
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 17 October 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1010490
This article is part of the Research TopicCase Reports in Surgical Oncology: 2022View all 56 articles
 Di Cao1,2
Di Cao1,2 Yu Gao3
Yu Gao3 Rong-xin Zhang1,2
Rong-xin Zhang1,2 Fu-long Wang1,2
Fu-long Wang1,2 Cong Li1,2
Cong Li1,2 Miao-qing Wu1,2
Miao-qing Wu1,2 Yi-fan Liu1,2
Yi-fan Liu1,2 Dan-dan Li1,4*
Dan-dan Li1,4* Gong Chen1,2*
Gong Chen1,2*Currently, immune checkpoint inhibitors (ICIs) are the mainstay of treatment for Lynch syndrome patients. However, the tumor regression features in radiology and pathology are inconsistent for patients who are treated with ICIs, which sometimes confuses surgical decision-making. Here, we report a case in which a 36-year-old patient suffering from infertility was diagnosed with Lynch syndrome-associated synchronous endometrial cancer and colon cancer, and persistently enlarged left iliac paravascular lymph nodes were detected after receiving sintilimab treatment, a programmed cell death 1 (PD-1) receptor inhibitor. Fortunately, when she was about to undergo hysterectomy and bilateral salpingo-oophorectomy, intraoperative pathology examination did not reveal any cancer cells in these lymph nodes, and therefore, her reproductive organs were preserved. Later, the patient successfully conceived and gave birth to a healthy male neonate with no immune-related adverse events (irAEs) during an 11-month follow-up. This case indicates that surgeons should carefully inspect the imaging characteristics after immunotherapy and that organ preservation is possible even for patients who fail to achieve complete clinical regression, which is especially important for female patients of childbearing age.
Lynch syndrome (LS), characterized by germline mutations of the DNA mismatch repair (MMR) system, is the most common inherited cancer syndrome (1). The global incidence of LS is approximately 1/279, and almost all LS-associated cancers present deficient MMR (dMMR) and high-level microsatellite instability (MSI-H). Colorectal cancer (CRC) and endometrial cancer (EC) are the two most common LS-associated cancers (2). There are approximately 6000 new LS-EC cases each year, accounting for 9% of all new EC cases worldwide (3, 4). Previously, the treatments for LS-EC and LS-CRC were the same as those of general EC and CRC. Surgery is the first choice if the tumor is resectable. For unresectable CRC or EC patients, receiving translational therapies is necessary, including chemotherapy, radiotherapy, targeted therapy and immunotherapy, which provides opportunities for radical resection. Understandably, surgery for LS-EC patients permanently destroys fertility. This situation hit a turning point when Le et al. demonstrated that dMMR patients were particularly sensitive to pembrolizumab, a representative ICI drug, in 2015 (5). Thus, the 2018 National Comprehensive Cancer Network (NCCN) guidelines recommended that pembrolizumab should be used in patients with MSI-H or dMMR diseases, greatly benefiting LS patients during immunotherapy.
However, the different tumor regression features in radiology and pathology of patients treated with ICIs have influenced clinical decision-making for surgeons. For female patients, reproductive organ preservation is an inevitable issue. If clinical complete regression (cCR) is not achieved according to preoperative images, patients are obliged to undergo radical resection, which leads to permanent infertility. However, if pathological complete regression (pCR) is achieved, radical surgery should be avoided.
Additionally, PD-1 inhibitors (IgG4 antibodies) may influence fetal development during pregnancy by crossing the human placenta. Using anti-PD-1 antibodies in mice weakens the immune tolerance mediated by regulatory T cells (Tregs) and significantly increases miscarriage rates (6). This finding revealed a key role of the PD-1/PD-L1 pathway in maintaining maternal-fetal immune tolerance. Thus, PD-1 antibody is classified as an FDA pregnancy Category D drug (7).
In general, this case suggests that surgeons should focus on reproductive organ preservation in female patients receiving ICI treatment rather than only considering preoperative images. In addition, our case provides additional experience in using PD-1 antibody in patients who plan to conceive. This study was reported in agreement with the principles of the CARE Guidelines (8) and includes a reporting checklist as Supplementary Material (Table S1) (9).
The patient was a 36-year-old woman who suffered from LS-EC and LS-CRC. She conceived naturally and successfully bore a male neonate after treatment with sintilimab, a PD-1 inhibitor. The timeline is shown in Figure 1. At the age of 32, she went to Fudan University Obstetrics and Gynecology Hospital because she had failed to conceive naturally for more than one year. She underwent hysteroscopy and was found to have atypical hyperplasia of the endometrium, so she was prescribed an initial regimen of megestrol 160 mg QD in combination with metformin 0.5 g TID. Seven months later, she underwent a second hysteroscopy, which detected that the endometrium had locally progressed to grade 1 endometrioid adenocarcinoma. Due to her strong desire to conceive, the patient refused the medical advice of hysterectomy. She was referred to Peking Union Medical College Hospital, and her medication regimen was changed to enantone 3.75 mg 1/28D + letrozole 2.5 mg QD. One year later, her endometrium had reversed, and she was advised to undergo in vitro fertilization (IVF). However, she did not adopt this suggestion for personal reasons, and over the following year, she still failed to conceive naturally without any contraception. In December 2019, she requested IVF at Xiangya Hospital of Central South University. Preoperative hysteroscopy did not show any endometrial abnormality, but she underwent additional pelvic magnetic resonance imaging (MRI) owing to her history of endometrial cancer, which showed that the left pelvic lymph nodes (LNs) were enlarged and considered metastases. In addition, her positron emission tomography-computed tomography (PET-CT) scan showed a hypermetabolic lesion in the transverse colon, which was later confirmed as a transverse colon (TC) adenocarcinoma by colonoscopy biopsy.
The patient came to our hospital in March 2020. We reexamined the colonoscopy results and identified the TC mass as a moderately to poorly differentiated adenocarcinoma. Of note, her immunohistochemistry (IHC) examination indicated the loss of MSH2 protein in both her EC and CRC lesions. Moreover, her father, aunt, and cousin all died of malignant tumors. Therefore, LS was highly suspected, and we conducted genetic testing, which revealed that both the EC and TC lesions were MSI-H and had somatic mutations of MSH2. More importantly, the patient also carried germline MSH2 mutations. The tumor mutation burden (TMB) of the EC lesion was 29.9 muts/Mb, and the TMB of the TC lesion was 77 muts/Mb. Based on her medical history and test results, the patient was diagnosed with LS.
Thoracoabdominal and pelvic CT were performed on March 16, 2020 (Figure 2A). CT images showed that the long diameter of the TC lesion was 32 mm, and there were enlarged LNs adjacent to the primary colon and left iliac vessels. The short diameters of the largest pelvic and pericolic LNs, which were considered metastatic LNs, were 12 mm and 18 mm, respectively. According to the results, we initiated neoadjuvant immunotherapy with sintilimab at a fixed dose of 200 mg every 3 weeks. From March to July 2020, the patient received 6 courses of immunotherapy. No significant immune-related adverse events (irAEs) were observed during the treatment. After the third course on May 5, 2020, the patient underwent enteroscopy and abdominal CT (Figure 2B). Both assessments suggested that the size of the primary and pericolic LNs had increased to 41 mm and 21 mm, respectively, compared with baseline, while the largest left pelvic LN had decreased to 8 mm. Because of our therapeutic goal of controlling the pelvic metastatic LNs, we decided to continue her neoadjuvant therapy. At the end of the sixth course on July 14, 2020, another abdominal CT was performed (Figure 2C) and showed that the TC lesion and pelvic LNs were stable, while the pericolic LNs had increased to 26 mm. In addition, her CEA serum level increased from 7.02 ng/ml to 19.17 ng/ml. At this time, we organized a multidisciplinary team (MDT) to discuss the case. According to the images, the enlarged left pelvic LNs most likely originated from EC metastasis. Although the TC lesion was confirmed as iCPD by the irRECIST method, it could reach R0 resection. Therefore, we decided to perform surgery. The surgical plan included radical TC resection, hysterectomy, bilateral salpingo-oophorectomy resection, pelvic LN dissection and retroperitoneal LN dissection.
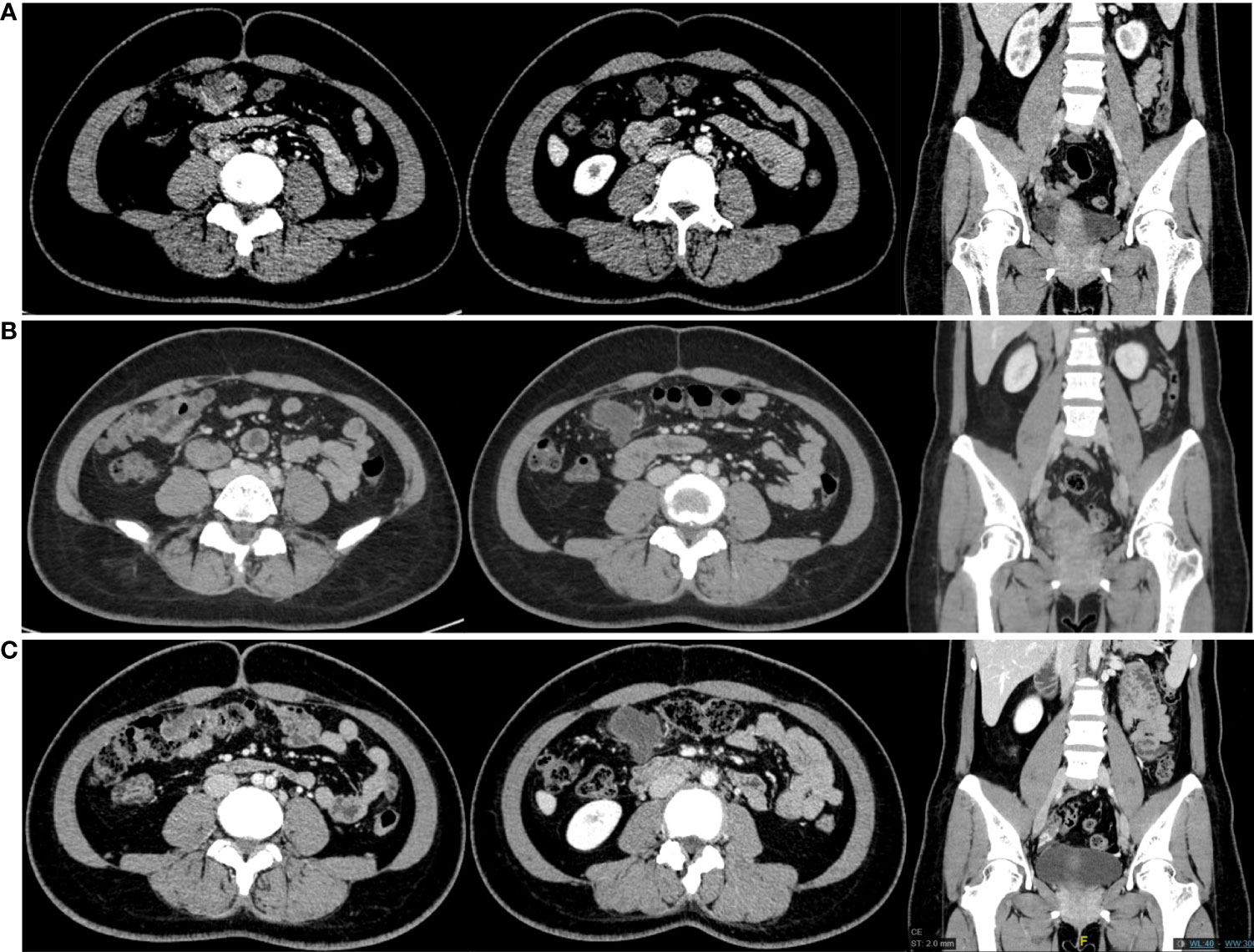
Figure 2 CT scans obtained from the patient. From left to right, each group of images shows the transverse colon lesion, para-transverse colon lymph nodes and the lymph nodes next to the left iliac vessels. (A) CT scans in March 2020. (B) CT scans in May 2020. (C) CT scans in July 2020.
On July 31, 2020, the patient underwent laparoscopic right hemicolectomy with radical lymphadenectomy. We did not observe any other lesions in the abdominal-pelvic cavity during intraoperative exploration. The tumor resided in the TC near the hepatic flexure and was approximately 6*5 cm in size. It possessed a tough texture and had invaded the serosa. The boundary between the tumor and the mesenteric LNs was unclear. There were several enlarged lymph nodes that extended from the left iliac vasculature to the left obturator area. Therefore, we performed an intraoperative gynecological consultation, and the gynecologist proposed total hysterectomy, bilateral salpingo-oophorectomy, and left pelvic lymphadenectomy. However, considering the patient’s desire to conceive and the possible effects of immunotherapy, we decided to send the left pelvic LNs for pathological examination before resection, and none of the 7 submitted pelvic LNs contained cancer cells. Subsequently, we communicated with her family about the possibility of pathological complete regression (pCR) and the risk of EC recurrence without hysterectomy. Ultimately, her family chose to preserve her uterus and bilateral adnexa and signed an informed consent document for the fertility preservation strategy. Postoperative pathological examination confirmed that the TC lesion was a poorly differentiated adenocarcinoma that had invaded through the muscularis propria into the pericolic tissues (pT3). Nerve tract invasion and intravascular cancer embolus were not observed. The resection margin was negative, and the tumor regression grade (TRG) was 2. No cancer tissue was observed in a total of 35 dissected LNs, including 28 pericolic LNs and 7 left pelvic LNs. The tumor deposits were also not detected.
After the surgery, the patient received adjuvant immunotherapy with a PD-1 inhibitor for 1 year. Although we told her to use contraception, she did not follow this advice because she had lost faith in natural conception. However, in December 2020, the patient unexpectedly found herself at 38 days’ gestation. Even though we informed the patient and her family of the possible adverse influences on the fetus, they still planned to continue with the pregnancy and complied with the obstetrician’s advice. There were no tumor-related conditions or gestational abnormalities, and in the 36th week of gestation, the patient successfully gave birth to a male neonate by cesarean section. The Apgar score of the male neonate was 9, and his serum levels of G6PD, TSH, 17-OHP and PHE were normal. There were no irAEs in either the mother or the neonate during an 11-month follow-up, and the patient is still being followed closely.
We report the first case in which a female LS patient suffering from both EC and CRC successfully conceived and delivered after receiving treatment with sintilimab, a PD-1 antibody. Our report mainly highlights the complexity of reproductive organ preservation for LS-EC patients. After PD-1 antibody treatment, uterine preservation is still feasible even in EC patients with pelvic LN metastases, which diverges from the previous therapeutic principle that hysterectomy is usually unavoidable for these patients.
In recent years, infertility treatment strategies have become established and various in number. Nontumorous female patients can undergo ovarian stimulation, oocyte vitrification, and IVF to treat infertility. However, these practices are still limited for patients suffering from EC, which is the most frequent gynecological malignancy and severely influences young patients without a childbearing history (10, 11). The early detection of EC is extremely important for the reproductive organ preservation of patients. Diagnostic tools for early-stage EC detection are emerging, such as relative telomere length in cell-free DNA, glandular cells detected at preoperative cervical smear, and transvaginal ultrasound (12–14). The treatment of early-stage EC is conservative and follows the conventional regimen of medroxyprogesterone acetate (MPA) and megestrol acetate (MA) (15). Previously, type I EC at the early stage was identified as the only histological type that can be addressed with a fertility-sparing approach. Nevertheless, with a deeper understanding of molecular oncology, the importance of molecular classification for EC treatment was gradually realized, and MSI was determined to be a fair prognostic factor for fertility-sparing treatment (16, 17).
Notably, LS-EC patients featuring MSI could benefit from PD-1 antibodies, and clinicians should take into account the possibility of CR, which means that non-early-stage young patients still have a chance to preserve their reproductive organs. It is worth noting that the pathological remission rate often exceeds the radiological remission rate in patients receiving PD-1 antibody treatment. For example, 13 patients with dMMR CRC achieved pCR after pembrolizumab treatment, but among them, only 1 patient achieved cCR (18). Furthermore, some patients may develop lesion enlargement during treatment, probably caused by T-cell infiltration into the tumor stroma. These studies suggest that imaging is insufficient as an absolutely accurate standard for evaluating PD-1 efficacy. Intraoperative pathological biopsy may provide additional information for better treatment decisions.
How PD-1 antibodies influence pregnancy has always been a topic of interest. Recently, several cases have reported successful pregnancy outcomes in patients treated with PD-1 antibodies in different cancer types, including metastatic melanoma, relapsed Hodgkin’s lymphoma and placental site trophoblastic tumors (6, 7, 19–27). Nevertheless, irAEs, such as intrauterine growth restriction, HELLP syndrome, placental insufficiency and low fetal heart rate, have occurred in some of these patients (28). Thus, whether conception is safe for patients after treatment with PD-1 antibody is still controversial. Current studies believe that blocking the PD-1/PD-L1 pathway will interfere with normal pregnancy because of its key role in maintaining maternal immune tolerance to the fetus, which is regarded as an allogeneic component. Fetal antigens can be processed and presented in the maternal body, leading to the activation and expansion of anti-fetus T cells (29). At the early stage of pregnancy, increased PD-L1 expression in trophoblast cells, decidual macrophages and decidual stromal cells on the maternal-fetal interface can inhibit the activity of T helper cells and the production of proinflammatory cytokines and facilitate the function of Treg cells, which mediate maternal-fetal immune tolerance. In mice, blockade of the PD-1/PD-L1 pathway inhibited Treg cell function and reduced the embryonic survival rate. In crab-eating monkeys, using high doses of nivolumab antibodies (10 mg/kg or more) resulted in significantly higher risks of fetal growth restriction and premature delivery.
However, the effect of PD-1 antibody on pregnancy is determined by multiple factors and needs comprehensive consideration. First, the administration doses and frequencies of PD-1 antibodies in clinical settings are much lower and less than those in experimental settings. Even when exposed to high doses of PD-1 antibody, animals did not present increasing risks of fetal malformations, immunodeficiencies or neurological complications (30). Second, the effect of PD-1 antibody on the fetus is subject to the interval between the last dose of the antibody and the start of pregnancy. PD-1 antibody, as an IgG antibody, requires active transport to enter the placenta where the neonatal Fc receptor (FcRn) resides. FcRn on syncytiotrophoblast cells can bind to IgG antibodies and allow them to pass through the placental barrier. However, FcRn is rarely detected during the first 14 weeks of pregnancy, which restricts the transfer of IgG antibody (31). Thus, the timing of the last administration and the pharmacokinetics of the PD-1 antibody are also involved in its effect on pregnancy. Finally, the patient’s fertility intention and physical condition need to be taken into full consideration. Currently, the National Comprehensive Cancer Network guidelines recommend that all patients of reproductive age should use effective contraception during immunotherapy and maintain its use for at least 5 months after the last course of immunotherapy, which lacks adequate evidence and needs further exploration.
In conclusion, our report indicates that for young female patients with LS-EC, surgeons should be aware of the possibility of reproductive organ preservation. Intraoperative pathological biopsy is more conducive to guiding surgical decision-making for these patients. In addition, we recommend that patients should use contraception for a sufficient period after receiving PD-1 antibody treatments to reduce the incidence of irAEs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DC collected the data and wrote the manuscript. DC, GY, CL, F-lW, R-xZ, D-dL, and GC was involved in the diagnosis and treatment of the disease. GY performed the histological obstetrical examination. D-dL and GC performed conception and design. GY, F-lW, R-xZ, CL, M-qW, Y-fL, D-dL, and GC reviewed and revised the manuscript. D-dL and GC supervised the review and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
We appreciated the gynecologist, Ting Wan and the department of pathology for consultations during treatment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1010490/full#supplementary-material
1. Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet (2009) 76(1):1–18. doi: 10.1111/j.1399-0004.2009.01230.x
2. Boland PM, Yurgelun MB, Boland CR. Recent progress in lynch syndrome and other familial colorectal cancer syndromes. CA Cancer J Clin (2018) 68(3):217–31. doi: 10.3322/caac.21448
3. Lu KH, Schorge JO, Rodabaugh KJ, Daniels MS, Sun CC, Soliman PT, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol (2007) 25(33):5158–64. doi: 10.1200/JCO.2007.10.8597
4. Gu B, Shang X, Yan M, Li X, Wang W, Wang Q, et al. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990-2019. Gynecol Oncol (2021) 161(2):573–80. doi: 10.1016/j.ygyno.2021.01.036
5. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
6. Menzer C, Beedgen B, Rom J, Duffert CM, Volckmar AL, Sedlaczek O, et al. Immunotherapy with ipilimumab plus nivolumab in a stage IV melanoma patient during pregnancy. Eur J Cancer (2018) 104:239–42. doi: 10.1016/j.ejca.2018.09.008
7. Gangadhar TC, Salama AK. Clinical applications of PD-1-based therapy: A focus on pembrolizumab (MK-3475) in the management of melanoma and other tumor types. Onco Targets Ther (2015) 8:929–37. doi: 10.2147/OTT.S53164
8. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
10. Prapas Y, Ravanos K, Petousis S, Panagiotidis Y, Papatheodorou A, Margioula-Siarkou C, et al. GnRH antagonist administered twice the day before hCG trigger combined with a step-down protocol may prevent OHSS in IVF/ICSI antagonist cycles at risk for OHSS without affecting the reproductive outcomes: a prospective randomized control trial. J Assist Reprod Genet (2017) 34(11):1537–45. doi: 10.1007/s10815-017-1010-7
11. Gullo G, Petousis S, Papatheodorou A, Panagiotidis Y, Margioula-Siarkou C, Prapas N, et al. Closed vs. open oocyte vitrification methods are equally effective for blastocyst embryo transfers: Prospective study from a sibling oocyte donation program. Gynecol Obstet Invest (2020) 85(2):206–12. doi: 10.1159/000506803
12. Benati M, Montagnana M, Danese E, Mazzon M, Paviati E, Garzon S, et al. Aberrant telomere length in circulating cell-free DNA as possible blood biomarker with high diagnostic performance in endometrial cancer. Pathol Oncol Res (2020) 26(4):2281–9. doi: 10.1007/s12253-020-00819-x
13. Casarin J, Bogani G, Serati M, Pinelli C, Laganà AS, Garzon S, et al. Presence of glandular cells at the preoperative cervical cytology and local recurrence in endometrial cancer. Int J Gynecol Pathol (2020) 39(6):522–8. doi: 10.1097/PGP.0000000000000642
14. Capozzi VA, Rosati A, Rumolo V, Ferrari F, Gullo G, Karaman E, et al. Novelties of ultrasound imaging for endometrial cancer preoperative workup. Minerva Med (2021) 112(1):3–11. doi: 10.23736/S0026-4806.20.07125-6
15. Gullo G, Etrusco A, Cucinella G, Perino A, Chiantera V, Laganà AS, et al. Fertility-sparing approach in women affected by stage I and low-grade endometrial carcinoma: An updated overview. Int J Mol Sci (2021) 22(21):11825. doi: 10.3390/ijms222111825
16. Cavaliere AF, Perelli F, Zaami S, D'Indinosante M, Turrini I, Giusti M, et al. Fertility sparing treatments in endometrial cancer patients: The potential role of the new molecular classification. Int J Mol Sci (2021) 22(22):12248. doi: 10.3390/ijms222212248
17. Tanos P, Dimitriou S, Gullo G, Tanos V. Biomolecular and genetic prognostic factors that can facilitate fertility-sparing treatment (FST) decision making in early stage endometrial cancer (ES-EC): A systematic review. Int J Mol Sci (2022) 23(5):2653. doi: 10.3390/ijms23052653
18. Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res (2009) 15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624
19. Anami Y, Minami S, Kumegawa A, Matsukawa H, Nishioka K, Noguchi T, et al. Malignant melanoma treated with pembrolizumab during pregnancy: A case report and review of the literature. Mol Clin Oncol (2021) 15(5):242. doi: 10.3892/mco.2021.2404
20. Xu W, Moor RJ, Walpole ET, Atkinson VG. Pregnancy with successful foetal and maternal outcome in a melanoma patient treated with nivolumab in the first trimester: case report and review of the literature. Melanoma Res (2019) 29(3):333–7. doi: 10.1097/CMR.0000000000000586
21. Bucheit AD, Hardy JT, Szender JB, Glitza Oliva IC. Conception and viable twin pregnancy in a patient with metastatic melanoma while treated with CTLA-4 and PD-1 checkpoint inhibition. Melanoma Res (2020) 30(4):423–5. doi: 10.1097/CMR.0000000000000657
22. Gambichler T, Susok L. Uncomplicated pregnancy and delivery under ongoing nivolumab therapy for metastatic melanoma. Melanoma Res (2022) 32(2):131–2. doi: 10.1097/CMR.0000000000000801
23. Burotto M, Gormaz JG, Samtani S, Valls N, Silva R, Rojas C, et al. Viable pregnancy in a patient with metastatic melanoma treated with double checkpoint immunotherapy. Semin Oncol (2018) 45(3):164–9. doi: 10.1053/j.seminoncol.2018.03.003
24. Haiduk J, Ziemer M. Pregnancy in a patient with metastatic uveal melanoma treated with nivolumab. J Dtsch Dermatol Ges (2021) 19(5):762–5. doi: 10.1111/ddg.14463
25. Le-Nguyen A, Rys RN, Petrogiannis-Haliotis T, Johnson NA. Successful pregnancy and fetal outcome following previous treatment with pembrolizumab for relapsed hodgkin's lymphoma. Cancer Rep (Hoboken) (2022) 5(1):e1432. doi: 10.1002/cnr2.1432
26. Hutson JR, Eastabrook G, Garcia-Bournissen F. Pregnancy outcome after early exposure to nivolumab, a PD-1 checkpoint inhibitor for relapsed hodgkin's lymphoma. Clin Toxicol (Phila) (2022) 60(4):535–6. doi: 10.1080/15563650.2021.1981361
27. Polnaszek B, Mullen M, Bligard K, Raghuraman N, Massad LS. Term pregnancy after complete response of placental site trophoblastic tumor to immunotherapy. Obstet Gynecol (2021) 138(1):115–8. doi: 10.1097/AOG.0000000000004434
28. Andrikopoulou A, Korakiti AM, Apostolidou K, Dimopoulos MA, Zagouri F. Immune checkpoint inhibitor administration during pregnancy: A case series. ESMO Open (2021) 6(5):100262. doi: 10.1016/j.esmoop.2021.100262
29. Poulet FM, Wolf JJ, Herzyk DJ, DeGeorge JJ. An evaluation of the impact of PD-1 pathway blockade on reproductive safety of therapeutic PD-1 inhibitors. Birth Defects Res B Dev Reprod Toxicol (2016) 107(2):108–19. doi: 10.1002/bdrb.21176
30. Borgers J, Heimovaara JH, Cardonick E, Dierickx D, Lambertini M, Haanen J, et al. Immunotherapy for cancer treatment during pregnancy. Lancet Oncol (2021) 22(12):e550–61. doi: 10.1016/S1470-2045(21)00525-8
Keywords: PD-1 inhibitor, organ preservation, lynch syndrome, pregnancy, case report
Citation: Cao D, Gao Y, Zhang R-x, Wang F-l, Li C, Wu M-q, Liu Y-f, Li D-d and Chen G (2022) Case report: Reproductive organ preservation and subsequent pregnancy for an infertility patient with lynch syndrome-associated synchronous endometrial cancer and colon cancer after treatment with a PD-1 checkpoint inhibitor. Front. Immunol. 13:1010490. doi: 10.3389/fimmu.2022.1010490
Received: 03 August 2022; Accepted: 03 October 2022;
Published: 17 October 2022.
Edited by:
Zhaolun Cai, Sichuan University, ChinaReviewed by:
Min Kyu Kim, Sungkyunkwan University, South KoreaCopyright © 2022 Cao, Gao, Zhang, Wang, Li, Wu, Liu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan-dan Li, bGlkZEBzeXN1Y2Mub3JnLmNu; Gong Chen, Y2hlbmdvbmdAc3lzdWNjLm9yZy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.