
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 24 November 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1015365
This article is part of the Research TopicNeoadjuvant and Adjuvant Immunotherapy in Thoracic CancerView all 9 articles
Background: The controlling nutritional status (CONUT) score, as an immune-nutritional index, has been reported to be related to prognosis in several cancers. Neoadjuvant immunochemotherapy (nICT) is an emerging pattern for cancer treatment in recent years. However, the usefulness of CONUT in esophageal squamous cell carcinoma (ESCC) with nICT has not been reported so far. This study attempted to clarify the usefulness of CONUT in predicting disease-free survival (DFS) in ESCC with nICT.
Methods: Two hundred sixteen ESCC patients receiving nICT between 2019 and 2021 were retrospectively enrolled. Based on CONUT, the patients were divided into two groups: low groups (score ≤ 2) and high (score ≥ 3) groups. The relationships between CONUT and clinical characteristics were estimated. Cox regression analyses with hazard ratios (HRs) and 95% confidence intervals (CIs) were also performed to evaluate the prognostic factors of DFS.
Results: Fifty-nine (27.3%) patients achieved pathologic complete response (pCR), and 30 (13.9%) cases had a recurrence. There were 150 cases (69.4%) in low CONUT group and 66 cases (30.6%) in high CONUT group, respectively. The results revealed that vessel invasion (P = 0.037), postoperative pneumonia (P = 0.001), advanced ypT stage (P = 0.011), cTNM stage (P = 0.007), and ypTNM stage (P < 0.001) were significantly related to patients with a high CONUT score. A high pCR rate was found in patients with a low CONUT score (33.3% vs. 13.6%, P = 0.003), and a high recurrence rate was found in patients with a high CONUT score (24.2% vs. 9.3%, P = 0.004), respectively. Patients with a low CONUT score had a better 1-year DFS than those with a high CONUT score (90.7% vs. 75.8%, P = 0.004). Multivariate analyses indicated that the pretreatment CONUT score was an independent predictor regarding DFS (HR = 2.221, 95% CI: 1.067–4.625, P = 0.033).
Conclusion: A better response and a lower recurrence were found in ESCC patients with a lower pretreatment CONUT. As a useful index for immune-nutritional status, the CONUT might be a reliable prognostic indicator in ESCC patients with nICT.
Esophageal cancer (EC) is one of the life-threatening diseases worldwide, which ranks the 10th and sixth, respectively, in terms of incidence and mortality (1, 2). More than 50% of EC occurs in China, which ranks the sixth and fourth, respectively, in morbidity and mortality (3). There are two main pathological types of ECs, and more than 90% of them in China are esophageal squamous cell carcinoma (ESCC) (3). Patients with ESCC are often diagnosed at locally advanced stages at the time of presentation. Although the therapeutic methods for ESCC have been improved in recent years, the treatment effect is still unsatisfactory (4). Therefore, there is a need to explore more and more reliable indicators with pretreatment variables to predict prognosis before treatment.
For those patients with locally advanced ESCC, the NCCN guidelines recommend neoadjuvant chemoradiotherapy (nCRT) as the golden standard treatment in recent years (5). Due to the differences of races and pathological types between the West and the East, neoadjuvant chemotherapy (nCT) is usually recommended in China and Japan (6). However, the treatment effect and prognosis of neoadjuvant therapy for patients with ESCC are still unsatisfactory. Recently, immunotherapy has become one of the important regimens and has achieved several remarkable results in patients with advanced EC (7, 8). Compared with chemotherapy, immunotherapy has achieved a better long-term survival based on the ATTRACTION-3 and KEYNOTE-181 studies. According to the CheckMate 577 study, moreover, adjuvant nivolumab is also recommended after nCRT in patients with EC (9). Following these encouraging results in advanced EC, several clinical studies were also conducted, and the results revealed that neoadjuvant immunochemotherapy (nICT) followed by surgery is safe and feasible (10–13).
Recently, more and more researchers focus on the relations between the immune-nutritional status and cancer (14). The controlling nutritional status (CONUT), as an immune-nutritional index deriving from peripheral blood variables of albumin (ALB), lymphocyte (LYM), and total cholesterol (TC), has been reported to be related to prognosis in various cancers, including ESCC (15–17). Moreover, pretreatment CONUT was also widely used as a prognostic index in other digestive tract cancers, such as gastric cancer (GC) and colorectal cancer (CRC) (18–20). These results clearly demonstrated that a poor prognosis in various cancers was related to a high CONUT. Moreover, a recent study analyzed the prognostic value of CONUT in advanced EC patients who were treated with immunotherapy (21). The results revealed that a high CONUT score was associated with a significantly worse prognosis in advanced EC. In addition, it has also been shown that CONUT correlates with neoadjuvant response to treatment in several cancers, such as GC and EC (22, 23).
To date, most published studies focus on the efficacy and safety of nICT in local advanced ESCC. No studies on cancer recurrence in ESCC after nICT have been reported. Moreover, prior to nICT treatment, there are no reliable and affordable indexes to predict recurrence in ESCC. Therefore, we herein aimed to verify the recurrence pattern after nICT and explore the predictive value of CONUT in predicting DFS in ESCC with nICT.
From 2019 to 2021, patients with local advanced ESCC with nICT in our department were enrolled. The inclusion criteria were as follows: (1) aged 18–75 years, (2) ESCC confirmed by histology, (3) ECOG-PS 0-1, (4) clinical TNM stage II-IVA, (5) radical R0 resection after nICT, and (6) complete clinical data and follow-up records over 6 months. The exclusion criteria were as follows: (1) non-ESCC, (2) R1 or R2 resection after nICT, (3) accompanied by other infection, hematologic, or autoimmune disease, (4) associated with other previous or synchronous cancers, or (5) combined with other anticancer treatment. All patients signed the written informed consent. The study was performed in accordance with the Helsinki Declaration. This study was reviewed and approved by the ethics committee of Zhejiang Cancer Hospital (IRB-2020-320).
The preoperative nICT treatment protocols were the same as in our previously published studies (13, 24). The patients were also notified of alternative treatment options (nCRT or nCT) when they signed the informed consent. All patients received two cycles of nICT every 3 weeks. The immunotherapy regimen was administered on day 1 with the following protocol: pembrolizumab—2 mg/kg, nivolumab—3 mg/kg, or sintilimab/tislelizumab/camrelizumab—200 mg. The chemotherapy regimen was albumin paclitaxel (days 1 and 8: 100 mg/m2) combined with carboplatin (day 1: area under the curve, AUC = 5 mg/ml/min). Surgical resection was usually performed 4–6 weeks after the last cycle of nICT. The McKeown or Ivor Lewis minimally invasive esophagectomy (MIE) with two‐ or three-field lymphadenectomy was the main surgical treatment in the current study (25). To date, the adjuvant treatment after nICT followed by surgery in patients with EC remains unclear. Adjuvant treatment in our institute included adjuvant immunotherapy and adjuvant radiotherapy with or without chemotherapy. According to published studies, adjuvant immunotherapy was recommended in EC patients after nCRT based on the CheckMate 577 study and the expert consensus in China (9, 26). Moreover, adjuvant radiotherapy with or without chemotherapy was also recommended in patients with advanced ypT stage (T3–T4) and/or ypN stage (N1-3) after radical resection (27, 28).
All patients were followed up periodically after completion of the treatments. The patients were followed up every 3 months during the first 2 years. Patients with no recurrence during the next 3–5 years were generally followed up every 6 months and once a year thereafter. The 8th AJCC/UICC staging system was used in this study (29). Pathological complete response (pCR) was defined as no evidence of residual tumor cells (30). Recurrence was regarded as any local, regional, or distant tumor recurrence. Disease-free survival (DFS) was defined as the time from surgery to the first documented recurrence. The last follow-up time was completed in June 2022.
The CONUT score, according to various published studies, was based on three hematological indicators, including LYM, TC, and ALB (15–20). Based on previously published studies, the CONUT was usually collected within 1 week before treatment (15, 16, 18–20). Therefore, data on the levels of the abovementioned three hematological variables within 1 week before nICT were extracted. Then, the patients were divided into two groups based on CONUT score: low group (score ≤2) and high group (score ≥3). The flowchart for CONUT construction is shown in Figure 1.
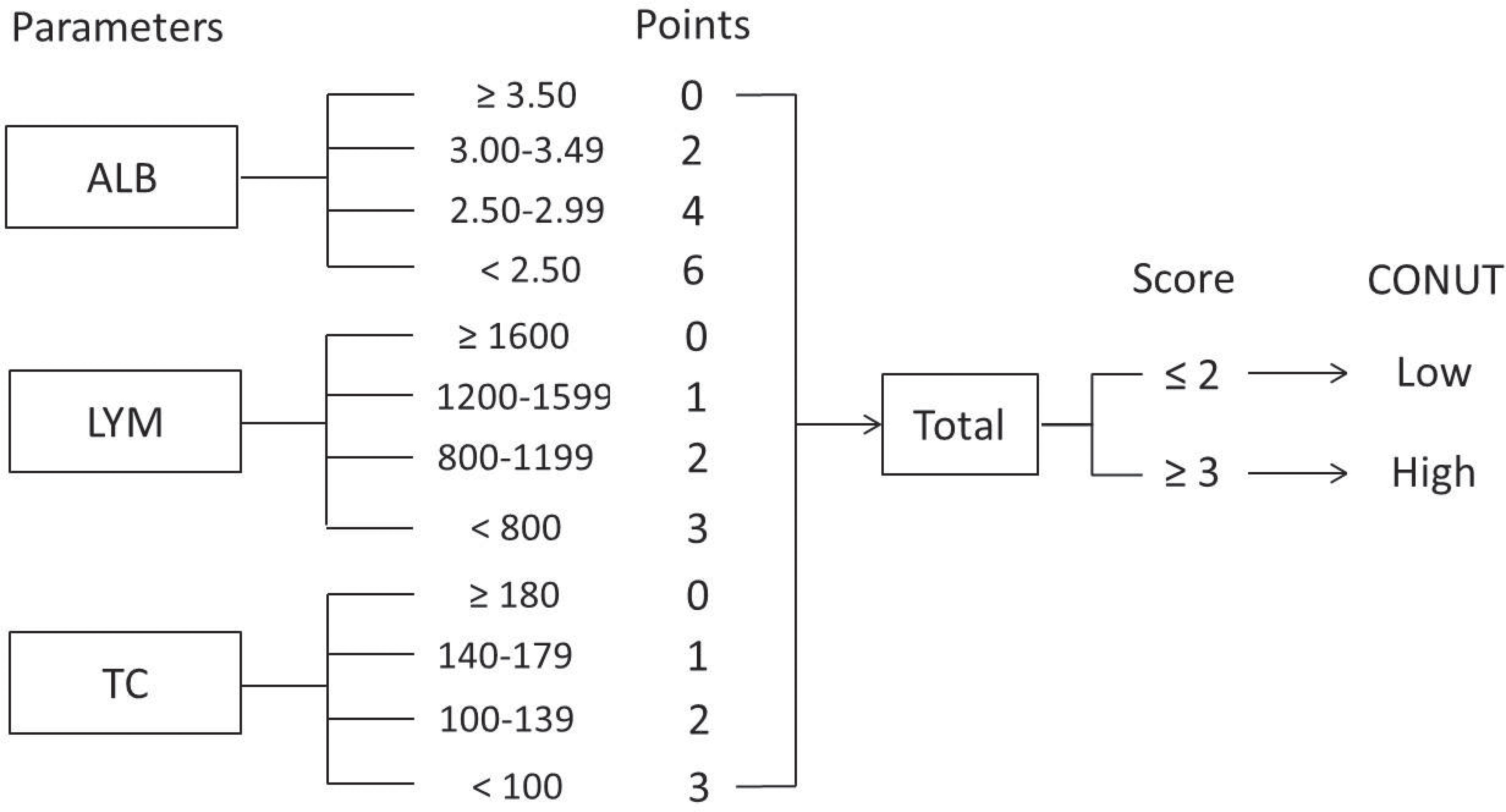
Figure 1 Flowchart for CONUT construction and risk stratification. The CONUT is calculated as the sum of the albumin, lymphocyte, and total cholesterol.
SPSS 20.0 and R software 4.1.2 were used to perform all statistical analyses. Student’s t-tests and chi-square or Fisher’s exact tests were carried out to analyze continuous and categorical variables, respectively. A three-dimensional scatter plot and a heat map were also drawn to explore the correlations. To better understand the predictive ability for pCR and recurrence, the AUCs were compared by receiver operating characteristic (ROC) curves. In the current study, clinical variables with statistical differences in the univariate analyses were then subjected to multivariate analyses by using a forward stepwise regression. Covariance analyses were also performed to avoid an interaction effect before Cox regression. Cox regression analyses with hazard ratios (HRs) and 95% confidence intervals (CIs) were used to identify the predictors of DFS. The DFS and overall survival (OS) differences were compared by log-rank tests in Kaplan–Meier curves. Finally, a novel nomogram model was also established to verify the prognostic value of independent prognostic factors. All statistical tests were two-sided, and a p-value <0.05 was considered to be statistically significant.
A total of 216 ESCC patients with nICT were enrolled. The mean age of all patients was 63.2 ± 6.6 years (range: 47–75 years). There were 13 (6.0%) female and 203 (94.0%) male patients. The median time of follow-up was 12 months (range: 6–29 months). The majority of the ESCC was located in the middle (57.9%) and lower (32.9%) segment of the esophagus. Most types of differentiation were moderate (46.7%) and poor (37.5%). The ypTNM stages were as follows: 59 had stage 0 (27.3%), 63 had stage I–II (29.2%), and 94 had stage III–IVa (43.5%). There were 59 (27.3%) patients who achieved pCR, and 30 (13.9%) cases had a recurrence. The detailed clinical characteristics are shown in Table 1.
The three-dimensional scatter diagram regarding the three variables is shown in Figure 2A. The mean values for LYM, ALB, and TC were 1,552 ± 565/mm3, 4.06 ± 0.40 mg/dl, and 172.9 ± 40.0 mg/dl, respectively. The heat map correlation diagram of CONUT and its components is shown in Figure 2B. According to our results, negative correlations were found between CONUT and LYM (r = -0.456, P < 0.001), ALB (r = -0.532, P < 0.001), and TC (r = -0.582, P < 0.001), respectively. A positive correlation was found between LYM and ALB (r = 0.164, P = 0.016). The number of cases based on the components of CONUT is shown in Figures 2C–F. The levels of LYM, ALB, and TC were significantly lower in the high CONUT group than those in the low CONUT group, respectively (P < 0.001, Figures 2G–I).
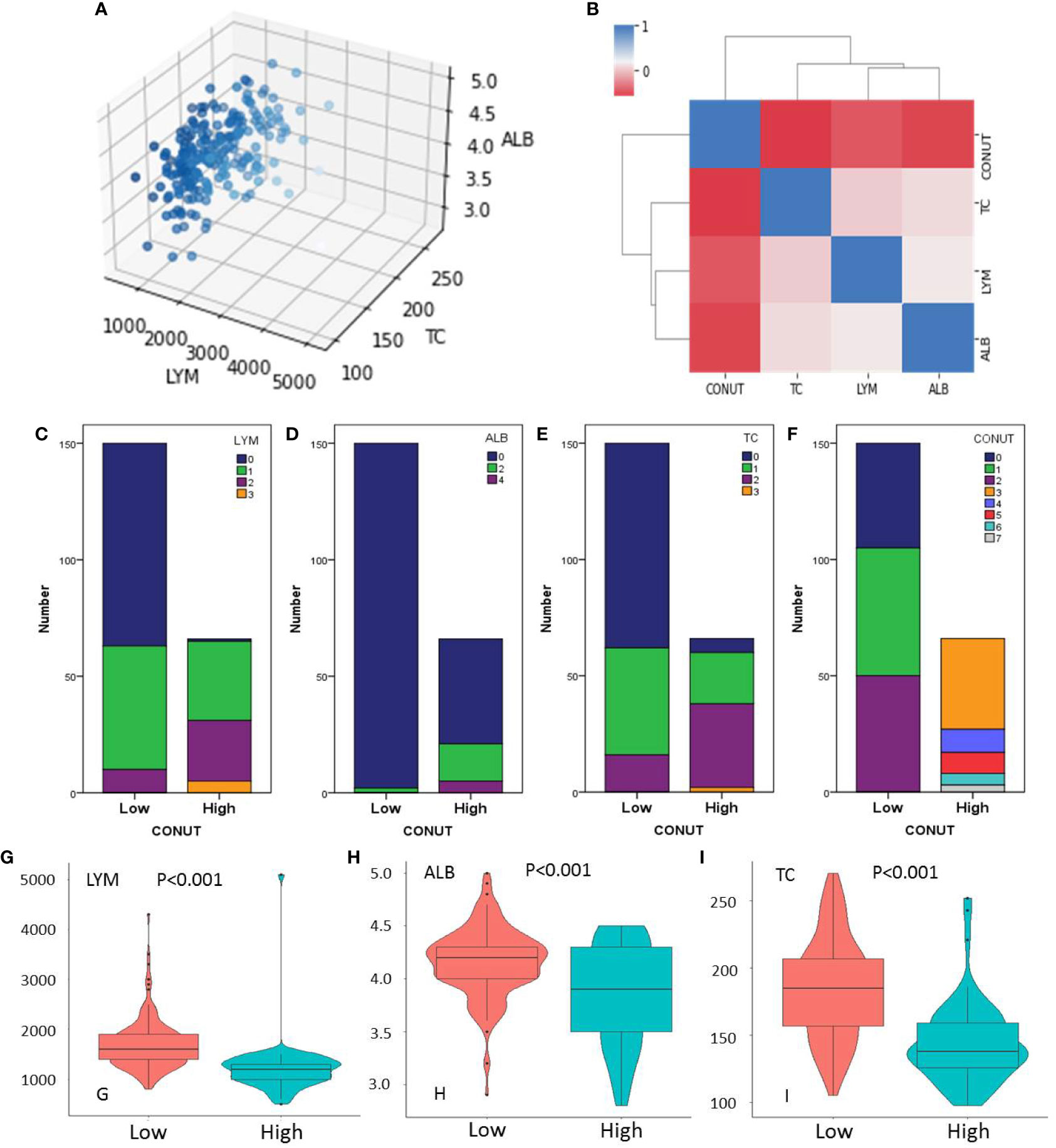
Figure 2 Three-dimensional scatter (A) and heat map diagram (B). Number of cases based on the lymphocyte (LYM) (C), albumin (ALB) (D), total cholesterol (TC) (E), and CONUT (F). The levels of LYM (G), ALB (H), and TC (I) grouped by CONUT.
The AUC comparisons between CONUT and components (LYM, ALB, and TC) according to the ROC curves in pCR and recurrence prediction are shown in Figure 3. Compared with its components of LYM, ALB, and TC, CONUT had the largest AUC (0.675 in pCR prediction and 0.725 in recurrence prediction) based on ROC curve analyses. As a comprehensive indicator, the CONUT can reflect host immune and nutritional status in a more extensive manner than other indicators. These results indicated a higher predictive ability of CONUT on pCR and recurrence prediction than other indicators.
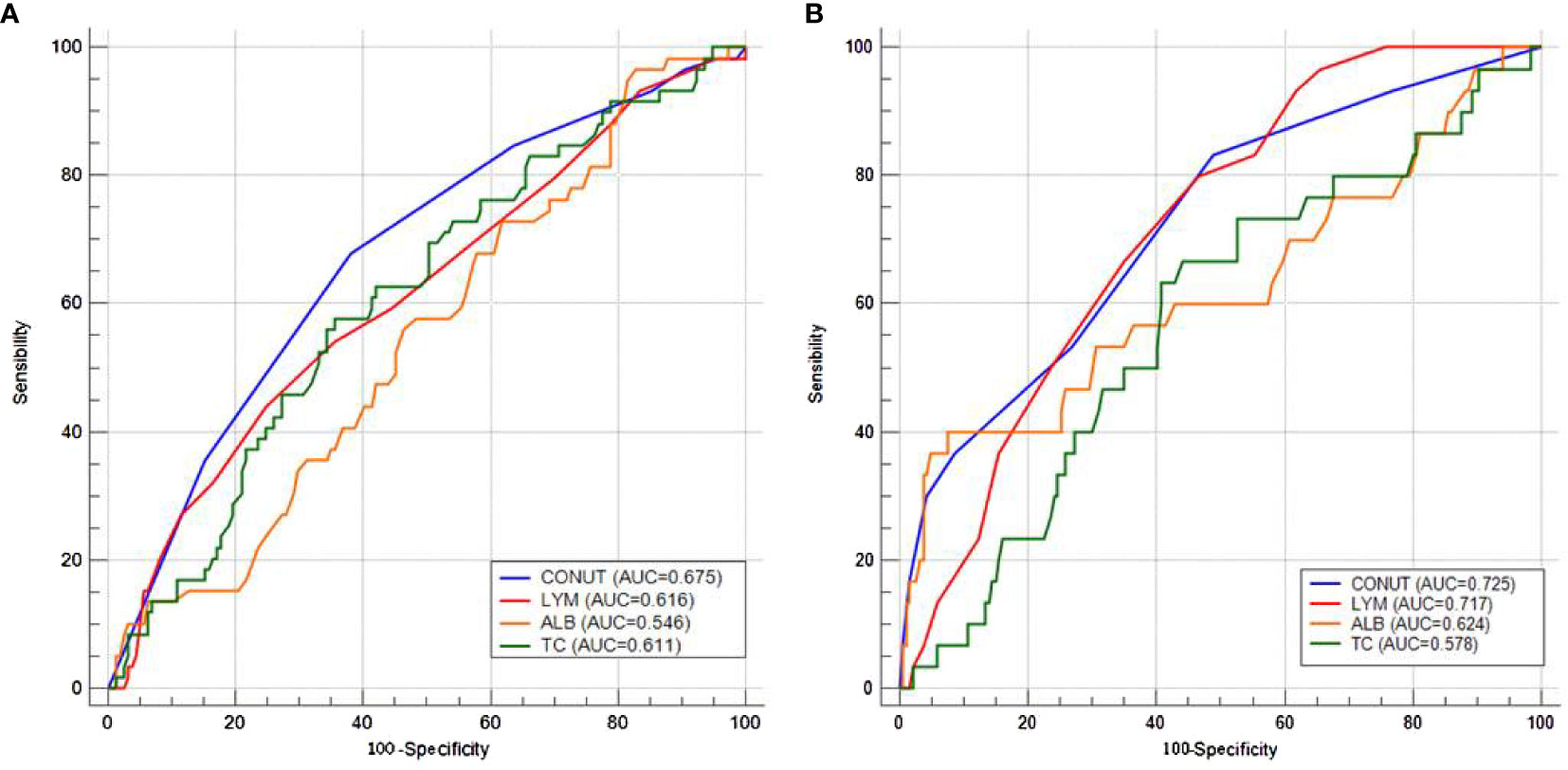
Figure 3 Area under the curve comparisons between CONUT and components (lymphocyte, albumin, and total cholesterol) according to the receiver operating characteristic curves in pathologic complete response (A) and recurrence (B) prediction.
One hundred fifty (69.4%) cases were enrolled in the low CONUT and 66 (30.6%) cases were enrolled in the high CONUT, respectively. Comparisons of the clinical characteristics grouped by CONUT are shown in Table 2. High CONUT was associated with vessel invasion (P = 0.037), postoperative pneumonia (P = 0.001), advanced ypT stage (P = 0.011), cTNM stage (P = 0.007), and ypTNM stage (P < 0.001). Moreover, a high pCR rate was found in the low CONUT group (33.3% vs. 13.6%, P = 0.003), and a high recurrence rate was found in the high CONUT group (24.2% vs. 9.3%, P = 0.004), respectively (Figures 4A, B). In addition, the pretreatment CONUT score was also significantly associated with operation time (P = 0.026) and hospital stay after the operation (P = 0.008) but not connected with blood loss (Figures 4C–E).
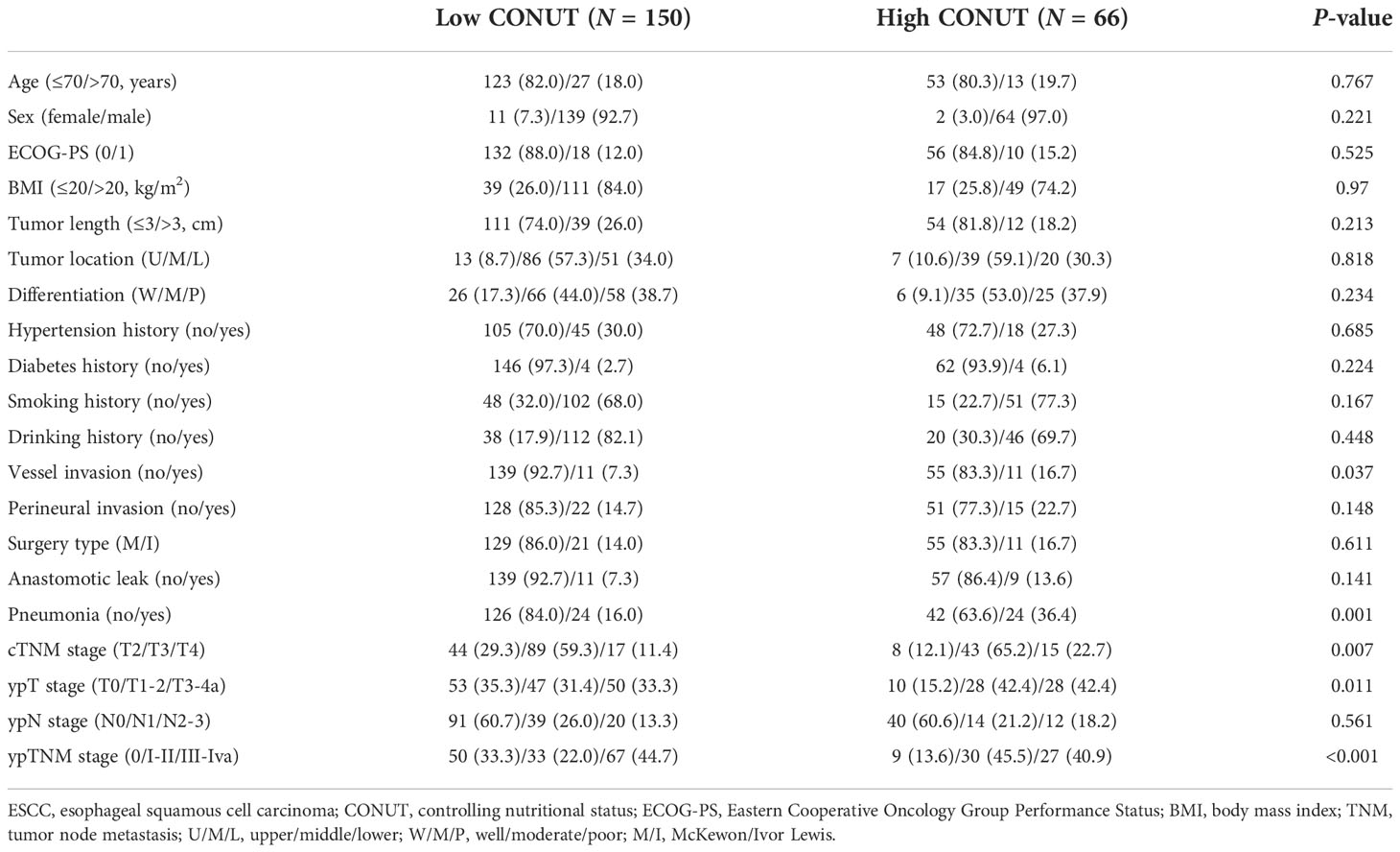
Table 2 Comparison of the clinical variables in esophageal squamous cell carcinoma grouped by CONUT.
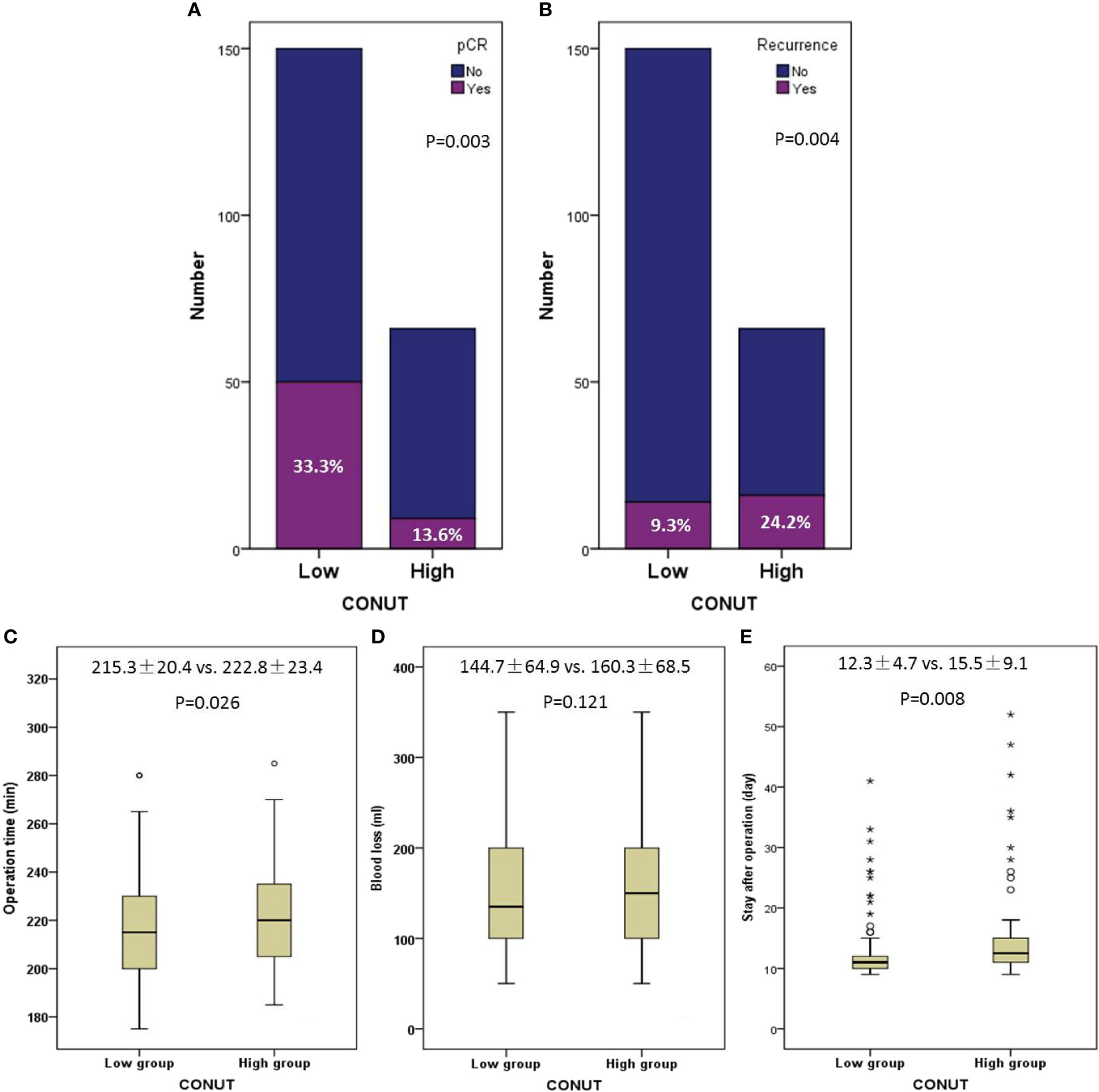
Figure 4 Comparisons of pathologic complete response (33.1% vs. 13.8%, P = 0.004) (A) and recurrence (24.6% vs. 9.3%, P = 0.003) (B) grouped by CONUT. Correlations between CONUT and operation time (P = 0.026) (C), blood loss (P = 0.141) (D), and hospital stay after operation (P = 0.008) (E). The symbols of “*, O” mean abnormal value.
Depending on the initial presentation, the patients were divided into local recurrence and distant recurrence, respectively. There were 19 (63.3%) patients with distant recurrence after treatment, including peritoneal metastasis and non-regional lymph node metastasis (LNM), while there were 11 (36.7%) cases with local recurrence, including locoregional LNM and anastomotic site recurrence. The detailed recurrence patterns are shown in Figure 5. The recurrence was confirmed by biopsy at the anastomotic site in two patients, and the remaining recurrences were scored by using imaging examinations.
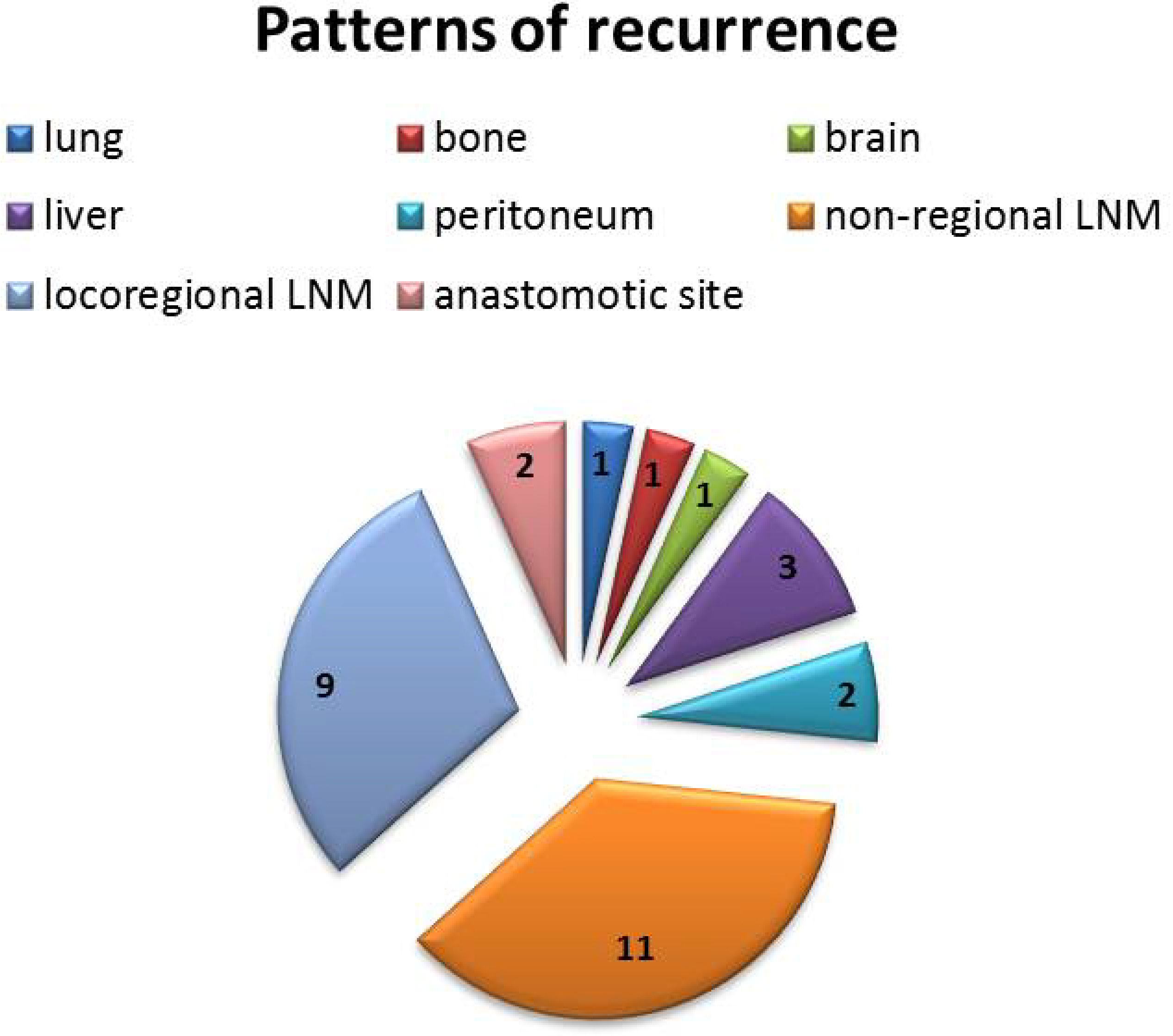
Figure 5 Detailed recurrence patterns after neoadjuvant immunochemotherapy. There were 19 (63.3%) cases with distant recurrence and 11 (36.7%) cases with local recurrence.
Clinical characteristics were used to perform Cox regression analyses (Table 3). There were nine variables, including location, tumor length, vessel invasion, perineural invasion, pCR, CONUT, ypT stage, ypN stage, and ypTNM stage, which were associated with DFS in the univariate analyses. The variables distinguished in the univariate analyses were then recruited in multivariate analyses. Multivariate Cox analyses indicated that tumor location (P = 0.006), ypN stage (P < 0.001), and CONUT (P = 0.033) were independent predictors regarding DFS. Patients in the high CONUT group had an HR of 2.221 (95% CI: 1.067–4.625) for DFS.
Patients with low CONUT had a better 1-year DFS than those with high CONUT (90.7% vs. 75.8%, P = 0.004, Figure 6A). Compared with patients with pCR, patients in the non-pCR group had a worse 1-year DFS (94.9% vs. 82.8%, P = 0.021, Figure 6B). Moreover, better OS curves were also found in patients with low CONUT (92.7% vs. 81.8%, P = 0.019, Figure 6C) and pCR (96.6% vs. 86.6%, P = 0.027, Figure 6D). No significant differences were found in this study between patients with or without postoperative radiotherapy. However, a subgroup analysis revealed that patients in the advanced stage with postoperative radiotherapy had a better 1-year DFS than those without radiotherapy (ypT3-4a: 90.3% vs. 70.2%, P = 0.050; ypN1-3: 86.5% vs. 60.4%, P = 0.013; ypTNM III-IVa: 86.8% vs. 66.1%, P = 0.030, respectively) (Figures 6E–G).
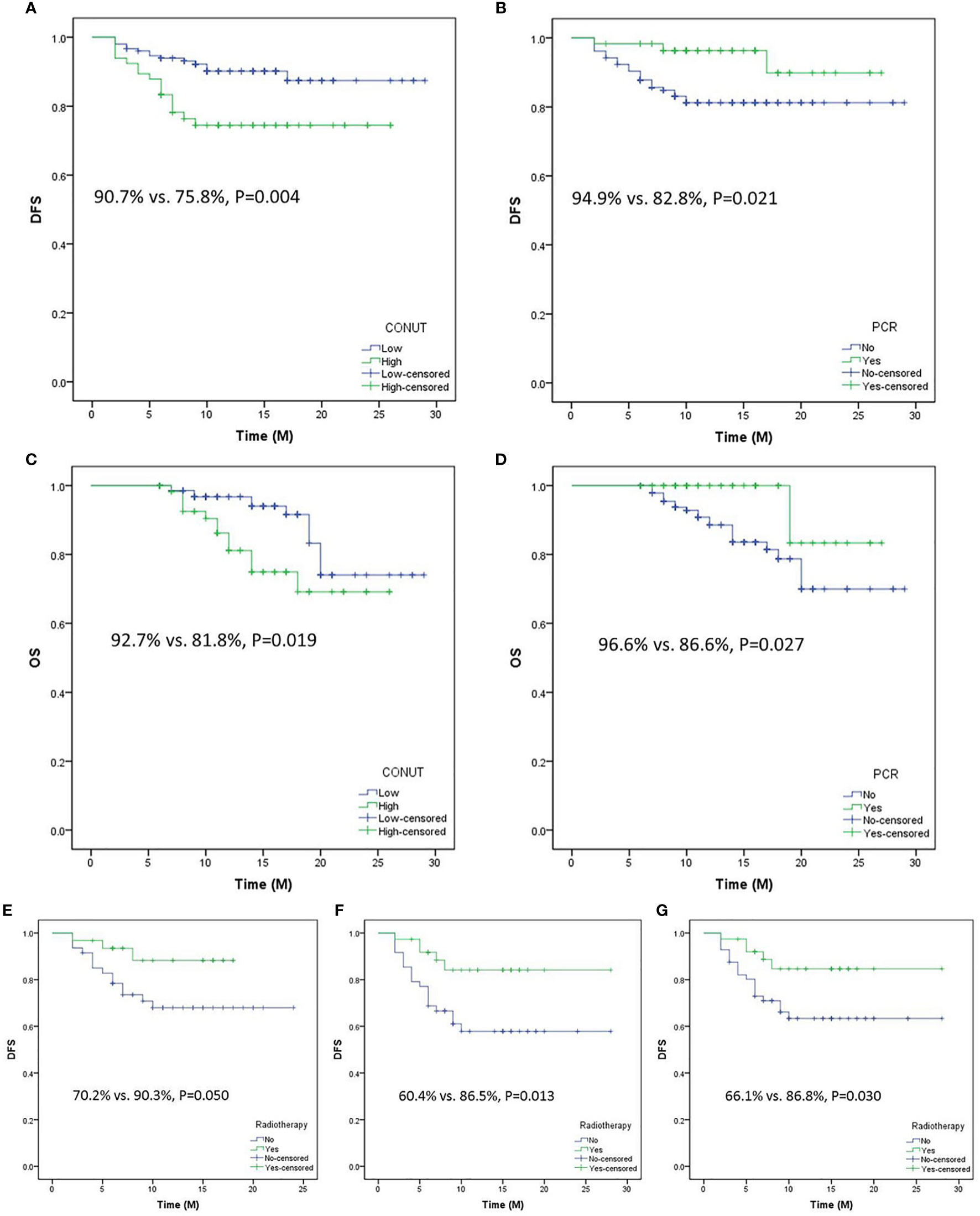
Figure 6 Disease-free survival (DFS) analyses grouped by CONUT (A) and pathologic complete response (pCR) (B). Overall survival analyses grouped by CONUT (C) and pCR (D). Subgroup analyses regarding DFS grouped by postoperative radiotherapy or not in ypT3-T4a (E), yp N1-3 (F), and ypTNM III-IVa (G).
A predictive nomogram of recurrence prediction in ESCC with nICT was established based on location, ypN stage, and CONUT (Figure 7A). The C-index for the nomogram model was 0.846. The model was confirmed through 1,000 bootstrapping internal validation, indicating an optimal agreement between the actual observation and model prediction (C-index: 0.838, Figure 7B). A good predictive ability regarding recurrence was also found according to the ROC and decision curve analyses (Figures 7C, D).
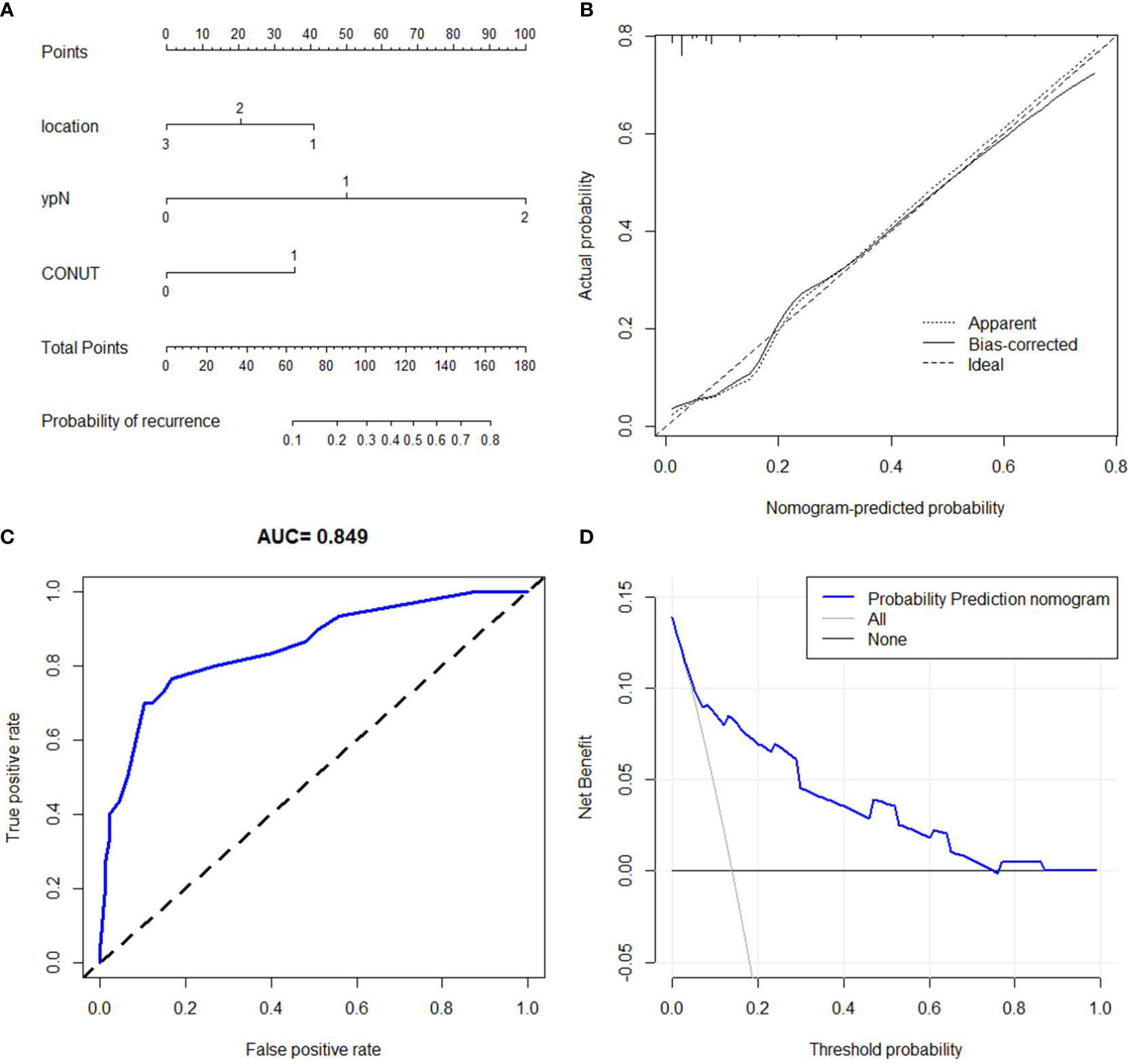
Figure 7 Nomogram for recurrence prediction. A predictive nomogram with the C-index of 0.846 was established (A). The calibration revealed an acceptable agreement of recurrence prediction internally (B). Receiver operating characteristic (C) and decision curve analyses (D) indicated a good clinical applicability of the model in predicting recurrence.
In the current study, we initially explored the role of pretreatment CONUT on recurrence prediction in ESCC with nICT. The results revealed that the CONUT score may be used as a predictor for DFS and OS. In addition, a nomogram was also developed to predict recurrence after nICT. To our knowledge, this is the first report that focused on the role of CONUT score in predicting recurrence after nICT in ESCC. Moreover, we initially proposed a predictive model to predict recurrence after nICT for ESCC. The results of our study will have an important significance to local advanced ESCC patients who were treated with nICT.
In recent years, immunotherapy has become the focus of cancer treatment. Recent studies revealed that immunotherapy significantly improved the prognosis in several randomized phase III studies and was approved for first-line treatment for advanced ESCC (7, 8). As an exploratory attempt, nICT has already been investigated for ESCC in several studies. The results as well as our previously published study indicated that a high R0 resection and pCR rate were found in patients with ESCC receiving nICT (10–13). However, most previously published studies focused on the efficiency and safety of nICT. There are no studies regarding recurrence after nICT in patients with ESCC so far. Therefore, it is important to understand the real treatment effect of nICT in patients with ESCC.
As a useful index for immune-nutritional status, recent studies revealed that CONUT score was an effective predictive and prognostic indicator in various cancers (15–17). Since 2016, a few studies on the CONUT score in patients with EC have been reported (16, 23, 31–33). A study including 352 ESCC patients with surgery reported that patients with moderate or severe CONUT score were at a high risk of postoperative complications (31). Another study revealed that patients with moderate or severe CONUT score were related to poor prognosis in 373 ESCC patients with radical resection (16). Compared with other indicators, several studies have also shown the superiority of the CONUT score in predicting the prognosis in ESCC patients undergoing surgery (32, 33). In addition, some studies have evaluated the treatment response of pretreatment CONUT for neoadjuvant therapy in GC and EC patients (22, 23). Recently, a meta-analysis with 952 patients including five studies verified the significant associations between the CONUT and prognosis (34). However, no relevant research has yet been reported regarding the predictive value of the CONUT for response to nICT in ESCC.
A recent study analyzed the prognostic value of CONUT in advanced EC patients who were treated with immunotherapy (21). The results revealed that a high CONUT score was associated with a significantly worse prognosis. Similar results were also found in our study for patients with local advanced ESCC in nICT. Based on these results, the significance of CONUT score as a prognostic index is clearer. The results will bring an important assessment of recurrence pattern before nICT and help clinicians provide a more personalized approach to adjuvant therapy in ESCC after nICT. We believe that patients with high CONUT in ESCC should be regarded with caution. Adjuvant therapy may be required for those with high CONUT.
There are several potential mechanisms that could explain the relationship between CONUT and cancer prognosis. It is well known that CONUT comes from three hematological variables, representing caloric consumption, protein reservation, and impaired immune defense, respectively. LYM, as a determinant of immunity, can inhibit the proliferation, migration, and invasion of cancer cells by initiating a cytotoxic immune response (35, 36). Lymphocytopenia causes an insufficient host immune response, resulting in a poor prognosis in cancers (37). ALB is used as a nutritional score to reflect the nutritional status. It has been reported that the mechanism of hypoproteinemia leading to poor prognosis may be through the release of a variety of inflammatory cytokines, such as IL-6 and TNF-α (38). TC, as a vital component of the cell membrane, participates in various biological signaling pathways. The main effect of hypocholesterolemia on the ability of transmembrane signaling may be due to the increased uptake of TC by tumor cells (39). Thus, combined with these three components, CONUT may provide a better balance of immunological and nutritional status.
Some limitations should be recognized. Firstly, this was a single-center study with retrospective characteristics. Secondly, the CONUT score, as a hematological index, may be affected by various other conditions. Thirdly, although internal validation was verified, there was a lack of an external validation cohort to validate the nomogram. Fourthly, the follow-up time for this study was too short. Therefore, some bias may exist in the prognostic factors. Fifthly, there is no special stratified analysis in the current study due to the short follow-up time. Finally, the basic biological mechanisms with regard to CONUT have not been thoroughly elucidated. However, it is believed that, with more and more studies regarding nICT in ESCC, the CONUT will be better elucidated.
These real-world data revealed that patients with a low score might have a better response and a lower recurrence. As a useful index for immune-nutritional status, the pretreatment CONUT score might be a reliable predictor in ESCC with nICT. The simple and easily obtained feature of the CONUT improves its application in daily clinical work.
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
This study was performed in accordance with the Helsinki Declaration. This study was reviewed and approved by the ethics committee of Zhejiang Cancer Hospital (IRB-2020-320). The patients/participants provided their written informed consent to participate in this study.
JF, QC, and XC conceived and designed the study. JF and LW collected the clinical baseline characteristics and drafted the manuscript. LW and XY carried out the follow-up. JF, LW, and XY performed the data analyses. QC and XC helped to draft the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Zhejiang TCM Science and Technology Project (2020ZB036, 2021ZB034, and 2022ZB051).
The authors thank all the patients and their families who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1015365/full#supplementary-material
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) (2021) 134(7):783–91. doi: 10.1097/CM9.0000000000001474
4. Du XX, Yu R, Wang ZF, Du DC, Liu QY, Wang RM, et al. Outcomes and prognostic factors for patients with cervical esophageal cancer undergoing definitive radiotherapy or chemoradiotherapy. Bosn J Basic Med Sci (2019) 19(2):186–94. doi: 10.17305/bjbms.2019.3873
5. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
6. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol (2012) 19(1):68–74. doi: 10.1245/s10434-011-2049-9
7. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888
8. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/S1470-2045(19)30626-6
9. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125
10. Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis (2021) 13(6):3518–28. doi: 10.21037/jtd-21-340
11. Yang G, Su X, Yang H, Luo G, Gao C, Zheng Y, et al. Neoadjuvant programmed death-1 blockade plus chemotherapy in locally advanced esophageal squamous cell carcinoma. Ann Transl Med (2021) 9(15):1254. doi: 10.21037/atm-21-3352
12. Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer (2022) 10(1):e003497. doi: 10.1136/jitc-2021-003497
13. Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol (2021) 12(1):1–10. doi: 10.21037/jgo-20-599
14. Ruan GT, Yang M, Zhang XW, Song MM, Hu CL, Ge YZ, et al. Association of systemic inflammation and overall survival in elderly patients with cancer cachexia - results from a multicenter study. J Inflammation Res (2021) 14:5527–40. doi: 10.2147/JIR.S332408
15. Li Y, Zhang C, Ji R, Lu H, Zhang W, Li LL, et al. Prognostic significance of the controlling nutritional status (CONUT) score in epithelial ovarian cancer. Int J Gynecol Cancer (2020) 30(1):74–82. doi: 10.1136/ijgc-2019-000865
16. Yoshida N, Harada K, Baba Y, Kosumi K, Iwatsuki M, Kinoshita K, et al. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch Surg (2017) 402(2):333–41. doi: 10.1007/s00423-017-1553-1
17. Nemoto Y, Kondo T, Ishihara H, Takagi T, Fukuda H, Yoshida K, et al. The controlling nutritional status CONUT score in patients with advanced bladder cancer after radical cystectomy. In Vivo (2021) 35(2):999–1006. doi: 10.21873/invivo.12343
18. Aoyama T, Komori K, Nakazano M, Hara K, Tamagawa H, Kazama K, et al. The clinical influence of the CONUT score on survival of patients with gastric cancer receiving curative treatment. In Vivo (2022) 36(2):942–8. doi: 10.21873/invivo.12784
19. Sun F, Zhang C, Liu Z, Ai S, Guan W, Liu S. Controlling nutritional status (CONUT) score as a predictive marker for short-term complications following gastrectomy of gastric cancer: A retrospective study. BMC Gastroenterol (2021) 21(1):107. doi: 10.1186/s12876-021-01682-z
20. Hayama T, Ozawa T, Okada Y, Tsukamoto M, Fukushima Y, Shimada R, et al. The pretreatment controlling nutritional status (CONUT) score is an independent prognostic factor in patients undergoing resection for colorectal cancer. Sci Rep (2020) 10(1):13239. doi: 10.1038/s41598-020-70252-2
21. Chang L, Cheng Q, Ma Y, Wu C, Zhang X, Ma Q, et al. Prognostic effect of the controlling nutritional status score in patients with esophageal cancer treated with immune checkpoint inhibitor. J Immunother (2022) 45(9):415–22. doi: 10.1097/CJI.0000000000000438
22. Jin H, Zhu K, Wang W. The predictive values of pretreatment controlling nutritional status (CONUT) score in estimating short- and long-term outcomes for patients with gastric cancer treated with neoadjuvant chemotherapy and curative gastrectomy. J Gastric Cancer (2021) 21(2):155–68. doi: 10.5230/jgc.2021.21.e14
23. Hikage M, Taniyama Y, Sakurai T, Sato C, Takaya K, Okamoto H, et al. The influence of the perioperative nutritional status on the survival outcomes for esophageal cancer patients with neoadjuvant chemotherapy. Ann Surg Oncol (2019) 26(13):4744–53. doi: 10.1245/s10434-019-07742-9
24. Feng J, Wang L, Yang X, Chen Q, Cheng X. Pathologic complete response prediction to neoadjuvant immunotherapy combined with chemotherapy in resectable locally advanced esophageal squamous cell carcinoma: Real-world evidence from integrative inflammatory and nutritional scores. J Inflammation Res (2022) 15:3783–96. doi: 10.2147/JIR.S367964
25. Zhang T, Hou X, Li Y, Fu X, Liu L, Xu L, et al. Effectiveness and safety of minimally invasive ivor Lewis and McKeown oesophagectomy in Chinese patients with stage IA-IIIB oesophageal squamous cell cancer: A multicentre, non-interventional and observational study. Interact Cardiovasc Thorac Surg (2020) 30(6):812–9. doi: 10.1093/icvts/ivaa038
26. Kang X, Qin Z, Zhang R, Wang Z, Zheng Q, Li Y, et al. 2021 NCC/CATS/CSTCVS/STM expert consensus on perioperative immunotherapy for esophageal cancer. Ann Esophagus (2021) 4:33. doi: 10.21037/aoe-21-64
27. Zhu Y, Li M, Kong L, Yu J. Postoperative radiation in esophageal squamous cell carcinoma and target volume delineation. Onco Targets Ther (2016) 9:4187–96. doi: 10.2147/OTT.S104221
28. Lin HN, Chen LQ, Shang QX, Yuan Y, Yang YS. A meta-analysis on surgery with or without postoperative radiotherapy to treat squamous cell esophageal carcinoma. Int J Surg (2020) 80:184–91. doi: 10.1016/j.ijsu.2020.06.046
29. Rice TW, Ishwaran H, Hofstetter WL, Kelsen DP, Apperson-Hansen C, Blackstone EH, et al. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus (2016) 29(8):897–905. doi: 10.1111/dote.12533
30. Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer (2005) 103(7):1347–55. doi: 10.1002/cncr.20916
31. Yoshida N, Baba Y, Shigaki H, Harada K, Iwatsuki M, Kurashige J, et al. Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg (2016) 40(8):1910–7. doi: 10.1007/s00268-016-3549-3
32. Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, et al. The pretreatment controlling nutritional status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: Results from a retrospective study. BMC Cancer (2016) 16(1):722. doi: 10.1186/s12885-016-2696-0
33. Hirahara N, Matsubara T, Hayashi H, Takai K, Nakada S, Tajima Y. Prognostic importance of controlling nutritional status in patients undergoing curative thoracoscopic esophagectomy for esophageal cancer. Am J Ther (2018) 25(5):e524–32. doi: 10.1097/MJT.0000000000000414
34. Takagi K, Buettner S, Ijzermans JNM, Wijnhoven BPL. Systematic review on the controlling nutritional status (CONUT) score in patients undergoing esophagectomy for esophageal cancer. Anticancer Res (2020) 40(10):5343–9. doi: 10.21873/anticanres.14541
35. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br J Cancer (2011) 105(1):93–103. doi: 10.1038/bjc.2011.189
36. Hao J, Li M, Zhang T, Yu H, Liu Y, Xue Y, et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on lymphocyte subsets in esophageal squamous cell carcinoma: An updated meta-analysis. Front Oncol (2020) 10:614. doi: 10.3389/fonc.2020.00614
37. Damen PJJ, Kroese TE, van Hillegersberg R, Schuit E, Peters M, Verhoeff JJC, et al. The influence of severe radiation-induced lymphopenia on overall survival in solid tumors: A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys (2021) 111(4):936–48. doi: 10.1016/j.ijrobp.2021.07.1695
38. Goh SL, De Silva RP, Dhital K, Gett RM. Is low serum albumin associated with postoperative complications in patients undergoing oesophagectomy for oesophageal malignancies? Interact Cardiovasc Thorac Surg (2015) 20(1):107–13. doi: 10.1093/icvts/ivu324
Keywords: controlling nutritional status, esophageal squamous cell carcinoma, pathologic complete response, recurrence, neoadjuvant immunochemotherapy, disease-free survival
Citation: Feng J, Wang L, Yang X, Chen Q and Cheng X (2022) The usefulness of pretreatment controlling nutritional status score for predicting recurrence in patients with esophageal squamous cell carcinoma undergoing neoadjuvant immunochemotherapy: A real-world study. Front. Immunol. 13:1015365. doi: 10.3389/fimmu.2022.1015365
Received: 09 August 2022; Accepted: 31 October 2022;
Published: 24 November 2022.
Edited by:
Wenwu He, Sichuan Cancer Hospital, ChinaReviewed by:
Guibin Qiao, Guangdong Provincial People’s Hospital, ChinaCopyright © 2022 Feng, Wang, Yang, Chen and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qixun Chen, Y2hlbnFpeEB5ZWFoLm5ldA==; Xiangdong Cheng, Y2hlbmd4ZDUxNkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.