
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 24 November 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1015081
This article is part of the Research TopicCombinational Immunotherapy of Cancer: Novel Targets, Mechanisms, and StrategiesView all 85 articles
 Xianggui Yuan1†
Xianggui Yuan1† Xian Li1†
Xian Li1† Yurong Huang1†
Yurong Huang1† Xueli Jin1
Xueli Jin1 Hui Liu1
Hui Liu1 Aiqi Zhao1
Aiqi Zhao1 Weiping Zhang2*
Weiping Zhang2* Wenbin Qian1,3*
Wenbin Qian1,3* Yun Liang1*
Yun Liang1*Introduction: Relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL) has poor clinical outcomes when treated with conventional salvage chemotherapy. Monotherapy using zanubrutinib, a selective Bruton’s tyrosine kinase (BTK) inhibitor, has achieved modest antitumor effect in R/R DLBCL. Here we aimed to evaluate the efficacy and safety of zanubrutinib plus salvage chemotherapy in R/R DLBCL patients.
Methods: We retrospectively reviewed R/R DLBCL patients who were administered with zanubrutinib plus salvage chemotherapy in our center between January, 2019 and December, 2021. Targeted panel sequencing of 11 lymphoma-related genes was performed on 8 patients with poor responses to zanubrutinib-based chemotherapy.
Results: 27 R/R DLBCL patients were enrolled. Median age at this study was 59 years (range, 15-72). The best overall response rate (ORR) was 74.1% and complete remission rate was 33.3%. With a median follow-up of 11 months (range, 1-17), the median progression-free survival (PFS) was 8.1 months, and the overall survival (OS) was not achieved. The most common grade-3/4 adverse events were neutropenia (70.4%), thrombocytopenia (66.7%), and febrile neutropenia (33.3%). In multivariate analysis, early treatment and overall response after chemotherapy were independent favorable prognostic factors for PFS. Overall response after chemotherapy was an independent favorable factor for OS. Among the 8 patients with poor response to zanubrutinib-based treatment, the majority of patients had NOTCH2 mutations (n=8, 100%) and TP53 mutations (n=7, 87.5%). However, these patients achieved an ORR of 75% at 3 months after CD19-CAR-T cell therapy (including 4 cases of complete remission and 2 cases of partial remission). With a median follow-up of 9 months from CAR-T cell infusion (range, 1-16 months), the median PFS was 14.5 months, and the median OS was not reached.
Conclusion: With high efficacy and manageable tolerability, zanubrutinib plus salvage chemotherapy may be a potential treatment option for R/R DLBCL. CAR-T cell therapy may be a priority strategy for these poor responders to BTKi-based treatment.
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive lymphoma, accounting for 30% to 40% of non-Hodgkin lymphomas (NHLs) (1, 2). R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard first-line treatment, achieving approximately 50%-60% of long-term remission. Unfortunately, up to 50% of patients are ultimately refractory to, or relapse after initial remission (3, 4). Salvage chemotherapy followed by autologous stem cell transplantation (auto-SCT) is effective for relapsed or refractory DLBCL (R/R DLBCL). But only 26% of patients respond to next-line salvage therapy and the median overall survival (OS) is only 6.3 months in the SCHOLAR-1 study (5). Currently there is no preferred salvage chemotherapy for R/R DLBCL.
Bruton tyrosine kinase (BTK) inhibitor has been proven highly effective for diverse B-cell malignancies. Ibrutinib, the first-in-class BTK inhibitor, has achieved an overall response rate (ORR) of 23% with modest activity in R/R DLBCL (6). Zanubrutinib (BGB-3111), a next-generation BTK inhibitor with minimal off-target effects, has demonstrated higher efficacy and safety for treating Waldenström macroglobulinemia, compared with ibrutinib (7). The phase 2 BGB-3111-207 study revealed that zanubrutinib monotherapy produced modest antitumor activity and favorable safety in R/R DLBCL, with an ORR of 29.3% and a complete remission (CR) rate of 17.1%. Developing mechanistically-based synergistic combinations may open a way to increase response rates and durability of zanubrutinib.
Over the last 2 years, our institution had integrated zanubrutinib into conventional salvage chemotherapy for R/R DLBCL. Therefore, we conducted a retrospective study to evaluate the efficacy and safety of zanubrutinib plus salvage chemotherapy for R/R DLBCL.
This was a retrospective study of R/R DLBCL patients who received zanubrutinib plus conventional chemotherapy at our center. Patients were enrolled from January 2019 to December 2021. Clinicopathological data were collected using electronic medical records. Follow-up data was obtained from patients’ records or by telephone.
Patients were enrolled in this study who met the criteria as follows: age ≥14 years and histologically diagnosed CD20-positive DLBCL with relapsed or refractory disease. The excluded criteria were: central nervous system lymphoma, HIV-positive DLBCL, post-transplant lymphoproliferative disorders, or prior exposure to a BTK inhibitor. Refractory disease was defined as progressive disease (PD) or stable disease (SD) as the best response to chemotherapy or relapse ≤12 months after auto-SCT. Primary refractory DLBCL was defined as non-responders to first-line treatment or patients who relapsed within 3 months of CR or partial remission (PR). Relapse was defined as recurrence of progressive disease after achieving a CR through last-line therapy. Patients with incomplete medical data or those lost to follow-up were excluded from this study. The day of the last follow-up was January, 6th, 2022. The Ethics Committee of the Second Affiliated Hospital, Zhejiang University approved this study, which was conducted in accordance with the Declaration of Helsinki.
Salvage chemotherapy regimens included the ICE regimen (ifosfamide, carboplatin and etoposide), GDP regimen (gemcitabine, dexamethasone, and cisplatin) and GemOx regimen (oxaliplatin and gemcitabine). Salvage chemotherapy was selected by treating investigator and the same salvage chemotherapy had not been ever applied before they were enrolled in this study. Patients received rituximab when the patients relapsed >6 months after rituximab-containing treatment or based on their willingness. The dose was reduced by 20%-50% after patients experienced grade-4 adverse events (AEs). Prophylactic pegylated granulocyte colony-stimulating factor (Peg-G-CSF) was administered if grade-4 neutropenia or grade-3 neutropenia with fever developed in previous cycles of treatment. Zanubrutinib,160 mg orally, twice a day was initially given and the dose was reduced by 50% after patients experienced grade-4 neutropenia or grade-3 neutropenia with fever again in previous cycles of treatment after prophylactic Peg-G-CSF used. The number of cycles was up to 6 cycles with response. Autologous stem cell transplantation (auto-SCT) consolidation was recommended for transplant-eligible patients who achieved remission from combination therapy. Prophylactic antifungal therapy was not routinely used.
Patients’ responses were assessed according to the revised response criteria for malignant lymphoma every two cycles (8). 18F-fluorodeoxyglucose positron-emission tomography and computed tomography (PET/CT) were used to assess responses after 4 cycles of treatment and upon suspected CR. Patients were regularly followed every 3 to 6 months thereafter. Overall response was defined as a PR or a CR. Covariates including disease stage, B symptoms, cell of origin, and results of immunohistochemical analysis were identified upon diagnosis. The nongerminal center B-cell like (non-GCB) or germinal center B-cell like (GCB) subtype was identified according to Hans’s algorithm. Eastern Cooperative Oncology Group (ECOG), Lactate dehydrogenase (LDH), extranodal sites, previous line of therapy, performance status, and disease status were determined before initiating treatment. Adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
Targeted panel sequencing was performed using a selected panel that contained 11 genes related to DLBCL (NOTCH2, TP53, KMT2D, CD79B, TRAF3, PRDM1, MYD88, CD79A, CXCR4, ARIDIA and LYN). Genomic DNA was extracted from the formalin-fixed paraffin-embedded tumor tissue samples at recurrence or refractory disease. The detailed methods were carried out as described previously (9). The aimed average sequencing depth for all targeted regions was 2000×. Targeted panel sequencing and sequencing data analysis were performed by Idtbio Biotechnology Co. LTD (Hangzhou, China).
Patients’ characteristics were summarized using descriptive statistical methods. Statistical analyses were performed using SPSS version 17. Statistical values were reported as medians. PFS was defined as initiation of zanubrutinib-based chemotherapy to disease progression or relapse, death of any cause, or last follow-up. OS was defined as initiation of zanubrutinib-based chemotherapy to death from any cause or last follow-up. PFS and OS were plotted according to the Kaplan-Meier method. Survival distributions were compared with the log-rank test. Multivariate analysis was performed using the Cox’s proportional hazards model. A two-sided P value <0.05 was considered a significant difference.
We identified 27 patients who received zanubrutinib combined with salvage chemotherapies between January 2019 and December 2021. Patients’ baseline data are presented in Table 1. At the time of diagnosis, 74.1% (n=20) patients were presented with Ann-Arbor stages III-IV, 82.5% (n=23) were identified with the non-GCB subtype, 51.9% (n=14) patients exhibited double expression, and 14.8% (n=4) patients exhibited double-hit status. At the time of this study, the median age was 59 (range, 15-72 years), 55.6% (n=15) had an ECOG score of 2-4, 70.5% (n=19) had elevated LDH levels, 66.7% (n=18) had extra-nodal disease, 14.8% (n=4) had relapsed disease, and 85.2% (n=23) had refractory diseases, among which 48.1% (n=13) had primary refractory diseases. The median lines of prior chemotherapies were 2 (range, 1-4). Two patients received prior auto-SCT and one received prior CD19-targeted chimeric antigen receptor T-Cell (CAR-T) immunotherapy.
Overall, 17, 7, and 3 patients received the ICE-based regimen, the GDP-based regimen, or the GemOx-based regimen, respectively. Swimmer plots of all patients evaluable for response are shown in Figure 1A. At the end of follow-up, 3 patients continued treatments, and 24 discontinued treatments. A total of 88 cycles of chemotherapy were administered. The median cycles of treatment were 4 (range, 1-6 cycles). 66.7% (n=18) patients received rituximab treatment.
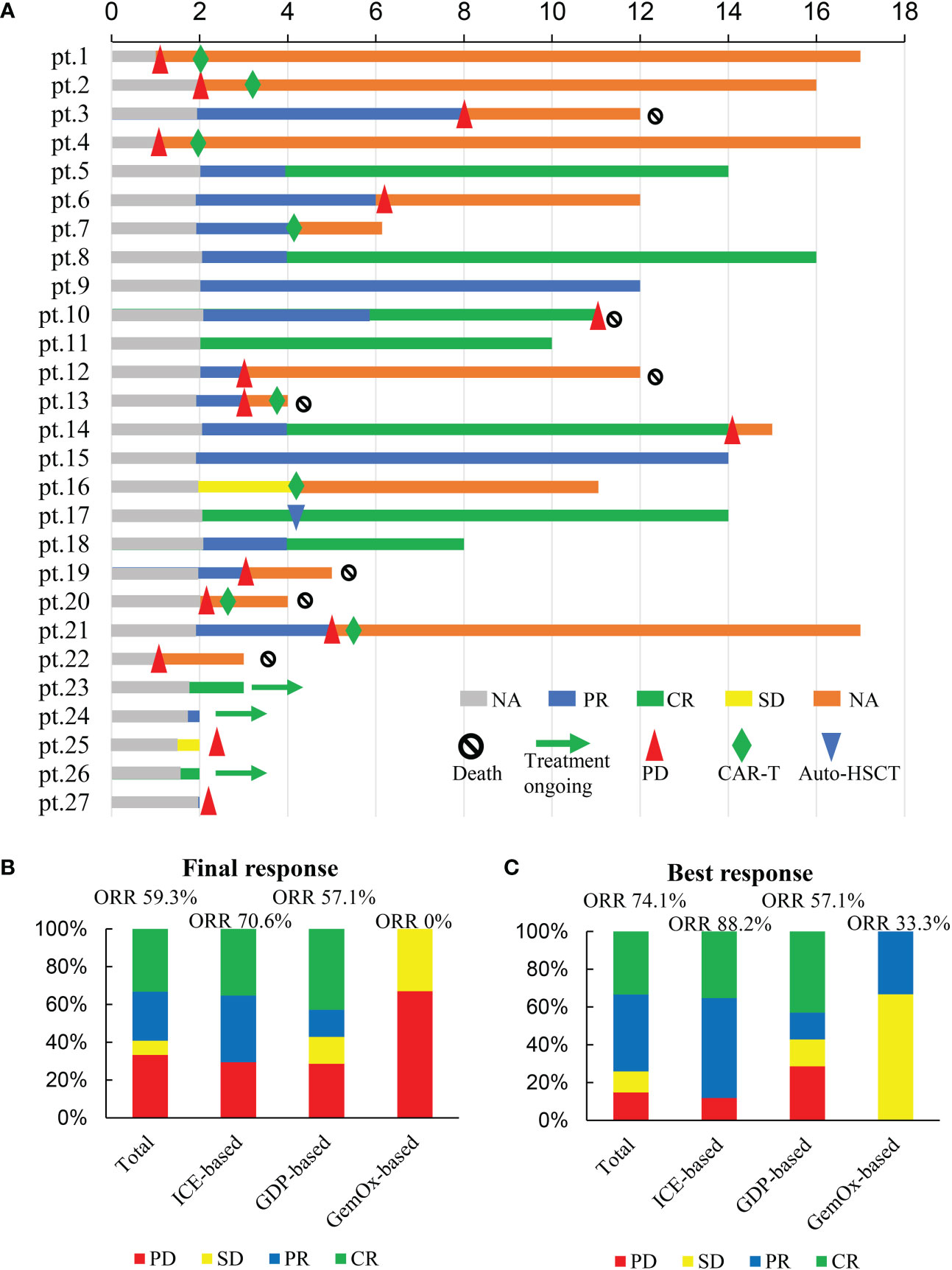
Figure 1 Responses to treatment. (A) swimmer plots of all patients evaluable for response; (B) Final responses to treatment; (C) Best responses to treatment.
The final responses and best responses to different combination regimens are shown in Figures 1B, C, respectively. At the end of treatment, 59.3% (n=16) patients had an overall response and 33.3% (n=9) achieved a CR. The best ORR was 74.1% (n=20) with 33.3% (n=9) of CR. Responses were observed in most subgroups (Figure 2), although there was a lower ORR trend in heavily pretreated patients (4-5 lines vs. 2-3 lines, 50.0% vs. 63.2%) and refractory patients (refractory vs. relapsed, 56.5% vs. 75%). Furthermore, 46.2% (6/13) patients with primary refractory DLBCL responded. The final ORR of the ICE-based, GDP-based, and GemOx-based groups were 70.6%, 57.1%, and 0%, respectively. The GemOx-based combination regimen was not as effective as the other two regimens. Overall, 7.1% (1/14) of transplant-eligible patients proceeded to auto-SCT, and 60% (6/10) of unresponsive patients as well as 2 PR patients proceeded to CD19-targeted CAR-T cell therapy with costimulatory 4-1BB endodomain (ClinicalTrials.gov ID: NCT04833504).
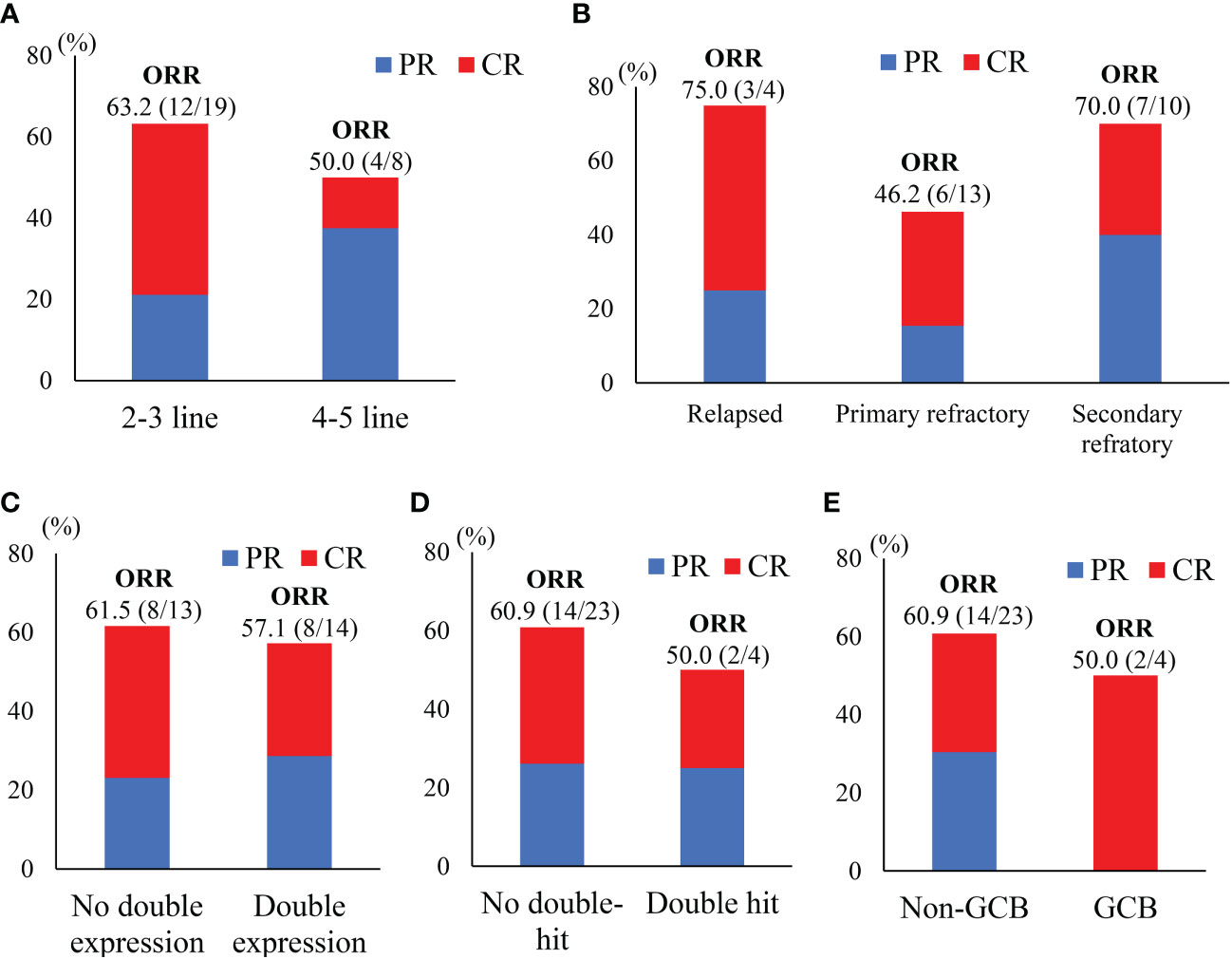
Figure 2 Subgroups responses to treatment. (A)Treatment lines; (B) disease status; (C) immunohistochemical analysis; (D) with or without double-hit; (E) cell of origin based on Hans’s algorithm.
With a median follow-up of 11 months (range, 1-17 months), 15 patients progressed and 7 died. The median PFS was 8.1 months (95%CI, 0.2-15.8) (Figure 3A), but the median OS was not reached (Figure 3B). Univariate and multivariate analyses of PFS and OS are described in Table 2. PFS and OS did not differ significantly regarding cell of origin, age, serum LDH, ECOG, disease status, combination regimen (Figures 3G, H), and subsequent treatment. Univariate analysis revealed that PFS was significantly longer in patients with early treatment (2-3 lines vs 4-5 lines, p=0.029) (Figure 3C) and in those with overall response after chemotherapy (p<0.001) (Figure 3E). Furthermore, univariate analysis revealed that OS was significantly longer in patients with overall response after chemotherapy (p=0.041) (Figure 3F) but not with prior lines of chemotherapies(p=0.819) (Figure 3D). Multivariate analysis revealed that early treatment (HR=0.27, p=0.032) and overall response after chemotherapy (HR=0.06, p<0.001) were independent factors for favorable PFS. Overall response after chemotherapy (HR=0.11, p=0.036) was an independent indicator for favorable OS.
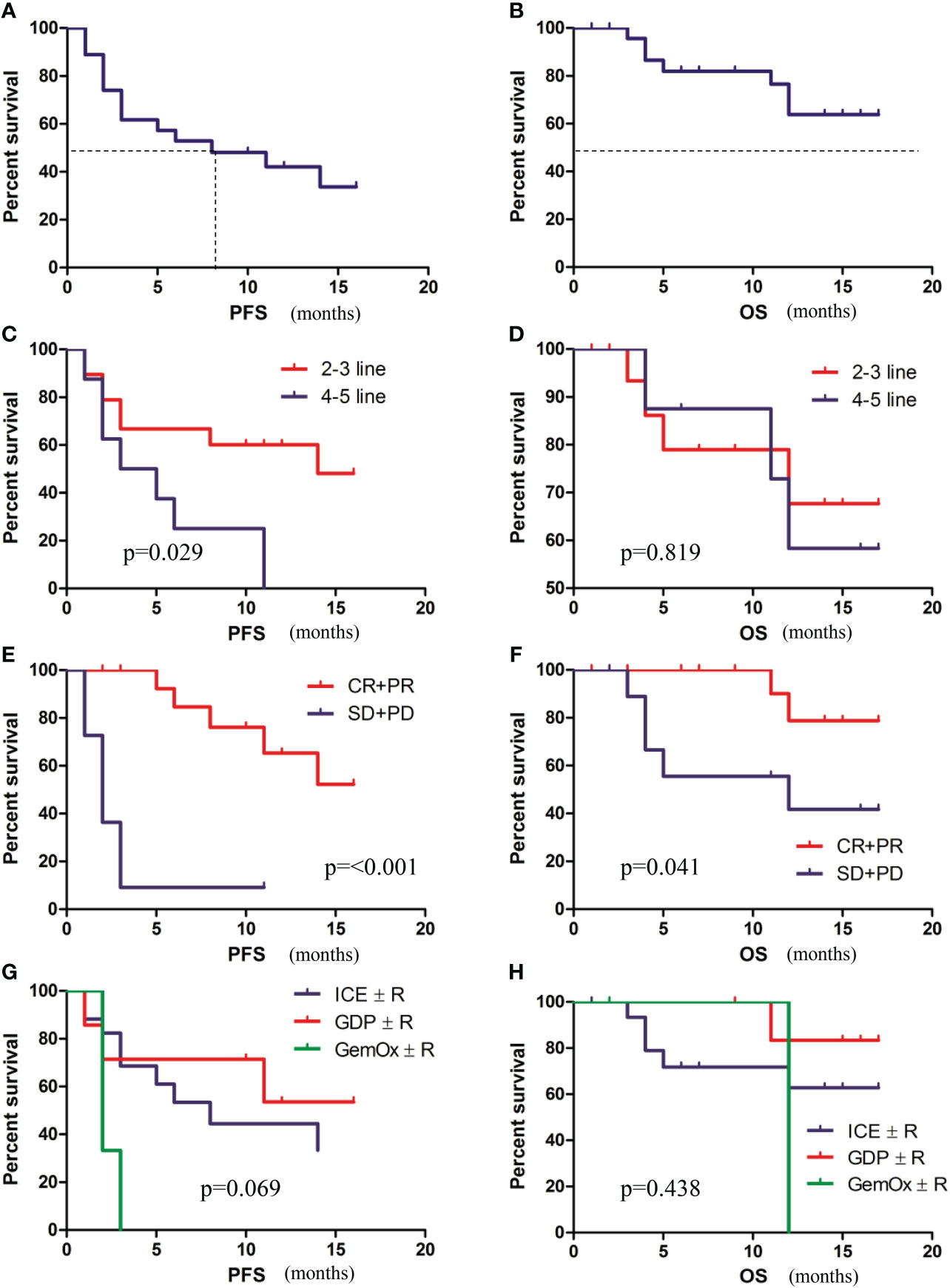
Figure 3 Survival analysis of patients. (A) PFS of patients. (B) OS of patients. (C) PFS of patients with early treatment (2-3 lines) versus late treatment (4-5 lines). (D) OS of patients with early treatment (2-3 lines) versus late treatment (4-5 lines). (E) PFS of patients with or without an overall response. (F) OS of patients with or without an overall response. (G) PFS according to combination regimens. (H) OS PFS according to combination regimens.
Treatment-related adverse events are described in Table 3. Neutropenia (n=19, 70.4%) and thrombocytopenia (n=18, 66.7%) were the most common grade 3/4 adverse events. Febrile neutropenia was observed in 33.3% patients (n=9). Platelet transfusion was required for 7.4% (n=2) of patients. The most non-hematologic adverse events were fatigue (n=13, 48.1%), nausea and vomiting (n=14, 51.9%), and bleeding (n=5, 18.5%). One patient developed Grade 4 thrombocytopenia and gastrointestinal bleeding, which were resolved with active symptomatic treatments. Two patients developed grade 1 hematuria and the remaining 2 patients developed grade 1 petechiae when the platelet count was normal. These bleeding disappeared after symptomatic treatments. Atrial fibrillation, aspergillosis, and tumor lysis syndrome were not observed. All toxicities were manageable and reversible. No treatment-related deaths were observed. The categories and severities of adverse events did not significantly vary among the different combination regimens.
The gene panel was performed on 8 patients (6 PD and 2 PR with prior zanubrutinib- based chemotherapy), who proceeded to CD19-CAR-T cell therapy. Genomic DNA was extracted from the formalin-fixed paraffin-embedded tumor tissue samples at recurrence or refractory disease. In total, 52 somatic alterations were detected. The patients presented a median of 6 mutations per sample (range 2–9). Missense mutations were the most frequent at 50/52 (96.2%). Figure 4A present the gene mutation frequencies. The most frequently mutated gene was NOTCH2 (8/8) and TP53 (7/8). Mutation location of NOTCH2 and TP53 at the protein level are shown in Figures 4D, E, respectively. The next were mutations of KMT2D and CD79B, which observed simultaneously in 5 cases. MYD88 mutation was identified in only one case, who achieved partial response with zanubrutinib-based chemotherapy. The ORR at 3 months after CAR-T cell therapy were 75% (including 4 cases of CR and 2 cases of PR). With a median follow-up of 9 months from CAR-T cell infusion (range, 1-16 months), the median PFS was 14.5 months (Figure 4B), but the median OS was not reached (Figure 4C).
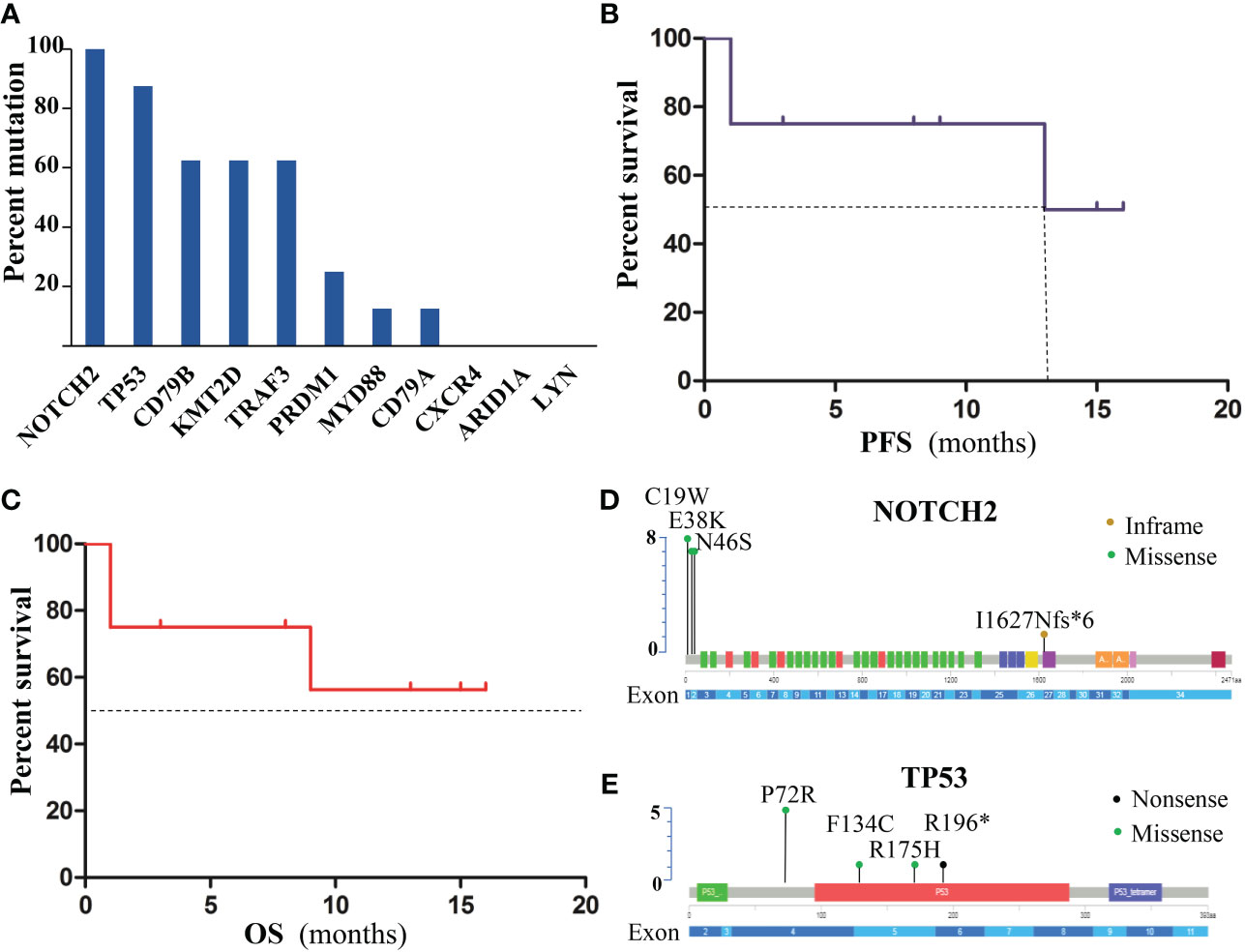
Figure 4 Gene mutations of patients with poor response to treatment. (A)Frequency of gene mutations. (B) PFS of patients. (C) OS of patients. (D) mutation location of NOTCH2 at the protein level. (E) TP53 Mutations at the protein level.
Zanubrutinib (BGB-3111) is a next-generation BTK inhibitor. Previous studies shows that zanubrutinib is more selective and active than ibrutinib in inhibiting BTK activity, with lower off-target activity against the follow protein tyrosine kinases: tyrosine kinase interleukin-2-inducible T-cell kinase (ITK), epidermal growth factor receptor (EGFR), and other kinases expressed in hepatocellular carcinoma (TEC) (10). In the present study, patients benefited from the encouraging efficacy of zanubrutinib plus salvage chemotherapy. The ORR of the present study was 74.1% and the CR rate was 33.3%. The median PFS was 8.1 months, but the median OS was not reached.
The responses and outcomes of BTK inhibitor monotherapy are unsatisfactory (6, 11). Developing mechanistically-based synergistic combinations may open a way to increase response rates and durability of BTK inhibitor. A study employing a high-throughput screening platform found that ibrutinib acted synergistically, additively, or both, with standard chemotherapeutic agents (12). A phase 1 study of ibrutinib plus R-ICE in R/R DLBCL demonstrated favorable tolerability and encouraging efficacies with 90% ORR and 55% CR (13). A phase 1/1b study of ibrutinib plus BR (rituximab, and bendamustine) induced 37% ORR and 31% CR in R/R DLBCL. Two patients with R/R DLBCL who received zanubrutinib plus R-DICE or R-DHAP respectively, also achieved a CR (14). Besides conventional salvage chemotherapy regimens, novel agents as BCL-2 inhibitors(venetoclax), immunomodulator(lenalidomide), PI3K inhibitors, XPO1 inhibitors(selinexor), IRAK4 inhibitors, immune checkpoint inhibitors, monoclonal/bispecific antibodies, CAR-T cell therapy and antibody-drug conjugates show strong synergistic activities with BTK inhibitors (12, 15, 16). A phase Ib study evaluated the combination of ibrutinib, lenalidomide, and rituximab for R/R DLBCL. The ORR was 44%, CR rate was 28%, and DOR was 15.9 months (17). Ibrutinib plus durvalumab achieved an ORR of 13% and 38% in GCB and non-GCB DLBCL, respectively (18). Ibrutinib plus venetoclax achieved an ORR of 53.8% after 4 cycles of treatment, and the median DOR、PFS and OS were 11 months, 5.6 months and 11.3 months, respectively (19). Acalabrutinib plus vistusertib (mTORC1/2 inhibitor) accomplished an ORR of 12% for R/R DLBCL (20). Ibrutinib plus buparlisib (a pan-PI3K inhibitor) for 37 patients with R/R DLBCL achieved an ORR of 31% (21). In the REAL-TREND study (22), a real-world retrospective analysis of treatment response of R/R DLBCL from 8 centers in China (including our center), the pooled ORR of salvage chemotherapy was 30% and the CRR was 9%. Our results with high efficacy indicated that zanubrutinib may act synergistically with conventional chemotherapeutic regimens.
DLBCL behaves genetic heterogeneity. Multiple studies have been made to identify sensitive patients who may potentially benefit from BTK inhibitors, based on tumor genetics, clinicopathology features, or both. Ibrutinib proves more effective in ABC-DLBCL (ORR=36.8%) than GCB (ORR=5%) (6). MCD, a genetic subtype of DLBCL with double mutant of CD79B and MYD88L265P, have inferior outcomes (23). The MCD subtype are more responsive to ibrutinib or zanubrutinib treatment (6, 11). CD79B and MYD88L265P double mutation are more responsive to ibrutinib, while single mutation is refractory (24, 25). Ibrutinib responders frequently harbor mutations in KLHL14, RNF213, and LRP1B, while non-responders commonly harbor mutations in EBF1, ADAMTS20, and AKAP9 (26). Furthermore, mutation of CARD11 and inactivation of TNFAIP3 (a negative regulator of NF-κB) predict no response to ibrutinib (6, 27). For non-responders to BTK inhibitors, BTK mutation are the best-described mechanisms (28). Third-generation non-covalent BTK inhibitors and CAR-T cell therapy, are promising strategies to overcome BTK inhibitor-resistance (29, 30). In the present study, the majority of the poor responders to zanubrutinib-based treatment had NOTCH2 mutations and TP53 mutations, while none was of MCD subtype, indicating that patients without MCD subtype maybe not benefit from BTKi-based treatment. TP53 is a tumor suppressor gene and TP53 mutation was an independent prognostic factor for survival in R/R DLBCL. In a retrospective study, in R/R DLBCL patients not treated with CAR-T cells, TP53 mutation was an independent inferior prognostic factor for OS, but in the CAR-T cell group, this significance could not be shown (31). CAR19/22 T-cell therapy combined with ASCT is efficacious in r/r aggressive B-NHL with TP53 alterations, producing a best ORR and CRR of 92.9% and 82.1%, respectively (32). However, in another retrospective study, TP53 alterations (mutations and/or copy number alterations) were still associated with inferior CR and OS rates in R/R DLBCL treated with CD19-CAR-T treatment (33). In our study, among the 7 patients with TP53 mutations, The ORR at 3 months after CAR-T cell therapy was 85.7% and the CRR was 57.1%. Compared with the results of TRANSCEND NHL 001study (Liso-cell) with the same costimulatory endodomain and similar follow-up time (ORR of 73% and CRR of 53%) (34), it seemed that the CAR-T cell therapy for patients with TP53 mutations was still highly effective. Although the prognostic value of TP53 mutations in R/R DLBCL patients receiving CAR-T cells is still undefined, CAR-T cell therapy may be a priority strategy for these patients.
As we expected, grade 3 and higher hematological toxicities were the major concern during and after zanubrutinib plus chemotherapy for patients with R/R DLBCL. Grade 3/4 neutropenia and thrombocytopenia of sole R-ICE regimen for R/R DLBCL occurred in 16% and 17.8% patients, respectively (35). Grade 3/4 neutropenia and thrombocytopenia of single zanubrutinib for R/R DLBCL occurred in 7.3% and 2.4% patients, respectively. In our present study, grade 3/4 neutropenia and thrombocytopenia of by zanubrutinib plus chemotherapy occurred in 70.4% and 66.7% of patients, respectively. Higher rates of hemorrhage were observed in 18.5% patients, which may be explained by thrombocytopenia and off-target activity of zanubrutinib. All above safety data showed that zanubrutinib plus chemotherapy increased myelosuppression. Fortunately, all hematological toxicities are manageable and reversible. There were no serious infectious complications or treatment-related mortality. To relieve bone marrow suppression, prophylactic Peg-G-CSF and recombinant human thrombopoietin (rhTPO) are required, and dose-modified salvage chemotherapy may also reduce hematologic toxicities. Regarding different BTK inhibitors, a phase 3 study demonstrated that the incidence and severity of BTK inhibitor toxicities were lower with zanubrutinib than ibrutinib in Waldenström macroglobulinemia (7). The efficacy and safety of zanubrutinib versus ibrutinib for CLL/SLL is ongoing in a head-to-head phase 3 study (36).
Our current study has several limitations. First, this study was insufficiently powered limited by the small sample size and short follow-up. Second, there were variations in prior therapies, which limit comparability. For example, only two patients received upfront auto-SCT in our study. Third, there were no genetic data available to demonstrate the underlying mechanisms of such synergistic combination. Nevertheless, the high activity of zanubrutinib combined with conventional chemotherapy, provides a new strategy for R/R DLBCL, and may serve as a bridge treatment to CAR-T cell therapy.
In conclusion, our study showed that zanubrutinib combined with salvage chemotherapy may serve as an effective salvage therapy for R/R DLBCL with manageable toxicity. Patients without MCD subtype maybe not benefit from BTKi-based treatment. CAR-T cell therapy may be a priority strategy for these poor responders to BTKi-based treatment. Further investigations of larger study populations are warranted to identify the most effective combination regimens and to precisely select patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by The Ethics Committee of the Second Affiliated Hospital, Zhejiang University. The patients/participants provided their written informed consent to participate in this study.
Conceptualization, YL and WQ; Methodology, XY; Software, YH and XL; Validation, XL; Formal Analysis, YH and HL; Investigation, XY, XL, and YH; Resources, YL and WQ; Data Curation, HL and AZ; Writing – Original Draft Preparation, XY; Writing -Review and Editing, WZ, WQ and YL: Visualization, XJ; Supervision, YL and WQ; Project Administration, XY; Funding Acquisition, WQ. All authors contributed to the article and approved the submitted version.
This work was supported by funds from Translational Research Grant of HCRCH (2020ZKZC01), the National Natural Science Foundation of China (No. 81830006), and the Natural Science Foundation of Zhejiang Province of China (No. LY15H160038).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. US Lymphoid malignancy statistics by world health organization subtypes. CA: Cancer J Clin (20162016) 66(6):443–59. doi: 10.3322/caac.21357
2. Sun J, Yang Q, Lu Z, He M, Gao L, Zhu M, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the world health organization classification. Am J Clin Pathol (2012) 138(3):429–34. doi: 10.1309/AJCP7YLTQPUSDQ5C
3. Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large b-cell lymphoma. J Clin Oncol (2006) 24(19):3121–7. doi: 10.1200/JCO.2005.05.1003
4. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the groupe d'Etudes des lymphomes de l'Adulte. Blood (2010) 116(12):2040–5. doi: 10.1182/blood-2010-03-276246
5. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large b-cell lymphoma: results from the international SCHOLAR-1 study. Blood (2017) 130(16):1800–8. doi: 10.1182/blood-2017-03-769620
6. Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting b cell receptor signaling with ibrutinib in diffuse large b cell lymphoma. Nat Med (2015) 21(8):922–6. doi: 10.1038/nm.3884
7. Tam CS, Opat S, D'Sa S, Jurczak W, Lee H-P, Cull G, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic waldenström macroglobulinemia: the ASPEN study. Blood (2020) 136(18):2038–50. doi: 10.1182/blood.2020006844
8. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol (2007) 25(5):579–86. doi: 10.1200/JCO.2006.09.2403
9. Jiang S, Qin Y, Jiang H, Liu B, Shi J, Meng F, et al. Molecular profiling of Chinese r-CHOP treated DLBCL patients: Identifying a high-risk subgroup. Int J Cancer (2020) 147(9):2611–20. doi: 10.1002/ijc.33049
10. Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in b-cell malignancies and safety and efficacy evaluation in CLL. Blood (2019) 134(11):851–9. doi: 10.1182/blood.2019001160
11. Yang H, Xiang B, Song Y, Zhang H, Zhao W, Zou D-H, et al. Zanubrutinib monotherapy for relapsed or refractory non-germinal center diffuse Large b-cell lymphoma. Blood Adv (2022) 6(6):1629–36. doi: 10.1182/bloodadvances.2020003698
12. Mathews Griner LA, Guha R, Shinn P, Young RM, Keller JM, Liu D, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated b-cell-like diffuse large b-cell lymphoma cells. P Natl Acad Sci USA (2014) 111(6):2349–54. doi: 10.1073/pnas.1311846111
13. Sauter CS, Matasar MJ, Schoder H, Devlin SM, Drullinsky P, Gerecitano J, et al. A phase 1 study of ibrutinib in combination with r-ICE in patients with relapsed or primary refractory DLBCL. Blood (2018) 131(16):1805–8. doi: 10.1182/blood-2017-08-802561
14. Zhang Y, Li Y, Zhuang Z, Wang W, Wei C, Zhao D, et al. Preliminary evaluation of zanubrutinib-containing regimens in DLBCL and the cerebrospinal fluid distribution of zanubrutinib: A 13-case series. Front Oncol (2021) 11:760405. doi: 10.3389/fonc.2021.760405
15. Schaffer M, Chaturvedi S, Davis C, Aquino R, Stepanchick E, Versele M, et al. Identification of potential ibrutinib combinations in hematological malignancies using a combination high-throughput screen. Leukemia Lymphoma (2018) 59(4):931–40. doi: 10.1080/10428194.2017.1349899
16. Sehn LH, Salles G. Diffuse Large b-cell lymphoma. N Engl J Med (2021) 384(9):842–58. doi: 10.1056/NEJMra2027612
17. Goy A, Ramchandren R, Ghosh N, Munoz J, Morgan DS, Dang NH, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center b-cell-like DLBCL. Blood (2019) 134(13):1024–36. doi: 10.1182/blood.2018891598
18. Herrera AF, Goy A, Mehta A, Ramchandren R, Pagel JM, Svoboda J, et al. Safety and activity of ibrutinib in combination with durvalumab in patients with relapsed or refractory follicular lymphoma or diffuse large b-cell lymphoma. Am J hematology (2020) 95(1):18–27. doi: 10.1002/ajh.25659
19. Zhou Z, Zhang L, Wang X, Li X, Li L, Fu X, et al. Ibrutinib combined with venetoclax for the treatment of relapsed/refractory diffuse large b cell lymphoma. Ann hematology (2021) 100(6):1509–16. doi: 10.1007/s00277-021-04535-7
20. Collins GP, Clevenger TN, Burke KA, Yang B, MacDonald A, Cunningham D, et al. A phase 1/2 study of the combination of acalabrutinib and vistusertib in patients with relapsed/refractory b-cell malignancies. Leukemia Lymphoma (2021) 62(11):2625–36. doi: 10.1080/10428194.2021.1938027
21. Stewart CM, Michaud L, Whiting K, Nakajima R, Nichols C, De Frank S, et al. Phase I/Ib study of the efficacy and safety of buparlisib and ibrutinib therapy in MCL, FL, and DLBCL with serial cell-free DNA monitoring. Clin Cancer Res (2022) 28(1):45–56. doi: 10.1158/1078-0432.CCR-21-2183
22. Wang S, Wang L, Hu J, Qian W, Zhang X, Hu Y, et al. Outcomes in refractory diffuse large b-cell lymphoma: results from a multicenter real-world study in China. Cancer Commun (Lond) (2021) 41(3):229–39. doi: 10.1002/cac2.12126
23. Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffuse Large b-cell lymphoma. New Engl J Med (2018) 378(15):1396–407. doi: 10.1056/NEJMoa1801445
24. Wang YL. MYD88 mutations and sensitivity to ibrutinib therapy. J Mol Diagn (2018) 20(2):264–6. doi: 10.1016/j.jmoldx.2017.11.006
25. Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center b-cell diffuse Large b-cell lymphoma. J Clin Oncol (2019) 37(15):1285–95. doi: 10.1200/JCO.18.02403
26. Hodkinson BP, Schaffer M, Brody JD, Jurczak W, Carpio C, Ben-Yehuda D, et al. Biomarkers of response to ibrutinib plus nivolumab in relapsed diffuse large b-cell lymphoma, follicular lymphoma, or richter's transformation. Transl Oncol (2021) 14(1):100977. doi: 10.1016/j.tranon.2020.100977
27. Bartlett NL, Costello BA, LaPlant BR, Ansell SM, Kuruvilla JG, Reeder CB, et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood (2018) 131(2):182–90. doi: 10.1182/blood-2017-09-804641
28. Woyach JA, Furman RR, Liu T-M, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the bruton's tyrosine kinase inhibitor ibrutinib. New Engl J Med (2014) 370(24):2286–94. doi: 10.1056/NEJMoa1400029
29. Ondrisova L, Mraz M. Genetic and non-genetic mechanisms of resistance to BCR signaling inhibitors in b cell malignancies. Front Oncol (2020) 10:591577. doi: 10.3389/fonc.2020.591577
30. Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood (2020) 135(19):1650–60. doi: 10.1182/blood.2019002936
31. Porpaczy E, Wohlfarth P, Königsbrügge O, Rabitsch W, Skrabs C, Staber P, et al. Influence of mutation on survival of diffuse Large b-cell lymphoma in the CAR T-cell era. Cancers (Basel) (2021) 13(22):5592. doi: 10.3390/cancers13225592
32. Wei J, Xiao M, Mao Z, Wang N, Cao Y, Xiao Y, et al. Outcome of aggressive b-cell lymphoma with TP53 alterations administered with CAR T-cell cocktail alone or in combination with ASCT. Signal Transduct Target Ther (2022) 7(1):101. doi: 10.1038/s41392-022-00924-0
33. Shouval R, Alarcon Tomas A, Fein JA, Flynn JR, Markovits E, Mayer S, et al. Impact of genomic alterations in Large b-cell lymphoma treated with CD19-chimeric antigen receptor T-cell therapy. J Clin Oncol (2022) 40(4):369–81. doi: 10.1200/JCO.21.02143
34. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large b-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet (London England) (2020) 396(10254):839–52. doi: 10.1016/S0140-6736(20)31366-0
35. Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large b-cell lymphoma in the rituximab era. J Clin Oncol (2010) 28(27):4184–90. doi: 10.1200/JCO.2010.28.1618
Keywords: relapsed or refractory diffuse large B-cell lymphoma, zanubrutinib, Bruton’s tyrosine kinase inhibitor, combination chemotherapy, TP53, chimeric antigen receptor T-cell (CAR-T)
Citation: Yuan X, Li X, Huang Y, Jin X, Liu H, Zhao A, Zhang W, Qian W and Liang Y (2022) Zanubrutinib plus salvage chemotherapy for relapsed or refractory diffuse large B-cell lymphoma. Front. Immunol. 13:1015081. doi: 10.3389/fimmu.2022.1015081
Received: 09 August 2022; Accepted: 07 November 2022;
Published: 24 November 2022.
Edited by:
Dianwen Ju, Fudan University, ChinaReviewed by:
Qi Deng, Tianjin First Central Hospital, ChinaCopyright © 2022 Yuan, Li, Huang, Jin, Liu, Zhao, Zhang, Qian and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Liang, bGlhbmd5dW5Aemp1LmVkdS5jbg==; Wenbin Qian, cWlhbndiQHpqdS5lZHUuY24=; Weiping Zhang, MTUzMjU3MTU1NjZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.