
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 19 October 2022
Sec. Molecular Innate Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1007382
This article is part of the Research Topic New Insights into the Role of Complement System in Liver Diseases View all 7 articles
As a main producer of complement, the environment in the liver is greatly affected by the complement system. Although the complement system is considered to have the ability of nonself discrimination, remarkable studies have revealed the tight association between improper complement activation in tumour initiation and progression. As complement activation predominantly occurs within the liver, the protumourigenic role of the complement system may contribute to the development of hepatocellular carcinoma (HCC). Improvement in the understanding of the molecular targets involved in complement-mediated tumour development, metastasis, and tumour-promoting inflammation in HCC would certainly aid in the development of better treatments. This minireview is focused on recent findings of the protumourigenic role of the complement system in HCC.
Liver cancer is a global health burden, and hepatocellular carcinoma (HCC), the most common type of liver cancer, is estimated to affect over one million people annually by 2025 (1). Omics-based technologies started the new chapter of the surveillance and treatment of HCC. The combined utilisation of proteomic- and genomic-based biomarkers contributed to the early detection and diagnosis of HCC and provided more options for the better management of HCC patients. Novel therapeutic opportunities have been revealed recently in successful clinical trials using immune checkpoint blockers, indicating that the immune system could be the most promising target to achieve a cure for cancer. However, interpatient variability is the major challenge for immunotherapy in HCC. A thorough understanding of the immune landscape will facilitate the identification of treatment targets and the design of therapeutic strategies.
It is well known that the body utilises the inflammatory system to exclude nonself or dead cells; however, cancer cells have been found to hijack the inflammatory system as a defence mechanism. In the majority of cancer types, cancerous cells become exclusively nonimmunogenic to avoid immunosurveillance. Although avoidance of immune destruction was not included in the six core hallmarks of cancer (2), it has been proven to be correlated with the development of carcinogenesis by numerous studies and should be considered a core hallmark of cancer. In addition, inflammation is involved in every critical step of tumourigenesis (3) and fosters other hallmarks of cancer, such as survival, proliferation and metastasis. Most recently, Douglas Hanahan stated that to better address the complexities of the pathogenesis of cancer, two additional enabling characteristics should be included in the hallmark system, and one of them is tumour-promoting inflammation (4, 5). As an important component of tumour-promoting inflammation, the complement system is involved in the formation of tumours not only by participating in the inflammatory response during tumourigenesis and metastasis but also by actively regulating the adaptive immune response and suppressing the function of T cells (6, 7).
The complement system is a network of soluble serum proteins, membrane-bound receptors and regulatory proteins that interacts with both the innate and adaptive immune system (8). However, there is not much evidence demonstrating the clearance of neoplasms being directly mediated by the complement system. Instead, a tumour-favouring environment arbitrated by the activation of the complement system has been reported (9–12).
The complement system can be activated by three distinct pathways: the classical pathway, lectin pathway and alternative pathway (13, 14) (Figure 1). Both the classical and lectin pathways are activated by the formation of binding complexes (antigen binds to the C1 complex in the classic pathway, and mannose-binding lectin binds to mannose in the lectin pathway), leading to the production of the C3 convertase C4bC2a and ultimately resulting in the assembly of the membrane attach complex (MAC) (15). However, in the alternative pathway, autoactivation occurs by slow hydrolysis of C3. C3 (H2O) then binds factor B to form C3 (H2O) Bb, which functions as the C3 convertase in the alternative pathway. The activation of the central component C3 in all three pathways leads to the complete stimulation of the complement cascade (16). Complement regulators are a group of inhibitors of both soluble (15, 17, 18) and membrane-bound forms (19–21). They regulate the complement system by inactivating proteins involved in the cascade, destroying C3 convertase and modulating MAC formation (22). The involvement of the complement system in HCC has been explored but is not thoroughly understood. There is some evidence indicating the therapeutic possibility of complement components as biomarkers or targets for immunotherapies. The involvement of each complement component in the development of HCC and the related therapeutic implications were comprehensively reviewed by Malik and colleagues in 2018 (23). Here, we summarise the recent findings of the complement system in the different hallmarks of HCC as well as its applications in clinical settings.
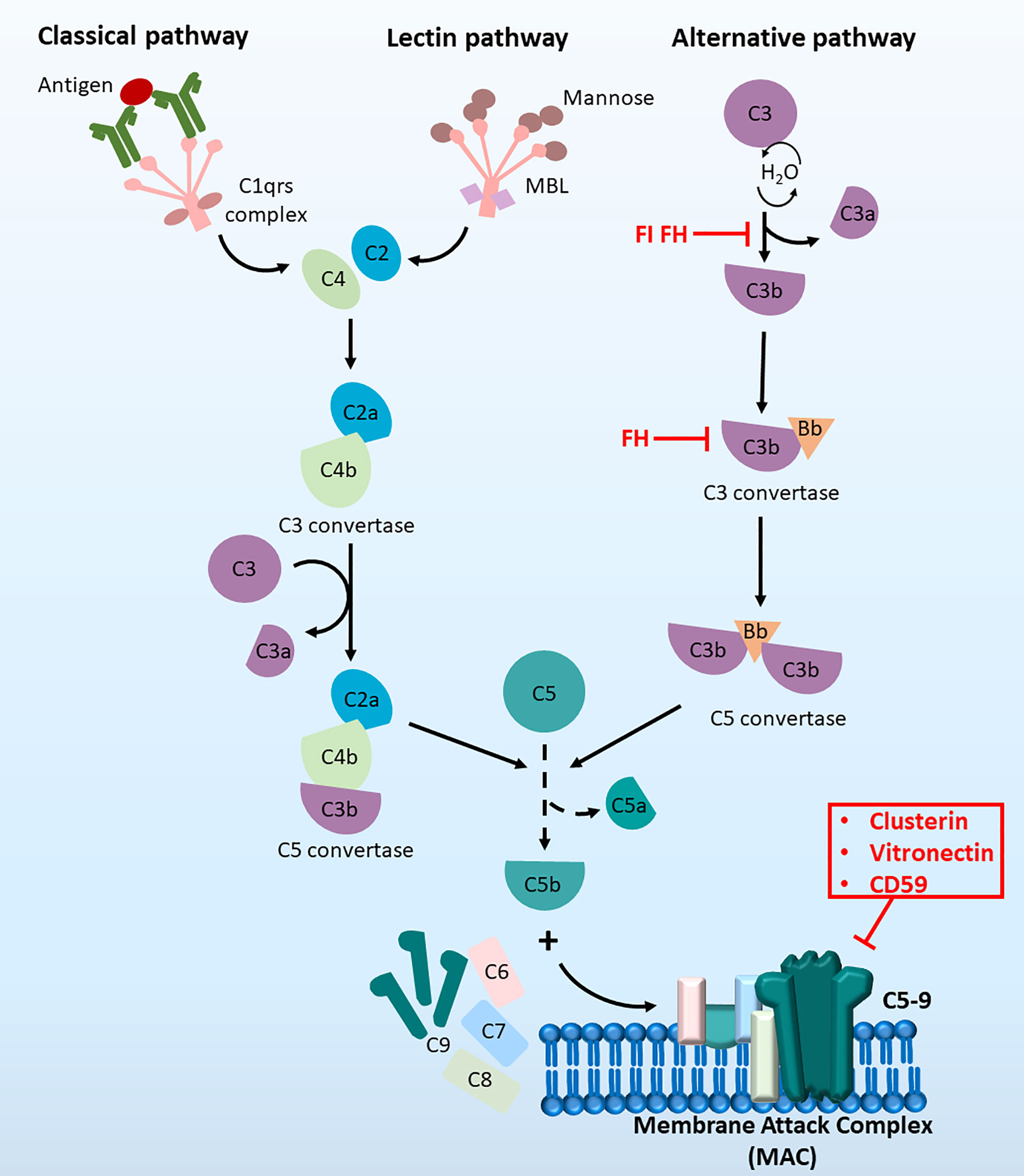
Figure 1 The complement cascade at a glance. The complement cascade can be activated by three pathways, (1) The classical pathway, (2) the lectin pathway and (3) the alternative pathway. Initiation of all three pathways can lead to the formation of C5 convertase to produce C5b, which then recruit C6, C7, C8 and C9 molecules. Together these components can form the terminal membrane attack complex (MAC), which induce cell lysis by inserting pores in cell membranes.
Malignancy develops from a complex biological process in which normal cells transform into cancer cells due to the accumulation of genetic and epigenetic alterations resulting in uncontrolled cell proliferation. The local microenvironment also provides a tumour-favouring niche and protective conditions for the growth of cancer cells. The complement system is generally considered a protective mechanism against tumour formation. Complement factor H-related 3 (CFHR3) is a complement regulator that belongs to the human factor H protein family (24). CFHR3 inhibited HCC proliferation and induced apoptosis via downregulation of Ki67, Bcl-2 and survivin and upregulation of Bax and active caspase-3 in an in vitro model (25). A circulating protein, apoptosis inhibitor of macrophages (AIM), was reported to inhibit steatosis-associated HCC by interfering with the accessibility of regulators of complement activation. By accumulating on the surface of HCC cells and activating complement cascades, AIM eliminated cancer cells by recruiting tumour cell-killing C3. The findings of that study also revealed a novel AIM-based therapeutic strategy for HCC in an animal model (26).
However, more studies revealed a protumourigenic potential of complement in humans. C3a and C5a are derived from the cleavage of complement C3/C5 involved in anaphylatoxic reactions and inflammatory responses via direct damage to the cell membrane or by indirect binding to the G protein-coupled receptor C3aR/C5aR on the cell surface. The inhibition of cell proliferation and epithelial-mesenchymal transition in HCC by downregulation of C3aR/C5aR indicated the critical role of complement in tumourigenesis (27). Complement factor H (CFH) regulates alternative pathway activation via its ability to bind to self-surface ligands. Laskowski and her research team noticed unexpected spontaneous liver tumour formation in CFH-deficient male mice when they studied complement-related kidney disease (28). CFH deficiency-related HCC may occur due to chronic activation of the alternative pathway, which leads to hepatocellular inflammation and injury and subsequent chronic liver damage and steatosis. Our group also reported that CFH-enriched small extracellular vesicles (sEVs) promoted HCC cell growth, migration, and invasiveness and enhanced liver tumour formation in mice (29). Moreover, our study also revealed the role of CFH-enriched sEVs in inhibiting complement-mediated cytotoxicity, thus facilitating the survival and proliferation of HCC cells. Hepatic stellate cells (HSCs) are one of the most important components in the tumour microenvironment of HCC (30). Active HSCs produce abundant cytokines, including C3 convertase, which plays a critical role in HCC (31). HSCs promoted HCC through C3-mediated suppression of dendritic cell differentiation and enhancement of myeloid-derived suppressor cells. In addition, T-cell apoptosis was exacerbated, and the proliferation of CD4+ and CD8+ T cells was inhibited under the influence of C3 produced by HSCs (32).
Liver cancer cells utilise their intrinsic properties to invade adjacent tissues and extravasate the vasculature, metastasize to distant organs and ultimately colonise those organs. The evolved migratory and invasive abilities of liver cancer cells are crucial for the successful liver tumourigenesis and metastasis. Complement participates in the progression of liver cancer and plays a dual role in this process.
All three pathways in the complement cascades converge towards the activation of the major component C3. C3a is generated by C2 cleavage and exhibits a proinflammatory function by binding to C3aR, which is an anaphylatoxin. Using an in vitro cell model, the activation of the C3a/C3aR in complement system was revealed to be involved in aristolochic acid I (AAI)-induced cell migration and invasion in HCC (33). The elevated expression of snail may serve as a downstream effect of C3a/C3aR signalling in AAI-induced cell migration and invasion. In addition to C3a, Lee JH’s team revealed that the first component of the classic pathway of complement, C1q, promotes HCC cell motility and invasiveness by directly binding to the collagen receptor discoidin domain receptor 1 (34). Upregulation of complement component C5a is associated with increased expression of TGFβR3 in HCC (35), and it contributes to poor clinical outcomes and promotes tumour progression by activating tumour-promoting macrophages.
A recent clinical study showed that compared with normal liver tissues, HCC samples contained significantly downregulated complement genes: C1R, C6, C7, CFP, and CFHR3 (36). Moreover, the downregulation of the mentioned complement genes was significantly correlated with overall survival, disease‐free survival, and progression‐free survival as well as advanced cancer stages and higher tumour grades in HCC patients. Using bioinformatics analysis of a dataset collected from The Cancer Genome Atlas database, Liu and colleagues revealed that low CFH expression was associated with poor overall survival and relapse-free survival, suggesting that low CFH expression was an independent predictor of poor prognosis in HCC (37). Significantly lower expression of complement component 2 (C2) was found in HCC patients than in healthy controls, and the expression of C2 was associated with TNM stage. Higher C2 expression was significantly associated with better prognosis, and multivariate analysis showed that C2 was also an independent factor for the prognosis of HCC (38). Complement C8 is a main component of the membrane attack complex, and high expression of one of its subunits, C8B, is correlated with better overall survival and recurrence-free survival in HBV-related HCC patients (39), suggesting that the expression of C8B could serve as a good prognostic indicator in this group of HCC patients. The multiple roles of the complement system in cancer progression make it a good candidate for targeted therapies to improve the management of cancer patients. However, there are inconsistent results from different studies, which may be due to the various parameters adopted, and more clinical and basic experiments are required to provide a clearer picture of the role of complement in HCC.
Cancer stem cells (CSCs) are the most resilient subset of cells that can undergo self-renewal and differentiation. Compared to their non-CSC counterparts, CSCs show an enhanced capacity for self-renewal, metastasis, drug resistance and immune tolerance (40, 41). Cancer cells display dynamic differentiation states, and the plasticity of CSCs relies on their interaction with various components of the tumour microenvironment (42). Given the involvement of the complement system in tumour microenvironment remodelling, complement proteins might also contribute to the maintenance of stemness in HCC. Seol and colleagues found that complement protein C7 and the complement regulatory protein CFH were upregulated in tumorspheres, a type of CSC surrogate, raised from both primary patient-derived liver tumour cells and liver cancer cell lines (43). Mechanistically, C7 and CFH maintained stemness properties and transactivated the expression of stemness genes by upregulating LSF-1. On the one hand, LSF-1 can transactivate the expression of CFH. On the other hand, osteopontin (OPN), another downstream target of LSF-1, can inhibit the lytic activity of the alternative complement pathway by binding to CFH on the cell surface and therefore prevent tumour cells from immune surveillance, indicating the complex interaction between LSF-1 and CFH (44, 45). CSCs share similar characteristics with normal stem cells, such as self-renewability. Transformation of liver progenitor cells was thought to be one of the origins of liver CSCs (46). One of the components of the C1 complex in the classical pathway, C1q, has been recently reported to support the stemness properties of hepatic progenitor cells. In a conditional β-catenin knockout mouse model, the depletion of β-catenin in hepatocytes created an inflammatory environment with increased secretion of complement C1q from macrophages, which in turn activated the β-catenin pathway of periportal hepatic progenitor cells and led to their expansion and dedifferentiation (47). This finding implicates the role of C1q in supporting the self-renewal ability of stem cells through the β-catenin pathway. C1q was also shown to activate the canonical Wnt signalling pathway through binding to frizzled receptors to promote the impairment of muscle regeneration related to ageing, further supporting the role of C1q in activating the Wnt/β-catenin pathway (48). C3a, the active form of C3, has been found to be elevated in HCV-related HCC patients and proposed to be a novel diagnostic marker for HCV-HCC, although its involvement in CSC regulation of HCC remains unknown (49, 50). The role of C3a in stemness has been implicated in cutaneous squamous cell carcinoma (cSCC). In cSCC cell lines and a xenograft model, C3a was reported to activate the expression of SOX2 and to support stemness by binding to its receptor, C3Ar, and activating the Wnt/β-catenin pathway (51). C5aR, the receptor of C5a, was found to be upregulated in HCC cell lines and tissues, which promoted HCC invasiveness by activating ERK1/2 signalling in vitro (52). In human induced pluripotent stem cells (hPSCs), the activation of C5aR1 by C5a stimulated the ERK1/2 signalling pathway and maintained the pluripotency states of OCT-4-positive hPSCs (53). In glioblastoma, C5a secreted from mesenchymal stem-like cells promoted aggressiveness by activating the p38 MAPK/ZEB1 pathway, as demonstrated by cell line models and xenograft models (54). While the role of C1a/C5a and their receptors in CSC maintenance of HCC remains to be explored, the results of these studies implied that anaphylatoxins have a supportive role in regulating stemness pathways and pluripotent genes via complement cascade-independent mechanisms by binding to their corresponding receptors. Elucidation of the role of anaphylatoxins in HCC stemness and exploration of complement cascade-related regulatory mechanisms in CSC maintenance would expedite our understanding of the role of the complement system in HCC pathogenesis.
Activation of the complement cascade is controlled by complement regulatory proteins (CRPs). Clusterin, one of the CRPs, has been shown to promote the CSC properties of HCC, including chemoresistance, metastasis and tumourigenesis, by activating the AKT/GSK-3β/β-catenin axis, as demonstrated by in vitro and in vivo assays (55). Clinically, coexpression of clusterin and β-catenin predicted poor survival. Consistently, by using HCC cell line models, Zhong and colleagues reported that suppression of clusterin sensitized HCC cells to sorafenib treatment by targeting ERK1/2 signalling (56). However, whether clusterin maintains the CSC phenotypes of HCC via suppression of complement cascade activation remains to be explored.
The complement system is a conventional defence mechanism that links the innate immune response to the adaptive immune response. Activation of complement proteins was thought to be a tumour surveillance mechanism, given the fundamental role of complement proteins against noxious pathogens and the clinical benefit of mAb-based immunotherapy by triggering complement-dependent cytotoxicity towards tumour cells (57, 58). However, studies in recent years have realised that the aberrant activation of the complement system plays an important role in creating an immunosuppressive tumour microenvironment by recruiting immunosuppressive immune cells, inducing immune cell differentiation, upregulating the expression of immune checkpoint molecules and suppressing T-cell toxicity.
Upregulation of C3- and C5-related complement components facilitates the creation of an immune-suppressive microenvironment. The active forms of complement proteins C3 and C5 (C3a and C5a) are potent anaphylatoxins that recruit immune cells, indicating their potential involvement in promoting immune cell infiltration in the tumour microenvironment. A pan cancer multiomics analysis showed that the expression of C3/C5/C3AR1/C5AR1 is associated with the immune evasion signature, indicating the possible immune modulating role of complement proteins in HCC (59). Myeloid-derived suppressor cells (MDSCs) are suppressive immune cells that protect cancer cells from T-cell toxicity. In both an in vitro model and an orthotopic HCC transplantation model, C3 from hepatic stellate cells was found to create an immune-suppressive microenvironment by inducing the expansion of MDSCs and promoting the apoptosis of T cells, which facilitated the development of HCC (32). In an orthotopic HCC mouse model, Wang and colleagues reported that PIWIL1-mediated increased secretion of C3 from HCC promoted the infiltration of MDSCs in the tumour microenvironment of HCC via the p38/MAPK pathway, which suppressed T-cell proliferation and facilitated HCC development (60). C3a has also been reported to recruit immune suppressive macrophages and neutrophils in other cancers. Tumour-derived C3a was reported to polarize tumour-associated macrophages (TAMs) towards an immunosuppressive M2-like phenotype and to mediate the suppression of T-cell proliferation through the C3a-C3aR axis in a syngeneic mouse model derived from mouse melanoma and in colon and lung cancer cells. Depletion of C3 in tumour cells sensitizes the tumour to anti-PDL1 treatment (61). Tumour-associated neutrophils (TANs) have been known to have protumourigenic effects by mediating immunosuppression to promote metastasis. Hsu et al. reported that C3a promoted the infiltration of immature low-density neutrophils (iLDNs), a protumourigenic neutrophil subtype, in breast cancer-derived liver metastases through the C3a-C3aR axis, as demonstrated in a liver metastasis mouse model created by intrasplenic injection of breast cancer cells (62). These findings imply the potential role of C3a in modulating macrophage and neutrophil function in HCC. While little is known about the immune modulating role of C5/C5a in HCC, the impact of C5/C5a on the immune microenvironment has been evident in other cancers. In colorectal cancer (CRC), C5a/C5aR1 promoted the initiation of CTCs by recruiting MDSCs and impairing CD8+ T-cell function (63). C5aR deficiency in mice impaired the liver metastasis of colon cancer by inhibiting the M2 polarisation of TAMs. In contrast, C5a stimulated the M2 polarisation of TAMs through C5aR/NF-κB (64). In clinical prostate cancer tissue, the expression of C5aR was upregulated, which correlated with the expression level of PD-L1. Treatment of prostate cancer cells with C5a stimulated PD-L1 expression (65). C5a and C5aR have been reported to have protumourigenic roles in HCC, indicating their potential involvement in immunomodulation of HCC (52).
In contrast to the possible immune suppressive role of C3 and C5, the expression of some of the complement components might be negatively correlated with the immune suppressive microenvironment. By analysing transcriptomic HCC data from the TCGA database, complement C2 was found to be downregulated in HCC. High expression of C2 was correlated with better HCC survival, with an increased infiltration of CD4+ T cells, while a low level of C2 expression was correlated with M0 macrophage infiltration (38). Since reduced infiltration of CD4+ cytotoxic T cells was correlated with poor survival of HCC (66) and the potential polarisation of M0 macrophages into protumourigenic M2 macrophages, downregulation of C2 may have an immunosuppressive role in HCC. Mannose-binding lectin (MBL) is an activator of the lectin pathway of the complement system. Using an orthotopic HCC model generated from MBL knockout mice, Li et al. reported that MBL deficiency in mice facilitated HCC tumourigenesis and increased MDSC and Treg infiltration with a reduced percentage of IFN-γ+CD8+ T cells. Mechanistically, MBL suppressed HCC progression by interacting with HSCs, thus preventing their activation by downregulating the ERK/COX-2/PGE2 pathway (67).
Collectively, dysregulation of the complement system exerts an immune-modulating effect during the progression of HCC. A better understanding of the regulatory mechanism of the complement system on the immune microenvironment of HCC would help to discover novel treatment strategies for HCC patients.
The aberrant expression of proteins in the complement system plays a complex role in the development of HCC by affecting multiple properties of cancer cells with both protumourigenic and antitumourigenic effects. The expression levels of C1q, C3/C3a, C5/C5a and the regulatory protein clusterin were upregulated in HCC, and this upregulation is responsible for aggressive tumour phenotypes, including tumourigenesis, metastasis, stemness and immune suppression, indicating the potential of these molecules as biomarkers and therapeutic targets for HCC. C1R, C2, C6, C8, MBL, CFP and CFHR were downregulated in HCC and exhibited tumour-suppressive effects; thus, they could serve as prognostic markers for HCC. However, the roles of some complement proteins, including C7 and CFH, are controversial. Some studies have reported their downregulation and antitumourigenic role in HCC, while others have illustrated their roles in supporting the invasiveness and stemness of cancer cells; thus, they have differential roles in the progression of HCC (Figure 2). While our knowledge of the detailed regulatory mechanisms of complement proteins in the pathogenesis of HCC is still limited, the reported findings implied multifaceted roles of the complement system in HCC. A deeper understanding of the mechanistic interaction between the complement system and HCC would foster the development of a novel therapeutic strategy for HCC targeting complement, either as a single treatment or in combination with traditional chemotherapies, targeted therapies or immunotherapies.
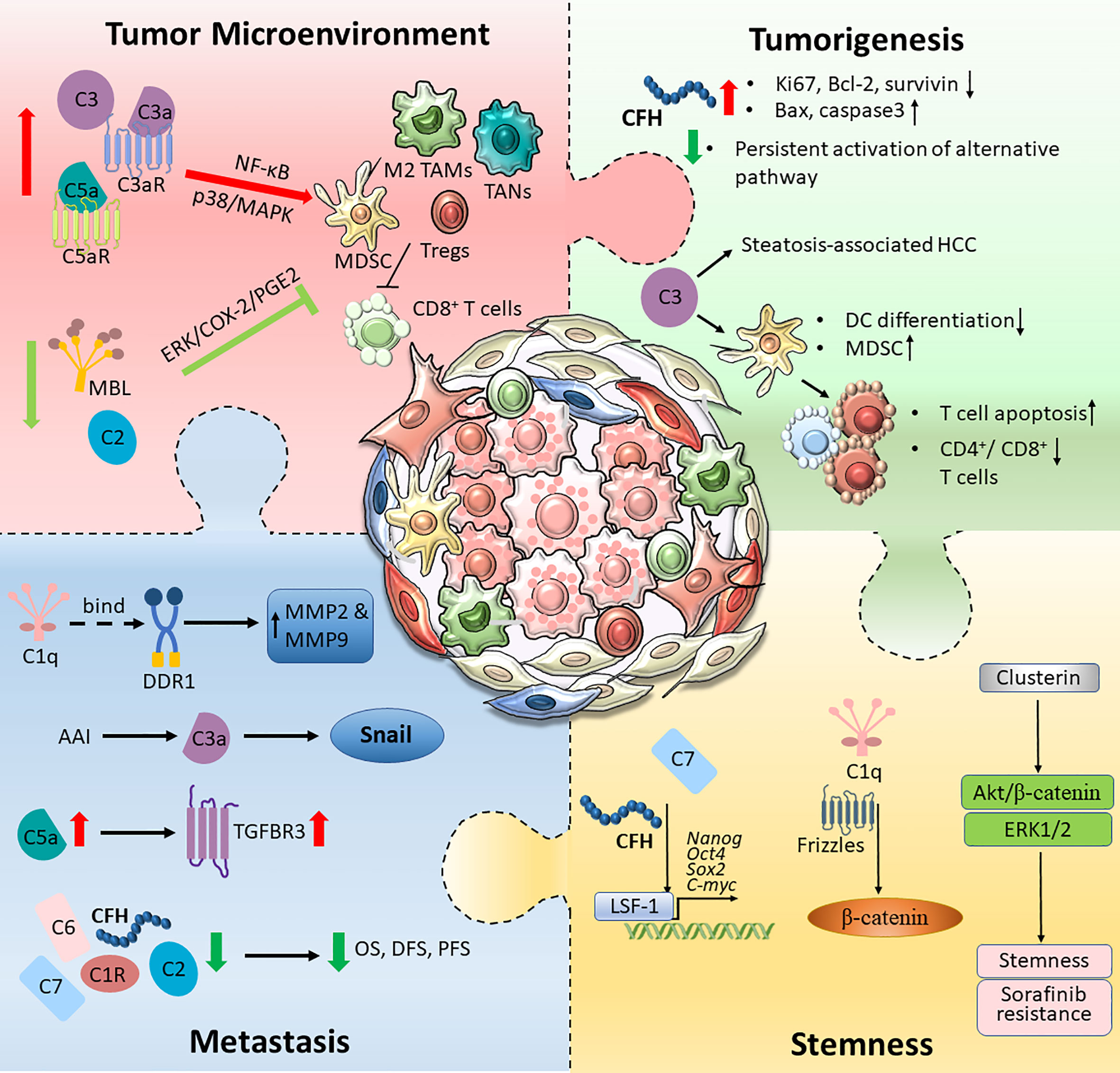
Figure 2 An overview of the complement activation in HCC tumour microenvironment. Tumour cells are capable of hijacking the immune system to cultivate a favourable TME. Different components of the complement pathway were found to be triggered and modified, forming an immunosuppressive environment. The schematic illustration summarised intricate interactions between tumour cells and the complement system, which promotes HCC tumourigenesis, metastatic and stemness.
ZJX contributed to the conception and design of the article, drafted the article and interpreting the relevant literature. CLSY drafted the figures and interpreting the relevant literature. JWPY revised the article critically for important intellectual content. XWM contributed to the conception and design of the article, drafted the article and interpreting the relevant literature. All authors contributed to the article and approved the submitted version.
National Natural Science Fund under National Natural Science Foundation of China [Project no. 81872340]. Research Start-up Fund of the Seventh Affiliated Hospital, Sun Yat-sen University [Project no. ZSQYBRJH0023].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Renne SL, Sarcognato S, Sacchi D, Guido M, Roncalli M, Terracciano L, et al. Hepatocellular carcinoma: a clinical and pathological overview. Pathologica (2021) 113(3):203–17. doi: 10.32074/1591-951X-295
2. Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer (2007) 7(2):139–47. doi: 10.1038/nrc2067
3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
4. Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discov (2022) 12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059
5. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
6. Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity (2012) 37(2):199–207. doi: 10.1016/j.immuni.2012.08.002
7. Coussens LM, Werb Z. Inflammation and cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322
8. Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I - molecular mechanisms of activation and regulation. Front Immunol (2015) 6:262. doi: 10.3389/fimmu.2015.00262
9. Agostinis C, Vidergar R, Belmonte B, Mangogna A, Amadio L, Geri P, et al. Complement protein C1q binds to hyaluronic acid in the malignant pleural mesothelioma microenvironment and promotes tumor growth. Front Immunol (2017) 8:1559. doi: 10.3389/fimmu.2017.01559
10. Markiewski MM, Lambris JD. Unwelcome complement. Cancer Res (2009) 69(16):6367–70. doi: 10.1158/0008-5472.CAN-09-1918
11. Ouyang Q, Zhang L, Jiang Y, Ni X, Chen S, Ye F, et al. The membrane complement regulatory protein CD59 promotes tumor growth and predicts poor prognosis in breast cancer. Int J Oncol (2016) 48(5):2015–24. doi: 10.3892/ijo.2016.3408
12. Riihila P, Nissinen L, Farshchian M, Kallajoki M, Kivisaari A, Meri S, et al. Complement component C3 and complement factor b promote growth of cutaneous squamous cell carcinoma. Am J Pathol (2017) 187(5):1186–97. doi: 10.1016/j.ajpath.2017.01.006
13. Walport MJ. Complement. first of two parts. N Engl J Med (2001) 344(14):1058–66. doi: 10.1056/NEJM200104053441406
14. Walport MJ. Complement. second of two parts. N Engl J Med (2001) 344(15):1140–4. doi: 10.1056/NEJM200104123441506
15. Blom AM, Villoutreix BO, Dahlback B. Complement inhibitor C4b-binding protein-friend or foe in the innate immune system? Mol Immunol (2004) 40(18):1333–46. doi: 10.1016/j.molimm.2003.12.002
16. Reis ES, Mastellos DC, Hajishengallis G, Lambris JD. New insights into the immune functions of complement. Nat Rev Immunol (2019) 19(8):503–16. doi: 10.1038/s41577-019-0168-x
17. Davis AE, Mejia P 3rd, Lu F. Biological activities of C1 inhibitor. Mol Immunol (2008) 45(16):4057–63. doi: 10.1016/j.molimm.2008.06.028
18. Jozsi M, Zipfel PF. Factor h family proteins and human diseases. Trends Immunol (2008) 29(8):380–7. doi: 10.1016/j.it.2008.04.008
19. Fischer E, Appay MD, Cook J, Kazatchkine MD. Characterization of the human glomerular C3 receptor as the C3b/C4b complement type one (CR1) receptor. J Immunol (1986) 136(4):1373–7.
20. Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol (2002) 3(7):659–66. doi: 10.1038/ni810
21. Abbott RJ, Spendlove I, Roversi P, Fitzgibbon H, Knott V, Teriete P, et al. Structural and functional characterization of a novel T cell receptor co-regulatory protein complex, CD97-CD55. J Biol Chem (2007) 282(30):22023–32. doi: 10.1074/jbc.M702588200
22. Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol (2013) 33(6):479–92. doi: 10.1016/j.semnephrol.2013.08.001
23. Malik A, Thanekar U, Amarachintha S, Mourya R, Nalluri S, Bondoc A, et al. “Complimenting the complement”: Mechanistic insights and opportunities for therapeutics in hepatocellular carcinoma. Front Oncol (2020) 10:627701. doi: 10.3389/fonc.2020.627701
24. Zipfel PF, Skerka C, Hellwage J, Jokiranta ST, Meri S, Brade V, et al. Factor h family proteins: on complement, microbes and human diseases. Biochem Soc Trans (2002) 30(Pt 6):971–8. doi: 10.1042/bst0300971
25. Liu H, Zhang L, Wang P. Complement factor hrelated 3 overexpression affects hepatocellular carcinoma proliferation and apoptosis. Mol Med Rep (2019) 20(3):2694–702. doi: 10.3892/mmr.2019.10514
26. Maehara N, Arai S, Mori M, Iwamura Y, Kurokawa J, Kai T, et al. Circulating AIM prevents hepatocellular carcinoma through complement activation. Cell Rep (2014) 9(1):61–74. doi: 10.1016/j.celrep.2014.08.058
27. Chen B, Zhou W, Tang C, Wang G, Yuan P, Zhang Y, et al. Down-regulation of C3aR/C5aR inhibits cell proliferation and EMT in hepatocellular carcinoma. Technol Cancer Res Treat (2020) 19:1533033820970668. doi: 10.1177/1533033820970668
28. Laskowski J, Renner B, Pickering MC, Serkova NJ, Smith-Jones PM, Clambey ET, et al. Complement factor h-deficient mice develop spontaneous hepatic tumors. J Clin Invest (2020) 130(8):4039–54. doi: 10.1172/JCI135105
29. Mao X, Zhou L, Tey SK, Ma APY, Yeung CLS, Ng TH, et al. Tumour extracellular vesicle-derived complement factor h promotes tumorigenesis and metastasis by inhibiting complement-dependent cytotoxicity of tumour cells. J Extracell Vesicles (2020) 10(1):e12031. doi: 10.1002/jev2.12031
30. Eggert T, Greten TF. Tumor regulation of the tissue environment in the liver. Pharmacol Ther (2017) 173:47–57. doi: 10.1016/j.pharmthera.2017.02.005
31. Hsieh CC, Chou HS, Yang HR, Lin F, Bhatt S, Qin J, et al. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood (2013) 121(10):1760–8. doi: 10.1182/blood-2012-06-440214
32. Xu Y, Huang Y, Xu W, Zheng X, Yi X, Huang L, et al. Activated hepatic stellate cells (HSCs) exert immunosuppressive effects in hepatocellular carcinoma by producing complement C3. Onco Targets Ther (2020) 13:1497–505. doi: 10.2147/OTT.S234920
33. Li Y, Zhu S, Xue M, Jing Y, Liu X, Cai D, et al. Aristolochic acid I promotes the invasion and migration of hepatocellular carcinoma cells by activating the C3a/C3aR complement system. Toxicol Lett (2020). doi: 10.1016/j.toxlet.2020.08.014
34. Lee JH, Poudel B, Ki HH, Nepali S, Lee YM, Shin JS, et al. Complement C1q stimulates the progression of hepatocellular tumor through the activation of discoidin domain receptor 1. Sci Rep (2018) 8(1):4908. doi: 10.1038/s41598-018-23240-6
35. Yeung OWH, Qi X, Pang L, Liu H, Ng KTP, Liu J, et al. Type III TGF-beta receptor down-regulation promoted tumor progression via complement component C5a induction in hepatocellular carcinoma. Cancers (Basel) (2021) 13(7). doi: 10.3390/cancers13071503
36. Qian X, Yang Z, Gao L, Liu Y, Yan J. The role of complement in the clinical course of hepatocellular carcinoma. Immun Inflamm Dis (2022) 10(3):e569. doi: 10.1002/iid3.569
37. Liu J, Li W, Zhao H. CFHR3 is a potential novel biomarker for hepatocellular carcinoma. J Cell Biochem (2020) 121(4):2970–80. doi: 10.1002/jcb.29551
38. Ning G, Huang YL, Zhen LM, Xu WX, Li XJ, Wu LN, et al. Prognostic value of complement component 2 and its correlation with immune infiltrates in hepatocellular carcinoma. BioMed Res Int (2020) 2020:3765937. doi: 10.1155/2020/3765937
39. Zhang Y, Chen X, Cao Y, Yang Z. C8B in complement and coagulation cascades signaling pathway is a predictor for survival in HBV-related hepatocellular carcinoma patients. Cancer Manag Res (2021) 13:3503–15. doi: 10.2147/CMAR.S302917
40. Batlle E, Clevers H. Cancer stem cells revisited. Nat Med (2017) 23(10):1124–34. doi: 10.1038/nm.4409
41. Bayik D, Lathia JD. Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer (2021) 21(8):526–36. doi: 10.1038/s41568-021-00366-w
42. Prager BC, Xie Q, Bao S, Rich JN. Cancer stem cells: The architects of the tumor ecosystem. Cell Stem Cell (2019) 24(1):41–53. doi: 10.1016/j.stem.2018.12.009
43. Seol HS, Lee SE, Song JS, Rhee JK, Singh SR, Chang S, et al. Complement proteins C7 and CFH control the stemness of liver cancer cells via LSF-1. Cancer Lett (2016) 372(1):24–35. doi: 10.1016/j.canlet.2015.12.005
44. Jain A, Karadag A, Fohr B, Fisher LW, Fedarko NS. Three SIBLINGs (small integrin-binding ligand, n-linked glycoproteins) enhance factor h’s cofactor activity enabling MCP-like cellular evasion of complement-mediated attack. J Biol Chem (2002) 277(16):13700–8. doi: 10.1074/jbc.M110757200
45. Santhekadur PK, Rajasekaran D, Siddiq A, Gredler R, Chen D, Schaus SE, et al. The transcription factor LSF: a novel oncogene for hepatocellular carcinoma. Am J Cancer Res (2012) 2(3):269–85.
46. Nio K, Yamashita T, Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer (2017) 16(1):4. doi: 10.1186/s12943-016-0572-9
47. Ho TC, Wang EY, Yeh KH, Jeng YM, Horng JH, Wu LL, et al. Complement C1q mediates the expansion of periportal hepatic progenitor cells in senescence-associated inflammatory liver. Proc Natl Acad Sci USA (2020) 117(12):6717–25. doi: 10.1073/pnas.1918028117
48. Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, et al. Complement C1q activates canonical wnt signaling and promotes aging-related phenotypes. Cell. (2012) 149(6):1298–313. doi: 10.1016/j.cell.2012.03.047
49. Lee IN, Chen CH, Sheu JC, Lee HS, Huang GT, Chen DS, et al. Identification of complement C3a as a candidate biomarker in human chronic hepatitis c and HCV-related hepatocellular carcinoma using a proteomics approach. Proteomics (2006) 6(9):2865–73. doi: 10.1002/pmic.200500488
50. Kanmura S, Uto H, Sato Y, Kumagai K, Sasaki F, Moriuchi A, et al. The complement component C3a fragment is a potential biomarker for hepatitis c virus-related hepatocellular carcinoma. J Gastroenterol (2010) 45(4):459–67. doi: 10.1007/s00535-009-0160-5
51. Fan Z, Qin J, Wang D, Geng S. Complement C3a promotes proliferation, migration and stemness in cutaneous squamous cell carcinoma. J Cell Mol Med (2019) 23(5):3097–107. doi: 10.1111/jcmm.13959
52. Hu WH, Hu Z, Shen X, Dong LY, Zhou WZ, Yu XX. C5a receptor enhances hepatocellular carcinoma cell invasiveness via activating ERK1/2-mediated epithelial-mesenchymal transition. Exp Mol Pathol (2016) 100(1):101–8. doi: 10.1016/j.yexmp.2015.10.001
53. Hawksworth OA, Coulthard LG, Taylor SM, Wolvetang EJ, Woodruff TM. Brief report: complement C5a promotes human embryonic stem cell pluripotency in the absence of FGF2. Stem Cells (2014) 32(12):3278–84. doi: 10.1002/stem.1801
54. Lim EJ, Kim S, Oh Y, Suh Y, Kaushik N, Lee JH, et al. Crosstalk between GBM cells and mesenchymal stemlike cells promotes the invasiveness of GBM through the C5a/p38/ZEB1 axis. Neuro Oncol (2020) 22(10):1452–62. doi: 10.1093/neuonc/noaa064
55. Zheng W, Yao M, Wu M, Yang J, Yao D, Wang L. Secretory clusterin promotes hepatocellular carcinoma progression by facilitating cancer stem cell properties via AKT/GSK-3beta/beta-catenin axis. J Transl Med (2020) 18(1):81. doi: 10.1186/s12967-020-02262-7
56. Zhong J, Yu X, Dong X, Lu H, Zhou W, Li L, et al. Downregulation of secreted clusterin potentiates the lethality of sorafenib in hepatocellular carcinoma in association with the inhibition of ERK1/2 signals. Int J Mol Med (2018) 41(5):2893–900. doi: 10.3892/ijmm.2018.3463
57. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol (2010) 11(9):785–97. doi: 10.1038/ni.1923
58. Taylor RP, Lindorfer MA. Cytotoxic mechanisms of immunotherapy: Harnessing complement in the action of anti-tumor monoclonal antibodies. Semin Immunol (2016) 28(3):309–16. doi: 10.1016/j.smim.2016.03.003
59. Lawal B, Tseng SH, Olugbodi JO, Iamsaard S, Ilesanmi OB, Mahmoud MH, et al. Pan-cancer analysis of immune complement signature C3/C5/C3AR1/C5AR1 in association with tumor immune evasion and therapy resistance. Cancers (Basel) (2021) 13(16). doi: 10.3390/cancers13164124
60. Wang N, Tan HY, Lu Y, Chan YT, Wang D, Guo W, et al. PIWIL1 governs the crosstalk of cancer cell metabolism and immunosuppressive microenvironment in hepatocellular carcinoma. Signal Transduct Target Ther (2021) 6(1):86. doi: 10.1038/s41392-021-00485-8
61. Zha H, Wang X, Zhu Y, Chen D, Han X, Yang F, et al. Intracellular activation of complement C3 leads to PD-L1 antibody treatment resistance by modulating tumor-associated macrophages. Cancer Immunol Res (2019) 7(2):193–207. doi: 10.1158/2326-6066.CIR-18-0272
62. Hsu BE, Roy J, Mouhanna J, Rayes RF, Ramsay L, Tabaries S, et al. C3a elicits unique migratory responses in immature low-density neutrophils. Oncogene. (2020) 39(12):2612–23. doi: 10.1038/s41388-020-1169-8
63. Ding P, Li L, Li L, Lv X, Zhou D, Wang Q, et al. C5aR1 is a master regulator in colorectal tumorigenesis via immune modulation. Theranostics. (2020) 10(19):8619–32. doi: 10.7150/thno.45058
64. Piao C, Zhang WM, Li TT, Zhang CC, Qiu S, Liu Y, et al. Complement 5a stimulates macrophage polarization and contributes to tumor metastases of colon cancer. Exp Cell Res (2018) 366(2):127–38. doi: 10.1016/j.yexcr.2018.03.009
65. Imamura R, Kitagawa S, Kubo T, Irie A, Kariu T, Yoneda M, et al. Prostate cancer C5a receptor expression and augmentation of cancer cell proliferation, invasion, and PD-L1 expression by C5a. Prostate. (2021) 81(3):147–56. doi: 10.1002/pros.24090
66. Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J, et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. (2013) 58(1):139–49. doi: 10.1002/hep.26054
Keywords: complement, hepatocellular carcinoma, tumourigenesis, metastasis, tumour microenvironment, stemness
Citation: Xiao ZJ, Yeung CLS, Yam JWP and Mao XP (2022) An update on the role of complement in hepatocellular carcinoma. Front. Immunol. 13:1007382. doi: 10.3389/fimmu.2022.1007382
Received: 30 July 2022; Accepted: 26 September 2022;
Published: 19 October 2022.
Edited by:
Guandou Yuan, The First Affiliated Hospital of Guangxi Medical University, ChinaReviewed by:
Kyle Poulsen, University of Texas Health Science Center at Houston, United StatesCopyright © 2022 Xiao, Yeung, Yam and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowen Mao, c3VzYW5tYW9AcGF0aG9sb2d5LmhrdS5oaw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.