cytokines and HBsAg loss
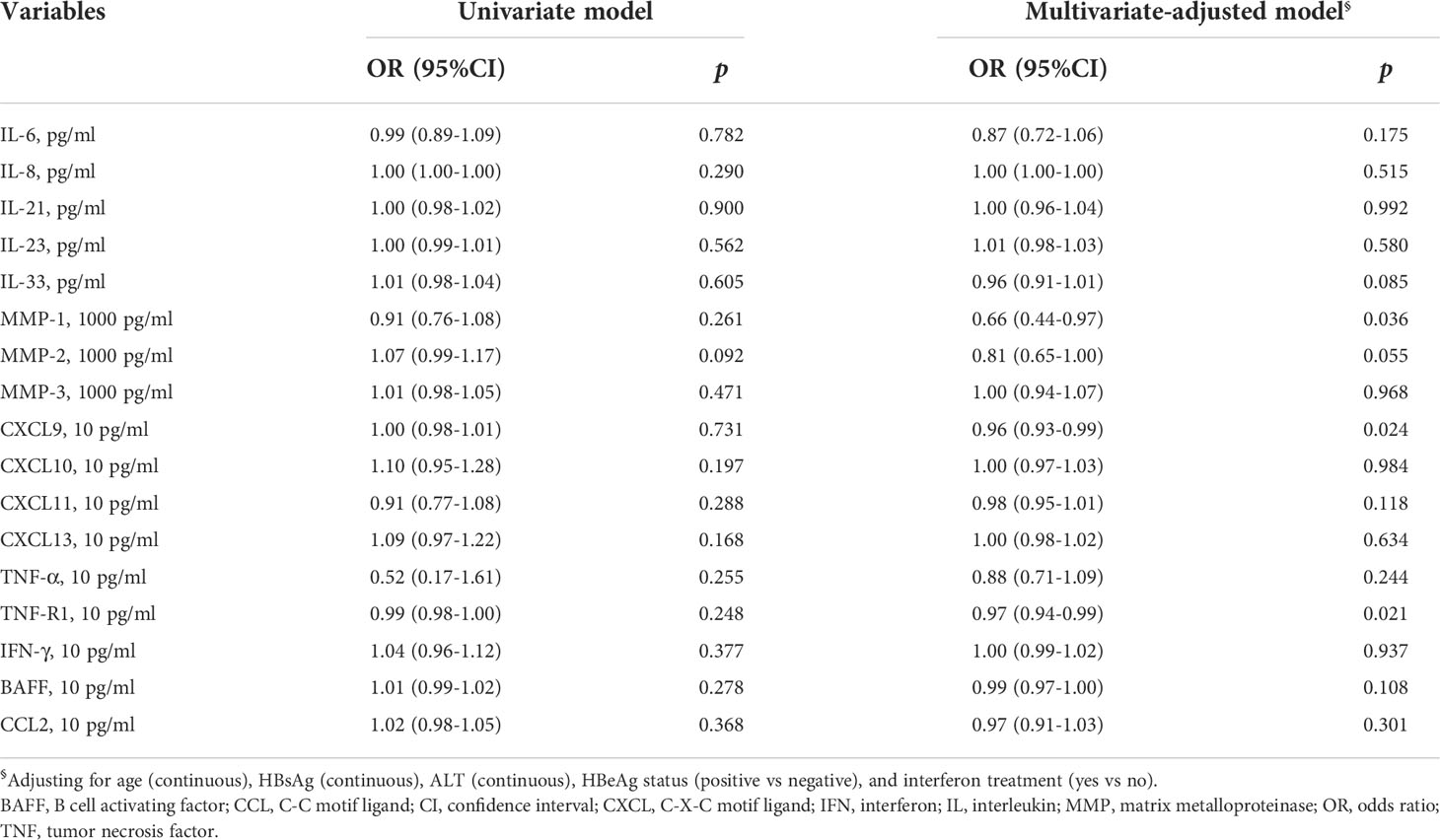
Cytokine levels at baseline between HBsAg consistency and HBsAg loss were compared. Participants who achieved HBsAg loss had lower TNF-R1 (Figure 2A and Supplementary Figure 2). The multivariate-adjusted logistic model showed that baseline MMP-1 (OR = 0.66, 95%CI = 0.44-0.97), CXCL9 (OR = 0.96, 95%CI = 0.93-0.99) and TNF-R1 (OR = 0.97, 95%CI = 0.94-0.99) levels negatively associated with HBsAg loss (Table 3).
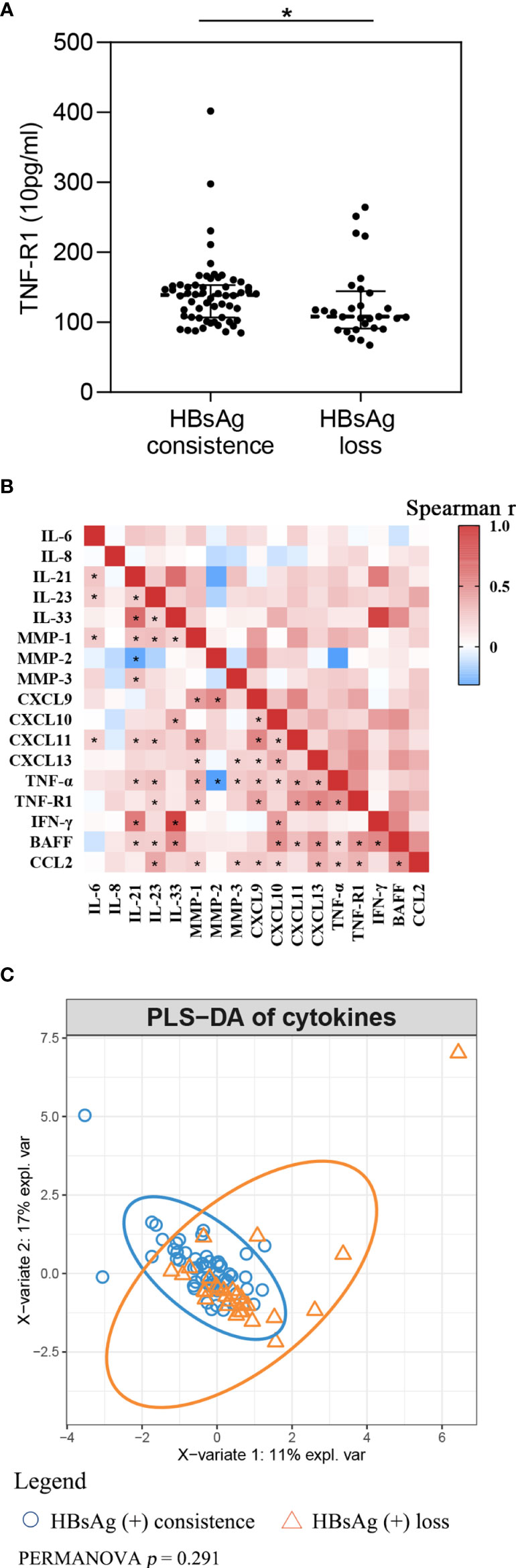
Figure 2 Cytokines comparation between HBsAg consistence and HBsAg loss group (A) TNF-R1 level between HBsAg consistence and HBsAg loss group; (B) Spearman correlation among cytokines. Red, means positive correlation; blue, means negative correlation; *p< 0.05 in Spearman correlation test. (C) Partial least squares discriminant analysis of cytokines.
In order to assess the linearity of relations between cytokines and HBsAg loss, we performed multivariate-adjusted RCS with three knots of P5, P50 and P95. We observed baseline MMP-1 (overall: p = 0.049; non-linear: p = 0.176) and TNF-R1 (overall: p = 0.047; non-linear: p = 0.072) had decreasing associations with HBsAg loss, but this correlation appears linear (Figures 1B, C). Baseline CXCL10 associated with HBsAg loss although the relationship appeared “U-shaped” (overall: p = 0.049; non-linear: p = 0.016). When CXCL10 < 500 pg/ml, CXCL10 had a negative relationship with HBsAg loss; and when CXCL10 ≥ 500 pg/ml, CXCL10 had an increasingly association with HBsAg loss (Figure 1D).
As can be seen in Figure 2B, spearman correlation analysis suggests that cytokines highly correlate, in general. Among the cytokines, IL-21 had a strong positive association with IL-33 (r = 0.711, p < 0.001) and IFN-γ (r = 0.635, p < 0.001) at baseline, and with IL-33 with IFN-γ (r = 0.909, p < 0.001). We took cytokines as integral and used PLS-DA to present the global difference (Figure 2C). PERMANOVA with Euclidean distance was used to compare fundamental differences between the two groups. Findings suggest there is no significant difference at baseline for integral cytokine levels (p = 0.222).
Cytokine SNPs and HBsAg loss
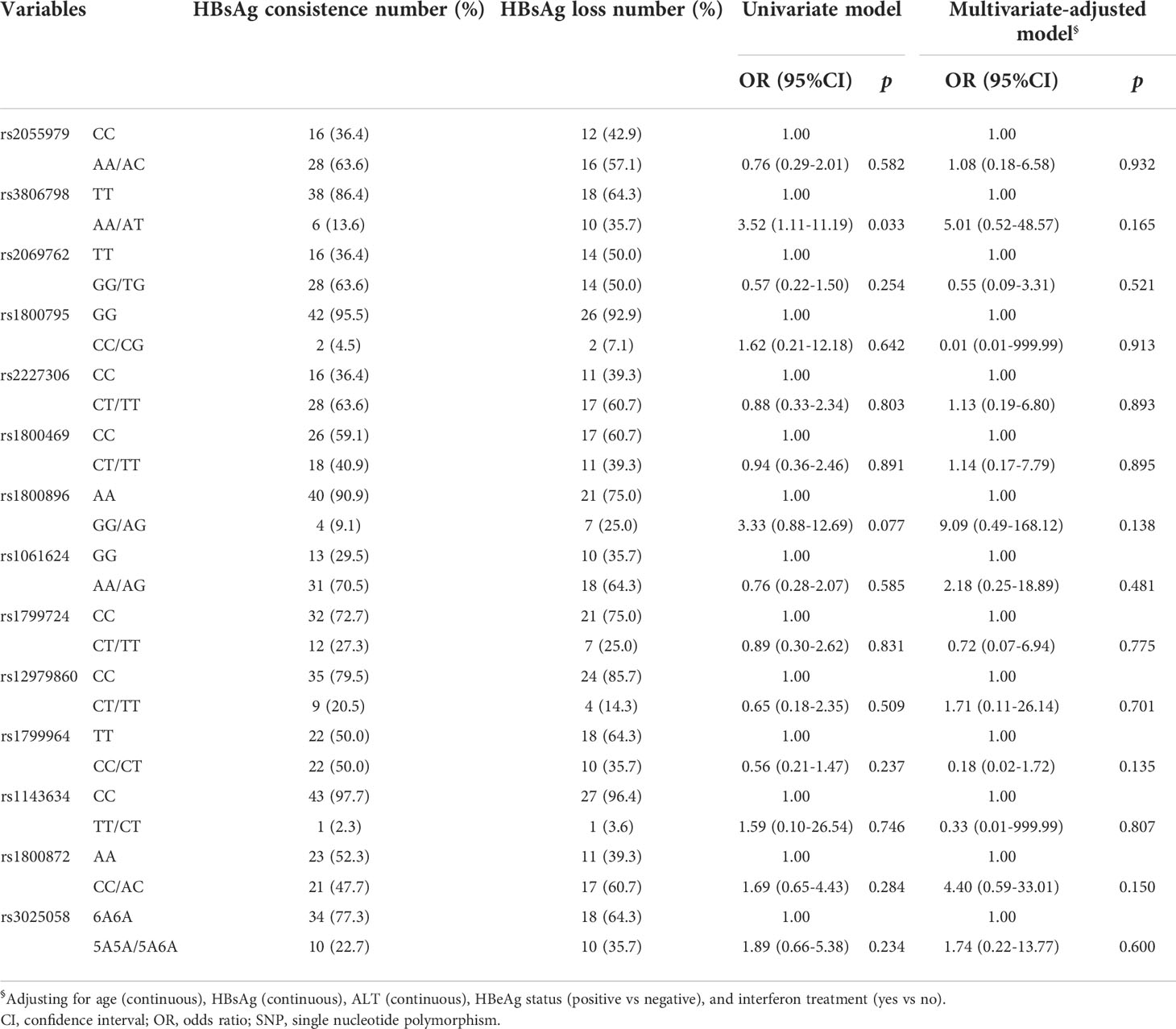
Percentages for SNPs related to cytokines have been provided in Supplementary Figure 3. The AT genotype for rs3806798 was significantly higher in those who encountered HBsAg loss compared to those with consistent HBsAg (Supplementary Figure 3B). Univariate logistic regression results showed the AT genotype of rs3806798 associated with an increased likelihood of HBsAg loss (OR = 3.52, 95%CI = 1.11-11.19). Please see Table 4 for further details. However, after adjusting for age, HBsAg, ALT, HBeAg status, and IFN treatment, statistically significant association disappeared (Table 4). SE, as a measure of genetic diversity, was calculated although no difference between the groups was observed using Wilcoxon’s test (p = 0.713; Supplementary Figure 3O).
Discussion
In this cohort study of HBV patients from Wuwei city, 2.41% encountered HBsAg loss and 0.82% achieved HBsAg seroclearance. In this instance, both scenarios were either spontaneous or initiated through antiviral treatments. During the nested case-control analysis of HBsAg carriers, lower HBsAg levels at baseline and IFN treatment were associated with an increased likelihood of encountering HBsAg loss. In addition, participants with lower levels of MMP-1, CXCL9 and TNF-R1 at baseline appear more likely to achieve HBsAg loss. Baseline CXCL10 appears to be associated with HBsAg loss although the relationship appears to be “U-shaped”.
We found that lower HBsAg levels at baseline is associated with an increased likelihood of encountering HBsAg loss. Lower HBsAg levels at baseline also appear to hold the greatest predictive value for HBsAg seroclearance, as participants with lower HBsAg levels achieve HBsAg loss easier and sooner (19, 20). We also found that IFN treatment was associated with an increased likelihood of HBsAg loss which may be indicative of other outcomes. A meta-analysis based on six clinical controlled trials recently reported that IFN could increase the likelihood of HBsAg seroclearance (21). In addition, IFN-based therapy can lead to more significant HBsAg reduction compared to therapies based on nucleoside and/or nucleotide analogues, leading to an increased likelihood of HBsAg seroclearance (22, 23). This provides evidence to support the use of IFN with (or without) NUC for both naïve and NUC-exposed patients (23). Although, local and regional experience tells us that the use of IFN therapies in the north-west of China remains uncommon. This is something which could be addressed relatively quickly with training or by updating the current Chinese guidelines.
A recent study found that baseline CXCL10/IP10 could be used to predict HBsAg decline in HBeAg-negative patients receiving Entecavir. This is because patients with baseline IP10 > 350 pg/ml were also found to achieve significantly more rapid HBsAg decline i.e. ≥ 0.5 log10 (HR = 4.39, 95%CI = 1.63-11.83) (24). Likewise, a cross-sectional study observed higher level of IP10 in CHB patients with HBsAg seroclearance (25). However, Wong et al. found that lower serum IP-10 levels at year zero (i.e. the time of achieving HBsAg seroclearance) is the only cytokine associated with HBsAg seroclearance (HR per 100 pg/mL = 0.82, 95% CI 0.85-0.99) (14).
Interestingly, we observed that CXCL10 was associated with HBsAg loss and this relationship appears “U-shaped” with the lowest point in the trough occurring at 500 pg/mL. That is to say, the plot highlights a substantial reduction in risk within the lower range of CXCL10, which is consistent with previous studies which have reported the lowest risk of around 500 pg/mL followed by an increase. However, the number of participants in our study was relatively small, especially for those with higher IP10 levels, therefore our study provides only a potential explanation for the aforementioned inconsistencies. Secondly, research suggests that CXCL10 declines before increasing over the treatment period (14, 24). Therefore, CXCL10 levels taken at different times may also become an important measure for assessing relationships.
We also observed an association between lower levels of plasma CXCL9 at baseline and a higher likelihood of HBsAg loss in CHB patients. Similar results have only been found in one acute hepatitis study, where CXCL9, CXCL10, CXCL11 and CXCL13 elevated during the acute phase but then decreased in conjunction with an HBsAg decline (26). However, some researchers have suggested this is not the case for CHB patients (26, 27). Such inconsistent results may be due to differences between study populations, limited sample sizes and variations in outcome definitions. Although, CXCL9 and CXCL10, also known as MIG and IP-10, are all members of CXCL family and could promote Th1 responses as well as initiate CD8+ and natural killer (NK) cell trafficking (28, 29). Increased NK cell functions and HBV-specific CD8+ T cell responses are also associated with HBsAg seroclearance (30). This of course requires further basic medical research but could be relatively easily confirmed.
MMP-1 is one metalloproteinase that has a role in both degrading and denaturing interstitial collagens, types I, II and III. In this study, we observed an association between lower levels of plasma MMP-1 and a greater likelihood of HBsAg loss, which has not yet been reported. On the other hand, the positive relationship between MMP-1 and adverse outcomes for CHB patients has been reported. For example, Flisiak et al. found that MMP-1 levels continue to rise during the first four weeks of acute viral hepatitis, which seems connected to hepatocytes damage (31). Yang et al. (32) also found that MMP-1 significantly increases in HCC patients and could therefore be useful biomarkers for the early HCC detection and in prognostics. Although, the role of MMP-1 during HBsAg loss is not fully understood and requires further investigation.
TNF-R1 appears on almost all cell types and mediates inflammatory responses, cytotoxic action, antiviral activity, and cell proliferation (33). Yang et al. (34) found that TNF-R1 is required to eliminate the natural cccDNA transcriptional template during natural HBV infection. Likewise, Tai et al. (35) observed soluble TNF-R1 levels are higher in CHB patients compared to healthy controls, and that TNF-R1 levels correlate with liver inflammation in CHB cases. In our study, we observed a negative association between plasma TNF-R1 at baseline and the likelihood of HBsAg loss. This may support the theory that participants with reduced inflammation are more likely to lose HBsAg although again further research is required.
In this study, we assumed that participants with HBsAg loss were more likely to present with an anti-inflammatory mode of cytokines, characterized by lower levels of MMP-1, CXCL9, CXCL10 and TNF-R1 at baseline. Therefore, we performed PLS-DA analysis to compare the fundamental differences between the two groups. We did not observe any significant differences although we did manage to develop a more comprehensive description of cytokines.
Before making any recommendations, it is important to first discuss the limitations involved. Firstly, we choose HBsAg loss rather than seroclearance as the main outcome of interest. One reason for this was that our cohort was based on only three years and 71.1% were followed less than twice. This meant we were unable to meet follow-up time criteria for HBsAg seroclearance. We also chose HBsAg loss hoping to gain insight into how HBsAg seroclearance is achieved. However, we accept that we still need to develop our understanding of the relationship between HBsAg loss and HBsAg seroclearance. Secondly, we only observed 62 participants who had achieved HBsAg loss and among these only 29 had complete data. This sample is small however we implemented a matching process (ratio 1:2) to increase inspection efficiency. Finally, we only examined the association between baseline characteristics and HBsAg loss. Some characteristics are time-specific and vary during follow-up. This variability may also influence HBsAg loss although further research is required.
Conclusion
This was a nested case-control study of HBsAg carriers from a cohort in Wuwei city, China. Lower levels of HBsAg, MMP-1, CXCL9 and TNF-R1 at baseline and IFN treatment were associated with an increased likelihood of HBsAg loss. Baseline CXCL10 also appears associated with HBsAg loss but the relationship appears U-shaped, which requires further investigation. It may be possible to develop a nomogram which intercalates these factors; however, researchers should consider immune processes involved in HBsAg loss. In order to improve interventions efficacy we should pay attention to immune activities related HBsAg loss.
Data availability statement
The datasets used in this study can be found in the Supplementary Material.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Committee of the Air Force Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZS and WlZ designed the study, reviewed, and revised the manuscript. XY and TF analyzed the data and wrote the manuscript. LX, ZH, HZ, XK, SZ, QZ and YL did laboratory tests. ZJ and SS revised the manuscript and helped to interpret the results. WhZ and YY managed the cohort. WJ, CL, HT and FW collected data. All authors read and approved the final manuscript.
Funding
This study was supported by China Special Grant for the Prevention and Control of Infection Diseases (2017ZX10105011), National Natural Science Foundation of China (81773488), and the Natural Science Foundation of Shaanxi Province (2021JQ-341).
Acknowledgments
The authors thank the participants who agreed to participate and made this cohort study possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1025654/full#supplementary-material
Glossary

References
1. GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis b, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol (2022) 7:796–829. doi: 10.1016/s2468-1253(22)00124-8
2. Wang H, Men P, Xiao Y, Gao P, Lv M, Yuan Q, et al. Hepatitis b infection in the general population of China: A systematic review and meta-analysis. BMC Infect Dis (2019) 19:811. doi: 10.1186/s12879-019-4428-y
3. Ji Z, Wang T, Shao Z, Huang D, Wang A, Guo Z, et al. A population-based study examining hepatitis b virus infection and immunization rates in Northwest China. PloS One (2014) 9:e97474. doi: 10.1371/journal.pone.0097474
4. Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the global burden of disease study 2013. Lancet (London England) (2016) 388:1081–8. doi: 10.1016/s0140-6736(16)30579-7
5. Anderson R, Choi H, Lenz O, Peters M, Janssen H, Mishra P, et al. Association between seroclearance of hepatitis b surface antigen and long-term clinical outcomes of patients with chronic hepatitis b virus infection: Systematic review and meta-analysis. Clin Gastroenterol Hepatol (2021) 19:463–72. doi: 10.1016/j.cgh.2020.05.041
6. European Association for the Study of the Liver.EASL 2017 clinical practice guidelines on the management of hepatitis b virus infection. J Hepatol (2017) 67:370–98. doi: 10.1016/j.jhep.2017.03.021
7. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis b: A 2015 update. Hepatol Int (2016) 10:1–98. doi: 10.1007/s12072-015-9675-4
8. Yip TC, Wong GL, Wong VW, Tse YK, Lui GC, Lam KL, et al. Durability of hepatitis b surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J Hepatol (2017) 6:32332–2. doi: 10.1016/j.jhep.2017.09.018
9. Yeo Y, Ho H, Yang H, Tseng T, Hosaka T, Trinh H, et al. Factors associated with rates of HBsAg seroclearance in adults with chronic HBV infection: A systematic review and meta-analysis. Gastroenterology (2019) 156:635–646.e639. doi: 10.1053/j.gastro.2018.10.027
10. Pfefferkorn M, Schott T, Böhm S, Deichsel D, Felkel C, Gerlich WH, et al. Composition of HBsAg is predictive of HBsAg loss during treatment in patients with HBeAg-positive chronic hepatitis b. J Hepatol (2021) 74:283–92. doi: 10.1016/j.jhep.2020.08.039
11. Cao J, Gong J, Tsia Hin Fong C, Xiao C, Lin G, Li X, et al. Prediction model of HBsAg seroclearance in patients with chronic HBV infection. BioMed Res Int (2020) 2020:6820179. doi: 10.1155/2020/6820179
12. Liu J, Lee M, Batrla-Utermann R, Jen C, Iloeje U, Lu S, et al. A predictive scoring system for the seroclearance of HBsAg in HBeAg-seronegative chronic hepatitis b patients with genotype b or c infection. J Hepatol (2013) 58:853–60. doi: 10.1016/j.jhep.2012.12.006
13. Yuan T, Jiang Y, Li M, Li W. Chronic hepatitis b surface antigen seroclearance-related immune factors. Hepatol Res (2017) 47:49–59. doi: 10.1111/hepr.12726
14. Wong G, Chan H, Chan H, Tse C, Chim A, Lo A, et al. Serum interferon-inducible protein 10 levels predict hepatitis b s antigen seroclearance in patients with chronic hepatitis b. Alimentary Pharmacol Ther (2016) 43:145–53. doi: 10.1111/apt.13447
15. Xia M, Liao G, Chen H, Wu Y, Fan R, Zhang X, et al. Plasma CXCL13 is a predictive factor for HBsAg loss and clinical relapse after discontinuation of nucleos(t)ide analogue treatment. Clin Immunol (Orlando Fla) (2019) 198:31–8. doi: 10.1016/j.clim.2018.11.016
16. Shannon CE. A mathematical theory of communication. Bell Syst Tech J (1948) 27:379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
17. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med (2010) 29:1037–57. doi: 10.1002/sim.3841
18. Rohart F, Gautier B, Singh A, KA LC. mixOmics: An r package for 'omics feature selection and multiple data integration. PloS Comput Biol (2017) 13:e1005752. doi: 10.1371/journal.pcbi.1005752
19. Mimura S, Fujita K, Takuma K, Nakahara M, Oura K, Tadokoro T, et al. Effect of pegylated interferon alfa-2a in HBeAg-negative chronic hepatitis b during and 48 weeks after off-treatment follow-up: The limitation of pre-treatment HBsAg load for the seroclearance of HBsAg. Internal Emergency Med (2021) 16:1559–1565. doi: 10.1007/s11739-020-02622-7
20. Lim SG, Phyo WW, Ling JZJ, Cloherty G, Butler EK, Kuhns MC, et al. Comparative biomarkers for HBsAg loss with antiviral therapy shows dominant influence of quantitative HBsAg (qHBsAg). Alimentary Pharmacol Ther (2021) 53:172–82. doi: 10.1111/apt.16149
21. Yang YF, Zhao W, Xia HM, Zhong YD, Huang P, Wen J. Long-term efficacy of interferon alpha therapy on hepatitis b viral replication in patients with chronic hepatitis b: A meta-analysis. Antiviral Res (2010) 85:361–5. doi: 10.1016/j.antiviral.2009.10.023
22. Yu Y, Hou J, Omata M, Wang Y, Li L. Loss of HBsAg and antiviral treatment: from basics to clinical significance. Hepatol Int (2014) 8:39–54. doi: 10.1007/s12072-013-9495-3
23. Lampertico P, Viganò M, Colombo M. Why do I treat HBeAg-negative chronic hepatitis b patients with pegylated interferon? Liv Int (2013) 33 Suppl 1:157–63. doi: 10.1111/liv.12064
24. Papatheodoridis G, Goulis J, Manolakopoulos S, Margariti A, Exarchos X, Kokkonis G, et al. Changes of HBsAg and interferon-inducible protein 10 serum levels in naive HBeAg-negative chronic hepatitis b patients under 4-year entecavir therapy. J Hepatol (2014) 60:62–8. doi: 10.1016/j.jhep.2013.08.023
25. Jaroszewicz J, Ho H, Markova A, Deterding K, Wursthorn K, Schulz S, et al. Hepatitis b surface antigen (HBsAg) decrease and serum interferon-inducible protein-10 levels as predictive markers for HBsAg loss during treatment with nucleoside/nucleotide analogues. Antivir Ther (2011) 16:915–24. doi: 10.3851/imp1866
26. Yoshio S, Mano Y, Doi H, Shoji H, Shimagaki T, Sakamoto Y, et al. Cytokine and chemokine signatures associated with hepatitis b surface antigen loss in hepatitis b patients. JCI Insight (2018) 3:e122268. doi: 10.1172/jci.insight.122268
27. Yoshikawa S, Yoshio S, Yoshida Y, Tsutsui Y, Kawai H, Yamazoe T, et al. Impact of immune reconstitution-induced hepatic flare on hepatitis b surface antigen loss in hepatitis b Virus/Human immunodeficiency virus-1 coinfected patients. J Infect Dis (2021) 223:2080–9. doi: 10.1093/infdis/jiaa662
28. Palomino DC, Marti LC. Chemokines and immunity. Einstein (Sao Paulo Brazil) (2015) 13:469–73. doi: 10.1590/s1679-45082015rb3438
29. Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, et al. International union of pharmacology. XXII. nomenclature for chemokine receptors. Pharmacol Rev (2000) 52:145–76.
30. Tout I, Loureiro D, Mansouri A, Soumelis V, Boyer N, Asselah T. Hepatitis b surface antigen seroclearance: Immune mechanisms, clinical impact, importance for drug development. J Hepatol (2020) 73:409–22. doi: 10.1016/j.jhep.2020.04.013
31. Flisiak R, Jaroszewicz J, Lapiński TW, Flisiak I, Rogalska M, Prokopowicz D. Plasma transforming growth factor beta1, metalloproteinase-1 and tissue inhibitor of metalloproteinases-1 in acute viral hepatitis type b. Regul peptides (2005) 131:54–8. doi: 10.1016/j.regpep.2005.06.002
32. Yang L, Rong W, Xiao T, Zhang Y, Xu B, Liu Y, et al. Secretory/releasing proteome-based identification of plasma biomarkers in HBV-associated hepatocellular carcinoma. Sci China Life Sci (2013) 56:638–46. doi: 10.1007/s11427-013-4497-x
33. Herbein G, O'Brien WA. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc Soc Exp Biol Med (2000) 223:241–57. doi: 10.1046/j.1525-1373.2000.22335.x
34. Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M, et al. . Proc Natl Acad Sci United States America (2010) 107:798–802. doi: 10.1073/pnas.0913498107
Keywords: HBsAg, interferon, chemokine, MMP-1, TNF-R1
Citation: Yuan X, Fu T, Xiao L, He Z, Ji Z, Seery S, Zhang W, Ye Y, Zhou H, Kong X, Zhang S, Zhou Q, Lin Y, Jia W, Liang C, Tang H, Wang F, Zhang W and Shao Z (2022) Describing immune factors associated with Hepatitis B surface antigen loss: A nested case-control study of a Chinese sample from Wuwei City. Front. Immunol. 13:1025654. doi: 10.3389/fimmu.2022.1025654
Received: 23 August 2022; Accepted: 20 September 2022;
Published: 11 October 2022.
Edited by:
Yuen Gao, Michigan State University, United StatesReviewed by:
Slim Fourati, Emory University, United StatesYongqiang Gao, Harvard Medical School, United States
Copyright © 2022 Yuan, Fu, Xiao, He, Ji, Seery, Zhang, Ye, Zhou, Kong, Zhang, Zhou, Lin, Jia, Liang, Tang, Wang, Zhang and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongjun Shao, MTM3NTk5ODE3ODNAMTYzLmNvbQ==; Weilu Zhang, emhhbmd3ZWlsdUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Xiaojie Yuan
Xiaojie Yuan Ting Fu
Ting Fu Lixin Xiao1,2
Lixin Xiao1,2 Zhen He
Zhen He Zhaohua Ji
Zhaohua Ji Samuel Seery
Samuel Seery Weilu Zhang
Weilu Zhang Zhongjun Shao
Zhongjun Shao