
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 10 October 2022
Sec. Vaccines and Molecular Therapeutics
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1021537
Background: Systemic therapy is an important treatment for psoriasis. Phosphodiesterase 4 (PDE4) inhibitors are new candidates for psoriasis therapy.
Objectives: To evaluate the efficacy and safety of PDE4 inhibitors in psoriasis.
Method: Randomized clinical trials with PDE4 inhibitors vs placebos in patients with psoriasis were identified from MEDLINE, Embase, Cochrane Controlled Register of Trials, ClinicalTrials.gov, from inception to July 14, 2022. The study was registered in PROSPERO (CRD42022345700).
Results: 18 studies were identified, 9 of which included moderate-to-severe plaque psoriasis, 2 mild-to-moderate plaque psoriasis, and 7 psoriatic arthritis. A total of 6036 patients were included. Only one oral PDE4 inhibitor, apremilast, met the inclusion criteria. Overall, compared with the placebo, apremilast was associated with higher response rates in PASI-75 (RR, 3.22; 95% CI, 2.59-4.01), ScPGA of 0 or 1 (RR, 2.21; 95% CI, 1.69-2.91), PPPGA of 0 or 1 (RR 2.33; 95%CI, 1.16-4.66), and a significant decrease in NPASI (SMD, -0.46; 95% CI, -0.58 to -0.33). There were no significant differences in serious adverse events. Subgroup analyses showed that significantly more patients achieved PASI-75 after 16 weeks of therapy with apremilast of 20 mg bid (RR, 2.82; 95% CI, 2.01-3.95) and 30 mg bid (RR, 4.08; 95% CI, 3.12-5.33). Heterogeneity was not significant across studies.
Conclusion: Apremilast is a safe and effective treatment for plaque psoriasis and psoriatic arthritis, especially for difficult-to-treat sites.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier (CRD42022345700).
Psoriasis is a common chronic relapsing non-infectious inflammatory disease. More than 60 million adults and children are affected worldwide (1). The clinical manifestations of psoriasis are extensive and recurring erythematous scales, especially on areas that affect aesthetics and function, such as the head, nails, palms, and soles, which seriously affect the patients’ mental health and quality of life (2). The lack of long-term and effective control means has brought a serious economic burden to individuals and society, and has become a public health problem of great concern (3).
Treatment strategies for psoriasis should take into account its severity, comorbidities, patient economic status, patient preference (oral or subcutaneous injection), and treatment expectations (1, 2, 4). With the exception of moderate to severe psoriasis, patients with mild psoriasis whose symptoms do not improve after topical therapy or who have psoriatic arthritis (PsA), psoriatic nail involvement, or involvement of the hands, feet, genital areas, or scalp may benefit from systemic therapy (5, 6).
Oral traditional systemic drugs used to treat psoriasis include methotrexate, cyclosporine and acitretin, which can exhibit drug-drug interactions and cumulative organ toxicity (7). Although biologics have shown good results in psoriasis, price may limit their use (8). Apremilast is a small molecule oral phosphodiesterase 4 (PDE4) inhibitor, which exerts anti-inflammatory effect by up-regulating the levels of anti-inflammatory cytokines (e.g., IL-10) (9), and reducing the levels of proinflammatory factors (e.g., TNFα and IL-8) (10). It has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of PsA and moderate to severe plaque psoriasis in 2014 (11).
A recently published meta-analysis evaluated the efficacy and safety of apremilast in the treatment of plaque psoriasis. This study included plaque psoriasis only, not other types of psoriasis, such as PsA. Patients with PsA also have psoriatic skin lesions. And this study only assessed general physical signs (Psoriasis Area Severity Index, PASI; Static Physician Global Assessment, sPGA), not localized lesions, such as nail and scalp (12).
Therefore, in order to further understand the application of PDE4 inhibitor in patients with various types of psoriasis, we used meta-analysis to quantitatively synthesize the current evidence on the efficacy and safety of PDE4 inhibitors in the treatment of various psoriasis, thereby providing a higher level of evidence-based medical evidence for clinical decision-making.
We registered the protocol in International Prospective Register of Systematic Reviews (PROSPERO CRD42022345700) and followed the standard methodological guidelines for meta-analysis of randomized clinical trials (RCTs) (13) and the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (PRISMA) (14).
We conducted searches in three electronic medical databases, including Medline, Embase and Cochrane Controlled Register of Trials (CENTRIL) from inception to July 14th, 2022. We also searched ClinicalTrials.gov for unpublished trials. No restrictions on languages were placed. The search strategies are provided in eTable 1.
Two investigators (HY and QK) independently assessed trial eligibility. We included RCTs comparing PDE4 inhibitors with placebo in the treatment of psoriasis, regardless of study design, age, race/ethnicity, dose, treatment duration, disease severity, and type of psoriasis. We excluded non-human studies, conference abstracts, protocols, studies that did not report outcomes of interest.
Three reviewers (HY, QK, and J-sC) independently extracted the following information: clinical trial registration number, age and number of subjects, type and severity of psoriasis, intervention details, timing of outcomes, and analyzed outcomes. Outcomes included treatment efficacy outcomes, including the percentage of participants who achieved at least 75% improvement in the PASI (PASI-75) from baseline, the percentage of participants who achieved sPGA, Scalp Physician’s Global Assessment (ScPGA), and Palmoplantar Psoriasis Physician Global Assessment (PPPGA) score of clear (0) or almost Clear (1), change from baseline in Nail Psoriasis Severity Index (NPASI), patient’s assessment of pain and pruritus in visual analogue scale (VAS), and Dermatology Life Quality Index (DLQI). And safety measures included the number of participants with any treatment-emergent adverse event (TEAE), any drug-related TEAE, any serious TEAE, and any TEAE leading to drug withdrawal. We had cross-checked the information to ensure accuracy and completeness of the content. Discrepancies in eligibility decisions were discussed at each stage of the process until the reviewers reached consensus.
Two reviewers (HY and QK) independently assessed study quality according to the Cochrane Handbook for Systematic Reviews of Interventions. Disagreements were resolved through discussions and consensus with the third author (J-sC), and a consensus was finally achieved. In addition, we used Begg’s test and Egger test to assess publication bias.
Dichotomous data were calculated by using relative risk (RR) with 95% confidence intervals (CIs). Continuous data were summarized by using the standardized mean difference (SMD) with 95% CIs. Statistical significance was assumed for P < 0.05. Data were pooled by means of meta-analyses carried out on the full intention-to-treat (ITT) population, using the software Review Manager 5.3 and STATA 12.0. When the information was not available, we followed cochrane handbook to calculate the unreported values from the reported pre- and post-intervention means, SDs, number of participants, and standard error.
Heterogeneity was assessed by using the Cochran Q test and quantified with the I2 statistic. A p value of 0.10 or less or an I2 of 50% or more indicated substantial heterogeneity. If statistical heterogeneity exists, we used sensitivity analyses to investigate the possible causes of heterogeneity and assess the robustness of the results. Subgroup analyses were used to further stratify the trials by psoriasis type, treatment dose, and duration. Based on the assumption that clinical and methodological heterogeneity may exist and influence the results, a random-effects meta-analysis model was performed to pool the data.
According to our search strategy, we initially identified 504 citations in three electronic databases (CENTRAL, MEDLINE, EMBASE). After excluding duplicated articles and screened titles and abstracts, we filtered out 33 RCTs that met the inclusion criteria. Finally, after further review of the full texts, we included 18 studies that could provide data for further analyses (Figure 1).
A total of 6036 patients were included in this meta-analysis. Nine of the 18 studies included moderate-to-severe plaque psoriasis, 2 mild-to-moderate plaque psoriasis, and 7 arthropathy psoriasis. All patients were adults and received the same test drug, apremilast, but with different doses and duration of treatment. The study characteristics of these 18 selected trials are summarized in Table 1 (15–32).
We chose proportion of PASI-75 as the primary outcome. 11 trials including 3536 patients were analyzed. Overall, a random-effects model meta-analysis favored apremilast over placebo in terms of it (RR, 3.22; 95% CI, 2.59-4.01; P < 0.00001). Heterogeneity was not significant between studies (I2 = 7%; P = 0.37) (Figure 2).
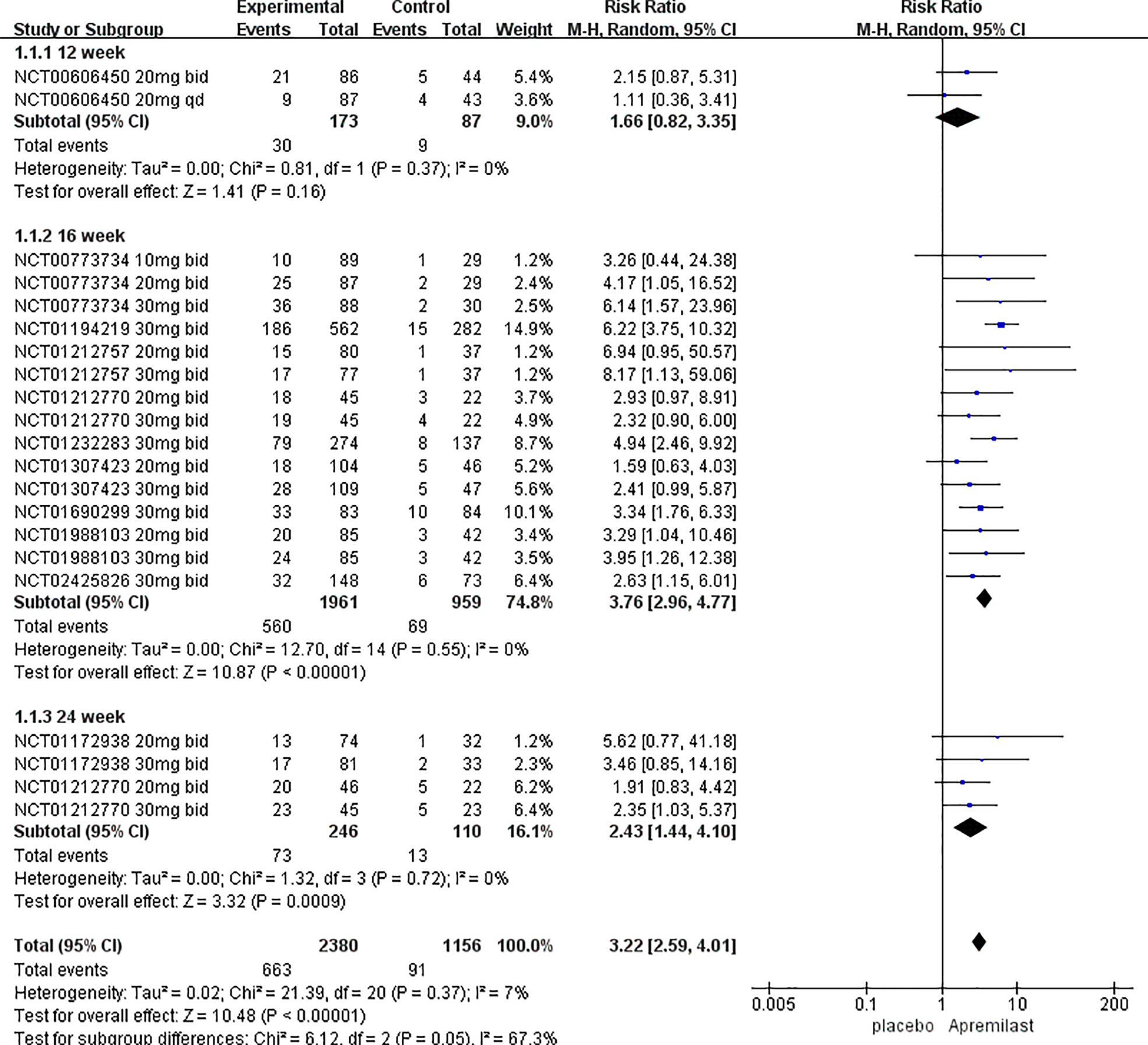
Figure 2 Meta-analysis of the association of phosphodiesterase 4 (PDE4) inhibitor vs placebos with the percentage improvement of PASI score by treatment duration.
Seven trials including 2800 patients with plaque psoriasis were analyzed. Overall, compared with placebo, apremilast was associated with a significantly higher percentage of participants with sPGA of 0/1 (RR, 3.57; 95% CI, 2.58-4.93; P < 0.00001), and no significant heterogeneity between studies was detected (I2 = 26%; P = 0.20).
In the assessment of pain symptom, 5 trials including 2166 patients with PsA analyzed improvement of joint pain, and one trial including 167 patients with plaque psoriasis analyzed improvement of skin pain. Of the 6 trials, 5 had a duration of 16 weeks and one had a 12-week duration. In the assessment of pruritus symptom, 6 trials including 2238 patients with plaque psoriasis analyzed the improvement in pruritus VAS at week 16. Overall, the pooled data found that apremilast was associated with a statistically significant decrease in pain VAS (SMD, -0.27; 95% CI, -0.36 to -0.18; P < 0.00001) and pruritus VAS (SMD, -0.51; 95% CI, -0.67 to -0.35; P < 0.00001) compared with placebo, respectively. However, significant heterogeneity between studies was detected in the analysis of pruritus score (I2 = 61%; P = 0.008), and no heterogeneity was detected in the analysis of pain score (I2 = 0%; P = 0.96).
The scalp, nails, palms, and soles are difficult-to-treat sites. Percentages of participants with ScPGA of 0/1, PPPGA of 0/1, and improvement of NAPSI were analyzed in 6 studies (including 2106 patients), 2 studies (including 142 patients), and 3 studies (including 1183 patients), respectively. All comparisons were apremilast 30 mg bid versus placebo at 16 weeks. The meta-analysis found that apremilast were associated with significantly higher scores in ScPGA of 0/1(RR, 2.21; 95% CI, 1.69-2.91; P < 0.00001), PPPGA of 0/1 (RR 2.33; 95%CI, 1.16-4.66; P =0.02) and significantly lower NAPSI (SMD, -0.46; 95% CI, -0.58 to -0.33; P < 0.00001). However, significant heterogeneity between studies was detected in ScPGA of 0/1 (I2 = 60%; P = 0.03), and no significant heterogeneity was detected in PPPGA of 0/1 (I2 = 0%; P = 0.55) or NAPSI (I2 = 1%; P = 0.37).
10 studies including 3341 patients analyzed improvements in patients’ quality of life. The pooled data found that apremilast was associated with a statistically significant reduction in DLQI compared with placebo (SMD, -0.58; 95% CI, -0.65 to -0.50; P < 0.00001). Heterogeneity was not significant between studies (I2 = 3%; P = 0.42).
Subgroup analyses of PASI-75 were performed by pooling trials with the same clinical phenotype, dosage, and duration of treatment, respectively. Table 2 shows that apremilast was associated with significantly higher response rates at the dosage of both 20 mg bid (RR, 2.82; 95% CI, 2.01 to 3.95; P < 0.00001) and 30 mg bid (RR, 4.08; 95% CI, 3.12 to 5.33; P < 0.00001), treatment course of both 16 weeks (RR, 3.78; 95% CI, 2.97 to 4.81; P < 0.00001) and 24weeks (RR, 2.47; 95% CI, 1.41 to 4.31; P=0.002), and clinical phenotype of plaque psoriasis (RR, 3.67; 95% CI, 2.72 to 4.97; P< 0.00001) and PsA (RR, 2.48; 95% CI, 1.76-3.50; P< 0.00001). However, at week 12 (RR, 1.66; 95% CI,0.82-3.35; P=0.16), dosage of 10 mg bid (RR, 1.98; 95% CI, 0.70-5.50; P=0.20) and 20mg qd (RR, 1.00; 95% CI, 0.42-2.40; P=1.00), no significant differences in PASI-75 were found between the apremilast groups and the placebo groups. Pooled studies across all subgroups were homogenous (I2<50%; P>0.1).
TEAEs led to study withdrawal of participants in 14 trials, including 283 patients. The results showed that apremilast was associated with a higher proportion of patients with TEAEs leading to withdrawal (RR, 1.41; 95% CI, 1.09-1.83; P=0.009), with no heterogeneity between studies (I2 = 0%; P =1.00).
15 trials including 3454 patients reported at least one TEAE. The pooled data showed that apremilast was associated with a higher proportion of patients with at least 1 TEAE (RR, 1.22; 95% CI, 1.17-1.28; P<0.00001), with no heterogeneity between studies (I2 = 0%; P =0.62).
There was no statistically difference in the results between apremilast and placebo treatments (RR,0.80; 95% CI, 0.56-1.15; P = 0.23). In these studies, 133 of 5839 patients had at least 1 serious TEAE, with apremilast group (82 of 3759 [2.18%]) and placebo group (51 of 2080 [2.45%]) were similar in incidence. No heterogeneity was detected (I2 = 0%; P = 0.86).
13 trials including 5228 patients reported on drug-related TEAEs. Results showed that compared with placebo (328 of 1905 [17.2%]), apremilast treatment (1156 of 3323 [34.79%]) was associated with a significantly higher incidence of any drug-related TEAEs (RR, 1.98; 95% CI, 1.77-2.20; P < 0.00001), and no heterogeneity was detected (I2 = 0%; P = 0.82). Drug-related TEAEs in the apremilast group included diarrhea, nausea, depression, not serious suicidal ideation, weight loss, dizziness, decreased blood pressure, sinusitis, hepatic enzyme increase, headache, and migraine. These TEAEs were reported to be mild to moderate, with some resolving after discontinuation and some resolving within 1 month despite continued treatment and no pharmacological intervention. Some serious apremilast-related TEAEs have been reported, including abdominal abscess, diverticulitis, pneumonia and gastrointestinal clostridial infection. However, the only reported event of diverticulitis resolved without any does change and medicinal intervention. The patients with pneumonia and gastrointestinal clostridial infections recovered after standard course of antibiotic therapy. Other serious adverse events, including malignant diseases, systemic vasculitis, major cardiovascular events, or death, were not considered treatment-related.
The included trials were determined to have a low and unclear risk of bias (eFigure 1 in the Supplement). For the outcome of proportion of PASI-75, no publication bias was detected by using an Egger’s test (bias, -0.98; 95% CI, -5.74 to 3.78; p = 0.67) or Begg’s test (pr> |z|=0.239).
In the pooled data on pruritus VAS improvement, we found that the heterogeneity came from trial NCT01194219. After excluding this study, no significant heterogeneity was detected (I2 = 30%; P = 0.19), and the effect on decreasing pruritus score was statistically unchanged in the meta-analysis (SMD, -0.46; 95% CI, -0.60 to -0.32; P < 0.00001). In meta-analysis of ScPGA of 0/1, we found that the heterogeneity came from trial NCT02425826, and after excluding this study, no heterogeneity was detected (I2 = 0%; P = 0.56), and the effect on the proportion of ScPGA of 0/1 was unchanged statistically (RR, 2.52; 95% CI, 2.09 to 3.04; P < 0.00001).
To our knowledge, this is the first study to systematically analyze the efficacy and safety of PDE4 inhibitor for the treatment of both PsA and plaque psoriasis using RCTs. Ultimately, only one oral PDE4 inhibitor, apremilast, met the inclusion criteria. Overall, the results of this meta-analysis of 18 studies showed that apremilast was effective in improving skin lesions and quality of life in patients with psoriasis, both PsA and plaque psoriasis, especially after 16 weeks of treatment. And apremilast was well tolerated.
PASI-75 is a classic tool for assessing the efficacy of psoriasis treatment (33). And 68.75% (11/16) of the trials included in this meta-analysis evaluated PASI-75, which is the most described tool in the trials included in this study. Therefore, we chose the proportion of PASI-75 as the primary outcome of this meta-analysis. In addition, sPGA of clear or almost clear skin was evaluated in 7/16 (43.75%) of included trials. Both PASI and sPGA are measures of general physical signs. Across all the included trials, apremilast significantly improved general physical signs in patients with psoriasis compared with placebo.
However, PASI and sPGA do not adequately reflect the severity of local lesions, such as scalp, nails, palms, and soles. These parts are more common at the onset of psoriasis and throughout the course of the disease (34), and are difficult to hide, which seriously affects the patients’ self-esteem and social ability (35). And worst of all, the treatments of psoriasis in these specific areas are very challenging, topical therapies are often ineffective (34, 36–38). Therefore, effective systemic treatment is required. Our study shows that apremilast can significantly improve all of these local signs. In addition, from a patient’s perspective, apremilast significantly reduced itching and pain and improved quality of life. Our conclusions are consistent with previous pooled studies (39, 40) and real-world findings (41, 42).
No publishing bias was detected in the measurement of PASI-75, and no significant heterogeneity was detected in the overall comparative study between apremilast and placebo. Although heterogeneity between studies was significantly higher for ScPGA 0/1 (I2 = 60%, p = 0.03) and pruritus scores (I2 = 61%; p=0.008), sensitivity analysis found that no effect estimate was statistically changed after excluding the source studies of heterogeneity. These findings suggest that the efficacy of apremilast in the treatment of psoriasis is robust and reproducible. Therefore, apremilast represents a new option for psoriasis therapy.
Furthermore, we found that apremilast 20 mg bid and 30 mg bid were more effective, while 10 mg bid and 20 mg qd were similarly effective with placebo, suggesting that the efficacy of apremilast was dose-dependent. Based on time-to-treatment analysis, the optimal treatment duration for apremilast to improve PASI of psoriasis was ≥16 weeks, suggesting that apremilast does not provide rapid relief of psoriatic symptoms. Therefore, apremilast is not suitable for monotherapy in critically ill patients who require rapid improvement of clinical symptoms and quality of life.
The safety analysis showed that while apremilast had higher rates of any TEAEs, drug-related TEAEs and withdrawal due to TEAEs than placebo, there were no significant differences in serious TEAEs, such as opportunistic infections, malignant disease, systemic vasculitis, major cardiovascular events, or death. In addition, although the incidence of drug-related TAEAs in apremilast group (34.79%) was almost double that in the placebo group (17.2%), most were mild to moderate, and most were self-limited or resolved after drug discontinuation. Infections were the most frequently reported serious TEAEs, including abdominal abscess, diverticulitis, pneumonia, and gastrointestinal infections, which either resolved spontaneously without intervention or with standard anti-infective therapy. In general, our study suggests that apremilast is well tolerated.
Available data were limited to the assessment of PPPGA 0/1, in which less than 200 patients were tested. The higher treatment goals of PASI-90 or PASI-100, which were not evaluated in this study, are another limitation. Furthermore, there are limited data available for subgroup analyses based on geography or ethnicity. Body weight or body surface area greatly affects the efficacy of systemic therapy (43). Asians are generally smaller in weight or body surface area than Europeans and Americans. However, there was only one study in the Japanese population in our study, the rest were from Europe and the Americas. In addition, the long-term efficacy, retention rate, cost and comparison with other drugs of apremilast in the treatment of psoriasis also need further study.
The results of this meta-analysis support the favorable efficacy and safety profile of apremilast in the treatment of plaque psoriasis and PsA, especially in difficult-to-treat areas. More studies are needed in the future in Asian populations and in difficult-to-treat areas such as nails, scalp, palms, and soles, as well as evaluation with higher goals, such as PASI 100.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
HY had the conception, collected and analyzed the data, wrote the manuscript, and revised the manuscript. QK collected and analyzed the data, and wrote the manuscript. J-sC collected and analyzed the data, and helped in the methods. All authors contributed to the article and approved the submitted version.
This work was supported by the joint project of Chongqing Municipal Health Commission and Chongqing Municipal Science and Technology Bureau (No. 2020MSXM070 to HY), and the second batch of Class A reserve talents of the Children’s Hospital Affiliated to Chongqing Medical University (No. RC05036 to HY).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1021537/full#supplementary-material
Supplementary Figure 1 | Assessment of risk bias of RCTs.
1. Griffiths CEM, Armstrong AW, Gudjonsson JE, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet (2021) 397(10281):1301–15. doi: 10.1016/S0140-6736(20)32549-6
2. Boehncke WH, Schön MP. Psoriasis. Lancet (2015) 386(9997):983–94. doi: 10.1016/S0140-6736(14)61909-7
3. AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis - comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol (2020) 59(5):566–71. doi: 10.1111/ijd.14864
4. Ramanunny AK, Wadhwa S, Singh SK, Sharma DS, Khursheed R, Awasthi A. Treatment strategies against psoriasis: Principle, perspectives and practices. Curr Drug Deliv (2020) 17(1):52–73. doi: 10.2174/1567201816666191120120551
5. Rigopoulos D, Baran R, Chiheb S, Daniel CR 3rd, Di Chiacchio N, Gregoriou S. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: A dermatologist and nail expert group consensus. J Am Acad Dermatol (2019) 81(1):228–40. doi: 10.1016/j.jaad.2019.01.072
6. Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: Nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther (2018) 31(3):e12589. doi: 10.1111/dth.12589
7. Balak DMW, Gerdes S, Parodi A, Salgado-Boquete L. Long-term safety of oral systemic therapies for psoriasis: A comprehensive review of the literature. Dermatol Ther (Heidelb) (2020) 10(4):589–613. doi: 10.1007/s13555-020-00409-4
8. Pourali SP, Nshuti L, Dusetzina SB. Out-of-Pocket costs of specialty medications for psoriasis and psoriatic arthritis treatment in the Medicare population. JAMA Dermatol (2021) 157(10):1239–41. doi: 10.1001/jamadermatol.2021.3616
9. Mavropoulos A, Zafiriou E, Simopoulou T, Brotis AG, Liaskos C, Roussaki-Schulze A, et al. Apremilast increases IL-10-producing regulatory b cells and decreases proinflammatory T cells and innate cells in psoriatic arthritis and psoriasis. Rheumatol (Oxford) (2019) 58(12):2240–50. doi: 10.1093/rheumatology/kez204
10. Jia Y, Chen X, Sun J. Apremilast ameliorates IL-1α-induced dysfunction in epidermal stem cells. Aging (Albany NY) (2021) 13(15):19293–305. doi: 10.18632/aging.203265
11. Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T (2015) 40(8):495–500.
12. Aljefri YE, Ghaddaf AA, Alkhunani TA, Alkhamisi TA, Alahmadi RA, Alamri AM, et al. Efficacy and safety of apremilast monotherapy in moderate-to-severe plaque psoriasis: A systematic review and meta-analysis. Dermatol Ther (2022) 35(7):e15544. doi: 10.1111/dth.15544
13. Higgins JPT, Green S eds. Cochrane handbook for systematic reviews of interventions. version 5.1.0. The Cochrane Collaboration. Available at: http://handbook-5-1.cochrane.org/.
14. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (2010) 8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007
15. American Academy of Dermatology Work Group, Menter A, Korman NJ, Elmets CA, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol (2011) 65(1):137–74. doi: 10.1016/j.jaad.2010.11.055
16. Dopytalska K, Sobolewski P, Błaszczak A, Szymańska E, Walecka I. Psoriasis in special localizations. Reumatologia (2018) 56(6):392–8. doi: 10.5114/reum.2018.80718
17. Kowalewska B, Krajewska-Kułak E, Sobolewski M. The impact of stress-coping strategies and the severity of psoriasis on self-esteem, illness acceptance and life satisfaction. Dermatol Ther (Heidelb) (2022) 12(2):529–43. doi: 10.1007/s13555-021-00669-8
18. Mosca M, Hong J, Hadeler E, Brownstone N, Bhutani T, Liao W. Scalp psoriasis: A literature review of effective therapies and updated recommendations for practical management. Dermatol Ther (Heidelb) (2021) 11(3):769–97. doi: 10.1007/s13555-021-00521-z
19. Sarma N. Evidence and suggested therapeutic approach in psoriasis of difficult-to-treat areas: Palmoplantar psoriasis, nail psoriasis, scalp psoriasis, and intertriginous psoriasis. Indian J Dermatol (2017) 62(2):113–22. doi: 10.4103/ijd.IJD_539_16
20. Hadeler E, Mosca M, Hong J, Brownstone N, Bhutani T, Liao W. Nail psoriasis: A review of effective therapies and recommendations for management. Dermatol Ther (Heidelb) (2021) 11(3):799–831. doi: 10.1007/s13555-021-00523-x
21. Thaçi D, Kimball A, Foley P, Poulin Y, Levi E, Chen R, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, improves patient-reported outcomes in the treatment of moderate to severe psoriasis: results of two phase III randomized, controlled trials. J Eur Acad Dermatol Venereol (2017) 31(3):498–506. doi: 10.1111/jdv.13918
22. Sobell JM, Foley P, Toth D, Mrowietz U, Girolomoni G, Goncalves J, et al. Effects of apremilast on pruritus and skin Discomfort/Pain correlate with improvements in quality of life in patients with moderate to severe plaque psoriasis. Acta Derm Venereol (2016) 96(4):514–20. doi: 10.2340/00015555-2360
23. Papadavid E, Rompoti N, Theodoropoulos K, Kokkalis G, Rigopoulos D. Real-world data on the efficacy and safety of apremilast in patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol (2018) 32(7):1173–9. doi: 10.1111/jdv.14832
24. Lanna C, Cesaroni GM, Mazzilli S, Vollono L, Gaziano R, Marino D, et al. Apremilast as a target therapy for nail psoriasis: a real-life observational study proving its efficacy in restoring the nail unit. J Dermatol Treat (2022) 33(2):1097–101. doi: 10.1080/09546634.2020.1801976
25. Pai MP. Drug dosing based on weight and body surface area: mathematical assumptions and limitations in obese adults. Pharmacotherapy (2012) 32(9):856–68. doi: 10.1002/j.187
26. Bissonnette R, Haydey R, Rosoph LA, Lynde CW, Bukhalo M, Fowler JF, et al. Apremilast for the treatment of moderate-to-severe palmoplantar psoriasis: results from a double-blind, placebo-controlled, randomized study. J Eur Acad Dermatol Venereol (2018) 32(3):403–10. doi: 10.1111/jdv.14647
27. Cutolo M, Myerson GE, Fleischmann RM, Lioté F, Díaz-González F, Van den Bosch F, et al. A Phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: Results of the PALACE 2 trial. J Rheumatol (2016) 43(9):1724–34. doi: 10.3899/jrheum.151376
28. Edwards CJ, Blanco FJ, Crowley J, Birbara CA, Jaworski J, Aelion J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis (2016) 75(6):1065–73. doi: 10.1136/annrheumdis-2015-207963
29. Stein Gold L, Bagel J, Lebwohl M, Jackson JM, Chen R, Goncalves J, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis: 52-week results of UNVEIL. J Drugs Dermatol (2018) 17(2):221–8.
30. Stein Gold L, Papp K, Pariser D, Green L, Bhatia N, Sofen H, et al. Efficacy and safety of apremilast in patients with mild-to-moderate plaque psoriasis: Results of a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol (2022) 86(1):77–85. doi: 10.1016/j.jaad.2021.07.040
31. Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis (2014) 73(6):1020–6. doi: 10.1136/annrheumdis-2013-205056
32. Nash P, Ohson K, Walsh J, Delev N, Nguyen D, Teng L, et al. Early and sustained efficacy with apremilast monotherapy in biological-naïve patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (ACTIVE). Ann Rheum Dis (2018) 77(5):690–8. doi: 10.1136/annrheumdis-2017-211568
33. Ohtsuki M, Okubo Y, Komine M, Imafuku S, Day RM, Chen P, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: Efficacy, safety and tolerability results from a phase 2b randomized controlled trial. J Dermatol (2017) 44(8):873–84. doi: 10.1111/1346-8138.13829
34. Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet (2012) 380(9843):738–46. doi: 10.1016/S0140-6736(12)60642-4
35. Papp KA, Kaufmann R, Thaçi D, Hu C, Sutherland D, Rohane P. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison study. J Eur Acad Dermatol Venereol (2013) 27(3):e376–383. doi: 10.1111/j.1468-3083.2012.04716.x
36. Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and safety trial evaluating the effects of apremilast in psoriasis [ESTEEM] 1). J Am Acad Dermatol (2015) 73(1):37–49. doi: 10.1016/j.jaad.2015.03.049
37. Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol (2015) 173(6):1387–99. doi: 10.1111/bjd.14164
38. Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol (2017) 31(3):507–17. doi: 10.1111/jdv.14015
39. Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey AR, et al. Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheumatol (2012) 64(10):3156–67. doi: 10.1002/art.34627
40. Strand V, Fiorentino D, Hu C, Day RM, Stevens RM, Papp KA. Improvements in patient-reported outcomes with apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of moderate to severe psoriasis: results from a phase IIb randomized, controlled study. Health Qual Life Outcomes (2013) 11:82. doi: 10.1186/1477-7525-11-82
41. Strand V, Schett G, Hu C, Stevens RM. Patient-reported health-related quality of life with apremilast for psoriatic arthritis: a phase II, randomized, controlled study. J Rheumatol (2013) 40(7):1158–65. doi: 10.3899/jrheum.121200
42. Van Voorhees AS, Stein Gold L, Lebwohl M, Strober B, Lynde C, Tyring S, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: Results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol (2020) 83(1):96–103. doi: 10.1016/j.jaad.2020.01.072
Keywords: psoriasis, PDE4 inhibitor, apremilast, meta-analysis, treatment strategies
Citation: Kang Q, Chen J-s and Yang H (2022) Efficacy and safety profile of phosphodiesterase 4 inhibitor in the treatment of psoriasis: A systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 13:1021537. doi: 10.3389/fimmu.2022.1021537
Received: 17 August 2022; Accepted: 22 September 2022;
Published: 10 October 2022.
Edited by:
Piero Pileri, Toscana Life Sciences, ItalyReviewed by:
Giampiero Girolomoni, University of Verona, ItalyCopyright © 2022 Kang, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Yang, ZGluYWJpbGxAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.