- 1Institute of Fundamental Medicine and Biology, Kazan (Volga Region) Federal University, Kazan, Russia
- 2Department of Neurology and Neurosurgery, Samara State Medical University, Samara, Russia
Multiple sclerosis (MS) is a heterogeneous disease where herpesvirus infection and genetic predisposition are identified as the most consistent risk factors. Serum and blood samples were collected from 151 MS and 70 controls and used to analyze circulating antibodies for, and DNA of, Epstein Barr virus (EBV), human cytomegalovirus (HCMV), human herpes virus 6 (HHV6), and varicella zoster virus (VZV). The frequency of selected single nucleotide polymorphisms (SNPs) in MS and controls were studied. Herpesvirus DNA in blood samples were analyzed using qPCR. Anti-herpesvirus antibodies were detected by ELISA. SNPs were analyzed by the allele-specific PCR. For statistical analysis, Fisher exact test, odds ratio and Kruskall–Wallis test were used; p<0.05 values were considered as significant. We have found an association between circulating anti-HHV6 antibodies and MS diagnosis. We also confirmed higher frequency of A and C alleles in rs2300747 and rs12044852 of CD58 gene and G allele in rs929230 of CD6 gene in MS as compared to controls. Fatigue symptom was linked to AC and AA genotype in rs12044852 of CD58 gene. An interesting observation was finding higher frequency of GG genotype in rs12722489 of IL2RA and T allele in rs1535045 of CD40 genes in patient having anti-HHV6 antibodies. A link was found between having anti-VZV antibodies in MS and CC genotype in rs1883832 of CD40 gene.
Introduction
Multiple sclerosis (MS) is a neurodegenerative disease of the central nervous system (CNS) where the breakdown of integrity of the myelin sheath is believed to be the main cause of nerve tissue damage (1). The disease has chronic progression characterized by the presence of demyelination areas in the brain, called plaques or lesions (2). These plaques are found predominantly in periventricular regions as well as in the temporal and parietal lobes of the brain (3). Destruction of grey and white brain matter was also associated with the disease (4, 5). Clinically, there are several forms of MS: relapsing remitting MS (RRMS), secondary progressing MS (SPMS), primary progressing MS (PPMS), and progressive relapsing (PRMS). The RRMS is characterized by episodes of neurological disability and recovery (4). Clinical symptoms of some RRMS could gradually exacerbate with a steady progression of symptoms, which is referred to as SPMS (6). PPMS is characterized by steady progression of the neurological symptoms without periods of recovery (7, 8).
Pathogenesis of MS is believed to be led by chronic inflammation, which is evident by increased serum mediators of inflammation (9–11). Leukocytes producing pro-inflammatory cytokines can also be found in the brain lesions (12, 13). This inflammation could be triggered by infection leading to activation of glial cells and stimulation of an autoimmune response (14, 15). The patient’s genetics could also contribute to inflammation by reducing control over the inflammatory pathways (16, 17). There are multiple identified risk factors for MS identified however, none of them is selected as a primary cause of the disease. This led to the recognition of MS as a heterogeneous disease where viral and genetic factors were frequently linked to the diagnosis.
The viral etiology of MS was suggested in several studies (18, 19) however, there are multiple viruses suggested to cause MS. Several studies have shown an association between antibodies to the Epstein Barr virus (EBV) and the risk of MS diagnosis (20–22). Further evidence of EBV’s role in MS pathogenesis emerges from finding more frequent disease diagnoses in children born to women with elevated levels of EBV IgG during pregnancy (23). Recently, a large cohort study demonstrated that the risk of MS is 32 folds higher after EBV infection (24). These data provide strong evidence in support of EBV’s role in MS pathogenesis. VZV and human HCMV also suggested contributing to MS risk (25). This suggestion was supported by finding a higher frequency of anti-VZV in MS as compared to controls (26). Interestingly, some studies report the association between anti-HCMV antibodies and reduced risk of MS (27). This protective effect of HCMV infection was explained by modified B cell differentiation and reduced production of pro-inflammatory cytokines in MS (28). Additionally, Human herpesvirus type 6 (HHV-6) was implicated to MS diagnosis (29). It was shown that an increased level of antibodies to HHV-6 directly correlates with a high probability of relapse in RRMS (30, 31). Also, HHV6 RNA was found in periventricular lesions supporting the involvement of HHV6 in MS pathogenesis (32, 33). It appears that there is solid evidence for a pathogenic role of herpesviruses in MS, where viruses could trigger autoimmunity and chronic inflammation (34, 35).
Genetic predisposition is also frequently identified in MS. The role of genetic factors could explain the high prevalence of MS in Northern Europe (36, 37). There are multiple studies suggesting the HLA allele DRB1* 15:01 as the most significant genetic risk factor for MS (38). Other genetic factors contributing to pathogenesis of MS are single nucleotide polymorphisms (SNPs) in genes coding for immune recognition factors, pro-inflammatory cytokines, and cell adhesion molecules. One of them is SNP rs4810485 in the CD40 gene which is linked to decreased expression of CD40 receptor in antigen presenting cells (APCs) (39). This could prevent ligand-receptor interaction and subsequent inhibition of APCs function (40). Presence of other SNPs, rs2104286 and rs11256593, in the IL2RA gene was shown to decrease the expression of the IL2RA receptor on CD4+ T-lymphocytes (41). This could lead to insufficient control over pathogenic CD4+ cells, which contributes to inflammation (41, 42). However, there is still limited data on the role of these and other SNPs on MS diagnosis, and little is known about the combined effect of herpesvirus infection and genetic markers of SNPs on MS diagnosis.
Accumulated clinical and epidemiological data provide evidence for herpesviruses as MS risk factors. The association between MS and herpesvirus infection was shown in Northern Europe (36, 43), Iran (44), the United States (24), Latvia (45) and India (46). MS is diagnosed in the Republic of Tatarstan, a part of the Volga Region of Russia. The prevalence and incidence rates are 36.7 and 5.5 per 100,000 population, which is within the average range in the European part of Russia (47). The role of infection in MS pathogenesis in Tatarstan is limited to a study demonstrating the higher expression of human endogenous retrovirus W in this cohort as compared to that in Great Britain (48). However, our understanding of herpesvirus’s role in MS in Tatarstan and other parts of the Volga Region remains limited. Likewise, our understanding of the association between herpesvirus infection and SNPs linked to MS in this region of Russia is incomplete. To address this knowledge gap, we sought to analyze herpesvirus infection and SNPs alleles in MS and controls from Tatarstan and Samara, two districts within the Volga region. We have found an association between detection of circulating anti-HHV6 antibodies and MS diagnosis. We also confirmed higher frequency of A and C alleles in rs2300747and rs12044852 of CD58 and G allele in rs929230 of CD6 gene in MS as compared to controls. We found higher frequency of rs1883832C and rs1535045T in MS female as compared to male in CD40. Fatigue was the only clinical symptom linked to AC and AA genotype in rs12044852 of CD58 gene. The interesting observation, that supports the role of HHV6 in MS pathogenesis, was finding higher frequency of GG genotype in rs12722489 of IL2RA and T allele in rs1535045 of CD40 genes in patient having anti-HHV6 antibodies. Also, a link between anti-VZV antibodies in MS and CC genotype in rs1883832 of CD40 gene.
Materials and methods
Human subjects
Venous blood and serum samples were collected from MS patients admitted to the Republican Clinical Neurological Center, Republic of Tatarstan, Russian Federation (18/34 male/female; 34.5 ± 10.6 years old) and the Samara State Medical University (SamSMU), the Department of Neurology and Neurosurgery, Samara Region, Russia (38/61 male/female; 38.1 ± 9.14 years old). All 151 MS samples were analyzed (herpesvirus seropositivity status, Expanded Disability Status Scale (EDSS), Multiple Sclerosis Severity Score (MSSS), and presence of fatigue) (Table 1). When MS patients were compared to controls, only age matched 124 MS samples were used for analysis (presence of herpesvirus DNA, seroprevalence, and SNPs) (Table 1). Age (22/48 male/female; 32.7 ± 13.1 years old) matched control samples from Tatarstan and Samara region were collected. Controls were neurological symptoms and/or MS symptoms free. Patients did not receive immune modified therapy. Demographic and clinical data are summarized in Table 1.
Ethics statement
This study was done in accordance with the recommendations of Biomedicine Ethic Expert Committee of Republican Clinical Neurological Center, Republic of Tatarstan, Russian Federation (N: 218, 15.11.2012). Signed written informed consent was collected from all subjects. The MS diagnosis was established by the primary physician according to the McDonald criteria (49).
Enzyme-linked immunosorbent assay
Herpes virus IgG antibodies were analyzed using HHV-6-IgG-ELISA-BEST (cat. D2166, Vector-Best, Russia), VectoVZV-IgG (cat. 2192, Vector-Best, Russia), VectoCMV-IgG (cat. 1554, Vector-Best, Russia), VectoVEB-NA-IgG (cat. 2170, Vector-Best, Russia) ELISA kits according to the instructions. Briefly, two wells of the strip were loaded with 100 μl of a negative control sample, while 100 μl of a positive control sample was added to a separate well. Diluted MS and control serum samples (100 μl; 1:100) were added to the remaining wells in duplicates. The plate was incubated (37 ± 1°C; 30 minutes), washed with 400 μl of washing solution (phosphate buffered saline and 0.5% Tween20 (PBS-T)) 5 times, followed by adding 100 μl of the conjugate. After incubation (37 ± 1°C; 30 minutes), the plate was washed (5x; PBS-T) and incubated with tetramethylbenzidine (TMB) solution (100 μl) for 25 minutes at room temperature. Stop reagent (100 μl) was added to each well before the optical density was measured using an Infinite 200 PRO vertical scanning spectrophotometer (Tecan Trading AG, Switzerland) at the main wavelength of 450 nm and a reference wavelength of 620 nm.
Each test system has a registration number, stating that the product is made specifically for clinical testing and in medical facilities (nevacert.ru). The specificity and sensitivity of each ELISA kit: 100% and 100% for HHV-6-IgG-ELISA-BEST, 100% and 100% VectoVZV-IgG, 100% and 100% for VectoCMV-IgG, 100% and 100% for VectoVEB-NA-IgG, respectively. The registration number is assigned for the product with testing characteristics, such as sensitivity and specificity, similar or superior to currently used tests for detecting anti-herpesvirus antibodies. VectoVEB-NA-IgG kit is marked as Conformité Européenne (CE), indicating that it complies with Harmonized Standards of the European Union (50).
DNA isolation
Total DNA was isolated using the ExtractDNA Blood & Cells kit (cat. # BC111M, Evrogen, Russia) according to the manufacturer’s manual. Briefly, 100 μl of whole blood was mixed with 100 μl of lysis buffer. GAUSS suspension (10 µl) was added, mixed and incubated at +56°C for 10 min. Then 100 μL of ethyl alcohol (96%) was added followed by mixing with 400 μl of binding solution. Supernatant was separated by centrifugation (1 min at 11000 g) and transferred on the column membrane. The membrane was washed by Washing buffer and DNA was eluted by Elution buffer and collected by centrifugation. Qualitative and quantitative analysis of the isolated DNA was done using Denovix DS-11+Spectrophotometer (DeNovix Inc., USA).
Quantitative polymerase chain reaction analysis
Herpes virus DNA analysis was done using RealBest DNA HHV-6 (cat. D-2150, Vector-Best, Russia) RealBest DNA VZV (cat. D-2185 Vector-Best, Russia) RealBest CMV DNA (cat. D-1598, Vector-Best, Russia) RealBest EBV DNA (cat. D-2198, Vector-Best, Russia) kits according to the manufacturer’s instructions. PCR-RT was carried out using Real-time CFX96 Touch (Bio-Rad, USA). For each reaction, 30 ng of total DNA was used. The PCR conditions were as follows: 50°C for 2 min, 95°C for 2 min, followed by 50 cycles of 94°C for 10 sec and 60°C for 20 sec. Fluorescence was measured at 60°C using Real-time CFX96 Touch (Bio-Rad, USA). The FAM detection channel was set to register the process of DNA amplification of the internal control sample and the ROX detection channel was set to detect EBV, VZV, HCMV and HHV-6 DNA.
Genotyping analysis
A total of 16 SNPs were selected in 16 genes: rs113516541, rs11575594 and rs11575584 (CCL27 gene), rs12722489 and rs2104286 (IL2RA gene), rs6897932 (IL7RA gene), rs12044852 and rs2300747 (CD58 gene), rs929230 (CD6 gene), rs9282860 (STK11 gene), rs12708716 and rs2041670 (CLEC16A gene), rs763361 (CD226 gene), and rs1883832 and rs1535045 (CD40 gene). The selection of genes and SNPs was based on data indicating their potential association with risk for MS (42, 51–57). SNPs analysis was done by allele-specific PCR (AS-PCR). Primers were synthesized by Evrogen (Russia). For AS-PCR, a “ScreenMix” reaction mix (Evrogen, Russia) and 20 ng of genomic DNA were used. The AS-PCR parameters were optimized for each set of SNPs primers. Typical AS-PCR results are presented in Supplemental Figure 1. The reaction was carried out using a C1000 Touch 96 amplifier (Bio-Rad, USA). Separation of AS-PCR products was done by gel electrophoresis. AS-PCR products were detected in the GelDoc XR+ gel-documenting system (Bio-Rad, USA).
Statistical analysis
Analysis of the genotype and allele frequencies in MS and control groups was done using the Fisher’s exact test. The strength of associations was assessed using the odds ratio (OR, (lower 95% confidence interval; upper 95% confidence interval). The qPCR and ELISA data analysis was carried out using the two-tailed Fisher’s exact test. Аanalysis of MSSS and EDSS was done using the Kruskal-Wallis test. p<0.05 values were considered as significant.
Results
MS and controls
One hundred fifty-one MS cases (56 males and 95 females; average age 36.8 ± 9.8) were included in this study. MS diagnosis was established according to the 2010 Revised MacDonald’s Diagnostic Criteria for MS (58). There were 123 RRMS cases, 4 cases with PPMS, 14 patients with SPMS (Table 1), and 10 patients diagnosed with CIS. The mean duration of disease was 8.82 ± 6.67 years. Expanded Disability Status Scale Score (EDSS) and Multiple Sclerosis Severity Score (MSSS) were 2.87 ± 1.60 and 4.28 ± 2.50, respectively. Additionally, 83 patients received disease modifying therapy (cladribine, natalisumab, glatiramer acetate, and pegylated IFN-β) while 68 patients had no treatment.
Herpesvirus antibody and DNA in blood of MS and controls
To demonstrate the prevalence of herpesvirus infection we selected age matched 124 MS and 70 control, serum antibodies for HCMV, HHV6, EBV, and VZV were analyzed (Table 2). We found that antibodies to HCMV, HHV6, EBV, and VZV were in 84.7, 63.7, 97.6 and 87.9% in MS patients, respectively. In controls, circulating antibodies for HCMV, HHV6, EBV, and VZV viruses were in 82.9, 45.7, 92.9, 80.0%, respectively. There was a higher prevalence of only anti-HHV6 antibodies in MS as compared to controls (OR=0.48 (0.26; 0.87), p = 0.02). There was no difference in MS and controls having antibodies to HCMV, EBV, and VZV. Next, we analyzed the herpesvirus seroprevalence in two groups of MS: age-matched (124 samples) and not matched (27 samples) with controls (Supplemental Table 1). The control age-matched MS was 33.7 ± 7.5 years old, while the other group was older, 51.5 ± 4.6 years old. We found no difference in seroprevalence between these two groups. These data suggest that the herpesvirus infection has a limited contribution to MS diagnosis in older age.
Herpesvirus DNA was analyzed in mononuclear leukocytes (Table 2) from 124 MS and 70 age matched controls. HCMV, HHV6, EBV, and VZV DNA was found in 0, 0.81, 0.81 and 23.4% MS, respectively. Interestingly, HCMV, HHV6, EBV, and VZV DNA was also demonstrated in 0, 1.7, 1.7 and 22.0% controls. We found no difference in herpesvirus DNA detection between MS and controls (Table 2).
Additional analysis revealed a lack of differences in herpesvirus DNA detection in MS and controls when groups were separated based on EDSS, MSSS, duration of the disease and treatment status (Supplemental Tables 2, 3). The frequency of anti-herpesvirus antibody detection did not differ in MS patients based on EDSS, MSSS and disease duration (Supplemental Table 2). Similarly, there was no association between the detection of herpesvirus DNA and MS patients’ treatment status (Supplementary Table 3).
Analysis of sex of MS and controls revealed a lack of difference in sero-prevalence to HCMV, HHV6, EBV, and VZV (Supplemental Tables 4A, B). To analyze the link between age and HHV6 antibody prevalence, patients and controls were divided into young (≤35 years old) and old (>35 years old) groups. We have found higher frequency of anti-HHV6 antibodies in older MS patients as compared to the same age controls (65.0% vs. 40.7%; p=0.04). We also found higher frequency of VZV DNA detection in MS patients as compared to controls in both age groups. We conducted an additional analysis using 45 years old cut-off to determine whether older age would be more associated with differences in herpesvirus prevalence. A significantly higher frequency of anti-HHV6 antibodies was found only in young (≤45 years old) as compared to the same age control. There was no difference in the detection of VZV DNA between MS and control in both age groups. These data indicate that VZV could play a role in younger MS.
SNPs analysis in MS and controls
In this analysis, we have used 124 MS samples and 70 age and sex matched controls. Genetic markers agreed to Hardy–Weinberg equilibrium proportions in the control population (p>0.05). Several SNPs were selected for analysis in MS and controls. We found a higher frequency of CC in rs12044852 and AA in rs2300747 genotypes in CD58, while genotype AC and AG in respective SNPs were more often found in controls (Figure 1A) (OR=2.92 (0.54; 24.51), p=0.05 and OR=0.41 (0.21; 0.80), p=0.01). AG genotype in rs929230 of CD6 gene was more frequent in controls, while in MS, GG was found more often (OR=0.86 (0.31; 2.37), p=0.0002).
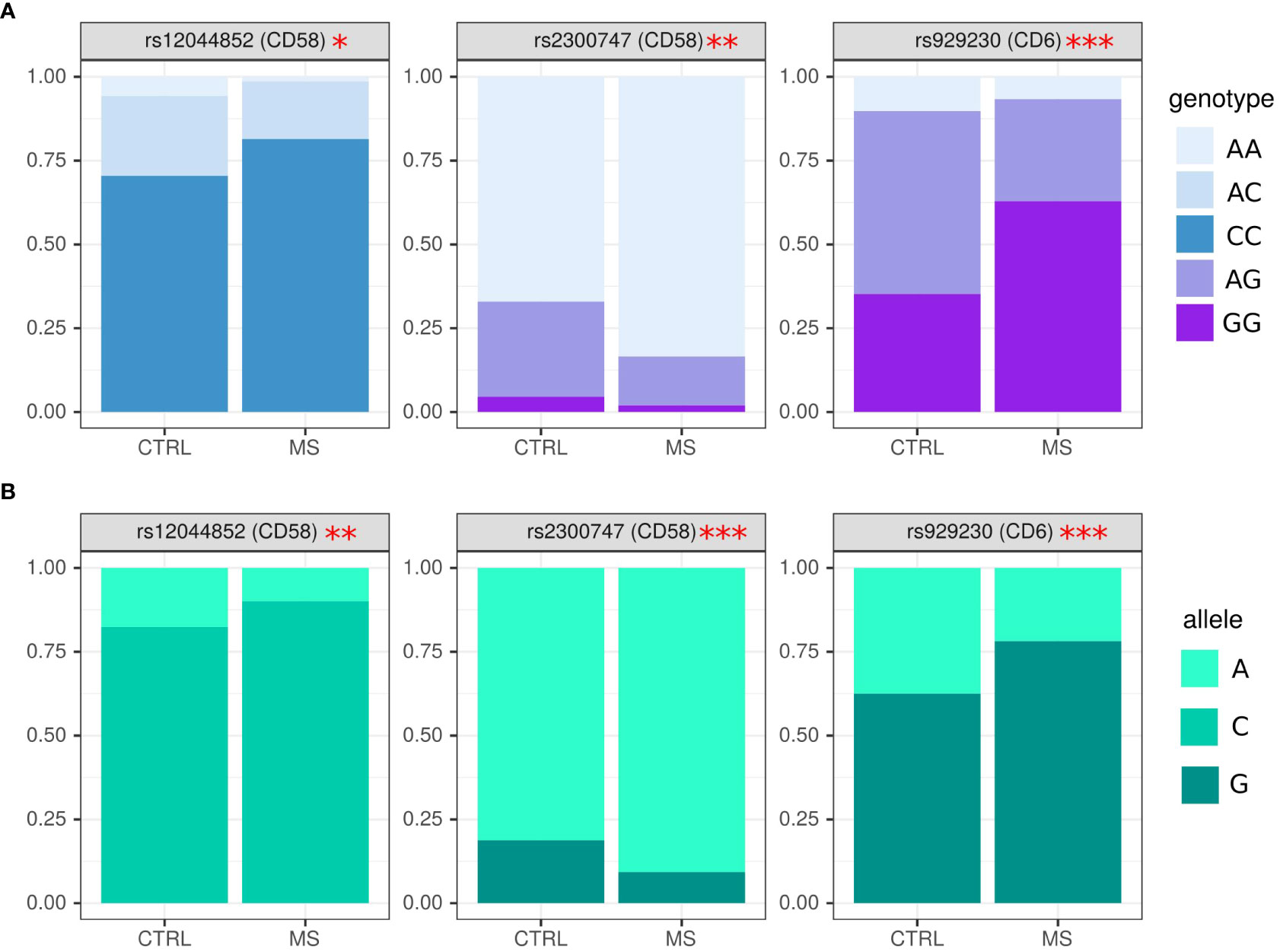
Figure 1 Analysis of genotype and allele frequency in rs2300747, rs929230 and rs12044852 SNPs in MS and controls. (A) – genotypes, (B) – alleles. SNPs analysis was done by AS-PCR. Data is presented as proportion of representation of genotype/allele. There were 151 MS and 70 controls samples used for the analysis. The analysis was done using Fisher’s exact test. *** - p<0.005, ** - p<0.05, * - p<0.1.
The frequency of alleles concurred with data on genotype where rs2300747A and rs12044852C in CD58 (OR=0.44 (0.26; 0.76), p=0.005, OR=1.94 (1.12; 3.34), p=0.02, respectively), and rs929230G in CD6 gene (OR=2.14 (1.42; 3.23), p=0.0003) were more often found in MS as compared to controls (Figure 1B).
When SNPs data was analyzed based on sex, CC genotype in rs1883832 of CD40 gene had a higher frequency in female MS (Figure 2A), while heterozygous CT genotype was often found in male MS (OR=2.40 (1.14; 5.09), p=0.05). We also found higher frequency of rs1883832C and rs1535045T in female as compared to male MS in CD40, (OR=1.72 (1.04; 2.85), p=0.04) and (OR=0.54 (0.31; 0.94), p=0.03), respectively (Figure 2B).
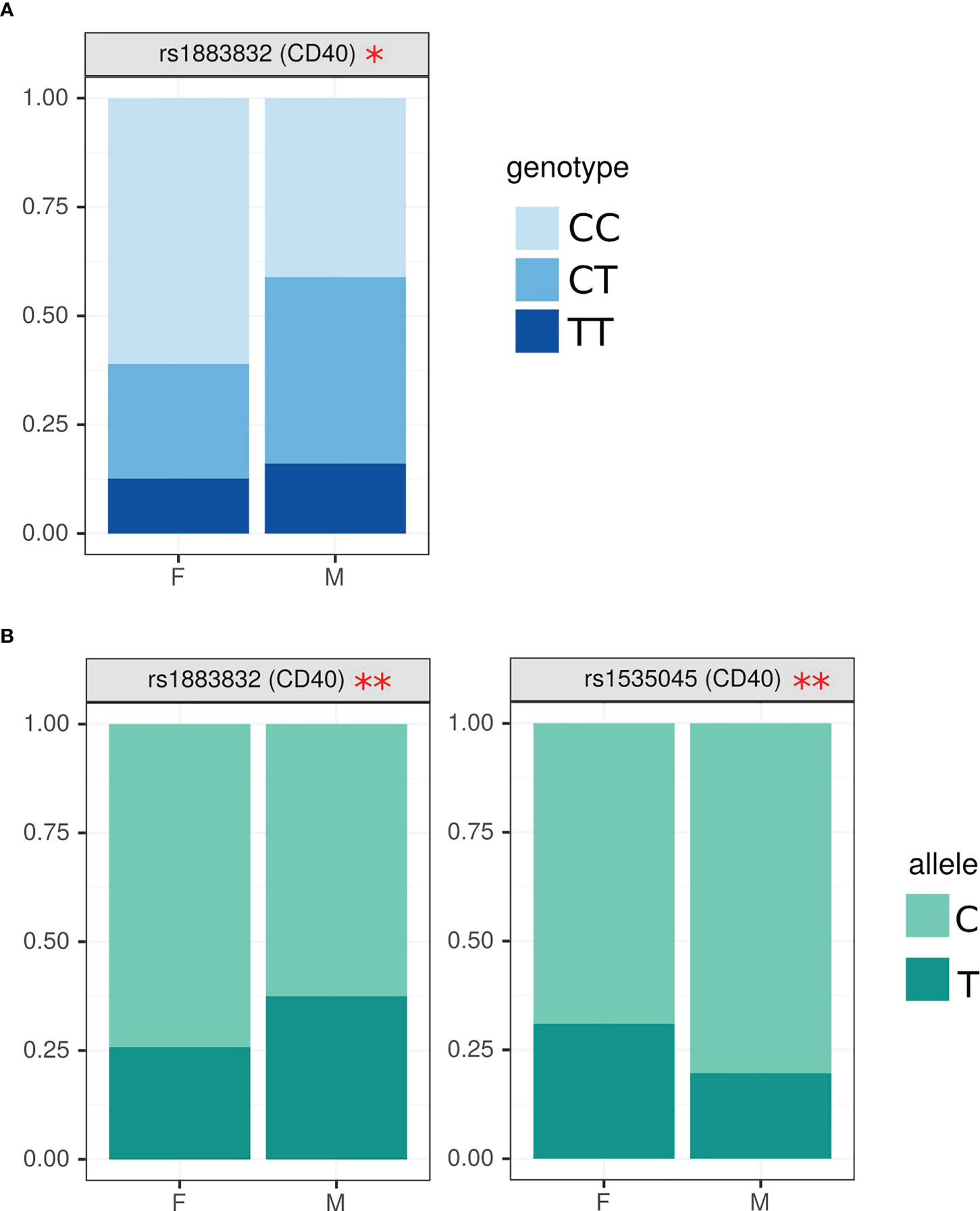
Figure 2 Analysis of SNPs genotype and allele frequency based on sex of MS patient. (A) – genotypes, (B) – alleles. SNPs analysis was done by AS-PCR. Data is presented as proportion of representation of genotype/allele. There were 151 MS patient samples used for the analysis. The analysis was done using Fisher’s exact test. ** - p<0.05, * - p<0.1.
Analysis of SNPs genotype and allele frequency in MS based on clinical presentation and presence of anti-herpesvirus antibodies
Next, we analyzed SNPs genotype and allele frequency in patients with fatigue. For these analyses, we used data on 151 MS patients and 70 controls since age have a limited effect on the genotype. We have found that MS with fatigue had higher frequency of AC and AA genotypes in rs12044852 of CD58 gene (OR=4.62 (1.17; 33.61), p=0.04) (Figure 3A). The higher frequency of allele A in patients with fatigue was also supported by the allele analysis in this SNP (Figure 3B).
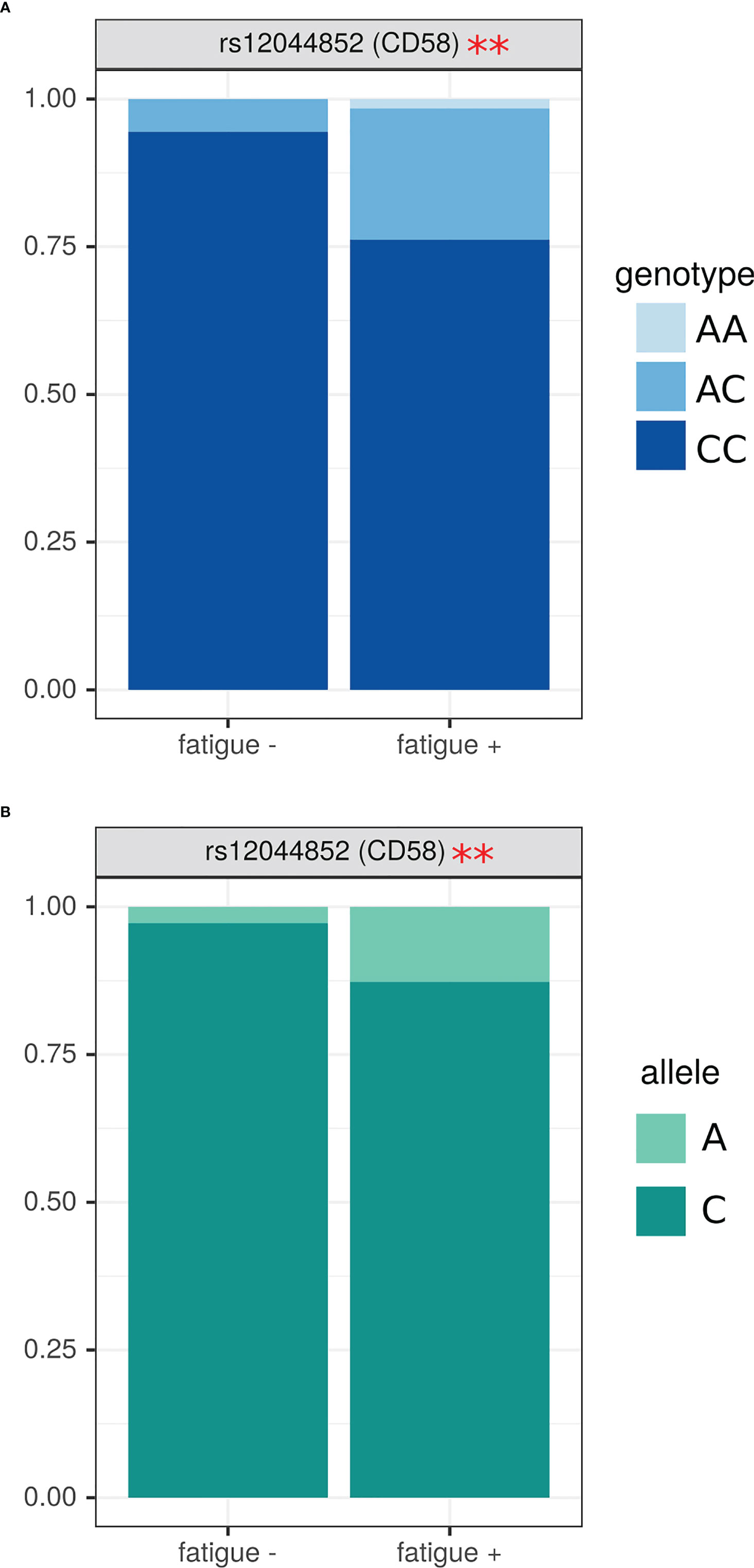
Figure 3 Analysis of SNPs genotype and allele frequency based on presence of fatigue in MS. (A) – genotypes, (B) – alleles. Data is presented as proportion of representation of genotype/allele. There were 151 MS patient samples used for the analysis. The analysis was done using Fisher’s exact test. ** - p<0.05.
Since our data demonstrated MS patients having higher HHV6 antibody prevalence as compared to controls, we sought to determine association between anti-herpesvirus antibodies and SNPs genotype. We have found that HHV6 antibody positive MS patients had more GG and less AG genotype in rs12722489 of IL2RA gene as compared to those without antibodies (Figure 4A) (OR=0.14 (0.005; 1.05), p=0.02). Also, the frequency of T allele in rs1535045 of CD40 gene was higher in anti-HHV6 antibody positive patients as compared to those without anti-herpesvirus antibodies (OR=1.77 (1.02; 3.14), p=0.04) (Figure 4B).
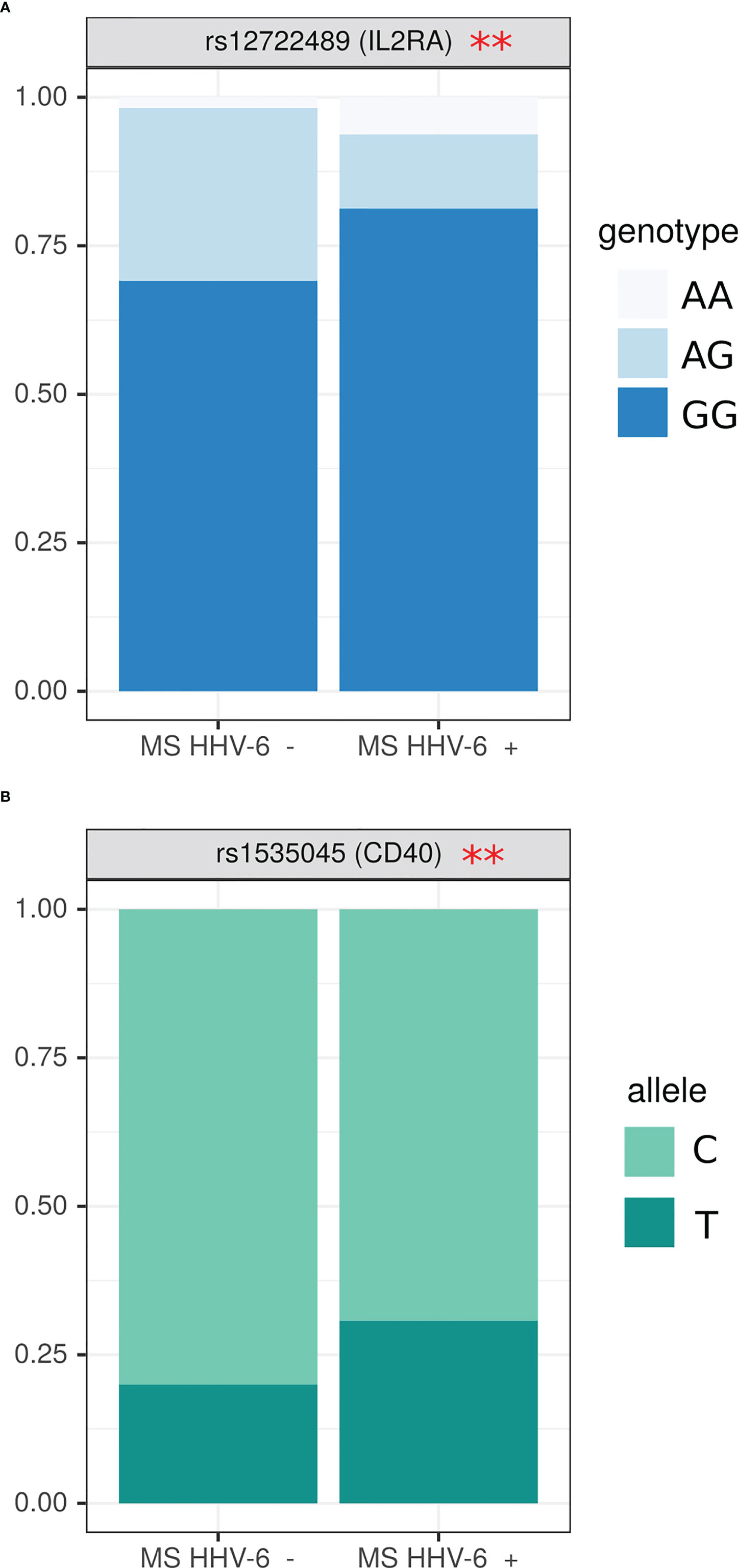
Figure 4 Analysis of SNPs genotype and allele frequency based on detection of circulating anti-HHV6 antibodies. (A) genotypes, (B) alleles. Data is presented as proportion of representation of genotype/allele. There were 151 MS patient samples used for the analysis. The analysis was done using Fisher’s exact test. ** - p<0.05.
MS positive for anti-VZV antibodies were having higher frequency of CC genotype in rs9282860 (STK11 gene) (OR=0.37, (0.07; 2.97) p=0.04) (Figure 5A). Interestingly, TT genotype in rs9282860 was absent in VZV seropositive. CC genotype was also more frequent in rs1883832 (CD40) of MS patients having anti-VZV antibodies as compared to those without (OR=0.24 (0.06; 0.79), p=0.003). Additionally, higher frequency of allele C in rs9282860 (STK11 gene) and rs1883832 (CD40 gene) was found in VZV seropositive as compared to seronegative MS (OR=0.19 (0.05, 0.84), p=0.03, OR=0.29 (0.14; 0.59) p=0.0006) (Figure 5B). T allele was more frequent in rs1535045 of CD40 gene of anti-VZV antibody positive patients as compared to those without antibodies (OR=2.60 (1.05; 7.94) p=0.049).
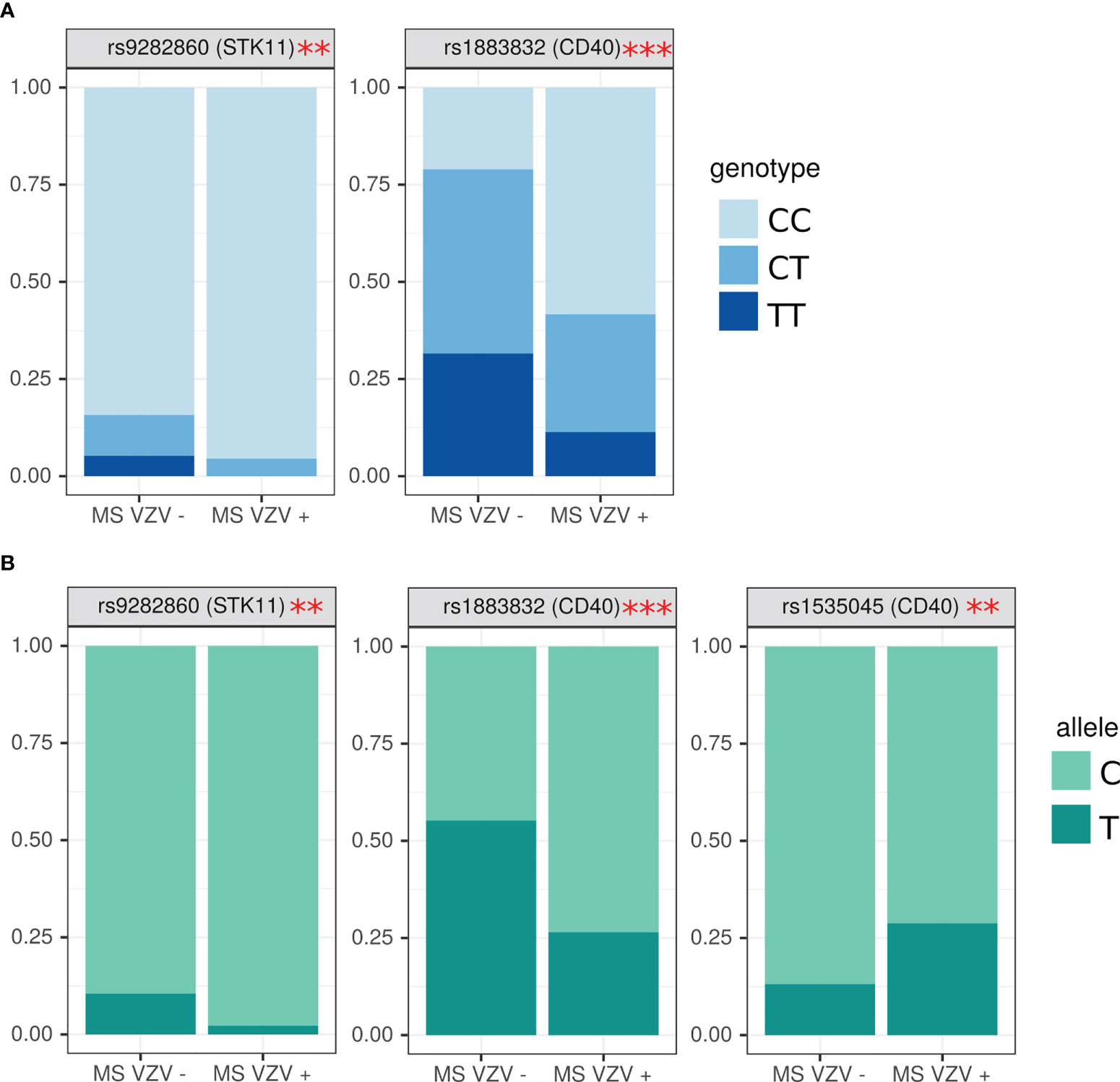
Figure 5 Analysis of SNPs genotype and allele frequency based on presence of circulating anti-VZV antibodies. (A) genotypes, (B) alleles. Data is presented as proportion of representation of genotype/allele. There were 151 MS patient samples used for the analysis. The analysis was done using Fisher’s exact test. *** - p<0.005, ** - p<0.05.
Discussion
Herpesvirus infections were linked to pathogenesis of MS in many studies. However, there is limited data on herpesvirus infection in the MS cohort from Russia, especially from the Volga Region of Russia. The population is a mix containing 66% Russians (Eastern Europeans), 13% Tatars and significant minorities of other groups such as the Bashkir (4.29%), Chuvash (4.26%), etc. Mixt ethnic ancestry is also frequent. This population diversity and composition differ substantially from that in Northern Europe, where high incidence and prevalence of MS are documented (59). MS is diagnosed in the Volga region of Russia, though the incidence (1.6-5.5 per 100,000 population) and prevalence (38-48 per 100,000 population) remain low (47) as compared to Northern Europe (59). Therefore, we sought to determine the prevalence of herpesvirus infection in MS and control cohorts from the Volga Region of Russia.
The current hypothesis of MS pathogenesis suggests multiple hits (60, 61), where one could be a herpesvirus infection, while the genetic risk factor could be the other. Therefore, we sought to analyze an association between herpesvirus infection and SNPs combination with MS diagnosis in the Volga Region of Russia cohort. Our knowledge of the MS associated SNPs in Russia is limited to several publications, including the patient’s cohort from Moscow and Novosibirsk (62–64). There is limited data on MS association with SNPs in the Volga Region of Russia cohort. Our data contribute to understanding the association between SNPs and MS from this region.
Our data confirms the potential link between herpesvirus infection and MS. We found significant association between HHV6 seropositivity and diagnosis of MS. This data corroborates previous observations where HHV6 infection was linked to MS (29, 65–67). Interestingly, EBV was also identified as a risk factor for MS (19, 24). In a comprehensive longitudinal study by Bjornevik et al., the association between EBV infection and MS diagnosis was thoroughly investigated (24) showing a 32 times higher risk of MS diagnosis after EBV seroconversion. Although our findings demonstrated a lack of connection between EBV and MS, we found more EBV seropositive samples (97.58%) in MS as compared to control (92.86%). This data supports a higher frequency of anti-EBV seropositivity in MS (68–70). A comprehensive analysis by Abrahamyan et al. revealed that most MS sera (93%) react with nuclear antigen (NA), while the remaining samples could react with viral capsid antigen (VCA) (22). The authors state that when reactivity to these viral antigens was analyzed, 100% EBV seropositivity was found in MS. We have used NA-based ELISA for anti-EBV antibody detection. Anti-NA antibodies are commonly used to establish EBV infection as they could be detected early (6-8 weeks after the exposure) and remain circulating for life (71). In contrast, anti-VCA antibodies indicate recent exposure as they could be found during the acute phase of the disease (72). Therefore, it appears that the detection of anti-NA antibodies would be a better indicator of the prior EBV infection. Still, anti-NA antibody negative results could not exclude the EBV infection, as some individuals’ serum could fail to react with anti-EBV NA (73).
Considering EBV as a risk factor for MS, it was interesting to find three EBV seronegative MS patients. Two patients were male, 41 and 42 years old. One patient was diagnosed with MS for one year and eight months. The patient did not receive treatment and had 6.5 and 9.47 scores for EDSS and MSSS, respectively. The other patient had a more extended history of MS diagnosis (22 years and three months). Unlike the first, this patient received disease-modifying treatment and had 4.0 and 1.64 scores for EDSS and MSSS, respectively. Interestingly, only this male patient was positive for HHV6 and VZV antibodies and VZV DNA. The third patient was a female with one year and ten months history of MS diagnosis. This patient did not receive disease-modifying treatment and had 6.5 and 1.77 scores for EDSS and MSSS, respectively. Interestingly, similar to the second male patient, antibodies to HHV6 and VZV as well as VZV DNA were detected in this female patient. These data support the role of herpesvirus infection in the pathogenesis of MS. It could be suggested to consider HHV6 and VZV as MS risk factors when an MS patient is negative for EBV antibodies and DNA.
Although herpesviruses were linked to MS diagnosis, the ubiquitous nature of these viruses and high seroprevalence could not entirely explain the MS global prevalence variations (74). Therefore, it could be suggested that additional factors, when combined with herpesvirus infection, contribute to the risk of MS. Genetic factors are often identified as markers of MS susceptibility, where multiple SNPs were shown to be associated with the likelihood of developing this disease. The study conducted by Zong-Li Xia et al., found correlation between genotypes in SNPs rs12722489 and rs2104286 (IL2RA gene) and MS diagnosis in the Hui and Han nationalities (75). The SNPs association rs6897932 was found in Jordanian MS patients (76). The rs9282860 in STK11 gene was linked to a high likelihood of MS in women in the United States (77). Using meta-analysis, rs763361 in the CD226 gene was shown to be linked to predisposition for having MS in Europe, Asia, Africa, and South America (78). In another study, SNP in the CD6 gene rs17824933 was associated with MS in India, Europe, and the United states (79, 80). In cohorts in Greece, rs17445836 located in the long intergenic non-coding RNA near IRF8 was identified as a risk factor for MS (81).
We have confirmed that the frequency of genotypes and alleles in selected genes differ between MS and controls. These data support the hypothesis of genetic risk factors of MS. We have found higher frequency of CC in rs12044852 and AA in rs2300747 genotypes in CD58 gene of MS. We also found that C allele in rs12044852 and A allele in rs2300747 in CD58 are risk factors for MS, while G allele in rs2300747 appears to be protective. These data corroborate de Jager, et al.’s findings, indicating protective role of rs2300747G allele in MS (82). It appears that presence of this protective G allele is associated with higher expression of CD58 RNA (82, 83). Interestingly, the higher frequency of rs2300747G allele in individuals with East Asian ancestry and associated increased level of CD58 expression demonstrated by Purcell et al. (84) could contribute to the lower incidence and prevalence of MS shown in Asia (85). Our data support Torbati et al. data, indicating CC genotype in rs12044852 in CD58 as an MS risk factor (86). Our results also support the previous observation that the rs12044852C allele is associated with MS in Caucasians (86), representing the majority in the Volga Region of Russia. Similar findings were reported by Hafler et al. in MS cohorts from the United States and Great Britain (87) as well as by Rubio et al. in Australian MS (88). In another study, d’Netto et al. presented data identifying rs12044852C as one of the non-HLA genetic risk factors (89). It could be suggested that the rs12044852C allele had a diagnostic value as its combination DRB1*15 appears to better differentiate MS from controls than the HLA marker alone (90). The CD58 gene encodes for a lymphocyte function-associated antigen 3 (LFA3), a glycosylated cell adhesion protein which is expressed on antigen presenting cells and function to enhance antigen presentation to T cells via interaction with CD2 (91, 92). This interaction was shown to be essential in antigen recognition and selection of primed T cells (93). Studies have shown that a protective G allele in rs2300747 genotype could cause an increased expression of CD58 which subsequently enhances function of CD4+CD25high regulatory T cells via expression of transcription factor FoxP3 (82).
Another finding was a higher frequency of GG genotype in rs929230 of CD6 in MS as compared to controls. The CD6 receptor is a transmembrane protein expressed on T-lymphocytes (94) and is involved in activation of lymphocytes by interacting with its ligand, the activated leukocyte cell adhesion molecule (ALCAM) [45]. Swaminathan et al. have demonstrated that rs17824933, located in the first intron of the gene, was more frequently found in MS within Spanish-Basque population (95). Similar association between this SNP and MS was demonstrated by de Jager et al. (82). We did not find the association between rs17824933 in CD6 and MS however, a higher frequency of allele G in rs929230 of the CD6 gene in MS was presented. Interestingly, this SNP is also located in the first intron similar to rs17824933 (96), suggesting the role of this region of the gene in pathogenesis of MS.
When sex differences between MS patients we analyzed, we found higher frequency of C and T alleles in females as compared to males in CD40 rs1883832 and rs1535045, respectively. CD40 is a member of the TNF superfamily which is expressed on activated T and B cells (97, 98). Studies have shown that SNPs in CD40 could be associated with MS depending on the ethnic and geographic location. In Australia, rs12044852 (CD40 gene) was found more common in MS (99). However, in cohort from Iran, this mutation was not associated with MS risk [50]. Similar conclusions were made based on the study results of this SNP in patient populations from the USA and Europe (100). Our finding of sex distribution of alleles in rs1883832 and rs1535045 of CD40 confirm the potential role of this gene in pathogenesis of MS (63, 101).
One interesting finding was a higher frequency of AC and AA genotypes in rs12044852 of the CD58 genes in patients with fatigue symptoms. This is the first observation suggesting a connection between CD58 gene SNPs and a symptom of MS. Fatigue is one most reported symptoms connected to an impaired quality of life (102, 103). Interestingly, altered CD58 expression was demonstrated in myalgic encephalitis, a disease characterizes by chronic fatigue (103).
An interesting observation made in this study was finding an association between frequency of alleles in IL2RA and CD40 as well as seropositivity to HHV6 and VZV. Higher frequency of GG genotype in rs12722489 of IL2RA was found to be linked to HHV6 seropositivity. Interestingly, the G allele was also found linked to a high risk of MS (87, 104). The analysis of an association between this SNP and MS is shown to be the result of a linkage disequilibrium with another SNP, rs2104286 (Refining genetic associations in multiple sclerosis. 2008). Both SNPs were shown to influence IL-2RA function. The IL-2RA receptor coded by a gene containing the rs12722489G allele had a higher affinity to estrogen receptor α (ERα) (105) Increased expression of IL-2RA was demonstrated in individuals having rs2104286 AA genotype (105), also known to be associated with MS. It was demonstrated that T helper cells from individuals carrying rs2104286 A SNP secrete more GM-CSF and have increased expression of IL-2RA. These data suggest that having a pathogenic allele in this SNP could support development of pathogenic GM-CSF expressing T helpers. IL-2RA is a component of the high affinity receptor for IL-2 (106). Therefore, the pathogenic effect of IL-2RA in MS could also be associated with IL-2 levels. Levels of IL-2 could be affected by HHV6 which suppress this cytokine production via expression of U54 protein (107). These data suggest that the role of IL-2RA in pathogenesis of MS could be more complex, and including regulatory cytokines, such as GM-CSF and IL-2, functions.
We also found an association between VZV sero-positivity and alleles C and T CD40 SNPs, rs1883832 and rs1535045, respectively. Interestingly, the same SNPs and alleles we found to be more frequent in females compared to male MS. CD40 SNP rs1883832 T>C was shown to be associated with increased MS risk (108). We found a higher frequency of CC genotype in females than in males in VZV infected MS. The CD40 rs1883832 was shown to have a substantial association with some form of autoimmunity. The association of rs1883832C with Grave’s disease and rs1883832T with MS was demonstrated in several studies (108–110). Interestingly, an association between rs1883832T and MS was shown in several distinct Russian MS cohorts from Moscow and Siberia (63). In this view, finding more VZV infected MS females carrying CC CD40 rs1883832 genotype compared to males is intriguing. C allele in this SNP appears to be protective, since the risk of MS increases from 1.5 times in CT to 2.5 times in TT genotype individuals (111). It was shown that the C allele could enhance the efficiency of corresponding gene translation (108). Interestingly, the lower expression f CD40 was demonstrated in individuals with rs1883832T compared to rs1883832C CD40 SNPs (108, 112). Also, reduced expression of CD40 was demonstrated in MS (108). This reduced CD40 expression was shown in B lymphocytes, dendritic cells and monocytes in MS (108). Field et al. suggested that failure to develop tolerogenic mechanisms to explain the role of the low CD40 expression in MS pathogenesis (108). This CD40 tolerogenic immune response could be mediated by the stimulation of Tregs and the production of interleukin 10 (IL-10) (108). The higher frequency of rs1883832C CD40 SNPs in VZV infected females MS as compared to males requires further investigation. Although we found no link between circulating VZV antibodies and MS diagnosis, which was due to high seroprevalence among controls and patients, confirming the ubiquitous nature of VZV infection. Still, there is much evidence suggesting VZV as a risk factor for MS [69-70]. Also, VZV could contribute to the progression of MS, as viral DNA was found in 100% of patients’ cerebrospinal fluid (CSF) samples during an exacerbation and in 31% of samples during remission [68]. Additionally more VZV DNA was found in CSF as compared to peripheral blood mononuclear cells in progressive MS [68].
In conclusion, we have found an association between HHV6 sero-positivity and MS diagnosis. This data contributes to our understanding the role of herpesvirus infection as risk factor of MS. Two viruses, EBV and HHV6, are frequently associated with MS (24, 67, 113, 114). Also, childhood herpesvirus infection increases the risk of MS diagnosis (115). Therefore, detection of anti-herpesvirus antibodies should be considered as a potential risk of MS diagnosis later. Also, studies demonstrated the association between HHV6 reactivation and MS relapse (116, 117). Therefore, the therapeutic potential of anti-herpesvirus drugs was evaluated in some studies showing limited efficacy (118, 119). However, these therapeutics could be used to control herpesvirus reactivation caused by alemtuzumab (120), a drug also used for MS treatment (121). We also confirmed higher frequency of A, C and G alleles in rs2300747 and rs12044852 of CD58 and rs929230 in CD6 in MS as compared to controls. We found higher frequency of C and T alleles in female as compared to male in CD40 rs1883832 and rs1535045, respectively. The herpesvirus infection and pathogenic SNPs combined mechanisms of MS pathogenesis are summarized in Figure 6. Fatigue symptoms were the only clinical symptoms linked to AC and AA genotype in rs12044852 of CD58 gene. The interesting observation, supporting the role of HHV6 in MS pathogenesis, was finding a higher frequency of GG genotype in rs12722489 of IL2RA and T allele in rs1535045 of CD40 genes in patient having anti-HHV6 antibodies as well as a link between having anti-VZV antibodies in MS and CC genotype in rs1883832 of CD40 gene.
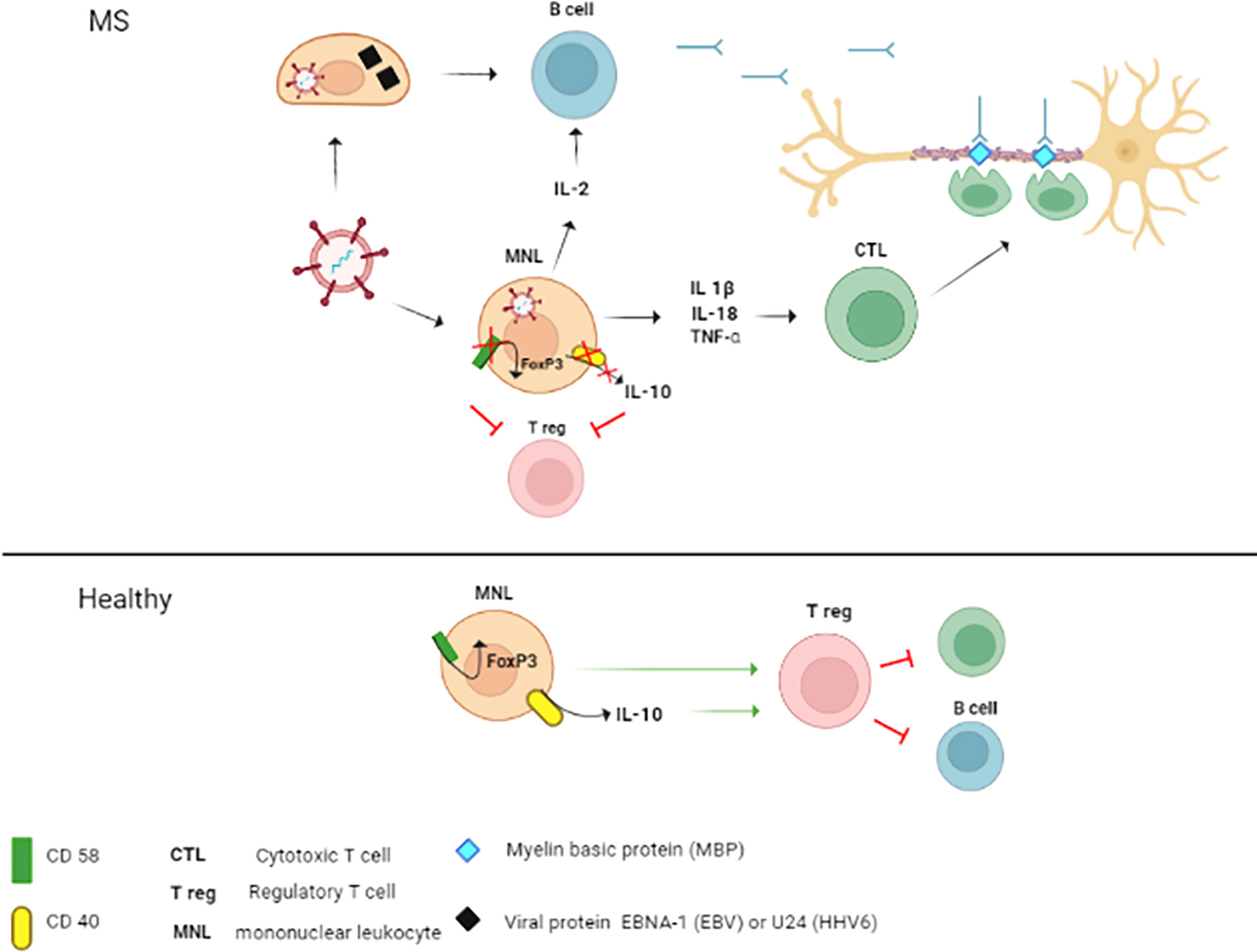
Figure 6 The mechanisms of the contribution of herpesvirus infection combined with CD58 and CD40 SNPs’ to MS pathogenesis. In MS, combined pathogenic effect of herpesviruses and SNPs in CD58 and CD40 could be explained by three potential mechanisms: 1. Molecular mimicry; 2. Activation and maintenance of inflammation; 3. Reduction of regulatory capacity of Tregs. 1. B cells primed with herpesvirus proteins (EBV nuclear antigen 1 (EBNA-1 and UL24) could produce antibody cross-reacting with myelin basic protein (MBP) epitopes (122,123). These antibodies could cross the blood-brain barrier (BBB) and target the axon myelin sheath. Also, cross-reactive cytotoxic T lymphocytes (CTL) could be generated and damage the axon myelin. 2. Herpesvirus infection could contribute to the pathogenesis of MS by activation of mononuclear leukocytes (MNL) and increasing the production of proinflammatory cytokines (IL-1β, IL-18 and TNF-α) (124–126). These cytokines could contribute to inflammation and promote activation and differentiation of pathogenic CTL (127,128).. Additionally, herpesviruses could activate autoreactive B cells directly and indirectly via the release of IL-2 by infected MNL (129,130). 3. The pathogenic role of SNPs in CD58 and CD40 could be associated with the reduction of Treg regulatory activity. This suggestion supports data demonstrating the association between the rs12044852A allele in CD58 and decreased expression of this surface marker (82). This low level of CD58 protein could consequently lead to the downregulation of FoxP3 (82), a regulatory element essential for the activation of Tregs (131). CD40 stimulates the production of IL-10 (132), a cytokine essential for Tregs signaling (133). Also, CD40 was shown to augment the tolerogenic effect of IL-10 (134). Therefore, rs1883832T associated reduction of CD40 expression could impact the Tregs function in MS. Also, suppression of Tregs could be caused by lower expression of CD40 in individuals with 1883832T allele (101,135). In healthy individuals, expression of CD40 and CD58 remains unaffected. Also, Tregs signaling would be supported by release of IL-10 and expression of FoxP3.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Biomedicine Ethic Expert Committee of Republican Clinical Neurological Center, Republic of Tatarstan, Russian Federation (N: 218, 15.11.2012). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
AZ, AM – investigation. MM - visualization. SK – conceptualization. VL, YD, EM, SK - writing, review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1010605/full#supplementary-material
References
1. Leray E, Moreau T, Fromont A, Edan G. Epidemiology of multiple sclerosis. Rev Neurol (Paris). (2016) 172(1):3–13. doi: 10.1016/j.neurol.2015.10.006
2. Höftberger R, Lassmann H. Inflammatory demyelinating diseases of the central nervous system. Handb Clin Neurol (2018) 145:263–83. doi: 10.1016/B978-0-12-802395-2.00019-5
3. Chataway J. Distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry (2020) 91(9):901–2. doi: 10.1136/jnnp-2019-322431
4. Tallantyre EC, Dixon JE, Donaldson I, Owens T, Morgan PS, Morris PG, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology (2011) 76(6):534–9. doi: 10.1212/WNL.0b013e31820b7630
5. Calabrese M, Favaretto A, Martini V, Gallo P. Grey matter lesions in MS. Prion (2013) 7(1):20–7. doi: 10.4161/pri.22580
6. Trapp BD, Nave K-A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu Rev Neurosci (2008) 31(1):247–69. doi: 10.1146/annurev.neuro.30.051606.094313
7. Khaleeli Z, Ciccarelli O, Manfredonia F, Barkhof F, Brochet B, Cercignani M, et al. Predicting progression in primary progressive multiple sclerosis: A 10-year multicenter study. Ann Neurol (2008) 63(6):790–3. doi: 10.1002/ana.21375
8. Koch M, Kingwell E, Rieckmann P, Tremlett H. The natural history of primary progressive multiple sclerosis. Neurology (2009) 73(23):1996–2002. doi: 10.1212/WNL.0b013e3181c5b47f
9. Khaiboullina SF, Gumerova AR, Khafizova IF, Martynova EV, Lombardi VC, Bellusci S, et al. CCL27: Novel cytokine with potential role in pathogenesis of multiple sclerosis. BioMed Res Int (2015) 2015:1–10. doi: 10.1155/2015/189638
10. Palle P, Monaghan KL, Milne SM, Wan ECK. Cytokine signaling in multiple sclerosis and its therapeutic applications. Med Sci (2017) 5(4):23. doi: 10.3390/medsci5040023
11. Goyal M, Khanna D, Rana PS, Khaibullin T, Martynova E, Rizvanov AA, et al. Computational intelligence technique for prediction of multiple sclerosis based on serum cytokines. Front Neurol (2019) 10. doi: 10.3389/fneur.2019.00781
12. Hofman FM, Hinton DR, Johnson K, Merrill JE. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med (1989) 170(2):607–12. doi: 10.1084/jem.170.2.607
13. Rasouli J, Ciric B, Imitola J, Gonnella P, Hwang D, Mahajan K, et al. Expression of GM-CSF in T cells is increased in multiple sclerosis and suppressed by IFN-β therapy. J Immunol (2015) 194(11):5085–93. doi: 10.4049/jimmunol.1403243
14. Peterson L, Fujinami R. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple sclerosis. J Neuroimmunol (2007) 184(1–2):37–44. doi: 10.1016/j.jneuroim.2006.11.015
15. Mameli G, Poddighe L, Mei A, Uleri E, Sotgiu S, Serra C, et al. Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: Inference for multiple sclerosis. PloS One (2012) 7(9):e44991. doi: 10.1371/journal.pone.0044991
16. Stampanoni Bassi M, Iezzi E, Drulovic J, Pekmezovic T, Gilio L, Furlan R, et al. IL-6 in the cerebrospinal fluid signals disease activity in multiple sclerosis. Front Cell Neurosci (2020) 14. doi: 10.3389/fncel.2020.00120
17. Buhelt S, Søndergaard HB, Mahler MR, Cobanovic S, Börnsen L, Ammitzbøll C, et al. Biomarkers of systemic inflammation, soluble IL-2Rα and the multiple sclerosis-associated IL2RA SNP rs2104286 in healthy subjects and multiple sclerosis patients. Mult Scler Relat Disord (2021) 54:103140. doi: 10.1016/j.msard.2021.103140
18. Jog NR, McClain MT, Heinlen LD, Gross T, Towner R, Guthridge JM, et al. Epstein Barr Virus nuclear antigen 1 (EBNA-1) peptides recognized by adult multiple sclerosis patient sera induce neurologic symptoms in a murine model. J Autoimmun (2020) 106:102332. doi: 10.1016/j.jaut.2019.102332
19. Engdahl E, Gustafsson R, Huang J, Biström M, Lima Bomfim I, Stridh P, et al. Increased serological response against human herpesvirus 6A is associated with risk for multiple sclerosis. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.02715
20. Langer-Gould A, Wu J, Lucas R, Smith J, Gonzales E, Amezcua L, et al. Epstein-Barr Virus, cytomegalovirus, and multiple sclerosis susceptibility. Neurology (2017) 89(13):1330–7. doi: 10.1212/WNL.0000000000004412
21. Levin LI, Munger KL, O’Reilly EJ, Falk KI, Ascherio A. Primary infection with the epstein-barr virus and risk of multiple sclerosis. Ann Neurol (2010) 67(6):824–30. doi: 10.1002/ana.21978
22. Abrahamyan S, Eberspächer B, Hoshi M-M, Aly L, Luessi F, Groppa S, et al. Complete Epstein-Barr virus seropositivity in a large cohort of patients with early multiple sclerosis. J Neurol Neurosurg Psychiatry (2020) 91(7):681–6. doi: 10.1136/jnnp-2020-322941
23. Munger KL, Hongell K, Cortese M, Åivo J, Soilu-Hänninen M, Surcel H, et al. Epstein–Barr virus and multiple sclerosis risk in the finnish maternity cohort. Ann Neurol (2019) 86(3):436–42. doi: 10.1002/ana.25532
24. Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science (2022) 375(6578):296–301. doi: 10.1126/science.abj8222
25. Karampoor S, Zahednasab H, Ramagopalan S, Mehrpour M, Etemadifar M, Alsahebfosoul F, et al. Cytomegalovirus and varicella zoster virus seropositivity of Iranian patients with multiple sclerosis: A population-based study. J Neuroimmunol (2017) 309:4–6. doi: 10.1016/j.jneuroim.2017.04.004
26. Kofahi RM, Kofahi HM, Sabaheen S, Al QM, Momani A, Yassin A, et al. Prevalence of seropositivity of selected herpesviruses in patients with multiple sclerosis in the north of Jordan. BMC Neurol (2020) 20(1):397. doi: 10.1186/s12883-020-01977-w
27. Grut V, Biström M, Salzer J, Stridh P, Jons D, Gustafsson R, et al. Cytomegalovirus seropositivity is associated with reduced risk of multiple sclerosis–a presymptomatic case–control study. Eur J Neurol (2021) 28(9):3072–9. doi: 10.1111/ene.14961
28. Zabalza A, Vera A, Alari-Pahissa E, Munteis E, Moreira A, Yélamos J, et al. Impact of cytomegalovirus infection on b cell differentiation and cytokine production in multiple sclerosis. J Neuroinflamm (2020) 17(1):161. doi: 10.1186/s12974-020-01840-2
29. Leibovitch EC, Jacobson S. Evidence linking HHV-6 with multiple sclerosis: an update. Curr Opin Virol (2014) 9:127–33. doi: 10.1016/j.coviro.2014.09.016
30. Simpson S, Taylor B, Dwyer DE, Taylor J, Blizzard L, Ponsonby A-L, et al. Anti-HHV-6 IgG titer significantly predicts subsequent relapse risk in multiple sclerosis. Mult Scler J (2012) 18(6):799–806. doi: 10.1177/1352458511428081
31. Keyvani H, Zahednasab H, Aljanabi HAA, Asadi M, Mirzaei R, Esghaei M, et al. The role of human herpesvirus-6 and inflammatory markers in the pathogenesis of multiple sclerosis. J Neuroimmunol (2020) 346:577313. doi: 10.1016/j.jneuroim.2020.577313
32. Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci (1995) 92(16):7440–4. doi: 10.1073/pnas.92.16.7440
33. Opsahl ML. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain (2005) 128(3):516–27. doi: 10.1093/brain/awh390
34. van Noort JM, Bajramovic JJ, Plomp AC, van Stipdonk MJB. Mistaken self, a novel model that links microbial infections with myelin-directed autoimmunity in multiple sclerosis. J Neuroimmunol (2000) 105(1):46–57. doi: 10.1016/S0165-5728(00)00181-8
35. Leibovitch EC, Caruso B, Ha SK, Schindler MK, Lee NJ, Luciano NJ, et al. Herpesvirus trigger accelerates neuroinflammation in a nonhuman primate model of multiple sclerosis. Proc Natl Acad Sci (2018) 115(44):11292–7. doi: 10.1073/pnas.1811974115
36. Tienari PJ, Salonen O, Wikstrom J, Valanne L, Palo J. Familial multiple sclerosis: MRI findings in clinically affected and unaffected siblings. J Neurol Neurosurg Psychiatry (1992) 55(10):883–6. doi: 10.1136/jnnp.55.10.883
37. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin d deficiency and insufficiency revisited. J Clin Endocrinol Metab (2012) 97(4):1153–8. doi: 10.1210/jc.2011-2601
38. Didonna A, Oksenberg JR. The genetics of multiple sclerosis. in: Multiple sclerosis: Perspectives in treatment and pathogenesis. Codon Publ (2017) 1:3–16. doi: 10.15586/codon.multiplesclerosis.2017.ch1
39. Smets I, Fiddes B, Garcia-Perez JE, He D, Mallants K, Liao W, et al. Multiple sclerosis risk variants alter expression of co-stimulatory genes in b cells. Brain (2018) 141(3):786–96. doi: 10.1093/brain/awx372
40. Eshel D, Toporik A, Efrati T, Nakav S, Chen A, Douvdevani A. Characterization of natural human antagonistic soluble CD40 isoforms produced through alternative splicing. Mol Immunol (2008) 46(2):250–7. doi: 10.1016/j.molimm.2008.08.280
41. Buhelt S, Søndergaard HB, Oturai A, Ullum H, von Essen MR, Sellebjerg F. Relationship between multiple sclerosis-associated IL2RA risk allele variants and circulating T cell phenotypes in healthy genotype-selected controls. Cells (2019) 8(6):634. doi: 10.3390/cells8060634
42. Wang X-X, Chen T. Meta-analysis of the association of IL2RA polymorphisms rs2104286 and rs12722489 with multiple sclerosis risk. Immunol Invest (2018) 47(5):431–42. doi: 10.1080/08820139.2018.1425699
43. Binzer M, Forsgren L, Holmgren G, Drugge U, Fredrikson S. Familial clustering of multiple sclerosis in a northern Swedish rural district. J Neurol Neurosurg Psychiatry (1994) 57(4):497–9. doi: 10.1136/jnnp.57.4.497
44. Ramroodi N, Sanadgol N, Ganjali Z, Niazi AA, Sarabandi V, Moghtaderi A. Monitoring of active human herpes virus 6 infection in Iranian patients with different subtypes of multiple sclerosis. J Pathog (2013) 2013:1–10. doi: 10.1155/2013/194932
45. Nora-Krukle Z, Chapenko S, Logina I, Millers A, Platkajis A, Murovska M. Human herpesvirus 6 and 7 reactivation and disease activity in multiple sclerosis. Medicina (Kaunas) (2011) 47(10):527–31. doi: 10.3390/medicina47100075
46. Pandit L, Malli C, D’Cunha A, Sudhir A. Role of viral infections in multiple sclerosis pathogenesis among Indian population. Neurol India (2021) 69(3):681. doi: 10.4103/0028-3886.319209
47. Boyko A, Melnikov M. Prevalence and incidence of multiple sclerosis in Russian federation: 30 years of studies. Brain Sci (2020) 10(5)22–31. doi: 10.3390/brainsci10050305
48. Tarlinton R, Wang B, Morandi E, Gran B, Khaiboullin T, Martynova E, et al. Differential expression of HERV-W in peripheral blood in multiple sclerosis and healthy patients in two different ethnic groups. Front Pharmacol (2020) 10. doi: 10.3389/fphar.2019.01645
49. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol (2018) 17(2):162–73. doi: 10.1016/S1474-4422(17)30470-2
51. Stefanović M, Životić I, Stojković L, Dinčić E, Stanković A, Živković M. The association of genetic variants IL2RA rs2104286, IFI30 rs11554159 and IKZF3 rs12946510 with multiple sclerosis onset and severity in patients from Serbia. J Neuroimmunol (2020) 347:577346. doi: 10.1016/j.jneuroim.2020.577346
52. Omraninava M, Mehranfar S, Vahedi P, Razi B, Imani D, Aslani S, et al. Association between IL7 receptor alpha (Il7ra) gene rs6897932 polymorphism and the risk of multiple sclerosis: A meta-regression and meta-analysis. Mult Scler Relat Disord (2021) 48:102687. doi: 10.1016/j.msard.2020.102687
53. Schmied MC, Zehetmayer S, Reindl M, Ehling R, Bajer-Kornek B, Leutmezer F, et al. Replication study of multiple sclerosis (MS) susceptibility alleles and correlation of DNA-variants with disease features in a cohort of Austrian MS patients. Neurogenetics (2012) 13(2):181–7. doi: 10.1007/s10048-012-0316-y
54. Liu J, Liu X, Liu Y, Deng S, Huang H, Chen Q, et al. Association of EVI5 rs11808092, CD58 rs2300747, and CIITA rs3087456 polymorphisms with multiple sclerosis risk: A meta-analysis. Meta Gene (2016) 9:97–103. doi: 10.1016/j.mgene.2016.04.005
55. Booth DR, Heard RN, Stewart GJ, Goris A, Dobosi R, Dubois B, et al. The expanding genetic overlap between multiple sclerosis and type I diabetes. Genes Immun (2009) 10(1):11–4. doi: 10.1038/gene.2008.83
56. Ghavimi R, Alsahebfosoul F, Salehi R, Kazemi M, Etemadifar M, Zavaran Hosseini A. High-resolution melting curve analysis of polymorphisms within CD58, CD226, HLA-G genes and association with multiple sclerosis susceptibility in a subset of Iranian population: A case–control study. Acta Neurol Belg (2020) 120(3):645–52. doi: 10.1007/s13760-018-0992-y
57. Chalmers SA, Ayilam Ramachandran R, Garcia SJ, Der E, Herlitz L, Ampudia J, et al. The CD6/ALCAM pathway promotes lupus nephritis via T cell–mediated responses. J Clin Invest (2022) 132(1):1288–92. doi: 10.1172/JCI147334
58. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol (2011) 69(2):292–302. doi: 10.1002/ana.22366
59. Kingwell E, Marriott JJ, Jetté N, Pringsheim T, Makhani N, Morrow SA, et al. Incidence and prevalence of multiple sclerosis in Europe: A systematic review. BMC Neurol (2013) 13(1):128. doi: 10.1186/1471-2377-13-128
60. Truini A, Prosperini L, Calistri V, Fiorelli M, Pozzilli C, Millefiorini E, et al. A dual concurrent mechanism explains trigeminal neuralgia in patients with multiple sclerosis. Neurology (2016) 86(22):2094–9. doi: 10.1212/WNL.0000000000002720
61. Kearns PKA, Casey HA, Leach JP. Hypothesis: Multiple sclerosis is caused by three-hits, strictly in order, in genetically susceptible persons. Mult Scler Relat Disord (2018) 24:157–74. doi: 10.1016/j.msard.2018.06.014
62. Favorova OO, Favorov AV, Boiko AN, Andreewski TV, Sudomoina MA, Alekseenkov AD, et al. Three allele combinations associated with multiple sclerosis. BMC Med Genet (2006) 7(1):63. doi: 10.1186/1471-2350-7-63
63. Sokolova EA, Malkova NA, Korobko DS, Rozhdestvenskii AS, Kakulya AV, Khanokh EV, et al. Association of SNPs of CD40 gene with multiple sclerosis in russians. PloS One (2013) 8(4):e61032. doi: 10.1371/journal.pone.0061032
64. Bashinskaya VV, Kulakova OG, Kiselev IS, Baulina NM, Favorov AV, Boyko AN, et al. GWAS-identified multiple sclerosis risk loci involved in immune response: Validation in russians. J Neuroimmunol (2015) 282:85–91. doi: 10.1016/j.jneuroim.2015.03.015
65. Cermelli C, Berti R, Soldan SS, Mayne M, D’ambrosia JM, Ludwin SK, et al. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J Infect Dis (2003) 187(9):1377–87. doi: 10.1086/368166
66. Derfuss T, Hohlfeld R, Meinl E. Intrathecal antibody (IgG) production against human herpesvirus type 6 occurs in about 20% of multiple sclerosis patients and might be linked to a polyspecific b–cell response. J Neurol (2005) 252(8):968–71. doi: 10.1007/s00415-005-0794-z
67. Voumvourakis KI, Fragkou PC, Kitsos DK, Foska K, Chondrogianni M, Tsiodras S. Human herpesvirus 6 infection as a trigger of multiple sclerosis: An update of recent literature. BMC Neurol (2022) 22(1):57. doi: 10.1186/s12883-022-02568-7
68. Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. part I: The role of infection. Ann Neurol (2007) 61(4):288–99. doi: 10.1002/ana.21117
69. Jacobs BM, Giovannoni G, Cuzick J, Dobson R. Systematic review and meta-analysis of the association between Epstein–Barr virus, multiple sclerosis and other risk factors. Mult Scler J (2020) 26(11):1281–97. doi: 10.1177/1352458520907901
70. Pakpoor J, Disanto G, Gerber JE, Dobson R, Meier UC, Giovannoni G, et al. The risk of developing multiple sclerosis in individuals seronegative for Epstein-Barr virus: A meta-analysis. Mult Scler J (2013) 19(2):162–6. doi: 10.1177/1352458512449682
71. Guan Y, Jakimovski D, Ramanathan M, Weinstock-Guttman B, Zivadinov R. The role of Epstein-Barr virus in multiple sclerosis: From molecular pathophysiology to in vivo imaging. Neural Regener Res (2019) 14(3):373. doi: 10.4103/1673-5374.245462
72. De Paschale M. Serological diagnosis of Epstein-Barr virus infection: Problems and solutions. World J Virol (2012) 1(1):31. doi: 10.5501/wjv.v1.i1.31
73. Bauer G. Simplicity through complexity: immunoblot with recombinant antigens as the new gold standard in Epstein-Barr virus serology. Clin Lab (2001) 47(5–6):223–30.
74. Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the atlas of MS, third edition. Mult Scler J (2020) 26(14):1816–21. doi: 10.1177/1352458520970841
75. Xia Z-L, Qin Q-M, Zhao Q-Y. A genetic link between CXCR5 and IL2RA gene polymorphisms and susceptibility to multiple sclerosis. Neurol Res (2018) 40(12):1040–7. doi: 10.1080/01616412.2018.1517110
76. AL-Eitan L, Al Qudah M, Al Qawasmeh M. Candidate gene association analysis of multiple sclerosis in the Jordanian Arab population: A case-control study. Gene (2020) 758:144959. doi: 10.1016/j.gene.2020.144959
77. Boullerne AI, Skias D, Hartman EM, Testai FD, Kalinin S, Polak PE, et al. A single-nucleotide polymorphism in serine-threonine kinase 11, the gene encoding liver kinase B1, is a risk factor for multiple sclerosis. ASN Neuro (2015) 7(1):175909141556891. doi: 10.1177/1759091415568914
78. Bai L, Jiang J, Li H, Zhang R. Role of CD226 Rs763361 polymorphism in susceptibility to multiple autoimmune diseases. Immunol Invest (2020) 49(8):926–42. doi: 10.1080/08820139.2019.1703737
79. D′Cunha M, Pandit L, Malli C. CD6 gene polymorphism rs17824933 is associated with multiple sclerosis in Indian population. Ann Indian Acad Neurol (2016) 19(4):491. doi: 10.4103/0972-2327.192384
80. The International Multiple Sclerosis Genetics Consortium. The genetic association of variants in CD6, TNFRSF1A and IRF8 to multiple sclerosis: A multicenter case-control study. PloS One (2011) 6(4):e18813. doi: 10.1371/journal.pone.0018813
81. Hadjigeorgiou GM, Kountra P-M, Koutsis G, Tsimourtou V, Siokas V, Dardioti M, et al. Replication study of GWAS risk loci in Greek multiple sclerosis patients. Neurol Sci (2019) 40(2):253–60. doi: 10.1007/s10072-018-3617-6
82. De Jager PL, Baecher-Allan C, Maier LM, Arthur AT, Ottoboni L, Barcellos L, et al. The role of the CD58 locus in multiple sclerosis. Proc Natl Acad Sci (2009) 106(13):5264–9. doi: 10.1073/pnas.0813310106
83. Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, Wong KCC, et al. A genome-wide association study of global gene expression. Nat Genet (2007) 39(10):1202–7. doi: 10.1038/ng2109
84. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet (2007) 81(3):559–75. doi: 10.1086/519795
85. Eskandarieh S, Heydarpour P, Minagar A, Pourmand S, Sahraian MA. Multiple sclerosis epidemiology in East Asia, South East Asia and south Asia: A systematic review. Neuroepidemiology (2016) 46(3):209–21. doi: 10.1159/000444019
86. Torbati S, Karami F, Ghaffarpour M, Zamani M. Association of CD58 polymorphism with multiple sclerosis and response to interferon ß therapy in a subset of Iranian population. Cell J (2015) 16(4):506–13. doi: 10.22074/cellj.2015.505
87. International Multiple Sclerosis Genetics Consortium, Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, et alRisk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med (2007) 357(9):851–62. doi: 10.1056/NEJMoa073493
88. Rubio JP, Stankovich J, Field J, Tubridy N, Marriott M, Chapman C, et al. Replication of KIAA0350, IL2RA, RPL5 and CD58 as multiple sclerosis susceptibility genes in australians. Genes Immun (2008) 9(7):624–30. doi: 10.1038/gene.2008.59
89. D’Netto MJ, Ward H, Morrison KM, Ramagopalan SV, Dyment DA, DeLuca GC, et al. Risk alleles for multiple sclerosis in multiplex families. Neurology (2009) 72(23):1984–8. doi: 10.1212/WNL.0b013e3181a92c25
90. Hecker M, Blaschke J, Blaschke P, Fitzner B, Koczan D, Thiesen H-J, et al. CD58 and multiple sclerosis: Genetic association, gene expression and prognostic value (P6.156). In: Neurology AAN Enterprises, Inc, vol. 82. (2014). Available at: http://n.neurology.org/content/82/10_Supplement/P6.156.abstract.
91. Davis SJ, van der Merwe PA. The structure and ligand interactions of CD2: implications for T-cell function. Immunol Today (1996) 17(4):177–87. doi: 10.1016/0167-5699(96)80617-7
92. Zhang Y, Yuan J, Yang X. A novel hantavirus detected in yunnan red-backed vole (Eothenomys miletus) in China. J Gen Virol (2011) 92(6):1454–7. doi: 10.1099/vir.0.030122-0
93. Teh S-J, Killeen N, Tarakhovsky A, Littman DR, Teh H-S. CD2 regulates the positive selection and function of antigen-specific CD4–CD8+ T cells. Blood (1997) 89(4):1308–18. doi: 10.1182/blood.V89.4.1308
94. Mori D, Grégoire C, Voisinne G, Celis-Gutierrez J, Aussel R, Girard L, et al. The T cell CD6 receptor operates a multitask signalosome with opposite functions in T cell activation. J Exp Med (2021) 218(2):84–92. doi: 10.1084/jem.20201011
95. Swaminathan B, Cuapio A, Alloza I, Matesanz F, Alcina A, García-Barcina M, et al. Fine mapping and functional analysis of the multiple sclerosis risk gene CD6. PloS One (2013) 8(4):e62376. doi: 10.1371/journal.pone.0062376
96. Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et aldbSNP short genetic variations. In: Nucleic Acids Res 29(1):308–11. doi: 10.1093/nar/29.1.308
97. Karpusas M, Hsu Y-M, Wang J, Thompson J, Lederman S, Chess L, et al. 2 å crystal structure of an extracellular fragment of human CD40 ligand. Structure (1995) 3(10):1031–9. doi: 10.1016/S0969-2126(01)00239-8
98. Carbone E, Ruggiero G, Terrazzano G, Palomba C, Manzo C, Fontana S, et al. A new mechanism of NK cell cytotoxicity activation: The CD40–CD40 ligand interaction. J Exp Med (1997) 185(12):2053–60. doi: 10.1084/jem.185.12.2053
99. Tang K, Cheng L, Zhang C, Zhang Y, Zheng X, Zhang Y, et al. Novel identified HLA-A*0201-restricted hantaan virus glycoprotein cytotoxic T-cell epitopes could effectively induce protective responses in HLA-A2.1/Kb transgenic mice may associate with the severity of hemorrhagic fever with renal syndrome. Front Immunol (2017) 8:1797. doi: 10.3389/fimmu.2017.01797
100. Coustet B, Agarwal SK, Gourh P, Guedj M, Mayes MD, Dieude P, et al. Association study of ITGAM, ITGAX, and CD58 autoimmune risk loci in systemic sclerosis: Results from 2 Large European Caucasian cohorts. J Rheumatol (2011) 38(6):1033–8. doi: 10.3899/jrheum.101053
101. Qin J, Xing J, Liu R, Chen B, Chen Y, Zhuang X. Association between CD40 rs1883832 and immune-related diseases susceptibility: A meta-analysis. Oncotarget (2017) 8(60):102235–43. doi: 10.18632/oncotarget.18704
102. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol (1988) 45(4):435–7. doi: 10.1001/archneur.1988.00520280085020
104. Maier LM, Lowe CE, Cooper J, Downes K, Anderson DE, Severson C, et al. IL2RA genetic heterogeneity in multiple sclerosis and type 1 diabetes susceptibility and soluble interleukin-2 receptor production. PloS Genet (2009) 5(1):e1000322. doi: 10.1371/journal.pgen.1000322
105. Afanasyeva MA, Putlyaeva LV, Demin DE, Kulakovskiy IV, Vorontsov IE, Fridman MV, et al. The single nucleotide variant rs12722489 determines differential estrogen receptor binding and enhancer properties of an IL2RA intronic region. PloS One (2017) 12(2):e0172681. doi: 10.1371/journal.pone.0172681
106. Chistiakov DA, Voronova NV, Chistiakov PA. The crucial role of IL-2/IL-2RA-mediated immune regulation in the pathogenesis of type 1 diabetes, an evidence coming from genetic and animal model studies. Immunol Lett (2008) 118(1):1–5. doi: 10.1016/j.imlet.2008.03.002
107. Iampietro M, Morissette G, Gravel A, Flamand L. Inhibition of interleukin-2 gene expression by human herpesvirus 6B U54 tegument protein. J Virol (2014) 88(21):12452–63. doi: 10.1128/JVI.02030-14
108. Field J, Shahijanian F, Schibeci S, Johnson L, Gresle M, Laverick L, et al. The MS risk allele of CD40 is associated with reduced cell-membrane bound expression in antigen presenting cells: Implications for gene function. PloS One (2015) 10(6):e0127080. doi: 10.1371/journal.pone.0127080
109. Australia and New Zealand Multiple Sclerosis Genetics Consortium (ANZgene). Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet (2009) 41(7):824–8. doi: 10.1038/ng.396
110. Gandhi KS, McKay FC, Cox M, Riveros C, Armstrong N, Heard RN, et al. The multiple sclerosis whole blood mRNA transcriptome and genetic associations indicate dysregulation of specific T cell pathways in pathogenesis. Hum Mol Genet (2010) 19(11):2134–43. doi: 10.1093/hmg/ddq090
111. Wagner M, Wisniewski A, Bilinska M, Pokryszko-Dragan A, Cyrul M, Kusnierczyk P, et al. Investigation of gene–gene interactions between CD40 and CD40L in polish multiple sclerosis patients. Hum Immunol (2014) 75(8):796–801. doi: 10.1016/j.humimm.2014.05.013
112. Jacobson EM, Concepcion E, Oashi T, Tomer Y. A graves’ disease-associated kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: A case for translational pathophysiology. Endocrinology (2005) 146(6):2684–91. doi: 10.1210/en.2004-1617
113. Soldan SS, Lieberman PM. Epstein–Barr Virus and multiple sclerosis. Nat Rev Microbiol (2022) 1:17–21. doi: 10.1038/s41579-022-00770-5
114. Oskari Virtanen J, Jacobson S. Viruses and multiple sclerosis. CNS Neurol Disord - Drug Targets (2012) 11(5):528–44. doi: 10.2174/187152712801661220
115. Xu Y, Hiyoshi A, Smith KA, Piehl F, Olsson T, Fall K, et al. Association of infectious mononucleosis in childhood and adolescence with risk for a subsequent multiple sclerosis diagnosis among siblings. JAMA Netw Open (2021) 4(10):e2124932. doi: 10.1001/jamanetworkopen.2021.24932
116. Álvarez-Lafuente R, De las Heras V, Bartolomé M, Picazo JJ, Arroyo R. Relapsing-remitting multiple sclerosis and human herpesvirus 6 active infection. Arch Neurol (2004) 61(10):1523. doi: 10.1001/archneur.61.10.1523
117. Chapenko S, Millers A, Nora Z, Logina I, Kukaine R, Murovska M. Correlation between HHV-6 reactivation and multiple sclerosis disease activity. J Med Virol (2003) 69(1):111–7. doi: 10.1002/jmv.10258
118. Bech E, Lycke J, Gadeberg P, Hansen HJ, Malmeström C, Andersen O, et al. A randomized, double-blind, placebo-controlled MRI study of anti–herpes virus therapy in MS. Neurology (2002) 58(1):31. doi: 10.1212/WNL.58.1.31
119. Friedman JE, Zabriskie JB, Plank C, Ablashi D, Whitman J, Shahan B, et al. A randomized clinical trial of valacyclovir in multiple sclerosis. Mult Scler J (2005) 11(3):286–95. doi: 10.1191/1352458505ms1185oa
120. Gill H, Hwang Y-Y, Chan TSY, Pang AWK, Leung AYH, Tse E, et al. Valganciclovir suppressed Epstein Barr virus reactivation during immunosuppression with alemtuzumab. J Clin Virol (2014) 59(4):255–8. doi: 10.1016/j.jcv.2014.01.005
Keywords: multiple sclerosis, single nucleotide polymorphism, herpesviruses, ebv, HHV6, risk factor
Citation: Lezhnyova V, Davidyuk Y, Mullakhmetova A, Markelova M, Zakharov A, Khaiboullina S and Martynova E (2022) Analysis of herpesvirus infection and genome single nucleotide polymorphism risk factors in multiple sclerosis, Volga federal district, Russia. Front. Immunol. 13:1010605. doi: 10.3389/fimmu.2022.1010605
Received: 03 August 2022; Accepted: 03 October 2022;
Published: 14 November 2022.
Edited by:
Jianhong Lu, Central South University, ChinaReviewed by:
Peter A. Maple, Nottingham University Hospitals NHS Trust, United KingdomAbdorreza Naser Moghadasi, Tehran University of Medical Sciences, Iran
Zuo Lielian, Central South University, China
Copyright © 2022 Lezhnyova, Davidyuk, Mullakhmetova, Markelova, Zakharov, Khaiboullina and Martynova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ekaterina Martynova, aWduaWV0ZmVycm8udmVuaXZlZGl2aWNpQGdtYWlsLmNvbQ==
 Vera Lezhnyova
Vera Lezhnyova Yuriy Davidyuk
Yuriy Davidyuk Asia Mullakhmetova1
Asia Mullakhmetova1 Maria Markelova
Maria Markelova Alexander Zakharov
Alexander Zakharov Svetlana Khaiboullina
Svetlana Khaiboullina Ekaterina Martynova
Ekaterina Martynova