
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 08 December 2022
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1025553
 Li-Chin Liao1,2
Li-Chin Liao1,2 Yi-Hsiu Fu2
Yi-Hsiu Fu2 Chieh-Mao Chuang2
Chieh-Mao Chuang2 Pei-Lun Liao3*
Pei-Lun Liao3* James Cheng-Chung Wei4,5,6*
James Cheng-Chung Wei4,5,6* Yun-Ching Fu2,7,8*
Yun-Ching Fu2,7,8*Objectives: Recent research has demonstrated the commonality of several biological markers between Kawasaki disease (KD) and juvenile idiopathic arthritis (JIA), including interleukin-1β and -6. Therefore, in this cohort study, we assessed whether KD increases the risk of JIA.
Methods: This study enrolled 7009 patients with and 56 072 individuals without KD in the period 2010–2018 from Taiwan’s National Health Insurance Research Database. On the basis of sex, age, and comorbidities, we executed propensity score matching at the ratio 1:8. The adjusted hazard ratio (aHR) for JIA was determined through multiple Cox regression. Stratified analysis and sensitivity tests were also employed.
Results: When adjusting for age, sex, and comorbidities, the JIA risk was noted to be 2.02-fold greater in children with KD than it was in those without (aHR: 2.02, 95% confidence interval: 1.12–3.67, p = 0.0205). The sensitivity test and subgroup analysis obtained consistent findings in the different sex and comorbidity subgroups.
Conclusion: Children’s risk of JIA is higher if they have KD. Pediatricians should consider the possibility of JIA in this population. More investigations are necessary to identify the pathological mechanisms that link JIA and KD.
Juvenile idiopathic arthritis (JIA) and KD are two distinct systemic inflammatory diseases. They have their own diagnostic criteria but do share numerous clinical features, including such biological markers as interleukin-1β (IL-1β) and IL-6 (1, 2).
For KD, the patients with which are usually children (3–5), intravenous immunoglobulin was discovered to lower the incidence of cardiovascular abnormalities from 25% to <5% (3).
A common complication of KD, arthritis was reported to occur in 30% of patients with this disease in a study conducted before intravenous immunoglobulin was widely used (3). Arthritis is a self-limiting condition and has no association with cartilage destruction (6); several cases of JIA have been reported (7). Diagnosis of KD or JIA is challenging owing to their shared features and the lack of knowledge on relevant biomarkers (7, 8). Kawasaki-disease-related arthritis is more frequently associated with coronary artery abnormalities than is non-Kawasaki-disease-related arthritis (9, 10).
Whether KD increases the risk of JIA in children has not yet been determined. Unfortunately, little evidence has been obtained regarding the correlation between KD and JIA. In addition, no study has yet employed a large national database to longitudinally assess this correlation. KD was hypothesized in this study to increase the JIA risk. Accordingly, Taiwan’s National Health Insurance Research Database (NHIRD) was employed to obtain a real-world, population-based retrospective cohort to test this hypothesis.
The NHIRD includes the health-care data of approximately 23 million National Health Insurance beneficiaries (>99% of the population). These data are claims data and include records relating to hospitalizations and outpatient and emergency visits. The Longitudinal Health Insurance Database, constituting an NHIRD subset, is composed of data on 1 million National Health Insurance beneficiaries for the period 2010–2018, a sample that was obtained through random sampling of all 23 million beneficiaries. Before researchers are given access to the database, all patient data are deidentified to ensure the patients’ privacy. We executed our current study after receiving ratification from Chung Shan Medical University Hospital’s Institutional Review Board (no. CS19159).
The cohort in this study comprised children who received a new diagnosis of KD (11–13) (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] code 446.1 and ICD-10-CM code M30.3) from 2010 to 2018. The diagnosis of KD in Taiwan would have been confirmed as American Heart Association diagnosis criteria of KD, which meet one principal diagnosis and up to four secondary diagnoses. Therefore, we exclude the incomplete type or atypical KD patients based on the criteria. To ensure diagnostic accuracy, children with any diagnosis of KD prior to 2010 were excluded from this study. Only children with at least 1 hospitalization were selected for the final analysis.
The non-Kawasaki-disease group contained children without a diagnosis of KD during 2010–2018. We considered the 30-day time point after KD diagnosis as the index date. To ensure that all of the included individuals had a new diagnosis of JIA, we excluded any pre-index-date diagnosis of JIA (ICD-9-CM codes 714 and 720; ICD-10-CM codes M05, M06, M08, and M45) (14).
A diagnosis of JIA, with the patient being hospitalized at least once or making one or more outpatient visits, was this study’s outcome variable. The follow-up period employed in this study was up to the occurrence of JIA; December 31, 2018; or withdrawal from the National Health Insurance system, whichever occurred first.
The following demographic and medical characteristics were considered in the present study: sex, level of urbanization of residence, age, and related comorbidities—autism (ICD-9-CM code 299.0; ICD-10-CM code F84.0), attention deficit hyperactivity disorder (ICD-9-CM code 314.0; ICD-10-CM codes F90, F98.8, and R41.840), asthma (ICD-9-CM code 493; ICD-10-CM codes J44 and J45), atopic dermatitis (ICD-9-CM code 691; ICD-10-CM codes L20 and L22), allergic rhinitis (ICD-9-CM code 477; ICD-10-CM code J30), and Salmonella infection (ICD-9-CM code 003; ICD-10-CM code A02). A patient was defined as having these comorbidities when they made at least one outpatient visit or had one hospitalization during the 12 months preceding the index date.
The two groups’ heterogeneity was balanced through matching of propensity scores, probabilities that are estimated through logistic regression. The groups were matched on the basis of sex, level of urbanization of residence, age, attention deficit hyperactivity disorder, allergic rhinitis, asthma, and atopic dermatitis. The binary variable was developing versus not developing KD.
The two groups were compared by utilizing absolute standardized differences. An absolute standardized difference < 0.1 indicated that the groups had similar characteristics. To estimate the hazard ratio for JIA in the Kawasaki-disease group versus the non-Kawasaki-disease group, we employed the Cox proportional-hazard model. The Kaplan–Meier method was employed to determine the JIA cumulative incidence; in addition, the log-rank test was next executed to test for significance.
As displayed in Figure 1, from the Longitudinal Health Insurance Database for the period 2010–2018, 7009 patients with KD and 56 072 matched controls could be identified. Table 1 shows that the sex and age distributions in the two groups were similar.
The intergroup differences after propensity score matching were discovered to be nonsignificant.
After making adjustments in the multivariate analysis, we discovered the JIA risk to be significantly greater for the children with KD than for those without (adjusted hazard ratio [aHR]: 2.02, 95% confidence interval [CI]: 1.12–3.67, p = 0.0205; Table 2). In terms of comorbidities, individuals with allergic rhinitis had a higher JIA risk (aHR: 3.06, 95% CI: 1.60–5.84, p < 0.001; Table 3). Children in the Kawasaki-disease group aged 6–18 years had a higher JIA risk than did those aged 0–3 years (aHR: 2.82, 95% CI: 1.49–5.34, p = 0.0015). Table 4 details the demographic-characteristic-based subgroup analyses of associations between KD and JIA. Those in the Kawasaki-disease group aged 0–3, 4–5, and 6–18 years had a 1.84-fold, 1.89-fold, and 3.1-fold greater risk of JIA, respectively (aHR: 1.84, 95% CI: 0.88–3.83, p = 0.104; aHR: 1.89, 95% CI: 0.20–17.94, p = 0.578; aHR: 3.1, 95% CI: 0.95–10.11, p = 0.061, respectively). The JIA risk was noted to be 3.01-fold greater in girls with KD than it was in girls without KD (aHR: 3.01, 95% CI: 1.38–6.54, p = 0.005), but it was noted to be 1.28-fold greater in boys with KD than it was in boys without KD (aHR: 1.28, 95% CI: 0.49–3.34, p = 0.612). Furthermore, the Kawasaki-disease group had a higher JIA risk regardless of comorbidities or level of urbanization of residence. We also observed the JIA risk to be greater in children with KD diagnosed through catastrophic disease certification than it was in those without KD (aHR: 3.11, 95% CI: 1.21–7.96, p = 0.0181; Table 5).
Figure 2 presents the Kaplan–Meier curves for our cohort. We determined the Kawasaki-disease group to exhibit a significantly higher cumulative incidence of JIA when compared with that in the non-Kawasaki-disease group. In the comparison of cumulative incidence curves, the log-rank test returned p = 0.007.
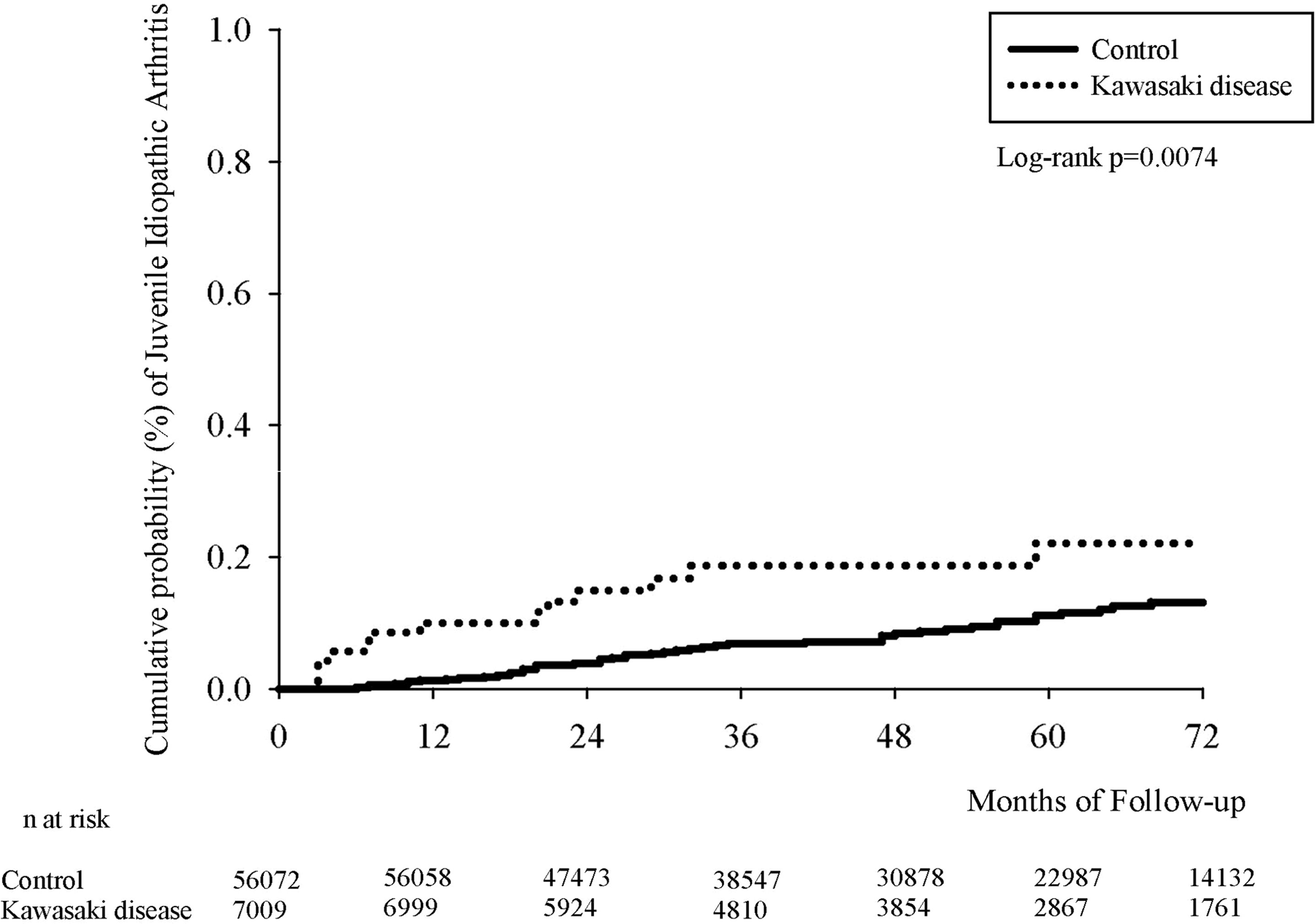
Figure 2 Kaplan–Meier curve of cumulative incidence proportion of JIA in the Kawasaki-disease and non-Kawasaki-disease groups.
In our current study, children with KD had a 2.02-fold higher JIA risk than did those without KD, regardless of sex, comorbidities, age, or level of urbanization of residence. This is the first research to analyze the association between KD and JIA in the setting of Taiwan. In addition, this appears to be the largest epidemiological study to probe the KD–JIA association by employing a nationwide longitudinal population-based dataset. The risk of JIA should be explained to the parents of children with KD, and these patients should be given appropriate management for JIA as required.
A study conducted recently in developed countries reported the prevalence of JIA to be up to 150 per 100 000 children (15). Because of differences in classification criteria, the studied population, and research methods, the findings related to JIA vary widely (16, 17). The JIA prevalence was 1.33 per 100 000 children in one study (18). The striking association of KD with JIA indicates that these two diseases have common immunobiology and similar cytokine signatures, biologic agents, and immune activation (19–23). In Taiwan, the JIA classification of the International League of Associations for Rheumatology (final revision) is employed to define diagnostic criteria (24).
IL-1β is a mediator of synovial inflammation and is released in response to injury or stress (25). Serum from patients with JIA was discovered to produce IL-1β after being stimulated, and IL-1β was relieved from peripheral blood cells (26). Data obtained in animal and genetic studies of KD indicate that IL-1β is involved in intravenous immune globulin, response to cardiac outcomes, and inflammation (27–29). Serum from patients with KD was found to persistently exhibit elevated IL-6 and IL-8 levels (22). IL-1β blockade in children with JIA may reduce the proportion of children with KD with excessive IL-1β production despite intravenous immunoglobulin therapy. The efficacy of canakinumab (30) and anakinra (31) is the same as that of IL-1 β blockade. In KD, anti-IL-1β treatment may help prevent coronary artery disease, although more studies are required on this topic. In JIA, blockade of IL-6 can reduce inflammation; thus, IL-6 can be concluded to play a major role in this disease (32, 33).
The bloodstream contains inflammatory cytokines, which continually infiltrate endothelial cells and structures by inducing inflammation (34). As KD progresses, C-reactive protein levels increase in tandem with increases in IL-1β and IL-6 Levels (35, 36). In 1991, Newburger proposed intravenous immunoglobulin therapy, which has become the conventional treatment for KD (37, 38). Although the mechanism behind this treatment remains unclear, intravenous immunoglobulin is thought to reduce the levels of inflammatory cytokines and thus achieve their balance. Intravenous immunoglobulin was reported by a previous study to reduce the level of IL-6 to within the normal range, although that study also noted the soluble IL-6 receptor and TNF-α levels to be unaffected by this therapy (39). Researchers have discovered that in KD’s pathogenesis, one critical factor is the presence in the blood of inflammatory cytokines (40, 41).
KD and JIA each have their own diagnostic criteria but initially have similar clinical manifestations. In one study, 0.5% of children with KD had JIA, whereas KD preceded JIA in 7% of cases (1). This percentage is higher than that reported in a study published in 2015, which revealed the KD–JIA incidence to be 0.2% among 6745 patients with KD, the data of whom were derived from a database containing patients’ health information (7). Patients satisfying both the KD and JIA diagnostic criteria were reported to exhibit a lower albumin level and higher leukocyte count and to be more refractory to standard intravenous immunoglobulin therapy (1). Arthritis was found in 7.5% of patients with KD, further highlighting the clinical similarity of these two diseases (42). The many clinical features shared by JIA and KD appear to be more persistent in JIA and more self-limiting in KD (1).
This study does have some limitations. First, in the NHIRD, KD and JIA diagnoses are both based on ICD-9-CM and ICD-10-CM codes. Therefore, our current study may have underestimated the incidence of JIA because patients who had mild symptoms may not have been hospitalized or brought to the outpatient department. Second, it was not possible to further analyze and discuss the results for JIA subtypes because the dataset did not contain related information. Third, the NHIRD does contain data on covariates, such as laboratory findings, environmental factors, social adversity, genetics, personal lifestyles, and family history. Although we performed propensity score matching and adjusted for various possible confounders, such as lifestyle-related comorbidities, unmeasured confounding factors may have influenced the present findings. Finally, considering that we derived our results from analyzing Taiwanese patient data, it may not be possible to directly generalize our findings to other ethnic groups. Scholars should conduct studies involving other nationalities and ethnicities to determine how generalizable is the relationship discovered in this study.
In conclusion, children with KD were at a 2.02-fold higher JIA risk than children without KD, regardless of sex, level of urbanization, age, or comorbidities. Therefore, when caring for a child with KD, the clinician should be aware of the possibility that other systemic inflammatory disorders, such as JIA, can develop in the future. The pathophysiological association between KD and JIA requires further study.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
L-CL and Y-HF drafted the manuscript. JW revised the manuscript critically. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Go E, van Veenendaal M, Manlhiot C, Schneider R, McCrindle BW, Yeung RSM. Kawasaki Disease and systemic juvenile idiopathic arthritis - two ends of the same spectrum. Front Pediatr (2021) 9:665815. doi: 10.3389/fped.2021.665815
2. Ko TM, Kuo HC, Chang JS, Chen SP, Liu YM, Chen HW, et al. CXCL10/IP-10 is a biomarker and mediator for Kawasaki disease. Circ Res (2015) 116(5):876–83. doi: 10.1161/CIRCRESAHA.116.305834
3. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American heart association. Circulation (2017) 135(17):e927–99. doi: 10.1161/CIR.0000000000000484
4. Hara T, Yamamura K, Sakai Y. The up-to-date pathophysiology of Kawasaki disease. Clin Transl Immunol (2021) 10(5):e1284. doi: 10.1002/cti2.1284
5. Wang CL, Wu YT, Liu CA, Kuo HC, Yang KD. Kawasaki Disease: infection, immunity and genetics. Pediatr Infect Dis J (2005) 24(11):998–1004. doi: 10.1097/01.inf.0000183786.70519.fa
6. Martins A, Conde M, Brito M, Gouveia C. Arthritis in Kawasaki disease: A poorly recognised manifestation. J Paediatr Child Health (2018) 54(12):1371–4. doi: 10.1111/jpc.14102
7. Dong S, Bout-Tabaku S, Texter K, Jaggi P. Diagnosis of systemic-onset juvenile idiopathic arthritis after treatment for presumed Kawasaki disease. J Pediatr (2015) 166(5):1283–8. doi: 10.1016/j.jpeds.2015.02.003
8. Jiang H, Yang Z. Severe recurrent fever episodes with clinical diagnosis of hemophagocytic lymphohistiocytosis, incomplete Kawasaki disease and systemic-onset juvenile idiopathic arthritis: A case report and literature review. Front Pediatr (2020) 8:93. doi: 10.3389/fped.2020.00093
9. Lefevre-Utile A, Galeotti C, Kone-Paut I. Coronary artery abnormalities in children with systemic-onset juvenile idiopathic arthritis. Joint Bone Spine (2014) 81(3):257–9. doi: 10.1016/j.jbspin.2013.09.004
10. Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int (2005) 47(2):232–4. doi: 10.1111/j.1442-200x.2005.02033.x
11. Weng KP, Cheng-Chung Wei J, Hung YM, Huang SH, Chien KJ, Lin CC, et al. Enterovirus infection and subsequent risk of Kawasaki disease: A population-based cohort study. Pediatr Infect Dis J (2018) 37(4):310–5. doi: 10.1097/INF.0000000000001748
12. Chen TY, Chou MC, Lai JN, Chiu LT, Chang R, Hung YM, et al. Non-typhoidal salmonella and the risk of Kawasaki disease: A nationwide population-based cohort study. Front Immunol (2021) 12:701409. doi: 10.3389/fimmu.2021.701409
13. Huang SH, Chen CY, Weng KP, Chien KJ, Hung YM, Hsieh KS, et al. Adenovirus infection and subsequent risk of Kawasaki disease: A population-based cohort study. J Chin Med Assoc (2020) 83(3):302–6. doi: 10.1097/JCMA.0000000000000266
14. Fung SG, Webster R, Kuenzig ME, Knight BD, Batthish M, Robinson C, et al. Incidence of chronic immune-mediated inflammatory diseases after diagnosis with Kawasaki disease: a population-based cohort study. Rheumatol (Oxford) (2022) 61(5):2095–103. doi: 10.1093/rheumatology/keab680
15. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet (2007) 369(9563):767–78. doi: 10.1016/S0140-6736(07)60363-8
16. Yu HH, Chen PC, Wang LC, Lee JH, Lin YT, Yang YH, et al. Juvenile idiopathic arthritis-associated uveitis: a nationwide population-based study in Taiwan. PloS One (2013) 8(8):e70625. doi: 10.1371/journal.pone.0070625
17. Huang JL, Yao TC, See LC. Prevalence of pediatric systemic lupus erythematosus and juvenile chronic arthritis in a Chinese population: a nation-wide prospective population-based study in Taiwan. Clin Exp Rheumatol (2004) 22(6):776–80.
18. Lin CH, Lin CL, Shen TC, Wei CC. Epidemiology and risk of juvenile idiopathic arthritis among children with allergic diseases: A nationwide population-based study. Pediatr Rheumatol Online J (2016) 14(1):15. doi: 10.1186/s12969-016-0074-8
19. Furukawa S, Matsubara T, Jujoh K, Yone K, Sugawara T, Sasai K, et al. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clin Immunol Immunopathol (1988) 48(2):247–51. doi: 10.1016/0090-1229(88)90088-8
20. Maury CP, Salo E, Pelkonen P. Circulating interleukin-1 beta in patients with Kawasaki disease. N Engl J Med (1988) 319(25):1670–1. doi: 10.1056/NEJM198812223192515
21. Leung DY, Cotran RS, Kurt-Jones E, Burns JC, Newburger JW, Pober JS. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet (1989) 2(8675):1298–302. doi: 10.1016/S0140-6736(89)91910-7
22. Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr (1992) 121(6):924–6. doi: 10.1016/S0022-3476(05)80343-9
23. Mellins ED, Macaubas C, Grom AA. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol (2011) 7(7):416–26. doi: 10.1038/nrrheum.2011.68
24. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol (2004) 31(2):390–2.
25. Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the koebner phenomenon: role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol (2008) 172(4):961–71. doi: 10.2353/ajpath.2008.070710
26. Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med (2005) 201(9):1479–86. doi: 10.1084/jem.20050473
27. Lee Y, Schulte DJ, Shimada K, Chen S, Crother TR, Chiba N, et al. Interleukin-1beta is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation (2012) 125(12):1542–50. doi: 10.1161/CIRCULATIONAHA.111.072769
28. Alphonse MP, Duong TT, Shumitzu C, Hoang TL, McCrindle BW, Franco A, et al. Inositol-triphosphate 3-kinase c mediates inflammasome activation and treatment response in Kawasaki disease. J Immunol (2016) 197(9):3481–9. doi: 10.4049/jimmunol.1600388
29. Schett G, Dayer JM, Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol (2016) 12(1):14–24. doi: 10.1038/nrrheum.2016.166
30. Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med (2012) 367(25):2396–406. doi: 10.1056/NEJMoa1205099
31. Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann Rheum Dis (2011) 70(5):747–54. doi: 10.1136/ard.2010.134254
32. Chen G, Deutsch GH, Schulert G, Zheng H, Jang S, Trapnell B, et al. Serum proteome analysis of systemic JIA and related lung disease identifies distinct inflammatory programs and biomarkers. Arthritis Rheumatol (2022) 74(7):1271–83. doi: 10.1101/2021.01.20.21250141
33. Aussems K, Schoemaker CG, Verwoerd A, Ambrust W, Cowan K, Dedding C. Research agenda setting with children with juvenile idiopathic arthritis: Lessons learned. Child Care Health Dev (2022) 48(1):68–79. doi: 10.1111/cch.12904
34. Matsubara T, Ichiyama T, Furukawa S. Immunological profile of peripheral blood lymphocytes and monocytes/macrophages in Kawasaki disease. Clin Exp Immunol (2005) 141(3):381–7. doi: 10.1111/j.1365-2249.2005.02821.x
35. Nishikawa T, Hagihara K, Serada S, Isobe T, Matsumura A, Song J, et al. Transcriptional complex formation of c-fos, STAT3, and hepatocyte NF-1 alpha is essential for cytokine-driven c-reactive protein gene expression. J Immunol (2008) 180(5):3492–501. doi: 10.4049/jimmunol.180.5.3492
36. Hagihara K, Nishikawa T, Sugamata Y, Song J, Isobe T, Taga T, et al. Essential role of STAT3 in cytokine-driven NF-kappaB-mediated serum amyloid a gene expression. Genes Cells (2005) 10(11):1051–63. doi: 10.1111/j.1365-2443.2005.00900.x
37. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med (1991) 324(23):1633–9. doi: 10.1056/NEJM199106063242305
38. Suzuki T, Michihata N, Yoshikawa T, Saito K, Matsui H, Fushimi K, et al. Low- versus high-concentration intravenous immunoglobulin for children with Kawasaki disease in the acute phase. Int J Rheum Dis (2022) 25(5):576–83. doi: 10.1111/1756-185X.14309
39. Gupta M, Noel GJ, Schaefer M, Friedman D, Bussel J, Johann-Liang R. Cytokine modulation with immune gamma-globulin in peripheral blood of normal children and its implications in Kawasaki disease treatment. J Clin Immunol (2001) 21(3):193–9. doi: 10.1023/A:1011039216251
40. Orenstein JM, Shulman ST, Fox LM, Baker SC, Takahashi M, Bhatti TR, et al. Three linked vasculopathic processes characterize Kawasaki disease: A light and transmission electron microscopic study. PloS One (2012) 7(6):e38998. doi: 10.1371/journal.pone.0038998
41. Galeotti C, Bayry J, Kone-Paut I, Kaveri SV. Kawasaki Disease: aetiopathogenesis and therapeutic utility of intravenous immunoglobulin. Autoimmun Rev (2010) 9(6):441–8. doi: 10.1016/j.autrev.2009.12.004
Keywords: Kawasaki disease, juvenile idiopathic arthritis, longitudinal health insurance database, interleukin-1β, interleukin-6
Citation: Liao L-C, Fu Y-H, Chuang C-M, Liao P-L, Wei JC-C and Fu Y-C (2022) Impact of Kawasaki disease on juvenile idiopathic arthritis in real-world patients: A population-based cohort study. Front. Immunol. 13:1025553. doi: 10.3389/fimmu.2022.1025553
Received: 23 August 2022; Accepted: 22 November 2022;
Published: 08 December 2022.
Edited by:
Ozgur Kasapcopur, Istanbul University-Cerrahpasa, TurkeyReviewed by:
Sezgin Sahin, Istanbul University-Cerrahpasa, TurkeyCopyright © 2022 Liao, Fu, Chuang, Liao, Wei and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Cheng-Chung Wei, amNjd2VpQGdtYWlsLmNvbQ==; Yun-Ching Fu, eXVuY2hpbmdmdUBnbWFpbC5jb20=; Pei-Lun Liao, bGlhb3BlaWx1bjA0MTBAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.